Abstract
Purpose
The prevalence of type 2 diabetes (T2D) is increasing in Asian populations. White rice is a common staple food in these populations and results from several studies suggest that high white rice consumption increases T2D risk. We assessed whether rice, noodles and bread intake was associated with T2D risk in an ethnic Chinese population.
Methods
We included data from 45,411 male and female Chinese participants of the Singapore Chinese Health Study cohort aged 45–74 years at baseline. Usual diet at baseline was evaluated by a validated 165-item semi-quantitative food frequency questionnaire. Physician-diagnosed T2D was self-reported during two follow-up interviews. Multivariable Cox regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
During a mean follow-up of 11 years, 5207 incident cases of T2D were documented. Rice intake was not associated with higher T2D risk [HR for extreme quintiles, 0.98 (95% CI 0.90, 1.08)] despite the large variation in intake levels (median intake for extreme quintiles: 236.5 g/day vs. 649.3 g/day), although the precise risk estimate depended greatly on the substitute food. Replacing one daily serving of rice with noodles [HR 1.14 (95% CI 1.07, 1.22)], red meat [HR 1.40 (95% CI 1.23, 1.60)] and poultry [HR 1.37 (95% CI 1.18, 1.59)] was associated with higher T2D risk, whereas the replacement of rice with white bread [HR 0.90 (95% CI 0.85, 0.94)] or wholemeal bread [HR 0.82 (95% CI 0.75, 0.90)] was associated with lower T2D risk.
Conclusions
Higher rice consumption was not substantially associated with a higher risk of T2D in this Chinese population. Recommendations to reduce high white rice consumption in Asian populations for the prevention of T2D may only be effective if substitute foods are considered carefully.
Clinical Trial Registry number and website
Keywords: Rice, Noodles, Bread, Type 2 diabetes, Grains, Refined grains
Introduction
Many Asian countries carry a heavy and rising burden of type 2 diabetes mellitus (T2D) [1, 2]. For example, it has been estimated that the South-East Asia region has nearly 78.3 million people with diabetes mellitus [3]. Because T2D is associated with a number of disabling complications and comorbidities, identifying risk factors as a means of prevention and control of the disease is a public health urgency [2–6]. A number of risk factors, including abdominal adiposity and lack of physical activity, are well-established [7, 8]; however, the role of certain foods commonly consumed in Asian populations in the development of T2D remain unclear.
White rice is the main source of carbohydrate in many Asian populations [9]. While brown rice has outer bran and germ portions containing fiber, vitamins and minerals that may confer protective effects against T2D [10], these have been removed in white rice. In addition, white rice generally has a high glycemic index (GI) and as it is consumed as a staple in Asian populations, it can be the main contributor of glycemic load (GL) in these populations [11–13]. Although the evidence is not entirely consistent [14], intakes of high GI foods and high GL diets have been associated with a higher incidence of T2D in several cohort studies [15]. Additionally, high intakes of refined grains, including white rice, have been associated with a higher risk of T2D in several [16–20], but not all [17, 19–21] cohort studies. In a meta-analysis of six prospective cohort studies, higher white rice consumption was associated with a higher risk of T2D in a dose-dependent manner [22]. However, this meta-analysis included only two prospective studies conducted in Asia, where white rice consumption is high [18, 20], while the other four studies included in the analysis were conducted in the U.S [17] and Australia [21], with relatively low levels of rice consumption.
In addition, the health impact of reducing rice consumption is likely to depend on the food that it is replaced with, and previous analyses did not conduct substitution analysis to study the effect of replacing rice with other food items in the Asian diet. To address this gap of knowledge, we prospectively examined the association between rice intake and risk of T2D in a large cohort of Chinese in Singapore. We also examined consumption of noodles, white and wholemeal bread, meats, soy and fruits as alternatives for rice, in relation to risk of T2D.
Methods
Study population
The Singapore Chinese Health Study is a population-based, prospective cohort for long-term study of dietary, genetic and environmental determinants of cancer and other chronic diseases in Singapore. It enrolled 63,257 ethnic Chinese men (n = 27,959) and women (n = 35,298) aged 45–74 years between 1993 and 1998 and who resided in government-built housing estates, where 86% of the Singapore population resided during the enrollment period. A trained interviewer elicited information on demographics, medical history and lifestyle factors from each consenting participant with the use of a structured questionnaire during home visits. The first and second rounds of follow-up interviews were conducted by telephone during the periods of 1999–2004 and 2006–2010, respectively. The design of the study has been described in detail previously [23]. All participants gave informed consent and the institutional review boards at the National University of Singapore and the University of Pittsburgh approved the study.
Dietary assessment
Before we embarked on recruitment for this cohort, we had first conducted a pilot study among 200 representative adults to obtain 24-h dietary recalls for the development of a structured food frequency questionnaire. Habitual diet over the past one year was then assessed using this interviewer-administered semi-quantitative food frequency questionnaire that contained 165 commonly consumed food and beverage items identified from the pilot study. Respondents were asked to report intakes from eight different frequencies (ranging from never or hardly ever to ≥ 2 times per day) as well as information on serving size (generally three options). For food items that could be easily counted, such as white bread and wholemeal bread, participants were asked to pick from three options for usual serving size (≤ 1, 2 or ≥ 3 slices). For other food items, options were based on colored photographs of food items on same-sized plates with portions representing the 15th, 50th and 85th percentiles of the range of portion sizes obtained from the pilot study data. Total rice intake was computed from the intake of eight separate rice-containing food dishes that included plain rice or porridge, flavored porridge, fried rice, coconut rice dishes, curry rice dishes, chicken rice and other types of flavored rice. White rice was the predominant form of rice consumed in Singapore during the period the food frequency questionnaire was administered. Total noodle intake was computed from the intake of five noodle items including fried vegetarian noodles, other fried noodles, soupy noodles, noodles with gravy and dry noodles.
The Singapore Food Composition Table was developed in conjunction with this cohort [24]. The intake of energy and nutrients calculated based on the food frequency questionnaire was subsequently validated against a series of 24-h dietary recalls among a randomly chosen subcohort of 810 participants [24]. Correlation coefficients for carbohydrate intake (percentage of energy) between the FFQ and 24-h recall surveys were 0.43 and 0.51 for Cantonese and Hokkien men, respectively, and the corresponding figures in women were 0.48 and 0.46 [24]. These results are similar to the accuracy reported for the assessment of carbohydrate intake in other Asian cohorts [20, 25]. In addition, the difference in mean nutrient intake measured by the food frequency questionnaire and 24-h recall for carbohydrates was < 10%, which suggested a good coverage of foods containing carbohydrate by the food frequency questionnaire.
Covariate assessment
The interviewer-administered questionnaire also collected data on other covariates including cigarette smoking, alcohol intake, education level, medical history, physical activity, and height and weight. BMI was calculated by taking the weight (kg) of a participant divided by the square of his height (m2). For those with missing weight and/or height (10,711), BMI was calculated using imputed weight and/or height derived from the linear regression equation: Weight = y-intercept + gradient X height, where values for the y-intercept and gradient were derived from gender-specific weight–height regression lines obtained from all cohort participants with known heights and weights. This method of imputed BMI was reported in detail previously [26]. The respondents were also asked about their engagement in physical activities of moderate and vigorous activities. Alcohol consumption was assessed from the intakes of beer, rice wine, other wines and hard liquor.
T2D assessment
Incidence of T2D was assessed by asking participants the following question, “Have you been told by a doctor that you have diabetes (high blood sugar)?” If participants answered yes, we asked the question “Please also tell me the age at which you were first diagnosed?” Cases were identified if they reported developing physician-diagnosed diabetes at any time between the baseline interview and the first (1994–2004) or second (2006–2010) follow-up telephone interviews.
We assessed the validity of using self-reported information on physician-diagnosed diabetes in this population among a subcohort (n = 1631) that reported incident diabetes at the first follow-up interview. The details of the validation have been published [27]. Briefly, we used two different methods to verify self-reported diabetes in a separate study of 1651 cohort subjects who self-reported a history of physician-diagnosed T2D at follow-up I: (1) linkage with a nationwide hospital-based discharge database; and (2) administration of a supplementary questionnaire about symptoms, diagnostic tests and treatment, and validated the accuracy (at 98.8%) of the self-reported diabetes in this cohort [27]. In addition, we measured glycated hemoglobin (HbA1c) in 2,625 randomly selected participants who reported having no diabetes. We calculated the negative predictive value to be 94% using the HbA1c diagnostic cutoff of ≥ 6.5% [27, 28].
Statistical analyses
We excluded participants without data for both follow-up interviews, due to non-response or death (n = 8916). In addition, we excluded participants who had self-reported physician-diagnosed diabetes (n = 5360), cancer (n = 1309), or heart disease (n = 1684) at baseline, and individuals who had implausible daily energy intakes (< 700 kcal or > 3700 kcal for men; < 600 kcal or > 3000 kcal for women) (n = 841). The participants may be excluded for one or more reasons. This resulted in a final number of 45,411 participants for the current analysis.
We computed the person-years for each participant from the year of recruitment to the year of reported T2D diagnosis or the year of the last completed follow-up interview for individuals who did not report diabetes diagnosis, whichever came first. All dietary variables were adjusted for total energy intake using the residual method [29] with the exception of white bread and wholemeal bread that were expressed as g/2000 kcal. Because a large proportion of participants did not consume bread and white (r = 0.18 with total energy intake) and wholemeal bread (r = 0.06) were only weakly correlated with total energy intake, the energy density method was used to avoid having a large number of participants with negative intake values due to the nature of the residual method. For the same reason, the analysis on rice and noodle subtypes also used intakes adjusted with the density method. Participants were then categorized into quintiles by their intakes of rice, noodles, total bread, carbohydrate and starch.
Distributions of demographic, lifestyle behaviors and dietary characteristics were compared across quintiles of rice and noodles intake for men and women separately. We also used partial correlations, adjusted for total energy intake, to assess the correlation between rice and noodle intakes, and major food groups and nutrients.
The Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for risk of T2D across quintile intakes of major sources of carbohydrate (rice, noodles, bread) as well as carbohydrate and starch intake, with the lowest quintile or category as the referent group. To examine linear trends, median values of the quintiles of each dietary component were entered as a continuous variable in the Cox proportional hazards model. We also analyzed the HRs and 95% CIs associated with a daily serving increment of rice, noodles or bread, or a 5% increase in energy contributed by carbohydrate or starch.
We considered age at recruitment (years) and total energy intake (kcal/day) as potential confounding variables in our basic age/energy-adjusted model. We additionally considered the following variables in our lifestyle-adjusted model: dialect group (Hokkien or Cantonese), year of interview (1993–1995, 1996–1998), cigarette smoking (never-smoker, former-smoker, current smoker), alcohol consumption (never, monthly, weekly, daily drinkers), education level (none, primary, secondary and above), physical activity (none, 0.5 to 4 h/week, ≥ 4 h/week), BMI (kg/m2) and self-reported history of physician-diagnosed hypertension (yes or no). White rice is consumed as a staple in Asian populations; thus, for a given calorie intake, participants who had higher rice intake consumed less of other foods. As it is difficult to compartmentalize completely different foods due to this high degree of intercorrelation, we also presented a model that adjusted for dietary pattern rather than individual foods in relation to T2D risk [30]. We chose the Alternative Healthy Eating Index (AHEI)-2010 that had previously been calculated for this study population [31] and was predictive of T2D risk [32]. We also performed sensitivity analysis on the associations of T2D risk with different types of rice (plain rice, plain porridge, flavored rice and flavored porridge) and noodles (dry, soupy, fried) using the AHEI-adjusted model.
There was no evidence for the violation of the proportional hazards assumption using Schoenfeld residuals (Ps > 0.050). We tested for heterogeneity in the associations among selected factors, including age groups (median age: ≥ 54 and < 54 years), sex and overweight status (using the Asian criteria of ≥ 23 or < 23 kg/m2), by including an product term for the interaction effect between one of these selected factors and median value of the quintile intake of rice or other dietary factors in the AHEI-adjusted model. We investigated the effect of substituting a serving of rice for other foods by including intakes of rice and the substitution food as continuous variables in the lifestyle-adjusted model, using a published method [33, 34]. The full AHEI-adjusted model was not used for this analysis because some of the substitute foods tested were components of the index [31]. The difference in their coefficients plus their covariance was used to estimate the HR and 95% CI for the substitution [33, 34]. One serving was defined as 100 g for rice or noodles, 90 g for red meat, fish or poultry, 130 g for fruit, 170 g for soy and two slices (equivalent to 60 g) for bread based on the serving size guide developed by the Singapore Health Promotion Board. We also modeled rice intake using a restricted cubic spline with 4 knots to evaluate the shape of the association based on the full (AHEI-adjusted) Cox regression model for men and women combined. We used the median intake of the first quintile (corresponding to the lowest risk) as the reference level. A P value for nonlinearity was calculated by testing the null hypothesis that the regression coefficients of the second, third and fourth spline transformations are jointly equal to zero [35].
STATA Software version 14 was used for all statistical analyses and 2-sided P values < 0.050 were considered statistically significant.
Results
During 494,741 person-years of follow-up (average of 11 years of follow-up), we observed 5207 cases of incident T2D, including 2195 cases in men (105 cases/10,000 person-years) and 3012 cases in women (106 cases/10,000 person-years). Rice consumption on average contributed to 40.7% of carbohydrate intake in our study population as compared with 8.2% for noodles and 7.2% for bread. This cohort also had a large variation in rice consumption; median of 649.3 g/day of rice in the highest quintile compared with 236.5 g/day in the bottom quintile. Men and women who had higher rice intake tended to be older and less educated, they were also more likely to be smokers and they consumed less alcohol at baseline (Table 1). They also generally consumed less of all other main food items including noodles, red meat, poultry, fish, soy, vegetables and fruits. Higher rice consumption was correlated with higher intakes of carbohydrate and starch, and lower intakes of protein, fat, fiber, calcium, magnesium and iron (Supplemental Table 1). Associations between noodle consumption and lifestyle and dietary factors are shown in Supplemental Tables 1 and 2.
Table 1.
Baseline characteristics according to quintiles of rice intake in men and women
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Quintile 1 (low) | Quintile 3 | Quintile 5 (high) | Quintile 1 (low) | Quintile 3 | Quintile 5 (high) | |
| n | 3244 | 3585 | 5713 | 5839 | 5497 | 3369 |
| Median rice intake (g/day) | 234.0 | 404.0 | 683.2 | 238.1 | 402.7 | 570.2 |
| Age at interview (year) | 54.3 ± 7.3 | 55.5 ± 7.6 | 55.5 ± 7.5 | 52.9 ± 6.9 | 55.5 ± 7.7 | 56.9 ± 7.9 |
| Cantonese [n (%)] | 1527 (47.1) | 1575 (44.0) | 2872 (50.3) | 2987 (51.2) | 2421 (44.0) | 1713 (50.9) |
| Higher education level [n (%)]a | 1728 (53.3) | 1483 (41.4) | 1881 (32.9) | 2117 (36.3) | 1031 (18.8) | 408 (12.1) |
| Current smokers [n (%)] | 1099 (33.9) | 1247 (34.8) | 2141 (37.5) | 220 (3.8) | 342 (6.2) | 254 (7.5) |
| Alcohol consumption [n (%)]b | 1060 (32.7) | 742 (20.7) | 1052 (18.4) | 414 (7.1) | 235 (4.3) | 123 (3.7) |
| Higher physical activity [n (%)]c | 1573 (48.5) | 1590 (44.4) | 2588 (45.3) | 1824 (31.2) | 1288 (23.4) | 731 (21.7) |
| Body mass index (kg/m2) | 23.1 ± 3.3 | 22.8 ± 3.1 | 23.0 ± 3.1 | 23.1 ± 3.4 | 23.1 ± 3.2 | 23.1 ± 3.4 |
| History of hypertension [n (%)] | 612 (18.9) | 698 (19.5) | 1041 (18.2) | 1082 (18.5) | 1086 (19.8) | 654 (19.4) |
| Energy intake (kcal) | 2004.0 ± 578.8 | 1601.2 ± 441.3 | 1986.1 ± 581.4 | 1630.8 ± 448.2 | 1322.5 ± 416.7 | 1484.8 ± 538.0 |
| Food intake (g/day) | ||||||
| Noodles | 71.9 ± 54.9 | 54.0 ± 38.0 | 36.6 ± 38.1 | 69.7 ± 48.1 | 53.3 ± 32.1 | 39.5 ± 29.8 |
| Bread | 38.7 ± 30.3 | 34.3 ± 24.1 | 24.2 ± 24.3 | 38.6 ± 25.4 | 33.9 ± 20.7 | 24.3 ± 19.7 |
| White bread | 31.9 ± 32.3 | 34.0 ± 35.2 | 25.3 ± 27.9 | 35.4 ± 34.3 | 38.9 ± 38.9 | 27.7 ± 30.9 |
| Wholemeal bread | 9.0 ± 22.2 | 8.1 ± 22.2 | 4.2 ± 14.0 | 11.8 ± 25.0 | 8.5 ± 22.6 | 4.3 ± 14.7 |
| Red meat | 37.0 ± 25.9 | 33.1 ± 18.3 | 22.4 ± 19.6 | 32.6 ± 19.9 | 30.7 ± 14.5 | 22.9 ± 14.7 |
| Poultry | 24.6 ± 22.3 | 21.5 ± 15.5 | 14.6 ± 16.6 | 23.9 ± 18.5 | 21.2 ± 13.2 | 15.8 ± 12.9 |
| Fish | 63.2 ± 33.7 | 56.6 ± 25.4 | 41.9 ± 26.3 | 65.1 ± 30.5 | 56.1 ± 21.4 | 43.7 ± 21.4 |
| Soy | 140.3 ± 113.9 | 104.6 ± 70.7 | 65.8 ± 71.8 | 158.3 ± 103.9 | 115.1 ± 61.9 | 80.3 ± 55.0 |
| Vegetables | 124.8 ± 71.3 | 107.5 ± 48.5 | 78.4 ± 47.9 | 147.8 ± 65.4 | 115.7 ± 43.0 | 90.2 ± 39.6 |
| Fruits | 260.1 ± 214.5 | 203.7 ± 143.4 | 128.4 ± 133.9 | 291.6 ± 193.1 | 204.1 ± 121.1 | 135.1 ± 101.5 |
Mean ± SD (all such values)
Secondary school and above
At least once weekly
At least 0.5 h per week of moderate or strenuous physical activity
Rice intake was not associated with T2D risk in the age-adjusted, lifestyle-adjusted or AHEI-adjusted models in either men or women. There was no evidence of gender heterogeneity (Table 2). Each daily serving increment of rice (100 g) was not associated with T2D risk. Restricted cubic analysis confirmed a null association between the wide range of rice intake and diabetes risk (P for nonlinearity = 0.514) (Fig. 1). We also evaluated, but did not detect any statistically significant association between specific type of rice (plain rice, plain porridge, flavored rice, and flavored porridge) and risk of T2D (results not shown).
Table 2.
HRs (95% CIs) of type 2 diabetes according to quintiles of rice, noodles and bread intake
| Quintiles of intake |
P-trend | Per serving increment (d) | |||||
|---|---|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 | 5 (high) | |||
| Rice | |||||||
| Men (cases/person-years) | 380/34,299 | 341/34,389 | 399/38,898 | 416/39,631 | 659/62,031 | – | – |
| Median intake (g/d) | 234.0 | 334.1 | 404.0 | 473.8 | 683.2 | – | – |
| Model 1 (age/energy-adjusted HR) | 1.00 | 0.90 (0.78, 1.05) | 0.94 (0.81, 1.08) | 0.96 (0.83, 1.11) | 0.96 (0.84, 1.08) | 0.898 | 1.00 (0.98, 1.02) |
| Model 2 (lifestyle-adjusted HR)a | 1.00 | 0.92 (0.79, 1.07) | 0.97 (0.84, 1.12) | 1.00 (0.87, 1.16) | 0.96 (0.84, 1.09) | 0.828 | 1.00 (0.97, 1.02) |
| Model 3 (AHEI-adjusted HR)b | 1.00 | 0.92 (0.79, 1.06) | 0.96 (0.83, 1.11) | 0.99 (0.86, 1.15) | 0.94 (0.83, 1.07) | 0.589 | 0.99 (0.97, 1.02) |
| Women (cases/person-years) | 620/63,109 | 697/65,007 | 641/60,658 | 616/59,598 | 438/37,121 | – | – |
| Median intake (g/d) | 238.1 | 333.7 | 402.7 | 474.4 | 570.2 | – | – |
| Model 1 (age/energy-adjusted HR) | 1.00 | 1.07 (0.96, 1.19) | 1.04 (0.93, 1.17) | 1.01 (0.90, 1.13) | 1.14 (1.01, 1.29) | 0.110 | 1.01 (0.98, 1.04) |
| Model 2 (lifestyle-adjusted HR) | 1.00 | 1.07 (0.96, 1.20) | 1.05 (0.93, 1.17) | 1.01 (0.90, 1.14) | 1.11 (0.98, 1.26) | 0.236 | 1.00 (0.97, 1.03) |
| Model 3 (AHEI-adjusted HR) | 1.00 | 1.05 (0.94, 1.18) | 1.02 (0.91, 1.14) | 0.97 (0.86, 1.09) | 1.03 (0.90, 1.17) | 0.903 | 0.98 (0.96, 1.01) |
| Men and women combinedc | |||||||
| Model 3 (AHEI-adjusted HR) | 1.00 | 1.00 (0.92, 1.10) | 1.00 (0.91, 1.09) | 0.98 (0.90, 1.08) | 0.98 (0.90, 1.08) | 0.608 | 0.99 (0.97, 1.01) |
| Noodles | |||||||
| Men (cases/person-years) | 527/53,840 | 385/38,930 | 396/36,483 | 411/38,197 | 476/41,798 | – | – |
| Median intake (g/day) | 10.5 | 31.5 | 46.8 | 66.1 | 108.2 | – | – |
| Model 1 (age/energy-adjusted HR) | 1.00 | 1.03 (0.90, 1.17) | 1.13 (0.99, 1.29) | 1.12 (0.98, 1.27) | 1.17 (1.03, 1.32) | 0.009 | 1.14 (1.04, 1.26) |
| Model 2 (lifestyle-adjusted HR) | 1.00 | 1.02 (0.90, 1.17) | 1.10 (0.96, 1.26) | 1.07 (0.94, 1.22) | 1.07 (0.94, 1.21) | 0.306 | 1.05 (0.95, 1.16) |
| Model 3 (AHEI-adjusted HR) | 1.00 | 1.02 (0.89, 1.16) | 1.09 (0.95, 1.25) | 1.06 (0.93, 1.21) | 1.05 (0.93, 1.20) | 0.405 | 1.04 (0.94, 1.15) |
| Women (cases/person-years) | 427/46,135 | 593/61,686 | 632/62,613 | 675/60,336 | 685/54,723 | – | – |
| Median intake (g/d) | 14.8 | 31.9 | 46.6 | 65.7 | 106.9 | – | – |
| Model 1 (age/energy-adjusted HR) | 1.00 | 1.03 (0.91, 1.17) | 1.10 (0.97, 1.25) | 1.23 (1.09, 1.39) | 1.39 (1.23, 1.57) | < 0.001 | 1.38 (1.26, 1.51) |
| Model 2 (lifestyle-adjusted HR) | 1.00 | 0.97 (0.85, 1.10) | 1.03 (0.91, 1.17) | 1.13 (1.00, 1.28) | 1.20 (1.06, 1.35) | < 0.001 | 1.22 (1.12, 1.34) |
| Model 3 (AHEI-adjusted HR) | 1.00 | 0.96 (0.84, 1.09) | 1.01 (0.89, 1.15) | 1.11 (0.98, 1.26) | 1.16 (1.02, 1.31) | < 0.001 | 1.19 (1.09, 1.30) |
| Men and women combined | |||||||
| Model 3 (AHEI-adjusted HR) | 1.00 | 0.98 (0.89, 1.07) | 1.04 (0.95, 1.14) | 1.09 (1.00, 1.19) | 1.11 (1.02, 1.22) | 0.001 | 1.12 (1.05, 1.20) |
| Bread | |||||||
| Men (cases/person-years) | 590/51,749 | 432/38,605 | 396/36,361 | 378/40,537 | 399/41,996 | – | – |
| Median intake (g/d) | 4.6 | 17.7 | 29.1 | 45.2 | 64.3 | – | – |
| Model 1 (age/energy-adjusted HR) | 1.00 | 1.00 (0.88, 1.13) | 0.97 (0.85, 1.10) | 0.82 (0.72, 0.94) | 0.84 (0.74, 0.95) | < 0.001 | 0.81 (0.73, 0.89) |
| Model 2 (lifestyle-adjusted HR) | 1.00 | 1.05 (0.92, 1.19) | 1.02 (0.89, 1.16) | 0.87 (0.76, 0.99) | 0.89 (0.78, 1.01) | 0.006 | 0.85 (0.77, 0.94) |
| Model 3 (AHEI-adjusted HR) | 1.00 | 1.05 (0.92, 1.19) | 1.02 (0.90, 1.16) | 0.88 (0.77, 1.01) | 0.90 (0.79, 1.02) | 0.013 | 0.86 (0.78, 0.95) |
| Women (cases/person-years) | 574/47,240 | 705/59,647 | 635/62,566 | 550/58,174 | 548/57,866 | – | – |
| Median intake (g/day) | 7.6 | 17.7 | 29.0 | 45.1 | 60.7 | – | – |
| Model 1 (age/energy-adjusted HR) | 1.00 | 0.98 (0.88, 1.10) | 0.84 (0.75, 0.94) | 0.79 (0.70, 0.88) | 0.78 (0.69, 0.88) | < 0.001 | 0.74 (0.67, 0.82) |
| Model 2 (lifestyle-adjusted HR) | 1.00 | 1.00 (0.89, 1.12) | 0.88 (0.78, 0.98) | 0.83 (0.74, 0.93) | 0.83 (0.74, 0.93) | < 0.001 | 0.79 (0.71, 0.87) |
| Model 3 (AHEI-adjusted HR) | 1.00 | 1.01 (0.91, 1.14) | 0.90 (0.80, 1.01) | 0.86 (0.76, 0.97) | 0.86 (0.77, 0.97) | 0.001 | 0.82 (0.74, 0.90) |
| Men and women combined | |||||||
| Model 3 (AHEI-adjusted HR) | 1.00 | 1.04 (0.95, 1.13) | 0.95 (0.87, 1.04) | 0.88 (0.80, 0.96) | 0.89 (0.81, 0.97) | < 0.001 | 0.84 (0.78, 0.90) |
| White bread | |||||||
| Men and women combined | |||||||
| Model 3 (AHEI-adjusted HR) | – | – | – | – | – | – | 0.90 (0.86, 0.95) |
| Wholemeal bread | |||||||
| Men and women combined | |||||||
| Model 3 (AHEI-adjusted HR) | – | – | – | – | – | – | 0.86 (0.78, 0.94) |
Estimates are hazard ratios (95% confidence intervals)
Lifestyle-adjusted model additionally controlled for father’s dialect, year of interview, cigarette smoking, alcohol consumption, education level, physical activity, body mass index and history of hypertension
In addition to model 2 with the exception of alcohol consumption, adjusted for Alternative Healthy Eating Index—2010 and sex (the latter only in the model with males and females combined) (31). We also mutually adjusted for white bread and wholemeal bread in the analysis involving white and wholemeal bread
P-interaction for sex in model 3, respectively, were as follows: rice 0.316; noodles 0.065; bread 0.567; white bread 0.765; wholemeal bread 0.609
Fig. 1.
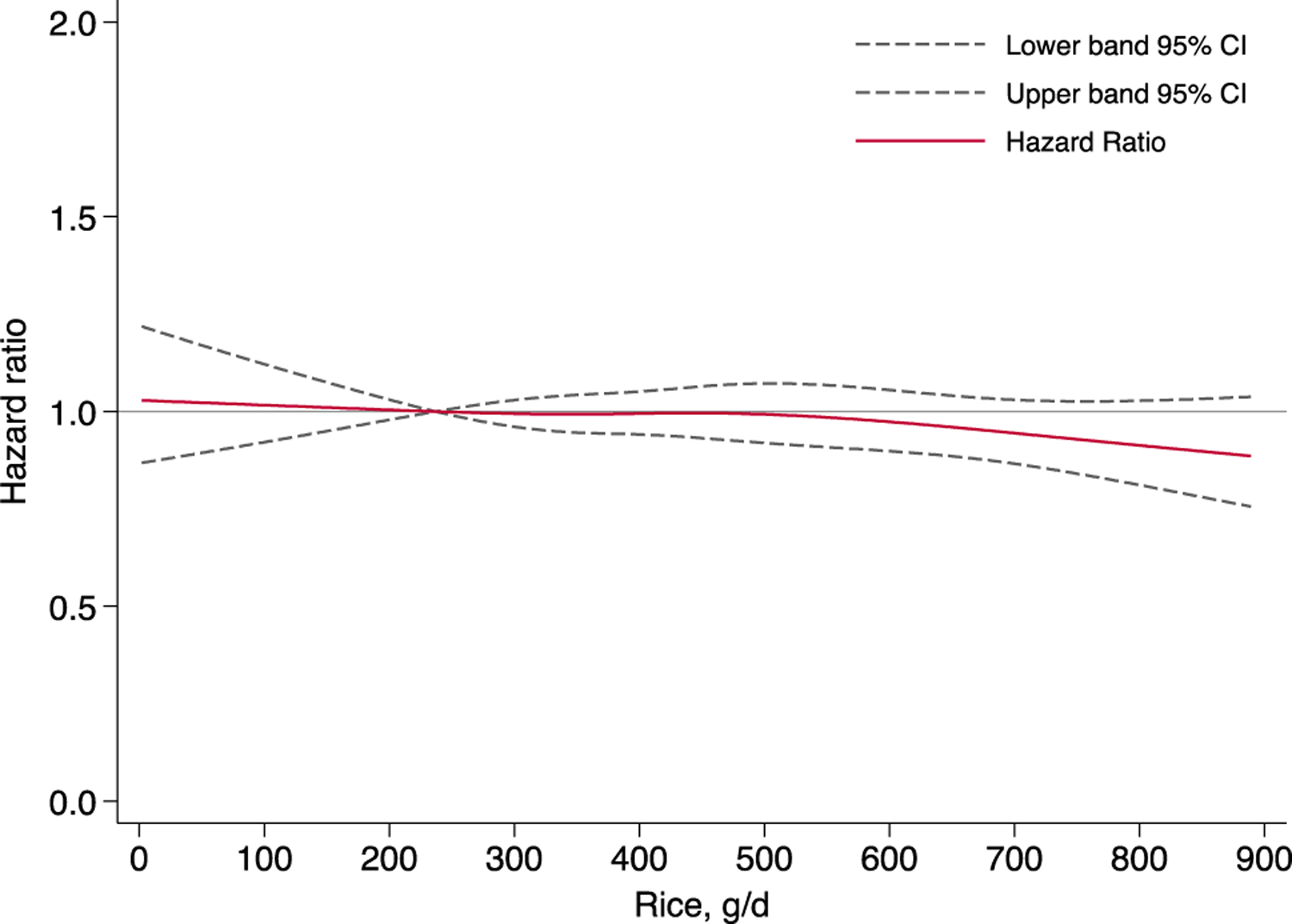
Dose–response relationship between rice intake and the risk of type 2 diabetes in men and women combined using restricted cubic analysis (P-interaction for sex = 0.316; P for nonlinearity = 0.514). Data were adjusted for potential confounders in Model 3 of Table 2
Noodle consumption was significantly associated with risk of T2D in both men and women after adjustment for age only (Table 2). Further adjustment for lifestyle factors and the AHEI-2010 index weakened the associations, although they remained statistically significant. Each daily serving increment of noodle intake was associated with 12.2% (95% CI 5.0%, 19.8%) higher risk of T2D. When we stratified the analysis by sex, associations between noodle consumption and risk of T2D appeared to be stronger in women [per serving increment: HR 1.19 (95% CI 1.09, 1.30) P-interaction with sex = 0.065].
The intake of bread in this population was low (median intake: 0.97 slices/day), but daily consumption of bread, regardless white or wholemeal, was associated with a lower T2D risk (Table 2). Adjustment for lifestyle factors and dietary index modestly weakened the associations but the inverse associations remained statistically significant with no suggestion of difference between two sexes. Each daily serving increment (2 slices, equivalent to 60 g) in white bread [men and women combined: HR 0.90 (95% CI 0.86, 0.95)] or wholemeal bread [HR 0.86 (95% CI 0.78, 0.94)] consumption was associated with a statistically significant lower T2D risk.
As it has been postulated that insulin resistant individuals may be particularly susceptible to a high carbohydrate load, we further examined possible effect modifications by over-weight status for the association between the examined foods and risk of T2D, but we did not observe any significant interaction (all P-interactions with overweight status > 0.050). There was also no evidence of effect modification by age.
Because dietary guidelines for the primary prevention of T2D often involve the selection of one food over another, we evaluated the magnitude in the risk change associated with the substitution of one serving of rice for a serving of another type of food (Fig. 2).
Fig. 2.
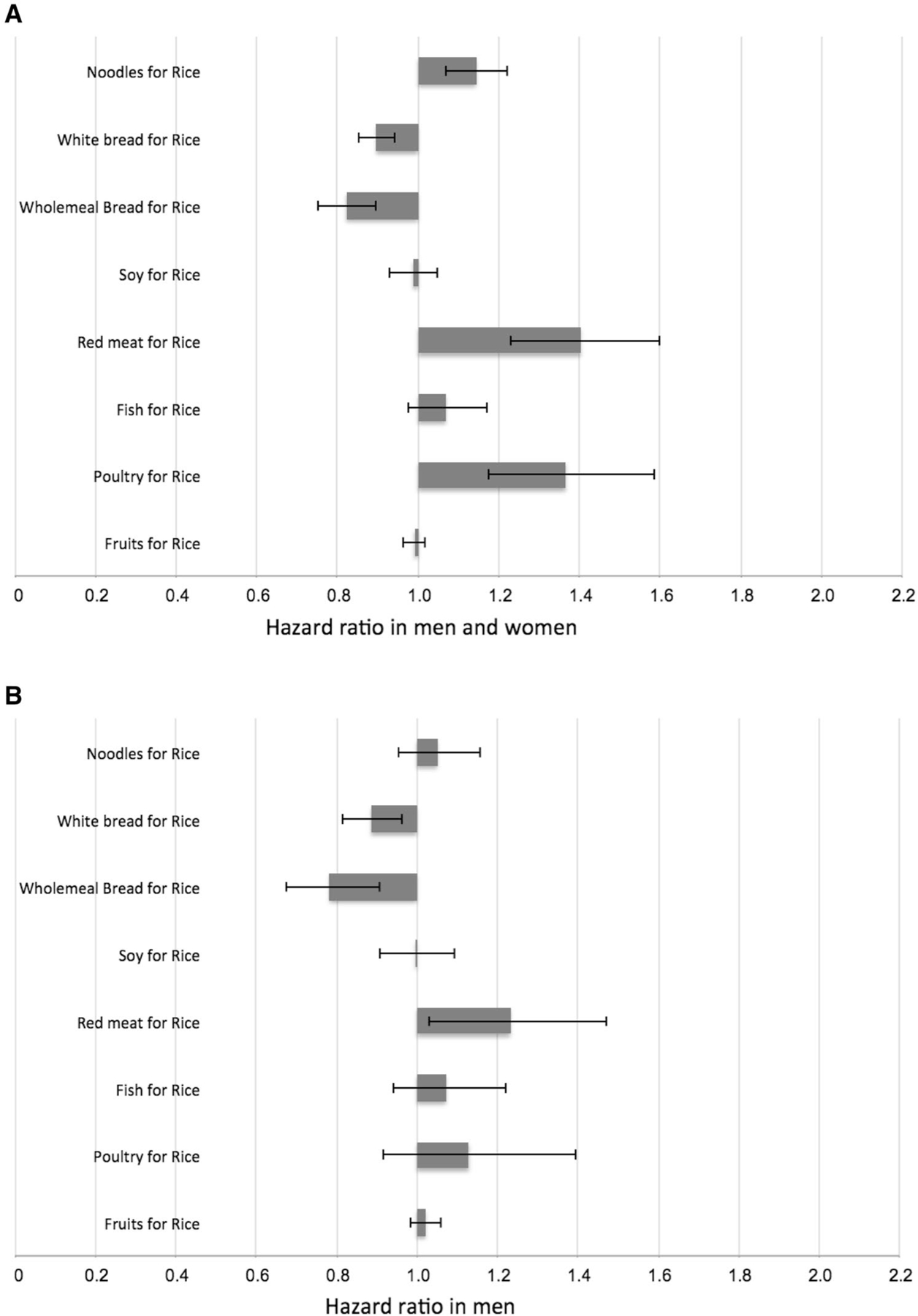
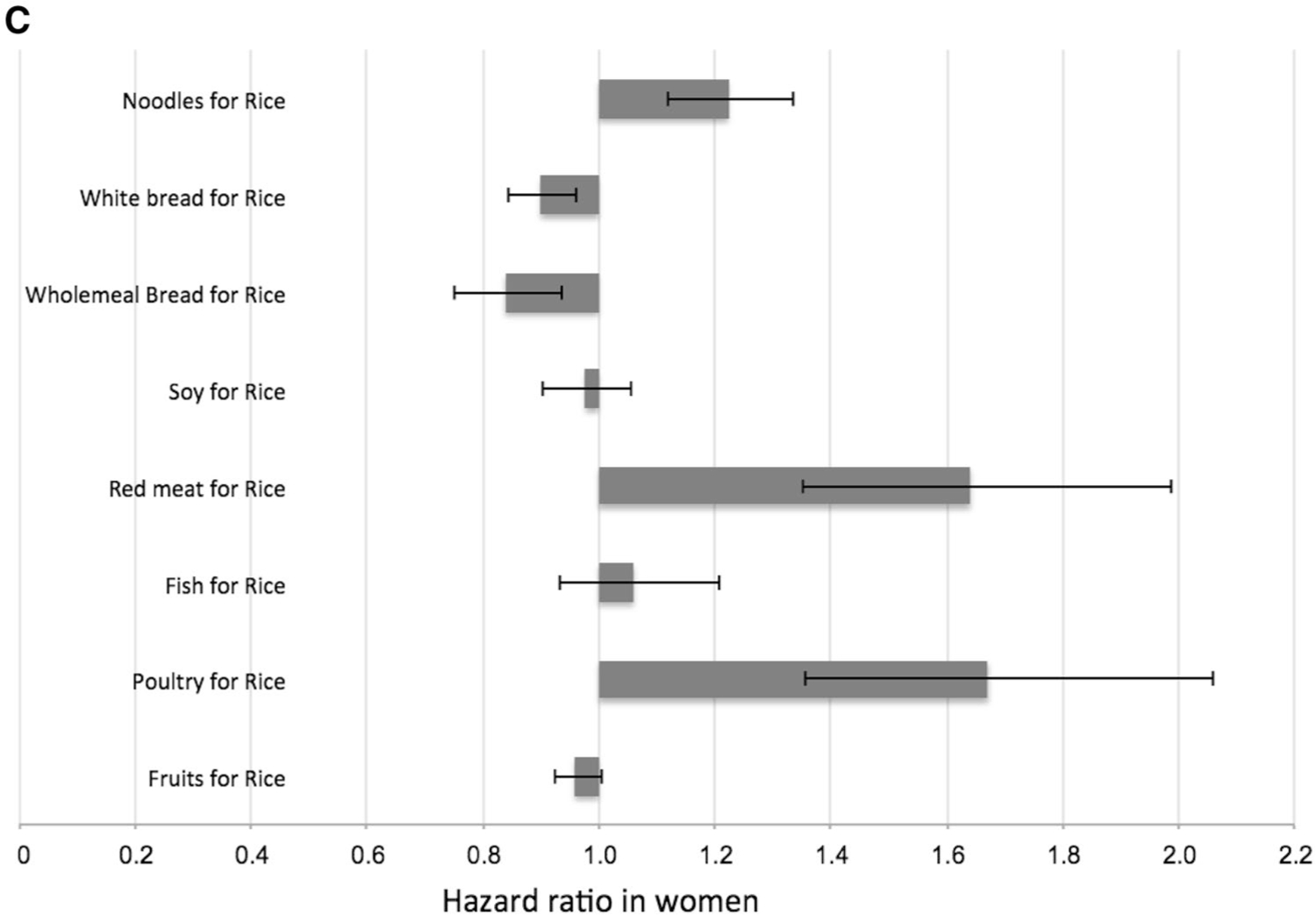
Estimated hazard ratio (95% confidence intervals) associated with the substitution of one serving of rice (100 g) for one serving of noodles (100 g), white bread (60 g), wholemeal bread (60 g), soy (170 g), red meat (90 g), fish (90 g), poultry (90 g) and fruits (130 g). Data are shown for all participants (a; n = 45,411), men (b; n = 19,409) and women (c; n = 26,002). Data were analyzed using the lifestyle-adjusted Cox proportional hazard regression model (see footnotes to Table 2), and substitution effects were estimated using a published method (33, 34)
We showed that the substitution of one daily serving of rice with noodles [HR 1.14 (95% CI 1.07, 1.22)], red meat [HR 1.40 (95% CI 1.23, 1.60)] and poultry [HR 1.37 (95% CI 1.18, 1.59)] was associated with a statistically significant higher risk of T2D, whereas the replacement of rice with white bread [HR 0.90 (95% CI 0.85, 0.94)] or wholemeal bread [HR 0.82 (95% CI 0.75, 0.90)] was associated with a lower risk. When we repeated the analysis separately for men and women, associations tended to be stronger in women than in men for noodles [HR 1.22 (95% CI 1.12, 1.26, P-interaction with sex = 0.056)] and poultry [HR 1.67 (95% CI 1.36, 2.06, P-interaction with sex = 0.091)]. Replacement of rice with soy, fish, or fruit was not significantly associated with T2D risk in either men or women. Substituting one daily serving of noodles with white bread [HR 0.82 (95% CI 0.76, 0.89)] or wholemeal bread [HR 0.76 (95% CI 0.69, 0.85)] was associated with lower T2D risk.
Discussion
Higher rice consumption was not associated with risk of T2D in this Chinese population. However, the precise risk estimate must be interpreted within the context of foods displaced as a result of high rice intake. Additionally, the benefit of replacing rice with other foods depended on how the substitute food could affect T2D risk. For example, replacing a serving of rice with a serving of red meat was associated with higher diabetes risk, whereas consumption of wholemeal bread in place of rice was associated with lower risk. Noodle consumption was associated with a higher risk of T2D in women but not men, while intake of bread was associated with lower risk in both men and women.
Higher white rice consumption was associated with a higher risk of T2D in a cohort of Chinese women in Shanghai and female participants of a Japanese cohort, but not among male participants of the same Japanese cohort [18, 20, 22]. In the Shanghai Women’s Health Study, women who were in the highest category of rice intake (≥ 300 g/day) had 1.78-fold higher risk of diabetes than that of women in the lowest category (< 200 g/day) [18]. However, this study did not consider a multivariable model adjusted for any dietary risk factors, such as red meat or poultry consumption [36, 37], and thus the direct comparison of results across different populations must be treated with caution. Indeed, in a more recent publication involving the Shanghai Women’s and Men’s Health Studies, high intake of total refined grains was not associated with risk of type 2 diabetes after adjusting for other dietary covariates [38]. Higher intake of white rice was also associated with metabolic risk factors in cross-sectional studies conducted in the Japanese [39, 40], Indian [41] and Singapore Chinese [42] populations. Among studies in western populations, white rice consumption was significantly associated with a higher risk of T2D in the Nurses’ Health Study II and the Pizarra Study [17, 43], but not in the Nurses’ Health Study I, the Health Professionals Follow-up Study and the Melbourne Collaborative Cohort Study [17, 21]. The lack of association observed in several cohort studies conducted in Western populations has often been attributed to the low intake of rice compared with Asian populations [17, 22]. However, the lack of association in our study (average rice intake 4.16 serv/d) and in Japanese men (average intake in quartile 4: 7.62 serv/day) [20] with high rice intakes suggests variation in population intake levels does not explain the inconsistent findings. In Iran, the intake of rice was between that of Western and East Asian populations and the authors of two prospective studies reported inconsistent results [44]. No association between rice intake and T2D risk was observed in the Golestan Cohort Study, whereas a 2.28-fold higher risk of T2D for higher rice consumption (> 250 g/d vs. <250 g/d) was observed in the Tehran Lipid and Glucose Study [44]. However, the small sample size and broad categories of rice intake in the latter study limits interpretation of the results. Overall, the association between rice consumption and T2D risk has been inconsistent even within different regions of the world and our results suggest that differences in substitution foods may have contributed to these inconsistencies.
Several biological mechanisms have been suggested for the detrimental effect of high white rice consumption on risk of T2D. First, the refining process of white rice results in a loss of fiber, vitamins, minerals such as magnesium and phytochemicals such as polyphenols that may reduce diabetes risk [10, 45–49]. However, white rice was almost exclusively consumed in Singapore until recently and we did not observe any association between high white rice intake and T2D risk. Second, white rice is a high GI food [11–13] and thus causes high postprandial glucose concentrations. Because white rice was the major contributor to carbohydrate intake in this population, participants with the highest consumption of white rice would also have the highest GL. High GI or GL diets elicit greater demand for insulin [50]. Coupled with insulin resistance determined primarily by other risk factors such as adiposity, this greater insulin demand may lead to hyperglycemia leading to loss of pancreatic β cell function and eventually diabetes [50]. In a meta-analysis using pooled data from cohort studies conducted in Western countries, high GI and high GL diets were associated with a higher risk of T2D [15]. Although this finding was supported by recent studies in Chinese and Japanese populations [18, 39], this was not consistent in all studies [14].
Staple foods such as rice are major contributors to energy intake and a substantially higher rice intake can thus be expected to be associated with substantially lower intakes of other foods. In our study, rice was the main contributor to energy intake (26.2%) and participants with high rice consumption had a different dietary pattern from those with a low rice consumption, exemplified by lower intakes of foods linked with a lower risk of T2D, such as whole grains [19], but also lower intakes of foods associated with a higher risk, such as red meat [36, 37]. Adjustment for AHEI-2010 (indicating adjustment for the overall healthfulness of the diet) did not alter the associations between rice intake and T2D risk, suggesting that although participants with high rice consumption had a less healthy dietary pattern, the T2D risk conferred by high rice intake depended on the foods displaced. Our substitution analysis directly modeled the replacement of rice by other foods, and for example, indicated that replacement of rice with wholemeal bread is associated with a substantially lower risk of T2D. However, experimental studies will be needed to elucidate whether the lower diabetes risk associated with this substitution reflects beneficial components of whole grains or independent properties of white rice.
It has been suggested that the adverse metabolic effects of high GL diets may be greater for insulin resistant individuals [50]. Because adiposity is a major predictor of insulin resistance, we evaluated whether the association between rice consumption and T2D was stronger in overweight as compared with leaner participants [50, 51]. However, in line with the results of a cohort of Chinese women [18], we did not observe a difference in the association between rice intake and risk of T2D by overweight status.
The association between higher noodle consumption and a higher risk of T2D observed in our study agrees with the cross-sectional association between higher noodle intake and insulin resistance and hyperglycemia in a different study in Singapore [42]. In contrast, higher noodle consumption was not substantially associated with risk of T2D in a Japanese cohort [20]. This difference in results may be due to the composition of the noodles and in the way the noodle dishes were prepared. Noodle items for the Japanese study included, for example, buckwheat noodles, which are not commonly consumed in Singapore. In addition, noodle dishes in Singapore are often cooked using palm oil or lard, large amounts of salt and with red meat or poultry, all of which may have adverse effects on risk of T2D [37, 52–54]. The content of food components such as saturated fat, cholesterol, sodium and fiber in noodle dishes, which are similar to Western fast foods, may have contributed to the different associations observed for rice and noodles [55]. Although there was suggestion of some differences in the association between noodle intake and risk of T2D by sex, these differences may have been chance findings. Further studies are required to confirm our findings on noodles.
We observed significant inverse associations between bread intake and risk of T2D, regardless of white or wholemeal. The benefits of consuming whole grains observed in our study were consistent with a recent meta-analysis in which three servings per day of total whole grains was associated with a 32% lower risk and three servings per day of whole grain bread with a 26% lower risk of T2D [19]. In contrast to our study, white bread intake was associated with a higher risk of T2D in an Australian study [21]. The reason for this discrepancy may be that although white bread has a lower fiber and phytochemical content than wholemeal bread, it still contains more fiber than white rice, the dominant starchy food in our population [56]. Alternatively, there may have been some misclassification between wholemeal, white, and intermediate versions of bread in our population resulting in more favorable associations for white bread than exists in reality. When we adjusted for the AHEI-2010 index, the associations were attenuated, suggesting that the beneficial effects observed from habitual bread consumption may be partly due to other foods commonly consumed together with bread [20].
Strengths of our study included the large sample size, the prospective design, the long follow-up period with minimal loss to follow-up and the use of a validated interviewer-administered food frequency questionnaire. Our study was also subject to several potential limitations. Food consumption was self-reported and this will have been prone to some inevitable measurement errors. However, we obtained reasonably good agreement between dietary estimates measured by the food frequency questionnaire and 24-h food recalls, and selected fatty acid biomarkers which was similar to other validation studies conducted in Western and Asian populations [20, 25, 57, 58]. In addition, dietary intakes were recorded only once at baseline which may not have fully reflected long-term intake. Given the prospective design of our study misclassification of dietary exposures is likely to have been non-differential and lead to in an underestimation of the risk estimates observed. As with all observational studies, we cannot rule out the possibility of residual confounding, although we carefully adjusted for known diabetes risk factors. Finally, we urge caution in generalizing our results to other ethnic groups.
In conclusion, our findings highlight that the impact of reducing white rice consumption on risk of T2D may depend on the replacement with other foods such as red meat and poultry. The increasing consumption of red meat with decreasing intake of rice, a dietary pattern shift that is occurring in Asian societies as a result of economic development and globalization, may contribute to the rising incidence of T2D in Asian populations. The results of the present study, if confirmed in clinical trials, are encouraging that the replacement of white rice with whole bread, but not red meat and poultry may substantially reduce incidence of T2D, especially in populations with high consumption of rice.
Supplementary Material
Acknowledgements
We are grateful to Siew-Hong Low of the National University of Singapore for supervising the fieldwork in the Singapore Chinese Health Study and Renwei Wang for the maintenance of the cohort study database. We also thank the founding principal investigator of the Singapore Chinese Health Study, Mimi C. Yu.
Funding
This study was supported by the National Institutes of Health, USA (R01 CA144034 and UM1 CA182876). JYHS is supported by the NGS Scholarship. W-PK is supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013).
Abbreviations
- T2D
Type 2 diabetes mellitus
- HR
Hazard ratio
- CI
Confidence interval
- GI
Glycemic index
- GL
Glycemic load
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00394-018-1879-7) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest All authors have no conflicts of interest.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87(1):4–14. 10.1016/j.diabres.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 2.Chan JCN, Malik V, Jia WP, Kadowaki T, Yajnik CS, Yoon KH, Hu FB (2009) Diabetes in Asia epidemiology, risk factors, and pathophysiology. J Am Med Assoc 301(20):2129–2140 [DOI] [PubMed] [Google Scholar]
- 3.Ogurtsova K, Fernandes J, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE (2017) IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50. 10.1016/j.diabres.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 4.Woodward M, Zhang X, Barzi F, Pan W, Ueshima H, Rodgers A, MacMahon S (2003) The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care 26(2):360–366 [DOI] [PubMed] [Google Scholar]
- 5.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, Investigators D (2006) Prevalence and risk factors for micro-albuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 69(11):2057–2063. 10.1038/sj.ki.5000377 [DOI] [PubMed] [Google Scholar]
- 6.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL (2008) Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus a systematic review and meta-analysis. J Am Med Assoc 300(23):2754–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY (2006) Epidemic obesity and type 2 diabetes in Asia. Lancet 368(9548):1681–1688. 10.1016/s0140-6736(06)69703-1 [DOI] [PubMed] [Google Scholar]
- 8.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J (2007) Active smoking and the risk of type 2 diabetes—a systematic review and meta-analysis. J Am Med Assoc 298(22):2654–2664. 10.1001/jama.298.22.2654 [DOI] [PubMed] [Google Scholar]
- 9.Muthayya S, Sugimoto JD, Montgomery S, Maberly GF (2014) An overview of global rice production, supply, trade, and consumption. Tech Consider Rice Fortificat Public Health 1324:7–14. 10.1111/nyas.12540 [DOI] [PubMed] [Google Scholar]
- 10.Slavin JL, Martini MC, Jacobs DR, Marquart L (1999) Plausible mechanisms for the protectiveness of whole grains. Am J Clin Nutr 70(3):459S–463S [DOI] [PubMed] [Google Scholar]
- 11.Atkinson FS, Foster-Powell K, Brand-Miller JC (2008) International tables of Glycemic Index and glycemic load values: 2008. Diabetes Care 31(12):2281–2283. 10.2337/dc08-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen HN, Rasmussen OW, Rasmussen PH, Alstrup KK, Biswas SK, Tetens I, Thilsted SH, Hermansen K (2000) Glycaemic index of parboiled rice depends on the severity of processing: study in type 2 diabetic subjects. Eur J Clin Nutr 54(5):380–385. 10.1038/sj.ejcn.1600969 [DOI] [PubMed] [Google Scholar]
- 13.Miller JB, Pang E, Bramall L (1992) Rice—a high or low Glycemic Index food. Am J Clin Nutr 56(6):1034–1036 [DOI] [PubMed] [Google Scholar]
- 14.Sluijs I, Beulens JWJ, van der Schouw YT, van der ADL, Buck-land, Kuijsten G, Schulze A, Amiano MB, Ardanaz P, Balkau E, Boeing B, Gavrila H, Grote D, Key VA, Li TJ, Nilsson KR, Overvad P, Palli K, Panico D, Quiros S, Rolandsson JR, Roswall O, Sacerdote N, Sanchez C, Sieri MJ, Slimani S, Spijkerman N, Tjonneland AMW, Tumino A, Sharp R, Langenberg SJ, Feskens C, Forouhi EJM, Riboli NG, Wareham E, InterAct NJ (2013) Dietary Glycemic Index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European Countries. J Nutr 143(1):93–99. 10.3945/jn.112.165605 [DOI] [PubMed] [Google Scholar]
- 15.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC (2008) Glycemic index, glycemic load, and chronic disease risk—a metaanalysis of observational studies. Am J Clin Nutr 87(3):627–637 [DOI] [PubMed] [Google Scholar]
- 16.Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V (2009) Dietary carbohydrates glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59). Br J Nutr 102(10):1498–1506. 10.1017/s0007114509990468 [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB (2010) White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 170(11):961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villegas R, Liu SM, Gao YT, Yang G, Li HL, Zheng W, Shu XO (2007) Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2, diabetes mellitus in middle-aged Chinese women. Arch Intern Med 167(21):2310–2316. 10.1001/archinte.167.21.2310 [DOI] [PubMed] [Google Scholar]
- 19.Aune D, Norat T, Romundstad P, Vatten LJ (2013) Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol 28(11):845–858. 10.1007/s10654-013-9852-5 [DOI] [PubMed] [Google Scholar]
- 20.Nanri A, Mizoue T, Noda M, Takahashi Y, Kato M, Inoue M, Tsugane S (2010) Rice intake and type 2 diabetes in Japanese men and women the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 92(6):1468–1477. 10.3945/ajcn.2010.29512 [DOI] [PubMed] [Google Scholar]
- 21.Hodge AM, English DR, O’Dea K, Giles GG (2004) Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 27(11):2701–2706. 10.2337/diacare.27.11.2701 [DOI] [PubMed] [Google Scholar]
- 22.Hu EA, Pan A, Malik V, Sun Q (2012) White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 344. 10.1136/bmj.e1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan JM, Stram DO, Arakawa K, Lee HP, Yu MC (2003) Dietary cryptoxanthin and reduced risk of lung cancer: The Singapore Chinese health study. Cancer Epidemiol Biomark Prevent 12(9):890–898 [PubMed] [Google Scholar]
- 24.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC (2001) Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer Int J 39(2):187–195. 10.1207/S15327914nc392_5 [DOI] [PubMed] [Google Scholar]
- 25.Eshak ES, Iso H, Date C, Yamagishi K, Kikuchi S, Watanabe Y, Wada Y, Tamakoshi A, Grp JS (2011) Rice intake is associated with reduced risk of mortality from cardiovascular disease in Japanese men but not women. J Nutr 141(4):595–602. 10.3945/jn.110.132167 [DOI] [PubMed] [Google Scholar]
- 26.Koh WP, Yuan JM, Wang R, Lee HP, Yu MC (2010) Body mass index and smoking-related lung cancer risk in the Singapore Chinese Health Study. Br J Cancer 102(3):610–614. 10.1038/sj.bjc.6605496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odegaard AO, Koh WP, Arakawa K, Yu MC, Pereira MA (2010) Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes. Am J Epidemiol 171(6):701–708. 10.1093/aje/kwp452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, Davidson MB, DeFronzo R, Genuth S, Holman RR, Ji L, Kirkman S, Knowler WC, Schatz D, Shaw J, Sobngwi E, Steffes M, Vaccaro O, Wareham N, Zinman B, Kahn R, Int Expert C (2009) International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32(7):1327–1334. 10.2337/dc09-9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65(4):1220–1228 [DOI] [PubMed] [Google Scholar]
- 30.Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13(1):3–9. 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- 31.Neelakantan N, Naidoo N, Koh WP, Yuan JM, van Dam RM (2016) The Alternative Healthy Eating Index is associated with a lower risk of fatal and nonfatal acute myocardial infarction in a Chinese adult population. J Nutr 146(7):1379–1386. 10.3945/jn.116.231605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang ML, Stampfer MJ, Willett WC (2012) Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 142(6):1009–1018. 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulldorff M, Sinha R, Chow WH, Rothman N (2000) Comparing odds ratios for nested subsets of dietary components. Int J Epidemiol 29(6):1060–1064. 10.1093/ije/29.6.1060 [DOI] [PubMed] [Google Scholar]
- 34.Halton TL, Willett WC, Liu SM, Manson JE, Stampfer MJ, Hu FB (2006) Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr 83(2):284–290 [DOI] [PubMed] [Google Scholar]
- 35.Orsini N, Li RF, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175(1):66–73. 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB (2011) Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 94(4):1088–1096. 10.3945/ajcn.111.018978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talaei M, Wang YL, Yuan JM, Pan A, Koh WP (2017) Meat, dietary heme iron, and risk of type 2 diabetes mellitus the Singapore Chinese Health Study. Am J Epidemiol 186(7):824–833. 10.1093/aje/kwx156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu D, Zheng W, Cai H, Xiang YB, Li H, Gao YT, Shu XO (2017) Long-term diet quality and risk of type 2 diabetes among urban Chinese adults. Diabetes Care 10.2337/dc17-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami K, Sasaki S, Takahashi Y, Okubo H, Hosoi Y, Horiguchi H, Oguma E, Kayama F (2006) Dietary glycemic index and load in relation to metabolic risk factors in Japanese female farmers with traditional dietary habits. Am J Clin Nutr 83(5):1161–1169 [DOI] [PubMed] [Google Scholar]
- 40.Nanri A, Mizoue T, Yoshida D, Takahashi R, Takayanagi R (2008) Dietary patterns and A1C in Japanese men and women. Diabetes Care 31(8):1568–1573. 10.2337/dc08-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V (2009) Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metab Clin Exp 58(5):675–681. 10.1016/j.metabol.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 42.Zuniga YLM, Rebello SA, Oi PL, Zheng HL, Lee J, Tai ES, Van Dam RM (2014) Rice and noodle consumption is associated with insulin resistance and hyperglycaemia in an Asian population. Br J Nutr 111(6):1118–1128. 10.1017/s0007114513003486 [DOI] [PubMed] [Google Scholar]
- 43.Soriguer F, Colomo N, Olveira G, Garcia-Fuentes E, Esteva I, de Adana MSR, Morcillo S, Porras N, Valdes S, Rojo-Martinez G (2013) White rice consumption and risk of type 2 diabetes. Clin Nutr 32(3):481–484. 10.1016/j.clnu.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 44.Golozar A, Khalili D, Etemadi A, Poustchi H, Fazeltabar A, Hosseini F, Kamangar F, Khoshnia M, Islami F, Hadaegh F, Brennan P, Boffetta P, Abnet CC, Dawsey SM, Azizi F, Malekzadeh R, Danaei G (2017) White rice intake and incidence of type-2 diabetes: analysis of two prospective cohort studies from Iran. BMC Public Health 10.1186/s12889-016-3999-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC (1997) Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 20(4):545–550. 10.2337/diacare.20.4.545 [DOI] [PubMed] [Google Scholar]
- 46.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC (1997) Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. J Am Med Assoc 277(6):472–477. 10.1001/jama.277.6.472 [DOI] [PubMed] [Google Scholar]
- 47.Meyer KA, Kushi LH, Jacobs DR, Slavin J, Sellers TA, Folsom AR (2000) Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 71(4):921–930 [DOI] [PubMed] [Google Scholar]
- 48.van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR (2006) Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in US black women. Diabetes Care 29(10):2238–2243. 10.2337/dc06-1014 [DOI] [PubMed] [Google Scholar]
- 49.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H (2007) Fiber and magnesium intake and incidence of type 2 diabetes—a prospective study and meta-analysis. Arch Intern Med 167(9):956–965. 10.1001/archinte.167.9.956 [DOI] [PubMed] [Google Scholar]
- 50.Willett W, Manson J, Liu SM (2002) Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr 76(1):274S–280S [DOI] [PubMed] [Google Scholar]
- 51.Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846. 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- 52.Chun YH, Han K, Kim DH, Park YG, Cho KH, Choi YS, Kim SM, Kim YH, Nam GE (2016) Association of urinary sodium excretion with insulin resistance in Korean adolescents results from the Korea National Health and Nutrition Examination Survey 2009–2010. Medicine 10.1097/md.0000000000003447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu G, Jousilahti P, Peltonen M, Lindstrom J, Tuomilehto J (2005) Urinary sodium and potassium excretion and the risk of type 2 diabetes: a prospective study in Finland. Diabetologia 48(8):1477–1483. 10.1007/s00125-005-1824-1 [DOI] [PubMed] [Google Scholar]
- 54.Marshall JA, Bessesen DH, Hamman RF (1997) High saturated fat and low starch and fibre are associated with hyperinsulinaemia in a non-diabetic population: the San Luis Valley Diabetes Study. Diabetologia 40(4):430–438. 10.1007/s001250050697 [DOI] [PubMed] [Google Scholar]
- 55.Rebello SA, Koh H, Chen C, Naidoo N, Odegaard AO, Koh WP, Butler LM, Yuan JM, van Dam RM (2014) Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: a prospective cohort study. Am J Clin Nutr 100(1):53–64. 10.3945/ajcn.113.076273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Energy and Nutrient Composition of Food (2017). https://focos.hpb.gov.sg/eservices/ENCF/. Accessed 8 Apr 2018
- 57.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE (1985) Reproducibility and validity of a Semiquantitative Food Frequency Questionnaire. Am J Epidemiol 122(1):51–65 [DOI] [PubMed] [Google Scholar]
- 58.Seah JYH, Gay GMW, Su J, Tai ES, Yuan JM, Koh WP, Ong CN, van Dam RM (2017) Consumption of red meat, but not cooking oils high in polyunsaturated fat, is associated with higher arachidonic acid status in Singapore Chinese adults. Nutrients 10.3390/nu9020101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


