Abstract
Objective:
People diagnosed with cancer have to deal with the debilitating psychological implications of this disease. Although the clinical efficacy of psychological interventions is well documented, relatively little has been written on the neural correlates of these treatments in the context of oncology. The present work is the first to provide an overall perspective of the existing literature on this topic. It also considers the potential directions for future research.
Methods:
This scoping review was carried out across 5 databases (EMBASE, PsycINFO, OVID MEDLINE, CINAHL, COCHRANE CENTRAL), from conception dates until 3 December 2021.
Results:
From an initial set of 4172 records, 13 papers were selected for this review. They consisted of 9 randomized controlled studies (RCTs), 1 quasi-experiment, 2 single case studies, and 1 secondary quantitative analysis. The studies were also heterogeneous in terms of the patient and control populations, psychological interventions, and neuroimaging methodologies used. The findings from these few studies suggest that psychological interventions in oncology patients may modulate both cortical and subcortical brain activity, consistent with the brain areas involved in distress reactions in general and to cancer specifically. The implications of this scoping review in terms of future research are also discussed.
Conclusions:
The literature on the neural correlates of psychological interventions in cancer patients is very limited, and thus requires further exploration. The provision of psychological interventions offers cancer patients a more integrated approach to care, which may in turn help preserve both the physical and the psychological wellbeing of individuals with cancer.
Keywords: cancer, neural correlates, neural signature, neuroimaging, oncology, psycho-oncology, psychological interventions, scoping review
Background
As stated by the World Health Organization, 1 the global cancer burden is increasing with major personal and social consequences. Treatment of the physical aspects of cancer generally takes center stage in the care of cancer patients; however, accumulating evidence is also starting to recognize the importance of considering the psychological implications of oncological disease. Between 30% and 45% of cancer patients experience high levels of health-related emotional distress, 2 as well as several physical and psychological issues that severely affect their quality of life. According to a recent review of epidemiological studies concerning psychiatric disorders in cancer patients, depression, anxiety, trauma or stress-related, somatic, neurocognitive disorders, and other subthreshold symptoms (such as an irritable mood, demoralization, or a sense of hopelessness) are the most common psychiatric conditions affecting cancer patients. 3 In particular, major depressive disorders and minor depressive episodes are very common in cancer patients, mainly related to pain and fatigue. Furthermore, phobias related to chemotherapy anticipatory nausea and vomiting are the most widespread anxiety issues, together with stress-related post-traumatic stress disorder (PTSD) sub-syndromic symptoms, which are estimated to affect 15% of cancer patients. Finally, the disease or its treatment (ie, chemotherapy) often impact several neurocognitive domains, including memory, attention, concentration, learning functions, calculation, and visual-spatial perception. 3 The concurrence of stress-related symptoms and disorders has been found to have a significant impact on a patients’ quality of life, and, as a consequence, on their global health outcomes. 4 Psychological distress in cancer patients can be reduced using psychosocial interventions, 5 although little has been written about the neurobiological mechanisms (such as brain networks, regions, or circuits involved) underpinning the clinical enhancements. One recent review, in addition to presenting the evidence on the effects of psychological interventions on clinical outcomes in cancer patients, reported on neuroimaging studies which correlate the negative psychological effects of cancer with major changes in brain activity. 2 Concerning the first point, the authors report on a number of stress management interventions (such as cognitive-behavioral approaches) which may reduce anxiety and depression, and in turn improve both quality of life and disease outcomes. Regarding the second point, they found that the distress states (mainly anxiety, depression, and PTSD) may be related to structural and functional alterations in the brain regions usually involved in emotional regulation, such as the amygdala (Amy), anterior cingulate cortex (ACC), hippocampus (Hy), hypothalamus (Hip), insula (Ins), prefrontal cortex (PFC), and thalamus (Th). It is noteworthy that neuroimaging studies have also indicated many of these areas (ie, ACC, PFC, Hy, and Amy) as being affected by psychotherapeutic interventions in depressed and PTSD patients.6,7 This is consistent with the idea that emotional dysregulation and abnormal brain activity, mainly within the cortico-limbic networks, are directly associated. 8 Investigations into the neural substrate of clinically observed changes may shine some light on the biological processes underpinning psychosocial interventions. The insights obtained may, in turn, help direct the further development of such interventions, and even establish why some patients respond to treatments better than others, as in the case of Cognitive Behavioral Therapy (CBT8,9) and Neurofeedback. 10 This is particularly relevant in the setting of cancer therapy, where acute and chronic stress can impact the onset of the disease as well as its progression.11-13
Thus, based on the findings of Reis and colleagues’, 2 who provide the neural description of psychological suffering within cancer patients, our work is a major stepping stone in the field which will ground the basis for future research. We propose that greater knowledge of the brain regions or circuits targeted by psychosocial interventions will be fundamental in paving the way to more targeted and effective treatments. For instance, describing the neural changes before and after a psychological treatment may overcome the intrinsic subjectivity of self-report measures and behavioral measures of psychopathology, 14 and this may help identify the specific factors of distinct interventions (eg, target brain area, duration of treatment, etc.) key to determining their clinical efficacy. Such evidence would undoubtedly support and promote the wider application of an integrated and comprehensive approach to cancer care as advocated by clinicians and researchers in the field of psycho-oncology. 4
The primary aim of this scoping review was to evaluate and summarize the available literature on the neural correlates of psychological interventions in the adult cancer population. Moreover, it aims to provide evidence on whether these structural and functional changes actually correlate with psychological outcome, as assessed using standardized and validated questionnaires.
Methods
Given that little has been written on the neurobiological correlates of psychological interventions in cancer patients, we adopted the scoping approach. This approach is designed to inform about the available evidence in a given field, the ways in which research has been conducted on a specific topic, and about the primary gaps in the current knowledge. 15 The scoping review framework set out by Arksey and O’Malley, 16 and formally reviewed by the Joanna Briggs Institute (JBI), 17 was used to guide the process. The 5 stages of the framework were: (i) identifying the research question(s); (ii) identifying the relevant studies; (iii) selecting the studies; (iv) extracting the data; (v) summarizing and discussing results. As suggested in the scoping framework of Sucharew and Macaluso, 18 a risk of bias appraisal regarding studies included is also provided.
Identifying relevant studies
A comprehensive research strategy was adopted in order to retrieve both published and unpublished studies. Electronic searches were performed on July 27, 2020, on 5 bibliographic databases (EMBASE, PsycINFO, OVID MEDLINE, CINAHL, COCHRANE CENTRAL) to identify potentially eligible studies. The search was updated on 3 December 2021, to identify and incorporate and further publications (see Supplemental Material). No restrictions were applied on language, gender, age, or publication date. The inclusion criteria are specified in Table 1. We targeted all forms of psychological intervention, including all forms of psychological, psychotherapeutic, or psychosocial intervention. Consistent with the inclusion criteria, we searched the oncology population as a whole. We confined our retrieval to a specific outcome, defined as any neuroimaging technique able to outline the neural correlates of the intervention’s effects.
Table 1.
Study Inclusion and Exclusion Criteria.
| Inclusion | Exclusion | |
|---|---|---|
| Design | Randomized and non-randomized controlled studies, case study/report, observational, case series, quasi-experiment, pre–post tests | Systematic reviews, trial protocols |
| Publication type | Peer-reviewed articles published in any language with available full text, conference proceedings, published abstracts, posters | |
| Participants | Adults aged ≥18, any cancer diagnosis and treatment phase | Participants <18, any other diagnosis |
| Interventions | Any psychological intervention | Any other type of intervention |
| Outcome | Any neurobiological technique | Any other technique |
Study Selection and Appraisal
Once all duplicates had been removed, the remaining titles were screened and studies with no clear relevance to the research topic were removed by 2 independent reviewers (PGR, LC), using a conservative approach as described by Higgins and colleagues. 19 The inclusion and exclusion criteria (Table 1) were then applied to the remaining abstracts in order to identify the eligible studies, for which the full-text papers were subsequently retrieved. If the full text was not available, the first or correspondence author was contacted. If no agreement could be reached regarding the inclusion of a study, even after full-text consultation, a third review author (FM) was involved to resolve the disagreement.
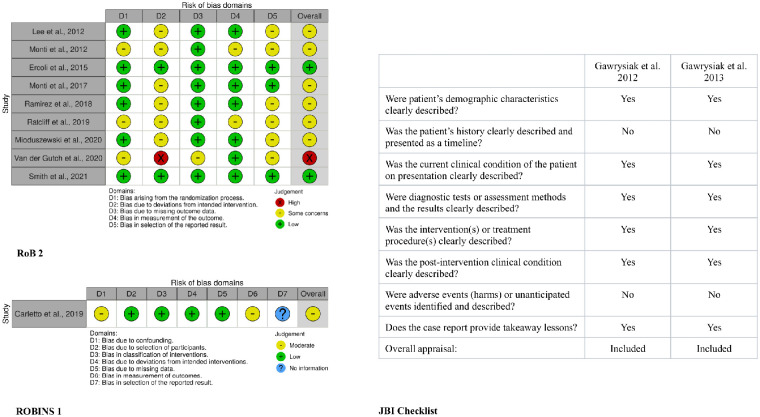
Two independents reviewers (PGR, FM) assessed the quality of the randomized controlled trials (RCTs) using the Cochrane RoB 2 Tool, 20 which can estimate the methodological weaknesses (risk of bias) on 5 specific domains: the randomization process (D1), the deviation from the intended intervention (D2), the missing outcomes data (D3), the measurement of the outcomes (D4), and the selection of reported results (D5) (Figure 2). An overall judgment of each RCT was thus obtained.
Figure 2.
Risk of bias assessment resume. The left-sided traffic light plots (ROB 2, ROBINS 1) provide an overview of the RCTs and quasi-experimental methodological quality, the right-sided table informs on the quality of single case studies.
The quality of the quasi-experimental study was assessed using the Cochrane ROBINS I Tool, 21 which can estimate the methodological weaknesses (risk of bias) on 7 specific domains: the confounding variables (D1), the selection of participants in the study (D2), the classification of interventions (D3), deviation from the intended interventions (D4), the missing outcomes data (D5), the measurements of outcomes (D6), and the selection of the reported results (D7) (Figure 2). An overall judgment of the quasi-experimental study was thus obtained.
The quality of case report studies was assessed using the only tool available: the JBI checklist for case reports, 22 which provides a quality assessment based on the patient’s demographic and history, the description of the current clinical condition, the treatment procedure explanation, the post-intervention outcomes reporting, the adverse events indication, and the presence of any takeaway lessons. An overall judgment of each case report regarding their inclusion or exclusion was thus obtained.
Data Extraction
After carefully reading the full-text versions of each study included in the scoping review, 2 reviewers (PGR, MCA) independently extracted the following data:
– Population: geographical location, sample size, and cancer type.
– Intervention/comparator: a short description of the psychological interventions and the control/comparator procedure used.
– Outcomes: the neuroimaging technique used to obtain the primary outcome; the name of the psychological distress assessment instruments used to obtain secondary clinical outcomes.
– Study design: a short description of the data collection procedures used.
Data were aggregated to answer the following scoping questions:
What are the most recurrent study designs?
What are the most recurrent oncological populations?
What are the most applied psychological interventions and comparators?
Which neuroimaging techniques are implemented the most to quantify the intervention outcomes?
What are the main intervention effects on neural outcome and clinical outcome, respectively?
Was it possible to correlate the neural and the clinical outcomes?
Results
Overview of the Included Articles
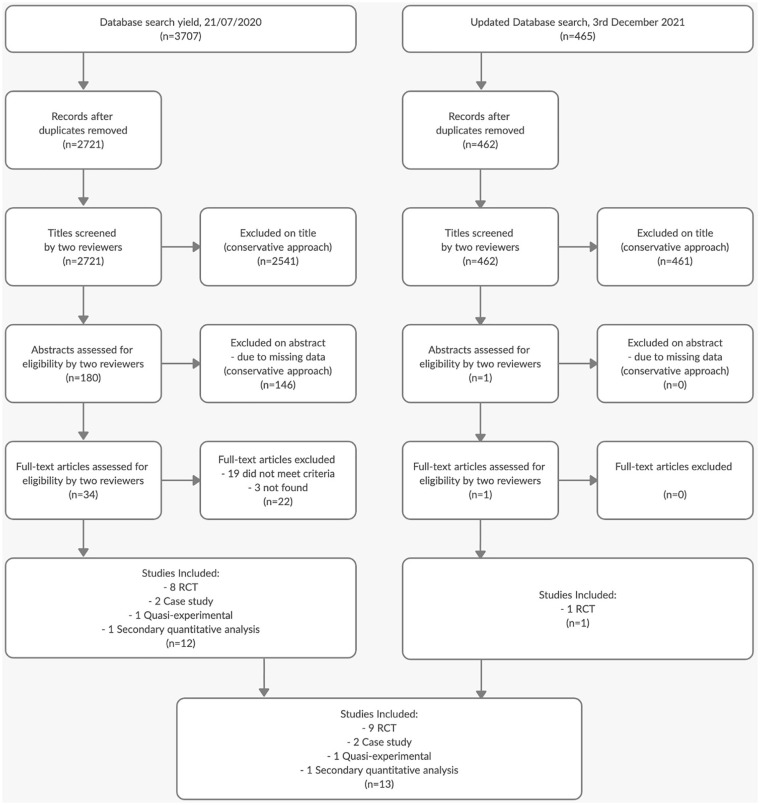
The study selection process is outlined in Figure 1 as a PRISMA flow diagram. 23 Thirteen manuscripts were considered eligible for inclusion in this review, and their characteristics are summarized in Table 2. All 13 records are articles published in journals and written in English. All studies were carried out in either North America or Europe. The oldest publication dated back to 2012, whereas the latest were published in 2021. The intervention groups of these studies amounted to a total of 182 patients, whereas the active control/waiting list group comprised 163 individuals. Neural data was obtained from a total of 148 patients, compared with 143 in the control/waiting group. Out of the 13 studies, 9 were randomized controlled trials, 1 was a quasi-experiment, 2 were single-case studies, and 1 constituted a quantitative secondary analysis of previously published data. In all studies, the neural and the clinical results for those in the experimental group are post-intervention compared to baseline. For studies with control conditions, there were no significant neural changes at post-study compared to baseline, with 1 exception. 24
Figure 1.
PRISMA flow chart of the studies selection process.
Table 2.
General Characteristics of the Included Studies.
| Authors | Study design | Population | Intervention (n) vs comparator (n)§ | Length of interventions | Neural outcome | Clinical outcome | Main results | Neural and clinical outcome correlations |
|---|---|---|---|---|---|---|---|---|
| Lee et al. 24 | RCT | Gynecologic cancer undergoing the first chemotherapy | MC (18); (16) vs PMR (19); (14) | MC: 34 min PMR: 34 min |
EEG: PSD | STAI-G, FB1 | MC and PMR: < anxiety and improved psychological states during chemotherapy + > posterior θ, < midfrontal β2 band MC: <α band posterior activity compared with PMR |
Not reported |
| Monti et al. 25 | RCT | Patients with breast cancer | MBAT (8) vs EC (10) | MBAT: 8-wk program | fMRI: CBF (rest, meditation, stress task) | SCL-90 (anxiety sub-scale) | MBAT: < anxiety + > CBF in left insula, right amygdala, right hippocampus, bilateral caudate during rest and meditation Stress task: < posterior cingulate |
MBAT: * > CBF left caudate and < anxiety score during rest condition |
| Ercoli et al. 26 | RCT | Breast cancer survivors + cognitive complaints | CR (29); (20) vs WL (16); (9) | CR: 5-wk, 2 h per wk | qEEG: PSD | PAOFI, BDI-II, cognitive batteries3 | CR: > PAOFI, RAVLT scores + <δ global power > frontal distribution of α power |
Not reported |
| Monti et al. 27 | RCT | Cancer patients + PTSD symptoms | NET (11) vs WL (12) | NET: 3 to 5 × 1-h sessions for ~1 mo | fMRI: BOLD (neutral, stress condition) | STAI, PTCI, BSI-18, IES | NET: < distress, anxiety and traumatic stress + < BOLD in right parahippocampus, brainstem, right anterior cingulate, left insula during stress condition (< reactivity) | Not reported |
| Monti et al. 28 | RCT (secondary quantitative analysis of Monti et al. 27 ) | Cancer patients + PTSD symptoms | NET (11) vs WL (12) | NET: 3 to 5 × 1-h sessions for ~1 mo | fMRI: FC + HR (stress condition) | STAI, PTCI, BSI-18, IES | NET: > functional connectivity between cerebellum and amygdala, parahippocampus, brainstem, < HR in response to distressing memories | NET: * < HR and < IES values |
| Ramirez et al. 29 | RCT | Terminally ill patients | MT (20) vs CG (20) | MT: 1 session ~30 min | EEG: PSD | ESAS | MT: < anxiety, tiredness, breathing difficultness, > well-being + > valence and arousal4 | Not reported |
| Ratcliff et al. 30 | RCT | Women undergoing SBB (partly breast cancer) | GM (30); (8) vs FB2 (30); (13) vs SC (16); (5) | GM: ~35 min FB2: ~35 min |
EEG: CSD | STAI, BPI, VAS | GM: >β activity during biopsy insula and ACC+ steeper reduction in anxiety during the biopsy compared with control groups (group × time effect on VAS anxiety ratings). FB2: similar trend to GM with >β during biopsy in insula and ACC GM, FB2: non-significant δ decrease precuneus |
* <δ precuneus and < VAS anxiety (not associated with groups) |
| Mioduszewski et al. 31 | RCT | Breast cancer survivors + CNP | MBSR (13) vs WL (10) | MBSR: 1-2.5-h sessions for 8 wk + 1 full day session during the fifth week | MRI: DTI-FA | BPI, FFMQ | MBSR: < pain severity and interference + > FA left uncinatum and fronto-occipital fasciculi, left amygdala, left hippocampus, left external capsule, left sagittal striatum | MBSR: * > FA values and < BPI scores |
| Van der Gucht et al. 33 | RCT | Breast cancer survivors + cognitive impairment | MBI (12) vs WL (13) | MBI: 4 × 3-h sessions over 8 wk | fMRI: FC | DASS, CIS (fatigue subscale), CIM, CFQ | MBI: < cognitive impairments on subjective measures, emotional distress, fatigue + > connectivity between IPS and ACC networks |
MBI: * > connectivity ACC-lIPS and < emotional distress |
| Smith et al. 32 | RCT | Breast cancer survivors + CNP | MBSR (13) vs WL (10) | MBSR: 1 × 2.5-h sessions for 8 wk + 1 full day session at the end | fMRI: FC | BPI, FFMQ | MBSR: < pain severity and interference + >FC PCC and ACC/medial PFC (FC1) + <FC PCC and bilateral precentral gyrus, right superior frontal gyrus and pons (FC2) | MBSR: * >FC1 and <Ps scores + * <FC2 and <Ps scores |
| Gawrysiak et al. 34 | Case report | Patient with breast cancer and depression | BATD (1) | BATD: 8 × 1-h sessions | fMRI: BOLD response (neutral, preferred music; music, silence) | BDI II, HRSD, EROS, BIS, BAS | BATD: < depression + preferred music, music > BOLD rdlPFC, rmoPFC, neutral music > BOLD subgenual cingulate cortex | Not reported |
| Gawrysiak et al. 35 | Case report | Patient with breast cancer and depression | PPP (1) | PPP: 8 × 1-h sessions over 13 wk | fMRI: BOLD response (neutral, preferred music) | BDI II, BAI, MSPSS, HRSD, EROS | PPP: < depression + preferred music > BOLD vmPFC and loPFC | Not reported |
| Carletto et al. 36 | Quasi Exp | Patients with breast cancer + PTSD | EMDR (14) vs TAU (15) | EMDR: 10 sessions over 2 to 3 mo | EEG: PSD | IES-R, SCID-5, CAPS-5, STAI-Y, BDI-II | EMDR: < depressive symptoms + >δ and θ power, left angular and right fusiform gyri | EMDR: * < clinical symptoms and >δ, θ bands |
Abbreviations: <: decrease; >: increase; *: correlation; §: when multiple sample size numbers are provided, the first indicates the total number of participants, while the second refers to the number of participants with analyzable neural outcome data; ACC: anterior cingulate cortex; Arousal4: ratio of β on α power over the prefrontal cortex; BADT: behavioral activation treatment for depression; BAI: beck anxiety inventory; BAS: behavioral activation scale; BDI II: beck depression inventory-II; BIS: behavioral inhibition scale; BOLD: blood oxygenation level dependence; BPI: brief pain inventory; BSI-18: brief symptom inventory; CAPS-5: clinician-administered PTSD scale; CBF: cerebral blood flow; CFQ: cognitive failure questionnaire; CG: control group; CIM: comprehensive inventory of mindfulness experiences; CIS: checklist individual strength; CNP: chronic neuropathic pain; cognitive batteries3: (brief visual memory test-revised) − (verbal fluency tests) − (paced auditory serial addition test) − (trail making tests) − (computerized CNS vital signs) − (WTAR: Wechsler test of adult reading); CR: cognitive rehabilitation; CSD: current source density; DASS: depression anxiety stress scales; DTI: diffusion tensor imaging; EC: educational control group; EEG: electroencephalography; EMDR: eye movement desensitization and reprocessing therapy; EROS: environmental reward observation scale; ESAS: Edmonton symptom assessment system; FA: fractional anisotropy; FB1: questionnaire about physical and psychological states and perception of the relaxation treatments; FB2: focused breathing; FC: functional connectivity; FFMQ: five facet mindfulness questionnaire; fMRI: functional magnetic resonance imaging; GM: mindfulness-based meditation; HR: heart rate; HRSD: Hamilton rating scale for depression; IES: impact of event scale; IES-R: impact of event scale-revised; IPS: intraparietal sulcus; lIPS: left intraparietal sulcus; loPFC: left orbital prefrontal cortex; MBAT: mindfulness-based art therapy; MBI: mindfulness-based intervention; MBSR: mindfulness-based stress reduction; MC: monochord (sounds); MR: magnetic resonance; MSPSS: multidimensional scale of perceived social support; MT: music therapy; NET: neuro emotional technique; Ps: pain severity; Pi: pain interference; PAOFI: patient’s assessment of own functioning inventory; PCC: posterior cingulate cortex; PFC: prefrontal cortex; PMR: progressive muscle relaxation; PPP: pragmatic psychodynamic psychotherapy; PSD: power spectral density; PTCI: post-traumatic cognitions inventory; PTSD: post-traumatic stress disorder; qEEG: quantitative electroencephalography; RAVLT: Rey auditory verbal learning test; RCT: randomized controlled trial; rdlPFC: dorsolateral prefrontal cortex (right-side); rmoPFC: medial orbital prefrontal cortex (right-side); rs: resting-state; SBB: stereotactic breast biopsy; SC: standard care; SCID-5: structured clinical interview for DSM-5; SCL-90: symptoms checklist-90-revised; STAI: state trait anxiety index; STAI-G: German version of the state anxiety inventory; STAI-Y: state-trait anxiety inventory Y; TAU: treatment as usual; Valence4: computed as the difference between the right and left frontal α power, that is αF4–αF3; VAS: visual analogue scales; vmPFC: ventral medial prefrontal cortex; WL: waiting list.
RCT Studies
Lee and colleagues 24 carried out an RCT to describe the relaxation effect of 30 minutes of monochord sounds (MC) on the power spectral density of the electroencephalography (EEG) signal and anxiety in gynecologic cancer patients during chemotherapy compared with a well-established relaxation technique (Progressive Muscular Relaxation [PMR]). The authors found increased posterior θ power and decreased midfrontal β2 power in response to the sound. However, the same effect was found in the control group treated with PMR. Both groups showed a reduction in anxiety level and an improved psychological state. A decrease in α power was observed in the MC intervention group only. No correlations or associations between neural and clinical outcomes were reported.
Monti and colleagues 25 evaluated changes in functional brain activation, using functional magnetic resonance imaging (fMRI), and anxiety scores following an 8-week mindfulness-based art therapy (MBAT) intervention in patients with breast cancer. Results were compared with those obtained in an educational control group, provided with support and resources to maximize QoL. The MBAT technique entails expressive art tasks and aims to enable self-expression and regulation. Changes in brain activation were assessed when the patients were in 5 different states: at rest (initial and final; no task demanded), during meditation (body scan, similar to those learnt during MBAT training), during a neutral task (passive listening), and during a stressful task (ie, counting backwards from 1000 for 7 minutes). The authors reported higher CBF (cerebral blood flow) signals in the left insula cortex, right amygdala, right hippocampus, and bilateral caudate nucleus during the initial rest period and during meditation compared to baseline (ie, brain activity pre MBAT intervention) in the experimental group. Furthermore, in the experimental group, the CBF signals detected during the final rest period and during the stressful task showed a tendency to be lower, although statistical significance was not achieved. Furthermore, a significantly lower CBF signal was found over the posterior cingulate in response to the stressful task, with a subsequent reduction in reactivity. Finally, the higher CBF signal detected during the initial rest period was correlated (r = .90; P < .002) with lower anxiety levels in the MBAT group. These results were not found in the educational control group.
Ercoli and colleagues 26 evaluated the effects of 5 weeks of cognitive rehabilitation (CR) for attention, executive, and memory functions on cognitive complaints and EEG signals in breast cancer survivors. The authors reported improved subjective measures of cognitive complaints and objective cognitive performance indexes. Furthermore, these improvements were accompanied by overall decrease in δ “slow-wave” power and an increase frontal distribution of α power with respect to baseline in the experimental group, which were not observed in the waiting list group (WL). These changes in α power were significantly associated with reduction in cognitive complaints as assessed 2 months after the rehabilitation program. Notably, the benefits of the interventions lasted up to 2 months.
Monti and colleagues 27 performed an RCT to evaluate the effect of a 1-hour Neuro Emotional Technique (NET) on functional brain activity, using fMRI, and traumatic stress symptoms in cancer patients (various diagnoses) with symptoms of PTSD, related to distressing memories (ie, the diagnosis itself, painful medical procedures, etc.). Importantly, since a full PTSD diagnosis occurs in just 3% to 10% of cancer cases, only patients with subsyndromal PTSD cancer-related symptoms were included in the study. NET is a form of complementary and alternative medicine (CAM). Its aim is to regulate the hyperactivation of the autonomic nervous system, reducing the impact of traumatic stress-related symptoms. To assess the effects of NET on traumatic stress responses, neutral and traumatic auditory stimuli (self-generated scripts related to the individual’s morning routine and to a distressing cancer-related event, respectively) were delivered to patients while fMRI assessments were performed. Patients who had undergone the NET intervention showed a decrease BOLD signal in the right parahippocampus, brainstem, right anterior cingulate gyrus, and left insula during the stress condition (ie, less reactivity to the distressing stimuli) compared to baseline together with a reduction in the clinical level of distress, anxiety, and traumatic stress. These results were not found in the waiting list control group. The reduction in neurophysiological reactivity was not significantly associated with clinical improvement. Moreover, secondary functional connectivity and autonomic reactivity (heart rate [HR]) analyses were performed in the same sample 28 in order to outline the neural correlates of the NET intervention better. The analysis revealed greater functional connectivity between the cerebellum and the amygdala and the parahippocampus and the brain stem in response to NET, together with a smaller rise in HR in response to distressing stimuli. This last decrease was correlated (r = .45; P = .047) with a smaller impact of the trauma.
Ramirez and colleagues 29 carried out an RCT to investigate the effect of 30 minutes of both active and receptive music therapy (MT) on the brain oscillatory activity (as assessed via EEG) and the emotional states (anxiety, anger, depression, stress) in terminally ill cancer patients. MT relies on the therapeutic effects of listening to sound on emotional, attentional, and cognitive regulation. The results reported an increased arousal state together with a more positive emotional valence in response to listening to music. MT was also found to decrease anxiety, tiredness, and the level of breathing difficulty and to increase well-being. These effects were not observed in the control group provided with company. No correlations or associations between neural and clinical outcomes were reported.
Using EEG current source density (CSD) analysis, Ratcliff and colleagues 30 compared the effect of 35 minutes of mindfulness-based guided meditation (GM), which entails an increase of self-awareness and regulation, with the effects of guided focused breathing (FB) and standard care (SC) control groups on cortical brain rhythms and levels of anxiety and pain in women scheduled for stereotactic breast biopsy (SBB). The results show greater values of β band power in the Ins (Cohen’s d = 1.4) and the ACC (d = 1.0) in the GM group compared with subjects in the SC group, together with lower anxiety levels. Notably, the authors report a group × time effect/interaction for visual analogue scale (VAS) anxiety ratings during the biopsy procedure compared with the control groups. The active control group (FB2) showed an increase in β band power in the Ins (d = 1.6) and the ACC (d = 0.70) similar to that observed in the GM group. Moreover, a non-significant δ band power decrease in the precuneus was found in both the GM and the FB group. Finally, the decrease in δ power in the precuneus was associated with decreased anxiety during the biopsy (VAS; r = .51, P = .009), although this was not associated with any specific group.
Mioduszewski and colleagues 31 used diffusion tensor imaging (DTI) to investigate the effects of an 8-week mindfulness-based stress reduction (MBSR) program on white matter integrity and pain levels in breast cancer patients. MBSR programs consist of training in self-awareness and stress management and are believed to help reduce chronic neuropathic pain. The MBSR training increased the fractional anisotropy (FA) in the left uncinatum and fronto-occipital fasciculus, left amygdala, left hippocampus, left external capsule, and left sagittal striatum as compared with the pre-MBSR scan. This was correlated with a decrease in the pain severity (r = −.49; P < .05) and pain interference scores (r = −.436; P < .05). This result was not found in the waiting list control group.
Smith and colleagues 32 used resting state fMRI to investigate the impact of an 8-week MBSR program on functional connectivity (FC), pain severity, and pain intensity in breast cancer survivors with comorbid chronic neuropathic pain as compared with a WL control group. In the MBSR group, FC was increased between the posterior cingulate cortex (PCC) and the ACC/medial prefrontal cortex and decreased between the PCC and the bilateral precentral gyrus, right superior frontal gyrus and pons. Moreover, the increase in FC was correlated with decrease in pain severity scores (r = −.57; P < .0005), and the decrease in FC was correlated (r = −.51; P = .014) with greater pain severity. These results were not found in the waiting list control group.
Finally, Van der Gucht and colleagues 33 used fMRI to compare the impact of an 8-week mindfulness-based intervention (MBI) on FC, emotional distress, and fatigue in breast cancer survivors with cognitive impairments with the same measures assessed in a WL control group. The fMRI routine was performed before commencing the intervention (T1), at the end of the 8-week treatment (T2), and 3 months post-intervention (T3). Increased connectivity was found between the ACC and both the left and the right intraparietal sulcus (IPS) networks at T2, along with improvements in distress, fatigue, and emotional distress symptoms. Moreover, the amelioration in distress symptoms was correlated (r = −.57; P = .004) with the increase in FC between the ACC and left IPS.
Non RCT Studies
Gawrysiak et al.34,35 reported on 2 cases of breast cancer patients with depression, where an fMRI music-based paradigm was applied to assess reward responsiveness in response to psychotherapies. Reward responsiveness is believed to be low in depressed individuals and may be involved in causing and maintaining the depressed state. Thus, the rationale behind Gawrysiak’s approach was to assess the impact of psychotherapies believed to reinforce the ability to react and enjoy positive experiences on the functional activation of several brain regions involved in reward responsiveness and on clinical symptoms.
In the first case study, following 8 sessions of behavioral activation treatment for depression (BATD), which aims to increase and reinforce healthy and positive behaviors, 34 brain activity was found to increase over the right dorsolateral prefrontal cortex (dlPFC) and medial orbital prefrontal cortex (moPFC) when the patient listened to either preferred or neutral music, whereas it decreased in response to neutral music over the subgenual cingulate cortex. The second case study 35 used fMRI to assess the effects of 8 sessions of pragmatic psychodynamic psychotherapy (PPP), which involved the identification of key relational and conflictual themes. The study revealed increased brain activity over the ventromedial prefrontal cortex (vmPFC) and the left orbital prefrontal cortex (loPFC) during preferred music listening. Both studies reported improvements in depressive symptoms, which were not correlated with changes in neural activity.
Finally, Carletto and colleagues, 36 in a quasi-experimental design study, compared the efficacy of 10 sessions of eye movement desensitization and reprocessing (EMDR) with 4 sessions of EMDR treatment in reducing PTSD symptoms in breast cancer patients. The impact of the 2 treatments on brain cortical oscillations (as assessed with EEG power spectral density) was also described. The authors reported an increase in δ and θ powers of the left angular and the right fusiform gyri in response to EMDR only. Moreover, authors reported significant correlations (P < .05) between δ power changes and decreases in clinical scores of depression and traumatic stress.
Risk of Bias Assessment
The result of the risk of bias appraisal is shown in Figure 2. In terms of methodology, RCT studies show a medium overall risk of bias, mainly due to participants’ non-adherence to their assigned intervention or a failure in observing the protocol. Results were partially selected according to their direction, magnitude, or statistical significance. Furthermore, subjects were often aware of the forthcoming treatment as often happens in psychological settings, where it is hard to leave the participant blind to procedures. Finally, major differences in equipment and methods were observed, leading to inconsistent results (this applies above all to EEG data). The quasi-experiment is marked by a moderate overall risk of bias, especially around the confounding variables domain, where the patients’ prognostic factor can predict their allocation; moreover, the outcome measure is at moderate risk due to the assessor’s awareness of the nature of the intervention. Finally, the case report studies were included in this scoping review since the JBI checklist highlighted methodological in 2 criteria only (patient’s history, adverse events identification).
Discussion
To the best of our knowledge, the present scoping review is the first to address the neural correlates of psychological interventions in the oncological population. Due to the detailed search strategy and the conservative screening approach applied, only 13 original studies were identified on this topic. Furthermore, the current literature is very heterogeneous with respect to intervention protocols and the types of neuroimaging methods adopted, the patient’s disease stage and type of cancer, the types of oncological treatments received, patient gender, the time elapsed since cancer diagnosis, and the concurrent assumption of psychoactive drugs, all of which this might contribute to inconsistent results. Moreover, our findings are mostly based on small and low powered samples, which does not allow us to draw any objective conclusions about the actual effects of the psychological interventions. Furthermore, most of the studies did not specify the duration of the observed effects, which is relevant for determining which interventions can exert long-lasting benefits. Finally, the majority of the studies (expect for Ratcliff et al. 30 ) quantify the impact of interventions in terms of P-values, rather than effect size.
The following discussion questions will address the scoping questions in order to give a broad appreciation of the available evidence.
What Are the Most Recurrent Study Designs?
Although the total number of studies considered was only 13, the majority of them were RCT’s (n = 9). The remaining studies adopted weaker methodologies, such as the quasi-experiment (n = 1), single case studies (n = 2), and secondary quantitative analysis (n = 1). More research adhering to methodologically strong approaches is essential to be able to draw more robust conclusions.
What Are the Most Recurrent Oncological Populations?
The most studied population consisted of breast cancer patients (9 studies): 7 studies reported on the neural correlates of psychological interventions and their effect on the clinical outcomes of various comorbid conditions (PTSD, cognitive complaints, cognitive impairments, chronic neuropathic pain, and depression); 1 study combined EEG studies with the assessment of parameters linked to anxiety and pain in women scheduled for medical procedure (stereotactic breast biopsy); and one did not address comorbidities at all. Regarding other patient populations, 1 study enrolled women with gynecological cancer (ovarian or cervical carcinoma), and another study enrolled terminally ill oncological patients. Finally, the remaining 3 studies enrolled patients with different forms of cancer and comorbid PTSD.
Since the majority of the studies were focused on breast cancer patients or survivors, the results obtained from the individual studies and scoping review cannot be generalized across oncological populations.
Which Are the Most Applied Psychological Interventions and Comparators?
Five studies applied a mindfulness-based technique to address anxiety, pain, and cognitive impairments. One study, which applied quantitative secondary analysis, implemented NET to regulate the hyperactivation of the autonomic nervous system and reduce the impact of PTSD symptoms. Two studies employed an intervention involving sound to impact anxiety and pain. Three studies applied structured psychotherapeutic interventions, namely an EMDR protocol, a form of psychodynamic therapy (PPP), and a behavioral therapy (BATD), to reduce depressive and PTSD symptoms. Finally, 1 study applied a form of cognitive rehabilitation with the aim of ameliorating cognitive complaints. Looking at the comparators, most of the studies included a WL control group. Other studies entailed support, company (ie, conversation), FB, SC, and PMR control groups.
Which Neuroimaging Techniques Are Implemented the Most to Quantify the Intervention Outcomes?
The literature considered in this scoping review mainly focused on EEG indexes and MRI measures. The power spectral density and the current source density, both of which estimate the frequency distribution of the EEG signal, were the measures most implemented to evaluate the cortical response to treatments. In relation to MRI imaging, on the other hand, and consistent with current trends in psychological research, 2 main pathways were followed 2 : the functional pathway, which was the most reported approach (7 studies), based on the role of local hemodynamic responses linked to changes in neural activity; and the structural pathway, such as diffusion tensor imaging (1 study) which provides a measure of the integrity of white matter between brain regions and their communication. Some of the key neurobiological outcomes assessed in the context of psychotherapy research are thought to seem to relate to spatial and temporal correlations between brain regions which may outline the modulation of large-scale brain networks, such as default, affective, and attentional networks. 6 In the oncological context, this indeed may prove to be a promising route of investigation; in fact, the present review revealed several studies that addressed measures of connectivity between brain regions. Furthermore, 1 study 28 assessed heart rate as a parameter of autonomic arousal and stress outcome. An alternative measure of this outcome could be heart rate variability (HRV; a measure of the fluctuation in the length of heartbeat intervals), shown to be a reliable bottom-up indicator of psychological distress 37 and ANS (autonomous nervous system)-heart interplay. 38 Finally, other biomarkers of treatment efficacy have been proposed, such as the output measures generated from the combination of EEG with transcranial magnetic stimulation (TMS), which may directly and reliably assess cortico-cortical excitability and connectivity, in a non-invasive way, in the context of psychological research. 39
What Are the Main Intervention Effects on Neural Outcome and Clinical Outcome, Respectively?
The main neural effects of treatments are illustrated in Figure 3. The results are discussed according to the type of psychological intervention and refer to the brain areas most involved (Table 3). The impact of mindfulness practices on neural activity is outlined in relation to several brain regions involved in high-level brain processes, such as attentional control and emotional regulation. In particular, the studies considered in this review looked at activity in the Ins, the ACC, and the PFC to investigate the impact of mindfulness practices at the neurobiological level. 40 Their results provide evidence that mindfulness strengthens prefrontal cognitive control mechanisms, with a concomitant downregulation of activity in regions relevant for affect processing, such as the amygdala and subcortical regions. 41 Conversely, the included studies report activation of both limbic and cortical brain regions in breast cancer patients treated with mindfulness,25,30-33 which is typical in untrained meditators. 42 Thus, although limbic activation is consistent with other fMRI studies, it needs to be further explored as these structures are expected to be less active in relaxation paradigms. 25 In this respect, future studies should consider the “expertise” variable, as the activation pattern in expert meditators entails cortical/prefrontal activation at the expenses of limbic components, which are primarily involved in emotional processing. 42
Figure 3.
Overview of the psychological interventions’ neural correlates. Each panel focuses on a specific intervention type.
Abbreviations: aCC, anterior cingulate cortex; aG: angular gyrus; Amy: amygdala; BS: brain steam; Cau: caudate; dlPFC: dorsolateral prefrontal cortex; ExC: external capsule; fG: fusiform gyrus; FOF: fronto-occipital fasciculus; Hip: hypothalamus; Ins: insula; iPS: intraparietal sulcus; moPFC: medial orbital prefrontal cortex; oPFC: orbital prefrontal cortex; pCC: posterior cingulate cortex; pHip: parahippocampus; PreC: precuneus; sCC: subgenual cingulate cortex; SS: sagittal striatum; Unc: uncinatum; vmPFC: ventromedial prefrontal cortex.
Table 3.
Overview of the Brain Regions Impacted by Each Intervention.
| Authors | Intervention | Brain areas |
|---|---|---|
| Mindfulness-based interventions | ||
| Monti et al. 25 | Mindfulness-based art therapy | ↑ Subcortical regions (insula, right amygdala, right hippocampus, bilateral caudate) |
| Ratcliff et al. 30 | Guided meditation Focused breathing |
↑ Subcortical regions (insula, ACC) ↓ Precuneus |
| Mioduszewski et al. 31 | Mindfulness-based stress reduction | ↑ Left subcortical regions (uncinate fasciculus, amygdala, hippocampus, external capsule) |
| Van der Gucht et al. 33 | Mindfulness-based intervention | ↑ Salience network (ACC), dorsal attention network (left and right IPS) |
| Smith et al. 32 | Mindfulness-based stress reduction | ↑↓ Default mode network: AAC, PCC |
| Neuroemotional technique | ||
| Monti et al. 27 | Neuroemotional technique | ↓ Subcortical regions (parahippocampus, brainstem, anterior cingulate, insula) |
| Monti et al. 28 | Neuroemotional technique | ↓ Subcortical regions (amygdala, parahippocampus, brain stem) |
| Structured psychotherapeutical interventions | ||
| Gawrysiak et al. 34 | Behavioral activation treatment for depression | ↑ Prefrontal regions (rmoPFC, rdlPFC), subgenual cingulate |
| Gawrysiak et al. 35 | Pragmatic psychodynamic psychotherapy | ↑ Prefrontal regions (loPFC, vmPFC) |
| Carletto et al. 36 | Eye movement desensitization and reprocessing therapy | ↑ Subcortical regions Left angular and right fusiform gyri |
| Music-based interventions | ||
| Lee et al. 24 | Monochord sound | ↑ Occipital, ↓ Frontal regions |
| Ramirez et al. 29 | Music therapy | ↑ Prefrontal regions |
| Cognitive rehabilitation | ||
| Ercoli et al. 26 | Cognitive rehabilitation | ↓ Generalized slow waves decrease; ↑ Frontal regions |
Abbreviations: ACC: anterior cingulate cortex; IPS: intraparietal sulcus; PCC: posterior cingulate cortex; rmoPFC: medial orbital prefrontal cortex (right-sided); rdlPFC: dorsolateral prefrontal cortex (right-sided); loPFC: left orbital prefrontal cortex; vmPFC: ventral medial prefrontal cortex.
Although research into the effects of NET is still in its early days,27,28 the evidence obtained thus far support the potential of this tool for investigating the subsyndromal traumatic stress symptoms reported by cancer patients and survivors. This may be done by investigating the role of the cerebellar functional pathway to the brainstem and limbic areas (mainly the parahippocampus, Ins, and ACC) in mediating the traumatic response to cancer. NET (as well as other mind-body therapies) seems to normalize the reactivity of these regions, enhancing self-regulation and favoring less-aroused states. 43 In particular, the involvement of these structures seems to be consistent with the findings from investigations into the neural features of PTSD in cancer patients, 37 and certainly needs to be investigated further.
Evidence from psychotherapeutic interventions are scarce. The 2 case studies of patients with both depression and cancer34,35 report that BATD and PPP trigger the hyperactivation of areas of the PFC (dlPFC, moPFC, oPFC) and cingulate cortex. This is consistent with other studies that investigated the neurobiological outcomes of therapeutic interventions in anxiety and depression disorders, and which reported neural activity changes in the cortico-limbic brain regions implicated in emotion regulation. 8 Similarly, EMDR seems to increase activation of associative cortical areas at the expense of the limbic components, which might be considered a neural correlate of improvements in PTSD symptomatology.44-47 The role of each PFC area in mediating the beneficial effects of psychotherapy in cancer patients remains to be clarified, which will require the implementation of more methodologically robust study designs (such as RCTs).
Studies looking at the effects of music therapy on brain activity revealed a pattern of posterior θ and α modulation. 29 This prompts the hypothesis that the effects of music therapy on patterns of brain cortical oscillations are similar to those obtained with meditation, which similarly leads to an increase in δ rhythms. 48 Moreover, evidence suggests that β rhythm asymmetry in frontal regions may be a sign of a more positive emotional state. 49 Furthermore, music seems to determine more sustained arousal (ie, less depressed mood) and more positive emotions, which is relevant within the context of palliative care. 29 However, larger sample sizes are needed to describe this effect in more detail.
Just 1 study 26 addressed cognitive complaints in breast cancer patients undergoing cognitive rehabilitation. Chemotherapy is often associated with cognitive dysfunctions (the so-called “chemobrain”), which can negatively impact the quality of life. 50 The reported overall reduction in δ power at 2 months after the intervention may reflect an improvement in cognition since slow EEG waves are associated with mild cognitive impairments. 51 However, these are preliminary outcomes only, and therefore need be replicated in further, more powerful studies. It is also highly noteworthy that all the included studies reported improvements in clinical psychological measures, showing treatments to be effective in reducing anxiety, depression, stress-induced pain, fatigue, and cognitive complaints in people dealing with cancer.
Overall, it is interesting that most of the areas targeted by psycho-oncological interventions are coherent with those found to be involved in distress reactions to cancer, 2 such as the ACC, prefrontal cortices, Hy, Amy, and Ins. This may mean that different psychological interventions share a common neural mechanism of action, where the higher-level areas modulate the hyperactivation of subcortical regions, leading to better emotional regulation and balance.41,47 Future studies should consider each area’s role in mediating the beneficial effect of several therapies, especially from a functional point of view.
Was It Possible to Correlate the Neural and the Clinical Outcomes?
Seven studies reported a significant positive or negative correlation between neurobiological effects and clinical outcome. Only statistically significant correlations are reported herein. Inconsistencies in the results may be due to low sample sizes and differences in the statistical power. Investigating the correlations between observed neurobiological changes and clinical outcome is essential in order to be able to understand what changes in neural activity may underlie the clinical effect of an intervention. Thus, future studies investigating the effectiveness of psychological interventions in cancer patients should also endeavor to gather data on the neural correlates of their treatments, such that new insights may come to light.
Study Strengths and Limitations
This present review adhered to the established guidelines for a scoping review17,18 and adopted a comprehensive search strategy involving 5 databases. The study design stages were carried out by at least 2 independent reviewers to ensure the highest degree of objectiveness and to be as inclusive as possible. An important strength lies in the fact that the review overviewed the existing evidence on a barely investigated topic. Moreover, this work followed a study protocol established a priori but which is not registered, since this is not a requirement for scoping reviews. However, this work is not exempt from limitations. Firstly, despite our attempts to be as comprehensive as possible, it is not impossible that gray literature published outside the standard channels exists and was missed by the review. Secondly, this study is significantly limited in that it does not provide any concrete guidance from a clinical or policy-making point of view; however, this is not the objective of a scoping review. Thirdly, for the same reason, the present work does not infer the effect size of interventions from the original studies. Finally, the review is not reporting all statistically non-significant (P < .05) results from primary studies, which may also be considered to generate additional hypothesis in future studies.
Clinical Implications and Future Directions
The limited and heterogeneous nature of the results does not allow us to provide any recommendations regarding specific treatments or protocols for specific conditions, which again was not an aim of this scoping review. Despite this, the observed improvement of distress symptoms following psychological interventions is notable and of great value as it shows that such treatments are able to positively impact upon quality of life in these patients as well as oncological disease outcomes. 4 Furthermore, although psychopharmacology options are valuable tools for treating symptoms of distress related to oncological disease, several papers have called for the need for more integrated cancer care, including psychosocial/psychotherapeutic, psychopharmacological, and complementary therapies. 52 In order for such strategies to become endorsed, determining whether non-pharmacological treatment options can exert significant changes on clinical symptoms and on brain activity is fundamental. Indeed, several studies have reported the effectiveness of psychotherapy in modulating the brain functioning across psychopathological conditions,8,53,54 and the same might be true for psychological interventions in the oncological context, where the distress burden has been shown to worsen the prognosis.11-13 Before recommending psychological interventions as evidence-based treatment options, much more research will be required in order to systematically assess their clinical and neural efficacy. Moreover, future studies should compare the aforementioned neural correlates with those found to underlie psychopharmacological treatments for emotional distress in cancer, as suggested by the literature on the effectiveness of psychotherapy. 53 Also, future research should include a follow-up assessment to describe the long-term effects of treatments, to control for covariates such as gender, cancer treatment type, and cancer stage, and provide a methodologically sound estimation of sample size to obtain sufficient power. Finally, since the P-value is hugely impacted by the sample size, 55 future studies should consider estimating the effect of intervention in terms of effect size.
Conclusions
The aim of this scoping review was to provide an early overview of the available evidence on the neural correlates of treatments for psychological distress in cancer patients. These preliminary findings indicate that the cortical and limbic areas targeted by psychological interventions may be coherent with those involved in the individual’s distress reaction to cancer. Furthermore, the evidence supports the use of both the neural and clinical outcomes for assessing the impact of a psychosocial treatment. Further research with larger and more heterogeneous populations is needed to draw stronger conclusions.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354221096808 for The Neural Signature of Psychological Interventions in Persons With Cancer: A Scoping Review by Pierre Gilbert Rossini, Luca Ostacoli, Marco Pagani, Francesca Malandrone, Francesco Oliva, Luca Cominu, Maria Chiara Annetta and Sara Carletto in Integrative Cancer Therapies
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Francesca Malandrone  https://orcid.org/0000-0002-9438-8637
https://orcid.org/0000-0002-9438-8637
Maria Chiara Annetta  https://orcid.org/0000-0003-3112-421X
https://orcid.org/0000-0003-3112-421X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. World Health Organization (WHO). Latest global cancer data. Press release no. 263. 2018. https://www.iarc.who.int/wp-content/uploads/2018/09/pr263_E.pdf. Accessed April 29, 2022.
- 2. Reis JC, Antoni MH, Travado L. Emotional distress, brain functioning, and biobehavioral processes in cancer patients: a neuroimaging review and future directions. CNS Spectr. 2020;25(1):79-100. doi: 10.1017/S1092852918001621 [DOI] [PubMed] [Google Scholar]
- 3. Caruso R, Breitbart W. Mental health care in oncology. Contemporary perspective on the psychosocial burden of cancer and evidence-based interventions. Epidemiol Psychiatr Sci. 2020;29:e86. doi: 10.1017/S2045796019000866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grassi L. Psychiatric and psychosocial implications in cancer care: the agenda of psycho-oncology. Epidemiol Psychiatr Sci. 2020;29:e89. doi: 10.1017/S2045796019000829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grassi L, Spiegel D, Riba M. Advancing psychosocial care in cancer patients. F1000Research. 2017;6:2083. doi: 10.12688/f1000research.11902.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weingarten CP, Strauman TJ. Neuroimaging for psychotherapy research: current trends. Psychother Res. 2015;25(2):185-213. doi: 10.1080/10503307.2014.883088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13(11):769-787. doi: 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalsi N, Altavilla D, Tambelli R, et al. Neural correlates of outcome of the psychotherapy compared to antidepressant therapy in anxiety and depression disorders: a meta-analysis. Front Psychol. 2017;8:927. doi: 10.3389/fpsyg.2017.00927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Månsson KNT, Lueken U, Frick A. Enriching CBT by neuroscience: novel avenues to achieve personalized treatments. Int J Cogn Ther. 2021;14(1):182-195. doi: 10.1007/s41811-020-00089-0 [DOI] [Google Scholar]
- 10. Linden DEJ. How psychotherapy changes the brain – the contribution of functional neuroimaging. Mol Psychiatry. 2006;11(6):528-538. doi: 10.1038/sj.mp.4001816 [DOI] [PubMed] [Google Scholar]
- 11. Jia Y, Li F, Liu YF, Zhao JP, Leng MM, Chen L. Depression and cancer risk: a systematic review and meta-analysis. Public Health. 2017;149:138-148. doi: 10.1016/j.puhe.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 12. Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer. 2019;19(1):943. doi: 10.1186/s12885-019-6181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang YH, Li JQ, Shi JF, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020;25(7):1487-1499. doi: 10.1038/s41380-019-0595-x [DOI] [PubMed] [Google Scholar]
- 14. Sankar A, Melin A, Lorenzetti V, Horton P, Costafreda SG, Fu CHY. A systematic review and meta-analysis of the neural correlates of psychological therapies in major depression. Psychiatry Res Neuroimaging. 2018;279:31-39. doi: 10.1016/j.pscychresns.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 15. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. doi: 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19-32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 17. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. doi: 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 18. Sucharew H, Macaluso M. Progress notes: methods for research evidence synthesis: the scoping review approach. J Hosp Med. 2019;14(7):416-418. doi: 10.12788/jhm.3248 [DOI] [PubMed] [Google Scholar]
- 19. Higgins JPT, Cochrane Collaboration, eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Wiley-Blackwell; 2020. [Google Scholar]
- 20. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 21. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- 23. Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264. [DOI] [PubMed] [Google Scholar]
- 24. Lee EJ, Bhattacharya J, Sohn C, Verres R. Monochord sounds and progressive muscle relaxation reduce anxiety and improve relaxation during chemotherapy: a pilot EEG study. Complement Ther Med. 2012;20(6):409-416. doi: 10.1016/j.ctim.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 25. Monti DA, Kash KM, Kunkel EJS, et al. Changes in cerebral blood flow and anxiety associated with an 8-week mindfulness programme in women with breast cancer. Stress Health. 2012;28(5):397-407. doi: 10.1002/smi.2470 [DOI] [PubMed] [Google Scholar]
- 26. Ercoli LM, Petersen L, Hunter AM, et al. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psychooncology. 2015;24(11):1360-1367. doi: 10.1002/pon.3769 [DOI] [PubMed] [Google Scholar]
- 27. Monti DA, Tobia A, Stoner M, et al. J Neuro emotional technique effects on brain physiology in cancer patients with traumatic stress symptoms: preliminary findings Cancer Surviv. 2017;11(4):438-446. doi: 10.1007/s11764-017-0601-8 [DOI] [PubMed] [Google Scholar]
- 28. Monti DA, Tobia A, Stoner M, et al. Changes in cerebellar functional connectivity and autonomic regulation in cancer patients treated with the Neuro Emotional Technique for traumatic stress symptoms. J Cancer Surviv. 2018;12(1):145-153. doi: 10.1007/s11764-017-0653-9 [DOI] [PubMed] [Google Scholar]
- 29. Ramirez R, Planas J, Escude N, Mercade J, Farriols C. EEG-based analysis of the emotional effect of music therapy on palliative care cancer patients. Front Psychol. 2018;9. doi: 10.3389/fpsyg.2018.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ratcliff CG, Prinsloo S, Chaoul A, et al. A randomized controlled trial of brief mindfulness meditation for women undergoing stereotactic breast biopsy. J Am Coll Radiol. 2019;16(5):691-699. doi: 10.1016/j.jacr.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mioduszewski O, Hatchard T, Fang Z, et al. Breast cancer survivors living with chronic neuropathic pain show improved brain health following mindfulness-based stress reduction: a preliminary diffusion tensor imaging study. J Cancer Surviv. 2020;14(6):915-922. doi: 10.1007/s11764-020-00903-w [DOI] [PubMed] [Google Scholar]
- 32. Smith AM, Leeming A, Fang Z, et al. Mindfulness-based stress reduction alters brain activity for breast cancer survivors with chronic neuropathic pain: preliminary evidence from resting-state fMRI. J Cancer Surviv. 2021;15(4):518-525. doi: 10.1007/s11764-020-00945-0 [DOI] [PubMed] [Google Scholar]
- 33. Van der Gucht K, Melis M, Ahmadoun S, et al. A mindfulness-based intervention for breast cancer patients with cognitive impairment after chemotherapy: study protocol of a three-group randomized controlled trial. Trials. 2020;21(1):1-11. doi: 10.1186/s13063-020-4204-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gawrysiak MJ, Carvalho JP, Rogers BP, Nicholas CRN, Dougherty JH, Hopko DR. Neural changes following behavioral activation for a depressed breast cancer patient: a functional MRI case study. Case Rep Psychiatry. 2012;2012:152916-152916. doi: 10.1155/2012/152916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gawrysiak MJ, Swan SA, Nicholas CRN, Rogers BP, Dougherty JH, Hopko DR. Pragmatic psychodynamic psychotherapy for a patient with depression and breast cancer: functional MRI evaluation of treatment effects. Am J Psychother. 2013;67(3):237-255. doi: 10.1176/appi.psychotherapy.2013.67.3.237 [DOI] [PubMed] [Google Scholar]
- 36. Carletto S, Porcaro C, Settanta C, et al. Neurobiological features and response to eye movement desensitization and reprocessing treatment of posttraumatic stress disorder in patients with breast cancer. Eur J Psychotraumatol. 2019;10(1):1600832. doi: 10.1080/20008198.2019.1600832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: a review. EXCLI J. 2017;16:1057–1072. doi: 10.17179/EXCLI2017-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valenza G, Citi L, Saul JP, Barbieri R. Measures of sympathetic and parasympathetic autonomic outflow from heartbeat dynamics. J Appl Physiol (1985). 2018;125(1):19-39. doi: 10.1152/japplphysiol.00842.2017 [DOI] [PubMed] [Google Scholar]
- 39. Tremblay S, Rogasch NC, Premoli I, et al. Clinical utility and prospective of TMS-EEG. Clin Neurophysiol. 2019;130(5):802-844. doi: 10.1016/j.clinph.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 40. Young KS, van der Velden AM, Craske MG, et al. The impact of mindfulness-based interventions on brain activity: a systematic review of functional magnetic resonance imaging studies. Neurosci Biobehav Rev. 2018;84:424-433. doi: 10.1016/j.neubiorev.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 41. Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16(4):213-225. doi: 10.1038/nrn3916 [DOI] [PubMed] [Google Scholar]
- 42. Lutz J, Brühl AB, Scheerer H, Jäncke L, Herwig U. Neural correlates of mindful self-awareness in mindfulness meditators and meditation-naïve subjects revisited. Biol Psychol. 2016;119:21-30. doi: 10.1016/j.biopsycho.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 43. Monti DA, Sufian M, Peterson C. Potential role of mind-body therapies in cancer survivorship. Cancer. 2008;112(11 Suppl):2607-2616. doi: 10.1002/cncr.23443 [DOI] [PubMed] [Google Scholar]
- 44. Carletto S, Pagani M. Neurobiological impact of EMDR in cancer. J EMDR Pract Res. 2016;10(3):153-161. doi: 10.1891/1933-3196.10.3.153 [DOI] [Google Scholar]
- 45. Pagani M, Di Lorenzo G, Verardo AR, et al. Neurobiological correlates of EMDR monitoring: an EEG study. PloS One. 2012;7(9):e45753. doi: 10.1371/journal.pone.0045753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Landin-Romero R, Moreno-Alcazar A, Pagani M, Amann BL. How does eye movement desensitization and reprocessing therapy work? A systematic review on suggested mechanisms of action. Front Psychol. 2018;9:1395. doi: 10.3389/fpsyg.2018.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pierce ZP, Black JM. The neurophysiology behind trauma-focused therapy modalities used to treat post-traumatic stress disorder across the life course: a systematic review. Trauma Violence Abuse. Published online December 6, 2021. doi: 10.1177/15248380211048446 [DOI] [PubMed] [Google Scholar]
- 48. Lomas T, Ivtzan I, Fu CHY. A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neurosci Biobehav Rev. 2015;57:401-410. doi: 10.1016/j.neubiorev.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 49. Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40(5):655-665. doi: 10.1111/1469-8986.00067 [DOI] [PubMed] [Google Scholar]
- 50. Moore HCF. An overview of chemotherapy-related cognitive dysfunction, or “chemobrain”. Oncology (Williston Park). 2014;28(9):797-804. [PubMed] [Google Scholar]
- 51. Anderson ND. State of the science on mild cognitive impairment (MCI). CNS Spectr. 2019;24(1):78-87. doi: 10.1017/S1092852918001347 [DOI] [PubMed] [Google Scholar]
- 52. Grassi L, Caruso R, Hammelef K, Nanni MG, Riba M. Efficacy and safety of pharmacotherapy in cancer-related psychiatric disorders across the trajectory of cancer care: a review. Int Rev Psychiatry. 2014;26(1):44-62. doi: 10.3109/09540261.2013.842542 [DOI] [PubMed] [Google Scholar]
- 53. Messina I, Sambin M, Palmieri A, Viviani R. Neural correlates of psychotherapy in anxiety and depression: a meta-analysis. PLoS One. 2013;8(9):e74657. doi: 10.1371/journal.pone.0074657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A. The effects of psychotherapy on brain function: a systematic and critical review. Prog Neurobiol. 2014;114:1-14. doi: 10.1016/j.pneurobio.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 55. Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J Thorac Dis. 2016;8(9):E928-E931. doi: 10.21037/jtd.2016.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354221096808 for The Neural Signature of Psychological Interventions in Persons With Cancer: A Scoping Review by Pierre Gilbert Rossini, Luca Ostacoli, Marco Pagani, Francesca Malandrone, Francesco Oliva, Luca Cominu, Maria Chiara Annetta and Sara Carletto in Integrative Cancer Therapies