Abstract
Investigators were continuously creating novel nanotechnologies to address unmet requirements throughout the administration of therapeutic medicines & imaging agents for cancer treatment & diagnostics, appropriately. LNPs(Lipid nanoparticles) are legitimate particulates (approx. 100 nm in size) gathered from various lipid as well as other biochemical compounds which overall functionality to resolve biological barriers (biobarriers), allowing LNPs to selectively collect somewhere outside of disease-target cells again for responsive therapeutics. Most pharmaceutically important compounds were insoluble throughout water solutions, were chemical & physiologically unstable, or have toxicities. Among the most potential drug carrier for bioactive organic compounds is LBNPs (Lipid based nanoparticles) technologies. Its present use in chemotherapy have transformed treatment for cancer by increasing the antitumor effect of a number of chemotherapeutics. Because they may be created using naturally occurring sources, LBNPs have great temporal and thermal stability, maximum load potential, simplicity of preparations, cheap manufacturing costs, & big manufacturing output. Furthermore, combining chemotherapeutic drugs with LNPs reduces active therapeutic dosage and toxicities, lowers treatment resistance, & raises drug concentration in tumour cells while reducing concentrations in normal tissue. LBNPs were widely studied in cancer treatment, both in vitro and in vivo, with encouraging outcomes in certain clinical trials. This study provides an overview of the many types of LBNPs which have been created in latest years and their applications and contributions in different types of cancers.
Keywords: Lipid based Nanopartcles, Tumour, Clinical trials, Liposomes, Solid lipid nanoparticles
Lipoid based nanoparticles; Tumour; Clinical trials; Liposomes; Solid lipid nanoparticles.
1. Introduction
Tumour is a category of illness that are explained as irregular cell development with ability to spread toward other cells or areas of the body. It is among the biggest killers, of over 100 distinct forms of cancer [1]. Inside the undeveloped nation, diseases like H. pylori, hbv, hep C, hpv infection, Epstein–Barr viruses, and HIV cause 15% of malignancies [2]. Those variables function, at least in part, through altering a cell's genes. Many genetic alterations are often necessary before cancer starts [3]. Cancers are caused by inherited genetic abnormalities in 5–10% of cases [4]. Various indications & indicators, as well as medical tests, can help identify malignancy. It would then be generally explored forward with diagnostic imaging & verified with a biopsy [5]. During 2015, around 90.5 million individuals were diagnosed with cancer [6]. Annually, nearly 18 million new cases are recorded in 2019 [7]. It was blamed for almost 8.8 million deaths each and every year [8]. Lung cancer, prostate cancer, colorectal cancer, and stomach cancer are the most prevalent kinds of cancer in men [9]. Breast cancer, colorectal cancer, lung cancer, and cervical cancer are the most prevalent kinds of cancer in women [10]. Skin cancers other than melanoma would account for around 40percent of new cases of cancer per year if total new cancer cases had been included [11, 12]. Acute lymphoblastic & brain cancer seem to be the most frequent in youngsters, other than in African, wherein non-Hodgkin cancer is more frequent [13].
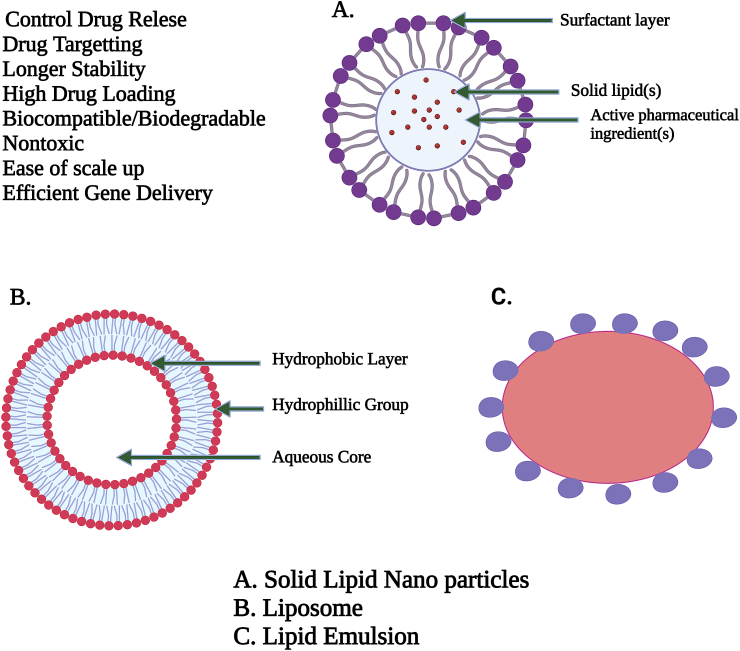
"Fat" seems to be another term for "lipid." The lipids seem to be a material which is unable to dissolve insoluble in h2o but having ability to dissolve in alcoholic, (C2H5)2O, & chcl3 [14]. Lipids were essential components of human cells. Lipids, along with Cx (H2O)y & proteins, were its primary components of plant and animal cells. Triglycerides and cholesterol are both lipids. Lipids are rapidly achieved and retained within system. It serves as an energy reference and is an important component of cell composition. Lipids include fatty acids, neutral fats, waxes, and steroids (like cortisone). Compound lipids include lipoproteins, glycolipids, and phospholipids (lipids complexed with another type of chemical molecule). Lipids are sometimes defined as aquaphobic or amphipathic small molecules; the amphiphilic characteristic of certain lipids enables us to build formations in an aqueous environment like vesicle, large uniflagellar liposomes, or membrane. Biological lipid is made up of two types of biochemical subunits or "building blocks": ketoacyl and isoprene groups. Structure of SLN, Liposomes and Lipid Emulsion are shown in Figure 1.
Figure 1.
The overall arrangement of solid lipid nanoparticles, which have benefits over liposomes and lipid emulsions, is represented schematically. Adopted from [80].
Because of their biocompatibility as transporters, lipids have gotten a lot of attention since the beginning of the pharmacological era. They have little oral absorption due to their highly hydrophobic nature [15]. As a consequence, the desire to extend the range of applications for such carriers was unsatisfactory, and they're not employed in propulsion systems till 1900, while they were encased into colloidal delivery systems [16, 17, 18, 19]. Lipid nanoparticles (LNPs) were seen to be better beneficial than polymeric nanoparticles in the creation of nanoparticle-based delivery systems, and they've been widely employed for drug delivery [20]. Because LNPs are made from physiologic and/or biodegradable lipids, these lipid-based carrier systems are also referred to as “Nano safe” carriers [21]. The very well LNP synthesis is solid lipid nanoparticles (SLNs), that were created in the early 1990s [22]. Because of the various benefits of prior carriers like emulsifiers, liposomes, & polymeric nanoparticles, that delivering method was introduced [23]. The feasibility of the production procedures and levelling-up process, the GRAS (generally recognised as safe) quality of all formulations, and the lack of polar compounds are the characteristics that distinguish SLNs from liposomes [24].
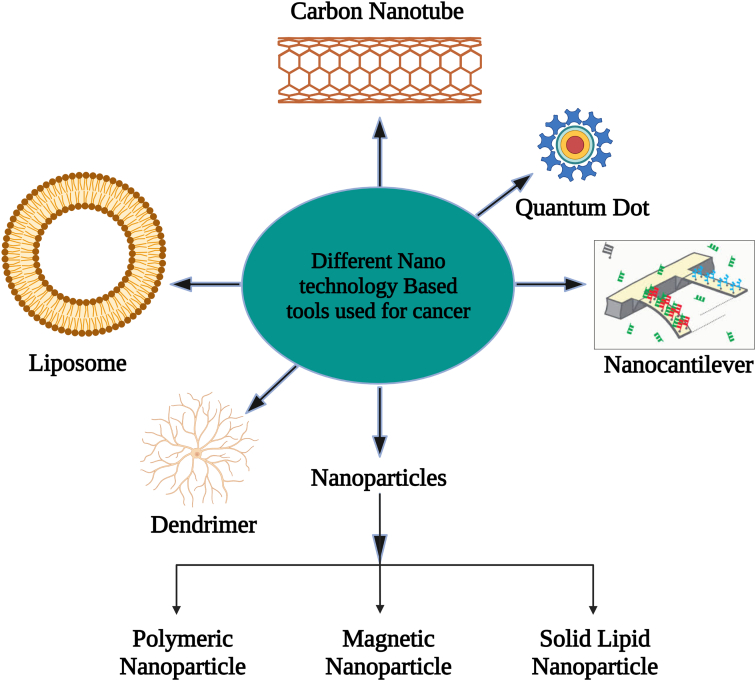
Tumor nanotechnology has now been created as a potential cancer therapeutic method for antitumor drug delivery [25]. Nanoparticles have diameters ranging from 1 to 1000 nm and boost therapeutic bioavailability as well as anticancer drug specificity [26]. Numerous nanoparticles (NPs) and nanotech methods to cancer treatment have recently been presented shown in Figure 2. Because of their distinctive optical characteristics, broad excitation spectrum, and overly limited symmetric intensity distribution, semiconductor quantum dots (QDs) may now be used as a flexible material system with tremendous promise for biological applications. Semiconductor QDs are an exciting new class of fluorescence components. They are employed in bioimaging, biolabeling, and biosensing applications. QDs have a greater impact than ordinary fluorophores. They are brighter, have more fluorescence intensity controllability, and are less photobleached. Different colored QDs may be excited by a single source of light and have broad absorption and narrow emission spectra. The aforementioned QDs appear to be the best alternative for screening cell receptors. To generate effective fluorescence probes, the surface of QDs must be changed utilising various biological substances [27].
Figure 2.
Various Nanotechnology based tools utilized in treatment of cancer. Adopted from [81].
Among the various nano formulations utilised in cancer shown in Figure 2., we emphasize those based on lipid formulations since substantial breakthroughs in preparation and alternative compositions have been realised in recent decades. Chemical changes to lipid nano systems can be used to evade immune system detection or to increase medication availability. These could also be manufactured in pH-sensitive compositions to increase release of the drug in an acidic condition, and they can be coupled along antibodies those detect tumour cells & their receptor like (FoA) folic acid [28]. Nano drugs can potentially be utilised in conjunction with other treatment approaches to increase patient ‘s response.
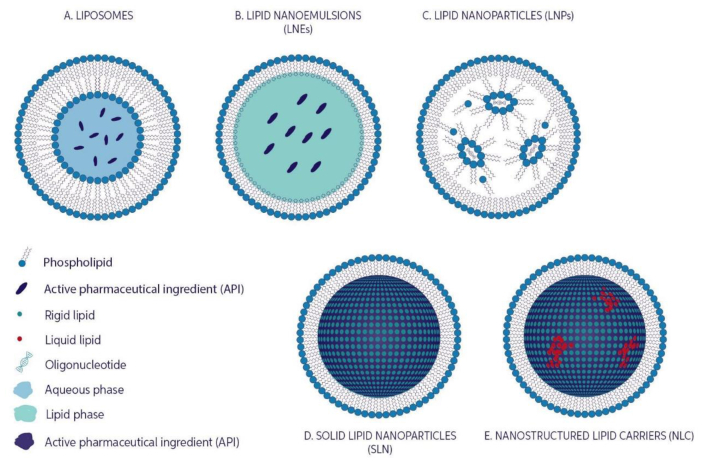
Numerous antitumour drugs, including cisplatin, irinotecan (IRI), paclitaxel (PTX), doxorubicin (DOX), oxaliplatin, daunorubicin, cytarabine, and vincristine, have already been analysed in nano formulations, and some were investigated in clinical studies and/or are commercially present for their clinical utilization [29]. In fact, first commercially utilised anticancer drug NanoSystems was Doxil®, a liposome formulation containing DOX. provides an overview of the many types of LBNPs (Figure 3) which have been created in latest years and their applications and contributions in different types of cancers.
Figure 3.
Lipid based nanoparticles showing A) LIPOSOMES, B) LIPID NANOEMULSIONS C) LIPID NANOPARTICLES, D) SOLID LIPID NANOPARTICLES, E) NANOSTRUCTURED LIPID CARRIERS. Adopted from [83].
2. Solid lipid nanoparticles
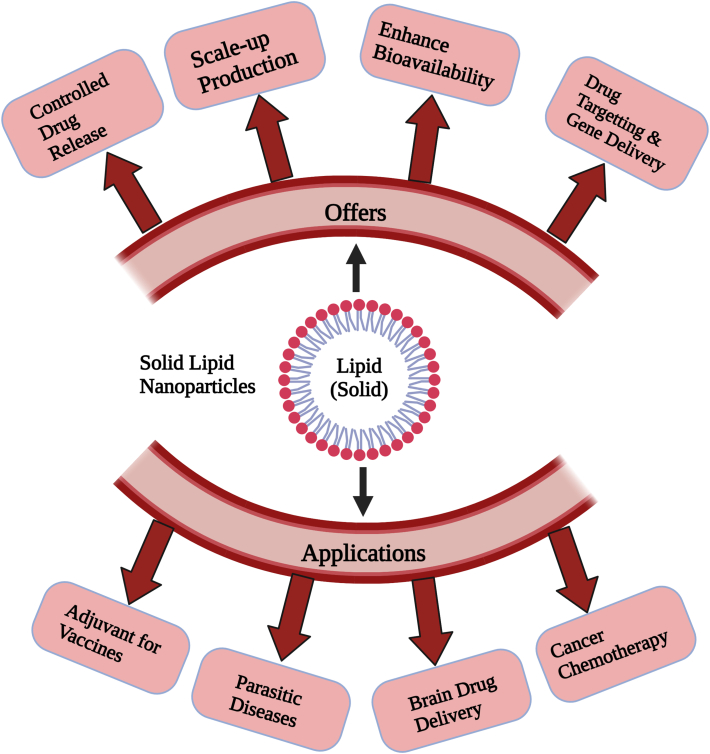
These are hard sized ranged between 1-1000nanometer. The size of particles mostly between 150-300nanometer. SLNs are solid, submicronic colloidal nanocarriers with a size range of 1e1000 nm. The particle size is mainly between 150 and 300 nm. Such delivery of drugs methods, like polymeric nanoparticles, provide a framework for regulated releases [30]. Their solid matrix of SLNs allows them to restrict medication motility & offer better stabilization, allowing them to combine the benefits of polymeric nanoparticles, liposomes, and micronized emulsifiers [31]. Moreover, tests show that SLNs were highly advantageous in a variety of aspects (Figure 4), such as the prevention of utilising organic solvent while manufacturing, possible scaling [32], as well as the inclusion both of lipophilic and hydrophilic medicines in significant quantities [33]. SLNs are created by replacing a solid lipid or even a combination of solid lipids for the liquid lipid (oil) in the structure of an oil in water emulsion. One important feature of SLNs is that they have been solid at both room as well as temperature of body [34]. Such drug transport systems are composed of 0.1–30% (w/w) solid lipid dispersed in an aqueous medium. SLNs are typically composed of solid form lipid such as higher purity of triglycerides, free fatty acids, free fatty alcohols, complex glyceride blends, and even wax (typically well-known physiologic lipids) [35]. It is also feasible to use more complicated structures [36].
Figure 4.
The Benefits and uses of SLN are depicted schematically. Adopted from [82].
2.1. Limitations of SLN and way to overcome
Although SLNs are most often made up of solid lipid, degradation and instability may become a concern. Factors that must be considered are including high pressure–induced drug degradation, the coexistence of different lipid modifications and colloidal species, the minimal drug-loading potential, and the kinetics of delivery process.
2.2. High pressure–induced drug degradation
Molecular size & structure are the primary causes of drug degradation, and high pressure homogeneity has also been shown to reduce polymeric molecular weight. High molecular weight composites or chain length elements are much more vulnerable than low molecular compounds with a spherical form, despite the fact that several studies show that high-pressure homogenization–induced drug degradation is not a concern for the overwhelming bulk of bioactive metabolites. Nevertheless, large molecular weight chemicals such as DNA, albumin, and dextrose are particularly vulnerable to breakage; hence, a separate approach must be used to integrate these elements into SLNs.
2.3. Lipid crystallization and drug incorporation
A further critical factor to take into account is lipid crystallisation. For past decade, researchers have been studying the relationship among lipid alteration and medication administration. The study of lipid changes is well known. The majority of the methods rely on X-ray and differential scanning calorimetric measurements. Nevertheless, the majority of the information has come from studies on huge quantities lipids. Due to the obvious nanosize of the carrier and the huge number of interface active participants needed to maintain the colloidal lipid dispersal, the effectiveness of SLNs may vary significantly. As a result, lipid crystallisation and drug inclusion have an impact on lipid particle properties. The following important considerations must be taken into consideration while discussing drugs capture within SLNs: (1) the occurrence of super-cooled melts; (2) the occurrence of multiple lipid alterations; (3) the morphology of lipid nanodispersions; and (4) gelation processes.
2.4. Several colloidal species coexist
The cohabitation of numerous nanoparticles within SLNs has received little attention from researchers, despite the fact that it is a crucial aspect to address. Surfactants are incorporated on both the lipid surface and the interior. In glycocholate/lecithin stabilised and related systems, heterogeneous micelles must be acknowledged. Because micelles, combined micelles, and liposomes are known to dissolve pharmaceuticals, they can be used as alternative therapeutic inclusion targets. The presence of various heterogeneous entities alone is insufficient to characterize the structure of colloidal lipid phase separation, because dynamic processes are critical for drug stabilization and releasing. As a result, the kinetics of distribution processes must be taken into account. For illustrate, hydrolytic medications will degrade quicker in water dissolved & interface localised compounds than in lipid compounds.
The rates of breakdown will be regulated by: (1) the medication's chemical nature, and (2) the drug concentration in the aqueous phase or at the lipid/water boundary. Volatile medications will undergo hydrolysis quickly when they come into touch with liquid, causing the drug's dispersion balance between various habitats to be disrupted. Carriers are only beneficial if they prohibit the medication from being redistributed. Naturally, enhancing the matrix thickness reduces the diffusion coefficient of the medication inside the transporter, hence SLNs are projected to outperform lipid nanoemulsions. To create an effective delivery mechanism, comprehensive transparency about bits' in vitro and in vivo destiny must be provided.
3. Nanostructured carriers of lipid (SLN & NLC)
Although their protection and effectiveness, SLNs have many major disadvantages, including higher moisture concentration (70–99.9%), poor drug content owing to crystalline form, drug ejection while preservation, and potential polymorphism transitions and particles development while storing. As a result, changes to the Solid Lipid Nanoparticles organization are necessary to reach these constraints. Ongoing research led to development of a "2nd gen" of LNPs at the millennium's turn: the NLCs [37]. Following that, Dr. Rimpler's (Wedemark, Germany) developed the very first NLC concepts: Nano repair Q10 cream, Nano repair Q10 serum, and Chemisches Laboratorium's Nano lipid CLR Restore (Berlin, Germany). With numerous possible applications as well as a brief period among discovery & commercial launch, Solid Lipid Nanoparticles will be at the leading edge of nanotechnology innovation [38]. The existing information suggests that SLNs & NLCs were ideal for the integration of lipophilic substances, despite the fact that drug loading with aquaphilic molecules is rather modest [39]. Early research on the topic shows that only very powerful aquaphilic medicines with modest quantities of efficacy could be fully incorporated throughout the solid lipid matrix [40].
4. Medical applications of SLN
4.1. Cancer chemoimmunotherapy
Tumour chemoimmunotherapy would be a medication which combines all beneficial effects of chemotherapeutic with immunotherapeutic. Chemo usually entails use of such traditional cytotoxic medicines as well as new molecularly targeted treatments. Immunotherapy, on the other hand, is a relatively new kind of cancer treatment which employs the sufferer's native immune system to fight cancer cells. It includes the Immune checkpoint inhibitors, adoptive cell therapy, cancer vaccinations, & cytokine therapies are all being used.
4.2. Nanoparticles based on lipids in cancer immunotherapy
Due to specific benefits, nanotech has received a lot of interest in cancer therapy [41]. Nanoparticle, in illustration, such as Polymeric micelles, lipid-based nanoparticles, gold nanoparticles, and inorganic nanoparticles are all examples of nanoparticles, are frequently employed to transport therapeutics including small molecules (either hydrophilic or hydrophobic), protein, & heredity materials for chemotherapeutic agents. Those nanomaterials can transport therapeutic drugs to certain cells via passively focusing techniques like the EPR impact or active targeting techniques like specific ligand [42].
Lipid-based nanoparticles, in particular, have appealing pharmacological & multifunctional properties, such as bio-compatibility, bio-degradability, as well as the potential to intimidate both aquaphilic and aquaphobic therapies [43, 44, 45]. Furthermore, the surface characteristics of lipid-based nanoparticles may be readily changed by changing the lipid components or altering the surface. Presently some of that are under preclinical trails such as hybrid lipid-based nanoparticles, nano discs, & liposomes, some of these are given in Table 1.
Table 1.
Lipid based nanoparticles in clinical trials.
| Composition | Chemotherapy | Immunotherapy | Type of Cancer | Mode of Operations | References |
|---|---|---|---|---|---|
| Liposomes | |||||
| PEGylated liposomes | Doxorubicin | Alendronate | Breast cancer | i.v. | [46] |
| Charge-reversal cell penetrating peptide-modified liposomes | Paclitaxel | PD-L1 antibody | Melanoma | i.v. | [47] |
| pH-responsive liposomes | Mitoxantrone | Indoximod | Breast cancer and renal cancer | i.v. | [48] |
| Enzyme/pH dual-sensitive micelle-liposomes | Paclitaxel | HY19991 | Metastatic breast cancer | i.v. | [49] |
| Hybrid lipid-based nanoparticles | |||||
| Thermo-sensitive exosome-liposome hybrid nanoparticles | Docetaxel | GM-CSF | Metastatic peritoneal carcinoma | i.v. | [50] |
| Lipid-coated calcium nanoparticles | Zoledronate | Zoledronate | Lung cancer | i.v. | [51] |
| Liposome-coated mesoporous silica nanoparticles | All-trans retinoic acid + doxorubicin | IL-2 | Melanoma | i.v. | [52] |
| Nano discs | |||||
| HDL-Nano disc | Doxorubicin | αPD-1 | Colorectal cancer | i.v. | [53] |
| Docetaxel Colon | Cholesterol modified CpG | carcinomas | Intra-tumoral | [54] | |
4.3. Liposomes
Liposomes are nanosized particles composed largely of cholesterol and phospholipids that have proven improvements in bio-compatibility and increased directed payloads distribution with little harm. With its lipid soluble ends, amphiphilic phospholipids self-assemble it into circular lipid bilayer form, allowing water insoluble medicines to be enclosed. Water - soluble head of phospholipids, but at the other hand, form an external surface as well as a watery centre which can contain aquaphilic substances. Numerous s medicinal compounds can be encapsulated into liposomes via charge–charge interactions or interactions with chemical linkers on the liposomal surface. Liposomes, which allow for the administering drugs including both lipid and water-soluble therapeutic drugs while maintaining effectiveness, are one of the most effective nanotech medications in cancer treatment. Although PEGylated liposomal DOX (Doxil®) has become the first Food and drug administration approved nano-drug in 1995, the FDA has authorised more than six liposomal medicines to be used in cancer treatment. Liposomes were used as among the most appealing targeted delivery in chemoimmunotherapy, building on the success of liposomes in chemo. Liposomes are the first and mostly explored nanocarriers for cancer drug delivery, which have shown great promise in clinical applications, but their limited accumulation and penetration into the tumor interstitial space, significantly reduce the therapeutic efficacy [55].
4.4. Nano disc
Nano discs are a synthetic model membrane system comprised of a phospholipid bilayer with the hydrophobic edge filtered by two amphipathic proteins are known as membranes scaffolding proteins (MSP). The MSP in certain nano discs is enhanced apolipoprotein A1 (apoA1), which would be the primary component of high-density lipoproteins (HDL). Nano discs have a shape comparable to discoidal HDL, which simulates a more natural environment then liposomes and micelles. In immunotherapy, this biomimicking delivery method appears to be more successful. Schwendeman's group completed extensive research on nano disc-based chemoimmunotherapy. Originally, they created an HDL-mimicking nano-disc that was attached to draining lymph nodes with a neoantigen (Ag peptide) and adjuvant (CpG). The nano-disc evoked upwards to 47-fold more neoantigen specific CTLs then solubilized vaccines and 31-fold more than the clinical trial adjunct. Those findings reinforced a novel potent strategy to cancer immunotherapy [56].
4.5. Nanoparticles based on a hybrid of lipids
Lipid-based hybrid nanoparticles with flexible configurations are appealing for chemoimmunotherapy. Towards effective therapeutic dosage, several inorganic nanoparticles with a lipids coating are being created. Kong et al., created lipid-coated bio-degradable hollow mesoporous silica nanoparticles (dHMLB) with all-trans retinoic acid (ATRA) co-encapsulation for chemoimmunotherapy [57]. A lipid component of hybrid nanoparticles is also being utilised as dosage form during chemoimmunotherapy. Zhang et al. created TCNs for synchronized bio-distribution & selective administration of SF & IMD-0354 to cancerous cells & TAMs, accordingly, to improve cancer-localized chemoimmunotherapy [58].
5. Applications in cancer therapy
Lipid-Based NPs (LBNPs) are a vast and diversified class of nanoparticles that are especially important in BreC therapy [59]. But besides their diversity, liposomes are widely employed because to their great biocompatibility and ability to encapsulate a wide range of cargos. LBNPs are now being employed in a number of studies, and a few of them (for example, Doxil® or Abraxane®) have previously been licenced for BreC therapy [6, 38]. This part presents the most recent major breakthroughs in the use of LBNPs in the treatment of the most common kinds of cancer.
5.1. Bowel cancer
Bowel cancer is a major health concern because of its high death rate (it is the second leading cause of death) and the recent increase in its incidence [60, 61]. LBNPs provide a possible method for improving existing treatments, particularly in advanced colorectal cancer where chemotherapy (5-FU alone or in combination with other medicines) or monoclonal antibodies (bevacizumab, trastuzumab, cetuximab) are ineffective. In comparison to a 5-FU thermosensitive gel-mediated microemulsion (ME), a thermosensitive gel-mediated 5-FU microemulsion (ME) was able to enhance Caco-2 permeability and cell uptake, as well as its accumulation in rectal tissue in vivo.
Low et al. [62] created a sophisticated device based on Pickering emulsions (PE) that consists of a magnetic cellulose nanocrystal loaded with CUR and is capable of regulated drug release when exposed to an external magnetic field. In both monolayer and multicellular spheroids, this approach inhibited the development of HCT116 cells. Furthermore, Ektate et al. [63] activated macrophages in the tumour environment using lipopolysaccharide (LPS) from attenuated Salmonella bacteria coated with DOX-thermosensitive liposomes and high-intensity focused ultrasonic waves.
Through changes in membrane fluidity, this approach was able to enhance DOX internalisation and reduce tumour development in vivo. Liposome characterisation is also being utilised to enhance CRC therapy. As a result, Moghimipour et al. [64] utilised FoA to enhance 5-FU uptake in CT-26 cells, lowering its IC50 & reducing tumour volume. Kaseem et al. [65] created niosomes containing imatinib mesylate (IM), which bring down the IC50 of the free drug (16-fold) in HCT-116 cells.
5.2. Stomach cancer
It is the globe's 5th another very frequent malignancy as well as the major cause of malignancy mortality [ 60,61]. Only stomach malignancy that has not spread to the lymph nodes could be managed via surgical removal only. Severe stomach carcinoma, should be managed with combination chemotherapy, which have significant adverse impacts. New treatments relying upon nano formulation are currently being explored to enhance patient responses. Liposomes were broadly applied in GC therapy, either alone or in combination with compounds including the Arg-Gly-Asp peptides [66], SATB1 siRNA/CD44 antibodies [67], or in the formation of DNA complexes [68]. Their use enhanced drug deposition in cancerous cells of any animals grafted with SGC7901 cells expressing high levels of integrin 51 [66]. Liposomes also exhibited increased targeting accuracy and were able to suppress SATB1 gene expression by about 80% in CD44 + GC starting cells [67]. Furthermore, liposomes recognised peritoneally dispersed GC MKN-45P cells, decreasing their accumulation in the liver. Initial studies using SLNs in GC [69] revealed that etoposide (VP16) had increased action in SGC-7901 cell, increasing inhibition of growth, causing cell arrests in the G2/M stage (17.13 percent), & triggering mitochondria-involved apoptotic. Li et al. [70] created an SLN for use in conjunction with in ATRA and sorafenib as well as miR-542-3p. This method improved the absorption of both anticancer medicines and had a synergistic activity on MGC-803 cells.
5.3. Breast tumour
It is the leading cause of mortality in women [60] and is experiencing significant shifts as a result of the development of NPs, notably in the treatment of metastatic cancer. Throughout the tolerant MCF-7/ADR cancer cell, NEs preloaded wit DOX & bromo tetra trandrine (W198, P-glycoprotein (P-gp) inhibitor) have been examined. That increased cellular absorption and deposition of DOX in cancer cells. DOX, on the other hand, reduced stomach & cardiac damage [71]. In contrast, DOX-liposome-based compositions were evaluated in clinical studies.
PLD in conjunction to lapatinib have been recently utilised in HER2-positive BreC sufferers (stage Ib) to find the best conjunction including both therapies at the highest acceptable dosage [72]. Furthermore, a phase 3 trials of Myocet in combination with cyclophosphamide (CM) or vinorelbine (MV) in cancer patients BreC has also been established [73].
SLNs are another type of LBNP employed in BreC investigations. Yu et al. [74] suggested a method for combining PTX & derivatized DNA delivery with such a pH-sensitive ligand. In vivo, this approach is responsible for reducing tumour volume while also lowering PTX deposition in all further organs. Furthermore, Garg et al. [75] created a fucose-methotrexate SLN that accumulated preferentially in tumour tissue just 2 h after treatment, as opposed to free methotrexate, that accumulates throughout the kidney, liver, & spleen.
5.4. Glandular carcinoma
Presently, the primary LBNPs under investigated as potential treatment methods in prostate cancer include NEs, liposomes, and solid-lipid NPs (SLNs) (PrC). Ahmad et al. [76] recently created an oil-in-water NE containing a toxoid therapeutic agent linked to an omega-3 fatty acid. That NE is effective to lessen the toxoid IC50 of PPT2 cell types 12-fold, enabling for a larger tumour size decrease in tumour-bearing rats than AbraxaneTM. In PC-3 cells, similar antitumor effects were seen when NE was loaded with catechin extract (flavanols having anticancer activities) [77]. Regarding liposomes, 22Rv1 PrC cells were treated with PEG-folate-targeted-oleuropein-liposomes.
In in vivo models, these nanoplatforms enhanced 22Rv1 cell apoptosis, oleuropein bioavailability, and survival [78]. Hua et al. [79] also created NPs that contains diversified liposome burdened with docetaxel and a gold nanorod, demonstrating 100 percent suppression of PrC cell growing by merge the mode with the use of a radiation.
And there are lots of application in all other types of cancer including lung cancer, nervous system cancer, liver cancer, pancreatic cancer etc. In recent years there are lot of modification and newly synthesized nanoparticles are introduced in cancer treatment.
6. Conclusion and future challenges
Lipid Based Nanoparticles are a varied and comprehensive category of compounds that have been utilised to treat various diseases, most notably malignancy. Liposomes are now the most often utilised Lipid Based Nanoparticles because to its excellent biocompatibility & flexibility; although, SLNs as well as NLCs have lately gained popularity.
Nonetheless, studies are just not focused primarily on such Nanoparticles, and there are several papers focusing on novel techniques for utilising Lipid Based Nanoparticles to heal other kinds of cancer. Some of it has already progressed towards the next stage & began new careers in clinical studies.
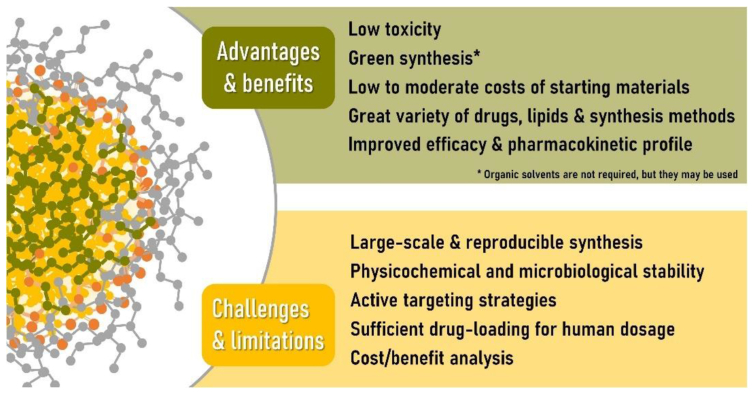
SLNs and nanostructured lipid carriers have received a lot of interest in the recent decade as prospective delivery of drugs (nano)systems. One ‘s main benefit may be the utilisation of biomaterial, environmentally safe ingredients and techniques of production (Figure 5). It really should be stressed, however, that prior to mass producible & distribution of these schemes, rigorous clinical and environmental safety analysis must be done. The establishment of standardised processes for assessing possible dangers of nano - materials consumption, as well as the corresponding regulatory environment, is considered necessary. As with other nanosized drug carriers, melanoma therapy is an important research area in which SLNs can be used, which might also represent both large level of funding in the field as well as the appropriateness of nanostructures for such delivering of cytotoxic drugs, owing to the direct and indirect attacking prompted by malignant cellular level. Nonetheless, there are numerous clinical sectors which derive value from using lipid nanoparticles. Regrettably, more research, extra effort & working capital facilities should be provided for SLN/NLC to be shown therapeutically effective in real-world circumstances. For said time being, the rarity of SLNs which have advanced to medical studies suggests that it would be at least a few years before such innovations approach the national or international drug market.
Figure 5.
Studying with solid lipid nanoparticles for drug carriers: benefits, present obstacles, and limits. Adopted from [84].
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
We deeply acknowledge Dr. Aparna Banerjee, Chile for her support providing the Biorender tool for image drawing.
Footnotes
This article is a part of the “Lipid-Based Nanoparticles in Diagnosis and Treatment” Special issue.
Contributor Information
Sumit Sheoran, Email: sheoran080897@gmail.com.
Sugunakar vuree, Email: sugunakar.24344@lpu.co.in.
References
- 1.Underwood J.C., Cross S.S. Elsevier heath science; Amsterdam, Netherlands: 2009. General and Systematic Pathology. [Google Scholar]
- 2.Cancer. World Health Organization; 12 September 2018. [Google Scholar]
- 3.World Cancer Report 2014. World Health Organization; 2014. Chapter 1.1. [Google Scholar]
- 4.Rehder H. Family cancer syndromes. Hered. Tumors. 2009:41–86. [Google Scholar]
- 5.How Is Cancer Diagnosed? American Cancer Society; 29 January 2013. [Google Scholar]
- 6.GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 8 October 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sciacovelli Marco, Schmidt Christina, Maher Eamonn R., Frezza Christian. Metabolic drivers in hereditary cancer syndromes. Annu. Rev. Cell Biol. 2020;4:77–97. [Google Scholar]
- 8.GBD 2015 Mortality and Causes of Death, Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 8 October 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Cancer Report 2014. World Health Organization; 2014. Chapter 1.1. [Google Scholar]
- 10.World Cancer Report 2014. World Health Organization; 2014. Chapter 1.1. Archived from the original on 12 July 2017. [Google Scholar]
- 11.Dubas L.E., Ingraffea A. Nonmelanoma skin cancer. Fac. Plastic Surg. Clin. North Am. February 2013;21(1):43–53. doi: 10.1016/j.fsc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Cakir B.Ö., Adamson P., Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Fac. Plastic Surg. Clin. North Am. November 2012;20(4):419–422. doi: 10.1016/j.fsc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 13.World Cancer Report . Vol. 2014. 2014. World Health Organization. pp. Chapter 1.3. Archived from the original on 12 July 2017. [Google Scholar]
- 14.IUPAC . second ed. The "Gold Book"; 1997. Compendium of Chemical Terminology. Online corrected version: (2006–) "lipids". [Google Scholar]
- 15.Jingling Tang J.S. Self-emulsifying drug delivery systems: strategy for improving oral delivery of poorly soluble drugs. Curr. Drug Ther. 2007;2 [Google Scholar]
- 16.Humberstone A.J., Charman W.N. Lipid-based vehicles for the oral delivery of poorly water-soluble drugs. Adv. Drug Deliv. Rev. 1997;25 [Google Scholar]
- 17.Kuentz M. Lipid-based formulations for oral delivery of lipophilic drugs. Drug Discov. Today Technol. 2012;9 doi: 10.1016/j.ddtec.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Patel A.R., Velikov K.P. Colloidal delivery systems in foods: a general comparisonwith oral drug delivery. Food Sci. Technol. 2011;44 [Google Scholar]
- 19.Pouton C.W., Porter C.J. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv. Drug Deliv. Rev. 2008;60 doi: 10.1016/j.addr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Müller R.H., Karsten K., Mäder S.G. Solid lipid nanoparticles (SLN) for controlled drug delivery e a review of the state of the art. Eur. J. Pharm. Biopharm. 2000;50(1) doi: 10.1016/s0939-6411(00)00087-4. 66 CHAPTER 3 Lipid-Based Nanoparticles for Drug Delivery Systems. [DOI] [PubMed] [Google Scholar]
- 21.Pardeike J., Hommoss A., Müller R.H. Vol. 366. 2009. Lipid Nanoparticles (SLN, NLC) in Cosmetic and Pharmaceutical Dermal Products; pp. 170–184. [DOI] [PubMed] [Google Scholar]
- 22.Gasco M.R. Method for producing solid lipid microspheres having a narrow size distribution. U.S. Patent No. 5. 1993;250:236. [Google Scholar]
- 23.Müller R.H., Lucks J.S. 1996. Medication Vehicles Made of Solid Lipid Particles (Solid Lipid Nanospheres SLN) [Google Scholar]
- 24.Doktorovova S., Souto E.B., Silva A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers e a systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014;87:1–18. doi: 10.1016/j.ejpb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Bor G., Mat Azmi I.D., Yaghmur A. Nanomedicines for cancer therapy: current status, challenges and future prospects. Ther. Deliv. 2019;10:113–132. doi: 10.4155/tde-2018-0062. [DOI] [PubMed] [Google Scholar]
- 26.Miele E., Spinelli G.P., Miele E., Di Fabrizio E., Ferretti E., Tomao S., Gulino A. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int. J. Nanomed. 2012;7:3637–3657. doi: 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fatima I., Rahdar A., Sargazi S., Barani M., Hassanisaadi M., Thakur V.K. Quantum dots: synthesis, antibody conjugation, and HER2-receptor targeting for breast cancer therapy. J. Funct. Biomater. 2021;12:75. doi: 10.3390/jfb12040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rama A.R., Jimenez-Lopez J., Cabeza L., Jimenez-Luna C., Leiva M.C., Perazzoli G., Hernandez R., Zafra I., Ortiz R., Melguizo C., et al. Last advances in nanocarriers-based drug delivery systems for colorectal cancer. Curr. Drug Deliv. 2016;13:830–838. doi: 10.2174/1567201813666151203232852. [DOI] [PubMed] [Google Scholar]
- 29.Alavi M., Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019;34 doi: 10.1515/dmpt-2018-0032. [DOI] [PubMed] [Google Scholar]
- 30.Muller R.H., Shegokar R., Keck C.M. 20 years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Curr. Drug Discov. Technol. 2011;8(3):207–227. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- 31.Kathe N., Henriksen B., Chauhan H. Vol. 9045. 2014. Physicochemical Characterization Techniques for Solid Lipid Nanoparticles: Principles and Limitations; pp. 1–11. [DOI] [PubMed] [Google Scholar]
- 32.Teeranachaideekul V., Muller R.H., Junyaprasert V. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC) e effects of formulation parameters on physicochemical stability. Int. J. Pharm. 2007;340:198–206. doi: 10.1016/j.ijpharm.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Jaiswal P., Gidwani B., Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cell Nanomed. Biotechnol. 2014:1–14. doi: 10.3109/21691401.2014.909822. [DOI] [PubMed] [Google Scholar]
- 34.Lima A.M., Pizzol C.D., Monteiro F.B., Creczynski-Pasa T.B., Andrade G.P., Ribeiro A.O., et al. Hypericin encapsulated in solid lipid nanoparticles: phototoxicity and photodynamic efficiency. J. Photochem. Photobiol., B. 2013;125:146–154. doi: 10.1016/j.jphotobiol.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Wissing S.A., Kayser O., Mu R.H. Vol. 56. 2004. Solid Lipid Nanoparticles for Parenteral Drug Delivery; pp. 1257–1272. [DOI] [PubMed] [Google Scholar]
- 36.Svilenov H., Tzachev C. Solid lipid nanoparticles - a promising drug delivery. Nanomedicine. 2009:187–237. [Google Scholar]
- 37.Muller R.H., Shegokar R., Keck C.M. 20 years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Curr. Drug Discov. Technol. 2011;8(3):207–227. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- 38.Iqbal M.A., S Md, Sahni J.K., Baboota S., Dang S., Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J. Drug Target. 2012;20(10):813–830. doi: 10.3109/1061186X.2012.716845. [DOI] [PubMed] [Google Scholar]
- 39.Almeida A.J., Souto E. Vol. 59. 2007. Solid Lipid Nanoparticles as a Drug Delivery System for Peptides and Proteins; pp. 478–490. [DOI] [PubMed] [Google Scholar]
- 40.Almeida A.J., Runge S., Muller R. Peptide-loaded solid lipid nanoparticles (SLN): influence of production parameters. Int. J. Pharm. 1997;149:255–265. [Google Scholar]
- 41.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 42.Zafar A., Alruwaili N.K., Imam S.S., Alharbi K.S., Afzal M., Alotaibi N.H., Yasir M., Elmowafy M., Alshehri S. Novel nanotechnology approaches for diagnosis and therapy of breast, ovarian and cervical cancer in female: a review. J. Drug Deliv. Sci. Technol. 2020;61:102198. [Google Scholar]
- 43.Ramishetti S., Kedmi R., Goldsmith M., Leonard F., Sprague A.G., Godin B., Gozin M., Cullis P.R., Dykxhoorn D.M., Peer D. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano. 2015;9:6706–6716. doi: 10.1021/acsnano.5b02796. [DOI] [PubMed] [Google Scholar]
- 44.Khan A., Imam S.S., Aqil M., Ahad A., Sultana Y., Ali A., Khan K. Brain targeting of temozolomide via the intranasal route using lipid-based nanoparticles: brain pharmacokinetic and scintigraphic analyses. Mol. Pharm. 2016;13:3773–3782. doi: 10.1021/acs.molpharmaceut.6b00586. [DOI] [PubMed] [Google Scholar]
- 45.Khan A., Aqil M., Imam S.S., Ahad A., Sultana Y., Ali A., Khan K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018;116:1260–1267. doi: 10.1016/j.ijbiomac.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 46.Shmeeda H., Amitay Y., Gorin J., Tzemach D., Mak L., Stern S.T., Barenholz Y., Gabizon A. Coencapsulation of alendronate and doxorubicin in pegylated liposomes: a novel formulation for chemoimmunotherapy of cancer. J. Drug Target. 2016;24:878–889. doi: 10.1080/1061186X.2016.1191081. [DOI] [PubMed] [Google Scholar]
- 47.Li M., Yang Y., Xu C., Wei J., Liu Y., Cun X., Yu Q., Tang X., Yin S., Zhang Z., et al. Tumor-targeted chemoimmunotherapy with immune-checkpoint blockade for enhanced anti-melanoma efficacy. AAPS J. 2019;21:18. doi: 10.1208/s12248-018-0289-3. [DOI] [PubMed] [Google Scholar]
- 48.Mei K.C., Liao Y.P., Jiang J., Chiang M., Khazaieli M., Liu X., Wang X., Liu Q., Chang C.H., Zhang X., et al. Liposomal delivery of mitoxantrone and a cholesteryl indoximod prodrug provides effective chemo-immunotherapy in multiple solid tumors. ACS Nano. 2020;14:13343–13366. doi: 10.1021/acsnano.0c05194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang T., Liu Y., Zheng Z., Ran W., Zhai Y., Yin Q., Zhang P., Li Y. Cocktail strategy based on spatio-temporally controlled nano device improves therapy of breast cancer. Adv. Mater. 2019;31 doi: 10.1002/adma.201806202. [DOI] [PubMed] [Google Scholar]
- 50.Wang G., Wu B., Li Q., Chen S., Jin X., Liu Y., Zhou Z., Shen Y., Huang P. Active transportation of liposome enhances tumor accumulation, penetration, and therapeutic efficacy. Small. 2020;16:2004172. doi: 10.1002/smll.202004172. [DOI] [PubMed] [Google Scholar]
- 51.Lv Q., Cheng L., Lu Y., Zhang X., Wang Y., Deng J., Zhou J., Liu B., Liu J. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv. Sci. 2020;7:2000515. doi: 10.1002/advs.202000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zang X., Zhou J., Zhang X., Chen D., Han Y., Chen X. Dual-targeting tumor cells and tumor associated macrophages with lipid coated calcium zoledronate for enhanced lung cancer chemoimmunotherapy. Int. J. Pharm. 2021;594:120174. doi: 10.1016/j.ijpharm.2020.120174. relay drug delivery for amplifying antitumor efficiency. Biomaterials 2018, 185, 205–218. [CrossRef] 138. [DOI] [PubMed] [Google Scholar]
- 53.Kong M., Tang J., Qiao Q., Wu T., Qi Y., Tan S., Gao X., Zhang Z. Biodegradable hollow mesoporous silica nanoparticles for regulating tumor microenvironment and enhancing antitumor efficiency. Theranostics. 2017;7:3276–3292. doi: 10.7150/thno.19987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuai R., Yuan W., Son S., Nam J., Xu Y., Fan Y., Schwendeman A., Moon J.J. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheetz L.M., Yu M., Li D., Castro M.G., Moon J.J., Schwendeman A. Synthetic HDL nanoparticles delivering docetaxel and CpG for chemoimmunotherapy of colon adenocarcinoma. Int. J. Mol. Sci. 2020;21:1777. doi: 10.3390/ijms21051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong M., Tang J., Qiao Q., Wu T., Qi Y., Tan S., Gao X., Zhang Z. Biodegradable hollow mesoporous silica nanoparticles for regulating tumor microenvironment and enhancing antitumor efficiency. Theranostics. 2017;7:3276–3292. doi: 10.7150/thno.19987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang T., Zhang J., Hou T., Yin X., Zhang N. Selective targeting of tumor cells and tumor associated macrophages separately by twin-like core-shell nanoparticles for enhanced tumor-localized chemoimmunotherapy. Nanoscale. 2019;11:13934–13946. doi: 10.1039/c9nr03374b. [DOI] [PubMed] [Google Scholar]
- 59.García-Pinel B., Porras-Alcalá C., Ortega-Rodríguez A., Sarabia F., Prados J., Melguizo C., López-Romero J.M. Lipid-based nanoparticles: application and recent advances in cancer treatment. Nanomaterials. 2019;9(4):638. doi: 10.3390/nano9040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 61.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 62.Low L.E., Tan L.T.-H., Goh B.-H., Tey B.T., Ong B.H., Tang S.Y. Magnetic cellulose nanocrystal stabilized Pickering emulsions for enhanced bioactive release and human colon cancer therapy. Int. J. Biol. Macromol. 2019;127:76–84. doi: 10.1016/j.ijbiomac.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 63.Ektate K., Munteanu M.C., Ashar H., Malayer J., Ranjan A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots) Sci. Rep. 2018;8:13062. doi: 10.1038/s41598-018-30106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moghimipour E., Rezaei M., Ramezani Z., Kouchak M., Amini M., Angali K.A., Dorkoosh F.A., Handali S. Folic acid-modified liposomal drug delivery strategy for tumor targeting of 5-fluorouracil. Eur. J. Pharmaceut. Sci. 2018;114:166–174. doi: 10.1016/j.ejps.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Kassem M.A., El-Sawy H.S., Abd-Allah F.I., Abdelghany T.M., El-Say K.M. Maximizing the therapeutic efficacy of imatinib mesylate–loaded niosomes on human colon adenocarcinoma using box-behnken design. J. Pharm. Sci. 2017;106:111–122. doi: 10.1016/j.xphs.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Ding J., Feng M., Wang F., Wang H., Guan W. Targeting effect of PEGylated liposomes modified with the Arg-Gly-Asp sequence on gastric cancer. Oncol. Rep. 2015;34:1825–1834. doi: 10.3892/or.2015.4142. [DOI] [PubMed] [Google Scholar]
- 67.Yang F., Zheng Z., Zheng L., Qin J., Li H., Xue X., Gao J., Fang G. SATB1 siRNA-encapsulated immunoliposomes conjugated with CD44 antibodies target and eliminate gastric cancer-initiating cells. OncoTargets Ther. 2018;11:6811–6825. doi: 10.2147/OTT.S182437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wonder E., Simón-Gracia L., Scodeller P., Majzoub R.N., Kotamraju V.R., Ewert K.K., Teesalu T., Safinya C.R. Competition of charge-mediated and specific binding by peptide-tagged cationic liposome–DNA nanoparticles in vitro and in vivo. Biomaterials. 2018;166:52–63. doi: 10.1016/j.biomaterials.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Zhu R., Sun X., Zhu Y., Liu H., Wang S.-L. Intracellular uptake of etoposide-loaded solid lipid nanoparticles induces an enhancing inhibitory effect on gastric cancer through mitochondria-mediated apoptosis pathway. Int. J. Nanomed. 2014;9:3987–3998. doi: 10.2147/IJN.S64103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li T., Zhang Y., Meng Y.-P., Bo L.-S., Ke W.-B. miR-542-3p appended sorafenib/all-trans retinoic acid (ATRA)-Loaded lipid nanoparticles to enhance the anticancer efficacy in gastric cancers. Pharm. Res. (N. Y.) 2017;34:2710–2719. doi: 10.1007/s11095-017-2202-7. [DOI] [PubMed] [Google Scholar]
- 71.Cao X., Luo J., Gong T., Zhang Z.-R., Sun X., Fu Y. Coencapsulated doxorubicin and bromotetrandrine lipid nanoemulsions in reversing multidrug resistance in breast cancer in vitro and in vivo. Mol. Pharm. 2015;12:274–286. doi: 10.1021/mp500637b. [DOI] [PubMed] [Google Scholar]
- 72.Rocca A., Cecconetto L., Passardi A., Melegari E., Andreis D., Monti M., Maltoni R., Sarti S., Pietri E., Schirone A., et al. Phase Ib dose-finding trial of lapatinib plus pegylated liposomal doxorubicin in advanced HER2-positive breast cancer. Cancer Chemother. Pharmacol. 2017;79:863–871. doi: 10.1007/s00280-017-3279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorusso V., Giotta F., Bordonaro R., Maiello E., Del Prete S., Gebbia V., Filipelli G., Pisconti S., Cinieri S., Romito S., et al. Non-pegylated liposome-encapsulated doxorubicin citrate plus cyclophosphamide or vinorelbine in metastatic breast cancer not previously treated with chemotherapy: a multicenter phase III study. Int. J. Oncol. 2014;45:2137–2142. doi: 10.3892/ijo.2014.2604. [DOI] [PubMed] [Google Scholar]
- 74.Yu D., Li W., Zhang Y., Zhang B. Anti-tumor efficiency of paclitaxel and DNA when co-delivered by pH responsive ligand modified nanocarriers for breast cancer treatment. Biomed. Pharmacother. 2016;83:1428–1435. doi: 10.1016/j.biopha.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 75.Garg N.K., Singh B., Jain A., Nirbhavane P., Sharma R., Tyagi R.K., Kushwah V., Jain S., Katare O.P. Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Colloids Surf. B Biointerfaces. 2016;146:114–126. doi: 10.1016/j.colsurfb.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 76.Ahmad G., El Sadda R., Botchkina G., Ojima I., Egan J., Amiji M. Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth. Cancer Lett. 2017;406:71–80. doi: 10.1016/j.canlet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen B.-H., Tsai Y.J. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016;11:1907. doi: 10.2147/IJN.S103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nassir A.M., Ibrahim I.A.A., Md S., Waris M., Ain M.R., Ahmad I., Shahzad N. Surface functionalized folate targeted oleuropein nano-liposomes for prostate tumor targeting: invitro and invivo activity. Life Sci. 2019;220:136–146. doi: 10.1016/j.lfs.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 79.Hua H., Zhang N., Liu D., Song L., Liu T., Li S., Zhao Y. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo- and chemotherapy. Int. J. Nanomed. 2017;12:7869–7884. doi: 10.2147/IJN.S143977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tekade Rakesh K., Maheshwari Rahul, Tekade Muktika, Chougule Mahavir B. Academic Press; 2017. Solid lipid nanoparticles for targeting and delivery of drugs and genes; pp. 256–286. [Google Scholar]
- 81.Mohanty Chandana. Improvement of cancer therapy by nanotechnology. Virol. Immunol. J. 2017;1 [Google Scholar]
- 82.Tekade Rakesh K., Maheshwari Rahul, Tekade Muktika, Chougule Mahavir B. Academic Press; 2017. Chapter 8 - solid lipid nanoparticles for targeting and delivery of drugs and genes, nanotechnology-based approaches for targeting and delivery of drugs and genes; pp. 256–286. [Google Scholar]
- 83.InProcess-LSP . AZoNano; 2021, September 30. Lipid-based nanoparticles: manufacturing and inline size characterization.https://www.azonano.com/article.aspx?ArticleID=5646 Retrieved on May 19, 2022 from. [Google Scholar]
- 84.Montoto Sebastián Scioli, Giuliana Muraca, Ruiz María, Esperanza Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.587997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.