Abstract
Purpose
Reactive arthritis is acute aseptic arthritis occurring 1 to 4 weeks after a distant infection in a genetically predisposed individual. It may occur after COVID-19 infection.
We summarize, in this article, the current findings of reactive arthritis following COVID-19 infection.
Methods
A literature search has been performed from December 2019 to December 2021. We included case reports of reactive arthritis occurring after COVID-19 infection. We collected demographic, clinical, and paraclinical data.
Results
A total of 22 articles were reviewed. There were 14 men and 11 women with a mean age of 44.96 + 17.47 years. Oligoarticular involvement of the lower limbs was the most frequent clinical presentation. The time between arthritis and COVID infection ranged from 6 to 48 days. The diagnosis was based on clinical and laboratory findings. The pharmacological management was based on non-steroidal anti-inflammatory drugs in 20 cases. Systemic or local steroid therapy was indicated in 13 patients. Sulfasalazine was indicated in two cases. Alleviation of symptoms and recovery were noted in 22 cases. The mean duration of the clinical resolution was 16 + 57 days.
Conclusion
The diagnosis of reactive arthritis should be considered in patients with a new onset of arthritis following COVID-19 infection. Its mechanism is still unclear.
Keywords: Reactive arthritis, COVID-19, Non-steroidal anti-inflammatory drugs, Oligoarthritis
Introduction
Reactive arthritis (ReA) is acute aseptic arthritis occurring 1 to 4 weeks after a distant infection in a genetically predisposed individual [1].
Frequent well-known triggers of reactive arthritis are bacterial infections of the genitourinary and gastrointestinal tracts. The incidence of ReA ranged from 1 to 1.5% after gastrointestinal infection and 4 to 8% after genital Chlamydia infection [2]. ReA presents, typically, as asymmetric oligoarticular arthritis of the lower limbs. Clinical manifestations may also include enthesitis, dactylitis, bursitis, and inflammatory back pain [1–3]. In addition to bacteria, viral triggers such as parvovirus B19, Chikungunya virus, and human immunodeficiency virus (HIV) have been reported [1]. It can also occur after BCG therapy [4].
Several cases of ReA have also been reported after COVID-19 infection.
Indeed, apart from fever and respiratory symptoms, emerging COVID-19 can be responsible for articular manifestations. Although non-specific arthralgia is a common feature of acute infection, several cases of ReA following COVID-19 have been reported [5]
This manuscript aims to review and summarize current findings on ReA occurring after COVID-19 infection.
Methods
Publication search
We performed a literature search from December 2019 (when severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) was first reported) to December 2021 in SCOPUS and MEDLINE using the following keywords from the Medical Subject Headings (MeSH): (COVID-19 [Mesh]) AND (Arthritis [Mesh]), (COVID-19 [Mesh]) AND (Arthritis, Reactive [Majr]).
Inclusion criteria
We included published articles in English on reactive arthritis occurring after COVID-19 infection. We included patients having either peripheral or axial articular manifestation with a delay between the onset (or diagnosis) of COVID-19 and the onset of rheumatological symptoms.
Non-inclusion criteria
Non-inclusion criteria were cases of arthritis due to crystal flares, systemic lupus erythematosus, dermatomyositis, and rheumatoid arthritis.
We excluded patients with a history of rheumatic diseases, concomitant COVID-19 infection arthritis, and cases of arthralgia, a defined axial spondyloarthritis, and psoriatic arthritis occurring after COVID-19.
Collection data
The following pieces of information were collected from each case: age, gender, COVID-19 infection severity, the delay between COVID-19 symptoms and arthritis, involved joints, associate signs, inflammatory biomarkers, immunological tests, genetic predisposition, diagnosis, and arthritis management.
Results
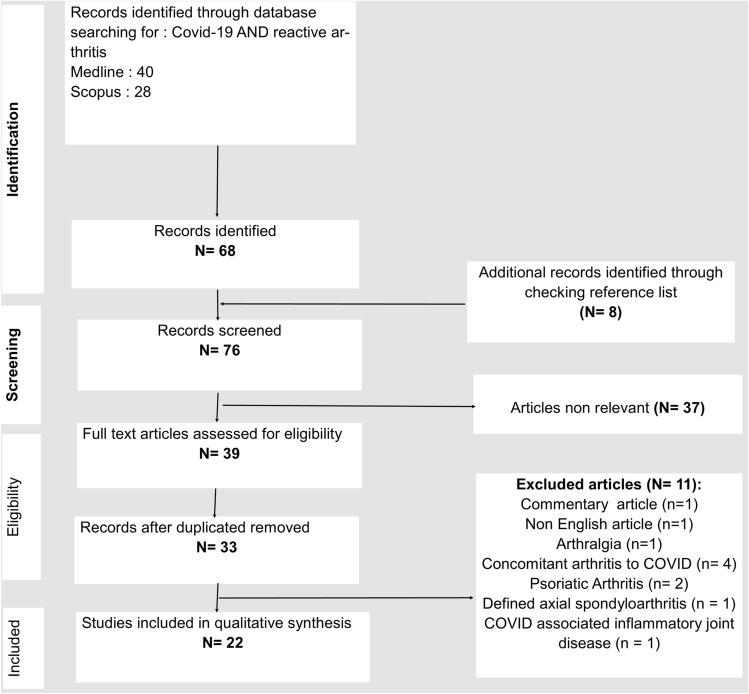
The initial search yielded 68 articles. Non-relevant and duplicated articles were removed (Fig. 1).
Fig. 1.
Flow chart for the study selection process
We excluded one case of arthralgia occurring after COVID-19 [6], four articles describing concomitant arthritis to COVID-19 infection [7–10], one case of a defined axial spondyloarthritis [11], and two cases of psoriatic arthritis occurring after COVID-19 [12, 13].
Another article was excluded because the authors described clinical and laboratory features of 35 patients with SARS-CoV-2-associated inflammatory joint disease without specifying the definite diagnosis for each patient. Moreover, the synovial fluid analysis was not performed, making the exclusion of crystal arthritis difficult [14].
Twenty-five cases (22 articles) of ReA occurring after COVID-19 infection are summarized in (Table 1) [15–36].
Table 1.
Cases of reactive arthritis occuring after COVID-19
| Reference | Age (years)/Sex | COVID-19 infection severitya/Treatment | Delay between COVID-19 and arthritis (days)/RT-PCR at onset of arthritis | Involved joints/Associate signs | Inflam- Matory Biomarkers | Immunological tests | HLA genotyping | Arthritis management | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Liew et al. [15] | 47/Male | Asymptomatic/None | 6/Positive | Right knee/Balanitis | N.S | N.S | N.S | NSAIDs IASI | N.S |
| Schenker et al. [16] | 65/Female | Moderate/NS | 10/Negative | Ankles, wrists and knee joints/Purpura of the calves | CRP: 34.7 mg/L | FR, ACPA and ANA negative | HLA-B27: positive | Prednisolone | Recovered |
| Saricaoglu et al. [17] | 73/Male | Moderate/Ceftriaxone Hidroxychloroquine Azitromycin | 8/Negative | Toes dactylitis | CRP:25 mg/L | RF and ACPA negative | N.S | NSAIDs | Symptoms resolution after 22 days of NSAIDs |
| Ono et al. [18] | 50/Male | Critical/Favipiravir cefepime vancomycin | 21/NS | Ankles Calcaneal enthesitis | CRP: 7.4 mg/dL | Negative | HLA -B27:negative | NSAIDs IASI | Moderate improvement |
| Sureja et al. [19] | 27/Female | Moderate/Methylprednisolone Favipiravir | 14/Negative | Knees, ankles, midfoot joints, small joints of the right hand | N.S | RF: positive in low titres. ACPA and ANA: negative | HLA-B27: negative | NSAIDs Opioid analgesic. Steroid | Recovered within 3 weeks |
| Honge et al.[20] | 53/male | Severe/dexamethasone | 16/Negative | Right knee and ankles | CRP: 327 mg/L | RF, ACPA, AAN: negative | HLA-B27: negative | NSAIDs Prednisolone (25 mg daily) | Recovered |
| Shokraee et al. [21] | 58/female | Severe/Interferon β1 Dexamethasone Ceftriaxone | 15 /NS | Right hip | CRP: 6.5 mg/L ESR: 45 mm | N.S | N.S | NSAIDs Prednisolone (80 mg) | Recovered within 5 days |
| Coath et al.[22] | 53/male | Mild/None | N.S | Sacroiliac costovertebral and costotransverse joints | CRP: 13 mg/L | NS | HLA-B27: positive | NSAIDs Methylprednisolone 120 mg | Recovered |
| Danssaert et al. [23] | 37/female | Moderate/NS | 12/Negative | Wrist,MCP,IP joints/Extensor tendinosis | CRP and ESR within normal limits | RF: negative ANA: speckled | N.S | NSAIDs topical Gabapentin Opioid | Improvement without recovery |
| Sinaei et al. [24] | 8/male | Mild/NS | 7/NP | Left hip | ESR: 3 mm CRP: 3.5 mg/L | RF and ANA: negative | N.S | NSAIDs | Recovered within one week |
| 6/female | Moderate/NS | 7/Positive | Knees, wrists, left hip | ESR = 39 mm CRP = 12 mg/L | RF and ANA: negative | N.S | NSAIDs | Recovered within 4 days | |
| Jali et al. [25] | 39/female | Moderate/NS | 21/Negative | Proximal and distal IP joints of the hands | ESR and CRP within the normal range | RF, ANA and ACPA: negative | N.S | NSAIDs | Recovered |
| Parisi et al. [26] | 58/female | Moderate/Paracetamol | 25/Negative | Ankle / Tendinosisof the Achilles tendon | CRP: 7.36 mg/L | RF, ANA, Antiextractable nuclear antigen, Antidouble-strand DNA: negative | N.S | NSAIDs | Clinical recovery within one month |
| Di-Carlo et al. [27] | 55/male | Mild/NS | 42/NS Negative 15 days after COVID infection | Right ankle | ESR: 67 mm CRP: 5.6 mg/L | N.S | HLA-B27: negative | Steroid | Recovered |
| Salvatierra J et al. [28] | 16/female | Mild/NS | 21/Negative | Dactylitis | NS | RF and ANA negative | HLA B27 negative | NSAIDs | Recovered within 5 days |
| Kocyigit BF et al. [29] | 53/female | Moderate/Favipiravir Hydroxychloroquine Azithromycin | 41/NS | Knee | CRP:10.8 mg/L | RF,ACPA,ANA negative | HLA B27 negative | NSAIDs | Recovered within 10 days |
| Ouedraogo F et al. [30] | 45/male | Critical/Azithromycin Ceftriaxone Hydroxychloroquine Tocilizumab | 48/Negative | Shoulders, Elbow, knee | CRP:187 mg/L ESR:136 mm/h | RF,ACPA:negative | NS | Steroid | Improvement |
| Gasparotto et al. [31] | 60/male | Severe/Azithromycin CeftriaxoneHydroxychloroquine | 32/Negative | Ankle, knee, hip | CRP:237 mg/L ESR:111 mm/h | RF, ACPA, ANA:negative | HLA B27 negative | NSAIDs | Recovered within 40 days of NSAIDs Follow up of 6 months |
| Apaydin H et al. [32] | 37/male | Mild/Hydroxychloroquine | 9/ Positive | Wrists, knees,ankles, elbows,MTP | CRP:117 mg/L ESR:63 mm/h | RF,ACPA,ANA: negative | HLA B27 positive | Methylprednisolone 16 mg/day | Persistant arthralgia 1 month later Initiation of Sulfasalazine |
| Yokogawa N et al.[33] | 57/male | Moderate/No specific treatment | 17 / Positive | Wrist, Shoulder, the bilateral knees | CRP:48 mg/L | RF,ACPA,ANA:negative | N.S | N.S | Spontaneous reolution after 10 days |
| Ghauri M et al. [34] | 34/male | Mild/AzithromycinZincMultivitamin | 16 /NS | Knee | N.S | N.S | N.S | NSAIDs IASI | Recoverd within 10 days |
| Colatutto et al. [35] | 58/female | Mild/Azithromycin Hydroxychloroqine | 28/ Negative | Shoulders, Sacroiliac | Normal | ANA,RF:negative | HLA-B8 and B57 positive HLA-B27 negative | NSAIDs | Persistance of sacroiliac joint pain at 7 months |
| 53/female | Mild/Azithromycin Hydroxychloroqine | 15/ Negative | Sacroiliac | CRP:19 mg/L | ANA,RF:negative | N.S | NSAIDs | Recovered (9 months of follow-up) | |
| El Hasbani et al. [36] | 25/male | Mild/NS | 19/ Negative | Left ankle, right elbow, low back pain, sacroiliac | CRP: 207 mg/dL | RF,ACPA,ANA:negative | HLA-B27 positive | NSAIDs Prednisolone (40 mg daily) Sulfasalazine | Improvement within 1 month |
| 57/male | Mild/NS | 30/ Negative | Left wrist Synovial thickening of the common extensor bursa | CRP: 28.9 mg/dL | RF,ACPA,ANA:negative | HLA-B27 positive | NSAIDs Prednisolone (30 mg daily) | Rapid improvement within 2 weeks |
RF rheumatoid factor, ACPA anti-cyclic citrullinated peptide antibodies, ANA antinuclear antibodies, NS not specified, NP not performed, MCP metacarpophalangeal joints, MTP metatarsophalangeal joints, IP interphalangeal joints, CRP C-reactive protein, ESR erythrocyte sedimentation rate, HLA human leucocyte antigen, NSAIDs non steroidal anti inflammatory drugs, IASIintra articular steroid injection
aSymptoms of COVID-19 infection were collected and severity was classified as: asymptomatic, mild, moderate, severe, and critical
There were 14 men and 11 women with a mean age of 44.96 + 17.47 years. The mean delay between infection and arthritis was 20 + 11.67 days, ranging from 6 to 48 days. COVID-19 severity ranged from asymptomatic to critical form. During COVID-19 infection, the following treatment was prescribed: Favipiravir (n = 3), Interferon β1 (n = 1), Hydroxychloroquine (n = 7), Azithromycin (n = 7), Tocilizumab (n = 1), and Ceftriaxone (n = 4).
Peripheral articular manifestations were noted in 20 cases. The affected peripheral joints were knees (n = 11), foot (n = 2), ankles (n = 9), hips (n = 3), wrists (n = 6), hands (n = 3), elbows (n = 3), and shoulders (n = 2).
The sacroiliac joints were affected in four cases and costotransverse joints in one case.
Patients developed Achilles tendinosis (n = 1), tenosynovitis of the common extensor tendons (n = 1), and extensor tendinosis (n = 1).
Dactylitis was reported in 2 cases and calcaneal enthesitis in one case. Two patients had cutaneous manifestations: peripheral purpura (n = 1) and balanitis (n = 1). Human Leukocyte Antigen (HLA) B27 was investigated in 13 subjects and was positive in five cases (38%).
The mean C reactive protein (CRP) was 61.89 + 94.92 mg/L. The CRP was normal in 3 cases and not specified in 3 others.
Immunological tests, including rheumatoid factor, anti-citrullinated protein antibodies, and antinuclear antibodies, were searched in 17 patients and were negative in all of them, except for one case with positive rheumatoid factor in low titres [19].
The synovial fluid was analyzed in eight patients. Synovial fluid COVID-19 PCR, done in three patients, was negative.
A radiograph of the affected joints was performed in six cases. It did not reveal erosions in [15, 17, 18, 20, 25, 36]. Articular ultrasound (US), done in three cases, revealed synovitis in all patients [21, 24, 26].
Seven patients underwent magnetic resonance imaging (MRI). It showed bone marrow oedema of the sacroiliac joints in four cases, sacroiliac joint effusion in two patients [21, 24, 34–36], and wrist joint synovitis with mild tenosynovitis of the flexor tendons in one patient [36].
The pharmacological management was based on non-steroidal anti-inflammatory drugs (NSAIDs) in 20 cases. Systemic or local steroid therapy was indicated in 13 patients. Sulfasalazine was indicated in two cases [32, 36]. Alleviation of symptoms and recovery were noted in 22 cases. The mean duration of the clinical resolution was 16 + 57 days. Persistent arthralgia was reported in two patients [32, 35]. Clinical follow-up was not available in one case.
Discussion
ReA is acute aseptic arthritis occurring 1 to 4 weeks after a distant infection, typically in a genetically predisposed individual [1]. There is no agreement on the diagnostic criteria of ReA.
[37]. ReA belongs to the group of spondyloarthritis (SpA) [38].
In 1996, diagnostic criteria for ReA have been proposed [39]. According to these criteria, the diagnosis of ReA can be established in patients with asymmetric oligoarthritis affecting predominantly the lower limbs associated with evidence of preceding infection (clear clinical diarrhoea or urethritis within the preceding 4 weeks) [39]. Laboratory confirmation of infection is essential if no clear preceding infection is noted. Other known causes of mono/oligoarthritis, such as defined spondyloarthropathies, septic arthritis, crystal arthritis, and Lyme disease, should be excluded before making the ReA diagnosis. Besides, the diagnosis of ReA does not require the presence of HLA-B27 [39].
In 2009, the Assessment of SpondyloArthritis International Society (ASAS) classification criteria for SpA subdivided SpA into axial and peripheral SpA. ReA corresponds to peripheral SpA with preceding infection. The latter was defined as urethritis, cervicitis, or diarrhoea occurring within one month before arthritis, enthesitis, or dactylitis [38].
In 2014, more recent diagnostic criteria were introduced. The diagnosis of probable ReA can be made if mono or oligoarthritis occurs 3 days to 6 weeks after enteritis or urethritis. Evidence of the triggering infection is necessary for a definite diagnosis of ReA [3].
Recently, the definition of ReA evolved, encompassing any arthritis occurring after mucosal infection [40]. Frequent well-known triggers of ReA are bacterial infections of the genitourinary and gastrointestinal tracts. Many infectious agents have also been reported to cause ReA [41]. COVID-19 is accepted as one of the new causative agents of ReA [40]. Post-COVID ReA diagnosis can be made based on a delayed occurrence of rheumatological manifestations after COVID-19 clinical recovery or nasopharyngeal Real-time polymerase chain reaction (RT-PCR) negativation [42]. Of note, RT-PCR positivity can be observed in subjects who have already been in remission [43].
There is no consensus regarding the status of HLA-B27 positivity for post-viral ReA [40].
ReA should be distinguished from viral infection-related arthritis. The latter is associated with prodromal symptoms or features of disseminated viral infection [44].
Epidemiological information regarding ReA post-COVID is scarce. In this review, a total number of 25 cases was included.
The pathophysiology is still unclear. Several mechanisms have been suggested: the arthritogenic potential of SARS-CoV-2 [14], molecular mimicry [45], immune complex formation [46], and auto-reactive T cells activation [47].
Indeed, COVID-19 infection can induce the recruitment of inflammatory cells and the secretion of pro-inflammatory cytokines such as interleukins (IL-1, IL-6, IL-17) and tumor necrosis factor (TNF)-α [48]. Cytokine levels are increased in synovial tissue of patients with ReA, inducing arthritis [49]. Interleukin 17 could represent a link between viral infection and ReA since this interleukine is involved in the pathogenesis of ReA [50].
This auto-inflammatory dysregulation may induce auto-immune disorders in predisposed patients. Some auto-immune diseases can occur during COVID-19 infection, such as Guillain–Barré syndrome, dermatomyositis, and auto-immune hemolytic anemia [51–53]. A post-COVID auto-immunity has also been suggested since cases of rheumatoid arthritis have been triggered after the infection [54–59].
Several studies demonstrated that the SARS-CoV-2 shares molecular epitopes with human proteins, which may cross-react and trigger auto-immune disorders [45]. Mimicking epitopes may be present in the synovial membrane and induce, therefore, an acute local inflammation. Acute arthritis can also result from circulated auto-immune complexes deposition in peripheral articular joints [46].
Like gastrointestinal or genitourinary system infection, COVID-19 has been reported to activate autoreactive immune T cells, especially in patients with HLA B27 [60].
In our review, HLA B27 was tested only in 13 patients and was positive in five cases. The authors suggested that HLA B27 is responsible for chronic synovial inflammation. In these cases, Conventional Disease-modifying Anti Rheumatic drugs such as sulfasalazine have been indicated [32].
These findings indicate that SARS-CoV-2 infection can trigger ReA, and further research is needed to delineate whether SARS-CoV-2 itself is an arthritogenic agent.
Associated factors with ReA are not well established. The severity of COVID-19 infection seems unlikely. Genetic predisposition appears to be the prominent factor that determines the immune response of the SARS-CoV-2 host.
The mean delay between COVID-19 infection and the onset of ReA was variable, ranging from 6 to 48 days. Clinical manifestations are not different from other causes of ReA, including often oligoarthritis of large joints, mainly of lower limbs. Nevertheless, axial skeletal involvement such as sacroiliitis, lumbar spine manifestations, and enthesitis has been reported [18, 22, 28, 35].
Radiological findings are not specific, but they are necessary to rule out differential diagnoses. Radiographs of involved joints are commonly unremarkable [15–18, 20, 25]. Joint ultrasound and MRI may show articular effusion, synovial thickness, and soft tissue edema [24, 26, 34, 35].
SARS-CoV-2 PCR is usually negative given the delay between the systemic infection and the articular involvement. Synovial fluid PCR may also be performed to confirm the sterile nature of the disease [5, 18, 30]. Inflammatory markers are commonly raised and may reach significant values, mimicking septic arthritis [20, 31, 32].
These findings suggest that the diagnosis of ReA should be considered in patients with rheumatological manifestations occurring after a COVID-19 infection. Thus, it is necessary to rule out differential diagnoses such as septic arthritis, crystal-associated arthritis, or another inflammatory rheumatic disease onset. Arthrocentesis with bacterial and crystal analysis should be performed before retaining the ReA diagnosis.
Microcrystalline arthritis can occur in patients with COVID-19. It has been diagnosed in four cases among 306 (1.3%) [61]. Three had a previous diagnosis of gout [61].In these cases, arthritis started 1 to 4 weeks after the COVID infection [61].
Besides, viral arthritis related to COVID-19 is possible [10, 62]. In this situation, arthritis is typically concomitant with fever and respiratory symptoms. In the case reported by Kushner et al., SARS CoV-2 synovial fluid PCR was also positive [10]. The mechanism of viral inoculation remains unclear.
Some drugs prescribed during COVID may be responsible for arthritis that could be confounded with ReA. Favipiravir is a pyrazine derivative that inhibits viral RNA-dependent RNA polymerase. This drug, prescribed in three cases among patients included in this review [18, 19, 29], may induce hyperuricemia and acute gouty arthritis [63]. However, the authors of these articles did not report hyperuricemia in included cases.
Interferon β1 treatment may be responsible for inflammatory rheumatic diseases in non-COVID patients [64, 65]. Only one patient included in our review received Interferon β1 treatment and showed hip involvement without signs of psoriasis or rheumatic arthritis, with a recovery within 5 days [21].
The management of COVID-19 ReA did not differ from classic forms. NSAIDs remain the first-line treatment leading to the alleviation of articular manifestations. Systemic or intraarticular steroid injections can also be indicated.
This updated review summarizes the different clinical, biological, and imaging features of ReA related to COVID-19. The main limitations are related to the paucity of series with large sample size and the limited duration of follow-up after COVID-19. A longer follow-up may clarify the effect of COVID-19 disease on the frequency of rheumatic diseases.
Conclusion
The diagnosis of ReA should be considered in patients with a new onset of arthritis following COVID-19 infection. Despite its rarity, ReA may occur with different clinical manifestations such as arthritis, enthesitis, or dactylitis. Synovial fluid analysis remains essential to exclude differential diagnoses. Clinical management is similar to other forms of ReA. Coronavirus should be added to the list of agents associated with ReA.
A longitudinal follow-up of these patients is needed to assess the evolution of these cases to radiographic axial spondyloarthritis.
Acknowledgements
None.
Author contributions
Dr. MS: methodology and writing-review and editing. Dr. MA: roles/writing-original draft. Dr. TM: data curation and formal analysis. Dr. RD: data analysis. Dr. LM: conceptualization. Dr. IG: supervision. Dr. BL: validation.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
References
- 1.García-Kutzbach A, Chacón-Súchite J, García-Ferrer H, Iraheta I. Reactive arthritis: update 2018. Clin Rheumatol. 2018;37(4):869–874. doi: 10.1007/s10067-018-4022-5. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt SK. Reactive arthritis. Infect Dis Clin North Am. 2017;31(2):265–277. doi: 10.1016/j.idc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13(4–5):546–549. doi: 10.1016/j.autrev.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Slouma M, Chammakhi M, Dhahri R, Metoui L, Boussetta N, Ajili F, et al. Unusual evolution of reactive arthritis induced by BCG therapy. Therapie. 2019;74(6):685–688. doi: 10.1016/j.therap.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Eastin C, Eastin T. Clinical characteristics of coronavirus disease 2019 in China. J Emerg Med. 2020;58(4):711–712. doi: 10.1016/j.jemermed.2020.04.004. [DOI] [Google Scholar]
- 6.Fragata I, Mourão AF. Coronavirus disease 19 (COVID-19) complicated with post-viral arthritis. Acta Reumatol Port. 2020;45(4):278–280. [PubMed] [Google Scholar]
- 7.Alivernini S, Cingolani A, Gessi M, Paglionico A, Pasciuto G, Tolusso B, et al. Comparative analysis of synovial inflammation after SARS-CoV-2 infection. Ann Rheum Dis. 2020;6:annrheumdis2020 218315. doi: 10.1136/annrheumdis-2020-218315. [DOI] [PubMed] [Google Scholar]
- 8.Houshmand H, Abounoori M, Ghaemi R, Bayat S, Houshmand G. Ten-year-old boy with atypical COVID-19 symptom presentation: a case report. Clin Case Rep. 2020;9:304–8. doi: 10.1002/ccr3.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talarico R, Stagnaro C, Ferro F, Carli L, Mosca M. Symmetric peripheral polyarthritis developed during SARS-CoV-2 infection. Lancet Rheumatol. 2020;2(9):e518–e519. doi: 10.1016/S2665-9913(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuschner Z, Ortega A, Mukherji P. A case of SARS-CoV-2-associated arthritis with detection of viral RNA in synovial fluid. J Am Coll Emerg Physicians Open. 2021;2:e12452. doi: 10.1002/emp2.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saikali W, Gharib S. The first non-radiographic axial spondyloarthrits with COVID-19. Immun Inflamm Dis. 2021 doi: 10.1002/iid3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cincinelli G, Di Taranto R, Orsini F, Rindone A, Murgo A, Caporali R. A case report of monoarthritis in a COVID-19 patient and literature review: Simple actions for complex times. Medicine (Baltimore) 2021;100:e26089. doi: 10.1097/MD.0000000000026089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Stefano L, Rossi S, Montecucco C, Bugatti S. Transient monoarthritis and psoriatic skin lesions following COVID-19. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218520. [DOI] [PubMed] [Google Scholar]
- 14.Ursini F, Ruscitti P, D’Angelo S, Cacciapaglia F, De Angelis R, Campochiaro C, et al. Broad clinical spectrum of SARS-CoV-2-associated inflammatory joint disease in adults: a report of 35 cases from the COVID-19 and Autoimmune systemic disease Italian study group. Ann Rheum Dis. 2021;80(11):1498–1501. doi: 10.1136/annrheumdis-2021-220606. [DOI] [PubMed] [Google Scholar]
- 15.Liew IY, Mak TM, Cui L, Vasoo S, Lim XR. A case of reactive arthritis secondary to coronavirus disease 2019 infection. JCR J Clin Rheumatol. 2020;26(6):233–233. doi: 10.1097/RHU.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schenker HM, Hagen M, Simon D, Schett G, Manger B. Reactive arthritis and cutaneous vasculitis after SARS-CoV-2 infection. Rheumatology. 2021;60(1):479–480. doi: 10.1093/rheumatology/keaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saricaoglu EM, Hasanoglu I, Guner R. The first reactive arthritis case associated with COVID-19. J Med Virol. 2021;93(1):192–193. doi: 10.1002/jmv.26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono K, Kishimoto M, Shimasaki T, Uchida H, Kurai D, Deshpande GA, et al. Reactive arthritis after COVID-19 infection. RMD Open. 2020;6:e001350. doi: 10.1136/rmdopen-2020-001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sureja NP, Nandamuri D. Reactive arthritis after SARS-CoV-2 infection. Rheumatol Adv Pract. 2021;5:rkab001. doi: 10.1093/rap/rkab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hønge BL, Hermansen MLF, Storgaard M. Reactive arthritis after COVID-19. BMJ Case Rep. 2021;14:e241375. doi: 10.1136/bcr-2020-241375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shokraee K, Moradi S, Eftekhari T, Shajari R, Masoumi M. Reactive arthritis in the right hip following COVID-19 infection: a case report. Trop Dis Travel Med Vaccines. 2021;7(1):18. doi: 10.1186/s40794-021-00142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coath FL, Mackay J, Gaffney JK. Axial presentation of reactive arthritis secondary to COVID-19 infection. Rheumatol Oxf Engl. 2021;20:keab009. doi: 10.1093/rheumatology/keab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danssaert Z, Raum G, Hemtasilpa S. Reactive arthritis in a 37-year-old female with SARS-CoV2 infection. Cureus. 2020;12:e9698. doi: 10.7759/cureus.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinaei R, Pezeshki S, Parvaresh S, Sinaei R, Shiari R, Hassas Yeganeh M, et al. Post SARS-CoV-2 infection reactive arthritis: a brief report of two pediatric cases. Pediatr Rheumatol. 2021;19(1):89. doi: 10.1186/s12969-021-00555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jali I. Reactive arthritis after COVID-19 infection. Cureus. 2020;12:e11761. doi: 10.7759/cureus.11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parisi S, Borrelli R, Bianchi S, Fusaro E. Viral arthritis and COVID-19. Lancet Rheumatol. 2020;2:e655. doi: 10.1016/S2665-9913(20)30348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Carlo M, Tardella M, Salaffi F. Can SARS-CoV-2 induce reactive arthritis? Clin Exp Rheumatol. 2021;128:25–6. [PubMed] [Google Scholar]
- 28.Salvatierra J, Martínez-Peñalver D, Salvatierra-Velasco L. COVID-19 related dactyitis. Joint Bone Spine. 2020;87(6):660. doi: 10.1016/j.jbspin.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kocyigit BF, Akyol A. Reactive arthritis after COVID-19: a case-based review. Rheumatol Int. 2021;41(11):2031–2039. doi: 10.1007/s00296-021-04998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouedraogo F, Navara R, Thapa R, Patel KG Reactive arthritis post-SARS-CoV-2. Cureus. 2021 Sep 20 [cited 2022 May 8]. Available from: https://www.cureus.com/articles/64634-reactive-arthritis-post-sars-cov-2 [DOI] [PMC free article] [PubMed]
- 31.Gasparotto M, Framba V, Piovella C, Doria A, Iaccarino L. Post-COVID-19 arthritis: a case report and literature review. Clin Rheumatol. 2021;40:3357–3362. doi: 10.1007/s10067-020-05550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apaydin H, Guven SC, Kucuksahin O, Omma A, Erten S. A case of human leukocyte antigen B27 positive reactive arthritis associated with severe acute respiratory syndrome coronavirus 2 infection. North Clin Istanb. 2021;8:423–424. doi: 10.14744/nci.2020.88965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokogawa N, Minematsu N, Katano H, Suzuki T. Case of acute arthritis following SARS-CoV-2 infection. Ann Rheum Dis. 2021;80:e101–e101. doi: 10.1136/annrheumdis-2020-218281. [DOI] [PubMed] [Google Scholar]
- 34.Ghauri MI, Mukarram MS, Riaz K, Syeda U. Post covid-19 reactive arthritis: an emerging existence in the spectrum of musculoskeletal complications of SARSCoV-2 infection. Int J Clin Rheumatol. 2020;0:198. [Google Scholar]
- 35.Colatutto D, Sonaglia A, Zabotti A, Cereser L, Girometti R, Quartuccio L. Post-COVID-19 arthritis and sacroiliitis: natural history with longitudinal magnetic resonance imaging study in two cases and review of the literature. Viruses. 2021;13:1558. doi: 10.3390/v13081558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Hasbani G, Jawad A, Uthman I. Axial and peripheral spondyloarthritis triggered by sars-cov-2 infection: a report of two cases. Reumatismo. 2021;73:59–63. doi: 10.4081/reumatismo.2021.1374. [DOI] [PubMed] [Google Scholar]
- 37.Sieper J, Braun J, Kingsley GH. Report on the fourth international workshop on reactive arthritis. Arthritis Rheum. 2000;43:720–734. doi: 10.1002/1529-0131(200004)43:4<720::AID-ANR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 39.Kingsley G, Sieper J. Third international workshop on reactive arthritis. 23–26 september 1995, Berlin, Germany report and abstracts. Ann Rheum Dis. 1996;55:564–84. doi: 10.1136/ard.55.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bekaryssova D, Yessirkepov M, Zimba O, Gasparyan AY, Ahmed S. Reactive arthritis before and after the onset of the COVID-19 pandemic. Clin Rheumatol. 2022;41:1641–1652. doi: 10.1007/s10067-022-06120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeidler H. Post-COVID‑19-Arthritis. Manifestation unter dem klinischen Bild einer reaktiven Arthritis. Z Für Rheumatol. 2021 Jul 9 [cited 2021 Jul 27]. Available from: https://link.springer.com/10.1007/s00393-021-01045-9 [DOI] [PMC free article] [PubMed]
- 42.Wendling D, Prati C, Chouk M, Verhoeven F. Reactive arthritis: treatment challenges and future perspectives. Curr Rheumatol Rep. 2020;22:29. doi: 10.1007/s11926-020-00904-9. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Galiana J, De Lucas RP, García-Botella A, García-Lledó A, Gómez-Pavón J, González Del Castillo J, et al. Persistence and viability of SARS-CoV-2 in primary infection and reinfections. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter. 2022;35:1–6. doi: 10.37201/req/129.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma V, Sharma A. Infectious mimics of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2022;36:101736. doi: 10.1016/j.berh.2021.101736. [DOI] [PubMed] [Google Scholar]
- 45.Kanduc D, Shoenfeld Y. On the molecular determinants of the SARS-CoV-2 attack. Clin Immunol. 2020;1:108426. doi: 10.1016/j.clim.2020.108426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yazdanpanah N, Rezaei N. Autoimmune complications of COVID-19. J Med Virol. 2022;94:54–62. doi: 10.1002/jmv.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen RL, Gillespie GM, Hall F, Edmonds S, Hall MA, Wordsworth BP, et al. Multiple T cell expansions are found in the blood and synovial fluid of patients with reactive arthritis. J Rheumatol. 1997;24:1750–1757. [PubMed] [Google Scholar]
- 48.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaurasia S, Shasany AK, Aggarwal A, Misra R. Recombinant Salmonella typhimurium outer membrane protein A is recognized by synovial fluid CD8 cells and stimulates synovial fluid mononuclear cells to produce interleukin (IL)-17/IL-23 in patients with reactive arthritis and undifferentiated spondyloarthropathy. Clin Exp Immunol. 2016;185:210–218. doi: 10.1111/cei.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wendling D, Prati C, Chouk M, et al. Reactive arthritis: treatment challenges and future perspectives. Curr Rheumatol Rep. 2020;22:29. doi: 10.1007/s11926-020-00904-9. [DOI] [PubMed] [Google Scholar]
- 51.Kilinc D, van de Pasch S, Doets AY, Jacobs BC, van Vliet J, Garssen MPJ. Guillain-Barré syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27:1757–1758. doi: 10.1111/ene.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gokhale Y, Patankar A, Holla U, Shilke M, Kalekar L, Karnik ND, et al. Dermatomyositis during COVID-19 pandemic (a case series): is there a cause effect relationship? J Assoc Physicians India. 2020;68:20–24. [PubMed] [Google Scholar]
- 53.Jacobs J, Eichbaum Q. COVID-19 associated with severe autoimmune hemolytic anemia. Transfusion (Paris) 2021;61:635–640. doi: 10.1111/trf.16226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah S, Danda D, Kavadichanda C, Das S, Adarsh MB, Negi VS. Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol Int. 2020;40:1539–1554. doi: 10.1007/s00296-020-04639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derksen VFAM, Kissel T, Lamers-Karnebeek FBG, van der Bijl AE, Venhuizen AC, Huizinga TWJ, et al. Onset of rheumatoid arthritis after COVID-19: coincidence or connected? Ann Rheum Dis. 2021;1:annrheumdis 2021 219859. doi: 10.1136/annrheumdis-2021-219859. [DOI] [PubMed] [Google Scholar]
- 56.Perrot L, Hemon M, Busnel JM, Muis-Pistor O, Picard C, Zandotti C, et al. First flare of ACPA-positive rheumatoid arthritis after SARS-CoV-2 infection. Lancet Rheumatol. 2021;3:e6–8. doi: 10.1016/S2665-9913(20)30396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slouma M, Mhemli T, Abbes M, Triki W, Dhahri R, Metoui L, et al. Rheumatoid arthritis occurring after coronavirus disease 2019 (COVID-19) infection: case based review. Egypt Rheumatol. 2022;44:275–278. doi: 10.1016/j.ejr.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roongta R, Chattopadhyay A, Ghosh A. Correspondence on ‘Onset of rheumatoid arthritis after COVID-19: coincidence or connected?’ Ann Rheum Dis. 2021 Apr 27 [cited 2021 Jul 25]. Available from: https://ard.bmj.com/content/early/2021/04/26/annrheumdis-2021-220479 [DOI] [PubMed]
- 59.Baimukhamedov C, Barskova T, Matucci-Cerinic M. Arthritis after SARS-CoV-2 infection. Lancet Rheumatol. 2021;3:e324–e325. doi: 10.1016/S2665-9913(21)00067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maggi E, Azzarone BG, Canonica GW, Moretta L. What we know and still ignore on COVID-19 immune pathogenesis and a proposal based on the experience of allergic disorders. Allergy. 2022;77:1114–1128. doi: 10.1111/all.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.del LópezGonzález MC, PeralGarrido ML, Calabuig I, TovarSugrañes E, Jovani V, Bernabeu P, et al. Case series of acute arthritis during COVID-19 admission. Ann Rheum Dis. 2021;80:e58–e58. doi: 10.1136/annrheumdis-2020-217914. [DOI] [PubMed] [Google Scholar]
- 62.Alivernini S, Cingolani A, Gessi M, Paglionico A, Pasciuto G, Tolusso B, et al. Comparative analysis of synovial inflammation after SARS-CoV-2 infection. Ann Rheum Dis. 2021;80:e91–e91. doi: 10.1136/annrheumdis-2020-218315. [DOI] [PubMed] [Google Scholar]
- 63.Hase R, Kurata R, Ishida K, Kurita T, Muranaka E, Mito H. Acute gouty arthritis during favipiravir treatment for coronavirus Disease 2019. Intern Med Tokyo Jpn. 2020;59(18):2327–2329. doi: 10.2169/internalmedicine.5377-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hojjati SMM, Heidari B, Babaei M. Development of rheumatoid arthritis during treatment of multiple sclerosis with interferon beta 1-a. Coincidence of two conditions or a complication of treatment: a case report. J Adv Res. 2016;7:611–3. doi: 10.1016/j.jare.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toussirot E, Béreau M, Bossert M, Malkoun I, Lohse A. Occurrence of psoriatic arthritis during interferon beta 1a treatment for multiple sclerosis. Case Rep Rheumatol. 2014;2014:949317. doi: 10.1155/2014/949317. [DOI] [PMC free article] [PubMed] [Google Scholar]