Abstract
Objectives
Abnormal vaginal discharge (Sayalan al-Rahim) is a common public health problem that significantly disrupts the health-related quality of life (HRQoL). Syndromic management infers the concurrent treatment of two or more infections. Hence, a comparative, single-blind study was planned to determine the efficacy of Acacia (Acacia nilotica Linn.) pod's sitz bath (Abzan) plus vaginal pessary (Farzaja) vs. placebo in abnormal vaginal discharge syndromic management, its associated symptoms, and women's HRQoL.
Methods
Diagnosed patients (n = 66) were randomly divided into Acacia (n = 33) and placebo (n = 33) group. Acacia group received Sitz bath with Acacia pod powder (30g) solution followed by vaginal cotton pessary (5 ml of the same solution) once daily for 10 days. The placebo group received palm sugar powder (30g) solution for Sitz bath plus vaginal cotton pessary same as the Acacia group. Primary outcomes included clinical cure assessed with VAS for symptoms and Modified McCormack Pain Scale (McPS) for pelvic tenderness. The secondary outcomes included were the EQ-5D-5 L questionnaire, TSQM questionnaire, sachet count, and microbiological cure. Overall, therapeutic cure included clinical and microbiological cure after treatment.
Results
The overall therapeutic cure for bacterial vaginosis, cervicitis, and uncomplicated pelvic inflammatory disease was 100% (n = 7/7), 45.45% (n = 10/22), and 71.42% (n = 5/7), respectively, in the Acacia group, while in the placebo group none of the patients had responded. The VAS score for symptoms was significantly reduced in Acacia than in the placebo group. At each follow-up, the improvement in the EQ-5D-5 L level of HRQoL was significantly higher in the Acacia group than in the placebo group.
Conclusion
Acacia would be an effective and safe alternative in syndromic management of abnormal vaginal discharge, associated symptoms, and improved women's HRQoL. Trial registration. This trial was registered in the Clinical Trials Registry of Indian Trials Website and given the identification no. CTRI/2018/02/012175 (dated: 27/02/2018).
1. Introduction
Abnormal vaginal discharge (Sayalan al-Rahim) is the commonest gynaecological problem in women [1] reported (21.8%) in reproductive tract infection (RTI) after abnormal uterine bleeding (AUB). Almost ten million clinical visits each year are ascribed to vaginal discharge complaints. It is more common in women in developing countries, and available evidence suggests that about one-fourth of these women are having this complaint [2]. The ICMR conducted a hospital-based study in Delhi and reported the prevalence of abnormal vaginal discharge (AVD) is about 30% in women with RTIs. Gardnerella vaginalis, Trichomonas vaginalis, Candida, aerobic and anaerobic microbes, viruses, Chlamydia, etc. are various organisms that cause RTIs [1]. Lower reproductive tract infections are more common in Indian reproductive age women [3]. These infections can lead to long-term complications in women such as infertility, ectopic pregnancy, pelvic inflammatory disease, and propensity towards neoplasia with noteworthy morbidity that affects the quality of life and leads to a substantial burden on the healthcare system [4]. Abnormal white/vaginal discharge in women is associated with various other symptoms such as pruritus vulvae, backache, dysuria, and burning micturition. The syndromic approach (WHO 2005) is employed to manage vaginal infections by healthcare providers. Syndromic management is established on the patient's symptoms and infers the concurrent treatment of two or more infections [5]. Clinically, depending on the pathogenic infection, the first choice to treat infective vaginal discharge is antibiotics. To cure or prevent infections, no single broad-spectrum formulations are currently available for intravaginal use. Most of the drugs used to treat infective vaginal discharge cause side effects. Furthermore, antimicrobial resistance is increasing, rendering some regimens ineffective in several infections [3]. In the current scenario, adverse drug reactions of a chemical drug are one of the reasons for an increased drive observed towards the consumption of herbal drugs in many disease conditions. One of the suggested herbal drugs is Acacia (Acacia nilotica Linn.) pod for abnormal vaginal discharge.
A. arabica is a moderate-sized, spiny evergreen tree. It is a popular ornamental multipurpose avenue tree from the family Fabaceae and is commonly known as kikar. In India, its parts are used in ethnomedicinal practice for the prevention and treatment of various illnesses for many years [6]. In classical Unani medical texts, it has been mentioned that A. arabica pods are useful in abnormal vaginal discharge as it possesses anti-inflammatory (Muhallil al-Waram), astringent (Qabiz), and antiseptic (Daf'-i-Taffun) properties [6,7]. Satish et al. (2008) demonstrated the activity of pod A. arabica against a few strains of bacteria and fungi [8]. Kalaivani and Methew (2010) also demonstrated the maximum activity of A. arabica against Candida albicans and anaerobic bacteria [9]. Pharmacologically, this medicinal plant is proven for astringent, antimicrobial, anti-inflammatory, analgesic, antioxidant, and diuretic properties [10–14]. Although a few studies have been carried out on complementary and alternative medicines for abnormal vaginal discharge, bacterial vaginosis and cervicitis are available [3,5,15–20]. However, to date, none of the studies has determined the efficacy of Acacia pod's Sitz bath and vaginal pessary in syndromic management of abnormal vaginal discharge, associated symptoms, and women's health-related quality of life (HRQoL). Hence, this study was planned to validate its efficacy.
2. Materials and Methods
2.1. Trial Design
A parallel, single-blind, prospective, single-centre, simple randomized, placebo-controlled trial was carried out with approval from Institutional Ethical Committee (IEC No.NIUM/IEC/2016-17/014/ANQ/06). The study was conducted based on the GCP guidelines, Ministry of AYUSH, Govt. of India, and the Helsinki Declaration. All randomized patients received written and verbal information about the aims and procedures of the research and then signed a consent form to participate in the study. Patients at any point in time had the option to withdraw from the study.
2.2. Participants
Sixty-six patients with abnormal vaginal discharge were recruited from the outpatients and inpatients of our hospital
2.2.1. Inclusion and Exclusion Criteria
Married women between 18 and 50 years of age who had abnormal vaginal discharge and/or associated with low backache, burning micturition, lower abdomen pain, dysuria, dyspareunia, vulvar itching, and irritation were eligible for inclusion.
Patients were excluded from the study, who had undiagnosed uterine or vaginal bleeding, ulceration, vaginal douches, and genital malignancies. Pregnant, lactating, and unmarried women who were suspicious or clinically manifested with venereal disease were also excluded.
2.2.2. Demographic and Clinical Assessment
A general questionnaire including demographic characteristics and relevant history was completed for each patient. The patient's socio-economic status was recorded as per Kuppuswamy's socio-economic scale. At visit 1 (Day 0), the VAS score was calculated for abnormal vaginal discharge and its associated symptoms. The per speculum and vaginum examination were performed to note the Modified McCormack tenderness scale for pelvic pain, the nature, colour, quantity, and consistency of vaginal discharge and other associated clinical features of infections (vaginitis, cervicitis, cervical ectopy, and uncomplicated pelvic inflammatory disease (uPID). A vaginal wet mount test to diagnose bacterial vaginosis, candidiasis and trichomoniasis, and uPID was performed. All laboratory procedures were performed in the pathological laboratory of the National Institute of Unani Medicine. BV was diagnosed with Amsel criteria. Cervicitis was diagnosed with the presence of congestion, hypertrophy, erosion of the cervix along with the presence of uterine/cervical motion tenderness, thick yellowish or greyish discharge, and the presence of >10 WBC per HPF on saline microscopy. Uncomplicated PID (uPID) was diagnosed as per CDC guidelines the presence of at least one of the following, i.e., uterine tenderness, cervical motion tenderness, or adnexal tenderness, and one or more of the following additional criteria, i.e., abnormal cervical or vaginal thick discharge; the presence of >10 WBCs per HPF or abundant number of WBCs with saline microscopy of vaginal fluid and elevated erythrocyte sedimentation rate were noted. The severity of cervical ectopy was graded as 2a (1/3rd) if it was involved 1/3rd portion of the cervix around the os and 2b (2/3rd) if the involved portion was 2/3rd of the cervix. Routine investigations at baseline were performed to exclude general diseases and sexually transmitted diseases. Wet mount and pap's smears were done at visit 1 (Day 0) and postintervention (days 11–13) for the assessment of the efficacy of the test drug. Pelvic ultrasonography was performed to exclude genital malignancies and other pathologies, respectively, at baseline.
2.3. Intervention
After a thorough literature survey, Acacia pod's Sitz bath plus vaginal cotton pessary for abnormal vaginal discharge was selected from the classical Unani literature based on its Unani and pharmacological properties such as antimicrobial, anti-inflammatory, and astringent. The pharmacognosist, Dr S. Noorunnisa Begum (Senior Assistant Professor, Centre for Repository of Medicinal Resources, Trans-Disciplinary University, Bengaluru), authenticated and identified the test drug as pods of Acacia nilotica Linn. belonging to the family Fabaceae with specimen number FRLHT Acc. No. 5008. The common name is Acacia. The test drug has been deposited in the Department of Pharmacology of our Institute with voucher specimen number 56/UQ/Res/2019 for future reference [Figure 1]
Figure 1.
Depiction of test drug (Acacia nilotica): (a) tree, (b) pod, (c) powder of aqueous extract.
2.3.1. Extract Preparation
Joshanda of pods was modified into dry powder. Dried pods of A. arabica Linn. were coarsely powdered and sieved under aseptic precautions. To obtain the extract, the sieved powder was soaked in water (at the ratio of 1 : 4) and boiled at 100°C for an hour and filtered. The filtrate was dried in a hot air oven for four hours at 60°C, and the dry powder was obtained. All procedures were carried out in our pharmacy under the direct supervision of the pharmacist from our research team.
2.3.2. Dispensing of Drugs
To avoid exposure to humidity, 30g extract powder or placebo (palm sugar powder) was packed and dispensed in airtight aluminium sachets. Ten aluminium sachets were dispensed to each patient. One placebo capsule filled with edible cellulose (250 mg) was administered orally in the morning after meal for 10 days in both groups to increase the compliance of the patients.
2.3.3. Dosage and Methods
Patients were advised for a Sitz bath with powder followed by per vaginum cotton pessary soaked in 5 ml of the same solution once daily for 10 days in both groups. Patients were taught verbally how to use their medication for sitz bath and vaginal pessary insertion. All patients were instructed to add and mix the powder of one sachet in 250 ml of lukewarm water, and from this, 5 ml of solution was kept aside for the vaginal pessary and the remaining solution was added in 5 litres of water in a sitz bath for 20 min. After the sitz bath, patients were instructed to insert a vaginal pessary per vagina soaked in 5 ml of solution (which was kept aside) and to remove it the next morning.
2.4. Follow-Up
To minimize the dropout rate, patients were instructed to visit the hospital on Day 3 and days 11–14 during treatment and two follow-ups on days 30–34 and Day 45 without treatment. If patients were not able to visit on Day 3, they were called on mobile to enquire regarding the clinical features and compliance of the research drug. Moreover, they were asked to deliver empty and unused sachets. The side effects were assessed by the researcher, who determined whether the event was study related or not.
2.5. Outcomes
The primary outcomes (clinical and symptomatic response) included a change in VAS score for symptoms (abnormal vaginal discharge, lower abdominal pain, dysuria, burning micturition, dyspareunia, vulvar irritation, and itching) and Modified McCormack Pain Scale for abdominal pain and rebound tenderness at Day 11 and Day 45 from baseline.
The secondary outcome included changes in quality of life assessed by the EQ-5D-5 L health survey questionnaire from baseline to days 11–14, days 30–34, and Day 45. Treatment Satisfaction Questionnaire for Medication (TSQM), Ver II for satisfaction with medications, sachets count for compliance were assessed on days 11–14 after completion of the treatment and microbiological cure (vaginal wet mount test, pH and Pap's smear) was assessed on days 11–14 from baseline.
2.6. Sachet's Count
All patients were given ten sachets at baseline. Patients were instructed to return any unused and empty sachets, and the number of unused sachets returned was counted and recorded on days 11–14. A measure of patch adherence was calculated as the number of sachets dispensed minus the number returned, divided by 10 (i.e., the total number of prescribed doses). If a patient dropped out of the treatment and failed to return dispensed sachets, the sachets were assumed not to have been used and were treated in the same way as returns. For the patient who dropped out of treatment in the first postrandomization of the study and never returned any sachets, this variable was coded as 0% adherence. This method of data collection for the measurement of sachet adherence is similar to pill count [21].
2.7. Overall Therapeutic Cure
Overall, the therapeutic cure was defined as meeting the criteria for both clinical and microbiological cure/investigational cure after treatment on days 11–14 from baseline. The microbiological test included vaginal saline wet mount test, pH determination, pus cell count, and whiff test with 10% KOH to confirm bacterial vaginosis with Amsel criteria, trichomoniasis, candidiasis, and uPID.
Clinical cure was defined as a patient who had normal vaginal discharge, negative KOH test, normal pH, and no uterine/cervical motion. Microbiological/investigational cure were defined as a normal vaginal cytology on vaginal smear and normal Pap smear report.
2.8. Randomization, Allocation, and Masking
A simple random sampling was used to randomly assign the patients into two groups. The allocation sequence was generated by random allocation software (RAS) with a single block with an allocation ratio of 1 : 1. An open list of random numbers was used through the order of randomization and until the interventions were assigned to the patient, it was concealed from the first researcher. The matching and masking were done by supplying the medicine in the same aluminium sachets.
2.9. Sample Size
The sample size of a total of 67 participants (n1 = 33, n2 = 34) was required that was calculated based on the proportion of cure 34% and 50% obtained from the previous study [22]. Hence, the sample size was taken as 66 including 10% dropout.
2.10. Statistical Analysis
For analysis of the data, the statistical software SPSS 22.0 ver 3.2.2 were used. Mean ± SD was used for results from continuous measurements and number (%) for categorical measurements. For all statistical tests, the test of significance was 5%, 95% confidence interval, and 80% power of the study, a two-sided p value. The Chi-square test or Fisher's tests were utilized for comparison of the proportions. For intragroup comparisons, a paired Student's t-test or Wilcoxon matched paired test depends on the skewness of data. The intergroup comparison using Student's t-test and Mann–Whitney U test for normally distributed data and skewed data, respectively, was performed. The ITT principle was performed for all efficacy variables using data from all randomized subjects with at least one postrandomization outcome measure. The last observation carried forward method was used to impute the missing data.
3. Results
3.1. Recruitment and Follow-Up
The recruitment of patients was initiated on 1 March 2018 and completed on 8 November 2018. Initially, a total of 130 patients were screened, of which 64 were excluded (31 were ineligible, and 33 patients refused to participate). Thus, 33 patients were randomly allocated to each group. The flowchart for the enrolment of patients is shown in Figure 2.
Figure 2.
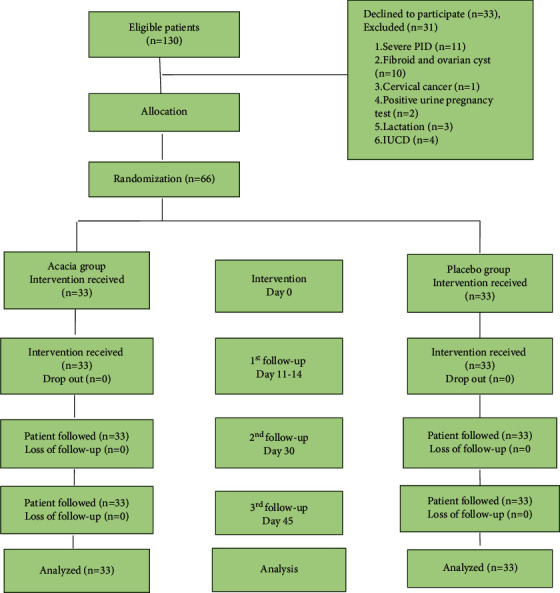
Flow chart of patients through the study according to consort statement.
3.2. Participant
The majority of the patients (96.96%) in each group were from an urban area. The mean age was 28.66 ± 5.76 and 30.57 ± 5.47 years in the Acacia and placebo group. The socio-demographic and reproductive characteristics are summarized in Table 1.
Table 1.
Baseline and socio-economic characteristics.
| Variables | Acacia group (n = 33) | Placebo group (n = 33) | p value |
|---|---|---|---|
| Age (year) | 28.66 ± 5.76 | 30.57 ± 5.47 | 0.17c |
| Urban | 32 (96.96) | 32(96.96) | 1.00a |
| Past menstrual cycle | 0.282a | ||
| Irregular | 6(18.18) | 3(9.09) | |
| Regular | 27(81.81) | 30(90.90) | |
| Duration of abnormal Vaginal discharge (days) | 15.51 ± 11.85 | 14.72 ± 13.74 | 0.65b |
| Socio-economic status | 0.71a | ||
| Upper middle (II) | 13 (39.39) | 16(48.48) | |
| Lower middle (III) | 13 (39.39) | 12(36.36) | |
| Upper lower (IV) | 7 (21.21) | 5(15.15) | |
| Height(cm) | 156.28 ± 5.40 | 154.22 ± 4.73 | 0.051c |
| Weight(Kg) | 61.09 ± 13.42 | 26.2 ± 5.89 | 0.714c |
| BMI (kg/m2) | 24.98 ± 5.19 | 26.2 ± 5.89 | 0.376c |
Data presented were as follows: mean ± SD or no (%); p > 0.05, considered not significant; tests used were as follows: aFisher's exact test; bChi-squared test; cMann–Whitney U test.
3.3. Primary Outcomes
3.3.1. VAS Score of Abnormal Vaginal Discharge (AVD) and Its Associated Symptoms
Acacia group showed improvement in the mean VAS score for AVD and its associated symptoms after treatment. The intergroup comparison at each follow-up was statistically significant, p < 0.0001 (Table 2).
Table 2.
Primary Outcome: VAS scoring of symptoms and the Modified McCormack Pain Scale in the Acacia and the control group.
| Primary outcome | Acacia group (n = 33) | Placebo group (n = 33) | p value |
|---|---|---|---|
| VAS for LAP and LBA | |||
| Day 0 | 4.66 ± 1.68 | 5.18 ± 1.26 | 0.26 |
| Days 11–14 | 1.36 ± 1.72 | 5.06 ± 1.27 | <0.0001 |
| Day 45 | 0.63 ± 1.02a | 4.90 ± 1.33b | <0.0001 |
| VAS for abnormal vaginal discharge | |||
| Day 0 | 6.39 ± 0.70 | 6.12 ± 0.59 | 0.39 |
| Days 11–14 | 1.51 ± 2.04 | 6 ± 0.61 | <0.0001 |
| Day 45 | 0.60 ± 1.19a | 6 ± 0.61b | <0.0001 |
| VAS for dyspareunia | |||
| Day 0 | 1.54 ± 2.32 | 1.75 ± 2.34 | 0.83 |
| Days 11–14 | 0.15 ± 0.44 | 1.72 ± 2.34 | 0.02 |
| Day 45 | 0.03 ± 0.17a | 1.60 ± 2.20b | 0.007 |
| VAS for dysuria | |||
| Day 0 | 1.69 ± 2.43 | 1.84 ± 2.43 | 0.74 |
| Days 11–14 | 1.69 ± 2.43 | 1.81 ± 2.39 | 0.04 |
| Day 45 | 0.09 ± 0.29a | 1.66 ± 2.21b | 0.008 |
| VAS for burning micturition | |||
| Day 0 | 3.48 ± 2.48 | 3 ± 2.44 | 0.38 |
| Days 11–14 | 0.75 ± 1.03 | 2.84 ± 2.34 | 0.0007 |
| Day 45 | 0.33 ± 0.64a | 2.69 ± 2.24b | 0.0001 |
| VAS for vulvar irritation | |||
| Day 0 | 3.18 ± 2.55 | 3 ± 2.72 | 0.94 |
| Days 11–14 | 0.87 ± 1.34 | 2.87 ± 2.64 | 0.003 |
| Day 45 | 0.18 ± 0.46a | 2.84 ± 2.60b | <0.0001 |
| VAS for vulvar itching | |||
| Day 0 | 4.63 ± 2.14 | 3.90 ± 2.45 | 0.18 |
| Days 11–14 | 1.42 ± 1.73 | 3.78 ± 2.23 | <0.0001 |
| Day 45 | 0.39 ± 0.65a | 3.63 ± 2.35b | <0.0001 |
| Modified McCormack Pain Scale (McPS) for abdominal tenderness | |||
| Day 0 | 2.36 ± 1.43 | 2.36 ± 1.43 | 0.43 |
| Days 11–14 | 1.06 ± 1.36 | 1.96 ± 1.28 | 0.01 |
| Day 45 | 0.33 ± 0.76a | 1.93 ± 1.24b | <0.0001 |
Data presented were as follows: mean ± SD; ap < 0.0001 considered extremely significant on Day 11 and Day 30 from Day 0 in the Acacia group. bp > 0.05 considered not significant on days 11–14 and Day 45 from Day 0 in the placebo group; tests used were as follows: Wilcoxon matched paired test VAS : visual analogue scale; LAP : lower abdominal pain; LBA : low backache.
3.3.2. Modified McCormack Pain Scale for Abdominal Pain and Rebound Tenderness
The mean score for the Modified McCormack Pain Scale (McPS) for abdominal pain and rebound tenderness on Day 11 was 1.06 ± 1.36 in the acacia and 1.96 ± 1.28 in the placebo group (P=0.01, statistically significant) (Table 2).
3.4. Secondary Outcome Measures
3.4.1. EQ-5D-5L Health Questionnaire for HRQoL
At baseline, index level in the Acacia and placebo group was statistically insignificant (p=0281). The Acacia group showed statistically significant improvement than the placebo group in EQ-5D-5L (p < 0.001). The Acacia group showed a statistically significant difference at posttreatment compared to baseline (p < 0.001), whereas it was insignificant in the placebo group (p > 0.05). The tests used were Mann–Whitney U test and Wilcoxon matched paired test for intergroup and intragroup comparison, respectively (Figure 3).
Figure 3.
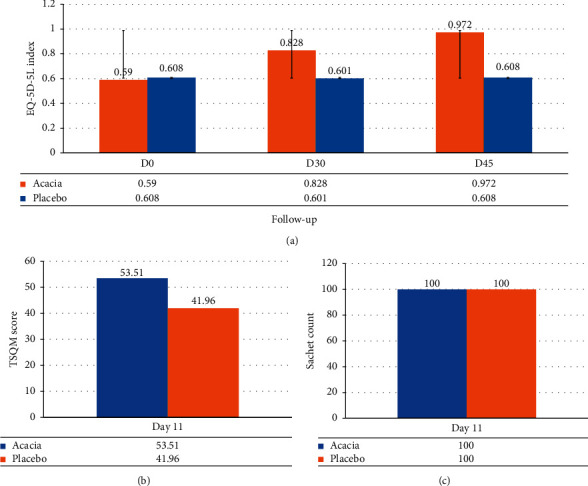
Depiction of secondary outcomes: (a) EQ-5D-5 L score, (b) TSQM score, and (c) Sachet count.
3.4.2. Treatment Satisfaction Questionnaire for Medication (TSQM)
On Day 11, the mean score of TSQM was 53.51 ± 4.34 and 41.96 ± 3.64 in the Acacia and placebo groups, respectively, statistically extremely significant (p < 0.001) in the Acacia group (Figure 3).
3.4.3. Sachet's Count for Compliance
The compliance in both groups was 100%
3.4.4. Microbiological Test
The microbiological test on Day 11 was statistically significant (p < 0.0001) when compared to Day 0 in the Acacia group; however, in the placebo group, it was insignificant (p > 0.05) (Table 3).
Table 3.
Microbiological evaluation in the Acacia and placebo groups.
| Investigation | Acacia group (n = 33) | p value | Placebo group (n = 33) | p value | ||
|---|---|---|---|---|---|---|
| Day 0 | Day 11–14 | Day 0 | Day 11-14 | |||
| Amsel's criteria | ||||||
| Whiff test | 0.02 | 1.00 | ||||
| Negative | 24 (72.72) | 31 (93.93) | 25 (75.75) | 25 (75.75) | ||
| Positive | 9 (27.27) | 2 (6.06) | 8 (24.24) | 8 (24.24) | ||
| Clue cells | 0.02 | 1 | ||||
| Negative | 24 (72.72) | 31 (93.93) | 24 (72.72) | 24(72.72) | ||
| Positive | 9 (27.27) | 2 (6.06) | 9 (27.27) | 9 (27.27) | ||
| Vaginal pH | 4.54 ± 0.46 | 3.93 ± 0.48a | <0.0001 | 4.57 ± 0.47 | 4.59 ± 0.44b | 0.78 |
| Vaginal wet mount test for microscopic investigation of vaginal discharge | ||||||
| KOH slide for hyphae | 1.00 | 1.00 | ||||
| Present | 0 (0) | 0 (0) | 1 (3.03) | 1 (3.03) | ||
| Absent | 33 (100) | 33 (100) | 32 (96.96) | 32 (96.96) | ||
| Normal saline test for trichomonas | 1.00 | 1.00 | ||||
| Absent | 33 (100) | 33 (100) | 33 (100) | 33( 100) | ||
| Present | 0 | 0 | 0 | 0 | ||
| Pus cells (hpf) | <0.001 | 0.93 | ||||
| <10 | 3 (9.09) | 24 (72.72) | 5 (15.15) | 5 (15.15) | ||
| 11–20 | 6 (18.18) | 6 (18.18) | 8 (24.24) | 7 (21.21) | ||
| 21–30 | 21 (63.63) | 2 (6.06) | 15 (45.45) | 14 (42.42) | ||
| >30 | 3 (9.09) | 1 (3.03) | 5 (15.15) | 7 (21.21) | ||
| Pus cells (hpf) | 22.96 ± 8.48 | 8.21 ± 7.85 | <0.001 | 23.15 ± 7.32 | 22.15 ± 8.7 | 0.47 |
| Pap smear | ||||||
| Normal | 4 (12.12) | 20 (60.60) | <0.001 | 4 (12.12) | 8 (24.24) | 0.63 |
| Inflammatory | 22 (66.66) | 11 (33.33) | 22 (66.66) | 18 (54.54) | ||
| BV | 7 (21.21) | 2 (6.06) | 6 (18.18) | 6 (18.18) | ||
| Candidiasis | 0(0) | 0(0)a | 1 (3.03) | 1(3.03)b | ||
Data presented were as follows: no (%) or mean ± SD; aP < 0.0001 considered extremely significant on day 11 from day 0 in the Acacia group; bP > 0.05 considered not significant on day 11 from day 0 in the Placebo group; Tests used were as follows: fisher's exact test and Wilcoxon matched paired test.
3.4.5. Overall Therapeutic Outcome
In the Acacia group, the therapeutic cure for bacterial vaginosis, cervicitis, and PID was 100% (n = 7/7), 45.45% (n = 10/22), and 71.42% (n = 5/7), respectively, whereas in the control group none of the patients had responded to the treatment (Table 4).
Table 4.
Overall therapeutic outcome (clinical cure, microbiological cure, and therapeutic cure in both groups).
| Variables | Bacterial vaginosis | Candidiasis | Cervicitis | uPID | |||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | AG | PG | AG | PG | AG | PG | AG | PG | |
| No. of Pt | 7(100) | 6(100) | 0 | 1(100) | 22(100) | 22(100) | 7(100) | 14(100) | |
| Clinical cure | |||||||||
| Colour of discharge | |||||||||
| D0 | White | 7(100) | 4(66.6) | 0 | 1(100) | 4(66.6) | 6(27.27) | 2(28.57) | 4(28.57) |
| Greyish | 0(0) | 0 | 15(68.18) | 14(63.63) | 2(28.57) | 6(42.85) | |||
| Yellowish | 0(0) | 2(33.33) | 3(13.63) | 2(9.09) | 3(42.85) | 4(28.57) | |||
| Greenish | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | |||
| D11-14 | Responded | 7(100) | 0 | 0 | 0 | 17(77.27) | 0 | 5(71.42) | 0 |
| Odour of discharge | |||||||||
| D0 | No-smell | 0(0) | 0(0) | — | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Foul-smell | 7(100) | 0(0) | — | 1(100) | 19(86.36) | 21(95.45) | 5(71.42) | 8(57.14) | |
| Fishy | 0(0) | 6(100) | 3(13.63) | 1(4.54) | 2(28.57) | 6(42.85) | |||
| D11-14 | Responded | 7(100) | 0 | 0 | 0 | 20(90.90) | 0 | 7(100) | 0 |
| Amount of discharge | |||||||||
| D0 | Present | 7(100) | 6(100) | — | 1(100) | 22(100) | 22(100) | 7(100) | 14(100) |
| D11-14 | Responded | 7(100) | 0 | — | 0 | 10(45.45) | 0 | 5(71.42) | 0 |
| D0 | Ut. motion tenderness | — | — | — | — | 17(77.27) | 18(81.81) | 7(100) | 14(100) |
| D11-14 | Responded | — | — | — | — | 10(58.82) | 0 | 5(71.42) | 0 |
| D0 | McPS | — | — | — | — | — | — | 7(100) | 14(100) |
| D11 | Responded | — | — | — | — | — | — | 5(71.42) | 0 |
| Microbiological and investigational cure (vaginal smear, pH, pap smear) | |||||||||
| D0 | pH > 4.5 | 6(85.7) | 5(83.33) | — | — | — | — | — | — |
| D11-14 | Responded | 6(100) | 0 | ||||||
| D0 | Clue cells | 7(100) | 6(100) | ||||||
| Hyphae | — | — | — | 1(100) | — | — | — | — | |
| Pus cells >10 | 22(100) | 21 (95.45) | 6(85.71) | 13(92.8) | |||||
| D11-14 | Responded | 7(100) | 0 | 0 | 13(59.09) | 0 | 6 | 7(100) | |
| D0 | Whiff test | 7(100) | 6(100) | — | — | — | — | — | 7(100) |
| D11-14 | Responded | 7(100) | 0 | — | — | — | — | — | 7(100) |
| D0 | Pap smear | 7(100) | 6(100) | - | 1(100) | 22(100) | 22(100) | 7(100) | |
| D11-14 | Responded | 7(100) | 0 | — | — | 10(45.45) | 0 | 7(100) | |
| Therapeutic cure | 7(100) | 0 | 0 | 0 | 10(45.45) | 0 | 5(71.42) | 0 | |
Data presented were as follows: no (%); AG : Acacia group; PG : placebo group; McPS : Modified McCormack Pain Scale; Pt : patient; uPID (uncomplicated pelvic inflammatory disease)
Data presented were as follows: no (%) or mean ± SD; ap < 0.0001 considered extremely significant on Day 11 from Day 0 in the Acacia group; bp > 0.05 considered not significant on Day 11 from Day 0 in the placebo group; tests used were as follows: Fisher's exact test and Wilcoxon matched paired test.
4. Discussion
4.1. Major Findings
This study is the first of its kind as none of the studies until date as per the researcher's knowledge has conducted a trial on sitz bath and vaginal pessary of A. arabica pod powder in abnormal vaginal discharge syndromic management, its associated symptoms, and improving women's health-related quality of life (HRQoL). The interpretation of the results supports that there was a significant reduction in abnormal vaginal discharge, its associated symptoms, and improvement in HRQoL in patients in the Acacia group. The present study showed a significant reduction in all symptoms in the Acacia group; similarly, Salhan et al. also reported a significant reduction in lower abdominal pain, dysuria, and vaginal itching with Praneem vaginal tablets [3]. In the Acacia group, the therapeutic cure for bacterial vaginosis, cervicitis, and PID was 100% (n = 7/7), 45.45% (n = 10/22), and 71.42% (n = 5/7), respectively, and none had trichomoniasis and a significant reduction in the symptoms. Likewise, Patel et al. showed that Ginlac-V pessary showed 100% efficacious in bacterial vaginosis and overall symptomatic relief was 82% in symptomatic vaginal discharge [17] and Motlagh et al. reported a 94% response with oral metronidazole plus Prangos ferulacea vaginal cream [19]. The present study showed a significant decrease in VAS score for low backache, vaginal discharge, and lower abdominal pain in the Acacia group; similarly, Bhat and Begum (2017) also reported a significant reduction in VAS score of symptoms for syndromic management of abnormal vaginal discharge with Unani formula [5].
4.2. Interpretation and Justification
Abnormal vaginal discharge is the commonest symptom for women in India to pursue care. Acacia pod extract was efficacious in the syndromic management of AVD, its associated symptoms, and improved HRQoL as it has anti-inflammatory (Muhallil al-Waram), astringent (Qabiz), and antiseptic (Daf'-i-Taffun) properties [6, 7]. Furthermore, in vitro and in vivo pharmacological studies have also proven anti-inflammatory [10, 11, 14], analgesic [10, 11], antimicrobial [23, 24], antispasmodic [25], diuretics [11], antioxidant properties [9, 13, 26], and antiseptic properties [10–14] of the pods. The aforementioned pharmacological properties are credited to the presence of phytoconstituents such as tannins, alkaloids, organic acids, flavonoids, polyphenolic compounds, volatile oils, glycosides, and coumarins [8, 9, 11, 13]. The methanolic pod extract of A. arabica showed significant inhibition against Gram-positive and Gram-negative species in vitro study [23]. Similarly, Satish et al. also reported antibacterial and antifungal activity of the methanolic extracts of A. arabica pods, and the highest activity was against S. aureus, E. coli, and A. Niger [8]. Another study also showed that the methanolic extract of pods had inhibitory activity against P. aeruginosa, E. coli, and S. aureus [24]. A study showed that the antimicrobial activity of the Acacia pods is probably due to polyphenolic compounds, and/or volatile oils cause inhibition of various microorganisms [8]. Phenol is established as a chemical antiseptic. The astringent effect of the pods is due to the presence of tannins [11]. Another study reported that the antioxidant and antibacterial activities are attributed to the presence of proteins and/or flavonoids and high total phenolic content [27]. The pods contain phytochemicals such as catechin, catechin 5-O-gallate, gallic acid, methyl gallate, 1-O-galloyl-β-D-glucose, gallocatechin 5-O-gallate, 1-6-di-O-galloyl-β-D-glucose, and digallic acid [28]. These phytochemicals are possibly accountable for the experiential activity. For example, tannins therapeutically have antiseptic properties and their precipitating activity is used in detecting alkaloids, proteins, and gelatin. Flavonoids and phenolic compounds are frequently found effective in vitro as antimicrobial substances against various microorganisms. They are plant metabolites with at least one hydroxyl group. Gallic acid by the mechanism of action in E. coli, S. aureus, P. aeruginosa, and Listeria monocytogenes led to “permanent changes in membrane properties through the decrease of negative surface charge, hydrophobicity changes, and pore formation in the cell membranes or local rupture with resulting leakage of essential intracellular constituents.” Oladous et al. (2019) reported that although gallic acid and methyl gallate have better activity than the crude extract, catechin was the most active compound against S. aureus, E. coli, P. aeruginosa, and clinical isolates of K. pneumonia, Candida albicans, S. typhi, and B. subtilis organisms [29].
The infective vaginal discharge not only affects women's routine physical and social activities but also their mental health and all aspects of a woman's life, thereby affecting HRQoL negatively. The positive effect of A. arabica pods on mood, headache, fatigue, and energy level is due to its antioxidant properties [9, 13, 26]. Furthermore, to explain that the reduction of associated symptoms may be attributed to its reported scientifically proven pharmacological activities such as astringent, anti-inflammatory, analgesic, and diuretic properties [8–14]. The Acacia group did not show any adverse effects during and after the completion of this trial.
4.3. Strengths of the Study
This is the first of its kind single-blind, randomized, placebo-controlled study using traditional regimen methods, sitz bath, and vaginal pessary of Acacia pods were efficacious for abnormal vaginal discharge syndromic management, its associated symptoms, and women's HRQoL. There were good patient's retention and compliance with the protocol. Overall therapeutic cure for the disease was also seen.
4.4. Limitations and Future Recommendations
Because of time constraints and lack of infrastructure, laboratory tests such as vaginal and cervical swab culture, nucleic acid amplification test (NAAT), and endometrial biopsy to verify the efficacy of the results were not possible. Furthermore, double-blind, phase IV clinical trials with larger samples are recommended.
5. Conclusion
The result of this study indicates that an Acacia pod's sitz bath and vaginal pessary were effective for syndromic management of abnormal vaginal discharge, its associated symptoms, and improving women's HRQoL. Furthermore, it is safe, well-accepted, and tolerated by the patients.
Acknowledgments
The authors are thankful to the Director of our Institute for providing all the best facilities and resources to accomplish this research work. This research was supported by the fund from the Ministry of AYUSH as an unrestricted grant for the dissertation work. UN and Hindawi under Research4Life policy have financially supported for publication.
Contributor Information
Arshiya Sultana, Email: drarshiya@yahoo.com.
Mumuni Ishawu, Email: ishawu.mumuni1975@gmail.com.
Data Availability
The research is approved by the Institutional Ethics Committee (same has been added in the manuscript). The findings of this study are available from the corresponding author upon request.
Disclosure
No competing financial interests exist.
Conflicts of Interest
The authors declared that they have no conflicts of interest.
Authors' Contributions
All authors equally contributed in drafting, designing, critically reviewing, analysing the data, and proofreading the manuscript.
References
- 1.Farhan A. M., Eldesouky E. A., Gaballah E. A., Soltan M. E. Comparison of visual, clinical, and microbiological diagnosis of symptomatic vaginal discharge in the reproductive age group. Benha Medical Journal . 2017;34(1):43–48. [Google Scholar]
- 2.Venugopal S., Gopalan K., Devi A., Kavitha A. Epidemiology and clinico-investigative study of organisms causing vaginal discharge. Indian Journal of Sexually Transmitted Diseases and AIDS . 2017;38(1):69–75. doi: 10.4103/2589-0557.203433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salhan S., Tripathi V., Sehgal R., Kumar G., Talwar G. P., Chatterjee A. A phase II randomized controlled trial to evaluate the safety and efficacy of Praneem polyherbal vaginal tablets compared with betadine vaginal pessary in women with symptoms of abnormal vaginal discharge. Asia-Pacific Journal of Public Health . 2009;21(4):461–468. doi: 10.1177/1010539509344610. [DOI] [PubMed] [Google Scholar]
- 4.Valsangkar S., Selvaraju D., Rameswarapu R., Kamutapu S. Impairment of quality of life in symptomatic reproductive tract infection and sexually transmitted infection. Journal of Reproduction and Infertility . 2014;15(2):87–93. [PMC free article] [PubMed] [Google Scholar]
- 5.Rabiu K. A., Adewunmi A. A., Akinlusi F. M., Akinola O. I. Female reproductive tract infections: understandings and care seeking behaviour among women of reproductive age in Lagos, Nigeria. BMC Women’s Health . 2010;10(1):8–7. doi: 10.1186/1472-6874-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rather L. J., Salam S., Mohammad F. Acacia arabica (L.): a review of its traditional uses, phytochemistry, and pharmacology. Sustainable Chemistry and Pharmacy . 2015;2 [Google Scholar]
- 7.Khare C. P. Indian Medicinal Plants (an illustrated dictionary) Berlin, Germany: Springer Publishers; 2007. [Google Scholar]
- 8.Satish S., Raghavendra M. P., Raveesha K. A. Evaluation of the antibacterial potential of some plant plants against human pathogenic bacteria. Advances in Biological Research . 2008;2(3-4):44–48. [Google Scholar]
- 9.Kalaivani T., Mathew L. Free radical scavenging activity from leaves of Acacia arabica (L.) Wild. ex Delile, an Indian medicinal tree. Food and Chemical Toxicology . 2010;48 doi: 10.1016/j.fct.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Sokeng S. D., Koubé J., Dongmo F., et al. Acute and chronic anti-inflammatory effects of the aqueous extract of Acacia arabica (L.) Del. (Fabaceae) pods. Academia Journal of Medicinal Plants . 2013;1(1):1–5. [Google Scholar]
- 11.Abeer M., Haj A., Yagoub S. O. Anti-microbial activity of Acacia arabica extracts against some bacteria isolated from clinical specimens. Research Journal of Medicinal Plant . 2007;1(1):25–28. [Google Scholar]
- 12.Bansal V. K., Goel R. K. Gastroprotective effect of Acacia arabica young seedless pod extract: role of polyphenolic constituents. Asian Pacific Journal of Tropical Medicine . 2012;5(7):523–528. doi: 10.1016/S1995-7645(12)60092-3. [DOI] [PubMed] [Google Scholar]
- 13.Singh B. N., Singh B. R., Singh R. L., Prakash D., Sarma B. K., Singh H. B. Antioxidant and anti-quorum sensing activities of green pod of Acacia arabica L. Food and Chemical Toxicology . 2009;47(4):778–786. doi: 10.1016/j.fct.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Alli L. A., Nafiu M. O., Adesokan A. A., Akanji M. A., Tijani A. Y., Salawu Q. A. Antipyretic and analgesic activities of aqueous extract of Acacia arabica root. Biokemistri . 2014;26(2):55–62. [Google Scholar]
- 15.Simbar M., Azarbad Z., Mojab F., Alavi Majd H. A comparative study of the therapeutic effects of the Zataria multiflora vaginal cream and metronidazole vaginal gel on bacterial vaginosis. Phytomedicine . 2008;15(12):1025–1031. doi: 10.1016/j.phymed.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Bhat T. A., Begum W. Efficacy of Tamarindus indicus, Melia azadirach and Santalum album in syndromic management of abnormal vaginal discharge: a single-blind randomised controlled trial. Journal of Complementary and Integrative Medicine . 2018;15(2):1–8. doi: 10.1515/jcim-2015-0023. [DOI] [PubMed] [Google Scholar]
- 17.Patel Y., Gopalan S., Bagga R., Sharma M., Chopra S., Sethi S. A randomized trial comparing a polyherbal pessary (a complementary and alternative medicine) with Ginlac-V pessary (containing clotrimazole, tinidazole and lactobacilli) for treatment of women with symptomatic vaginal discharge. Archives of Gynecology and Obstetrics . 2008;278(4):341–347. doi: 10.1007/s00404-008-0568-9. [DOI] [PubMed] [Google Scholar]
- 18.Balogun J. A., Okonofua F. E. Management of chronic pelvic inflammatory disease with shortwave diathermy: a case report. Physical Therapy . 1988;68(10):1541–1545. [PubMed] [Google Scholar]
- 19.Azadpour Motlagh A., Dolatian M., Mojab F., et al. The effect of Prangos Ferulacea vaginal cream on accelerating the recovery of Bacterial Vaginosis: a randomized controlled clinical trial. International Journal of Community Based Nursery and Midwifery . 2018;6(2):100–110. [PMC free article] [PubMed] [Google Scholar]
- 20.Mao X., Zhao R., Yao R., et al. Chinese herbal formula Feilin vaginal gel prevents the cervicitis in mouse model. Evidence Based Complementart Alternative Medicine . 2019;2019:10. doi: 10.1155/2019/4168126.4168126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam W. Y., Fresco P. Medication adherence measures: an overview. BioMed Research International . 2015;2015:1–12. doi: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moghtadaei P., Sardari F., Esmaeilian S. Comparison of ceftriaxone plus weekly azithromycin or daily ofloxacin for outpatient treatment of pelvic inflammatory disease: a randomized clinical trial. Journal of Family and Reproductive Health . 2008;2(2):87–94. [Google Scholar]
- 23.Gmaraldeen S. M., Magzoub A. A., Badri A. M., Garbi M. I., Saleh M. Antibacterial activity of Acacia nilotica fruits extract against pathogenic bacteria. Int J Appl Res . 2016;2(6):103–106. [Google Scholar]
- 24.Oladosu P., Isu N. R., Ibrahim K., et al. Time kill-kinetics antibacterial study of Acacia nilotica. African Journal of Microbiology Research . 2013;7(46):5248–5252. [Google Scholar]
- 25.Chaubal R., Pawar P. V., Hebbalkar G. D., et al. Larvicidal activity of Acacia nilotica extracts and isolation of D- Pinitol- A bioactive carbohydrate. Chemistry and Biodiversity . 2005;2:684–688. doi: 10.1002/cbdv.200590044. [DOI] [PubMed] [Google Scholar]
- 26.Abuelgassim O. A. Antioxidant potential of date palm leaves and Acacia nilotica fruit in comparison with other four common Arabian medicinal plants. Life Science Journal . 2013;10(4):3405–3410. [Google Scholar]
- 27.Sadiq M. B., Hanpithakpong W., Tarning J., Anal A. K. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Industrial Crops and Products . 2015;77:873–882. doi: 10.1016/j.indcrop.2015.09.067. [DOI] [Google Scholar]
- 28.Karim A. A., Azlan A. Fruit pod extracts as a source of nutraceuticals and pharmaceuticals. Molecules . 2012;17(10):11931–11946. doi: 10.3390/molecules171011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oladosu O. P., Isu N. R., Aboh I. M., Okhale S. E., Orishadipe A. T., Egharevba H. O. Antibacterial activity of bioflavonoid from fruit pulps of Acacia nilotica Willd. Microbiology Research Journal International . 2019;1:1–12. doi: 10.9734/mrji/2019/v28i430139. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research is approved by the Institutional Ethics Committee (same has been added in the manuscript). The findings of this study are available from the corresponding author upon request.


