Abstract
Digital health management is increasingly pivotal in the care of patients with diabetes. The aim of this review was to evaluate the clinical benefits of using smart insulin pens with connectivity for diabetes management. The search was performed using PubMed and PubMed Central on May 15, 2019, to identify publications investigating the use of insulin pens. Studies evaluating insulin pens with connectivity via Bluetooth/Near Field Communication, with an associated electronic device enabling connectivity, or with a memory function were included in the review. Nine studies were identified in the search. Overall, these studies lacked data on smart insulin pens with a connectivity function, with eight of the available studies investigating only pens with a memory function. The studies focused primarily on assessing patient preference, usability, and technical accuracy. The number of studies assessing clinical outcomes was small (n = 3). However, the majority of studies (n = 8) reported that patients preferred smart insulin pens because they increased confidence with regard to diabetes self-management. These results suggest a lack of published data regarding smart insulin pens with connectivity for the management of diabetes. However, the available published data on usability and patient preference suggest that the use of smart insulin pens holds promise for improving and simplifying diabetes self-management.
Keywords: diabetes, digital technologies, insulin therapy, pens, review, smart insulin pen
Introduction
Digital health management plays an increasing role in the care of individuals with diabetes at all stages of the disease journey and has the potential to simplify the complex process of diabetes self-management. 1 Among the established tools and devices in diabetes management, smart insulin pens have the potential to fulfill some of the unmet needs of people with diabetes through the accurate administration of bolus doses, the simplification of documentation relating to diabetes therapy, and the improvement of communication and the quality of advice given to patients.
Multiple technological innovations, including continuous glucose monitoring (CGM) and insulin pumps, have sought to ease the burden of diabetes self-management and improve patient outcomes.2-4 A major step forward in the simplification of insulin delivery was the development of insulin pens,5,6 such that, by 2018, the majority of individuals requiring insulin in Germany were using pens for injection. 7 Insulin pens first became available in 1985 8 and eliminated the need to draw up insulin from a vial, improving the convenience of administration for users. 5 Both disposable and reusable insulin pens are available. Insulin pens have shown improved dosing accuracy and consistency compared with syringes. 9 The improvements conferred by insulin pens over syringes may be improved further with the use of motor-driven smart pens, with potential benefits including improved adherence, memory support, and reduced costs.10,11
A pen with a memory function was first marketed in 2007. 12 In 2014, the first “enhanced” insulin pen cap became available in the United States, 13 the use of which could inform the user of a regular disposable insulin pen how much time had passed since their last injection. The US Food and Drug Administration (FDA) approved the first reusable smart insulin pen in 2017. 14 Different kinds of smart insulin pens and associated devices, such as smart pen caps, 15 are on the market, and smart insulin pens with connectivity are defined as those with built-in interface technology (Bluetooth or Near-Field Communication [NFC]). Bluetooth connectivity enables automatic and immediate transmission of data from the pen to a corresponding medical smartphone application (app), currently via Bluetooth Low Energy (BLE). BLE and NFC connectivity enable the patient or healthcare center to “scan” insulin data manually into digital storage or a logbook so the stored data can then be analyzed and shared with either healthcare providers or caregivers. Smart pen caps function as add-on modules to insulin pens and enable similar connectivity. The original pen cap is overlaid or replaced with a pen cap that counts the number of “clicks.” This enables the number of insulin doses to be displayed on the pen cap and this information to be transmitted via Bluetooth or NFC.
Recent diabetes guidelines acknowledge the role of real-time monitoring technologies and telemedicine in improving patient health, 16 highlighting the need for an evidence-based evaluation of the functionality of smart insulin pens with connectivity. Such an evaluation will assist in the placement of these devices in the management of diabetes and encourage further research of these devices in areas where evidence is missing or limited.
The aim of this review was to elucidate the potential clinical benefits of using smart insulin pens with connectivity in diabetes management through an examination of published peer-reviewed literature. Currently, a common, globally confirmed definition or name for insulin pens with connectivity is lacking. To establish a common term for such insulin pens, the term “smart insulin pens with connectivity” is used throughout this review.
Methods
Data Sources and Literature Search
Two independent researchers (Masem Research Institute GmbH) performed a literature search using PubMed and PubMed Central on May 15, 2019. The search focused on publications from 2006 in English or German. Keyword search terms and details of the hand search performed can be found in the supplementary materials.
Study Selection and Quality Assessment
The studies selected for inclusion were those including people with type 1 or type 2 diabetes mellitus receiving insulin treatment using a smart insulin pen with connectivity via Bluetooth/NFC, an insulin pen with an associated electronic device enabling connectivity, or an insulin pen with a memory function. Only publications in peer-reviewed journals were considered. Exclusion criteria were studies primarily investigating non-connected pens, pen needles, other insulin delivery devices (eg, pumps), CGM systems, apps, or insulin. Reviews or expert comments were excluded. Further details on the review process and quality assessment can be found in the supplementary material.
Data Extraction
Data from the publications identified were extracted into an Excel spreadsheet. Types of data collected are summarized in the supplementary material.
Results
The literature search identified 286 publications overall (Figure 1). Successive rounds of screening identified only one article on the use of a smart pen with connectivity; this study investigated an electronic device that connected to the insulin pens and provided connectivity capabilities (ie, an insulin pen cap). 15 Nine studies meeting the inclusion criterion of investigating an insulin pen with a memory function were also identified.17-25 Two publications presented data from the same study22,25; the duplicate 22 was discarded, leaving nine studies for the qualitative analysis (Table 1).
Figure 1.
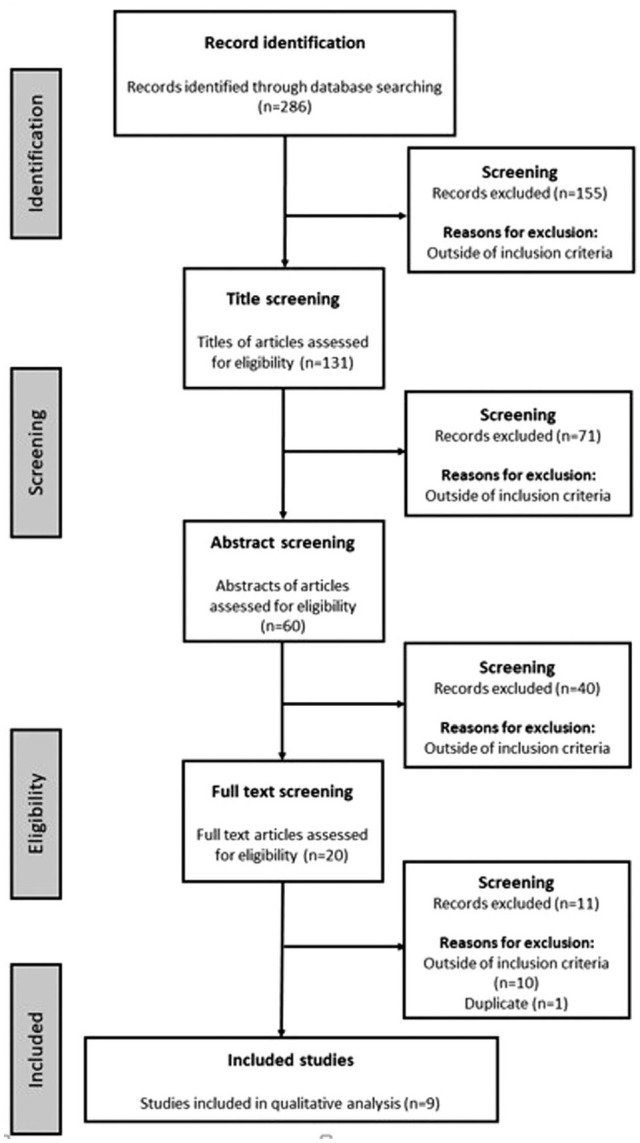
Study identification.
Table 1.
Design Details of the Identified Studies.
| Study reference; countries | Study design (duration) | Study device | Diabetes type (% population) | Study subjects (N) | Aspects of smart/connected pen or device use examined | Assessment tools used |
|---|---|---|---|---|---|---|
| Adolfsson et al 17 ; Canada, Finland, Israel, Sweden | Open-label, observational, multicenter study (12-18 weeks) | Novo Pen Echo | T1DM (100) | Pts (358) | Technical complaints related to adverse reactions, usability, HbA1c | Questionnaire, case report forms |
| Asakura and Jensen 18 ; Japan | Randomized, open-label, crossover (60-90 minutes) | Groups used both Levemir FlexPen and Lantus OptiClik pen | T2DM (100) | Pts (61), split into intuitiveness and instruction time groups | Injection time with and without instruction a , pt preference and usability | Questionnaire |
| Cerna and Maresova 19 ; Czech Republic | Online survey (NA) | None | T1DM (45) T2DM (55) | Pts (313) | Attitudes about diabetes treatment, data recording, use of mobile applications | Questionnaire |
| Danne et al 20 ; Germany | Randomized, open-label, parallel-group, multicenter (24 weeks) | HumaPen Memoir vs HumaPen Luxura | T1DM (100) | Pts (257) | Change from baseline in HbA1c, a hypoglycemia, pen acceptance | Blood samples, interviews, questionnaire |
| Gomez-Peralta et al 15 ; Spain | Main functionalities and performance test (NR) | Humalog KwikPen with Insulclock b | T1DM (100) | Pts with diabetes (9-49) | Insulin type detection, dose detection, injection duration, temperature sensing | Insulclock measuring tools and database |
| Guo et al 21 ; China, Germany, UK | Randomized, open-label, crossover, multicenter (90 minutes) | Two pens without memory function (Humapen Luxura & ClikSTAR) vs two pens with memory function (NovoPen 5 & Humapen Memoir) | T1DM (26) T2DM (74) | Pts (278) HCPs (102) | Pt/HCP preference a and usability | Face-to-face interview, questionnaire |
| Klausmann et al 23 ; Canada, China, Germany | Randomized, crossover, multicenter study (60 minutes) | NovoPen 5 vs HumaPen Luxura | T1DM (25) T2DM (75) | Pts (300) HCPs (150) | Pt and HCP preference a and usability | Face-to-face interview, questionnaire |
| Olsen et al 24 ; Canada, France, Germany | Randomized, open-label, crossover, multinational, multicenter (45-90 minutes) | NovoPen Echo vs NovoPen Junior and HumaPen Luxura | T1DM (100) | Pediatric pts (79) Parents (78) HCPs (48) | Pediatric pts, parents, and HCP preference and usability | Face-to-face interview, rating scales |
| Venekamp et al 25 ; 21 countries in Europe, India, South Africa | Multicenter, single-arm, open-label, observational study (6-10 weeks) | HumaPen Memoir vs pre-study delivery system for glargine (if used) | T1DM (38) T2DM (62) | Pts (304) | Pen functionality, a glycemic control, pt preference, acceptance, and confidence in delivery system, HCP acceptance | Laboratory procedures, pt questionnaire, pt diary |
Stated primary endpoint.
Connected smart cap for insulin pens.
Abbreviations: HbA1c, glycosylated hemoglobin; HCPs, healthcare providers; NA, not applicable; NR, not reported; pt(s) patient(s); T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UK, United Kingdom.
Quality of Evidence Supporting Digital Diabetes Management
Overall, the quality of published evidence for digital diabetes management using smart insulin pens was low with a wide heterogeneity in study design and quality. Only five of the identified studies used a control group.18,20,21,23,24 Eight of the nine studies used face-to-face interviews and/or questionnaires to gather data,17-21,23-25 and some had a limited sample size (n = 9-79; Table 1).15,18,24
In addition, the few studies meeting the inclusion criteria were generally older, published between 2006 and 2016, with only one study published in the year the literature search was conducted 15 (Table 1).
Qualitative Overview of Included Studies
Six studies investigated patient preferences regarding smart versus non-smart insulin pens or smart insulin pens versus insulin delivery methods used prior to the study baseline,17,18,21,23-25 and five of these studies also investigated smart insulin pen usability (eg, ease of use, ease of handling, convenience).17,18,21,23,24 One non-comparative observational study investigated the safety of a smart insulin pen. 17 One study investigated the performance of a smart pen cap enabling connectivity. 15 Two studies assessed patient or healthcare provider acceptance of a smart insulin pen versus a non-smart alternative.20,25 One study investigated patient attitudes about diabetes treatment, data recording, and use of mobile apps. 19 Only three studies assessed clinical endpoints, such as glycemic control and hypoglycemia,17,20,25 with the change in glycosylated hemoglobin (HbA1c) from baseline being the primary outcome in one of these studies. 20 Other primary endpoints stated were injection time, patient/healthcare provider preference, and pen functionality. The duration of the studies, where stated, ranged from 45 minutes to 18 weeks (Table 1). Two studies included participants aged <18 years.17,24
Overall, most studies investigating patient preference reported that the smart insulin pen (primarily involving a memory function) was preferred over the alternative (Table 2).17,21,23-25 Only one study did not report a preference for the insulin pen that included a memory function; this study did not analyze the digital features of the pen—the primary endpoint being injection time of a specific dose with and without instruction—therefore, definitive smart pen-related conclusions could not be drawn. 18 The two comparative studies that investigated glycemic control with pens with a memory function found no impact of smart pen use on glycemic control compared with a conventional insulin device, and, in both studies, the incidence of hypoglycemic events did not differ between insulin treatment groups.20,25 The non-comparative study in children and adolescents with type 1 diabetes found a very small numerical increase in mean, but not median, HbA1c levels and decreases in the incidence of hypoglycemia over the study period with the use of a smart pen. 17
Table 2.
Demographics and Outcomes of Participants in the Identified Studies.
| Study reference | Pt age, mean a years; female sex (%) | Pt adherence | Confidence ratings | Pt preference outcomes | Other outcomes |
|---|---|---|---|---|---|
| Adolfsson et al 17 | Median 12.0; 47.5 | • Proportion of children reporting self-injection with
NovoPen Echo was higher than those using their previous
device (71% vs 66%;
p = .006) • Proportion of pts reporting forgotten injections was significantly lower (27% vs 51%; p < .0001) |
• Confidence increased: more pts reported the NovoPen Echo increased their confidence about not missing injections (85.9%) and managing daily injections (73.3%) (both p < .0001) | • 75.7% found NovoPen Echo better looking; easier to depress (72.7%), prepare (70.7%), and use for injection (71.1%); and easier to use overall (75.1%) than their previous device (all p < .0001) | • Mean HbA1c increased from 8.4% to 8.6% during the study.
Proportion of pts achieving HbA1c <7.5% decreased (23.4%
vs 17.8%) • Major hypoglycemic events reported in the four weeks prior decreased from 6.3% of pts using NovoPen Echo vs 10% (335.3 events per 100 PY) with previous devices |
| Asakura and Jensen 18 | 61.9; 42.6 | NR | • Confidence in setting and injecting the correct dose was extremely important in 44% and 48% of pts, respectively | • 82% of pts preferred FlexPen over OptiClik | • FlexPen vs OptiClik, required less instruction time and was rated as simpler to use (82% vs 12%; p < .001) and more convenient (71% vs 12%; p < .001) |
| Cerna and Maresova 19 | NR (48.6% aged >50); 57.8 | NR | NR | NR | • Pts had a low level of knowledge about using technologies
for diabetes treatment • Only 25% of pts knew of any diabetes-related apps • Positive correlation between technical skills and methods of entering data |
| Danne et al 20 | 39.8; NR | NR | NR | • 76.7% and 78.1% of pts were mostly or definitely willing to continue using the HumaPen Memoir or the HumaPen Luxura, respectively | • No significant difference between the two insulin pens
regarding mean change in HbA1c up to week
24 • Overall incidence of hypoglycemia was not significantly different between the two insulin pen treatment groups • Memory function of the smart insulin pen might be helpful for certain pt populations, eg, children or forgetful pts |
| Gomez-Peralta et al 15 | NA; NA | NA | NA | NA | • 97% of injections performed were correctly
detected • Relative error was 2.9%-6.8% for dose accuracy detection across all dose groups • Strong correlation between time detected by Insulclock and an external chronometer (R2 = 0.99) and a correlation between temperatures detected by Insulclock and an external thermometer (R2 = 0.90) |
| Guo et al 21 | 49.8; 54.0 | NR | • More pts would be “very confident” using NovoPen 5 (64%)
vs HumaPen Memoir (43%), HumaPen Luxura (49%), and ClikSTAR
(45%) • For use of a pen with memory function vs a conventional pen: 46% of pts would be “very confident” |
• 49% of pts preferred NovoPen 5 | • Significant differences between NovoPen 5 and other pens with respect to design, ease of learning, and confidence |
| Klausmann et al 23 | NR (65% aged 31-64); 50.0 | NR | • Significantly more pts would have more confidence using the NovoPen 5 for managing daily injections vs HumaPen Luxura (82 vs 11%; p < .001) | • Significantly more pts preferred the NovoPen 5 to the HumaPen Luxura (82% vs 17%; p < .001) | • Pts indicated the memory function would be most helpful
with increasing confidence about timing and amount of their
last insulin dose • Pts gave higher ratings to NovoPen 5 than to HumaPen Luxura on ease of handling, satisfaction, convenience for daily use, pen quality, and extent to which the pen met their needs (p < .05 for all) |
| Olsen et al 24 | NR (56% aged 13-18); 52.0 | NR | • Feeling secure regarding complete injection of the dose contributed to participants’ overall high satisfaction with the NovoPen Echo | • 80% of participants preferred NovoPen Echo (vs 7% and 12%
for NovoPen Junior and HumaPen Luxura; both
p < .0001) • 74% of pediatric pts, 88% of parents favored NovoPen Echo |
• Features of the NovoPen Echo that may lead to successful use in the pediatric setting include the simple memory function, half-increment units, ease of use, and options for appearance customization |
| Venekamp et al 25 | 51.7; 42.0 | NR | NR | • 81.4% of pts preferred HumaPen Memoir over their pre-study device | • No serious concerns regarding functionality of HumaPen
Memoir • Where differences between HumaPen Memoir and the pre-study device were significant (p < .05), the majority favored the HumaPen Memoir • HumaPen Memoir was considered easier and more convenient to use than the pre-study device • Glycemic control was maintained; hypoglycemic events reported were not considered related to the HumaPen Memoir |
Unless otherwise noted.
Abbreviations: HbA1c, glycosylated hemoglobin; NA, not applicable; NR, not reported; pt(s) patient(s); PY, patient-years; R2, coefficient of determination.
Discussion
The literature search identified only a small number of studies that met the inclusion criteria. The studies presented were heterogeneous with respect to study design and study quality. Only one identified study was published in 2019, the year of the literature search, and only this study investigated a device with actual connectivity capabilities. The data from these trials were generally of low quality, with some including only a limited number of patients and many lacking a control group. This demonstrates a significant lack of evidence, especially from high-quality studies investigating the current generation of smart insulin pens with connectivity.
To date, the literature on smart insulin pens primarily focuses on assessing patient preference, usability, and technical accuracy. The majority of studies identified in the literature search concluded that the smart pens investigated were the preferred choice for people with diabetes.15,17,19-21,23-25 Many studies also noted increased confidence in not missing injections and managing daily injections when using smart devices,18,21,23,24 as well as increases in adherence/decreases in missed doses, 17 factors likely to lead to improved diabetes self-management and general well-being. Children and adolescents with diabetes often have difficulty with diabetes self-management, 17 and the two studies that included pediatric participants reported that the use of smart pens is likely to improve adherence in this population.17,20
Only two comparative studies investigated HbA1c reduction in users of classic insulin pens versus smart insulin pens with a memory function.20,25 In both studies, glycemic control was not impacted by the use of a smart pen versus the comparator device; however, Danne and colleagues 20 concluded that the memory function might be helpful for specific populations, such as children, adolescents, people with impaired memory, or the elderly. Venekamp and colleagues 25 concluded that the insulin pen with a memory function had a favorable benefit/risk profile when safety, user complaints, and patient/healthcare professional acceptance were concerned. A non-comparative study in children and adolescents with type 1 diabetes reported a very small increase in mean, but not median, HbA1c levels over the study period with use of a smart pen; however, the authors noted that, because of the short-term, observational nature of the study, these results should be interpreted with caution. 17
The peer-reviewed published literature on smart insulin pens with connectivity is currently limited in number; however, non-peer-reviewed information and other research published outside of traditional academic channels can provide some additional insights, and this was searched to provide additional context to the published literature search. A 2019 scientific evaluation of a reusable smart insulin pen with a telemonitoring system found that, in people with diabetes treated with insulin with poor glycemic control despite participation in a disease management program, mean HbA1c decreased by 0.9% overall and by 2% in people with type 2 diabetes. 26 Despite lowering HbA1c, there was no increased use of insulin or higher incidence of hypoglycemia. 26
The limitations of HbA1c in describing both short- and long-term glycemic control have recently been recognized. 27 Recent studies have shown that percent time in range (TIR) may have associations with diabetes microvascular complications similar to those of HbA1c level.28,29 A study published in 2020 reported improved insulin adherence through a reduction in the number of missed bolus doses, better mealtime dosing, and increased TIR in people with type 1 diabetes using smart insulin pens with connectivity in a real-world setting. 30 These data suggest the use of smart insulin pens with connectivity is likely to result in improved glycemic control through decreased HbA1c, enhanced TIR, absence of an increase in the incidence of hypoglycemia, closer adherence to diabetes treatment guidelines, 31 and reductions in diabetes-related complications.28,29,32 However, these assumptions will need to be confirmed in well-designed clinical trials and via collection of real-world evidence.
In clinical reality, glucose data alone are often not sufficient to safely adjust insulin doses and to change insulin prescriptions. However, when used in conjunction with exact information about the type of insulin, the injected insulin doses, and the time of injection, more appropriate and safer dose adjustments are possible. For the patient, smart pens offer the possibility to see calculated “insulin on board” via an appropriate app, 33 which is crucial for multiple daily therapy decisions (eg, doses of correctional insulin, therapy adjustment before and during exercise, etc.). Of note, the data output will vary depending on the device; those that measure the displacement of the plunger report both the injected dose and any priming dose(s) as one dose, whereas devices that measure lead screw rotation can differentiate between multiple small doses, providing the opportunity to distinguish between priming and administered dose(s), and a more accurate measure of actual injected doses.
As seen with CGM devices and insulin pumps, the creation of robust, reliable databases and overviews may help to facilitate an engaging and open patient-healthcare provider dialogue, which has been identified as highly important for optimal disease management.34,35 A recent study assessing the association between the timing of insulin administration and pre- and post-prandial glucose levels found that the use of a smart insulin pen with connectivity and CGM provided data that may help healthcare providers and patients understand how the timing of mealtime insulin impacts glucose levels. 36 Having access to robust sources of insulin data will provide opportunities for clinicians to conduct more informed discussions with insulin users, thereby improving patient-healthcare provider communication and potentially leading to the implementation of strategies to improve glycemic control through fine tuning therapy and self-management plans and configuring the tool to match the individual’s therapy plan and preferences. Optimization of this type of health technology so it works as intended is crucial to its success; thus, when initiating smart insulin pens, patient education strategies need to be adjusted, and all trials of smart insulin pens should report how and with what content patient education and coaching were performed.
Taken together, the peer-reviewed published literature and the gray literature suggest that smart pens with connectivity have the potential to improve adherence, with lack of adherence currently a significant problem in diabetes management. Smart pens with connectivity also have the potential to improve dosing accuracy and lead to more appropriate and/or safer dosage decisions. Insulin doses can be missed for a number of reasons: forgetfulness, embarrassment, dose complexity, cost, and deliberately missing doses for weight control. 37 Munshi and colleagues 38 demonstrated that non-adherence to insulin dosing and timing can be objectively assessed by smart insulin pens with connectivity, and missed bolus doses were associated with poor glycemic control. The authors suggested that use of a smart pen with connectivity may help close the gap between patient-reported and actual adherence. 38 A smart pen with connectivity also allows for the potential to send reminders in the case of missed doses when paired with an appropriate mobile app. 33
Despite the general lack of data in the literature on smart insulin pens, there are some obvious scenarios where smart insulin pens are likely to be beneficial. The overall benefits of smart insulin pens may be particularly useful for certain subpopulations, such as young and elderly individuals with diabetes, and people with additional physical conditions or disabilities that may hinder self-management. 37 Smart insulin pens with connectivity improve communication with healthcare providers through data sharing, resulting in robust transparency; thus, people for whom these devices will likely be beneficial include those starting insulin who present with the potential for hypoglycemia and/or excess weight gain; those for whom hypoglycemia is a recurrent problem or in whom there is hypoglycemia unawareness; those with frequent episodes of uncontrolled diabetes requiring unscheduled visits to healthcare providers; those with glycemic variability that causes psychological distress; those for whom forgetfulness is frequent or in whom deliberate insulin omission is suspected 39 ; those whose numeracy makes dose calculations difficult or who tend to give similar doses for very different meals; children with type 1 diabetes; older insulin-treated individuals living on their own; and women with gestational diabetes requiring insulin.24,40 These benefits may also extend to caregivers of people with diabetes and healthcare workers managing patients with diabetes in the inpatient setting.
Optimal features of a smart insulin pen with connectivity include a low level of complexity, with automatic recording, dose recommendations and reminders, convenience (no need for the patient to wear an additional device, long battery life, automatic changes to time zones), and data integration capabilities. 41 Integration of dose data with other diabetes and lifestyle data adds value and allows for the possibility of remote patient monitoring and a more continuous, data-driven therapy approach well suited to a chronic condition such as diabetes. When considering possibilities around remote monitoring of insulin dosing and blood glucose data, the individual with diabetes using a smart pen with connectivity has the added security of knowing their data are being monitored by another person who can alert them or their healthcare provider if any aspect of their diabetes management needs to be improved. Remote monitoring across a broader population also has the potential to identify specific groups of individuals who might benefit from specific diabetes management interventions.
Smart pens with connectivity require the use of an app to collect the data sent from the pen, but standards for the interoperability of smart diabetes devices are currently lacking. Simple and reliable technical solutions are needed so that all kinds of smart insulin devices can be easily read by medical practice software and hospital management software. Smart devices have been tailored to other chronic conditions such as asthma and hypertension,42,43 demonstrating that chronic disease management can adapt to new technologies.
Conclusion
This analysis has shown that the published literature on smart insulin pens with connectivity is limited. Most papers focus on insulin pens with a memory function rather than devices with connectivity capabilities. The majority of the current peer-reviewed literature on smart insulin pens focuses on patient preferences, adherence, and usability, and robust data on the impact of smart pens on clinical endpoints are lacking. However, the development of new smart insulin pens with connectivity is a promising approach for improving and simplifying the management of type 1 or type 2 diabetes for individuals, including children and adolescents. These devices may offer the potential for improved satisfaction, adherence, administration, safety, and quality of care, as well as an approach that can be individualized to the needs of the person with diabetes.
Supplemental Material
Supplemental material, sj-pdf-1-dst-10.1177_1932296820983863 for Digital Diabetes Management: A Literature Review of Smart Insulin Pens by Lutz Heinemann, Oliver Schnell, Bernhard Gehr, Nanette C. Schloot, Sven W. Görgens and Christoph Görgen in Journal of Diabetes Science and Technology
Acknowledgments
Sheridan Henness, PhD, provided medical writing assistance in the preparation of this article on behalf of Rx Communications.
Footnotes
Abbreviations: app, application; BLE, Bluetooth Low Energy; CGM, continuous glucose monitoring; FDA, US Food and Drug Administration; HbA1c, glycosylated hemoglobin; HCPs, healthcare providers; NA, not applicable; NFC, near-field communication; NR, not reported; pt(s) patient(s); PY, patient-years; R2, coefficient of determination; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TIR, time in range; UK, United Kingdom; USA, United States of America.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LH reports that he supports a number of companies that are developing novel diagnostic and therapeutic options for the treatment of patients with diabetes. He is a shareholder of Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany, and Prosciento, San Diego, USA. OS reports personal fees from Eli Lilly, outside the submitted work. BG reports personal fees from Lilly, Novo Nordisk, Sanofi, Dexcom, Roche, Medtronic, and IME-DC, outside the submitted work. NS, SG, and CG are employees of Lilly Deutschland GmbH, Germany. All authors meet authorship criteria and take responsibility for the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The data collection, analysis, and preparation of this manuscript were funded by Eli Lilly and Company.
ORCID iDs: Lutz Heinemann  https://orcid.org/0000-0003-2493-1304
https://orcid.org/0000-0003-2493-1304
Oliver Schnell  https://orcid.org/0000-0003-4968-2367
https://orcid.org/0000-0003-4968-2367
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Heintzman ND. A digital ecosystem of diabetes data and technology: services, systems, and tools enabled by wearables, sensors, and apps. J Diabetes Sci Technol. 2015;10(1):35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choudhary P, Campbell F, Joule N, Kar P, Diabetes UK. A type 1 diabetes technology pathway: consensus statement for the use of technology in type 1 diabetes. Diabet Med. 2019;36(5):531-538. [DOI] [PubMed] [Google Scholar]
- 3. Fagherazzi G, Ravaud P. Digital diabetes: perspectives for diabetes prevention, management and research. Diabetes Metab. 2019;45(4):322-329. [DOI] [PubMed] [Google Scholar]
- 4. Lindpointner S, Korsatko S, Kohler G, et al. Use of the site of subcutaneous insulin administration for the measurement of glucose in patients with type 1 diabetes. Diabetes Care. 2010;33(3):595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pisano M. Overview of insulin and non-insulin delivery devices in the treatment of diabetes. P T. 2014;39(12):866-876. [PMC free article] [PubMed] [Google Scholar]
- 6. Sorli C, Heile MK. Identifying and meeting the challenges of insulin therapy in type 2 diabetes. J Multidiscip Healthc. 2014;7:267-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deutsche Diabetes Gesellschaft [Internet]. German Health Report Diabetes 2018. https://www.diabetesde.org/system/files/documents/gesundheitsbericht_2018.pdf. Accessed September 17, 2020.
- 8. Hyllested-Winge J, Sparre T, Pedersen LK. NovoPen Echo® insulin delivery device. Med Devices (Auckl). 2016;9:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luijf YM, DeVries JH. Dosing accuracy of insulin pens versus conventional syringes and vials. Diabetes Technol Ther. 2010;12(Suppl 1):S73-S77. [DOI] [PubMed] [Google Scholar]
- 10. Hall RL, Willgoss T, Humphrey LJ, Kongso JH. The effect of medical device dose-memory functions on patients’ adherence to treatment, confidence, and disease self-management. Patient Prefer Adherence. 2014;8:775-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah RB, Patel M, Maahs DM, Shah VN. Insulin delivery methods: past, present and future. Int J Pharm Investig. 2016;6(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eli Lilly and Company [Internet]. Lilly introduces world’s first digital insulin pen with memory. 2007. https://investor.lilly.com/static-files/27d4d6a5-4235-4cd0-82fb-7e764f3c2c6a. Accessed December 6, 2019.
- 13. Timesulin [Internet]. Timesulin announces successful FDA registration and partnership with Facet Technologies. 2014. https://timesulin.com/timesulin-announces-successful-fda-registration-and-partnership-with-facet-technologies/. Accessed December 6, 2019.
- 14. Companion Medical [Internet]. Companion medical announces U.S. commercial launch of smart insulin pen system. 2017. https://www.prnewswire.com/news-releases/companion-medical-announces-us-commercial-launch-of-smart-insulin-pen-system-300571413.html. Accessed December 12, 2019.
- 15. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Ruiz L. Insulclock: a novel insulin delivery optimization and tracking system. Diabetes Technol Ther. 2019;21(4):209-214. [DOI] [PubMed] [Google Scholar]
- 16. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adolfsson P, Veijola R, Huot C, Hansen HD, Lademann JB, Phillip M. Safety and patient perception of an insulin pen with simple memory function for children and adolescents with type 1 diabetes–the REMIND study. Curr Med Res Opin. 2012;28(9):1455-1463. [DOI] [PubMed] [Google Scholar]
- 18. Asakura T, Jensen KH. Comparison of intuitiveness, ease of use, and preference in two insulin pens. J Diabetes Sci Technol. 2009;3(2):312-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cerna L, Maresova P. Patients’ attitudes to the use of modern technologies in the treatment of diabetes. Patient Prefer Adherence. 2016;10:1869-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danne T, Forst T, Deinhard J, Rose L, Moennig E, Haupt A. No effect of insulin pen with memory function on glycemic control in a patient cohort with poorly controlled type 1 diabetes: a randomized open-label study. J Diabetes Sci Technol. 2012;6(6):1392-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo X, Sommavilla B, Vanterpool G, Qvist M, Bethien M, Lilleore SK. Evaluation of a new durable insulin pen with memory function among people with diabetes and healthcare professionals. Expert Opin Drug Deliv. 2012;9(4):355-356. [DOI] [PubMed] [Google Scholar]
- 22. Ignaut DA, Venekamp WJ. HumaPen memoir: a novel insulin-injecting pen with a dose-memory feature. Expert Rev Med Devices. 2007;4(6):793-802. [DOI] [PubMed] [Google Scholar]
- 23. Klausmann G, Hramiak I, Qvist M, Mikkelsen KH, Guo X. Evaluation of preference for a novel durable insulin pen with memory function among patients with diabetes and health care professionals. Patient Prefer Adherence. 2013;7:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olsen BS, Lilleore SK, Korsholm CN, Kracht T. Novopen Echo® for the delivery of insulin: a comparison of usability, functionality and preference among pediatric subjects, their parents, and health care professionals. J Diabetes Sci Technol. 2010;4(6):1468-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Venekamp WJ, Kerr L, Dowsett SA, et al. Functionality and acceptability of a new electronic insulin injection pen with a memory feature. Curr Med Res Opin. 2006;22(2):315-325. [DOI] [PubMed] [Google Scholar]
- 26. Emperra E-Health Technologies. Scientific evaluation of the ESYSTA® S-T-A-R-T project 2019. German. https://www.emperra.com/wp-content/uploads/2019/08/Whitepaper-START_RZ_web_2019.pdf. Accessed December 12, 2019.
- 27. Hirsch IB, Sherr JL, Hood KK. Connecting the dots: validation of time in range metrics with microvascular outcomes. Diabetes Care. 2019;42(3):345-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370-2376. [DOI] [PubMed] [Google Scholar]
- 30. Adolfsson P, Hartvig NV, Kaas A, Moller JB, Hellman J. Increased time in range and improved insulin adherence after introduction of a smart connected insulin pen [published online ahead of print March 11, 2020]. Diabetes Technol Ther. doi: 10.1089/dia.2019.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. International Diabetes Federation [Internet]. IDF clinical practice recommendations for managing type 2 diabetes in primary care. 2017. https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html. Accessed December 12, 2019.
- 32. Munshi MN, Segal AR, Suhl E, et al. Assessment of barriers to improve diabetes management in older adults: a randomized controlled study. Diabetes Care. 2013;36(3):543-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gildon BW. InPen smart insulin pen system: product review and user experience. Diabetes Spectr. 2018;31(4):354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17(4):243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ritholz MD, Beverly EA, Brooks KM, Abrahamson MJ, Weinger K. Barriers and facilitators to self-care communication during medical appointments in the United States for adults with type 2 diabetes. Chronic Illn. 2014;10(4):303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toschi E, Slyne C, Greenberg JM, et al. Examining the relationship between pre- and postprandial glucose levels and insulin bolus timing using Bluetooth-enabled insulin pen cap technology and continuous glucose monitoring. Diabetes Technol Ther. 2020;22(1):19-24. [DOI] [PubMed] [Google Scholar]
- 37. Klonoff DC, Kerr D. Smart pens will improve insulin therapy. J Diabetes Sci Technol. 2018;12(3):551-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munshi MN, Slyne C, Greenberg JM, et al. Nonadherence to insulin therapy detected by Bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care. 2019;42(6):1129-1131. [DOI] [PubMed] [Google Scholar]
- 39. Brod M, Kongso J, Bushnell DM. The impact of memory problems on diabetes treatment in Germany. Value Health. 2013;16(7):A449-A450. [Google Scholar]
- 40. Smallwood C, Lamarche D, Chevrier A. Examining factors that impact inpatient management of diabetes and the role of insulin pen devices. Can J Diabetes. 2017;41(1):102-107. [DOI] [PubMed] [Google Scholar]
- 41. Kerr D, Warshaw H, Choi NY. Smart insulin pens will address unmet needs for people with diabetes using insulin. Endocrine Today. 2019;17(5):20-21. [Google Scholar]
- 42. Anderson WC, 3rd. Incorporating technology to advance asthma controller adherence. Curr Opin Allergy Clin Immunol. 2017;17(2):153-159. [DOI] [PubMed] [Google Scholar]
- 43. Kitt J, Fox R, Tucker KL, McManus RJ. New approaches in hypertension management: a review of current and developing technologies and their potential impact on hypertension care. Curr Hypertens Rep. 2019;21(6):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dst-10.1177_1932296820983863 for Digital Diabetes Management: A Literature Review of Smart Insulin Pens by Lutz Heinemann, Oliver Schnell, Bernhard Gehr, Nanette C. Schloot, Sven W. Görgens and Christoph Görgen in Journal of Diabetes Science and Technology


