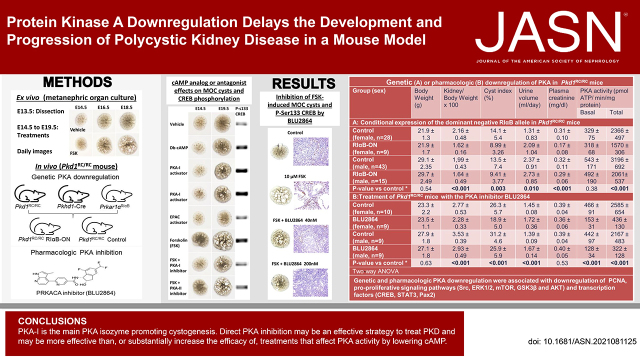
Significance Statement
The only treatment approved for PKD inhibits production of cAMP, the main PKA activator. It is only partially effective, likely because side effects restrict dosing and because other sources of cAMP and mechanisms of cAMP-independent PKA activation exist. Which PKA isozyme(s) promotes PKD is uncertain and selective PKA inhibitors usable in vivo have not been available. Experiments in a mouse model show PKA-I is the main PKA isozyme promoting cystogenesis and that constitutive PKA-I downregulation and a novel, highly selective PKA inhibitor ameliorate PKD. The dose of PKA inhibitor used had no detectable adverse effects. This information provides a strong rationale for a strategy that may be more effective, or substantially increase the efficacy of the currently approved treatment.
Keywords: polycystic kidney disease, ADPKD, cyclic AMP, cell signaling, protein kinase A
Visual Abstract
Abstract
Background
Upregulation of cAMP-dependent and cAMP-independent PKA signaling is thought to promote cystogenesis in polycystic kidney disease (PKD). PKA-I regulatory subunit RIα is increased in kidneys of orthologous mouse models. Kidney-specific knockout of RIα upregulates PKA activity, induces cystic disease in wild-type mice, and aggravates it in Pkd1RC/RC mice.
Methods
PKA-I activation or inhibition was compared with EPAC activation or PKA-II inhibition using Pkd1RC/RC metanephric organ cultures. The effect of constitutive PKA (preferentially PKA-I) downregulation in vivo was ascertained by kidney-specific expression of a dominant negative RIαB allele in Pkd1RC/RC mice obtained by crossing Prkar1αR1αB/WT, Pkd1RC/RC, and Pkhd1-Cre mice (C57BL/6 background). The effect of pharmacologic PKA inhibition using a novel, selective PRKACA inhibitor (BLU2864) was tested in mIMCD3 3D cultures, metanephric organ cultures, and Pkd1RC/RC mice on a C57BL/6 × 129S6/Sv F1 background. Mice were sacrificed at 16 weeks of age.
Results
PKA-I activation promoted and inhibition prevented ex vivo P-Ser133 CREB expression and cystogenesis. EPAC activation or PKA-II inhibition had no or only minor effects. BLU2864 inhibited in vitro mIMCD3 cystogenesis and ex vivo P-Ser133 CREB expression and cystogenesis. Genetic downregulation of PKA activity and BLU2864 directly and/or indirectly inhibited many pro-proliferative pathways and were both protective in vivo. BLU2864 had no detectable on- or off-target adverse effects.
Conclusions
PKA-I is the main PKA isozyme promoting cystogenesis. Direct PKA inhibition may be an effective strategy to treat PKD and other conditions where PKA signaling is upregulated. By acting directly on PKA, the inhibition may be more effective than or substantially increase the efficacy of treatments that only affect PKA activity by lowering cAMP.
Autosomal dominant polycystic kidney disease (ADPKD), the most common inherited renal cystic disease, is responsible for 5%–10% of kidney failure worldwide. It is characterized by development and growth of cysts, causing progressive kidney enlargement and functional decline, hypertension, cyst hemorrhage, gross hematuria, nephrolithiasis, cyst infection, pain, and reduced quality of life.1–5
Reduced function of the ADPKD gene (PKD1 and PKD2) products polycystin-1 and -2 (PC1 and PC2) is thought to cause dysregulation of intracellular calcium, activation of adenylyl cyclases 5 and 6, inhibition of phosphodiesterase 1, and upregulation of cAMP and protein kinase A (PKA) signaling.6–10 These alterations increase cell proliferation and chloride-driven fluid secretion and underlie cyst development and growth. This hypothesis pointed to the arginine vasopressin V2 receptor (V2R), the major adenylyl cyclase agonist in the collecting duct and distal nephron, as a therapeutic target for ADPKD, and led to the approval of a V2R antagonist (tolvaptan) for the treatment of rapidly progressive ADPKD.11–13 V2R antagonists affect PKA activity indirectly by lowering the production of cAMP, their efficacy is less than that achieved by genetic elimination of vasopressin,9 and PKA activation in ADPKD may also occur by cAMP independent mechanisms.14 Therefore, interventions directly targeting PKA have potential for improving or exceeding the efficacy V2R antagonists.
PKA holoenzymes consist of two catalytic subunits that bind a dimer of identical regulatory (R) subunits. Binding of the regulatory subunit dimer to catalytic subunits keeps the PKA inactive.15–17 Two major forms of PKA, PKA-I and PKA-II, are determined by their regulatory subunits (RI and RII). RI and RII are classified into α- and β-forms (RIα, RIβ, RIIα, and RIIβ) encoded by separate genes (PRKAR1A, PRKAR1B, PRKAR2A, and PRKAR2B). Regulatory subunits contain two cAMP binding domains (CBD), CBD A and CBD B. The binding of two molecules of cAMP to each regulatory subunit causes conformational changes that result in release of the catalytic subunits, which phosphorylate target proteins.
PKA-RIα is ubiquitously expressed. Its knockout, but not those of other R subunits, is embryonically lethal.18–20 We observed that RIα is increased in the kidneys of orthologous mouse models of ADPKD (Pkd1RC/RC and Pkd2WS25/-).21,22 Because it has been proposed that R1α upregulation may be a compensatory mechanism to control unrestrained PKA catalytic activity,23–25 we generated a kidney-specific knockout of Prkar1a encoding RIα to test this hypothesis in ADPKD. This knockout induced renal cystic disease on a wild-type genetic background, and markedly aggravated the cystic disease on a Pkd1 hypomorphic background.22
The studies reported here were designed to further ascertain the role or RIα in ADPKD, whether the expression of a dominant negative RIα allele partially inhibiting PKA-I is protective in ADPKD, and whether pharmacologic downregulation of the PKA alpha catalytic subunit (PRKACA), the most ubiquitous and important PKA catalytic subunit, could be a novel treatment strategy for this disease.
Materials and Methods
Study Approval
These experiments were conducted under the Institutional Animal Care and Use Committee protocols A4412–19 and A5227–20. These protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee in accordance with National Institutes of Health, United States Department of Agriculture, and American Association for Laboratory Animal Science regulations. Mice were housed with free access to regular food and water.
In vitro Three-dimensional Cystogenesis
mIMCD3 cells (American Type Culture Collection) were cultured in Matrigel in the presence of 100 μM N6,2′-O-dibutyryladenosine-3′,5′–cAMP or 10 μM forskolin to induce cyst formation, in the presence or absence of BLU2864. The compounds or vehicle were added daily. Images were collected every 3 hours using the IncuCyteS3 system, which automatically measures the average cyst areas.
Ex Vivo Cystogenesis
Pkd1RC/RC metanephric kidneys were dissected at embryonic day 13.5, and placed on a 0.4 µm transparent Falcon membrane. Images were taken daily and analyzed using an automated tool that segments kidneys, detects the cysts, and measures total and cystic areas. Metanephroi cultured from Pkd1RC/RC mouse embryos develop cysts in the absence of Forskolin, and form more and larger cysts in response to 10 µM FSK than metanephroi cultured from wild-type mouse embryos, thus supporting the relevance of this system as a model for PKD.
Generation of Pkd1RC/RC Mice Heterozygous for a Kidney-specific dominant negative RIαB Allele
The Cre recombinase inducible dominant-negative PKA subunit (RIαB) mouse line was generated and characterized previously.26 Prkar1αR1αB/WT, Pkd1RC/RC, and Pkhd1-Cre mice (expressing cre recombinase in the collecting duct), all on a C57BL/6 genetic background, were crossed to generate Pkd1RC/RC mice expressing the RIαB repressive allele (Pkd1RC/RC; Pkhd1-cre; Prkar1αRIαB/WT) and controls (Pkd1RC/RC; Pkhd1-cre; Prkar1αWT/WT, Pkd1RC/RC; Prkar1αWT/WT and Pkd1RC/RC; Prkar1αRIαB/WT). Only female RIαB heterozygotes were used for breeding because male RIαB heterozygotes have reduced fertility.26 Then 24-hour urine outputs were collected in metabolic cages. At 4 months of age the mice were anesthetized (ketamine 90 mg/kg intraperitoneally and xylazine 10 mg/kg intraperitoneally). Blood was obtained by cardiac puncture. The right kidney and part of the liver were placed into preweighed vials containing 10% formaldehyde in phosphate buffer and weighed. The left kidney and remaining liver were immediately frozen in liquid nitrogen and weighed. Kidney and liver weights were recorded.
Genotyping
Tissue samples for genotyping were collected by tail-clipping at 2 weeks of age into labeled microfuge tubes. Genomic DNA was extracted using QIAamp DNA Mini kit (Qiagen Inc. Valencia, CA). Pkhd1-Cre and Pkd1 genotyping was performed by PCR. The primers used were:
Pkhd1-Cre: forward-AGGTTCGTTCACTCATGGA; reverse-TCGACCAGTTTAGTTACCC.
Pkd1RC: forward-CAAAGGTCTGGGTGATAACTGGTG, reverse-CAGGACAGCCAAATAGACAGGG.
PKA R1αB genotyping was performed using Custom TaqMan® Assays. The primers used were:
PKA R1aB forward-GCTTCCATAGGTGATAGTCGTTACTG; reverse-GCCCGAGGACGATTCATCAG;
WT Taqman probe: VIC-CTTTTCCAGGTGAAATT-MGB.
RIaB Taqman probe: FAM-CTTTCCAGATGAAATT-MGB.
Description of BLUE2864
BLU2864 is a highly selective, ATP-competitive inhibitor of the PRKACA, identified and optimized by Blueprint Medicines from a library of >10,000 agnostically designed kinase inhibitors.27 The chemical structure is shown in Supplemental Figure 1. Its cLogP value is 3.4, topological polar surface area 90.9 angstroms, plasma protein binding fraction 98%, and it is compliant with Lipinski’s Rule of 5. Kinome-wide selectivity of BLU2864 was assessed at 3 μM concentration across a panel of 400 kinases using the KINOMEscan methodology.28 BLU2864 had excellent overall kinome selectivity with an (S[0]) selectivity score of 0.057. BLU2864 had the most potent binding affinity to PRKACA with a dissociation constant (Kd) of 3.3 nM and exhibited a Kd value <100 nM for only 10 kinases identified in the kinome screening assay (Supplemental Table 1).27 BLU2864 inhibited PRKACA catalytic activity with an IC50 of 0.3 nM, compared with 12.7 nM for ROCK2, 2120 nM for AKT1, 4910 nM for AKT2, and 475 nM for AKT3 (Supplemental Table 2).27 The pharmacokinetic profile was assessed in NOD-SCID after administration by oral gavage (30 mg/kg). Plasma concentrations peaked within 2–4 hours of daily dosing.27 The pharmacodynamic profile was assessed in NOD-SCID mice with xenografts derived from patients with subcutaneous fibrolamellar carcinoma. Phosphorylated VASP levels in the xenografts, indicative of PKA activity, were reduced to 27% of baseline 2 hours after dosing and were fully recovered by 24 hours postadministration.27
Preclinical trial of BLU2864 in F1 129S6/Sv x C57BL/6B6 Pkd1RC/RC Mice
Hypomorphic Pkd1RC/RC mice inbred (>99%) into two genetic backgrounds, 129S6/Sv or C57BL/6, were crossed to generate the F1 129S6/Sv x C57BL/6B6 mice. Kidney magnetic resonance scans were obtained at 3–4 weeks of age and kidney volumes were measured. Mice were randomized into treatment and control groups on the basis of sex and the initial total kidney volume to ensure similar baseline disease severity in the two groups. BLU2864 (45 or 30 mg/kg body weight) or vehicle (10% DMSO, 10% Kolliphor HS 15, 16% HPBCD) alone was administered daily between 9 and 10 AM by oral gavage for 5 days, or from 4 to 16 weeks of age. Body weights were measured weekly. Kidney MR scans were again obtained at 15–16 weeks of age. Before sacrifice at 4 months of age, 24-hour urine outputs were collected in metabolic cages.
Magnetic Resonance Imaging
Ultrahigh field magnetic resonance imaging (16.4 T) was performed in all animals under isoflurane anesthesia using a Bruker Advance 700 Mhz (16.4T) vertical bore nuclear magnetic resonance spectrometer and following procedures described in detail elsewhere29 (https://www.jove.com/video/52757/use-ultra-high-field-mri-small-rodent-models-polycystic-kidney). TKV was calculated from axial slices of the kidney using Analyze 12.0 software and using an automated method.30
Measurement of Plasma BLU2864 Levels
Plasma levels of BLU2864 were measured using liquid chromatography with tandem mass spectrometry.
Cyclic AMP Content of Whole Kidneys
The kidneys were weighed and ground to a fine powder under liquid nitrogen in a stainless-steel mortar and homogenized in 10 volumes of 0.1 M HCl. The protein concentration was measured by using BCA Protein Assay Kit (Pierce, IL). After centrifugation at 600 g for 10 minutes, the supernatant was further diluted in the 0.1 M HCl. cAMP content was assessed by enzyme-linked immunosorbent assay, according to the manufacturer’s instructions (Enzo Life Sciences, Exeter, UK). Samples were taken in triplicate. The results were expressed in pmol/mg of protein.
PKA Activity in Whole Kidney Lysates
The PKA activity was assayed using a 32P base and the biotinylated form of peptide substrate to measure basal and total PKA activities from kidney protein lysates. Briefly, 2 μg of protein lysate containing γ32P-labeled phosphate was incubated with biotinylated Kemptide, a PKA-specific substrate, in the absence (basal activity) or the presence of added 0.01 mM cAMP (total activity). The 32P-labeled biotinylated substrate is recovered from the reaction mix using perforated SAM2 Biotin Capture Membranes that bind to and immobilize the substrate. PKA activity is then measured using standard scintillation to determine radioactive counts/minute, reflecting the amount of phosphorylated substrate. All determinations of PKA activity were performed in duplicate, corrected for protein concentration, and an average value was calculated for each experiment.
Plasma BUN and Creatinine
Measurements of plasma urea and creatinine were performed using the QuantiChrom urea or creatinine assay kits (Bioassay Systems, Hayward, CA, USA).
Histology and Histomorphometric Analysis
Next, 5 µm transverse tissue sections of the kidney, including cortex, medulla, and papilla, and the liver were stained with hematoxylin-eosin to measure cystic indices. Image analysis procedures were performed with NIS-Elements AR software (Universal Imaging, West Chester, PA). Digital images were acquired using a light microscope with a high-resolution Nikon Digital camera (Nikon DXM 1200). The observer interactively applied techniques of enhancement for better definition of interested structures and to exclude fields too damaged to be analyzed. A colored threshold was applied at a level that separated cysts from noncystic tissue to calculate the surface area of cysts as percentages of kidney section area (cystic index). Histomorphometric analyses were performed blindly, without knowledge of group assignment. Immunohistochemistry for P-Ser133 CREB was performed using a P-Ser133 CREB antibody (Cell Signaling Technology, Inc., Danvers, MA) and Goat Anti-Rabbit IgG H&L (HRP polymer, Abcam, Waltham, MA) following the manufacturer’s protocol.
Western Blotting in Whole Kidney Lysates and Nuclear Fractions
Kidney protein was extracted using Pierce subcellular protein fractionation kit (Pierce 87790. Thermo Fisher Scientific Inc., Wyman, MA) according to the manufacturer’s protocol. Protein concentrations were determined with BCA protein assay kit (Pierce Chemical Co.). Kidney protein was heated in a sample buffer, electrophoresed on SDS-polyacrylamide gel, and transferred to PVDF membrane. The blots were blocked with 5% milk in TBS-T (0.1% tween-20) at room temperature for 1 hour, then incubated with the primary antibody overnight at 4°C. After incubation, the membrane was washed and incubated with secondary antibody for 1 hour at room temperature. Detection was performed using enhanced ECL (Pierce Chemical Co.). For loading control and quantification, membranes were stained using the Swift Membrane Stain Kit (catalog 786–677; Geno Technology Inc., St. Louis, MO) according to the manufacturer’s protocol. Total protein stain was used as a loading control, and multiple proteins of a thin strip through the center of the lane running from top to bottom were used for quantification. Bands were analyzed using Image J software, level of protein was normalized to respective total protein, the control group was normalized to 1 and experimental group was compared with this. Nuclear protein was used for CREB, P-CREB, 4E-BP1, P-4E-BP1, and PAX2. Antibodies used were from Santa Cruz Biotechnology (PCNA), BD Transduction Laboratories (PKA-R1α, PKA-R2α, PKA-R2β), Cell Signaling Technology (PKA-Cα, Src, P-Ser17 Src, ERK1/2, P-Thr202/Tyr204 ERK1/2, CREB, P-Ser133 CREB, STAT3, P-Tyr705 STAT3, P-Ser9 GSK3β, S6K, P-Thr389 S6K, 4E-BP1, P-Thr37/46 4E-BP1, GSK3β, Akt, P-Ser473 Akt, and P-Thr308 Akt), Abcam (Pax2, Gli1, P-Ser261 AQP2), Novus (Gli2, AQP2), and GeneTex (Gli3-A and Gli3-R).
Statistical Methods
Each experimental or treatment group included 9–15 animals. Ten male and ten female animals in each experimental or treatment group provides 90% power to detect a reduction in cystic index from 25% to 20% assuming a cyst volume density of 25±5% in the control group. We have found this sample size to be adequate in previous preclinical studies using ADPKD rodent models. Data were expressed as mean±SD. Two-way ANOVA and two-sided t test were used for comparisons between the groups.
Results
PKA-I Activation Promotes Ex Vivo Cystogenesis in Pkd1RC/RC Metanephric Organ Culture
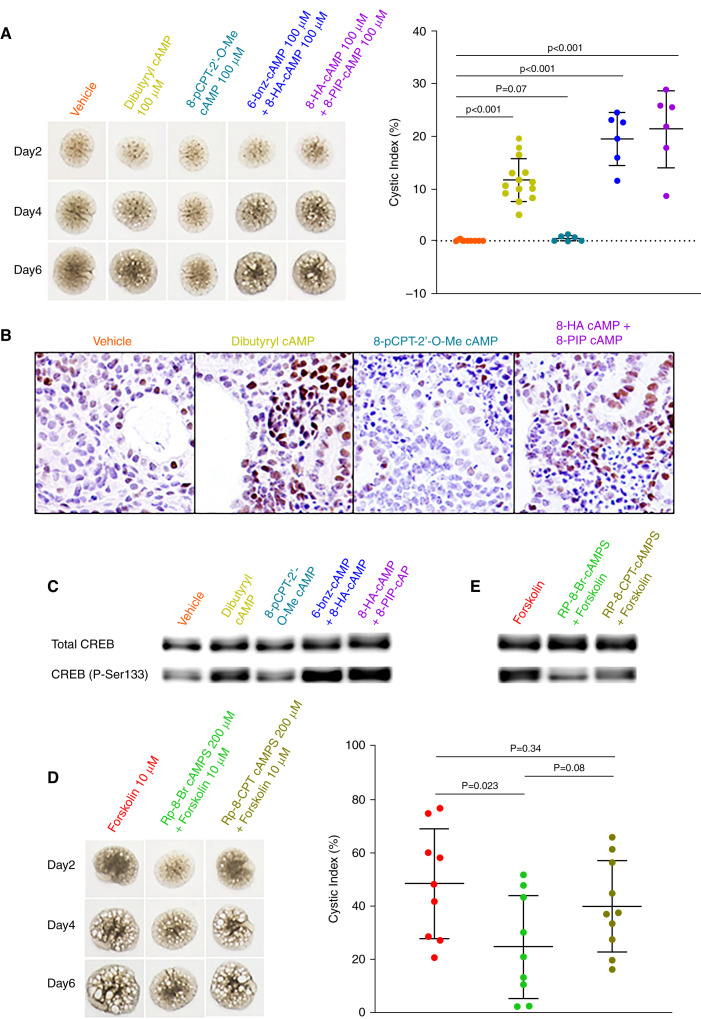
It is generally accepted that enhanced cAMP signaling promotes cystogenesis through PKA activation,31 whereas the role of the Epac pathway is less clear.32 Previous work suggested that PKA-I rather than PKA-II is most important in PKD.22 We treated Pkd1RC/RC metanephric organ cultures with N6,2′-O-dibutyryladenosine-3′,5′–cAMP (cell permeant cAMP analog), 8-pCPT-2’-O-Me–cAMP (Epac selective cAMP analog),33 and cAMP analog combinations, which preferentially activate PKA-I, 6-Benz-cAMP or 8-PIP–cAMP (binding to CBD A of PKA RI) and 8-HA–cAMP (binding to CBD B of PKA RI).34–38 PKA-I but not Epac activation increased CREB phosphorylation and promoted ex vivo cystogenesis (Figure 1, A–C). We also examined the effect of Rp-8-Br–cAMPS, a synthetic diastereomeric phosphorothioate competitive antagonist of cAMP, which preferentially inhibits PKA-I,38,39 and of Rp-8-CPT–cAMPS, which inhibits PKA-II more effectively than PKA-I.39,40 Rp-8-Br–cAMPS inhibited forskolin induced CREB phosphorylation and cystogenesis more effectively than Rp-8-CPT–cAMPS (Figure 1, D–E).
Figure 1.
Treatment of E13.5 Pkd1RC/RC metanephric kidneys with cAMP analogs shows that PKA-I preferentially modulates ex vivo cystogenesis. Cultures were either left untreated (addition of vehicle only) or were treated with various cAMP analogs. (A–C) N6,2′-O-dibutyryladenosine-3′,5′-cAMP (dibutyryl cAMP, a cell permeant cAMP analog) and cAMP analog combinations that preferentially activate PKA-I, 6-Benz–cAMP or 8-PIP–cAMP (binding to CBD A of PKA RI) and 8-HA–cAMP (binding to CBD B of PKA RI), induced cyst formation and increased the expression of P-Ser133-CREB, whereas the Epac selective cAMP analog 8-pCPT-2’-O-Me–cAMP had no effect. (D–E) Rp-8-Br-cAMPS, a PKA inhibitor that preferentially blocks PKA-I, inhibited forskolin induced cystogenesis and expression of P-Ser133–CREB to a larger extent than Rp-8-CPT CAMPS, which is a better inhibitor of PKA-II compared with PKA-I. All kidneys are at the same scale.
Heterozygous Kidney-specific Expression of a Dominant Negative RIαB Allele Inhibits PKA Activity and Ameliorates PKD in Pkd1RC/RC Mice
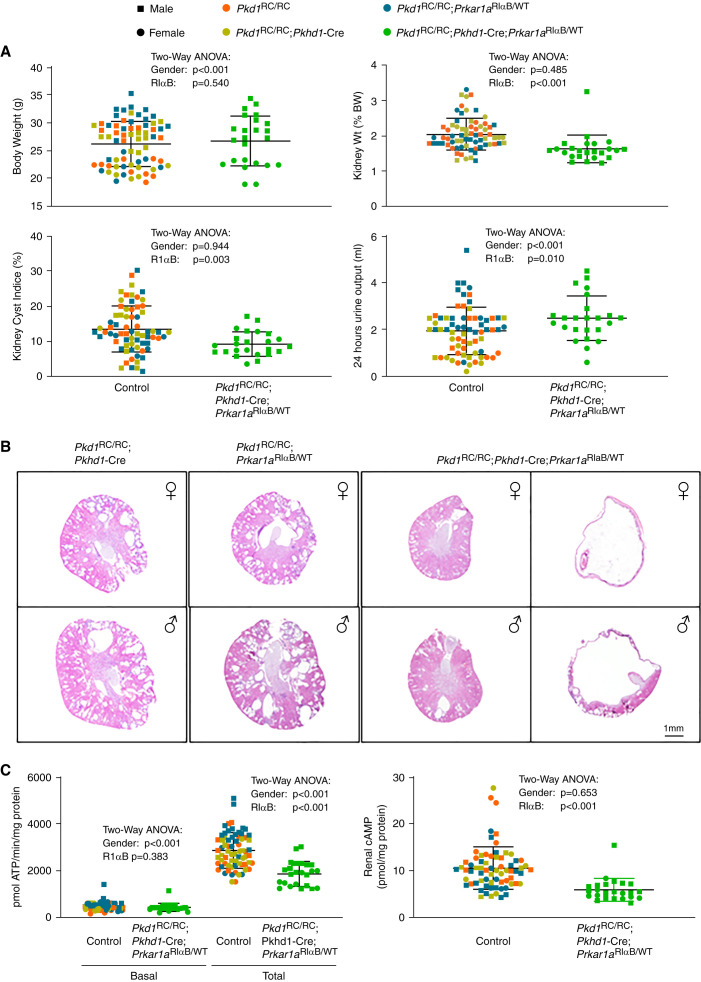
To determine the effect of PKA-I downregulation in vivo, we used a mouse line that carries a mutant Prkar1α allele with a glycine to aspartate substitution in CBD B,26,41–43 a mutation identified in a cAMP resistant lymphoma cell line.41 This mutation produces a constitutively repressive RIα regulatory subunit (RIαB) that cannot release the catalytic subunits in the presence of cAMP at physiologic concentrations. Insertion of a loxP-flanked neomycin cassette in the intron preceding the mutation prevents expression of the RIαB until the cassette is excised by Cre recombinase. We crossed these mice with Pkhd1-Cre mice (expressing cre recombinase in the collecting duct)44 and with Pkd1RC/RC mice,45 all on a C57BL/6 genetic background, to generate Pkd1RC/RC mice expressing the RIαB repressive allele (Pkd1RC/RC;Pkhd1-cre;Prkar1αRIαB/WT or Pkd1RC/RC RIαB-ON) and controls (Pkd1RC/RC;Pkhd1-cre;Prkar1αWT/WT, Pkd1RC/RC;Prkar1αWT/WT and Pkd1RC/RC;Prkar1αRIαB/WT). There were no significant differences between the control groups and they therefore were analyzed as a single group (Figure 2A, Table 1A). Body weight, general appearance, and activity of the RIαB expressing Pkd1RC/RC and control mice were not different (Figure 2A). RIαB expressing Pkd1RC/RC had lower kidney weights, kidney weights as percent of body weights, cyst indices, and higher urine outputs (Figure 2, A and B, Supplemental Figure 2, A and B). Three RIαB expressing Pkd1RC/RC mice (one male, two females) had unilateral hydronephrosis (Figure 2B, Supplemental Figure 2, A and B) and their data were excluded from the analysis (Table 1A). No other abnormalities of the collecting system were observed. Plasma creatinine and urea concentrations were lower in the RIαB-expressing Pkd1RC/RC mice (Table 1A). Liver volumes were not different.
Figure 2.
Heterozygous kidney specific expression of a dominant negative RIαB allele partially inhibits PKA activity and ameliorates PKD in Pkd1RC/RC mice. (A) Body weights, relative kidney weights, cyst indices, and urine outputs of RIαB-expressing mice and controls. (B) Cross-sections stained with hematoxylin and eosin of representative kidneys of RIαB-expressing (Pkd1RC/RC;Pkhd1-cre;Pkrar1αRIαB/WT mice and Pkd1RC/RC;Pkrar1αRIαB/WT controls; two RIαB expressing mice had unilateral hydronephrotic kidneys. (C) PKA activities and cAMP levels in kidney tissue lysates of RiαB-expressing mice and controls.
Table 1.
Genetic or pharmacologic downregulation of PKA in Pkd1RC/RC mice
| Group (sex) | Body Weight (g) | Kidney Weight (g) | Kidney/Body Weight × 100 | Cyst Index (%) | Urine Volume (ml/day) | Kidney cAMP (pmol/mg protein) | Plasma Creatinine (mg/dl) | Plasma Urea (mg/dl) | PKA Activity (pmlol ATP/min per mg protein) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Basal | Total | |||||||||
| Conditional expression of the dominant negative RIαB allele in Pkd1RC/RC mice | ||||||||||
| Control (female, n=28) | 21.9±1.3 | 0.47±0.11 | 2.16±0.48 | 14.1±5.4 | 1.31±0.83 | 12.13±5.8 | 0.31±0.10 | 57.2±12.9 | 329±75 | 2366±497 |
| RIαB-ON (female, n=9) | 21.9±1.7 | 0.35±0.04 | 1.62±0.16 | 8.99±3.26 | 2.09±1.04 | 4.98±1.39 | 0.17±0.08 | 39.0±12.5 | 318±68 | 1570±306 |
| P valuea | 0.99 | <0.001 | <0.001 | 0.002 | 0.07 | <0.001 | <0.001 | 0.002 | 0.67 | <0.001 |
| Control (male, n=43) | 29.1±2.35 | 0.58±0.14 | 1,99±0.43 | 13.5±7.4 | 2.37±0.91 | 9.74±3.4 | 0.32±0.11 | 56.1±13.3 | 543±171 | 3196±692 |
| RIαB-ON (male, n=15) | 29.7±2.49 | 0.49±0.14 | 1.64±0.49 | 9.41±3.77 | 2.73±0.85 | 6.47±2.82 | 0.29±0.06 | 50.6±9.12 | 492±190 | 2061±537 |
| P valuea | 0.41 | 0.037 | 0.023 | 0.009 | 0.18 | 0.001 | 0.14 | 0.09 | 0.37 | <0.001 |
| Group effect P valueb | 0.54 | <0.001 | <0.001 | 0.003 | 0.010 | <0.001 | <0.001 | <0.001 | 0.38 | <0.001 |
| Sex effect, P valueb | <0.001 | <0.001 | 0.48 | 0.94 | <0.001 | 0.65 | 0.008 | 0.09 | <0.001 | <0.001 |
| Treatment of Pkd1RC/RC mice with the PKA inhibitor BLU2864 | ||||||||||
| Control (female, n=10) | 23.3±2.2 | 0.65±0.17 | 2.77±0.53 | 26.3±5.7 | 1.45±0.08 | 28.9±7.2 | 0.39±0.04 | 54.6±9.6 | 466±91 | 2585±654 |
| BLU2864 (female, n=9) | 23.5±1.1 | 0.54±0.07 | 2.28±0.33 | 18.9±5.0 | 1.72±0.36 | 27.6±7.7 | 0.36±0.06 | 60.2±14.6 | 153±31 | 436±130 |
| P valuea | 0.80 | 0.069 | 0.029 | 0.007 | 0.057 | 0.71 | 0.30 | 0.34 | <0.001 | <0.001 |
| Control (male, n=9) | 27.9±1.8 | 0.98±0.12 | 3.53±0.39 | 31.2±4.6 | 1.39±0.09 | 34.4±6.4 | 0.39±0.04 | 58.3±5.4 | 442±97 | 2167±483 |
| BLU2864 (male, n=9) | 27.1±1.8 | 0.80±0.16 | 2.93±0.49 | 25.9±5.9 | 1.67±0.14 | 31.1±5.7 | 0.40±0.05 | 60.6±5.5 | 128±34 | 322±128 |
| P valuea | 0.38 | 0.012 | 0.011 | 0.049 | <0.001 | 0.26 | 0.76 | 0.40 | <0.001 | <0.001 |
| Drug effect P valueb | 0.63 | 0.003 | <0.001 | <0.001 | <0.001 | 0.31 | 0.53 | 0.22 | <0.001 | <0.001 |
| Sex effect, P valueb | <0.001 | <0.001 | <0.001 | 0.002 | 0.41 | 0.053 | 0.21 | 0.52 | 0.31 | 0.07 |
Values are mean±SD.
t test.
Two-way ANOVA.
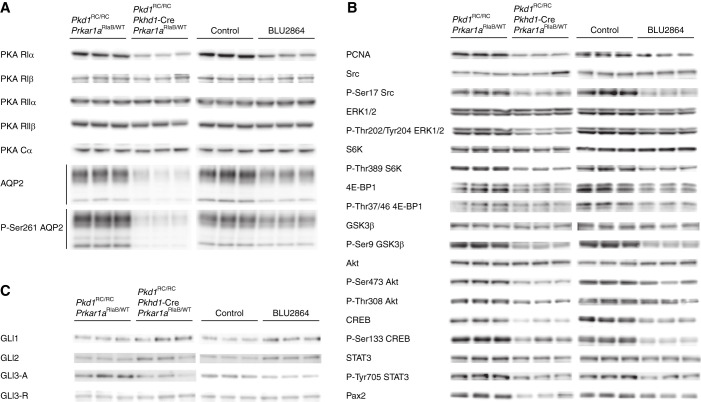
Total kidney PKA activities were lower in whole kidney lysates of RIαB-expressing Pkd1RC/RC mice than in controls, whereas the basal PKA levels were not significantly different (Figure 2C). The concentrations of cAMP (Figure 2C) and RIα, but not RIβ, RIIα, RIIβ, or CIα (Figure 3), were also lower. Consistent with the role of PKA regulating the expression46 and phosphorylation of aquaporin-2 (AQP-2),47 the levels of total AQP-2 and pAQP-2 were reduced in RIαB-expressing Pkd1RC/RC mice (Figure 3). Proliferating cell nuclear antigen (a marker of cell proliferation, PCNA), proproliferative signaling pathways (Src, ERK1/2, mTOR, GSK3β, and AKT), and transcription factors, (CREB, STAT3, Pax2), downstream from PKA activation, were downregulated (Figure 3, Supplemental Table 3). Gli1 and Gli2 were higher, whereas Gli3A and Gli3R were unchanged in RIαB expressing Pkd1RC/RC mice (Figure 3, Supplemental Table 3).
Figure 3.
Genetic or pharmacologic downregulation of PKA reduce cell proliferation and the expression of AQP-2, and of pro-proliferative signaling proteins and transcription factors. Western blots of regulatory and catalytic PKA subunits, and of (A) glycosylated (upper bands) and unglycosylated (lower bands) AQP-2 and pAQP-2, (B) PCNA and pro-proliferative signaling proteins and transcription factors,and (C) Gli proteins, in RiαB-expressing (left panels) and BLU2864-treated mice (right panels) and their respective controls.
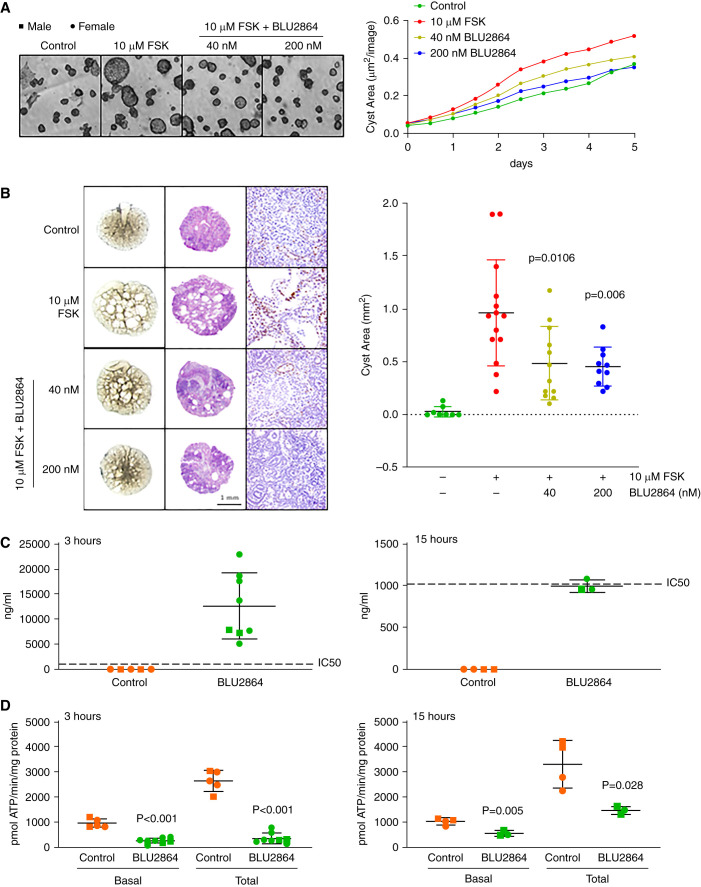
BLU2864, a Small Molecule PKA Inhibitor, Inhibits In Vitro and Ex Vivo Cystogenesis
BLU2864 inhibited forskolin induced in vitro cystogenesis of mIMCD3 cells cultured in Matrigel by 72% and 100% at 40 and 200 nM concentrations, respectively, relative to control (Figure 4A). Similar concentrations also inhibited forskolin induced ex vivo cystogenesis by 50% and 53% (Figure 4B) and CREB phosphorylation (Figure 4C) in Pkd1RC/RC metanephric organ cultures without any evidence of toxicity macro or microscopically (Figure 4D).
Figure 4.
BLU2864 inhibits in vitro and ex vivo cystogenesis, and administration of BLU2864 by oral gavage inhibits kidney PKA activity. (A) Forskolin induced cyst formation in a three-dimensional Matrigel culture of mIMCD3 cells untreated or treated with BLU2864. (B) Forskolin induced cyst formation (left two columns) and expression of P-Ser133 CREB (right column) in metanephric organ cultures untreated or treated with BLU2864. (C) Plasma concentrations of BLU2864 3 and 15 hours after the last of five daily doses (45 mg/kg body weight [b.w.]) by oral gavage. (D) Kidney PKA activities 3 and 15 hours after the last of five daily doses of BLU2864 (45 mg/kg b.w.) by oral gavage.
BLU2864 Inhibits Renal PKA Activity in Pkd1RC/RC Mice
Pkd1RC/RC mice were treated daily for 5 days with BLU2864 (45 mg/kg body weight a dose found in preliminary studies to be tolerated in mice) or vehicle (10% DMSO, 10% Kolliphor HS 15, 16% HPBCD) by oral gavage. They were sacrificed 3 or 15 hours after the last dose. Plasma concentrations of BLU2864 at these times were undetectable in the controls and close to the IC90 and IC50 values in the BLU2864-treated mice (Figure 4C). Kidney basal and total PKA activities were suppressed by 74% and 87% at 3 hours and by 46% and 56% at 15 hours, respectively, in the BLU2864-treated mice compared with controls (Figure 4D).
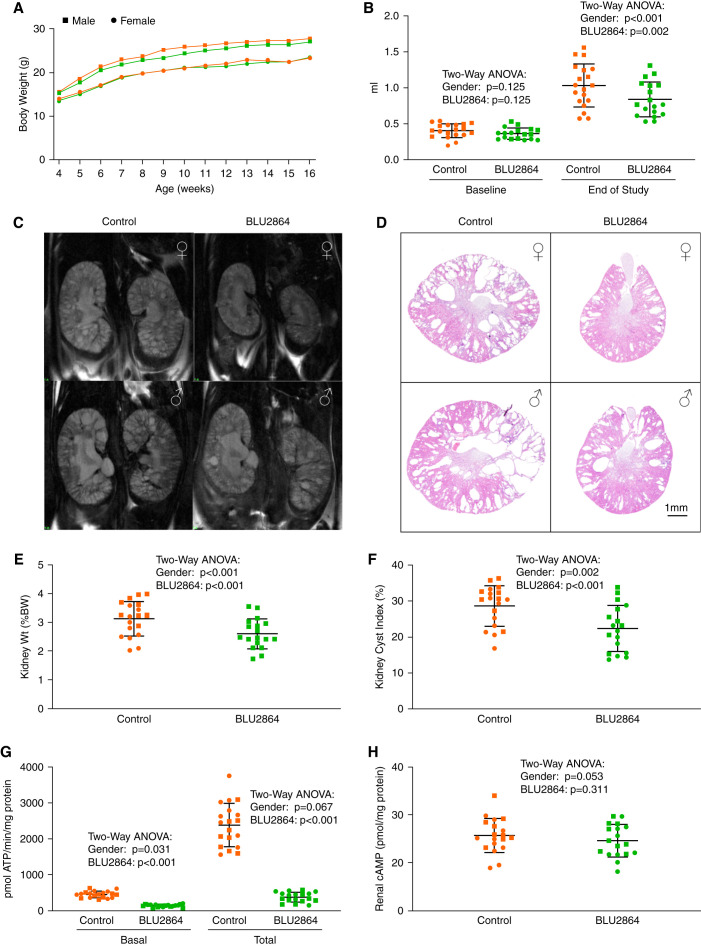
BLU2864 Inhibits PKA Activity and Ameliorates PKD in Pkd1RC/RC Mice
Because weanling mice could be more susceptible than adult mice to drug toxicity, a dose of 30 mg/kg body weight was used to test the efficacy of BLU2864 in F1 mice from C57BL/6 × 129S6/Sv crosses. This genetic background was chosen because the course of the disease in the F1 mice is more similar to that of human ADPKD and most appropriate for therapeutic trials.48 BLU2864 was administered daily by oral gavage from 4–16 weeks of age, whereas the controls received vehicle alone (10% DMSO, 10% Kolliphor HS 15, 16% HPBCD). Weekly body weights were similar in both groups (Figure 5A, Table 1). Urine outputs at 15 weeks of age were higher in the BLU2864-treated mice than in the controls (Table 1). Kidney volumes at baseline measured by magnetic resonance imaging were similar in both groups, but they were lower in the BLU2864-treated mice at 15 weeks of age (Figure 5, B and C, Supplemental Figure 3). The mice were euthanized at 16 weeks of age 1–2 hours after oral gavage. BLU2864-treated mice had lower kidney weights, kidney volumes as percent of body weights, and cyst indices (Figure 5, D–F, Supplemental Figure 4). Plasma creatinine and urea concentrations were not significantly different (Table 1). Liver volumes in the control and treated mice were not different (absence of macroscopic liver cysts and hepatomegaly in this model45).
Figure 5.
Administration of BLU2864 by oral gavage ameliorates PKD in vivo in Pkd1RC/RC mice. (A) Similar growth rates in BLU2864 treated and control mice. (B) Kidney volumes measured by magnetic resonance imaging in the treated and control group were similar at randomization, but significantly lower in the treated mice at the end of treatment at 16 weeks of age. (C) Representative coronal magnetic resonance images in BLU2864-treated and control mice at the end of treatment. (D) Cross-sections stained with hematoxylin and eosin of representative kidneys from BLU2864 treated and control mice at sacrifice. (E and F) Relative kidney weights and cyst indices in BLU2864-treated and control mice at sacrifice. (G and H) Kidney PKA activities and cAMP levels at sacrifice.
Renal basal and total PKA activities at sacrifice, 1–2 hours after oral gavage, were 69% and 84% lower in the BLU2864-treated mice compared with controls (Figure 5G). Cyclic AMP levels also trended to be lower, but not significantly (Figure 5H). Kidney AQP-2 and P-AQP-2, PCNA, proproliferative signaling pathways (Src, ERK1/2, mTOR, GSK3β, and AKT) and transcription factors, (CREB, STAT3, Pax2), downstream from PKA activation, were downregulated in BLU2864-treated mice (Figure 3, Supplemental Table 3). Gli1 and Gli2 were higher, whereas Gli3A and Gli3R were unchanged (Figure 3, Supplemental Table 3).
Discussion
A wealth of evidence supports that upregulation cAMP signaling promotes cystogenesis in ADPKD. The previous observation that RIα is increased in the kidneys of orthologous mouse models of ADPKD (Pkd1RC/RC and Pkd2WS25/-)21,22 and that a kidney specific knockout of RIα induced renal cystic disease in wild-type mice and markedly aggravated the cystic disease in Pkd1RC/RC mice22 pointed to the importance of PKA-I and RIα. This study shows that cAMP analogs activating PKA-I, but not those activating Epac, promote cystogenesis in Pkd1RC/RC metanephric organ cultures, and that Rp-8-Br–cAMPS, which preferentially inhibits PKA-I, blunts forskolin-induced ex vivo cystogenesis. Kidney-specific expression of a dominant negative RIαB allele and the systemic administration of BLU2864 downregulated PKA activity and reduced the severity of the renal cystic disease in Pkd1RC/RC mice. Both caused a small increase in urine output associated with reduced AQP-2 expression and phosphorylation as previously reported in mice with single-minded homolog 1 (Sim1)-Cre driven expression of RIαB expression in AQP2-expressing principal cells.43
PKA R-subunits have different patterns of tissue and subcellular expression and biologic functions.49 PKA RIα is ubiquitously expressed and its knockout18,19 is the only embryonic lethal R subunit knockout.20 RI subunits associate to the cytosolic fraction of cellular homogenates, possibly due to their low affinity to binding partners, whereas RII subunits associate to particulate and membrane fractions.50,51 PKA holoenzymes localize to cellular microdomains through interactions with A kinase anchoring proteins (AKAPs).52 Most AKAPs display high affinity for RII but low or no affinity for RI, although AKAPs with high affinity for RI subunits have been identified. RI subunits preferentially regulate nuclear C-subunit entry, phosphorylation of transcription factors, and induction of gene expression.53–55 RII subunits preferentially regulate rapid PKA phosphorylation cycles, such as ion channel regulation.56 Recently, it has been shown that RIα undergoes liquid-liquid phase separation and form condensates (membraneless organelles) that sequester cAMP, restrict cAMP diffusion, and serve as a dynamic buffering system.57 Loss of RIα phase separation disrupts cAMP compartmentalization, and leads to increased cell proliferation.
Several observations in the study deserve comment. The cystic disease severity of the control groups for the RIαB expressing and the BLU2864 treated Pkd1RC/RC mice was different. The genetic backgrounds of the mice, C57BL/6 and C57BL/6 x 129S6/Sv F1, respectively, likely account for the disparity, because the severity of the renal cystic disease in Pkd1RC/RC is strongly affected by the genetic background (more severe in the 129S6/Sv than in the C57BL/6 background).48 Furthermore, cAMP levels in kidney lysates of Pkd1RC/RC mice on 129S6/Sv were higher than those on C57BL/6 background. The observation that the genetic background of different mouse strains may affect the metabolism of cAMP has been reported in other tissues, particularly in the brain.58–61
Total PKA activity was reduced in the kidney homogenates of RIαB-expressing Pkd1RC/RC mice, whereas both basal and total PKA activities were reduced in the homogenates of BLU2864-treated Pkd1RC/RC mice. This may be due to the low level of basal PKA activity and that measurements were made in whole kidney homogenates instead of homogenates of the inner medulla (RIαB is expressed only in the collecting ducts and possibly the distal nephron). The reduced PKA activity likely accounts for the lower RIα protein expression in RIαB-expressing and BLU2864 treated Pkd1RC/RC mice,because Pkrar1a transcription is upregulated by cAMP signaling.46 Reduced RIα expression (Figure 5) may also account for the lower kidney cAMP level of the RIαB expressing mice, because their kidneys likely contain fewer RIα condensates sequestering cAMP at high concentrations.57 Plasma creatinine and urea concentrations were significantly lower in RIα expressing but not in the BLU2864-treated mice compared with controls. The earlier suppression of PKA activity in the genetic compared with the pharmacologic approach, the more selective suppression of PKA-I and other disparities in the experimental protocol (e.g., oral gavage) may account for the difference.
The most common inherited diseases of PKA signaling are caused by PRKAR1A mutations and provide insights into the parhogenesis of ADPKD. Inactivating mutations cause Carney complex, a multiple endocrine neoplasia syndrome.62–67 PRKAR1A was initially thought to be a tumor-suppressor gene, with tumors exhibiting germline mutations and subsequent loss of heterozygosity at the PRKAR1A locus.62 Later, PRKAR1A haploinsufficiency was shown to increase the concentration of free PKA catalytic subunits and PKA activity was sufficient to promote the development of certain tumors.68,69 The pathogenetic roles of loss of heterozygosity and haploinsufficiency, the proliferative response of cells isolated from affected tissues to cAMP, the activation of similar proproliferative signaling pathways, and the rarity of malignant transformation despite the proliferative phenotype are features shared by the Carney complex and ADPKD.70–79 The high frequency of multiple kidney and liver cysts recently reported in patients with Carney complex also supports the similarity of pathogenic mechanisms for both conditions.22
Acrodysostosis type 1 is caused by dominant negative mutations of PRKAR1A and consists of skeletal abnormalities, hormonal resistance, and extraskeletal manifestations including hydronephrosis.80–82,81,83,84 PRKA1A mutations causing acrodysostosis type 1, like the RIαB allele in this study, impair the ability of cAMP to dissociate the RIα and catalytic subunits and dampen the PKA response to cAMP.85 Gain of function mutations to phosphodiesterase 4D also inhibit PKA signaling and cause acrodysostosis type 2.86 Because the expression of RIαB allele in this study was under the control of the Pkhd1 promoter (expressed in the collecting duct and ureteric bud),87 no skeletal abnormalities were observed. Hydronephrosis was observed in three mice expressing RIαB.
We have previously shown that kidney specific, constitutive activation of PKA activates proproliferative signaling pathways (Src, Ras, MAPK/ERK, mTORC1) and transcription factors (CREB, STAT3, Pax2).22 As expected, genetic suppression or pharmacologic inhibition of PKA had the opposite effects. In this study we also assessed the effect of PKA downregulation on AKT because this kinase is thought to play an important role in the pathogenesis of PKD88 and is inhibited by BLU2864.27 Because both genetic and pharmacologic inhibition reduced the phosphorylation of AKT, the effect of BLU2864 may not be a direct off-target effect. The higher IC50 values of BLU2864 for AKT1, AKT2 and AKT3 (2120, 4910, and 475 nM) compared with that for PRKACA (0.3 nM, which is also the plasma level measured at 15 hours post gavage) make a direct off-target effect of BLU2864 on AKT and PKD improbable. It seems more likely that PKA downregulation (genetic or pharmacologic) inhibits AKT through interference in one or more pathways associated with AKT activation downstream from PKA. For example, PKA phosphorylates c-Src at Ser307 and active c-Src drives tyrosine phosphorylation of AKT with subsequent phosphorylation at Thr308 by phosphoinositide-dependent kinase 1 and at Ser473 by mTORC2.89 PKA also phosphorylates KRAS4B at Ser181 and RHOA at Ser188, forming an active complex with mTORC2, which phosphorylates AKT at Ser473.90 PKA activation increases the expression of GSK3β,91 which is constitutively active, phosphorylates phosphatase and tensin homolog at Thr366, and results in loss of phosphatase and tensin homolog activity, accumulation of phosphatidylinositol 3,4,5-trisphosphate, activation of phosphoinositide-dependent kinase 1, and AKT phosphorylation at Thr308.92 On the other hand, PKA induced phosphorylation at Ser9 deactivates GSK3β, stabilizes β-catenin, and may also promote cystogenesis.93 Enhanced superoxide anion generation and Ras-SIN1 mediated activation of mTORC294 or increased cytokine generation and IKK-mediated activation of mTORC295 may also contribute to AKT activation downstream from PKA. Although the precise identification of the mechanism(s) responsible for the attenuation of AKT signaling by PKA downregulation in PKD is beyond the scope of this study, it may contribute to its protective effect in PKD.
The effects of genetic or pharmacologic suppression of PKA on Hedgehog signaling were opposite to those associated with constitutive activation.22 Constitutive targeted activation of PKA-I inhibited Hedgehog signaling in the kidney, consistent with earlier observations in Prkar1a deficient embryonic mouse fibroblasts96 and the role of PKA as the main repressor of Hedgehog signaling, globally and in the ciliary compartment.97,98 The suppression of Hedgehog was accompanied by hydronephrosis in 31% of mice, possibly due to PKA triggered upregulation of GLI3R.22 This is consistent with the occurrence of hydronephrosis in the Pallister–Hall syndrome,99,100 a rare autosomal dominant disorder caused by GLI3 mutations that result in a truncated protein acting as an obligate transcriptional repressor,101,102 in mice engineered to constitutively express GLI3R,103,104 and in Shh-deficient mice.105 In this study, genetic suppression of PKA-I increased Gli1 and Gli2 without changing Gli3R protein levels. Paradoxically, three mice expressing the suppressive RIaB allele had unilateral hydronephrosis. Although this observation could be due to chance, targeted inactivation of Patched1 has been shown to stimulate Hedgehog signaling in ureteric bud tips and disrupt ureteric branching.106 Possibly, excessive or insufficient Hedgehog activity may be deleterious for normal development.107
The key role that protein kinases play in cellular signaling pathways and the pathogenesis of many human diseases has stimulated the search for inhibitors.108 Although PKA was one of the first protein kinases to be discovered, identification of specific PKA inhibitors without potential off-target effects has been challenging.109,110 We have used a novel highly selective PKA/PRKACA inhibitor.27 In addition to off-target effects, PKA inhibition could have many potential on target adverse effects. Nevertheless, therapeutic effectiveness may be achieved without undue adverse effects. Marked upregulation of PKA activity, as seen in ADPKD and other pathophysiological conditions (e.g., Carney complex, McCune-Albright syndrome, adrenocortical tumors with PKA R or C somatic mutations, fibrolamellar hepatocellular carcinoma, and an increasing number of epithelial cell cancers, adrenal carcinomas, and lymphomas),111–113 may be more sensitive to PKA inhibition and downregulation of PKA signaling may be achievable and effective, while avoiding or minimizing on-target adverse effects. The heterozygous knockout of PRKACA is viable.114 In this study, no evidence of toxicity was associated with the genetic or pharmacologic PKA downregulation. In addition, targeted delivery of PKA inhibitors to the site of interest or combination with other site-specific therapies such as vasopressin V2 receptor antagonists may increase tolerability and efficacy. Other strategies such as development of selective PKA-I inhibitors (e.g., targeting RI-specific AKAPS) also deserve consideration.
Strategies directly targeting PKA, rather than targeting cAMP through inhibition of adenylyl cyclases or activation of phosphodiesterases may be more effective in conditions where PKA activation occurs independently from cAMP. Many of these conditions may occur in ADPKD, for example115–118: (1) activation of NF-kB signaling dissociates the NF-κB:IκB complex, which under basal conditions interacts and holds PKA C in its inactive state.119,120 (2) Activation of TGF-β signaling results in the formation of heterodimeric complexes of Smad2 and Smad3 with Smad4, which bind to the PKA R subunits and activate PKA C activity.121,122 (3) Pro-oxidant conditions promote the formation of a disulfide bond between N-terminal cysteines of RI subunits and PKA activation.123 (4) Phosphorylation of the RING-H2 protein praja2 induce ubiquitin proteasome system mediated PKA R subunit proteolysis and sustained PKA C activation.124–126
Disclosures
K. Hoeflich and T. LaBranche are employees and shareholders of Blueprint Medicines Corporation. M. Palmer is an employee of and has a patent in conjunction with Blueprint Medicines Corporation; reports employment with C4 Therapeutics; and reports having an ownership interest C4 Therapeutics. P. Harris reports consultancy Mitobridge, Otsuka, Regulus, Vertex; reports receiving research funding from Acceleron, Jemincare, Navitor, and Otsuka Pharmaceuticals; and reports having patents or royalties Amgen, Bayer, Genzyme, GlaxoSmithKline, Millipore, Mitobridge, and Vertex. K. Hoeflich reports having an employer Nested Therapeutics; reports being a consultant for Turbine AI; and ownership interest Blueprint Medicines, and Nested Therapeutics. S. Schalm is an employee and shareholder of and has a patent in conjunction with Blueprint Medicines Corporation. V. Torres reports receiving grants and/or other fees from Blueprint Medicines, Mironid, Otsuka Pharmaceuticals, Palladio Biosciences, Reata, Regulus Therapeutics, and Sanofi Genzyme, all outside the submitted work; reports having advisory or leadership roles with Mironid, Otsuka Pharmaceuticals, Palladio, Reata, and Sanofi-Genzyme. All remaining authors have nothing to disclose.
Funding
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-44863 and DK-90728, by the Mayo Clinic Robert M. and Billie Kelley Pirnie Translational PKD Research Center, funding number 91384127, and by Blueprint Medicines Inc, funding number 91384148.
Supplementary Material
Acknowledgments
We thank Dr. Slobodan I. Macura and the Nuclear Magnetic Resonance Core Facility for assistance in animal imaging.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
P. Harris, K. Hoeflich, T. LaBranche, M. Palmer, S. Schalm, G. Stanley McKnight, C. Sussman, V. Torres, and X. Wang conceptualized the study; L. Jiang, K. Thao, V. Torres, and X. Wang were responsible for the data curation; L. Jiang, S. Schalm, G. Stanley McKnight, V. Torres, and X. Wang were responsible for the formal analysis; L. Jiang, K. Thao, and X. Wang were responsible for the investigation; G. Stanley McKnight was responsible for the resources; X. Wang was responsible for the project administration; X. Wang was responsible for the validation; V. Torres and X. Wang wrote original draft; and P. Harris, K. Hoeflich, L. Jiang, T. LaBranche, M. Palmer, S. Schalm, G. Stanley McKnight, C. Sussman, K. Thao, V. Torres, and X. Wang reviewed and edited the manuscript.
Data Sharing Statement
All data is included in the manuscript and/or supporting materials. More detail, data and material can be provided upon request.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021081125/-/DCSupplemental.
Supplemental Figure 1. Chemical structure of BLU2864.
Supplemental Figure 2. Cross-sections of left kidneys from all Pkd1RC/RC mice expressing RIαB and controls.
Supplemental Figure 3. Coronal MR images of kidneys from all control and BLU2864-treated Pkd1RC/RC mice at randomization and end of study.
Supplemental Figure 4. Cross-sections of left kidneys from all control and BLU2864-treated Pkd1RC/RC mice at end of study.
Supplemental Table 1. Kinases with the highest binding affinity for BLU2864.
Supplemental Table 2. Enzymatic inhibition of PRKACA and closely related kinases by BLU2864.
Supplemental Table 3. Western blot quantification normalized to loading control.
References
- 1.Harris PC, Torres VE: Polycystic kidney disease, autosomal dominant. In: GeneReviews(R), edited by Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, et al., University of Washington, Seattle, WA, 1993 [PubMed] [Google Scholar]
- 2.Ong AC, Devuyst O, Knebelmann B, Walz G; ERA-EDTA Working Group for Inherited Kidney Diseases : Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet 385: 1993–2002, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, et al. ; Conference Participants : Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 88: 17–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE: Polycystic kidney disease. Nat Rev Dis Primers 4: 50, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornec-Le Gall E, Alam A, Perrone RD: Autosomal dominant polycystic kidney disease. Lancet 393: 919–935, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Gattone VH 2nd, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH 2nd: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Gattone V 2nd, Harris PC, Torres VE: Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16: 846–851, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE: Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aihara M, Fujiki H, Mizuguchi H, Hattori K, Ohmoto K, Ishikawa M, et al. : Tolvaptan delays the onset of end-stage renal disease in a polycystic kidney disease model by suppressing increases in kidney volume and renal injury. J Pharmacol Exp Ther 349: 258–267, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. ; TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. ; REPRISE Trial Investigators : Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, et al. : A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol 29: 2458–2470, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baro Graf C, Ritagliati C, Stival C, Luque GM, Gentile I, Buffone MG, et al. : Everything you ever wanted to know about PKA regulation and its involvement in mammalian sperm capacitation. Mol Cell Endocrinol 518: 110992, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Skalhegg BS, Tasken K: Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci 5: D678–D693, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Taskén K, Aandahl EM: Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84: 137–167, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Yu B, Ragazzon B, Rizk-Rabin M, Bertherat J: Protein kinase A alterations in endocrine tumors. Horm Metab Res 44: 741–748, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Amieux PS, Howe DG, Knickerbocker H, Lee DC, Su T, Laszlo GS, et al. : Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J Biol Chem 277: 27294–27304, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH 2nd, Carney JA, Westphal H, et al. : A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65: 4506–4514, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kirschner LS, Yin Z, Jones GN, Mahoney E: Mouse models of altered protein kinase A signaling. Endocr Relat Cancer 16: 773–793, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Ward CJ, Harris PC, Torres VE: Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int 77: 129–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye H, Wang X, Constans MM, Sussman CR, Chebib FT, Irazabal MV, et al. : The regulatory 1α subunit of protein kinase A modulates renal cystogenesis. Am J Physiol Renal Physiol 313: F677–F686, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhler MD, McKnight GS: Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem 262: 15202–15207, 1987 [PubMed] [Google Scholar]
- 24.Amieux PS, Cummings DE, Motamed K, Brandon EP, Wailes LA, Le K, et al. : Compensatory regulation of RIalpha protein levels in protein kinase A mutant mice. J Biol Chem 272: 3993–3998, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Amieux PS, McKnight GS: The essential role of RI alpha in the maintenance of regulated PKA activity. Ann N Y Acad Sci 968: 75–95, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Willis BS, Niswender CM, Su T, Amieux PS, McKnight GS: Cell-type specific expression of a dominant negative PKA mutation in mice. PLoS One 6: e18772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schalm SS, O’Hearn E, Wilson K, LaBranch TP, Silva G, Zhang Z, et al. : Evaluation of PRKACA as a therapeutic target for fibrolamellar carcinoma. bioRxiv: 2022.2001.2031.477690, 2022.
- 28.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, et al. : Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29: 1046–1051, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Irazabal MV, Mishra PK, Torres VE, Macura SI: Use of ultra-high field MRI in small rodent models of polycystic kidney disease for in vivo phenotyping and drug monitoring. J Vis Exp : e52757, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards ME, Periyanan S, Anaam D, Gregory AV, Kline TL: Automated total kidney volume measurements in pre-clinical magnetic resonance imaging for resourcing imaging data, annotations, and source code. Kidney Int 99: 763–766, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, et al. : cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF: The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology 49: 160–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, et al. : A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4: 901–906, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Beebe SJ, Blackmore PF, Chrisman TD, Corbin JD: Use of synergistic pairs of site-selective cAMP analogs in intact cells. Methods Enzymol 159: 118–139, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Døskeland SO, Ogreid D: Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem 265: 10484–10491, 1990 [PubMed] [Google Scholar]
- 36.Calebiro D, de Filippis T, Lucchi S, Martinez F, Porazzi P, Trivellato R, et al. : Selective modulation of protein kinase A I and II reveals distinct roles in thyroid cell gene expression and growth. Mol Endocrinol 20: 3196–3211, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Lucchi S, Calebiro D, de Filippis T, Grassi ES, Borghi MO, Persani L: 8-Chloro-cyclic AMP and protein kinase A I-selective cyclic AMP analogs inhibit cancer cell growth through different mechanisms. PLoS One 6: e20785, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gjertsen BT, Mellgren G, Otten A, Maronde E, Genieser HG, Jastorff B, et al. : Novel (Rp)-cAMPS analogs as tools for inhibition of cAMP-kinase in cell culture. Basal cAMP-kinase activity modulates interleukin-1 beta action. J Biol Chem 270: 20599–20607, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Schwede F, Maronde E, Genieser H, Jastorff B: Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol Ther 87: 199–226, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Maronde E, Wicht H, Taskén K, Genieser HG, Dehghani F, Olcese J, et al. : CREB phosphorylation and melatonin biosynthesis in the rat pineal gland: Involvement of cyclic AMP dependent protein kinase type II. J Pineal Res 27: 170–182, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Yang L, Gilbert ML, Zheng R, McKnight GS: Selective expression of a dominant-negative type Iα PKA regulatory subunit in striatal medium spiny neurons impairs gene expression and leads to reduced feeding and locomotor activity. J Neurosci 34: 4896–4904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nedvetsky PI, Zhao X, Mathivet T, Aspalter IM, Stanchi F, Metzger RJ, et al. : cAMP-dependent protein kinase A (PKA) regulates angiogenesis by modulating tip cell behavior in a Notch-independent manner. Development 143: 3582–3590, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilbert ML, Yang L, Su T, McKnight GS: Expression of a dominant negative PKA mutation in the kidney elicits a diabetes insipidus phenotype. Am J Physiol Renal Physiol 308: F627–F638, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams SS, Cobo-Stark P, Hajarnis S, Aboudehen K, Shao X, Richardson JA, et al. : Tissue-specific regulation of the mouse Pkhd1 (ARPKD) gene promoter. Am J Physiol Renal Physiol 307: F356–F368, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, et al. : Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122: 4257–4273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solberg R, Sandberg M, Natarajan V, Torjesen PA, Hansson V, Jahnsen T, et al. : The human gene for the regulatory subunit RI alpha of cyclic adenosine 3′, 5′-monophosphate-dependent protein kinase: two distinct promoters provide differential regulation of alternately spliced messenger ribonucleic acids. Endocrinology 138: 169–181, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Salhadar K, Matthews A, Raghuram V, Limbutara K, Yang CR, Datta A, et al. : Phosphoproteomic identification of vasopressin/cAMP/PKA-dependent signaling in kidney. Mol Pharmacol 99: 358–369, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arroyo J, Escobar DL, Wells HH, Constans MC, Thao K, Smith JM, et al. : Genetic background significantly impacts the severity of renal cystic disease in the pkd1rc/rc model of ADPKD. Kidney Int : 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bossis I, Stratakis CA: Minireview: PRKAR1A: Normal and abnormal functions. Endocrinology 145: 5452–5458, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Brunton LL, Hayes JS, Mayer SE: Functional compartmentation of cyclic AMP and protein kinase in heart. Adv Cyclic Nucleotide Res 14: 391–397, 1981 [PubMed] [Google Scholar]
- 51.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, et al. : Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res 103: 836–844, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Torres-Quesada O, Mayrhofer JE, Stefan E: The many faces of compartmentalized PKA signalosomes. Cell Signal 37: 1–11, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Stakkestad Ø, Larsen AC, Kvissel AK, Eikvar S, Ørstavik S, Skålhegg BS: Protein kinase A type I activates a CRE-element more efficiently than protein kinase A type II regardless of C subunit isoform. BMC Biochem 12: 7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kopperud R, Christensen AE, Kjarland E, Viste K, Kleivdal H, Døskeland SO: Formation of inactive cAMP-saturated holoenzyme of cAMP-dependent protein kinase under physiological conditions. J Biol Chem 277: 13443–13448, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Ilouz R, Lev-Ram V, Bushong EA, Stiles TL, Friedmann-Morvinski D, Douglas C, et al. : Isoform-specific subcellular localization and function of protein kinase A identified by mosaic imaging of mouse brain. eLife 6: e17681, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker-Gray R, Stengel F, Gold MG: Mechanisms for restraining cAMP-dependent protein kinase revealed by subunit quantitation and cross-linking approaches. Proc Natl Acad Sci U S A 114: 10414–10419, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang JZ, Lu TW, Stolerman LM, Tenner B, Yang JR, Zhang JF, et al. : Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. Cell 182: 1531–1544, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sattin A: Cyclic AMP accumulation in cerebral cortex tissue from inbred strains of mice. Life Sci 16: 903–913, 1975 [DOI] [PubMed] [Google Scholar]
- 59.Hindin D, Erickson RP: Regional and strain variation in brain 3′:5′ cyclic adenosine monophosphate of inbred mice. Experientia 35: 1025–1026, 1979 [DOI] [PubMed] [Google Scholar]
- 60.Erickson RP, Butley MS, Martin SR, Betlach CJ: Variation among inbred strains of mice in adenosine 3′:5′ cyclic monophosphate levels of spermatozoa. Genet Res 33: 129–136, 1979 [DOI] [PubMed] [Google Scholar]
- 61.Newman ME, Hamburger-Bar R: Vasopressin inhibition of cyclic AMP accumulation and effects on the learned response in inbred mouse strains. Life Sci 37: 2037–2042, 1985 [DOI] [PubMed] [Google Scholar]
- 62.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, et al. : Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 26: 89–92, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Casey M, Vaughan CJ, He J, Hatcher CJ, Winter JM, Weremowicz S, et al. : Mutations in the protein kinase A R1alpha regulatory subunit cause familial cardiac myxomas and Carney complex. J Clin Invest 106: R31–R38, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL: The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64: 270–283, 1985 [DOI] [PubMed] [Google Scholar]
- 65.Stratakis CA, Kirschner LS, Carney JA: Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 86: 4041–4046, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Stratakis CA: Carney complex: A familial lentiginosis predisposing to a variety of tumors. Rev Endocr Metab Disord 17: 367–371, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamilaris CDC, Faucz FR, Voutetakis A, Stratakis CA: Carney complex. Exp Clin Endocrinol Diabetes 127: 156–164, 2018 [DOI] [PubMed] [Google Scholar]
- 68.Groussin L, Kirschner LS, Vincent-Dejean C, Perlemoine K, Jullian E, Delemer B, et al. : Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet 71: 1433–1442, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Veugelers M, Wilkes D, Burton K, McDermott DA, Song Y, Goldstein MM, et al. : Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci U S A 101: 14222–14227, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson-White AJ, Hsiao HP, Leitner WW, Greene E, Bauer A, Krett NL, et al. : Protein kinase A-independent inhibition of proliferation and induction of apoptosis in human thyroid cancer cells by 8-Cl-adenosine. J Clin Endocrinol Metab 93: 1020–1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horvath A, Mathyakina L, Vong Q, Baxendale V, Pang AL, Chan WY, et al. : Serial analysis of gene expression in adrenocortical hyperplasia caused by a germline PRKAR1A mutation. J Clin Endocrinol Metab 91: 584–596, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Mavrakis M, Lippincott-Schwartz J, Stratakis CA, Bossis I: Depletion of type IA regulatory subunit (RIalpha) of protein kinase A (PKA) in mammalian cells and tissues activates mTOR and causes autophagic deficiency. Hum Mol Genet 15: 2962–2971, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, et al. : Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/- or Rb1+/- backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet 19: 1387–1398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pringle DR, Yin Z, Lee AA, Manchanda PK, Yu L, Parlow AF, et al. : Thyroid-specific ablation of the Carney complex gene, PRKAR1A, results in hyperthyroidism and follicular thyroid cancer. Endocr Relat Cancer 19: 435–446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beristain AG, Molyneux SD, Joshi PA, Pomroy NC, Di Grappa MA, Chang MC, et al. : PKA signaling drives mammary tumorigenesis through Src. Oncogene 34: 1160–1173, 2015 [DOI] [PubMed] [Google Scholar]
- 76.Keith DS, Torres VE, King BF, Zincki H, Farrow GM: Renal cell carcinoma in autosomal dominant polycystic kidney disease. [Review] J Am Soc Nephrol 4: 1661–1669, 1994 [DOI] [PubMed] [Google Scholar]
- 77.Stewart JH, Buccianti G, Agodoa L, Gellert R, McCredie MR, Lowenfels AB, et al. : Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 14: 197–207, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Wetmore JB, Calvet JP, Yu AS, Lynch CF, Wang CJ, Kasiske BL, et al. : Polycystic kidney disease and cancer after renal transplantation. J Am Soc Nephrol 25: 2335–2341, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karami S, Yanik EL, Moore LE, Pfeiffer RM, Copeland G, Gonsalves L, et al. : Risk of renal cell carcinoma among kidney transplant recipients in the United States. Am J Transplant 16: 3479–3489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maroteaux P, Malamut G: [Acrodysostosis]. Presse Med 76: 2189–2192, 1968 [PubMed] [Google Scholar]
- 81.Robinow M, Pfeiffer RA, Gorlin RJ, McKusick VA, Renuart AW, Johnson GF, et al. : Acrodysostosis. A syndrome of peripheral dysostosis, nasal hypoplasia, and mental retardation. Am J Dis Child 121: 195–203, 1971 [PubMed] [Google Scholar]
- 82.Michot C, Le Goff C, Blair E, Blanchet P, Capri Y, Gilbert-Dussardier B, et al. : Expanding the phenotypic spectrum of variants in PDE4D/PRKAR1A: from acrodysostosis to acroscyphodysplasia. Eur J Hum Genet 26: 1611–1622, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson LC, Oude Luttikhuis ME, Baraitser M, Kingston HM, Trembath RC: Normal erythrocyte membrane Gs alpha bioactivity in two unrelated patients with acrodysostosis. J Med Genet 34: 133–136, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindstrand A, Grigelioniene G, Nilsson D, Pettersson M, Hofmeister W, Anderlid BM, et al. : Different mutations in PDE4D associated with developmental disorders with mirror phenotypes. J Med Genet 51: 45–54, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Linglart A, Menguy C, Couvineau A, Auzan C, Gunes Y, Cancel M, et al. : Recurrent PRKAR1A mutation in acrodysostosis with hormone resistance. N Engl J Med 364: 2218–2226, 2011 [DOI] [PubMed] [Google Scholar]
- 86.Briet C, Pereda A, Le Stunff C, Motte E, de Dios Garcia-Diaz J, de Nanclares GP, et al. : Mutations causing acrodysostosis-2 facilitate activation of phosphodiesterase 4D3. Hum Mol Genet 26: 3883–3894, 2017 [DOI] [PubMed] [Google Scholar]
- 87.Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, et al. : Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113: 814–825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Margaria JP, Campa CC, De Santis MC, Hirsch E, Franco I: The PI3K/Akt/mTOR pathway in polycystic kidney disease: A complex interaction with polycystins and primary cilium. Cell Signal 66: 109468, 2020 [DOI] [PubMed] [Google Scholar]
- 89.Lodeiro M, Theodoropoulou M, Pardo M, Casanueva FF, Camiña JP: c-Src regulates Akt signaling in response to ghrelin via beta-arrestin signaling-independent and -dependent mechanisms. PLoS One 4: e4686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Senoo H, Murata D, Wai M, Arai K, Iwata W, Sesaki H, et al. : KARATE: PKA-induced KRAS4B-RHOA-mTORC2 supercomplex phosphorylates AKT in insulin signaling and glucose homeostasis. Mol Cell 81: 4622–4634.e8, 2021 [DOI] [PubMed] [Google Scholar]
- 91.Tao S, Kakade VR, Woodgett JR, Pandey P, Suderman ED, Rajagopal M, et al. : Glycogen synthase kinase-3β promotes cyst expansion in polycystic kidney disease. Kidney Int 87: 1164–1175, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao C, Yuan X, Jiang Z, Gan D, Ding L, Sun Y, et al. : Regulation of AKT phosphorylation by GSK3β and PTEN to control chemoresistance in breast cancer. Breast Cancer Res Treat 176: 291–301, 2019 [DOI] [PubMed] [Google Scholar]
- 93.Li A, Xu Y, Fan S, Meng J, Shen X, Xiao Q, et al. : Canonical Wnt inhibitors ameliorate cystogenesis in a mouse ortholog of human ADPKD. JCI Insight 3: e95874, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lone MU, Miyan J, Asif M, Malik SA, Dubey P, Singh V, et al. : Direct physical interaction of active Ras with mSIN1 regulates mTORC2 signaling. BMC Cancer 19: 1236, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu Y, Lai E, Liu J, Lin J, Yang C, Jia C, et al. : IKK interacts with rictor and regulates mTORC2. Cell Signal 25: 2239–2245, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Jacob LS, Wu X, Dodge ME, Fan CW, Kulak O, Chen B, et al. : Genome-wide RNAi screen reveals disease-associated genes that are common to Hedgehog and Wnt signaling. Sci Signal 4: ra4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gorojankina T: Hedgehog signaling pathway: A novel model and molecular mechanisms of signal transduction. Cell Mol Life Sci 73: 1317–1332, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee RT, Zhao Z, Ingham PW: Hedgehog signalling. Development 143: 367–372, 2016 [DOI] [PubMed] [Google Scholar]
- 99.Pallister PD, Hecht F, Herrman J: Three additional cases of the congenital hypothalamic “hamartoblastoma” (Pallister-Hall) syndrome. Am J Med Genet 33: 500–501, 1989 [DOI] [PubMed] [Google Scholar]
- 100.Hall JG, Pallister PD, Clarren SK, Beckwith JB, Wiglesworth FW, Fraser FC, et al. : Congenital hypothalamic hamartoblastoma, hypopituitarism, imperforate anus and postaxial polydactyly--a new syndrome? Part I: clinical, causal, and pathogenetic considerations. Am J Med Genet 7: 47–74, 1980 [DOI] [PubMed] [Google Scholar]
- 101.Johnston JJ, Olivos-Glander I, Killoran C, Elson E, Turner JT, Peters KF, et al. : Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: Robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet 76: 609–622, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Démurger F, Ichkou A, Mougou-Zerelli S, Le Merrer M, Goudefroye G, Delezoide AL, et al. : New insights into genotype-phenotype correlation for GLI3 mutations. Eur J Hum Genet 23: 92–102, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Böse J, Grotewold L, Rüther U: Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum Mol Genet 11: 1129–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 104.Blake J, Hu D, Cain JE, Rosenblum ND: Urogenital development in Pallister-Hall syndrome is disrupted in a cell-lineage-specific manner by constitutive expression of GLI3 repressor. Hum Mol Genet 25: 437–447, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, et al. : GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development 133: 569–578, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Cain JE, Islam E, Haxho F, Chen L, Bridgewater D, Nieuwenhuis E, et al. : GLI3 repressor controls nephron number via regulation of Wnt11 and Ret in ureteric tip cells. PLoS One 4: e7313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.D’Cruz R, Stronks K, Rowan CJ, Rosenblum ND: Lineage-specific roles of hedgehog-GLI signaling during mammalian kidney development. Pediatr Nephrol 35: 725–731, 2020 [DOI] [PubMed] [Google Scholar]
- 108.Roskoski R Jr: A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol Res 100: 1–23, 2015 [DOI] [PubMed] [Google Scholar]
- 109.Murray AJ: Pharmacological PKA inhibition: All may not be what it seems. Sci Signal 1: re4, 2008 [DOI] [PubMed] [Google Scholar]
- 110.Saad NS, Elnakish MT, Ahmed AAE, Janssen PML: Protein kinase A as a promising target for heart failure drug development. Arch Med Res 49: 530–537, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rotman M, Hamdy NAT, Appelman-Dijkstra NM: Clinical and translational pharmacological aspects of the management of fibrous dysplasia of bone. Br J Clin Pharmacol 85: 1169–1179, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berthon AS, Szarek E, Stratakis CA: PRKACA: The catalytic subunit of protein kinase A and adrenocortical tumors. Front Cell Dev Biol 3: 26, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tomasini MD, Wang Y, Karamafrooz A, Li G, Beuming T, Gao J, et al. : Conformational landscape of the PRKACA-DNAJB1 chimeric kinase, the driver for fibrolamellar hepatocellular carcinoma. Sci Rep 8: 720, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang Y, Roelink H, McKnight GS: Protein kinase A deficiency causes axially localized neural tube defects in mice. J Biol Chem 277: 19889–19896, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Sun L, Hu C, Zhang X: TRAF3 delays cyst formation induced by NF-κB signaling. IUBMB Life 69: 170–178, 2017 [DOI] [PubMed] [Google Scholar]
- 116.Zhou J, Ouyang X, Schoeb TR, Bolisetty S, Cui X, Mrug S, et al. : Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement. J Am Soc Nephrol 23: 1161–1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hama T, Nakanishi K, Sato M, Mukaiyama H, Togawa H, Shima Y, et al. : Aberrant Smad3 phosphoisoforms in cyst-lining epithelial cells in the cpk mouse, a model of autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol 313: F1223–F1231, 2017 [DOI] [PubMed] [Google Scholar]
- 118.Liu D, Wang CJ, Judge DP, Halushka MK, Ni J, Habashi JP, et al. : A Pkd1-Fbn1 genetic interaction implicates TGF-β signaling in the pathogenesis of vascular complications in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 25: 81–91, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S: The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89: 413–424, 1997 [DOI] [PubMed] [Google Scholar]
- 120.Dulin NO, Niu J, Browning DD, Ye RD, Voyno-Yasenetskaya T: Cyclic AMP-independent activation of protein kinase A by vasoactive peptides. J Biol Chem 276: 20827–20830, 2001 [DOI] [PubMed] [Google Scholar]
- 121.Zhang L, Duan CJ, Binkley C, Li G, Uhler MD, Logsdon CD, et al. : A transforming growth factor beta-induced Smad3/Smad4 complex directly activates protein kinase A. Mol Cell Biol 24: 2169–2180, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang H, Lee CJ, Zhang L, Sans MD, Simeone DM: Regulation of transforming growth factor beta-induced responses by protein kinase A in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 295: G170–G178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schröder E, Wait R, et al. : Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem 281: 21827–21836, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Lignitto L, Carlucci A, Sepe M, Stefan E, Cuomo O, Nisticò R, et al. : Control of PKA stability and signalling by the RING ligase praja2. Nat Cell Biol 13: 412–422, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Rinaldi L, Sepe M, Donne RD, Feliciello A: A dynamic interface between ubiquitylation and cAMP signaling. Front Pharmacol 6: 177, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lignitto L, Arcella A, Sepe M, Rinaldi L, Delle Donne R, Gallo A, et al. : Proteolysis of MOB1 by the ubiquitin ligase praja2 attenuates Hippo signalling and supports glioblastoma growth. Nat Commun 4: 1822, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.