Abstract
We developed a thermal-gelling, erodible hydrogel system for localized delivery of viable mitochondria in vivo, as well as labeled transplanted mitochondria with specific dyes and/or genetically modified mitochondria tagged with red fluorescence protein (RFP). We also employed cell lines to optimize a hydrogel composed of methylcellulose and hyaluronic acid designed to preserve bioenergetics while facilitating mitochondrial release. We further investigated how transplantation of allogeneic or xenogeneic mitochondria into respective cell lines affects host cellular metabolism, as measured by MTS assay. We found that 70% of mitochondria are released from the hydrogel within 20 min at 37 °C, that the respiratory capacity of hydrogel-released mitochondria over 60 min was greater than those without gel, and that MTR-labeling of mitochondria is not indelible. RFP-tagged transgenic mitochondria isolated from modified SH-SY5Y human neuroblastoma cells showed effective uptake into both naïve SH-SY5Y cells and rat PC-12 cells, notably when released from hydrogel. The hydrogel both protected the mitochondria at physiological conditions in vitro while solidifying and diffusing within 60 min locally in situ. To assess metabolic effects, both cell lines were transplanted with different concentrations of SH-SY5Y or PC-12 cell line-derived mitochondria and all resulted in significant increases in metabolism at 6- and 24-hour after transplantation. Alternatively, transplanted mitochondria at highest concentration from rat brain and spinal cord tissues reduced metabolic activities after 24-hour. Along with hydrogel refinements, we are further investigating whether such metabolic changes are due to alterations in cell proliferation or the number of exogenous mitochondria incorporated into individual host cells.
Keywords: Mitochondrial transplantation, MTR, MTS, Metabolism, Hydrogel, Spinal cord
1. Introduction
Our collective work in pre-clinical neurotrauma therapeutics has tested and evaluated, in particular, pharmacological agents or delivery techniques that target more directly the mitochondrion, the origin(s) of oxidative damage in the injured central nervous system (CNS). We have recently reviewed evidence that ‘MitoCeuticals’ promote neuroprotection following both traumatic brain and spinal cord injury (TBI and SCI) (Rabchevsky et al., 2020; Semple, 2014). Alternatively, the evolution of techniques for mitochondrial isolation and transplantation has led to strategies for replacing damaged or dysfunctional ones with exogenous healthy mitochondria (see (Gollihue and Rabchevsky, 2017)). This is based on evidence that mitochondria released into extracellular space after acute CNS injury are transferred among cells to support oxidative phosphorylation in recipient cells (Hayakawa et al., 2018; Hayakawa et al., 2016; Nakamura et al., 2020). This concept has been extended to explain how transplanted cells (such as stem-cell therapy) may manifest their effects by “donating” mitochondria to compromised host cells, especially in light of generally poor graft cell survival. The mode of transplantation, however, has been approached in different ways, by 1) direct injection of exogenous mitochondria into injured tissues (Cowan et al., 2016; Gollihue et al., 2018; Gollihue et al., 2017; Hayakawa et al., 2016; Masuzawa et al., 2013; McCully et al., 2009), 2) utilizing cell-to-cell contact and transfer (Islam et al., 2012), or 3) vesicle mediated cell–cell donation (Hayakawa et al., 2016). Notably, while mitochondrial transplantation into contused rat spinal cords significantly maintains acute bioenergetics, such early preservation did not manifest in long-term functional recovery or tissue sparing (Gollihue et al., 2018).
It remains equivocal whether direct focal circumferential injections of mitochondria, alone, in the parenchymal penumbra of contusion sites is adequate to maintain sufficient bioenergetic integrity in the injured spinal cord to spare compromised tissues (Gollihue et al., 2018). Moreover, the intraspinal injection boluses compromised localized local tissue, though not functional recovery (Gollihue et al., 2018). We are, therefore, currently developing thermogelling erodible hydrogels to protect the isolated mitochondria from the extracellular environment while being delivered in a controlled manner to the injured spinal cord, as premise for delivering mitochondria intrathecally after SCI. Herein, we transplanted different concentrations of allogeneic or xenogeneic mitochondria into and from two different cell lines or tissue sources to assess the effects of host cell metabolism. We further assessed the ability of hydrogels to release and maintain integrity of isolated mitochondria. Collectively, we present data describing the properties of the hydrogels, the equivocation of voltage-dependent dyes used to indelibly label and track transplanted mitochondria in host cells, the ability of thermogelling hydrogel to maintain mitochondrial integrity ex vivo and release in situ, and that mitochondria transplantation in vitro increases the metabolism of recipient cell lines, according to their source.
2. Methods
2.1. Cell culture
SH-SY5Y human neuroblastoma cells, with and without RFP-tagged mitochondria (gifted by Dr. Joe Springer, SCoBIRC, University of Kentucky), were cultured individually with Dulbecco’s modified eagle medium (DMEM) (Thermofisher, Massachusetts, USA), 10% fetal bovine serum (FBS) (R&D systems, Minneapolis, USA) and Penicillin-Streptomycin antibiotic solution (100 μg/ml) (Corning Inc. Corning, NY). Adherent variant of rat pheochromocytoma cells (PC-12 cells) (PC-12 Adh ATCC CRL-1721.1) ATCC Manassas, VA) were maintained in F12K medium (Thermofisher, Waltham, MA) with 15% horse serum (Thermofisher, Massachusetts, USA), 2.5% FBS and Penicillin-Streptomycin antibiotic solution (100 μg/ml).
2.2. Mitochondrial isolation
Cell culture-derived mitochondria were isolated from SH-SY5Y and PC-12 cells, as described with modification (Gollihue et al., 2017). Briefly, 95% confluent cells were manually dissociated and collected from the plate by using a cell scrapper and washed in mitochondria isolation buffer (1 mM EGTA, 215 mM mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, and pH adjusted to 7.2 with KOH) and centrifuged at 1300 rcf for 3 min to remove media and serum. Essential ingredients used in isolation buffer are mannitol and sucrose to maintain osmolarity, and HEPES to maintain pH; which is similar to the buffers used in ischemia models with slight modifications (McCully et al., 2022). EGTA is used in isolation buffer as calcium chelator to prevent possible influx of calcium into mitochondria in order to maintain their viability during isolation. Mannitol and sucrose help maintain osmolarity and HEPES maintains pH. EGTA is used in isolation buffer as calcium chelator to prevent possible influx of calcium into mitochondria in order to maintain their viability during isolation. Mannitol and sucrose help maintain osmolarity and HEPES maintains pH. The cell membrane was then disrupted by incubating the cells in a pressure chamber filled with nitrogen gas at 1200 psi for 10 min (nitrogen bombing), followed by passing them through a 27G × ½” needle thrice. The procedure was repeated 4 times to release maximum mitochondria from cells to increase yield. The cellular debris was removed by centrifugation at low speed 1500 rcf for 3 min. The supernatant with the mitochondria was collected in a separate tube and pelleted by centrifugation at 13,000 rcf for 10 min. The resultant mitochondrial pellet was resuspended and further purified by passing through a discontinuous ficoll gradient (7.5% and 10%) by ultracentrifugation at 32000 rpm for 30 min at 4 °C. To remove ficoll, the purified mitochondria were washed and resuspended in EGTA-free isolation buffer to prevent EGTA’s influence on host cells before quantifying using Pierce™ BCA Protein Assay Kit (Thermofisher, Massachusetts, USA) according to manufacturer’s recommendations. Alternatively, isolating mitochondria from rat brain, spinal cord and soleus muscle (female Sprague–Dawley rats (n = 4) (Harlan Labs, IN) weighing 225–250g) was performed using mitochondrial isolation buffer and purified in a discontinuous ficoll gradient (7.5% and 10%) (Sigma Aldrich St. Louis, MO), as we’ve reported (Gollihue et al., 2018; Patel et al., 2009a; Patel et al., 2010; Patel et al., 2009b). Prior to transplantation, viability of isolated mitochondrial samples was confirmed by assessing their respiratory capacity using Seahorse Bioscience XFe24 Extracellular Flux Analyzer (Agilent Technology, Santa Clara, CA, USA), as described previously (Patel et al., 2014; Sauerbeck et al., 2011).
3. HA-MC hydrogel preparation:
HA-MC hydrogel was prepared using a modified procedure (Delplace et al., 2019). Sodium hyaluronate (Acros Organics, Geel, Belgium) and methylcellulose (Tokyo Chemical Industry, Chikusei, Japan) were mixed with mitochondrial isolation buffer to make the hydrogel using a Vortex Mixer (Fisher Scientific, Waltham, MA) until a homogeneous gel solution was obtained, which was then kept at 4 °C for 30 min. Afterwards, the mixture was centrifuged at 12,000 rpm for 2 min to flatten the gel solution. Solution was again stirred and mixed with a spatula and kept at 4 °C for 30 min. The mixing, centrifuging, and refrigeration process was repeated two more times. A metal spatula was used after each mixing process to manually mix the bottom of the tube, ensuring homogeneity. After the third refrigeration cycle any layer of foam on top of the gel was discarded and the gel stored at 4 °C.
4. Parallel plate shear rheology:
To perform a rheological analysis assessing the thermal gelation points of the material, a Discovery HR-2 Rheometer (TA Instruments) was used to perform a temperature sweep from 0 °C to 50 °C with 5 °C temperature steps and a 10 s soak time on 25 mm parallel plates. Soak time is defined here as the waiting time before taking a measurement once the desired temperature is reached. The angular frequency and strain were held constant at 0.1 Hz and 1%, respectively. 0.5 mL of gel was injected between the plates with a 1 mm gap. Excess gel was removed before testing. The storage modulus (G’), loss modulus (G”), and loss tangent (tan δ) were measured as a function of temperature. The loss tangent is defined as the ratio of the loss modulus to the storage modulus, tan(δ) = G”/G’, with the gel point described as the point where the storage modulus equals the loss modulus (tan δ = 1). High tan δ (tan(δ) > 1) indicates predominant liquid-like behavior and demonstrates the gel to be in an injection-feasible state, whereas a low value (tan(δ)< 1) indicates more solid-like behavior. This transition is observed at basal body temperature (37 °C), and demonstrates it becoming localization-feasible.
To measure the swelling pressure of the polymer gel, the Discovery HR-2 Rheometer was used with parallel plates and an immersion cup. Immersion cups partially enclose the bottom plate of a rheometer to allow samples to be immersed in a liquid. Tests were conducted at a set static gap distance of 1 mm and axial force was measured as a function of time with a temperature of 37 °C and no soak time. Test parameters were set to 1% strain and 0.1 Hz frequency. Gel was loaded between the plates and excess was removed before starting the experiment. Samples were allowed to equilibrate over 200 s of small amplitude oscillation with no buffer solution. Once equilibrated, mitochondria isolation buffer at 37 °C was added to the immersion cup.
5. Quantification of mitochondrial release from hydrogel
We have previously documented that the MitoTracker Green FM (MTG) probe used for the current mitochondrial release assay is not irreversibly bound to exogenous mitochondria (Gollihue et al., 2017), and its fluorescence intensity is partially dependent upon membrane potential (Keij et al., 2000). Because MTG is essentially non-fluorescent in aqueous solutions and becomes fluorescent only once it accumulates in the lipid layers of mitochondria, the leaky nature of dye does not affect outcomes of the release assay. Therefore, mitochondria were resuspended in EGTA-free isolation buffer to yield 10 μg/μl mitochondrial protein, and we used 2 μl of MTG mitochondria (20 μg) for the release assays. Specifically, 20 μg of MTG-labeled mitochondria solution in isolation buffer was transferred into 400 μl gel with a pipette and a fine point stirring rod was used to carefully mix the mitochondria and gel until homogeneously mixed, ensuring no bubbles were created. Mitochondria containing gel was kept at 37 °C for 15 min for complete gelification and 1 mL of isolation buffer (already equilibrated at 37 °C) was added. 100 μl of the supernatant was collected after a series of dissolution time for analysis and replaced by 100 μl fresh isolation buffer each time. The collected supernatant was analyzed for fluorescent intensity using a Biotek 96 well-plate reader. The amount of mitochondria released was determined using a calibration curve prepared using known concentrations of mitochondria solution in isolation buffer and expressed as Mt/M∞, where Mt is the released mass at certain time and M∞ is mass release after infinite time.
5.1. Impact of hydrogel on mitochondrial integrity
Mitochondrial integrity was assessed by measuring mitochondrial membrane potential using rhodamine 123 (Zhao et al., 2010). Prior to the assay, isolated mitochondria were kept on ice (4 °C) or in water bath (37 °C) for a predetermined time interval in isolation buffer. After which 5 μg of mitochondria was added to respiration buffer containing 5 mM pyruvate, 2.5 mM malate, 1 μM oligomycin and 1 μM rhodamine 123. The fluorescence intensity was compared to the buffer control. Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone) (FCCP) was added (1 μM) as a positive control to intentionally break any membrane potential. For hydrogels, the above experimental procedures were repeated with mitochondria added to 100 μl of hydrogel in well-plate and mixed gently. Fluorescent intensity was recorded after incubation at either 4 °C or 37 °C. Membrane potential was expressed as the difference between fluorescence intensity with and without mitochondria, normalized to initial difference in fluorescence intensity (Zhao et al., 2010).
where, FIR123+mito, FIR123, (IR123+mito)0, (FIR123)0 are the fluorescent intensity with mitochondria, without mitochondria, initial fluorescent intensity with mitochondria, and initial fluorescent intensity without mitochondria, respectively.
5.2. Respiration of mitochondria released from hydrogel
To test the integrity of exogenous mitochondria released from hydrogel, 100 μg of isolated mitochondria was homogenously mixed in 200 μl in liquid hydrogel consisting of 1% methylcellulose (MC) and 1% hyaluronic acid (HA) in mitochondrial isolation buffer, and incubated at 37 °C for 10 min to solidify the hydrogel. After which, 500 μl of isolation buffer was added on top of hydrogel-mitochondria mixture as release medium. The mitochondria released into the buffer were collected at 20-minute intervals for up to 1 h. The released mitochondrial suspension was centrifuged at 10,000 RCF for 10 min. Resultant mitochondrial pellet was resuspended in isolation buffer. After protein estimation using BCA kit, 5 μg of mitochondria from each sample in triplicate was used to assess their health in terms of their oxygen consumption rate (OCR) using Seahorse Bioscience XFe24 Extracellular Flux Analyzer, as we have described previously (Patel et al., 2014; Sauerbeck et al., 2011).
5.3. DNA isolation and PCR
Rat PC-12 cells were incubated with either 10, 25 or 50 μg mitochondria for 2 h. The cells were then washed twice with fresh media, before dissociating them with 0.25% trypsin (Thermofisher, Massachusetts, USA) for DNA isolation from the cell pellet using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), according to manufacturer’s recommendation. In brief, the cells were lysed using the lysis buffer and the DNA was purified with multiple washes in spin columns. The purified DNA was eluted into fresh tubes using the elution buffer supplied in the kit. The quality and concentration of isolated DNA was estimated using Nanodrop 2000c (Thermofisher Scientific, Massachusetts, USA). PCR to estimate the presence of mtDNA was carried out using Power-TrackTM SYBR green master mix (Applied biosystems, Massachusetts, USA) according to manufacturer’s protocol. The PCR reaction (hold 2 min at 95 °C, then 40 cycles of 15 s at 95 °C and 1 min at 60 °C, followed by melt curve analysis according to manufacturer’s instructions) was setup in QuantStudioTM 7 Flex Real-Time PCR system. The presence of mtDNA is represented as fold change with rat nuclear DNA as internal control. The primers used in the experiment are as follows.
| Rat nuclear DNA | FW Primer | 5′GGTGTACTTGAGCAGAGCGCTATAAAT3′ |
| REV primer | 5′CACTTACCCACGGCAGCTCTCTAC3′ | |
| Human mtDNA | FW Primer | 5′ACAGTTTGAACATACAAAACCCACC3′ |
| REV primer | 5′AAGGGGAGATAGGTAGGAGTAGCG3′ |
5.4. Hydrogel injection in vivo
Female Sprague–Dawley rats (n = 3) (Harlan Labs, IN) weighing 225–250 g were housed in the animal facility, Biomedical & Biological Science Research Building, University of Kentucky and allowed ad libitum access to water and food. All animal procedures were approved by the Institutional Animal Care and Use Committee, University of Kentucky and according to NIH guidelines. Rats were anesthetized with ketamine (80 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (10 mg/kg, Lloyd Laboratories, Shenandoah, IA). A dorsal laminectomy was performed to completely remove the 12th, and partially remove the 13th thoracic vertebra. A 27G needle curved tip (see Fig. 5A) was carefully inserted into the subdural space, and 2 min later 100 μl of 1:1% (MC:HA) hydrogel solution containing 2% dextran blue (Spectrum Chemical, New Brunswick, NJ) was injected slowly over ~ 2 min time. Degradation of hydrogel in terms of disappearance of dextran blue was documented with intermittent photography over a 1-hour time period.
Fig. 5.
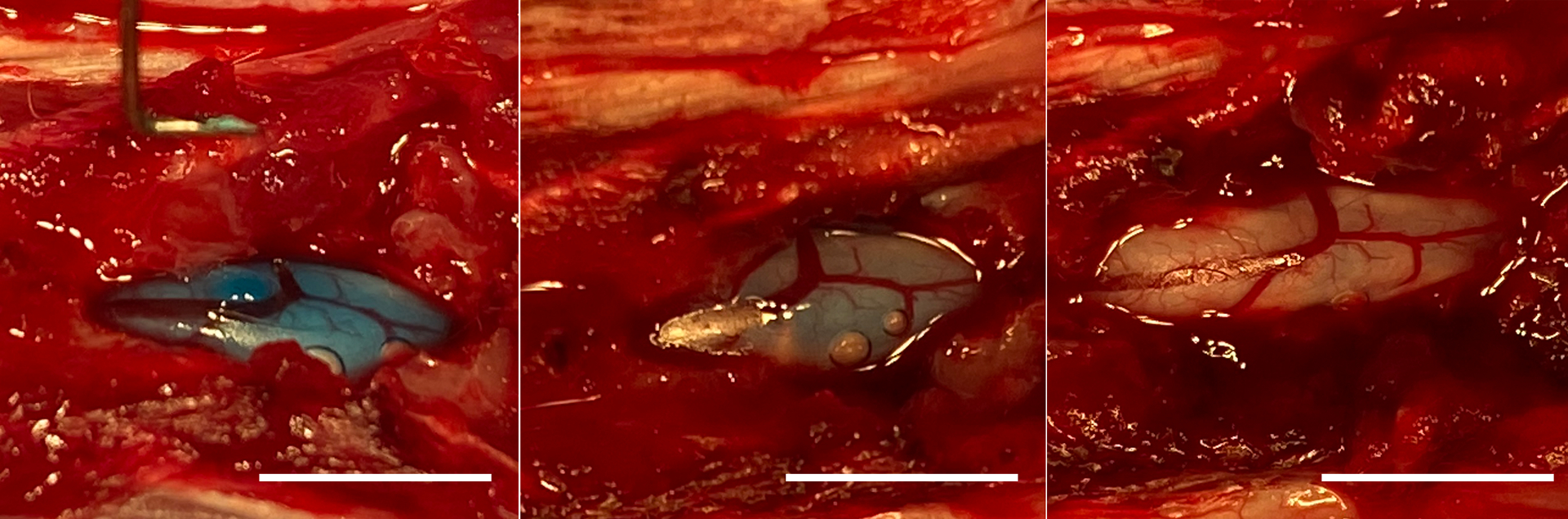
(A, B, C) Representative images in situ showing dissolution of hydrogel mixed with dextran blue within 60 min following subdural delivery into the spinal cord of naïve rat. Scale bars = 10 mm.
5.5. Tracking uptake of exogenous mitochondria in vitro
Mitochondria isolated from SH-SY5Y cells, PC-12 cells or rat brain were labelled with 100 nM MitoTracker™ Red CMXRos (MTR) dye (Molecular Probe, Eugene, OR) for 30 min at 4 °C. Mitochondria were then washed with isolation buffer twice. A subset of mitochondria was heat-inactivated by incubating at 95 °C for 5 min prior to labelling with MTR. To test for potential dye leakage from isolated mitochondria, cultures of both SH-SY5Y and PC-12 cells were incubated with 100 nM MTR dye for 30 min, washed twice, and then processed for mitochondrial isolation. In addition, a subset of collected mitochondria were filtered through a 0.22 μm syringe filter and the filtrate was then added to cells. The labelled mitochondria (10, 20 or 50 μg) or filtrate were incubated with SH-SY5Y or PC-12 cells (1 × 105cells/well) plated on 12-well glass bottomed plates for a total incubation period of 2 h, 6 h or 24 h. The cells were placed on a rocker inside a humidified CO2 incubator at 37 °C with 5% CO2 for the first 2 h to ensure optimal uptake, after which they were incubated for the remaining incubation period without shaking. The cells were washed twice prior to imaging with confocal Nikon Ti-e inverted microscope (Nikon Instruments, Melville, NY) to document intracellular uptake of exogenous mitochondria by host cells.
Integrated fluorescence densities of images were quantified using ImageJ software (National Institutes of Health, Bethesda, MD). For imaging the intensity of MTR labeling, 4 equal and non-overlapping regions of interest (ROI; 150 × 150 pixels, 200 μm × 200 μm) were randomly selected in each image (n = 3 per group), and the integrated density was calculated using the measure tool in ImageJ (see Figs. 6 and 7).
Fig. 6.

(A, B) Representative confocal images showing labeling of mitochondria in host PC-12 and SH-SY5Y cells following 2 h co-incubation with (A, E) Live or (B, F) heat-inactivated (Dead) rat brain mitochondria labelled with MTR. (C, D, G, H) Non-specific labeling of host mitochondria persisted following filtration of MTR-labelled (C, G) Live and (D, H) Dead mitochondria through a 0.2 μm filter. Four non-overlapping regions of interest (ROI) shown in panel A were used to measure fluorescence intensities. (I, J) Bar graphs show mean integrated densities of MTR fluorescence in PC-12 and SH-SY5Y cells co-incubated with Live or Dead rat brain mitochondria labelled with MTR and mitochondrial filtrates. Bars are means ± SD. * p < 0.05 vs Mitochondria-Live and Dead groups. Scale bars = 100 μm For imaging the intensity of MTR labeling, 4 equal and) were randomly selected in each image (n = 3 per group) and the integrated density.
Fig. 7.
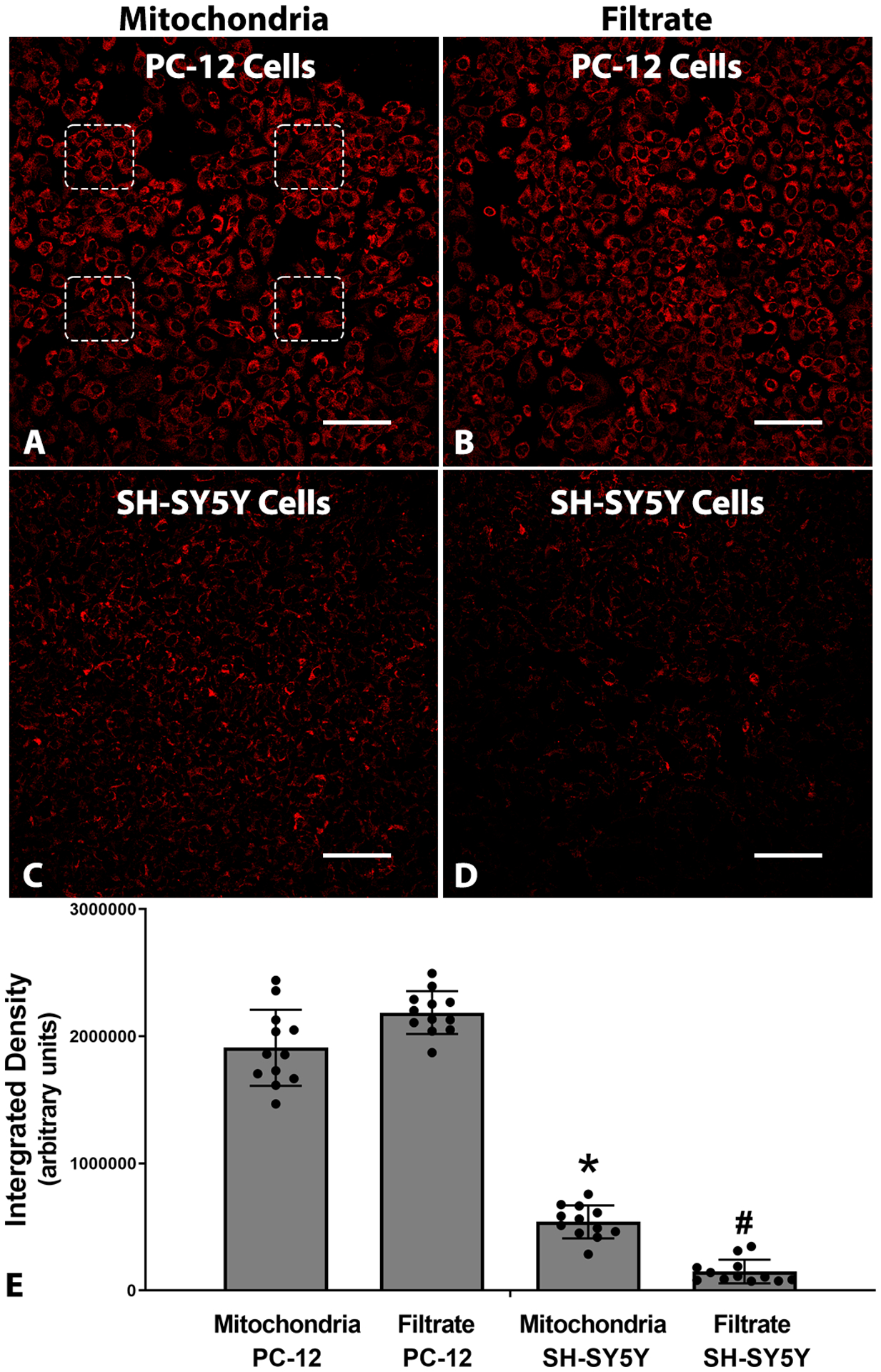
(A, B) Representative confocal images showing nonspecific labeling of mitochondria in host PC-12 and SH-SY5Y cells following 2 h co-incubation with (A, C) Live or (B, D) heat-inactivated (Dead) PC-12 syngeneic mitochondria labelled with MTR prior to mitochondrial isolation. Non-specific labelling was reduced but not eliminated when MTR labeling was carried out on cells prior to mitochondrial isolation. Four non-overlapping regions of interest (ROI) used to measure fluorescence intensities are shown in panel A. (E) Graphs showing integrated density of MTR fluorescence in cells labelled with MTR prior to mitochondrial isolation. Bars are means ± SD. *p < 0.05 vs Mitochondria in PC-12 cells and #p < 0.05 vs Mitochondria in SH-SY5Y cells. Scale bars = 100 μm.
To verify hydrogel-mediated mitochondrial uptake, 50 μg RFP-tagged mitochondria isolated from SH-SY5Y cells were mixed with 200 μl hydrogel (1:1% (MC:HA) and co-incubated with recipient PC-12 and SH-SY5Y cells for 2 h prior to imaging, as above.
5.6. MTS assay
SH-SY5Y (1 × 104cells/well) and PC-12 (1 × 104cells/well) cells were plated in 96-well plates and cultured for 24 h. The cells were then incubated for 6hrs or 24hrs with either 10 or 50 μg mitochondria from syngeneic (SH-SY5Y or PC-12 cells) or xenogeneic (SH-SY5Y, PC-12 cells, rat brain, rat spinal cord, rat muscle) sources. Afterwards, 20 μl of CellTiter 96® AQueous One Solution reagent (MTS) (Promega Madison, WI) was added to the cells and incubated for 3 h at 37 °C, and then the absorbance was measured at 490 nm using a Biotek Synergy HT plate reader (Winooski, Vermont).
5.7. Statistics
Endpoint data are presented as means ± SD. In the experimental analysis, groups were compared using analysis of variance (ANOVA) followed by Tukey’s post hoc test, when appropriate (GraphPad Prism 8 software package, GraphPad Software, Inc. La Jolla, CA or GB-Stat, Dynamic Microsystems, Inc., Silver Spring, MD). Statistical significance was set at p < 0.05.
6. Results
To begin addressing whether isolated mitochondria can be delivered through erodible and diffusible hydrogel polymers subdurally into spinal cords, we sought to better understand the thermogelling nature of the polymer system employed.
To study the gelling behavior, we use parallel plate shear rheology to track the mechanical response at different temperatures. As illustrated in Fig. 1, the loss tangent gradually decreased as the temperature increased, reaching a value of ~ 0.5 at 50 °C starting from ~ 1.6 at 10 °C. High tanδ indicates predominant liquid-like behavior, demonstrating the gel to be injection-feasible at 4 °C, whereas its low value (showing solid-like behavior) at basal body temperature (37 °C) demonstrates it becoming localization-feasible.
Fig. 1.

Thermal gelation assessment 1–1 HAMC performed at angular frequency of 0.1 Hz.
This data set confirms the inverse thermal-gelation properties of the hydrogel, showing more liquid-like behavior (tan(δ) > 1) at cold temperatures and more solid-like behavior (tan(δ) < 1) at basal body temperature (37 °C). This result provided confidence in the nature of the gelation mechanism as well as the reproducibility of the effect.
In addition to the gelation point, we were concerned regarding the potential swelling of the gel once implanted near the spinal cord. As gels swell, they are expected to exhibit a degree of swelling pressure on the surrounding tissue, which may pose a concern regarding their biocompatible nature. In this experiment, an initial “baseline” pressure is applied (20 Pa) as the platen is lowered onto the gel. In Fig. 2, after subtracting the baseline pressure of the gel (20 Pa) and the calculated hydrostatic pressure of the solutions (~90 Pa), the gel in isolation buffer showed an initial swelling pressure of 60 Pa within 2 h. This pressure plateaued at the max pressure after 3 h in the isolation buffer. The observed decrease in pressure afterwards is believed to be a result of both the evaporation of solution during the course of the experiment and potentially dissolution of the hydrogel. While we did not measure spinal cord pressure in situ, we report that the external pressure the gel exerts on the surroundings due to swelling is well below the normal and post-trauma intraspinal pressures (Khaing et al., 2016). This means that the gel itself is not expected to induce and further pressure onto the surrounding tissues beyond that of the liquid volume. While we are not able to say for certain if pressure has an impact on mitochondrial viability, it is possible that the hydrogel’s swelling pressure may aide in the stability of mitochondria by counteracting the mitochondria’s osmotic swelling. Importantly, as observed in Fig. 4B, the 1:1% MC:HA hydrogel does not affect mitochondrial respiration assessed by oxygen consumption rate over time.
Fig. 2.
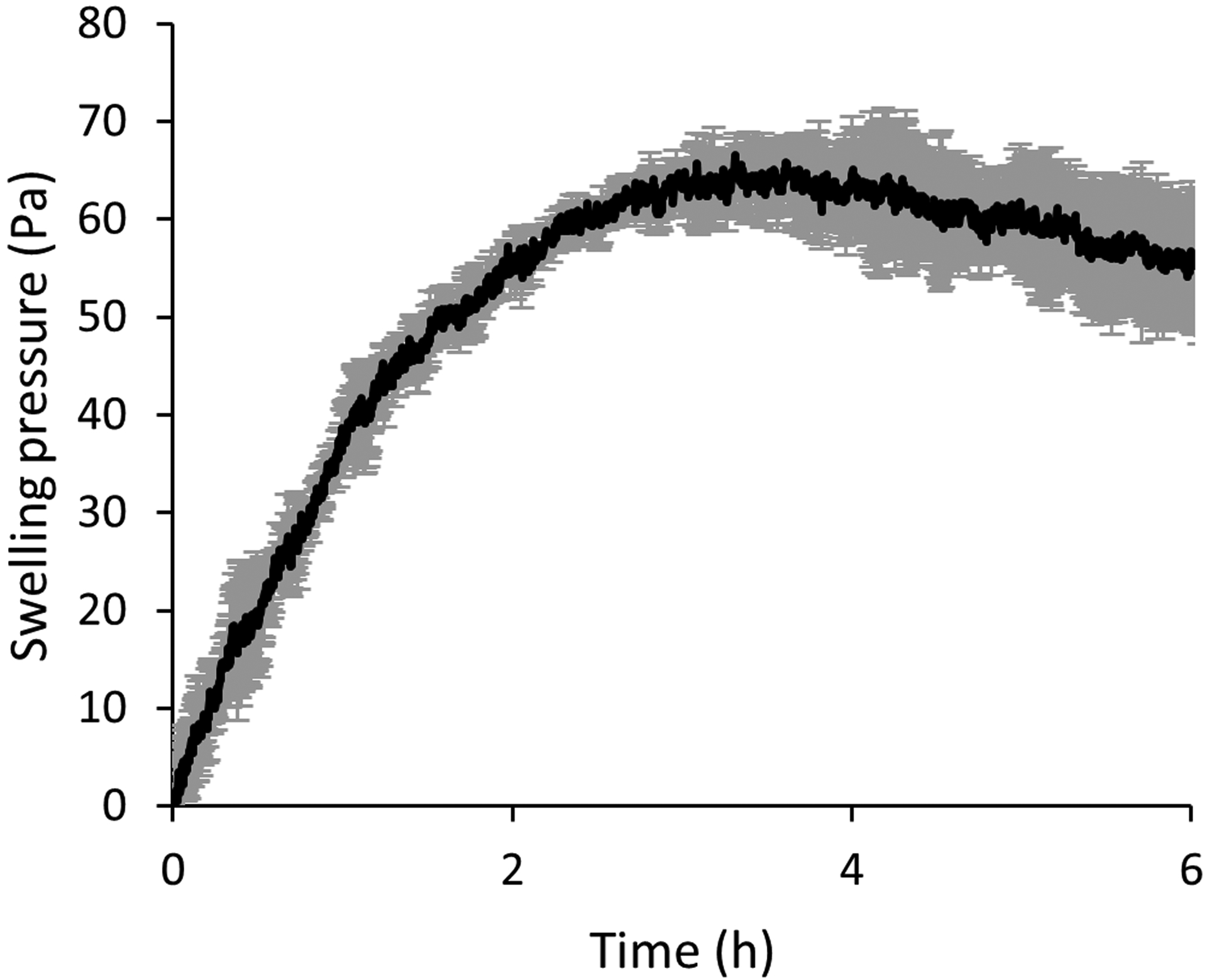
Swelling pressure of 1%:1% HA:MC gel submerged in mitochondria isolation buffer using an immersion cup at 0.1 Hz, 1% strain, and a 1 mm gap.
Fig. 4.
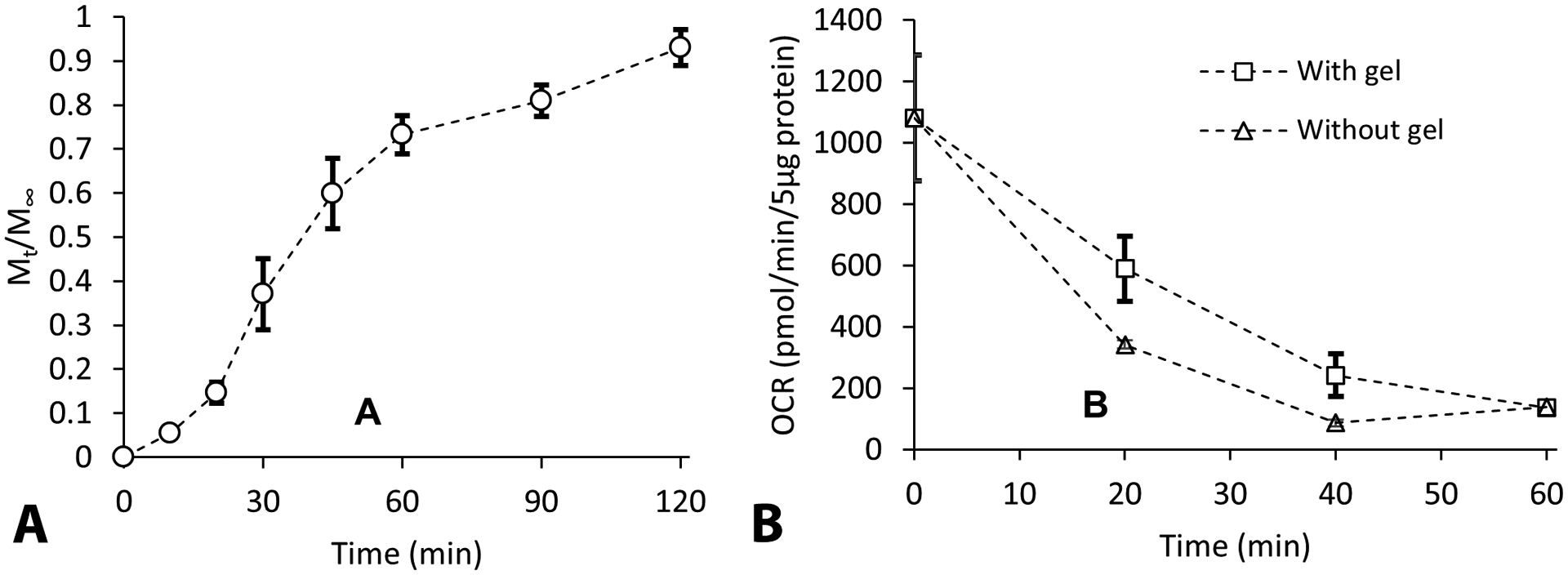
(A) Representative time course graphs showing that the rate of mitochondrial release from the hydrogel is ~ 70% within 60 min. (B) Mitochondria released from the gel after both 20 min and 40 min had comparably higher OCR than the mitochondria incubated at 37 °C in mitochondrial isolation buffer without gel. Symbols are means ± SD.
In order to evaluate the impact of hydrogel on mitochondrial integrity, mitochondrial membrane potential was measured using rhodamine 123, with or without hydrogel. Prior to the assay, isolated muscle mitochondria were kept on ice (4 °C) or in water bath (37 °C) for a predetermined time interval in isolation buffer. In the presence of viable mitochondria with intact membrane potential, R123 is sequestered and stacked in the mitochondrial matrix, resulting in reduced fluorescence intensity compared to buffer without mitochondria. When the membrane potential is compromised deliberately (by adding FCCP), R123 fluorescent intensity returns to levels of buffer without mitochondria (Fig. 3A). Mitochondrial integrity at different temperatures (4 °C and 37 °C) is presented in Fig. 3 B,C for mitochondria suspended in isolation buffer only or in hydrogel, respectively. Integrity was maintained at 4 °C for over 100 min, whereas intensity rapidly decreased over time at 37 °C. However, no significant differences in normalized fluorescence intensities was observed in the presence of hydrogel, indicating no adverse effects of hydrogel on mitochondrial integrity.
Fig. 3.
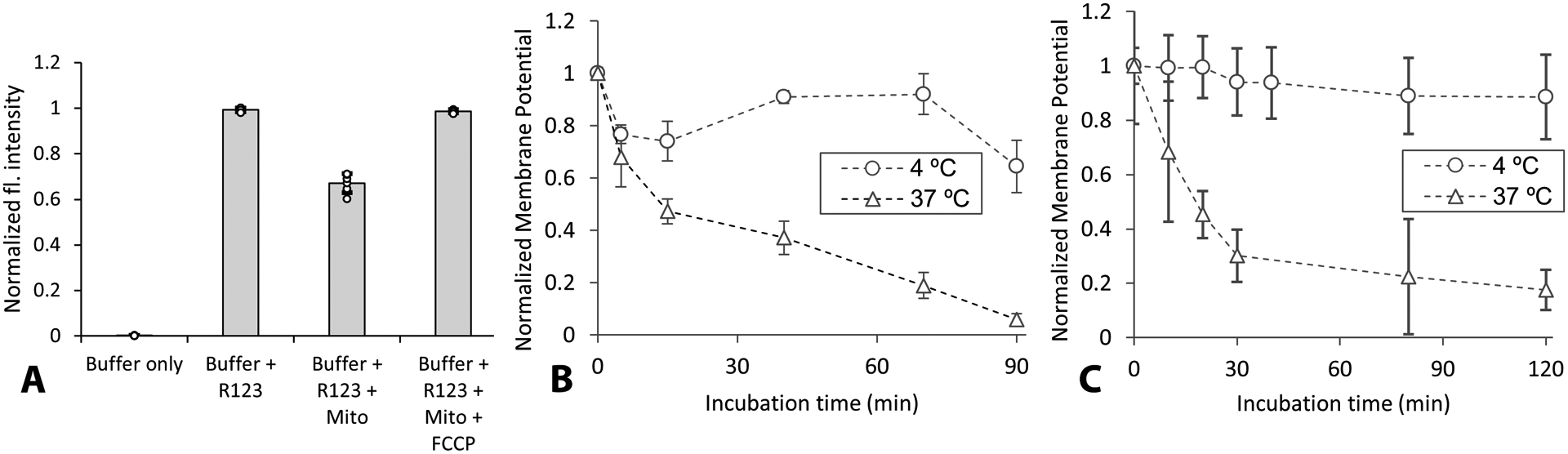
(A) Representative normalized fluorescence (fl.) intensities in presence or absence of mitochondria (Mito) for first 5 min of measurement, showing decreased intensity with Mito addition. (B) Comparison of mitochondrial membrane potential at 4 °C vs 37 °C for different incubation times without hydrogel. (C) Comparison of mitochondrial integrity overtime measured in hydrogel at 4 °C vs 37 °C. Note that in B and C, 1 indicates maximum membrane potential. Symbols are means ± SD.
When we tested the rate of release and integrity of exogenous mitochondria released from hydrogel (Fig. 4), mitochondria release was noted as early as 20 min post incubation and ~ 70% of the incubated mitochondria were released by 60 min (Fig. 4A). The mitochondria that were collected were assessed by Seahorse to estimate their oxygen consumption rates (OCR). It was observed that mitochondria released from the gel after both 20 min and 40 min had higher OCR than the mitochondria incubated at 37 °C in mitochondrial isolation buffer without gel (Fig. 4B). Thus, a hydrogel composition composed of 1% MC and 1% HA have protective properties which can be utilized for mitochondrial delivery in vivo. While we are not certain of the source of this protective effect, it is possible that the hydrogel serves as an osmolyte (as suggested by the swelling data in Fig. 2), which will counteract mitochondrial swelling. Alternatively, the hydrogels may help inhibit mitochondria aggregation, and thereby enhance the exchange of substrate and toxic by-products from the surface of mitochondria.
When we performed feasibility experiments using inert dextran blue dye to assess the gross diffusion and degradation of blue hydrogel, the rate of degradation was monitored (pictured) every 10 min to document the temporal disappearance of dextran blue from injection site. The hydrogel rapidly congealed in situ (Fig. 5A) and did not visually dissolute until after 30 min (Fig. 5B), before disappearing within 60 min without causing any damage to the spinal cord (Fig. 5C). The average core body temperature of a rat is 37 °C, and after SCI it reduces by approximately 0.9 °C (Laird et al., 2006). While we did not measure the temperature of spinal cord dura, the surface temperature of the spinal cord is reported to be 34.5 to 35.5 °C after injury (see Supplementary Fig. 1 in (Bazley et al., 2014)). As we show in Fig. 1, the temperature-dependent changes of the thermogel are within this temperature range.
7. Spatio-temporal tracking of exogenous mitochondria
MTR labelled rat brain mitochondria were co– incubated with naïve rat PC-12 or human SH-SY5Y cells to track mitochondrial uptake. Despite being a membrane potential-dependent, mitochondria-specific dye, we observed non-specific labelling of both host PC-12 (Fig. 6 A) and human SH-SY5Y (Fig. 6 E), including labelling after coincubation with heat-inactivated mitochondria (Fig. 6 B,F). To rule out labelling due to untagged MTR residue in the mitochondrial solution, the mitochondria were further filtered through a 0.2 μm filter prior to co-incubation with host cells. Despite filtering the mitochondria, nonspecific labelling was still observed in PC12 cells (Fig. 6 C,D) and human SH-SY5Y cells (Fig. 6 G,H).
In another series of experiments, the mitochondria within cultured cells were labelled, prior to mitochondrial isolation, in an attempt to transplant only MTR-bound mitochondria (Fig. 7) (Sun et al., 2019; Yao et al., 2021). It was found that non-specific labelling of recipient cells was reduced, although not eliminated, when MTR labeling was carried out prior to mitochondrial isolation from both PC-12 (Fig. 7 A,B) and SH-SY5Y (Fig. 7 C,D) cells. This non-specific labelling of MTR is due to dye leakage predominantly from live mitochondria, suggesting that MTR dye used in this study requires rigorous testing prior to its use to track exogenous mitochondria in vitro and in vivo.
We used mitochondria isolated from genetically modified SH-SY5Y cells with mitochondria-tagged fusion protein, red fluorescent protein (RFP), as the source. RFP-tagged mitochondria transplanted onto either naïve PC-12 or SH-SY5Y recipient cells showed evidence of mitochondrial uptake into cells as early as 2 h and after 24 h incubation, in both cell lines (Fig. 8). There was no non-specific labelling observed in cells transplanted with the filtrate from RFP-tagged mitochondria (data not shown). Unequivocally, mtDNA serves as a valuable surrogate marker for determining mitochondrial uptake into cells, and qPCR demonstrated a dose-dependent increase in the amount of mtDNA internalized by PC-12 cells following 2-hour incubation with RFP-tagged mitochondria isolated from human SH-SY5Y cells (Fig. 8E).
Fig. 8.
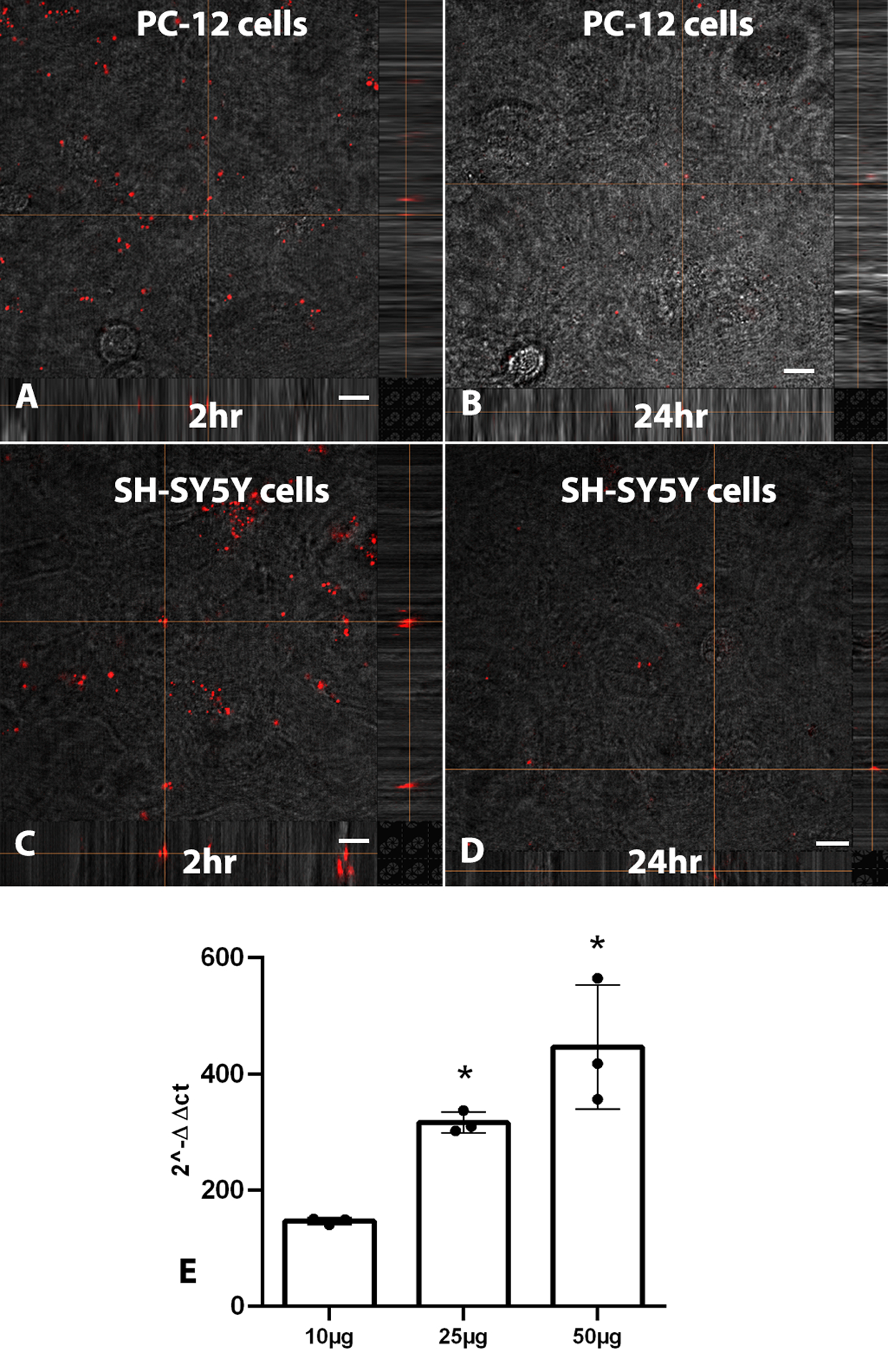
Confocal images show RFP-labeled mitochondrial uptake into naïve PC-12 and SH-SY5Y cells after 2 h (A,C) and 24 h (B,D). E) qPCR results show presence of human mtDNA in PC-12 cells following 2-hour incubation with isolated mitochondria from human SH-SY5Y in a dose-dependent manner. Scale bars = 10 μm. Bars are means ± SD. *p < 0.05 compared to 10 μg group.
This lays the precedence to deliver RFP mitochondria into the spinal cord for purposes of indelible tracking. It must be noted that successful incorporation of tagged mitochondria into the host network is likely to cause dilution or “watering down” of the fluorescence signal. While we have not tested the efficiency of RFP-tagged vs -untagged mitochondria, we intend to use RFP-tagged mitochondria only for visualization purposes in situ, because it is more clinically relevant to deliver unlabeled autologous mitochondria rather than genetically modified mitochondria (Masuzawa et al., 2013).
To establish hydrogel-mediated mitochondrial uptake in vitro, 50 μg RFP-tagged mitochondria from SH-SY5Y cells were mixed with 200ul 1:1% (MC:HA) hydrogel and co-incubated with PC-12 and SH-SY5Y recipient cells for 2 h. Confocal images show uptake of RFP-tagged mitochodria released from hydrogels by both recipient cell lines (Fig. 9 A,B).
Fig. 9.
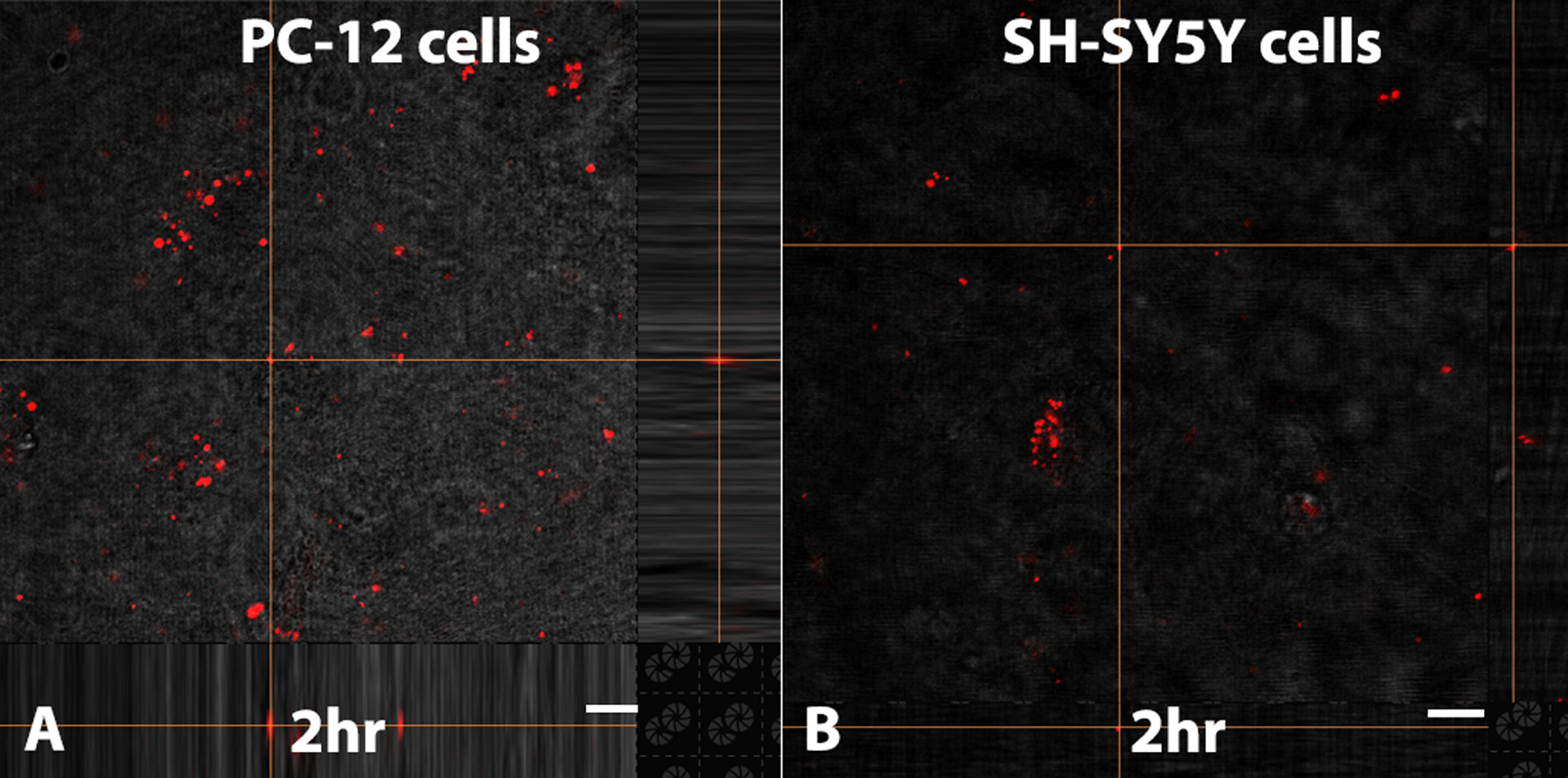
Confocal images show hydrogel-mediated RFP mitochondrial uptake by naïve PC-12 (A) and SH-SY5Y (B) cells after 2 h. Scale bars = 10 μm.
7.1. Effects of mitochondrial transplantation on recipient cell redox activities:
With the intent to track RFP-tagged mitochondria isolated from SH-SY5Y cells in rat spinal cords in vivo, we tested the efficacy of mitochondrial transplantation to increase metabolic activity of recipient cells, and whether xenogeneic mitochondrial sources affected this. Using the MTS assay, a fluorometric indicator of metabolic activity, we found that the source of exogenous mitochondria significantly altered the metabolism of recipient PC-12 and SH-SY5Y cells (Fig. 10). Recipient SH-SY5Y cells transplanted with SH-SY5Y or PC-12 cell line-derived mitochondria underwent dose-dependent increases in metabolic activity (Fig. 10 A–D). Syngeneic mitochondrial transplantation (SH-SY5Y mitochondria into SH-SY5Y cells) resulted in ~ 50% increased intensity compared to controls, notably after 24 h. Alternatively, exogenous mitochondria isolated from rat brain or spinal cord tissues (Fig. 10 E–J) transplanted into either SH-SY5Y or PC-12 cells reduced intensities in both cell lines, notably at the highest concentration of mitochondria delivered.
Fig. 10.

Mitochondria source influences cellular redox activity: A, C. Transplantation of syngeneic SH-SY5Y mitochondria on SH-SY5Y cells or PC-12 mitochondria on PC-12 cells resulted in increased optical densities due to the conversion of tetrazolium salts into colored formazan dye by NAD(P)H-dependent dehydrogenase enzymes in metabolically active cells, indicative of higher metabolic activities. B, D. Xenogeneic transplantation of either SH-SY5Y mitochondria on PC-12 cells or PC-12 mitochondria on SH-SY5Y cells resulted in significant increases in metabolic activity, though lower in magnitude in PC-12 cells co-incubated with SH-SY5Y mitochondria. E-J. Transplantation of exogenous, tissue-derived mitochondria resulted in significant decreases in metabolic activity in both cell lines, notably at the highest concentration delivered. Bars are means ± SD; n = 3–9/group; *p ≤ 0.05 vs 6 h control and #p < 0.05 vs 24 h.
8. Discussion
The burgeoning body of literature assessing various mitochondrial transplantation techniques in many organs and the CNS demands validated methods to verify their localization after transplantation. While studies use MTR to determine mitochondrial viability and track them both in vitro and in vivo (Masuzawa et al., 2013; Sun et al., 2019), we present evidence that indelible tracking of transplanted mitochondria requires genetically tagging them. Nevertheless, it must be considered that as mitochondria undergo fusion and fission, it is reasonable to assume that any fluorescent tag would also be dispersed across the host cell network. Additionally, mitochondria undergo turnover, and as the transplanted mitochondria age, the fluorescent tag, along with the mitochondria, could undergo mitophagy and, therefore, loss of signal.
To track and confirm cellular uptake, seminal mitochondrial transplantation studies have used direct fluorescent labeling (Cowan et al., 2016; Kaza et al., 2017; Masuzawa et al., 2013; McCully et al., 2009), compartmental fluorescent labeling (Pacak et al., 2015), xenogeneic immunofluorescent labeling (Cowan et al., 2016; Kaza et al., 2017; Masuzawa et al., 2013), baculovirus-mediated transfer of mammalian fusion genes (Cowan et al., 2016) and DsRed (MitoTimer), a fluorescent reporter targeted to the mitochondrial matrix (Kesner et al., 2016). We previously used transgenic labeled turbo green fluorescent protein mitochondria transfected into PC-12 cells targeting mitochondria subunit VIII of cytochrome C oxidase to indelibly track transplanted mitochondria in naïve and injured rat spinal cords, showing uptake and integration of mitochondria in a variety of host cell types (Gollihue et al., 2018; Gollihue et al., 2017). While many of our studies transplant unlabeled mitochondria, others have transplanted human mitochondria for unambiguous results in uptake studies (Cowan et al., 2016). Accordingly, we found that RFP-tagged mitochondria isolated from human SH-SY5Y cells successfully integrate into syngeneic and allogeneic cell lines, notably after being released from protective hydrogel.
While MitoTracker probes are widely used, MitoSOX Red is taken up passively by cells and oxidized by superoxide in the mitochondria, resulting in the emission of red fluorescence (Puleston, 2015). Reports of mitochondria colocalization in neurons in situ using MTR-labelling showed they were localized inside the injured parenchyma, but intracellular localization was equivocal (Li et al., 2019). Controlled studies in vitro have used it to demonstrate mitochondrial transfer after 4 h of co-culturing bone marrow-derived mesenchymal stem cells and T cells, and MTR CMXRos-labelled astrocytic extracellular mitochondrial particles injected directly into the peri-infarct cortex are reported to be present in neurons (Hayakawa et al 2016). Based on our findings, we contend that MTR is a useful surrogate marker for the visual presence of mitochondria, but only as a surrogate marker that is not indelible in vitro or in vivo. After stringent washing of the pellets and supernatants, naïve host cells still label non-specifically, even after the labelled mitochondria are heat-killed and filtered through 0.02 μm filter prior to transplantation. Moreover, when cultured cells are labelled and their mitochondria isolated afterwards, this does not avert non-specific host staining. Accordingly, for validation of in vitro or in vivo effects of mitochondrial transplantation, we corroborate our results obtained using voltage-dependent dyes with direct measures of oxygen consumption (OCR) using the SeaHorse flux analyzer.
Localization of mitochondria to the site of injury was believed to be a significant hurdle to the effective delivery and uptake of mitochondria. In addition, it was theorized that effectiveness of this therapy will be enhanced if the delivered mitochondria maintain an effective oxygen consumption rate. Given the size of the mitochondria, it was determined that an eroding gel system would be needed to regulate delivery, as diffusion through most hydrogels would be too slow to be effective. As such, to test this concept, we selected the thermal gelling, erodible system composed of hyaluronic acid and methyl cellulose. By tuning the concentration of the HA and MC, this system allows for tunable release window from several hours to weeks, which provides enough control to tune release for this application. However, as concentration increases, viscosity of the solution also increases, increasing the pressure required for localized injection.
Another limitation to this delivery approach is that the gel is not able to protect or maintain mitochondrial function once release occurs. If it is determined that release and uptake is still too slow for functional delivery, alternative strategies (e.g., coating, coupling agents to mitochondria) will be employed to maintain integrity. Experiments in vitro show that mitochondria are internalized by the cells within minutes after co-incubation, so we expect the mitochondria can be incorporated by host cells within a similar time-span in vivo. Our main goal using hydrogel is to enhance localization of mitochondria at the intended site of delivery, at and around injury epicenter, and we are working to modify hydrogel fabrication to stabilize mitochondrial integrity for longer duration extracellularly.
We observed that isolated mitochondria lose their viability with at 37 °C over-time in isolation buffer, with complete loss of respiration by 3 h (unpublished observations; AGR). Therefore, in the current experiments, to facilitate cellular uptake and internalization in situ, the thermogelling hydrogel system was uniquely developed to set up and erode over the course of 1 to 3 h, facilitating mitochondrial release for localized host uptake. Importantly, this shortened time-window was selected to address mitochondrion’s touted limited functional lifespan outside of the cytosol in vivo (Bertero et al., 2018).
As expected, mitochondria were too large to diffuse through the hydrogel mesh network, and therefore release was purely determined by the erosion rate of the hydrogel network. The gel completely disappeared in situ within 60 min without causing any damage to the cord. One concern was whether or not the swelling hydrogel would create local osmotic pressure that could perturb the injury site. While water uptake into the gel did result in an increased pressure, the magnitude of this force was lower than the expected pressure already existing in the spinal cord (Khaing et al., 2016). We have not comparatively assessed various populations of mitochondria, but based on our microbead studies, we believe that mitochondria from sources such as brain, spinal cord or cell cultures will have similar rates of release compared to muscle mitochondria. Since mitochondria size and shape exceed the mesh size of the hydrogel, drug release is dictated by hydrogel dissolution. The advantage of this approach is that as long as mitochondria (>300 nm) are larger than the network mesh size (typically < 50 nm), the release rate is likely to be independent of variations in the mitochondria size and shape (Canal and Peppas, 1989; Logan, 2003). This means that release would not be determined by the source (muscle, nerve, etc.) of the mitochondria.
We found that the mitochondrial source (syngeneic vs xenogeneic or cell line- vs rat tissue-derived) influenced cellular redox activity of recipient cells differentially, assessed by MTS assay (Mosmann, 1983). Compared to controls, MTS values were significantly higher (25–50%) in SH-SY5Y cells after mitochondrial transplantation from cell line-derived sources [syngeneic (SH-SY5Y) or xenogeneic (PC-12) mitochondria] compared to rat tissue-derived mitochondria (rat brain, spinal cord and muscle tissue). Similar alterations were observed in PC-12 cells, albeit at a lower intensity. Such differences in metabolic activity between cell line- and tissue-derived mitochondria is likely due to the heterogenous nature of tissue mitochondria compared to those isolated from rather homogenous cell cultures. Notably, we did not further investigate differences among species in this proof-of-principle study, but both preclinical and clinical evidence indicates that there are no detectable immune, autoimmune or inflammatory responses when autologous, allogeneic or xenogeneic mitochondria are transplanted (Emani and McCully, 2018; Kaza et al., 2017; Masuzawa et al., 2013; McCully et al., 2009; Moskowitzova et al., 2020; Ramirez-Barbieri et al., 2019).
In is important to note that the widely used MTS assay is an index of cellular metabolic activity. MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)–2H-tetrazolium, inner salt] is reduced by NAD(P)H-dependent dehydrogenase enzymes in metabolically active cells into violet-colored formazan product, the optical density of which is directly proportional to the number of cultured cells (Mosmann, 1983). However, redox changes driven by ROS induction can also increase conversion of MTS, which restricts interpretation of the current results and is a limitation of this study. Nevertheless, we are engaging in studies aimed at refining our hydrogel constructs to include mitochondrial protectants to combat ROS and a robust localized subdural delivery method in the injured spinal cord to obviate further damage and localized inflammation accompanying focal injections (Gollihue et al., 2018; Gollihue et al., 2017).
Herein, we demonstrate the feasibility of the hydrogel to both protect the mitochondria at physiological conditions and allow their local diffusion and incorporation into host cells. While we did not measure MTS values when assessing the hydrogel-released mitochondria, our findings indicate that the metabolic activity of cells is augmented following mitochondrial transplantation. While the cause for this is not yet certain, ongoing investigations are employing flow cytometry, automated cell counting and proliferation markers to determine if the observed increase in MTS activity is due to either enhanced cell proliferation or the incorporation of greater numbers of exogenous mitochondria into individual host cells. While one could also interpret the lack of change in some cases to indicate that the addition of mitochondria did not increase redox or increase bioenergetic demand in cell lines, our controlled studies more support incorporation of greater numbers of exogenous mitochondria into individual host cells. Future studies will employ mitochondrial inhibitors of ATP production, uncouplers and oxymetric assessments to address these important questions more in depth. We will also consider using OCR and non-cell permeant substrates to measure respiration outside cells and then inside cells.
Supplementary Material
Acknowledgements
Financial Support: Chair Endowments from the Spinal Cord & Brain Injury Research Center, University of Kentucky (AGR & PGS), DoD W81XWH2010347 (AGR) and NIH R01 NS119337 (AGR & SPP). RFP was gifted by Dr. Joe Springer, SCoBIRC, University of Kentucky-UK). We would like to thank Hope Vaught and Job Tharappel at UK SCo-BIRC for expert technical assistance, and Kelley Wiegman (UK Chemical Engineering) for her helpful feedback during the development of the mitochondria release studies.
Abbreviations:
- HA-MC
hyaluronic acid-methylcellulose
- MTG
MitoTracker Green
- MTR
MitoTracker™Red CMXRos
- MTS
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)–2H-tetrazolium, inner salt]
- OCR
oxygen consumption rate
- RFP
red flourecent protein
- ROS
reactive oxygen species
- SCI
spinal cord injury
- TBI
traumatic brain injury
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mito.2022.04.002.
References
- Bazley FA, Pashai N, Kerr CL, All AH, 2014. The effects of local and general hypothermia on temperature profiles of the central nervous system following spinal cord injury in rats. Ther. Hypother. Temp. Manag 4, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero E, Maack C, O’Rourke B, 2018. Mitochondrial transplantation in humans: “magical” cure or cause for concern? J. Clin. Invest 128, 5191–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal T, Peppas NA, 1989. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res 23, 1183–1193. [DOI] [PubMed] [Google Scholar]
- Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, Zurakowski D, Ericsson M, Friehs I, Wu Y, Levitsky S, Del Nido PJ, Packard AB, McCully JD, 2016. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One 11, e0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delplace V, Ortin-Martinez A, Tsai ELS, Amin AN, Wallace V, Shoichet MS, 2019. Controlled release strategy designed for intravitreal protein delivery to the retina. J. Control. Release 293, 10–20. [DOI] [PubMed] [Google Scholar]
- Emani SM, McCully JD, 2018. Mitochondrial transplantation: applications for pediatric patients with congenital heart disease. Transl. Pediatr 7, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue JL, Patel SP, Eldahan KC, Cox DH, Donahue RR, Taylor BK, Sullivan PG, Rabchevsky AG, 2018. Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J. Neurotrauma 35, 1800–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue JL, Patel SP, Mashburn C, Eldahan KC, Sullivan PG, Rabchevsky AG, 2017. Optimization of mitochondrial isolation techniques for intraspinal transplantation procedures. J. Neurosci. Methods 287, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue JL, Rabchevsky AG, 2017. Prospects for therapeutic mitochondrial transplantation. Mitochondrion 35, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Bruzzese M, Chou SH, Ning M, Ji X, Lo EH, 2018. Extracellular mitochondria for therapy and diagnosis in acute central nervous system injury. JAMA Neurol. 75, 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH, 2016. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J, 2012. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med 18, 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, Ericsson M, Yao R, Thedsanamoorthy JK, Zurakowski D, Levitsky S, Del Nido PJ, Cowan DB, McCully JD, 2017. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J. Thorac. Cardiovasc. Surg 153, 934–943. [DOI] [PubMed] [Google Scholar]
- Keij JF, Bell-Prince C, Steinkamp JA, 2000. Staining of mitochondrial membranes with 10-nonyl acridine orange, MitoFluor Green, and MitoTracker Green is affected by mitochondrial membrane potential altering drugs. Cytometry 39, 203–210. [DOI] [PubMed] [Google Scholar]
- Kesner EE, Saada-Reich A, Lorberboum-Galski H, 2016. Characteristics of mitochondrial transformation into human cells. Sci. Rep 6, 26057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaing ZZ, Ehsanipour A, Hofstetter CP, Seidlits SK, 2016. Injectable hydrogels for spinal cord repair: A focus on swelling and intraspinal pressure. Cells Tissues Organs 202, 67–84. [DOI] [PubMed] [Google Scholar]
- Laird AS, Carrive P, Waite PM, 2006. Cardiovascular and temperature changes in spinal cord injured rats at rest and during autonomic dysreflexia. J. Physiol 577, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang C, He T, Zhao T, Chen YY, Shen YL, Zhang X, Wang LL, 2019. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics 9, 2017–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DC, 2003. Mitochondrial dynamics. New Phytol. 160, 463–478. [DOI] [PubMed] [Google Scholar]
- Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C, Seth P, Bloch DB, Levitsky S, Cowan DB, McCully JD, 2013. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol 304, H966–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S, 2009. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart Circ. Physiol 296, H94–H105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully JD, Del Nido PJ, Emani SM, 2022. Mitochondrial transplantation for organ rescue. Mitochondrion 64, 27–33. [DOI] [PubMed] [Google Scholar]
- Moskowitzova K, Orfany A, Liu K, Ramirez-Barbieri G, Thedsanamoorthy JK, Yao R, Guariento A, Doulamis IP, Blitzer D, Shin B, Snay ER, Inkster JAH, Iken K, Packard AB, Cowan DB, Visner GA, Del Nido PJ, McCully JD, 2020. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol 318, L78–L88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T, 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Park JH, Hayakawa K, 2020. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol 324, 113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak CA, Preble JM, Kondo H, Seibel P, Levitsky S, Del Nido PJ, Cowan DB, McCully JD, 2015. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open 4, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Gamboa JL, McMullen CA, Rabchevsky A, Andrade FH, 2009a. Lower respiratory capacity in extraocular muscle mitochondria: evidence for intrinsic differences in mitochondrial composition and function. Invest. Ophthalmol. Vis. Sci 50, 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Lyttle TS, Rabchevsky AG, 2010. Acetyl-L-carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J. Neurochem 114, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Goldstein GA, VanRooyen JL, Yonutas HM, Eldahan KC, Morehouse J, Magnuson DS, Rabchevsky AG, 2014. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp. Neurol 257, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Rabchevsky AG, 2009b. Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J. Neurosci. Res 87, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleston D, 2015. Detection of mitochondrial mass, damage, and reactive oxygen species by flow cytometry. Cold Spring Harb. Protoc 2015 pdb prot086298. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Michael FM, Patel SP, 2020. Mitochondria focused neurotherapeutics for spinal cord injury. Exp. Neurol 330, 113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Barbieri G, Moskowitzova K, Shin B, Blitzer D, Orfany A, Guariento A, Iken K, Friehs I, Zurakowski D, Del Nido PJ, McCully JD, 2019. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion 46, 103–115. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Pandya J, Singh I, Bittman K, Readnower R, Bing G, Sullivan P, 2011. Analysis of regional brain mitochondrial bioenergetics and susceptibility to mitochondrial inhibition utilizing a microplate based system. J. Neurosci. Methods 198, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, 2014. Early preservation of mitochondrial bioenergetics supports both structural and functional recovery after neurotrauma. Exp. Neurol 261, 291–297. [DOI] [PubMed] [Google Scholar]
- Sun C, Liu X, Wang B, Wang Z, Liu Y, Di C, Si J, Li H, Wu Q, Xu D, Li J, Li G, Wang Y, Wang F, Zhang H, 2019. Endocytosis-mediated mitochondrial transplantation: Transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics 9, 3595–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Ma Y, Zhou W, Liao Y, Jiang Z, Lin J, He Q, Wu H, Wei W, Wang X, Björklund M, Ouyang H, 2021. In-cytoplasm mitochondrial transplantation for mesenchymal stem cells engineering and tissue regeneration. Bioeng. Transl. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ye L, Liu H, Xia Q, Zhang Y, Yang X, Wang K, 2010. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J. Inorg. Biochem 104, 371–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


