Abstract
The placenta facilitates the transport of nutrients to the fetus, removal of waste products from the fetus, immune protection of the fetus and functions as an endocrine organ, thereby determining the environment for fetal growth and development. Additionally, the placenta is a highly metabolic organ in itself, utilizing a majority of the oxygen and glucose derived from maternal circulation. Consequently, optimal placental function is required for the offspring to reach its genetic potential in utero. Among ruminants, pregnant sheep have been used extensively for investigating pregnancy physiology, in part due to the ability to place indwelling catheters within both maternal and fetal vessels, allowing for steady-state investigation of blood flow, nutrient uptakes and utilization, and hormone secretion, under non-stressed and non-anesthetized conditions. This methodology has been applied to both normal and compromised pregnancies. As such, our understanding of the in vivo physiology of pregnancy in sheep is unrivalled by any other species. However, until recently, a significant deficit existed in determining the specific function or significance of individual genes expressed by the placenta in ruminants. To that end, we developed and have been using in vivo RNA interference (RNAi) within the sheep placenta to examine the function and relative importance of genes involved in conceptus development (PRR15 and LIN28), placental nutrient transport (SLC2A1 and SLC2A3), and placenta-derived hormones (CSH). A lentiviral vector is used to generate virus that is stably integrated into the infected cell’s genome, thereby expressing a short-hairpin RNA (shRNA), that when processed within the cell, combines with the RNA Induced Silencing Complex (RISC) resulting in specific mRNA degradation or translational blockage. To accomplish in vivo RNAi, day 9 hatched and fully expanded blastocysts are infected with the lentivirus for 4 to 5 h, and then surgically transferred to synchronized recipient uteri. Only the trophectoderm cells are infected by the replication deficient virus, leaving the inner cell mass unaltered, and we often obtain ~70% pregnancy rates following transfer of a single blastocyst. In vivo RNAi coupled with steady-state study of blood flow and nutrient uptake, transfer and utilization can now provide new insight into the physiological consequences of modifying the translation of specific genes expressed within the ruminant placenta.
Keywords: blood flow, Fick principle, nutrients, placenta, RNA interference, ruminant
In vivo modification of the abundance of specific gene products within the placenta, coupled with steady-state assessment of nutrient uptake, transfer and utilization, as well as placental hormone secretion, can provide new insight and direct assessment of ruminant placental function.
Introduction
In livestock production, considerable emphasis has been placed on improving postnatal growth rates, thereby maximizing meat production. However, it has become increasingly clear that altered fetal growth trajectories negatively impact both muscle mass and carcass composition within those offspring (Du et al., 2010). Indeed, 8-mo-old lambs born from ewes nutrient restricted from early through mid-gestation were fatter, with reduced lean-to-fat ratio and fewer myofibers in the longissimus dorsi muscle (Zhu et al., 2006). Interestingly, lambs derived from both nutrient restricted and over-fed pregnancies exhibited reduced cross-sectional area of the semitendinosus muscle, compared to controls, at 3 mo of age (Reed et al., 2014). These data are supported by the observation that fetal myogenesis is impaired (Tong et al., 2009) and adipocyte numbers are greater in mature lamb semitendiosus muscle (Yan et al., 2011), suggesting that both relative fetal undernutrition or overnutrition are detrimental to subsequent carcass composition.
Once formed, the placenta is responsible for transporting oxygen and nutrients, including glucose and amino acids, from the mother to the fetus, as well as serving as an active endocrine organ (Anthony et al., 1998; Barry and Anthony, 2008; Roberts and Anthony, 1994). Robust assessment of maternal-placental-fetal interactions requires the ability to repetitively sample blood from both the maternal and fetal sides of the placenta. While feasible in cattle, during the past 50+ yr, the pregnant sheep has been primarily used to investigate maternal-placental-fetal interactions, in part due to our ability to surgically place catheters allowing the repetitive and simultaneous sampling of both maternal and fetal arterial and venous blood from non-anesthetized/non-stressed pregnancies (Meschia et al., 1965; Battaglia et al., 1968). Since these early studies (Meschia et al., 1965; Battaglia et al., 1968), the pregnant sheep has provided considerable insight into uteroplacental uptake, transfer, and utilization of nutrients. For example, these approaches were used to demonstrate that the placenta is not just a conduit of nutrients to the fetus, but is in itself a highly metabolic organ. At mid-gestation, the placenta utilizes ~80% of the oxygen and glucose taken up from the maternal circulation (Bell et al., 1986), and even during late gestation, when demand by the fetus is much greater, the placenta continues to consume 45% of the oxygen and 72% of the glucose (Meschia et al., 1980).
While considerable insight into in vivo maternal-placental-fetal interactions has been gained from studies in pregnant sheep, the lack of approaches to generate placenta-specific functional gene ablation, needed to assess the role of specific gene products during the course of gestation, has limited the direct assessment of “cause and effect” relationships. However, what is now feasible is the use of in vivo RNA interference (RNAi) to reduce specific gene products within the placenta to determine the ramification of the generated deficiency (Dunlap et al., 2006; Purcell et al., 2009; Baker et al., 2016). In vivo RNAi within sheep placenta is now being used to generate unique models of altered placental function, when coupled with the long-standing approaches to assess maternal-placental-fetal interactions under steady-state conditions (Meschia et al., 1965; Battaglia et al., 1968), allowing the direct assessment of the effect of specific gene product deficiencies during the course of gestation (Tanner et al., 2021a,b). It is our goal to review not only the methodology, but what can be learned from steady-state in vivo assessment of maternal-placental-fetal interactions, in vivo RNAi and how the combination of these approaches are providing new insight into ruminant placental function.
In Vivo Assessment of Placental Function
Fick principle and application for assessing placental physiology
In order to assess placental function under non-stressed, non-anesthetized conditions, a technique employing the surgical placement of indwelling catheters was developed (Meschia et al., 1967; Meschia et al., 1980). Surgical catheter placement allows for the infusion of a tracer molecule (transplacental diffusion technique) until steady-state concentrations of the tracer are achieved (Meschia et al., 1967; Meschia et al., 1980). Once this occurs, blood is collected from maternal and fetal catheters simultaneously which allows for the calculation of vessel specific blood flow and arteriovenous differences in substrates (Meschia et al., 1967; Meschia et al., 1980).
While the complexity of the surgical preparations varies, a minimal surgical prep for measuring both uterine and umbilical blood flows includes the placement of vascular catheters for blood sampling (Figure 1) into the umbilical vein, fetal descending aorta (proxy umbilical artery), the uterine vein, and the maternal femoral artery (proxy uterine artery; Meschia et al.,1965; Meschia et al., 1980). An additional catheter for tracer infusion into a fetal limb vein is also required. By infusing a tracer molecule which is freely diffusible and cannot be metabolized or produced within the exchanger, the transplacental diffusion rate is blood flow dependent and is calculated by the difference between the infusion and escape rates of the tracer (Battaglia and Meschia, 1986). The tracer is infused into a fetal limb vein and then undergoes mixing with other venous blood in the atria and ventricles of fetal heart. Thus, concentrations of the tracer molecule are highest in the umbilical artery, followed by the umbilical vein, uterine vein, and uterine artery, respectively. Using the Fick Principle, blood flow is calculated by dividing the transplacental diffusion rate by the arteriovenous differences in tracer concentrations (Meschia et al., 1980). Once blood flow is known, one can calculate specific substrate uptake rates.
Figure 1.
Schematic representation of the placenta, uterine, and umbilical circulation, identifying catheter placement for sampling maternal and fetal blood under steady-state non-anesthetized/non-stressed conditions.
The Fick Principle is defined as the quantity of a substrate entering an organ (arterial blood) must equal the quantity of the substrate being extracted from the blood by the organ plus the quantity of substrate leaving an organ (venous blood; Battaglia and Meschia, 1986). As summarized in Table 1, to calculate uptake rate of a substrate by the uterus (uteroplacental unit) or by umbilical circulation (fetal), the rate of blood flow is multiplied by the arteriovenous differences in the substrate (Meschia et al., 1980; Battaglia and Meschia, 1986). To calculate what the uteroplacental unit is utilizing, the difference between uterine and umbilical uptakes is calculated (Meschia et al., 1980; Battaglia and Meschia, 1986). From a research perspective, the Fick Principle is the gold standard for investigating placental and fetal physiology as it not only addresses rates of blood flow but also permits the calculation of the transplacental transport of nutrients.
Table 1.
Calculations1 for blood flow, nutrient uptake, nutrient utilization, and quotients
| Blood flow (3H2O tracer) | |
| Rinf3H2O (dpm/min) | Pump rate ∗ [infusate] |
| Racc(f) (dpm/min) | αpl slope ∗ (0.8 ∗ fetal weight) |
| Racc(m) (dpm/min) | Racc(f) + [αpl slope ∗ 0.8(uterine weight)] |
| Umbilical Blood Flow (UBF; mL/min) | (Rinf − Racc(f))/ ([3H2O]α(WB) − [3H2O]γ(WB)) |
| Umbilical Plasma Flow (UPF; mL/min) | UBF ∗ [1 − Hctf(avg)] |
| Uterine Blood Flow (UtBF; mL/min) | (Rinf − Racc(m))/ ([3H2O]V(WB) − [3H2O]A(WB)) |
| Uterine Plasma Flow (UtPF; mL/min) | UtBF ∗ [1 − Hctm(avg)] |
| Nutrient uptake and utilization rates | |
| Umbilical Oxygen Uptake (fetal oxygen utilization; UOU; mmol/min) | UBF ∗ ([O2]γ(WB) − [O2]α(WB)) |
| Uterine Oxygen Uptake (UtOU; mmol/min) | UtBF ∗ ([O2]A(WB) − [O2]V(WB)) |
| Uteroplacental Oxygen Utilization (mmol/min) | UtOU − UOU |
| Plasma to WB Glucose Conversion | [G]pl ∗ [1 − (0.24 ∗ Hct)] − (3.3 ∗ Hct) |
| Umbilical Glucose Uptake (UGU; µmol/min) | UBF ∗ ([G]γ(WB) − [G]α(WB)) |
| Uterine Glucose Uptake (UtGU; µmol/min) | UtBF ∗ ([G]A(WB) − [G]V(WB)) |
| Uteroplacental Glucose Utilization (µmol/min) | UtGU − UGU |
| Umbilical Lactate Uptake (µmol/min; ULU) | UBF ∗ ([L]γ(pl) − [L]α(pl)) |
| Uterine Lactate Secretion (µmol/min; UtLS) | UtBF ∗ ([L]V(pl) − [L]A(pl)) |
| Uteroplacental Lactate Production (µmol/min) | ULU + UtLS |
| Umbilical AA Uptake (µmol/min; UAAU) | UPF ∗ ([AA]γ(pl) − [AA]α(pl)) |
| Uterine AA Uptake (µmol/min; UtAAU) | UtPF ∗ ([AA]A(pl) − [AA]V(pl)) |
| Umbilical AA Carbon Uptake (µmol/min; UCU) | (#AA carbons) ∗ UAAU |
| Uterine AA Carbon Uptake (µmol/min; UtCU) | (#AA carbons) ∗ UtAAU |
| Umbilical AA Nitrogen Uptake (µmol/min; UNU) | (#AA nitrogens) ∗ UAAU |
| Uterine AA Nitrogen Uptake (µmol/min; (UtNU) | (#AA nitrogens) ∗ UtAAU |
Calculations are derived from Cilvik et al. (2021).
Use of chronically catheterized pregnant sheep to assess placental physiology
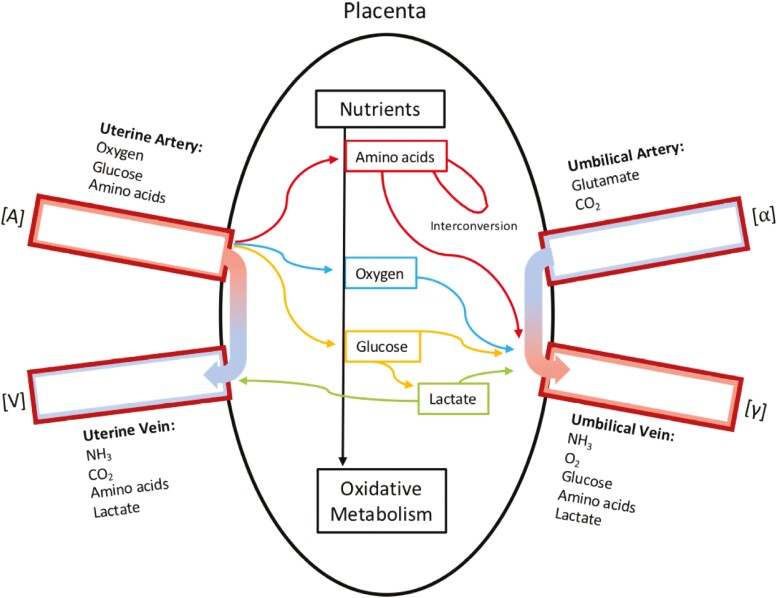
Considerable insight into the uterine uptake, umbilical uptake, and placental utilization of various nutrients has been obtained using the chronically catheterized pregnant sheep. As depicted in Figure 2, the transfer and utilization of various nutrients is complex as a result of the placenta being a metabolically active organ in itself. How this impacts the uptake and utilization of specific nutrients is described below.
Figure 2.
Schematic representation of the uterine uptake, umbilical uptake, and placental metabolism of nutrients during late gestation in sheep, as assessed under steady-state non-anesthetized/non-stressed conditions.
Oxygen
While the Fick Principle has been employed to assess organ specific blood flow in a variety of organs, one of its earliest uses to investigate placental physiology was a series of experiments intended to assess placental oxygen transfer (Meschia et al., 1965). Using chronically catheterized sheep, blood oxygen tension was measured by determining the arteriovenous differences in the partial pressure of oxygen (pO2) based on equations documented in Meschia et al. (1961). The rate of oxygen transfer across the placenta was calculated, requiring the rate of specific vessel blood flow and the arteriovenous difference of oxygen (Meschia et al., 1965). Using these methods, Meschia et al. (1965) determined that the transplacental oxygen gradient was relatively constant over gestation, but the placental uptake of oxygen (rate of transfer) increased with fetal weight.
These methods were also utilized to better understand how gestation impacted placental oxygen permeability and uptake. To this end, Meschia et al. (1965) determined the oxygen dissociation curves for each vessel, based on the oxygen content, oxygen capacity and saturation, and the type of hemoglobin. Furthermore, they calculated maternal and fetal “effective oxygen pressures” (pO2 mm Hg) to determine the change in pO2 which is necessary to calculate the diffusing capacity of the placenta (O2 uptake/change in pO2;Meschia et al., 1965). The results of this study revealed that placental oxygen permeability increases as gestation progresses (increases 10-fold) in proportion to fetal weight, which appears to be the main driver of increased oxygen transfer (Meschia et al., 1965).
While these studies represent some of the earliest reports of in vivo placental nutrient exchange in ruminants, much of our knowledge of placental physiology is derived from these techniques. Using the sheep, Bell et al. (1986) determined that during mid-gestation, the placenta consumes approximately 83% of the oxygen it takes up from the uterine circulation. Meschia et al. (1980) found that from mid-to late gestation, there is a proportional decrease in oxygen demands by the placenta as it only consumes approximately 45% of the oxygen it takes up from maternal circulation near-term. However, on a total oxygen volume basis, the late gestation placenta actually consumes approximately 75% more oxygen compared to the mid-gestation placenta (Meschia et al., 1980). On a weight-specific basis, the oxygen consumption by the placenta is four to five times greater than the oxygen consumption of the fetus (Hay, 1991), with approximately 90% of the oxygen utilized by the placenta going to oxidative phosphorylation (Meschia et al., 1980). Taken together, these experiments determined that placental oxygen transport (diffusion) capacity across the placenta is incumbent upon rates of blood flow (uterine and umbilical), the oxygen carrying capacity of maternal and fetal blood, hemoglobin oxygen binding efficiency, placental permeability, placental surface area, and oxygen consumption by the placenta. When any of these factors are perturbed, deficits in oxygen delivery to the fetus can occur and lead to fetal hypoxemia and subsequent fetal growth restriction.
Glucose
One of the most critical substrates for fetal and placental growth is glucose.
Placental glucose transfer is characterized by saturable, carrier mediated transport that is dependent on the maternofetal concentration gradient (Widdas et al., 1952). Glucose must be transported by facilitative glucose transporters, from maternal circulation across the maternal facing microvillous membrane of the trophoblast, then across the cytoplasm, before being transported across the fetal facing basal membrane of the trophoblast to reach fetal circulation (Barry and Anthony, 2008).
Similar to the investigation of placental oxygen transfer, chronically catheterized sheep have been used to better understand the role of maternal glucose concentrations on the partitioning of glucose to the fetus. Hay et al. (1983), using fed or fasted ewes during late gestation, found that the proportion of maternal glucose supplying the uteroplacental unit and the fetus was constant in spite of the drastic reductions in maternal glucose levels. To understand how maternal glucose concentrations alter glucose consumption by the placenta, Hay and Meznarich (1989) clamped maternal glucose concentrations at various concentrations to setup varying maternofetal glucose gradients, to directly assess uteroplacental glucose consumption. They found that uteroplacental glucose transport and uptake/consumption were directly related to maternal arterial glucose concentrations and reach maximal transport and uptake at the same threshold (Hay and Meznarich, 1989). Subsequently, Hay et al. (1990) investigated the impact of fetal glucose concentrations on uteroplacental glucose transfer, by clamping umbilical glucose at varying concentrations. Collectively, these studies demonstrated that both maternal and fetal glucose concentrations can effectively impact the maternofetal glucose gradient, thereby impacting the net transfer of glucose across the placenta.
Other key insights into fetal and placental glucose oxidative needs have been derived from chronically catheterized pregnant sheep. One critical concept regarding transfer of glucose to the fetus that is often overlooked is the consumption of glucose by the placenta which constitutes a significant portion of the transplacental glucose gradient (Hay, 1991). Bell et al. (1986) determined that at mid-gestation, the placenta oxidizes approximately 83% of the glucose it takes up from the uterine circulation. Between mid-to late gestation, the placenta increases its glucose demands 6-fold to support a 10-fold increase in fetal mass. Placental oxidative needs during late gestation continue to utilize 72% of the glucose taken up from uterine circulation (Meschia et al., 1980). While this is a proportionally lower degree of glucose consumption compared to mid-gestation, the absolute glucose consumption by the placenta increases by 86% compared to mid-gestation (Meschia et al., 1980). Direct changes to uteroplacental glucose consumption can thereby impact the concentration of glucose in the umbilical vein (Hay and Meznarich, 1989). In spite of the metabolic demands by the placenta, it can shift oxidative substrates in times of maternal hypoglycemia to divert limited glucose supply to sustain fetal viability (Hay, 1991). This shift in oxidative substrate utilization is critical or the highly metabolic placenta would almost entirely block the net transport of glucose to the fetus (Hay, 1991).
The combination of investigations into placental glucose transfer in vivo using the chronically catheterized pregnant ewe has provided some of the most fundamental insights into placental physiology. Ultimately, glucose transport is dependent on 1) the availability of transporters to shuttle glucose across the placenta, 2) the maternofetal glucose concentration gradient, as well as 3) the placenta’s inordinate metabolic demands.
Amino acids
Amino acids also function as critical oxidative substrates supporting appropriate fetoplacental growth and development. The mechanisms of placental amino acid transfer are much more complex than that for glucose transfer, due to the redundancy of multiple transport systems for neutral, anionic, and cationic amino acids resulting in at least 15 different transporter proteins (Regnault et al., 2005). Much of placental amino acid uptake is dependent on energy availability as many amino acid transporters require energy to concentrate amino acids within the trophoblast intercellular matrix for transport into fetal circulation (Hay, 1991). Lemons et al. (1976) first utilized cannulated pregnant sheep to investigate umbilical amino acid uptake. They determined that while the fetus takes up a variety of neutral and basic amino acids, far in excess of growth requirements (suggesting degradation and transamination by the fetus), the fetus did not take up aspartate and glutamate, and was in fact a net glutamate producer for the placenta to use (Lemons et al., 1976). Additionaly, the placenta itself can produce amino acids through interconversion of metabolically related amino acids, and transfer those to the fetus, as demonstrated in vivo in sheep (Cetin, 2001).
Despite these complexities, the use of labeled amino acids in combination with chronically catheterized pregnant sheep has permitted the study of bidirectional amino acid flux, and improved our understanding of placental amino acid transport capacity. Furthermore, this approach can be used to demonstrate the incorporation of amino acids into placental proteins or fetal tissue (Barry and Anthony, 2008). Chronically catheterized pregnant sheep have been used to examine the relationship between maternal amino acid concentration, and the umbilical uptake of amino acids (Józwik et al., 1999a, b; 2001). Józwik et al. (1999a, b; 2001) infused a mixture of amino acids into maternal circulation, and found that increasing maternal amino acid concentrations lead to varying impacts on umbilical uptakes of specific amino acids. In some instances, the umbilical uptakes increased, stayed the same, or decreased, likely due to competitive inhibition of transport mechanisms (Józwik et al., 1999a, b; 2001). However, the infusion of a single amino acid into maternal circulation positively increases the fetal uptakes of that amino acid (Battaglia, 2002). Thus, in spite of the complexities of investigating placental amino acid flux, the chronically catheterized pregnant sheep has helped provided clarity.
Other substrates
The chronically catheterized sheep has also been utilized to explore the importance of other carbon sources for placental and fetal physiology. For instance, fructose, a hexose present in high concentrations in fetal and neonatal circulation (Battaglia and Meschia, 1986; Hugget et al., 1951) was revealed to play little role in meeting fetal oxidative demands (Warnes et al. 1977a, b). Warnes et al (1977a, b) infused [14C] fructose into the fetal lamb and documented low fetal utilization of fructose (0.12 mg/kg/min) in contrast to glucose (4–5 mg/kg/min). This is further evidenced by no significant umbilical arteriovenous difference in fructose concentrations (Battaglia and Meschia, 1986).
The role of fructose during compromised pregnancies is less clear. While little metabolism or turnover of [14C] fructose was documented by Alexander et al. (1970) in the fetal lamb, the quantity of 14CO2 increased when glucose concentrations decreased. This may suggest that fructose plays a larger role as a fetal oxidative substrate in cases of prolonged fetal hypoglycemia. This is corroborated by low maternal and fetal glucose during maternal fasting, where the concentrations of fructose decreased slowly during this period, indicating some utilization of fructose by the fetus under these conditions (Battaglia and Meschia, 1986), or that hypoglycemia results in less glucose for the placenta to convert into sorbitol.
Lactate, on the other hand, which was originally misinterpreted as an indicator of fetal hypoxia and the end-product of anaerobic metabolism (Battaglia and Meschia, 1986) was determined to be second only to glucose as a major carbohydrate source for the fetus, accounting for 25% of fetal oxygen consumption (Battaglia and Meschia, 1986). This was first evidenced by investigating late gestation, chronically catheterized pregnant sheep, where umbilical vein lactate concentrations were determined to be persistently higher than umbilical artery concentrations (Burd et al., 1975). The placenta is a net producer of lactate, secreting into both fetal and maternal circulation. Despite higher lactate concentrations in fetal circulation, the placenta secretes more lactate into the umbilical circulation as compared to maternal circulation (Battaglia and Meschia, 1986). While the utility and versatility of the chronically catheterized pregnant sheep in investigating normal placental physiology cannot be overstated, these methods have proved equally valuable for investigating compromised pregnancies.
Examining compromised placental nutrient flux
The study of cannulated sheep pregnancies compromised by fetal growth restriction (FGR) has proven invaluable for 1) understanding perturbations in placental nutrient transfer, 2) determining functional glucose transfer capacity, and 3) determining the ramification of infusing hormones or growth factors into fetal circulation. One of the most robustly investigated sheep models of fetal growth restriction, resulting from placental insufficiency (PI), is induced via maternal hyperthermia. This approach was initially developed by Alexander and Williams (1971), and works by exposing pregnant sheep to elevated ambient temperatures (35–40 °C with 30–40% relative humidity) across specific timepoints in pregnancy. The degree of growth restriction varies based on the duration of maternal hyperthermia, with up to ~60% reduction in placental weight and ~50-60% reduction in fetal weight if exposed from early to-late gestation (Anthony et al., 2003).
By strategically combining the generation of compromised pregnancies and surgical cannulations of those pregnancies, the ramifications of placental insufficiency on blood flow and nutrient transfer have been investigated. Using this approach, Thureen et al. (1992) determined that PI-FGR pregnancies exhibited reduced uterine and umbilical blood flows, resulting in diminished uterine, umbilical, and uteroplacental oxygen and glucose uptakes. The impact of maternal hyperthermia on fetal amino acid concentrations appears to be more variable, likely depending on severity of FGR (de Vrijer et al., 2004). For example, in hyperthermia-induced moderate FGR, fetal amino acid concentrations were reduced but conversely, in instances of severe FGR, fetal amino acid concentrations were elevated compared to healthy pregnancies (de Vrijer et al., 2004). They attribute this to less severe reductions in glucose and oxygen concentrations in moderate growth restricted sheep and to more severe reductions in severely growth restricted sheep (de Vrijer et al., 2004). By combining labeled amino acids (leucine or threonine) with catheterized, hyperthermia-induced severe FGR pregnancies, Ross et al. (1996) and Anderson et al. (1997) reported that transplacental flux of both amino acids was markedly reduced.
Another sheep model of compromised fetal growth was developed by Wallace et al (1999a, b), that being consistent maternal overnutrition of adolescent, singleton-bearing ewe lambs can result in fetal growth impairment. This is thought to be due to the prioritization of maternal growth over supplying adequate nutrients to the gravid uterus. To generate this model, embryos derived from mature ewes are transferred into adolescent ewes immediately following puberty, then the pregnant ewes are fed at 200% of their daily requirements (Carr et al., 2012). If this approach is maintained over the duration of pregnancy, and those pregnancies are studied under steady-state conditions, both uterine and umbilical blood flows are reduced, as well as umbilical oxygen and glucose uptakes (Wallace et al., 2002). While this maternal overnutrition model might initially show commonalities with maternal hyperthermia in terms of blood flow and nutrient uptake reductions, subtler critical distinctions exist which can be further elucidated by manipulating glucose concentrations in those pregnancies.
Assessing placental glucose transfer in FGR pregnancies
The functional power of the chronically catheterized sheep pregnancy in investigating placental pathophysiology is highlighted by the ability to clamp either maternal or fetal glucose at varying concentrations. Using the hyperthermia-induced FGR model, Thureen et al. (1992) sought to ascertain whether the reductions in the umbilical uptake of glucose was due to 1) reduced placental mass, 2) placental perfusion, 3) placental glucose consumption, or 4) reduced placental transfer capacity. To accomplish this, maternal glucose was clamped at 70 mg/dL by maternal dextrose infusion, followed by a basal study in which fetal glucose concentrations were left unperturbed. The basal study was then followed by both fetal hyperglycemic clamps (fetal dextrose infusion) and hypoglycemic clamps (fetal insulin infusion). After determining umbilical glucose uptakes under the three resulting fetal glucose concentrations (Thureen et al., 1992), linear regression analysis of umbilical glucose uptake (y-axis) versus the transplacental arterial glucose concentration gradient (x-axis; Thureen et al., 1992) was conducted. This revealed reduced placental glucose transfer capacity in FGR pregnancies, as differences in placental mass, perfusion, and glucose consumption did not account for the differences in umbilical glucose uptake (Thureen et al., 1992). By contrast, using a similar approach, generating three distinct maternal-fetal glucose concentration gradients, Wallace et al. (2003) demonstrated that the reduced umbilical glucose uptake in the overnourished adolescent ewe model of FGR was primarily a function of reduced placental mass. The utility of these methods to explicate the mechanistic distinctions in placental glucose transfer cannot be overstated.
Assessing the ramifications of fetal hormone infusion
A strength of chronic catheterization is the ability for both simultaneous sampling and infusion within either maternal or fetal vessels. This lends itself to study the ramification of infusing specific hormones on subsequent fetal, placental, and/or maternal physiology. A recent example utilizing this approach was studies conducted by Stremming et al. (2021), which employed short term (1 wk) fetal infusions of an IGF1 analogue (LR3 IGF1) during late gestation (126–134 d of gestational age; dGA). In response to LR3 IGF1 infusion, uterine and umbilical blood flows were not impacted, nor were the umbilical uptakes of glucose, oxygen, and lactate (Stremming et al., 2021). However, umbilical amino acid uptakes were reduced in LR3 IGF1 infused fetuses, as were net leucine uptake and the flux of leucine into fetal blood from the placenta (Stremming et al., 2021). As a result of these findings, these investigators hypothesize that IGF1 aids in fetal nutrient utilization in an organ-specific manner rather than through targeting blood flow or transplacental nutrient uptake (Stremming et al., 2021).
In PI-FGR pregnancies, umbilical glucagon concentrations are elevated ~2 fold (Limesand et al., 2006), leading Cilvik et al. (2021) to investigate the impact of chronic (8–10 d) fetal infusion during late gestation in normal pregnancy. Enhanced fetal glucagon concentrations suppressed uterine and umbilical uptake of amino acids, fetal leucine flux, fetal protein synthesis and accretion, and umbilical insulin concentrations (Cilvik et al. 2021). Suprisingly, while umbilical blood flow was not impacted, uterine blood flow was reduced 33%. Similarly, umbilical concentrations of chorionic somatomammotropin (CSH)/placental lactogen (PL) were not impacted by fetal glucagon infusion, but maternal concentrations of CSH/PL were reduced 75%. Not only did this study emphasize the negative impact of hyperglucagonmeia on fetal physiology, but also stresses the distinction between maternal and fetal responses to fetal hormones, as well as the impact a fetal origin hormone can have on placental function and maternal physiology (Cilvik et al., 2021).
In Vivo RNA Interference Impact on Placental Function
Methods to modify ruminant placenta function
Assessing the function of specific genes expressed by the placenta is difficult, since classical ablation-replacement strategies are not feasible with just the placenta. While CRISPR-Cas9 genome editing methods are currently being applied to livestock, as yet it is only feasible to generate “global” gene ablation, and not in a tissue/organ specific manner. However, for the past 15 yr, in vivo RNAi has been used in sheep as an approach to alter trophoblast function (Dunlap et al., 2006; Purcell et al., 2009). Dunlap et al. (2006) successfully infused morpholino oligonucleotides into the uterine lumen, to examine the impact of inhibiting the expression of the endogenous retrovirus enJSRV env within sheep conceptuses. Antisense morpholino oligonucleotides incorporate morpholine rings in their structure in place of deoxyribose and are designed to hybridize with RNA sequences encompassing the translational start site, or an exon/intron splice-acceptor site (Summerton, 1999). Hybridization at these sites provides steric blocking of translation and/or pre-mRNA splicing in an RNase H independent fashion (Summerton 1999). Using morpholino oligonucleotide infusion, Dunlap et al. (2006) demonstrated that enJSRV env plays an important role in trophectoderm growth and differentiation in elongating sheep conceptuses. Since their initial report, this approach has been used to determine the impact of creating deficiencies in specific gene products expressed by the elongating sheep conceptus, including interferon-tau (Brooks and Spencer, 2015; Brooks et al., 2015a, b). This has proven to be a useful approach to study early events in conceptus growth and placentation. However, since the approach relies on transient transfection, efficacy of morpholino oligonucleotide mediated gene “knockdown” is lost as the conceptus/placenta proliferates.
To circumvent the transient nature of morpholino oligonucleotide-mediated RNAi, Purcell et al. (2009) infected the trophectoderm cells of hatched sheep blastocysts with a lentivirus expressing a short-hairpin RNA (shRNA) to obtain RNAi of proline-rich 15 (PRR15), a nuclear protein expressed during conceptus elongation. Use of a replication-deficient lentivirus accomplishes two critical aspects of obtaining long-term placenta-specific RNAi. Since the lentivirus is incapable of replicating within the host cell, only the trophectoderm cells of the blastocyst are infected during culture with the virus, and the inner cell mass is not impacted. Additionally, since lentivirus is stably integrated into the host genome, expression of the shRNA will continue throughout gestation in a placenta-specific fashion. Purcell et al. (2009) were able to use this approach to demonstrate the requirement of PRR15 for conceptus elongation in sheep. Subsequently, this methodology has been refined and applied to a number of genes expressed within either the developing conceptus (LIN28; Ali et al., 2020a), or later on in gestation by the fully formed and functional placenta (CSH; Baker et al., 2016; Jeckel et al., 2018).
Lentiviral-mediated RNAi methods
The principle behind shRNA-mediated RNAi is based on our knowledge of how endogenous micro-RNA (miRNA) are generated, processed, and function (He and Hannon, 2004). Primary miRNA transcripts are processed in the nucleus by the RNase III activity of Drosha, yielding pre-miRNA with a characteristic hairpin-loop structure, that are exported out of the nucleus. The pre-miRNA is then processed in the cytoplasm by the RNase III activity of Dicer, yielding an ~22-base pair duplex, mature miRNA. The mature miRNA serves as a guide sequence incorporated into the RNA-induced silencing complex (RISC). When RISC complexes with the target transcript, Argonaute, a component of RISC that possesses RNase H activity, cleaves the mRNA, thus targeting it for nuclease-mediated degradation. Argonaute does not always cleave the target mRNA, especially if there is incomplete complementarity between the target mRNA and the guide sequence. Rather, Argonaute may recruit additional proteins that ultimately inhibit translation of the target mRNA. Many endogenous miRNA target the 3ʹ-untranslated regions of mRNA, such that the miRNA-RISC complex represses translation, rather than resulting in targeted mRNA degradation (He and Hannon, 2004).
When shRNA is introduced into cells, either in vitro or in vivo, the shRNA is processed in the cell like endogenous miRNA (Paddison et al., 2002), providing for targeted-degradation or translational repression of that mRNA. Expression cassettes encoding a shRNA consist of inverted repeats separated by a loop sequence, followed by a short poly(T) track to terminate transcription (Paddison et al., 2002). The shRNA-encoding cassette is inserted downstream of a RNA polymerase III promoter (Paddison et al. 2004), within the targeting vector. Purcell et al. (2009) choose to use a replication-deficient lentiviral construct generated by Rubinson et al. (2003), such that when hatched blastocysts were exposed to the virus, only the outer trophectoderm was infected. The construct used, Lentilox 3.7 (LL3.7; Rubinson et al., 2003), contains an EGFP expression cassette driven by the cytomegalovirus promoter, and an insertion site for the shRNA cassette downstream of the mouse U6 promoter, a RNA polymerase III promoter. Alternatively, lentiviral vectors which contain RNA polymerase II promoters can be used, to drive the expression of miRNA mimics, i.e., the expression cassette is designed to more closely mimic a miRNA in its structural properties, rather than just a simple stem-loop structure used in shRNA (Baker et al., 2016). Regardless of the vector “backbone,” multiple shRNA/miRNA mimics should be designed and tested in vitro before moving to in vivo experiments. Sufficient validation is needed to verify that adequate repression of the specific gene products is obtained, and that the effects are not a result of inducing generalized repression of multiple mRNA, or that the viral infection is triggering interferon induction and an innate immune response. Both in vitro and in vivo, infection with a virus that is expressing a “control” shRNA/miRNA mimic should be used to verify that the effects observed are specific in nature. The “target” sequence can be scrambled and used as a control (SC or scrambled control), or a validated “non-targeting” sequence (NTS). One potential issue with a SC is that it may or may not interact with Dicer and RISC, thereby diminishing its value as a true RNAi control. A variety of NTS sequences have been reported, and may simply be a target sequence that does not exist in the species of interest.
With the in vitro assessment of the various viral constructs, we routinely infect trophoblast cells at a “multiplicity of infection” (MOI) of 100 and 500. The rationale for this is two-fold. Trophectoderm cells appear to be resistant to lentiviral infection, such that we infect hatched blastocysts with 100–150,000 transducing units of virus/blastocyst, giving an effective MOI of ≥500. Second, it is necessary to get adequate copies of the construct integrated into the host genome, such that the expression of the shRNA/miRNA mimic is sufficiently high to allow effective competition with endogenous miRNA for Dicer and RISC. As such, the accuracy of the viral titer is imperative for both in vitro and in vivo experiments. Since LL3.7 contains an EGFP expression cassette (Rubinson et al., 2003), we titer LL3.7 virus at 48 hrs following infection of HEK293 cells by visually counting those cells with robust green fluorescence (Purcell et al., 2009; Baker et al., 2016). With the RNA polymerase II vectors reported in Baker et al. (2016), since they contained a puromycin expression cassette, we used a TCID50 calculation to determine the viral titer. It should be noted that the relative deficiency in specific-gene products obtained in vitro always appears to be more robust than what is obtained in vivo. Additionally, while the RNA polymerase II based lentiviral vectors, expressing miRNA mimics, worked extremely well in vitro, when we used them in vivo (Baker et al., 2016), their overall efficacy was not nearly as good as the simpler LL3.7 based vector. As reported in Baker et al. (2016), we modified LL3.7, replacing the mouse U6 promoter with the human U6 promoter, and have continued to use this approach for generating the LL3.7-based targeting vectors as well as NTS vector since (Jeckel et al., 2018, Ali et al., 2020a; Tanner et al., 2021a, b).
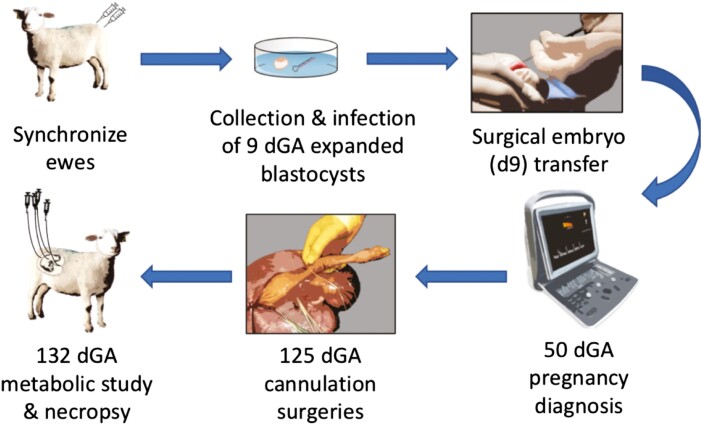
While our methodologies (Figure 3) for generating RNAi pregnancies are extensively described in Baker et al. (2016), the success or failure of these experiments depends on several factors. It has been our experience that selecting only fully hatched and fully expanded blastocysts for infection and transfer is key to the establishment and maintenance of these RNAi pregnancies, especially when only transferring a single blastocyst. Beginning with the studies of Baker et al. (2016) through our current ongoing studies, the singleton pregnancies generated using these techniques result in 50% to 80% success, as measured by viable pregnancies near-term (135 dGA), and typically is ~70%. Beyond the quality of the blastocysts at the time of transfer, use of pre-tested media, medical grade incubator gases, quality plasticware, etc., have all been noted to improve our overall success. As mentioned earlier, having an accurate functional titer of the viral transducing units is critical. While not reported in Purcell et al. (2009), preliminary studies demonstrated that 104 transducing units did not result in uniform infection of the blastocyst trophectoderm, whereas 106 transducing units were detrimental to blastocyst viability and 107 transducing units were lethal to the blastocysts. This is corroborated by recent work in mice (Vaughn et al., 2021), where they found that infecting groups of 5–10 blastocysts with 5 × 106 transducing units resulted in 1% viable fetuses and an 8% implantation rate, whereas infecting with 5 × 105 transducing units resulted in 27% viable fetuses and a 33% implantation rate.
Figure 3.
Schematic of the work flow for generating in vivo RNAi pregnancies, and their subsequent study.
As mentioned earlier, the efficacy of RNAi is usually much more robust in vitro than it is in vivo. In vitro, it is typical to obtain ≥90% reduction in the targeted mRNA, and an 80%–90% reduction in the specific protein. However, in vivo, in both sheep and mice, the reduction in mRNA concentrations ranges from 50% to 60%, and placental protein concentration is reduced 30%–40% (Baker et al., 2016; Tanner et al., 2021b; Vaughn et al., 2021). The reduction in “knockdown” efficiency could result from a variety of factors such as integration rate, diminished and/or silencing of shRNA expression over time, and not the least of which is the transcription and translation of the target mRNA in non-trophectoderm derived cells within the placenta. Regardless, this degree of gene product reduction within the placenta has led to significant phenotypic changes (Baker et al., 2016; Tanner et al., 2021b; Vaughn et al., 2021) near-term in both sheep and mice.
As with most in vivo experiments, this methodology does not guarantee a significant phenotypic effect in vivo. In ongoing work examining the relative importance of the two main facilitative glucose transporters expressed by the ruminant placenta (Wooding et al., 2005), divergent results have been obtained, as assessed at mid-gestation (75 dGA). With SLC2A3 (GLUT3) RNAi, significant phenotypic changes were observed in these pregnancies (Lynch et al., 2020), including fetal hypoglycemia and hypoinsulinemia, as a result of a 37% reduction in placental GLUT3 (Lynch et al., unpublished data). With SLC2A1 (GLUT1) RNAi (Kennedy et al., unpublished results), no phenotypic changes were discernable, and no significant reductions in the placental concentration of GLUT1 have been detected. With both SLC2A1 and SLC2A3 RNAi, in vitro infection of immortalized sheep trophoblast cells (iOTR cells; Ali et al., 2020a) with the same virus used in vivo, the reduction in either GLUT1 or GLUT3 exceeded 80%. Since GLUT1 is so abundant in the late gestation placenta, we overexpressed SLC2A1 mRNA in iOTR cells and was still able to obtain an 85% reduction in GLUT1 abundance following SLC2A1 RNAi (Kennedy et al., unpublished data). While this research is ongoing, the unexpected lack of effect of SLC2A1 RNAi may have resulted from in vivo silencing of the shRNA construct, or it was unable to counter the abundance of SLC2A1 transcription and translation. The later possibility is supported by evidence reported for human FGR pregnancies (Janzen et al., 2013), in which SLC2A1 mRNA concentration was reduced ~50%, with no change in placental GLUT1 concentration. If it is determined that our SLC2A1 RNAi resulted in significant reductions in SLC2A1 mRNA without impacting the concentration of GLUT1 at 75 dGA, it would support the concept that there is an excess of SLC2A1 transcription in order to maintain a high concentration of GLUT1 for glucose transfer to the fetus.
Combining lentiviral-mediated RNAi with in vivo assessment of placental function
Baker et al. (2016) was able to demonstrate that in vivo RNAi could be used to assess the function of an abundantly expressed gene throughout gestation. The focus of that work (Baker et al., 2016) was on chorionic somatomammotropin (CSH)/placental lactogen (PL), which is one of the most abundantly expressed genes by the sheep placenta (Kappes et al., 1992, Anthony et al., 1995). Near-term (135 dGA) CSH RNAi resulted in two different phenotypes, pregnancies that exhibited placental and fetal weights (PI-FGR) that were 2 SD below the mean of the NTS RNAi controls, and those that did not meet that criteria (non-FGR). Baker et al. (2016) focused on characterizing those pregnancies that exhibited significant placental and fetal growth restriction. In those PI-FGR pregnancies, placental weight was reduced 52% and fetal weight by 32%, relative to the control pregnancies, and was associated with fetal hypoinsulinemia and a 62% reduction in umbilical artery concentrations of IGF1 (Baker et al., 2016). It had been hypothesized for some time that CSH/PL impacted both maternal and fetal metabolisms, by stimulating maternal and fetal IGF1 production and maternal insulin resistance (Handwerger 1991), and the data presented by Baker et al. (2016) supported the hypothesis regarding the fetus, but did not support impacts on maternal metabolism. The impact of CSH RNAi on placental mass was unexpected, suggesting that CSH RNAi was impacting placental growth and development, thereby leading to PI-FGR in these pregnancies. Subsequently, Jeckel et al. (2018) examined the impact of CSH RNAi at 50 dGA and reported an ~40% reduction in uterine vein CSH/PL concentrations with significant reductions in both fetal weight and fetal liver weight, with a lesser effect on placental mass. Jeckel et al. (2018) also reported reductions in placental IGF1, IGF2, SLC2A1, and SLC2A3 mRNA concentrations and were able to demonstrate a significant reduction in placental concentration of GLUT3 at 135 dGA.
As reported in Baker et al. (2016), not all of the pregnancies resulted in reductions in placental and fetal growth. There is precedence for the two distinct phenotypes obtained by CSH RNAi, as a number of human case studies reporting deletions or mutations in the CSH loci either resulted in apparent normal infant outcomes, or resulted in FGR (Alexander et al., 1982; Barbeiri et al., 1986; Borody and Carlton, 1981; Rygaard et al., 1998; Sideri et al., 1983; Simon et al., 1986). Daikoku et al. (1979) reported that suppressed maternal concentrations of CSH/PL was associated with FGR in some pregnancies, and not in others. This was corroborated by Lindberg and Nilsson (1973), who reported that ~40% of pregnancies with suppressed maternal CSH/PL concentrations had no birth weight abnormalities. As the uterine artery:uterine vein glucose concentration gradient was significantly elevated in both phenotypes of CSH RNAi pregnancies from the Baker et al. (2016) study, we questioned if the normal fetal weight pregnancies (non-FGR) were truly normal. Ali et al. (2020b) reported that in the non-FGR CSH RNAi pregnancies that umbilical artery IGF1 concentrations were significantly reduced (33%), as was the fetal liver mRNA concentrations of IGF1, IGF2, IGFBP2, and IGFBP3, similar to what was reported by Baker et al. (2016) for the CSH RNAi PI-FGR pregnancies.
Unfortunately, with the CSH RNAi pregnancies reported by Baker et al. (2016), Jeckel et al. (2018), and Ali et al. (2020b), the samples were collected at terminal surgery, under isoflurane anesthesia following an 18 h fast, precluding a steady-state assessment of placental function. We have recently reported the steady-state assessment of blood flow, uterine, and umbilical nutrient uptakes and uteroplacental utilization, under non-anesthetized/non-stressed conditions (Tanner et al., 2021a, b). In Tanner et al. (2021a), we reported the in vivo assessment of CSH RNAi pregnancies in which fetal weight near-term (132 dGA) was not impacted (non-FGR), placental weight was only ~12% less, and empty uterine weight was unchanged. As blood flow data were not obtained in Baker et al. (2016) and Ali et al. (2020b), it was surprising to find that both absolute (mL/min) and relative (mL/min/kg fetus) uterine blood flows were significantly reduced in these non-FGR pregnancies as a result of CSH RNAi (Tanner et al., 2021a). The ~23% reduction in uterine blood flow was associated with a 24% reduction in the uterine caruncle concentration of NOS3, corroborating the report by Gonzalez et al. (2015), who demonstrated that rat PL induced nitric oxide-dependent vasodilation of rat aortic rings in vitro. While uterine blood flow was reduced in these non-FGR pregnancies (Tanner et al., 2021a), umbilical blood flow was not impacted.
In the non-FGR pregnancies reported by Tanner et al. (2021a), little change was noted in uterine uptake, umbilical uptake, and uteroplacental utilization of oxygen and most amino acids. As noted in Baker et al. (2016) and Ali et al. (2020b) with the original CSH RNAi pregnancies, the non-FGR pregnancies reported by Tanner et al. (2021a) also exhibited significantly increased uterine artery:uterine vein glucose gradients. However, due to the reduction in uterine blood flow, uterine uptake of glucose was not impacted by CSH RNAi in these non-FGR pregnancies, nor was umbilical glucose uptake. In contrast, relative uteroplacental glucose utilization (μmol/min/kg placenta) was significantly increased, and as such, the fraction of uterine glucose uptake utilized by the placenta was increased, while the fraction of uterine glucose uptake transferred to the fetus was decreased. This study of non-FGR pregnancies as a result of CSH RNAi highlights the fact that in vivo physiological changes can occur in the absence of altered birth weights, which could still predispose the offspring for altered production traits. Furthermore, Tanner et al. (2021a) coupled with Ali et al. (2020b), highlight the utility of direct in vivo assessment of maternal-placental-fetal physiology under steady-state conditions to uncover novel impacts on pregnancy progression.
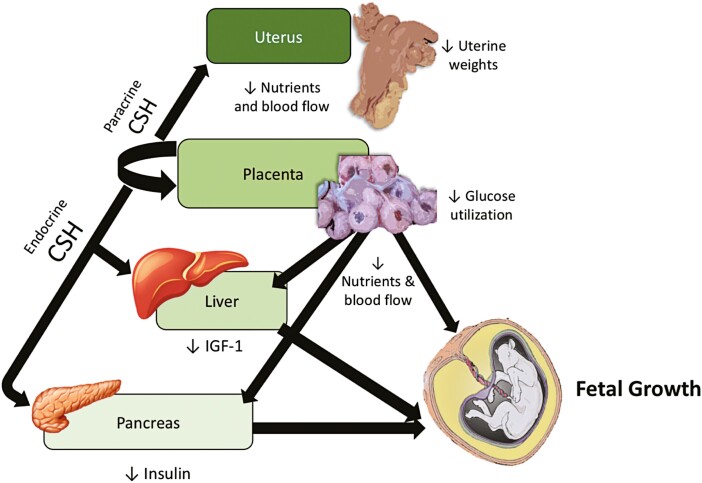
Tanner et al. (2021b) recently reported the in vivo assessment of a cohort of CSH RNAi pregnancies with FGR, with the major findings represented in Figure 4. In this cohort, fetal weight was reduced (30%) comparable to what was reported by Baker et al. (2016), yet placental weight was only reduced 21%. What had not been assessed in the original Baker et al. (2016) study was empty uterine weight, which in this study (Tanner et al., 2021b) was reduced 43%. The reduction in uterine weight mirrored the reduction in uterine blood flow (42%), and even though placental weight was not as severely impacted as it was in the Baker et al. (2016) study, umbilical blood flow was also significantly reduced (40%). Furthermore, umbilical blood flow relative to fetal weight was also reduced in these CSH RNAi pregnancies. The consistent effect of CSH RNAi on uterine blood flow (Tanner et al., 2021a,b), regardless of the presence or absence of an effect on uterine, placental, or fetal weight, may well infer that one of CSH/PLs direct actions is regulating uterine blood flow. As mentioned earlier, fetal infusion of glucagon over an 8- to 10-d period in late gestation (Cilvik et al., 2021) resulted in a 33% reduction in uterine blood flow and significant reductions in fetal weight, without effects on placental weight or umbilical blood flow. Somewhat surprising, was the finding that CSH/PL secretion rate (ng/min) into maternal circulation, but not fetal circulation, was reduced 80% as a result of fetal glucagon infusion. This may infer that the CSH/PL secretion at the maternal-fetal interface is the mechanism by which uterine blood flow was also diminished. Since these pregnancies (Cilvik et al., 2021) were catheterized, we were able to recently assess CSH/PL concentrations in the samples collected over the glucagon infusion period. As evidenced in Figure 5, it took 3 to 4 d of fetal glucagon infusion before there was a significant effect on maternal CSH/PL concentrations, at the same time not impacting concentrations of estradiol. Collectively, these data suggest that fetal glucagon may be impacting chorionic binucleate cell (origin of CSH/PL) formation or migration and fusion with the maternal-fetal syncytium. The in vivo relationship uncovered from these studies (Cilvik et al., 2021; Tanner et al., 2021a, b) between maternal CSH/PL, fetal glucagon, and uterine blood flow would not have been made without the ability to apply the Fick Principle under non-stressed/non-anesthetized conditions.
Figure 4.
Schematic representation of the major physiological changes that occur in CSH RNAi pregnancies near-term that results in fetal growth restriction.
Figure 5.
Impact of fetal glucagon infusion on uterine artery concentrations of CSH/PL and estradiol. Samples were derived from the study reported by Cilvik et al. (2021), and demonstrate the timecourse by which fetal glucagon infusion impacts maternal CSH/PL (A) without impacting estradiol (B).
While the Jeckel et al. (2018) data suggested that CSH RNAi may be impacting the abundance of placental glucose transporters, Tanner et al. (2021b) was able to directly assess glucose uptake, utilization, and transfer in CSH RNAi FGR pregnancies (Figure 4). As a result of CSH RNAi, uterine glucose uptake, umbilical glucose uptake, and uteroplacental glucose utilization were all reduced 44%–47% (Tanner et al., 2021b). This was accompanied by significant reductions in oxygen uptakes and utilization, as well as significant reductions in uterine uptakes of the essential amino acids (Tanner et al., 2021b). Due to the global impact on placental glucose transfer, an obvious question is whether this is merely a function of placental mass or due to functional changes in the placenta, as discussed earlier (Thureen et al., 1992; Wallace et al., 2003). We have begun to address this question in ongoing studies. While still ongoing, we have been able to study a small group of CSH RNAi pregnancies that exhibited PI-FGR comparable to what was reported in Baker et al. (2016), with both placental and fetal weights 2 SD below the mean of the controls (52% and 30% reductions, respectively). These CSH RNAi pregnancies exhibited significant reductions in uterine and umbilical blood flows, and global nutrient transfer deficiencies similar to that reported in Tanner et al. (2021b; Figure 4). Following the baseline study, either a maternal hyperglycemic clamp (dextrose infusion) or a hypoglycemic clamp (insulin infusion) was conducted to alter the maternofetal glucose gradient. Due to various catheters failing during the study periods, the current dataset is limited, but as evidenced in Figure 6, CSH RNAi clearly impacted placental glucose transfer to the fetus. The elevation/intercept of the two regression lines are different (P ≤ 0.01) and the slopes of the two regression lines tend to differ (P = 0.13) as well, implicating a deficit in placental glucose transport capacity. This is highlighted when examining CSH RNAi umbilical glucose uptake, following the maternal hyperglycemic clamp designed to maximize facilitative placental glucose transfer (greatest maternofetal glucose gradients), which is still significantly reduced (P ≤ 0.05). Recognizing that this dataset is limited in number, we believe it still demonstrates the potential to address mechanistic questions in vivo in a fashion not previously feasible in ruminants.
Figure 6.
Impact of in vivo CSH RNAi resulting in PI-FGR on umbilical glucose uptake in relationship to the maternofetal arterial glucose gradient. The intercept/elevation of the two regression lines are different (P ≤ 0.01) and the slope of the two lines tend to differ (P = 0.13).
Conclusions
Optimal placental development and function is a prerequisite for the successful outcome of pregnancy and as such, compromised placental function can impact postnatal health and well-being, including production traits of ruminants. In this review, it was not our intent to provide an exhaustive literature review, but rather to present fundamental findings in our understanding of in vivo placental function and the methodologies used. The ability to conduct steady-state in vivo study of placental function in catheterized sheep pregnancies has provided considerable insight into pregnancy physiology. The advent of in vivo RNAi methods, which can impact the abundance of specific gene products in the ruminant placenta, provides another methodological approach to investigate placental function. Both methodologies are technically and resource demanding, but together provide the opportunity for in vivo studies that are no longer just correlative in nature, and are not compromised by anesthesia, fasting, etc., which can confound the data being collected. Consequently, these two investigative approaches can now be applied together in a fashion that will provide insight into ruminant placental function that was not previously feasible.
Acknowledgments
Previously unpublished data presented in this review were supported by National Institutes of Health grants DK088139, HD093701, and HD094952. We want to thank the numerous students and staff at the Colorado State University Animal Reproduction and Biotechnology Laboratory and the University of Colorado Perinatal Research Center for animal care and technical support. This work was supported by National Institutes of Health grants DK088139, HD093701, and HD094952. All animal procedures described herein were approved by the Colorado State University Institutional Animal Care and Use Committee (Protocols KP1483 and KP1576) and the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee (Protocol 00714).
Glossary
Abbreviations
- CRISPR-Cas9
clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9
- CSH/PL
chorionic somatomammotropin/placental lactogen
- EGFP
enhanced green fluorescent protein
- GLUT1
facilitative glucose transporter 1
- GLUT3
facilitative glucose transporter 3
- FGR
fetal growth restriction
- IGF1
insulin like growth factor 1
- IGF2
insulin like growth factor 2
- IGFBP
insulin like growth factor binding protein
- iOTR
immortalized ovine trophoblast cells
- LL3.7
Lentilox 3.7
- LR3
human IGF1 analogue
- miRNA
micro RNA
- MOI
multiplicity of infection
- NTS
non-targeting sequence
- PI-FGR
placental insufficiency-fetal growth restriction
- pO2
partial pressure of oxygen
- PRR15
proline-rich 15
- RISC
RNA induced silencing complex
- RNAi
RNA interference
- RNase
ribonuclease
- SC
scrambled control
- shRNA
short-hairpin RNA
- SLC2A1
solute carrier family 2 member 1 (GLUT1)
- SLC2A3
solute carrier family 2 member 3 (GLUT3)
- TCID50
tissue culture infectious dose
Contributor Information
Amelia R Tanner, Department of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Victoria C Kennedy, Department of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Cameron S Lynch, Department of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Taylor K Hord, Department of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Quinton A Winger, Department of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Paul J Rozance, Department of Pediatrics, Division of Neonatology, College of Medicine, University of Colorado, Aurora, CO 80045, USA.
Russell V Anthony, Department of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Alexander, I., Anthony F., and Letchworth A. T.. . 1982. Placental protein profile and glucose studies in a normal pregnancy with extremely low levels of human placental lactogen. Case report. Br. J. Obstet. Gynaecol. 89:241–243. doi: 10.1111/j.1471-0528.1982.tb03623.x. [DOI] [PubMed] [Google Scholar]
- Alexander, D. P., Britton H. G., and Nixon D. A.. . 1970. The metabolism of fructose and glucose by the sheep foetus: studies on the isolated perfused preparation with radioactively labelled sugars. Q. J. Exp. Physiol. Cogn. Med. Sci. 55:346–362. doi: 10.1113/expphysiol.1970.sp002087. [DOI] [PubMed] [Google Scholar]
- Alexander, G., and Williams D.. . 1971. Heat stress and development of the conceptus in domestic sheep. J. Agric. Sci. 76:53–72. doi: 10.1017/S0021859600015616. [DOI] [Google Scholar]
- Ali, A., Stenglein M. D., Spencer T. E., Bouma G. J., Anthony R. V., and Winger Q. A.. . 2020a. Trophectoderm-specific knockdown of Lin28 decreases expression of genes necessary for cell proliferation and reduces elongation of sheep conceptus. Int. J. Mol. Sci. 21:2549. doi: 10.3390/ijms21072549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, A., Swanepoel C. M., Winger Q. A., Rozance P. J., and Anthony R. V.. . 2020b. Chorionic somatomammotropin RNA interference alters fetal liver glucose utilization. J. Endocrinol. 247:169–180. doi: 10.1530/JOE-20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, A. H., Fennessey P. V., Meschia G., Wilkening R. B., and Battaglia F. C.. . 1997. Placental transport of threonine and its utilization in the normal and growth retarded fetus. Am. J. Physiol. Endocrinol. Metab. 35:E892–E900. doi: 10.1152/ajpendo.1997.272.5.E892. [DOI] [PubMed] [Google Scholar]
- Anthony, R.V., Limesand S.W., Fanning M.D., and Liang R.. . 1998. Placental lactogen and growth hormone. In: The Endocrinology of Pregnancy, Bazer F.W., ed. Totowa (NJ):Humana Press. p.461–490. [Google Scholar]
- Anthony, R. V., Pratt S. L., Liang R., and Holland M. D.. . 1995. Placental-fetal hormonal interactions: impact on fetal growth. J. Anim. Sci. 73:1861–1871. doi: 10.2527/1995.7361861x. [DOI] [PubMed] [Google Scholar]
- Anthony, R. V., Scheaffer A. N., Wright C. D., and Regnault T. R.. . 2003. Ruminant models of prenatal growth restriction. Reprod. Suppl. 61:183–194. [PubMed] [Google Scholar]
- Baker, C. M., Goetzmann L. N., Canton J. D., Jeckel K. M., Winger Q. A., and Anthony R. V.. . 2016. Development of ovine chorionic somatomammotroin hormone-deficient pregnancies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R837–R846. doi: 10.1152/ajpregu.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeiri, F., Botticelli A., Consarino R., Genazzani A. R., and Volpe A.. . 1986. Failure of placenta to produce hPL in an otherwise uneventful pregnancy: a case report. Biol. Res. Pregnancy Perinatol. 7:131–133. [PubMed] [Google Scholar]
- Barry, J. S., and Anthony R. V.. . 2008. The pregnant sheep as a model for human pregnancy. Theriogenology. 69:55–67. doi: 10.1016/j.theriogenology.2007.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia, F. C. 2002. In vivo characteristics of placental amino acid transport and metabolism in ovine pregnancy—a review. Placenta. 23(Suppl A):S3–S8. doi: 10.1053/plac.2002.0812. [DOI] [PubMed] [Google Scholar]
- Battaglia, F.C., and Meschia G.. . 1986. An introduction to fetal physiology. Orlando (FL):Academic Press. p. 1–135. [Google Scholar]
- Battaglia, F. C., Meschia G., Makowski E. L., and Bowes W.. . 1968. The effect of maternal oxygen inhalation upon fetal oxygenation. J. Clin. Invest. 47:548–555. doi: 10.1172/JCI105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, A. W., Kennaugh J. M., Battaglia F. C., Makowski E. L., and Meschia G.. . 1986. Metabolic and circulatory studies of fetal lamb at midgestation. Am. J. Physiol. Endocrinol. Metab. 250:E538–E544. doi: 10.1152/ajpendo.1986.250.5.E538. [DOI] [PubMed] [Google Scholar]
- Borody, I. B., and Carlton M. A.. . 1981. Isolated defect in human placental lactogen synthesis in a normal pregnancy. Case report. Br. J. Obstet. Gynaecol. 88:447–449. doi: 10.1111/j.1471-0528.1981.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Brooks, K., Burns G. W., and Spencer T. E.. . 2015a. Peroxisome proliferator activator receptor gamma (PPARG) regulates conceptus elongation in sheep. Biol. Reprod. 92:1–13. doi: 10.1095/biolreprod.114.123877. [DOI] [PubMed] [Google Scholar]
- Brooks, K., Burns G., and Spencer T. E.. . 2015b. Biological roles of hydroxysteroid (11-Beta) dehydrogenase 1 (HSD11B1), HSD11B2, and glucocorticoid receptor (NR3C1) in sheep conceptus elongation. Biol. Reprod. 93:1–12. doi: 10.1095/biolreprod.115.130757. [DOI] [PubMed] [Google Scholar]
- Brooks, K., and Spencer T. E.. . 2015. Biological roles of interferon tau (IFNT) and type I IFN receptors in elongation of the ovine conceptus. Biol. Reprod. 92:1–10. doi: 10.1095/biolreprod.114.124156. [DOI] [PubMed] [Google Scholar]
- Burd, L. I., Jones M. D., Simmons M. A., Makowski E. L., Meschia G., and Battaglia F. C.. . 1975. Placental production and foetal utilisation of lactate and pyruvate. Nature. 254:710–711. doi: 10.1038/254710a0. [DOI] [PubMed] [Google Scholar]
- Carr, D. J., Aitken R. P., Milne J. S., David A. L., and Wallace J. M.. . 2012. Fetoplacental biometry and umbilical artery doppler velocimetry in the overnourished adolescent model of fetal growth restriction. Am. J. Obstet. Gynecol. 207:e6–e15. doi: 10.1016/j.ajog.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Cetin, I. 2001. Amino acid interconversions in the fetal-placental unit: the animal model and human studies in vivo. Ped. Res. 49:148–154. doi: 10.1203/00006450-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Cilvik, S. N., Wesolowski S. R., Anthony R. V., Brown L. D., and Rozance P. J.. . 2021. Late gestation fetal hyperglucagonaemia impairs placental function and results in diminished fetal protein accretion and decreased fetal growth. J. Physiol. 599:3403–3427. doi: 10.1113/JP281288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku, N. H., Tyson J., Graf E., Scott C., Smith R. B., Johnson J. W. C., and King T. M.. . 1979. The relative significance of human placental lactogen in the diagnosis of retarded fetal growth. Am. J. Obstet. Gynecol. 135:516–521. doi: 10.1016/0002-9378(79)90443-5. [DOI] [PubMed] [Google Scholar]
- Du, M., Tong J., Zhao J., Underwood K. R., Zhu M., Ford S. P., and Nathanielsz W.. . 2010. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 88:E51–E60. doi: 10.2527/jas.2009-2311. [DOI] [PubMed] [Google Scholar]
- Dunlap, K. A., Palmarini M., Varela M., Burghardt R. C., Hayashi K., Farmer J. L., and Spencer T. E.. . 2006. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc. Natl. Acad. Sci. U. S. A. 103:14390–14395. doi: 10.1073/pnas.0603836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, C., Rosas-Hernandez H., Jurado-Manzano B., Ramirez-Lee M. A., Salazar-Garcia S., Martiez-Cuevas P. P., Velarde-Salcedo A. J., Morales-Loredo H., Espinosa-Tanguma R., Ali S. F., . et al. 2015. The prolactin family hormones regulate vascular tone through NO and prostacyclin production in isolated rat aortic rings. Acta Pharmacol. Sin. 36:572–586. doi: 10.1038/aps.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger, S. 1991. Clinical counterpoint: the physiology of placental lactogen in human pregnancy. Endocr. Rev. 12:329–336. doi: 10.1210/edrv. [DOI] [PubMed] [Google Scholar]
- Hay, W. W. 1991. Energy and substrate requirements of the placenta and fetus. Proc. Nutr. Soc. 50:321–336. doi: 10.1079/pns19910042. [DOI] [PubMed] [Google Scholar]
- Hay, W. W., and Meznarich H. K.. . 1989. Effect of maternal glucose concentration on uteroplacental glucose consumption and transfer in pregnant sheep. Proc. Soc. Exp. Biol. Med. 190:63–69. doi: 10.3181/00379727-190-42830. [DOI] [PubMed] [Google Scholar]
- Hay, W. W., Molina R. A., DiGiacomo J. E., and Meschia G.. . 1990. Model of placental glucose consumption and glucose transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 258:R569–R577. doi: 10.1152/ajpregu.1990.258.3.R569. [DOI] [PubMed] [Google Scholar]
- Hay, W. W., Myers S. A., Sparks J. W., Wilkening R. B., Meschia, G. and Battaglia F. C.. . 1983. Glucose and lactate oxidation rates in the fetal lamb. Proc. Soc. Exp. Biol. Med. 173:553–563. doi: 10.3181/00379727-173-41686. [DOI] [PubMed] [Google Scholar]
- He, L., and Hannon G. J.. . 2004. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Huggett, A. S., Warren F. L., and Warren N. V.. . 1951. The origin of the blood fructose of the foetal sheep. J. Physiol. 113:258–275. doi: 10.1113/jphysiol.1951.sp004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen, C., Lei M. Y. Y., Cho J., Sullivan P., Shin B.-C., and Devaskar S. U.. . 2013. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta. 34:1072–1078. doi: 10.1016/j.placenta.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel, K. M., Boyarko A. C., Bouma G. J., Winger Q. A., and Anthony R. V.. . 2018. Chorionic somatomammotropin impacts early fetal growth and placental gene expression. J. Endocrinol. 237:301–310. doi: 10.1530/JOE-18-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józwik, M., Teng C., Battaglia F. C., and Meschia G.. . 1999a. Fetal supply of amino acids and amino nitrogen after maternal infusion of amino acids in pregnant sheep. Am. J. Obstet. Gynecol. 180:447–453. doi: 10.1016/s0002-9378(99)70230-9. [DOI] [PubMed] [Google Scholar]
- Józwik, M., Teng C., Battaglia F. C., and Meschia G.. . 1999b. Contribution of branched-chain amino acids to uteroplacental ammonia production in sheep. Biol. Reprod. 61:792–797. doi: 10.1095/biolreprod61.3.792. [DOI] [PubMed] [Google Scholar]
- Józwik, M., Teng C., Wilkening R. B., Meschia G., Tooze J., Chung M., and Battaglia F. C.. . 2001. Effects of branched-chain amino acids on placental amino acid transfer and insulin and glucagon release in the ovine fetus. Am. J. Obstet. Gynecol. 185:487–495. doi: 10.1067/mob.2001.116096. [DOI] [PubMed] [Google Scholar]
- Kappes, S. M., Warren W. C., Pratt S. L., Liang R., and Anthony R. V.. . 1992. Quantification and cellular localization of ovine placental lactogen messenger ribonucleic acid expression during mid- and late-gestation. Endocrinology. 131:2829–2838. doi: 10.1210/endo.131.6.1446621. [DOI] [PubMed] [Google Scholar]
- Lemons, J. A., Adcock E. W., Jones M. D., Naughton M. A., Meschia G., and Battaglia F. C.. . 1976. Umbilical uptake of amino acids in the unstressed fetal lamb. J. Clin. Invest. 58:1428–1434. doi: 10.1172/JCI108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand, S. W., Rozance P. J., Zerbe G. O., Hutton J. C., and W. W.Hay, Jr. 2006. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 147:1488–1497. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- Lindberg, B. S., and Nilsson B. A.. . 1973. Human placental lactogen (HPL) levels in abnormal pregnancies. Br. J. Obstet. Gynaecol. 80:1046–1053. doi: 10.1111/j.1471-0528.1973.tb02978.x. [DOI] [PubMed] [Google Scholar]
- Lynch, C. S., Ali A., Kennedy V. C., Tanner A. R., Winger Q. A., and Anthony R. V.. . 2020. Placental GLUT3 (SLC2A3) RNA interference: impact on fetal growth at mid-gestation. J. Anim. Sci. 98(Suppl. 4):378. doi: 10.1093/jas/skaa278.665. [DOI] [Google Scholar]
- Meschia, G., Battaglia F. C. P. D. Bruns, P. D.. 1967. Theoretical and experimental study of transplacental diffusion. J. Appl. Physiol. 22:1171–1178. doi: 10.1152/jappl.1967.22.6.1171. [DOI] [PubMed] [Google Scholar]
- Meschia, G., Battaglia F. C., Hay W. W., and Sparks J. W.. . 1980. Utilization of substrates by the ovine placenta in vivo. Federation Proc. 39:245–249. [PubMed] [Google Scholar]
- Meschia, G., Cotter J. R., Breathnach C. S., and Barron D. H.. . 1965. The diffusibility of oxygen across the sheep placenta. Q. J. Exp. Physiol. Cogn. Med. Sci. 50:466–480. doi: 10.1113/expphysiol.1965.sp001812. [DOI] [PubMed] [Google Scholar]
- Meschia, G., Hellegers A., Blechner J. N., Wolkoff A. S., and Barron D. H.. . 1961. A comparison of the oxygen dissociation curves of the bloods of maternal, fetal and newborn sheep at various pHs. Q. J. Exp. Physiol. Cogn. Med. Sci. 46:95–100. doi: 10.1113/expphysiol.1961.sp001520. [DOI] [PubMed] [Google Scholar]
- Paddison, P. J., Caudy A. A., Bernstein E., Hannon G. J., and Conklin D. S.. . 2002. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison, P. J., Caudy A. A., Sachidanandam R., and Hannon G. J.. . 2004. Short hairpin activated gene silencing in mammalian cells. Methods Mol. Biol. 265:85–100. doi: 10.1385/1-59259-775-0:085. [DOI] [PubMed] [Google Scholar]
- Purcell, S. H., Cantlon J. D., Wright C. D., Henkes L. E., Seidel G. E., and Anthony R. V.. . 2009. The involvement of proline-rich 15 in early conceptus development in sheep. Biol. Reprod. 81:1112–1121. doi: 10.1095/biolreprod.109.076190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, S. A., Raja J. S., Hoffman M. L., Zinn S. A., and Govoni K. E.. . 2014. Poor maternal nutrition inhibits muscle development in ovine offspring. J. Anim. Sci. Biotechnol. 5:43. doi: 10.1186/2049-1891-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault, T. R. H., Friedman J. E., Wilkening R. B., Anthony R. V., and Hay W. W.. . 2005. Fetoplacental transport and utilization of amino acids in IUGR—a review. Placenta. 26:S52–S62. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Roberts, R.M., and Anthony R.V.. . 1994. Molecular biology of the trophectoderm and placental hormones. In: Molecular Biology of the Female Reproductive System. Findlay J.K., ed. San Diego (CA):Academic Press. p. 395–440. [Google Scholar]
- Ross, J. C., Fennessey P. V., Wilkening R. B., Battaglia F. C., and Meschia G.. . 1996. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am. J. Physiol. Endocrinol. Metab. 270:E491–E503. doi: 10.1152/ajpendo.1996.270.3.E491. [DOI] [PubMed] [Google Scholar]
- Rubinson, D. A., Dillon C. P., Kwiatkowski A. V., Sievers C., Yang L., Kopinja J., Rooney D. L., Zhang M., Ihrig M. M., McManus M. T., . et al. 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Rygaard, K., Revol A., Esquivel-Escobedo D., Beck B. L., and Barrera-Saldana H. A.. . 1998. Absence of human placental lactogen and placental growth hormone (HGH-V) during pregnancy: PCR analysis of the deletion. Hum. Genet. 102:87–92. doi: 10.1007/s004390050658. [DOI] [PubMed] [Google Scholar]
- Sideri, M., De Virgiliis G., Guidobono F., Borgese N., Sereni L. P., Nicolini U., and Remotti G.. . 1983. Immunologically undetectable human placental lactogen in a normal pregnancy. Case report. Br. J. Obstet. Gynaecol. 90:771–773. doi: 10.1111/j.1471-0528.1983.tb09309.x. [DOI] [PubMed] [Google Scholar]
- Simon, P., Decoster C., Brocas H., Schwers J., and Vassart G.. . 1986. Absence of human chorionic somatomammotropin during pregnancy associated with two types of gene deletion. Hum. Genet. 74:235–238. doi: 10.1007/BF00282540. [DOI] [PubMed] [Google Scholar]
- Stremming, J., Heard S., White A., Chang E. I., Shaw S. C., Wesolowski S. R., Jonker S. S., Rozance P. J., and Brown L. D.. . 2021. IGF-1 Infusion to fetal sheep increases organ growth but not by stimulating nutrient transfer to the fetus. Am. J. Physiol. Endocrinol. Metab. 320:E527–E538. doi: 10.1152/ajpendo.00453.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerton, J. 1999. Morpholino antisense oligomers: the case for an RNase h-independent structural type. Biochim. Biophys. Acta 1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Tanner, A. R., Lynch C. S., Ali A., Winger Q. A., Rozance P. J., and Anthony R. V.. . 2021a. Impact of chorionic somatomammotropin RNA interference on uterine blood flow and placental glucose uptake in the absence of intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 320:R138–R148. doi: 10.1152/ajpregu.00223.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner, A. R., Lynch C. S., Kennedy V. C., Ali A., Winger Q. A., Rozance P. J., and Anthony R. V.. . 2021b. CSH RNA Interference reduces global nutrient uptake and umbilical blood flow resulting in intrauterine growth restriction. Int. J. Mol. Sci. 22. doi: 10.3390/ijms22158150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thureen, P. J., Trembler K. A., Meschia G., Makowski E. L., and Wilkening R. B.. . 1992. Placental glucose transport in heat-induced fetal growth retardation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 263:R578–R585. doi: 10.1152/ajpregu.1992.263.3.R578. [DOI] [PubMed] [Google Scholar]
- Tong, J. F., Yan X., Zhu M. J., Ford S. P., Nathanielsz P. W., and Du M.. . 2009. Maternal obesity downregulates myogenesis and β-catenin signaling in fetal skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 296:E917–E924. doi: 10.1152/ajpendo.90924.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn, O. R., Maksym K., Silva E., Barentsen K., Anthony R. V., Brown T. L., Hillman S. L., Spencer R., David A. L., Rosario F. J., . et al. 2021. Placenta-specific Slc38a2/SNAT2 knockdown causes fetal growth restriction in mice. Clin. Sci. 135:2049–2066. doi: 10.1042/CS20210575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrijer, B., Regnault T. R. H., Wilkening R. B., Meschia G., and Battaglia F. C.. . 2004. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am. J. Physiol. Endocrinol. Metab. 287:E1114–E1124. doi: 10.1152/ajpendo.00259.2004. [DOI] [PubMed] [Google Scholar]
- Wallace, J. M., Bourke D. A., and Aitken R. P.. . 1999a. Nutrition and fetal growth: paradoxical effects in the overnourished adolescent sheep. J. Reprod. Fertil. Suppl. 54:385–399. [PubMed] [Google Scholar]
- Wallace, J. M., Bourke D. A., Aitken R. P., and Cruickshank M. A.. . 1999b. Switching maternal dietary intake at the end of the first trimester has profound effects on placental development and fetal growth in adolescent ewes carrying singleton fetuses. Biol. Reprod. 61:101–110. doi: 10.1095/biolreprod61.1.101. [DOI] [PubMed] [Google Scholar]
- Wallace, J. M., Bourke D. A., Aitken R. P., Leitch N., and Hay W. W.. . 2002. Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282:R1027–R1036. doi: 10.1152/ajpregu.00465.2001. [DOI] [PubMed] [Google Scholar]
- Wallace, J. M., Bourke D. A., Aitken R. P., Milne J. S., and Hay W. W.. . 2003. Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. J. Physiol. 547:85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes, D. M., Seamark R. F., and Ballard F. J.. . 1977a. Metabolism of glucose, fructose and lactate in vivo in chronically cannulated foetuses and in suckling lambs. Biochem. J. 162:617–626. doi: 10.1042/bj1620617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes, D. M., Seamark R. F., and Ballard F. J.. . 1977b. The appearance of gluconeogenesis at birth in sheep. Activation of the pathway associated with blood oxygenation. Biochem. J. 162:627–634. doi: 10.1042/bj1620627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdas, W. F. 1952. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J. Physiol. 118:23–39. doi: 10.1113/jphysiol.1952.sp004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding, F. B. P., Fowden A. L., Bell A. W., Ehrhardt R. A., Limesand S. W., and Hay W. W.. . 2005. Localisation of glucose transport in the ruminant placenta: implications for sequential use of transporter isoforms. Placenta. 26:626–640. doi: 10.1016/j.placenta.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Yan, X., Huang Y., Zhao J. X., Long N. M., Uthlaut A. B., Zhu M. J., Ford S. P., Nathanielsz P. W., and Du M.. . 2011. Maternal obesity-impaired insulin signaling in sheep and induced lipid accumulation and fibrosis in skeletal muscle of offspring. Biol. Reprod. 85:172–178. doi: 10.1095/biolreprod.110.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, M. J., Ford S. P., Means W. J., Hess B. W., Nathanielsz P. W., and Du M.. . 2006. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J. Physiol. 575:241–250. doi: 10.1113/jphysiol.2006.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]