Abstract
Recently, a new Listeria species, “Listeria swaminathanii”, was proposed. Here, we phenotypically and genotypically characterize two additional strains that were previously obtained from soil samples and compare the results to the type strain. Complete genomes for both strains were assembled from hybrid Illumina and Nanopore sequencing reads and annotated. Further genomic analysis including average nucleotide identity (ANI) and detection of mobile genetic elements and genes of interest (e.g., virulence-associated) were conducted. The strains showed 98.7–98.8% ANI with the type strain. The UTK C1-0015 genome contained a partial monocin locus and a plasmid, while the UTK C1-0024 genome contained a full monocin locus and a prophage. Phenotypic characterization consistent with those performed on the proposed type strain was conducted to assess consistency of phenotypes across a greater diversity of the proposed species (n = 3 instead of n = 1). Only a few findings were notably different from those of the type strain, such as catalase activity, glycerol metabolism, starch metabolism, and growth at 41 °C. This study further expands our understanding of this newly proposed sensu stricto Listeria species.
Subject terms: Bacterial genomics, Taxonomy
Introduction
Listeria spp. are small Gram-positive, motile, non-sporulating, and non-capsulated rods1–3. The Listeria genus consists of two clades, sensu stricto and sensu lato4,5, and currently contains 26 validly published species6. The sensu stricto clade includes the human and animal pathogen L. monocytogenes7. Listeria spp. are ubiquitous and commonly isolated from natural environments8 and the majority of novel species or subspecies described in recent years were originally isolated from these environments4,9–15.
Recently, a strain isolated from soil collected in the Great Smoky Mountains National Park (GSMNP)8 was proposed as a novel sensu stricto species, “Listeria swaminathanii”16. However, it was unable to be validly published due to culture collection deposition restrictions imposed by the National Park Service and rules on strain availability set forth by the International Committee on Systematics of Prokaryotes (ICSP).
In another study, Claxton and Hudson et al.17 obtained two distinct isolates, UTK C1-0015 and UTK C1-0024, from soil samples collected in the GSMNP that were not closely related to any published type species. Here, we show that these two isolates are additional members of the proposed species “L. swaminathanii”. We further characterized these two strains genotypically and phenotypically to expand our understanding of this newly proposed species by increasing the number of characterized strains from one to three. Additionally, we sequenced the isolates using both short- and long-read sequencing technologies and were able to produce complete closed genomes and further characterized the genomic features of each isolate.
Materials and methods
Genome sequencing and assembly
Genomic DNA was extracted using a Qiagen QIAamp DNA mini kit (Hilden, Germany) per manufacturer protocol, with the addition of an RNase treatment step18. For short-read sequencing, library preparation and sequencing were performed by the Microbial Genome Sequencing Center (MiGS; Pittsburgh, PA). Sequencing was performed on an Illumina NextSeq 2000 instrument with 151 bp paired-end read chemistry. For each, 2.5–2.6 million total paired sequencing reads were produced, with average lengths of 146.0–146.3 bp. Mean quality phred scores were > 32, indicating good quality calls.
For long-read sequencing, the SQK-RBK004 kit (Oxford Nanopore Technologies, Oxford, UK) was used for library preparation and a MinIon instrument with a FLO-MIN106 flow cell were used for sequencing, along with the MinKNOW software (v3.6.5, fast basecalling model). A total of 293,459 sequencing reads were produced for UTK C1-0015 with an average length of 5656 bp. A total of 86,653 sequencing reads were produced for UTK C1-0024 with an average length of 5862 bp.
Raw Illumina reads were trimmed using Trimmomatic19 (v0.39; with the following parameters: ILLUMINACLIP:NexteraPE-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36). Read quality statistics for both types of reads were generated with FastQC20 (v0.11.9). Both short- and long-reads were used to create hybrid assemblies using Unicycler21 (v0.4.8; default parameters). Assembly statistics were generated with QUAST22 (v5.0.2), BBMAP23, and SAMtools24 (v1.10). Assemblies were submitted to NCBI and annotated using the NCBI Prokaryotic Genome Annotation Pipeline25 (PGAP; v5.3).
Genomic characterization
Relatedness to other species and taxonomy were assessed using various genomic methods, including ANI, ribosomal multilocus sequence typing (rMLST), and dDDH. Assemblies for all currently described Listeria species type strains and representative strains were downloaded from the RefSeq or GenBank databases on NCBI or the ATCC genome portal, along with the assembly for the “L. swaminathanii” type strain (GCF_014229645.1). PYANI26 (v0.2.10) was used to calculate ANI between all strains and bactaxR27 was used to create an ANI dendrogram. The assemblies were also input into the rMLST28 tool (available on PubMLST) and the Type Strain Genome Server (TYGS)29. A whole-genome alignment was performed with the two strains and FSL L7-0020 in Geneious using the progressiveMauve30 algorithm (Mauve plugin v1.1.3) and visualized with Mauve30,31. For the alignment, the FSL L7-0020 assembly contigs reordered relative to the strain genomes and concatenated into a single sequence to form a pseudochromosome using the MCM algorithm in Geneious; the plasmid was also excluded from UTK C1-0015.
Genomes were evaluated for loci associated with antimicrobial resistance, virulence, motility, metal and disinfectants resistance, stress islands, and Listeria genomic islands using ResFinder32 (v4.1), KmerResistance33,34 (v2.2), VirulenceFinder35 (v2.0), and the relevant schemes on Pasteur36–39. Mobile genetic elements (MGEs) were identified and characterized using PlasmidFinder (v.2.0), PLSDB40 (v.2021_06_23), Phaster41, and PhageBoost42. BLAST and BLAST Ring Image Generator (BRIG) (v0.95)43 were used to create a plasmid map for comparison of similar plasmids. Genomic comparison of monocin loci and nucleotide and amino acid identity were determined using BLAST and EasyFig44 (v2.2.2).
Phenotypic characterization
The phenotypic characterization of UTK C1-0015 and UTK C1-0024 was performed as per the standardized methodology in the FDA Bacteriological Analytical Manual (BAM) Chapter 1045 and those described by Carlin et al.10,16. The following characteristics were assessed: growth at different temperatures (4, 7, 22, 30, 37, and 41 °C), growth under anaerobic conditions, colony morphology on selective and differential agar medium, motility, Gram stain, hemolysis, oxidase and catalase activity, nitrate reduction, and the biochemical tests included in three different commercial test kits (API Listeria, API 20 E, and API 50 CH). For each phenotypic analysis, from frozen stock, strains were streaked for isolation onto Brain Heart Infusion (BHI) agar (BD Biosciences, Franklin Lanes, NJ + Fisher Scientific Agar, Waltham, MA) and incubated aerobically at 30 °C for 24 h. From that plate, isolated colonies were either used directly or to inoculate a BHI broth (BD Biosciences, Franklin Lanes, NJ) tube, followed by aerobic incubation with shaking at 30 °C for 24 h. Unless otherwise specified, three biological replicates were performed for each test, each starting from a different single, isolated colony. Control strains included the well-characterized L. monocytogenes 10403S, L. monocytogenes ATCC 19115, L. ivanovii subsp. ivanovii ATCC 19119, L. innocua ATCC 33090, L. seeligeri ATCC 35967, and L. booriae FSL A5-0281T.
Growth temperature
To measure growth at different temperatures, BHI broth cultures were used to inoculate 5 mL BHI broth tubes to a concentration of 102–103 CFU/mL for each strain and temperature combination; L. monocytogenes 10403S was included as a positive control. The tubes were then aerobically incubated at 4, 7, 22, 30, 37, or 41 °C for up to 5 days. Enumerations were performed by spread plating onto BHI agar in duplicate at 24 and 48 h for 22, 30, 37, and 41 °C, at 11 and 14 days for 4 °C, and at 11 and 15 days for 7 °C. Enumeration plates were incubated for 24–36 h at 30 °C. If no growth occurred at 48 h, additional enumerations were performed daily for up to 5 days.
Anaerobic growth
To assess growth under anaerobic conditions, BHI agar plates were streaked from BHI broth cultures in duplicate; L. monocytogenes 10403S was included as a positive control. Plates were incubated at 30 °C, one aerobically and one anaerobically (in an anaerobic chamber with GasPak™ EZ Anaerobe Container System Sachets with Indicator [BD Difco, Franklin Lanes, NJ]). Growth was assessed at 24 and 48 h.
Selective and differential agars
For colony phenotypes on selective and differential agars, BHI broth cultures were streaked onto modified oxford agar (MOX; Remel Oxford Agar Base Modified, Lenexa, KS; BD Difco Supplement, Franklin Lanes, NJ) and Listeria CHROMagar (commercially prepared; BD Biosciences, Franklin Lanes, NJ) plates. Listeria monocytogenes 10403S, Listeria ivanovii ATCC 19119, L. seeligeri ATCC 35967, and Listeria innocua ATCC 33090 were included as controls. Plates were incubated aerobically at 30 °C and evaluated at 24 and 48 h.
Motility
Two methods were used to detect motility: microscopic observation and observation of growth in Motility Test Medium (MTM). L. monocytogenes 10403S and L. booriae FSL A5-0281T were included as positive and negative controls, respectively. BHI agar streak plates were incubated aerobically at 25 °C and 37 °C for 24 h. Wet mounts from both sets of plates were observed microscopically for tumbling motility. This assay was completed once for each strain and temperature combination. Additionally, MTM tubes (commercially prepared; Hardy Diagnostics, Santa Maria, CA) were stab inoculated from the 25 °C BHI agar plates, incubated at 25 °C, and observed daily for 7 d.
Oxidase and catalase
Oxidase and catalase tests were performed using isolated colonies from BHI agar plates and L. monocytogenes 10403S as a control. For the catalase test, 3% hydrogen peroxide (Medique Products, Fort Myers, USA) was added and observed for the formation of gas bubbles. For the oxidase test, an oxidase test strips (OxiStrips; Hardy Diagnostics, Santa Maria, CA) were used.
Hemolysis
Hemolysis was evaluated by stab inoculating sheep blood agar plates (SBA; commercially prepared; Hardy Diagnostics, Santa Maria, CA) from BHI agar plates. Listeria monocytogenes 10403S, Listeria monocytogenes ATCC 19115, Listeria ivanovii ATCC 19119, and Listeria seeligeri ATCC 35967 were included as positive controls and Listeria innocua ATCC 33090 as a negative control. SBA plates were incubated aerobically at 35 °C and checked at 24 and 48 h.
Nitrate reduction
Nitrate reduction was evaluated by inoculating nitrate broth with Durham tubes (commercially prepared; BD BBL, Franklin Lanes, NJ) with several colonies from BHI agar and incubating at 35 °C for up to 7 days. Reduction of nitrate to nitrite was evaluated daily by adding NIT1 and NIT2 reagents (bioMérieux, Marcy-l’Étoile, France) to 1 mL aliquots. If negative, zinc powder (bioMérieux, Marcy-l’Étoile, France) was added to confirm the presence of nitrate. Additionally, tubes were observed for the production of gas, which would indicate further reduction to gaseous nitrogen products. L. booriae FSL A5-0281T and L. monocytogenes 10403S were included as positive and negative controls, respectively.
Biochemical test kits
API Listeria, API 20 E and API 50 CH test kits (bioMérieux, Marcy-l’Étoile, France) were all performed following the manufacturer’s instructions. For the API 50 CH strips, the Bacillus methods in the instructions and API 50 CHB/E medium were used. L. monocytogenes 10403S and L. innocua ATCC 33,090 were used as controls for all three, in duplicate. Inoculum for the strips was prepared by suspending colonies from BHI agar in the appropriate suspension medium for each. API Listeria and API 20 E strips were incubated at 35 °C for 24 h and then interpreted. API 50 CH test strips were incubated at 30 °C and interpreted at 24 and 48 h.
Results/discussion
Since 2010, there have been multiple new species added to the Listeria genus, many originally isolated from natural environments16. This paper describes the genotypic and phenotypic characterization of two new Listeria isolates obtained from soil samples collected in the Great Smoky Mountains National Park along the North Carolina-Tennessee border17. Evaluation of genotypic and phenotypic characteristics of “L. swaminathanii” strains will aid in the characterization of this novel species and contribute to our knowledge of the diversity of Listeria spp. Here, we describe the newly isolated strains, UTK C1-0015 and UTK C1-0024, and compare with the “L. swaminathanii” type strain (FSL L7-0020T) and other Listeria spp.
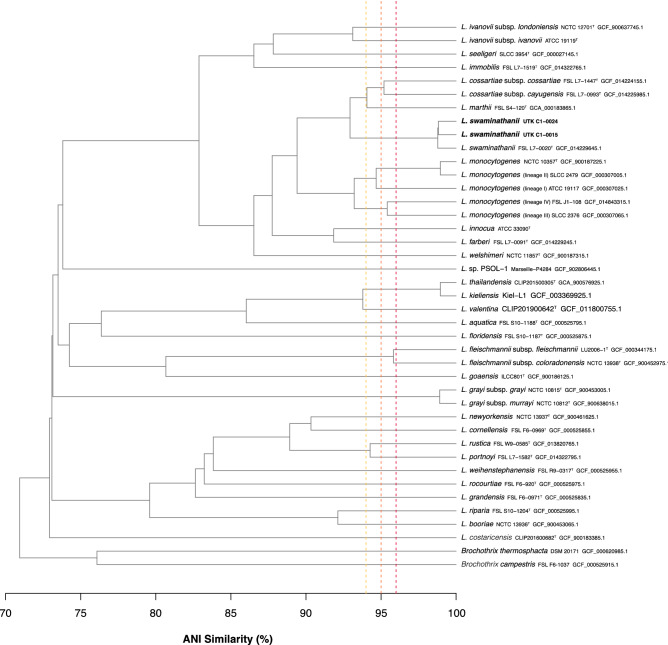
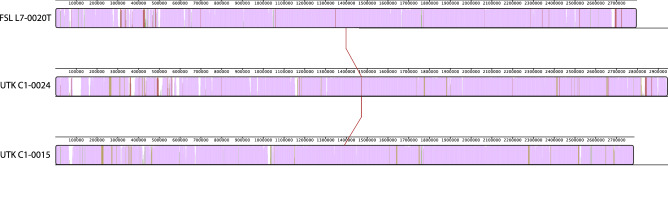
Both genomes were able to be assembled into complete closed genomes (contiguous sequences that comprise the entire genome). The genome of UTK C1-0015 consists of a 2.78 Mb chromosome and 55 Kb plasmid (total genome length of 2.84 Mb) with a G+C content of 38.7%; UTK C1-0024 consists of a 2.95 Mb chromosome with a G+C content of 38.6% (Table 1), which is consistent with FSL L7-0020T. Of the validly published type strains, the two isolates showed highest similarity to L. marthii (94.0–94.1%) (Fig. 1); however, they were most closely related to “L. swaminathanii” FSL L7-0020T, with 98.7–98.8% ANI, indicating that they belong to the same species. Examination of the chromosomal alignment of the two isolates and the type strain shows that, overall, there is a high level of conservation across the entire chromosome, with no large rearrangements or deletions (Fig. 2). However, there are some loci throughout that are present or absent in only one of the isolates.
Table 1.
Genome statistics.
| Strain | NCBI RefSeq and genome accessions | Assembly | Annotation | |||||
|---|---|---|---|---|---|---|---|---|
| Length (bp) | No contigs | GC (%) | Read coverage (x) | No. genes | No. RNA genes | No. pseudo genes | ||
| Total Coding |
Total rRNAs (5S, 16S, 23S) tRNAs ncRNAs |
|||||||
| UTK C1-0015 | GCF_021029855.2 | 2,840,389 | 2 | 38.7 | 260 |
2857 2741 |
89 18 (6, 6, 6) 67 4 |
27 |
| Chromosome | CP089090.1 | 2,784,409 | 1 | 38.8 | ||||
| Plasmid | CP089091.1 | 55,980 | 1 | 34.6 | ||||
| UTK C1-0024 |
GCF_021029705.2 |
2,947,729 | 1 | 38.6 | 250 |
2955 2849 |
89 18 (6, 6, 6) 67 4 |
17 |
| FSL L7-0020T 16 |
GCF_014229645.1 |
2,796,956 | 12 | 38.6 | 127 |
2797 2695 |
77 11 (5, 2, 4) 62 4 |
25 |
Genome statistics for the two “L. swaminathanii” strain hybrid assemblies described here and for the recently described type strain16.
Figure 1.
ANI Similarity Dendrogram. Average nucleotide Identity (ANI) dendrogram of the recently isolated “L. swaminathanii” strains (bold), along with all described Listeria spp. type strains and representative from each of the L. monocytogenes lineages (indicated in parentheses). Horizontal distance represents ANI similarity (%) and vertical dashed lines indicate ANI values of 96 (yellow), 95 (orange), and 94% (red).
Figure 2.
Chromosomal alignment of FSL L7-0020T, UTK C1-0024, and UTK C1-0015. Alignment shows three horizontal panels, one per strain. The colored portions inside each panel represents sequence similarity, with height corresponding to average conservation at that location. Regions that are conserved among all genomes are purple. Regions that are conserved among only two of the genomes are red (FSL L7-0020T and UTK C1-0024), green (FSL L7-0020T and UTK C1-0015), or yellow (UTK C1-0024 and UTK C1-0015). Regions without coloring were not aligned and likely contain loci that are present in only a single genome.
Both genomes contained the following antibiotic resistance genes: fosX, lin, norB, and sul. Virulence-associated genes involved with adherence (dltA, fbpA, lap, lapB, pdeE), bile-resistance (bsh, mdrM), immune modulation (lntA), intracellular survival (lplA1, oppA, pdeE, prsA2, purQ, svpA), invasion (iap, lpeA, pdeE), peptidoglycan modification (oatA, pdgA), regulation of transcription and translation (agrAC, cheAY, codY, fur, lisKR, stp, virRS), surface protein anchoring (lgt, lspA, srtAB), and teichoic acid biosynthesis (gltB, gtcA) were identified in both genomes, along with internalins inlGHJK, inlC2, and inlD (Supplementary Table S1). Genes associated with Listeria pathogenicity island LIPI-3 (llsABDGPXY) were only found in UTK C1-0024 (Supplementary Fig. S1), as well as gltA (teichoic acid biosynthesis). The internalin genes inlA and inlB and genes associated with Listeria pathogenicity islands LIPI-1, LIPI-2, or LIPI-4 were not detected in either.
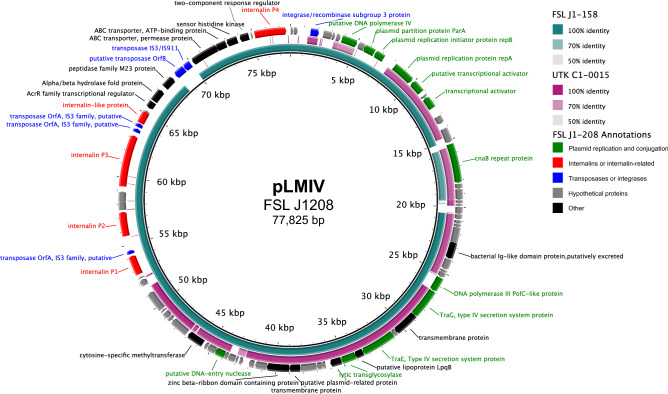
A 56 Kb plasmid was identified in UTK C1-0015. The plasmid has an Illumina read depth of 2.2× the overall median depth, indicating a copy number of two. The plasmid found in UTK C1-0015 shows a high similarity (86.03% nucleotide identity) to pLMIV from L. monocytogenes strain FSL J1-020846,47. However, pLMIV is approximately 21 Kb longer than the plasmid found in UTK C1-0015; this is due to the presence of a region encoding four complete internalins and one internalin-like protein in pLMIV, this region is absent in the plasmid in UTK C1-0015 (Fig. 3). Both plasmids are also similar to the plasmid in L. monocytogenes FSL J1-0158. Both FSL J1-0208 and FSL J1-0158 were originally isolated from clinical caprine sources46. Most genes in the plasmid found in UTK C1-0015 seem to encode proteins predicted to be involved in plasmid maintenance and conjugation46, with only a few putative cargo genes, most which are of unknown function and one encoding a DNA-methyltransferase.
Figure 3.
Comparison of plasmid found in UTK C1-0015 to plasmids from FSL J1-020 and FSL J1-158. Comparison of the plasmids found in UTK C1-0015, FSL J1-020, and FSL J1-158, using pLMIV from J1-208 as the reference. The innermost black ring represents pLMIV. The middle rings represent FSL J1-158 (teal) and UTK C1-0015 (purple), with BLAST identity indicated by shading (see legend). The outermost ring contains gene annotations from pLMIV that are colored by functional category: green (plasmid replication and conjugation), red (internalins or internalin-related), blue (transposases or integrases), gray (hypothetical proteins), and black (other).
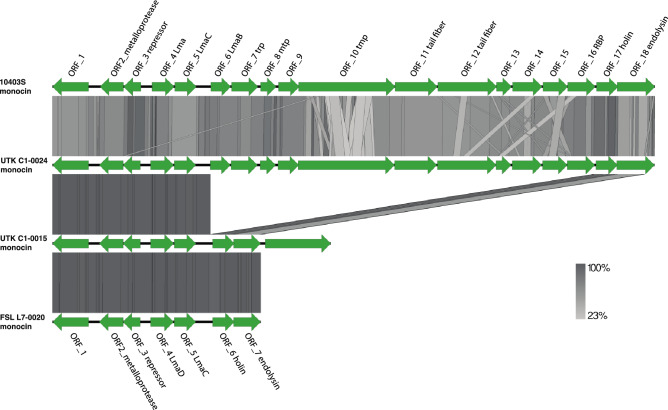
PHASTER and PhageBoost were used to predict prophage sequences in the genomes. The genome UTK C1-0024 was predicted to house a prophage integrated near a tRNA-Lys gene. Blastn results show the prophage from UTK C1-0024 has an 88.58% identity to Listeria phage A500 with 60% coverage. Prophages and other mobile genetic elements can contribute to genome diversity and have been used to distinguish epidemic clones of L. monocytogenes48–50. Strain UTK C1-0015 was predicted to house a partial monocin locus of eight open reading frames 51,52; structural genes such as those that code for the tail tape measure protein or tail fibers were absent from the locus. The monocin locus from strain UTK C1-0015 shares a 99.405% identity to the monocin locus from FSL L7-0020T (GCF_014229645.1). The UTK C1-0024 genome was predicted to house the full monocin locus of 18 open reading frames, similar to the monocin in L. monocytogenes strain 10403S (Fig. 4). Blastp queries using the monocin locus from UTK C1-0015 and UTK C1-0024 return hits to L. marthii, L. cossartiae, L. innocua, L. farberi, and L. monocytogenes strains with 100% coverage and > 89.90% identity, suggesting this is fairly dispersed across the sensu stricto clade of Listeria. Monocins are bacteriocins produced by the host that may be significant in establishing dominant strains in ecological niches, as they target closely related species, but remain inactive against the producing strain53.
Figure 4.
Nucleotide similarity of monocin regions. BLAST comparisons of monocin regions from L. monocytogenes 10403S, UTK C1-0015, UTK C1-0024, and the “L. swaminathanii” type strain FSL L7-0020T. Genes are represented by green arrows. The shaded regions represent nucleotide similarity (see scale at bottom right).
Listeria spp. grow at a wide range of temperatures from 0 to 45 °C7,8,14,16 and can survive at temperatures below freezing (− 7 °C)54. In the current study, we performed growth assessments at 4, 7, 22, 30, 37, and 41 °C. These temperatures were chosen to encompass the known growth temperature range, with 4 and 7 °C specifically included because some species are unable to grow well at low temperatures (< 7 °C) 4. Strain UTK C1-0015 exhibited growth at all temperatures tested and strain UTK C1-0024 exhibited growth at all termperatures except 41 °C (Supplementary Table S2). After 24 h of incubation, UTK C1-0015 and UTK C1-0024 showed optimal growth at 30 °C (9.2 and 9.4 log10 CFU/mL), followed by at 37 °C (8.9 and 9.0 log10 CFU/mL). At 41 °C, UTK C1-0024 was enumerated daily for up to five days and no growth was observed, which is dissimilar to both UTK C1-0015 and FSL L7-0020T. At 4 °C, the concentration increases of UTK C1-0015 and UTK C1-0024 after 11 d (6.4 and 6.8 log10 CFU/mL, respectively) were higher than the increases seen in FSL L7-0020T (4.1 log10 CFU/mL)16.
Listeria spp. are Gram-positive rods7; this was confirmed for UTK C1-0015 and UTK C1-0024. Both isolates were observed to grow under aerobic and anaerobic conditions at 30 °C after 24 h; this is another expected result, as Listeria spp. are facultative aerobes7. Both strains were oxidase negative (Supplementary Table S3), as expected7, indicating a lack of cytochrome c oxidase. Additionally, both were catalase positive, indicating they produce the catalase enzyme that converts hydrogen peroxide into oxygen gas and water; however, FSL L7-0020T is catalase negative16, a phenotype that has only been described in one other Listeria spp. (L. costaricensis)55. When kat gene from the reference, two isolates, and the type strain are aligned, there are nucleotide differences at 158 positions. 16 of the nucleotide differences differ between the type strain and one or both of the isolates. Four of those result in amino acid differences, with two between the type strain and both isolates. At amino acid position 72, the type strain has glutamic acid (polar, acidic) and the two isolates have lysine (polar, basic), a radical substitution. At amino acid position 92, the type strain has histidine and the other two arginine (both polar, basic), a conservative substitution. These amino acid differences may have an effect on the structure and function of the resulting protein, leading to the catalase-negative phenotype of FSL L7-0020T.
On MOX agar, UTK C1-0015 and UTK C1-0024 colonies were typical for Listeria spp.: gray to black colonies with sunken centers and black halos, indicating esculin hydrolysis. On Listeria CHROMagar, UTK C1-0015 and UTK C1-0024 were typical for Listeria spp.: blue colonies (indicating β-glucosiadase enzyme activity), but lacking opaque white halos typical for L. monocytogenes and L. ivanovii (indicating no phosphoatidylinositol-specific phospholipase C [PI-PLC] activity) (Supplementary Table S3).
API test kits were used to characterize metabolic function of UTK C1-0015 and UTK C1-0024.
The Listeria API kit is designed for species-level identification Listeria spp. based on enzymatic tests and sugar fermentations. For this test, both strains generated a code of 6110 (Supplementary Table S3), consistent with FSL L7-0020T 16 and indicates an 80% (t-value of 0.62) ID to L. monocytogenes according to the APIweb database. The control strains, L. monocytogenes 10403S and L. innocua ATCC 33090, generated the expected codes of 6510 and 7510, respectively.
The API 20 E kit is designed for identification of Enterobacteriaceae and other non-fastidious Gram-negative rods; however, this kit contains tests that can be used for genus-level identification of Listeria spp. and has been used previously in the characterization of novel Listeria spp.10,16. For this test, UTK C1-0015 and UTK C1-0024 were positive for acetoin production (Voges Proskauer) and D-glucose and amygdalin fermentation, which is consistent with L. monocytogenes 10403S, L. innocua ATCC 33090, and FSL L7-0020T 16 (Supplementary Table S3). UTK C1-0015 and UTK C1-0024 were negative for all other tests, including indole, urease, and H2S production 16. All API 20 E results were consistent with FSL L7-0020T 16. Nitrogen reduction was evaluated using both the API 20E kits and nitrogen broth; both strains were negative.
The API 50 CH kit is designed for the study of carbohydrate and carbohydrate-derivative metabolism and API 50 CHB/E medium is designed for use with Bacillus and related genera, Enterobacteriaceae, and Vibrionaceae. Results for this test were consistent between UTK C1-0015, UTK C1-0024 and FSL L7-0020T (Supplementary Table S3), with four differences. UTK C1-0015 yielded a negative result for D-lactose, a result that differs from UTK C1-0024, FSL L7-0020T, and most sensu stricto Listeria species16. Both strains tested negative for glycerol and starch (amidon); this differed from the type strain16, which is positive for both. UTK C1-0024 was positive for d-trehalose fermentation, while UTK C1-0015 and the type strain were negative. Examination of the genomes shows that a locus containing three genes associated with trehalose fermentation (treR, treC, and treP) is present in UTK C1-0024, but absent in the two other genomes. In L. monocytogenes, trehalose has been shown to increase biofilm formation56. The API 50CH test is a qualitative test and interpretation of results can vary, which is one major limitation of qualitative tests.
The complete lysis of red blood cells, β hemolysis, is associated with pathogenicity in Listeria spp.7 On SBA, UTK C1-0015 and UTK C1-0024 were non-hemolytic, which is consistent with the non-hemolytic FSL L7-0020T 16 and the negative control L. innocua ATCC 33090. β hemolysis is typically only observed in L. monocytogenes, L. ivanovii, and L. seeligeri7,45.
When observed microscopically, both UTK C1-0015 and UTK C1-0024 appeared motile at 25 °C and nonmotile at 37 °C (Supplementary Table S3). Motility at 25 °C was confirmed with MTM tubes; both strains were clearly motile after 5 days of incubation as evidenced by an umbrella-shaped growth pattern, characteristic of motile Listeria spp. These results were consistent with FSL L7-0020T 16 and other sensu stricto species, with the exception of L. immobilis (non-motile at 25 °C10). In L. monocytogenes, motility genes like flagellin are expressed at lower temperatures like 25 °C, but become restricted at 37 °C57.
Conclusions
In this study, we described two strains isolated from soil samples collected in the GSMNP, which belong to the recently proposed novel species “L. swaminathanii”. By the addition of two additional strains to this species (bringing the total number described to three), the diversity of this species can be further evaluated and the characteristics of these two strains can be compared to those of the type strain FSL L7-0020T. Additionally, we were able to provide complete closed genomes for both strains, including the plasmid found in UTK C1-0015, and further characterize genomic features. As the two strains described in this study were also isolated from the GSMNP, they are subject to the same restrictions as the proposed “L. swaminathanii” type strain.
Supplementary Information
Acknowledgements
We thank Martin Wiedmann (Cornell University) for providing the Listeria booriae strain FSL A5-0281T.
Author contributions
Conceptualization: L.K.H., C.R.C., H.C.B., T.G.D.; data curation: L.K.H., H.K.C., C.S., T.L.P.; formal analysis: L.K.H., T.L.P.; funding acquisition: T.G.D.; investigation: L.K.H., H.K.C., C.S., M.L.C., D.W.B., T.L.P., Y.S.; methodology: L.K.H., H.K.C., H.C.B., T.G.D.; project administration: L.K.H., D.W.B., T.G.D.; resources: T.G.D.; supervision: L.K.H., T.G.D.; visualization: L.K.H., H.K.C., C.S., T.L.P.; writing (original draft): L.K.H., H.K.C., C.S., T.L.P.; writing (review & editing): L.K.H., H.K.C., C.S., M.L.C., D.W.B., T.L.P., Y.S., C.R.C., H.C.B., T.G.D.
Funding
This study was funded by Multistate Project “Enhancing Microbial Food Safety by Risk Analysis” (S1077).
Data availability
Raw sequencing reads and genome assemblies are available on NCBI under BioProject PRJNA760531.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13119-y.
References
- 1.Barbuddhe S, Hain T, Doijad SP, Chakraborty T. Practical Handbook of Microbiology. CRC Press; 2021. pp. 411–442. [Google Scholar]
- 2.Hain T, Steinweg C, Chakraborty T. Comparative and functional genomics of Listeria spp. J. Biotechnol. 2006;126:37–51. doi: 10.1016/j.jbiotec.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 3.Nwaiwu, O. An overview of Listeria species in Nigeria. Int. Food Res. J. 22 (2015).
- 4.Orsi RH, Wiedmann M. Characteristics and distribution of Listeria spp., including Listeria species newly described since 2009. Appl. Microbiol. Biotechnol. 2016;100:5273–5287. doi: 10.1007/s00253-016-7552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiara M, et al. Comparative genomics of Listeria Sensu Lato: Genus-wide differences in evolutionary dynamics and the progressive gain of complex, potentially pathogenicity-related traits through lateral gene transfer. Genome Biol. Evol. 2015;7:2154–2172. doi: 10.1093/gbe/evv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LPSN—List of Prokaryotic names with Standing in Nomenclature. Genus Listeria. https://lpsn.dsmz.de/genus/listeria (2021). Accessed 6 Oct 2021.
- 7.McLauchlin, J. & Rees, C. E. D. Listeria in Bergey's Manual of Systematics of Archaea and Bacteria (Eds. M.E. Trujillo, Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B.) Wiley. 1–29 (2015).
- 8.Liao J, et al. Nationwide genomic atlas of soil-dwelling Listeria reveals effects of selection and population ecology on pangenome evolution. Nat. Microbiol. 2021;6:1021–1030. doi: 10.1038/s41564-021-00935-7. [DOI] [PubMed] [Google Scholar]
- 9.Linke K, et al. Reservoirs of Listeria species in three environmental ecosystems. Appl. Environ. Microbiol. 2014;80:5583–5592. doi: 10.1128/AEM.01018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlin CR, et al. Listeria cossartiae sp. nov., Listeria immobilis sp. nov., Listeria portnoyi sp. nov. and Listeria rustica sp. nov., isolated from agricultural water and natural environments. Int. J. Systematic Evolut. Microbiol. 2021 doi: 10.1099/ijsem.0.004795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Bakker HC, et al. Listeria floridensis sp. nov., Listeria aquatica sp. nov., Listeria cornellensis sp. nov., Listeria riparia sp. nov. and Listeria grandensis sp. nov., from agricultural and natural environments. Int. J. Systematic Evolut. Microbiol. 2014;64:1882–1889. doi: 10.1099/ijs.0.052720-0. [DOI] [PubMed] [Google Scholar]
- 12.Doijad SP, et al. Listeria goaensis sp. nov. Int. J. Systematic Evolut. Microbiol. 2018;68:3285–3291. doi: 10.1099/ijsem.0.002980. [DOI] [PubMed] [Google Scholar]
- 13.Graves LM, et al. Listeria marthii sp. nov., isolated from the natural environment, Finger Lakes National Forest. Int. J. Systematic Evolut. Microbiol. 2010;60:1280–1288. doi: 10.1099/ijs.0.014118-0. [DOI] [PubMed] [Google Scholar]
- 14.Lang Halter E, Neuhaus K, Scherer S. Listeria weihenstephanensis sp. nov., isolated from the water plant Lemna trisulca taken from a freshwater pond. Int. J. Systematic Evolut. Microbiol. 2013;63:641–647. doi: 10.1099/ijs.0.036830-0. [DOI] [PubMed] [Google Scholar]
- 15.den Bakker HC, Manuel CS, Fortes ED, Wiedmann M, Nightingale KK. Genome sequencing identifies Listeria fleischmannii subsp. coloradonensis subsp. nov., isolated from a ranch. Int. J. Systematic Evolut. Microbiol. 2013;63:3257–3268. doi: 10.1099/ijs.0.048587-0. [DOI] [PubMed] [Google Scholar]
- 16.Carlin, C. R. et al. Soil collected in the Great Smoky Mountains National Park yielded a novel Listeria sensu stricto species, L. swaminathanii. Microbio. Spec.10.1128/spectrum.00442-22 (2022). [DOI] [PMC free article] [PubMed]
- 17.Claxton ML, Hudson LK, Bryan DW, Denes TG. Listeria spp. isolated from soil samples collected in the great smoky mountains. bioRxiv. 2021 doi: 10.1101/2021.11.19.469259. [DOI] [Google Scholar]
- 18.Denes T, et al. Selection and characterization of phage-resistant mutant strains of Listeria monocytogenes reveal host genes linked to phage adsorption. Appl. Environ. Microbiol. 2015;81:4295–4305. doi: 10.1128/AEM.00087-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews, S. FastQC. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010). Accessed 23 May 2022.
- 21.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushnell, B. Bbtools: A suite of fast, multithreaded bioinformatics tools designed for analysis of DNA and RNA sequence data. https://jgi.doe.gov/data-and-tools/bbtools/ (2018). Accessed 23 May 2022.
- 24.Li H, et al. The sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatusova T, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard L, Glover RH, Humphris S, Elphinstone JG, Toth IK. Genomics and taxonomy in diagnostics for food security: Soft-rotting enterobacterial plant pathogens. Anal. Methods. 2016;8:12–24. doi: 10.1039/C5AY02550H. [DOI] [Google Scholar]
- 27.Carroll LM, Wiedmann M, Kovac J, Turner MS. Proposal of a taxonomic nomenclature for the Bacillus cereus group which reconciles genomic definitions of bacterial species with clinical and industrial phenotypes. MBio. 2020;11:e00034-00020. doi: 10.1128/mBio.00034-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jolley KA, et al. Ribosomal multilocus sequence typing: Universal characterization of bacteria from domain to strain. Microbiology. 2012;158:1005–1015. doi: 10.1099/mic.0.055459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier-Kolthoff JP, Göker M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019;10:2182. doi: 10.1038/s41467-019-10210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darling AE, Mau B, Perna NT. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darling ACE, Mau B, Blattner FR, Perna NT. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zankari E, et al. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clausen PTLC, Aarestrup FM, Lund O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 2018;19:307. doi: 10.1186/s12859-018-2336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clausen PTLC, Zankari E, Aarestrup FM, Lund O. Benchmarking of methods for identification of antimicrobial resistance genes in bacterial whole genome data. J. Antimicrob. Chemother. 2016;71:2484–2488. doi: 10.1093/jac/dkw184. [DOI] [PubMed] [Google Scholar]
- 35.Joensen KG, et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014;52:1501–1510. doi: 10.1128/JCM.03617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moura A, et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016;2:16185. doi: 10.1038/nmicrobiol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ragon M, et al. A new perspective on listeria monocytogenes evolution. PLoS Pathog. 2008;4:e1000146. doi: 10.1371/journal.ppat.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolley K, Bray J, Maiden M. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018;3:1. doi: 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jolley KA, Maiden MCJ. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galata V, Fehlmann T, Backes C, Keller A. PLSDB: A resource of complete bacterial plasmids. Nucleic Acids Res. 2018;47:D195–D202. doi: 10.1093/nar/gky1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arndt D, et al. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sirén K, et al. Rapid discovery of novel prophages using biological feature engineering and machine learning. NAR Genom. Bioinform. 2021 doi: 10.1093/nargab/lqaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan MJ, Petty NK, Beatson SA. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitchins, A. D., Jinneman, K. & Chen, Y. Chapter 10: Detection of Listeria monocytogenes in Foods and Environmental Samples, and Enumeration of Listeria monocytogenes in Foods in Bacteriological Analytical Manual (BAM) (eds. U.S. Food and Drug Administration) https://www.fda.gov/food/laboratory-methods-food/bam-chapter-10-detection-listeria-monocytogenes-foods-and-environmental-samples-and-enumeration (2017).
- 46.Bakker HC, Bowen BM, Rodriguez-Rivera LD, Wiedmann M. FSL J1–208, a virulent uncommon phylogenetic lineage IV listeria monocytogenes strain with a small chromosome size and a putative virulence plasmid carrying internalin-like genes. Appl. Environ. Microbiol. 2012;78:1876–1889. doi: 10.1128/AEM.06969-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hudson LK, et al. Complete genome sequences of three Listeria monocytogenes bacteriophage propagation strains. Microbiol. Resource Announcements. 2021;10:e01159–e11120. doi: 10.1128/MRA.01159-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Knabel SJ. Prophages in Listeria monocytogenes contain single-nucleotide polymorphisms that differentiate outbreak clones within epidemic clones. J. Clin. Microbiol. 2008;46:1478–1484. doi: 10.1128/JCM.01873-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orsi RH, et al. Short-term genome evolution of Listeria monocytogenes in a non-controlled environment. BMC Genom. 2008;9:539. doi: 10.1186/1471-2164-9-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vu HTK, Stasiewicz MJ, Benjakul S, Vongkamjan K. Genomic analysis of prophages recovered from Listeria monocytogenes lysogens found in seafood and seafood-related environment. Microorganisms. 2021;9:1354. doi: 10.3390/microorganisms9071354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee G, et al. F-type bacteriocins of Listeria monocytogenes: A new class of phage tail-like structures reveals broad parallel coevolution between tailed bacteriophages and high-molecular-weight bacteriocins. J. Bacteriol. 2016;198:2784–2793. doi: 10.1128/JB.00489-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zink R, Loessner MJ, Scherer S. Charaterization of cryptic prophages (monocins) in Listeria and sequence analysis of a holin/endolysin gene. Microbiology. 1995;141:2577–2584. doi: 10.1099/13500872-141-10-2577. [DOI] [PubMed] [Google Scholar]
- 53.Curtis GDW, Mitchell RG. Bacteriocin (monocin) interactions among Listeria monocytogenes strains. Int. J. Food Microbiol. 1992;16:283–292. doi: 10.1016/0168-1605(92)90030-7. [DOI] [PubMed] [Google Scholar]
- 54.Ramaswamy V, et al. Listeria-review of epidemiology and pathogenesis. J. Microbiol. Immunol. Infect. 2007;40:4. [PubMed] [Google Scholar]
- 55.Núñez-Montero K, et al. Listeria costaricensis sp. nov. Int. J. Systematic Evolut. Microbiol. 2018;68:844–850. doi: 10.1099/ijsem.0.002596. [DOI] [PubMed] [Google Scholar]
- 56.Kim KY, Frank JF. Effect of nutrients on biofilm formation by listeria monocytogenes on stainless steel. J. Food Prot. 1995;58:24–28. doi: 10.4315/0362-028x-58.1.24. [DOI] [PubMed] [Google Scholar]
- 57.Azizoglu RO, Osborne J, Wilson S, Kathariou S. Role of growth temperature in freeze-thaw tolerance of Listeria spp. Appl. Environ. Microbiol. 2009;75:5315–5320. doi: 10.1128/AEM.00458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing reads and genome assemblies are available on NCBI under BioProject PRJNA760531.