ABSTRACT
This review focuses on summarizing current knowledge on how time-restricted feeding (TRF) and continuous caloric restriction (CR) affect central neuroendocrine systems involved in regulating satiety. Several interconnected regions of the hypothalamus, brainstem, and cortical areas of the brain are involved in the regulation of satiety. Following CR and TRF, the increase in hunger and reduction in satiety signals of the melanocortin system [neuropeptide Y (NPY), proopiomelanocortin (POMC), and agouti-related peptide (AgRP)] appear similar between CR and TRF protocols, as do the dopaminergic responses in the mesocorticolimbic circuit. However, ghrelin and leptin signaling via the melanocortin system appears to improve energy balance signals and reduce hyperphagia following TRF, which has not been reported in CR. In addition to satiety systems, CR and TRF also influence circadian rhythms. CR influences the suprachiasmatic nucleus (SCN) or the primary circadian clock as seen by increased clock gene expression. In contrast, TRF appears to affect both the SCN and the peripheral clocks, as seen by phasic changes in the non-SCN (potentially the elusive food entrainable oscillator) and metabolic clocks. The peripheral clocks are influenced by the primary circadian clock but are also entrained by food timing, sleep timing, and other lifestyle parameters, which can supersede the metabolic processes that are regulated by the primary circadian clock. Taken together, TRF influences hunger/satiety, energy balance systems, and circadian rhythms, suggesting a role for adherence to CR in the long run if implemented using the TRF approach. However, these suggestions are based on only a few studies, and future investigations that use standardized protocols for the evaluation of the effect of these diet patterns (time, duration, meal composition, sufficiently powered) are necessary to verify these preliminary observations.
Keywords: circadian rhythms, light-entrainable oscillator, peripheral oscillators, hypothalamus, satiety, calorie restriction, time-restricted feeding
Statement of Significance: This is an in-depth review of current literature on the effects of time-restricted feeding and calorie restriction on central neuroendocrine systems involved in satiety. Our reviews weave together information on both central and peripheral systems of satiety, which tend to be examined separately, in hopes of guiding future research in this area.
Introduction
In the current obesity epidemic, calorie restriction (CR) is often prescribed to reduce calorie intake and induce weight loss. More recently, time-restricted feeding (TRF) has become a popular modality to achieve a caloric deficit to reduce body weight. Given that energy intake is a key determinant of body weight, studying the regulation of energy intake remains of high interest. Energy intake regulation is complex, and satiety factors regulating energy intake are under active investigation. Peripheral signals from the body (e.g., hormones, glucose, etc.) determine satiety, along with several other physiological parameters. The central nervous system integrates these signals (1), ultimately leading to sensations of satiety or hunger, and decisions to consume food or terminate a feeding episode. Central regulation of satiety and hunger involves several regions of the brain, including the hypothalamus, ventral tegmental area (VTA), parabrachial nucleus (PBN), nucleus accumbens (NAc), prefrontal cortex (PfC), central nuclei of the amygdala, dorsal vagal complex (DVC), and bed nuclei of stria terminalis (BNST), among several others (1–8). To date, no report exists that evaluates TRF or CR on satiety, summarizing their effects on central neural/neuroendocrine systems. To address this knowledge gap, here we discuss the current status of knowledge with regard to the effect of CR and TRF on central mechanisms involved in the regulation of satiety. In a companion article, we discuss the current status of knowledge with regard to changes in peripheral satiety hormones in response to CR and TRF (9).
Neural oscillators are common control processes that are involved in the regulation of various physiological systems. Recent evidence suggests that oscillators could involve transcription of genes or are neurons/groups of neurons with intrinsic rhythmic patterns of neural activity (10). There are several clocks or oscillators at the cellular, tissue, and systemic levels that use biological networks to generate 24-h (circadian; Latin: “circa” = about, “diem” = a day) and other rhythmic processes (11). The primary circadian rhythm of the body is set by the photic-oscillator (responds to visual light input) often called light-entrained oscillator (LEO), which is located in the suprachiasmatic nucleus (SCN) in the hypothalamus, and throughout this article we will refer to this as the “SCN-clock.” There are also peripheral oscillators that are cell-autonomous and maintained as a negative feedback network to track metabolism. The mechanisms for the 2 appear to work independently, but are also interdependent (12). The circadian rhythm genes [Clock, Brain and Muscle ARNT-Like 1(Bmal1), Cryptochrome 1/2(Cry1/2), Period Circadian Regulator 1/2(Per 1/2), Nuclear Receptor Subfamily 1 Group D Member 1(Rev-erbα)] are involved in this network responsible for molecular peripheral oscillators, and have been found in several organs (liver, pancreas, skeletal muscle, stomach) and cell types in humans (13). Hence, multiple oscillators direct the biological clock in humans. In humans and most mammals, the central LEO controls broad-stroke mechanisms of several metabolic processes (14), and the peripheral oscillators are responsible for 3–10% of all transcribed mRNA, suggesting a tissue-specific, temporal effect (12).
The interaction between peripheral clocks and SCN-clock under different diet conditions has been examined using gene expression studies (circadian rhythm genes) in animals and humans. When food availability is manipulated to produce vastly different cycles than the light-dark cycle, such as switching rodents to be diurnal from nocturnal, or in nightshift workers, there is a decoupling of central and peripheral clocks (15). Recent evidence suggests that TRF leads to modulation of SCN-clock and circadian rhythms through alterations in clock gene expression (16, 17). In TRF, hepatic circadian gene expression (Per1/2, Bmal1, Cry1/2, Clock1) increases and is time-locked (i.e., entrained by the timing of food) (18). Following CR there is a resetting of the circadian rhythm by the SCN-clock as seen by the increase in the amplitude of clock gene expression in peripheral tissues; however, the phasic pattern remained the same, unlike following TRF (19). Due to the complex interplay between metabolic factors and satiety, these hepatic time-locked genes may influence the circadian rhythm of the satiety hormones.
The concept of a central food or feeding entrainable oscillator (FEO) has been suggested but is highly debated since its location remains unclear (20). One proposed theory is that the FEO is an autonomous circadian oscillator, potentially a network of neurons in the hypothalamic and non-hypothalamic regions (21, 22). The consideration of the FEO as a central oscillator suggests that it may play a pertinent role in food-intake behavior. Predating the concept of the FEO is the food anticipatory activity (FAA), which in recent decades has been shown to be the output of FEO (20). FAA is a set of behaviors—including increased locomotion/activity for foraging and eating—which occur at specific times during the day, culminating in food consumption (23). The FAA is likely mediated both by FEO and LEO (21), both of which, in turn, communicate with peripheral oscillators (24). The circadian rhythm genes can influence the FEO, but it can and often does act independently of genetic circadian input. The LEO is influenced by CR, and LEO and FEO also interact and influence each other; however, the form and extent of this interaction remain unclear (25). Leptin and ghrelin have been suspected to be primary influences on the LEO, and more recent evidence also includes insulin (25). However, since the FEO is a more disparate network across the hypothalamic and non-hypothalamic regions of the brain, a greater number of signals [peptide YY (PYY), cholecystokinin (CCK), amylin, glucagon-like peptide 1 (GLP-1), etc.] could influence and entrain this.
It has been suggested that the FEO is recruited in TRF (with or without CR) but not in CR alone; however, this is debated (20, 26). Studies investigating TRF in both animals and humans do not use consistent protocols, and sometimes include CR, while other times it is an inadvertent outcome (27), making it challenging to compare and understand the role of LEO versus FEO on FAA, albeit, recent evidence suggests that TRF combined with CR sustains the metabolic and behavioral peripheral oscillator entrainment (28), leading to the possibility that the FEO learns and sustains activity with TRF and makes downstream adherence to CR more feasible (29).
Temporal patterns of feeding have been shown to affect the circadian rhythm. TRF (with and without CR) is an interesting model in this regard because it offers the potential to affect and synchronize peripheral clocks and even FEO, but without involving LEO (25, 30). Furthermore, there is a strong influence of TRF on peripheral clocks and modulation of LEO in the SCN. If there is an increase in the strength of coupling between the SCN-clock with the food-modulated clock (peripheral and potentially FEO), this could modulate satiety hormones and boost adherence to TRF, and potentially within it, CR.
Current Status of Knowledge
Central control of food intake
Studies were identified by searching PubMed and Google Scholar electronic databases for peer-reviewed, English-language publications. Search terms included the following: “hypothalamus,” “ARC/arcuate nucleus,” “DMH/dorsomedial hypothalamus/hypothalamic nucleus,” “VMH/ventromedial hypothalamus/hypothalamic nucleus,” “PVN/paraventricular nucleus,” “LHA/lateral hypothalamus/hypothalamic area,” “SCN/suprachiasmatic nucleus,” “mesolimbic system,” “NAc/nucleus accumbens,” “VTA/ventral tegmental area,” “Pfc/Prefrontal cortex,” “Amg/Amygdala,” “DVC/dorsal vagal complex,” “NTS/nucleus tractus solitarius,” “AP/area postrema,” and “DMNV/dorsal motor nucleus of the vagus nerve” with “calorie/caloric restriction” and “time-restricted feeding/TRF” and “weight loss.” Tables 1 and 2 present summaries of studies that have looked at either CR or TRF, respectively, and their effect on these brain regions. The central nervous systems regulating food intake are presented in Figure 1, along with peripheral signal interactions. It is important to keep in mind that some of the peripheral signals are synthesized and released both in the periphery and the brain (CCK, PYY, GLP-1, amylin), while others are exclusively produced in the periphery and cross the blood–brain barrier [ghrelin, leptin, insulin, gastrointestinal peptide (GIP), pancreatic polypeptide (PP)]. There is some debate about insulin and ghrelin being produced in the brain, adding controversy (31, 32). Since the central integration of satiety signals is an area that is under active study and review (33–37), this current article will present a brief overview of links between the gut hormone and central nervous system areas of regulation. We will also summarize current knowledge on the effect of CR and TRF (with or without CR) on brain regions, and circuits within and between the hypothalamic and non-hypothalamic regions in the brain.
TABLE 1.
Summary of central neuroendocrine regions in response to CR protocols included in this review1
| Brain area and region | Study (ref) | Animal or human model | Study duration | Calorie restriction | Weight change or difference? | Difference in protein/gene expression | Difference in activity of neurons | Interpretive comments/include behavior measures if any were made |
|---|---|---|---|---|---|---|---|---|
| Hypothalamus | ||||||||
| ARC | Derous et al. (55) | Animal; C57BL/6 mice (male, n = 8) | 3 mo | 10%, 20%, 30%, and 40% energy restriction | Not reported | Npy: ↑ in all CR groups | — | RNA-seq (transcriptome sequencing) used to determine differential gene expression |
| Agrp: ↑ in 30% and 40% CR | Expression of Agrp and Npy was negatively correlated with leptin, insulin, and IGF-I. Pomc was positively correlated with IGF-1 only | |||||||
| Pomc: ↓ in 40% CR only | ||||||||
| Rogers et al. (56) | Animal; C57BL/6 mice (male, n = 7) | 1 y | 40% kcal deficit | Weight and length: ↓ vs. controls | Npy: ↑ | — | Measured mRNA levels for gene expression (qPCR for transcription factors) | |
| Agrp: ↑ | Ghrelin and GHSR1a controls and ablation (+/+ and −/−). No difference in gene expression from CR between ghrelin/GHSR1a groups | |||||||
| Pomc: ↓ | ||||||||
| Hambly et al. (57) | Animal; MF1 mice (n = 10) | 50 d total: 25 d preintervention + 25 d intervention | 50% energy restriction vs. ad libitum control | ↓ Average 2.6 g lower vs. control | Npy: ↑ after CR, not significantly different after 4 d of ad libitum refeeding | — | Used in situ hybridization to measure mRNA levels for gene expression | |
| Agrp: ↑ after CR, not significantly different after 4 d of ad libitum refeeding | ||||||||
| Pomc: ↓ after CR, not significantly different after 4 d of ad libitum refeeding | ||||||||
| Bi et al. (58) | Animal; Sprague-Dawley rats (male, n = 6) | 14 d | 30% kcal deficit vs. ad libitum control | ↓ Rate of weight gain (27.3% lower) | Npy: ↑ 156% compared to control | — | Used in situ hybridization to measure mRNA levels for gene expression | |
| Agrp: slight increase but not significantly different than control | Significant reduction in plasma leptin concentrations, but normal glucose and insulin. No significant change in ObRb gene expression in the ARC | |||||||
| Pomc: ↓ 26.4% compared to control | ||||||||
| Kinzig et al. (59) | Animal; Long-Evans rats (male, n = 7) | 4 wk | 30% kcal deficit vs. ad libitum control | ↓ 19% lower than control | Npy: not significantly different than controls | — | Plasma insulin and leptin concentrations were significantly lower. Plasma ghrelin concentrations were significantly higher | |
| Agrp: not significantly different than controls | Used in situ hybridization to measure mRNA levels for gene expression | |||||||
| Pomc: ↓ 32.2% compared to control | ||||||||
| Jarvie et al. (64) | Animal; Background C57BL/6 mice (n = 7) | 2 wk | ∼30% kcal deficit vs. ad libitum control | Maintained at 80% of starting weight | Gad1 in POMC neurons: ↓ 39–44% compared to control | — | Gad1 correlates with GABA release from neurons | |
| Gad1 in ARC: not significantly different than control | Used in situ hybridization to measure mRNA levels for gene expression | |||||||
| Satoh et al. (62) | Animals; C57BL/6 mice and BRASTO (male, n = 2–6) | 14 d (short-term CR); 104 d (long-term CR) | 40% kcal deficit vs. ad libitum control | Not reported | SIRT1: no difference | c-FOS: no difference between CR and ad libitum | Immunostaining used to measure SIRT1 and c-FOS | |
| Peripheral ghrelin injection increased c-FOS-positive cells over 2 h | ||||||||
| Radler et al. (60) | Animals; C57BL/6J mice (male, n = 15–16) | 4 wk | 50% kcal deficit vs. ad libitum controls | Not reported | NPY: ↑ vs. control | — | Immunostaining used to measure NPY present in tissue samples | |
| DMH | Bi et al. (58) | Animal; Sprague-Dawley rats (male, n = 6) | 14 d | 30% kcal deficit vs. ad libitum control | ↓ Rate of weight gain (27.3% lower) | Npy: ↑ ∼50% compared to control | — | Used in situ hybridization to measure mRNA levels for gene expression |
| Signification reduction in plasma leptin concentrations, but normal glucose and insulin. No significant change in ObRb gene expression in the DMH | ||||||||
| Kinzig et al. (59) | Animal; Long-Evans rats (male, n = 7) | 4 wk | 30% kcal deficit vs. ad libitum control | ↓ 19% lower than control | Npy: ↑ 451.6% compared to control | — | Used in situ hybridization to measure mRNA levels for gene expression | |
| Satoh et al. (62) | Animals; C57BL/6 mice and BRASTO (male, n = 2–6) | 14 d (short-term CR); 104 d (long-term CR) | 40% kcal deficit vs. ad libitum control | Not reported | Ox2r: ↑ number of Ox2r-positive cells and total signal [14 d] | c-FOS: ↑ expression at 14 d and 104 d; ↑ 32% expression (14 d CR) in BRASTO mice | Immunostaining used to measure SIRT1 and c-FOS. Used in situ hybridization to measure mRNA levels for gene expression | |
| SIRT1: ↑ at 14 d and 104 d | Peripheral ghrelin injection increased c-FOS-positive cells over 2 h | |||||||
| VMH | Satoh et al. (62) | Animals; C57BL/6 mice and BRASTO (male, n = 2–6) | 14 d (short-term CR); 104 d (long-term CR) | 40% kcal deficit vs. ad libitum control | Not reported | Ox2r: no difference | c-FOS: no difference | Immunostaining used to measure SIRT1 and c-FOS. Used in situ hybridization to measure mRNA levels for gene expression |
| SIRT1: no difference | ||||||||
| PVN | Satoh et al. (62) | Animals; C57BL/6 mice and BRASTO (male, n = 2–6) | 14 d (short-term CR); 104 d (long-term CR) | 40% kcal deficit vs. ad libitum control | Not reported | SIRT1: no difference | c-FOS: no difference | Immunostaining used to measure SIRT1 and c-FOS |
| Saeed et al. (99) | Animals; B6D2F1 mice (female, n = not reported) | 22 mo total: 14 wk adjustment + 74 wk CR | 40% kcal deficit vs. ad libitum controls | Not reported | IGF-IR%: ↑ vs. controls | — | Immunostaining used to measure IGF-IR and all cells in the PVN | |
| Total PVN cell count: ↓ 13% vs. control | Compared to younger mice (7 wk old), both CR and control mice had lower PVN cells counts, but percentage of IGF-IR immunoreactive cells was not significantly different from the young mice and CR mice. Control mice has significantly less than both | |||||||
| Radler et al. (60) | Animals; C57BL/6J mice (male, n = 15–16) | 4 wk | 50% kcal deficit vs. ad libitum controls | Not reported | NPY: no difference | — | Immunostaining used to measure NPY present in tissue samples | |
| Kenny et al. (100) | Animals; Wistar rats (male, n = 36) | 3 wk | 25% kcal deficit vs. ad libitum control | ↓ vs. control | — | Fos-positive cells: increased with with stress, but not significantly different than controls | Neuronal activation measured by positive Fos immunoreactivity | |
| Significantly higher basal corticosterone levels but exhibited less grooming during open field tests | ||||||||
| LHA | Satoh et al. (62) | Animals; C57BL/6 mice and BRASTO (male, n = 2–6) | 14 d (short-term CR); 104 d (long-term CR) | 40% kcal deficit vs. ad libitum control | Not reported | Ox2r: ↑ number of Ox2r-positive cells and total signal | c-FOS: ↑ expression at 14 d and 104 d; ↑ 43% expression (14 d CR) in BRASTO mice | Immunostaining used to measure SIRT1 and c-FOS. Used in situ hybridization to measure mRNA levels for gene expression |
| SIRT1: ↑ at 14 d and 104 d | Peripheral ghrelin injection increased c-FOS-positive cells over 2 h | |||||||
| Valenzano et al. (114) | Human – 10 W, 10 M; BMI 32.19 ± 4.78 kg/m2; age: 48 ± 10 y | 8 wk | 700–900 kcal/d (ketogenic VLCD) | ↓ 12.6 kg weight loss | Plasma orexin-A: ↓ 6.33 pg/mL from baseline | — | Plasma orexin-A concentrations measured by ELISA. Blood samples were taken before and after intervention after a 12-h fast | |
| Pankevich et al. (115) | Animals; C57BL/6J mice (male, n = 37–47) | 3 wk | 25% kcal deficit vs. ad libitum control, then fed HFD for 1 wk | ↓ 4.6 g vs. control | Mch: ↑ in CR mice after HFD refeeding | — | Measured mRNA levels for gene expression (qPCR for transcription factors) | |
| Hcrt (orexin): ↑ in CR mice after HFD refeeding | Leptin was significantly lower and basal corticosterone was higher than control | |||||||
| SCN | Satoh et al. (62) | Animals; C57BL/6 mice and BRASTO (male, n = 2–6) | 14 d (short-term CR); 104 d (long-term CR) | 40% kcal deficit vs. ad libitum control | Not reported | SIRT1: ↑ at 14 d and 104 d | c-FOS: no difference | Immunostaining used to measure SIRT1 and c-FOS |
| Mesocorticolimbicsystem | ||||||||
| NAc | Diao et al. (143) | Animal; Fischer-344 rats (female, n = 5–7) | 4 mo | 40% kcal deficit vs. ad libitum control | Not reported | — | DA overflow: ↑ amount and duration in response/DA clearance | In vivo electrochemistry used to determine potassium-evoked dopamine overflow from the dorsal striatum across the ventral striatum/nucleus accumbens |
| Kolta et al. (144) | Animal; Fischer-344 rats (male and female, n = 5–10) | 18.75 mo | 40% kcal deficit vs. ad libitum controls | ↓ 46–48% vs. control | [DA] and [DOPAC]: not significantly different than control | — | Neurotransmitter assays using HPLC were used to determine concentration of monoamines and metabolites | |
| [5-HT] and [5-HIAA]: ↓ 37% and 55% than control in female rats only | ||||||||
| Maswood et al. (145) | Animal; rhesus monkeys (male, n = 6–7) | 6 mo | 30% kcal deficit vs. ad libitum control | ↓ 12% vs. control | [DA], [DOPAC], [HVA]: ↑ than control after injection of a Parkinson's-inducing neurotoxin | — | HPLC was used to determine concentrations of DA and metabolites in the striatal regions of the brain | |
| Similar doses of the neurotoxin given to both groups, causes selective degeneration of DA neurons | ||||||||
| Vialou et al. (146) | Animal; C57BL6/J background mice (n = 7) | 10 d | 40% kcal deficit (then 2 d ad libitum to return to baseline body weight) vs. ad libitum controls | ↓ 15–20% from baseline, then not different from control after 2 d ad libitum feeding | ΔFosB + cells in NAc shell: ↑ vs. control | — | Immunostaining methods used to count cells containing ΔFosB in the NAc | |
| ΔFosB + cells in NAc core: no difference | CR group had significantly high rewards earned than control using operant response tests using a high-fat pellet reward | |||||||
| VTA | Roseberry (151) | Animals: adult male C57BL/6J mice | 1 d | Acute 24-h fast vs. ad libitum fed | Not reported | None | ↑ Dopamine receptor (D2R) activity following fasting compared to fed | DA neuron in the VTA were recorded by electrophysiology |
| ↓ Release of low-calcium aCSF following fasting compared to fed | Forskolin was given to compare second messenger systems involved in dopamine release | |||||||
| ↓ Response to forskolin following fasting compared to fed | ||||||||
| No difference in response to l-DOPA between groups | ||||||||
| Maswood et al. (145) | Animal; rhesus monkeys (male, n = 6–7) | 6 mo | 30% kcal deficit vs. ad libitum control | ↓ 12% vs. control | TH-positive cells: ↑ 15% in VTA/SN vs. control after injection of a Parkinson's-inducing neurotoxin | — | PET scans and immunostaining used to measure DA neurons in VTA/SN | |
| Similar doses of the neurotoxin given to both groups, causes selective degeneration of DA neurons | ||||||||
| PfC | Siep et al. (157) | Human; 12 W; BMI 21.5 ± 1.9 kg/m2; age: 19.3 ± 0.9 y | 1 d | 18-h food deprivation vs. satiated | Not reported | — | Medial PfC: ↓ inhibition activity after presentation of high caloric foods | fMRI used to measure BOLD signal change in various regions of interest in the brain |
| Willette et al. (158) | Animal; rhesus monkeys (n = 18–26) | 13–16 y | 30% kcal deficit vs. ad libitum control | Not reported | — | Association between stress reactivity and PfC volume/tissue density was ↓ in CR group than control | MRI used to measure changes in volume and tissue density. Urinary cortisol was also measured | |
| Amg | Siep et al. (157) | Human; 12 W; BMI 21.5 ± 1.9 kg/m2; age: 19.3 ± 0.9 y | 1 d | 18-h food deprivation vs. satiated | Not reported | — | Left Amg: no difference between conditions with shown high- and low-calorie foods | fMRI used to measure BOLD signal change in various regions of interest in the brain |
| Willette et al. (158) | Animal; rhesus monkeys (n = 18–26) | 13–16 y | 30% kcal deficit vs. ad libitum control | Not reported | — | Association between stress reactivity and Amg volume/tissue density was ↓ in CR group compared with control | MRI used to measure changes in volume and tissue density. Urinary cortisol was also measured | |
| Zséli et al. (161) | Animal; Wistar rats (male, n = 24) | 2 d | 40-h food deprivation followed by ad libitum refeeding | Not reported | — | ↑ c-Fos from other regions to Amg: PVT and PBN | Immunocytochemistry used to map the location of refeeding-activated neurons projecting to the central Amg. C-Fos immunoreactivity for neuron activity | |
| Dorsal vagal complex | ||||||||
| NTS | Zséli G et al. (161) | Animal; Wistar rats (male, n = 24) | 2 d | 40-h food deprivation followed by ad libitum refeeding | Not reported | — | ↑ c-Fos from Amg to other regions: BNST, LHA, PVT, and NTS | Immunocytochemistry used to map the location of refeeding-activated neurons projecting to the central Amg. C-Fos immunoreactivity for neuron activity |
aCSF, artifical cerebrospinal fluid; Agrp, agouti-related peptide; Amg, amygdala; ARC, arcuate nucleus of the hypothalamus; BNST, bed nuclei of stria terminalis; BOLD, blood oxygenation level dependent; BRASTO, transgenic mice that overexpress SIRT1; CR, calorie restriction; DA, dopamine; DMH, dorsomedial hypothalamic nucleus; DOPAC, 3,4-dihydroxyphenylacetic acid (metabolite of dopamine); GABA, γ-aminobutyric acid; Gad1, glutamate decarboxylase 1; GHSR1a, growth hormone secretagogue receptor 1a; Hcrt, hypocretin/orexin; HFD, high-fat diet; HVA, homovanillic acid (metabolite of dopamine); IGF-I, insulin-like growth factor I; IGF-IR, insulin-like growth factor 1 receptor; l-DOPA, l-3,4-dihydroxyphenylalanine; LHA, lateral hypothalamic area; M, men; Mch, melanin-concentrating hormone; NAc, nucleus accumbens; Npy, neuropeptide Y; NTS, nucleus tractus solitarius; ObRb, leptin receptor (long isoform); OxR2, orexin receptor 2; PET, positron emission tomography; PfC, prefrontal cortex; Pomc, proopiomelanocortin; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular thalamus; ref, reference; SIRT1, sirtuin-1; TH, tyrosine hydroxylase; VLCD, very low-calorie diet; VMH, ventromedial hypothalamic nucleus; VTA/SN, ventral tegmental area/substantia nigra; W, women; 5-HIAA, 5-hydroxyindoleacetic acid (metabolite of serotonin); 5-HT, serotonin; ↓, significant decrease; ↑, significant increase.
TABLE 2.
Summary of central neuroendocrine regions in response to TRF protocols included in this review1
| Brain area and region | Study (ref) | Animal or human model | Study design | Study duration | Fasting: feeding window (h:h) | Calorie restriction? | Weight loss? | Difference in protein/gene expression | Difference in activity of neurons/neurotransmitter | Interpretive comments/include behavior measures if any were made |
|---|---|---|---|---|---|---|---|---|---|---|
| Hypothalamus | ||||||||||
| ARC | Lauzurica et al. (65) | Animal: female rats | Restricted feeding/refeeding protocol (RFR) | 7 d | 20 h:2 h | Yes | Yes | Npy: ↑ 57.7% in TRF compared to baseline, and remained elevated 5 d after ad libitum feeding | — | Rebound hyperphagia and reduced plasma leptin were observed following restricted feeding, which was seen just before animals were killed to evaluate ARC levels of NPY and POMC |
| Pomc: ↓ (no % reported) | ||||||||||
| Verhagen et al. (69) | Animals: female outbred Wistar WU rats, n = 16 | Restricted feeding with set feeding time (RF-S, n = 9) vs. random dark phase feeding times (RF-R, n = 7) | 3 d | 23 h:1 h | No | ↑ In RF-R compared to RF-S | — | ↓ c-FOS–positive cells in RF-R compared to RF-S | Total food intake was not different between RF-S and RF-R | |
| Sorrell et al. (67) | Animal: male mice—DIO vs. WT vs. lean | High-fat-fed DIO mice subjected to dark-restricted feeding protocol (DRF) | 2 wk | 12 h:12 h | Not reported | Yes | Agrp: ↑ compared to controls | — | DRF reduced hyperphagia seen in high-fat-fed DIO mice in control group, and comparable to WT and lean groups | |
| Pomc: No change | DRF increased effectiveness of leptin and ghrelin signaling in the hypothalamus, reinforcing the strength of hunger and satiety signals | |||||||||
| Arntl/Bmal1: No change | Higher plasma ghrelin in DRF vs. controls, but no difference in food intake | |||||||||
| Ghsr: ↑ compared to controls | ||||||||||
| Fos: ↑ compared to controls | ||||||||||
| Nr1d1/Rev-erbα: ↑ compared to controls | ||||||||||
| Per2: No change | ||||||||||
| Cry1: No change | ||||||||||
| Brady et al. (66) | Animal: male and female Sprague-Dawley rats | Restricted feeding (RF) vs. ad libitum control (C) | 2 wk | 23 h:1 h | 10 g/d for RF | Yes, ∼↓ 18.5% in RF | NPY: ↑ 45.6% in RF compared to C | — | — | |
| POMC: ↓11% in RF compared to C | ||||||||||
| GAL: ↓16.5% in RF compared to C | ||||||||||
| CRH: ↓24.5% in RF compared to C | ||||||||||
| Miñana-Solis et al. (68) | Animals: male Wistar rats | Restricted light-cycle feeding (RF) vs. ad libitum controls (C) | 3 wk | 22 h:2 h for RF | No | Not reported | Bmal1: No change | — | — | |
| Per1: ↑ in RF compared to C | ||||||||||
| Per2: No change | ||||||||||
| Lewis et al. (50) | Animals: male, lean (+/+) rats JCR:LA-cp strain, n = 10 | Restricted feeding (RF) vs. ad libitum control (C) | 40 d | 23 h:1 h | Yes, 15 g/d total food provided for RF only | Yes, RF 107 g ↓ vs. control | NPY: ↑ in RF compared to C | — | — | |
| DMH | Lewis et al. (50) | Animals: male, lean (+/+) rats JCR:LA-cp strain, n = 10 | Restricted feeding (RF) vs. ad libitum control (C) | 40 d | 23 h:1 h | Yes, 15 g/d total food provided for RF only | Yes, RF 107 g ↓ vs. control | NPY: ↑ in restricted group compared to controls | — | — |
| Verhagen et al. (69) | Animals: female outbred Wistar WU rats, n = 16 | Restricted feeding with set feeding time (RF-S, n = 9) vs. random dark phase feeding times (RF-R, n = 7) | 3 d | 23 h:1 h | No | ↑ In RF-R compared to RF-S | — | ↓ c-FOS positive cells in RF-R compared to RF-S | Total food intake was not different between RF-S and RF-R | |
| Miñana-Solis et al. (68) | Animals: male Wistar rats | Restricted light-cycle feeding (RF) vs. ad libitum controls (C) | 3 wk | 22 h:2 h | No | Not reported | Bmal1: No change | — | There was a phase advancement of clock gene expression in RF compared to C | |
| Per1: ↑ in RF compared to C | ||||||||||
| Per2: ↓ in RF compared to C | ||||||||||
| Angeles-Castellanos et al. (80) | Animals: adult male Wistar rats n = 6 | Restricted feeding (RF) vs. ad libitum control (C) | 3 wk | 22 h:2 h for RF | No | Not reported | — | No difference in c-FOS-IR between groups | ↑ c-FOS-IR in DMH at feed deprivation and mealtimes in both groups. Control animals that were evaluated after a 22-h acute feed deprivation showed similar activation/entrainment as the 3-wk feed-deprived rats, but this was not seen in rats that were not feed deprived | |
| VMH | Angeles-Castellanos et al. (80) | Animals: adult male Wistar rats n = 6 | Restricted feeding (RF) vs. ad libitum control (C) | 3 wk | 22 h:2 h for RF | No | Not reported | — | No difference in c-FOS-IR between groups | No effect of mealtime or fasting on c-FOS-IR VMH. Control animals that were evaluated after a 22-h acute feed deprivation showed similar activation/entrainment as the 3-wk feed-deprived rats, but this was not seen in rats that were not feed deprived |
| Lewis et al. (50) | Animals: male, lean (+/+) rats JCR:LA-cp strain, n = 10 | Restricted feeding (RF) vs. ad libitum control (C) | 40 d | 23 h:1 h | Yes, 15 g/d total food provided for RF only | Yes, RF 107 g ↓ vs. control | NPY: no difference between groups | — | — | |
| Kurumiya and Kawamura (92) | Animals: male albino Wistar rats, blinded with bilateral SCN lesions | Time-restriction (TR) followed by complete restriction | 10 d | 22 h:2 h | No | None reported | — | ↑ Multiple-unit activity 3-4 h prior to feeding time, and stayed 6-7 h after consumption, and lasted 4 d into the complete caloric restriction | Chronically implanted electrodes used to measure food entrainment in the absence of SCN and light entrainment | |
| Miñana-Solis et al. (68) | Animals: male Wistar rats | Restricted light-cycle feeding (RF) vs. ad libitum controls (C) | 3 wk | 22 h:2 h for RF | No | Not reported | Bmal1: No change | — | — | |
| Per1: No change | ||||||||||
| Per2: ↓ in RF compared to C | ||||||||||
| PVN | Brady et al. (66) | Animal: male and female Sprague-Dawley rats | Restricted feeding (RF) vs. control (C) | 2 wk | 23 h:1 h | 10 g/d for TR | Yes, ∼↓ 18.5% in TR | NPY: ↑ 45.6% in RF compared to C | — | — |
| POMC: ↓11% in RF compared to C | ||||||||||
| GAL: ↓16.5% in RF compared to C | ||||||||||
| CRH: ↓24.5% in RF compared to C | ||||||||||
| Angeles-Castellanos et al. (80) | Animals: adult male Wistar rats n = 6 | Restricted feeding (RF) vs. ad libitum control (C) | 3 wk | 22 h:2 h for RF | No | Not reported | — | No difference in c-FOS-IR between groups | Control animals that were evaluated after a 22-h acute feed deprivation showed similar activation/entrainment as the 3-wk feed-deprived rats, but this was not seen in rats that were not feed deprived | |
| Verhagen et al. (69) | Animals: female outbred Wistar WU rats, n = 16 | Restricted feeding with set feeding time (RF-S, n = 9) vs. random dark phase feeding times (RF-R, n = 7) | 3 d | 23 h:1 h | No | ↑ In RF-R compared to RF-S | — | No difference in c-FOS positive cells between RF-R and RF-S groups | Total food intake was not different between RF-S and RF-R | |
| Miñana-Solis et al. (68) | Animals: male Wistar rats | Restricted light-cycle feeding (RF) vs. ad libitum controls (C) | 3 wk | 22 h:2 h for RF | No | Not reported | Bmal1: No change | — | — | |
| Per1: No change | ||||||||||
| Per2: No change | ||||||||||
| Kurose et al. (103) | Animals: male Wistar rats, n = 3–6 | Restricted feeding (RF) vs. ad libitum control (C) | 3 wk | 22 h:2 h | No | ↓ In RF compared to C, but not significant | Oxr2: 16.6% ↓ in RF compared to AL-fed animals | — | — | |
| Lewis et al. (50) | Animals: male, lean (+/+) rats JCR:LA-cp strain, n = 10 | Restricted feeding (RF) vs. ad libitum control (C) | 40 d | 23 h:1 h | Yes, 15 g/d total food provided for RF only | Yes, RF 107 g ↓ vs. control | NPY: no difference between groups | — | — | |
| LHA | Verhagen et al. (69) | Animals: female outbred Wistar WU rats, n = 16 | Restricted feeding with set feeding time (RF-S, n = 9) vs. random dark phase feeding times (RF-R, n = 7) | 3 d | 23 h:1 h | No | ↑ In RF-R compared to RF-S | — | ↓ c-FOS–positive cells in RF-R compared to RF-S | Total food intake was not different between RF-S and RF-R |
| Angeles-Castellanos et al. (80) | Animals: adult male Wistar rats n = 6 | Restricted feeding (RF) vs. ad libitum control (C) | 3 wk | 22 h:2 h for RF | No | Not reported | — | ↑ c-FOS-IR at fasting and mealtimes in RF compared to C group | Control animals that were evaluated after a 22-h acute feed deprivation showed similar activation/entrainment as the 3-wk feed-deprived rats, but this was not seen in rats that were not feed deprived | |
| Lewis et al. (50) | Animals: male, lean (+/+) rats JCR:LA-cp strain, n = 10 | Restricted feeding (RF) vs. ad libitum control (C) | 40 d | 23 h:1 h | Yes, 15 g/d total food provided for RF only | Yes, RF 107 g ↓ vs. control | NPY: ↑ in RF compared to C | — | — | |
| Kurumiya and Kawamura (92) | Animals: male albino Wistar rats, blinded with bilateral SCN lesions | Time-restriction (TR) followed by food deprivation | 10 d | 22 h:2 h | No | None reported | — | Multiple-unit activity was higher 3-4 h prior to feeding time, and stayed 6–7 h after consumption, and lasted 4 d into the food deprivation | Chronically implanted electrodes used to measure food entrainment in the absence of SCN and light entrainment | |
| Kurose et al. (103) | Animals: male Wistar rats, n = 3–6 | Restricted feeding (RF) vs. ad libitum control (C) | 3 wk | 22 h:2 h | No | ↓ In RF compared to C, but not significant | — | c-FOS-LI: ↑ in RF (22.2%) compared to C (5.6%) | — | |
| SCN | Mendoza et al. (127) | Animals: C3H mice | Calorie and time Restricted (C-TR) vs. ad libitum (AL) controls | 3 wk | Food given once at 0600 h for C-TR group | Yes 4.6 g/d for AL vs. ∼3 g/d for C-TR | Yes, ↓ 20% in C-TR compared to AL | PER-1: phase advanced in C-TR compared to AL | — | — |
| PER-2: ↑ amplitude in C-TR compared to AL | ||||||||||
| CLOCK: ↓ amplitude in C-TR compared to AL | ||||||||||
| AVP: phase advanced in C-TR compared to AL | ||||||||||
| Sorrell et al. (67) | Animal: male mice—DIO vs. WT vs. lean | High-fat-fed DIO mice subjected to dark-restricted feeding protocol (DRF) | 2 wk | 12 h:12 h | Not reported | Yes | Agrp: No change | — | DRF reduced hyperphagia seen in high-fat-fed DIO mice in control group, and comparable to WT and lean groups | |
| Pomc: No change | DRF increased effectiveness of leptin and ghrelin signaling in the hypothalamus, reinforcing the strength of hunger and satiety signals | |||||||||
| Arntl/Bmal1: No change | Higher plasma ghrelin in DRF vs. controls, but no difference in food intake | |||||||||
| Ghsr: No change | ||||||||||
| Fos: No change | ||||||||||
| Nr1d1/Rev-erbα: No change | ||||||||||
| Per2: No change | ||||||||||
| Cry1: No change | ||||||||||
| Angeles-Castellanos et al. (80) | Animals: adult male Wistar rats, n = 6 | Restricted feeding (RF) vs. ad libitum control (C) | 3 wk | 22 h:2 h for RF | No | Not reported | — | No difference in c-FOS-IR between groups | Control animals that were evaluated after a 22-h acute feed deprivation showed similar activation/entrainment as the 3-wk feed deprived rats, but this was not seen in rats that were not feed deprived | |
| Verhagen et al. (69) | Animals: female outbred Wistar WU rats, n = 16 | Restricted feeding with set feeding time (RF-S, n = 9) vs. random dark phase feeding times (RF-R, n = 7) | 3 d | 23 h:1 h | No | ↑ In RF-R compared to RF-S | — | No difference in c-FOS–positive cells between RF-R and RF-S | Total food intake was not different between RF-S and RF-R | |
| Mesocorticolimbic system | ||||||||||
| NAc | Wallace et al. (147) | Animals: male and female C57BL/6 mice | High-fat diet vs. control-fed mice used in a 12-h fasting vs. ad libitum–fed protocol | 3 wk | 12 h:12 h | Not reported | No difference between groups | — | ↑ DA release and re-uptake in NAc after 12-h fast | |
| Olivo et al. (148) | New Zealand white rabbits, mother and pups | Mother rabbits entrained to 12-h:12-h LD cycle; pups, 24-h dark cycle | 7 d | Pups fed once a day for 2–4 min to feed | Not reported | Not reported | — | Cytochrome oxidase–based brain metabolic activity ↑ in NAc 2–3 h before feeding time, suggesting entrainment | Elevated FAA alongside increased brain metabolic activity | |
| PfC | Guerrero-Vargas et al. (159) | Animals: adult male Wistar rats, n = 48 | Control (C) vs. weekday shift work with ad libitum food intake (W-AL) vs. weekday shift work with time-restricted food intake (W-TR); weekend schedules were matched | 6 wk | 12 h:12 h (light cycle) | C: 16% during light and 84% in dark; W-AL: 30% during light and 70% during dark; W-TR: 100% in dark | ↑14% in W-AL vs. C | ↓ Microglia fibrillary acidic protein and IBA-1 positive cells in W-TR compared to W-AL in PfC | — | ↓ Anhedonia, hypoactivity in W-TR compared to W-AL; ↑ anxiety-like symptoms in open field tests in W-AL compared to W-TR |
| W-AL > W-TR; W-AL > C | ↓ 12% in W-TR vs. C | C and W-TR were not significantly different. These effects on the PfC suggests reduced neuroinflammation in W-TR compared to W-AL | ||||||||
| Amg | Olivo et al. (148) | New Zealand white rabbits, mother and pups | Mother rabbits entrained to 12-h:12-h LD cycle; pups, 24-h dark cycle | 7 d | Pups fed once a day for 2–4 min to feed | Not reported | Not reported | — | Cytochrome oxidase-based brain metabolic activity ↑ in Amg 2–3 h before feeding time | Elevated FAA alongside increased brain metabolic activity. Increased activity before feeding suggests entrainment |
| Guerrero-Vargas et al. (159) | Animals: adult male Wistar rats, n = 48 | Control (C) vs. weekday shift work with ad libitum food intake (W-AL) vs. weekday shift work with time-restricted food intake (W-TR); weekend schedules were matched | 6 wk | 12 h:12 h (light cycle) | C: 16% during light and 84% in dark; W-AL: 30% during light and 70% during dark; W-TRF: 100% in dark | ↑14% in W-AL vs. C | ↓ Microglia fibrillary acidic protein and IBA-1 positive cells in W-TR compared to W-AL in Amg | — | ↓ Anhedonia, hypoactivity in W-TR compared to W-AL; ↑ anxiety-like symptoms in open field tests in W-AL compared to W-TR | |
| W-AL > W-TRF; W-AL > C | ↓ 12% in W-TR vs. C | C and W-TR were not significantly different. These effects on the Amg suggest reduced neuroinflammation in W-TR compared to W-AL | ||||||||
| Dorsal vagal complex | ||||||||||
| NTS | Begriche et al. (164) | Animals: C57BL/6J mice WT and Mc3r−/− | Calorie and time restriction and ad libitum control | 3 d–2 wk | 12 h:12 h, single meal in time-restricted group at 1300 h | 30–40% Calorie restriction | Not reported | Bmal1: ↓ in Mc3r−/− compared to WT in restricted and control-fed mice | — | ↑Wakefulness and activity 2 h prior to “entrained” mealtime in WT, but absent in Mc3r−/− mice |
| Rev-erbα: ↓ in Mc3r−/− compared to WT in restricted and control-fed mice | ||||||||||
| Olivo et al. (148) | New Zealand white rabbits, mother and pups | Mother rabbits entrained to 12-h:12-h LD cycle; pups, 24-h dark cycle | 7 d | Pups fed once a day for 2–4 min to feed | Not reported | Not reported | — | Cytochrome oxidase-based brain metabolic activity ↑ in NTS 2–3 h before feeding time | Elevated FAA alongside increased brain metabolic activity. Increased activity before feeding suggests entrainment |
Agrp, agouti-related peptide; Amg, amygdala; ARC, arcuate nucleus of the hypothalamus; Arntl, Aryl Hydrocarbon Receptor Nuclear Translocator Like; Bmal1, brain and muscle ARNT-like 1; Clock, clock circadian regulator; Cry1/2, Cryptochrome 1/2; AVP, arginine vasopressin; CRH, corticotrophin-releasing hormone; DA, dopamine; DIO, diet-induced obesity; DMH, dorsomedial hypothalamic nucleus; FAA, food anticipatory activity; GAL, galanin; GHSR, growth hormone secretagogue receptor; IBA-1, ionized calcium binding adaptor molecule 1; LD, light:dark; LHA, lateral hypothalamic area; Mc3r, melanocortin 3 receptor; NAc, nucleus accumbens; Npy, neuropeptide Y; NTS, nucleus tractus solitarius; OxR2, orexin receptor 2; Per 1/2, Period Circadian Regulator 1/2; PfC, prefrontal cortex; Pomc, proopiomelanocortin; PVN, paraventricular nucleus of the hypothalamus; ref, reference;Rev-erba/Nr1d1, Nuclear Receptor Subfamily 1 Group D Member 1; VMH, ventromedial hypothalamic nucleus; VTA/SN, ventral tegmental area/substantia nigra; WT, wild-type; WU, Unilever Outbred; ↓, significant decrease; ↑, significant increase.
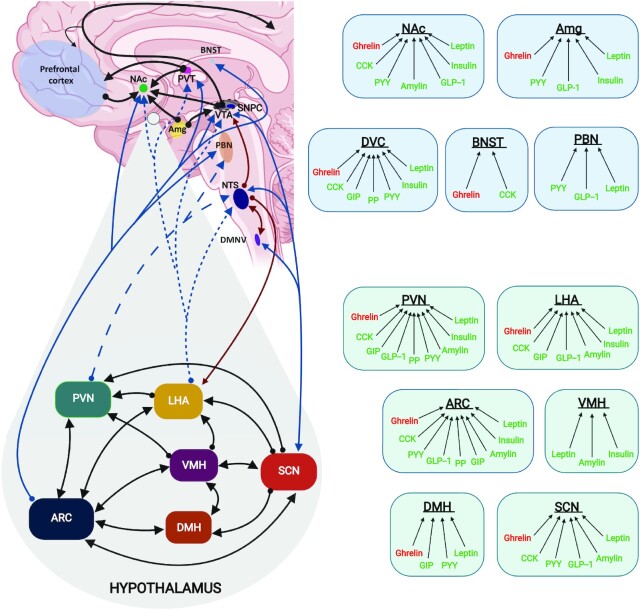
FIGURE 1.
An integrated overview of the brain regions and peripheral hormones involved in hunger and satiety. Right box panels depict the interaction of peripheral gut hormones with specific brain regions. Several hunger (ghrelin and orexin) and satiety (leptin, insulin, GLP-1, amylin, PYY, CCK, GIP) peptide hormones cross the blood–brain barrier and elicit responses in the regions alluded via unique receptor activation. Left panel: Black arrows in the non-hypothalamic region indicate neural projection connections in the mesocorticolimbic system (which includes the VTA/SNPC, Amg, NAc, PVT, and prefrontal cortex), dark-red arrows depict the DVC, made up of DMNC and NTS, in the hypothalamus: the blue dotted line indicates connections between the LHA and extrahypothalamic areas, the blue dashed line indicates the connections from the PVN to extrahypothalamic areas; black lines indicate intrahypothalamic connections between nuclei; bidirectional arrows suggest neural projections going both directions. Created with Biorender.com, Toronto, Ontario. Amg, amygdala; ARC, arcuate nucleus; BNST, bed nuclei of stria terminalis; CCK, cholecystokinin; DaMH, dorsal medial hypothalamus; DMNV, dorsal motor nucleus of the vagus; DVC, dorsal vagal complex; GIP, gastrointestinal peptide; GLP-1, glucagon-like-peptide 1; LHA, lateral hypothalamus; NAc, nucleus accumbens; NTS, nucleus tractus solitarius; PBN, parabrachial nucleus; PP, pancreatic polypeptide; PVN, paraventricular nucleus; PVT, paraventricular thalamus; PYY, peptide YY; SCN, suprachiasmatic nucleus; VMH, ventral medial hypothalamus; VTA/SNPC, ventral tegmental area/substantia nigra pars compacta.
Hypothalamic regions
Arcuate nucleus of the hypothalamus
The arcuate nucleus of the hypothalamus (ARC) is a major site for food intake and metabolic regulation (38). The ARC is located above the hypophyseal system and near the median eminence, an organ that is abundant in fenestrate capillaries and is ideally situated to be a “command center” between periphery and brain (39). The ARC receives information from other hypothalamic nuclei and extrahypothalamic regions to secrete various neuropeptides into the hypophyseal portal system to then modulate hormones released from the pituitary out to the periphery (40). Additionally, nutrient and hormonal input from the periphery can reach ARC neurons through the median eminence, which then relays this information to other areas of the brain (41). Receptors for ghrelin, leptin, amylin, insulin, CCK, PYY, GIP, GLP-1, and PP can be found in neurons throughout this hypothalamic nucleus (42). Two types of neurons found in the ARC with well-documented effects on food intake are the agouti-related peptide (AgRP) and neuropeptide Y (NPY)-expressing neurons and proopiomelanocortin (POMC)-expressing neurons (43). In addition to these peptide transmitters, both populations of neurons co-express glutamate and γ-aminobutyric acid (GABA) (44).
Stimulation of AgRP/NPY leads to hyperphagia and weight gain, but deletion of the genes encoding these neurons does not result in starvation or a lean phenotype in mice, possibly due to other pathways within the brain developing to ensure food consumption (45). AgRP/NPY project from the ARC to the paraventricular nucleus (PVN), lateral hypothalamus (LHA), and dorsal medial hypothalamus (DMH) within the hypothalamus, as well as other extrahypothalamic regions such as the PBN, paraventricular thalamus (PVT), and BNST. The release of NPY and GABA from these neurons induce a rapid feeding response by sending inhibitory signals to POMC neurons within the ARC, glutamatergic neurons in the PVN, and the PBN (46–48). The release of AgRP also stimulates food intake only after 4 h of stimulation by antagonizing melanocortin-4 (MCR4) receptors. The net effect of AgRP/NPY stimulation and release from the ARC is the inhibition of the PVN (46), ventral medial hypothalamus (VMH) (49), and DMH (50) and activation of the PVT (51), thus inducing food intake and feeding behaviors. In contrast, stimulated POMC neurons lead to satiety and reduction in food-seeking behaviors (52). POMC can be cleaved into α-melanocortin–stimulating hormone (α-MSH), adrenocorticotropic hormone (ACTH), and β-endorphin. Temporally, the influence of α-MSH on food intake via agonism of MCR4 receptors occurs after both NPY and AgRP, as 1 study demonstrated no change in food intake after 2 h of POMC activation (53). The binding of α-MSH to its receptors in the PVN and VMH stimulates second-order neurons within these nuclei to decrease food intake (47, 49, 54). Additionally, α-MSH influences postsynaptic glutamate transmission from glutamatergic, non–POMC-releasing neurons from ARC to PVN neurons that have been implicated in short-term satiety (47).
Chronic CR has been shown to increase NPY and AgRP mRNA expression and decrease POMC mRNA expression in mice (55–60). However, in Satoh et al., CR did not lead to c-Fos activation of the ARC or change in sirtuin 1 (SIRT-1) protein [NAD+ histone deacetylases and activation is associated with metabolic health (61)] after short-term (14 d) or long-term (104 d) CR compared with control (62). A 2-wk CR, providing approximately 30% of ad libitum food intake (to reduce weight by 20%), led to decreases in gene expression of glutamate decarboxylase 1 (Gad1; Gad1 is an enzyme needed to produce GABA) in POMC neurons. However, overall expression of glutamate decarboxylase in ARC was not significantly different between CR and control-fed mice, suggesting that CR selectively decreased GABAergic signaling in POMC neurons only (63). In a previous study by Jarvie et al. (64), an acute 17-h fast increased Gad1 expression in NPY/AgRP neurons and subsequent GABA release from NPY/AgRP neurons to POMC neurons. These studies indicate that CR differentially regulates GABA release in NPY/AgRP and POMC neurons that might be missed in the global assessment of GABA regulation from the ARC. Increases in NPY expression are not limited to the ARC as chronic CR in male rats resulted in increased NPY concentrations and expression in DMH as well (58).
After 7–40 d of TRF (with 1–2-h feeding windows/d) in rats, gene expression of ARC NPY mRNA and NPY protein was higher than in the nonrestricted controls (50, 65, 66). Additionally, POMC mRNA concentrations were high in the time-restricted group as well (65, 66), but no change was seen in 1 study (67). Restricting availability of a high-fat diet to the dark cycle for 12 h resulted in decreased caloric intake and weight loss compared with their ad libitum–fed littermates after 15 d (67). TRF resulted in higher circulating ghrelin concentrations, and greater ghrelin receptor gene expression in the ARC, but no increase in food intake. Increased signaling efficiency of leptin in the ARC was also reported, as seen by the increased weight loss following intracerebral injections of leptin in restricted animals compared with controls. In the ARC, these suggest that the higher sensitivity to leptin, along with the increases in ghrelin concentration and signaling strength, can improve energy balance signaling. The differences seen in TRF compared with ad libitum–fed controls in leptin and ghrelin signaling in the absence of change in NPY, AgRP, and POMC could likely be through differential GABAergic mechanisms, which have not been investigated in TRF. Hypothalamic gene expression was measured using qPCR and showed significantly higher AgRP expression in the intervention group when compared with the control group in the first hour of the feeding window; however, POMC was unaffected. When clock gene expression was measured in the ARC, an increase in Per1 and Nr1d1 was seen following 2- to 3-wk TRF protocols (1- to 12-h feeding windows) compared with controls; however, no changes were reported in Bmal1, Cry1, or Cry2 (67, 68). Similar to CR, a 3-d TRF protocol did not lead to changes in c-Fos activation of the ARC (69). Nevertheless, the melanocortin system still seems to play an important role in the beneficial effects of TRF on weight and food intake as knockout of MCR4 in obese mice led to no reduction in caloric intake (70). Based on the studies reported here, both CR and TRF increase NPY and decrease POMC in the ARC, suggesting amplified hunger and reduced satiety signaling. However, TRF appears to improve energy balance signals by strengthening the effect of leptin and ghrelin in the ARC, resulting in decreased food intake/hyperphagia, which has not been reported in CR.
Dorsomedial hypothalamic nucleus
The DMH is a cluster of neurons surrounding the poles of the VMH, although it is a poorly defined area in the human brain (71). DMH contains a high number of GABAergic neurons (72) and NPY-expressing neurons (58). Stimulated GABAergic neurons, which also express leptin receptors in ventral DMH, inhibit AgRP neurons in ARC and contribute to satiety and suppression of food intake (48). Interestingly, inhibition of total GABA release from the DMH resulted in a reduction in inhibitory tone on ARC POMC neurons, suggesting that the DMH may play a significant inhibitory role for POMC neurons through secretion of GABA (73). Previous research has shown in lean rats that overexpressing NPY in DMH leads to hyperphagia and weight gain, and a knockdown of NPY decreased food intake and weight gain (74). Additionally, knockdown of DMH NPY also led to a reduction in NPY signaling to DVC, reducing meal size and increasing satiety-inducing response to peripheral CCK administration. Unlike ARC, NPY-expressing neurons in this hypothalamic nucleus are not influenced by leptin signaling but are responsive to CR (58). Ghrelin can affect DMH in 2 ways: directly through ghrelin receptors and indirectly through activation of NPY/AgRP neurons in ARC, which project to DMH (75). The distribution of growth hormone secretagogue receptor 1a (GHSR1a; ghrelin receptor) through various neurons is not yet known; however, it has been shown that direct infusion of ghrelin into DMH increases food intake and weight gain in adult male mice. In addition to leptin and ghrelin, receptors for PYY and NPY are found in this hypothalamic area (76).
Chronic CR (given 70% of normal food intake) for 2–4 wk led to increased NPY expression in the DMH (58), which also persisted when weight was partially and fully restored to that of control rats (59). This suggests that CR has similar effects on NPY neurons in both the ARC and DMH. Interestingly, Satoh et al. (62) demonstrated that a 40% CR (60% caloric intake of controls) for 2 wk increased SIRT-1 concentration at 14 d and 104 d, which then increased orexin receptor type 2 gene expression in DMH and promoted physical activity/locomotion, which was not seen in the ARC. Additionally, male mice that overexpress SIRT-1 (BRASTO) had marginal increases in FAA versus SIRT-1–deficient mice when given 60% of their daily caloric intake for 14 d and for 104 d, suggesting that SIRT-1 may mediate the effects of CR on hypothalamic nuclei; however, additional studies are needed to confirm this. Taken together, the limited studies on the effects of CR on the DMH indicate increased hunger signaling through increased NPY expression and orexin sensitivity. However, longer-term and human studies are needed to verify this effect.
With regard to appetite or food intake, TRF (1-h feeding window) with CR for 40 d increased NPY concentrations in the DMH as well as the ARC and LHA of lean male rats (50). However, whether the increased NPY concentrations in DMH were due to increased NPY transport from ARC or increased synthesis within DMH was not established. Verhagen et al. (69) reported a decrease in c-FOS+ cells in DMH after a 3-d TRF (1-h feeding window), but this did not affect total food intake significantly. The DMH has direct connections to the SCN and plays a critical role in circadian rhythms. It was hypothesized to be the controller of the elusive FEO and previous research by Gooley and colleagues (77) showed that mice were unable to anticipate meal timing after lesioning of the DMH, suggesting that the DMH plays an important role in FEO and FAA. However, more recent studies have shown ablation of the DMH can influence food anticipatory behavior but does not completely abolish food-entrained rhythms or FAA, suggesting that the DMH is not the central regulator for FAA or FEO (69, 78, 79). A 2-h daily TRF regimen for 3 wk in adult Wistar rats demonstrated no difference in DMH activity via c-FOS immunoreactivity; however, non-TRF animals (controls) that were exposed to a 22-h feed deprivation showed entrainment in the DMH that was similar to the 3-wk TRF animals (80). Additionally, a similar TRF regimen reported an increase in Per1, a decrease in Per2, and no change in Bmal1 gene expression within the DMH (68). Further, research on the role of TRF and CR on DMH activity and clock gene expression is needed to improve our understanding of its role in food intake and satiety.
Ventromedial hypothalamic nucleus
The VMH is a pear-shaped nucleus located adjacent to the ARC (71). The VMH contains highly heterogeneous cells and thus is generally further divided into subnuclear regions: the ventrolateral VMH contains a large portion of estrogen receptors, somatostatin cells, and oxytocin receptors; the smaller dorsomedial and central VMH contains GHSR1a, leptin receptors, GABA receptors, steroidogenic factor 1 (SF-1), and brain-derived neurotrophic factor (BDNF) (81). Various studies have also shown that the VMH can influence energy balance and is sensitive to leptin, insulin, ghrelin, CCK, GLP-1, PYY, and orexin (82, 83). The VMH receives input from the ARC (49), DMH, and SCN, and sends signals to various brain regions including the ARC, DMH, SCN, PVN, BNST, NAc, amygdala, and brainstem (81). Interestingly, glutamatergic neurons lacking MCR4 receptors in the VHM stimulate POMC neurons in the ARC, and the activity of VMH glutamatergic neurons is reduced during fasting (54), suggesting a redundant pathway to communicate energy status to the ARC. Additionally, glutamatergic neurons from the VMH are thought to project to the LHA as well, which increase during fasting, possibly promoting food-seeking activity (82).
To our knowledge, only 1 study looking at the effects of CR on VMH has been reported. Unlike the DMH, 40% CR for 14 d did not lead to changes in SIRT-1, orexin receptor gene expression, or neuron activity (measured via c-FOS expression) in the VMH (62). BDNF has anorexigenic properties and its expression is influenced by starvation and the melanocortin system from the ARC (49). In mice, removing food on alternating days led to an overall decrease in caloric intake by 30–40% and increased overall BDNF expression (84). Similarly in humans, 25% CR for 3 mo in overweight and obese adults led to a 7% weight reduction and significantly increased serum BDNF concentrations (85). It should be noted that other brain regions (notably the hippocampus) express BDNF as well (86). However, selective deletion of the Bdnf gene in the VMH and DMH leads to hyperphagia and obesity in mice (87). Recent research suggests that BDNF plays an important role within the VMH to regulate inhibitory signaling to neurons expressing SF-1, a transcriptional factor that is exclusively found in the VMH (88). Evidence from mouse studies reports that VMH SF-1 is important for leptin-mediated reductions in meal size and increases energy expenditure (89). However, the mechanism by which dietary restriction leads to increased BDNF has not been elucidated, although it is hypothesized that it is a type of stress response to fasting and CR (90). Therefore, more targeted research on the effects of CR on satiety and food intake in VMH, focusing on hypothalamic BDNF or other VMH-specific factors, is needed.
As mentioned previously, the VMH influences FAA, and previous research has demonstrated that ablation of the VMH and silencing ghrelin receptors in the VMH attenuates FAA in male mice (82). Mice that were shifted to mid-light-cycle feeding had higher VMH activity following the meal shift than mice that were kept in the dark-cycle feeding (91). Projections from the VMH to the preoptic area (POA) in the hypothalamus could be how the VMH influences locomotor activity related to FAA (81, 82). A few studies have looked at activity and gene expression in the VMH using a TRF paradigm. Restricting feeding times to 2 h/d for 3 wk did not change c-FOS–measured neuronal activity or Bmal1 or Per1 clock gene expression, but a reduction in Per2 was reported (68, 80). NPY concentrations in VMH were not affected by 40 d of TRF (23 h feed deprivation:1 h feeding) in male rats, despite having lower body mass than ad libitum–fed controls (50). In a study by Kurumiya and Kawamura (92), adult mice with lesions in the SCN developed a food-entrained daily rhythm in VMH when placed in a 2-h feeding regimen for 10 d, which persisted 4 d after the TRF regimen had stopped. The available literature on the effects of CR and TRF on VMH is sparse, making it difficult to draw definitive conclusions. Due to its close ties to peripheral signals of appetite and energy expenditure, more research on the VMH is needed.
Paraventricular nucleus of the hypothalamus
The PVN is another cluster of neurons known to be important in energy balance and feeding behaviors. In addition to signals from within the hypothalamus from the ARC, LHA, and SCN, the PVN's activity is also influenced by circulating ghrelin, orexin, PYY (76), CCK, insulin, leptin, GLP-1, PP, and amylin (42, 93). The PVN has a higher concentration of MCR4 receptors than other areas of the brain (94) and electrical lesioning of this area leads to hyperphagia and obesity in rodents (95). α-MSH from the POMC neurons in the ARC binds to these MCR4 receptors, stimulating neurons in the PVN to synthesize and release several neuropeptides including corticotrophin-releasing hormone and oxytocin and potentiate signals to the PBN to induce satiety (38, 46). BDNF-expressing neurons also in the PVN have been shown to influence energy balance and can inhibit feeding (96). A subset of PVN neurons expressing thyrotropin-releasing hormone (TRH) and pituitary adenylate cyclase-activating peptide (PACAP) has been shown to stimulate AgRP neurons in the ARC, leading to increased hunger signaling, suggesting that the PVN can signal for both orexigenic and anorexigenic behaviors, but in differing neuronal pathways (97).
CR and food deprivation reduce core body temperature, which may contribute to beneficial effects of CR on aging and longevity. The ARC, PVN, and LHA contain high concentrations of neurons that express thermoregulatory neuropeptides [such as TRH, melanin-concentrating hormone (MCH), NPY, etc.] (98). CR led to increased insulin-like growth factor I (IGF-I) sensitivity in PVN, which is hypothesized to decline with aging (99). Interconnectivity of PVN to ARC and LHA suggests that changes related to CR in ARC and LHA activity will also affect PVN secondarily, but no studies have focused on satiety as the primary outcome. Radler et al. (60) demonstrated that 50% CR for 4 wk led to significant changes in NPY activity in the ARC, but not the PVN. Whether this relates to changes in other neuropeptides, appetite, or satiety was not the focus of this study, so further research is needed. A 25% CR for 3 wk increased basal glucocorticoid concentrations but did not significantly increase PVN activity and the mice displayed reduced anxiety-related behaviors (such as grooming) and normal stress response (100). In agreement with this, mice placed on 40% CR for 14 d had no differences in SIRT-1 and c-FOS immunoreactivity compared with ad libitum–fed controls (62). Taken together, this suggests that modest CR is not significantly stress-inducing or that other hormones that increase in response to CR (e.g., ghrelin) may help attenuate the stress response to CR. Gastrointestinal hormones were not measured in this study, so additional studies are needed to confirm this hypothesis.
Deletion of the clock gene Bmal1 in the PVN led to increased body weight and reduced feeding rhythmicity; however, overall daily food intake was not different than that of controls (101). Weight gain in these rats was primarily attributed to reduced energy expenditure, suggesting that disruptions in PVN rhythms affect metabolism but have less effect on feeding behaviors. Additionally, 3 wk of TRF (2-h feeding window) in male Wistar rats did not affect clock gene expression in the PVN, as no changes in Bmal1, Per1, or Per2 gene expression were reported (68). Two studies have reported no increases in PVN activation (via c-FOS immunoreactivity) following TRF with a 1- to 2-h feeding window in Wistar rats (69, 102) and 40 d of a 1-h TRF regimen in male rats reported no differences in NPY concentrations compared with ad libitum controls (50). In contrast, Brady et al. (66) demonstrated that TRF (23 h fasting:1 h feeding) for 14 d increased NPY and decreased POMC expression in the PVN of Sprague-Dawley rats. A restricted feeding period of 2 h for 3 wk led to reduced orexin receptor type 2 gene expression in the PVN in rodents (103). The evidence for the effect of TRF on PVN is mixed and additional research is necessary to fill this knowledge gap.
Lateral hypothalamic area
The LHA includes orexin, MCH, GABA, and glutamate neurons that innervate several areas in the brain including the VMH, PVN, ARC, BNST, NAc, and VTA (54, 104). The prevailing thought is that the LHA combines the information of body energy and fluid status, “reward-related learning” (hedonic), and cognition to then influence the motivated behaviors in the mesolimbic system (105). Increased activity in the LHA is indicative of hunger and satiety states in humans (106). Electrical lesioning of LHA in mice led to decreased adipose tissue in both obese and lean mice, but food intake remained adequate for growth (107) suggesting that LHA influences energy balance. Other pathways to drive hunger and food intake exist. Orexin neurons in LHA secrete orexin, leading to increased food intake among other intake behavior including locomotor activity and water intake. FAA is reduced when orexin neurons are ablated or in orexin-knockout models in LHA; however, FAA is not completely abolished. Locomotor activity is also increased during electrical stimulation of LHA (82). MCH, another orexigenic neuropeptide, is also produced and secreted from LHA. Stimulation of GABAergic neurons in LHA increases appetitive and consummatory behaviors in mice, whereas inhibition leads to attenuated weight gain and decreased food intake (108). These GABAergic neurons from LHA project to VTA to inhibit both PVN (109) and VTA neurons (110). Excitatory glutamatergic neurons from LHA also project to VTA to stimulate activity (110) and receive inhibitory signals from the BNST (111). Some of these glutamatergic neurons that express the leptin receptor also affect orexin neurons within LHA to reduce food intake (112). Ghrelin activates orexin neurons; however, prolonged and repeated activation of the orexin neurons may downregulate preproorexin mRNA expression in LHA, impairing the compensation capacity of orexin neurons (111). Although MCR4 is present in several LHA neurons, recent evidence suggests that AgRP antagonism in this region is not necessary to stimulate feeding behavior (113). Other peripheral hormones that influence LHA activity include insulin, GLP-1, GIP, amylin, and CCK (112).
CR led to a decrease in plasma orexin-A concentration in obese adults following a very-low-calorie ketogenic diet (114), suggesting sensitivity of orexin neurons in the LHA and PVN to energy status. Orexin and MCH gene expression in the LHA were higher after 3 wk in mice that were refed a high-fat diet following a 25% CR compared with mice that were refed a normal feed pellet diet or mice fed a high-fat diet ad libitum (115). Additionally, SIRT-1 expression increased in LHA after 14 d of CR (60% daily caloric intake of controls), leading to higher orexin receptor type 2 gene expression and locomotor activity in male mice (62). These data suggest that LHA is sensitive to CR and increases in LHA activity could directly affect other areas in the brain, including extra-hypothalamic regions such as the mesocorticolimbic system via connections to the VTA.
The role of the LHA in FAA/FEO is unclear, although a few TRF studies did investigate this. Wistar rats that were subjected to 1- to 2-h feeding windows for 3–21 d had higher LHA activation compared with ad libitum–fed controls (102, 103). In an earlier study by Kurumiya and Kawamura, adult mice with lesions in the SCN developed a food-entrained daily rhythm in LHA when placed in a 2-h feeding regimen for 10 d, which persisted days after the TRF regimen had stopped (92). A more recent study that used a 4-h TRF feeding window for 9 d in orexin knockout mice observed entrainment of locomotor activity and body temperature, but not in food intake compared with wild-type controls (116). Additionally, another study (3 d of a 23-h fasting:1-h feeding TRF regimen, with set 1 h vs. random 1 h feeding times) reported no differences in total food intake between the restricted-feeding group (69), suggesting that time restriction affects food intake regardless of whether food was given at the same time or not. A 1-h TRF feeding window with CR for 40 d in rats resulted in increased NPY protein in the LHA along with body-weight loss (50). However, future studies should focus on the effects of TRF on LHA without imposing CR to tease apart the effect of CR from TRF on NPY and downstream hunger. Furthermore, studies are required to confirm these reports of entrainment of neuronal activity in LHA.
Suprachiasmatic nucleus
The SCN within the hypothalamus drives and maintains the 24-h circadian rhythm by releasing “time-of-day” signals to the periphery, while also modulating its own sensitivity to peripheral signals (117). The LEO in the SCN responds to direct and indirect retinal ganglionic inputs and sets the body clock (25). The SCN regulates the secretion of melatonin, adrenocorticotrophic hormone, and arginine vasopressin, all of which constitute “time-of-day” signals (118, 119).
While the hypocretin system in the LHA receives direct and indirect input from the SCN, orexin receptors in the SCN can cause phase shifts in circadian rhythm, establishing a significant link between sleep–wake cycle, food intake, and activity (120). Similarly, ghrelin receptors are also found in the SCN and have been shown to influence the central circadian clock (121). In addition to these, the gastrointestinal clock—enforced by the migrating motor complex (MMC; the electrical rhythm leading to peristalsis and gastrointestinal movement)—is influenced by melatonin, and therefore the SCN (122). Starting in the stomach, MMC is generated by ghrelin and motilin (122) and may be “entrained” by TRF differently than non-TRF or CR, as observed from the peripheral hormone literature. This suggests a potential central–peripheral interplay mechanism for where the difference between the CR and TRF regimes plays out (Figure 2) (122). While it is unclear if there are insulin receptors on the SCN, the latter does have a significant impact on insulin resistance, which is mediated by neural links between the SCN, VMH, and PVN (123). Leptin (via leptin receptors) has also been observed to interact with and influence the circadian clock (124). Both leptin and insulin are central energy-sensing signals, and ghrelin is the primary peripheral orexigen, so it is reasonable to expect these to interact with the SCN.
FIGURE 2.
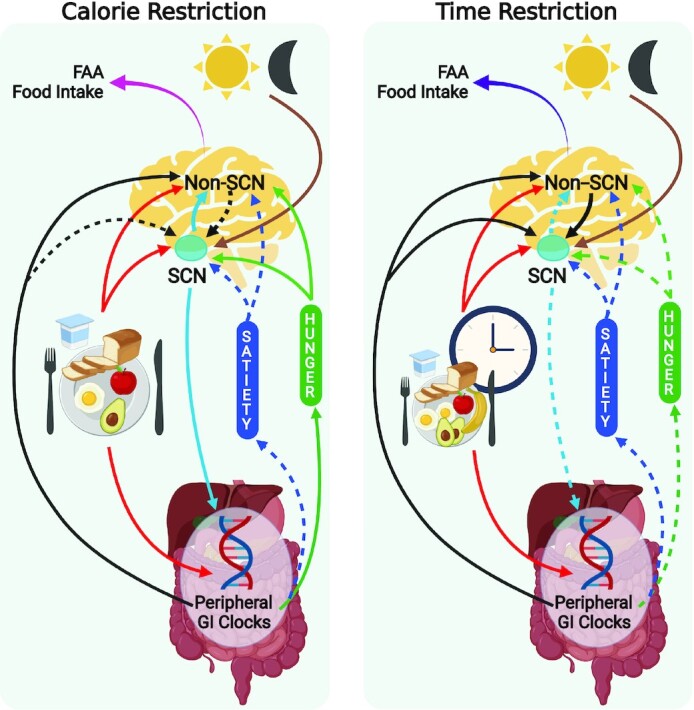
Effect of CR and TRF on peripheral and central clocks involved in hunger and satiety. Left: time restriction; right:—calorie restriction. An overview of mechanisms suggested to be involved in TRF and CR compared with ad libitum food consumption and their eventual effect on FAA and food choice (highly palatable foods, etc.). This depicts the integration of satiety and hunger signals (blue and green boxes originating from the gut) with the non-SCN and SCN clocks in the brain. During ad libitum feeding, the light-dark cycle entrains the light-entrainable oscillators in the SCN, while food cues and peripheral clocks are regulated and reset by the SCN-clock. Hunger systems may be less “stimulated” following TRF, in contrast to CR, while satiety systems are equally suppressed following both regimes. Following TRF, the food-entrainable and peripheral clocks become stronger, and induce resetting of the SCN-clock. In the case of CR, the SCN-clock resets the food-entrainable and peripheral clocks. Combined, the integrated mechanism suggests that the overall lack of increase in hunger and stronger regulation by peripheral metabolic and food-entrainable non-SCN clocks may mean that TRF could aid in adherence to specific food intake regimes such as CR. Different color arrows used to indicate entrainment (brown and red), feedback of peripheral satiety signals to the brain (blue and green), interactions between SCN, non-SCN, and peripheral clocks (black, light blue). Dashed arrows indicate a reduction in strength of physiological signal compared with no-CR or no-TRF. Created with Biorender.com. CR, calorie restriction; FAA, food anticipatory activity; GI, gastrointestinal; SCN, suprachiasmatic nucleus; TRF, time-restricted feeding.
Daily regulation of tissue- and organ-level functions appears to be regulated by peripheral clocks; however, large sweeping changes, such as complete phase shift in light-dark cycles and synchronization of peripheral clocks, are regulated by the central circadian clock in the SCN (12, 18), More importantly, non-SCN clocks (which may or may not constitute the FEO) that exist in the brain are entrained by timing of food intake and can influence downstream behavioral and endocrine functions, including satiety systems, independently of the SCN (24).
A few studies have looked at the effect of CR and TRF on the SCN. Satoh et al. (62) reported increases in SIRT-1 concentrations in the SCN following 14 d of CR (40% caloric reduction), although no changes in SCN c-FOS activity were seen. Despite the lack of change in neuronal activity, Froy et al. (18, 19) have shown that CR enhanced the synchronicity of biological rhythms and a pronounced SCN-clock, based on increases in hepatic clock gene expression in transgenic mice that restrict caloric intake naturally by 30–50%. However, time restriction has also been shown to reset the SCN and peripheral clocks through observed changes in hepatic clock gene expression (18, 125–128). While 2 studies that looked at the effect of TRF for 2–3 wk on clock gene expression in the SCN have reported contrasting effects, this is likely due to differences in their study design and research questions being nonuniform (67, 127). Mendoza et al. (127) conducted TRF with CR for 3 wk and reported an increase in PER2 and decrease in CLOCK proteins, as well as a phase advancement in PER1 and AVP in the TRF compared with controls. On the other hand, Sorrell et al. (67) conducted a shorter-term (2-wk) TRF regimen with a 12-h dark-restricted feeding window [in high-fat-fed DIO (diet-induced obesity) mice] and reported no effect on clock or melanocortin gene expression. However, this restricted feeding protocol strengthened leptin and ghrelin receptor signaling in the SCN, suggesting an effect of peripheral satiety signals of the central SCN-clock. Yet another study in male Wistar rats following a 3-wk TRF (2-h feeding window) also reported no difference in neuronal activity in the SCN based on c-FOS measurements (102). However, the TRF rats showed similar entrainment of the SCN as the control rats after an acute 22-h feed deprivation. In summary, CR may lead to pronounced SCN-clock signals and synchronization of biological rhythms, influencing the regulation of metabolic and satiety mechanisms. TRF likely resets the circadian rhythm, as seen by changes in the phasic expression of clock genes and neuronal activity in the SCN, albeit with some contradictory evidence, and influences leptin and ghrelin signaling, hinting at a peripheral–central integrated effect that needs further investigation.
Non-hypothalamic regions
Mesocorticolimbic system: NAc, VTA, PfC, and amygdala
The NAc along with the VTA, the medial PfC, amygdala, and hippocampus form what is known as the mesocorticolimbic system (129). The NAc is 1 of 2 primary nutrient-sensing regions in the brain and it regulates and participates in dopamine-mediated behavioral response following a rewarding stimulus (130). Dopaminergic inputs from VTA are received in the NAc, and low concentrations of dopamine promote lesser-effort/risk-involved reward-seeking behavior, compared with higher dopamine concentrations (131). In the context of satiety and food intake regulation, the nucleus accumbens shell (NAcSh) is reciprocally connected to feeding-related areas in the hypothalamus (D1R-neurons to the LH). The NAcSh is purported to be more involved in the hunger system responses compared with the nucleus accumbens core (NAcc), although alternate theories emphasize the role of dopamine signaling in the NAcc (132). When D1R in the NAc are activated, food consumption is reduced, via inhibition of LH GABA-transporter neurons, while D2R in the NAc appear to be involved in taste perception (133). In addition to this, ex vivo activation of NAcSh activity upon ghrelin injection has been reported recently (134), suggesting a link between peripheral and central food intake control systems that do not involve the mesocorticolimbic system. Similarly, CCK-8S, the sulfated form of CCK produced in the brain, activates CCKB receptors in the NAc and contributes to dopamine or GABA-induced downstream excitation in the NAc (135). This is independent, however, from the peripheral CCK, since CCK cannot cross the blood–brain barrier (136). Also, insulin receptors in the NAc directly respond to glucose (137), which is relevant to the systemic circulation and food intake drive, since insulin does get past the blood–brain barrier (138). Amylin, which also crosses the blood–brain barrier (139), activates amylin receptors in the NAcSh to suppress feeding (140). PYY3-36 and GLP-1 also influence activity in the NAc, but most likely via dopaminergic inputs originating from receptors for these hormones in the VTA (141). A recent systematic review that summarized 349 human fMRI studies that probed the link between peripheral and neural circuits reported that, with the exception of glucose, no other peripheral satiety hormone was associated with activity in the NAc (142). This is in contrast with our earlier reports of independent studies that report receptor activity in the NAc. The receptor activity studies were done directly on rat/animal brain slices, and evaluated either electrical or chemical activity downstream, and are hence more reliable. However, their translation to the human brain, and at the systemic level, could be questioned.
CR in female Fischer-344 rats (from 4 to 26 mo of age, 40% CR vs. ad libitum–fed control group) resulted in increased dopamine overflow in the ventral striatum and NAc, countering the age-related decline seen in dopamine neuronal function (143). In contrast, a different study (also using Fischer-344 rats, both male and female, with 40% CR, from 3.5 to 22.5 mo of age) did not see any increase in dopamine or related metabolites in the NAc compared with the ad libitum–fed control group (144). Instead, they observed a reduction in 5-hydroxyindoleacetic acid (a serotonin metabolite). In a more recent investigation, male rhesus monkeys were placed on a 6-mo 30% CR and compared with an ad libitum–fed control group (145). They observed an increase in dopamine and dopamine metabolites (3,4-dihydroxyphenylacetic acid and homovanillic acid) in the striatal region and NAc compared with the control group. In mice subjected to CR (40% CR, for 10 d), there was an accumulation of ΔFosB (a protein that plays a role in addiction development and maintenance) in the NAcSh, suggesting a link between addiction pathways and seeking behavior of high-energy foods as a reward (146). Overall, it appears that, while CR in the acute time frame (days) may result in increased reward-seeking behavior, over the long run it may mean higher stimulated dopamine release in the NAc region. This would increase the reward-seeking behavior—in this case, eating food. TRF for 3 wk with a 12-h feeding window in mice has been shown to alter tonic and phasic dopamine release by increasing dopamine secretion and re-uptake in the NAc, which affects both motivation and food-consumption drive (147). In rabbit pups fed in a time-restricted manner (feeding once a day for 2–4 min), FAA and cytochrome oxidase–based brain metabolic activity in the NAcc is elevated 2–3 h before scheduled food intake, due to the entrainment, and this elevation influences the amount of food consumed (148).
The VTA (mostly talked about along with the substantia nigra pars compacta) is caudal to the posterior hypothalamus, bracing the third ventricle (130). The generally accepted notion is that VTA largely uses dopaminergic systems (while it also has GABAergic and glutamatergic projections) to direct motivated behavior in response to inputs that are rewarding or aversive (149) and works closely with the NAc for these processes. As mentioned earlier, dopaminergic neurons are involved in estimating the cost associated with obtaining a reward in the form of food, following exposure to a food cue (150). The VTA, along with the NAc, is also involved in FAA. Both acute 24-h feed deprivation (in mice) and 6 mo CR (30% deficit, in rhesus monkeys) have been shown to increase dopamine release into the VTA (145, 151), which is closely linked to the NAc via the mesocorticolimbic circuit. The VTA has ghrelin, insulin, and leptin receptors, and activation of all are involved in modulating dopaminergic reward-seeking behaviors originating in the mesocorticolimbic system. Ghrelin increases reward-based food intake (152, 153), while insulin and leptin suppress this (154). Furthermore, hypothalamic ghrelin may also increase the release of GABA, leading to the hyperpolarization of POMC neurons (155). Ghrelin also targets the VTA and influences taste sensations and reward-seeking behavior, through stimulation of the “cholinergic-dopaminergic” reward link (156). And yet, to our knowledge, no studies to date have investigated the effect of TRF on VTA.
The mesocorticolimbic pathway extends to the PfC and the central nucleus of the amygdala. Studies have shown that hunger influences activity in various portions of the cortex—posterior cingulate cortex, caudate-putamen, fusiform gyrus, and amygdala (157). Moreover, food deprivation resulting in CR increased reward processing in these areas following the presentation of energy-dense foods, while satiated states resulted in elevated rewards processing when exposed to low-energy foods, along with reduced stress reactivity (157, 158). A reduction in stress reactivity in the PfC and amygdala also resulted in reduced anxiety and depression-like behaviors following TRF (for example nightshift workers) (159). However, the primary effect of CR on the PfC appears to be related to increased reward for high-energy foods. The amygdala is known to inhibit food intake and, for learning aversive cues, to limit food intake (160). In particular, the activity of the amgygdala was found to be involved in curbing food intake during an eating episode, following a fast (161). This suggests that the amgygdala activation following a fasting–refeeding regimen, such as in TRF, helps limit subsequent food intake. The effect of CR on the amgygdala and TRF on the PfC remains uninvestigated to date.
Based on the few studies that have evaluated the mesocorticolimbic system, both CR and TRF appear to increase dopamine and reward-seeking behavior, which aligns with the reduced satiety seen in the peripheral systems, not to mention the increase in palatable food intake and craving with CR. Intricate differences between TRF and CR, if any, in the non-hypothalamic regions do not seem to be an active area of study; however, TRF studies do indicate entrainment of food timing synchronized with change in FAA. Further, dopaminergic systems in the mesocorticolimbic circuit, along with food aversion capabilities of the amgygdala, warrant further investigations into these areas, keeping CR and TRF in mind.
Dorsal vagal complex
The DVC comprises the nucleus tractus solitarius (NTS), the area postrema (AP), and the dorsal motor nucleus of the vagus nerve (DMNV) (162) and is involved in determining long-term feeding adaptation (163). This network comprising NTS, AP, and DMNV is part of the central melanocortin system, which is involved in nutrient sensing, and interacts with hypothalamic, limbic, and hindbrain regions (164). The melanocortin system consists of the melanocortin-3 (MC3R) and melanocortin-4 (MC4R) receptors. While MC4R helps regulate peripheral satiety, MC3R mostly works within the hypothalamic and limbic structures (i.e., the DVC) (165). There exists an antagonistic interaction between, and co-localization of, melanocortin and mu-opioid receptors in the NTS, DMNV, and the vagus; however, the exact mechanisms involved in this are still under investigation (166, 167). The mu-opioid receptors are key players that respond to endogenous opioids and modulate hedonic eating behaviors (168). When the AP/NTS region was surgically ablated in rats, their overall food intake decreased, suggesting a reduction in both physiological and hedonic signal interruption (169).
The NTS is the site of integration of signals related to satiety and forms an important junction between peripheral and central (hypothalamic and non-hypothalamic) signals. In mice placed on TRF protocols (presented with food briefly once every 24 h), there was increased wakefulness and FAA, and the expression of MC3R receptor in the NTS was associated with and involved in these behaviors by influencing the clock genes Bmal1 and Rev-erbα (164). Rats placed on time-restricted access to food (both normal feed pellets and sweetened palatable foods, given access for 2 h in a 24-h cycle) displayed reduced expression of mu-opioid mRNA in the NTS, suggesting that the hedonic pathways may also be dampened under restricted-feeding regimes (170).
The DMNV is intricately involved in pancreatic secretory function and has receptors for ghrelin, insulin, CCK, PP, and PYY3-36 (162). Ghrelin is produced in the DVC, and ghrelin protein and mRNA expression increased during feed deprivation (24 and 48 h feed deprivation) conditions; longer periods of feed deprivation meant greater increases in DVC (primarily in the DMNV) in rats (31). Therefore, while ghrelin can cross the blood–brain barrier, endogenously produced ghrelin in the DVC responds to nutrient status (mechanisms for which remain yet unclear), and likely elicits downstream behavior via its projections to the central amygdala within the mesocorticolimbic system (171). When access to food is restricted (3–4 h per 24-h cycle), rodents exhibit FAA. However, recent evidence suggests that neural peripheral postprandial signals (from leptin and insulin), which are important elements in the “food entrainment” system, play a larger role in entrainment-based appetite regulation during time-restricted access to food (102) and that the DMNV is involved in this process. The DVC appears to be a poorly investigated area with regard to CR and TRF.
If the peripheral signals elicited by feeding contribute to the food entrainment system, this may suggest that the FAA may be balanced by the food entrainment aspects of appetite control, offering a system check. Since FAA is associated with energy intake, the entrainment may offer control on energy intake.
Conclusions
CR and TRF affect various hypothalamic and non-hypothalamic regions associated with appetite and satiety. Short-term studies (ranging from days to weeks) indicate that both CR and TRF increased NPY expression (which is associated with increased hunger) and decreased POMC expression (suggesting reduced satiety). CR also increases mRNA expression of AgRP and GABA, which also stimulate hunger and signal for food intake. Additionally, CR increases the amplitude of clock gene expression in SCN-controlled peripheral tissues, indicating that there is both strengthening/pronouncement of the SCN-clock, and increased synchronicity with peripheral tissues (18, 19). Further research on the effects of CR on other hypothalamic regions associated with appetite and satiety and the integration of signals between each of these regions is needed.
Unlike CR, TRF has been shown to improve both energy balance signals in the hypothalamus and the ability of leptin to preserve body fat mass, resulting in blunted hyperphagia leading to weight loss. In a companion paper where we summarized the results from CR and TRF protocols and their effect on peripheral circulating hormones, we report that CR results in elevated fasting ghrelin while TRF results in decreased or unchanged ghrelin (9). Taken together, these suggest that in TRF the central and peripheral hunger and satiety mechanisms act via hypothalamic melanocortin systems to regulate energy balance, and mitigate hyperphagia seen in CR.
Several studies report that a few days of TRF results in entrainment of neuronal activity in several hypothalamic regions, including the ARC, LHA, VMH, and DMH, which dictates both food anticipatory behavior and food intake (68, 92, 102, 125). Further, this entrainment lasts as long as the behavior is sustained, or even in complete abstinence from feeding. Many TRF studies examining changes in the hypothalamus largely focus on FAA, entrainment, and neuronal activity with feeding windows that were extremely short, resulting in increased locomotor activity and weight loss (50, 69, 80, 103). While this provides insight into intra-hypothalamic networks and mechanisms that synchronize FAA, FEO, and food timing, more research is needed on the effect of TRF or slight shifts in meal timing (without CR or increased energy expenditure) on these brain regions. The addition of circulating appetite/satiety-related hormone concentrations and food intake measurements in these studies would provide valuable insight into this area.
In looking at non-hypothalamic regions, both short-term CR and TRF increased dopamine secretion and activity in the mesocorticolimbic system; however, TRF regimens also result in entrainment of FAA and reduced neuroinflammation. Additionally, the DVC is poorly investigated in CR and TRF conditions, and it is difficult to draw any conclusions based on the available literature at this time.
There are not enough long-term studies done on CR and TRF to understand their effects on central mechanisms related to satiety. It is known that the SCN is plastic due to changes at the level of DNA methylation and network interactions (172, 173), induced by external factors such as season and time-of-day (174). Thus, TRF and potentially CR might indirectly lead to neuroplastic changes in the SCN and peripheral oscillators (175, 176). In addition, plasticity of brain regions involved in food-related behavior and satiety (such as PfC and VTA) could potentially be involved in long-term changes in response to CR and TRF (177). Future studies are required to examine the nature of such alterations to identify the long-term effects of CR and TRF.
Another aspect to consider when evaluating CR and TRF is the effect of sleep on LEO/peripheral clocks/FEO. The LEO is responsible for regulating sleep cycles, both CR and TRF have been shown to increase sleep (178–180), and on the flip side, lack of adequate sleep has been shown to increase caloric intake (181). Since satiety mechanisms involving ghrelin and orexin are critical to how the brain regulates food intake, and orexin influences both hunger and sleep, it is clear that timing, duration, quality, and type of sleep affect these mechanisms, and need to be factored in for a clear picture to evolve. Qian et al. (182) reported that a complete phase shift (as seen in nightshift workers) of both sleep patterns and food intake influenced metabolic health by increasing circulating lipids and catecholamines. More such studies that look at the effect of sleep, along with food intake, are necessary to better understand underlying mechanisms.
Limitations and Directions for Future Research
Animal studies that looked at the effect of CR and TRF on brain regions used nonuniform caloric and time-restriction protocols (20–50% CR, 24-h–40-d TRF regimes) along with differing study designs (CR vs. non-CR, TRF vs. non-TRF, CR vs. TRF, TRF + CR vs. ad libitum intake), diets (regular feed pellets, high-fat), animal models (rats, mice–wild-type/transgenic, primates, rabbits, human), and light-dark entrainment (light feeding, dark feeding, all-time food access, randomly selected times for food access), making it challenging to identify clear effects. Moreover, very few studies were able to minimize the effect of SCN-clocks while looking at non-SCN and peripheral clock entrainment effects of TRF, and only 1 study mimicked the effect of weekday compared with weekend schedule misalignment as seen in shift workers. These variations impact our ability to draw clear conclusions, and more studies in this area addressing these sources of variation will help us better understand the effect of CR and TRF. Animal studies that looked at central satiety mechanisms and brain region receptor activation failed to report on circulating hormone concentrations. This makes it difficult to draw connections between the 2. Given that some peripheral hormones that act as satiety and hunger signals may also be produced centrally, a measure of these hormones in peripheral circulation could answer important questions. More imaging-based studies in humans that also measure and report circulating hormones are necessary if we are to gain more insight into physiological satiety systems that play a role in regulating food intake. Satiety and hunger systems play a significant role in facilitating long-term dietary modifications and weight loss (27). The study of these systems would fill the current knowledge gap and elevate the mechanistic basis of obesity research.
Acknowledgments
We are grateful to Fanny Lee for her review of the manuscript. The authors’ responsibilities were as follows—SK and GPK: conceived the idea; SK, GPK, DKMT, APT, CER, and WFH: collected the literature; DKMT, SK, and GPK: critically assessed the identified literature/manuscripts; DKMT, APT, CER, GPK, and SK: developed the early manuscript versions; WFH and NLK: helped to critically assess the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors report no funding received for this work.
Author disclosures: The authors have no conflicts of interest to report.
Abbreviations used: AgRP, agouti-related peptide; AP, area postrema; ARC, arcuate nucleus of the hypothalamus; BDNF, brain-derived neurotrophic factor; Bmal1, brain and muscle ARNT-like 1; BNST, bed nuclei of stria terminalis; CCK, cholecystokinin; CR, calorie restriction; Cry1/2, Cryptochrome 1/2; DMH, dorsal medial hypothalamus; DMNV, dorsal motor nucleus of the vagus; DVC, dorsal vagal complex; FAA, food anticipatory activity; FEO, food/feeding entrainable oscillator; GABA, γ-aminobutyric acid; Gad1, glutamate decarboxylase 1; GHSR1a, growth hormone secretagogue receptor 1a; GIP, gastrointestinal peptide; GLP-1, glucagon-like peptide 1; IGF-I, insulin-like growth factor I; LEO, light-entrained oscillator; LHA, lateral hypothalamus; MCH, melanin-concentrating hormone; MC3R, melanocortin-3; MC4R, melanocortin-4; MMC, migrating motor complex; NAc, nucleus accumbens; NAcc, nucleus accumbens core; NAcSh, nucleus accumbens shell; NTS, nucleus tractus solitarius; NPY, neuropeptide Y; PBN, parabrachial nucleus; Per 1/2, Period Circadian Regulator 1/2; PfC, prefrontal cortex; POMC, proopiomelanocortin; PP, pancreatic polypeptide; PVN, paraventricular nucleus; PVT, paraventricular thalamus; PYY, peptide YY; Rev-erba, Nuclear Receptor Subfamily 1 Group D Member 1; SCN, suprachiasmatic nucleus; SF-1, steroidogenic factor 1; SIRT-1, sirtuin 1; TRF, time-restricted feeding; TRH, thyrotropin-releasing hormone; VMH, ventral medial hypothalamus; VTA, ventral tegmental area; α-MSH, α-melanocortin–stimulating hormone.
Contributor Information
Debra K M Tacad, Obesity and Metabolism Research Unit, USDA–Western Human Nutrition Research Center, Davis, CA, USA; Department of Nutrition, University of California, Davis, Davis, CA, USA.
Ashley P Tovar, Department of Nutrition, University of California, Davis, Davis, CA, USA.
Christine E Richardson, Department of Nutrition, University of California, Davis, Davis, CA, USA.
William F Horn, Obesity and Metabolism Research Unit, USDA–Western Human Nutrition Research Center, Davis, CA, USA.
Nancy L Keim, Obesity and Metabolism Research Unit, USDA–Western Human Nutrition Research Center, Davis, CA, USA; Department of Nutrition, University of California, Davis, Davis, CA, USA.
Giri P Krishnan, Department of Medicine, School of Medicine, University of California, San Diego, San Diego, CA, USA.
Sridevi Krishnan, Department of Pediatrics, School of Medicine, University of California, San Diego, San Diego, CA, USA.
References
- 1. Chambers AP, Sandoval DA, Seeley RJ. Integration of satiety signals by the central nervous system. Curr Biol. 2013;23(9):R379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Konturek PC, Konturek JW, Czesnikiewicz-Guzik M, Brzozowski T, Sito E, Konturek SJ. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol. 2005;56(Suppl 6):5–25. [PubMed] [Google Scholar]
- 3. Benelam B. Satiation, satiety and their effects on eating behaviour. 2009;34(2):126–73. [Google Scholar]
- 4. Berthoud HR, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav. 2006;89(4):517–24. [DOI] [PubMed] [Google Scholar]
- 5. McMinn JE, Baskin DG, Schwartz MW. Neuroendocrine mechanisms regulating food intake and body weight. Obes Rev. 2000;1(1):37–46. [DOI] [PubMed] [Google Scholar]
- 6. Asarian L, Bächler T. Neuroendocrine control of satiation. Horm Mol Biol Clin Investig. 2014;19(3):163–92. [DOI] [PubMed] [Google Scholar]
- 7. Maljaars J, Peters HP, Masclee AM. Review article: the gastrointestinal tract: neuroendocrine regulation of satiety and food intake. Aliment Pharmacol Ther. 2007;26:241–50. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz GJ. Brainstem integrative function in the central nervous system control of food intake. Forum Nutr. 2010;63:141–51. [DOI] [PubMed] [Google Scholar]
- 9. Tacad DK, Tovar AP, Richardson CE, Horn WF, Krishnan GP, Keim NL, Krishnan S. Satiety associated with calorie restriction and time-restricted feeding: peripheral hormones. Adv Nutr. 2022. doi: 10.1093/advances/nmac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stiefel KM, Ermentrout GB. Neurons as oscillators. J Neurophysiol. 2016;116(6):2950–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saini R, Jaskolski M, Davis SJ. Circadian oscillator proteins across the kingdoms of life: structural aspects. BMC Biol. 2019;17(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35(1):445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J. 2012;26(9):3602–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Suppl 2):R271–7. [DOI] [PubMed] [Google Scholar]
- 15. Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. 2019;20(4):227–41. [DOI] [PubMed] [Google Scholar]
- 16. Mendoza J. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005;25(6):1514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Froy O, Chapnik N, Miskin R. The suprachiasmatic nuclei are involved in determining circadian rhythms during restricted feeding. Neuroscience. 2008;155(4):1152–9. [DOI] [PubMed] [Google Scholar]
- 19. Froy O, Chapnik N, Miskin R. Long-lived αMUPA transgenic mice exhibit pronounced circadian rhythms. Am J Physiol Endocrinol Metab. 2006;291(5):E1017–24. [DOI] [PubMed] [Google Scholar]
- 20. Pendergast JS, Yamazaki S. The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J Biol Rhythms. 2018;33(5):458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carneiro BT, Araujo JF. Food entrainment: major and recent findings. Front Behav Neurosci. 2012;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol Metab. 2014;3(7):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci. 2009;106(16):6808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oosterman JE, Kalsbeek A, la Fleur SE, Belsham DD. Impact of nutrients on circadian rhythmicity. Am J Physiol Regul Integr Comp Physiol. 2015;308(5):R337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Challet E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J Comp Physiol B. 2010;180(5):631–44. [DOI] [PubMed] [Google Scholar]
- 26. Tahara Y, Shibata S. Chronobiology and nutrition. Neuroscience. 2013;253:78–88. [DOI] [PubMed] [Google Scholar]
- 27. O'Connor SG, Boyd P, Bailey CP, Shams-White MM, Agurs-Collins T, Hall K, Reedy J, Sauter ER, Czajkowski SM. Perspective: time-restricted eating compared with caloric restriction: potential facilitators and barriers of long-term weight loss maintenance. Adv Nutr. 2021;12(2):325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. García-Gaytán AC, Miranda-Anaya M, Turrubiate I, López-De Portugal L, Bocanegra-Botello GN, López-Islas A, Díaz-Muñoz M, Méndez I. Synchronization of the circadian clock by time-restricted feeding with progressive increasing calorie intake. Resemblances and differences regarding a sustained hypocaloric restriction. Sci Rep. 2020;10(1):10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu FB. Calorie restriction in an obesogenic environment: reality or fiction?. Lancet Diabetes Endocrinol. 2019;7(9):658–9. [DOI] [PubMed] [Google Scholar]
- 30. Sunderram J, Sofou S, Kamisoglu K, Karantza V, Androulakis IP. Time-restricted feeding and the realignment of biological rhythms: translational opportunities and challenges. J Transl Med. 2014;12(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang L, Qiu B, Yuan L, Zheng L, Li Q, Zhu S. Influence of fasting, insulin and glucose on ghrelin in the dorsal vagal complex in rats. J Endocrinol. 2011;211(3):257–62. [DOI] [PubMed] [Google Scholar]
- 32. Blázquez E, Velázquez E, Hurtado-Carneiro V, Ruiz-Albusac JM. Insulin in the brain: its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front Endocrinol. 2014;5:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37(4):811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes. 2009;33(S2):S8–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abdalla MM. Central and peripheral control of food intake. Endocr Regul. 2017;51(1):52–70. [DOI] [PubMed] [Google Scholar]
- 36. Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57(5):359–72. [DOI] [PubMed] [Google Scholar]
- 37. Timper K, Bruning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Models Mechanisms. 2017;10(6):679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):s37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodríguez EM, Blázquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31(4):757–76. [DOI] [PubMed] [Google Scholar]
- 40. Fekete C, Singru PS, Sanchez E, Sarkar S, Christoffolete MA, Riberio RS, Rand WM, Emerson CH, Bianco AC, Lechan RM. Differential effects of central leptin, insulin, or glucose administration during fasting on the hypothalamic-pituitary-thyroid axis and feeding-related neurons in the arcuate nucleus. Endocrinology. 2006;147(1):520–9. [DOI] [PubMed] [Google Scholar]
- 41. Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6:1–11. [DOI] [PubMed] [Google Scholar]
- 42. Wynne K, Stanley S, Mcgowan B, Bloom S. Appetite control. J Endocrinol. 2005;184(2):291–318. [DOI] [PubMed] [Google Scholar]
- 43. Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Models Mechanisms. 2017;10(6):679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521(14):3287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman Aet al. . Agouti-related peptide—expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8(10):1289–91. [DOI] [PubMed] [Google Scholar]
- 46. Krashes J, Michael SP, Bhavik KS, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18(4):588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fenselau H, Campbell JN, Verstegen AMJ, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, Mandelblat-Cerf Y, Livneh Yet al. . A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat Neurosci. 2017;20(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TEet al. . Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 2016;19(12):1628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lewis DE, Shellard L, Koeslag DG, Boer DE, McCarthy HD, McKibbin PE, Russell JC, Williams G. Intense exercise and food restriction cause similar hypothalamic neuropeptide Y increases in rats. Am J Physiol Endocrinol Metab. 1993;264(2):E279–84. [DOI] [PubMed] [Google Scholar]
- 51. Betley JN, Cao Zhen Fang H, Ritola Kimberly D, Sternson Scott M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhan C, Zhou J, Feng Q, Zhang J-E, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus. J Neurosci. 2013;33(8):3624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sternson SM, Shepherd GMG, Friedman JM. Topographic mapping of VMH → arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8(10):1356–63. [DOI] [PubMed] [Google Scholar]
- 55. Derous D, Mitchell SE, Green CL, Chen L, Han JDJ, Wang Y, Promislow DEL, Lusseau D, Speakman JR, Douglas A. The effects of graded levels of calorie restriction: VI. Impact of short-term graded calorie restriction on transcriptomic responses of the hypothalamic hunger and circadian signaling pathways. Aging. 2016;8(4):642–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rogers NH, Walsh H, Alvarez-Garcia O, Park S, Gaylinn B, Thorner MO, Smith RG. Metabolic benefit of chronic caloric restriction and activation of hypothalamic AGRP/NPY neurons in male mice is independent of ghrelin. Endocrinology. 2016;157(4):1430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hambly C, Mercer JG, Speakman JR. Hunger does not diminish over time in mice under protracted caloric restriction. Rejuvenation Res. 2007;10(4):533–42. [DOI] [PubMed] [Google Scholar]
- 58. Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1030–6. [DOI] [PubMed] [Google Scholar]
- 59. Kinzig KP, Hargrave SL, Tao EE. Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am J Physiol Endocrinol Metab. 2009;296(2):E282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Radler ME, Wright BJ, Walker FR, Hale MW, Kent S. Calorie restriction increases lipopolysaccharide-induced neuropeptide Y immunolabeling and reduces microglial cell area in the arcuate hypothalamic nucleus. Neuroscience. 2015;285:236–47. [DOI] [PubMed] [Google Scholar]
- 61. Kupis W, Pałyga J, Tomal E, Niewiadomska E. The role of sirtuins in cellular homeostasis. J Physiol Biochem. 2016;72(3):371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S-I. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30(30):10220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dicken MS, Hughes AR, Hentges ST. Gad1mRNA as a reliable indicator of altered GABA release from orexigenic neurons in the hypothalamus. Eur J Neurosci. 2015;42(9):2644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jarvie BC, King CM, Hughes AR, Dicken MS, Dennison CS, Hentges ST. Caloric restriction selectively reduces the GABAergic phenotype of mouse hypothalamic proopiomelanocortin neurons. J Physiol. 2017;595(2):571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lauzurica N, García-García L, Pinto S, Fuentes JA, Delgado M. Changes in NPY and POMC, but not serotonin transporter, following a restricted feeding/repletion protocol in rats. Brain Res. 2010;1313:103–12. [DOI] [PubMed] [Google Scholar]
- 66. Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52(5):441–7. [DOI] [PubMed] [Google Scholar]
- 67. Sorrell J, Yates E, Rivir M, Woods SC, Hogenesch JB, Perez-Tilve D. The central melanocortin system mediates the benefits of time-restricted feeding on energy balance. Physiol Behav. 2020;227:113132. [DOI] [PubMed] [Google Scholar]
- 68. Miñana-Solis MC, Ángeles-Castellanos M, Feillet C, Pévet P, Challet E, Escobar C. Differential effects of a restricted feeding schedule on clock-gene expression in the hypothalamus of the rat. Chronobiol Int. 2009;26(5):808–20. [DOI] [PubMed] [Google Scholar]
- 69. Verhagen LAW, Luijendijk MCM, De Groot J-W, Van Dommelen LPG, Klimstra AG, Adan RAH, Roeling TAP. Anticipation of meals during restricted feeding increases activity in the hypothalamus in rats. Eur J Neurosci. 2011;34(9):1485–91. [DOI] [PubMed] [Google Scholar]
- 70. Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor—regulated energy homeostasis. Nat Neurosci. 2016;19(2):206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hofman MA, Swaab DF. Neuroplasticity in the human hypothalamus during ageing. In: Straub RH, Mocchegiani Eeditors. Neuroimmune biology. Amsterdam, Netherlands: Elsevier; 2004. p. 105–21. [Google Scholar]
- 72. Vong L, Ye C, Yang Z, Choi B, Chua S Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rau AR, Hentges ST. GABAergic inputs to POMC neurons originating from the dorsomedial hypothalamus are regulated by energy state. J Neurosci. 2019;39(33):6449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29(1):179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hyland L, Park S-B, Abdelaziz Y, Abizaid A. Ghrelin infused into the dorsomedial hypothalamus of male mice increases food intake and adiposity. Physiol Behav. 2020;220:112882. [DOI] [PubMed] [Google Scholar]
- 76. Gustafson EL, Smith KE, Durkin MM, Walker MW, Gerald C, Weinshank R, Branchek TA. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Mol Brain Res. 1997;46(1-2):223–35. [DOI] [PubMed] [Google Scholar]
- 77. Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9(3):398–407. [DOI] [PubMed] [Google Scholar]
- 78. Landry GJ, Kent BA, Patton DF, Jaholkowski M, Marchant EG, Mistlberger RE. Evidence for time-of-day dependent effect of neurotoxic dorsomedial hypothalamic lesions on food anticipatory circadian rhythms in rats. PLoS One. 2011;6(9):e24187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, Shibata S. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci. 2009;29(7):1447–60. [DOI] [PubMed] [Google Scholar]
- 80. Angeles-Castellanos M, Aguilar-Roblero R, Escobar C. c-Fos expression in hypothalamic nuclei of food-entrained rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R158–65. [DOI] [PubMed] [Google Scholar]
- 81. Mcclellan K, Parker K, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol. 2006;27(2):193–209. [DOI] [PubMed] [Google Scholar]
- 82. Ribeiro AC, Lesauter J, Dupré C, Pfaff DW. Relationship of arousal to circadian anticipatory behavior: ventromedial hypothalamus: one node in a hunger-arousal network. Eur J Neurosci. 2009;30(9):1730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87(2):221–44. [DOI] [PubMed] [Google Scholar]
- 84. Duan W, Lee J, Guo Z, Mattson MP. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci. 2001;16(1):1–12. [DOI] [PubMed] [Google Scholar]
- 85. Araya AV, Orellana X, Espinoza J. Evaluation of the effect of caloric restriction on serum BDNF in overweight and obese subjects: preliminary evidences. Endocrine. 2008;33(3):300–04. [DOI] [PubMed] [Google Scholar]
- 86. Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet J-Let al. . Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148(9):4318–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of BDNF in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27(52):14265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kamitakahara A, Xu B, Simerly R. Ventromedial hypothalamic expression of BDNF is required to establish normal patterns of afferent GABAergic connectivity and responses to hypoglycemia. Mol Metab. 2016;5(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kim KW, Zhao L, Donato J, Kohno D, Xu Y, Elias CF, Lee C, Parker KL, Elmquist JK. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci. 2011;108(26):10673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Manchishi SM, Cui RJ, Zou XH, Cheng ZQ, Li BJ. Effect of caloric restriction on depression. J Cell Mol Med. 2018;22(5):2528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ribeiro AC, Sawa E, Carren-Lesauter I, Lesauter J, Silver R, Pfaff DW. Two forces for arousal: pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci. 2007;104(50):20078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kurumiya S, Kawamura H. Damped oscillation of the lateral hypothalamic multineuronal activity synchronized to daily feeding schedules in rats with suprachiasmatic nucleus lesions. J Biol Rhythms. 1991;6(2):115–27. [DOI] [PubMed] [Google Scholar]
- 93. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37(4):811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, Mcgovern RA, Kenny CDet al. . Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. [DOI] [PubMed] [Google Scholar]
- 95. Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27(6):1031–40. [DOI] [PubMed] [Google Scholar]
- 96. An J, Juan L G-Y, Kinney E, Clint S N, Xu B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 2015;22(1):175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida Net al. . An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507(7491):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bartfai T, Conti B. Molecules affecting hypothalamic control of core body temperature in response to calorie intake. Front Genet. 2012;3:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Saeed O, Yaghmaie F, Garan SA, Gouw AM, Voelker MA, Sternberg H, Timiras PS. Insulin-like growth factor-1 receptor immunoreactive cells are selectively maintained in the paraventricular hypothalamus of calorically restricted mice. Int J Dev Neurosci. 2007;25(1):23–8. [DOI] [PubMed] [Google Scholar]
- 100. Kenny R, Dinan T, Cai G, Spencer SJ. Effects of mild calorie restriction on anxiety and hypothalamic-pituitary-adrenal axis responses to stress in the male rat. Physiol Rep. 2014;2(3):e00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kim ER, Xu Y, Cassidy RM, Lu Y, Yang Y, Tian J, Li D-P, Van Drunen R, Ribas-Latre A, Cai Z-Let al. . Paraventricular hypothalamus mediates diurnal rhythm of metabolism. Nat Commun. 2020;11(1):3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Angeles-Castellanos M, Mendoza J, Díaz-Muñoz M, Escobar C. Food entrainment modifies the c-Fos expression pattern in brain stem nuclei of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(3):R678–84. [DOI] [PubMed] [Google Scholar]
- 103. Kurose T, Ueta Y, Yamamoto Y, Serino R, Ozaki Y, Saito J, Nagata S, Yamashita H. Effects of restricted feeding on the activity of hypothalamic orexin (OX)-A containing neurons and OX2 receptor mRNA level in the paraventricular nucleus of rats. Regul Pept. 2002;104(1-3):145–51. [DOI] [PubMed] [Google Scholar]
- 104. Petrovich GD. Lateral hypothalamus as a motivation-cognition interface in the control of feeding behavior. Front Syst Neurosci. 2018;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hurley SW, Johnson AK. The role of the lateral hypothalamus and orexin in ingestive behavior: a model for the translation of past experience and sensed deficits into motivated behaviors. Front Syst Neurosci. 2014;8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Talakoub O, Paiva RR, Milosevic M, Hoexter MQ, Franco R, Alho E, Navarro J, Pereira JF, Popovic MR, Savage Cet al. . Lateral hypothalamic activity indicates hunger and satiety states in humans. Ann Clin Translat Neurol. 2017;4(12):897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Milam KM, Stern JS, Storlien LH, Keesey RE. Effect of lateral hypothalamic lesions on regulation of body weight and adiposity in rats. Am J Physiol Regul Integr Comp Physiol. 1980;239(3):R337–43. [DOI] [PubMed] [Google Scholar]
- 108. Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo Ket al. . Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160(3):516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wu Z, Kim ER, Sun H, Xu Y, Mangieri LR, Li D-P, Pan H-L, Xu Y, Arenkiel BR, Tong Q. GABAergic projections from lateral hypothalamus to paraventricular hypothalamic nucleus promote feeding. J Neurosci. 2015;35(8):3312–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Lepla CA, Wichmann R, Neve R, Wildes CP, Tye KM. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160(3):528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341(6153):1517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Berthoud H-R, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DPet al. . A neural basis for melanocortin-4 receptor—regulated appetite. Nat Neurosci. 2015;18(6):863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Valenzano A, Polito R, Trimigno V, Di Palma A, Moscatelli F, Corso G, Sessa F, Salerno M, Montana A, Di Nunno Net al. . Effects of very low calorie ketogenic diet on the orexinergic system, visceral adipose tissue, and ROS production. Antioxidants. 2019;8(12):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J Neurosci. 2010;30(48):16399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kaur S, Thankachan S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, Shiromani PJ. Entrainment of temperature and activity rhythms to restricted feeding in orexin knock out mice. Brain Res. 2008;1205:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gillette MU, Tischkau SA. Suprachiasmatic nucleus: the brain's circadian clock. Recent Prog Horm Res. 1999;54:33–58.; discussion, 9. [PubMed] [Google Scholar]
- 118. Mieda M. The network mechanism of the central circadian pacemaker of the SCN: do AVP neurons play a more critical role than expected?. Front Neurosci. 2019;13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ma MA, Morrison EH. Neuroanatomy, nucleus suprachiasmatic. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 120. Klisch C, Inyushkin A, Mordel J, Karnas D, Pévet P, Meissl H. Orexin A modulates neuronal activity of the rodent suprachiasmatic nucleus in vitro. Eur J Neurosci. 2009;30(1):65–75. [DOI] [PubMed] [Google Scholar]
- 121. Lamont EW, Bruton J, Blum ID, Abizaid A. Ghrelin receptor-knockout mice display alterations in circadian rhythms of activity and feeding under constant lighting conditions. Eur J Neurosci. 2014;39(2):207–17. [DOI] [PubMed] [Google Scholar]
- 122. Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol. 2011;62(2):139–50. [PubMed] [Google Scholar]
- 123. Radziuk JM. The suprachiasmatic nucleus, circadian clocks, and the liver. Diabetes. 2013;62(4):1017–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Inyushkin AN, Bhumbra GS, Dyball RE. Leptin modulates spike coding in the rat suprachiasmatic nucleus. J Neuroendocrinol. 2009;21(8):705–14. [DOI] [PubMed] [Google Scholar]
- 125. Mendoza J, Drevet K, Pévet P, Challet E. Daily meal timing is not necessary for resetting the main circadian clock by calorie restriction. J Neuroendocrinol. 2008;20(2):251–60. [DOI] [PubMed] [Google Scholar]
- 126. Froy O, Chapnik N, Miskin R. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech Ageing Dev. 2009;130(3):154–60. [DOI] [PubMed] [Google Scholar]
- 127. Mendoza J, Pévet P, Challet E. Circadian and photic regulation of clock and clock-controlled proteins in the suprachiasmatic nuclei of calorie-restricted mice. Eur J Neurosci. 2007;25(12):3691–701. [DOI] [PubMed] [Google Scholar]
- 128. Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. Eur J Neurosci. 2009;30(9):1707–17. [DOI] [PubMed] [Google Scholar]
- 129. Ferrario CR, Labouèbe G, Liu S, Nieh EH, Routh VH, Xu S, O'Connor EC. Homeostasis meets motivation in the battle to control food intake. J Neurosci. 2016;36(45):11469–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cassidy RM, Tong Q. Hunger and satiety gauge reward sensitivity. Front Endocrinol. 2017;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sternson SM, Eiselt AK. Three pillars for the neural control of appetite. Annu Rev Physiol. 2017;79(1):401–23. [DOI] [PubMed] [Google Scholar]
- 132. Aitken TJ, Greenfield VY, Wassum KM. Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. J Neurochem. 2016;136(5):1026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27(7):1594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vestlund J, Bergquist F, Eckernäs D, Licheri V, Adermark L, Jerlhag E. Ghrelin signalling within the rat nucleus accumbens and skilled reach foraging. Psychoneuroendocrinology. 2019;106:183–94. [DOI] [PubMed] [Google Scholar]
- 135. Kombian SB, Ananthalakshmi KV, Parvathy SS, Matowe WC. Cholecystokinin activates CCKB receptors to excite cells and depress EPSCs in the rat rostral nucleus accumbens in vitro. J Physiol. 2004;555(1):71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hommer DW, Palkovits M, Crawley JN, Paul SM, Skirboll LR. Cholecystokinin-induced excitation in the substantia nigra: evidence for peripheral and central components. J Neurosci. 1985;5(6):1387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Woods CA, Guttman ZR, Huang D, Kolaric RA, Rabinowitsch AI, Jones KT, Cabeza de Vaca S, Sclafani A, Carr KD. Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal. Physiol Behav. 2016;159:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19(5):883–9. [DOI] [PubMed] [Google Scholar]
- 140. Baisley SK, Baldo BA. Amylin receptor signaling in the nucleus accumbens negatively modulates μ-opioid-driven feeding. Neuropsychopharmacology. 2014;39(13):3009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JDet al. . The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14(5):700–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zanchi D, Depoorter A, Egloff L, Haller S, Mählmann L, Lang UE, Drewe J, Beglinger C, Schmidt A, Borgwardt S. The impact of gut hormones on the neural circuit of appetite and satiety: a systematic review. Neurosci Biobehav Rev. 2017;80:457–75. [DOI] [PubMed] [Google Scholar]
- 143. Diao LH, Bickford PC, Stevens JO, Cline EJ, Gerhardt GA. Caloric restriction enhances evoked DA overflow in striatum and nucleus accumbens of aged Fischer 344 rats. Brain Res. 1997;763(2):276–80. [DOI] [PubMed] [Google Scholar]
- 144. Kolta MG, Holson R, Duffy P, Hart RW. Effect of long-term caloric restriction on brain monoamines in aging male and female Fischer 344 rats. Mech Ageing Dev. 1989;48(2):191–8. [DOI] [PubMed] [Google Scholar]
- 145. Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MAet al. . Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc Natl Acad Sci. 2004;101(52):18171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Vialou V, Cui H, Perello M, Mahgoub M, Yu HG, Rush AJ, Pranav H, Jung S, Yangisawa M, Zigman JMet al. . A role for ΔFosB in calorie restriction-induced metabolic changes. Biol Psychiatry. 2011;70(2):204–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wallace CW, Loudermilt MC, Fordahl SC. Effect of fasting on dopamine neurotransmission in subregions of the nucleus accumbens in male and female mice. Nutr Neurosci. 2020:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Olivo D, Caba M, Gonzalez-Lima F, Rodríguez-Landa JF, Corona-Morales AA. Metabolic activation of amygdala, lateral septum and accumbens circuits during food anticipatory behavior. Behav Brain Res. 2017;316:261–70. [DOI] [PubMed] [Google Scholar]
- 149. Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18(2):73–85. [DOI] [PubMed] [Google Scholar]
- 150. Reichelt AC, Westbrook RF, Morris MJ. Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses. Br J Pharmacol. 2015;172(22):5225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Roseberry AG. Acute fasting increases somatodendritic dopamine release in the ventral tegmental area. J Neurophysiol. 2015;114(2):1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wei XJ, Sun B, Chen K, Lv B, Luo X, Yan JQ. Ghrelin signaling in the ventral tegmental area mediates both reward-based feeding and fasting-induced hyperphagia on high-fat diet. Neuroscience. 2015;300:53–62. [DOI] [PubMed] [Google Scholar]
- 153. Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. [DOI] [PubMed] [Google Scholar]
- 154. Figlewicz DP. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat: historical perspective. Brain Res. 2016;1645:68–70. [DOI] [PubMed] [Google Scholar]
- 155. Gualillo O, Lago F, Gómez-Reino J, Casanueva FF, Dieguez C. Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003;552(2-3):105–09. [DOI] [PubMed] [Google Scholar]
- 156. Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340(1):80–7. [DOI] [PubMed] [Google Scholar]
- 157. Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198(1):149–58. [DOI] [PubMed] [Google Scholar]
- 158. Willette AA, Coe CL, Colman RJ, Bendlin BB, Kastman EK, Field AS, Alexander AL, Allison DB, Weindruch RH, Johnson SC. Calorie restriction reduces psychological stress reactivity and its association with brain volume and microstructure in aged rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):903–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Guerrero-Vargas NN, Zárate-Mozo C, Guzmán-Ruiz MA, Cárdenas-Rivera A, Escobar C. Time-restricted feeding prevents depressive-like and anxiety-like behaviors in male rats exposed to an experimental model of shift-work. J Neurosci Res. 2021;99(2):604–20. [DOI] [PubMed] [Google Scholar]
- 160. Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M. Central, but not basolateral, amygdala is critical for control of feeding by aversive learned cues. J Neurosci. 2009;29(48):15205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zséli G, Vida B, Szilvásy-Szabó A, Tóth M, Lechan RM, Fekete C. Neuronal connections of the central amygdalar nucleus with refeeding-activated brain areas in rats. Brain Struct Funct. 2018;223(1):391–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mussa BM, Verberne AJ. The dorsal motor nucleus of the vagus and regulation of pancreatic secretory function. Exp Physiol. 2013;98(1):25–37. [DOI] [PubMed] [Google Scholar]
- 163. Moyse E, Bédard K, Segura S, Mahaut S, Tardivel C, Ferland G, Lebrun B, Gaudreau P. Effects of aging and caloric restriction on brainstem satiety center signals in rats. Mech Ageing Dev. 2012;133(2-3):83–91. [DOI] [PubMed] [Google Scholar]
- 164. Begriche K, Sutton GM, Butler AA. Homeostastic and non-homeostatic functions of melanocortin-3 receptors in the control of energy balance and metabolism. Physiol Behav. 2011;104(4):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Holland J, Sorrell J, Yates E, Smith K, Arbabi S, Arnold M, Rivir M, Morano R, Chen J, Zhang Xet al. . A brain-melanocortin-vagus axis mediates adipose tissue expansion independently of energy intake. Cell Rep. 2019;27(8):2399–2410.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Vrinten DH, Gispen WH, Kalkman CJ, Adan RA. Interaction between the spinal melanocortin and opioid systems in a rat model of neuropathic pain. Anesthesiology. 2003;99(2):449–54. [DOI] [PubMed] [Google Scholar]
- 167. Starowicz K, Przewlocki R, Gispen WH, Przewlocka B. Modulation of melanocortin-induced changes in spinal nociception by mu-opioid receptor agonist and antagonist in neuropathic rats. Neuroreport. 2002;13(18):2447–52. [DOI] [PubMed] [Google Scholar]
- 168. Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14(3):370–8. [DOI] [PubMed] [Google Scholar]
- 169. Kott JN, Ganfield CL, Kenney NJ. Area postrema/nucleus of the solitary tract ablations: analysis of the effects of hypophagia. Physiol Behav. 1984;32(3):429–35. [DOI] [PubMed] [Google Scholar]
- 170. Bello NT, Patinkin ZW, Moran TH. Opioidergic consequences of dietary-induced binge eating. Physiol Behav. 2011;104(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. García-Cabrerizo R, Carbia C, Riordan KJO, Schellekens H, Cryan JF. Microbiota-gut-brain axis as a regulator of reward processes. J Neurochem. 2021;175(5):1495–524. [DOI] [PubMed] [Google Scholar]
- 172. Aton SJ, Block GD, Tei H, Yamazaki S, Herzog ED. Plasticity of circadian behavior and the suprachiasmatic nucleus following exposure to non-24-hour light cycles. J Biol Rhythms. 2004;19(3):198–207. [DOI] [PubMed] [Google Scholar]
- 173. Azzi A, Evans JA, Leise T, Myung J, Takumi T, Davidson AJ, Brown SA. Network dynamics mediate circadian clock plasticity. Neuron. 2017;93(2):441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Meijer JH, Michel S, Vanderleest HT, Rohling JHT. Daily and seasonal adaptation of the circadian clock requires plasticity of the SCN neuronal network. Eur J Neurosci. 2010;32(12):2143–51. [DOI] [PubMed] [Google Scholar]
- 175. Van Der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V, Hut RA. Cold and hunger induce diurnality in a nocturnal mammal. Proc Natl Acad Sci. 2014;111(42):15256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Aguilar-Arnal L, Zocchi L, Masri S, Katada S, Sassone-Corsi P. Plasticity of the circadian system: linking metabolism to epigenetic control. In: Christen Yeditor. Research and perspectives in neurosciences. Berlin, Heidelberg (Germany): Springer; 2012. p. 23–30. [Google Scholar]
- 177. Masri S, Sassone-Corsi P. Plasticity and specificity of the circadian epigenome. Nat Neurosci. 2010;13(11):1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Collet TH, van der Klaauw AA, Henning E, Keogh JM, Suddaby D, Dachi SV, Dunbar S, Kelway S, Dickson SL, Farooqi ISet al. . The sleep/wake cycle is directly modulated by changes in energy balance. Sleep. 2016;39(9):1691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kesztyüs D, Fuchs M, Cermak P, Kesztyüs T. Associations of time-restricted eating with health-related quality of life and sleep in adults: a secondary analysis of two pre-post pilot studies. BMC Nutr. 2020;6(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hou T, Wang C, Joshi S, O'Hara BF, Gong MC, Guo Z. Active time-restricted feeding improved sleep-wake cycle in db/db mice. Front Neurosci. 2019;13:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4(1):2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Qian J, Morris CJ, Caputo R, Wang W, Garaulet M, Scheer F. Sex differences in the circadian misalignment effects on energy regulation. Proc Natl Acad Sci. 2019;116(47):23806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]