Abstract
Retinol-binding protein 2-deficient (Rbp2–/–) mice are more prone to obesity, glucose intolerance, and hepatic steatosis than matched controls. Glucose-dependent insulinotropic polypeptide (GIP) blood levels are dysregulated in these mice. The present studies provide new insights into these observations. Single cell transcriptomic and immunohistochemical studies establish that RBP2 is highly expressed in enteroendocrine cells (EECs) that produce incretins, either GIP or glucagon-like peptide-1. EECs also express an enzyme needed for all-trans-retinoic acid (ATRA) synthesis, aldehyde dehydrogenase 1 family member A1, and retinoic acid receptor-alpha, which mediates ATRA-dependent transcription. Total and GIP-positive EECs are significantly lower in Rbp2–/– mice. The plasma transport protein for retinol, retinol-binding protein 4 (RBP4) is also expressed in EECs and is cosecreted with GIP upon stimulation. Collectively, our data support direct roles for RBP2 and ATRA in cellular processes that give rise to GIP-producing EECs and roles for RBP2 and RBP4 within EECs that facilitate hormone storage and secretion.
Keywords: retinol-binding protein 2, retinoids, enteroendocrine cells, incretins, glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1
Since its original description by Ong and colleagues, retinol-binding protein 2 (RBP2) (previously known as cellular retinol–binding protein, type 2 or CRBP2) has been studied as an intracellular binding protein with the exclusive function of mediating dietary retinoid (vitamin A and its metabolites) uptake and metabolism within the enterocyte (1-3). A member of the superfamily of fatty acid–binding proteins, RBP2 is reported to be expressed only along the absorptive surface (villus) of the human and mouse intestine, specifically in enterocytes (1-3). Expression is more abundant in the proximal portion of the small intestine, with the vast majority of investigations having a focus on RBP2 actions in the jejunum, where most retinoid absorption takes place (1-3). RBP2 is highly abundant, accounting for 0.4% to 1% of total cytosolic protein in the proximal small intestine (4, 5). Within enterocytes, RBP2 binds retinol or retinaldehyde, facilitating dietary retinoid absorption, metabolism, and packaging as retinyl ester into nascent chylomicrons (see Fig. 1A) (6, 7). It has long been thought that RBP2 sole function is to assure optimal uptake and accumulation of dietary retinoids (8, 9). However, we reported recently that RBP2 binds with very high affinity the canonical endocannabinoid, 2-arachidonoylglycerol, and to other endocannabinoid-like 2-monoacylglycerols (2-MAGs) (10, 11). This suggests a previously unsuspected role for RBP2 in mediating the metabolism/signaling of bioactive 2-MAGs (10, 11).
Figure 1.
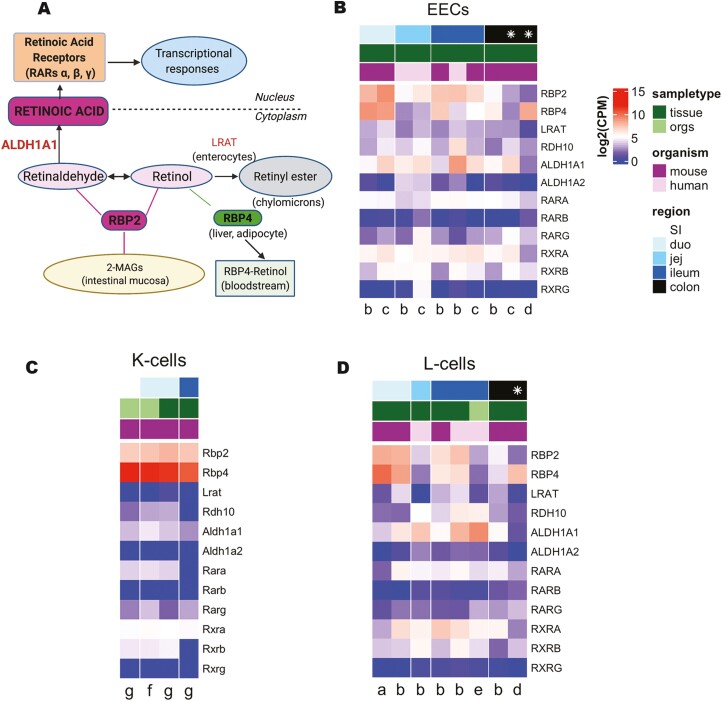
Expression of retinoid-related genes in human and mouse enteroendocrine cells. (A) Schematic representation of retinoid metabolism. (B-D) Heat maps plotting expression of retinoic acid (RA) metabolism-related genes in published single cell and bulk RNA sequencing (RNAseq) datasets for (B) enteroendocrine cells (EECs), (C) K-cells, and (D) L-cells. Top 3 rows label region of the gut (darker blue labels more distal gut, white = whole small intestine (SI), * = combined colon and rectum); sample type (dark green = primary tissue, light green = organoids); and organism (pink = human, purple = mouse). Letters at the bottom label publication (a = Glass et al 2017, b = Roberts et al 2018, c = Larraufie et al 2019, d = Billing et al 2019, e = Goldspink et al 2020, f = Beumer et al 2018, g = Gehart et al 2019). Gene expression is plotted in log2 (counts per million). Expression for single cell RNAseq datasets (a, d, f, g) were calculated as the average across the cell type of interest.
Retinoids are required to maintain normal cell proliferation and differentiation (12, 13). They act critically in the maintenance of barrier functions, immunity, normal tissue growth and development, and normal reproduction, as well as in many other physiologic processes (13, 14). These effects all involve retinoid actions as potent transcriptional regulators. This transcriptional regulatory activity requires the metabolite all-trans-retinoic acid (ATRA) and its binding to 1 of 3 distinct nuclear hormone receptors, retinoic acid receptor-α, -β, or -γ (RARα, -β, or -γ) (12-16). The ATRA-RAR complex specifically binds retinoic acid response elements within genes, affecting rates of transcription for more than 500 ATRA-responsive genes (17).
The intestinal epithelium undergoes complete renewal every 3 to 5 days (18, 19). This fast regeneration rate happens owing to the existence of stem cells in the bottom of the crypts, which differentiate to give rise to the absorptive and secretory cell types present in the intestinal epithelium (20). Among the secretory cell types are enteroendocrine cells (EECs), an important hormone-producing cell population that accounts for <1% of the epithelium, but 1 that plays a key role in modulating local and systemic energy metabolism (21, 22). Traditionally, EECs are classified based on their hormone products; a classification scheme that has proven to be increasingly problematic given the accumulating evidence for overlapping hormone synthesis/secretion by a given cell (23). Identification and understanding of biological factors that may impact EEC physiology, distribution, and their commitment to a specific hormone product are important research areas in need of further exploration. This is especially important for the incretin-producing K- and L-cell EEC subtypes, which secrete glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like polypeptide-1 (GLP-1), respectively. These hormones act pivotally in diabetes physiopathology and are therapeutic targets for treatment of metabolic disease (22, 24-27).
Six- to 7-month-old Rbp2-deficient (Rbp2–/–) mice fed a chow diet throughout life gain more body weight, become glucose intolerant, and develop hepatic steatosis more quickly than matched littermate controls (10). These same metabolic phenotypes quickly develop when mutant mice are fed a high-fat diet starting at 2 months of age. When subjected to an oral fat challenge, within 2 hours Rbp2–/– mice show a 2-fold elevation in circulating GIP levels compared with controls (10). These data suggest that RBP2 acts either in a paracrine and/or autocrine manner to modulate EEC actions within the small intestine, especially those of the GIP-producing (GIP+) cells. The present studies represent a first step toward identifying how RBP2 acts to modulate GIP levels in the circulation. We hypothesize that either the retinoid and/or the 2-MAG ligands of RBP2 are responsible for the elevated GIP levels. We further hypothesize that elevated GIP levels directly contribute to the development of the metabolic phenotypes observed for Rbp2–/– mice.
Materials and Methods
Animals and Animal Husbandry
We studied age- and gender-matched male Rbp2–/– and wild-type mice congenic in the C57BL/6J genetic background. The Rbp2–/– mice were originally described by Li and colleagues (28). For cell sorting studies, we used NeuroD1 Cre mice crossed with Rosa26-EYFP (NeuroD1YFP mice), a model previously described by Li et al (29) and used for this purpose as previously published (30, 31). This reporter mouse model allows isolation of the total population of EECs present in the intestine. Mice were maintained on a 12:12-hour dark–light cycle and provided with ad libitum access to water and standard rodent chow diet (PicoLab Rodent Diet 5053) in a conventional barrier facility. All animal experiments were conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (32) and were approved by the Columbia University Institutional Animal Care and Use Committee.
Bulk and Single Cell Mouse and Human Enteroendocrine RNA Sequencing Datasets Analysis
All RNA sequencing (RNA-seq) data were analyzed using R (version 4.0.3). Datasets in this analysis include both genders and are available under Gene Expression Omnibus ID GEO: GSE114913, GSE114853, GSE121490, GSE148224, GSE114988, and GSE113561 (20, 23, 30, 31, 33-35), further details are listed elsewhere (Table 2 (36)).
Bulk RNA-seq datasets were converted into DESeq2 format (1.30.0; Bioconductor). Raw counts were manually converted to transcripts per million by dividing by the total number of millions of counts per sample. Bulk RNA-seq data presented in heat maps are log2 (average transcripts per million across samples).
Single-cell datasets were converted into Seurat format (4.0.2; Bioconductor), filtering only for cells with counts for >2000 genes for the Gehart et al (20) dataset, >400 genes for the Beumer et al dataset, and >200 genes for all other datasets (23). Only genes with counts for >3 cells were included in downstream analysis. Data were normalized to counts per million (CPM) using Seurat’s in-built function (NormalizeData). For L-cell data from Billing et al, all cells were first clustered to obtain 5 populations as originally published, and data filtered to the 2 L-cell populations (Insl5 and Nts) (31). For K-cell data from Beumer et al, cells were first clustered to obtain the Gip-, Sst-, and Vmn2r55-expressing clusters, data filtered to the K-cell population (23). For the Gehart et al dataset, the following genes responsible for clustering artifacts were first removed from analysis, as in the original paper: ERCC-00092, Rn45s, Malat1, Kcnq1ot1, A630089N07Rik, Gm17821 (20). Gehart et al K-cells were extracted after clustering and separated into upper proximal small intestine (duodenum), distal small intestine (ileum), and organoid (small intestine; SI) sample sets. All single-cell RNA-seq data presented in heat maps are log2 (average CPM across cells).
Tissue Preparation for Histology
Small intestines from 35-40 day old male Rbp2–/– and wild-type mice (n = 4 for each group) were employed in our studies. Animals were sacrificed according to Columbia University guidelines and the small intestine (from pylorus to cecum) was removed. The lumen was rinsed with phosphate-buffered saline (PBS). Each intestine sample was then divided into 3 sections: duodenum (proximal 10 cm from the pylorus), jejunum (the next 12 cm), and ileum (the rest of small intestine) and opened longitudinally. The tissue was fixed as a Swiss roll in 4% paraformaldehyde for 24 hours and afterwards switched to 70% ethanol for embedding in paraffin blocks. Slides were then prepared and stained by the Columbia University Histology Core Facility.
Flow Cytometry
Single-cell digests of mouse proximal small intestine were prepared for fluorescence-activated cell sorting (FACS) as previously described (37). Briefly, proximal small intestine from a wild-type control NeuroD1YFP and a NeuroD1YFP × Rbp2–/– mouse were enzymatically and mechanically digested to single cells. Cells were resuspended in a wash solution containing Hanks’ balanced salt solution (without Ca2+ or Mg2+) supplemented with 10 μM Rho-kinase (ROCK) inhibitor Y-27632 and 10% fetal bovine serum. Live DAPI-negative and DRAQ5-positive (Biolegend) single cells were sorted based on yellow fluorescent protein (YFP) fluorescence applying side scatter, forward scatter and pulse width gates using a FACSAria cell sorter (BD Biosciences) at the Columbia Stem Cell Initiative Flow Cytometry Core Facility (Fig. 1 (36)). NeuroD1-expressing cells represented 0.43% of the parent population. YFP-positive and -negative cells were collected in LoBind tubes (Eppendorf) containing the wash solution described above. Cells were pelleted by centrifugation at 500g for 5 minutes at 4°C and kept at –80°C prior to their use for Western blot (WB) analyses.
Histology and Immunostaining Studies
Villi length, crypt depth, and muscularis layer thickness were assessed making greater than 70 measurements per animal using hematoxylin and eosin–stained slides in mouse jejunum sections. Alcian Blue (pH 2.5)–stained slides were used to assess goblet cells.
For immunostaining studies, 5-μm intestinal sections were deparaffinized and hydrated using standard methods using citrate buffer (pH 6.0) for antigen retrieval and blocked with MOM mouse Ig blocking reagent (MOM Elite immunodetection kit peroxidase, Vector laboratories Cat# PK 2200) for 1 hour and/or with 10% goat serum for 25 minutes at room temperature. The samples were then incubated with primary antibodies (see details in Table 1 (36)). We assessed Paneth cells using antilysozyme antibody. Double-labeled immunostaining was used for colocalization of chromogranin A (ChgA), a marker of EECs, with RBP2, RBP4, ALDH1A1, or RARα. Colocalization was also evaluated for ChgA and GLP-1, ChgA and GIP, RBP2 and GLP-1, as well as RBP2 and GIP. Tissue sections were incubated for 30 minutes at room temperature with either a goat antirabbit horseradish peroxidase–conjugated secondary antibody for 30 minutes at room temperature or with horse biotinylated antimouse secondary antibody for 10 minutes at room temperature. For immunofluorescence, the signal was amplified for the rabbit anti-ChgA, anti-RBP2, anti-RBP4, and anti-ALDH1A1 with the streptavidin Alexa Fluor 488 conjugate while the streptavidin Alexa fluor 594 conjugate was used for the mouse anti-ChgA, anti-GLP-1, anti-GIP, anti-RARα, and antilysozyme. Images were acquired using a Zeiss LSM710 confocal laser-scanning microscope equipped with a Motorized Stage, an oil-immersion ×40 or ×63 objective lens and argon laser.
Paneth cells, goblet cells, ChgA+, GIP+, and GLP-1+ cells were quantified in mouse jejunum sections in Rbp2–/– mice and their control littermates. Quantification of the number/location of EECs and number of Paneth cells on intestinal section images was performed using Zeiss Zen software on the raw, unprocessed images. Quantification of the number/location of Goblet cells was performed using Aperio Image Scope 12.3 (Leica) software. Number of cells was normalized by intestinal section length in mm which was measured using ImageJ.
Cells sorted by FACS as previously described (37) were assessed for RBP2 protein expression by WB. For this experiment, we used an equal amount of YFP-positive (EECs) and YFP-negative cells (1.5 × 103) in wash media obtained from a NeuroD1YFP mouse and a NeuroD1YFPx Rbp2–/– mouse. Purified RBP2 protein (see Table 1 (36)) was used at different concentrations (10 ng, 1 ng and 100 pg) to allow for an approximate estimate to be made from the WB of the quantity of RBP2 present in the cells. Samples were combined with a similar volume of 2× Laemmli sample buffer (BioRad) and separated on a hand-cast 12% sodium dodecyl sulfate polyacrylamide gel. Following electrophoresis, samples were transferred onto a polyvinylidene difluoride membrane (Millipore) and subsequently blocked with 1% bovine serum albumin in Tris-buffered saline with 0.05% Tween. The membrane was probed with anti-RBP2 rabbit monoclonal antibody and later with anti-Beta-actin (see details in Table 1 (36)). The incubation time for the primary antibodies was at least 2 hours. After incubation with each primary antibody, the membrane was washed and incubated with the appropriate secondary antibody for another hour. After the final washing period, chemiluminescence images were obtained using an Azure c600 Imager (Azure Biosystems). Band densitometry analysis was performed using Image J software (National Institutes of Health)
3D Structured Illumination Microscopy
3D structured illumination microscopy (SIM) was performed with a Nikon N-SIM based on an Eclipse TiE inverted microscope using an SR Apo-TIRF 100×/1.49 oil immersion objective and an Andor iXon 3 EMCCD camera. Images were acquired in 3D SIM mode using excitation at 405, 488, and 561 nm and the following emission bandpasses: 425-475 nm for DAPI; 500-550 nm for the green channel; 570-630 nm for the red channel. Image z-stacks were collected with a z interval of 125 nm. Focus stabilization (Perfect Focus System, Nikon) was used to maintain focus during image acquisition. SIM image reconstruction, channel alignment and 3D reconstruction were performed using NIS-Elements AR software.
GIP and RBP4 Secretion Assays
Mouse primary small intestinal culture and secretion assays were performed as previously described (38), under UK Home Office license PE5OF6065. Briefly, the proximal half (15-20 cm) of the small intestine of 4-month-old (C57BL/6) mice was digested in 0.3 mg/mL collagenase XI for 40 minutes, centrifuged at 100g, and resuspended in Dulbecco’s modified Eagle’s medium (25 mM glucose), supplemented with 10% v/v fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 10 µM Y-27632 (Tocris). Tissue digests were passed through a 70-μm cell strainer and plated onto 24-well plates precoated with 2% Matrigel (BD Biosciences) for 18 to 20 hours at 37°C/5% CO2. Basal saline solution (138 mM NaCl, 4.5 mM KCl, 4.2 mM NaHCO3, 1.2 mM NaH2PO4, 2.6 mM CaCl2, 1.2 mM MgCl2, 10 mM HEPES, 0.1% w/v fatty acid–free bovine serum albumin; pH 7.4), or saline supplemented with 10 µM forskolin, 10 µM 3-isobutyl-1-methylxanthine, and 10 mM glucose (termed F/I/10G), was added to wells for 2 hours at 37°C. Supernatants were collected, centrifuged at 2000g and snap frozen on dry ice before measurement of GIP (Millipore, EZRMGIP-55K) and RBP4 (Abcam, ab202404) by enzyme-linked immunosorbent assay, following the manufacturer’s instructions.
Intestinal Permeability Studies
Intestinal permeability was assessed by administering as an oral gavage fluorescein isothiocyanate (FITC) dextran 4000 in a modified version of the protocol described by Woting and Blaut (39). Briefly, after a 10-hour fast, mice were given an oral gavage consisting of 150 μL of FITC-dextran (catalog no. 60842-46-8; 100 mg/mL, Millipore Sigma) and whole blood was collected 4 hours later in EDTA-containing Eppendorf tubes. Plasma was then obtained following centrifugation at 3000g for 10 minutes, then kept in the dark as 100-µL samples were dispensed onto a 96-well plate in duplicate. FITC-dextran standards ranged between 1 and 100 µg/mL and were used to create a standard curve to determine sample FITC concentrations. Fluorescence intensities of the standards and samples were measured using an excitation of 485 nm and an emission of 528 nm.
RBP4 Measurement and Ligand Specificity
Plasma levels of RBP4 were determined using a sensitive and specific radioimmunoassay for rodent RBP4, according to procedures we have described earlier (40).
Human RBP4 was expressed in Escherichia coli and purified in the complex with retinol as described by Golczak et al (41). To identify ligands having a high affinity for RBP4, we employed essentially the same approach that we have used earlier to identify novel ligands for RBP1 and RBP2 (10, 42). Briefly, 200 μL of 20 mM Tris-HCl (pH 7.4), 250 mM KIO3, 5% glycerol, and 0.5 μM holo-RBP4 was placed into wells of UV transparent 96-well plates (Corning). Cayman Chemicals (Bioactive Lipid I Screening) and Enzo Life Science (Screen-Well Bioactive Lipid) libraries were screened by transferring 1 μL of each lipid compound (dissolved in dimethyl sulfoxide) into the wells to a final concentration of 5 μM. The intrinsic fluorescence of RBP4 excited at 285 nm and the retinoid moiety was recorded using Synergy H1 microplate reader (Agilent BioTeK). The ratio between the fluorescence intensity at 470 and 345 nm was calculated for each examined compound. The threshold for a positive high throughput screening hit was set at the F470/F350 value of 0.5.
Statistical Analysis
All data are presented as means ± SEM. Data were analyzed for statistical significance using standard procedures consisting of an unpaired t test for comparisons of 2 groups. For secretion studies, data were analyzed using a Wilcoxon test on relative secretion data compared with basal for each secretion product. All RNA-seq data were analyzed using R (version 4.0.3) and specific methods of data analysis are specified under the respective subheadings. All data obtained from measures were included in statistical calculations. Statistically significant differences were noted for P < .05.
Results
The Absence of RBP2 Does not Markedly Affect Small Intestine Morphology or Integrity
Although at the time of their first description, the Rbp2–/– mice employed in our studies were reported to have a normal small intestine, this was not explored extensively in adult animals (28). Consequently, due to the well-established role of ATRA in regulating cell proliferation, differentiation, and apoptosis (12, 13), we carried out a more in-depth analysis of whether the absence of RBP2 in the adult has an effect on intestinal morphology and/or integrity. Adult Rbp2–/– mice show no significant differences in the overall length of the small intestine when compared with littermate controls, even after adjusting for body weight (Fig. 2B (36)). Hematoxylin and eosin–stained sections of jejunum from Rbp2–/– compared with littermate controls (Fig. 2A (36)) showed no differences in villus length (Fig. 2C (36)) or muscularis thickness (Fig. 2F (36)). However, Rbp2–/– mice have deeper crypts (P = .02) (Fig. 2D (36) ). We also assessed whether RBP2 absence influenced either goblet or Paneth cell numbers or distribution. Goblet and Paneth cells, identified respectively in Alcian blue/PAS and lysozyme-stained sections (Fig. 2E and 2H (36)), showed no differences in numbers or location for either cell type when normalized for intestinal section length (Fig. 2G and 2I) (36)). Intestinal permeability was determined using a direct permeability test assessing 4 kDa FITC-dextran uptake into the circulation (39, 43). The uptake of FITC-dextran into the circulation failed to reveal any differences in intestinal permeability between Rbp2–/– and matched littermate controls (Fig. 2J (36)).
RBP2 and Other Retinoid-related Genes and Proteins are Highly Expressed in EECs Present in the Small Intestine
Total and single-cell transcriptomics in EECs
Because Rbp2–/– mice show a significantly elevated GIP response following an oral fat challenge (10), we asked whether Rbp2 is expressed in incretin-secreting EECs within the mouse small intestine. We carried out an analysis of bulk (30, 33, 34) and single-cell (20, 23, 31, 35) transcriptomic databases created for EECs isolated from either intestinal tissue or EEC-enriched organoid cultures. This analysis showed the presence of relatively high expression levels (defined as log2 [CPM] ≥5) of Rbp2 and other retinoid-related genes in EECs in small intestine sections of human (from jejunum and ileum of both males and females) and mouse (from duodenum and ileum) origin. This was true for both human and mouse small intestine and intestinal organoids for the different databases we searched (30, 33), with substantial levels of expression across databases for Aldh1a1, Rara, and Rbp4 (Fig. 1B). Interestingly, Rbp2 also was found to be expressed in mouse colon tissue in 1 dataset while Rbp4 was expressed in mouse colon in 2 (1 bulk and 1 single cell) databases.
Analysis of databases for the incretin-secreting EEC subtypes revealed relatively high levels of Rbp2 expression, as well as relatively high expression levels for other retinoid-related genes. Specifically, analysis of K-cell single-cell transcriptomic databases evidenced high expression levels for Rbp2 (Fig. 1C). Surprisingly, given that the literature does not indicate that Rbp4 is expressed in the small intestine, all of the K-cell databases showed high levels of Rbp4 expression (20, 23). Moreover, K-cells express the retinoid-related Aldh1a1 and Rara genes (20, 23) (Fig. 1C). Analysis of L-cell databases indicates that Rbp2 as well as Rbp4, Aldh1a1, and Rara are also expressed in this EEC subtype (30, 31, 34, 35) (Fig. 1D). Lrat expression was very low in all of the transcriptomic databases we searched (Fig. 1B-1D).
Since RBP2 binds ligands other than retinol and retinaldehyde with high affinity, specifically long-chain unsaturated 2-MAGs including the canonical endocannabinoid 2-arachidonoylglycerol (10, 11), we also searched the databases for genes related to fatty acid and monoacylglycerol metabolism. Fabp1, Fabp2, Dagla, and Mgll expression were all enriched in both human and mouse EECs (Fig. 3A (36)). Relatively high expression levels for each of these genes were found to be expressed in upon searches of mouse and human K-cells (Fig 3B (36)) and mouse L-cells databases as well (Fig. 3C) (36) ).
RBP2 and other retinoid-related proteins immunolocalization in EECs
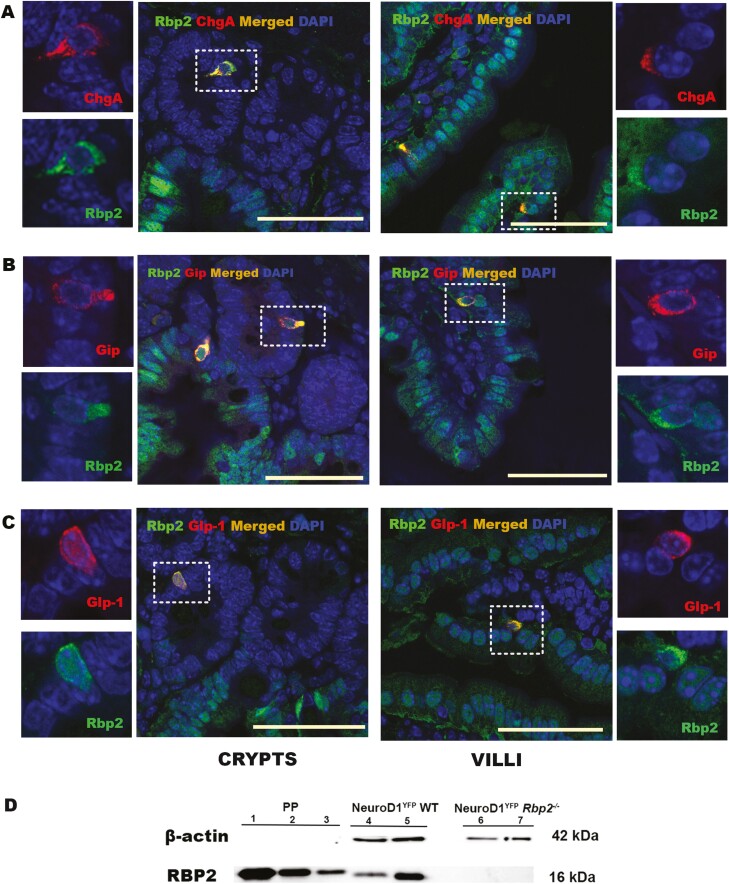
To confirm these findings, we assessed RBP2, ALDH1A1, RARα, and RBP4 presence by immunofluorescence in mouse jejunum sections. The jejunum is the anatomic region associated with the greatest vitamin A absorption and RBP2 abundance (1, 2, 44). In addition to its established localization in the absorptive small intestine epithelial cells along the villus, RBP2 is expressed in cells positive for ChgA (ChgA+), a marker for all EECs (Fig. 2A) (45). RBP2 was also found in ChgA+ cells located in intestinal crypts. This was unexpected since RBP2 has been reported to be expressed only along the villi (1-3). RBP2 was present in GIP+ cells present in both crypts and along the villi (Fig. 2B). In addition, RBP2 was present in GLP-1-positive (GLP-1+) cells in both crypts and villi (Fig. 2C). Hence, RBP2 is expressed in both incretin hormone secreting EEC types within the small intestine. We also confirmed RBP2 presence by WB in 1500 EECs isolated by FACS from a mouse model where a floxed YFP fluorescent reporter was activated upon crossing of this mouse with a mouse where Cre expression was driven by the NeuroD1 promoter (NeuroD1 also being a marker for all EECs) (Fig. 2D). When compared with an identical amount of YFP– cells, which represent mainly enterocytes since goblet and Paneth cells do not express RBP2 as corroborated by the lack of immunofluorescence staining in these cells, it is obvious that the EECs express RBP2, albeit at a level lower than the YFP– cells (Fig. 2D, Lanes 4 and 5).
Figure 2.
RBP2 is expressed in enteroendocrine cells present in wild-type C57BL/6J mouse jejunum. (A) Immunofluorescence (IF) confocal imaging establishing colocalization of RBP2 in ChgA+ cells, a marker for all EECs. (B) IF confocal imagining establishing colocalization of RBP2 with GIP. (C) IF confocal imaging establishing colocalization of RBP2 with GLP-1. The proteins colocalize in crypts (center left with higher resolution insets to the left) as well as in villi (center right with higher resolution insets to the right). Selected cells shown in the insets are highlighted by rectangles in the main micrograph. Color code: red indicates ChgA, GIP or GLP-1 IF; green indicates RBP2 IF; blue indicates DAPI staining; and merged images (ChgA, GIP or GLP-1 with RBP2) are in orange. All bars = 50 µm. (D) Western blot of EECs lysates confirming the presence of RBP2. PP: purified protein at a concentration of 10 ng (lane 1), 1ng (lane 2) and 100 pg (lane 3); NeuroD1YFP wild type: NeuroD1 expressing cells sorted by FACS using YFP fluorescence from proximal small intestine of a C57BL/6J mouse consisting of 1,500 YFP+ cells (lane 4) representing the total EEC population and 1,500 YFP– cells (lane 5) representing mostly enterocytes (since goblet and Paneth cells do not present positive IF for RBP2); NeuroD1YFPRbp2–/–: NeuroD1 expressing cells obtained by FACS sorting using YFP fluorescence from proximal small intestine of a Rbp2–/–mouse to serve as a negative control for RBP2 for 1500 YFP+ cells (lane 6) representing the total EEC population and 1500 YFP– cells (lane 7). Bands from beta-actin are only present in the sorted cell samples (lanes 4-7) at 42 kDa.
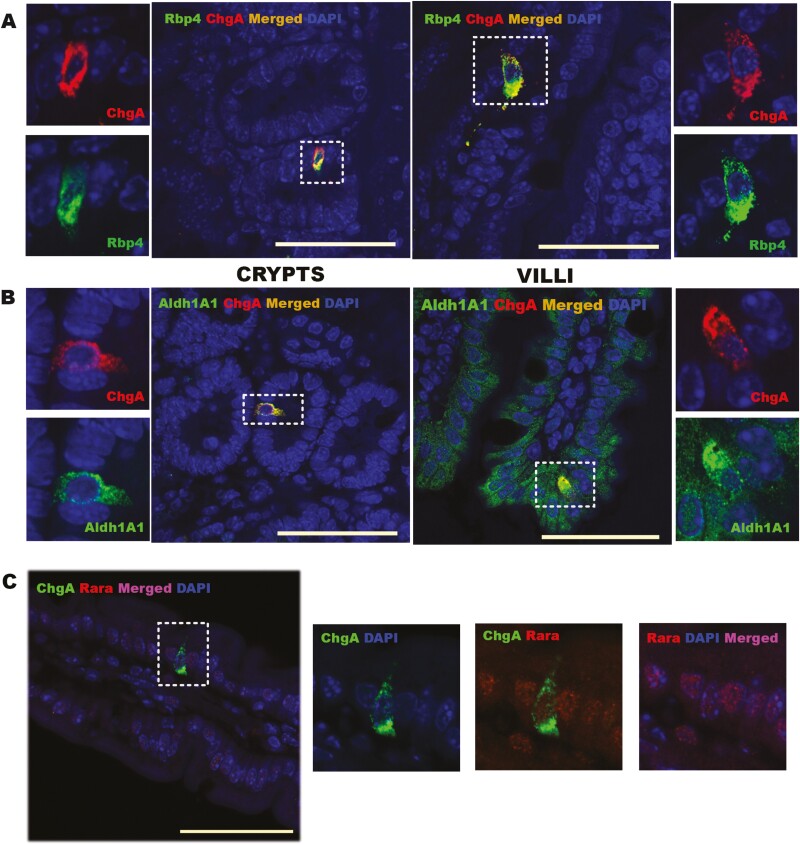
ALDH1A1, a key enzyme involved in retinoic acid formation, and one which is known to be present within enterocytes (46), also colocalizes to ChgA+ cells (Fig. 3B). Thus, like RBP2, ALDH1A1 is expressed in EECs present in both crypts, where its presence was previously unknown, and along the villi (Fig. 3B). ALDH1A1 expression levels in EECs are relatively high compared with neighboring enterocytes (Fig. 4B (36)). Similarly, RARα is highly expressed in ChgA+ cells in both crypts and along the villus (Fig. 3C). Thus, ChgA+ cells have the capacity to synthesize ATRA and for ATRA-responsive regulation of gene expression.
Figure 3.
RBP4, ALDH1A1 and RARα are all expressed in ChgA+ cells within the jejunums of male wild-type C57BL/6J mice. (A) Immunofluorescence (IF) confocal imaging establishing colocalization of RBP4 in ChgA+ cells. RBP4 and ChgA colocalize in crypts (center left with higher resolution insets to the left) as well as in villi (center right with higher resolution insets to the right). (B) IF confocal imagining establishing colocalization of ALDH1A1 and ChgA. The proteins colocalize in crypts (center left with higher resolution insets to the left) as well as in villi (center right with higher resolution insets to the right). Selected cells shown in the insets of A and B are outlined by rectangles in the main micrograph. Color code for A and B: red indicates ChgA; green indicates RBP4 or ALDH1A1; blue indicates DAPI staining; and merged images (ChgA with RBP4 or ALDH1A1) in orange. (C) IF confocal imaging establishing colocalization of RARα with ChgA. The left panel gives the merged image showing RARα and ChgA expression in a DAPI-stained villus. The subsequent 3 images (left to right) show ChgA expression in a DAPI-stained villus tissue, ChgA and RARα overlap, and RARα expression in DAPI-stained villus tissue. Color code for C: red indicates RARα; green indicates ChgA; blue indicates DAPI staining; and merged images (RARα and ChgA) are in magenta. All bars = 50 µm.
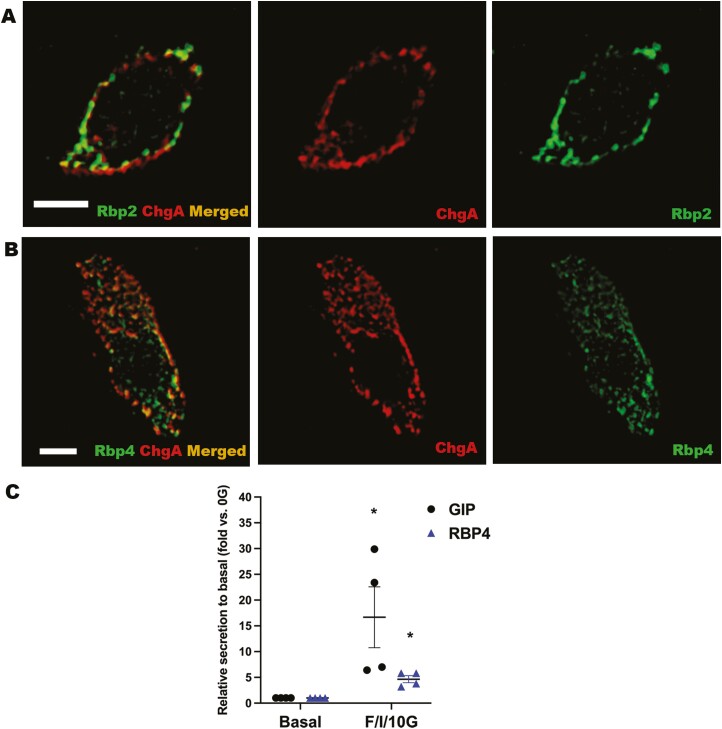
Although the small intestine has not been identified as a tissue site of RBP4 expression, a strong presence of RBP4 in ChgA+ cells was detected for both crypts and villi of mouse jejunum (Fig. 3A). The distribution of RBP4 within ChgA+ cells displayed a granular appearance that seemed to be in close proximity or possibly within the secretory vesicles. We therefore further investigated the association of RBP4 and RBP2 (which also displayed a similar distribution pattern) with the secretory vesicles containing ChgA using 3D structural illumination microscopy (3D SIM) (Fig. 4A and 4B). Surprisingly, we found a partial overlap of both RBP4 and RBP2 with ChgA present in vesicles located around the periphery of the cells. We take this to indicate that RBP4 and RBP2 are localized either within or immediately around ChgA-containing secretory granules. When primary cultures of mouse intestinal cells obtained from the proximal small intestine were treated with a cocktail containing forskolin, 3-isobutyl-1-methylxanthine, and glucose, one which is known to stimulate secretory granule release from EECs (38), both GIP and RBP4 were cosecreted into the medium (Fig. 4C). We take this to indicate that RBP4 is localized within EEC secretory granules and that it has a role in hormone secretion from these cells.
Figure 4.
RBP2 and RBP4 are in close relationship with chromogranin A vesicles in EECs within the jejunums of male wild-type C57BL/6J mice. (A) 3D SIM images of an EEC showing partial overlap of ChgA-containing vesicles and RBP2 (left) and individual distribution of ChgA vesicles (center) and RBP2 (right). (B) 3D SIM images of an EEC showing overlap of ChgA-containing vesicles and RBP4 (left) and individual distribution of ChgA vesicles (center) and RBP4 (right). Color code for A and B: red indicates ChgA IF; green indicates RBP2 or RBP4 IF; and merged images (ChgA with RBP2 or RBP4) are in orange. Scale bars = 3 µm. (C) GIP and RBP4 were cosecreted from murine proximal small intestine cultures upon stimulation with forskolin/-isobutyl-1-methylxanthine/10 mM glucose (F/I/10G). Samples were obtained from mouse primary intestinal cultures prepared for 4 different animals, each treated in duplicate. (C) Data represent mean ± SEM (GIP in black, RBP4 in blue) showing increased media levels after treatment compared with basal (0G) levels. Statistical significance: *P < .05.
Absence of RBP2 Influences EEC Numbers and Distribution
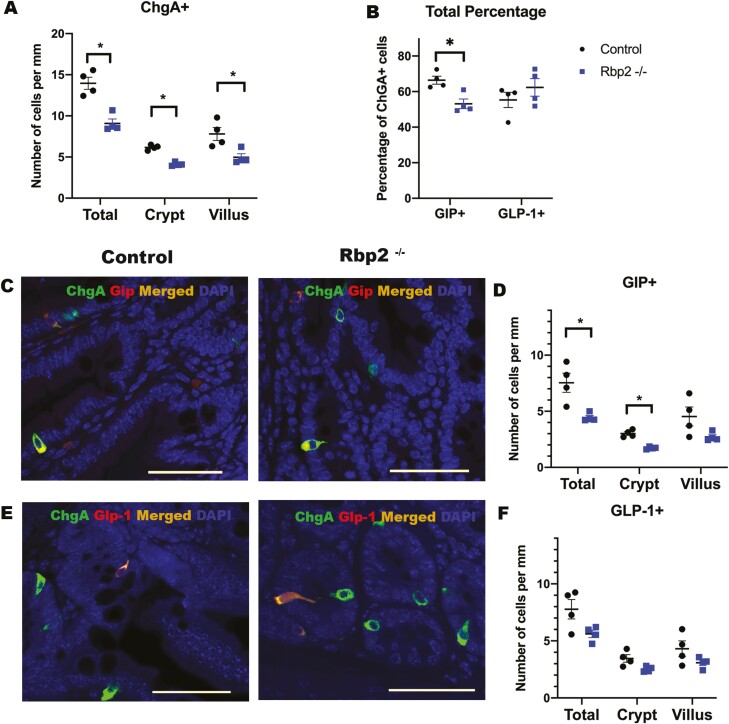
Considering the known actions of retinoids in regulating cell proliferation and differentiation, the finding that RBP2, as well as an enzyme needed for the synthesis of ATRA, and RARα, all colocalize to EECs, led us investigate possible differences in EEC numbers and distributions within the jejunum. To this end, we assessed whether there are differences in ChgA+ cell numbers for Rbp2–/– compared with control mice. When expressed as number of cells per millimeter, to adjust for section length, we found that Rbp2–/– mice have fewer ChgA+ cells (P = .002), both in total (crypts plus villi) and individually in crypts and in villi (crypts P < .001; villi P = .02) (Fig. 5A). We next analyzed the impact that the absence of RBP2 specifically has on GIP+ and GLP-1+ cell distribution (Fig. 5C and 5E). Rbp2–/– mice displayed significantly fewer total (P = .03) GIP+ cells and fewer GIP+ cells in crypts (P = .002) than in controls (Fig. 5D). Moreover, Rbp2–/– mice displayed both fewer total GIP+ cells and GIP+ cells in the crypts when expressed as a percentage of ChgA+ cells (P = .009 and 0.001 respectively) (Fig. 5B and Fig. 5A (36)). Thus, the total number of ChgA+ cells is diminished in Rbp2–/– jejunum and a reduction of GIP+ cells is also observed. Both the number and distribution of GLP-1+ cells showed no significant differences either in crypts or along the villi for Rbp2–/– mice when compared with littermate controls (Fig. 5F). Nor did we detect any difference in GLP-1+ cells as a percentage relative to ChgA+ cells (Fig. 5B and Fig. 5B (36)). However, GLP-1+ cells were more predominant than GLP-1– ChgA+ cells in Rbp2–/– intestinal crypts (2.52 ± 0.26 vs 1.60 ± 0.19 cells per mm, P = .009).
Figure 5.
Effects of RBP2 deficiency on EEC numbers and distribution in the jejunum compared with matched littermate controls. (A) Small intestines of Rbp2–/– mice showed lower numbers of ChgA+ cells for both the total crypt–villus axis as well as for crypts and villi alone compared with matched control mice. (B) Quantification of the total percentage of ChgA+ cells that coexpress GIP and GLP-1. (C,D) Immunostaining of matched control (wild type) and Rbp2–/– mouse jejunum identified both a lower GIP+ total cell number and a lower number for crypts of Rbp2–/– mice compared with controls. (E,F) Immunostaining of Rbp2–/– mouse jejunum showed no differences in GLP-1+ cell number compared with controls. A, B, D, and F provide mean values and error bars represent SEM for 4 mice in each panel. Black dots represent wild-type C57BL/6J littermate controls and blue squares represent Rbp2–/– mice. Statistical significance: *P < .05. Scale bar = 50 µm.
RBP2 Absence Does not Affect Circulating RBP4 Levels
Owing to the unexpected findings that both RBP2 and RBP4 are expressed in ChgA+ cells within the proximal small intestine, we investigated possible connections between RBP2 and RBP4 in the development of the metabolic disorders observed in Rbp2–/– mice. The absence of RBP2 does not bring about differences in circulating levels of RBP4 in 2-month-old Rbp2–/– mice maintained on a chow diet (2.2 ± 0.4 μM, N = 16) compared with controls (2.4 ± 0.4 μM, N = 15). Nor does Rbp2 absence affect Rbp4 mRNA levels in the liver. Since elevated circulating RBP4 levels are associated with metabolic disease development (47), the lack of a difference in RBP4 levels between Rbp2–/– and control mice indicates that RBP4 does not causally contribute to the metabolic phenotypes observed for Rbp2–/– mice, although it is highly expressed in ChgA+ cells. Since RBP2 and RBP4 both bind retinol with high affinity, we wondered whether, like RBP2, RBP4 may also tightly bind 2-MAGs or any other class of endogenous signaling lipids beside retinoids. Thus, we carried out a high throughput screening of 2 chemical libraries comprising more than 1000 individual lipid compounds. Using this approach, we earlier identified that 2-MAGs are high-affinity ligands for RBP2 (10, 11). We obtained no evidence that RBP4 binds 2-MAGs with high affinity, unlike RBP2. We did however identify several fatty acids and fatty acid derivatives that bind RBP4 (48), including ones that were not previously known to have a strong affinity for RBP4 (Fig. 6 (36)), specifically 2 18-carbon unsaturated fatty acids (linolenic acid and γ-linolenic acid), as well as 3 20-carbon unsaturated fatty acids.
Discussion
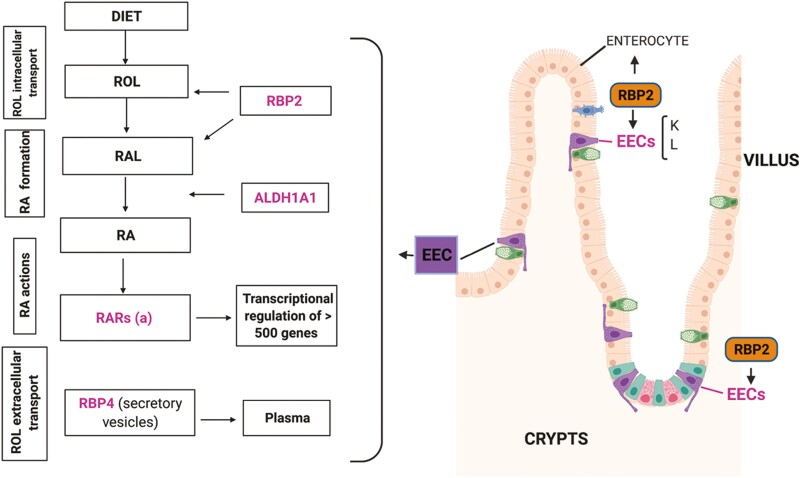
We wished to understand molecular relationships between RBP2 and GIP production by EECs present within the proximal small intestine due to our previous finding that Rbp2–/– mice display an age- and diet-related phenotype of obesity, glucose intolerance, and elevated hepatic (10). These phenotypes are associated with significantly elevated circulating GIP levels, both fasting and following an oral fat challenge (10). RBP2 is highly expressed in enterocytes lining the villus, but not in goblet cells (1, 2). However, whether the other 2 major intestinal secretory cell types, the Paneth cells and EECs, may express RBP2 was unknown. The immunohistochemical studies provided no evidence supporting the possibility that Paneth cells express RBP2. Given the fact that the elevated GIP levels in Rbp2–/– mice are observed early in life, we hypothesize a role for RBP2 and/or its retinoid and/or 2-MAG ligands in GIP synthesis and/or secretion. Thus, the question is whether RBP2’s role involves paracrine effects and signals originating from enterocytes or whether there may be also a direct effect originating within EECs. Our transcriptomics data and immunohistochemical data establish that RBP2 is expressed in ChgA+ cells, including in both GIP+ and GLP-1+ cells. Thus, RBP2 effects on GIP are likely to include direct mechanisms affecting EEC biology yet to be unraveled (Fig. 6).
Figure 6.
A diagram showing RBP2 and other retinoid-related proteins present in enteroendocrine cells. Proteins of interest are in magenta and their known roles in retinoid metabolism are described in the panels on the left (rectangles). A diagram showing the cell lineages in the small intestine and their location in the crypt-villus axis is shown on the right side. Since enterocytes are only located in the villi, RBP2 is only found in the crypts when associated with EECs.
Our assessment of transcriptomics databases obtained from total EEC populations as well as from isolated K and L cells indicates high expression of a number of genes importantly involved in ATRA signaling, in addition to Rbp2. However, the gene encoding LRAT, which catalyzes retinyl ester synthesis and which is needed for packaging dietary retinoid into chylomicrons for uptake into the body, is poorly expressed. We take this to indicate that RBP2 within these cells metabolically channels retinol and retinaldehyde toward ATRA formation and not toward retinyl esters and retinoid uptake. Indeed, ALDH1A1, which catalyzes the final enzymatic step needed for ATRA formation (46), and RARα are highly expressed in ChgA+ cells. This suggests that RBP2 found within ChgA+ cells is metabolically channeling retinol and retinaldehyde toward the formation of ATRA, which is needed to facilitate ATRA-regulated transcription.
Because ATRA is a potent regulator of cell proliferation, differentiation, and apoptosis (12, 13), we investigated whether the absence of RBP2 might influence the integrity and/or the cellularity of the small intestine in adult mice. Although we found no differences in intestinal permeability and in most morphological parameters, aside from a greater jejunal crypt depth, total ChgA+ and GIP+ cell numbers were significantly lower within the proximal intestine of Rbp2–/– mice. However, no differences in GLP-1+, goblet, or Paneth cell numbers were detected. Our finding regarding ChgA+ cells is similar to what has been reported for the distal small intestine of Raravillin mice (49). These mice displayed a diminished number of EECs compared with controls. This provides further support for our conclusion that Rbp2 absence specifically affects ATRA-dependent signaling and EEC numbers. Interestingly, the Raravillin mice also show increased numbers of goblet and Paneth cells (49). We did not observe this for Rbp2–/– mice. Thus, it appears that ATRA-RARα actions are needed to assure normal differentiation and proliferation by all 3 major secretory lineages, but RBP2 action is needed only by the EEC lineage.
Since we identified elevated circulating GIP levels in Rbp2–/– mice (10), our present finding of diminished GIP+ cell numbers in this mouse model seems contradictory. Given that the elevated GIP levels in Rbp2–/– mice cannot be explained by increased cellularity, other possible mechanistic explanations may involve enhanced GIP synthesis and/or secretion. This notion is not unprecedented in the literature, where it is reported that elevated expression of transcriptional regulatory factor X6 (Rfx6) in K-cells contributes to GIP hypersecretion in response to high fat diet-induced obesity without an increase in K-cell number (50). Interestingly, inducible deletion of Rfx6 in the adult mouse is reported to result in extensive loss of EECs expressing peptide hormones (51). We also note that earlier we demonstrated that RBP2 binds tightly 2-MAGs (10), which can stimulate the actions of the cell surface receptor GPR119, which is expressed on K-cells (52, 53), resulting in increased GIP release. Consequently, the absence of RBP2 results in elevated 2-MAG levels following a fatty meal. Thus, although the absence of RBP2 is associated with a decreased number of GIP+ cells, it also results in an increase in intestinal 2-MAG levels (10) possibly resulting in the differences that we have observed.
Paradoxically, GIP has long been considered to be a hormone that promotes obesity (54). The hormone is secreted in response to dietary lipid intake and enhances postprandial lipid clearance and its deposition in adipose tissue (54). At the molecular level, GIP increases adipocyte lipid accrual by affecting insulin-independent stimulation of lipoprotein lipase (LPL) activity and fat incorporation into adipose tissue (50, 51). With the colocalization of RBP2 to GIP-producing K-cells and the known elevated GIP response to lipid for Rbp2–/–mice, this led us to speculate that this may help explain the excess weight gain phenotype observed for Rbp2–/– mice. However, as noted in 2 recent reviews (55, 56), whether GIP contributes to the development of obesity, or rather to weight loss, is now controversial. Thus, this hypothesis and its implications will need to be compellingly established by future investigations.
Under physiological conditions, GIP and GLP-1 signaling account for up to 70% of β-cell insulin secretion after an oral glucose challenge (57). Having said that, GIP displays a weak or absent insulinotropic response in insulin resistant conditions like type 2 diabetes, with GLP-1 responsiveness still preserved at some level in β-cells (24, 54, 57). Nevertheless, there has been renewed interest in this incretin as therapeutic agent thanks to the recent development of a dual GIP and GLP-1 agonist, which is showing more than promising results for glucose and weight control. This drug, called tirzepatide, showed a synergistic and superior effect in reducing body weight and in glycemia control when compared with a GLP-1 agonist alone in a phase III trial in patients with type 2 diabetes (55, 58). Given the potential therapeutic benefits of GIP, factors affecting its levels and conditions that could influence its beneficial vs its less desirable systemic effects are worth exploring.
A surprising finding from our studies is the identification of RBP4 expression in EECs, in both K- and L-cells. It had been thought that RBP4, which is the sole specific transporter for retinol in the circulation, is not expressed in the small intestine. Indeed, RBP4 is not expressed in enterocytes, but it is present in secretory granules found in ChgA+ cells. Moreover, we have shown RBP4 is cosecreted with GIP. RBP4 is synthesized and secreted by liver and adipose tissue and its elevated levels in the circulation have been associated with the pathophysiology of insulin-resistant states with several research studies targeting its actions on muscle, liver, and especially adipose tissue through its proinflammatory effects (59, 60). Transcriptomic data have suggested the presence of Rbp4 in pancreatic delta cells (61). It has been recently proposed that Rbp4 may signal a defective glucose-stimulated insulin secretion in β-cells through its actions on its receptor STRA6 (62). However, why is RBP4 expressed in EECs since it has no known direct effects on ATRA biosynthesis, nor does it bind 2-MAGs? One possible explanation for this may be that RBP4 is needed to export retinol from these cells to prevent its overaccumulation and uncontrolled use for ATRA biosynthesis. Alternatively, our observation of a partial overlap within the EEC cytoplasm of RBP4 with ChgA+ secretory vesicles, which also overlaps with RBP2, raises a question of the possible direct involvement of these proteins in hormone secretory vesicle formation, integrity, and/or release. This possibility clearly merits further investigation.
In summary, our data establish the presence of RBP2 in EECs, especially in incretin-secreting K- and L-cells. This provides support for our hypothesis that direct effects of RBP2 within K-cells may account for the metabolic phenotypes exhibited by Rbp2–/– mice through mechanisms yet to be identified. The high expression levels in K- and L-cells of ALDH1A1 as well as RARα, but not Lecithin Retinol Acyltransferase (LRAT), indicates to us that ATRA actions contribute to K- and L-cell biology within the intestine. The diminished number of K-cells present in the jejunums of Rbp2–/– mice is consistent with the notion that this difference may involve ATRA-mediated transcriptional effects on cell proliferation and differentiation. Both genes involved in ATRA formation as well as in 2-MAG transport and metabolism are expressed in K- and L-cells based on single-cell transcriptomics analyses. One of our working hypotheses for explaining why we see elevated circulating GIP levels in the face of diminished K-cell numbers in the Rbp2-deficient intestine is that this results from dysregulated 2-MAG formation and/or catabolism, elevating 2-MAG levels, and giving rise to increased GIP release from GIP+ cells. However, this needs to be definitively established. The discovery that RBP4, the only extracellular retinol-binding protein, is present within ChgA+ cells raises a number of questions regarding retinoid processing within these cells. The association of RBP2, and to a greater degree RBP4, with ChgA-containing secretory vesicles suggests actions of these proteins that may affect GIP levels in the circulation which are independent of direct ATRA actions. This too requires further investigation.
Acknowledgments
The authors wish to thank Drs. Munemasa Mori and Wellington Cardoso for their assistance and guidance that allowed the undertaking of our studies. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission
Glossary
Abbreviations
- 2-MAG
2-monoacylglycerol
- ALDH1A1
aldehyde dehydrogenase 1 family member A1
- ATRA
all-trans-retinoic acid
- ChgA
chromogranin A
- CPM
counts per million
- EEC
enteroendocrine cell
- FACS
fluorescence-activated cell sorting
- FITC
fluorescein isothiocyanate
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- RAR
retinoic acid receptor
- RBP
retinol-binding protein
- SIM
structured illumination microscopy
- WB
Western blot
- YFP
yellow fluorescent protein.
Contributor Information
Rossana M Calderon, Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Christopher A Smith, Institute of Metabolic Sciences and MRC-Metabolic Diseases Unit, University of Cambridge, Cambridge CB0 0QQ 44106, UK.
Emily L Miedzybrodzka, Institute of Metabolic Sciences and MRC-Metabolic Diseases Unit, University of Cambridge, Cambridge CB0 0QQ 44106, UK.
Josie A Silvaroli, Department of Pharmacology, Case Western Reserve University, Cleveland, OH, USA.
Marcin Golczak, Department of Pharmacology, Case Western Reserve University, Cleveland, OH, USA; Cleveland Center for Membrane and Structural Biology, School of Medicine, Case Western Reserve University, Cleveland, OH, USA.
Fiona M Gribble, Institute of Metabolic Sciences and MRC-Metabolic Diseases Unit, University of Cambridge, Cambridge CB0 0QQ 44106, UK.
Frank Reimann, Institute of Metabolic Sciences and MRC-Metabolic Diseases Unit, University of Cambridge, Cambridge CB0 0QQ 44106, UK.
William S Blaner, Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Financial Support
National Institutes of Health grants R01 DK068437 and R21 AA028110 (W.S.B.), National Institutes of Health grant R01 DK122071 (W.S.B. and M.G.). National Institutes of Health grant T32 HL007343 (R.M.C.). Wellcome Trust grant WT220271 (F.M.G., F.R.), Medical Research Council UK grant MRC_MC_UU_12012/3 (F.M.G./F.R.), Wellcome Trust grant to support dissertation research (E.L.M.).
Disclosure Summary
The authors declare that they have no conflicts of interest with the contents of this article.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Crow JA, Ong DE. Cell-specific immunohistochemical localization of a cellular retinol-binding protein (type two) in the small intestine of rat. Proc Natl Acad Sci USA. 1985;82(14):4707-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ong DE, Newcomer M, Chytil F. Cellular retinoid-binding proteins. In: The Retinoids: Biology, Chemistry and Medicine. 2nd ed. Raven Press; 1994: 283-318. [Google Scholar]
- 3. Blaner WS, Brun PJ, Calderon RM, Golczak M. Retinol-binding protein 2 (RBP2): biology and pathobiology. Crit Rev Biochem Mol Biol. 2020;55(2):197-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ong DE. Vitamin A-binding proteins. Nutr Rev. 1985;43(8):225-232. [DOI] [PubMed] [Google Scholar]
- 5. Cheng L, Qian SJ, Rothschild C, et al. Alteration of the binding specificity of cellular retinol-binding protein II by site-directed mutagenesis. J Biol Chem. 1991;266(36):24404-24412. [PubMed] [Google Scholar]
- 6. Napoli JL. Functions of intracellular retinoid binding-proteins. Subcell Biochem. 2016;81:21-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Napoli JL. Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: effects on retinoid metabolism, function and related diseases. Pharmacol Ther. 2017;173:19-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herr FM, Wardlaw SA, Kakkad B, Albrecht A, Quick TC, Ong DE. Intestinal vitamin A metabolism: coordinate distribution of enzymes and CRBP(II). J Lipid Res. 1993;34(9):1545-1554. [PubMed] [Google Scholar]
- 9. Wongsiriroj N, Piantedosi R, Palczewski K, et al. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem. 2008;283(20):13510-13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee SA, Yang KJZ, Brun PJ, et al. Retinol-binding protein 2 (RBP2) binds monoacylglycerols and modulates gut endocrine signaling and body weight. Sci Adv. 2020;6(11):eaay8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silvaroli JA, Plau J, Adams CH, et al. Molecular basis for the interaction of cellular retinol binding protein 2 (CRBP2) with nonretinoid ligands. J Lipid Res. 2021;62:100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9(7):541-553. [DOI] [PubMed] [Google Scholar]
- 13. Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345-364. [DOI] [PubMed] [Google Scholar]
- 14. Blaner WS. “Vitamin A” in Present Knowledge in Nutrition. 11th ed. Academic Press, Cambridge MA; 2020:73-92. [Google Scholar]
- 15. Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res. 2013;54(7):1761-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benbrook DM, Chambon P, Rochette-Egly C, Asson-Batres MA. History of retinoic acid receptors. Subcell Biochem. 2014;70:1-20. [DOI] [PubMed] [Google Scholar]
- 17. Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43(11):1773-1808. [DOI] [PubMed] [Google Scholar]
- 18. Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16(1):19-34. [DOI] [PubMed] [Google Scholar]
- 19. Bonis V, Rossell C, Gehart H. The intestinal epithelium – fluid fate and rigid structure from crypt bottom to villus tip. Front Cell Dev Biol. 2021;9:661931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gehart H, van Es JH, Hamer K, et al. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell. 2019;176(5):1158-1173.e16. [DOI] [PubMed] [Google Scholar]
- 21. Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12 (Suppl 2):S63-68. [DOI] [PubMed] [Google Scholar]
- 22. Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15(4):226-237. [DOI] [PubMed] [Google Scholar]
- 23. Beumer J, Artegiani B, Post Y, et al. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol. 2018;20(8):909-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4(6):525-536. [DOI] [PubMed] [Google Scholar]
- 25. Petersen N, Reimann F, van Es JH, et al. Targeting development of incretin-producing cells increases insulin secretion. J Clin Investig. 2015;125(1):379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willard FS, Douros JD, Gabe MB, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5(17):e140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calanna S, Christensen M, Holst JJ, et al. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care. 2013;36(10):3346-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xueping E, Zhang L, Lu J, et al. Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J Biol Chem. 2002;277(39):36617-36623. [DOI] [PubMed] [Google Scholar]
- 29. Li HJ, Kapoor A, Giel-Moloney M, Rindi G, Leiter AB. Notch signaling differentially regulates the cell fate of early endocrine precursor cells and their maturing descendants in the mouse pancreas and intestine. Dev Biol. 2012;371(2):156-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roberts GP, Larraufie P, Richards P, et al. Comparison of human and murine enteroendocrine cells by transcriptomic and peptidomic profiling. Diabetes. 2019;68(5):1062-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Billing LJ, Larraufie P, Lewis J, et al. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice - Identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol Metab. 2019;29:158-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Research Council. Guide for the Care and Use of Laboratory Animals. 8 ed. Washington DC: National Academies Press (US). 2011. [Google Scholar]
- 33. Larraufie P, Roberts GP, McGavigan AK, et al. Important role of the GLP-1 axis for glucose homeostasis after bariatric surgery. Cell Rep. 2019;26(6):1399-1408.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldspink DA, Lu VB, Miedzybrodzka EL, et al. Labeling and characterization of human GLP-1-secreting L-cells in primary ileal organoid culture. Cell Rep. 2020;31(13):107833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glass LL, Calero-Nieto FJ, Jawaid W, et al. Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Mol Metab. 2017;6(10):1296-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calderon RM, Smith CA, Miedzybrodzka EL, Silvaroli JA, Golczak M, Gribble FM, Reimann F, Blaner WS. Supplementary information for: intestinal enteroendocrine cell signaling: retinol-binding protein 2 (RBP2) and retinoid actions. Acad Commons. 2022. 10.7916/seyp-0s14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Galvin SG, Larraufie P, Kay RG, et al. Peptidomics of enteroendocrine cells and characterisation of potential effects of a novel preprogastrin derived-peptide on glucose tolerance in lean mice. Peptides. 2021;140:170532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Psichas A, Tolhurst G, Brighton CA, Gribble FM, Reimann F. Mixed primary cultures of murine small intestine intended for the study of gut hormone secretion and live cell imaging of enteroendocrine cells. J Vis Exp. 2017;(122):55687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woting A, Blaut M. Small intestinal permeability and gut-transit time determined with low and high molecular weight fluorescein isothiocyanate-dextrans in C3H mice. Nutrients 2018;10(6):685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blaner WS. Radioimmunoassays for retinol-binding protein, cellular retinol-binding protein, and cellular retinoic acid-binding protein. Methods Enzymol. 1990;189:270-281. [DOI] [PubMed] [Google Scholar]
- 41. Golczak M, Maeda A, Bereta G, et al. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem. 2008;283(15):9543-9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silvaroli JA, Widjaja-Adhi MAK, Trischman T, et al. Abnormal cannabidiol modulates vitamin A metabolism by acting as a competitive inhibitor of CRBP1. ACS Chem Biol. 2019;14(3):434-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lackey AI, Chen T, Zhou YX, et al. Mechanisms underlying reduced weight gain in intestinal fatty acid-binding protein (IFABP) null mice. Am J Physiol Gastrointest Liver Physiol. 2020;318(3):G518-G530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goncalves A, Roi S, Nowicki M, et al. Fat-soluble vitamin intestinal absorption: absorption sites in the intestine and interactions for absorption. Food Chem. 2015;172:155-160. [DOI] [PubMed] [Google Scholar]
- 45. Yan KS, Gevaert O, Zheng GXY, et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell. 2017;21(1):78-90.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiang CP, Wu CW, Lee SP, et al. Expression pattern, ethanol-metabolizing activities, and cellular localization of alcohol and aldehyde dehydrogenases in human small intestine. Alcohol Clin Exp Res. 2012;36(12):2047-2058. [DOI] [PubMed] [Google Scholar]
- 47. Blaner WS. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. 2019;197:153-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perduca M, Nicolis S, Mannucci B, Galliano M, Monaco HL. Human plasma retinol-binding protein (RBP4) is also a fatty acid-binding protein. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(4):458-466. [DOI] [PubMed] [Google Scholar]
- 49. Jijon HB, Suarez-Lopez L, Diaz OE, et al. Intestinal epithelial cell-specific RARalpha depletion results in aberrant epithelial cell homeostasis and underdeveloped immune system. Mucosal Immunol. 2018;11(3):703-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suzuki K, Harada N, Yamane S, et al. Transcriptional regulatory factor X6 (Rfx6) increases gastric inhibitory polypeptide (GIP) expression in enteroendocrine K-cells and is involved in GIP hypersecretion in high fat diet-induced obesity. J Biol Chem. 2013;288(3):1929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Piccand J, Vagne C, Blot F, et al. Rfx6 promotes the differentiation of peptide-secreting enteroendocrine cells while repressing genetic programs controlling serotonin production. Mol Metab. 2019;29:24-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ekberg JH, Hauge M, Kristensen LV, et al. GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40). Endocrinology. 2016;157(12):4561-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reimann F, Diakogiannaki E, Moss CE, Gribble FM. Cellular mechanisms governing glucose-dependent insulinotropic polypeptide secretion. Peptides. 2020;125:170206. [DOI] [PubMed] [Google Scholar]
- 54. Holst JJ, Rosenkilde MM. Recent advances of GIP and future horizons. Peptides. 2020;125:170230. [DOI] [PubMed] [Google Scholar]
- 55. Del Prato S, Gallwitz B, Holst JJ, Meier JJ. The incretin/glucagon system as a target for pharmacotherapy of obesity. Obes Rev. 2022;23(2):e13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miedzybrodzka EL, Reimann F, Gribble FM. The enteroendocrine system in obesity. In: Handbook of Experimental Pharmacology. Springer; 2022. [DOI] [PubMed] [Google Scholar]
- 57. Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297(1-2):127-136. [DOI] [PubMed] [Google Scholar]
- 58. Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515. [DOI] [PubMed] [Google Scholar]
- 59. Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356-362. [DOI] [PubMed] [Google Scholar]
- 60. Moraes-Vieira PM, Yore MM, Sontheimer-Phelps A, et al. Retinol binding protein 4 primes the NLRP3 inflammasome by signaling through Toll-like receptors 2 and 4. Proc Natl Acad Sci USA. 2020;117(49):31309-31318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. DiGruccio MR, Mawla AM, Donaldson CJ, et al. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab. 2016;5(7):449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang R, Bai X, Li X, Wang X, Zhao L. Retinol-binding protein 4 activates STRA6, provoking pancreatic beta-cell dysfunction in type 2 diabetes. Diabetes. 2021;70(2):449-463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.