Abstract
Background and Objectives
Blood biomarkers may allow earlier identification of Parkinson disease (PD), parkinsonism, and poor PD-related outcomes, such as physical functioning. Neurofilament light (NfL), a neuronal cytoplasmic protein, is a biomarker of neurodegeneration measurable in biofluids. Our objective was to examine the association of serum NfL at baseline with clinically diagnosed PD, parkinsonian signs, and physical functioning change over 16 years in a population-based sample of older adults.
Methods
Data came from 1,327 older participants from the Chicago Health and Aging Project, a longitudinal population-based study. Clinical evaluations included assessing parkinsonian signs in 4 domains—bradykinesia, parkinsonian gait, rigidity, and tremors—using a structured version of the Unified Parkinson's Disease Rating Scale. Board-certified neurologists diagnosed PD. Physical functioning was assessed using chair stands, tandem walk, and timed walk. An ultrasensitive immunoassay was used to measure the concentration of NfL in blood.
Results
Of the 1,254 participants examined for clinical PD, 77 (6.1%) developed clinical PD and parkinsonian signs were on average 9.5 (range 0–66.0). After adjusting for demographic characteristics, APOE ε4 allele, and global cognition, a 2-fold higher concentration of serum NfL was associated with incident clinical PD (odds ratio [OR] 2.54, 95% CI 1.70, 3.81) and global parkinsonian signs (OR 2.44, 95% CI 1.94, 2.94). This association was significant >5 years before diagnosis. Compared with participants with levels below 18.5 pg/mL of serum NfL at baseline, participants with levels between 18.5 and 25.4 pg/mL, between 25.4 and 37.3 pg/mL, and above 37.3 pg/mL had a higher OR of clinical PD at all time intervals from the time of diagnosis to >5 years before diagnosis. A higher concentration of serum NfL was associated with a faster rate of physical functioning decline. In participants with 2-fold higher concentrations of serum NfL, the annual rate of decline in physical functioning increased by 0.15 units (95% CI 0.21, 0.08).
Dicussion
Serum NfL was associated with incident clinical PD, parkinsonian signs, and physical functioning decline in a population-based sample. Our findings suggest that NfL may serve as a potential biomarker for neurodegeneration, including PD outcomes.
Classification of Evidence
This study provides Class II evidence that serum NfL levels are associated with incident PD, parkinsonian signs, and physical functioning decline.
Clinical diagnosis of Parkinson disease (PD) traditionally involves extensive and time-intensive workups with neurologic and physical examinations, most often after the appearance of physical parkinsonian signs (e.g., bradykinesia, parkinsonian gait, rigidity, tremors).1 Promising blood biomarkers may allow for the early identification of patients at increased risk for PD or those at greater risk for poor symptoms and physical functioning.2 Neurofilament light (NfL), a neuronal cytoplasmic protein, is a known biomarker of neurodegeneration most commonly measured in CSF or blood.3 CSF and blood levels of NfL increase due to damage to connecting axonal fibers in the brain and thus can serve as an early indicator of cognitive decline and other neurodegenerative disorders.4-7 There is some promise that higher concentrations of NfL may be a biomarker for PD. Moreover, higher concentrations of NfL may indicate disease severity or progression in PD, including PD-related physical functioning outcomes. Thus, NfL may be used to detect PD earlier in the disease trajectory, allowing for the implementation of disease-slowing or disease-modifying therapeutics and interventions.3
Diagnosis of PD early in the disease trajectory is difficult due to overlapping symptoms with other motor disorders (e.g., dementia with Lewy bodies, progressive supranuclear palsy). However, NfL as measured in blood and CSF is markedly more elevated early in disease trajectories in these other disorders compared with PD. Some studies have indicated that NfL levels and trends can be used to discriminate PD from other motor disorders and examine PD severity over time.8-14 However, there is a lack of studies with blood measures of NfL (2 that we identified10,12), with no study utilizing population-based samples. Less is known on the long-term relations between blood NfL, parkinsonian signs, and the related physical functioning outcomes. Although it is well-established that physical functioning is closely related to the overall functional trajectory of PD,15 there is no examination of NfL with physical functioning outcomes. Additional investigation is needed to elucidate associations between NfL, PD, and PD-related outcomes. We examined the associations of serum NfL at baseline with clinically diagnosed PD, parkinsonian signs, and changes in physical functioning over 16 years in a population-based study of older Black and White adults to address these gaps. The primary research question addressed is as follows: Are serum NfL levels associated with incident PD, parkinsonian signs, and physical functioning decline?
Methods
This study followed a longitudinal, retrospective design, using data from the Chicago Health and Aging Project (CHAP), a longitudinal population-based study of common chronic aging conditions between 1993 and 2012.16,17 Participants were recruited and enrolled based on a door-to-door census of 4 Chicago neighborhoods with large proportions of residents who were either Black or White. The only inclusion criteria were residence in the designated neighborhood area and age 65 years or older. Eighty percent of all older adults in the neighborhood areas participated in the study.
Data collection cycles for CHAP are each approximately 3 years in duration. Between 1993 and 2012, there were up to 6 triennial cycles of data collection, and 4 cohorts of new participants who reached 65 years of age during the study were additionally enrolled. Each cycle consists of 2 phases: (1) an in-home interview and (2) detailed clinical evaluation of Alzheimer disease, other neurodegenerative conditions, and illnesses that impair cognitive function from a random stratified sample of persons.16,17 The clinical evaluation includes a neurologic examination that incorporates a modified version of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) and is fully specified and structured. The examinations are conducted by nurse clinicians who have undergone structured training protocols that included extensive supervised practice and performance-based certification. Blood collection is completed at the clinical evaluation, including approximately one-third of participants16,17 and limited to a subsample due to budgetary and resource considerations.7
Immunoassays were completed in 3,000 blood samples collected throughout the study. For the current analysis, participants who had undergone a clinical assessment of PD and provided multiple blood samples longitudinally were selected to maximize the power to detect time-varying associations. First, we selected all 454 participants who had 4 or more blood draws and underwent clinical assessments. Then we randomly selected from the 3,148 participants those who had 3 or fewer blood draws and underwent clinical examinations. We employed a random selection of participants due to budgetary considerations of completing the immunoassays. Thus, we included a total of 1,327 participants in this analysis.
Blood Biomarker Analysis
Blood samples were collected between 1994 and 2012 during home assessments, transported to the Rush Biorepository freezer, and frozen at −80°C. Previously unthawed blood samples were shipped to Quanterix Corporation, and serum NfL, a neuronal cytoskeletal biomarker of neurodegeneration, was assayed. The Quanterix ultrasensitive immunoassays were assessed in duplicate using the bead-based HD platform and the Neurology 4Plex A kit. For data analysis, the mean concentration of the duplicated measurement was used. The variation coefficient between duplicates was 3.0%.
Clinical Diagnosis of PD
The diagnosis of clinical PD was made at the time of clinical evaluation. The structured neurologic examination and medical history were performed by trained nurse clinicians. A board-certified neurologist reviewed all data and reexamined each participant with emphasis on findings considered to be clinically important or atypical. The diagnosis of PD required a review of the clinical history, evaluation of the parkinsonian signs, self-reported diagnosis for PD for which the participant received l-dopa or a dopamine agonist, and review of current medications to assess whether the participant was currently taking medications for treatment of PD, medications for PD-related symptoms, or medications that potentially induce parkinsonian signs (e.g., neuroleptics, antiemetics, antidepressants, blood pressure medications such as calcium channel blockers). The review of medications was conducted by the nurse clinician at every clinical evaluation and reviewed by the neurologist.
Parkinsonian Signs
A modified version of the UPDRS with 26 items was used to evaluate 4 parkinsonian signs: bradykinesia, parkinsonian gait, rigidity, and tremors. The global parkinsonian score was the average of all 4 parkinsonian signs (0–100, with higher scores indicating more parkinsonian signs).18,19 The global parkinsonian score, bradykinesia, and gait were reported as continuous scores; rigidity and tremors were reported as the presence (yes or no) of those traits.
Physical Functioning
A series of performance-based tests assessed physical functioning. The performance-based tests occurred during clinical evaluations and assessed function of lower extremities and included the chair stand (ability to move from a standing position from a chair), tandem stand, tandem walk, and timed walk.20 Summary scores of each test are created and range from 0 to 15, with lower scores indicating potential impairment or inability to conduct tasks of independent living, contributing to physical disability. This series of performance-based tests had strong internal consistency (Cronbach α = 0.76) and test–retest reliability (0.88–0.92).21
Covariates
Covariates used in these analyses were demographics (age, sex, race/ethnicity, and education level), activities of daily living (ADL), global cognition, and clinical diagnosis of Alzheimer dementia. ADL were the summary score of the Katz Index of Activities of Daily Living, a well-established measure of a participant's ability to perform 6 basic tasks for self-care.22 Higher scores indicate better ability to perform ADL; lower scores indicate potential physical disability or increased reliance on others for care.23-25 Global cognition was the average of 4 standardized cognitive tests (2 episodic memory tests, 1 executive function test, and the Mini-Mental State Examination) administered during the home-based assessment. Individual scores were centered and scaled to the average of the cohort at baseline.26
Clinical diagnosis of Alzheimer dementia followed the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) guidelines, which are detailed elsewhere.7,17 Briefly, NINCDS-ADRDA requires a history of cognitive function declines, with 2 cognitive domains with impairment. Cut points for tests of 5 cognitive domains (orientation, attention, memory, language, and perception) were adjusted for education. First, a neuropsychologist reviewed the test scores and ratings for each cognitive domain. The neuropsychologist had access to cognitive data, education, occupation, sensorimotor problems, and motivation to inform ratings and diagnoses. Then, a board-certified neurologist, who had access to clinical data, reviewed the ratings and diagnoses. If there was a disagreement, the neurologist provided a revised diagnosis.
Statistical Analysis
Baseline characteristics of the study population are shown as mean and SD, percentages of participants, or medians and quartiles. The first blood draw was treated as the baseline time for time-dependent NfL values for analyses examining clinical PD, as well as analyses that examined NfL values and physical functioning over time. The mean level of serum NfL was skewed and was log-transformed for regression analyses, and 2-fold levels were used in analyses to consider the scale of NfL. Global parkinsonian scores and continuous traits were square root-transformed for regression analyses. All generalized linear models and graphical representations were performed using the R program.27 Models that examined physical functioning were completed in the complete sample of 1,327 participants; models that examined clinical PD and parkinsonian signs were completed in a subsample of 1,254 participants.
First, we conducted regression analyses to examine associations between serum NfL values and clinical PD and parkinsonian signs (scores and continuous traits). A quasibinomial logistic regression model was performed with time-dependent log-transformed NfL to examine the association of serum NfL at baseline with the clinical diagnosis of PD (considering the number of years between blood draw and the annual clinical assessment), presence of rigidity, and presence of tremor.28 A person-specific random-effects regression model was conducted to examine the association between time-dependent log-transformed NfL and the global parkinsonian, bradykinesia, and gait scores. In this first series of models, covariates included age at baseline blood draw, female sex, Black race/ethnicity, education, global cognition, and ADL. We then performed secondary analyses with participants categorized based on quartiles of serum NfL values to examine whether associations were graded.
Second, we conducted regression analyses to examine associations between serum NfL values and changes in physical functioning scores (composite score, chair stand, tandem stand, and timed walk). A linear mixed-effects model with person-specific intercept and slope was conducted to examine the association of time-dependent log-transformed NfL on longitudinal changes in composite and individual physical functioning scores.29 In secondary analyses, we used quartiles of concentrations of NfL at the first blood draw and examined associations with longitudinal changes in physical functioning. Covariates in this second series of models included age, sex, race/ethnicity, education, global cognition, and ADL.
Third, we stratified the first series of models tested by the time between blood draw and clinical diagnosis during 3 separate intervals: at the time of diagnosis, at a blood draw within the first 5 years (0–5 years) before the diagnosis of PD, and at a blood draw more than 5 years (up to 16 years) before the diagnosis of PD. Models testing the associations between serum NfL and parkinsonian signs (global score, bradykinesia score, gait score, presence of rigidity, and presence of tremors) were similarly stratified with the same time intervals.
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Board of the Rush University Medical Center approved the study protocols and all participants provided written consent for blood collection, population interviews, and clinical evaluations.
Data Availability
Anonymized data that support study findings are available by request from qualified investigators through our research data portal (riha.rush.edu/dataportal.html).
Results
This analysis included 1,327 older adult participants who underwent 3,000 total blood draws performed between 1995 and 2011. In addition, this analysis has complete data for the study variables of the current analyses for the physical functioning outcomes. For models examining clinical PD and parkinsonian signs, a subsample of 1,254 participants was used (Table 1). At baseline, participants were on average 73.5 (SD 6.4) years of age, had 12.5 (SD 3.6) years of education, 62% were women, and 61% were Black. During the longitudinal study, 6.1% (n = 77) developed clinical PD, and the time from baseline clinical evaluation to clinical PD development was on average 3.3 years (range, baseline–16.2). Baseline NfL ranged from 0.99 (limit of detection) to 755 pg/mL (mean, 25.7 pg/mL) with 7-fold higher concentration from the 5th to the 95th percentile.
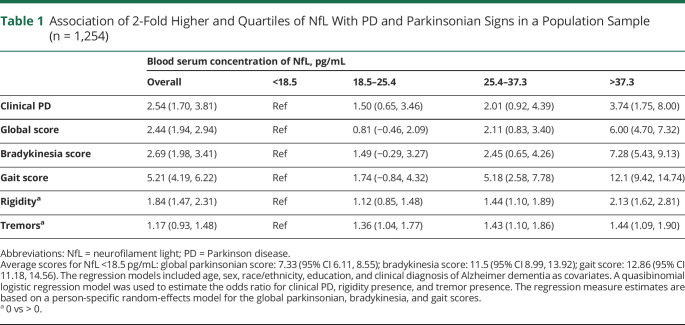
Table 1.
Association of 2-Fold Higher and Quartiles of NfL With PD and Parkinsonian Signs in a Population Sample (n = 1,254)
Baseline NfL and Incident Clinical PD
A 2-fold higher concentration of serum NfL was significantly associated with incident clinical PD in adjusted models (OR 2.54, 95% CI 1.70, 3.81; Table 1), with a significant graded association with clinical PD in the highest quartile (37.3 pg/mL or higher) of NfL (OR 3.74, CI 1.75, 8.00) compared with participants below 18.5 pg/mL. A higher concentration of NfL was associated with a higher OR for scores of global parkinsonian signs, bradykinesia, and gait, and presence of rigidity (b = 2.44, CI 1.94, 2.94; b = 2.69, CI 1.98, 3.41; b = 5.21, CI 4.19, 6.22; OR 1.84, CI 1.47, 2.31, respectively). There was a significant graded association with parkinsonian signs and all domains in the third quartile (25.4–37.3 pg/mL) and fourth quartile (37.3 pg/mL or higher) of NfL. Participants in the third and fourth quartiles of NfL concentration had a higher OR of higher global parkinsonian scores, bradykinesia scores, and gait scores, as well as the presence of rigidity and tremors, when compared with the participants with NfL concentrations <18.5 pg/mL.
Baseline NfL and Physical Functioning Scores
A 2-fold higher concentration of serum NfL was associated with both lower composite and individual (chair stand, tandem stand, and timed walk) physical functioning scores at baseline (Table 2). There was a significant graded association with the composite and individual physical functioning scores at baseline in the highest quartiles of NfL. Participants in the lowest quartile of NfL had higher levels of overall physical functioning at baseline (b = 11.03, CI 10.60, 11.46) compared with the third (b = −0.52, CI −0.99, −0.05) and fourth quartiles (b = −1.28, CI −1.75, −0.81). Participants in the fourth quartile of NfL concentration had lower scores for the all individual tests of physical functioning when compared with the first quartile.
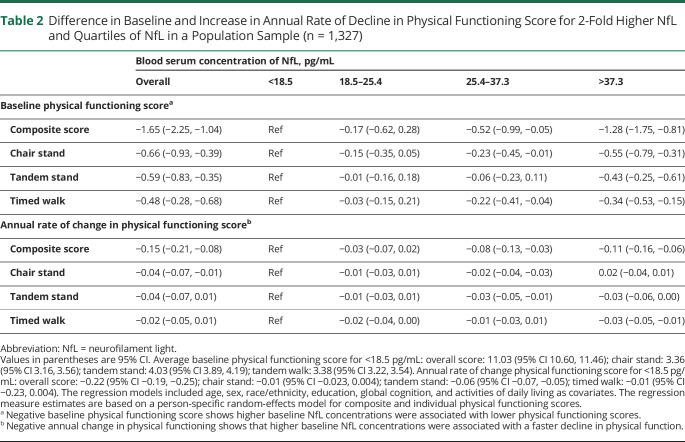
Table 2.
Difference in Baseline and Increase in Annual Rate of Decline in Physical Functioning Score for 2-Fold Higher NfL and Quartiles of NfL in a Population Sample (n = 1,327)
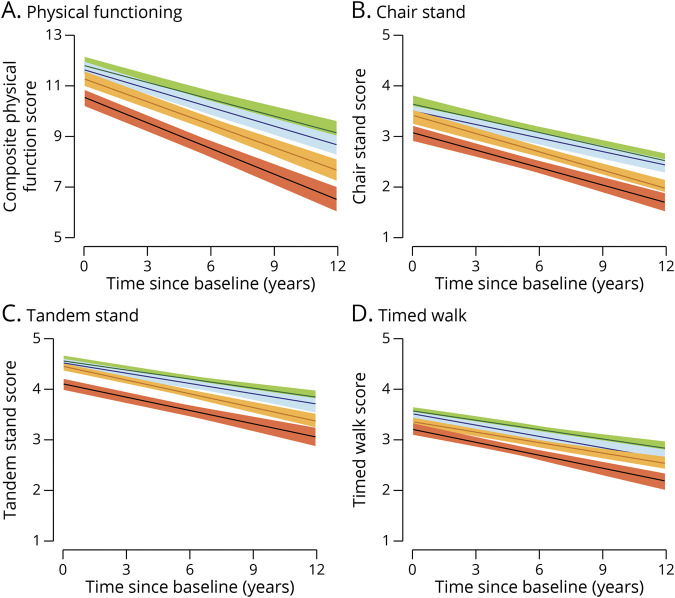
A higher concentration of 2-fold NfL at baseline was significantly associated with declines in composite and individual scores of physical functions over time (Table 2). For every 2-fold higher concentration of NfL, overall physical functioning (composite) decreased by 0.15 SD units (SDU) per year. Significant associations were found for individual scores (chair stand, tandem stand, and timed walk) for the chair stand scores only, with an annual rate of change of −0.04 SDU. The Figure illustrates that NfL in quartiles was also associated with more significant declines in physical functioning. The annual rate of change for overall physical functioning was −0.22 SDU for those in the first quartile (indicated by green). In addition, there were more significant declines in the third quartile, as indicated by orange (−0.30 SDU or 26.67% rate of faster decline), and the fourth quartile, as indicated by red (−0.33 SDU or 33.33% more rapid rate of decline). For the chair stand score, the third quartile showed a more significant reduction (−0.06 SDU or 33.33% faster rate of decline) when compared with the lowest quartile (−0.01 SDU).
Figure. Annual Rate of Change for Physical Functioning by Quartiles of Neurofilament Light in a Population Sample (n = 1,327).
(A) Physical functioning. (B) Chair stand. (C) Tandem stand. (D) Timed walk.
NfL and Incident Clinical PD at Specific Time Intervals
The association of NfL with clinical PD was significant more than 5 years before diagnosis (Table 3). Serum NfL collected at the time of clinical PD diagnosis, between 0 and 5 years prior to clinical PD diagnosis, and more than 5 years prior to clinical PD diagnosis were all significantly associated with incident clinical PD. At the time of diagnosis, a 2-fold higher concentration of NfL was associated with a higher OR of clinical PD (OR 2.89, 95% CI 1.63, 5.04), at 0–5 years prior to diagnosis the OR of clinical PD was 3.68 (95% CI 1.99, 6.79), and at more than 5 years prior to diagnosis 1.27 (95% CI 0.26, 6.09). Similarly, at all specific time intervals (at the time of diagnosis, 0–5 years, >5 years), 2-fold higher concentration of NfL was significantly associated with global parkinsonian scores, bradykinesia scores, and gait scores. For presence of rigidity, NfL was significantly associated with higher OR at diagnosis and the 0–5 years time intervals only.
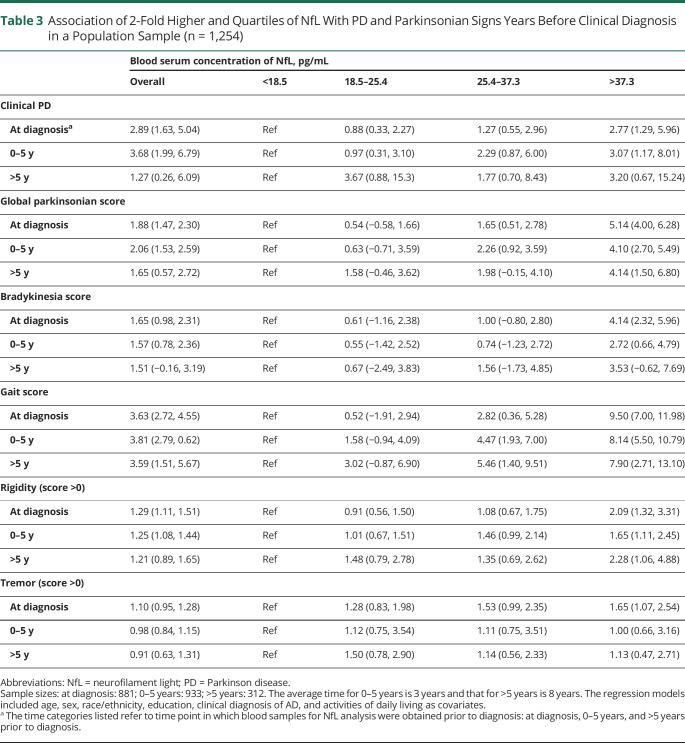
Table 3.
Association of 2-Fold Higher and Quartiles of NfL With PD and Parkinsonian Signs Years Before Clinical Diagnosis in a Population Sample (n = 1,254)
There was a graded association with clinical PD and all parkinsonian signs (global score, bradykinesia score, gait score, presence of rigidity, and presence of tremors) in each higher quartile of NfL (Table 3). Compared with participants with levels below 18.5 pg/mL, participants in the fourth quartile had a significantly higher OR of clinical PD at all time intervals ranging from diagnosis to >5 years. Moreover, across all time intervals, participants with 2-fold higher NfL concentration in the third and fourth quartiles had higher ORs of higher scores of global parkinsonian, bradykinesia, and gait when compared with participants in the lowest quartile. For the presence of rigidity and tremors, participants in higher quartiles (second, third, and fourth) showed higher ORs when compared with participants in the lowest quartile. This study provides Class II evidence that serum NfL levels are associated with incident PD, parkinsonian signs, and physical functional decline.
Discussion
We examined associations of blood NfL level with clinically diagnosed PD and changes in physical functioning over 16 years in a population-based sample of community-dwelling older adults. In adjusted models, we found significant associations between NfL and odds of clinical diagnosis of PD, global score of parkinsonian signs, and physical functioning outcomes, with graded associations in each higher quartile of NfL.
Our analysis focused on the association of NfL with clinical PD and related physical functioning outcomes, despite the utility of NfL as a nonspecific, although sensitive, biomarker of general axonal neurodegeneration. In PD, neuronal deposits of α-synuclein are related to higher levels of NfL,8 and higher NfL is associated with neuroimaging markers of cortical neurodegeneration.30 Yet NfL levels can rise in response to damage to neuronal axons caused by a variety of injuries, including neurodegenerative, inflammatory, vascular, or traumatic disease processes.3 As such, NfL has been shown to have prognostic value in multiple neurodegenerative diseases (e.g., PD, Alzheimer's disease,6,7 traumatic brain injury31), so increases in NfL may be due to various etiologies. Thus, neurologic assessments and other blood biomarkers should be considered to complement serum NfL levels in the diagnosis of PD in those with increased risk (i.e., family history or carriers of genetic mutations that increase risk of PD), rather than a precise diagnostic. NfL may help identify those at higher risk for a variety of neurodegenerative diseases, including PD,2 which may inform early evaluations. To account for the potential influence of other conditions known to decrease NfL levels, we adjusted for clinical Alzheimer disease in models with clinical PD outcomes and adjusted for general cognitive function in models with physical functioning outcomes in our analyses.
In our examination of the effects of serum NfL on physical functioning outcomes, longitudinal results across individual physical functioning tests were mixed, despite significant findings with the composite measure of overall physical function. The composite measure of overall physical functioning may be a more robust representation of functional status than an individual test (e.g., gait speed). Regardless, in our analysis, higher levels of NfL corresponded with higher scores of individual physical functioning tests, but these associations did not reach significance. These mixed findings may be related to the notion that a composite measure of overall physical functioning obtained from an entire battery of performance tests may have advantages for research compared with a single test alone. In an earlier examination of a similar performance battery, a single measure (e.g., gait speed) showed benefit as a clinical assessment of functional status. However, a composite measure was a more exact exemplification of overall physical functioning.32 Thus, the composite measure over a single measure can be considered for research use.
Our results build on earlier studies that previously examined NfL, more commonly CSF, as a clinical PD or PD severity biomarker.8-14 Our study is the first to examine the easy-to-collect and scalable serum NfL from ultrasensitive immunoassays in a biracial, population-based sample in a longitudinal study. Our findings that serum NfL was associated with clinical PD up to 5 years prior to incidence, with those in the higher quartiles of NfL showing more severe parkinsonian signs, support the hypothesis that PD development involves neuropathologic processes that occur long before symptoms manifest and the eventual diagnosis. The associations of serum NfL with incident clinical PD, parkinsonian signs, and poor physical functioning over time point to the utility of serum NfL for clinical and public health populations to help inform the diagnosis of neurodegenerative disease and the early implementation of interventions.2,3,10
Findings from our analysis may be limited. First, this sample represented a biracial population-based cohort in the Chicago metropolitan area, so the results may not generalize to other populations. Second, the neuropathologic development of PD may begin in early adult or midlife periods that were not captured in the CHAP study of older adults. An earlier examination of blood biomarkers of PD over time would help elucidate the early stages of preclinical development. Third, a potential limitation is method of diagnosing clinical PD. At the time of the clinical evaluation, the neurologist rendered a diagnosis of clinical PD based on clinical history, evaluation of the parkinsonian signs, self-reported diagnosis for PD for which the participant received l-dopa or a dopamine agonist, and review of medications. Because these diagnostic criteria may not wholly align with other accepted definitions, our rate of clinical PD diagnosis may differ from other reports in the general population. Furthermore, the recruitment timeline of the CHAP study spanned 16 years, which may have resulted in variability of diagnosis over time. Fourth, there was a small number of patients diagnosed with PD in this cohort (n = 77), which affects our findings with clinical PD as the outcome. Fifth, although performance-based physical function tests are preferred over self-report measures, there still may be variation across raters. Finally, we examined only one biomarker of PD, which only assesses neurodegeneration related to axonal loss and does not definitively discriminate PD from other neurodegenerative diseases in asymptomatic individuals. In future work, other biomarkers of PD could be considered, such as α-synuclein (a marker of Lewy body neuropathology) or inflammatory factors (e.g., tumor necrosis factor–α).33 Our study did not obtain neuropathologic autopsy data, which should be considered for future studies.
Our study indicates that a blood-based cytoskeletal biomarker, NfL, may be an informative and convenient biomarker associated with incidence of clinical symptoms, severity of parkinsonian signs, and PD-related physical functioning outcomes. Furthermore, the significant graded associations suggest potential clinical applicability for assessment over time of PD and PD-related outcomes in older adults. Future work should consider analyzing other biomarkers of clinical PD and physical functioning and neuropathologic data obtained from autopsy. The examination of NfL and other blood biomarkers should be regarded in younger populations, including midlife age groups, to help advance the understanding of early detection, development, and prognosis of PD across the lifespan and inform potential early interventions.
Glossary
- ADL
activities of daily living
- CHAP
Chicago Health and Aging Project
- NfL
neurofilament light
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association
- PD
Parkinson disease
- SD
USD units
- UPDRS
Unified Parkinson’s Disease Rating Scale
Appendix. Authors
Footnotes
Editorial, page 911
Class of Evidence: NPub.org/coe
Study Funding
This study was supported by NIH R01 grants R01AG051635, RF1AG057532, and R01AG058679.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Marras C, Beck JC, Bower JH, et al. . Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis. 2018;4(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parnetti L, Gaetani L, Eusebi P, et al. . CSF and blood biomarkers for Parkinson's disease. Lancet Neurol. 2019;18(6):573-586. [DOI] [PubMed] [Google Scholar]
- 3.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870-881. [DOI] [PubMed] [Google Scholar]
- 4.Mielke MM, Syrjanen JA, Blennow K, et al. Comparison of variables associated with cerebrospinal fluid neurofilament, total-tau, and neurogranin. Alzheimers Dement. 2019;15(11):1437-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedet AL, Ashton NJ, Pascoal TA, et al. . Plasma neurofilament light associates with Alzheimer's disease metabolic decline in amyloid-positive individuals. Alzheimers Dement Diagn Assess Dis Monit. 2019;11(1):679-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin M, Cao L, Dai Y. Role of neurofilament light chain as a potential biomarker for Alzheimer's disease: a correlative meta-analysis. Front Aging Neurosci. 2019;11:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajan KB, Aggarwal NT, McAninch EA, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88(6):1065-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bäckström D, Linder J, Jakobson S, et al. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology. 2020;95(7):e827-e838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantinescu R, Rosengren L, Johnels B, Zetterberg H, Holmberg B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson's disease and atypical parkinsonian disorders. Parkinsonism Relat Disord. 2010;16(2):142-145. [DOI] [PubMed] [Google Scholar]
- 10.Hansson O, Janelidze S, Hall S, et al. Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janelidze S, Lindqvist D, Francardo V, et al. Increased CSF biomarkers of angiogenesis in Parkinson disease. Neurology. 2015;85(21):1834-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CH, Li CH, Yang KC, et al. Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology. 2019;93(11):e1104-e1111. [DOI] [PubMed] [Google Scholar]
- 13.Lin YS, Lee WJ, Wang SJ, Fuh JL. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep. 2018;8(1):17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollenhauer B, Zimmermann J, Sixel-Döring F, et al. . Baseline predictors for progression 4 years after Parkinson's disease diagnosis in the de novo Parkinson cohort (DeNoPa). Mov Disord. 2019;34(1):67-77. [DOI] [PubMed] [Google Scholar]
- 15.DiPietro L, Campbell WW, Buchner DM, et al. Physical activity, injurious falls, and physical function in aging: an umbrella review. Med Sci Sports Exerc. 2019;51(6):1303-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis. 2003;5(5):349-355. [DOI] [PubMed] [Google Scholar]
- 17.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60(2):185-189. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses' ratings of parkinsonian signs with a modified Unified Parkinson's Disease Rating Scale. Neurology. 1997;49(6):1580-1587. [DOI] [PubMed] [Google Scholar]
- 19.Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 1999;54(4):M191-M196. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94. [DOI] [PubMed] [Google Scholar]
- 21.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM; Women’s Health and Aging Study. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women's Health and Aging Study. J Clin Epidemiol. 2002;55(9):916-921. [DOI] [PubMed] [Google Scholar]
- 22.Katz S, Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Index of activities of daily living. J Am Med Assoc. 1963;185(12):914-919. [Google Scholar]
- 23.Courtney MD, Edwards HE, Chang AM, et al. Improved functional ability and independence in activities of daily living for older adults at high risk of hospital readmission: a randomized controlled trial. J Eval Clin Pract. 2012;18(1):128-134. [DOI] [PubMed] [Google Scholar]
- 24.Katz S, Yeh SCJ, Lo SK. Index of activities of daily living. J Nurs Care Qual. 2004;19:58-66. [DOI] [PubMed] [Google Scholar]
- 25.Mograbi DC, Faria CdeA, Fichman HC, Paradela EM, Lourenço RA. Relationship between activities of daily living and cognitive ability in a sample of older adults with heterogeneous educational level. Ann Indian Acad Neurol. 2014;17(1):71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):P155-P160. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018. R-project.org/ [Google Scholar]
- 28.Hosmer. Applied Logistic Regression, 3rd ed. Wiley; 2013. [Google Scholar]
- 29.Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of Longitudinal Data. 2nd ed. Oxford University Press; 2013. [Google Scholar]
- 30.Sampedro F, Pérez-González R, Martínez-Horta S, Marín-Lahoz J, Pagonabarraga J, Kulisevsky J. Serum neurofilament light chain levels reflect cortical neurodegeneration in de novo Parkinson's disease. Parkinsonism Relat Disord. 2020;74:43-49. [DOI] [PubMed] [Google Scholar]
- 31.Korley FK, Yue JK, Wilson DH, et al. . Performance evaluation of a multiplex assay for simultaneous detection of four clinically relevant traumatic brain injury biomarkers. J Neurotrauma. 2019;36(1):182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seino S, Kim Mji, Yabushita N, et al. . Is a composite score of physical performance measures more useful than usual gait speed alone in assessing functional status? Arch Gerontol Geriatr. 2012;55(2):392-398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data that support study findings are available by request from qualified investigators through our research data portal (riha.rush.edu/dataportal.html).