ABSTRACT
Infants born preterm (<37 weeks of gestation) often experience feeding problems during hospitalization. Whether difficulties persist or have long-term sequelae on childhood eating is unclear. We aimed to describe the oromotor eating skills (e.g., chewing/swallowing), eating behaviors (e.g., food neophobia), food parenting practices (e.g., pressure to eat), and dietary patterns of preterm children during late infancy (6–12 mo) and early childhood (>12 mo–7 y) and to determine whether these differed from those of term-born peers. We identified 67 articles (57 unique studies) for inclusion. We used random-effects meta-analysis of proportions to examine the prevalence of oromotor eating skill and eating behavior challenges among preterm children, standard meta-analysis for comparisons with term-born peers, and the Grading of Recommendations, Assessment, Development and Evaluation approach to assess the certainty of evidence. Forty-three percent (95% CI: 24%, 62%) of infants and 25% (95% CI: 17%, 33%) of children born preterm experienced oromotor eating difficulties and 16% (95% CI: 4%, 27%) and 20% (95% CI: 11%, 28%), respectively, exhibited challenging eating behaviors. During late infancy and early childhood, oromotor eating difficulties (OR: 2.86; 95% CI: 1.71, 4.77; I2 = 67.8%) and challenging eating behaviors (OR: 1.52; 95% CI: 1.11, 2.10; I2 = 0.0%) were more common in those born preterm than in those born term: however, the certainty of evidence was very low. Owing to the low number and heterogeneity of studies, we narratively reviewed literature on food parenting and dietary patterns. Mothers of preterm infants appeared to have heightened anxiety while feeding and utilized coercive food parenting practices; their infants reportedly received less human milk, started solid foods earlier, and had poorer diet quality than term-born peers. In conclusion, meta-analyses show preterm children experience frequent oromotor eating difficulties and challenging eating behaviors throughout the early years. Given preterm birth increases risk of later obesity and diet-related chronic disease, research examining the effects of caregiver–child interactions on subsequent diet is warranted.
This review was registered at www.crd.york.ac.uk/prospero/ as CRD42020176063.
Keywords: preterm birth, feeding and eating disorders of childhood, picky eating, feeding skills, oromotor skills, eating behaviors, parent–child interaction, food parenting, diet quality, meta-analysis
Statement of Significance: Preterm children continue to experience frequent oromotor eating difficulties and challenging eating behaviors throughout the early years, more commonly than term-born peers.
Introduction
Infants born preterm (<37 weeks of gestation) and at a very low birth weight (VLBW; <1500 g) experience high rates of feeding problems in hospital (1, 2). Eating is a neurodevelopmental process that requires motor organization, rhythmic sucking, and a coordinated suck-swallow-breathe pattern while integrating incoming sensory components of both the food and the feeding environment (2, 3). Organ immaturity, birth complications, and medical interventions such as tube feeding interrupt the development of these necessary skills and progression to complete oral feeding. Although often essential for survival, procedures in the neonatal intensive care unit (NICU) may limit opportunities for positive oral experiences and parent–child interaction, causing parental distress and negative feeding experiences (1, 4).
There is little consensus about the persistence of eating difficulties into late infancy and early development among children born preterm (5). Ross and Browne (2) conducted a systematic review qualitatively synthesizing the literature on breastfeeding, eating problems, and growth in preterm infants at, and post, NICU discharge, and concluded that preterm infants are slow to develop eating skills and that parental reports of eating challenges are prevalent. More recently, Pados et al. (6) conducted a meta-analysis examining the prevalence of problematic feeding (e.g., choking, food refusal) in preterm children and reported 42% (95% CI: 33%, 51%) exhibit such challenges in the first 4 y. Notably, neither of these reviews used exhaustive literature searches, included a term-born comparison group, or critiqued the certainty of evidence across studies. To our knowledge, this is the first systematic review and meta-analysis to explore the prevalence of oromotor eating difficulties and behavioral eating challenges among children born preterm, as well as the first to compare with term-born peers.
Early food and eating experiences have a powerful influence on food preferences, including interactions with others (7, 8). The current food parenting guidance is based on term-born children and, as such, may not be sufficient or appropriate for families with a child born preterm. When problematic behaviors and poor dietary intake recur without support or intervention, restrictive preferences and maladaptive habits become entrenched. The impact of eating and feeding challenges on preterm children's dietary patterns or nutritional status during the early years is relatively unknown (9, 10). As such, it can also be difficult for clinicians to determine whether parental concerns regarding preterm children's feeding/eating are truly problematic or, rather, reflective of the typical development of children's eating behaviors and food preferences (7, 11). Given that children born preterm experience higher rates of adiposity and chronic disease during young adulthood than their term-born peers (12), understanding the eating behaviors, food parenting practices, and dietary patterns of these children is crucial to supporting their long-term health. Elucidating whether the eating and feeding challenges experienced by preterm infants are unique will help create tailored anticipatory guidance and nutritional supports for this vulnerable population.
We aimed to describe the oromotor eating skills (e.g., chewing, swallowing), eating behaviors (e.g., food neophobia), food parenting practices (e.g., pressure to eat), and dietary patterns of preterm children during late infancy (6–12 mo) and early childhood (>12 mo–7 y) and to determine whether these differed from those of children born at term. For clarification, throughout this review, “eating” refers to the child's actions and behaviors, whereas “feeding” refers to those of the parent.
Methods
We conducted a systematic review and meta-analyses according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13); protocol registration can be found on the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020176063).
Eligibility and literature search
We outlined our eligibility criteria using the Participants, Intervention (or Exposure), Comparator, Outcomes, Study Design (PICOS) format (Table 1). We formulated our PICOS statement a priori before systematically searching the electronic databases Medline, Embase, and PsycINFO (OVID); Cochrane (Wiley); CINAHL (EBSCO); and Scopus (Elsevier) using subject headings specific to each database and keywords on 22 April, 2020, with support from a research librarian (QM). We also searched the gray literature using Google Advanced Search, ProQuest Dissertation and Theses Global, and the clinicaltrials.gov registry (14). We updated all searches on 16 September, 2021. Search terms were related to 1) oromotor eating skills (e.g., eating skill, oromotor) and eating behaviors (e.g., food refusal, food neophobia), 2) food parenting (e.g., parent–child interactions, pressure to eat), 3) dietary patterns (e.g., diet quality), and 4) prematurity or low birth weight. The full Embase search is available in Supplemental Table 1 (See Supplemental Methods). After completing our initial screening, we reviewed the reference lists of all eligible studies to identify additional publications for inclusion.
TABLE 1.
PICOS criteria for inclusion and exclusion of studies examining the oromotor eating skills and eating behaviors, food parenting, and dietary patterns of children born preterm between the ages of 6 mo and 7 y
| Criteria | Definition |
|---|---|
| Participants | Infants and children born preterm (<37 weeks of gestation) and aged 6 mo postnatal or corrected age to 7 y at the time of data collection |
| Intervention (or Exposure) | Preterm birth |
| Comparator | Full-term birth (>37 weeks of gestation), excluding those born small for gestational age |
| Outcomes (eligible studies had to examine ≥1) | Oromotor eating skills (e.g., chewing and swallowing) and Eating behaviors (e.g., food refusal, food neophobia) |
| Food parenting (e.g., parent–child interactions, pressure to eat) | |
| Dietary patterns (e.g., diet quality) | |
| Study design | Observational studies and intervention trials were included. Case studies, review articles and commentaries, studies that focused solely on children born full-term but small for gestational age, and studies examining nutrient intakes provided by mother's milk, donor human milk, or infant formula were excluded. We did not include any search restrictions related to publication language or date |
Outcomes
Oromotor eating skills and eating behaviors
Oromotor eating skills refer to the movement, strength, and coordination of the jaw, tongue, lips, and cheeks. These skills lay the foundation for eating-related tasks, including sucking, chewing, and swallowing. Oromotor eating difficulties among children may include chewing, swallowing or gagging concerns, excessive drooling, oral sensitivity, and avoidance of particular food textures (5). Eating behaviors refer to how one eats. During late infancy and early childhood examples include food responsiveness, emotional under-or-over-eating, enjoyment of food, desire to drink, satiety responsiveness, slowness in eating, food fussiness, food neophobia, or food selectivity (15). Eating behaviors may be disruptive (e.g., tantrums, food refusal, throwing or pushing food away) or undesirable, like extended bottle use past the age of 2 y.
Food parenting
This encompasses parents’ knowledge, experiences, emotions, and responsivity related to feeding their child as well as behavioral strategies to influence what, how much, or whether their child eats (16, 17). For the current review, we explored parental concern and emotions during feeding, specific feeding practices, and parent–child interactions during mealtimes. Examples of feeding practices include coercive food parenting (e.g., pressuring a child to eat, restricting intake of or access to specific foods or food groups, using food as a reward for eating or behavioral control) and noncoercive food parenting (e.g., modeling and monitoring of intake, positive commenting, age-appropriate nutrition education, offering a variety of foods, frequent family meals).
Dietary patterns
According to the Dietary Guidelines for Americans 2020–2025, a dietary pattern refers to the entirety of what individuals habitually eat and drink (18). The components of the pattern act synergistically to affect health (18). For this review, we chose to focus on aspects of dietary patterns pertinent to infants and young children, including human milk (mother's own or donor human milk) feeding duration and exclusivity, age at introduction to solid foods, food group consumption, and overall diet quality. The composition of human milk (e.g., macro- and micronutrients in mother's milk or human donor milk) fed to infants born preterm was outside the scope of this review.
Data management and extraction
Using Covidence online software (19), 2 independent reviewers (KW and AID) screened articles for inclusion based first on titles and abstracts, followed by a full-text review. Reasons for excluding articles were documented and discrepancies between reviewers resolved in all instances.
Using a data extraction sheet created in the Research Electronic Data Capture (REDCap) software (20), the 2 reviewers independently extracted data from eligible studies including publication year; country; study setting (i.e., hospital, community, home); study design (i.e., cross-sectional, prospective cohort, retrospective cohort, other); sample size; child age; gestational age at birth; birth weight; sex; relationship of caregiver to child; and results related to child oromotor eating skills and eating behaviors, food parenting, and dietary patterns and details of how these data were obtained (e.g., direct observation, parental report, 24-h dietary recall). In articles with a term-born comparison, data were reported separately for preterm and term-born children. After reviewing for accuracy, extracted data from the 2 reviewers were combined.
Statistical analysis
We analyzed data in Stata version 16 (StataCorp LP). We conducted 3 different random-effects meta-analyses of proportions using metaprop and a maximum likelihood (ML) estimator to explore the prevalence of oromotor eating difficulties and eating behavior challenges among children born preterm in studies that examined 1) only oromotor eating difficulties; 2) only eating behavior challenges; or 3) any eating challenge, not specified (articles that did not differentiate oromotor difficulties and behavioral eating challenges) within the age categories 6–12 mo and >12 mo–7 y. Percentages and 95% CIs are displayed to represent the prevalence for each meta-analysis of proportions. We also conducted random-effects meta-analysis using a restricted maximum likelihood (REML) random-effects model to evaluate the odds of eating problems in children born preterm compared with those born at term. The ORs and 95% CIs are presented. We used random-effects models because they assume that study effect sizes differ and that the studies included represent a random sample from a larger set of studies (i.e., a population of studies). We estimated statistical heterogeneity based on I2 and considered values of 25%, 50%, and 75% as low, moderate, and high heterogeneity, respectively (21).
In prospective studies with 2 measurement points within the same age group (e.g., 6 and 8 mo corrected age), we used the earlier time point to allow for maximum sample size. We excluded articles with children that spanned the 2 age groups and could not be disaggregated (n = 3) (22–24). We also excluded studies from the meta-analysis that did not report prevalence of challenges, or where prevalence could not be discerned (n = 12) (25–36).
Risk of bias and certainty of evidence assessment
We utilized the Newcastle-Ottawa Scale (NOS) for nonrandomized analyses (37) to assess the risk of bias of each eligible study. Two independent reviewers (KW and AID) achieved consensus 100% of the time. In our NOS-guided review, the following features of each study were assessed: representativeness of the study sample; method by which preterm birth was confirmed; how outcomes were assessed (e.g., blind independent observer, parental report); adequacy of cohort follow-up (e.g., study retention); and the comparability of the study (37). For the comparability category (whether individual studies adjusted for similar important confounding variables), we decided a priori to award 2 points to studies adjusting for parental educational attainment, family income (or a composite of socioeconomic status), parental weight status, gestational age and/or birth weight, and child sex, and 1 point to studies that adjusted for ≥4 other covariates (37). For our review, a sufficient follow-up period to observe our outcomes of interest was not required, and in cohort studies, feeding problems may have been present at the start of the study. Thus, we adapted the NOS such that scores for case-control studies ranged from 0 to 9 and for cohort studies from 0 to 7, with scores ≥7 and ≥5 indicating low risk of bias, respectively (37).
We assessed the certainty of evidence across studies included in the respective meta-analyses (as opposed to at the individual study level) using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach (38). Evidence started at moderate quality because of the observational nature of studies included in this systematic review, and was downgraded based on the following 4 GRADE criteria: 1) risk of bias of individual studies included in the meta-analysis (study design or failing to blind birth groups for analysis could affect rating); 2) inconsistency (unexplained statistical heterogeneity in meta-analysis results); 3) indirectness [not using validated tools or assessments or failing to distinguish between types of eating problems (oromotor compared with behavioral)]; and 4) imprecision (wide CIs around the estimates or ORs in meta-analysis). An overall score for each outcome (high, moderate, low, or very low) was determined by considering certainty across all GRADE criteria (38).
Results
Our search of 6 electronic databases, the gray literature, and reference lists identified 6221 abstracts after the removal of duplicates (Figure 1). We examined the eligibility of 232 full-text articles. In cases where we could not locate full-text articles (n = 4), we attempted to contact the corresponding authors but did not receive any responses. Of the full texts reviewed, 67 articles reporting on 57 unique studies met our inclusion criteria for this systematic review (Table 2); in 10 cases, articles (i.e., published manuscripts) originated from the same study sample but reported on different outcomes. Supplemental Table 2 provides relevant results from each article. These 57 unique studies reported on a total of 11,728 children born preterm, with mean gestational age at birth ranging from 25.9 to 36.6 wk and mean birth weight ranging from 453 to 2526 g. Fifteen articles (22% of articles; n = 13 unique studies) reported exclusively on children born preterm and VLBW. Twenty-six articles (39%) provided data on infants between 6 and 12 mo of age, 28 (42%) on children >12 mo–7 y of age, and 13 (19%) spanned both age groups. Few articles included data on children older than 2 y of age (n = 14; 21%). Where articles included information on who the caregiver was (n = 60, 90%), most identified the child's mother as the caregiver.
FIGURE 1.
Systematic review study selection process to examine the oromotor skills, eating behaviors, food parenting, and dietary patterns of children born preterm compared with term-born peers.
TABLE 2.
Summary of included articles examining the oromotor skills, eating behaviors, food parenting, and dietary patterns of children born preterm between the ages of 6 mo and 7 y1
| Authors, citation | Country | Study design | Preterm sample characteristics | Age group | Term comparison? | Outcomes assessed | Measures |
|---|---|---|---|---|---|---|---|
| Abily-Donval et al. (39) | France | Prospective cohort | n = 536 infants born at <33 weeks of gestation; n = 431 followed to 2 y | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | Study-developed questionnaire. Eating problems categorized as no problem; small problem, easily solved; moderate difficulties requiring special involvement of parents to manage; major difficulties: daily concern to tolerable limits for parents |
| Adams-Chapman et al. (40) | USA | Prospective cohort | n = 1477 preterm infants participating in a clinical trial with follow-up to 18 mo CA. Infants with congenital anomalies or infection excluded | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | Neonatal unit–developed questionnaire (parent report). Dysfunctional eating was defined as any of the following: 1) physician order not to ingest feedings by mouth; 2) need for gastrostomy or tube feedings; 3) gags/chokes or coughs with oral feeds; 4) documented history of aspiration; 5) excessive drooling during feeds; or 6) difficulty swallowing. Type and consistency of food were also recorded |
| Adams-Chapman et al. (41) | USA | Retrospective cohort | n = 467 infants born at <1000 g and without major congenital anomalies or infection. Follow-up at 18 and 30 mo CA | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | Eating behaviors assessed by a certified examiner. Abnormal eating behaviors were coded when the child 1) was unable to tolerate foods by mouth; 2) required nasogastric or G-tube feeds for >50% of intake; 3) choked, gagged, coughed, or gasped with solids; or 4) drooled continuously. History of dysphagia or aspiration in fluoroscopic swallow studies was coded as abnormal |
| Amarger et al. (42) | France | Prospective cohort | n = 217 infants born at <33 weeks of gestation with eating behavior data at 2 y CA.Same sample as Migraine et al. (30) | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | Child Eating Difficulties Questionnaire (parent report) which assesses 4 constructs of eating difficulties: neophobia, pickiness, low appetite, and low enjoyment of food. Neophobia and pickiness were combined to create a “narrow food repertoire” dimension. Low appetite and enjoyment of food were combined for a low drive to eat dimension. The 2 dimensions were then summed for an “Eating Difficulty”; higher scores indicate more difficulties (range: 2–10) |
| Anderson et al. (43) | USA | Cross-sectional | n = 41 preschool children (mean age: 47 mo) who were singletons born at ≤30 weeks of gestation to English-speaking parents. Children that were blind, deaf, or tube fed were excluded | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | “Problematic Mealtime Behaviours” domain of the Meals in Our Household Questionnaire (parent report), which measures the frequency and intensity of 10 problematic mealtime behaviors (e.g., refuses what is served) over the past 3 mo. Responses to 20 statements are summed to generate a problematic mealtime behavior score; higher scores indicate more severe problems |
| Barnard et al. (25) | USA | Prospective cohort | n = 88 infants born at ≤34 weeks of gestation without Down syndrome, central nervous system dysfunction, and not requiring assisted respiration, to mothers with no history of late-pregnancy substance addiction. Observed at 4 and 8 mo of age | 6–12 mo | n = 166 term-born infants were recruited from a parallel cohort study that began in 1973. Infants were born to mothers with “excellent prenatal and postnatal care.” This sample was a comparatively well-educated, healthy, and middle-class sample | Oromotor eating skills and eating behaviorsFood parenting | Infant eating behavior (e.g., state while eating, affect, visual attentiveness to mother, infant control) was observed during clinic visits for preterm infants and at home for term infants.Maternal food parenting was observed including adequacy of positioning of the infant; attentiveness to the infant and to feeding; kinesthetic, visual, and tactile stimulation; amount/variety of verbal stimulation; level of affect expressed; and responsiveness to the infant's distress or satiation cues |
| Bilgin and Wolke (44) | United Kingdom | Prospective cohort | n = 73 infants born at ≤32 weeks of gestation at <1500 g. Exclusion criteria: transfer out of unit before discharge, living ≥2 h from the hospital, foster care. Followed up at 3, 6 and 18 mo CA.Same sample as Bilgin and Wolke (45) | 6–12 mo and >12 mo–7 y | n = 105 infants born between 37 and 42 weeks of gestation at the same hospitals. Matched to preterm infants by SES, sex, and multiple birth. Exclusion included a neonatal medical problem recorded at recruitment | Oromotor eating skills and eating behaviorsDietary patterns | A structured interview of child eating behaviors was developed for the study. Questions assessed oromotor eating problems (e.g., excessive dribbling/difficulty swallowing). At 6 mo, faddy eating/food refusal was measured with 1 item (fighting against the bottle/breast) and at 18 mo, children's eating behaviors (e.g., eats too little, poor appetite, picky eater).Breastfeeding was measured by maternal report at each time point |
| Bilgin and Wolke (45) | United Kingdom | Prospective cohort | n = 73 infants. Same sample as Bilgin and Wolke (44) | >12 mo–7 y | n = 105 term infants. Same hospitals. See Bilgin and Wolke (44) | Oromotor eating skills and eating behaviors | See Bilgin and Wolke (44) |
| Burklow et al. (46) | USA | Retrospective cohort | n = 83 children born preterm (<38 weeks of gestation) attending an initial outpatient clinic appointment with an interdisciplinary feeding team. Mean ± SD age: 31.8 ± 20.2 mo | >12 mo–7 y | n = 60 children born at >38 weeks of gestation presenting to the same feeding clinic | Oromotor eating skills and eating behaviorsDietary patterns | History of bottle feeding and breastfeeding, solid food introduction, previous and current eating difficulties (i.e., poor sucking skills, emesis), and current eating (i.e., tube feeding supplementation) by parent report.Medical record documentation of nonexclusive problems including structural/mechanical, neurologic, behavioral, cardiorespiratory, and metabolic conditions causing eating issues |
| Buswell et al. (47) | United Kingdom | Retrospective cohort | n = 15 infants born at <37 weeks of gestation. Infants were free of congenital problems, significant parenchymal hemorrhage or leukomalacia, visual impairment, aspiration risk precluding oral feeding, and foster care. Age 10 mo at time of study | 6–12 mo | No | Oromotor eating skills and eating behaviors | Age at which infants were weaned (parent report).An independent blind observer coded videos of infants eating during home visits using the SOMA to measure infants’ oral motor skills |
| Cerro et al. (8) | South Australia | Cross-sectional | n = 95 children born at <32 weeks of gestation or weighing <1500 g without congenital abnormalities or neurologic impairment. Aboriginal and Torres Strait Islander children were excluded (questionnaire was not culturally appropriate). Children were recruited from the NICU follow-up clinic and 1.5–3 y old at time of study | >12 mo–7 y | Every 7th name was selected from the 1996 birth registry at the same hospital. Recruitment letters were sent to the parents of those who were born singletons at >37 weeks of gestation and without congenital abnormalities (n = 143 responded). At the time of the study, term children were significantly younger than preterm (CA; difference of ∼8 mo) | Oromotor eating skills and eating behaviorsFood parentingDietary patterns | Questionnaire developed for study (parent report) assessing 1) Eating behaviors (e.g., vomiting, reflux requiring medication, picky/fussy eating, food refusal, ability to self-feed); 2) Food parenting (perceptions of when children should eat, how much they should eat, what they should eat, mealtime environment, duration of meals, restriction of specific foods, parent feelings when child refuses food, pressure to eat/coaxing, use of nonfood reward and food reward, and bribes and threats to prompt child to eat); and 3) Dietary patterns (range and textures of foods eaten, age at |
| introduction to solids, breastfeeding duration, amount of junk food eaten, amount of food eaten as snacks and meals) | |||||||
| Cho et al. (48) | Korea | Cross-sectional | n = 390 infants born at <37 weeks of gestation at <2500 g without chromosomal aberrations, congenital metabolic/endocrine disorders, major congenital anomalies, or surgery for gastrointestinal anomalies. Data extracted for n = 263 infants aged ≥8 mo at the time of data collection (8, 12, or 18 mo CA) | 6–12 mo and >12 mo–7 y | No | Oromotor eating skills and eating behaviorsFood parenting | Parents’ “concerns” about their preterm child using an open-ended question on a survey developed for the study. Concerns were subsequently coded by the research team |
| Chung et al. (49) | USA | Cross-sectional | n = 76 infants at 2–23 mo CA attending NICU follow-up in New York. Non-English-speaking families were excluded | 6–12 mo and >12 mo–7 y | No | Oromotor eating skills and eating behaviorsFood parenting | Child's developmental readiness to start solids (e.g., trouble controlling head and neck) (parent report) and avoidant eating behaviors (e.g., push food away). Children categorized as developmentally ready or not for starting solid foods.Parental feeding attitudes/satisfaction on the Delighted-Terrible scale (e.g., “When you first introduced solids, how comfortable did you feel feeding your infant?”) |
| Crapnell et al. (1) | USA | Prospective cohort | n = 80 consecutive infants born at ≤30 weeks of gestation without congenital anomalies in a level 3 NICU. Recruited by day 3 of life and followed until 2 y of age.Same sample as Crapnell et al. (50) | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | Eating subscale of the Infant-Toddler Social Emotional Assessment (ITSEA) at 2 y of age (parent report): gagging and choking on food, refusing to eat, refusing to eat foods that require chewing, spitting out food, accepting foods right away, whether child is considered a good eater or a picky eater, refusing to eat certain foods for ≥2 d, and holding food in cheeks |
| Crapnell et al. (50) | USA | Prospective cohort | n = 86 infants. Same sample as Crapnell et al. (1) | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | See Crapnell et al. (1) |
| Davenport et al. (51) | United Kingdom | Cross-sectional | n = 38 infants born at ≤37 weeks of gestation at ≤2499 g. Recruited at 3–4 y of age from a previous case-control study examining the relation between periodontal disease and delivery of a preterm LBW infant. Participants lived close to the dental institute and most were of Bengali origin | >12 mo–7 y | n = 62 infants born at >37 weeks of gestation and weighing >2500 g were recruited from the same maternal periodontal disease study | Dietary patterns | Children's dietary patterns were measured by 24-h recall to collect information about the numbers and types of snacks children consumed per day, their amount of sugar consumption, and the types of beverages consumed.Parents reported on the duration of breastfeeding |
| DeMauro et al. (52) | USA | Prospective cohort | n = 890 infants born at between 25 and 36 weeks of gestation without any major congenital or chromosomal abnormalities. Followed up at 3, 6, and 12 mo CA | 6–12 mo | No | Oromotor eating skills and eating behaviorsFood parenting | Infant's appetite; trouble with sucking, swallowing, choking; length of feeding and whether the infant is feeding enough; frequency infant pushes food away, turns head, closes mouth, gags, holds food in mouth, spits, and cries when food is offered; and if infant had been seen in a specialty feeding clinic (yes/no) (all parent-report) |
| Food parenting (parent report): Are feeding times (very relaxed, relaxed, average, stressful, very stressful); How comfortable are you in feeding baby? | |||||||
| den Boer and Schipper (53) | Netherlands | Cross-sectional | n = 47 infants born at <32 weeks of gestation and/or weighing <1500 g. Infants were 9 mo CA at time of assessment | 6–12 mo | n = 52 infants born at >37 weeks of gestation without any complications recruited randomly from the hospital database | Oromotor eating skills and eating behaviors | Infants were observed by a Speech Language Pathologist while eating in the clinic at 9 mo CA. Observations included sufficient postural balance, drinking independently from bottle, choking while drinking, gagging during meal, eating bread with crust, accepting teeth brushing, keeping tongue in mouth, lip closure while eating, lateral tongue movements, and drooling |
| Dodrill et al. (26) | Australia | Cross-sectional | n = 20 infants born at between 32 and 37 weeks of gestation. Infants living within a 50-km radius of the study hospital were recruited from the hospital database at 11–17 mo CA. Excluded if any history of structural lesions to the swallowing mechanism; or respiratory, cardiac, gastrointestinal, or neurologic conditions | >12 mo–7 y | n = 10 infants born at >37 weeks of gestation were recruited using noticeboards in childcare centers and playgroups within a 50-km radius of the hospital. Matched to preterm infants on gender and CA at assessment | Oromotor eating skills and eating behaviors | Observations using the Royal Children's Hospital OSC and the PSAS. The OSC measures 1) child's facial defensiveness and 2) oral sensitivity indicated by response to stimulation in the oral region. The PSAS provides a rating of a child's functional feeding abilities. Each subtest of the PSAS lists a variable number of rankings based on normal developmental milestones |
| Duran et al. (27) | Turkey | Cross-sectional | n = 79 children born at 32–36.6 weeks of gestation and treated in the recruiting hospital's NICU. Infants were excluded if they had major structural or chromosomal anomalies. Infants were followed up at 2 y CA | >12 mo–7 y | n = 79 infants born at 37–41.6 weeks of gestation at the same hospital and treated in the NICU over the same time frame. Term-born infants were also excluded if they had structural or chromosomal abnormalities | Oromotor eating skills and eating behaviorsDietary patterns | The Children's Eating Difficulties Questionnaire (CEDQ) was used to assess neophobia, pickiness, low appetite, and low enjoyment of food.Frequency of vegetable, fruit, meat, fish, ready meals, desserts, cereals, potatoes, milk, and cheese consumption over previous month (parent report) |
| Ericson et al. (54) | Sweden | Prospective cohort | n = 281 infants born at <37 weeks of gestation recruited from 6 NICUs 48 h after birth. Born to mothers who provided breast milk regardless of amount or method. Excluded if terminally ill, transferred, or where language barriers existed. Followed at 6 and 12 mo postnatal age | 6–12 mo | No | Dietary patterns | Parental report of breastfeeding duration by telephone call and mailed surveys. Exclusive breastfeeding defined as feeding with breast milk only regardless of feeding method (e.g., breast, bottle, tube, or cup) in previous 24 h. Partial breastfeeding was defined as feeding with breast milk in combination with formula and/or solid food in previous 24 h |
| Ernst et al. (55) | USA | Prospective cohort | n = 122 infants born at ≤35 weeks of gestation, weighing <1500 g, and admitted to the NICU at <48 h of age. Feeding information was collected among n = 89 infants at term, 3, 6, 9, and 12 mo postnatal age | 6–12 mo | No | Dietary patterns | During interview with Registered Dietitian, parents were asked the child's age at introduction to solid foods, cow milk introduction, when chopped table foods were introduced, and the types of foods that were introduced at each time |
| Flacking et al. (56) | Sweden | Prospective cohort | n = 2093 infants born singletons at ≤37 weeks of gestation identified from the Child Health Centre registers on breastfeeding in Örebro and Uppsala counties with data in the Medical Birth Registry in Sweden. Followed at 2, 4, 6, 9, and 12 mo postnatal age | 6–12 mo | n = 35,250 infants born singletons at >37 weeks of gestation also with data both in the same Child Health Centre registers and in the Medical Birth Registry in Sweden | Dietary patterns | Mothers were asked at each visit to the Community Health Centre whether their infant was being breastfed (exclusively or partially) |
| Forcada-Guex et al. (28) | Switzerland | Prospective cohort | n = 47 infants born at <34 weeks of gestation and admitted to the NICU over a 12-mo period at a university hospital. Followed at 6 and 18 mo CA.Same sample as Pierrehumbert et al. (32) | 6–12 mo and >12 mo–7 y | n = 25 infants born at >37 weeks of gestation recruited from the maternity ward in the same hospital | Oromotor eating skills and eating behaviors | At 6 mo CA, mother–infant 10-min play interactions were coded using the Care Index by 2 blinded, independent coders to assess the mother's (sensitivity, control, and unresponsiveness) and the child's (cooperation, compliance, difficult, and passivity) interactional behavior. At 18 mo CA, The SCL was completed by a semistructured interview with the mother. The SCL focuses on eating problems (refusal to eat, meal as a negative experience, vomiting, and the overall consequence of these problems) |
| Francis et al. (9) | Canada | Prospective cohort | n = 300 infants born weighing <1500 g, admitted to the NICU, and expected to begin enteral feeding within 7 d of birth. Infants with chromosomal abnormalities, severe birth asphyxia, or who were transferred to a nonparticipating NICU were excluded. Same sample as McGee et al. (57) | 6–12 mo | No | Dietary patterns | Parents were contacted by telephone monthly after discharge from the NICU and were asked whether they were providing mother's milk, infant formula, or cow milk and the frequency of provision. If mother's milk was discontinued, they were asked to recall the last date provided. Date solid foods were introduced was also collected |
| Gibson and DeWolfe (58) | Canada | Prospective cohort | n = 33 infants (mean birth weight: 1800 g; mean gestational age: 33 wk). Infants were followed at 1, 3, 6, and 12 mo of age | 6–12 mo | n = 29 infants born at between 38 and 42 weeks of gestation and weighing >2500 g were recruited from the same hospital | Dietary patterns | At 6 and 12 mo, 3-d food records were used to assess infants’ diets |
| Hawdon et al. (59) | United Kingdom | Prospective cohort | n = 35 infants admitted to the NICU at University College London Hospital over a 3-mo period and followed up at 6 and 12 mo | 6–12 mo | No | Oromotor eating skills and eating behaviorsFood parentingDietary patterns | A feeding assessment by a trained observer using the Neonatal Oral Motor Assessment Scale to categorize infants as normal, disorganized, or dysfunctional feeders. At 6 and 12 mo of age, parents were interviewed about breastfeeding; age at introduction to solids; food eaten; coughing, spitting, or vomiting during drinking or solid feeds; both the parent's and infant's enjoyment of feeding; feeding environment; and concerns about feeding |
| Hill (60) | USA | Prospective cohort | n = 120 infants born at between 20 and 32 weeks of gestation and weighing <1500 g. | 6–12 mo | No | Oromotor eating skills and eating behaviors | Parents completed the Pediatric Assessment Scale for Severe Feeding Problems to assess progress and development of oral eating skills |
| Infants were born to English-speaking mothers who could attend follow-up at 2, 4, 6, and 12 mo of age. Excluded if infant had craniofacial abnormalities | including nutrition, oral sensory, oral motor, behavioral feeding, and quality of life issues | ||||||
| Holditch-Davis et al. (61) | USA | Cross-sectional | n = 29 infants born weighing <1500 g and/or mechanically ventilated in the NICU | 6–12 mo | No | Oromotor eating skills and eating behaviorsFood parenting | Mother–child interactions were observed over the span of 1 h during feeding and nonfeeding interactions by a single observer when infants were 6 mo of age. The coding scheme was developed for the study and examined both maternal (e.g., feeding, playing, talking, touching, positive affect) and child (e.g., movement, vocalization, positive affect, playing, self-feeding, interactive states, alertness) behaviors |
| Hoogewerf et al. (62) | Netherlands | Prospective cohort | n = 251 infants born at <36 weeks of gestation admitted to the NICU for ≥4 d. Infants with chromosomal abnormalities were excluded | >12 mo–7 y | n = 127 born at between 37 and 42 weeks of gestation and treated in the NICU over the same time. Same exclusion criteria.A reference population of n = 771 term-born, healthy Dutch children who were between 8 and 30 mo old at the time of assessment was also included to compare the NICU sample (preterm and term-born infants treated in the NICU) with the healthy term-born sample | Oromotor eating skills and eating behaviors | When infants were 1–2 y of age, parents completed the 14-item SEP, a Dutch translation of the Montreal Children's Hospital Feeding Scale which examines both oromotor and behavioral eating challenges |
| Howe et al. (29) | Taiwan | Cross-sectional | n = 239 children born at <37 weeks of gestation and weighing <1500 g attending NICU follow-up appointments at a large urban hospital. Children were recruited for the study when they were <2 y of age | 6–12 mo and >12 mo–7 y | n = 181 children born at >37 weeks of gestation and recruited from well-baby visits at the same hospital | Oromotor eating skills and eating behaviorsDietary patterns | Child eating behaviors/skills were measured by the Behavior Based Feeding Questionnaire (parent-report), which assesses feeding endurance, Gatrointestinal-related issues, muscle tone, oral motor function, respiration/sensory regulation, and frequency and duration of feeding. Parents also reported duration of breastfeeding and types of foods fed to child at introduction to solids |
| Hubl et al. (63) | Germany | Prospective cohort | n = 40 infants born at between 24 and 34 weeks of gestation. Infants were excluded if they had congenital malformations, chromosomal abnormalities, or acute illness or had not started oral feeds by 34 wk. Assessments were conducted at 6, 9, 12, and 24 mo PMA | 6–12 mo and >12 mo–7 y | No | Oromotor eating skills and eating behaviorsDietary patterns | Children's eating behaviors/skills were measured by observation by 2 independent observers using the Neonatal Oral Motor Assessment Scale at 34, 37, and 44 wk PMA; the Observation List Spoon Feeding (OSF) at 6, 9, and 12 mo PMA; and the Mastication Observation and Evaluation Instrument (MOE) at 9, 12, and 24 mo PMA |
| Husk and Keim (64) | USA | Cross-sectional | n = 189 children participating in 1 of 2 ongoing clinical trials. All children had been previously admitted to the NICU or scheduled for a neonatal follow-up appointment. Children in trial 1 were born at ≤29 weeks of gestation and in trial 2 at ≤35 weeks of gestation. Data for the current study were collected when infants were 1–3 y of age | >12 mo–7 y | No | Dietary patterns | Parents completed the Harvard Semi-quantitative FFQ which examines food intake over the last month. Parents also reported breastfeeding duration |
| Johnson et al. (65) | United Kingdom | Prospective cohort | n = 628 infants born at between 32 and 36.6 weeks of gestation to mothers living in the East Midlands (England). Excluded if major structural or chromosomal abnormalities including cardiovascular malformations and neurosensory impairments. Assessed at 2 y of age | >12 mo–7 y | n = 759 infants born at 37.0–42.6 weeks of gestation were recruited during the same time and from the same geographical region. Term infants were selected by random sampling of dates/times in computerized birth records during the previous year | Oromotor eating skills and eating behaviors | Children's eating behaviors/skills were measured using a questionnaire previously validated by the group (parent report). Questionnaire assessed the presence of eating difficulties across 4 domains: 1) refusal/picky; 2) oral motor problems; 3) oral hypersensitivity; 4) eating behavior problems (tantrums or makes a mess during meal). Scores at the 90th percentile of term-born scores were used to identify clinically significant eating difficulties overall |
| Jonsson et al. (66) | Sweden | Prospective cohort | n = 27 infants born at between 28 and 33 weeks of gestation and cared for in the Umeå University Hospital. At the time of assessment, infants were aged 34–42 mo | >12 mo–7 y | n = 29 healthy infants born at between 38 and 41 weeks of gestation. Matched to preterm infants on gender and birth date (within 3 d) | Oromotor eating skills and eating behaviorsDietary patterns | Parents reported their child's eating habits on a questionnaire developed by the authors. Parents were also asked to report on their experiences with mealtimes from hospital discharge through to introduction to solids |
| Kirk et al. (67) | Rwanda | Cross-sectional | n = 86 infants born at <37 weeks of gestation and weighing <2000 g, identified in patient registers. Excluded if they had genetic dysmorphologies, congenital heart disease, or birth asphyxia. Assessed at 17.5–30.5 mo of age | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | Child eating skills were measured by parental report on a questionnaire developed for the study. Parents were asked whether the child displayed any eating difficulties including choking, coughing, or gagging |
| Kmita et al. (68) | Poland | Prospective cohort | n = 40 infants born at <35 weeks of gestation. Secondary data analysis, infants were included if | 6–12 mo | No | Oromotor eating skills and eating behaviorsDietary patterns | Mothers and fathers were interviewed together when infants were 1, 4, 6, and 12 mo CA using a semistructured interview developed for the study. |
| they had full data and both the mother and father participated. Exclusion criteria included having a teenage parent and being born with a congenital malformation or genetic syndrome | Questions were related to concerns about child's eating behaviors and skills as well as breastfeeding | ||||||
| Litt et al. (69) | USA | Prospective cohort | n = 50 infants born singletons at ≤35 weeks of gestation recruited from an academic hospital. Excluded if parents did not speak/read English or child was in foster care. Infants were followed up at 1, 3, and 6 mo of age | 6–12 mo | No | Oromotor eating skills and eating behaviors | Feeding Difficulty Scale (parent report). Scores from the 17 items represent 6 domains related to appetite, oromotor skills, avoidant eating behaviors, and family distress related to the child's eating to create an overall composite score |
| Mathisen et al. (70) | Australia | Cross-sectional | n = 20 infants born at 23–29 weeks of gestation weighing <1000 g. Recruited through the Growth and Development clinic at 2 hospitals in Brisbane. Excluded if they had intraventricular hemorrhage, necrotizing enterocolitis, bronchopulmonary dysplasia, chromosomal abnormalities, were born small for gestational age, or were still receiving supplemental oxygen at the time of recruitment | 6–12 mo | n = 20 infants born at >38 weeks of gestation were recruited from well-baby visits at the same hospitals. Term infants were matched to preterm infants on age, race, gender, and SES and excluded if they had a history of feeding problems, reflux, or neurologic impairments. Infants had to be eating solids for ≥2 wk at time of recruitment | Oromotor eating skills and eating behaviorsFood parentingDietary patterns | Infant Feeding Questionnaire (maternal report) which asks about maternal enjoyment, anxiety, and special concerns regarding the feeding of the infant.Direct observation using The Feeding Environment Checklist to assess contextual features such as lunch timing, light, noise, distraction, and the suitability of positioning and the food and equipment used to feed the infant. The Feeding Assessment Schedule (FAS) and SOMA assessed oromotor skills |
| McComish (71) | USA | Prospective cohort | n = 42 African-American infants born at <35 weeks of gestation and weighing <1500 g or requiring mechanical ventilation. Exclusion criteria: congenital neurologic problems, substance exposure, hospitalized for >2 mo postterm, mothers <15 y old with substance abuse challenges, who did not have custody of the infant, had history of a mental health diagnosis, or did not speak English | 6–12 mo | No | Oromotor eating skills and eating behaviors | Children's eating skills were measured by observation using a modified version of the SOMA. Mealtime communication “red flags” (e.g., lack of infant engagement, responding, vocalizing, or imitating) and oral motor “red flags” (e.g., choking, coughing, or gagging; liquid or food loss; panic reactions) during feeding interactions were coded |
| McGee et al. (57) | Canada | Cross-sectional | n = 158 children born at <37 weeks of gestation and weighing <1500 g. This was a 5.5-y follow-up of a previous RCT. See Francis et al. (9) | >12 mo–7 y | No | Dietary patterns | At 5.5 y of age, diet quality [Healthy Eating Index (HEI)-2010] and usual intakes of fruits and vegetables and added sugars were determined from 2 dietary recalls analyzed using the Automated Self-Administered 24-hour (ASA24®) Dietary Assessment Tool |
| Menezes et al. (22) | Brazil | Cross-sectional | n = 38 infants born at <37 weeks of gestation recruited from an outpatient clinic following high-risk newborns. Infants with craniofacial malformations, heart disease, and severe respiratory diseases that prevented them from eating safely or infants with oropharyngeal or esophageal dysphagia were excluded. Infants were 6 mo–2 y old at time of data collection | 6–12 mo and >12 mo–7 y | No | Oromotor eating skills and eating behaviorsDietary patterns | Structured interview developed for the study (parent report) on child's behavior and oromotor skills. An affirmative response to any item on the checklist was considered to indicate eating difficulty. For the food refusal item, if the infant presented a refusal at any meal of the day during the last month, it was considered difficulty in the introduction of complementary feeding. Parents also reported on breastfeeding duration |
| Milton and King (72) | United Kingdom | Prospective cohort | n = 169 infants born at <34 weeks of gestation and admitted to an NICU. Infants were between 12 and 18 mo old at the time of data collection | >12 mo–7 y | No | Oromotor eating skills and eating behaviorsDietary patterns | Parents completed a questionnaire that asked about age at cup introduction, frequency of cup and bottle use, types of drinks consumed, cups used, and vitamin supplements given. Appropriateness of vitamin use was assessed by examining combination of cow milk provision, formula provision, and supplementation use |
| Migraine et al. (30) | France | Prospective cohort | n = 234 infants born at <33 weeks of gestation. At follow-up, infants were 2 y of age.Same sample as Amarger et al. (42) | >12 mo–7 y | n = 245 infants were participating in a different RCT (Opaline trial) where mothers were recruited before delivery through advertisements in maternity waiting rooms | Oromotor eating skills and eating behaviorsDietary patterns | Mothers completed the Children's Eating Difficulties Questionnaire. Children in the upper 2 quintiles for each of the 2 overall dimensions were defined as having eating disorders.Mothers also completed an FFQ. Children's preferences for each food on the FFQ were assessed on a 4-point Likert scale (1 = child turns head away/spits out; 4 = child accepts food/asks for more) |
| Mokhlesin et al. (73) | Iran | Retrospective cohort | n = 38 children born at between 28 and 32 weeks of gestation. Those with oral or neurologic problems or whose mothers had depression or other psychological problems were excluded. Children were 2 y old at the time of assessment | >12 mo–7 y | n = 38 two-year-old children born at between 38 and 41 weeks of gestation, recruited from the same hospital by random sampling, and matched to preterm infants on age, gender, SES, and maternal education level | Oromotor eating skills and eating behaviors | Parents reported their children's eating behaviors/skills on the Children's Feeding Disorder Questionnaire. The questionnaire examines eating behaviors, eating tension, physical eating challenges, food variety, and mother's satisfaction with child eating |
| Nieuwenhuis et al. (74) | Netherlands | Cross-sectional | n = 35 children born before 32 weeks of gestation admitted to the NICU. At the time of | >12 mo–7 y | n = 248 infants born at >37 weeks of gestation recruited from local health care | Oromotor eating skills and eating behaviors | Parents reported their children's eating challenges on the 14-item SEP, a Dutch translation of the Montreal Children's Hospital Feeding Scale |
| assessment, children were 3 y of age | centers. Exclusion criteria: mothers had severe complications during pregnancy, born by emergency cesarean delivery, resuscitated at birth, tube fed, or had any congenital anomalies and syndromes | which examines both oromotor and behavioral eating challenges | |||||
| Park et al. (31) | USA | Cross-sectional | n = 256 children born at <37 weeks of gestation. Parents of children with and without feeding problems were recruited from a variety of sources including feeding clinics, online parent support groups, and research registries. Children were between the ages of 6 mo and 7 y at the time of data collection | 6–12 mo and >12 mo–7 y | n = 979 children born at ≥37 weeks of gestation recruited using the same methods. Exclusion criteria included if the child had any language or development delay; hearing or vision impairment; structural abnormality of the mouth, face, or gastrointestinal tract; or significant health conditions (e.g., congenital heart disease, autism, cerebral palsy) | Oromotor eating skills and eating behaviors | Parents reported their children's eating behaviors and skills using the PediEAT. PediEAT is a 78-item questionnaire with 4 subscales: Physiologic Symptoms (27 items), Problematic Mealtime Behaviors (23 items), Selective/Restrictive Eating (15 items), and Oral Processing (13 items) |
| Philip and Vijay Kumar (75) | India | Cross-sectional | n = 25 infants born at between 30 and 34 weeks of gestation with a birth weight appropriate for gestational age. Excluded if they had any craniofacial abnormalities. Infants were 6 mo CA at time of data collection | 6–12 mo | n = 25 term infants born with an appropriate birth weight and without craniofacial abnormalities or any neurologic, cardiology, or vital organ issues | Oromotor eating skills and eating behaviors | Questionnaire developed for the study (parent report) asking about child's feeding history, current eating status, age of solid food introduction, current eating behavior, eating difficulties, and duration of meals |
| Pierrehumbert et al. (32) | Switzerland | Prospective cohort | n = 50 infants born at <33 weeks of gestation and admitted to an NICU. Excluded if any malformations, chromosomal abnormalities, fetopathy, neurodevelopmental complications, parental psychiatric illness and/or drug abuse, or their parents had difficulty speaking French. Same sample as Forcada-Guex et al. (28) | >12 mo–7 y | n = 25 term infants were recruited from the maternity ward of the same hospital. Infants were excluded if there were any problems during pregnancy or delivery, the infant had somatic abnormalities, or the parents had psychiatric problems or language difficulties | Oromotor eating skills and eating behaviors | At 18 mo, the SCL was completed by a semistructured interview with the infant's mother |
| Pridham et al. (33) | USA | Cross-sectional | n = 47 born at <32 weeks of gestation with weight appropriate for gestation and a history of lung disease. Infants were recruited from 3 NICUs in a Midwestern city. Infants with congenital or medical conditions that could interfere with oral intake (e.g., heart defect or gastrointestinal system defect requiring surgery) were excluded or if their mothers were <17 y of age, not able to read English, or were not involved in care. Followed up at 12 mo postnatal age.Same sample as other Pridham et al. articles (76, 77) | 6–12 mo | n = 52 full-term infants were recruited from family practice clinics, a pediatric primary care clinic, and a Women, Infants and Children (WIC) clinic in the area | Oromotor eating skills and eating behaviorsFood parenting | The Parent-Child Early Relational Assessment tool was used to observed child eating behaviors and food parenting. This tool assesses both positive (e.g., responsiveness) and negative affect (e.g., avoiding or averting behavior) behaviors of the infant during feeding as well as the positive (e.g., warmth) and negative (e.g., frustration) affect of the mother during feeding |
| Pridham et al. (76) | USA | Prospective cohort | n = 45 infants born singletons and weighing <1250 g and without medical complications.See other Pridham et al. articles (33, 77) | 6–12 mo | No | Oromotor eating skills and eating behaviorsFood parenting | Mother–infant dyads were observed during feeding interactions. Infant eating skills were measured on the Child Feeding Skills Checklist, which was created for the study to quantify skills observed during the observation or reliably reported by the mother. Mother–infant interactions were coded by the Parent-Child Early Relational Assessment tool |
| Pridham et al. (77) | USA | Prospective cohort | n = 41 infants born at <32 weeks of gestation and with a weight appropriate for their gestational age. See the previous Pridham et al. (33, 76) studies. In the current study, infants were followed up at 1 and 8 mo of age | 6–12 mo | No | Food parenting | Maternal food parenting was observed using the Parent-Child Early Relational Assessment tool. Mothers’ feeding competencies were structured into 2 factors: Parental Positive Affective Involvement, Sensitivity, and Responsiveness (PPAISR; e.g., warmth toward infant) and Parental Negative Affect and Behavior (PNAB; e.g., ability to regulate negative tone toward infant) |
| Ribas et al. (78) | Brazil | Cross-sectional | n = 108 infants born VLBW and at <32 weeks of gestation. Infants were followed up at 2 y CA from a specialized outpatient clinic. Exclusion criteria included conditions that affect fetal growth or situations that may interfere with anthropometric and dietary parameters (e.g., major malformations, chromosomal disorders) as well as missing dietary records after introduction of solids | 6–12 mo and >12 mo–7 y | No | Dietary patterns | Dietary data were collected from 24-h recalls completed during clinic visits between 4 and 24 mo CA. Data on type and time of exclusive and total breastfeeding and composition of complementary foods were also collected. Diet quality was assessed using a tool created by the authors, the IMQCF, which is based on the Brazilian Food Guide for children aged <2 y. The IMQCF has 9 items which are scored from the 24-h recalls providing an overall score out of 100; higher scores indicate higher diet quality |
| Saleska et al. (79) | USA | Cross-sectional | n = 331 children born at <35 weeks of gestation and enrolled into the Omega-Tots RCT. Children were mean (SD) 15.1 (1.7) mo CA at baseline. Only data from the 60-d time point are used in the current study | 6–12 mo and >12 mo–7 y | No | Food parenting | Chadn |
| Salvatori et al. (34) | Italy | Prospective cohort | n = 27 infants born at ≤32 weeks of gestation or with a birth weight < 1500 g. Excluded if major cerebral damage (intraventricular hemmorage > III or IV grade), periventricular leukomalacia, retinopathy of prematurity, and hydrocephalus or genetic syndrome, if parent had a past/present psychiatric history, neurologic disorders, disordered eating, or was not fluent in Italian. Measured at 18 and 24 mo of age | >12 mo–7 y | n = 20 term-born infants were recruited from preschools in the same area. Excluded if they had birth complications, cerebral damage, disabilities, or genetic syndromes | Oromotor eating skills and eating behaviorsFood parenting | The Feeding Scale—Italian version (direct observation; blinded by birth group), which assesses mother and child dysfunctional behaviors during the meal via 46 items across 4 dimensions: Affective State of the Mother (e.g., presence of sadness, anger, distress), Interactional Conflict (e.g., forcing child to eat), Food Refusal Behavior of the Child (e.g., spitting out food), and Affective State of the Dyad (e.g., level of reciprocity, mother not supporting child's autonomy) |
| Samara et al. (80) | United Kingdom and Ireland | Cross-sectional | n = 223 children born at ≤25.6 weeks of gestation and known to be alive at 30 mo of age owing to their participation in the EPICure study [see Wood et al. (81)]. Children were 6 y of age | >12 mo–7 y | n = 148 age- and sex-matched term-born children were recruited from mainstream schools | Oromotor eating skills and eating behaviors | Questionnaire developed for the study (parent report) assessing problems related to food refusal/faddy eating (e.g., refuses food; 7 items), oral-motor problems (e.g., dribbles when drinking; 6 items), oral hypersensitivity (e.g., does not like things put in mouth; 2 items), and behavioral problems during meals (e.g., makes a mess, has tantrums; 4 items) |
| Sanchez et al. 2016 (82) and 2017 (83) | Australia | Prospective cohort | n = 90 infants born at <30 weeks of gestation. Infants with congenital abnormalities known to affect neurodevelopment or non-English-speaking parents were excluded. Infants were followed until 12 mo CA.Same sample as Sanchez et al. (5) | 6–12 mo | n = 137 term infants recruited from the maternity wards in the same hospitals over the same time period. The same exclusion criteria were applied | Oromotor eating skills and eating behaviors | At 12 mo of age, infant eating skills were measured by observation using the SOMA tool |
| Sanchez et al. (5) | Australia | Prospective cohort | n = 111 children born at <30 weeks of gestation. Infants with congenital abnormalities known to affect neurodevelopment or non-English-speaking parents were excluded. Infants were followed until 36 mo CA.See other Sanchez et al. studies (82, 83) | >12 mo–7 y | n = 106 term infants were recruited from the maternity wards in the same hospitals over the same time period. The same exclusion criteria were applied | Oromotor eating skills and eating behaviorsFood parenting | BPFAS (parent report). The BPFAS consists of 35 questions related to child eating (e.g., takes longer than 20 min to finish a meal; enjoys eating; has problems chewing foods) and food parenting behaviors (e.g., I get frustrated and/or anxious when feeding my child; I feel confident my child gets enough to eat) |
| Sauve and Geggie (84) | Canada | Prospective cohort | n = 118 infants born in Alberta weighing <1500 g or requiring mechanical ventilation during initial hospitalization. All birth weights were appropriate for gestational age. Infants with evidence of chronic lung disease were excluded. Infants were followed from 4 to 24 mo CA | 6–12 mo and >12 mo–7 y | n = 114 term infants matched to preterm infants on age, sex, and year of birth recruited from well-baby visits over the same time period. Term infants had to be born at a birth weight appropriate for their gestational age | Oromotor eating skills and eating behaviors | Parents reported on their infants’ eating behaviors and skills during a structured interview at each time point. Details of the questions asked were not provided. Eating problems were considered present only if they were a major concern to parents or if they led to some form of therapy or investigation |
| Silberstein et al. (85) | Israel | Prospective cohort | n = 76 low-risk infants born preterm at <36 weeks of gestation. Recruited within the first 2 wk of life from a tertiary NICU. Excluded if intraventricular hemorrhage (grade 3 and 4); perinatal asphyxia; metabolic, genetic, or syndromic disease; or if parents met any psychosocial criteria: teenage pregnancy, single parenthood, or unemployment of both parents. Followed for the first 12 mo of life | 6–12 mo | No | Oromotor eating skills and eating behaviorsFood parenting | Structured interview (parent report) at 12 mo, about children's eating behaviors, mealtime environment (e.g., where meals are eaten, screen use), rules (e.g., routines), picky eating, feeding interaction, and maternal/child enjoyment during meals.Direct observation of mother–child feeding interactions at 12 mo using the Coding of Interactive Behavior- Newborn tool, which assesses mother's touch, gaze to infant, and infant feeding performance and maternal adaptation (e.g., degree to which the mother adjusts to infant signals) |
| Singer et al. (35) | USA | Prospective cohort | n = 171 infants born at <36 weeks of gestation weighing <1500 g. Infants were divided into high risk (lung concerns; n = 103) and low risk (no lung concerns; n = 68). Excluded if evidence of drug exposure, major congenital malformations, mothers with psychiatric or physical illness, HIV, mental disabilities, or if living >2 h from hospital. Followed at term, 8, and 12 mo CA | 6–12 mo | n = 117 infants born at >36 weeks of gestation weighing >2500 g from the newborn nurseries at the same hospital. Infants were included if they had no diagnosis of medical abnormalities or illness at birth. The same exclusion criteria applied | Oromotor eating skills and eating behaviorsFood parenting | Direct observation using the NCFAS. The NCFAS includes 76 dichotomous items to quantify the presence or absence of parent or infant behaviors including parent “Sensitivity to Cues,” “Response to Distress,” “Social-emotional Growth Fostering,” “Cognitive Growth Fostering,” and Infant's behaviour (“Responsivity to Parent” and “Clarity of Cues”) |
| Steinberg et al. (23) | Brazil | Cross-sectional | n = 62 infants born preterm without metabolic diseases or medical diagnosis of neuropathies, syndromes, craniofacial malformations, heart disease, or severe respiratory disease that prevented safe oral eating. Infants were recruited from an outpatient high-risk infant follow-up clinic if <24 mo CA and had started solid foods | 6–12 mo and >12 mo–7 y | No | Oromotor eating skills and eating behaviorsFood parentingDietary patterns | Checklist (maternal report) of infant's defensive behaviors during mealtimes (e.g., arching back, crying, mealtimes lasting ≥40 min, texture/food selectivity, food refusal, difficulty chewing or swallowing). Oromotor skills observed by an adapted version of the Protocol for Pediatric Dysphagia (PAD-PED) while mother fed infant.Parent report of feelings of difficulty feeding child and age of infant at introduction of solids |
| Weber and Harrison (36) | USA | Prospective cohort | n = 61 infants born at <32 weeks of gestation with respiratory distress syndrome. All birth weights were appropriate for gestational age. Excluded if infants had physiological conditions that could interfere with eating or digestion, if mothers didn't speak English, or if mothers were <18 y old. Followed at 1, 4, 8, and 12 mo CA | 6–12 mo | n = 53 healthy infants born at 38–42 weeks of gestation with Apgar scores ≥7 at 1 min and >9 at 5 min and no medical complications during hospitalization. Recruited from the same hospital | Oromotor eating skills and eating behaviorsFood parenting | Direct observation using the Child Feeding Skills Checklist, which is based on the presence of 4 subsets of skills (oral-motor, hand-eye, head/trunk, and communicative-social) at 1, 4, 8, and 12 mo.Direct observation using the Parent-Child Early Relational Assessment tool. Only the PNAB scale was used |
| Wood et al. (81) | United Kingdom and Ireland | Prospective cohort | n = 283 infants born at ≤25 weeks of gestation. Infants were excluded if they had lethal congenital abnormalities. Data collected at 30 mo CA. Same sample as Samara et al. (80) | >12 mo–7 y | No | Oromotor eating skills and eating behaviors | Semistructured interview (parent report) about child's eating behaviors and skills including swallowing issues and food refusal |
| Yatziv et al. (86) | Israel | Prospective cohort | n = 70 infants born singletons at between 28 and 34 weeks of gestation with a birth weight >1000 g and low medical risk on the Nursery Neurobiological Risk Score. All infants were born to 2-parent Hebrew-speaking homes. Data collected at 6 and 12 mo CA | 6–12 mo | n = 64 infants born at >37 weeks of gestation were recruited from maternity wards in the same medical center. The same inclusion and exclusion criteria applied | Food parentingDietary patterns | “Pressure to eat” and “Concern about child undereating or becoming underweight” subscales of the Child Feeding Questionnaire (parent report at 6 mo CA). Mothers reported if they breastfed their infants at 6 mo CA and the duration at 12 mo CA.Direct observation using the Mother-Infant/Toddler Feeding Scale at 12 mo CA |
| Zimmerman and Rosner (24) | USA | Cross-sectional | n = 37 infants born preterm by (mean ± SD) 2.47 ± 1.70 wk. Recruited by convenience using study flyers, social media, and pregnancy-related groups. Exclusion criteria: nonmother caregivers or children older than 3 y | 6–12 mo and >12 mo–7 y | n = 167 term-born infants were recruited by the same methods | Oromotor eating skills and eating behaviors | Online questionnaire developed for the study (parent report). Eating difficulties were categorized if children were reported to have ≥1 of the following: difficulties in sucking, difficulties in food transitions, gastroesophageal reflux, food selectivity, salivary control issues, or poor growth |
n = 67 articles, 57 unique studies. BPFAS, Behavioural Pediatrics Feeding Assessment Scale; CA, corrected age; IMQCF, Index for Measuring the Quality of Complementary Feeding; LBW, low birth weight; NCFAS, Nursing Child Assessment Feeding Scale; NICU, neonatal intensive care unit; OSC, Oral Sensitivity Checklist; PNAB, Parental Negative Affect and Behavior Scale; PediEAT, Pediatric Eating Assessment Scale; PMA, postmenstrual age; RCT, Randomized controlled trial; PSAS, Pre-Speech Assessment Scale; SCL, Symptoms Checklist; SEP, Screeningslijst Eetgedrag Peuters; SES, socioeconomic status; SOMA, Schedule for Oral Motor Assessment; VLBW, very low birth weight (<1500 g).
Thirty-four of the 67 articles (51% of articles; n = 30 unique studies) included a term-born comparison group. These studies reported on a total of 40,257 children born at ≥37 weeks of gestation, with mean birth weight >3000 g. Of the articles with a term-born comparison group, 19 (56%) were prospective cohorts, 13 (38%) cross-sectional, and 2 (6%) retrospective cohorts.
Based on the available studies, we conducted our meta-analysis with articles that provided data on children's oromotor eating skills and eating behaviors. The methodology and outcomes of studies examining food parenting and dietary patterns were highly varied and were thus only discussed narratively.
Oromotor eating skills and eating behaviors
We included 32 articles (28 unique studies) in our meta-analysis of children's oromotor eating skills and eating behaviors (Figure 1). The majority (n = 21, 66%) of these articles were based on caregiver report (survey or interview) to quantify eating skills and behaviors. Next, we discuss our findings related to oromotor eating difficulties and eating behavior challenges.
Oromotor eating difficulties
Twenty-two articles (20 unique studies) were included in our exploration of oromotor eating difficulties. Areas of oromotor concern identified among infants included coordination of the suck-swallow-breathe pattern, lip, and jaw movements; coughing while eating purees; and difficulties self-feeding (e.g., coordinating utensils with the lips and mouth, particularly with pureed foods) (70, 75). Among children, common challenges included food loss/dribbling and coughing or gagging (5, 45, 66).
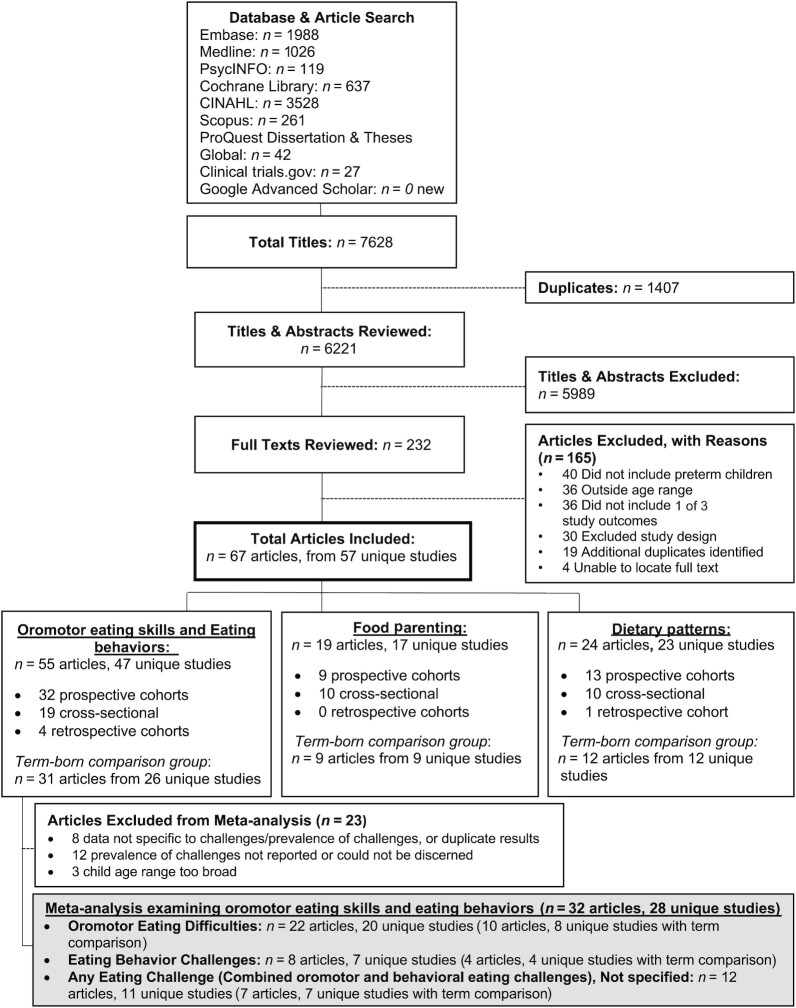
Pooled results showed that 34% (95% CI: 27%, 40%) (Figure 2) of children born preterm experienced oromotor eating difficulties between 6 mo and 7 y of life. Among infants aged 6–12 mo, 43% (95% CI: 24%, 62%) exhibited an oromotor eating difficulty. The prevalence decreased among children >12 mo–7 y old with 25% (95% CI: 17%, 33%) experiencing oromotor eating difficulties. The heterogeneity between studies was high (I2 values > 97%) in both subgroups.
FIGURE 2.
Prevalence of oromotor eating difficulties among infants and children born preterm. Random-effects meta-analysis of proportions using a maximum likelihood estimator. Results are expressed as prevalence (95% CI). The weighting for each study is the inverse of the total variance. I2 = the percentage of variation across studies that is due to heterogeneity rather than chance. Values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively.
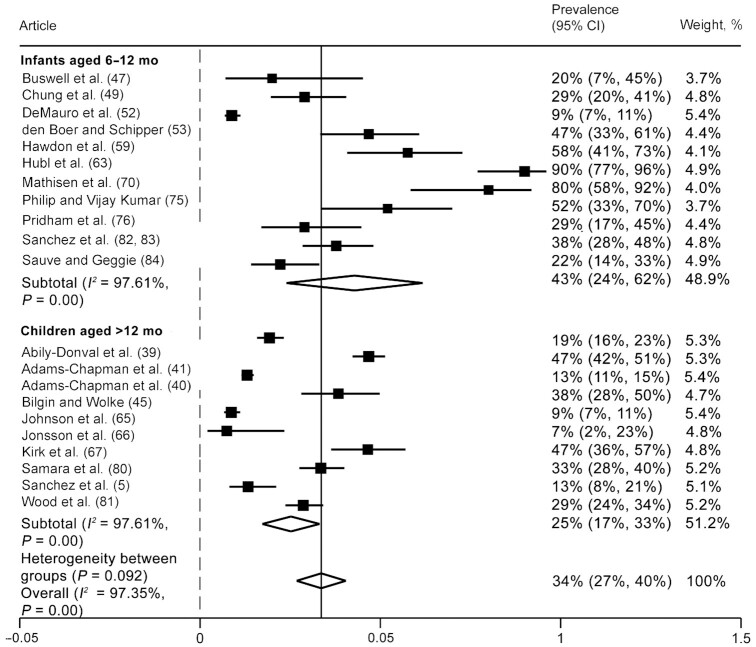
Compared with term-born counterparts (Figure 3), children born preterm were 2.86 times more likely to experience oromotor eating difficulties between the ages of 6 mo and 7 y (OR: 2.86; 95% CI: 1.71, 4.77; I2 = 67.8%). Findings were generally consistent between both age groups. Heterogeneity between studies was moderate to high (I2 values ranging from 67% to 70%). Among articles that were excluded from the meta-analysis because prevalence of oromotor eating difficulties was not reported (n = 4), all suggested that children born preterm had significantly worse oromotor eating skills than children born at term across both age groups (26, 29, 31, 36).
FIGURE 3.
Odds of oromotor eating difficulties: comparison of infants and children born preterm and at term. Random-effects meta-analysis using a REML model. Results are presented as ORs (95% CIs). The weighting for each study is the inverse of the total variance. I2 = the percentage of variation across studies that is due to heterogeneity rather than chance. Values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively. REML, restricted maximum likelihood.
Eating behavior challenges
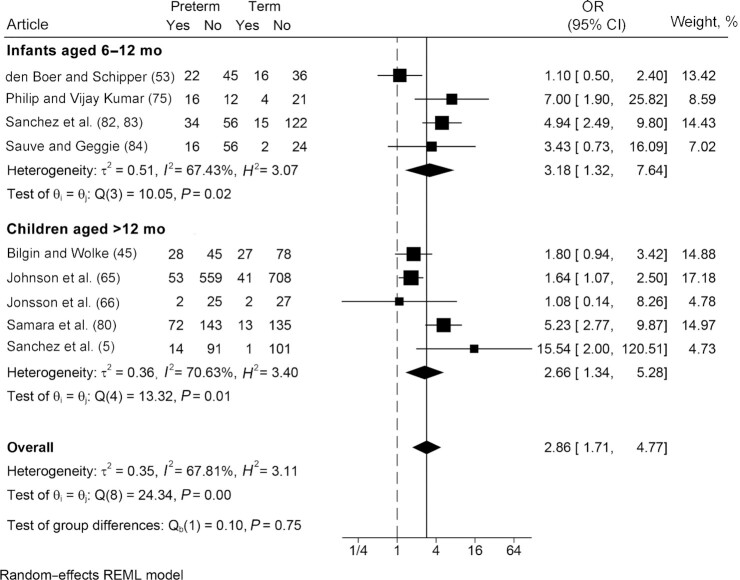
We included 8 articles (7 unique studies) in our meta-analysis of eating behavior challenges. Eighteen percent (95% CI: 12%, 24%) (Figure 4) of children born preterm exhibited eating behavior challenges in the early years, such as picky eating, food refusal, or tantrums during meals; however, heterogeneity between studies was high (I2 values >92%). These estimates were consistent between the 2 age groups.
FIGURE 4.
Prevalence of eating behavior challenges among infants and children born preterm. Random-effects meta-analysis of proportions using a maximum likelihood estimator. Results are expressed as prevalence (95% CI). The weighting for each study is the inverse of the total variance. I2 = the percentage of variation across studies that is due to heterogeneity rather than chance. Values of 25%, 50%, and 75% were considered as low, moderate, and high, respectively.
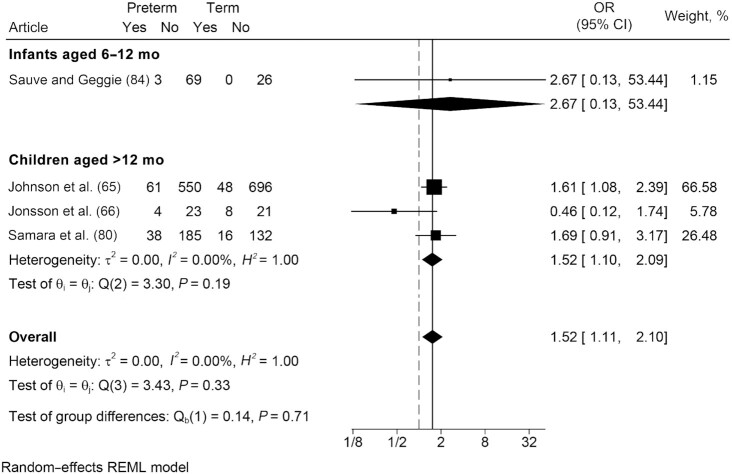
In comparison with term-born peers, results showed that those born preterm were 1.5 times more likely to present with challenging eating behaviors (OR: 1.52; 95% CI: 1.11, 2.10; I2 = 0.0%), with homogeneity between individual studies (Figure 5). Only 1 study of infants compared the odds of eating behavior challenges among those born preterm with term-born peers. Results showed no significant difference between the groups. Among children, results were similar to the pooled estimate. Nine studies presented mean differences, rather than prevalence of eating behavior challenges. Six articles spanning both age groups reported that children born preterm had significantly higher eating behavior challenge scores than term-born peers, indicating more eating behavior challenges such as food refusal and tantrums (27, 28, 31, 34, 35, 70), and 3 found no significant differences (25, 30, 33).
FIGURE 5.
Odds of eating behavior challenges: comparison of infants and children born preterm and at term. Random-effects meta-analysis using a REML model. Results are presented as ORs (95% CIs). The weighting for each study is the inverse of the total variance. I2 = the percentage of variation across studies that is due to heterogeneity rather than chance. Values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively. REML, restricted maximum likelihood.
Any eating challenge, not specified
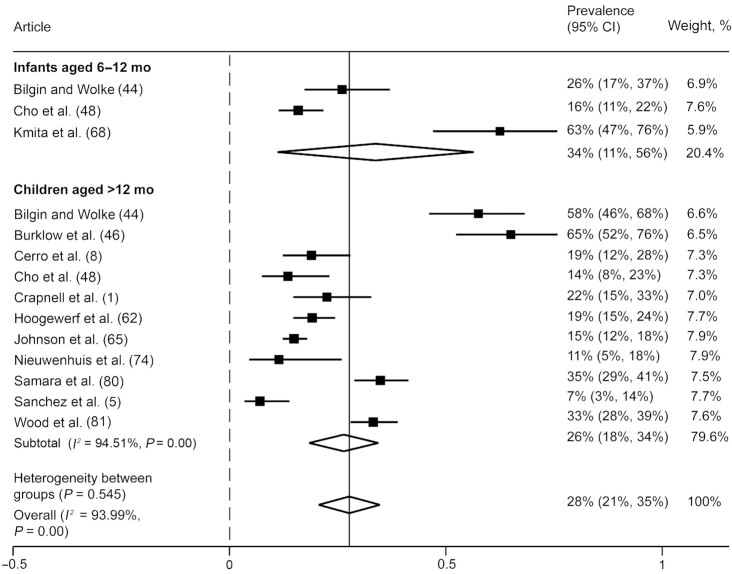
We included 12 articles (11 unique studies) in our analysis that reported on any eating challenge (i.e., studies that did not differentiate between oromotor or eating behavior challenges). Results showed that, overall, 28% (95% CI: 21%, 35%) of those born preterm experienced eating challenges during the early years, although heterogeneity between studies in all groups was high (I2 values >93%) (Figure 6). Subgroup analyses showed that 34% (95% CI: 11%, 56%) of infants and 26% (95% CI: 18%, 34%) of children born preterm experienced some type of eating challenge.
FIGURE 6.
Prevalence of any eating challenge, not specified among infants and children born preterm. Any eating challenge refers to articles that did not differentiate between oromotor eating difficulties or eating behavior challenges. Random-effects meta-analysis of proportions using a maximum likelihood estimator. Results are expressed as prevalence (95% CIs). The weighting for each study is the inverse of the total variance. I2 = the percentage of variation across studies that is due to heterogeneity rather than chance. Values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively.
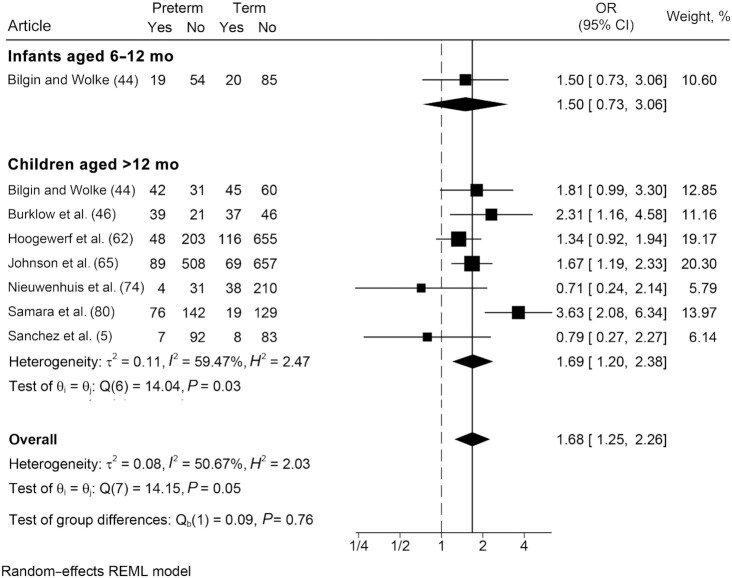
In comparison with term-born peers, those born preterm were nearly 1.7 times more likely (OR: 1.68; 95% CI: 1.25, 2.26; I2 = 50.7%) to exhibit some type of eating challenge (Figure 7). Results were similar in subgroup analyses; however, only 1 article reported on any eating challenges among infants. Two articles presented mean differences, rather than prevalence, of any type of eating challenge experienced by those born preterm and at term. One study spanning both age groups reported that preterm children experienced significantly more eating challenges than those born at term (31). The other study, conducted among children at 18 mo of age, reported no statistically significant differences (32).
FIGURE 7.
Odds of any eating challenge, not specified: comparison of infants and children born preterm and at term. Any eating challenge refers to studies that did not differentiate between oromotor eating difficulties or eating behavior challenges. Random-effects meta-analysis using a REML model. Results are presented as ORs (95% CIs). The weighting for each study is the inverse of the total variance. I2 = the percentage of variation across studies that is due to heterogeneity rather than chance. Values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively. REML, restricted maximum likelihood.
Results suggest that children born preterm commonly experienced difficulties with oromotor eating skills and eating behaviors across the early developmental years, and more frequently than term-born peers. The findings related to behavior lacked consistency.
Food parenting
Nineteen articles (17 unique studies) reported on food parenting. Nine articles were based on parental report of feeding, 7 articles used direct observation, and 3 used a mixed-methods approach. In most articles (n = 13; 68%), “parent” referred to the child's mother. Table 3 provides a narrative summary of findings related to parental concern and emotions during feeding, specific feeding practices, and parent–child interactions during mealtimes. Results suggested mothers of children born preterm had significant concerns about their children's eating, experienced negative emotions, and used coercive feeding practices during mealtimes, which in many cases contextualized negative feeding interactions overall. When compared with term-born mother–child dyads, these food parenting factors seemed to be more frequently reported as more severe among preterm-born mother–child dyads.
TABLE 3.
Summary of food parenting results1
| Food parenting subtheme | Findings for infants and children born preterm2 | Term-born comparison3 |
|---|---|---|
| Parental concern and emotions during feeding: 9 articles, 9 unique studies (5, 8, 23, 48, 49, 52, 59, 70, 86) |
|
|
| Specific feeding practices: 5 articles, 5 unique studies (8, 61, 70, 79, 85) |
|
|
| Parent–child interactions during mealtimes: 10 articles, 8 unique studies (25, 33–36, 61, 76, 77, 85, 86) |
|
|
n = 19 articles, 17 unique studies. CA, corrected age.
Summary of results for all articles reporting on food parenting (articles with and without a term-born comparison group), highlighting findings relevant to preterm infants/children only.
Summary of results for articles reporting on food parenting that included a term-born comparison group, highlighting birth group comparisons (preterm compared with term) only.
Dietary patterns
Twenty-four articles (23 unique studies) explored the dietary patterns of those born preterm. Almost all articles relied on parent report using nonstandardized methods to assess dietary patterns. Table 4 summarizes results related to human milk feeding duration and exclusivity, age at introduction to solids, food group consumption, and overall diet quality. Results suggested that infants and children born preterm were not meeting nutritional recommendations and that their dietary patterns were poorer than for those born at term.
TABLE 4.
Summary of dietary patterns results1
| Dietary pattern subtheme | Findings for infants and children born preterm2 | Term-born comparison3 |
|---|---|---|
| Human milk feeding duration and exclusivity: 10 articles, 10 unique studies (9, 22, 44, 54, 56, 58, 64, 68, 78, 86) |
|
|
| Age at introduction to solids: 11 articles, 11 unique studies (8, 9, 23, 29, 46, 55, 59, 63, 66, 78, 84) |
|
|
| Solid food group consumption and overall diet quality: 7 articles, 7 unique studies (27, 30, 51, 57, 64, 72, 78) |
|
|
n = 24 articles, 23 unique studies. CA, corrected age; NICU, neonatal intensive care unit; VLBW, very low birth weight (<1500 g).
Summary of results for all articles reporting on dietary patterns (articles with and without a term-born comparison group), highlighting findings relevant to preterm infants/children only.
Summary of results for articles reporting on dietary patterns that included a term-born comparison group, highlighting birth group comparisons (preterm compared with term) only.
Risk of bias and certainty of evidence assessment
Risk of bias assessed by the NOS suggested that 0 of 14 articles from case-control studies and 10 of 53 articles from cohort studies had low risk of bias (Supplemental Table 3). Limitations included recruiting only children presenting to a feeding clinic, using nonstandardized or unvalidated assessments for outcomes of interest, not controlling for clinically important covariates, follow-up rates <80% in prospective cohorts, and failing to blind assessors to birth groups in studies with a term-born comparison group.
Our GRADE assessment (38) indicated that both the proportion of infants and children born preterm with oromotor eating difficulties and eating behavior challenges and the odds of children born preterm experiencing such problems compared with term-born peers are based on very-low-quality evidence (Supplemental Table 4). Individual study risk of bias was serious or very serious for all outcomes. Where we could compute I2 values, we found a substantial amount of unexplained statistical heterogeneity, leading to a downgrade in the certainty of evidence. Other concerns included a large proportion of studies using nonstandardized questionnaires to quantify eating challenges, different methodologies for assessing behavioral eating challenges, and individual studies not distinguishing between types of eating challenges (oromotor compared with behavioral).
Discussion
This systematic review and meta-analysis revealed that infants born preterm continue to experience high rates of oromotor eating difficulties and behavioral eating challenges throughout the early developmental years. Although it was not possible to conduct meta-analyses examining food parenting practices or dietary patterns owing to the small number of studies and heterogeneity of outcome assessment, our narrative review of the literature showed that mealtimes with preterm children are characterized by parental anxiety and coercive, negative feeding interactions. Consistent with these findings, we also found the dietary patterns of children born preterm to be poor and often falling short of recommendations. Overall, we found a higher incidence of eating, feeding, and dietary challenges among children born preterm than among those born at term. To our knowledge, this is the first systematic review and meta-analysis to explore the prevalence of oromotor eating difficulties and behavioral eating challenges among children born preterm, as well as the first to compare with term-born peers. Further, this was the first systematic review we know of to consider the context in which children born preterm are fed (food parenting) and associated dietary patterns.
Our meta-analysis found that children born preterm experienced high rates of oromotor eating difficulties during late infancy and throughout childhood (34%) and had 2.86 times the odds (95% CI: 1.71, 4.77) of experiencing such difficulties compared with term-born peers, although the quality of evidence was found to be very low. The American Academy of Pediatrics’ (AAP's) Textbook of Pediatric Care suggests that those with cardiorespiratory or chronic lung disease, neurologic impairments, repeated intubations, extended tube feeding, and severe reflux are at highest risk of feeding problems (87). This guidance is general, however, and does not focus on types of eating challenges. Our research has real-world implications for NICU follow-up care, in that it highlights the prevalence of oromotor eating difficulties in children born preterm. Our findings lay the groundwork for future research to determine what should be screened at critical points in time, including coordination of the suck-swallow-breathe pattern, lip, and jaw movements; coughing while eating purees; difficulties self-feeding (e.g., coordinating utensils with the lips and mouth); and coughing or gagging. Our findings also revealed that the prevalence of oromotor eating difficulties likely drops as children age, suggesting that all children learn to eat. Those born preterm appear to take longer to master the skills necessary for successful oral eating, which would require more support to earlier close this gap between them and peers and to ensure difficulties do not persist. This information has bearing on clinician education practices, because it emphasizes specific areas for surveillance and oral sensorimotor intervention in this population. Future research utilizing standardized assessments will be beneficial in identifying those at highest risk as well as the specific eating challenges experienced.
Our meta-analysis found that 18% of those born preterm experienced eating behavior challenges such as food refusal or tantrums during mealtimes during the developmental years and children born preterm had 1.5 times higher odds (OR: 1.52; 95% CI: 1.11, 2.10) of such difficulties than term-born peers, although the quality of the evidence was very low. This highlights the need to specifically consider the impact of birth gestation on the development of eating habits among children and warrants future exploration into strategies to both prevent and reduce challenging mealtime behaviors given the unique challenges this vulnerable population may experience. In the only other meta-analysis known to explore eating behaviors among preterm children, Pados et al. (6) estimated the prevalence of “problematic feeding” to be 42% among preterm children <4 y of age; a comparison with term-born peers was not conducted. Notably, Pados et al. (6) did not differentiate between eating challenges (oromotor compared with behavioral). By contrast, our results looking at “any eating challenge” found 28% of children born preterm experienced some type of oromotor eating difficulty or behavioral eating challenge. Reasons for these differences in estimates are likely attributable to definition differences and methods, because our review used a more exhaustive literature review. Consistently defining eating challenges and differentiating between concerns will be an important step in advancing both research and clinical practice (88). Importantly, most studies included in our review were based on parental report of child eating behaviors. Parent report is susceptible to bias, and many of the behaviors parents consider challenging are developmentally appropriate (e.g., initial food refusal as children discern preferences for flavor and textures) (7, 89). Future research should utilize observational methods to elucidate the extent and severity of challenging eating behaviors among children born preterm.
We found that mothers of preterm infants expressed more concern, experienced heightened anxiety, and presented with more coercive and negative interactions while feeding compared with term mother–child dyads. Considering the likely stress experienced when giving birth to a child preterm, there is significant potential for sustained impact on parental mental health and parent–child attachment, and an overall negative outlook on a child's health and developmental outcomes in the parent (60). Indeed, from a bidirectional perspective, early child oromotor eating difficulty and lower infant engagement likely reinforce stress and negative feelings about feeding. Heightened negative parental expectations may then influence specific feeding practices (e.g., pressure to eat due to concerns about weight) and, in turn, influence child outcomes, perpetuating the cycle. Our narrative review also showed that parental concerns (e.g., choking) may delay introduction to solids and texture progression, inadvertently contributing to further oromotor difficulties (49, 63). However, positive parent–child interactions may be protective against eating challenges (28). Future research and intervention initiatives should focus on positive parent–child relationships as children learn to eat and as parents learn to feed to help children born preterm meet oromotor milestones and reduce challenging mealtime interactions. Although the AAP's Textbook of Pediatric Care highlights including evaluations of parent–child interaction as part of ongoing surveillance for preterm infants, no guidance for supporting families is provided, nor is the association between feeding outcomes and nutrition discussed (87). Further research is crucial for informing and updating these guidelines to educate clinicians and in turn, to support these children and their families. Updates may include information on oromotor eating milestones and best practices for child feeding, as well as anticipatory feeding guidance for families. Importantly, only 1 study in our review examined parent–child interactions among children older than 12 mo of age. Given that we found the prevalence of challenging eating behaviors to be high in this age group, future research should explore parent–child mealtime interactions of children, and not just infants, born preterm.
Our narrative review suggested that the dietary patterns of children born preterm were poor from late infancy throughout early childhood; this finding is not surprising given the high prevalence of both oromotor eating difficulties and eating behavior challenges found. Low human milk feeding initiation, duration, and exclusivity are multifactorial but are likely due in part to early oromotor eating difficulties, parental frustration, and anxiety over child growth and development. Similarly, early introduction to solid foods may be due to parental concern over child growth. Findings from Francis et al. (9) highlight low adherence to infant feeding recommendations overall and underscore the importance of including nutrition as a focus in neonatal follow-up. Few studies have explored the dietary patterns of children born preterm. Still, our results showed inadequate dietary patterns present in infants born preterm persist throughout childhood and may be worse than those of term-born peers. Although not included in this review owing to our age restriction, limited research suggests that inadequate dietary intake among children born preterm tracks into young adulthood (90, 91). Infants born preterm are at high risk of metabolic disturbances and chronic disease in adulthood (12). Thus, providing parents of preterm infants with anticipatory guidance and support to promote healthy dietary patterns is essential, focusing on improving children's eating and parents’ feeding.
Limitations
Despite many strengths, this systematic review has limitations to consider. First, most individual articles (84%) were deemed to have a high risk of bias and the certainty of evidence for our meta-analysis of oromotor eating difficulties and eating behavior challenges was deemed very low. Second, several articles did not differentiate between oromotor or eating behavior challenges. It is imperative that future research clearly and consistently defines eating difficulties. A consensus statement was recently published presenting a comprehensive definition of pediatric feeding disorders (88). Like our study, this definition explores feeding skills, psychosocial factors (including parent–child interactions), and nutritional factors separately. Consistent language will help identify those most at risk and create tailored interventions to support the healthy development of eating habits among the vulnerable preterm population. Third, owing to the limited number of and heterogeneity among studies, we narratively reviewed the literature on food parenting and dietary patterns. Fourth, most articles utilized maternal report, which is susceptible to bias. Focusing solely on the maternal perspective is not reflective of a typical home environment because mothers are not the only caregivers engaging in child feeding. Finally, it is also possible that differences found between those born preterm and their term-born peers may be attributed to sociodemographic disparities. At an individual article level, however, many matched their birth groups on sociodemographic characteristics, used random sampling to recruit term-born comparisons, or used similar inclusion and exclusion criteria to assess eligibility for each group. Future research should use rigorous recruitment and matching methods to enroll term-born comparison groups.
Conclusions
Our results demonstrate higher rates of oromotor difficulties and behavioral eating challenges during late infancy and early childhood among children born preterm than among their term-born peers. However, the certainty of this evidence is very low. Based on narrative review, children born preterm seemed to experience more coercive and negative feeding interactions with caregivers, and these interactions are associated with high rates of maternal anxiety. We also narratively observed poorer dietary patterns among preterm children including low human milk feeding duration and exclusivity, early introduction to solids, and poor diet quality. Poor dietary patterns likely contribute to the heightened risk of presenting with chronic disease in adulthood, thus following the nutrition outcomes of this population post–NICU discharge throughout the developmental years is essential. Research examining the role of caregiver–child interactions in eating, feeding, and nutrition/dietary outcomes in this vulnerable population is needed.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—KW, AID, and QM: conducted the research; KW and AID: analyzed the data; KW: wrote the paper; DLO: has primary responsibility for the final content; and all authors: conceptualized the study, helped interpret findings, and read and approved the final manuscript.
Notes
Supported by a Canadian Institutes of Health Research (CIHR) Postdoctoral Fellowship (to KW) and a CIHR Doctoral Research Award (to AID). The sources of support had no role in the conception or design of this review and were not involved in the conduct of the review, statistical analyses, data interpretation, or writing of the manuscript.
Author disclosures: The authors report no conflicts of interest.
Supplemental Methods and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
SLU and DLO contributed equally to this work as senior authors.
Abbreviations used: AAP, American Academy of Pediatrics; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; NICU, neonatal intensive care unit; NOS, Newcastle-Ottawa Scale; PICOS, Participants, Intervention (or Exposure), Comparator, Outcomes, Study Design; VLBW, very low birth weight.
Contributor Information
Kathryn Walton, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada.
Allison I Daniel, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada; Centre for Global Child Health, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada.
Quenby Mahood, Hospital Library & Archives, Learning Institute, The Hospital for Sick Children, Toronto, Ontario, Canada.
Simone Vaz, Department of Pediatrics, William Osler Health System, Brampton, Ontario, Canada.
Nicole Law, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada.
Sharon L Unger, Department of Paediatrics, University of Toronto, Toronto, Ontario, Canada; Paediatrics, Sinai Health, Toronto, Ontario, Canada; Division of Neonatology, The Hospital for Sick Children, Toronto, Ontario, Canada.
Deborah L O'Connor, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Paediatrics, Sinai Health, Toronto, Ontario, Canada.
Data Availability
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Crapnell TL, Rogers CE, Neil JJ, Inder TE, Woodward LJ, Pineda RG. Factors associated with feeding difficulties in the very preterm infant. Acta Paediatr. 2013;102(12):e539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ross ES, Browne JV. Feeding outcomes in preterm infants after discharge from the neonatal intensive care unit (NICU): a systematic review. Newborn Infant Nurs Rev. 2013;13(2):87–93. [Google Scholar]
- 3. Browne JV, Ross ES. Eating as a neurodevelopmental process for high-risk newborns. Clin Perinatol. 2011;38(4):731–43. [DOI] [PubMed] [Google Scholar]
- 4. Silberstein D, Geva R, Feldman R, Gardner JM, Karmel BZ, Rozen H, Kuint J. The transition to oral feeding in low-risk premature infants: relation to infant neurobehavioral functioning and mother–infant feeding interaction. Early Hum Dev. 2009;85(3):157–62. [DOI] [PubMed] [Google Scholar]
- 5. Sanchez K, Boyce JO, Morgan AT, Spittle AJ. Feeding behavior in three-year-old children born <30 weeks and term-born peers. Appetite. 2018;130:117–22. [DOI] [PubMed] [Google Scholar]
- 6. Pados BF, Hill RR, Yamasaki JT, Litt JS, Lee CS. Prevalence of problematic feeding in young children born prematurely: a meta-analysis. BMC Pediatr. 2021;21(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walton K, Kuczynski L, Haycraft E, Breen A, Haines J. Time to re-think picky eating?: a relational approach to understanding picky eating. Int J Behav Nutr Phys Act. 2017;14(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cerro N, Zeunert S, Simmer KN, Daniels LA. Eating behaviour of children 1.5–3.5 years born preterm: parents’ perceptions. J Paediatr Child Health. 2002;38(1):72–8. [DOI] [PubMed] [Google Scholar]
- 9. Francis J, Unger S, Bando N, Vance A, Gibbins S, Kiss A, Church P, Sellen D, O'Connor DL, GTA-DoMINO Feeding Group . Postdischarge feeding of very-low-birth-weight infants: adherence to nutrition guidelines. J Pediatr Gastroenterol Nutr. 2018;67(3):401–8. [DOI] [PubMed] [Google Scholar]
- 10. Lapillonne A, O'Connor DL, Wang D, Rigo J. Nutritional recommendations for the late-preterm infant and the preterm infant after hospital discharge. J Pediatr. 2013;162(3):S90–S100. [DOI] [PubMed] [Google Scholar]
- 11. Kerzner B. Clinical investigation of feeding difficulties in young children: a practical approach. Clin Pediatr (Phila). 2009;48(9):960–5. [DOI] [PubMed] [Google Scholar]
- 12. Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. J Pediatr. 2019;210:69–80.e5. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 14. Bonato S. Searching the grey literature: a handbook for searching reports, working papers, and other unpublished research. Lanham (MD): Rowman & Littlefield, Medical Library Association; 2018. [Google Scholar]
- 15. Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42(7):963–70. [DOI] [PubMed] [Google Scholar]
- 16. Vaughn AE, Ward DS, Fisher JO, Faith MS, Hughes SO, Kremers SP, Musher-Eizenman DR, O'Connor TM, Patrick H, Power TG. Fundamental constructs in food parenting practices: a content map to guide future research. Nutr Rev. 2016;74(2):98–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musher-Eizenman D, Holub S. Comprehensive Feeding Practices Questionnaire: validation of a new measure of parental feeding practices. J Pediatr Psychol. 2007;32(8):960–72. [DOI] [PubMed] [Google Scholar]
- 18. USDA and US Department of Health and Human Services (DHHS) . Dietary Guidelines for Americans, 2020–2025. 9th ed. Washington (DC): USDA and US DHHS; 2020. [Google Scholar]
- 19. Veritas Health Innovation . Covidence Software Platform. [Internet]. Melbourne (Australia): Veritas Health Innovation; 2018; [version as of 16 September, 2021]. [Accessed 2020 Apr 22]. Available from: http://covidence.org. [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Menezes LVP, Steinberg C, Nóbrega AC. Complementary feeding in infants born prematurely. Codas. 2018;30(6):e20170157. [DOI] [PubMed] [Google Scholar]
- 23. Steinberg C, Menezes L, Nóbrega AC. Oral motor disorder and feeding difficulty during the introduction of complementary feeding in preterm infants. Codas. 2021;33(1):e20190070. [DOI] [PubMed] [Google Scholar]
- 24. Zimmerman E, Rosner A. Feeding swallowing difficulties in the first three years of life: a preterm and full-term infant comparison. J Neonatal Nurs. 2018;24(6):331–5. [Google Scholar]
- 25. Barnard KE, Bee HL, Hammond MA. Developmental changes in maternal interactions with term and preterm infants. Infant Behav Dev. 1984;7(1):101–13. [Google Scholar]
- 26. Dodrill P, McMahon S, Ward E, Weir K, Donovan T, Riddle B. Long-term oral sensitivity and feeding skills of low-risk pre-term infants. Early Hum Dev. 2004;76(1):23–37. [DOI] [PubMed] [Google Scholar]
- 27. Duran S, Duran R, Acunaş B, Cesur G, Çiftdemir NA. Eating behaviors of late and moderately preterm infants at two years of age and their associations with mothers’ mental health. J Pediatr Gastroenterol Nutr. 2021;72(2):311–5. [DOI] [PubMed] [Google Scholar]
- 28. Forcada-Guex M, Pierrehumbert B, Borghini A, Moessinger A, Muller-Nix C. Early dyadic patterns of mother–infant interactions and outcomes of prematurity at 18 months. Pediatrics. 2006;118(1):e107–14. [DOI] [PubMed] [Google Scholar]
- 29. Howe T-H, Sheu C-F, Wang T-N. Feeding patterns and parental perceptions of feeding issues of preterm infants in the first 2 years of life. Am J Occup Ther. 2019;73(2):7302205030p1–10. [DOI] [PubMed] [Google Scholar]
- 30. Migraine A, Nicklaus S, Parnet P, Lange C, Monnery-Patris S, Des Robert C, Darmaun D, Flamant C, Amarger V, Rozé J-C. Effect of preterm birth and birth weight on eating behavior at 2 y of age. Am J Clin Nutr. 2013;97(6):1270–7. [DOI] [PubMed] [Google Scholar]
- 31. Park J, Thoyre SM, Pados BF, Gregas M. Symptoms of feeding problems in preterm-born children at 6 months to 7 years old. J Pediatr Gastroenterol Nutr. 2019;68(3):416–21. [DOI] [PubMed] [Google Scholar]
- 32. Pierrehumbert B, Nicole A, Muller-Nix C, Forcada-Guex M, Ansermet F. Parental post-traumatic reactions after premature birth: implications for sleeping and eating problems in the infant. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F400–F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pridham KF, Brown R, Clark R, Sondel S, Green C. Infant and caregiving factors affecting weight-for-age and motor development of full-term and premature infants at 1 year post-term. Res Nurs Health. 2002;25(5):394–410. [DOI] [PubMed] [Google Scholar]
- 34. Salvatori P, Andrei F, Neri E, Chirico I, Trombini E. Pattern of mother–child feeding interactions in preterm and term dyads at 18 and 24 months. Front Psychol. 2015;6:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singer LT, Fulton S, Davillier M, Koshy D, Salvator A, Baley JE. Effects of infant risk status and maternal psychological distress on maternal-infant interactions during the first year of life. J Dev Behav Pediatr. 2003;24(4):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weber AM, Harrison TM. Maternal behavior and infant physiology during feeding in premature and term infants over the first year of life. Res Nurs Health. 2014;37(6):478–89. [DOI] [PubMed] [Google Scholar]
- 37. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet]. Ottawa (Canada): Ottawa Hospital Research Institute; [accessed 2020 Feb 21]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 38. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abily-Donval L, Pinto-Cardoso G, Chadie A, Guerrot A-M, Torre S, Rondeau S, Marret S. Comparison in outcomes at two-years of age of very preterm infants born in 2000, 2005 and 2010. PLoS One. 2015;10(2):e0114567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adams-Chapman I, Bann CM, Vaucher YE, Stoll BJ. Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development-Third Editio n. J Pediatr. 2013;163(3):680–5.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adams-Chapman I, Bann C, Carter SL, Stoll BJ. Language outcomes among ELBW infants in early childhood. Early Hum Dev. 2015;91(6):373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amarger V, Bouvagnet A, Moyon T, Vaiman D, Darmaun D, de Lauzon-Guillain B, Robitaille J, Flamant C, Rozé J-C, Parnet P. A common genetic variant in the insulin receptor gene is associated with eating difficulties at 2 years of age in a cohort of preterm infants. J Nutrigenet Nutrigenomics. 2015;8(4–6):153–63. [DOI] [PubMed] [Google Scholar]
- 43. Anderson SE, McNamara K, Andridge R, Keim SA. Executive function and mealtime behavior among preschool-aged children born very preterm. Eat Behav. 2015;19:110–4. [DOI] [PubMed] [Google Scholar]
- 44. Bilgin A, Wolke D. Regulatory problems in very preterm and full-term infants over the first 18 months. J Dev Behav Pediatr. 2016;37(4):298–305. [DOI] [PubMed] [Google Scholar]
- 45. Bilgin A, Wolke D. Associations between feeding problems and maternal sensitivity across infancy: differences in very preterm and full-term infants. J Dev Behav Pediatr. 2017;38(7):538–44. [DOI] [PubMed] [Google Scholar]
- 46. Burklow KA, McGrath AM, Valerius KS, Rudolph C. Relationship between feeding difficulties, medical complexity, and gestational age. Nutr Clin Pract. 2002;17(6):373–8. [DOI] [PubMed] [Google Scholar]
- 47. Buswell CA, Leslie P, Embleton ND, Drinnan MJ. Oral-motor dysfunction at 10 months corrected gestational age in infants born less than 37 weeks preterm. Dysphagia. 2009;24(1):20–5. [DOI] [PubMed] [Google Scholar]
- 48. Cho J-Y, Lee J, Youn YA, Kim SJ, Kim SY, Sung IK. Parental concerns about their premature infants’ health after discharge from the neonatal intensive care unit: a questionnaire survey for anticipated guidance in a neonatal follow-up clinic. Korean J Pediatr. 2012;55(8):272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chung J, Lee J, Spinazzola R, Rosen L, Milanaik R. Parental perception of premature infant growth and feeding behaviors: use of gestation-adjusted age and assessing for developmental readiness during solid food introduction. Clin Pediatr (Phila). 2014;53(13):1271–7. [DOI] [PubMed] [Google Scholar]
- 50. Crapnell TL, Woodward LJ, Rogers CE, Inder TE, Pineda RG. Neurodevelopmental profile, growth, and psychosocial environment of preterm infants with difficult feeding behavior at age 2 years. J Pediatr. 2015;167(6):1347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davenport ES, Litenas C, Barbayiannis P, Williams CES. The effects of diet, breast-feeding and weaning on caries risk for pre-term and low birth weight children. Int J Paediatr Dent. 2004;14(4):251–9. [DOI] [PubMed] [Google Scholar]
- 52. DeMauro SB, Patel PR, Medoff-Cooper B, Posencheg M, Abbasi S. Postdischarge feeding patterns in early- and late-preterm infants. Clin Pediatr (Phila). 2011;50(10):957–62. [DOI] [PubMed] [Google Scholar]
- 53. den Boer SL, Schipper JA. Feeding and drinking skills in preterm and low birth weight infants compared to full term infants at a corrected age of nine months. Early Hum Dev. 2013;89(6):445–7. [DOI] [PubMed] [Google Scholar]
- 54. Ericson J, Eriksson M, Hoddinott P, Hellström-Westas L, Flacking R. Breastfeeding and risk for ceasing in mothers of preterm infants—long-term follow-up. Matern Child Nutr. 2018;14(4):e12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ernst JA, Bull MJ, Rickard KA, Brady MS, Lemons JA. Growth outcome and feeding practices of the very low birth weight infants (less than 1500 grams) within the first year of life. J Pediatr. 1990;117(2):S156–66. [DOI] [PubMed] [Google Scholar]
- 56. Flacking R, Nyqvist KH, Ewald U. Effects of socioeconomic status on breastfeeding duration in mothers of preterm and term infants. Eur J Public Health. 2007;17(6):579–84. [DOI] [PubMed] [Google Scholar]
- 57. McGee M, Unger S, Hamilton J, Birken CS, Pausova Z, Kiss A, Bando N, O'Connor DL. Associations between diet quality and body composition in young children born with very low body weight. J Nutr. 2020;150(11):2961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gibson RS, DeWolfe MS. The food consumption patterns and nutrient intakes of some Canadian low birth-weight infants during the first twelve months of infancy. Can J Public Health. 1981;72(4):273–81. [PubMed] [Google Scholar]
- 59. Hawdon JM, Beauregard N, Slattery J, Kennedy G. Identification of neonates at risk of developing feeding problems in infancy. Dev Med Child Neurol. 2000;42(4):235–9. [DOI] [PubMed] [Google Scholar]
- 60. Hill AS. Mothers’ perceptions of child vulnerability in previous preterm infants. ABNF J. 2015;26(1):11–16. [PubMed] [Google Scholar]
- 61. Holditch-Davis D, Miles MS, Belyea M. Feeding and non-feeding interactions of mothers and prematures. West J Nurs Res. 2000;22(3):320–34. [DOI] [PubMed] [Google Scholar]
- 62. Hoogewerf M, ter Horst HJ, Groen H, Nieuwenhuis T, Bos AF, van Dijk MWG. The prevalence of feeding problems in children formerly treated in a neonatal intensive care unit. J Perinatol. 2017;37(5):578–84. [DOI] [PubMed] [Google Scholar]
- 63. Hubl N, Costa SPD, Kaufmann N, Oh J, Willmes K. Sucking patterns are not predictive of further feeding development in healthy preterm infants. Infant Behav Dev. 2020;58:101412. [DOI] [PubMed] [Google Scholar]
- 64. Husk JS, Keim SA. Breastfeeding and dietary variety among preterm children aged 1–3 years. Appetite. 2016;99:130–7. [DOI] [PubMed] [Google Scholar]
- 65. Johnson S, Matthews R, Draper ES, Field DJ, Manktelow BN, Marlow N, Smith LK, Boyle EM. Eating difficulties in children born late and moderately preterm at 2 y of age: a prospective population-based cohort study. Am J Clin Nutr. 2016;103(2):406–14. [DOI] [PubMed] [Google Scholar]
- 66. Jonsson M, van Doorn J, van den Berg J. Parents’ perceptions of eating skills of pre-term vs full-term infants from birth to 3 years. Int J Speech Lang Pathol. 2013;15(6):604–12. [DOI] [PubMed] [Google Scholar]
- 67. Kirk CM, Uwamungu JC, Wilson K, Hedt-Gauthier BL, Tapela N, Niyigena P, Rusangwa C, Nyishime M, Nahimana E, Nkikabahizi Fet al. Health, nutrition, and development of children born preterm and low birth weight in rural Rwanda: a cross-sectional study. BMC Pediatr. 2017;17(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kmita G, Urmańska W, Kiepura E, Polak K. Feeding behaivour problems in infants born preterm: a psychological perspective. Preliminary report. Med Wieku Rozwoj. 2011;15(3):216–23. [PubMed] [Google Scholar]
- 69. Litt JS, Ho T, Obregon E, Patel P, Ziyeh T, McCormick MC. Characterizing early state regulation in preterm infants. J Dev Behav Pediatr. 2019;40(4):293–300. [DOI] [PubMed] [Google Scholar]
- 70. Mathisen B, Worrall L, O'Callaghan M, Wall C, Shepherd RW. Feeding problems and dysphagia in six-month-old extremely low birth weight infants. Adv Speech Lang Pathol. 2000;2(1):9–17. [Google Scholar]
- 71. McComish CS. The relationship of mealtime communication and oral-motor feeding skills to later language skills in premature infants. Diss Abstr Int B Sci Eng. 2008;69(4-B):2288. [Google Scholar]
- 72. Milton J, King C. Cup introduction, drink type and vitamin supplementation in preterm babies at 11–25 months. J Hum Nutr Diet. 2012;25(2):148–54. [DOI] [PubMed] [Google Scholar]
- 73. Mokhlesin M, Mirmohammadkhani M, Nooripour S, Rashidan S, Ahmadizadeh Z. Feeding problems score and its related factors in two-year-old children born very-preterm and full-term. Iran J Nurs Midwifery Res. 2019;24(4):256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nieuwenhuis T, Verhagen EA, Bos AF, van Dijk MW. Children born preterm and full term have similar rates of feeding problems at three years of age. Acta Paediatr. 2016;105(10):e452–7. [DOI] [PubMed] [Google Scholar]
- 75. Philip AK, Vijay Kumar KV. Comparison of feeding behaviours in term infants and preterm infants (30 to 34 weeks) at six months corrected age. J Nepal Paediatr Soc. 2016;35(2):202–5. [Google Scholar]
- 76. Pridham K, Steward D, Thoyre S, Brown R, Brown L. Feeding skill performance in premature infants during the first year. Early Hum Dev. 2007;83(5):293–305. [DOI] [PubMed] [Google Scholar]
- 77. Pridham K, Melby JN, Brown R, Clark R. The contribution of infant, maternal, and family conditions to maternal feeding competencies. Parenting. 2010;10(1):18–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ribas SA, de Rodrigues MCC, Mocellin MC, Marques ES, da Rosa GPC, Maganha CR. Quality of complementary feeding and its effect on nutritional status in preterm infants: a cross-sectional study. J Hum Nutr Diet. 2021;34(1):3–12. [DOI] [PubMed] [Google Scholar]
- 79. Saleska JL, Sheppard K, Turner AN, Boone KM, Keim SA. Parental perceptions and behaviors regarding child weight status among toddlers born preterm. Am J Perinatol. 2020;37(5):525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Samara M, Johnson S, Lamberts K, Marlow N, Wolke D. Eating problems at age 6 years in a whole population sample of extremely preterm children. Dev Med Child Neurol. 2010;52(2):e16–22. [DOI] [PubMed] [Google Scholar]
- 81. Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR, EPICure Study Group . The EPICure study: growth and associated problems in children born at 25 weeks of gestational age or less. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F492–F500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sanchez K, Spittle AJ, Slattery JM, Morgan AT. Oromotor feeding in children born before 30 weeks’ gestation and term-born peers at 12 months’ corrected age. J Pediatr. 2016;178:113–18.e1. [DOI] [PubMed] [Google Scholar]
- 83. Sanchez K, Morgan AT, Slattery JM, Olsen JE, Lee KJ, Anderson PJ, Thompson DK, Doyle LW, Cheong JLY, Spittle AJ. Neuropredictors of oromotor feeding impairment in 12 month-old children. Early Hum Dev. 2017;111:49–55. [DOI] [PubMed] [Google Scholar]
- 84. Sauve RS, Geggie JH. Growth and dietary status of preterm and term infants during the first two years of life. Can J Public Health. 1991;82(2):95–100. [PubMed] [Google Scholar]
- 85. Silberstein D, Feldman R, Gardner JM, Karmel BZ, Kuint J, Geva R. The mother–infant feeding relationship across the first year and the development of feeding difficulties in low-risk premature infants. Infancy. 2009;14(5):501–25. [DOI] [PubMed] [Google Scholar]
- 86. Yatziv T, Gueron-Sela N, Meiri G, Marks K, Atzaba-Poria N. Prematurity and maladaptive mealtime dynamics: the roles of maternal emotional distress, eating-related cognitions, and mind-mindedness. J Abnorm Child Psychol. 2020;48(8):1089–103. [DOI] [PubMed] [Google Scholar]
- 87. Bernbaum JC. Follow-up care of the graduate from neonatal intensive care. In: McInerny TK, Adam HM, Campbell DE, DeWitt TG, Foy JM, Kamat DM, Baum R, Kelleher KJ, editors. American Academy of Pediatrics textbook of pediatric care. Itasca (IL): American Academy of Pediatrics; 2016. 1068–84. [Google Scholar]
- 88. Goday PS, Huh SY, Silverman A, Lukens CT, Dodrill P, Cohen SS, Delaney AL, Feuling MB, Noel RJ, Gisel Eet al. Pediatric feeding disorder: consensus definition and conceptual framework. J Pediatr Gastroenterol Nutr. 2019;68(1):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Birch L, Marlin DW. I don't like it; I never tried it: effects of exposure on two-year-old children's food preferences. Appetite. 1982;3(4):353–60. [DOI] [PubMed] [Google Scholar]
- 90. Kaseva N, Wehkalampi K, Hemiö K, Hovi P, Järvenpää A-L, Andersson S, Eriksson JG, Lindström J, Kajantie E. Diet and nutrient intake in young adults born preterm at very low birth weight. J Pediatr. 2013;163(1):43–8. [DOI] [PubMed] [Google Scholar]
- 91. Matinolli H-M, Hovi P, Levälahti E, Kaseva N, Silveira PP, Hemiö K, Järvenpää A-L, Eriksson JG, Andersson S, Lindström Jet al. Neonatal nutrition predicts energy balance in young adults born preterm at very low birth weight. Nutrients. 2017;9(12):1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.