Abstract
Background
The dementias are long-term, chronic conditions caused by progressive neurological degeneration. Current literature suggests that cardiovascular disease risk factors may contribute to the onset of dementia; however, evidence of these associations is inconsistent.
Objectives
This study aimed to examine the impact of risk factors on dementia onset in older adults diagnosed and managed in Canadian primary care settings.
Methods
A retrospective cohort study was employed utilizing electronic medical records data in the Canadian Primary Care Sentinel Surveillance Network (CPCSSN). Patients aged 65+ years with no dementia diagnosis at baseline who were followed from 2009 to 2017 with a run-in year to exclude existing undiagnosed dementia cases. Multivariate Cox proportional hazard models were used to estimate risk.
Results
Age was associated with an increased incidence risk of dementia in both examined age groups: 65–79 years (13%) and 80+ years (5%). History of depression increased dementia risk by 38% and 34% in the age groups. There were significant associations with lower social deprivation area quintile, smoking history, osteoarthritis, and diabetes mellitus in patients aged 65–79 years but not in those aged 80+ years. Sex, hypertension, obesity, dyslipidemia, and the use of antihypertensive medications and statins were not associated with risk of incident dementia diagnosis.
Conclusions
The association between chronic health conditions and dementia onset is complicated. Primary care electronic medical record data might be useful for research in this topic, though follow-up time is still relatively short to observe a clear causal relationship. Future studies with more complete data may provide evidence for dementia preventive strategies within primary care practice.
Keywords: dementia, electronic medical records, mental health, primary care, risk factors, survival analysis
Key messages.
The association between dementia and its risk factors are complicated.
Primary care data might be useful to study dementia and its comorbidities.
Age, smoking, depression, osteoarthritis, diabetes may increase risk of dementia.
Background
Over the last 30 years, dementia has gained the attention of researchers resulting in an increase in the quality and quantity of studies assessing the impact of associated risk factors and identifying areas of preventability.1 Individual longitudinal studies show that cardiovascular risk factors (e.g. physical inactivity, smoking, hypertension, obesity, diabetes) may be associated with dementias.2,3 Many of these risk factors are modifiable, which presents opportunities to prevent dementia onset by controlling these risk factors. However, meta-analyses and systematic reviews have mixed conclusions about whether evidence indicates a clear causal relationship between these factors and subsequent dementia incidence.4–6
This study explores the occurrence of risk factors prior to a diagnosis of dementia using pan-Canadian primary care data to quantify the potential effect of modifiable risk factors, comorbidities, and demographic characteristics on the incidence of dementia in community-dwelling Canadians aged 65 years and older. The use of routinely collected clinical data, though subject to care-seeking bias, will overcome difficulties clinical trials and prospective cohort designs have faced in finding representative study samples.7 It is to minimize selection bias where patients with more serious health conditions or who are at risk for dementia may be excluded, and information bias where patients with cognitive decline may have difficulty recalling their symptoms and prior experience, or not respond at all to direct questioning.
Methods
Study design
This was a retrospective cohort design using primary care EMR data obtained from the Canadian Primary Care Sentinel Surveillance Network (CPCSSN). Participants included people aged 65+ years at any time in 2009, whose records included demographic data (sex, age, postal code, and deprivation index) and a minimum of 6 years of recorded data from 2008 January 1 to 2013 December 31.
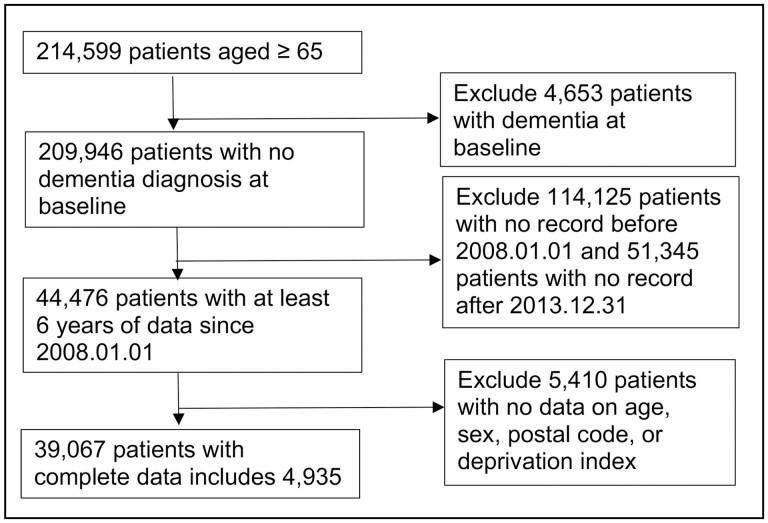
To ensure the accuracy of incident cases, we identify 2008 as a wash-out year; patients with a diagnosis of dementia at any time before 2008 December 31 were considered prevalent cases and were excluded from the analysis. The index date for the study was 2009 January 1, and the end date was the earliest of either the date of dementia diagnosis, the time point 2 years after the last recorded visit, or 2017 December 31. Figure 1 present a flowchart of the cohort development.
Figure 1.
Flowchart of cohort identification.
Data source
CPCSSN is the largest pan-Canadian data platform for EMR-based primary care research. A copy of deidentified, patient-level clinical information, including diagnoses, prescribed medications, demographics, medical examinations, laboratory test results, referrals, and risk factors, is extracted every 6 months from primary care EMRs supported by a variety of vendors. It is cleaned and processed into a standard format.8
As of 2017, CPCSSN included 373,373 patients aged 65+ years (20.4% of its total population) compared to 14.8% in the Canadian general population 2011 census.9 These numbers may reflect that older adults have more health concerns and hence are more likely to visit their family physicians to receive care. The ratio of sex in people aged 65+ years in the CPCSSN was 0.79 males to females, approximating the census ratio of 0.80.10
Case definition
Dementia
The CPCSSN case definition for dementia is a comprehensive algorithm, designed to include all types of the condition. It requires evidence from the health condition (or problem list), encounter diagnosis, or billing tables for an ICD-9 code of 290.∗, 331.∗, 294.1, 294.8, 797.∗, or 438.∗, or a prescription for a cholinesterase inhibitor (Rivastigmine, Galantamine, or Donepezil) or an N-methyl-D-aspartate (NMDA) receptor antagonist (Memantine). The date of dementia diagnosis was defined as a clinical encounter recorded in the EMR at which a patient first met the validated CPCSSN case criteria for the condition. All CPCSSN case definitions have been validated using either EMR chart or CPCSSN record review as the reference standard.11 Validation metrics are presented in Table 1 for the dementia and selected covariate (hypertension, diabetes mellitus, osteoarthritis, depression) CPCSSN case definitions.
Table 1.
Validity metrics of CPCSSN case definition.11
| Condition | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive predictive value % (95% CI) | Negative predictive value % (95% CI) |
|---|---|---|---|---|
| Dementiaa | 96.8 (93.3–100.0) | 98.1 (97.5–98.7) | 72.8 (65.0–80.6) | 99.8 (99.6–100.0) |
| Hypertension | 84.9 (82.6–87.1) | 93.5 (92.0–95.1) | 92.9 (91.2–94.6) | 86.0 (83.9–88.2) |
| Diabetes | 95.6 (93.4–97.9) | 97.1 (96.3–97.9) | 87.0 (83.5–90.5) | 99.1 (98.6–99.6) |
| Depression | 81.1 (77.2–85.0) | 94.8 (93.7–95.9) | 79.6 (75.7–83.6) | 95.2 (94.1–96.3) |
| Osteoarthritis | 77.8 (74.5–81.1) | 94.9 (93.8–96.1) | 87.7 (84.9–90.5) | 90.2 (88.7–91.8) |
| Dyslipidemia12(p)b | 98 | 100 | 100 | 93 |
The case definition of dementia by CPCSSN includes Alzheimer’s disease, frontotemporal dementia, Pick’s disease, senile degeneration of the brain, corticobasal degeneration, cerebral degeneration, dementia with Lewy bodies, mild cognitive impairment, senile dementia, presenile dementia, vascular dementia, and senility without mention of psychosis.14
95% CIs for validity are not provided in the cited paper.
Covariates
Dyslipidemia cases were defined in this analysis as having a combination of one prescription of lipid-lowering medications (ATC code C10) and one abnormal blood lipid reading for any type of cholesterol, or one occasion of being diagnosed with dyslipidemia (ICD-9 code 272.4).12
Being underweight was defined as median prior to baseline (2009 January 1) body mass index (BMI) less than 20.0; and being obese was defined as median prior to baseline BMI greater than or equal to 30.0 or a physician diagnosis of obesity (ICD-9 code 278.00, 278.01, 278.03). Patients who were not identified as being either underweight or obese, including those without a BMI measurement, were assumed not to have these conditions. Any record of BMI less than 10.0 or greater than 70.0 was assumed to be a recording error, values of these magnitudes were therefore removed.
Patients were identified as smokers if they had at least one indicator of smoking in their EMR (e.g. documented in CPCSSN as being a current or past smoker prior to baseline). Non-smokers were those documented as having never smoked before baseline or with no record of smoking.
Social and material deprivation indices were calculated using the six-digit postal code recorded in CPCSSN and the Canadian Index of Multiple Deprivation derived from the Canadian 2011 census data. Deprivation indices are calculated as quintiles, with 1 being least deprived and 5 being most deprived. Given that scores are calculated for small areas (e.g. dissemination areas containing approximately 400–700 people in each),13 this method has shown to be adequate for community research.
Medication use was defined as having received at least one prescription within the 2 years prior to baseline. Relevant medications included antihypertensives (ATC code C02∗); diuretics (C03∗) except for furosemide (C03CA01), bumetanide (C03CA02), and metolazone (C03BA08), those used primarily for heart failure or other oedematous states; peripheral vasodilators (C04∗); beta-blocking agents (C07∗); calcium channel blockers (C08∗); agents acting on the renin-angiotensin system (C09∗); and statins (atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin [C10AA∗ and C10B∗]).
Statistical analysis
Incidence of dementia was compared among those exposed and those unexposed to the selected risk factors to identify demographic and health characteristics of patients who did and did not develop incident dementia during follow-up. Survival analysis using Cox proportional hazard model was performed to estimate hazard ratios (HRs). Denominators of person-years at risk were the total of the number of years since the index date to the last date eligible of each participant. All steps of the analysis from data management to inferential analysis were conducted using SQLite14 and STATA 14.15
Ethics
The CPCSSN database has received ethics approval from each contributing local Practice Based Research Network’s local Research Ethics Board, including waivers of individual patient consent for their deidentified data to be used for surveillance and research. This study received specific approval from the University of Alberta Research Ethics Board (Pro00083659).
Results
Over a follow-up period of 9 years (2009–2017), 39,067 eligible patients contributed a total of 329,731 person-years at risk, with 4,935 (12.6%) incident cases of dementia identified. Since previous evidence found a sharp increase in dementia incidence after the age of 80 years,16 this analysis examined two age groups, patients aged 65–79 years and those aged 80 years and over at baseline.
There were 31,185 (79.8%) patients aged 65–79 years and 7,881 (20.2%) aged 80 years and over. The older group included more females (63.8% versus 56.0%, P < 0.001) and those who resided in an urban postal code (79.1% versus 75.3%, P < 0.001) than patients aged 65–79 years. The percentage of patients recorded as having ever smoked was 21.4% of those 80 years and older and 26.8% (P < 0.001) of the 65–79 years. Except for hypertension and osteoarthritis, which had higher prevalence in the older group, lower prevalence was found among the 80+ years for other chronic conditions, including diabetes mellitus, dyslipidemia, obesity, and depression. Table 2 presents baseline characteristics of the two age groups, and of the entire cohort under study.
Table 2.
Demographic and health characteristics at baseline of patients by age group (2009–2017).
| All patients | 65–79 years old | 80+ years old | |
|---|---|---|---|
| n | 39,066 | 31,185 | 7,881 |
| Person-years at risk | 329,731 | 267,996 | 61,737 |
| Age at baseline (mean ± SD) | 74.1 ± 6.2 | 71.6 ± 4.0 | 83.8 ± 3.4 |
| Female, n (%) | 22,484 (57.6) | 17,454 (56.0) | 5,031 (63.8) |
| Urban, n (%) | 29,699 (76.0) | 23,467 (75.3) | 6,232 (79.1) |
| Smokers, n (%) | 10,042 (25.7) | 8,355 (26.8) | 1,687 (21.4) |
| Social index | |||
| 1 (least deprived) | 5,853 (15.0) | 4,795 (15.4) | 1,058 (13.4) |
| 2 | 9,215 (23.6) | 7,540 (24.2) | 1,675 (21.3) |
| 3 | 8,230 (21.1) | 6,660 (21.4) | 1,570 (19.9) |
| 4 | 6,919 (17.7) | 5,503 (17.7) | 1,416 (18.0) |
| 5 (most deprived) | 8,849 (22.7) | 6,687 (21.4) | 2,162 (27.4) |
| Material index | |||
| 1 (least deprived) | 8,314 (21.3) | 6,504 (20.9) | 1,810 (23.0) |
| 2 | 6,502 (16.6) | 5,302 (17.0) | 1,200 (15.2) |
| 3 | 6,778 (17.4) | 5,485 (17.6) | 1,293 (16.4) |
| 4 | 9,188 (23.5) | 7,364 (23.6) | 1,824 (23.1) |
| 5 (most deprived) | 8,284 (21.2) | 6,530 (20.9) | 1,754 (22.3) |
| Comorbidities | |||
| Depression, n (%) | 2,274 (5.8) | 1,848 (5.9) | 426 (5.4) |
| Osteoarthritis, n (%) | 6,220 (15.9) | 4,707 (15.1) | 1,513 (19.2) |
| Hypertension, n (%) | 13,900 (35.6) | 10,638 (34.1) | 3,262 (41.4) |
| Diabetes mellitus, n (%) | 5,644 (14.5) | 4,586 (14.7) | 1,058 (13.4) |
| Dyslipidemia, n (%) | 15,013 (38.4) | 12,277 (39.4) | 2,736 (34.7) |
| Underweight, n (%) | 249 (0.6) | 178 (0.6) | 71 (0.9) |
| Obesity, n (%) | 3,858 (9.9) | 3,326 (10.7) | 516 (6.6) |
Patients aged 65–79 years included 267,996 person-years at risk with 3,012 dementia cases. After adjusting for comorbidities and demographic characteristics, the risk of dementia onset increased by 13% for every additional year in age, HR = 1.13 (95% confidence interval [CI], 1.12–1.14). Patients living in more socially deprived postal codes (deprivation quintile 3, 4, and 5) had a higher risk of a dementia diagnosis than patients in the least deprived quintiles (1 and 2; P = 0.001), with no statistically significant relationship between quintile 1 and 2. Depression, osteoarthritis, and diabetes mellitus significantly increased the risk of incident dementia (P-value < 0.01). Sex, material deprivation, hypertension, obesity, underweight, and dyslipidemia had no statistically significant association with dementia onset.
In the older group, 7,881 patients contributed 61,737 person-years at risk; only three variables significantly predicted dementia onset. Age increased the risk at a lower rate of 5% for every one additional year in age, HR = 1.05 (95% CI, 1.03–1.06). Patients with a diagnosis of depression were at higher risk, HR = 1.33 (95% CI, 1.11–1.60) than those without, and older underweight adults were at higher risk than those of normal weight, HR = 1.84 (95% CI, 1.26–2.67). Table 3 presents adjusted HRs of dementia onset for the two groups.
Table 3.
HRs of dementia onset by age groups at baseline (2009–2017).
| Characteristics | 65–79 years old | 80+ years old | ||
|---|---|---|---|---|
| AHR [95% CI] | P-value | AHR [95% CI] | P-value | |
| Age | 1.13 [1.12–1.14] | < 0.001 | 1.05 [1.03–1.06] | < 0.001 |
| Sex | ||||
| Male | 1.01 [0.94–1.09] | 0.747 | 0.95 [0.86–1.04] | 0.254 |
| Living area | ||||
| Urban | 1.05 [0.95–1.16] | 0.359 | 1.08 [0.95–1.24] | 0.239 |
| Smoking history | ||||
| Smokers | 1.09 [1.01–1.18] | 0.037 | 1.01 [0.91–1.13] | 0.807 |
| Social index | ||||
| 1 (least deprived) | 1.00 | 1.00 | — | |
| 2 | 1.12 [0.98–1.27] | 0.101 | 0.86 [0.73–1.02] | 0.086 |
| 3 | 1.27 [1.12–1.45] | < 0.001 | 1.15 [0.98–1.36] | 0.082 |
| 4 | 1.35 [1.18–1.54] | < 0.001 | 1.12 [0.95–1.33] | 0.180 |
| 5 (most deprived) | 1.41 [1.24–1.60] | < 0.001 | 0.95 [0.81–1.12] | 0.573 |
| Material index | ||||
| 1 (least deprived) | 1.00 | 1.00 | — | |
| 2 | 1.00 [0.89–1.13] | 0.958 | 0.80 [0.69–0.92] | 0.003 |
| 3 | 0.98 [0.87–1.10] | 0.699 | 0.82 [0.71–0.94] | 0.006 |
| 4 | 1.08 [0.97–1.21] | 0.173 | 0.94 [0.82–1.08] | 0.407 |
| 5 (most deprived) | 0.98 [0.87–1.10] | 0.763 | 0.75 [0.65–0.87] | < 0.001 |
| Comorbidities | ||||
| Depression | 1.38 [1.20–1.58] | < 0.001 | 1.33 [1.11–1.60] | 0.002 |
| Osteoarthritis | 1.15 [1.05–1.26] | 0.004 | 1.10 [0.98–1.23] | 0.108 |
| Hypertension | 0.96 [0.89–1.04] | 0.305 | 0.94 [0.86–1.03] | 0.207 |
| Diabetes | 1.19 [1.08–1.32] | < 0.001 | 1.14 [1.00–1.30] | 0.053 |
| Obesity | 0.93 [0.82–1.05] | 0.254 | 0.92 [0.76–1.12] | 0.381 |
| Underweight | 1.11 [0.72–1.70] | 0.642 | 1.84 [1.26–1.67] | 0.001 |
| Dyslipidemia | 1.04 [0.96–1.12] | 0.329 | 0.96 [0.87–1.06] | 0.403 |
AHR, adjusted hazard ratio.
Two subanalyses, replacing hypertension and dyslipidemia with antihypertensive and statin use, one at a time, were performed. After controlling for demographic characteristics and other chronic diseases, no significant association was found in either age group (Table 4).
Table 4.
Subanalysis to access the association between related medication and the risk of dementia onset (2009–2017).
| 65–79 years old | 80+ years old | |||
|---|---|---|---|---|
| Characteristics | AHR [95% CI] | P-value | AHR [95% CI] | P-value |
| Antihypertensive | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.02 [0.94–1.10] | 0.714 | 0.91 [0.82–1.00] | 0.052 |
| Statin | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.07 [0.98–1.17] | 0.110 | 0.93 [0.83–1.04] | 0.219 |
AHR, adjusted hazard ratio.
Testing the proportional hazard assumptions (HR is constant throughout follow-up) using log-minus-log plots (LML plots) and phtest command (# estat phtest, detail) showed violation of assumption for depression and hypertension. Therefore, interactions of these two predictors with time were included in the model to account for the change of effect produced by time. However, these interactions did not significantly change the main effect of depression and hypertension on incidence of dementia.
Discussion
Overall, after a mean follow-up of 8.4 ± 1.4 years, in 39,066 patients aged 65 years and over, this study found that incidence of dementia was associated with age but not with sex or rurality in either age groups. There was a positive association with history of smoking, higher levels of social deprivation, depression, osteoarthritis, and diabetes mellitus among patients aged 65–79 years at baseline. However, among those aged 80 years and over at baseline, only associations with higher material deprivation and depression were statistically significant. Compared to Statistics Canada reports,17,18 the prevalence of chronic conditions in this cohort, including smoking, hypertension, diabetes, dyslipidemia, and obesity, was slightly lower in those living in urban settings. A possible explanation is that the case definitions used for these conditions in this study were more stringent and thus more conservative. Using multiple criteria such as clinical diagnosis or prescription of relevant medications may lead to a more valid estimate than those employed by Statistics Canada which mainly used self-reported data.18 Also, a gap of 8 years in the two sets of statistics (2008 for the study cohort and 2016 for the Statistics Canada sample) may be significant since the proportion of Canadian people aged 65 years and older increased from 13.9% in 2009 to 16.9% in 2017. This might be associated with an increased prevalence of other chronic diseases in the community.
Hypertension
Evidence regarding the effect that hypertension has on dementia incidence is conflicting. Some studies suggest a positive association19,20 while others found negative effects,21,22 both directions having statistically and non-statistically significant associations. We found a non-significant association between hypertension and subsequent dementia diagnosis for patients in all ages. This concurs with several other longitudinal studies on both vascular dementia and Alzheimer’s disease.6 A potential explanation may be that most hypertensive patients recorded in primary care data are treated and have reasonably well-controlled blood pressure which reduces damage and complications to a point where hypertension ceases to be a risk factor; the same scenario has been claimed for stroke.23 However, among uncontrolled hypertensive patients, previous studies have found that more severe high blood pressure was not a statistically significant predictor.24,25 The relationship between hypertensive medication and dementia is also unclear as meta-analyses have found inconsistent evidence for the association between blood pressure drug treatment and dementia incidence.26 Antihypertensive medication prescription during the last 2 years before baseline had no significant association with subsequent dementia incidence.
Diabetes mellitus
Evidence for an increased risk of incident dementia in patients with diabetes is consistent with most published studies.27 We found that patients in the younger group with a diabetes diagnosis before baseline were 1.19 times at higher risk of dementia than those without diabetes compared to 1.33 times found in a British study which also used health data from primary care.28 Our study found a non-significant association between diabetes and dementia onset in the oldest group. This finding may be due to an effect of survival bias for those who were considered “healthier” until the age of 80 were less likely to develop dementia in their later years regardless to be diagnosed with diabetes or not.
Dyslipidemia
Consistent with other studies, patients with dyslipidemia experienced a non-significant 4% greater risk of incident dementia in people aged 65–79 years and also a non-significant 4% lower risk in people aged 80 and older.7,29 However, some studies report a lower risk of dementia in people older than 85 years associated with higher cholesterol level, while others show increment in risk.30 As dyslipidemia case definitions are not consistent between studies, different classification may lead to different findings. This study applied a relatively broad (although validated) case definition, but blood cholesterol levels are relatively easily changed according to dietary variation, and this may have overestimated the prevalence of dyslipidemia.
The use of statins was not significantly associated with dementia onset, replicating the finding from the UK.20 This is unsurprising as 98% of patients with dyslipidemia in this cohort were treated with a statin.
Obesity and underweight
Median baseline BMI ≥ 30.0 and/or having a diagnosis of being obese of any type recorded in the medical records was negatively, though non-significantly, associated with dementia onset. It was associated with a reduction in risk of 7% and 8%, respectively, in the younger and older age groups. This contrasts with evidence that obese patients have a greater risk of dementia than those who have a “normal” BMI.31
However, we found that BMI lower than 20.0 in patients older than 79 years old significantly increased the risk of subsequent dementia by 84%; this association was not statistically significant in the younger group. This aligns with previous studies which found that lower BMI increased the risk of dementia32 and patients who were underweight had a relatively higher risk of subsequent dementia than those of normal weight.33 This could become apparent years before diagnosis since dementia has a relatively long latency without perceptible symptoms.34 Under-diagnosis of dementia is common due to limited resources in primary care, where physicians are often reluctant to commit to a diagnosis prematurely,35 and/or patients and their families may also be hesitant to identify a problem,36 which may lead to the suggestion that dementia has already occurred (though not been diagnosed) among the oldest old whose weight is significantly lowered. It is of interest to understand whether losing weight in old age might be an early sign of latent dementia or vice versa.
Other comorbidities
Even though the pathological mechanism of the association between depression and subsequent dementia is unclear, the association itself is known37 and replicated in this study. As for osteoarthritis, a recent meta-analysis which included patients from a variety of age ranges found that its occurrence increases the risk of dementia.38 Our study found the same result for patients aged 65–79 but not for the very old group.
Strengths and limitations
In conducting a retrospective longitudinal follow-up study using available and accessible information with CPCSSN-processed EMR data, the challenge of a non-representative sample of the Canadian population is minimized; while implementing a dementia-free cohort allows us to have a highly precise denominator for incidence rate calculation and survival analysis.
However, secondary use of clinical data is limited to the availability and quality of data, which was collected by health care providers for non-research purposes. This may introduce information bias due to misclassification as dementia might have been misdiagnosed, while case definitions rarely achieve validation metrics of 100%. Conversely, other information biases, such as recall bias or interviewer bias, are expected to occur at very low frequency as information was routinely entered by clinicians at the time of providing health care. Our study is subjected to risk of unmeasured confounding, where the current database limits the opportunity of accurately evaluating competing risks from other possible factors in dementia incidence, such as inherited genetic conditions, physical inactivity, mental illnesses other than depression, as well as mortality. Other medications, including prescription opioids and benzodiazepines, are potential confounders39,40 but were not included in this analysis. Further investigation into the relationship between the use of drugs to treat chronic pain or anxiety and dementia onset in primary care will be beneficial.
Another limitation is that dementia subtypes are not distinguished in this study due to the current lack of subtype case definitions. Being able to separate vascular dementia, Alzheimer’s disease and other dementias may contribute to the improved understanding of risk factors.
Conclusion
This study found that diabetes mellitus, depression, osteoarthritis, and being underweight were associated with an increased risk for incident dementia diagnosis, but hypertension, obesity, or dyslipidemia managed in primary care were not. Used with caution, routinely collected clinical data are useful for studies with models that include more related variables to provide a more complete picture of dementia risk and protective factors. We have focused on cardiovascular-related comorbidities in this study. Further work to investigate the relationship between laboratory test results, medications, or other treatment activity recorded in primary care might be useful to better understand the apparent association between other chronic diseases and incidence of dementia.
Acknowledgements
ANQP has received the 2019 Dr. Peter N. McCracken Legacy Scholarship to support this work.
Declaration
Ethics approval
The CPCSSN database has received ethics approval from each contributing network’s local Research Ethics Board, including waivers of individual patient consent for their deidentified data to be used for surveillance and research. This study also was the subject of a formal data sharing agreement with the CPCSSN and received specific approval from the Research Ethics Board at the University of Alberta (Pro00083659).
Funding
This work was supported by the Canadian Institute of Health Research (SPN139524); and Alberta Innovates (201500394).
Conflict of interest
The authors declare that there is no conflict of interest.
Data availability
The data underlying this article were provided by the Canadian Primary Care Sentinel Surveillance Network by permission (No. 2018SRSC111). Data will be shared on request to the corresponding author with permission of the Canadian Primary Care Sentinel Surveillance Network.
References
- 1. Mangialasche F, Kivipelto M, Solomon A, Fratiglioni L.. Dementia prevention: current epidemiological evidence and future perspective. Alzheimers Res Ther. 2012;4(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A.. A population-based study of dementia in 85-year-olds. N Engl J Med. 1993;328(3):153–158. [DOI] [PubMed] [Google Scholar]
- 3. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C.. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. [DOI] [PubMed] [Google Scholar]
- 4. Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D.. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22(5):646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharp SI, Aarsland D, Day S, Sønnesyn H, Ballard C; Alzheimer’s Society Vascular Dementia Systematic Review Group . Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry. 2011;26(7):661–669. [DOI] [PubMed] [Google Scholar]
- 6. Purnell C, Gao S, Callahan CM, Hendrie HC.. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2009;23(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20(2):93–100. [DOI] [PubMed] [Google Scholar]
- 8. Nicholson K, Terry AL, Fortin M, Williamson T, Thind A.. Understanding multimorbidity in primary health care. Can Fam Physician. 2015;61(10):e489. [PMC free article] [PubMed] [Google Scholar]
- 9. Statistics Canada. Analysis: population by age and sex. Statistics Canada. 2020. [accessed 2021 Aug 1] . https://www150.statcan.gc.ca/n1/pub/91-215-x/2017000/sec2-eng.htm.
- 10. Queenan JA, Williamson T, Khan S, Drummond N, Garies S, Morkem R, Birtwhistle R.. Representativeness of patients and providers in the Canadian Primary Care Sentinel Surveillance Network: a cross-sectional study. CMAJ Open. 2016;4(1):E28–E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson T, Green ME, Birtwhistle R, Khan S, Garies S, Wong ST, Natarajan N, Manca D, Drummond N.. Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med 2014;12(4):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aref-Eshghi E, Oake J, Godwin M, Aubrey-Bassler K, Duke P, Mahdavian M, Asghari S.. Identification of dyslipidemic patients attending primary care clinics using electronic medical record (EMR) data from the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) database. J Med Syst. 2017;41(3):45. [DOI] [PubMed] [Google Scholar]
- 13. Statistics Canada. Dissemination area: detailed definition. 2018. [accessed 2021 Nov 12]. https://www150.statcan.gc.ca/n1/pub/92-195-x/2011001/geo/da-ad/def-eng.htm.
- 14. Hipp RD. SQLite [Computer Software]. Version 3.31.1. Public Domain; 2020. [Google Scholar]
- 15. STATA [Computer software]. Version: 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 16. van der Flier WM, Scheltens P.. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76:v2–v7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Statistics Canada. Table 13-10-0096-10 smokers, by age group. 2020. [accessed 2021 Aug 1]. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009610.
- 18. Statistics Canada. Table 13-10-0096-01 Canadian health characteristics, annual estimates. 2020. [accessed 2021 Aug 1]. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009601.
- 19. Ogunniyi A, Lane KA, Baiyewu O, Gao S, Gureje O, Unverzagt FW, Murrell JR, Smith-Gamble V, Hall KS, Hendrie HC.. Hypertension and incident dementia in community-dwelling elderly Yoruba Nigerians. Acta Neurol Scand. 2011;124(6):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu C, Xu W, Winblad B, Fratiglioni L.. Vascular risk profiles for dementia and Alzheimer’s disease in very old people: a population-based longitudinal study. J Alzheimers Dis. 2010;20(1):293–300. [DOI] [PubMed] [Google Scholar]
- 21. Corrada MM, Hayden KM, Paganini-Hill A, Bullain SS, DeMoss J, Aguirre C, Brookmeyer R, Kawas CH.. Age of onset of hypertension and risk of dementia in the oldest-old: the90+ study. Alzheimers Dement. 2017;13(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sibbett A, Russ C, Deary J, Starr M.. Risk factors for dementia in the ninth decade of life and beyond: a study of the Lothian birth cohort 1921. BMC Psychiatry. 2017;17(1):205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katsanos AH, Filippatou A, Manios E, Deftereos S, Parissis J, Frogoudaki A, Vrettou AR, Ikonomidis I, Pikilidou M, Kargiotis O, et al. Blood pressure reduction and secondary stroke prevention: a systematic review and metaregression analysis of randomized clinical trials. Hypertension. 2017;69(1):171–179. [DOI] [PubMed] [Google Scholar]
- 24. Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L.. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol. 2003;60(2):223–228. [DOI] [PubMed] [Google Scholar]
- 25. Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA.. A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol. 2010;67(7):835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ligthart SA, Moll van Charante EP, Van Gool WA, Richard E.. Treatment of cardiovascular risk factors to prevent cognitive decline and dementia: a systematic review. Vasc Health Risk Manag. 2010;6:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, Yu JT.. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55:100944. [DOI] [PubMed] [Google Scholar]
- 28. Walters K, Hardoon S, Petersen I, Iliffe S, Omar RZ, Nazareth I, Rait G.. Predicting dementia risk in primary care: development and validation of the dementia risk score using routinely collected data. BMC Med. 2016;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mielke MM, Zandi PP, Sjögren M, Gustafson D, Ostling S, Steen B, Skoog I.. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64(10):1689–1695. [DOI] [PubMed] [Google Scholar]
- 30. Reitz C. Dyslipidemia and dementia: current epidemiology, genetic evidence, and mechanisms behind the associations. J Alzheimers Dis. 2012;30(suppl 2):S127–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beydoun MA, Beydoun HA, Wang Y.. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9(3):204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Power BD, Alfonso H, Flicker L, Hankey GJ, Yeap BB, Almeida OP.. Changes in body mass in later life and incident dementia. Int Psychogeriatr. 2013;25(3):467–478. [DOI] [PubMed] [Google Scholar]
- 33. Nourhashémi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P; PAQUID study. Personnes Agées Quid . Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60(1):117–119. [DOI] [PubMed] [Google Scholar]
- 34. Barrett E. Dementia revealed: what primary care needs to know. London: NHS England; 2014. [Google Scholar]
- 35. Szymczynska P, Innes A, Mason A, Stark C.. A review of diagnostic process and postdiagnostic support for people with dementia in rural areas. J Prim Care Community Health. 2011;2(4):262–276. [DOI] [PubMed] [Google Scholar]
- 36. Koehn S, Badger M, Cohen C, McCleary L, Drummond N.. Negotiating access to a diagnosis of dementia: implications for policies in health and social care. Dementia (London). 2016;15(6):1436–1456. [DOI] [PubMed] [Google Scholar]
- 37. Mejía S. Promoting multilevel primary prevention of depression and diabetes during midlife may protect against dementia. Evid Based Ment Health. 2016;19:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weber A, Mak SH, Berenbaum F, Sellam J, Zheng YP, Han Y, Wen C.. Association between osteoarthritis and increased risk of dementia: a systemic review and meta-analysis. Medicine (Baltimore). 2019;98(10):e14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen PA, Poly TN, Masud JH, Li YJ, Shabbir SA.. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. 2016;47(3–4):181–191. [DOI] [PubMed] [Google Scholar]
- 40. Lucchetta RC, da Mata BPM, Mastroianni PC.. Association between development of dementia and use of benzodiazepines: a systematic review and meta-Analysis. Pharmacotherapy. 2018;38(10):1010–1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article were provided by the Canadian Primary Care Sentinel Surveillance Network by permission (No. 2018SRSC111). Data will be shared on request to the corresponding author with permission of the Canadian Primary Care Sentinel Surveillance Network.