ABSTRACT
Oral microbiota is associated with human diseases including cancer. Emerging evidence suggests that proton pump inhibitors (PPIs), which allow the oral microbiome to translocate into the gut, negatively influence the efficacy of immune checkpoint blockade (ICB) in cancer patients. However, currently there is no effective treatment that restores the decreased efficacy. To address this issue, we retrospectively evaluated 118 advanced or recurrent non-small cell lung cancer (NSCLC) patients treated with ICB and analyzed 80 fecal samples of patients with lung cancer by 16S metagenomic sequencing. Clostridium butyricum therapy using C. butyricum MIYAIRI 588 (CBM588), a live biotherapeutic bacterial strain, was shown to improve the ICB efficacy in lung cancer. Thus, we investigated how CBM588 affects the efficacy of ICB and the gut microbiota of lung cancer patients undergoing PPI treatment. We found that PPI treatment significantly decreased the efficacy of ICB in NSCLC patients, however, CBM588 significantly restored the diminished efficacy of ICB and improved survival. In addition, CBM588 prolonged overall survival in patients receiving PPIs and antibiotics together. The fecal analysis revealed that PPI users had higher abundance of harmful oral-related pathobionts and lower abundance of beneficial gut bacteria for immunotherapy. In contrast, patients who received CBM588 had lesser relative abundance of potentially harmful oral-related bacteria in the gut. Our research suggests that manipulating commensal microbiota by CBM588 may improve the therapeutic efficacy of ICB in cancer patients receiving PPIs, highlighting the potential of oral-related microbiota in the gut as a new therapeutic target for cancer immunotherapy.
KEYWORDS: Antibiotics, CBM588, gut microbiome, immune checkpoint blockade, clostridium butyricum, lung cancer, oral microbiota, proton pump inhibitors (PPIs)
Introduction
Immune checkpoint blockade (ICB) has emerged as a new pillar of cancer treatment and opened a new era for cancer therapy.1,2 However, only a minority of patients with advanced lung cancer responds to ICB that targets the programmed death 1 (PD-1) receptor or programmed death ligand 1 (PD-L1). To overcome the resistance to ICB therapy, extensive research efforts have been undertaken.3 Accumulating evidence revealed that gut microbiota influences the response of tumors to ICB in cancer patients.4–7 Modulation of gut microbiota has been investigated in preclinical murine tumor models and cancer patients to treat cancer.4,5,8,9 Intriguingly, clinical studies have reported that manipulating the gut microbiota by fecal microbiota transplantation using stool collected from patients who had response to ICB allow advanced melanoma patients to overcome resistance to ICB.10,11 These findings support the concept of overcoming primary and acquired resistance to ICB by modulating the gut microbes using live biotherapeutic bacteria.4,5
A number of studies have reproducibly shown that unfavorable changes in gut microbial composition caused by antibiotics, which are referred to as “dysbiosis”, impairs response to ICB, suggesting that an intact gut microbiota is essential to improve the efficacy of ICB.4,6,12,13 For this, the gut microbiota can be an attractive therapeutic target for cancer therapy. Proton pump inhibitors (PPIs) also affect the integrity of the intestinal microbiota and have been associated with gut dysbiosis.14–16 PPIs are drugs used to suppress gastric-acid production and treat gastrointestinal disorders such as gastro-esophageal reflux and gastric ulcers. They have been considered low-risk and widely adopted; thus, PPIs are often over-prescribed across the world.14,15,17 It has been reported that multiple oral bacteria that related cancer development were found in the feces of PPI users.14 Importantly, several retrospective studies have identified a possible association between PPI use and poor overall survival (OS) of non-small cell lung cancer (NSCLC) patients treated with ICB.4,18,19 However, the mechanisms underlying the association between PPI use and the detrimental effects on ICB efficacy have not been elucidated.4,13,17–20 Also, there is no effective treatment that restores the decreased therapeutic efficacy of ICB in cancer patients receiving PPIs or concomitant use of PPIs and antibiotics.4,5,13,20
The clinical value of modulating gut microbiota by administration of specific bacterial species in cancer patients receiving ICB remains largely unknown.4,8,20–22 C. butyricum is a butyrate-producing, spore-forming anaerobic bacterium and found in healthy human and animal intestines, and also in environments, including soil and vegetables.8 C. butyricum has been investigated for potential protective effects in dysbiosis-associated diseases, including gut infection, irritable bowel syndrome, inflammatory bowel disease, and metabolic disease and safely used in the clinical setting for decades in Japan.8 In addition, accumulating evidence has shown C. butyricum modulates host-immunity through producing short-chain fatty acids (SCFAs), butyrate, and acetate.8,23 In 2020, we reported that manipulating commensal microbiota by C. butyricum therapy using a single microbial, live biotherapeutic bacterium, C. butyricum MIYAIRI 588 strain (CBM588, MIYA-BM®) has the potential to enhance the efficacy of ICB in patients with advanced NSCLC.20 A randomized phase IB clinical trial comparing nivolumab/ipilimumab with or without CBM588 in patients with metastatic renal cell carcinoma is ongoing (NCT03829111) and the preliminary results suggested that CBM588 has the capacity to modulate the gut microbiome and enhance the response to ICB.22
In the current study, we hypothesized that PPI use may impact on the gut microbial composition in advanced NSCLC and CBM588 may restore the diminished therapeutic efficacy of ICB in cancer patients receiving PPIs. To test this, we retrospectively evaluated the impact of PPI use and CBM588 on survival in 118 advanced or recurrent NSCLC patients treated with ICBs. In addition, we investigated the association between PPI usage and the oral-gut microbiome axis of patients with thoracic cancer using 16S rRNA sequencing of gene amplicons of 80 fecal samples. We show that CBM588 improved the decreased efficacy of ICB in NSCLC patients who received PPIs. Bacterial 16S rRNA sequencing and taxonomic analyses revealed that PPI users had higher abundance of oral-related pathobionts and cancer patients who received CBM588 had lesser relative abundance of harmful oral-related bacteria for immunotherapy. These findings support the hypothesis that manipulating commensal microbiota by CBM588 has the potential to improve the efficacy of ICB and highlight the potential of oral-related microbiota in the gut as a new therapeutic target for cancer immunotherapy.
Materials and methods
Patients
We retrospectively evaluated 118 patients with NSCLC consecutively treated with ICB therapy in routine clinical practice at Kumamoto University Hospital between January 1, 2016 and May 31, 2019. The patients are the same cohort as who have been previously reported.20 A total of 99 men and 19 women [median (range) age, 68 (37–83) years] with advanced or recurrent NSCLC were included in this study. The clinical information for all NSCLC patients has been fully updated and reanalyzed for the analyses. Patients with NSCLC received anti-PD-(L)1 antibody alone or in combination with chemotherapy. The medical records of patients who had received nivolumab, pembrolizumab, or atezolizumab were reviewed. Treatments were provided until disease progression, unacceptable toxicity, or consent withdrawal. All patients enrolled in this study were Japanese. The following data were extracted from the database: date, type of treatment, age, sex, histology, PD-L1 status, stage at initial diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking status, driver mutations, the response to ICB, any oral or intravenous PPIs used within a period of 30 days prior and 30 days after initiation of ICB therapy, any oral or intravenous antibiotics used within the 60 days before the start of ICB therapy, and C. butyricum therapy using CBM588 (MIYA-BM®, Miyarisan Pharmaceutical Co., Ltd., Tokyo, Japan) prescribed within 6 months before beginning ICB therapy and/or concurrently with ICB therapy until cessation. The time frames for PPI use, antibiotic use and C. butyricum therapy were analyzed based upon prior analyses.17–20 Patient characteristics by PPI use within the 60-day window are summarized in Table 1. The histories of PPI use, antibiotic use, and C. butyricum therapy were extracted by using prescription database and also manually checked from medical records. Attending physician and pharmacists confirmed that patients had taken CBM588 as prescribed. This study was conducted in accordance with the amended Declaration of Helsinki. The present study was performed after approval by the Kumamoto University Institutional Review Board (IRB number, 1825, Approval Date, July 2, 2021), which also waived the need to obtain informed consent because the data were analyzed retrospectively and anonymously.
Table 1.
Summary of patient characteristics by PPI use within the 60-day window.
| PPI user N = 72 | PPI non-user N = 46 | P value | |
|---|---|---|---|
| Median age (IQR) | 67.0 (61.0–72.0) | 68.0 (60.5–72.0) | 0.83 |
| Sex, N (%) | |||
| Male | 61 (85%) | 38 (83%) | 0.80 |
| Female | 11 (15%) | 8 (17%) | |
| ECOG performance status, N (%) | |||
| 0 | 16 (22%) | 17 (37%) | 0.21 |
| 1 | 36 (50%) | 23 (50%) | |
| 2 | 16 (22%) | 6 (13%) | |
| 3 | 3 (4%) | 0 (0%) | |
| 4 | 1 (1%) | 0 (0%) | |
| Smoking history, N (%) | |||
| Current | 7 (10%) | 7 (15%) | 0.45 |
| Former | 56 (78%) | 31 (67%) | |
| Never | 9 (12%) | 8 (17%) | |
| Stage at initial diagnosis, N (%) | |||
| I–III | 32 (44%) | 16 (35%) | 0.34 |
| IV | 40 (56%) | 30 (65%) | |
| Histology, N (%) | |||
| Adenocarcinoma | 51 (71%) | 30 (65%) | 0.55 |
| Squamous/NOS | 21 (29%) | 16 (35%) | |
| EGFR mutation status, N (%) | |||
| wild-type | 54 (75%) | 35 (76%) | 0.53 |
| mutant | 5 (7%) | 1 (2%) | |
| Unknown | 13 (18%) | 10 (22%) | |
| PD-L1 status, N (%) | |||
| TPS ≥50% | 26 (36%) | 14 (30%) | 0.27 |
| TPS 1–49% | 10(14%) | 9 (20%) | |
| TPS <1% | 21 (29%) | 8 (17%) | |
| Unknown/Undeterminable | 15 (21%) | 15 (33%) | |
| ICB therapy line, N (%) | |||
| 1st line | 24 (33%) | 13 (28%) | 0.27 |
| 2nd line | 26 (36%) | 17 (37%) | |
| ≥3rd line | 22 (31%) | 16 (35%) | |
| Immune checkpoint inhibitor, N (%) | |||
| Nivolumab | 29 (40%) | 22 (48%) | 0.33 |
| Pembrolizumab | 34 (47%) | 22 (48%) | |
| Atezolizumab | 9 (13%) | 2 (4%) | |
|
ICB monotherapy/ combination therapy, N (%) |
|||
| Monotherapy | 66 (92%) | 41 (89%) | 0.75 |
| Combination therapy | 6 (8%) | 5 (11%) | |
| Antibiotic use within 60 days before the start of ICB therapy, N (%) | 32 (44%) | 14 (30%) | 0.18 |
| C. butyricum therapy using CBM588, N (%) | N = 25 (35%) | N = 14 (30%) | 0.69 |
| Before ICB initiation, N (%) | 3 (12%) | 5 (36%) | 0.22 |
| During ICB therapy, N (%) | 9 (36%) | 3 (21%) | |
| Before and during ICB therapy, N (%) | 13 (52%) | 6 (43%) | |
| Response to ICB, N (%) | N = 62 | N = 44 | 0.12 |
| CR | 3 (5%) | 1 (2%) | 0.85 |
| PR | 20 (32%) | 12 (27%) | |
| SD | 23 (37%) | 19 (43%) | |
| PD | 16 (26%) | 12 (27%) | |
| ORR | 37% | 30% | 0.73 |
| DCR | 74% | 73% | 1.00 |
Pembrolizumab/pemetrexed/platinum (N = 6), pembrolizumab/nab-paclitaxel/carboplatin (N = 4), and atezolizumab/bevacizumab/carboplatin/paclitaxel (N = 1) were used as combination therapies with ICBs and chemotherapies. Tumor responses of 108 patients were objectively assessed by pulmonary physicians according to Response Evaluation Criteria in Solid Tumors, version 1.1. For recurrent NSCLC, clinical stages at initial diagnosis were recorded. Abbreviations: IQR, interquartile; CR, complete response; ECOG, Eastern Cooperative Oncology Group; EGFR, Epidermal Growth Factor Receptor; DCR, disease control rate; ICB, immune checkpoint blockade; N., number; ORR, objective response rate; PD, progression disease; PD-L1, Programmed cell death ligand 1; PR, partial response; SD, stable disease; TPS, tumor proportion score.
Fecal sample collection from cancer patients
A total of 80 fecal samples were collected from 52 patients with thoracic cancer, who visited Kumamoto University Hospital, according to a protocol approved by The Kumamoto University Institutional Review Board (IRB number, 2287; approval date, January 23, 2018) from November 1, 2020 to April 23, 2021. Written informed consent from eligible patients willing to participate in this study was obtained. Fecal samples consecutively collected were all analyzed and there were no excluded samples in this study. A total of 38 men and 14 women with thoracic cancer were included. Median age was 70.5 years. The cohorts comprise 52 patients with locally advanced or metastatic thoracic cancer, including 43 NSCLC, 8 small cell lung cancer (SCLC), and 1 malignant mesothelioma (Supplementary Table S1). Around 0.1 g of fecal samples were collected and suspended in 900 μL of DNA extraction buffer (4 M guanidium thiocyanate, 100 mM Tris-HCl (pH 9.0), 40 mM EDTA (pH 8.0)), which can stabilize fecal samples under room temperature for up to 30 days, and transported to the laboratory within 5 days. Then, fecal samples were stored at −80°C. Fecal samples were analyzed by two experienced researchers who were blinded to the patients’ detailed clinical statuses. Patient records were reviewed to determine any oral or intravenous PPI use within 60 days before the time of stool collection, any oral or intravenous antibiotic use within 60 days before the time of stool collection, and C. butyricum therapy within 6 months before the time of stool collection.
Bacterial 16S rRNA gene sequencing and taxonomic analyses
Total DNA was extracted from fecal samples and purified using the High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany). The variable V3-4 regions of the 16S rRNA gene were amplified by PCR using a TaKaRa Ex Taq® Hot Start Version (Takara Bio Inc., Shiga, Japan) and the universal 16S primer set 341 F and 805 R, which contain the Illumina index and sequencing adapter overhangs. The pooled samples were sequenced using MiSeq Reagent Kit v3 (600-cycle; Illumina, Inc., CA, USA) on a MiSeq platform according to the manufacturer’s instructions. The raw sequence data were processed using quantitative insights into microbial ecology 2 (QIIME 2 2019.10) pipeline with DADA2 plugin and SILVA 138.1 rRNA database. For diversity analysis, the alpha-diversities were calculated using the Chao1 and Shannon index. For the beta-diversity analysis, unweighted UniFrac distance matrices were calculated and used to determine the distance between samples, and principal coordinate analysis (PCoA) was applied to generate two-dimensional plots. Statistical differences in alpha-diversities between the non-treatment group and treatment group were tested using the Mann-Whitney U-test. The significances of the groups in beta-diversities were tested using permutational multivariate analysis of variance (PERMANOVA). Relative abundances of genera between the groups were tested using Mann-Whitney U-test. Detailed methods are provided in Supplementary Materials and Methods.
Quantitative PCR
TB Green® Premix Ex Taq™ II (Takara Bio) was used to conduct quantitative PCR according to the manufacturer’s protocol. The following primer sets were used: Total bacteria, 5’-CGGYCCAGACTCCTACGGG-3’ and 5’-TTACCGCGGCTGCTGGCAC-3’, Clostridium butyricum, 5- AGTGATTGTCAGTAGTAGACGAGCG −3’ and 5- CATGCGCCCTTTGTAGC −3’. A quantitative PCR reaction was performed on a Thermal Cycler Dice Realtime System II (Takara Bio). The PCR conditions were 95°C for 30sec, followed by 38 cycles of 95°C for 5 s, and 60°C for 30 s. To create PCR controls, the number of CBM588, cultured independently, was counted under a phase-contrast microscope. The DNA was then extracted from the bacteria. The relative abundance of C. butyricum were calculated from the Ct values on the basis of the calibration curves made by serial dilution of the PCR controls. The significance of the groups was tested using Mann-Whitney U-test.
Statistical analysis
Patient characteristics were described according to the status of PPI use (PPI user versus PPI non-user) and compared using Fisher’s exact test for categorical data and Wilcoxon rank sum test for continuous data. We presented patient characteristics as medians as appropriate. PFS and OS were evaluated using the Kaplan–Meier method, with differences being estimated using the two-tailed log-rank tests. PFS was measured from the date ICB started to the date of documented progression or death. Patients who were alive and not known to have progressed were censored. OS was measured from the date ICB started to the date of death or last follow-up. The data cutoff date was April 30, 2020. For additional analyses, the Kaplan–Meier method was used to estimate 3-year PFS and OS rate, in which the data cutoff date was December 31, 2021. Survival analysis was conducted using univariate analyses and multivariate Cox proportional hazards regression models using propensity score to correct for potential confounding factors that may affect the treatment assignment. For multivariable modeling, we used propensity score adjustment for sex, age, ECOG performance status, histology, smoking history, PD-L1 status, initial stage, ICB therapy line, ICI monotherapy/combination therapies, antibiotic use, and C. butyricum therapy. Each factor was categorized as shown in Table 1. The method of propensity score adjustment preserved statistical power by reducing covariates into a single variable. To evaluate the adjusted effect of CBM588 or PPI, propensity scores were estimated through a binary logistic regression providing the predicted probability with making CBM588 or PPI have a function above background factors. Next, we performed survival analyses using multivariate Cox proportional hazard models with inverse probability of treatment weighting (IPTW) using the propensity score that balances the relevant characteristics between CBM588 group vs no CBM588 group, or between PPI user group vs PPI non-user group. To confirm the statistical robustness, we performed another method using the propensity score as covariate in multivariate Cox proportional hazard models. Statistical analyses were performed with R version 3.5.3 (The R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was indicated by P < .05.
Results
Patient characteristics
Of the 118 patients with NSCLC treated with anti–PD-1/PD-L1 antibody therapy, 72 (61%) received a PPI within the 60-day window. Table 1 presents patient characteristics by PPI use status. Of the 72 NSCLC patients using a PPI, 41 were using esomeprazole (56.9%), 17 lansoprazole (23.6%), 9 vonoprazan fumarate (12.5%), 3 omeprazole (4.2%), and 2 rabeprazole (2.8%) (Supplementary Table S2).
Among 118 NSCLC patients, 39 (33%) received CBM588 within 6 months before beginning ICB and/or concurrently with ICB (Table 1). Twenty five of 72 patients (35%) received CBM588 in PPI user group. Fourteen of 46 patients (30%) received CBM588 in PPI non-user group. The indications and characteristics of C. butyricum therapy by PPI use status are shown in Supplementary Table S3.
A total of 46 (39%) patients received antibiotic therapy within a period of 60 days prior to ICB therapy initiation. Characteristics of antibiotic therapy by PPI use status are shown in Supplementary Table S4. Thirty two of 72 patients (44%) received antibiotic therapy in PPI user group. Fourteen of 46 patients (30%) received antibiotic therapy in PPI non-user group. Quinolone and β-lactam–based antibiotic therapy were the most common antibiotics used for both groups. Among 32 patients who had received both PPIs and antibiotic therapy within the treatment windows, 16 (50%) received CBM588. Among 40 patients who had received PPIs but not received antibiotic therapy, 9 (23%) received CBM588.
PPI use associates with worse survival outcome in NSCLC patients treated with ICB
In the 118 patients with NSCLC treated with anti-PD-1/PD-L1 antibody therapy, PPI use was significantly associated with worse OS on univariable analysis (HR 2.47, 95% CI 1.28–4.74, P = .007; Log-rank test P = .005, median, 361 days versus NR, Figure 1(a)). We applied multivariate Cox proportional hazard models with IPTW using the propensity score. Antibiotic use and C. butyricum therapy were used as the background factors in addition to other clinical factors. The propensity score analysis confirmed that PPI use was independently associated with worse OS (IPTW-adjusted HR 3.39, 95% CI 1.62–7.11, P = .001). No significant differences in PFS were found for PPI use (Supplementary Figure S1A and Supplementary Table S5). To confirm statistical robustness, we performed another method using the propensity score as covariate in Cox proportional hazards regression models, which also confirmed that PPI use was independently associated with shorter OS (HR 2.18, 95% CI 1.09–4.34, P = .027). Subgroup analyses were conducted based on various clinicopathological factors (Figure 1(b) and Supplementary Figure S1B). The results were consistent with those of the whole-cohort analyses, with the OS, but not the PFS, being superior in the PPI non-user group in most of the analyses. We also confirmed that PPI use within the 60-day window was consistently associated with shorter 3-year OS (IPTW-adjusted HR 1.89, 95% CI 1.17–3.07, P = .009) (Supplementary Figure S2).
Figure 1.
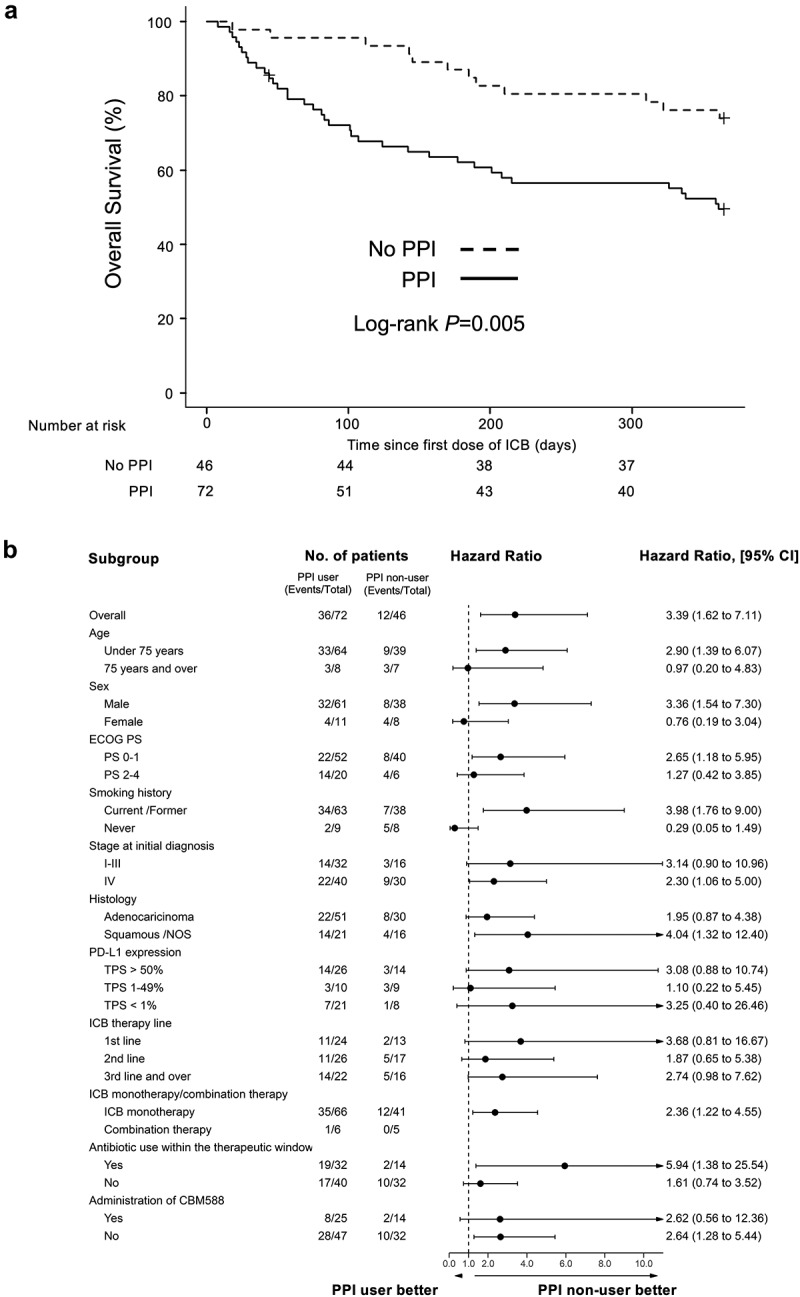
Kaplan-Meier estimate of survival outcome for PPI users vs non-users in NSCLC patients treated with ICB. (a) OS in NSCLC patients treated with ICB, stratified by PPI usage within a period of 30 days prior and 30 days after initiation of anti-PD-(l)1 antibody therapy is shown. (b) Subgroup analysis of OS among all patients.
C.butyricum therapy restores the decreased efficacy of ICB in PPI users.
There is no effective treatment that restores the decreased efficacy of ICB in cancer patients receiving PPIs. We hypothesized that C. butyricum therapy using CBM588 may improve the therapeutic outcomes of ICB in NSCLC patients who receive PPIs. We evaluated the impact of CBM588 on survival in those who received or those who did not receive PPIs within the 60-day window. Patients who received PPIs had improved PFS (HR 0.52, 95% CI 0.29–0.94, P = .030, Log-rank test P = .030, median PFS, 250 days versus 88 day) and OS (HR 0.42, 95% CI 0.19–0.92, P = .030, Log-rank test P = .030, median OS, NR versus 208 days) when given CBM588 compared to those not given CBM588 (Figure 2(a) and Figure 3(a)). We applied multivariate Cox proportional hazard models with IPTW using the propensity score. Antibiotic usage was used as the background factors in addition to other clinical factors. The propensity score analysis confirmed that PFS (IPTW-adjusted HR 0.42, 95% CI 0.23–0.76, P = .004) and OS (IPTW-adjusted HR 0.25 95% CI 0.10–0.61, P = .003) were significantly longer for patients who had received CBM588 compared with those who had not. To confirm statistical robustness, we performed another method using the propensity score as covariate in Cox proportional hazards regression models, which confirmed that CBM588 was independently associated with longer PFS (HR 0,40, P = .008, 95% CI 0.21–0.79) and OS (HR 0.3, P = .008, 95% CI 0.12–0.73). Subgroup analyses were conducted based on various clinicopathological factors (Figure 2(b) and Figure 3(b)). The results were consistent with those of the whole-cohort analyses, with the PFS and OS, being superior in the CBM588 group in most of the analyses. In patients with no PPIs, CBM588 did not improve PFS (median, 192 days versus 152 days, P = .32) and OS (median, NR versus NR, P = .23) (Supplementary Table 6–7). We also confirmed that CBM588 was consistently associated with longer 3-year PFS (IPTW-adjusted HR 0.48, 95% CI 0.27–0.87, P = .016) and OS (IPTW-adjusted HR 0.39, 95% CI 0.19–0.80, P = .011) (Supplementary Figure S3). These results suggest that CBM588 have the potential to improve the decreased efficacy of ICB in NSCLC patients who received PPIs.
Figure 2.
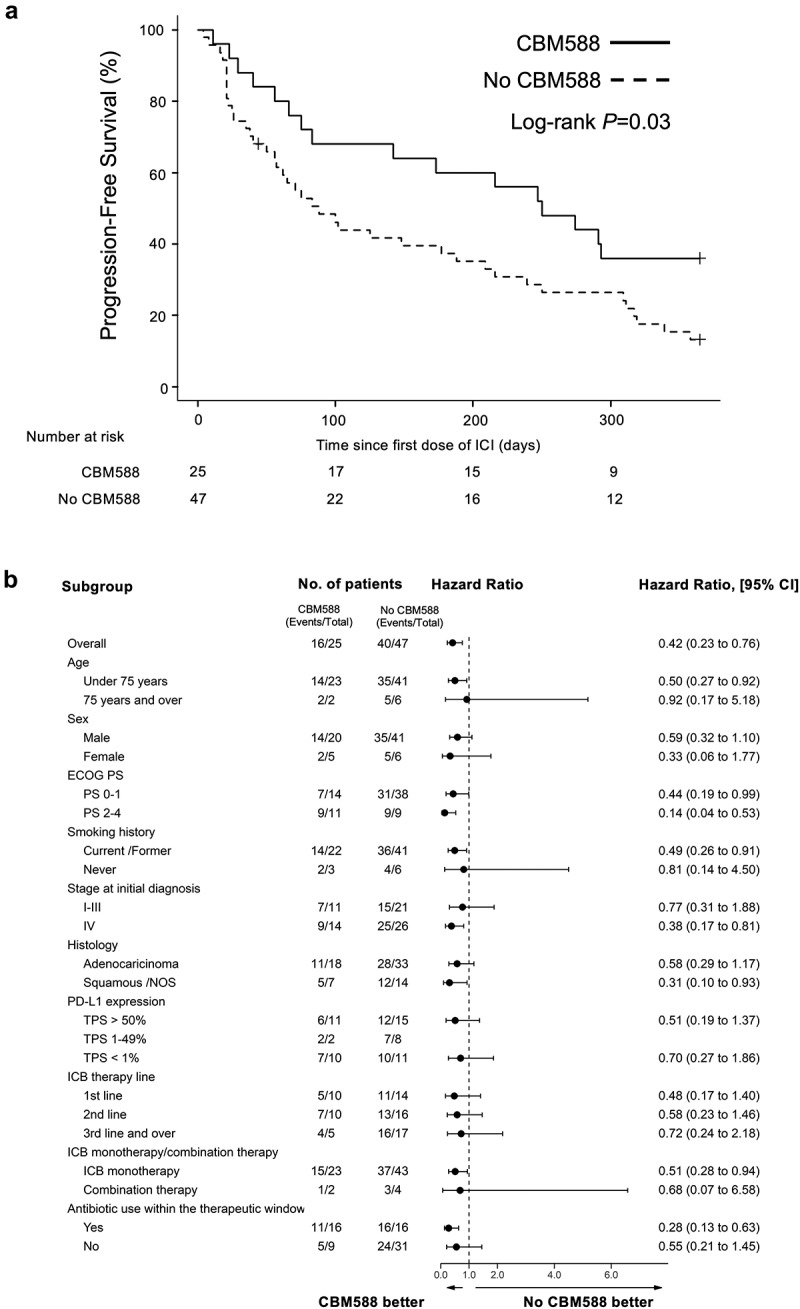
Kaplan-Meier estimate of survival outcome in NSCLC patients received PPIs within the 60-day window. (a) PFS in NSCLC patients who received PPIs, stratified by administration of CBM588 is shown. (b) Subgroup analysis of PFS among PPI users.
Figure 3.
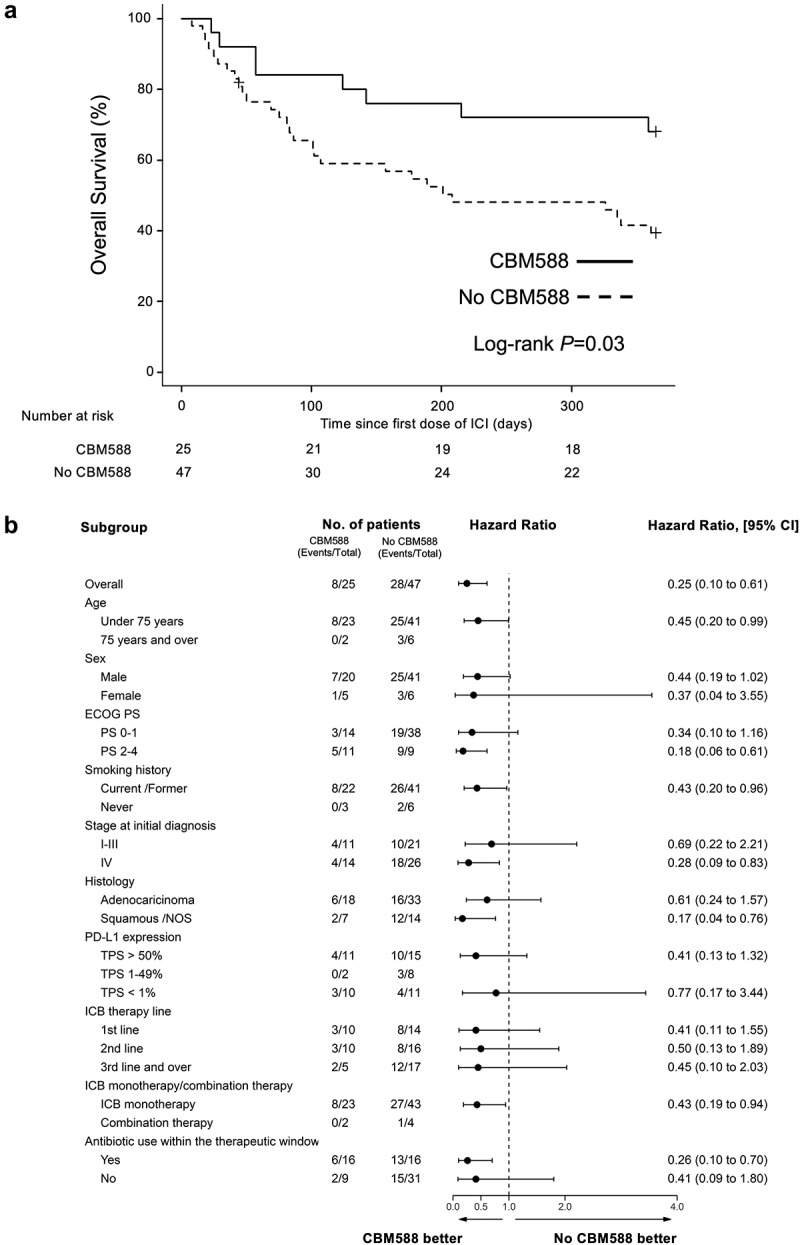
Kaplan-Meier estimate of survival outcome in NSCLC patients received PPIs within the 60-day window. (a) OS in NSCLC patients who received PPIs, stratified by administration of CBM588 are shown. (b) Subgroup analysis of OS among PPI users.
C.butyricum therapy restores the decreased efficacy of ICB in NSCLC patients who received PPIs plus antibiotics.
Retrospective studies have reproducibly shown that exposure to antibiotics prior to receiving ICB therapy is especially detrimental to the clinical outcome.4,6,9,24,25 Thus, we investigated the influence of antibiotic therapy within 60 days before the start of ICB therapy on clinical outcomes in patients who received or those who did not receive PPIs.
Thirty two of 72 PPI users (44%) received antibiotic therapy in the 60 days prior to ICB initiation. Patients who received PPIs plus antibiotics showed a trend toward shorter PFS (HR 1.65, 95% CI 0.97–2.80, P = .07; Log-rank test P = .06; median, 75 days versus 209 days) and OS (HR 1.62, 95% CI 0.84–3.11, P = .15; Log-rank test P = .15; median, 211 days versus NR) compared with patients who received only PPIs (Supplementary Figure S4). Fourteen of 46 PPI non-users (30%) received antibiotic therapy in the 60 days prior to ICB initiation. No differences in PFS (median, 164 days versus 178 days, P = .80) and OS (median, NR versus NR, P = .27) were found for antibiotic therapy in PPI non-user group, suggesting that the combination of PPIs with antibiotics is more detrimental than only PPI or antibiotic use to the clinical outcome.
Next, we evaluated the impact of CBM588 on survival in 32 patients who received both PPIs and antibiotics within the therapeutic windows. Patients who received antibiotics plus PPIs had significantly improved PFS (HR 0.28, 95% CI 0.13–0.63, P = .002; Log-rank test P = .001; median PFS, 194 days versus 32 days) and OS (HR 0.26 95% CI 0.10–0.70, P = .008; Log-rank test P = .005; median OS, NR versus 79 days) when given CBM588 compared to those not given CBM588 (Figure 4). We also confirmed that CBM588 was consistently associated with longer 3-year PFS (HR 0.48, 95% CI 0.27–0.87, Log-rank test P = .001) and OS (HR 0.39, 95% CI 0.19–0.80, Log-rank test P = .005) in patients who received antibiotics plus PPIs (Supplementary Figure S5), suggesting CBM588 restores the decreased therapeutic efficacy of ICB even in patients who received antibiotics plus PPIs.
Figure 4.
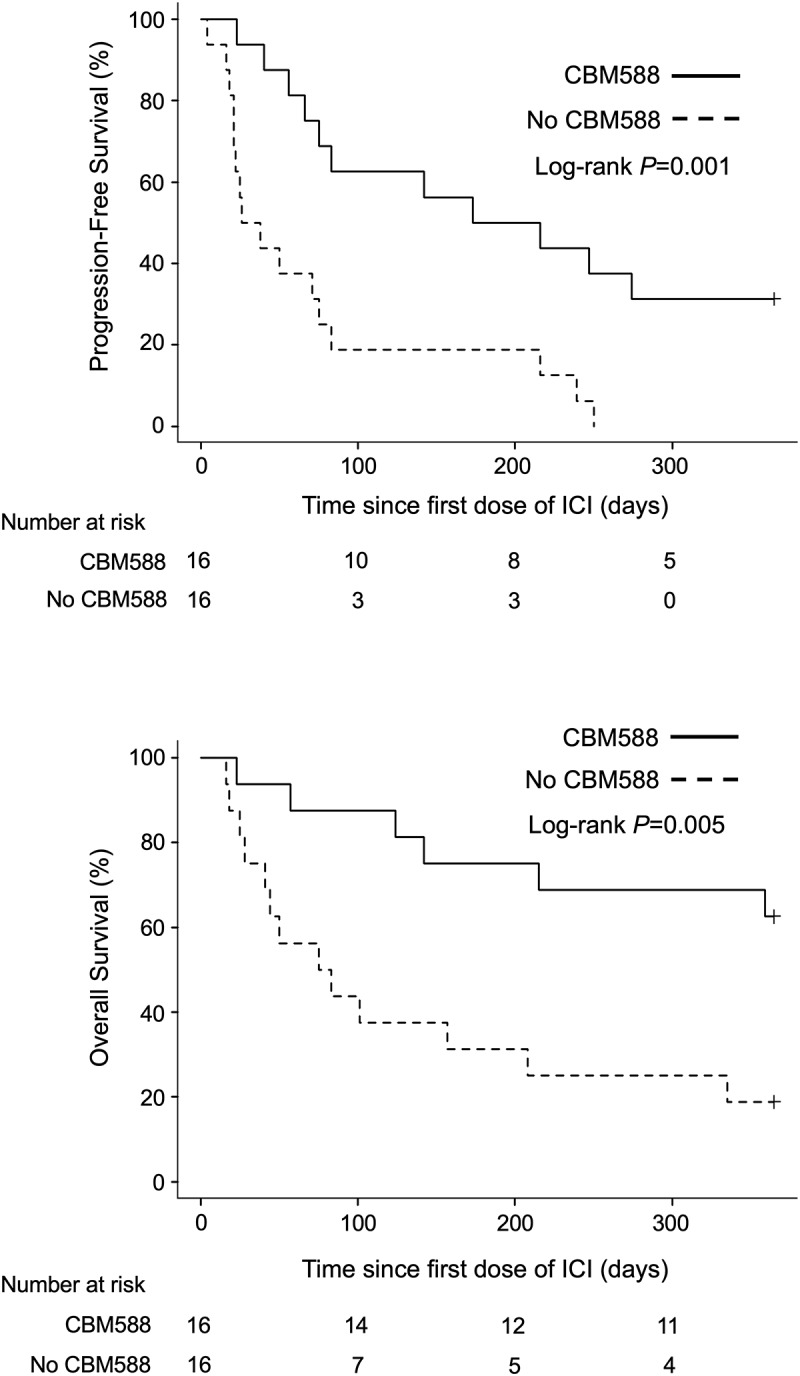
Kaplan-Meier estimate of survival outcomes in NSCLC patients received PPIs and antibiotics. PFS (upper panel) and OS (lower panel) in NSCLC patients who received PPIs and antibiotics, stratified by administration of CBM588 are shown.
Influence of PPI on the gut microbiome in cancer patients
PPIs are known to induce gut microbiota changes in non-cancer individuals.14,15 However, the impact of PPI use on gut microbial composition in patients with thoracic cancer has not yet been investigated. We collected a total of 80 fecal samples from the 52 patients with locally advanced or metastatic thoracic cancer and investigated the influences of PPI monotherapy (PPI; n = 22, 27.5%), antibiotic monotherapy (ATB; n = 19, 23.7%), and concomitant use of PPIs and antibiotics (ATB+PPI; n = 13, 16.3%) on gut microbiome. In 26 of 80 fecal samples, neither PPIs nor antibiotics were used (non-treatment, n = 26, 32.5%) (Supplementary Table 1).
We first compared the median alpha-diversity between four groups. 16S metagenomic sequencing showed there were no significant differences across multiple diversity metrics (Shannon and Chao1, Figure 5(a)). PCoA for microbial beta-diversity between groups was performed and the two-dimensional plot for unweighted UniFrac distance matrices was depicted. There was a significant different taxonomy structure between non-treatment group and PPI group, and a trend of difference between non-treatment group and ATB+PPI group (Figure 5(b)). Next, we assessed the relative abundance of gut microbiome taxa. Potentially beneficial bacteria for immunotherapy,4 Lachnospiraceae uncultured and Ruminococcus were significantly decreased or tended to decrease in PPI users, respectively (Figure 5(c)).
Figure 5.
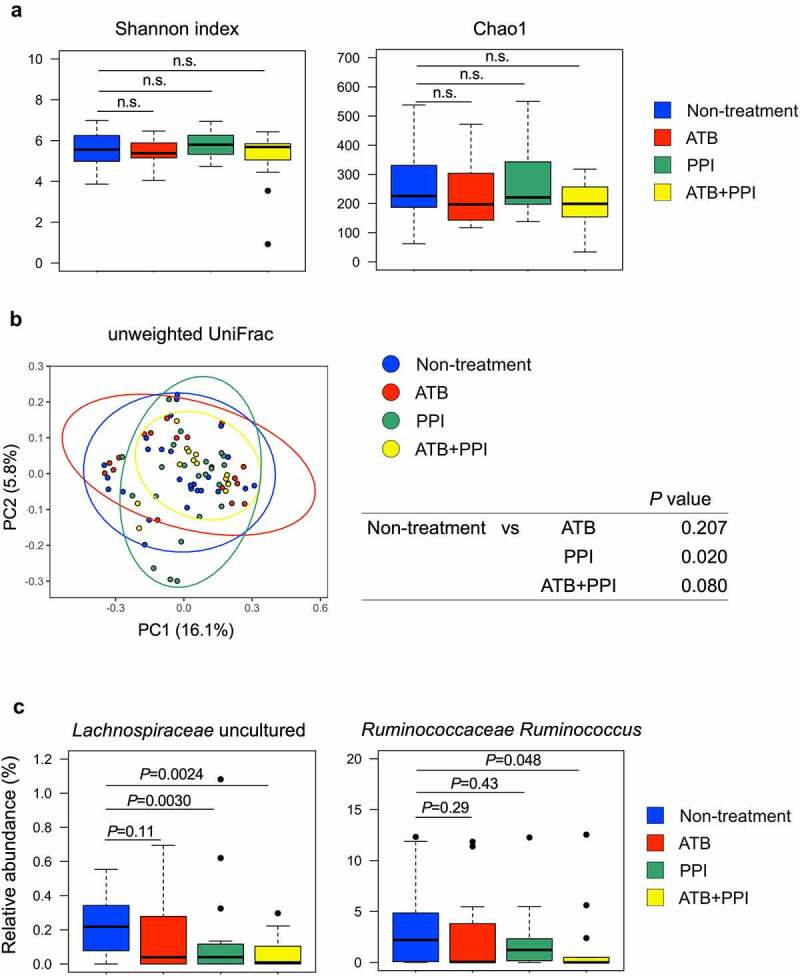
16S Metagenomic analyses of fecal samples in patients with locally advanced or metastatic thoracic cancer. (a) Alpha-diversity for 80 fecal samples from 52 cancer patients stratified according to the four groups; non-treatment (n = 26), antibiotic use (ATB; n = 19), PPI use (PPI; n = 22), concomitant use of antibiotics and PPIs (ATB+PPI; n = 13). Shannon or Chao1 indices are shown. The bold line represents the median. The bottom and top hinges correspond to the first and third quartiles (the 25th and 75th percentiles). n.s. indicates not significant. (b) PCoA plot of the unweighted UniFrac distance for beta-diversity stratified according to the four groups. (c) Relative abundance of potentially beneficial genera for immunotherapy, Lachnospiraceae uncultured and Ruminococcus, are shown. A Mann-Whitney U test was used to assess statistical differences compared to the non-treatment group.
Accumulating pieces of evidence suggests several oral-related pathobionts translocated into the gut may negatively influence the efficacy of ICB in cancer patients26–28 or promote the development of lung cancer.29–32 Thus, we focused on the bacterial genera typically found in the oral microbiome.33 Twenty-nine oral-related bacteria were detected in cancer patients’ fecal samples and relative abundance of oral-related bacteria were significantly enriched in PPI group and tended to increase in ATB+PPI group (Figure 6(a)). Overall compositions of gut microbiota at each taxonomy level were showed in the Supplementary Figure S6. In contrast, the composition of resident gut microbiota was not altered by PPI use (Supplementary Figure S7). The oral-related bacteria which is known to be associated with cancer development and/or detrimental effects on the efficacy of ICB26–32 were significantly increased in PPI group (Granulicatella, Haemophlus, Actinomyces, Gemella, Rothia, Streptococcus, Atopobium, and Veillonella) and ATB+PPI group (Granulicatella, Haemophlus, Actinomyces, Gemella, Rothia, and Streptococcus) (Figure 6(b)). These results suggest that PPI use leads to unhealthy gut microbiome in lung cancer patients.
Figure 6.
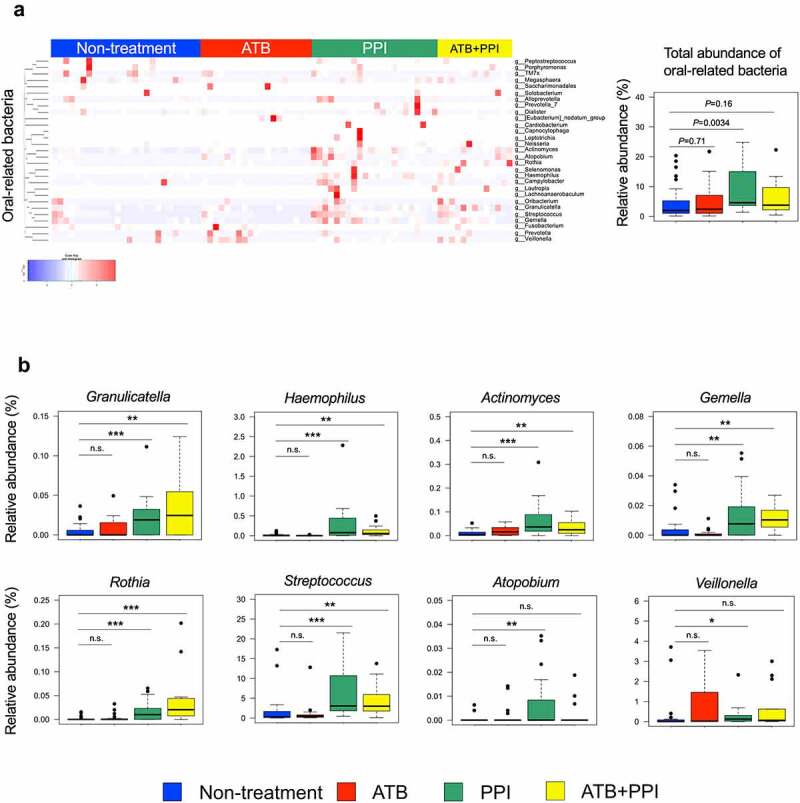
Fecal microbiota differences in patients with thoracic cancer who receiving PPIs and/or antibiotics. (a) Heatmap of scaled relative abundances of oral-related bacteria detected in the gut of patients with thoracic cancer. Right box plot indicates comparing total relative abundance of the 29 genera of oral-related bacteria. A Mann-Whitney U test was used to assess statistical differences between indicated two groups. (b) Box plots comparing the relative abundance of the genera typically found in the oral microbiota (Granulicatella, Haemophilus, Actinomyces, Gemella, Rothia, Streptococcus, Atopobium, and Veillonella) are shown. A Mann-Whitney U test was used to assess statistical differences between indicated two groups (* p < .05, ** p < .01, *** p < .001, n.s. not significant). The number of samples in four groups; non-treatment (n = 26), antibiotic use (ATB; n = 19), PPI use (PPI; n = 22), concomitant use of antibiotics and PPIs (ATB+PPI; n = 13).
Impact of CBM588 on the gut microbial composition in cancer patients
Next, we investigated the impact of CBM588 on the gut microbial composition in cancer patients. CBM588 had been administered in patients for whom 31 samples could be harvested and analyzed. The duration of CBM588 administration before the time of stool collection is shown in Supplementary Table S8. Overall fecal microbiota compositions at each taxonomy level compared between in the patients who treated with CBM588 and did not were shown in the Supplementary Figure S8 and Figure 7a. Patients who received CBM588 had greater relative abundance of C. butyricum than those did not receive CBM588 (Figure 7(b)). CBM588 has been shown to increase resident Bifidobacterium, which is known as a potentially beneficial bacteria for immunotherapy.4,22,34,35 16S rRNA gene sequencing of fecal samples showed a 1.8-fold non-significant increase in Bifidobacterium in cancer patients who received CBM588 (Figure 7(c)). Patients who received CBM588 had lesser relative abundance of potentially harmful oral-related bacteria for immunotherapy, Atopobium (Figure 7(d)).4,27 The relative abundance of a lung cancer-associated oral-related bacteria, Streptococcus, tended to be reduced in the subset of patients who took CBM588 compared with the subset who did not.29,32 These results suggest that the presence of a live biotherapeutic bacterium CBM588 in the gut of cancer patients may provide a beneficial impact on gut commensals.
Figure 7.
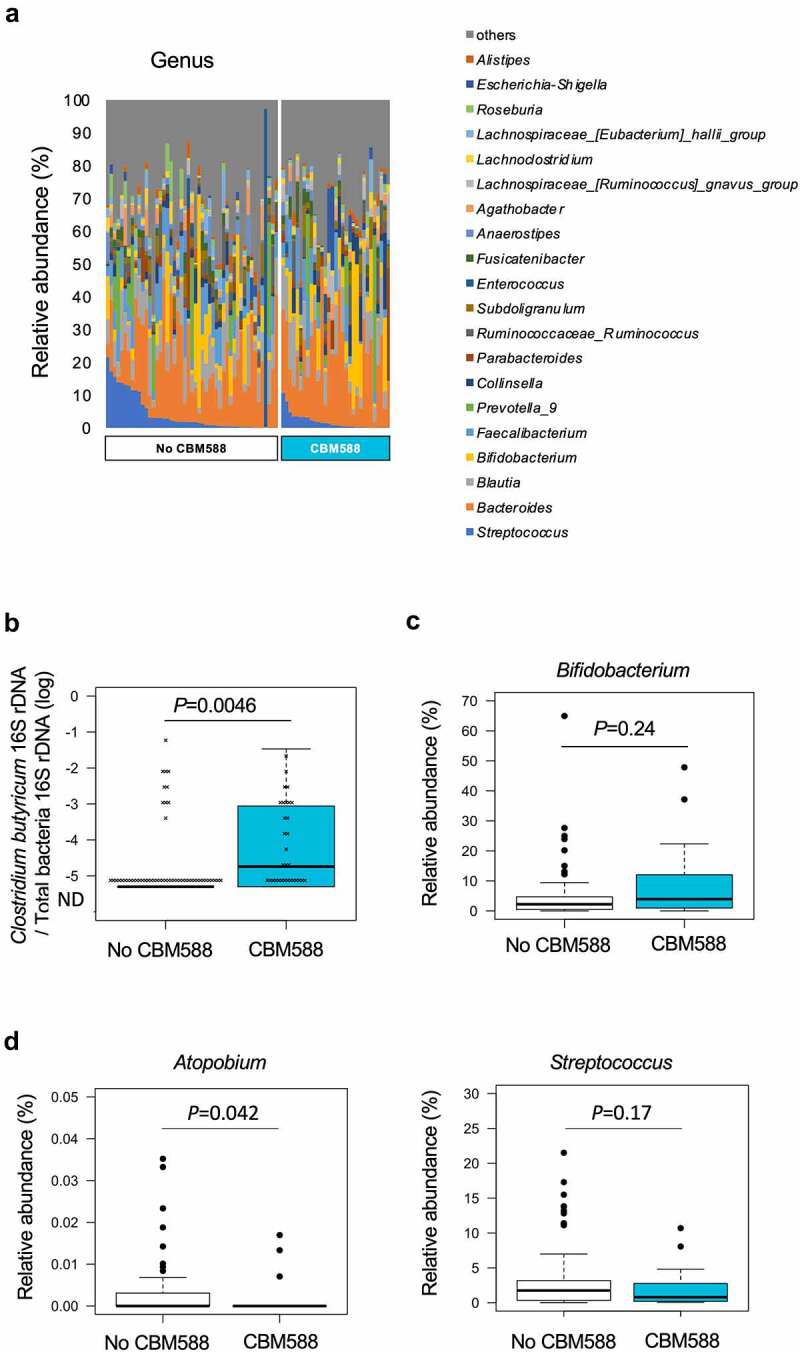
Fecal microbiota differences in patients with thoracic cancer treated with CBM588. (a) Stacked bar charts indicate the gut microbiota composition compared in the two groups; No CBM588, n = 49, CBM588, n = 31 at genus level. The data are sorted by the richness of the taxonomic category which includes genus Streptococcus. Only the 15 or 20 most abundant bacterial genera are shown. (b) Box plots comparing the abundance of C. butyricum in 80 fecal samples. Quantitative PCR analysis of C. butyricum in 80 fecal samples was performed. A Mann-Whitney U-test was used to assess statistical differences between indicated two groups. No CBM588, n = 49, CBM588, n = 31. (c) Relative abundance of Bifidobacterium is shown. (d) Relative abundance of Atopobium and Streptococcus are shown.
Discussion
The gut microbiota represents a complex ecosystem essential for maintaining intestinal immune homeostasis.7,36–38 The interactions between the gut microbiota and host immunity play a key role in human health and disease.39 A number of studies have reproducibly shown that a disruption of the homeostatic balance within the gut microbiome due to antibiotic exposure impairs response to ICB in advanced or recurrent NSCLC, suggesting a causal link between antibiotics use, dysbiosis, and poor ICB efficacy.4,9 Similarly, significant negative prognostic associations of PPI use on survivals in patients with NSCLC, melanoma, and urothelial carcinoma treated with ICB were shown.17–19 However, there had been no evidence to show the association of microbial alterations in the gut associated with PPI use or with concomitant use of PPIs and antibiotics in lung cancer. In addition, no treatment to restore the decreased therapeutic efficacy of ICB in patients receiving PPIs is available.4,5,13 In the current study, we showed PPI use was independently associated with shorter OS in NSCLC patients treated with ICB, which is consistent with the findings of previous studies.18,19 PPIs tend to be used for extended time periods in cancer patients, which may result in potential long-lasting detrimental effects on the efficacy of ICB.17–19 Given the widespread use of PPIs, clinicians should take into consideration the negative influence of PPIs on the efficacies of ICB.
We have previously shown that CBM588 has the potential capacity to restore the clinical activity of ICB in NSCLC patients who took antibiotics before the initiation of ICB.20 The impact of CBM588 on patients’ survival was more significant in cancer patients who received antibiotic therapy than those who did not received antibiotic therapy,20 suggesting that a beneficial impact of CBM588 on gut microbiome may be enhanced under the condition of drug-induced gut dysbiosis. Based on these findings, we hypothesized that C. butyricum therapy using CBM588 may reduce the negative effects of PPIs on ICB efficacy. In this study, we demonstrated for the first time that CBM588 restored the decreased efficacy of ICB in cancer patients who received PPIs.
A recent study highlighted the impact of concomitantly using two dysbiosis-inducing drugs, PPIs and antibiotics. The magnitude of the negative association between PPI use and OS was greater in patients with urothelial carcinoma who received antibiotics in the 60 days prior to ICB therapy.17 In consistent with the result, we demonstrated that the concomitant use of antibiotics plus PPIs was more detrimental than only PPI use to the clinical outcome in NSCLC treated with ICB therapy. Importantly, in the subgroup analysis of patients who received both PPIs and antibiotics, CBM588 restored the decreased efficacy of ICB. These results suggest that manipulating commensal microbiota by CBM588 has the potential to reduce the negative effects of concomitant use of two major dysbiosis-inducing drugs, PPIs, and antibiotics, on ICB efficacy.
Emerging evidence suggests a detrimental effect of PPIs on ICB efficacy.4,13,18,19 There is a postulated link between microbiome dysbiosis induced by PPIs and poor ICB efficacy, however, the mechanism by which this occurs has not been elucidated. Although PPIs are known to induce gut microbiota changes in non-cancer individuals,14,15 it remains unclear whether PPI use or concomitant use of PPIs and antibiotics indeed impact on the gut microbial composition in cancer patients. Therefore, we investigated the effect of PPI use or concomitant use of PPIs and antibiotics on the gut microbial composition in patients with thoracic cancer. It has been reported that the changes in the gut microbiome associated with PPI use are caused by reduced acidity of the stomach and the subsequent survival of more bacteria that are ingested with food and oral mucus.14,15 In our study, beta-diversity between PPI users and PPI non-users was significantly different, indicating that the taxonomy community structure differs in cancer patients. In consistent with the findings previously reported in healthy individuals,14,15 we found a significantly higher abundance of oral-related commensals in the gut of PPI users with lung cancer. Veillonella, Gemella, Atopobium, Streptococcus, Actinomyces, and Haemophlus have been shown to be enriched in the gut of NSCLC, hepatocellular carcinoma, and melanoma patients with unfavorable response to ICB.26–28 Rothia, Veillonella, Streptococcus, Gemella, Atopobium, Haemophlus, Granulicatella, and Actinomyces have been shown to be abundant in patients with NSCLC, pancreatic cancer, and colorectal cancer compared with healthy individuals or nonmalignant control. These findings suggest that PPIs significantly alter the composition of gut microbiota by allowing the oral microbiome to translocate into the gut, which may lead to poor ICB efficacy and cancer progression in PPI users.29–32,40,41
Recent preliminary results of clinical studies have shown the ability of live biotherapeutic bacterium to induce compositional shifts in the gut microbiome and to provide the positive impact on the clinical benefit to ICB.4,20,22,42 However, the impact of CBM588 on gut microbiota in patients with thoracic cancer had remained unknown. In the current study, we found patients who received CBM588 had a lesser abundance of potentially harmful oral-related bacterial genera, Atopobium and Streptococcus,26,27 suggesting that CBM588 may have the potential to shift the gut dysbiosis to a favorable microbiota.
It is reported that Akkermansia muciniphila was associated with clinical benefit of ICI in patients with NSCLC cancer, whereas the genus Clostridium including Clostridium innocuum and Hungatella hathewayi was associated with resistance to ICI by Derosa et al.43 However, in our study, there were no significant differences in the relative abundances of Akkermansia and Clostridium between PPI users and PPI non-users (data not shown).
Mager et al. reported Bifidobacterium pseudolongum produces inosine and enhances the efficacy of ICB.34,35 It has been shown that CBM588 modulates composition of gut microbiome and increases resident Bifidobacterium in a murine model and cancer patients.8,22 In consistent with the results reported by Dizman and Meza et al.,22 our study showed that resident Bifidobacterium tends to increase in the gut of lung cancer patients who received CBM588 of the presence of C. butyricum in the gut microbiome. These lines of evidence support the hypothesis that CBM588 may have the potential to improve the therapeutic efficacy of ICB through the modulation of gut microbiota.
Retrospective and prospective clinical studies have reported CBM588 significantly enhanced the efficacy of ICB,20,22 however, the underlining mechanism remains unknown. Dietary fiber is fermented to butyrate by C. butyricum,8 and the butyrate promotes the epithelial barrier function and has potent epigenetic regulatory activity.8,23 Bachem et al. revealed the microbiota-derived butyrate enhanced the memory potential of activated CD8+ T cells in a murine model.44 In addition, it has been shown that high concentration of fecal butyrate in cancer patients treated with ICB was significantly associated with longer PFS.45 C. butyricum produces a robust amount of butyrate,8 which might have played a key role in improving the efficacy of ICB in PPI users. The association of fecal butyrate with survival benefits in patients treated with ICB in combination with or without CBM588 need to be assessed in prospective studies.
IFN-γ-producing CD4+ T helper 1 (Th1) cells play a key role in antitumor immunity,46,47 and IFN-γ itself is tumoricidal and stimulates tumor-specific cytotoxic T cell.47,48 In a recent preclinical study, we demonstrated that CBM588 has immunomodulatory effects and increases Th1 cells during Clostridioides difficile infection.23 We speculate that CBM588 may influence the T cells unleashed by ICB and promote the induction of Th1 cells, resulting in enhanced clinical activity of ICB in cancer patients receiving dysbiosis-inducing drugs. Accumulating evidence warrants further study in clinical setting.
Our study has limitations in view of the retrospective nature, a small sample size, and heterogeneity of a study cohort. It has been shown that diet including dietary fiber intake, lifestyle, or genetics can affect the composition of the gut microbiota.21,49,50 We did not assess these possible factors impacting patients’ gut microbiome. Although the ethnic origins of individuals are also an important factor to consider in microbiome research,49 only Japanese patients were analyzed in our study. We speculate that CBM588 may modulate gut microbiota and shift an unfavorable to a favorable microbiota, leading to increase the clinical activity of ICB. However, we did not assess the dose–response relationship and characterize the mechanism by which CBM588 exerts a positive effect on clinical outcomes in cancer patients who received PPIs or PPIs plus antibiotic therapy. We could only observe the trend of the positive effects of CBM588 on the putative unfavorable oral-related microbiota due to its small numbers of the patients. Profiling of the gut microbiome and systemic immunity pre and post ICB therapy with or without CBM588 in a prospective study is essential to elucidate the mechanism of how CBM588 impact on clinical outcomes of ICB in lung cancer patients.
In conclusion, our findings support the hypothesis that C. butyricum therapy using CBM588 may restore the decreased clinical efficacy of ICB in patients who receive PPIs, providing a rationale for combining CBM588 with immunotherapies, especially in cancer patients who receive PPIs or PPIs plus antibiotic therapy within the particular therapeutic windows. The oral-related microbiota in the gut could be a potential new therapeutic target for cancer immunotherapy. Despite the acknowledged limitations, our findings provide the first evidence that manipulating commensal microbiota by CBM588 may improve the therapeutic efficacy of ICB in cancer patients receiving dysbiosis-inducing drugs.
Supplementary Material
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 18K15928, 22K08256, and 20K16449.
Abbreviations
| CBM588 | Clostridium butyricum MIYAIRI 588 |
| CI | confidence interval |
| HR | hazard ratio |
| ICB | Immune checkpoint inhibitors |
| NSCLC | Non-small cell lung cancer |
| PPI | proton pump inhibitor |
Acknowledgments
We are very grateful to our patients for participation in this study.
Disclosure statement
Takuro Sakagami received research funding from Miyarisan Pharmaceutical Co., Ltd. to his institution. Ayaka Minemura, Kentaro Oka, Atsushi Hayashi, and Motomichi Takahashi are employees of Miyarisan Pharmaceutical Co., Ltd. Yusuke Tomita received fundings from the JSPS KAKENHI (Grant Number 18K15928 and 22K08256). Shinya Sakata received funding from the JSPS KAKENHI (Grant Number 20K16449).
The other authors have no conflicts of interest to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [YT], upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2022.2081010
References
- 1.Wolchok J. Putting the immunologic brakes on cancer. Cell. 2018;175(6):1452–16. doi: 10.1016/j.cell.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 3.Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 2021;19(1):37–50. doi: 10.1038/s41571-021-00552-7. [DOI] [PubMed] [Google Scholar]
- 4.Derosa L, Routy B, Desilets A, Daillere R, Terrisse S, Kroemer G, Zitvogel L. Microbiota-centered interventions: the next breakthrough in immuno-oncology? Cancer Discov. 2021;11(10):2396–2412. doi: 10.1158/2159-8290.CD-21-0236. [DOI] [PubMed] [Google Scholar]
- 5.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–388. doi: 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- 6.Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, Segata N, Desnoyer A, Pietrantonio F, Ferrere G, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. 2020;78(2):195–206. doi: 10.1016/j.eururo.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Fluckiger A, Daillere R, Sassi M, Sixt BS, Liu P, Loos F, Richard C, Rabu C, Alou MT, Goubet A-G, et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science. 2020;369(6506):936–942. doi: 10.1126/science.aax0701. [DOI] [PubMed] [Google Scholar]
- 8.Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M, McMurdie PJ, Kolterman O, Eid J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021;13(1):1–28. doi: 10.1080/19490976.2021.1907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 10.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 11.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaifaoui M, Mimpen I, Terrisse S, Derosa L, Zitvogel L, Routy B, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. 2020;8(10):1243–1250. doi: 10.1158/2326-6066.CIR-20-0196. [DOI] [PubMed] [Google Scholar]
- 13.Lee KA, Shaw HM, Bataille V, Nathan P, Spector TD. Role of the gut microbiome for cancer patients receiving immunotherapy: dietary and treatment implications. Eur J Cancer. 2020;138:149–155. doi: 10.1016/j.ejca.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJM, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins AM, Kichenadasse G, Karapetis CS, Rowland A, Sorich MJ. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin Cancer Res. 2020;26(20):5487–5493. doi: 10.1158/1078-0432.CCR-20-1876. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins AM, Kichenadasse G, McKinnon RA, Abuhelwa AY, Logan JM, Badaoui S, Karapetis CS, Rowland A, Sorich MJ. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: post hoc analysis of IMpower150. Br J Cancer. 2021;126(1):42–47. doi: 10.1038/s41416-021-01606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalabi M, Cardona A, Nagarkar DR, Dhawahir Scala A, Gandara DR, Rittmeyer A, Albert ML, Powles T, Kok M, Herrera FG, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31(4):525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S, et al. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res. 2020;8(10):1236–1242. doi: 10.1158/2326-6066.CIR-20-0051. [DOI] [PubMed] [Google Scholar]
- 21.Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022;28(4):704–712. doi: 10.1038/s41591-022-01694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi A, Nagao-Kitamoto H, Kitamoto S, Kim CH, Kamada N. The butyrate-producing bacterium Clostridium butyricum suppresses clostridioides difficile infection via neutrophil- and Antimicrobial cytokine-dependent but GPR43/109a-independent mechanisms. J Immunol. 2021;206(7):1576–1585. doi: 10.4049/jimmunol.2000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang XZ, Gao P, Song YX, Xu Y, Sun JX, Chen XW, Zhao J-H, Wang Z-N. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 2019;8(12):e1665973. doi: 10.1080/2162402X.2019.1665973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung MW, Kim MJ, Won EJ, Lee YJ, Yun YW, Cho SB, Joo YE, Hwang JE, Bae WK, Chung IJ, et al. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J Gastroenterol. 2021;27(42):7340–7349. doi: 10.3748/wjg.v27.i42.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, Koh AY. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19(10):848–855. doi: 10.1016/j.neo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song P, Yang D, Wang H, Cui X, Si X, Zhang X, Zhang L. Relationship between intestinal flora structure and metabolite analysis and immunotherapy efficacy in Chinese NSCLC patients. Thorac Cancer. 2020;11(6):1621–1632. doi: 10.1111/1759-7714.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H, Gao NL, Tong F, Wang J, Li H, Zhang R, Ma H, Yang N, Zhang Y, Wang Y, et al. Alterations of the human lung and gut microbiomes in non-small cell lung carcinomas and distant metastasis. Microbiol Spectr. 2021;9(3):e0080221. doi: 10.1128/Spectrum.00802-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu H, Ren Z, Li A, Li J, Xu S, Zhang H, Jiang J, Yang J, Luo Q, Zhou K, et al. Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. J Oral Microbiol. 2019;11(1):1563409. doi: 10.1080/20002297.2018.1563409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Druzhinin VG, Matskova LV, Demenkov PS, Baranova ED, Volobaev VP, Minina VI, Minina VI, Apalko SV, Churina MA, Romanyuk SA, et al. Taxonomic diversity of sputum microbiome in lung cancer patients and its relationship with chromosomal aberrations in blood lymphocytes. Sci Rep. 2020;10(1):9681. doi: 10.1038/s41598-020-66654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Druzhinin VG, Matskova LV, Demenkov PS, Baranova ED, Volobaev VP, Minina VI, Larionov AV, Titov VA, Fucic A. Genetic damage in lymphocytes of lung cancer patients is correlated to the composition of the respiratory tract microbiome. Mutagenesis. 2021;36(2):143–153. doi: 10.1093/mutage/geab004. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama H, Masago A, Nambu T, Mashimo C, Takahashi K, Okinaga T. Inter-site and interpersonal diversity of salivary and tongue microbiomes, and the effect of oral care tablets. F1000Res. 2020;9:1477. doi: 10.12688/f1000research.27502.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Man Lei Y, Jabri B, Alegre M-L, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 36.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nat. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell. 2016;165(2):276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Zitvogel L, Kroemer G. Cross-reactivity between cancer and microbial antigens. Oncoimmunology. 2021;10(1):1877416. doi: 10.1080/2162402X.2021.1877416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, Leeming E, Gibson R, Le Roy C, Khatib HA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021;27(2):321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leng Q, Holden VK, Deepak J, Todd NW, Jiang F. Microbiota biomarkers for lung cancer. Diagno (Basel). 2021;11: doi 10.3390/diagnostics11030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cameron SJS, Lewis KE, Huws SA, Hegarty MJ, Lewis PD, Pachebat JA, Mur LAJ. A pilot study using metagenomic sequencing of the sputum microbiome suggests potential bacterial biomarkers for lung cancer. PLoS One. 2017;12(5):e0177062. doi: 10.1371/journal.pone.0177062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takada K, Shimokawa M, Takamori S, Shimamatsu S, Hirai F, Tagawa T, Okamoto T, Hamatake M, Tsuchiya‐Kawano Y, Otsubo K, et al. Clinical impact of probiotics on the efficacy of anti-PD-1 monotherapy in patients with nonsmall cell lung cancer: a multicenter retrospective survival analysis study with inverse probability of treatment weighting. Int J Cancer. 2021;149(2):473–482. doi: 10.1002/ijc.33557. [DOI] [PubMed] [Google Scholar]
- 43.Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, Silva CAC, Terrisse S, Bonvalet M, Scherpereel A, et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315–324. doi: 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, Whitney PG, Fernandez-Ruiz D, Dähling S, Kastenmüller W, et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity. 2019;51(2):285–97 e5. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, Matsumoto S, Inoue K, Muto M. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3(4):e202895. doi: 10.1001/jamanetworkopen.2020.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 47.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70(21):8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 49.Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, Tremaroli V, Bakker GJ, Attaye I, Pinto-Sietsma S-J, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med. 2018;24(10):1526–1531. doi: 10.1038/s41591-018-0160-1. [DOI] [PubMed] [Google Scholar]
- 50.Xavier JB, Young VB, Skufca J, Ginty F, Testerman T, Pearson AT, Macklin P, Mitchell A, Shmulevich I, Xie L, et al. The cancer microbiome: distinguishing direct and indirect effects requires a systemic view. Trends Cancer. 2020;6(3):192–204. doi: 10.1016/j.trecan.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [YT], upon reasonable request.


