Abstract
The human leukocyte antigen (HLA) system is one of the most crucial host factors influencing disease progression in bacterial and viral infections. This review provides the basic concepts of the structure and function of HLA molecules in humans. Here, we highlight the main findings on the associations between HLA class I and class II alleles and susceptibility to important infectious diseases such as tuberculosis, leprosy, melioidosis, Staphylococcus aureus infection, human immunodeficiency virus infection, coronavirus disease 2019, hepatitis B, and hepatitis C in populations worldwide. Finally, we discuss challenges in HLA typing to predict disease outcomes in clinical implementation. Evaluation of the impact of HLA variants on the outcome of bacterial and viral infections would improve the understanding of pathogenesis and identify those at risk from infectious diseases in distinct populations and may improve the individual treatment.
1. Introduction
The cell-mediated adaptive immune response is regulated by the major histocompatibility complex (MHC) or human leukocyte antigen (HLA) in humans [1]. HLA molecules are cell surface glycoproteins whose primary function is to present endogenous and exogenous antigens to T lymphocytes for recognition and response [2]. The HLA molecules that present antigen to T lymphocytes are divided into two main classes: HLA class I and HLA class II molecules. HLA class I molecules play an essential role in the immune defense against intracellular pathogens, whereas HLA class II molecules are predominantly involved in displaying peptides from extracellular pathogens [3]. The HLA region is highly polymorphic, and polymorphisms in the HLA molecules result in variability in amino acid sequences of HLA molecules and thus affect the peptide binding specificity [4]. HLA molecules encoded by different alleles have different peptide-binding repertoires [5]. The polymorphisms in the HLA locus contribute to the genetic diversity of humans and the differences in susceptibility to diseases among genetically distinct groups, thus offering evolutionary advantages of a diverse immunological response to a wide range of infectious pathogens [6]. The associations between HLA alleles and susceptibility to or protection from infectious diseases have been well documented. However, the molecular mechanism underlying host HLA function to infection remains far from understood. Infectious disease continues to affect poor and marginalized populations; therefore, it is essential to utilize the increasing knowledge and technological advances in HLA typing to study the pathogenesis and development of novel therapeutic targets in infectious diseases of public health concerns.
Genetic variations at the loci encoding HLA genes are associated with susceptibility or protection to infectious diseases. Genetic studies have found an association between the HLA alleles or haplotypes and bacterial infectious diseases, including tuberculosis, leprosy, and melioidosis [7–9]. Identifying risk and protective HLA alleles will provide critical insights into the mechanisms that influence the pathogenesis of infections and protection. HLA typing can identify associations between HLA alleles and infections in an individual [10]. Patients exhibit different immune responses to bacterial and viral infections, and HLA molecules play an essential role in regulating the host's immune response. Therefore, the reported HLA alleles contributing to the susceptibility or protective effect to bacterial and viral infections will aid in elucidating the immunological mechanisms in disease outcomes [11, 12].
This review will provide the basic concepts of HLA and the current status of the HLA associations with bacterial and viral infections across world populations. This review primarily focuses on predisposing risk and protective HLA alleles among several populations in major infectious diseases, including bacterial infections (tuberculosis, leprosy, melioidosis, and Staphylococcus aureus infections) and viral infections (human immunodeficiency virus (HIV) infection, coronavirus disease 2019 (COVID-19), hepatitis B, and hepatitis C). Many studies on these infections have shown HLA-associated susceptibility in many populations, but the association with melioidosis and S. aureus infections is less characterized. We also discuss the challenges of complicating disease outcome prediction through HLA typing. A deeper understanding of the genetic basis of susceptibility to these infections will aid in understanding the pathogenesis of the disease, identify new molecular targets for prophylactic and therapeutic interventions, and develop a potential tool to identify those at risk of rapid disease progression.
2. Structure and Function of Human Leukocyte Antigen
The HLA molecule is the name for the human MHC, which orchestrates immune regulation by antigen presentation to T cells [13]. The HLA system resides in a region that spans approximately 4,000 kilobases (kb) of DNA on the short arm of chromosome 6 (6p21). This region encodes three major classes of proteins, HLA class I (HLA-A, HLA-B, and HLA-C), class II (HLA-DP, HLA-DQ, and HLA-DR), and class III (components of the complement system, 21-hydroxylase, heat shock protein, and tumor necrosis factors) (Figure 1) [14, 15].
Figure 1.
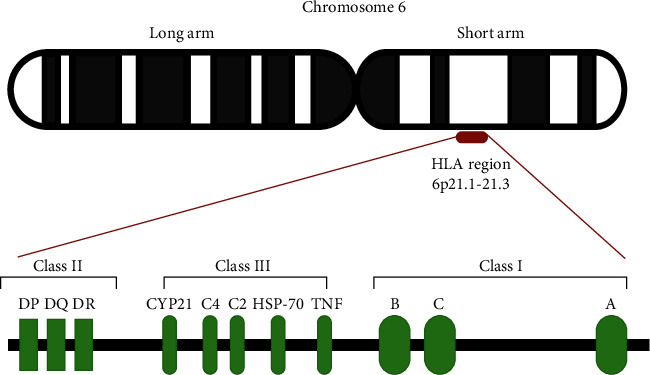
Schematic representation of the HLA locus on human chromosome 6.
HLA class I molecules are present as transmembrane glycoproteins on the surface of nearly all nucleated cells. These molecules present intracellular self- or non-self-antigens to CD8+ cytotoxic T cell receptors and killer cell immunoglobulin-like receptors (KIR) [16]. HLA class I molecules consist of two heterodimer polypeptide chains, a heavy α chain, and a lighter β2-microglobulin chain. The α chain has three extracellular domains (α1, α2, and α3), a transmembrane region, and a C-terminal cytoplasmic tail. The two domains, α1 and α2, fold to form a peptide-binding groove and are referred to as the recognition region. The β2-microglobulin chain is primarily associated with the α3 domain and is responsible for HLA stability (Figure 2(a)) [17, 18].
Figure 2.
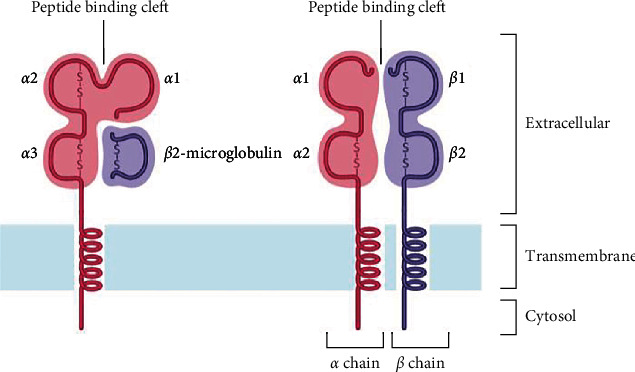
Schematic presentation of the structure of HLA class I (a) and class II (b) molecules.
HLA class II molecules are present on the surface of antigen-presenting cells (APC), such as macrophages, B cells, and dendritic cells, and display short antigen peptides to CD4+ helper T cells and their receptors [19]. HLA class II molecules consist of two polypeptide chains (an α and a β chain), and each chain is folded into two separate domains: α1 and α2 and β1 and β2, respectively. A peptide-binding groove is formed by the distal α1 and β1 domains. The proximal domains, α2 and β2, are highly conserved to which the T cell receptor (TCR) binds (Figure 2(b)) [18].
Unlike HLA class I and HLA class II regions, whose functions in the immune response are well defined, the HLA class III region encodes for various inflammatory molecules, complement, and heat shock protein [11]. The HLA class III region spans 700 kb of DNA and is located between the centromeric class II (HLA-DRA) and the telomeric class I regions (MICB) (Figure 1) [20].
3. HLA Nomenclature
The WHO Nomenclature Committee for Factors of the HLA System is responsible for the formal naming of HLA alleles and has reported the names through two websites, Immuno Polymorphism Database-International ImMunoGeneTics project/HLA (IPD-IMGT/HLA) database (https://www.ebi.ac.uk/ipd/imgt/hla/) and HLA Nomenclature (http://hla.alleles.org/nomenclature/naming.html) [21, 22]. The current HLA nomenclature system uses a unique number corresponding to up to four sets of digits separated by colons (Figure 3). The HLA-prefix signifies the human MHC gene complex. The next portion after the HLA-prefix indicates the specific HLA genomic region. The first two digits of the number (field 1) show the allele group (or allele family). The second field provides the specific HLA allele (HLA protein). The third field names the alleles that differ only by synonymous nucleotide substitutions within the coding region. The fourth field names the alleles that vary only by sequence polymorphisms in introns, 3′-untranslated regions, and 5′-untranslated regions. Last is the suffix consisting of a letter that denotes alleles with changes in expression levels of the HLA protein products. The suffix “N” is used for null alleles with no HLA protein expression. Other letters have been used to designate an allele to indicate its expression status: L: low expression, S: secreted, and Q: questionable. A standardized HLA nomenclature has contributed to the understanding of the HLA system and proved to be an essential resource to address HLA typing ambiguity in the clinical applications of HLA [23].
Figure 3.
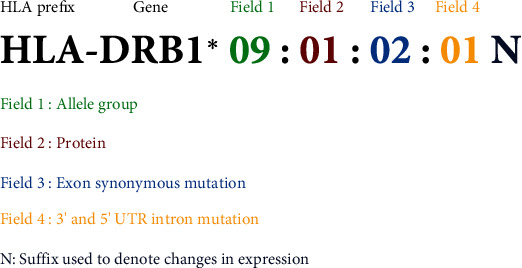
HLA molecule nomenclature with information between prefix (HLA) and suffix (N).
4. Genetic Association between HLA Loci and Infectious Diseases
The HLA family of genes is one of the most polymorphic genes in the human genome [24]. The IPD-IMGT/HLA Database is a repository for the variant sequences of HLA alleles. As of April 2022, the IPD-IMGT/HLA Database has reported 33,490 HLA alleles. Of the 24,308 HLA class I alleles, 7,452, 8,849, and 7,393 alleles are counted in HLA-A, HLA-B, and HLA-C genes. Of 9,182 HLA class II alleles, 32, 4,018, 442, 40, 2,230, 406, 5, 1,958, and 6 alleles are counted in HLA-DRA, HLA-DRB, HLA-DQA1, HLA-DQA2, HLA-DQB1, HLA-DPA1, HLA-DPA2, HLA-DPB1, and HLA-DPB2 genes, respectively (https://www.ebi.ac.uk/ipd/imgt/hla/about/statistics/) (Table 1) [21]. Research in infectious diseases has not described the strongest association with HLA class III in different ethnic groups. Our review will assess the association studies focusing on HLA class I and class II variants associated with susceptibility or protection to infectious diseases.
Table 1.
HLA class I and class II genes and number of alleles (April 2022).
| HLA locus | Number of alleles | Number of expressed proteins |
|---|---|---|
| HLA-A | 7,742 | 4,355 |
| HLA-B | 8,849 | 5,343 |
| HLA-C | 7,393 | 4,095 |
| HLA-DRA | 32 | 5 |
| HLA-DRB | 4,018 | 2,736 |
| HLA-DQA1 | 442 | 205 |
| HLA-DQA2 | 40 | 11 |
| HLA-DQB1 | 2,230 | 1,407 |
| HLA-DPA1 | 406 | 173 |
| HLA-DPA2 | 5 | 0 |
| HLA-DPB1 | 1,958 | 1,223 |
| HLA-DPB2 | 6 | 0 |
4.1. HLA Associations with Tuberculosis
Tuberculosis (TB), caused by Mycobacterium tuberculosis (M. tuberculosis), is an infectious disease posing a significant public health threat primarily in low- and middle-income countries [25]. The World Health Organization reported an estimated 10.0 million TB cases, 1.2 million TB deaths among HIV-negative people, and an additional 208,000 TB deaths among HIV-positive people in 2019 [26]. M. tuberculosis can modulate the HLA class II pathway by inhibiting phagosome maturation and thus preventing the formation of bacterial peptide-MHC-II (HLA class II) complexes and subsequent T cell responses to bacterial antigens [27]. M. tuberculosis also inhibits MHC-II expression and antigen processing resulting in decreased recognition by T cells [28].
Several genetic polymorphisms of HLA have been implicated in individuals' genetic susceptibility to tuberculosis in distinct populations (Table 2). A study on 31 pulmonary tuberculosis patients in Poland showed a higher frequency of HLA-DRB1∗16 in patients when compared to the 58 healthy controls. In comparison, the frequency of the HLA-DRB1∗13 allele was significantly lower in the patient group than in the healthy controls [29]. In Iranian patients with pulmonary tuberculosis, HLA-DRB1∗07 and HLA-DQA1∗01:01 alleles appeared to be the risk alleles, and HLA-DQA1∗03:01 and HLA-DQA1∗05:01 alleles were the protective alleles [30]. Wamala et al. investigated HLA class II gene polymorphisms in susceptibility to pulmonary tuberculosis in Uganda and observed that the HLA-DQB1∗03:03 allele was associated with resistance to pulmonary tuberculosis [31]. In the study performed in South India, the frequencies of HLA-DRB1∗15:01 and HLA-DQB1∗06:01 alleles were higher in pulmonary tuberculosis patients than in the control group. In contrast, the frequency of the HLA-DPB1∗04 allele was highly prevalent among the control group and was deemed to be a protective allele against pulmonary tuberculosis [32]. A study by Sveinbjornsson et al. in Icelanders demonstrated HLA-DQA1∗03 (represented by p.Ala210Thr) and a noncoding variant, rs557011, located between HLA-DQA1 and HLA-DRB1 contributing to genetic susceptibility to tuberculosis [33]. They also demonstrated the association of rs9271378 with a reduced risk of pulmonary TB in Icelanders. A first genome-wide association study (GWAS) on tuberculosis in Han Chinese revealed HLA loci, rs41553512 (a missense mutation in HLA-DRB5), significantly associated with tuberculosis [34]. Strain-based association analysis between HLA class II genes and tuberculosis in the Thai population identified a significant association of HLA-DRB1∗09:01 and HLA-DQB1∗03:03 with a modern strain of M. tuberculosis (absence of M. tuberculosis-specific deletion 1 (TbD1) region) [35].
Table 2.
Associations between HLA and tuberculosis.
| Population | Study design | Sample size | Serotype, allele, SNP, or haplotype | Type of association | Ref. |
|---|---|---|---|---|---|
| Polish | Case-control | 31 pulmonary TB patients and 58 healthy controls | HLA-DRB1∗16 | Susceptibility | [29] |
| HLA-DRB1∗13 | Protection | ||||
| Iranian | Case-control | 40 pulmonary TB patients and 100 healthy controls | HLA-DRB1∗07 and HLA-DQA1∗01:01 | Susceptibility | [30] |
| HLA-DQA1∗03:01 and HLA-DQA1∗05:01 | Protection | ||||
| Uganda | Case-control | 43 pulmonary TB patients and 42 healthy controls | HLA-DQB1∗03:03 | Protection | [31] |
| Indian | Case-control | 126 pulmonary TB patients and 87 healthy controls | HLA-DRB1∗15:01 and HLA-DQB1∗06:01 | Susceptibility | [32] |
| HLA-DPB1∗04 | Protection | ||||
| Icelander | Case-control | 3,686 pulmonary TB patients, 14,723 patients with M. tuberculosis infection, 8,162 patients with any other forms of TB, and 277,643 healthy controls | rs557011[T] located between HLA-DQA1 and HLA-DRB1 | Susceptibility to pulmonary TB and M. tuberculosis infection | [33] |
| HLA-DQA1∗03 | Susceptibility to M. tuberculosis infection | ||||
| rs9271378[G] located between HLA-DQA1 and HLA-DRB1 | Reduced risk of pulmonary TB | ||||
| Han Chinese | Case-control | 4,310 TB patients and 6,386 healthy controls | HLA-DRB5 rs41553512 | Susceptibility | [34] |
| Thai | Case-control | 682 TB patients and 836 healthy controls | HLA-DRB1∗09:01 and HLA-DQB1∗03:03 | Susceptibility to TB caused by modern M. tuberculosis strains | [35] |
4.2. HLA Associations with Leprosy
Leprosy is a chronic infectious disease caused by Mycobacterium leprae. Based on clinical, histopathological, microbiological, and immunological features, Ridley and Jopling classified the leprosy spectrum into five groups: tuberculoid (TT), borderline-tuberculoid (BT), borderline-borderline (BB), borderline-lepromatous (BL), and lepromatous (LL) [36].
Both HLA class I and class II genes have been implicated in susceptibility to leprosy and its subtypes in different populations (Table 3). In a study in India, the frequencies of HLA-A∗02:06, HLA-A∗11:02, HLA-B∗40:16, HLA-B∗51:10, HLA-Cw∗04:07, and HLA-Cw∗07:03 alleles were significantly higher in leprosy patients compared to healthy controls, while the frequencies of HLA-A∗0101, HLA-Cw∗04011, and HLA-Cw∗06:02 alleles were markedly lower in leprosy patients compared to healthy controls [37]. Another study of genetic susceptibility to leprosy in India found rs1071630 located in HLA-DQA1 and rs9270650 in HLA-DRB1 associated with susceptibility to leprosy [38]. A genome-wide association study in 706 patients with leprosy and 1225 unaffected controls in Han Chinese found a single-nucleotide polymorphism (SNP) rs602875 at the HLA-DR-DQ locus associated with susceptibility to leprosy [39]. HLA-DR molecules activate T cells by presenting M. leprae peptide antigens to CD4+ T cells and activate various pathways. Anomalies in those pathways could cause HLA-associated leprosy [39]. The HLA-DRB1∗15 allele was associated with leprosy, while HLA-DRB1∗09 was significantly protective against leprosy in Han Chinese [40]. A meta-analysis by Zhang et al. identified HLA-DQA1∗03:03 and HLA-C∗08:01 as causal variants to leprosy susceptibility in the Han Chinese population [41]. In the association study between HLA-DRB1 and leprosy among Brazilian and Vietnamese people, the HLA-DRB1∗04 allele was associated with protection against leprosy, and the HLA-DRB1∗10 allele was found to be associated with susceptibility to leprosy [42]. A recent study was conducted to investigate the association of HLA class I and II genes with leprosy in a Brazilian population. The study identified the association of HLA-C∗12 and HLA-DPB1∗105 with susceptibility to leprosy, while HLA-C∗08, HLA-DPB1∗04, and HLA-DPB1∗18 were protective against leprosy [43]. Dallmann-Sauer et al. performed next-generation sequencing to genotype three HLA class I and eight class II genes in 1,155 individuals from a Vietnamese leprosy case-control sample. The HLA-DQA1∗01:05 and HLA-DRB1∗10:01 alleles in complete linkage disequilibrium (LD) were associated with leprosy, whereas the HLA-C∗07:06 allele was shown to be protective against leprosy in the Vietnamese population [44]. A study of the association of HLA-DRB1 alleles in 71 leprosy patients and 81 healthy controls in Argentina found a higher frequency of HLA-DRB1∗14:01 and HLA-DRB1∗14:06 alleles in leprosy patients compared to controls. In contrast, the frequency of HLA-DRB1∗08:08 and HLA-DRB1∗11:03 was highly prevalent among the healthy controls compared to the leprosy patients hence indicating resistance to leprosy [45]. Interestingly, a study in Taiwan assessing the leprosy association with HLA class I and class II alleles found a protective effect of HLA-DRB1∗04:05 on multibacillary leprosy [46].
Table 3.
Associations between HLA and leprosy.
| Population | Study design | Sample size | Serotype, allele, SNP, or haplotype | Type of association | Ref. |
|---|---|---|---|---|---|
| Indian | Case-control | 32 leprosy patients and 67 healthy controls | HLA-A∗02:06, HLA-A∗11:02, HLA-B∗40:16, HLA-B∗51:10, and HLA-Cw∗04:07 | Susceptibility | [37] |
| HLA-A∗0101, HLA-Cw∗04011, and HLA-Cw∗06:02 | Protection | ||||
| HLA-A∗11-B∗40, HLA-A∗11:02-B∗40:06-Cw∗04:07, and HLA-A∗11:02-B∗40:06-Cw∗15:02 | Susceptibility to lepromatous leprosy | ||||
| Indian | Case-control and family-based | 258 leprosy patients, 161 families, and 300 healthy controls | HLA-DQA1 rs1071630 and HLA-DRB1 rs9270650 | Susceptibility | [38] |
| Han Chinese | Case-control | 3,254 leprosy patients and 5,955 healthy controls | HLA-DR-DQ rs602875 | Susceptibility | [39] |
| Han Chinese | Case-control | 305 leprosy patients and 527 healthy controls | HLA-DRB1∗15 | Susceptibility | [40] |
| HLA-DRB1∗09 | Protection | ||||
| Han Chinese | Meta-analysis | Four imputed data sets | HLA-DQA1∗03:03 and HLA-C∗08:01 | Susceptibility | [41] |
| Brazilian | Case-control | 578 leprosy patients and 691 healthy controls | HLA-DRB1∗10 | Susceptibility | [42] |
| HLA-DRB1∗04 | Protection | ||||
| Brazilian | Case-control | 411 leprosy patients and 415 healthy controls | HLA-C∗12 and HLA-DPB1∗105 | Susceptibility | [43] |
| HLA-C∗08, HLA-DPB1∗04 and HLA-DPB1∗18 | Protection | ||||
| Vietnamese | Family-based | 194 families | HLA-DRB1∗10 | Susceptibility | [42] |
| HLA-DRB1∗04 | Protection | ||||
| Vietnamese | Case-control | 687 leprosy patients and 468 healthy controls | HLA-DQA1∗01:05 and HLA-DRB1∗10:01 | Susceptibility | [44] |
| HLA-C∗07:06 | Protection | ||||
| Argentinean | Case-control | 142 leprosy patients and 162 healthy controls | HLA-DRB1∗14 :01 and HLA-DRB1∗14:06 | Susceptibility | [45] |
| HLA-DRB1∗08:08 and HLA-DRB1∗11:03 | Protection | ||||
| Taiwanese | Case-control | 65 multibacillary leprosy patients and 190 healthy controls | HLA-DRB1∗04:05 | Protection against multibacillary leprosy | [46] |
4.3. HLA Associations with Melioidosis
Melioidosis is an infectious disease caused by the Gram-negative bacillus Burkholderia pseudomallei. Melioidosis is widely endemic in Southeast Asia, especially in Thailand, and northern Australia [47]. The disease is highly seasonal, and the organism is commonly found in soil and water in the endemic areas [48]. Risk factors include diabetes mellitus, chronic kidney disease, chronic lung disease, alcohol abuse, and steroid therapy [49, 50]. Diabetes mellitus is the major underlying risk factor occurring in 60-75% of patients diagnosed with melioidosis [50–52]. Clues to the mechanisms involved between diabetes mellitus and melioidosis might be explained by the role of HLA alleles in both diabetes mellitus and melioidosis. HLA class II alleles have been documented to have prominent effects on diabetes mellitus in distinct populations [53–57].
Studies in Thailand have reported the risk of HLA alleles associated with melioidosis (Table 4). In 1998, Dharakul et al. investigated the associations between HLA class II alleles and melioidosis in 79 melioidosis patients and 105 healthy controls in Northeast Thailand [8]. The study demonstrated a significant association between the DRB1∗16:02 allele and the susceptibility to melioidosis in the Thai population. In addition, associations were observed with the DRB1∗16:02 allele for severe melioidosis and septicemic melioidosis when various clinical groups of melioidosis patients were compared with healthy controls. In another study in Northeast Thailand, HLA-B∗46 and HLA-C∗01 (HLA class I alleles) were associated with increased mortality from acute melioidosis compared to the survived patients from acute melioidosis [58].
Table 4.
Associations between HLA and melioidosis.
| Population | Study design | Sample size | Serotype, allele, SNP, or haplotype | Type of association | Ref. |
|---|---|---|---|---|---|
| Thai | Case-control | 79 melioidosis patients and 105 healthy controls | HLA-DRB1∗16:02 | Susceptibility and poor prognosis | [8] |
| Thai | Case-control | 183 acute melioidosis patients and 21 healthy controls | HLA-B∗46 and HLA-C∗01 | Increased mortality | [58] |
In melioidosis, suppressed HLA-DR expression on classical monocytes was associated with poor outcomes [59]. A transcriptomic analysis of changes in gene expression of nonsurvivors from melioidosis in Northeast Thailand found the downregulation of HLA class II genes, including HLA-DPB1, HLA-DRA, HLA-DOA, and HLA-DOB [60]. The role of HLA in the pathogenesis and poor prognosis of melioidosis is not fully understood. Reynolds et al. demonstrated a strong binding affinity of alkyl hydroperoxide reductase (AhpC), a highly dominant B. pseudomallei antigen, with HLA-DR alleles, HLA-DR1, HLA-DR3, HLA-DR4, HLA-DR7, HLA-DR9, HLA-DR11, HLA-DR13, HLA-DR15:01, and HLA-DR15:02, and the HLA-DQ alleles, HLA-DQB1∗06:02 and HLA-DQB1∗03:02 [61]. In addition, the study also reported that among patients with acute melioidosis in Northeast Thailand, survival was associated with a strong HLA class II-restricted T cell response to AhpC.
4.4. HLA Associations with Staphylococcus aureus Infections
S. aureus is an opportunistic pathogen and a leading cause of morbidity and mortality in hospital and community settings [62]. The S. aureus superantigens, toxic shock syndrome toxin-1 (TSST-1) and S. aureus enterotoxin B (SEB), bind to the HLA class II molecule HLA-DR1 [63]. Genetic variations within the host are associated with susceptibility to S. aureus infections suggesting why one-third of humans are known to be colonized with S. aureus in their anterior nares, but most avoid clinically significant S. aureus infections [64].
Several HLA alleles are proposed as susceptibility factors to S. aureus infection (Table 5). A GWAS conducted to identify specific genetic variants that underlie susceptibility to infections caused by S. aureus in white subjects reported three SNPs, rs4321864 located in the HLA-DRA gene and rs115231074 and rs35079132 located in HLA-DRB1 genes, associated with S. aureus infection. The study also found an association between HLA-DRB1∗04 serotype and S. aureus infection [65]. Cyr et al. evaluated the role of genetic variation on susceptibility to S. aureus bacteremia in African Americans. They found the genetic association of one region on chromosome 6 in the HLA class II region with susceptibility S. aureus bacteremia [66].
Table 5.
Associations between HLA and S. aureus infections.
| Population | Study design | Sample size | Serotype, allele, SNP, or haplotype | Type of association | Ref. |
|---|---|---|---|---|---|
| White | Case-control | 4,701 culture-confirmed S. aureus cases and 45,344 healthy controls | HLA-DRA, rs4321864, HLA-DRB1, rs115231074 and rs35079132, and HLA-DRB1∗04 | Susceptibility | [65] |
| African American | Case-control | 390 cases and 175 healthy controls | 52 SNPs from physical position 32377284 to 32660943 (hg19) in the HLA class II region | Susceptibility | [66] |
4.5. HLA Associations with HIV Infection
HIV infection is a major global public health issue with a devastating impact on social and economic indicators [67–69]. HLAs play a complex role in immunomodulation during HIV infection, and variations at the HLA class I locus have been linked to the efficiency of CD8+ T cell control of viremia [70].
Polymorphisms within HLA class I and II loci have been identified as the host genetic modifier of HIV disease progression in several populations (Table 6). In the Argentinian population, the frequency of the HLA-B∗39 allele was significantly higher in HIV-1-positive subjects than in controls, whereas the HLA-B∗44 allele was absent among the HIV-1-positive subjects [71]. Claiborne et al. identified four HLA class I alleles (B∗14:01, B∗57, B∗58:01, and B∗81) and two HLA class II alleles (DQB1∗02 and DRB1∗15) associated with the protection from rapid CD4+ T cell decline without controlling early plasma viral load in a Zambian early infection cohort [72]. A GWAS in HIV-1 infected Caucasian subjects showed HLA-B∗57:01 rs2395029 and HLA-C rs9264942 associated with HIV-1 disease progression [73]. Analysis of the HLA-B allele frequencies among HIV-1-infected individuals classified as rapid progressors (RPs), typical progressors (TPs), and long-term nonprogressors (LTNPs) in the Brazilian population revealed the higher frequency of the HLA-B∗52 allele in the LTNP group than in either the TP group or the RP group, and thus, the presence of the HLA-B∗52 allele is favorable to slow AIDS progression [74]. A study involving treatment-naive patients with chronic HIV-1 infection from (i) Warsaw, Poland; (ii) Athens, Greece; (iii) Mexico City, Mexico; (iv) Bonn, Germany; (v) Boston, MA; (vi) Barcelona, Spain; and (vii) Thames Valley, UK, suggested that HLA-B∗27:02 was associated with slower progression to HIV disease [75]. In a study among the HIV clade B-infected ART-naïve individuals from Mexico and Central America, several HLA alleles were identified as protective (A∗03:01, B∗15:39, B∗27:05, B∗39:02, B∗57:01/02/03, and B∗58:01) and risk (A∗68:03/05, B∗15:30, B∗35:02, B∗35:12/14, B∗39:01/06, B∗39:05, and B∗40:01) factors for disease progression [76].
Table 6.
Associations between HLA and HIV infection.
| Population | Study design | Sample size | Serotype, allele, SNP, or haplotype | Type of association | Ref. |
|---|---|---|---|---|---|
| Argentinian | Case-control | 56 HIV-1-positive patients and 56 healthy individuals | HLA-B∗39 | Susceptibility | [71] |
| HLA-B∗44 | Protection | ||||
| Zambian | Longitudinal | 127 subjects with acute HIV-1 infections | HLA-B∗14:01, B∗57, B∗58:01 and B∗81, and HLA-DQB1∗02 and DRB1∗15 | Slow disease progression | [72] |
| Caucasian | Longitudinal | 2,554 HIV-1 infected subjects | HLA-B∗5701, rs2395029 and HLA-C, rs9264942 | Accelerated disease progression | [73] |
| Brazilian | Retrospective observational | 218 HIV-1 infected subjects | HLA-B∗52 | Slow disease progression | [74] |
| Mexican and Central American | Multicenter cross-sectional | 3,213 HIV clade B-infected patients | HLA-A∗68:03/05, HLA-B∗15:30, B∗35:02, B∗35:12/14, B∗39:01/06, B∗39:05, and B∗40:01 | Accelerated disease progression | [76] |
| HLA-A∗03:01, HLA-B∗15:39, B∗27:05, B∗39:02, B∗57:01/02/03, and B∗58:01 | Slow disease progression |
4.6. HLA Associations with COVID-19
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is a worldwide pandemic with 198,778,175 confirmed cases, including 4,235,559 deaths (as of 3 August 2021) [77]. Patients with severe COVID-19 have been found to exhibit immune dysregulation characterized by IL-6-mediated low HLA-DR expression [78].
Several HLA polymorphisms are associated with susceptibility and severity to COVID-19 in different populations (Table 7). In a study among individuals of European descent experiencing variable clinical outcomes following COVID-19 infection, the frequency of HLA-DRB1∗04:01 was higher among asymptomatic COVID-19 patients than the severe COVID-19 patients and suggested the protective effects of the HLA-DRB1∗04:01 allele against developing severe complications from COVID-19 [79]. The DRB1∗09:01 allele was associated with risk for severe COVID-19 in Japanese [80]. Novelli et al. analyzed the HLA allele frequency distribution in Italian COVID-19 patients to identify potential markers of susceptibility to the disease and observed that the frequencies of HLA-DRB1∗15:01, HLA-DQB1∗06:02, and HLA-B∗27:07 alleles were higher among the severe affected COVID-19 patients compared to healthy controls [81]. In a comprehensive in silico analysis, HLA-B∗46:01 had the fewest predicted binding peptides for SARS-CoV-2, indicating that the individuals with this allele may be particularly vulnerable to COVID-19 [82]. Another in silico analysis found the association between HLA-A∗02:01 and an increased risk for COVID-19, and HLA-A∗02:01 was predicted to present the lower SARS-CoV-2 antigens and subsequent lower T cell-mediated antiviral responses compared to HLA-A∗11:01 or HLA-A∗24:02 alleles [83]. In a study conducted by Shkurnikov et al. in deceased patients with COVID-19 in Russia, HLA-A∗01:01 was associated with early COVID-19 deaths among the high-risk patients, and HLA-A∗02:01 and HLA-A∗03:01 alleles were associated with early COVID-19 deaths among the low-risk patients [84].
Table 7.
Associations between HLA and COVID-19.
| Population | Study design | Sample size | Serotype, allele, SNP, or haplotype | Type of association | Ref. |
|---|---|---|---|---|---|
| European | Case-control | 49 severe COVID-19 patients and 69 asymptomatic COVID-19 patients | HLA-DRB1∗04:01 | Protection against disease severity | [79] |
| Japanese | Case-control | 73 severe COVID-19 patients and 105 nonsevere COVID-19 patients | HLA-DRB1∗09:01 | Risk of severe COVID-19 | [80] |
| Italian | Case-control | 99 severe COVID-19 patients and 1,017 healthy controls | HLA-B∗27:07, HLA-DQB1∗06:02, and HLA-DRB1∗15:01 | Risk of severe COVID-19 | [81] |
| Russian | Case-control | 111 deceased patients with confirmed COVID-19 and 428 healthy controls | HLA-A∗01:01, HLA-A∗02:01, and HLA-A∗03:01 | Early COVID-19 deaths | [84] |
4.7. HLA Associations with Hepatitis B
Hepatitis B is a significant public health problem putting people at high risk of death from cirrhosis and liver cancer [85]. Hepatitis B is caused by the hepatitis B virus (HBV). HBV-specific CD8+ cytotoxic T lymphocytes play a critical role in viral clearance and liver injury, and HLA polymorphisms have been reported to alter CD8+ cytotoxic T lymphocyte responses [86].
Multiple population association studies have provided evidence of an association between HLA locus variations and hepatitis B virus infection (Table 8). A study comparing the distribution of HLA alleles between persistent and transient HBV infection in children and adults in the Gambia found HLA-DRB1∗13:02 associated with protection against persistent HBV infection among children and adults [87]. A Chinese study by Fan et al. showed an association between the HLA-DQ rs9275319C allele and decreased HBV infection risk and an increased HBV clearance [88]. Another Chinese study showed that HLA-DQB1∗06:03 protected against HBV infection [89]. An association analysis performed among the Turkish population revealed the association of the HLA-DPB1 rs9277535A allele with the risk of persistent HBV infection [90]. A Caucasian study showed that HLA-A∗03:01 was associated with viral clearance, and HLA-B∗8 was associated with viral persistence [91]. Al-Qahtani et al. demonstrated an association of HLA-DQ alleles (rs2856718A and rs9275572A) and HLA-DP alleles (rs3077G and rs9277535G) with HBV infection in Saudi Arabian patients [92]. Nishida et al. showed that HLA-DQB1∗06:01 was associated with chronic HBV infection in Japanese patients [93].
Table 8.
Associations between HLA and hepatitis B.
| Population | Study design | Sample size | Serotype, allele, SNP, or haplotype | Type of association | Ref. |
|---|---|---|---|---|---|
| Gambian | Case-control | 185 children with persistent HBV infection, 218 children with transient HBV infection, 40 adults with persistent infection, and 195 adults with transient HBV infection | HLA-DRB1∗13:02 | Protection against persistent HBV infection | [87] |
| Chinese | Case-control | 397 chronic hepatitis B subjects, 434 HBV spontaneous clearance subjects, and 238 healthy controls | HLA-DQ, rs9275319C | Decreased HBV infection risk and an increased HBV clearance | [88] |
| Chinese | Case-control | 256 patients with HBV infection and 433 healthy controls | HLA-DQB1∗06:03 | Protection against chronic HBV infection | [89] |
| Turkish | Case-control | 294 chronic HBV infection patients and 234 persons with HBV natural clearance | HLA-DPB, rs9277535A | Risk of persistent HBV infection | [90] |
| Caucasian | Nested case-control | 194 persistent HBV infection individuals and 342 controls with viral clearance | HLA-A∗03:01 | Increased HBV clearance | [91] |
| HLA-B∗8 | Risk of persistent HBV infection | ||||
| Saudi Arabian | Case-control | 488 inactive HBV carriers, 208 active HBV carriers, 85 HBV-infected patients suffering from cirrhosis or cirrhosis and hepatocellular carcinoma, 304 HBV-cleared individuals and 587 healthy uninfected controls | HLA-DQ, rs2856718A, and HLA-DP, rs3077G, and rs9277535G | Risk of HBV infection | [92] |
| HLA-DQ, rs9275572A | Protective effect against HBV infection and increased HBV clearance | ||||
| Japanese | Case-control | 805 HBV patients and 2,278 healthy controls | HLA-DQB1∗06:01 | Risk of chronic HBV infection | [93] |
4.8. HLA Associations with Hepatitis C
Hepatitis C virus (HCV) infection is a significant cause of acute and chronic hepatitis. Chronic hepatitis leads to liver cirrhosis and hepatocellular carcinoma (HCC) [94, 95]. HCV persistence or clearance is proposed to depend on the response of the HLA class I-restricted HCV-specific CD8+ cytotoxic T cell-mediated lysis of virus-infected host cells [96].
Depending on ethnicity, a significant association has been suggested between HLA alleles and HCV persistence or spontaneous clearance (Table 9). In the Thai population, the frequency of HLA- DRB1∗03:01 and HLA-DQB1∗02:01 was higher in the persistent HCV infection group than in the transient HCV infection group, revealing their susceptibility effect on persistent HCV infection [97]. Genotyping a large multiracial cohort of US women to evaluate associations between HLA alleles and HCV viremia indicated some HLA alleles (B∗57:01, B∗57:03, Cw∗01:02, and DRB1∗01:01) were associated with the absence of HCV RNA. At the same time, the presence of HCV RNA was observed for HLA-DRB1∗03:01 [98]. Huang et al. reported the association of HLA-A∗02:01 and HLA-DRB1∗11:01 with HCV spontaneous clearance in the Chinese population [99]. In Egyptian HCV patients and their families or close household contacts, HLA-DRB1 allele associations with HCV were reported (DRB1∗03:01:01 and DRB1∗13:01:01 alleles and the risk of progression to chronic hepatitis C infection and DRB1∗04:01:01, DRB1∗04:05:01, DRB1∗07:01:01, and DRB1∗11:01:01 and protection against HCV infection) [100].
Table 9.
Associations between HLA and hepatitis C.
| Population | Study design | Sample size | Serotype, allele, SNP, or haplotype | Type of association | Ref. |
|---|---|---|---|---|---|
| Thai | Case-control | 57 subjects with persistent HCV infection and 43 subjects with transient HCV infection | HLA- DRB1∗03:01 and HLA-DQB1∗02:01 | Persistent HCV infection | [97] |
| HLA-DRB1∗03:01-HLA-DQA1∗05:01-HLA-DQB1∗02:01 | Persistent HCV infection | ||||
| Multiracial US women | Case-control | 622 HCV RNA positive women and 136 HCV RNA negative women | HLA-B∗57:01, B∗57:03, HLA-Cw∗01:02, and HLA-DRB1∗01:01 | HCV clearance | [98] |
| HLA-DRB1∗03:01 | Persistent HCV infection | ||||
| Chinese | Case-control | 429 subjects with persistent HCV infection and 231 subjects with HCV clearance | HLA-A∗02:01 and HLA-DRB1∗11:01 | HCV clearance | [99] |
| Egyptian | Family-based and case-control | 162 Egyptian families (255 subjects with chronic hepatitis C, 108 persons who spontaneously cleared the virus, and 588 persons in the control group) | HLA-DRB1∗03:01:01 and HLA-DRB1∗13:01:01 | Persistent HCV infection | [100] |
| HLA-DRB1∗04:01:01, DRB1∗04:05:01, DRB1∗07:01:01, and DRB1∗11:01:01 | HCV clearance |
5. Challenges in HLA Typing to Predict Disease Outcomes
5.1. HLA Diversity
The genetic diversity of HLA within each population can be explained or measured by allelic richness (ar) and the expected heterozygosity (H). The allelic richness of a population at a particular locus is the expected number of alleles present in the population at that locus [101]. The expected heterozygosity is defined as the average proportion of heterozygotes per locus in a randomly mating population [102]. Pathogen richness is the number of pathogens within a defined geographical region [103]. Sanchez-Mazas et al. reported a significant positive correlation between genetic diversity and pathogen richness at HLA-A and HLA-B and a significant negative correlation at HLA-DQB1 [104].
Identifying the most clinically relevant HLA variant is necessary for facilitating improvements in the diagnosis and treatment of human disease. The identifiable HLA variants provide opportunities to refine medical management to optimize patient health and medical outcomes. However, genetic diversity within and between populations poses a challenge in HLA genomics to become a standard component of health care. Many rare HLA variants are identified, and they are likely to contribute to interindividual differences in risk or protection to disease. HLA data from various populations have been collected, but populations of African and Asian descent have limited representation to provide insight into HLA disease associations [105]. HLA genetic diversity among Europeans is well documented [106–108]. Hurley et al. recently reported global frequencies of common, intermediate, and well-documented HLA alleles and highlighted the HLA diversity in world populations [109].
5.2. HLA Genotyping
Different DNA-based molecular techniques represent the modern methods used for HLA typing in clinical applications. Depending on their power to discriminate between HLA alleles, DNA-based HLA typing methods are characterized by low resolution (result at the level of the digits composing the first field in the HLA nomenclature) and high resolution (result at the level of four digits) [110]. The most widely used DNA-based methods in conjunction with PCR for HLA typing include sequence-specific oligonucleotides (SSO), sequence-specific primers (SSP), and sequence-based typing (SBT) [111]. In PCR-SSO, PCR products are hybridized into sequence-specific oligonucleotide probes. In PCR-SSP, primers complementary to particular HLA allele sequences are used, and amplification with sequence-specific primers yields only a product if the target sequences are present in the DNA sample. In SBT, HLA genetic regions are amplified by PCR using locus-specific primers, followed by direct sequencing of the PCR products [112].
Although SSO and SSP methods are widely used, they are not practical and capable of detecting all known HLA polymorphisms and novel HLA alleles [113]. SSO and SSP typing methods struggle to resolve the major allele groups [114]. The SBT provides high-resolution HLA genotyping and can identify new alleles. While SBT allows for a detailed interpretation of HLA alleles, it has limitations, including time-consuming protocols, low throughput, and ambiguities in HLA typing results [115–117]. SNP-based HLA typing on microarray produces a high-resolution HLA type but has not been used in clinical typing due to its tendency to miss several HLA variants [117].
Next-generation sequencing- (NGS-) based HLA typing methods allow high-throughput sequencing, massively parallel analysis, and high-resolution HLA typing with minimal ambiguity [118, 119]. NGS-based HLA typing has been implemented with better accuracy compared to traditional HLA typing methods [120]. NGS-based HLA typing approaches are promising but are not yet ready to be implemented in routine clinical care settings due to the higher cost and complex protocol [111].
5.3. Implementing Therapeutic Approaches Using Genomic Knowledge of Specific Targets and Their Roles in Disease
Utilization of HLA typing can identify alleles associated with disease risks and improve clinical outcomes. However, genomic literacy among clinicians is a low to moderate level which presents a challenge in adopting genomic services by clinicians in clinical practices [121]. To overcome this challenge, high-quality results in HLA association studies must be disseminated among the health workforce, including the policymakers and the personnel on the ground. Awareness about the implications of HLA typing into mainstream clinical practice must be raised by educating the health workforce. There is a need for more reporting on the clinical validity and clinical utility of genetic testing used for screening of risk and protective HLA alleles in diseases [122, 123]. Integrating genomic services utilizing HLA-specific tests face challenges and barriers to widespread adoption. These include the lack of a single standard approach to achieve HLA typing by NGS data, integrating electronic health records (EHR) of genomic results and clinical decision support (CDS), ensuring confidentiality for patients and families and lack of reimbursement [124–127].
6. Conclusions
This article summarized the findings from association studies of HLA variants with bacterial and viral infections. It is important to note that there were no overlaps in the HLA variants associated with susceptibility or protection to infectious diseases in populations worldwide. Despite the evidence of association of HLA variants with disease susceptibility in our review, a consistent genetic HLA locus has not been demonstrated within the population.
The global pathogens will expand to new geographical locations with genetic mutations in the future [128]. New sequencing techniques that allow faster, cheaper, and less intensive sequencing need to be developed to effectively implement HLA typing in routine clinical care. Advancements in HLA typing technologies are enabling a more accurate linking of HLA genotypes to disease outcomes. NGS technologies will provide a deeper insight into disease mechanisms and biological processes of HLA. Combining HLA sequencing with the expression levels of HLA genes can provide a clearer picture of the role of HLA genes in the pathogenesis of diseases. To understand HLA evolution, HLA expression levels must be integrated with information on HLA genetic variation in diverse populations. At the same time, large data sets need to be generated to reinterpret information to understand pathogen spread in the future [129].
In light of the current evidence, the goal of HLA typing should aid in the realization of “precision medicine” that will benefit patients in diverse populations. Significant research on HLA and infectious diseases is needed in subjects of all ethnic origins to achieve optimum therapeutic outcomes for broader clinical implementation.
Acknowledgments
This work was supported by the Mahidol University Postdoctoral Fellowship 2021.
Data Availability
The data availability is not declared. Our manuscript is a review paper.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Robson K. J., Ooi J. D., Holdsworth S. R., Rossjohn J., Kitching A. R. HLA and kidney disease: from associations to mechanisms. Nature Reviews. Nephrology . 2018;14(10):636–655. doi: 10.1038/s41581-018-0057-8. [DOI] [PubMed] [Google Scholar]
- 2.Ryan S. O., Cobb B. A. Roles for major histocompatibility complex glycosylation in immune function. Seminars in Immunopathology . 2012;34(3):425–441. doi: 10.1007/s00281-012-0309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D., Sun K., Zhao Y., et al. Polymorphism in the major histocompatibility complex (MHC class II B) genes of the rufous-backed bunting (Emberiza jankowskii) PeerJ . 2017;5, article e2917 doi: 10.7717/peerj.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janeway C. A., Jr., Travers P., Walport M., et al. Immunobiology: The Immune System in Health and Disease . 5th. New York: Garland Science; 2001. The major histocompatibility complex and its functions. [Google Scholar]
- 5.Al Naqbi H., Mawart A., Alshamsi J., et al. Major histocompatibility complex (MHC) associations with diseases in ethnic groups of the Arabian Peninsula. Immunogenetics . 2021;73(2):131–152. doi: 10.1007/s00251-021-01204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Frontiers in Zoology . 2005;2(1) doi: 10.1186/1742-9994-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh N., Agrawal S., Rastogi A. K. Infectious diseases and immunity: special reference to major histocompatibility complex. Emerging Infectious Diseases . 1997;3(1):41–49. doi: 10.3201/eid0301.970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dharakul T., Vejbaesya S., Chaowagul W., Luangtrakool P., Stephens H. A. F., Songsivilai S. HLA-DR and -DQ associations with melioidosis. Human Immunology . 1998;59(9):580–586. doi: 10.1016/S0198-8859(98)00052-4. [DOI] [PubMed] [Google Scholar]
- 9.Jarduli L. R., Sell A. M., Reis P. G., et al. Role of HLA, KIR, MICA, and cytokines genes in leprosy. BioMed Research International . 2013;2013:17. doi: 10.1155/2013/989837.989837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Profaizer T., Pole A., Monds C., Delgado J. C., Lázár-Molnár E. Clinical utility of next generation sequencing based HLA typing for disease association and pharmacogenetic testing. Human Immunology . 2020;81(7):354–360. doi: 10.1016/j.humimm.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Crux N. B., Elahi S. Human leukocyte antigen (HLA) and immune regulation: how do classical and non-classical HLA alleles modulate immune response to human immunodeficiency virus and hepatitis C virus infections? Frontiers in Immunology . 2017;8:p. 832. doi: 10.3389/fimmu.2017.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi Z., Liu H., Zhang F. Advances in the immunology and genetics of leprosy. Frontiers in Immunology . 2020;11:p. 567. doi: 10.3389/fimmu.2020.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosaad Y. M. Clinical role of human leukocyte antigen in health and disease. Scandinavian Journal of Immunology . 2015;82(4):283–306. doi: 10.1111/sji.12329. [DOI] [PubMed] [Google Scholar]
- 14.Choo S. Y. The HLA system: genetics, immunology, clinical testing, and clinical implications. Yonsei Medical Journal . 2007;48(1):11–23. doi: 10.3349/ymj.2007.48.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matzaraki V., Kumar V., Wijmenga C., Zhernakova A. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biology . 2017;18(1):p. 76. doi: 10.1186/s13059-017-1207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyatt R. C., Lanzoni G., Russell M. A., Gerling I., Richardson S. J. What the HLA-I!-classical and non-classical HLA class I and their potential roles in type 1 diabetes. Current Diabetes Reports . 2019;19(12):p. 159. doi: 10.1007/s11892-019-1245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulsson K. M. Evolutionary and functional perspectives of the major histocompatibility complex class I antigen-processing machinery. Cellular and Molecular Life Sciences . 2004;61(19-20):2446–2460. doi: 10.1007/s00018-004-4113-0. [DOI] [PubMed] [Google Scholar]
- 18.Tapias P. C., Castiblanco J., Anaya J. M. Autoimmunity: From Bench to Bedside . Bogota (Colombia): El Rosario University Press; 2013. Major histocompatibility complex: antigen processing and presentation. [PubMed] [Google Scholar]
- 19.Chen B., Khodadoust M. S., Olsson N., et al. Predicting HLA class II antigen presentation through integrated deep learning. Nature Biotechnology . 2019;37(11):1332–1343. doi: 10.1038/s41587-019-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valdes A. M., Thomson G., Barcellos L. F., the T1DGC Genetic variation within the HLA class III influences T1D susceptibility conferred by high-risk HLA haplotypes. Genes and Immunity . 2010;11(3):209–218. doi: 10.1038/gene.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson J., Barker D. J., Georgiou X., Cooper M. A., Flicek P., Marsh S. G. E. IPD-IMGT/HLA database. Nucleic Acids Research . 2020;48(D1):D948–D955. doi: 10.1093/nar/gkz950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh S. G., Albert E. D., Bodmer W. F., et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens . 2010;75(4):291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurley C. K. Naming HLA diversity: a review of HLA nomenclature. Human Immunology . 2021;82(7):457–465. doi: 10.1016/j.humimm.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y., Wang J., Bachtiar M., Chong S. S., Lee C. G. L. Architecture of polymorphisms in the human genome reveals functionally important and positively selected variants in immune response and drug transporter genes. Human Genomics . 2018;12(1):p. 43. doi: 10.1186/s40246-018-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miggiano R., Rizzi M., Ferraris D. M. Mycobacterium tuberculosis pathogenesis, infection prevention and treatment. Infection Prevention and Treatment. Pathogens . 2020;9(5):p. 385. doi: 10.3390/pathogens9050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Global tuberculosis report 2020: executive summary . Geneva: WHO; 2020. [Google Scholar]
- 27.Harding C. V., Ramachandra L., Wick M. J. Interaction of bacteria with antigen presenting cells: influences on antigen presentation and antibacterial immunity. Current Opinion in Immunology . 2003;15(1):112–119. doi: 10.1016/S0952-7915(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 28.Noss E. H., Pai R. K., Sellati T. J., et al. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. Journal of Immunology . 2001;167(2):910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 29.Dubaniewicz A., Lewko B., Moszkowska G., Zamorska B., Stepinski J. Molecular subtypes of the HLA-DR antigens in pulmonary tuberculosis. International Journal of Infectious Diseases . 2000;4(3):129–133. doi: 10.1016/S1201-9712(00)90073-0. [DOI] [PubMed] [Google Scholar]
- 30.Amirzargar A. A., Yalda A., Hajabolbaghi M., et al. The association of HLA-DRB, DQA1, DQB1 alleles and haplotype frequency in Iranian patients with pulmonary tuberculosis. The International Journal of Tuberculosis and Lung Disease . 2004;8(8):1017–1021. [PubMed] [Google Scholar]
- 31.Wamala D., Buteme H. K., Kirimunda S., Kallenius G., Joloba M. Association between human leukocyte antigen class II and pulmonary tuberculosis due to mycobacterium tuberculosis in Uganda. BMC Infectious Diseases . 2016;16(1) doi: 10.1186/s12879-016-1346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravikumar M., Dheenadhayalan V., Rajaram K., et al. Associations of HLA-DRB1, DQB1 and DPB1 alleles with pulmonary tuberculosis in South India. Tubercle and Lung Disease . 1999;79(5):309–317. doi: 10.1054/tuld.1999.0213. [DOI] [PubMed] [Google Scholar]
- 33.Sveinbjornsson G., Gudbjartsson D. F., Halldorsson B. V., et al. HLA class II sequence variants influence tuberculosis risk in populations of European ancestry. Nature Genetics . 2016;48(3):318–322. doi: 10.1038/ng.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi H., Zhang Y. B., Sun L., et al. Discovery of susceptibility loci associated with tuberculosis in Han Chinese. Human Molecular Genetics . 2017;26(23):4752–4763. doi: 10.1093/hmg/ddx365. [DOI] [PubMed] [Google Scholar]
- 35.Toyo‐oka L., Mahasirimongkol S., Yanai H., et al. Strain-based HLA association analysis identified HLA-DRB1∗09:01 associated with modern strain tuberculosis. HLA . 2017;90(3):149–156. doi: 10.1111/tan.13070. [DOI] [PubMed] [Google Scholar]
- 36.Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. International Journal of Leprosy and Other Mycobacterial Diseases . 1966;34(3):255–273. [PubMed] [Google Scholar]
- 37.Shankarkumar U., Ghosh K., Badakere S., Mohanty D. Novel HLA class I alleles associated with Indian leprosy patients. Journal of Biomedicine & Biotechnology . 2003;2003(3):208–211. doi: 10.1155/S1110724303210019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong S. H., Gochhait S., Malhotra D., et al. Leprosy and the adaptation of human toll-like receptor 1. PLoS Pathogens . 2010;6(7, article e1000979) doi: 10.1371/journal.ppat.1000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F. R., Huang W., Chen S. M., et al. Genomewide association study of leprosy. The New England Journal of Medicine . 2009;361(27):2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 40.Zhang F., Liu H., Chen S., et al. Evidence for an association of HLA-DRB1∗15 and DRB1∗09 with leprosy and the impact of DRB1∗09 on disease onset in a Chinese Han population. BMC Medical Genetics . 2009;10(1) doi: 10.1186/1471-2350-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X., Cheng Y., Zhang Q., et al. Meta-analysis identifies major histocompatiblity complex loci in or Near HLA-DRB1, HLA-DQA1, HLA-C as associated with leprosy in Chinese Han population. The Journal of Investigative Dermatology . 2019;139(4):957–960. doi: 10.1016/j.jid.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 42.Vanderborght P. R., Pacheco A. G., Moraes M. E., et al. HLA-DRB1∗04 and DRB1∗10 are associated with resistance and susceptibility, respectively, in Brazilian and Vietnamese leprosy patients. Genes and Immunity . 2007;8(4):320–324. doi: 10.1038/sj.gene.6364390. [DOI] [PubMed] [Google Scholar]
- 43.Covolo de Souza-Santana F., Querino G. A., Mendes Camargo R., et al. HLA-DPB1 and HLA-C alleles are associated with leprosy in a Brazilian population. Human Immunology . 2021;82(1):11–18. doi: 10.1016/j.humimm.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Dallmann-Sauer M., Fava V. M., Gzara C., et al. The complex pattern of genetic associations of leprosy with HLA class I and class II alleles can be reduced to four amino acid positions. PLoS Pathogens . 2020;16(8, article e1008818) doi: 10.1371/journal.ppat.1008818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borrás S. G., Cotorruelo C., Racca L., et al. Association of leprosy with HLA-DRB1 in an Argentinean population. Annals of Clinical Biochemistry . 2008;45(1):96–98. doi: 10.1258/acb.2007.007156. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh N. K., Chu C. C., Lee N. S., Lee H. L., Lin M. Association of HLA-DRB1∗0405 with resistance to multibacillary leprosy in Taiwanese. Human Immunology . 2010;71(7):712–716. doi: 10.1016/j.humimm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Peacock S. J., Limmathurotsakul D. Infectious Diseases . Elsevier; Melioidosis; pp. 1073–1077.e. [DOI] [Google Scholar]
- 48.Wiersinga W. J. Melioidosis. Nature Reviews Disease Primers . 2018;4(1) doi: 10.1038/nrdp.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Currie B. J., Jacups S. P., Cheng A. C., et al. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Tropical Medicine & International Health . 2004;9(11):1167–1174. doi: 10.1111/j.1365-3156.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 50.Zueter A., Yean C. Y., Abumarzouq M., Rahman Z. A., Deris Z. Z., Harun A. The epidemiology and clinical spectrum of melioidosis in a teaching hospital in a north-eastern state of Malaysia: a fifteen-year review. BMC Infectious Diseases . 2016;16(1):p. 333. doi: 10.1186/s12879-016-1583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassan M. R. A., Pani S. P., Peng N. P., et al. Incidence, risk factors and clinical epidemiology of melioidosis: a complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infectious Diseases . 2010;10 doi: 10.1186/1471-2334-10-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chien J. M., Saffari S. E., Tan A. L., Tan T. T. Factors affecting clinical outcomes in the management of melioidosis in Singapore: a 16-year case series. BMC Infectious Diseases . 2018;18(1):p. 482. doi: 10.1186/s12879-018-3393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Z. J., Sun P., Guo G., Zhang R., Chen L. M. Association of the HLA-DQA1 and HLA-DQB1 alleles in type 2 diabetes mellitus and diabetic nephropathy in the Han ethnicity of China. Journal Diabetes Research . 2013;2013, article 452537:5. doi: 10.1155/2013/452537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobi T., Massier L., Klöting N., et al. HLA class II allele analyses implicate common genetic components in type 1 and non-insulin-treated type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism . 2020;105(3):e245–e254. doi: 10.1210/clinem/dgaa027. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L. P., Alshiekh S., Zhao M., et al. Next-generation sequencing reveals that HLA-DRB3, -DRB4, and -DRB5 may be associated with islet autoantibodies and risk for childhood type 1 diabetes. Diabetes . 2016;65(3):710–718. doi: 10.2337/db15-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noble J. A., Valdes A. M. Genetics of the HLA region in the prediction of type 1 diabetes. Current Diabetes Reports . 2011;11(6):533–542. doi: 10.1007/s11892-011-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santos D. C., Porto L. C., Oliveira R. V., et al. HLA class II genotyping of admixed Brazilian patients with type 1 diabetes according to self-reported color/race in a nationwide study. Scientific Reports . 2020;10(1):p. 6628. doi: 10.1038/s41598-020-63322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunachie S. J., Jenjaroen K., Reynolds C. J., et al. Infection with Burkholderia pseudomallei - immune correlates of survival in acute melioidosis. Scientific Reports . 2017;7(1):p. 12143. doi: 10.1038/s41598-017-12331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kronsteiner B., Chaichana P., Sumonwiriya M., et al. Diabetes alters immune response patterns to acute melioidosis in humans. European Journal of Immunology . 2019;49(7):1092–1106. doi: 10.1002/eji.201848037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yimthin T., Cliff J. M., Phunpang R., et al. Blood transcriptomics to characterize key biological pathways and identify biomarkers for predicting mortality in melioidosis. Emerging Microbes & Infections . 2021;10(1):8–18. doi: 10.1080/22221751.2020.1858176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds C., Goudet A., Jenjaroen K., et al. T cell immunity to the alkyl hydroperoxide reductase of Burkholderia pseudomallei: a correlate of disease outcome in acute melioidosis. Journal of Immunology . 2015;194(10):4814–4824. doi: 10.4049/jimmunol.1402862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monaco M., Pimentel de Araujo F., Cruciani M., Coccia E. M., Pantosti A. Worldwide epidemiology and antibiotic resistance of Staphylococcus aureus. Current Topics in Microbiology and Immunology . 2017;409:21–56. doi: 10.1007/82_2016_3. [DOI] [PubMed] [Google Scholar]
- 63.Kim J., Urban R. G., Strominger J. L., Wiley D. C. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science . 1994;266(5192):1870–1874. doi: 10.1126/science.7997880. [DOI] [PubMed] [Google Scholar]
- 64.Shukla S. K., Rose W., Schrodi S. J. Complex host genetic susceptibility to Staphylococcus aureus infections. Trends in Microbiology . 2015;23(9):529–536. doi: 10.1016/j.tim.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 65.DeLorenze G. N., Nelson C. L., Scott W. K., et al. Polymorphisms in HLA class II genes are associated with susceptibility to Staphylococcus aureus infection in a White population. The Journal of Infectious Diseases . 2016;213(5):816–823. doi: 10.1093/infdis/jiv483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cyr D. D., Allen A. S., du G. J., et al. Evaluating genetic susceptibility to Staphylococcus aureus bacteremia in African Americans using admixture mapping. Genes and Immunity . 2017;18(2):95–99. doi: 10.1038/gene.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boutayeb A. The impact of HIV/AIDS on human development in African countries. BMC Public Health . 2009;9(Supplement 1):p. S3. doi: 10.1186/1471-2458-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ji G., Li L., Lin C., Sun S. The impact of HIV/AIDS on families and children--a study in China. AIDS . 2007;21(Supplement 8):S157–S161. doi: 10.1097/01.aids.0000304712.87164.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gayle H. D., Hill G. L. Global impact of human immunodeficiency virus and AIDS. Clinical Microbiology Reviews . 2001;14(2):327–335. doi: 10.1128/CMR.14.2.327-335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kloverpris H. N., Stryhn A., Harndahl M., et al. HLA-B∗57 micropolymorphism shapes HLA allele-specific epitope immunogenicity, selection pressure, and HIV immune control. Journal of Virology . 2012;86(2):919–929. doi: 10.1128/JVI.06150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Sorrentino A. H., Marinic K., Motta P., Sorrentino A., López R., Illiovich E. HLA class I alleles associated with susceptibility or resistance to human immunodeficiency virus type 1 infection among a population in Chaco Province, Argentina. The Journal of Infectious Diseases . 2000;182(5):1523–1526. doi: 10.1086/315854. [DOI] [PubMed] [Google Scholar]
- 72.Claiborne D. T., Scully E. P., Palmer C. D., et al. Protective HLA alleles are associated with reduced LPS levels in acute HIV infection with implications for immune activation and pathogenesis. PLoS Pathogens . 2019;15(8, article e1007981) doi: 10.1371/journal.ppat.1007981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fellay J., Ge D., Shianna K. V., et al. Common genetic variation and the control of HIV-1 in humans. PLoS Genetics . 2009;5(12, article e1000791) doi: 10.1371/journal.pgen.1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teixeira S. L., de Sá N. B. R., Campos D. P., et al. Association of the HLA-B∗52 allele with non-progression to AIDS in Brazilian HIV-1-infected individuals. Genes and Immunity . 2014;15(4):256–262. doi: 10.1038/gene.2014.14. [DOI] [PubMed] [Google Scholar]
- 75.Adland E., Hill M., Lavandier N., et al. Differential immunodominance hierarchy of CD8(+) T-cell responses in HLA-B∗27:05- and -B∗27:02-mediated control of HIV-1 infection. Journal of Virology . 2018;92(4) doi: 10.1128/JVI.01685-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valenzuela-Ponce H., Alva-Hernández S., Garrido-Rodríguez D., et al. Novel HLA class I associations with HIV-1 control in a unique genetically admixed population. Scientific Reports . 2018;8(1):p. 6111. doi: 10.1038/s41598-018-23849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.World Health Organization. https://covid19.who.int/
- 78.Giamarellos-Bourboulis E. J., Netea M. G., Rovina N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host & Microbe . 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Langton D. J., Bourke S. C., Lie B. A., et al. The influence of HLA genotype on the severity of COVID-19 infection. HLA . 2021;98(1):14–22. doi: 10.1111/tan.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anzurez A., Naka I., Miki S., et al. Association of HLA-DRB1∗09:01 with severe COVID-19. HLA . 2021;98(1):37–42. doi: 10.1111/tan.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novelli A., Andreani M., Biancolella M., et al. HLA allele frequencies and susceptibility to COVID-19 in a group of 99 Italian patients. HLA . 2020;96(5):610–614. doi: 10.1111/tan.14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen A., David J. K., Maden S. K., et al. Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. Journal of Virology . 2020;94(13) doi: 10.1128/JVI.00510-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomita Y., Ikeda T., Sato R., Sakagami T. Association between HLA gene polymorphisms and mortality of COVID-19: an in silico analysis. Immunity, Inflammation and Disease . 2020;8(4):684–694. doi: 10.1002/iid3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shkurnikov M., Nersisyan S., Jankevic T., et al. Association of HLA class I genotypes with severity of coronavirus disease-19. Frontiers in Immunology . 2021;12, article 641900 doi: 10.3389/fimmu.2021.641900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuen M. F., Chen D. S., Dusheiko G. M., et al. Hepatitis B virus infection. Nature Reviews. Disease Primers . 2018;4(1, article 18035) doi: 10.1038/nrdp.2018.35. [DOI] [PubMed] [Google Scholar]
- 86.Wang L., Zou Z. Q., Wang K. Clinical relevance of HLA gene variants in HBV infection. Journal of Immunology Research . 2016;2016:7. doi: 10.1155/2016/9069375.9069375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thursz M. R., Kwiatkowski D., Allsopp C. E. M., Greenwood B. M., Thomas H. C., Hill A. V. S. Association between an MHC class II allele and clearance of hepatitis B virus in the Gambia. The New England Journal of Medicine . 1995;332(16):1065–1069. doi: 10.1056/NEJM199504203321604. [DOI] [PubMed] [Google Scholar]
- 88.Fan J. H., Hou S. H., Qing-Ling L., Hu J., Peng H., Guo J. J. Association of HLA-DQ and IFNL4 polymorphisms with susceptibility to hepatitis B virus infection and clearance. Annals of Hepatology . 2016;15(4):532–539. [PubMed] [Google Scholar]
- 89.Ou G., Xu H., Yu H., et al. The roles of HLA-DQB1 gene polymorphisms in hepatitis B virus infection. Journal of Translational Medicine . 2018;16(1):p. 362. doi: 10.1186/s12967-018-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akgöllü E., Bilgin R., Akkız H., et al. Association between chronic hepatitis B virus infection and HLA-DP gene polymorphisms in the Turkish population. Virus Research . 2017;232:6–12. doi: 10.1016/j.virusres.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 91.Thio C. L., Thomas D. L., Karacki P., et al. Comprehensive analysis of class I and class II HLA antigens and chronic hepatitis B virus infection. Journal of Virology . 2003;77(22):12083–12087. doi: 10.1128/JVI.77.22.12083-12087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Qahtani A. A., Al-Anazi M. R., Abdo A. A., et al. Association between HLA variations and chronic hepatitis B virus infection in Saudi Arabian patients. PLoS One . 2014;9(1, article e80445) doi: 10.1371/journal.pone.0080445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishida N., Ohashi J., Khor S. S., et al. Understanding of HLA-conferred susceptibility to chronic hepatitis B infection requires HLA genotyping-based association analysis. Scientific Reports . 2016;6(1):p. 24767. doi: 10.1038/srep24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saito H., Umemura T., Joshita S., et al. KIR2DL2 combined with HLA-C1 confers risk of hepatitis C virus-related hepatocellular carcinoma in younger patients. Oncotarget . 2018;9(28):19650–19661. doi: 10.18632/oncotarget.24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manns M. P., Buti M., Gane E., et al. Hepatitis C virus infection. Nature Reviews. Disease Primers . 2017;3(1, article 17006) doi: 10.1038/nrdp.2017.6. [DOI] [PubMed] [Google Scholar]
- 96.Cerny A., Chisari F. V. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology . 1999;30(3):595–601. doi: 10.1002/hep.510300312. [DOI] [PubMed] [Google Scholar]
- 97.Vejbaesya S., Songsivilai S., Tanwandee T., Rachaibun S., Chantangpol R., Dharakul T. HLA association with hepatitis C virus infection. Human Immunology . 2000;61(3):348–353. doi: 10.1016/S0198-8859(99)00131-7. [DOI] [PubMed] [Google Scholar]
- 98.Kuniholm M. H., Kovacs A., Gao X., et al. Specific human leukocyte antigen class I and II alleles associated with hepatitis C virus viremia. Hepatology . 2010;51(5):1514–1522. doi: 10.1002/hep.23515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang J., Huang K., Xu R., et al. The associations of HLA-A∗02:01 and DRB1∗11:01 with hepatitis C virus spontaneous clearance are independent of IL28B in the Chinese population. Scientific Reports . 2016;6(1):p. 31485. doi: 10.1038/srep31485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.El-Bendary M., Neamatallah M., Elalfy H., et al. HLA class II-DRB1 alleles with hepatitis C virus infection outcome in Egypt: a multicentre family-based study. Annals of Hepatology . 2019;18(1):68–77. doi: 10.5604/01.3001.0012.7864. [DOI] [PubMed] [Google Scholar]
- 101.Leberg P. L. Estimating allelic richness: effects of sample size and bottlenecks. Molecular Ecology . 2002;11(11):2445–2449. doi: 10.1046/j.1365-294X.2002.01612.x. [DOI] [PubMed] [Google Scholar]
- 102.Luo Z., Brock J., Dyer J. M., et al. Genetic diversity and population structure of a Camelina sativa spring panel. Frontiers in Plant Science . 2019;10:p. 184. doi: 10.3389/fpls.2019.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guernier V., Hochberg M. E., Guegan J. F. Ecology drives the worldwide distribution of human diseases. PLoS Biology . 2004;2(6, article e141) doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanchez-Mazas A., Lemaitre J. F., Currat M. Distinct evolutionary strategies of human leucocyte antigen loci in pathogen-rich environments. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences . 2012;367(1590):830–839. doi: 10.1098/rstb.2011.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burke W. Utility and diversity: challenges for genomic medicine. Annual Review of Genomics and Human Genetics . 2021;22(1):1–24. doi: 10.1146/annurev-genom-120220-082640. [DOI] [PubMed] [Google Scholar]
- 106.Sanchez-Mazas A., Nunes J. M., Middleton D., et al. Common and well-documented HLA alleles over all of Europe and within European sub-regions: a catalogue from the European Federation for Immunogenetics. HLA . 2017;89(2):104–113. doi: 10.1111/tan.12956. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez-Mazas A., Buhler S., Nunes J. M. A new HLA map of Europe: regional genetic variation and its implication for peopling history, disease-association studies and tissue transplantation. Human Heredity . 2014;76(3-4):162–177. doi: 10.1159/000360855. [DOI] [PubMed] [Google Scholar]
- 108.Tokić S., Žižkova V., Štefanić M., et al. HLA-A, -B, -C, -DRB1, -DQA1, and -DQB1 allele and haplotype frequencies defined by next generation sequencing in a population of East Croatia blood donors. Scientific Reports . 2020;10(1):p. 5513. doi: 10.1038/s41598-020-62175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hurley C. K., Kempenich J., Wadsworth K., et al. Common, intermediate and well-documented HLA alleles in world populations: CIWD version 3.0.0. HLA . 2020;95(6):516–531. doi: 10.1111/tan.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nunes E., Heslop H., Fernandez-Vina M., et al. Definitions of histocompatibility typing terms. Blood . 2011;118(23):e180–e183. doi: 10.1182/blood-2011-05-353490. [DOI] [PubMed] [Google Scholar]
- 111.Bravo-Egana V., Sanders H., Chitnis N. New challenges, new opportunities: next generation sequencing and its place in the advancement of HLA typing. Human Immunology . 2021;82(7):478–487. doi: 10.1016/j.humimm.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 112.Marino S., Jaramillo A., Fernande M. The human major histocompatibility complex and DNA based typing of human leukocyte antigens for transplantation. In: O’Gorman M. R. G., Donnenberg A. D., editors. Handbook of Human Immunology . Boca Raton, Florida: CRC Press; 2008. pp. 541–564. [DOI] [Google Scholar]
- 113.Baxter-Lowe L. A. The changing landscape of HLA typing: understanding how and when HLA typing data can be used with confidence from bench to bedside. Human Immunology . 2021;82(7):466–477. doi: 10.1016/j.humimm.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 114.Dunckley H. HLA typing by SSO and SSP methods. Methods in Molecular Biology . 2012;882:9–25. doi: 10.1007/978-1-61779-842-9_2. [DOI] [PubMed] [Google Scholar]
- 115.Adams S. D., Barracchini K. C., Chen D., et al. Ambiguous allele combinations in HLA class I and class II sequence-based typing: when precise nucleotide sequencing leads to imprecise allele identification. Journal of Translational Medicine . 2004;2(1):p. 30. doi: 10.1186/1479-5876-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Voorter C. E., Palusci F., Tilanus M. G. Sequence-based typing of HLA: an improved group-specific full-length gene sequencing approach. Methods in Molecular Biology . 2014;1109:101–114. doi: 10.1007/978-1-4614-9437-9_7. [DOI] [PubMed] [Google Scholar]
- 117.Kishore A., Petrek M. Next-generation sequencing based HLA typing: deciphering immunogenetic aspects of sarcoidosis. Frontiers in Genetics . 2018;9:p. 503. doi: 10.3389/fgene.2018.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu C., Duffy B. F., Weimer E. T., et al. Performance of a multiplexed amplicon-based next-generation sequencing assay for HLA typing. PLoS One . 2020;15(4, article e0232050) doi: 10.1371/journal.pone.0232050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Do M. D., le L. G. H., Nguyen V. T., et al. High-resolution HLA typing of HLA-A, -B, -C, -DRB1, and -DQB1 in Kinh Vietnamese by using next-generation sequencing. Frontiers in Genetics . 2020;11:p. 383. doi: 10.3389/fgene.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Profaizer T., Dibb K., Bethers H., et al. Comparison of next-generation sequencing-based human leukocyte antigen typing with clinical flow cytometry and allele-specific PCR melting assays for HLA-B27 genotyping. The Journal of Applied Laboratory Medicine . 2021;6(5):1221–1227. doi: 10.1093/jalm/jfab046. [DOI] [PubMed] [Google Scholar]
- 121.Ha V. T. D., Frizzo-Barker J., Chow-White P. Adopting clinical genomics: a systematic review of genomic literacy among physicians in cancer care. BMC Medical Genomics . 2018;11(1):p. 18. doi: 10.1186/s12920-018-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jansen M. E., Rigter T., Rodenburg W., et al. Review of the reported measures of clinical validity and clinical utility as arguments for the implementation of pharmacogenetic testing: a case study of statin-induced muscle toxicity. Frontiers in Pharmacology . 2017;8:p. 555. doi: 10.3389/fphar.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Franceschini N., Frick A., Kopp J. B. Genetic testing in clinical settings. American Journal of Kidney Diseases . 2018;72(4):569–581. doi: 10.1053/j.ajkd.2018.02.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klasberg S., Surendranath V., Lange V., Schöfl G. Bioinformatics strategies, challenges, and opportunities for next generation sequencing-based HLA genotyping. Transfusion Medicine and Hemotherapy . 2019;46(5):312–325. doi: 10.1159/000502487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williams M. S., Taylor C. O., Walton N. A., et al. Genomic information for clinicians in the electronic health record: lessons learned from the clinical genome resource project and the electronic medical records and genomics network. Frontiers in Genetics . 2019;10:p. 1059. doi: 10.3389/fgene.2019.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burton H., Cole T., Lucassen A. M. Genomic medicine: challenges and opportunities for physicians. Clinical Medicine (London, England) . 2012;12(5):416–419. doi: 10.7861/clinmedicine.12-5-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manolio T. A., Chisholm R. L., Ozenberger B., et al. Implementing genomic medicine in the clinic: the future is here. Genetics in Medicine . 2013;15(4):258–267. doi: 10.1038/gim.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baker R. E., Mahmud A. S., Miller I. F., et al. Infectious disease in an era of global change. Nature Reviews. Microbiology . 2022;20(4):193–205. doi: 10.1038/s41579-021-00639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meyer D., Aguiar V. R., Bitarello B. D., Brandt D. Y., Nunes K. A genomic perspective on HLA evolution. Immunogenetics . 2018;70(1):5–27. doi: 10.1007/s00251-017-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data availability is not declared. Our manuscript is a review paper.


