Abstract
Cuscuta campestris is an obligate parasitic plant that requires a host to complete its life cycle. Parasite–host connections occur via a haustorium, a unique organ that acts as a bridge for the uptake of water, nutrients, and macromolecules. Research on Cuscuta is often complicated by host influences, but comparable systems for growing the parasite in the absence of a host do not exist. We developed an axenic method to grow C. campestris on an artificial host system (AHS). We evaluated the effects of nutrients and phytohormones on parasite haustoria development and growth. Haustorium morphology and gene expression were also characterized. The AHS consists of an inert, fibrous stick that mimics a host stem, wicking water and nutrients to the parasite. It enables C. campestris to exhibit a parasitic habit and develop through all stages of its life cycle, including production of new shoots and viable seeds. The phytohormones 1-naphthaleneacetic acid and 6-benzylaminopurine affect haustoria morphology and increase parasite fresh weight and biomass. Unigene expression in AHS haustoria reflects processes similar to those in haustoria on living host plants. The AHS is a methodological improvement for studying Cuscuta biology by avoiding specific host effects on the parasite and giving researchers full control of the parasite environment.
An artificial host system supports growth of an obligate parasitic plant, establishing the minimum requirements for parasitism and challenging the concepts of host specificity and dependence.
Introduction
Dodders are parasitic plants of the genus Cuscuta (Convolvulaceae) that can invade a wide range of dicot plants. Cuscuta spp. are characterized by their vining growth habit and lack of roots and expanded leaves. They use an organ unique to parasitic plants, the haustorium, to invade host stems to gain access to essential nutrients and water resources. Cuscuta spp. exhibit compromised or no photosynthetic activity (Van Der Kooij et al., 2000), so they rely at various levels on their hosts as their only carbon source. Thus, their survival depends on parasitic features like the ability to find a host, produce functional haustoria and efficiently take up resources.
Cuscuta spp. are obligate parasites, so must have a host to complete their life cycles (Westwood et al., 2010). The Cuscuta spp. life cycle comprises the stages seed, seedling, host colonization, haustoria development, vegetative growth, flower production, and fruit and seed set. Cuscuta seeds germinate on the soil without any chemical stimulant (Benvenuti et al., 2005), and the thread-like seedlings must find and parasitize a suitable plant host to avoid death due to exhausting their limited resources (Shimizu and Aoki, 2019). After the parasite has found a host, its shoot coils around the host stem, and the haustorium starts to develop from within the internal face of the parasite stem. Coiling and haustorium formation respond positively to exposure to enriched far-red light (700–800 nm) and tactile stimuli (Lane and Kasperbauer, 1965; Tada et al., 1996). These stimuli provoke an increase in internal levels of the phytohormone cytokinin, leading to haustoria induction (Haidar et al., 1998; Furuhashi et al., 2011).
Haustorium development involves tissue dedifferentiation and redifferentiation, and can be divided into three phases: adhesive, intrusive, and conductive (Heide-Jørgensen, 2008). In the adhesive phase, epidermal cells redifferentiate into trichome-like cells that form a holdfast or adhesive disc (Vaughn, 2002; Shimizu and Aoki, 2019). Holdfast cells secrete pectin and other compounds that mediate the adhesion of parasite to host tissues. Meanwhile, meristematic cells in the inner cortex behind the holdfast generate the prehaustorium (Shimizu and Aoki, 2019).
During the intrusive phase, the prehaustorium becomes the haustorium (Heide-Jørgensen, 2008). The haustorium, formed by intrusive cells, elongates through the center of the holdfast and penetrates the host stem epidermis and cortex (Vaughn, 2003). Then terminal digitate cells, later termed searching hyphae, elongate to encounter host vascular elements. Initial haustoria formation is independent of host factors, however, such factors may be required for further differentiation of xylem vessels (Kaga et al., 2020). Searching hyphae cells adjacent to host xylem elements become xylem hyphae, while those adjacent to sieve tubes become absorbing hyphae. Subsequently, cortex cells in the haustorium also differentiate into phloem or xylem elements as appropriate (Vaughn, 2006; Shimizu et al., 2018; Shimizu and Aoki, 2018).
The conductive phase starts after searching hyphae differentiate into vascular conductive elements in the mature haustoria, completing the vascular connection between the host and the parasite (Heide-Jørgensen, 2008). At this point, the parasite becomes a strong sink competing with the host for resources as exemplified by C. reflexa, which obtains 99% of its carbon from the host (Jeschke et al., 1994). Once the connections are established, the Cuscuta shoot resumes growth to produce new shoots that reach other host stems, makes additional points of connection, and ultimately produces flowers, fruits, and seeds.
Recent studies have described interesting features of the host–Cuscuta system including the reciprocal exchange of large amounts of macromolecules such as proteins (Liu et al., 2020), messenger RNAs (mRNAs) (Kim et al., 2014), and microRNAs (Shahid et al., 2018). Some of the exchanged microRNAs have been shown to function by affecting gene expression in the host plant (Shahid et al., 2018). There is an urgent need to develop new methods to study Cuscuta in ways that can distinguish parasite processes from host processes in order to define the contributions of each to the interaction.
One approach to studying Cuscuta in the absence of a host is through tissue culture. Published methodologies exist for obtaining callus and shoot regeneration for Cuscuta trifolii (Bakos et al., 1995; Borsics et al., 2002), Cuscuta europea (Švubová and Blehová, 2013), and Cuscuta reflexa (Srivastava and Dwivedi, 2001), and floral induction for C. reflexa (Baldev, 1962). Other studies reported the production of haustoria by applying mechanical pressure to shoots of Cuscuta spp. held between two surfaces, and supplemented with a far-red light stimulus (Tada et al., 1996; Furuhashi et al., 1997; Kaga et al., 2020; Lachner et al., 2020). These systems have certain advantages, but tissue culture does not allow investigations related to haustorial form or function, and none of them accurately reflect normal Cuscuta parasitic growth habit with production of new shoots and viable seeds.
Here we report the development of an axenic system to grow Cuscuta campestris on an artificial host (AH) that provides water, nutrients, and phytohormones to the parasite. We demonstrate that C. campestris growing in this AH system (AHS) exhibits all the stages observed during its typical lifecycle, including flower, fruit, and production of viable seeds. Functional characterization and transcriptomic analyses of the haustorial region of C. campestris growing in the AHS suggest that growth is similar to that on a living host.
Results
An AHS for growing C. campestris under axenic conditions
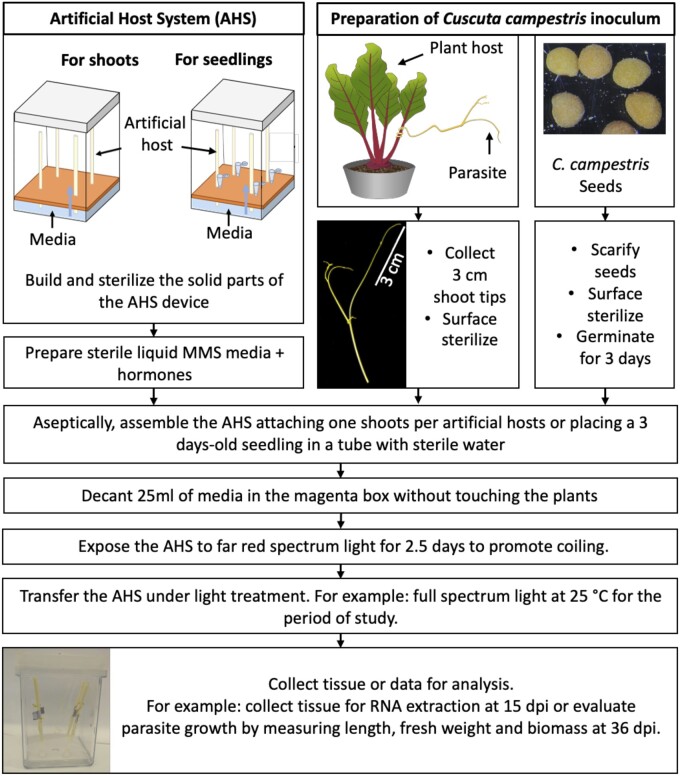
We developed a system that provides the essential components for C. campestris growth in a way that mimics growth on a plant host. The main elements of the AHS are a solid vertical support with capillary capacity, liquid media with proper nutrients and phytohormones, and a translucent container to hold these together under aseptic conditions (Figure 1; Supplemental Figure S1). We evaluated several materials for use as AHs, and found the best to be the paper spindle from a cotton swab, which has a firm structure, yet sufficient porosity to allow the movement of liquid media by capillary action. This vertical AH “stem” provides a tactile stimulus that, when combined with far-red enriched light, induces the parasite to coil and form haustoria (Tada et al., 1996; Furuhashi et al., 2011). The AHS works with C. campestris shoots originating from either vegetative cuttings or seedlings (Figure 1). However, shoot tips exhibited a higher rate of coiling and holdfast production (84% and 82%, respectively) than seedlings (74% and 49%, respectively). Therefore, we used shoot tips for further experiments.
Figure 1.
Schematic depiction of the AHS and the key steps needed to grow Cuscuta campestris on the AH. dpi are counted from the day of assembly when the parasite was attached to the AH.
Cuscuta campestris displays all life cycle stages under AHS conditions
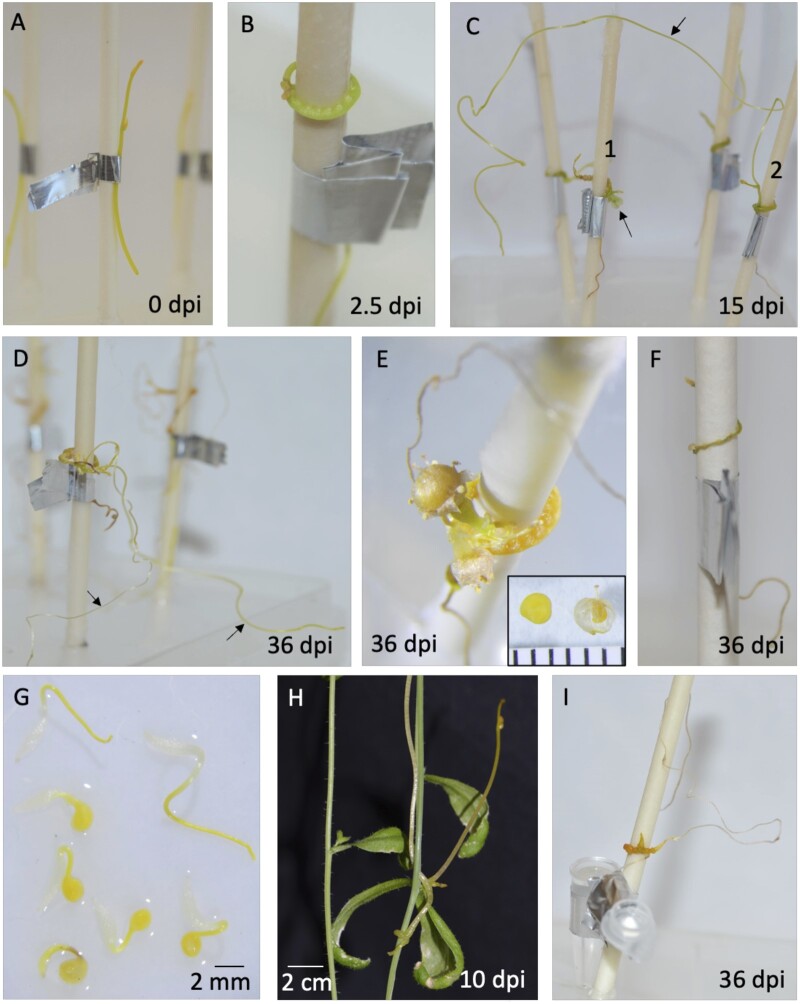
Cuscuta species are obligate parasites, so normally cannot grow beyond the seedling stage without a plant host (Kaiser et al., 2015), but the AHS adequately substituted for the host, enabling C. campestris to complete all life stages. We attached 3-cm shoot tips to AHs and evaluated their development up to 36-d postinoculation (dpi). At 2.5 dpi, parasites had coiled and formed holdfast structures on the shoot in contact with the AH (Figure 2, A and B). We consider the presence of a holdfast to be a sign of initiation of haustorial development. At 15 and 36 dpi, parasites had conspicuous enlargements in the haustorial region and produced new vegetative shoot growth, flowers, or both (Figure 2, C–E). After 36 dpi, some plants were still elongating (Figure 2D; Supplemental Figure S2A), whereas others had long shoots that had died back to a small section (<3 cm) of green turgid tissue at the tip, which we considered to be senescent shoots (Supplemental Figure S2A). In the cases where AHs were supplied with pure water or no media at all, C. campestris was not able to grow (Figure 2F; Supplemental Figure S3). Generally, no healthy holdfasts developed under these treatments, but some formed when just water was applied, resulting in a slight increase in fresh weight. However, addition of nutrient media led to significant gains in length and biomass (Supplemental Figure S3).
Figure 2.
Cuscuta campestris grows without a plant host under the controlled conditions of the AHS. A–F, Cuscuta campestris 3 cm shoot tips were used as inoculum. A, 3 cm shoot tips attached to an AH at 0 dpi. B, Parasite coiled with holdfasts after 2.5 dpi. C, Parasite at 15 dpi, growth is evident as flower development (bottom arrow) in plant No. 1 and production of a new shoot (top arrow) in plant No. 2. D, New shoots elongated, 36 dpi. E, Parasite flowered and produced seeds (inset; scale is in millimeters). F, Cuscuta campestris attached to an AH without any media or water at 36 dpi. Parasite did not develop swollen holdfast or subsequent growth. G, Seedlings after scarification and germination of seeds obtained from C. campestris grown in the AHS. H, Seedling from seed obtained through AHS colonizing Arabidopsis 10 dpi. I, Seedling growing 36 dpi after being used as inoculum in a second AHS.
Cuscuta campestris colonizes new hosts through either vegetative shoot branches or seedlings coming from germinated seeds. To be useful, the AHS should be able to support growth of C. campestris from both sources. We confirmed vegetative propagation using shoot cuttings from AHS-grown plants at 36 dpi by taking active shoots of at least 3 cm and applying them to new plant hosts (Supplemental Figure S2B) as well as new AHs. These shoots were viable, coiled, produced holdfasts, and grew on both types of hosts. For propagation by seed, we scarified the AHS-produced seeds and determined that they are able to germinate (Figure 2G), with a germination rate of 28% (n = 47). We attached these seedlings to new plant hosts as well as new AHs and found that they are able to grow on plant hosts as well as on AHs (Figure 2, H and I).
The parasite in the AHS feeds through the haustorial region
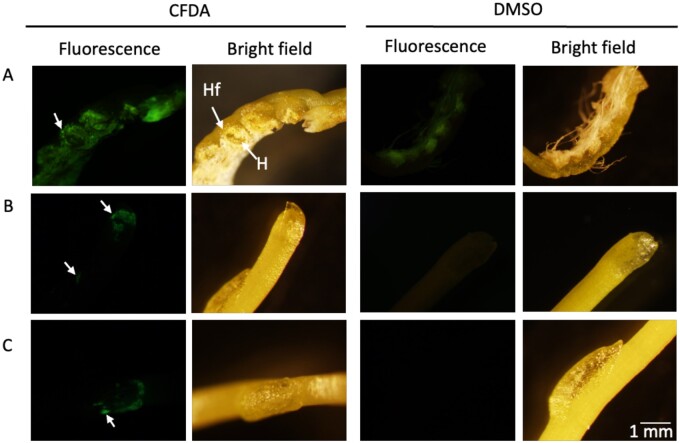
The haustorium is the signature organ of parasitism in parasitic plants and is essential for uptake of water and nutrients from the hosts (Kuijt, 1977). To characterize the ability of haustoria to take up resources, we added the symplasm-mobile dye carboxyfluorescein diacetate (CFDA; Grignon et al., 1989) to the media and monitored its uptake and translocation into the parasite. CFDA is not fluorescent, but once inside a cell it is cleaved to carboxyfluorescein, which is fluorescent. We observed the assimilation of fluorescent signal in the parasite tissues, both in association with the holdfast/haustorium region and in scale leaves that cover apical and lateral meristems, which are located away from the haustorial region (Figure 3). These observations indicate that the haustoria of C. campestris growing on the AHS function in uptake of small molecules.
Figure 3.
Cuscuta campestris growing in the AHS exposed to the fluorescent dye CFDA or DMSO solvent control from the AH. A, Haustorial region. Cells of the holdfast show fluorescence. Holdfast (Hf), Haustorium (H). B and C, Scale leaves protecting apical and lateral meristems. Scale bar in the bottom right panel refers to all panels.
The AHS allows study of C. campestris under controlled axenic conditions
Establishment: parasite establishment is independent of the media composition, although holdfast morphology is sensitive to external phytohormones
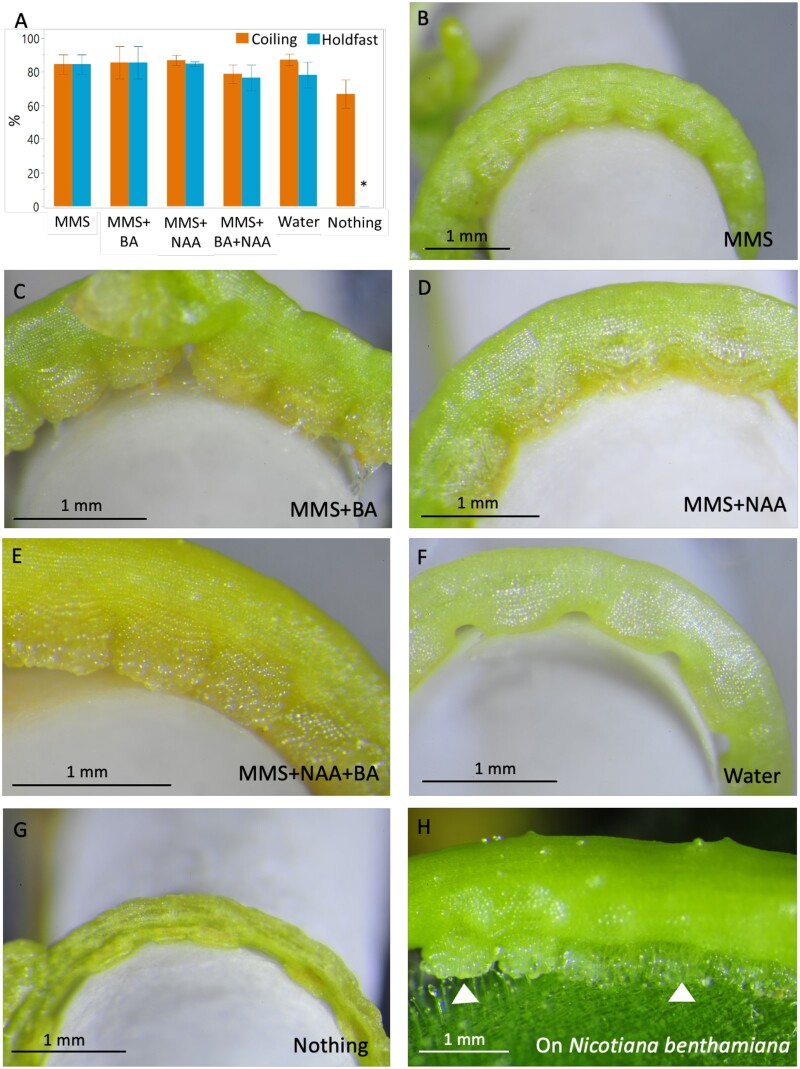
To evaluate the impact of media composition on parasite establishment (here defined as stem coiling and formation of the holdfast), we applied C. campestris shoot tips to AHs supplied with six different media treatments: nothing (no media at all), pure water, Modified Murashige and Skoog (MMS) media, MMS with 1-naphthaleneacetic acid (NAA), MMS with the 6-benzylaminopurine (BA), and MMS with NAA plus BA. The coiling phase occurred by 1 dpi and we found no significant differences in the coiling rates among the treatments (Figure 4A). The holdfast formation phase occurred by 2 dpi, and the percentage of shoots forming holdfasts was similar among all treatments except for the dry spindle (Figure 4G). The media composition affected holdfast morphology. At 8 dpi, holdfasts showed altered phenotypes in response to the different media compositions (Figure 4, B–G). Parasite holdfasts exposed to MMS media with NAA and/or BA (Figure 4, C–E) exhibited a more elaborate structure, with greater protrusions toward the host than holdfasts developing in the presence of just MMS media or water (Figure 4, B and F). Inclusion of BA produced the greatest effect, leading to more developed and thicker holdfasts (Figure 4, C and E) that more closely resembled those formed on a plant host such as Nicotiana benthamiana (Figure 4H).
Figure 4.
Characterization of C. campestris coiling and Hf morphology in the AHS. A, Frequencies of coiling and Hf production evaluated at 36 dpi. The experiment was repeated three independent times, with an average of 15 replicates. Statistical differences were detected by ANOVA. A post hoc Tukey’s Honest Significant Difference (HSD) test was applied for frequency of Hf production. Differences with P < 0.05 are indicated with an asterisk. Graph presents means between the three experiments and ±se. B–G, Hf morphology as influenced by phytohormones in the media. Holdfasts of parasites growing with MMS media (B), MMS + BA (C), MMS + 1-NAA (D), MMS + NAA+BA (E) or just water (F). G, Parasite growing on an AH without any media, “Nothing.” H, Holdfasts (arrow heads) of C. campestris growing on N. benthamiana. Pictures were taken at 8 dpi.
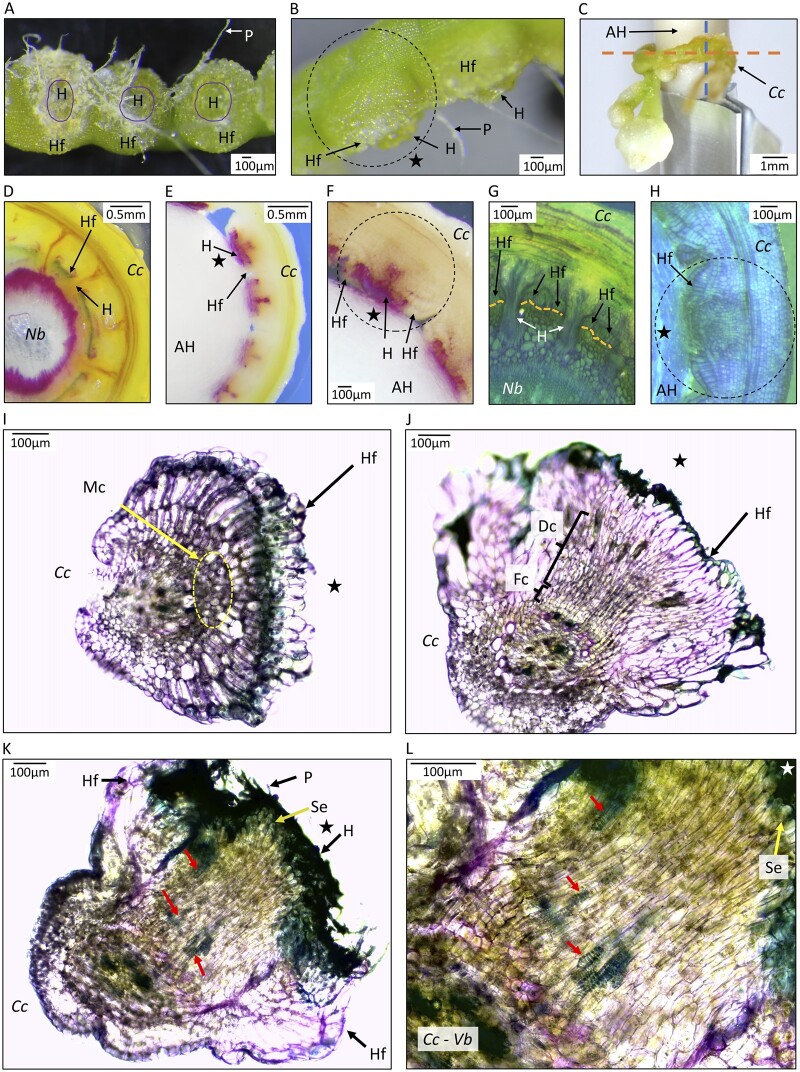
To further examine the parasitic organ produced on the AH, we inspected the anatomy of the haustorial region. The holdfast cells form an adhesion disc on the outer section of the haustoria that adhered firmly to the AH and resisted removal as evidenced by fibers from the swab spindle remaining attached to the holdfast cells (Figure 5, A and B). Cross-sections of the holdfasts (Figure 5C) revealed that haustorial organs on AHs show typical early development, but do not achieve the vascular continuity associated with haustoria on living hosts. Haustoria of C. campestris growing on N. benthamiana form connections to the vascular tissue of the host (Figure 5, D and G). In contrast, haustoria do not penetrate substantially beyond the surface of the AH (Figure 5, E, F, and H). Staining with phloroglucinol revealed specific deposition of lignin components in the developing parasitic organ adjacent to the AH surface (Figure 5, E and F). When grown on N. benthamiana, this stain revealed xylem of host and parasite, including the haustorial bridge, and also traces of lignin at the holdfast surfaces (Figure 5D). Staining in the center of parasitic organs was consistently observed and may be associated with xylem development initiated at the point of host contact (Vaughn, 2006). Sections through parasite structures grown on AHs show development that is typical of haustoria in association with living hosts (Lee, 2007; Hong et al, 2011; Švubová et al, 2017; Shimizu and Aoki, 2019), including organized cells extending toward the host (Figure 5H) and a group of meristem cells (Figure 5I) in immature haustoria. More developed haustoria have file cells and digitate cell elongating toward the host (Figure 5J). Mature organs with the most developed haustoria on the AHs had searching hyphae reaching to the AH interface, and these organs showed evidence of tracheary element differentiation identifiable by their ringed secondary walls (Figure 5, K and L). The organization of these putative xylem elements seemed discontinuous, suggesting that vascularization in the AHS may not be complete, despite the acquisition of water by the parasite that is sufficient to enable growth and reproduction.
Figure 5.
Anatomy of parasitic organs produced by C. campestris growing on an AH with MMS media with NAA and BA. A, Haustorial region inner face view. Haustoria (H) are observed in the center of the parasitic organ surrounded by the adhesion disk or holdfast (Hf). Upon removal from the AH, paper fibers (P) remain attached to the haustorial region. B, Lateral view of haustorial region with visible holdfast and protruding haustoria. Dotted line circle indicates a parasitic organ. C, Haustorial region attached to an AH. Orange dotted line indicates the sagittal plane of the haustorial region, sectioning direction used for (D–H). Dotted blue line indicates the transverse plane of the haustorial region, sectioning direction used for (I–L). D and G, Sections of C. campestris (Cc) growing on stems of N. benthamiana (Nb), with orange dotted lines in (G) indicating the epidermis of the host. E, F, and H, Cuscuta campestris growing on AHs. (D–F) were stained with phloroglucinol–HCl and fuchsia color indicates components of lignin. (G, H, and I–L) were stained with toluidine blue-O. H–L, Organized growth of haustorial cells on AH. H, Prehaustorial growth attached to AH, with star indicating outer layer of spindle. For (I–L), C. campestris was detached from the AH before sectioning, with the star indicating the face of C. campestris haustoria that had been in contact with the AH. I, Parasitic organ in an early developmental stage. Holdfast epidermal cells on the face were in contact with the host, so represent the adhesion disk. Yellow dotted line circles meristematic cells (Mc). J, A more developed parasitic organ showing File cells (Fc) and Digitate cells (Dc) positioned toward the side of the parasite facing the AH. K, Mature parasitic organ with elongated cells protruding through the middle of the Hf toward the AH. Searching hyphae are visible in the surface of the haustorium. L, Higher magnification of section of C. campestris in (K). Segments of tracheary elements (ring structures, indicated by arrows) are observed pointing in the direction of the haustorium tip that faces the AH. Sections in (E) and (F) were 20 dpi, (H) was 16 dpi and (I–L) were 36 dpi. Different stages of parasitic organ development were observed at 36 dpi in the same haustorial regions.
Parasite growth and development: phytohormones affect C. campestris weight, length, and fitness
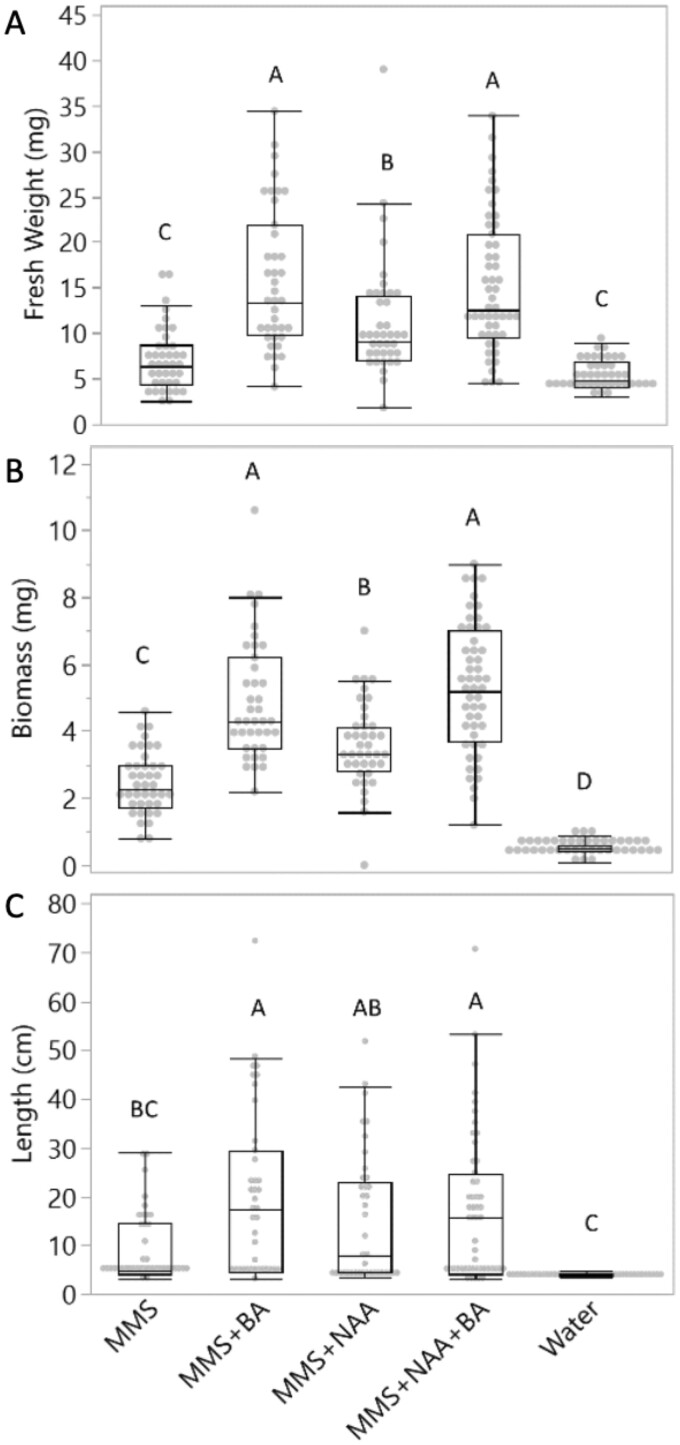
To evaluate the effect of the AHS media composition on parasite growth, we inoculated C. campestris shoot tips on AHs and evaluated changes in parasite fresh weight, biomass, and total length at 36 dpi. The parasite exhibited significantly higher fresh weight and biomass when exposed to MMS media containing phytohormones as compared to only MMS media or water (Figure 6, A and B). The greatest gain in fresh weight and biomass occurred in treatments containing BA compared to MMS alone or containing only NAA. Parasites exposed to only MMS or water treatments showed comparable fresh weights, while MMS allowed significantly higher biomass accumulation than just water. Fewer differences were found when measuring shoot length. Parasites exposed to media with MMS and BA attained the greatest length but did not differ significantly from plants with NAA-containing media. No significant length gain was found in parasites growing on AHS media without phytohormones (Figure 6C). Our results show that the fresh weight, biomass, and total length of C. campestris are significantly enhanced by externally supplied phytohormones.
Figure 6.
Cuscuta campestris growth in the AHS supplied with different media. Parasite fresh weight (A), parasite biomass (B), and parasite total length (C) were measured at 36 dpi. Different media were tested: MMS media, MMS with BA, MMS with NAA, MMS with NAA and BA, and water alone. Statistical differences were detected by ANOVA with post hoc Tukey’s HSD test. Differences were considered statistically significant at P < 0.05 and indicated with different letters. Data are pooled from three independent experiments. Analysis included only plants that coiled and developed healthy holdfasts. Number of samples ranged from 39 to 51 per treatment. The center line in the boxes of the boxplot shows the median. The bottom and top of the boxes correspond to the 25th and 75th quantiles, respectively. Whiskers extend 1.5*IQR (interquartile range). Dots are observations, with those above or below the end of the whiskers being outliers.
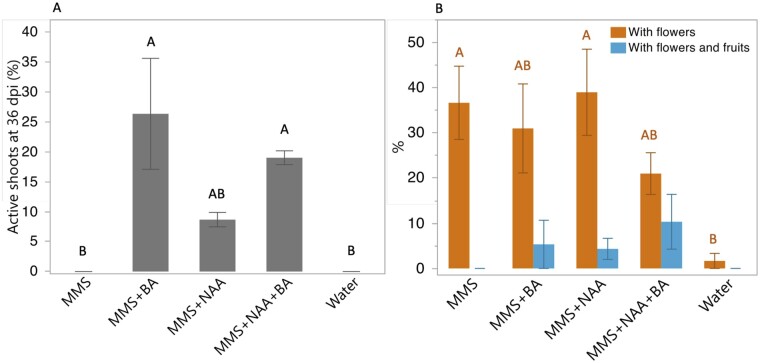
Cuscuta campestris propagation occurs through seeds and shoots, so measuring these aspects of parasite growth in the AHS provides an indication of C. campestris fitness. We assessed the percentage of new active shoots and fruits as indicators of the parasite potential to propagate and colonize new hosts. We consider actively growing shoots to be those with turgid shoot tips of at least 3 cm and able to colonize new hosts (Supplemental Figure S2). Only plants growing with media containing BA showed a significant increase in the percentage of active shoots (Figure 7A). With respect to sexual reproduction, flowers were formed under all MMS media conditions, regardless of phytohormones (Figure 7B). Parasites with fruits generally produced one fruit with one to two viable seeds. Only C. campestris exposed to media containing phytohormones produced seed. These results suggest that externally supplied phytohormones BA and NAA enhance parasite fitness in the AHS.
Figure 7.
Effect of AHS media composition on the ability of C. campestris to generate parasitically competent shoots, flowers, and fruits. A, The percentage of plants with active shoots at 36 dpi. Active shoots were defined as those with tips of at least 3 cm of healthy, turgid shoot, which are characteristic of shoots capable of forming new host connections. B, The percentage of plants with flowers only or with fruit production. Analyses included only plants that coiled and developed a healthy holdfasts. Graphs present means ± se between three independent experiments, each experiment with 6–19 plants per treatment. Statistical differences were detected by ANOVA with post hoc Tukey’s HSD test. Differences were considered statistically significant at P < 0.05 and indicated with different letters.
In measuring C. campestris fitness and growth, we noted a frequent tradeoff in the type of growth. Some plants grew long vegetative shoots, while others would grow thicker stems and produce flowers. In order to understand parasite growth parameters, we evaluated the relationship between biomass, fresh weight, and total length. Data from each parameter were grouped, regardless of the media, and Pearson’s coefficient of correlation was calculated between the pairs of parameters (Supplemental Figure S4). We also analyzed the distribution of plants with flowers and fruits in relation to biomass and total length, and this confirmed that most parasites with flowers or flowers and fruits displayed reduced total length and elevated biomass compared to the parasites without flowers or fruits (Supplemental Figure S5). We conclude that reliance on any single parameter can be misleading and recommend the use of more than one of these three growth parameters to capture the phenotypic response of C. campestris.
Haustorial function: characterization of the unigene expression profile of a functional haustorial region in the absence of a plant host
In order to explore features associated with a functional haustoria in the absence of a plant host, we analyzed differential unigene expression between the haustorial region and the newly developed stem of plants growing in the AHS (Supplemental Figure S6). We grew C. campestris in the AHS (MMS media with NAA and BA at 15 dpi) and sequenced mRNA extracted from the haustorial region and vegetative shoots. On average, 90% of the reads uniquely mapped to a previously published C. campestris genome (Vogel et al., 2018). A total of 36,342 unigenes mapped against 44,303 predicted high confidence gene loci reported by Vogel et al. (2018). From this set we identified 20,749 differentially expressed unigenes (DEUs), with 9,939 upregulated and 10,810 downregulated in the haustorial region compared to the stem, using a P-adj < 0.05 (Supplemental Figure S6).
We performed a gene ontology (GO) enrichment analysis to determine which biological processes were associated with AHS-grown haustorial development and function. Among the overrepresented processes in the haustorial region are lignin biosynthesis, xylem development, and response to hormones, all of which may be related to haustoria development (Supplemental Figure S7A; Supplemental Table S1). Other enriched processes include transport (ions, carbohydrates, and amino acids), hormone biosynthesis, response to abiotic stress, and defense, which may be associated with haustorium interaction with a host. On the other hand, unigenes underrepresented in the haustorial region compared to the shoot include those functioning in organ and stomatal morphogenesis, gravitropism, response to hormones, cuticle and cell wall-related biosynthetic processes, auxin efflux, cell differentiation, DNA-related processes, and translation (Supplemental Figure S7B; Supplemental Table S1).
Unigenes upregulated in haustorial regions of plants in the AHS are also expressed during parasitism of actual plant hosts
Finally, we compared the DEU found in the haustorial region of a parasite growing in the AHS with other published gene expression datasets from haustorial tissues associated with living plant hosts. We used datasets from two categories of haustoria, “functional” or “immature.” Functional haustoria come from studies that report established haustoria that allow parasite growth on compatible host plants. Data in the “functional” category were derived from a union of data from analysis of C. campestris growing on Arabidopsis (Arabidopsis thaliana) floral stems (Kim et al., 2014) and on tomato (Solanum lycopersicum) and tobacoo (Nicotianna tabacum) stems (Ranjan et al., 2014). Immature haustoria have visible holdfasts, but growth of new shoots or flowers is not reported. Data in the “immature” category are from C. campestris haustoria 87 h after haustorial induction in contact with a leaf of Arabidopsis (Kaga et al., 2020).
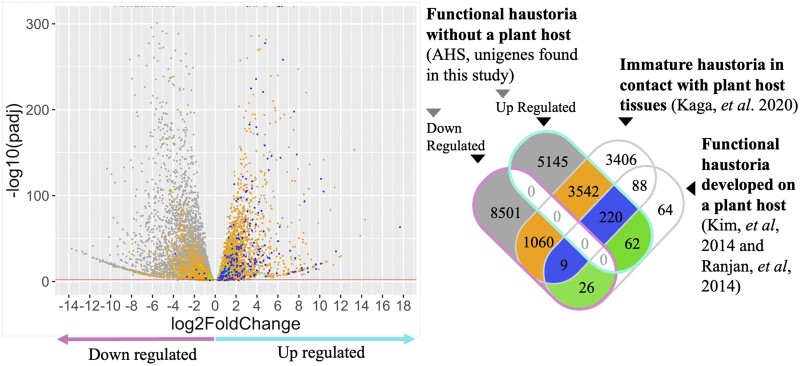
Comparison of DEUs from the AHS-grown haustoria to the immature and mature haustoria in contact with a plant host revealed that 220 genes are induced in all three datasets regardless of haustorial functional status or contact with actual plant tissues (Figure 8; Supplemental Table S2). This stands in contrast to 88 unigenes that were shared between the groups that had a contact with live plant hosts, but not found in the AHS haustoria. Another 62 upregulated unigenes were uniquely shared by functional haustoria even though AHS-grown plants lacked contact with a real plant host. To assess the functions of the 220 and 62 shared genes, we searched for homologues of the C. campestris genes in Arabidopsis, finding 148 and 45 genes, respectively (Supplemental Figure S8). Genes present in all haustoria were grouped in biological process categories related to anatomical structure development, multicellular organism development, and response to endogenous stimulus, biotic stimulus, external stimulus, abiotic stimulus, and stress (Supplemental Figure S8A). Genes shared by just functional haustoria were grouped in biological processes related to anatomical structure development, response to abiotic stimulus and response to stress (Supplemental Figure S8B).
Figure 8.
DEUs found in the haustorial region of C. campestris growing in the AHS, and comparison with upregulated unigenes reported in other haustoria developmental conditions. Left: Volcano plot showing the distribution of upregulated and downregulated DEUs (20,749 DEU, P-adj < 0.05) found in the haustorial region of C. campestris growing in the AHS. The red line corresponds to a P-adj of 0.01. Colors of data points correspond to those of the Venn diagram at right. Right: Venn diagram showing the intersection of DEUs reported for three categories of haustoria developmental conditions. First category includes upregulated and downregulated unigenes in functional haustorial regions of parasites growing in AHS (18,565 DEU with P-adj < 0.01 were included). The second category corresponds to up regulated unigenes in immature haustorial tissue, 87 h after induction of haustoria triggered by contact with a host leaf (Kaga et al., 2020). The third category includes upregulated unigenes expressed in functional haustorial regions of C. campestris growing on plant hosts (Kim et al., 2014; Ranjan et al., 2014).
Discussion
Features of the AHS
One of the primary challenges of studying obligate parasitic plants is the difficulty in separating the biology of the parasite from that of its host. Although Cuscuta species have previously been grown under tissue culture conditions (Loo, 1946; Baldev, 1962; Bakos et al., 1995; Srivastava and Dwivedi, 2001; Borsics and Lados, 2002; Švubová and Blehová, 2013), the phenotype of the parasite under such conditions does not correspond to the coiling and haustorial feeding habit that characterizes normal growth. To overcome this limitation, we developed an AHS to grow the obligate parasite C. campestris without a plant host. The AHS allows growth of C. campestris under axenic conditions while exhibiting a parasitic habit including haustorial function that approximates natural mechanisms of nutrient acquisition and growth of the parasite. Additionally, the AHS supports production of viable seeds and new shoots under in vitro conditions (Figure 2), which may be useful in the genetic manipulation of Cuscuta.
Cuscuta will coil around almost any solid object of the appropriate size, shape, and orientation, which is usually anything resembling a vertical plant stem (Furuhashi et al., 2011). During the optimization of the AHS, we tested different diameters and types of AH materials, including paper straws, rolled filter papers, and solid paper spindles. The best substrates were cylindrical with a relatively small diameter and a firm structure. The material also must deliver water and nutrients to the parasite. We tested liquid and solid media inside straws, but ultimately recognized that substrates with capillary capacity provided sufficient delivery of liquid media to the parasite. It is possible that other materials can be used for AHs, but we found these to be simple and effective. It may be advantageous that cellulose is a major component of the spindle, as C. campestris may recognize the material as chemically similar to a host plant. It is likely that C. campestris secretes lytic enzymes when interacting with the AH just as it would during interactions with a plant host (Kaiser et al., 2015). The AHS would be a good system for addressing this aspect of parasite–host interactions.
Haustorial structures developed in the AHS appear to function in uptake similar to natural haustoria. In this system, C. campestris interacts with the AH to take up water and small molecules as demonstrated by the uptake of CFDA and phytohormones (Figures 3, 4, 6 and 7). Unigene expression in AHS-grown haustorial regions also showed an alignment with its function in nutrient uptake and interaction with hosts, with similarities to unigenes induced in haustoria of parasites on actual plant hosts (Ranjan et al., 2014). For example, we found that haustorial regions had enhanced expression of unigenes categorized as associated with increased response to stress, catabolism, and transport (carbohydrates, amino acids, and ions), but reduced expression for morphogenesis and cell wall-associated genes (Supplemental Figure S7). Haustorial regions also showed activation of unigenes involved in response to abiotic and biotic stress.
Insights into parasitism
The AHS provides a system in which to explore other aspects of Cuscuta biology. For example, what is the nature of host specificity for C. campestris when it grows relatively normally on a nonliving host? Cuscuta species are recognized as having broad host ranges (Mishra, 2009), but the bases for host specificity are uncertain and have been variously attributed to host anatomy (Dawson et al., 1994), host chemical composition (Honaas et al., 2013), or perhaps some type of passive or active defense mechanism (Kaiser et al., 2015). The AHS described herein suggests that a minimum host provides water, sugar, mineral nutrients, and at least one cytokinin, with no requirement for specific anatomical features. On the other hand, it should be recognized that Cuscuta in the AHS does not grow as vigorously as one on a preferred plant host, so the AHS lacks certain aspects of the parasite–host interaction that contribute to optimal parasite growth. But the ability of the parasite to subsist and produce viable seeds in such a system serves as a strong reminder that this parasite is extremely adaptable and can complete its life cycle with few resources.
A notable aspect of the AHS is how well the haustorial development appears to parallel that of normal haustoria on living host plants. We found evidence for an effect of the hormones NAA and BA on the parasite, with holdfast phenotype (Figure 4) and parasite growth (Figure 6) responding to hormone treatment applied through the AH. Elongation of cells at the holdfast surface (Figure 5) agrees with reports on the effect of injected auxin in parasite tissues (Löffler et al., 1999). On the other hand, our results contrast with those of another system for artificial haustoria induction where no visible alterations in haustoria structures were observed after 48 h in contact with a solid media containing phytohormones and without contact with the host tissues (Kaga et al., 2020). We postulate that the longer time allowed for haustorial development in the AHS or different media compositions could explain the conflicting results.
The addition of the cytokinin BA to the medium had a pronounced effect on parasite development and growth. Parasites in the AHS with BA-containing medium showed more robust holdfast morphology and greater biomass accumulation, as well as a capacity to form shoots capable of making new host connections and setting viable seeds (Figures 4, 6, and 7). Gene expression data showed “response to cytokinin” as an enriched process in parasite shoots as compared to haustoria (Supplemental Figure S7B). In a previous study, BA applied to cut ends of C. reflexa stem sections induced haustoria in the absence of the host and in darkness, with the maximum number of individual haustoria formed close to the apex (Ramasubramanian et al., 1988). Although we did not monitor the number of individual haustoria (holdfasts in our case), we did not note differences in holdfast formation in parasites receiving diverse treatments such as water or media, including media with BA (Supplemental Figure 4A).
Another example of the minimum host requirements for C. campestris growth is the formation of putative vascular tissue in the haustorial structure. The development of the C. pentagona xylem has been associated with differentiation of haustorial searching hyphae that contact host xylem (Vaughn, 2006). In the AHS, tracheary elements formed in the haustorial structure in contact with the AH, host-derived auxin (NAA) and cytokinin (BA), sugar (glucose), and other nutrients in the media, suggesting that these are sufficient to induce differentiation in at least some cells (Supplemental Figure S5, K and L). This agrees with the crucial role for auxin and cytokinin in vascular differentiation (Wetmore and Rier, 1963; Aloni, 1987) and vascular reconnection during formation of grafting unions (Melnyk et al., 2015). Additionally, our observation of xylem elements and lignin accumulation (Figure 5) is supported by transcriptional data that show an enrichment in xylem development and lignin biosynthetic process in haustorial regions compared to stems at 15 dpi (Supplemental Figure S7A). Kaga et al. (2020) report the expression of genes related to xylem differentiation in C. campestris at 57-h postinoculation without penetrating a plant host, indicating that haustoria acquire potential for xylem differentiation at early stages.
Other topics where the AHS provides insight into parasitism include defense and reproduction. We found genes related to defense induced in the haustorial region relative to the stem, which may indicate a preparation of the parasite for the biological interaction with a host. It is clear that a complex molecular interaction occurs between parasite and host (Hegenauer et al., 2016, 2020), leading to outcomes ranging from host susceptibility to resistance (Hegenauer et al., 2016; Jhu et al., 2019). The AHS will be useful in dissecting general mechanisms of host invasion from host-specific processes. The issue of flowering is another exciting topic, with recent work suggesting that the parasite relies on its host for regulation of flower induction (Shen et al., 2020). The hypothesis is that C. campestris has lost many of its own flowering regulator genes through evolutionary reduction (Vogel et al., 2018), so it must therefore rely on flowering signals obtained from a host plant that serve to synchronize Cuscuta flowering time with that of the host. However, we observed C. campestris flowering and producing seeds in the absence of a flowering—or even a living—plant host. Further study is needed to understand flowering dynamics in Cuscuta, and the AHS may be a useful tool.
Conclusions
Historically, pure microbial cultures and axenic cultures have facilitated deeper understanding of the biology of microorganisms and parasites. For example, the growing of human parasites in vitro has enabled the study of their biological features and requirements without the influence or contamination of the host. It has also facilitated their identification and the assessment of control methods (Visvesvara and Garcia, 2002). The AHS presents a valuable approach to studying Cuscuta. In this system, it is possible to manipulate “host” chemical composition such as phytohormones (e.g. Figures 4, 6, and 7), or to test hypotheses involving environmental conditions that would otherwise be impractical with a live host (e.g. growth in darkness). The AHS is versatile and may be modified to explore different substrate materials or compare specific host requirements of different Cuscuta species, although the AHS was optimized for C. campestris. We expect this tool to be a valuable addition to the workbench of Cuscuta researchers.
Materials and methods
Preparation of the AHS
The AHS was assembled using a magenta GA-7 plant culture box (Sigma, St Louis, MO, USA) and a plastic autoclavable lamina (Polypropylene No. 5, 0.4-mm thick) cut to fit the bottom of the box and serve as a support to hold the AHs (Figure 1; Supplemental Figure S1). The paper spindle from a cotton swab (Q-tips, USA) was used as the “host stem.” Each magenta box held four spindles. When Cuscuta seedlings were used as inoculum, 0.2-mL microcentrifuge tubes were attached to the base of each spindle to hold the seedling. All the system components, magenta boxes, sheets with AHs, and aluminum foil strips (used to attach the plant to the AH) were autoclaved before assembly.
Media preparation
MMS liquid media (Srivastava and Dwivedi, 2001) was used as the base media for most of the experiments. It contained 300-mg L−1 NH4NO3, 800-mg L−1 KNO3, 250-mg L−1 CaCl2.2H2O, 260-mg L−1 MgSO4.7H2O, 120-mg L−1 KH2PO4, and 3% (w/v) glucose, pH 5.7. Sterile 1× MS micronutrients (Phytotechlab, Lenexa, KS, USA) were added after autoclaving. This MMS media was supplemented with filter-sterilized phytohormones NAA (3 mg L−1) or BA (1 mg L−1) for specific experiments.
Preparation of parasitic plant material
Vegetative C. campestris shoot tips were used as inoculum for most of the experiments. Nurseries of the parasite were established on beets (Beta vulgaris, Detroit Dark Red) as follows. Cuscuta campestris seeds were scarified by immersion in sulfuric acid (95%) for 20 min, followed by 3 washes with water for 10 min. Seeds were germinated on wet filter paper in Petri dishes for 4 d, then seedlings were attached to petioles of 1-month-old beet plants using a small piece of tape. Host and parasite were placed under direct full spectrum light (Mercury Vapor lamp) and far-red enriched light (Sylvania Spot-Gro bulb, enriched in >750 nm wavelength, 35 µmol m−2s−1) under a 14-h light/10-h dark photoperiod. Plants were watered as needed and fertilized with All Purpose Plant Food (Miracle-Gro, Marysville, OH, USA). Once the C. campestris plants produced large numbers of shoots (around 1.5 months after inoculation), 3 cm shoot tips were harvested for inoculum. Shoot tips were surface sterilized with 3.5% (v/v) sodium hypochlorite and 0.04% (v/v) Tween-20 for 10 min, followed by 5 washes with sterile water just before attaching them to the AH.
When seedlings were used as inoculum, parasite seeds were scarified as described above and surface sterilized for 5 min with 3.5% (v/v) sodium hypochlorite and 0.1% (v/v) sodium dodecyl sulfate (SDS) followed by five washes with sterile water. Sterilized seeds were germinated as described above under sterile conditions. For the germination of seeds collected from plants growing in the AHS, mature seeds were scarified and germinated as described above, except the scarification period was 7 min. Seedlings and new shoots were attached to Arabidopsis (A. thaliana) inflorescences under light conditions described above. Aracones (Arasystems, Belgium) were used to increase relative humidity in the parasite–host microenvironment and to avoid the parasite growing onto neighboring plants in later stages.
AHS assembly
Working under aseptic conditions, surface-sterilized C. campestris shoots were attached to a spindle using a sterile strip of aluminum foil that held the parasite shoot securely to the spindle with a gentle twist of the foil (e.g. Figure 2, A and B). The foil was placed approximately 1 cm from the tip of the C. campestris shoot, and each parasite shoot was placed high enough on the spindle to keep its lowest extremity away from any contact with media in the bottom of the box. After attaching the shoots, liquid media was added to the box, avoiding touching the plants. Magenta boxes were sealed with parafilm and placed under a Sylvania Spot-Gro bulb for 2.5 d with a 14-h light/10-h dark photoperiod at 33°C. Boxes were then transferred to a growth chamber under full spectrum light (61-µmol m−2s−1, 25°C). Relative humidity in the boxes with liquid media reached 100%, whereas boxes without media were at 43%. AHS boxes were kept under full spectrum light at 25°C for 33.5 d in the case of growth assays, this time was reduced to 12.5 d for other assays like RNA-seq. When seedlings were used as inoculum, the procedure was the same except a 3-d-old C. campestris seedling was placed in a 0.2-mL microcentrifuge tube containing 150 µL of sterile water to keep the seedling hydrated and the tube was attached to the base of each spindle.
Measuring parasite growth
For evaluating parasite growth, AHS boxes were opened, plants were detached from AHs, extended on a white surface with a ruler, and photographed. Pictures were analyzed using ImageJ version 1.51j8 (National Institutes of Health, USA) to obtain the total length of the plant, including all branches and the initial 3-cm shoot. Shoots were then weighed to obtain fresh weight and dried at 50°C for 4 d to before reweighing to obtain biomass data (dry weight). Data were collected from three independent experiments with a total of 96, 105, and 76 plants, respectively. Only plants that coiled and produced healthy haustoria were considered for growth analyses. Statistical analysis used JMP Pro 15 (SAS Institute Inc. Cary, NC, USA).
CFDA assay
Cuscuta campestris plants were grown for 15 d in AHS containing MMS media with NAA and BA. The spindle “hosts,” along with the connected parasites, were then transferred to a 1.5-mL microcentrifuge tube containing the same media plus 0.3-mg mL−1 CFDA (initially dissolved in dimethyl sulfoxide; DMSO) and held inside a closed sterile glass tube. Negative controls consisted of the equivalent concentration of DMSO without CFDA. In this way, the spindle conducted media containing CFDA to the point of the parasite attachment that was the only point of transfer to the parasite. After 4 d, plants were detached, rinsed thoroughly with water, and examined for CFDA fluorescence using a stereoscope under UV light with an EGFP filter.
Microscopy
Cuscuta campestris was grown in the AHS with MMS, NAA, and BA. Plants were collected at various times postinoculation (dpi), hand-sectioned, and stained with toluidine blue-O or phloroglucinol-HCl. Cross-sections were evaluated and imaged under either a dissecting microscope or an inverted microscope (Olympus CKX53) equipped with a camera (Olympus EP50).
RNA-seq and transcriptomic analysis
Vegetative parasite shoot tips were introduced to the AHS containing MMS media with NAA and BA. After 15 dpi, tissue was collected for RNA extraction (Supplemental Figure S4). Plants with healthy holdfasts and newly developed shoots were detached from the AH. For haustorial samples, regions harboring visibly healthy holdfasts (identified by formation of new shoots) were separated from any shoot or lateral meristem. For nonhaustorial stem samples, new healthy and turgid shoots were collected after removing 5 mm of the stem connecting to the haustorial region to avoid tissue contamination. All tissues were immediately frozen in liquid nitrogen. Samples from three to four plants were pooled for each replicate and four replicates were used for each type of tissue. For RNA extraction, frozen tissue was ground using glass beads in a bead beater and extracted using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). RNA was precipitated using 3-M NaOAc and Ethanol 100%, and resuspended in RNAse-free water. RNA concentration and purity were evaluated using a Nanodrop OneC (Thermo Fisher Scientific, Waltham, MA, USA), and quality was confirmed using an RNA TapeStation System (Agilent Technologies, Santa Clara, CA, USA). An mRNA library was prepared using Poly-A enrichment, and sequencing was performed by Novogene using Illumina NovaSeq 6000 platform with PE150.
Bioinformatic analysis
RNA-seq raw data quality was initially evaluated using FASTQC version 0.11.9 (Braham Bioinformatics Cambridge, UK). Then, FASTP version 1.0.0 (Chen et al., 2018) was used to detect and trim adaptors, and filter out sequences shorter than 50 nt, with low quality or low complexity. Clean reads were mapped with STAR version 2.5.2b (Dobin et al., 2013) against the genome of C. campestris (Vogel et al., 2018). Unigene counts obtained using STAR version 2.5.2b were processed by DESeq2 version 1.30.1 (Love et al., 2014) using R (https://www.r-project.org/), with differential expression analysis using the default settings and a threshold of unigenes with more than 10 counts. To increase the stringency of DEU detection and filter out biases related to the structured distribution of the samples (haustoria vs stem from the same plant pool), unigene counts were also processed by edgeR version 3.32.1 (Robinson et al., 2010; McCarthy et al., 2012) using R to perform a second DEU analyses accounting for plant pool as blocking factor using the option of generalized linear model-likelihood ratio test. Unigenes with low counts were filtered using count-per million and normalization was done using trimmed mean of M-values according to default settings. DEUs were determined by the unigenes in the intersection from the two approaches and considering thresholds at adjusted P-value (P-adj) < 0.05 for DESeq2 or false discovery rate (FDR) < 0.05 for edgeR.
Protein sequences reported for C. campestris (Vogel et al., 2018) were used for a functional annotation using eggNOG-mapper version 2 (Huerta-Cepas et al., 2017, 2019) to obtain their associated GO terms. Then a Kolmogorov–Smirnov like enrichment test (also known as GSEA) was performed. Enrichment analysis was done independently for downregulated and upregulated unigenes. DEUs, their expression P-value according to DESeq2, and their GO terms were analyzed by topGO R package version 2.42.0 (Alexa and Rahnenfuhrer, 2021) considering the settings weight 01 and NodeSize 10. Top enriched GO terms (P ≤ 0.001) were plotted using REVIGO (Supek et al., 2011) under default conditions.
Comparison with other C. campestris expression datasets
Unigenes found to be upregulated in the haustorial region of C. campestris growing in AHS (with a P-adj < 0.01) were compared to other published unigene expression datasets (Supplemental Figure S4). The first selected dataset corresponds to upregulated unigenes in micro-dissected haustoria tissue, 87 h after the forced induction of haustoria while in contact with a leaf of Arabidopsis (Kaga et al., 2020). The second dataset corresponds to upregulated unigenes in fully formed haustorial region of C. campestris (formerly published as C. pentagona) growing on two hosts, tomato (S. lycopersicum) and tobacco (Nicotiana tabacum; Ranjan et al., 2014). A third dataset was taken from Kim et al. (2014) that used functional connections between C. campestris (also formerly published as C. pentagona) and Arabidopsis. In this case, we used clean unigene counts of total expressed unigenes in the interface (corresponding to the haustorial region) and parasite (stem tissue) to perform DEU analyses. Data corresponding to three replicates were processed by edgeR version 3.32.1 (Robinson et al., 2010; McCarthy et al., 2012) to perform a DEU analysis accounting for batch effect and using default settings. DEUs were determined by FDR < 0.05.
Unigenes IDs used by Vogel et al. (2018) for C. campestris were used as a convention. A BlastX (-evalue 1e-50, -max_target_seqs 1, -max_hsps 1) against the proteins reported by Vogel et al. (2018) was used to retrieve the unigene IDs that correspond to the data obtained from Ranjan et al. (2014) and Kim et al. (2014). Gene IDs utilized by Kaga et al. (2020) were used directly as they correspond to Vogel et al. (2018) unigene IDs. We grouped the datasets of Ranjan et al. (2014) and Kim et al. (2014) based on their study of functional haustorial regions of C. campestris growing on a plant host.
Once the shared upregulated unigenes in haustorial regions among the selected studies were identified, we used the C. campestris protein sequences in Vogel et al. (2018) to identify their homologues in Arabidopsis TAIR10 (Phytozome version 12.1.6) using BlastX (-e value 1e-50, -max_target_seqs 1, -max_hsps 1). Candidate Arabidopsis homologs were subjected to Single Enrichment Analysis (SEA) using agriGO version 2.0 (Tian et al., 2017) using Go slim and Phytozome version 11 as reference, with default settings.
Accession numbers
Sequence data from this article can be found in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) archive, reference number GSE178396, and in the supplemental data.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Materials for building the AHS.
Supplemental Figure S2. New parasite shoots produced in the AHS are viable.
Supplemental Figure S3. Role of the holdfast (Hf) in C. campestris development.
Supplemental Figure S4. Relationship among biomass, length, and fresh weight in C. campestris.
Supplemental Figure S5. Relationship among biomass and length with respect to the formation of flowers and fruits in C. campestris.
Supplemental Figure S6. Differential unigene expression analysis on haustorial region and stem tissues collected from C. campestris growing in the AHS.
Supplemental Figure S7. Enriched GO terms associated with Biological Process corresponding to DEUs in the haustorial region of a parasite growing in the AHS.
Supplemental Figure S8. SEA of C. campestris upregulated unigenes in haustorial regions under different conditions.
Supplemental Table S1. Enriched GO terms for upregulated and downregulated genes.
Supplemental Table S2. DEGs shared by datasets considering haustoria functional status.
Supplementary Material
Acknowledgments
Hannah Ambrose helped with the AHS experiments.
Funding
This project was supported by the US National Science Foundation (IOS-1645027) and National Institute of Food and Agricultural award 160111 to J.H.W.
Conflict of interest statement. The authors declare no conflict of interest.
V.B.G. and J.H.W. conceived the project. V.B.G. developed the system, conducted the experiments, analyzed the data, and drafted the manuscript. V.B.G. and K.B. contributed histological images. J.H.W. supervised the project and edited the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is James Westwood (westwood@vt.edu).
References
- Alexa A, Rahnenfuhrer J (2021) topGO: Enrichment analysis for gene ontology. R package version 2.46.0. DOI: 10.18129/B9.bioc.topGO
- Aloni R (1987) Differentiation of vascular tissues. Annu Rev Plant Physiol 38: 179–204 [Google Scholar]
- Bakos A, Fari M, Toldi O, Lados M (1995) Plant regeneration from seedling-derived callus of dodder (Cuscuta trifolii Bab. et Giggs). Plant Sci 109: 95–101 [DOI] [PubMed] [Google Scholar]
- Baldev B (1962) In vitro studies of floral induction on stem apices of Cuscuta reflexa Roxb.-a short-day plant. Ann Bot 26: 173–180 [Google Scholar]
- Benvenuti S, Dinelli G, Bonetti A, Catizone P (2005) Germination ecology, emergence and host detection in Cuscuta campestris. Weed Res 45: 270–278 [Google Scholar]
- Borsics T, Lados M (2002) Dodder infection induces the expression of a pathogenesis-related gene of the family PR-10 in alfalfa. J Exp Bot 53: 1831–1832 [DOI] [PubMed] [Google Scholar]
- Borsics T, Mihálka V, Oreifig AS, Bárány I, Lados M, Nagy I, Jenes B, Toldi O (2002) Methods for genetic transformation of the parasitic weed dodder (Cuscuta trifolii Bab. et Gibs) and for PCR-based detection of early transformation events. Plant Sci 162: 193–199 [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J (2018) Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34: i884–i890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JH, Musselman LJ, Wolswinkel P, Dörr I (1994) Biology and control of Cuscuta. Rev Weed Sci 6: 265–317 [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) Sequence analysis STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi K, Tada Y, Okamoto K, Sugai M, Kubota M, Watanabe M (1997) Phytochrome participation in induction of haustoria in Cuscuta japonica, a holoparasitic flowering plant. Plant Cell Physiol 38: 935–940 [Google Scholar]
- Furuhashi T, Furuhashi K, Weckwerth W (2011) The parasitic mechanism of the holostemparasitic plant Cuscuta. J Plant Interact 6: 207–219 [Google Scholar]
- Grignon N, Touraine B, Durand M (1989) 6(5)Carboxyfluorescein as a tracer of phloem sap translocation. Am J Bot 76: 871–877 [Google Scholar]
- Haidar MA, Orr GL, Westra P (1998) The response of dodder (Cuscuta spp.) seedlings to phytohormones under various light regimes. Ann Appl Biol 132: 331–338 [Google Scholar]
- Hegenauer V, Fürst U, Kaiser B, Smoker M, Zipfel C, Felix G, Stahl M, Albert M (2016) Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science 353: 478–481 [DOI] [PubMed] [Google Scholar]
- Hegenauer V, Slaby P, Körner M, Bruckmüller JA, Burggraf R, Albert I, Kaiser B, Löffelhardt B, Droste-Borel I, Sklenar J, et al. (2020) The tomato receptor CuRe1 senses a cell wall protein to identify Cuscuta as a pathogen. Nat Commun 11: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide-Jørgensen HS (2008) Parasitic Flowering Plants. Brill Academic, Leiden, NL [Google Scholar]
- Honaas LA, Wafula EK, Yang Z, Der JP, Wickett NJ, Altman NS (2013) Functional genomics of a generalist parasitic plant: laser microdissection of host-parasite interface reveals host-specific patterns of parasite gene expression. BMC Plant Biol 13: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L, Shen H, Chen H, Li L, Hu X, Xu X, Ye W, Wang Z (2011) The morphology and anatomy of the haustoria of the holoparasitic angiosperm Cuscuta campestris. Pak J Bot 43: 1853–1859 [Google Scholar]
- Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, von Mering C, Bork P (2017) Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Mol Biol Evol 34: 2115–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J, Szklarczyk D, Heller D, Hernández-Plaza A, Forslund SK, Cook H, Mende DR, Letunic I, Rattei T, Jensen LJ, et al. (2019) EggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res 47: D309–D314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke WD, Räth N, Bäumel P, Czygan F-C, Proksch P (1994) Modelling the flow and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb and its host Lupinus albus L. J Exp Bot 45: 791–800 [Google Scholar]
- Jhu MY, Farhi M, Wang L, Philbrook R, Belcher M, Nakayama H, Zumstein K, Rowland S, Ron M, Shih P, et al. (2019) Lignin-based resistance to Cuscuta campestris parasitism in Heinz resistant tomato cultivars. bioRxiv 706861 DOI: 10.1101/706861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga Y, Yokoyama R, Sano R, Ohtani M, Demura T, Kuroha T, Shinohara N, Nishitani K (2020) Interspecific signaling between the parasitic plant and the host plants regulate xylem vessel cell differentiation in haustoria of Cuscuta campestris. Front Plant Sci 11: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser B, Vogg G, Fürst UB, Albert M (2015) Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front Plant Sci 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, LeBlanc M, Wafula EK, DePamphilis CW, Westwood JH (2014) Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 345: 808–811 [DOI] [PubMed] [Google Scholar]
- Kuijt J (1977) Haustoria of phanerogamic parasites. Annu Rev Phytopathol 15: 91–118 [Google Scholar]
- Lachner LA, Galstyan L, Krause K (2020) A highly efficient protocol for transforming Cuscuta reflexa based on artificially induced infection sites. Plant Direct 4: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HC, Kasperbauer MJ (1965) Photomorphogenic responses of dodder seedlings. Plant Physiol 40: 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KB (2007) Structure and development of the upper haustorium in the parasitic flowering plant Cuscuta japonica (Convolvulaceae). Am J Bot 94: 737–745 [DOI] [PubMed] [Google Scholar]
- Liu N, Shen G, Xu Y, Liu H, Zhang J, Li S, Li J, Zhang C, Qi J, Wang L, et al. (2020) Extensive inter-plant protein transfer between Cuscuta parasites and their host plants. Mol Plant 13: 573–585 [DOI] [PubMed] [Google Scholar]
- Löffler C, Czygan FC, Proksch P (1999) Role of indole-3-acetic acid in the interaction of the phanerogamic parasite Cuscuta and host plants. Plant Biol 1: 613–617 [Google Scholar]
- Loo SW (1946) Cultivation of excised stem tips of dodder in vitro. Am J Bot 33: 295–300 [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk CW, Schuster C, Leyser O, Meyerowitz EM (2015) A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr Biol 25: 1306–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra JS (2009) Biology and management of Cuscuta species. Indian J Weed Sci 41: 1–11 [Google Scholar]
- Ramasubramanian TS, Paliyath G, Rajagopal I, Maheshwari R, Mahadevan S (1988) Hormones and Cuscuta development: in vitro induction of haustoria by cytokinin and its inhibition by other hormones. J Plant Growth Regul 7: 133–144 [Google Scholar]
- Ranjan A, Ichihashi Y, Farhi M, Zumstein K, Townsley B, David-Schwartz R, Sinha NR (2014) De novo assembly and characterization of the transcriptome of the parasitic weed dodder identifies genes associated with plant parasitism. Plant Physiol 166: 1186–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid S, Kim G, Johnson NR, Wafula E, Wang F, Coruh C, Bernal-Galeano V, Phifer T, Depamphilis CW, Westwood JH, et al. (2018) MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 553: 82–85 [DOI] [PubMed] [Google Scholar]
- Shen G, Liu N, Zhang J, Xu Y, Baldwin IT, Wu J (2020) Cuscuta australis (dodder) parasite eavesdrops on the host plants’ FT signals to flower. Proc Natl Acad Sci USA 117: 23125–23130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Aoki K (2019) Development of parasitic organs of a stem holoparasitic plant in genus Cuscuta. Front Plant Sci 10: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Aoki K (2018) Differentiation of vascular elements in haustoria of Cuscuta japonica. Plant Signal Behav 13: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Hozumi A, Aoki K (2018) Organization of vascular cells in the haustorium of the parasitic flowering plant Cuscuta japonica. Plant Cell Physiol 59: 715–723 [DOI] [PubMed] [Google Scholar]
- Srivastava S, Dwivedi UN (2001) Plant regeneration from callus of Cuscuta reflexa – an angiospermic parasite – and modulation of catalase and peroxidase activity by salicylic acid and naphthalene acetic acid. Plant Physiol Biochem 39: 529–538 [Google Scholar]
- Supek F, Bošnjak M, Škunca N, Šmuc T (2011) REVIGO Summarizes and visualizes long lists of gene ontology terms. PLoS One 6: e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Švubová R, Blehová A (2013) Stable transformation and actin visualization in callus cultures of dodder (Cuscuta europaea). Biologia (Bratisl) 68: 633–640 [Google Scholar]
- Švubová R, Lukacova Z, Kastier P, Blehová A (2017) New aspects of dodder–tobacco interactions during haustorium development. Acta Physiol Plant 39: 66 [Google Scholar]
- Tada Y, Sugai M, Furuhashi K (1996) Haustoria of Cuscuta japonica, a holoparasitic flowering plant, are induced by the cooperative effects of far-red light and tactile stimuli. Plant Cell Physiol 37: 1049–1053 [Google Scholar]
- Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z (2017) AgriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res 45: W122–W129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kooij TAW, Krause K, Dörr I, Krupinska K (2000) Molecular, functional and ultrastructural characterisation of plastids from six species of the parasitic flowering plant genus Cuscuta. Planta 210: 701–707 [DOI] [PubMed] [Google Scholar]
- Vaughn KC (2002) Attachment of the parasitic weed dodder to the host. Protoplasma 219: 227–237 [DOI] [PubMed] [Google Scholar]
- Vaughn KC (2003) Dodder hyphae invade the host: a structural and immunocytochemical characterization. Protoplasma 220: 189–200 [DOI] [PubMed] [Google Scholar]
- Vaughn KC (2006) Conversion of the searching hyphae of dodder into xylic and phloic hyphae: a cytochemical and immunocytochemical investigation. Int J Plant Sci 167: 1099–1114 [Google Scholar]
- Visvesvara GS, Garcia LS (2002) Culture of protozoan parasites. Clin Microbiol Rev 15: 327–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A, Schwacke R, Denton AK, Usadel B, Hollmann J, Fischer K, Bolger A, Schmidt MH-W, Bolger ME, Gundlach H, et al. (2018) Footprints of parasitism in the genome of the parasitic flowering plant Cuscuta campestris. Nat Commun 9: 2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, Depamphilis CW (2010) The evolution of parasitism in plants. Trends Plant Sci 758: 1–9 [DOI] [PubMed] [Google Scholar]
- Wetmore RH, Rier JP (1963) Experimental induction of vascular tissues in callus of angiosperms. Am J Bot 50: 418–430 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.