Abstract
Several gram-negative bacteria employ type III secretion systems (T3SS) to inject effector proteins into eukaryotic host cells directly from the bacterial cytoplasm. The export gate SctV (YscV in Yersinia) binds substrate:chaperone complexes such as YscX:YscY, which are essential for formation of a functional T3SS. Here, we present structures of the YscX:YscY complex alone and bound to nonameric YscV. YscX binds its chaperone YscY at two distinct sites, resembling the heterotrimeric complex of the T3SS needle subunit with its chaperone and co-chaperone. In the ternary complex the YscX N-terminus, which mediates YscX secretion, occupies a binding site within one YscV that is also used by flagellar chaperones, suggesting the interaction’s importance for substrate recognition. The YscX C-terminus inserts between protomers of the YscV ring where the stalk protein binds to couple YscV to the T3SS ATPase. This primary YscV–YscX interaction is essential for the formation of a secretion-competent T3SS.
Subject terms: X-ray crystallography, Proteins, Pathogens, Bacterial secretion, Bacterial pathogenesis
Recruitment of substrates to virulent bacterial type III secretion systems remains enigmatic. Here, a crystal structure reveals two binding sites between a secretion substrate in complex with its chaperone and the cytosolic domain of the export gate.
Introduction
Type III secretion systems (T3SSs; also: injectisomes, virulence-associated vT3SS) are a common device of bacterial virulence that is present in gram-negative bacteria such as Salmonella, Pseudomonas, or Yersinia1. The macromolecular complex is composed of approximately 20 different protein species, many of which are present in multiple copies, and spans both bacterial membranes. Injectisomes share high structural similarity and likely a common ancestor with the secretion system found in bacterial flagella (fT3SS). With the needle-like character of purified injectisomes2 and their capability to secrete effector proteins directly into a host cell, the T3SS can also be described as a ‘molecular syringe’ and may be a useful protein delivery tool3.
Secretion through the Yersinia T3SS is strictly regulated by the inner membrane-anchored export apparatus containing YscRSTUV and can be divided into three distinct phases: (i) ‘early’ substrates, which self-assemble into the extracellular needle; (ii) ‘intermediate’ substrates, hydrophobic translocator proteins that insert themselves into the host membrane, thereby forming a direct connection between bacterial and host cytosols; and (iii) ‘late’ substrates or effector proteins that are then directly funneled through the injectisome into the host cytoplasm4. Many substrates of the T3SS are transported to the export apparatus in complex with a type III secretion chaperone (T3SC), which keeps the substrate in a partially unfolded and secretion-competent conformation. The ATPase complex then separates the complex under ATP-hydrolysis5 and allows for the secretion of the substrate in an unfolded or partially helical state6.
T3SCs can further be subdivided into three classes. Class I chaperones bind effector proteins before injection. Class II binds to hydrophobic translocator proteins. Class III is a heterogeneous group and contains chaperones of flagellin in fT3SS or the injectisomal needle subunit SctF (YscF). Class II chaperones contain three tetratricopeptide repeat (TPR) motifs7 where one TPR is made up of two antiparallel α-helices. The tandem arrangement of TPRs generates a convex outer side that forms extensive interactions with the translocator and a concave binding groove where a short stretch of the substrate molecule binds in an extended conformation8–11. Additionally, a gatekeeper protein is involved to ensure timely secretion of translocators before effector proteins12. For the class III chaperone of the needle component SctF, two structures (YscEFG from Yersinia and PscEFG from Pseudomonas) have been published13,14. Both show heterotrimeric complexes of SctF with the two chaperones YscG/PscG and YscE/PscE, where SctF binds into the concave groove of the TPR protein YscG/PscG via a short α-helix. Interestingly, the second chaperone YscE/PscE, which mainly consists of two α-helices, caps the N-terminus of YscG/PscG but barely interacts with the substrate.
Injectisomes can be divided into seven families15, such as the Inv-Mxi-Spa family present in Salmonella enterica, Shigella sp. or Escherichia coli, or the Ysc family associated with Yersinia sp., Pseudomonas aeruginosa, Aeromonas sp. or Photorhabdus luminescens. Ysc family T3SS produce two proteins, SctX and SctY (YscX and YscY in Yersinia), that are not found in other T3SS families. Functionally, little is known about these two proteins except that they are required for the export of early substrates and hence the assembly of a functional T3SS16–18. YscX and its Aeromonas homolog AscX are secreted with YscY acting as chaperone for YscX prior to secretion17,19. YscX is not an early substrate, since its secretion occurs after the YscP-mediated substrate specificity switch18. It has been shown that the N-terminus of YscX harbors its secretion signal, while its C-terminus is essential for the formation of a secretion-competent T3SS17. While no experimental structures are available for either protein, YscY can be assigned a role as T3SC based on its sequence which predicts 3 TPRs20. The structure of YscX remains elusive, given that its only homologs are other uncharacterized SctX proteins. The binding mode appears to be conserved between different SctX and SctY, as heterologous complexes from different species have been observed via yeast two-hybrid assays21. After separation from YscX, YscY probably serves a regulatory function within the bacterial cell due to its interaction with SycD22,23, the chaperone escorting the hydrophobic translocator proteins YopB and YopD.
The interaction between YscX, YscY, and the major export apparatus protein YscV has been established to be a prerequisite for the timely secretion of early substrates and the tripartite complex SctVXY is conserved between different T3SS-carrying species18,21. SctVs belong to the FlhA superfamily and as such are structurally similar to their flagellar FlhA counterparts. The export gate may receive substrates previously bound by a cytosolic sorting platform24,25. Over the years, several structures of FlhA and SctVs have been published (FlhA:26–28 SctV:29–34), recently amended by the electron microscopy (EM) structure of Yersinia YscV as a homo-nonamer35. In general, SctVs contain an N-terminal transmembrane domain followed by a linker and a large cytosolic domain. It has been shown that SctVs form oligomeric structures in vivo that are consistent with the nonameric ring observed in different crystal and EM structures36. The cytosolic domain can further be subdivided into four subdomains (SD1–SD4), where SD2 is an insertion in SD1. SD3 and SD4 are necessary for the formation of the nonameric ring30,37. NMR data of a ternary complex composed of the cytosolic domain of Salmonella FlhA, the substrate FliC and its chaperone FliS has demonstrated that for a flagellar T3SS, recognition of the substrate:chaperone complex is facilitated via the chaperone and a conserved region in SD2 of FlhA38. It is noted, however, that the recognition site is not conserved in injectisomal SctVs and deletion of the entire SD2 did not abolish secretion of translocator and tip proteins for Shigella MxiA30. Therefore, the binding of substrate:chaperone complexes might occur at a different position in virulence-associated T3SS than in fT3SS.
Here, we report the structure of the YscX:YscY complex alone, and as a tripartite complex with the cytosolic domain of YscV. Our results demonstrate that the binding mode of this injectisomal substrate:chaperone complex is distinct from the binding of flagellar chaperones to the export gate and they explain why the C-terminus of YscX is essential for its function as injectisomal component.
Results
Structure determination of the YscX:YscY complex
To structurally characterize the two proteins YscX and YscY, we first attempted to express them independently as YscX fused to maltose-binding protein (MBP-YscX) and His6-YscY in E. coli. While YscY was expressed insolubly, MBP-YscX was observed in the soluble fraction but showed heavy precipitation after cleaving off the solubility-promoting MBP-tag. We, therefore, tried co-expression of the two constructs, which yielded soluble protein. Each protein could pull down the other during affinity chromatography and YscY and YscX co-eluted in a symmetrical peak when subjected to size exclusion chromatography. Since stored samples of the complex showed clear signs of proteolytic digestion of YscX, limited proteolysis coupled with N-terminal sequencing and mass spectrometric analysis was conducted to identify a more stable N-terminally truncated version of YscX (YscX32). Neither the C-terminal region of YscX nor YscY were sensitive towards proteolysis. A study by Day and Plano17 further suggested that only the region YscX50–110 is necessary for it to bind YscY, so further constructs truncating the N-terminus (YscX50), the C-terminus (YscX32–110), or both (YscX50–110) were created.
Our crystallization constructs contained N-terminally truncated YscX50 and full-length YscY with an N-terminal hexahistidine tag. The complex crystallized in space group P21212 with the asymmetric unit (AU) containing two heterodimers related via translational non-crystallographic symmetry. We solved the structure by molecular replacement (MR) using a model generated by AlphaFold 2 (Supplementary Fig. 1). Data collection and refinement statistics are reported in Supplementary Table 1. The two dimers in the AU are very similar with an r.m.s.d. value of 0.744 Å between the Cα residues of the complex. Differences lie mostly within YscX—where the r.m.s.d. between the two molecules is 0.904 Å—caused by a slight shift in the second helix (α2) of the protein.
YscX and YscY form an entwined heterodimer
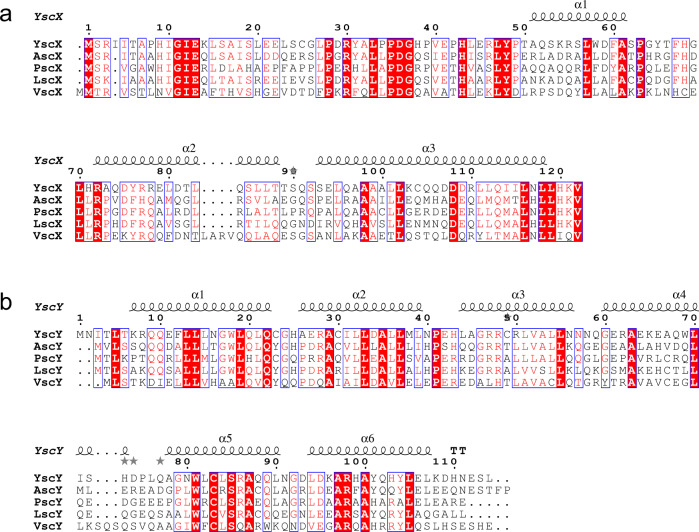
The AlphaFold 2 model correctly predicted the overall fold of the complex with YscX binding to YscY at two distinct sites connected via a short linker, resulting in YscX entwining its chaperone (Fig. 1a). Similar to other T3SCs the concave face of YscY forms an interaction site to which YscX binds via a short helix α1 (S53–A61). The area of interaction for this site is approximately 500 Å2. Moreover, this groove is largely lined by hydrophobic residues and allows for the binding of YscX’s L57, W58, F60, A61, and P63 into distinct binding pockets. Hydrophilic residues such as K54, R55, and D59 point toward the solvent (Fig. 1b). S53 and S56 of YscX are 3.8 and 3.9 Å apart from Y101 and Y104 of YscY, respectively, making their contribution to the binding interface via hydrogen bonds unlikely. The importance of the amphipathic character of the binding helix in YscX is further underlined by the high degree of sequence conservation for the hydrophobic residues that interact with the chaperone when compared to the otherwise low conservation of helix α1 (Fig. 2a). The linker between the binding helix and the C-terminal helices of YscX is only resolved in one of the two heterodimers in the AU, indicating the flexibility of the loop.
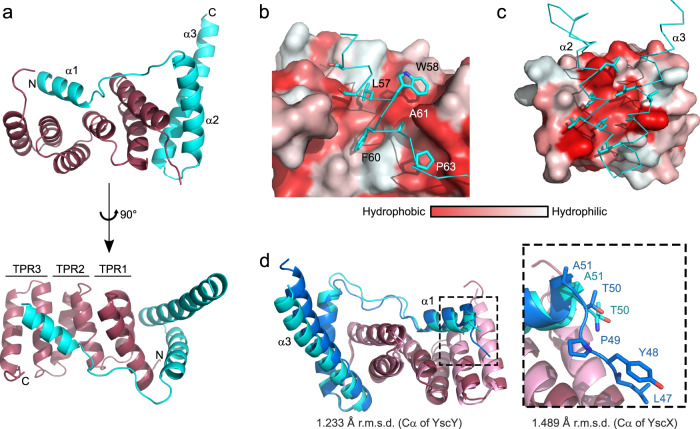
Fig. 1. Overall structure of the YscX:YscY heterodimer.
a Cartoon representation of YscX (cyan) and YscY (red). YscX binds to YscY at two independent sites. b, c Surface hydrophobicity gradient of YscY shown from hydrophobic (red) to hydrophilic (white) using the normalized consensus hydrophobicity scale65. b YscX binds the groove of the TPR protein YscY via the hydrophobic side of a short amphipathic helix. c Two leucine-rich helices of YscX interact with the largely hydrophobic surface of the N-terminal TPR in YscY. d Superposition of YscX50:YscY (cyan and red) and YscX32:YscY (blue and pink) models. The alignment was calculated using the Cα atoms of YscY. Zoom-in shows the YscX N-terminus before α1 for both structures when aligned using the Cα atoms of YscX.
Fig. 2. Sequence alignment of SctX and SctY.
a Alignment of SctX. b Alignment of SctY. Alignments were calculated using ClustalW66 and visualized with ESPript3 (espript.ibcp.fr,67). SctX and SctY sequences from Yersinia enterocolitica (YscX: AAD16820.1, YscY: AAK69217.1), Aeromonas hydrophila (AscX: AAS91814.1, AscY: AAS91815.1), Pseudomonas aeruginosa (PscX: AAY17109.1, PscY: AAY17110.1), Photorhabdus luminescens (LscX: CAE16135.1, LscY: CAE16134.1), and Vibrio parahaemolyticus (VscX: UJX28051.1, VscY: EDM61220.1) were used to generate the alignment. Definition of colors and symbols: red box, white character: strict identity; red character: similarity in a group; blue frame: similarity across groups; gray star: residues with alternate conformations.
A second interface is formed via the interaction of YscY’s N-terminal TPR and the two longer C-terminal α-helices (α2 and α3) of YscX. The helices of YscX are rotated by about 90° relative to YscY with the binding site containing the hydrophobic residues I3, L5, F12, L13, I31, L32, A35, and L39 for YscY as well as L80, L83, L86, L87, L95, A98, L101 and L102 for YscX (Fig. 1c). The total area of interaction averages out at 1436.7 Ų for both YscXY heterodimers (PISA39).
The C-terminal helix of YscX extends about 26 Å further out from where it last interacts with its chaperone, as measured from L102 to H120. A hydrogen bond of Q106 to D81 is the last intramolecular interaction between α2 and α3 of YscX. Therefore, the remaining residues (about 20 Å of the C-terminal helix) are not forming interactions within the YscX:YscY heterodimer. In the crystal, this helix is stabilized by interacting with helix α3 of the neighboring molecule as well as the same helix from symmetry-related molecules, forming a 4-helix interface. B-factors for the protruding part of helix α3 are relatively high in chain B, interestingly however not in chain D despite its generally higher B-factors, especially in the poorly defined loop and helix α2 (Supplementary Fig. 2). Furthermore, K121 and V122 are not ordered in the crystal, suggesting that the C-terminus of YscX is somewhat flexible.
The YscX N-terminus is disordered
In addition to our structure of YscX50:YscY, we also crystallized a longer construct containing residues 32–122 of YscX (YscX32:YscY). Crystallization of this complex required the reductive methylation of primary amines40. The resolution was considerably poorer, with the protein adopting a loose packing in space group I4122 with a solvent content of 64.3% (Supplementary Table 1). The overall fold is identical to that of the shorter construct and the structures align well with r.m.s.d. values of 2.033 Å between YscX32:YscY and chain A and B of YscX50:YscY or 1.388 Å with chain C and D of the latter. This is compared to an r.m.s.d. of 0.744 Å between the two complexes in the AU of the YscX50:YscY crystal. In general, the r.m.s.d. values were a bit higher for YscX (1.489 Å or 1.383 Å for chain B or chain D, respectively) than for YscY (1.233 Å or 1.078 Å) and the models differ the most in the position of the loop between helices α1 and α2 of YscX and in a slightly increased bending at the C-terminus of α3 in the model for YscX32:YscY (Fig. 1d). Even though this YscX construct is 18 residues longer than YscX50, only L47-P49 of these are resolved, suggesting that the N-terminus is largely disordered. Moreover, the C-terminus in this model is fully resolved and is helical albeit with high B-factors for the last residues (Supplementary Fig. 2).
Structure determination of YscX:YscY bound to nonameric YscV
We then attempted to co-purify the cytosolic domain of of YscV (YscVC) with complexes of YscX and YscY from E. coli. Yields were considerably higher for the ternary complex (30 mg per liter of culture) than for the heterodimer (1–5 mg per liter). During gel filtration, the complex eluted as a species of high molecular weight. The assembly crystallized readily and conditions were identical regardless of which YscX construct (YscXfull-length, YscX32, or YscX50) was used. Diffraction measurement of the ternary complex containing YscVC, YscX32, and His6-YscY gave data to a resolution of 4.1 Å (Supplementray Table 1). We tested approximately 100 crystals of this complex with most not diffracting further than 8 Å if at all. The phase problem was solved via MR with an EM model of nonameric YscV (PDB: 7ALW). Two nonamers are stacked upside-down with their membrane-distal sides interacting in an assembly probably lacking biological significance also described by Kuhlen et al.35 (Fig. 3a). We then attempted to place the model of YscX50:YscY via MR as well, which failed most likely due to its relatively low scattering mass. Instead, 18 binary complexes were placed manually into helical density present outside of the nonameric YscV rings (Supplementary Fig. 3). The crystal described here is packed loosely with a solvent content of 67.2% that originates both from the central 5 nm-wide pore of the cyclic YscVC nonamer and the fact that the stacked nonamers hardly interact with one another, leaving a large volume of bulk solvent between the 18-mers (Supplementary Fig. 4). Instead, almost all crystal contacts occur between YscY molecules or between an YscY molecule and SD2 of YscVC which also points out from the ring.
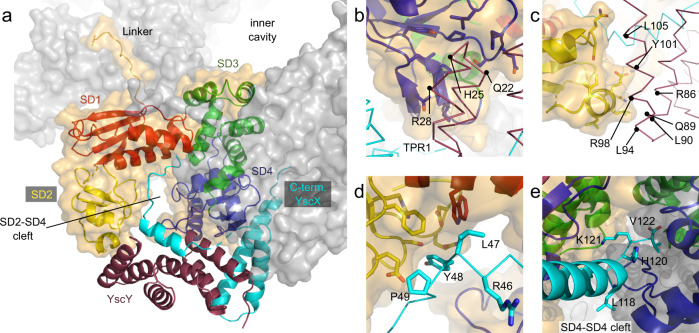
Fig. 3. Overall structure of the ternary YscVC:YscX32:YscY complex.
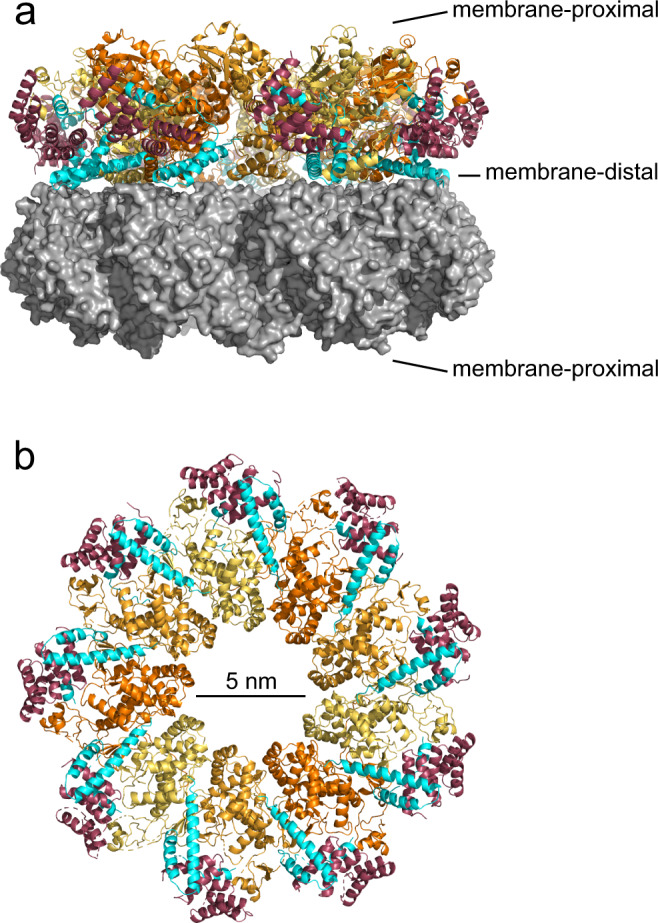
a Side View of the two stacked nonameric rings of YscVc (orange, three shades for clarity). Half of the chains are shown as gray surface. YscX32 (cyan) and YscY (red) bind on the periphery of the nonamer. b View on the membrane-distal side of one nonamer. The C-terminal helix of YscX protrudes into the ring between two adjacent YscVC protomers.
Overall structure of nonameric YscVC
In general, the electron density was reasonably well defined for subdomains 3 and 4 (SD3 and SD4), which are mostly buried within the ring. The average area of the YscV-YscV interaction is approximately 1600 Å2 and is stabilized by four salt bridges between E431-R560, D511-R592, E517-R563, and R519-E531. The electron density of SD1 was interpretable, even though the central 4-stranded β-sheet was only visible as a continuous sheet of electron density. In contrast, SD2 showed the lowest map quality with broader variations between the protomers with regard to connectivity of the density. The cleft formed between SD2 and SD4 is in an open conformation, with a distance of roughly 10 Å between the two domains at the cleft’s entrance. Parts of the linker between transmembrane and cytoplasmic domain are also visible (residues 359–371) and fold onto the neighboring protomer, where it runs along a defined groove, as previously observed35. We confirmed the sequence register of YscVc via anomalous difference density from a selenomethionine derivative (Supplementary Fig. 5).
The individual chains of the complex superimpose well (Supplementary Fig. 6a), especially the rigid SD3 of YscVC. More flexibility can be observed for SD2 and at the outer regions of SD1 and SD4. YscX chains are very similar except for the linker between helices α1 and α2, which was poorly defined (Supplementary Fig. 6e). The binding of YscX:YscY to YscVC seems to allow some leeway, since an alignment of all substrate or all chaperone chains results in fanning out of the YscVC chains (Supplementary Fig. 6d−g).
YscY and the YscX N-terminus bind the SD2-SD4 cleft
Each YscX:YscY complex binds to the SD2-SD4 cleft of an individual YscVC protomer. The PISA server39 reports interactions between (A) YscY’s first TPR and SD4, (B) the third TPR and SD2, as well as (C) a putative interaction between the extended N-terminus (L47 and Y48) of YscX with SD1 from inside the cleft between SD2 and SD4 (Fig. 4b–d). The latter interaction was only observed for chains FB, GB, KB as well as LB and may be biologically relevant since these residues are conserved between SctX. Due to the poor resolution, the exact nature of these interactions could not be determined as density was usually absent for side chains in YscX and YscY. For the aforementioned site (A) hydrophobic interactions could originate from I604, P606, L652, I655, P659, and L699 of YscV, and hydrogen bonds could arise between Y608, Q656, S657, and Q697 of YscV with Q22, H25, and R28 of YscY. Several leucines and isoleucines can be found in site (B), which may be complemented by polar interactions between Q452, E454, S486, and Q487 of SD2 as well as R86, Q89, R98, and Y101 of YscY. In case of site (C), the conserved Y48 of YscX might be interacting with aromatic residues of YscV in vicinity, such as F491 and W499 of SD1. Furthermore, Y48 is pointing towards a patch of different possible hydrogen bonding partners, such as R447, S449, D469, Q470, and E489 of YscV.
Fig. 4. Interactions of YscV, YscX, and YscY.
One YscVC protomer is highlighted (orange surface) within the nonameric ring (gray surface). The cartoon representation of YscVC is divided into the four subdomains: SD1 (red, residues 372–438 and 493–513), SD2 (yellow, residues 439–492), SD3 (green, residues 514–600), and SD4 (blue, residues 601–704). For clarity, YscX (cyan) and YscY (dark red) are shown only for this heterotrimer and are depicted as ribbon in (b–d). a The overall structure of the heterotrimer within the assembly shows that all interactions, except for the insertion of YscX’s C-terminus, are confined within one heterotrimer, i.e., YscY does not interact with a neighboring YscV subunit. b YscY interacts with SD4 via the loop of its first TPR (site A) and c with SD2 via the face of its C-terminal TPR (site B). d In chains FB, GB, KB, and LB, YscX exhibits density for an extended N-terminus which binds to SD1 and SD2 via a conserved 46RLYP49 motif (site C). Note that the exact positions of the side chains could not be determined at this resolution and are only included for completeness. e The C-terminus of YscX is inserted into the SD4–SD4 cleft. Both involved YscVC subunits are shown as cartoon colored by subdomain. YscX is shown as ribbon with sidechains starting from L118.
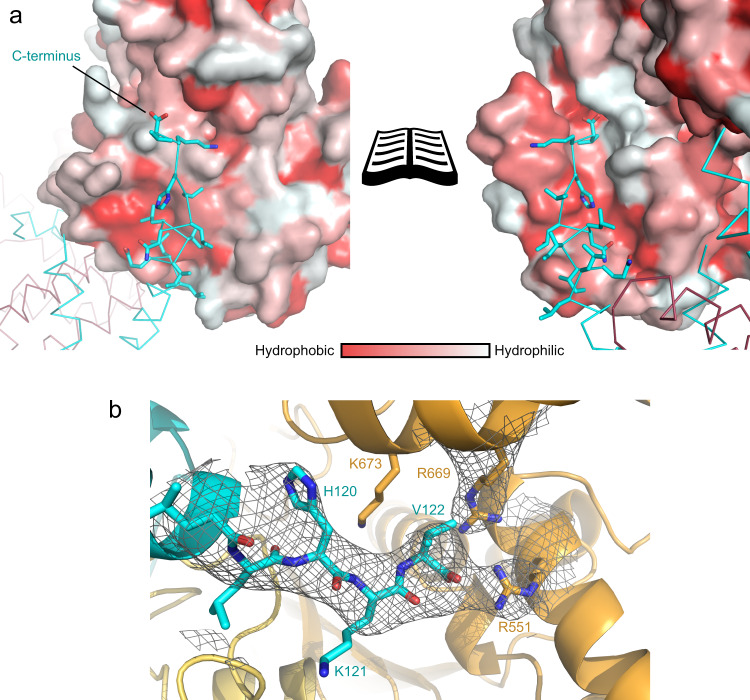
The C-terminus of YscX binds between YscV protomers
The C-terminal α3 helix—which is the extended helix seen in the YscX:YscY structures—is inserted between two YscVC protomers (Fig. 3b) at the cleft formed between two adjacent SD4 (hereafter referred to as SD4–SD4 cleft). This is the major binding interface between YscV and YscX and involves SD3 and SD4 of YscV. Except for a salt bridge formed between D108 of YscX and R701 of YscVC directly at the entry point for the helix, the interaction is largely hydrophobic (Fig. 5a) with most residues involved being highly conserved (Fig. 2a). The C-terminal helix of YscX does not extend all the way into the inner hole of the YscV nonamer but instead ends contacting the neighboring YscV molecule. The negative charge of the carboxy-terminus at V122 is presumably neutralized by R551 and R669 of a second YscV molecule (Figs. 4e and 5). Mutating R551 to alanine (R551A) or glutamate (R551E) reduced or abolished the ability of YscVC to be pulled down by MBP-YscX50:His6-YscY in MBP pull-down assays (Fig. 6a), indicating the importance of charge neutralization for recognition of YscX by the export gate.
Fig. 5. The C-terminus of YscX binds between two YscV protomers.
a Surface hydrophobicity of YscV (left) and the neighboring protomer (right) from hydrophobic (red) to hydrophilic (white) with a ribbon and stick representation of YscX (cyan). Helix α3 inserts between two YscV molecules and binds via hydrophobic interactions. The C-terminus is pointing towards a positively charged patch of the neighboring subunit, which likely stabilizes the carboxylate. b 2mFO-DFC density contoured at 1σ at the C-Terminus of YscX suggests positioning of the carboxylate towards R551 of YscV (orange). The density of the arginines is exemplary and varies between the chains.
Fig. 6. Recognition of YscXY by YscVC depends on the C-terminus of YscX as well as SD3 and SD4 of YscVC.
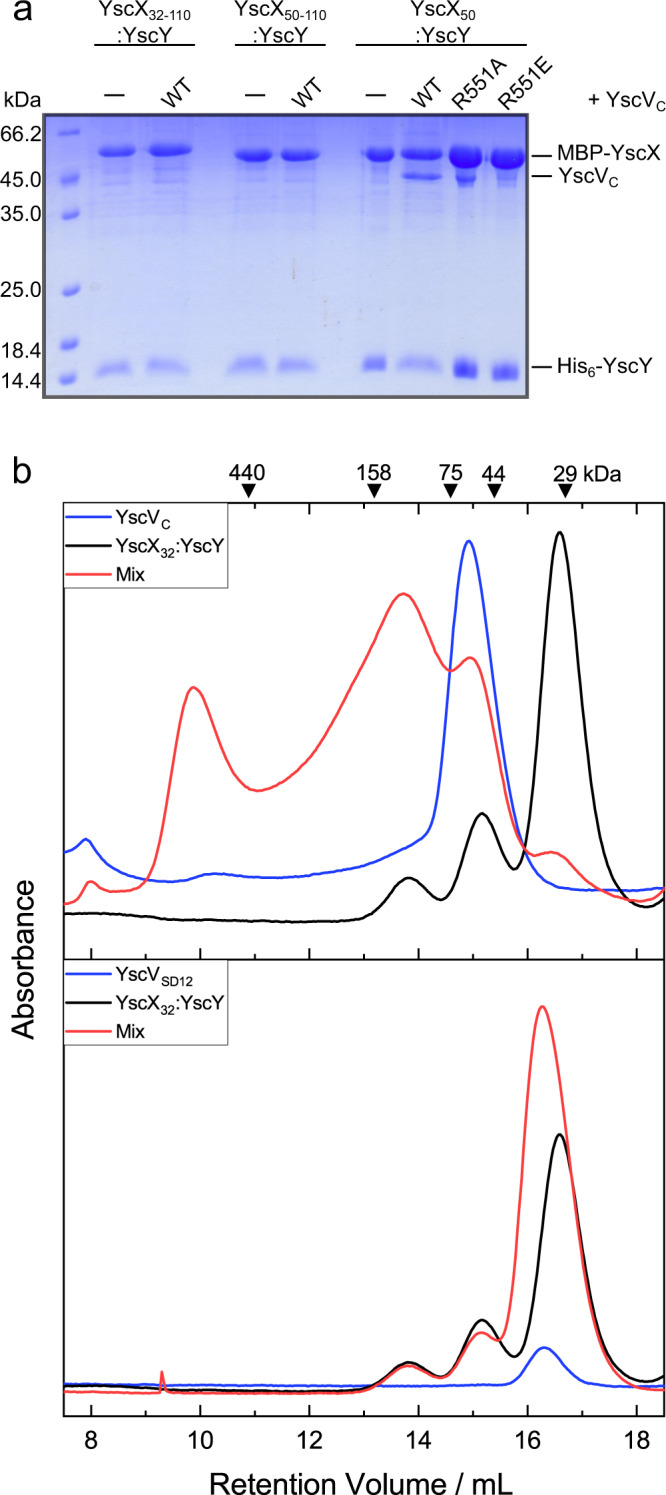
a Coomassie-stained SDS-PAGE of MBP pull-downs. N- and C-terminally truncated MBP-YscX constructs were co-expressed with His6-YscY and YscVC. Lysates were loaded onto amylose resin and the washed matrix loaded onto the gel. The pull-downs generated consistent results over three replications. Source data are provided as a Source Data file. b Analytical size exclusion chromatography with 10 µM YscVC, YscVSD12 (both blue), YscX32:YscY (black), or mixtures (red) of the proteins. Absorbance at 280 nm shows a shift in retention volume for a mixture of YscVC with YscX32:YscY, but not YscVSD12 with YscX32:YscY. Chromatographies involving YscVSD12 or YscVC were performed twice or four times, respectively, with consistent results. A SDS-PAGE and Western blot analyzing contents of gel filtration peaks are shown in Supplementary Fig. 8.
YscV requires the C-terminus of YscX for its recognition
From the ternary complex structure, it became clear that the major mode of binding between the YscX:YscY heterodimer and the nonameric YscV is via an insertion of YscX’s C-terminus between two YscV monomers within the ring (Figs. 3 and 4a) with further interactions involving the chaperone and SD2 or SD4 as well as residues L47 and Y48 of YscX. To test whether the inserting helix of YscX is necessary for binding to YscV, an MBP pull-down assay was employed, where MBP-YscX:YscY complexes were immobilized from lysate either with or without YscVC present. The SDS-PAGE (Fig. 6a) clearly demonstrated that the N-terminal truncation YscX50 has no impact on binding, whereas deletion of the C-terminal 12 amino acids (YscX32–110:YscY or YscX50–110:YscY) abolished the interaction. This was underlined by analytical gel filtrations done with mixtures of either YscVC or a truncated version lacking SD3 and SD4 (YscVSD12, residues 356–513)—which therefore does not contain the binding site for the C-terminal helix of YscX—with YscX32:YscY. Since YscX32:YscY contains the putative binding region 46RLYP49, it should be able to bind to SD1 via YscX and to SD2 via YscY.
On its own, YscX32:YscY tends to produce a three-peak pattern during gel filtration, most likely due to the formation of oligomers in solution (Fig. 6b). At a concentration of 10 µM, YscVC forms a single peak at around 60 kDa apparent molecular mass. As discussed later, the shift from the expected weight of 40.5 kDa was attributed to dynamic assembly and disassembly of the nonameric ring during the run (Fig. 7). With a mixture of YscVC and the binary complex, a clear shift towards higher apparent molecular masses can be seen as a peak around 10 mL retention volume (Fig. 6b top, Supplementary Table 2, Supplementary Fig. 8), which we attributed to a nonameric ring of YscVC with an unknown quantity of YscX:YscY bound to it. For the ternary complex, two more peaks were observed at approximately 14 and 15 mL, the latter of which could be attributed to free YscVC in solution or a 1:1:1 ternary complex for which a molecular mass of 65 kDa would be expected. The large and broad signal at around 14 mL might also indicate other oligomeric forms and a dynamic equilibrium between different states. The shortened YscVSD12 construct elutes after approximately 16 mL, which corresponds to around 36 kDa, potentially representing a dimer (Fig. 6b, bottom). Here, no shift in mass was observed when the protein was mixed with YscX32:YscY in an equimolar fashion and thus we concluded that SD1 and SD2 alone do not bind YscX:YscY with high affinity. A construct of YscVC only containing SD3 and SD4 (residues 514–704) could not be analyzed as it was insoluble upon expression in E. coli.
Fig. 7. Concentration-dependent nonamerization of YscVC.
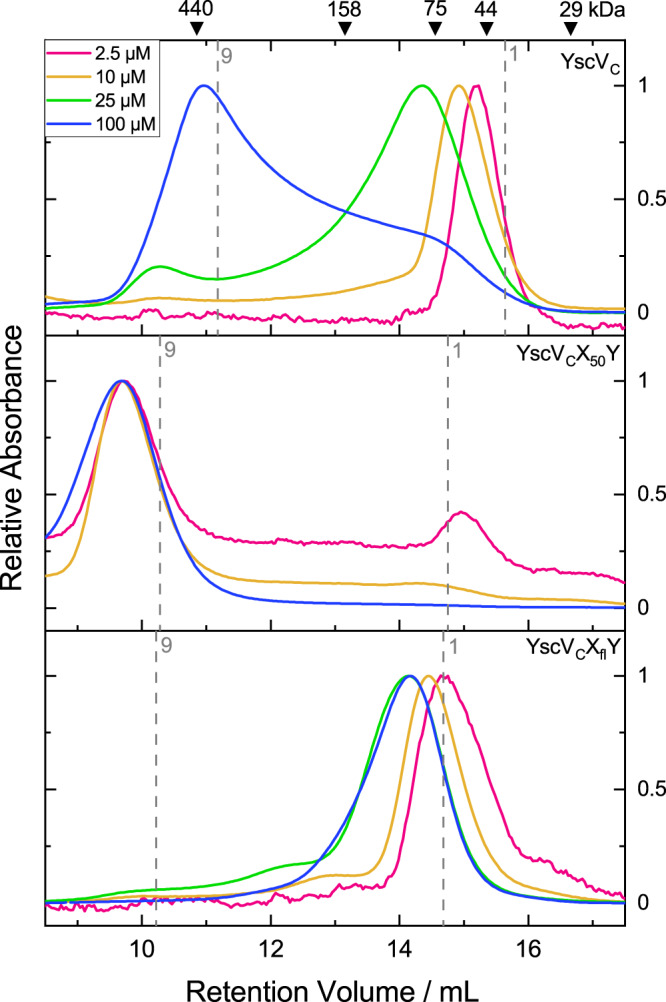
For comparability, absorbances were normalized to the highest peak. From top to bottom: YscVC, a ternary complex of YscVC co-expressed with YscX50 and His6-YscY, and the same ternary complex but with full-length YscX (YscXfl) were loaded at 2.5 µM (pink) to 100 µM (blue) onto a Superdex 200 column. For reference, the expected retention volumes for the monomeric species (or 1:1:1 heterotrimer) and nonameric species (assuming 9:9:9 stoichiometry and a globular shape) are shown as dashed gray lines. The weak auto-nonamerization of YscVC is enhanced by the presence of YscX50 but severely impeded by full-length YscX. Experiments were performed three times with consistent results.
The termini of YscX oppositely influence nonamerization of YscVC
YscV forms higher oligomers in Yersinia regardless of whether YscX and YscY are present36,37 and micelle-embedded YscV assembles into nonamers in vitro35. For the isolated cytosolic domain of other SctV proteins, both monomeric, as well as nonameric species, have been observed in solution31,32,34. With the major recognition site of YscX:YscY and YscVC being located at the far C-terminus of YscX and between two YscV protomers, we aimed at investigating a possible influence of YscX on the nonamerization of the export gate’s cytosolic domain. Size exclusion chromatography of YscVC at different concentrations revealed a concentration-dependent dynamic equilibrium between different states with the nonameric form being stabilized at higher concentrations (Fig. 7, top). At 2.5 µM initial concentration of YscVC, the protein appears to be largely monomeric as indicated by a symmetrical peak at around the expected molecular mass of 40.5 kDa. At 10 or 25 µM, we observed a shift in the apparent mass and significant fronting, indicating the dynamic association and dissociation of higher oligomeric species on the column. At 100 µM the main peak is shifted to a high apparent molecular mass corresponding to a nonamer, but there is still notable tailing, i.e., incomplete conversion to the nonameric ring. We then compared the oligomerization behavior in the presence of two different YscX:YscY constructs, which were co-purified with YscVC. At 10 µM, YscVCX50Y showed almost-complete conversion to higher masses. Interestingly, however, the full-length YscX-containing ternary complex (YscVCXflY) favored the monomeric species at the same molar concentration of protein (Fig. 7, middle and bottom).
Using the same concentration series as for YscVC, the nonameric ring of YscVCX50Y is stable even at an initial concentration of 2.5 µM, whereas full-length YscX seems to inhibit ring formation even at high concentrations (Fig. 7, bottom). We concluded that the N-terminus of YscX is not necessary for binding YscV, but it decreases the propensity of YscVC to nonamerize and therefore must interact in some other way.
Discussion
YscX and YscY are largely uncharacterized proteins from the T3SS of Yersinia with a yet unknown function despite being essential for a functional injectisome. With YscX belonging to a unique and elusive protein family without any homologous structures, we were unable to solve the phase problem via MR. After laborious attempts at experimental phasing, we attempted to predict the structure of the two proteins computationally using I-TASSER41, tFold (to be published), RoseTTAfold42, and AlphaFold 243. YscY was always predicted as a TPR protein and the general fold was very similar between programs (Supplementary Fig. 1b). YscX on the other hand produced models that were only similar for the C-terminal α2 and α3 helices (Supplementary Fig. 1a). Finally, we attempted to predict the complex structure using the same programs but supplying both sequences fused together by a 33-residue linker. While the other predictions were disregarded, we were intrigued by how compatible the AlphaFold 2 model was to our biochemical and crystallographic observations. Indeed, we could solve the phase problem for our YscX:YscY crystals with a truncated version of the AlphaFold 2 complex model. In retrospect, models of YscY were also sufficient for MR when generated by AlphaFold 2, tFold or RoseTTAfold, but not I-TASSER.
Here, we provide the structure of a SctX-family protein. YscX and YscY depend on each other for soluble expression in E. coli, which is understandable given how entwined the two proteins are in their complex. Lacking YscX, YscY would present a large hydrophobic face at its N-terminal TPR that could explain why it is insoluble in isolation. After secretion of its substrate, this face might be responsible for recognizing SycD, an interaction previously observed22,23. We found that the N-terminal 31 residues of YscX are prone to proteolysis, indicating that they may not exhibit a tertiary structure. It is possible that the N-terminus carries a proteinaceous signal for YscX secretion, especially considering how conserved the sequence is among the SctX family (Fig. 2a). The smallest YscX fragment to direct secretion (amino acids 1–60) contains helix α117, leaving the possibility that YscY bound to helix α1 of YscX is part of the secretion signal. This does not seem to be the case for the structurally similar YscEFG complex, for which the transport of YscF to the injectisome depends on its co-chaperone YscE, which structurally fulfills the role of α2 and α3 in YscX44. Alternatively, the structurally unresolved N-terminus of YscX might bind other T3S proteins and only fold in their presence, as was observed for YopN in presence of TyeA in the same secretion system45. It is unclear whether YscX serves a hitherto unknown function outside the bacterial cell after its secretion or if its absence from the export apparatus signals a switch in substrate recognition, as was postulated by Day, et al.17.
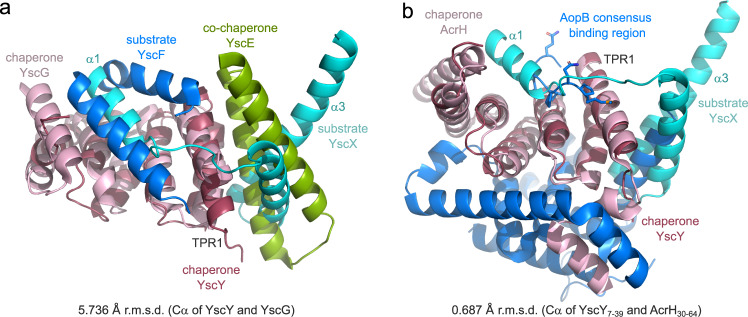
When compared to other T3S substrate:chaperone complexes, the structural similarities between YscXY and the complex of the needle protein with its two chaperones become obvious (Fig. 8a). Two structures of such a complex, one from Pseudomonas (PscEFG, PDB: 2UWJ14) and the other from Yersinia (YscEFG, PDB: 2P5813) have been published, both revealing an amphipathic SctF helix that binds into a hydrophobic groove formed by the concave side of the TPR protein YscG/PscG. The co-chaperone YscE/PscE was found to bind the hydrophobic face of the N-terminal TPR of YscG/PscG with its two helices that contribute several leucine residues to the binding interface. Thus, it appears as if YscX fulfills the roles of both “co-chaperone” and “substrate” in this assembly reminiscent of the YscEFG complex. In contrast, class II substrate:chaperone complexes—which are hydrophobic translocator proteins and their chaperones—form soluble 1:1 heterodimers like YscXY, but exhibit vastly different binding behaviors. A short stretch close to the N-terminus of the substrate binds to the groove formed by the chaperone’s concave side in extended conformation (Fig. 8b, refs. 8–11,46). The structure of Aeromonas AcrH with translocator protein AopB shows further interactions between the two proteins. Analogous to the YscEFG and YscXY complex, AopB binds the N-terminal TPR of AcrH via an α-helix. In addition, AopB extensively wraps around the convex side of the chaperone, a feature neither present in YscXY nor YscEFG11. Thus, despite its heterodimeric nature, the YscXY complex more closely resembles the heterotrimeric YscEFG complex, suggesting structural similarity to class III chaperones.
Fig. 8. Comparison of YscXY with other T3S substrate:chaperone complexes.
a Superposition of YscX50:YscY with YscEFG (PDB: 2P58). The alignment was done using the chaperones YscY (red) and YscG (pink) with an r.m.s.d. of 5.736 Å. The substrates YscX (cyan) and YscF (blue) as well as the co-chaperone YscE (green) are also shown. b Superposition of YscXY with the complex of class II chaperone AcrH (pink) and the major translocator protein AopB (blue), both from Aeromonas (PDB: 3WXX). The alignment was calculated using the first TPR of YscY (residues 7–39) and the first TPR of AcrH (residues 30–64) with an r.m.s.d. of 0.687 Å to obtain the right register. For clarity, sticks are shown for side chains of AopB where it binds the TPR groove of AcrH.
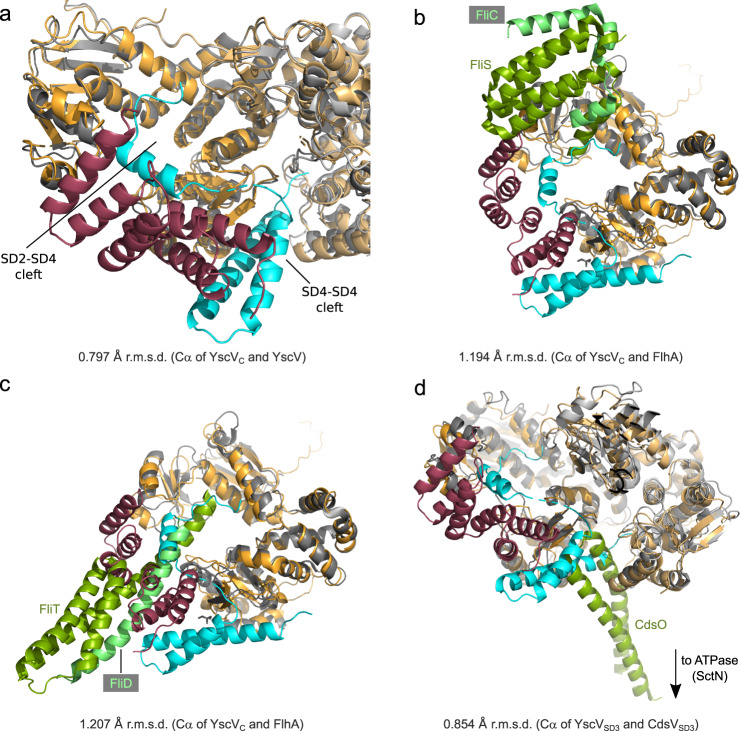
Since structures of FlhA were solved in 2010, several flagellar and injectisomal export gates were deposited in the PDB (FlhA:26–28 SctV:29–33,35). Some discussion is dedicated to elucidating differences in how the subdomains are arranged relative to each other, especially considering the cleft between SD2 and SD4 that is responsible for the recognition of the flagellar FliD:FliT and FliC:FliS substrate:chaperone complexes38. The structure of YscVC was previously reported as adopting the “open” conformation with space between SD2 and SD435 when present without additional binding partners. Our structure of the ternary complex YscVC:YscX32:YscY exhibits the same open conformation showing that no major domain rearrangements are necessary to accommodate YscXY (Fig. 9a). Minor movements are observed at the binding site between YscY and SD4 as well as helix α3 of YscX and SD4 but these do not seem to affect the export gate globally (Supplementary Fig. 7). Our model also establishes that a highly conserved 46RLYP49 motif of YscX binds within the aforementioned SD2-SD4 cleft. While we could model this region only in some of the chains, considerable difference electron density is present in the remaining chains indicating that this binding is a consistent feature. Interestingly, the binding site coincides with the recognition site of flagellar FliT and FliS chaperones (Fig. 9b, c). The binding of YscXY to YscV as seen in our structure might block access of other chaperone-bound secretion substrates to the SD2-SD4 cleft and thereby impede their secretion.
Fig. 9. Superposition of export gate complexes with YscVXY.
YscV, YscX, and YscY are shown in orange, cyan, and red, respectively. a Superposition with the cryo-EM structure of YscVC (gray, PDB: 7ALW), which is shown as cartoon representation. YscVC protomers are aligned with an r.m.s.d. of 0.797 Å. b, c Superposition with FlhA (gray) bound to the flagellar chaperone:substrate complexes (green:light green). b FlhA:FliS:FliC (PDB: 6CH3). c FlhA:FliT:FliD (PDB: 6CH2). Alignments were obtained using SD3 of YscVC (residues 514–600) with an r.m.s.d. of 1.194 Å (PDB: 6CH2) and 1.207 Å (PDB: 6CH3). d YscVXY superimposed with Chlamydia pneumoniae CdsV in complex with the stalk protein CdsO (PDB: 6WA9). The alignment was calculated using SD3 of YscVC with an r.m.s.d. of 0.854 Å. Two subunits of YscV and CdsV (gray) are shown, CdsO is colored in green.
The major binding site for YscX lies between the fourth subdomain of two adjacent subunits of the YscV nonamer, where the C-terminal α3 helix of YscX inserts, pointing towards the inner cavity of the ring but not reaching it. While we initially thought that this hints at YscX stabilizing an otherwise dissociation-prone YscVC nonamer in vivo, our gel filtration results indicate that full-length YscX actually disfavors nonamerization of the export gate (Fig. 7 bottom). The importance of the YscX C-terminus for recognition by YscV explains why complementation of yscX deletion strains with C-terminally FLAG-tagged YscX does not produce an injectisome competent to secrete Yops17. The same construct, however, was secreted by a functional T3SS17, suggesting that the interaction of YscXY with the SD2-SD4 cleft is sufficient for the secretion of YscX.
The binding site of the ATPase stalk protein SctO, as published for the complex of Chlamydia CdsV:CdsO32, is likewise located in the SD4-SD4 cleft and would not allow for simultaneous binding of SctO and SctXY (Fig. 9d). It should be noted that the Chlamydia T3SS does not belong to the Ysc family and therefore lacks SctX and SctY. It is still poorly understood how different factors work together in the regulation of SctV. These include the binding of interacting proteins such as SctO, SepL:SepD in enteropathogenic E. coli47, or substrate:chaperone complexes as well as local pH33, and the linker between transmembrane and cytosolic domain34,48,49. As such, further studies should investigate the potential interplay between YscV, YscO, and YscXY. We argue that, based on the lack of rearrangement after YscXY binding and the nonamer destabilization in vitro, YscX (and its chaperone YscY) may contribute to those dynamics by destabilizing the nonameric ring of YscV, most likely via YscX’s yet uncharacterized N-terminus. This may contribute to facilitating the substrate switch from needle components to hydrophobic translocators.
Methods
Cloning
The vectors pETM-40_yscX and pACYCDuet-1_yscY were generated for the expression of YscX:YscY binary complexes via PCR and restriction cloning using NcoI and NotI. Furthermore, pACYCDuet-1 containing both yscY in its first and yscV356 in its second multiple cloning site was prepared using the same method but employing NdeI and XhoI. For the expression of YscVC alone, pETM-11_yscV356 was used. From this vector, a deletion mutant lacking SD3 and SD4 was generated (YscVSD12). All genes were cloned from the Yersinia enterocolitica pYVe227 virulence plasmid (G. Cornelis, Basel) via PCR. Deletions and mutations were introduced via ‘Round the horn mutagenesis. For a full overview of all constructs used, refer to Supplementary Table 3.
Expression and purification of YscX:YscY complexes
Complexes of YscX with its chaperone YscY were expressed in E. coli BL21 (DE3) transformed with both pETM-40_yscX and pACYCDuet-1_yscY vectors. Cell cultures were grown at 37 °C to OD600 = 0.5 in LB medium and cooled to 20 °C before induction with 0.25 mM isopropyl β-D-1-thiogalactopyrsanoside at OD600 = 0.8–0.9. After incubation over night, cells were spun down at 4000 × g and pellets stored at −20 °C. Cell pellets were resuspended in 50 mM Tris pH 8, 150 mM NaCl and 10 mM β-mercaptoethanol supplemented with one tablet of cOmplete protease inhibitor cocktail (Roche) and 0.6 mg DNase I per liter of expression culture and then passed through a pressure cell homogenizer (Stansted FPG12800) at 120 MPa.
Lysates were cleared by centrifugation (60 min, 30,000 × g, 4 °C). Amylose affinity chromatography (10 mL Amylose Resin HighFlow, New England Biolabs) was employed to capture MBP-YscX:His6-YscY. After 60 min of light pivoting at 4 °C, the resin was washed by raising the NaCl concentration to 300 mM. The protein-loaded amylose was re-equlibrated with 50 mM Tris pH 8, 150 mM NaCl, and 1 mM dithiothreitol. To remove the MBP-tag of YscX, 1 mg of TEV-protease was added per 50 mg of target protein and the resin was incubated at 20 °C over night on a seesaw shaker. The flow-through and successive washing fractions were collected, pooled and imidazole was added to a final concentration of 15 mM before being loaded onto 5 mL of NiNTA resin (Protino Ni-NTA Agarose, Macherey-Nagel).
After 60 min of incubation, unbound protein was washed from the column by increasing the imidazole and NaCl concentrations to 100 and 300 mM, respectively. YscX:YscY eluted from the resin when adding elution buffer (20 mM Tris pH 8, 150 mM NaCl, 250 mM imidazole, 1 mM DTT). Finally, the protein was purified via size exclusion chromatography on a HiLoad 16/60 Superdex 75pg (Cytiva) where the buffer was exchanged to 20 mM Tris pH 8, 150 mM NaCl. After concentrating the complex to approximately 10 mg/mL, 5 mM tris(2-carboxyethyl)phosphine (TCEP) was added and the solution was flash-frozen at −80 °C.
Reductive methylation of YscX:YscY
NiNTA-purified protein was first dialyzed against 2×2 L 50 mM Hepes pH 8, 300 mM NaCl, 2 mM DTT and then diluted to approximately 1 mg/mL. Borane dimethylamine complex (97%, Alfa Aesar) as well as formaldehyde were added to 1.2 mg/mL and 1.2% (v/v), respectively, and the mixture incubated on ice. After three and six hours, the same amounts of the two reagents were added and the protein was further incubated over night. The reaction was stopped with the addition of 250 mM Tris pH 8, the protein was concentrated and purified via gel filtration as described above.
Expression and purification of YscVCXY
For the ternary complex of YscX32:YscY with YscVC, pETM-40_yscX32 and pACYCDuet-1_yscY_yscV356 were co-transformed into E. coli BL21 (DE3). Expression and purification steps were identical until the protein was eluted from the amylose resin after TEV-digestion. Instead of successive NiNTA affinity chromatography, the complex was loaded onto 5 mL amylose resin to remove the remaining MBP-tagged protein. The flow-through and washing fractions were combined, concentrated, and purified via size exclusion chromatography on a HiLoad 16/60 Superdex 200 pg (Cytiva). Before freezing the concentrated protein complex, 5 mM TCEP was added.
Expression and purification of His6-YscV constructs
For the stand-alone purification of the cytosolic domain of YscV (YscVC, residues 356–704) or YscVSD12 (residues 356–513), the proteins were expressed as described above from pETM-11 with an N-terminal and TEV-cleavable hexahistidine tag. The cleared lysate was loaded onto 10 mL NiNTA resin and incubated for 60 min with 10 mM imidazole. The flow-through was collected and the resin was washed first with 50 mM Tris pH 8, 300 mM NaCl, 10 mM imidazole, and 10 mM β-mercaptoethanol and then with 30 mM imidazole in the same buffer. An increase to 250 mM imidazole eluted the target protein, which was then dialyzed against 20 mM Tris pH 8, 150 mM NaCl, 1 mM DTT after adding 1:50 w/w TEV protease. Re-chromatographic NiNTA cleared the target of uncleaved His6-YscV before the protein was concentrated and polished via size exclusion chromatography on a HiLoad 16/60 Superdex 200pg (YscVC) or HiLoad 16/60 Superdex 75pg (YscVSD12) (Cytiva) with 20 mM Tris pH 8, 150 mM NaCl as running buffer. After concentration to 10–15 mg/mL, 5 mM TCEP was added and the protein was flash-frozen.
MBP-pull-down assay
To test the binding of different YscX truncations to YscVC, both proteins and YscY were co-expressed as described for the ternary complex but at a scale of 100 mL culture. The cleared lysate was applied to 1 mL of amylose resin and unbound protein was washed off. Detection of amylose-bound protein was achieved by resuspending the resin in washing buffer and loading it on an SDS-PAGE.
Limited proteolysis
YscXfull-length:YscY was subjected to limited proteolysis by trypsin, chymotrypsin, proteinase K, and thermolysin to identify stable constructs for expression. Purified protein (1 mg/mL) was mixed with 1:100 (w/w) protease in 50 mM Tris pH 8, 150 mM NaCl, and 1 mM DTT or 0.5 mM CaCl2 (for thermolysin) and incubated on ice for 3 h. Samples were analyzed by SDS-PAGE and sequenced at their N-terminus via Edman degradation (Proteome Factory, Berlin, DE) after transfer to PVDF membrane (Immobilon, Merck) in a semi-dry set-up. Total mass was determined in-house using MALDI-TOF after dialysis against 10 mM acetate buffer pH 4.6.
Analytical size exclusion
Size exclusion chromatography on a Superdex 200 10/300GL was utilized to analyze protein oligomerization, dissociation, and association. The proteins were thawed on ice, combined for protein mixtures, or diluted for concentration gradients, and incubated for at least 30 min on ice before centrifugation and application to the column. All runs were carried out at 0.5 mL/min flowrate with 20 mM Tris pH 8, 150 mM NaCl as running buffer.
Crystallization and data collection
Crystals of YscX50:YscY grew in 0.1 M BisTris pH 6.0, 0.2 M MgCl2 and 21% PEG 3,350 with 5 mg/mL protein and a drop ratio of 0.33 µL reservoir to 0.66 µL protein solution at 20 °C in a sitting drop setting over 60 µL of reservoir. Microseeding of these into the same condition but with 13% PEG 3350 and 3–5 mg/mL protein was necessary to obtain single crystals. Drops were equilibrated for 6 h or over night before adding the seeds. Crystal growth was poorly reproducible and depended on purification batch. For cryoprotection, the crystals were transferred to a drop containing 0.1 M BisTris pH 6.0, 0.2 M CaCl2, 19% PEG 3350 supplemented with 5% glycerol. Measurements were carried out at the BL14.2. beamline at the BESSY II electron storage ring operated by the Helmholtz-Zentrum Berlin für Materialien und Energie50. Data were collected using the local installation of MxCube51.
For crystallization of the methylated complex YscX32:YscY, the protein buffer was exchanged to 10 mM Tris pH 8, 50 mM NaCl, 5% (w/v) sucrose, 2.5 mM TCEP. Crystals were obtained at 20–25 °C with 0.1 M sodium succinate pH 6–6.5, 4.2–4.4 M NaCl as reservoir solution using 5 mg/mL protein but could be optimized by the addition of 4% acetonitrile (Hampton Research Additive Screen). The drop ratio was 1 µL protein plus 0.5 or 1 µL reservoir solution. For harvesting, trays were transferred from 20 to 4 °C, and crystals were cryoprotected with 10 % (w/v) sucrose. Data collection took place at beamline X06SA at SLS (Villigen, Switzerland) using 1.00 Å wavelength and 0.25° of oscillation between frames.
The ternary complex of YscVC, YscX32, and His6-YscY was crystallized in a sitting drop setting with 0.1 M Hepes pH 6.8–7.5, 1% PEG 2000 MME, and 0.7–1.0 M sodium succinate as reservoir solution. The drop contained 0.33 µL reservoir solution and 0.66 µL of 5 mg/mL protein. Large crystals between 200 and 1000 µm in length grew after 2–3 days of incubation at 20 °C. As these crystals were very fragile and tended to crumble when touched, the plates were transferred to 4 °C the evening before harvesting. Cryoprotection was achieved by gradually transferring the crystals to solutions containing 0.1 M Hepes, 1% PEG 2000 MME, and 1.1 M sodium succinate as well as 5, 10, 15, and finally 22.5% glycerol. Data were collected through the local installation of MxCube51 at beamline P13 at DESY (Hamburg, Germany) using a helical scan with 0.066 s exposure time per image and 100% transmission52.
Data reduction, model building, and refinement
Diffraction data were indexed, integrated, and scaled using XDS53 or XDSAPP54 for YscX50:YscY. The MR model was generated using a local installation of AlphaFold 243, supplying the sequences of YscX and YscY with a 33 residue linker between them. The phase problem was initially solved for the YscX50:YscY data with a truncated model of the complex using Phaser55 with the tNCS option disabled. After initial model building using BUCCANEER56 in the CCP4i GUI57 successive rounds of manual model building in Coot58 and refinement in phenix.refine59,60 generated the final model. TLS parameters were determined by the “find TLS” option in phenix.refine.
For YscX32:YscY a model of the shorter YscX50:YscY construct was employed as a search model during MR. Secondary structure restraints and reference model restraints from the aforementioned structure were used during refinement in phenix.refine. The ternary complex structure was solved via MR with a cryo-EM model of YscVC35 (PDB: 7ALW (YscVC nonamer)). After an initial round of refinement in phenix.refine, the YscX and YscY chains were placed manually into the density and refined with reference model restraints. To account for the low resolution data, group B-factors, secondary structure restraints, Ramachandran restraints, and torsion-angle NCS restraints were enabled in refinement. The resolution cutoff for the YscVCX32Y dataset was determined using paired refinements61 as implemented in PAIREF62,63.
Molecular graphics and alignments were generated in PyMOL. The r.m.s.d. value of alignments was calculated in PyMOL using only Cα atoms without outlier rejection. The entire residue range was considered unless stated otherwise.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank the Helmholtz-Zentrum für Materialien und Energie for the allocation of synchrotron radiation beamtime. We acknowledge the Paul Scherrer Institut, Villigen, Switzerland for provision of synchrotron radiation beamtime at beamline X06SA (PX1) of the SLS and would like to thank Tomizaki Takashi for assistance. The synchrotron data were collected at beamline P13 operated by EMBL Hamburg at the PETRA III storage ring (DESY, Hamburg, Germany). We would like to thank Isabel Bento for the assistance in using the beamline. We acknowledge the help of Dr. Jens Sproß (Bielefeld University) in preparing, collecting, and analyzing mass spectrometry data as well as Dr. Gregor Hagelueken (University of Bonn) for generating the AlphaFold 2 models. We thank Martin Malý (Czech Technical University) for his help in running PAIREF. D.G. acknowledges funding from the Bielefelder Nachwuchsfonds. H.H.N. thankfully acknowledges the financial support by HZB (travel grant “BESSY 2 MX: 17”).
Source data
Author contributions
H.H.N., D.G. and M.S. conceived the project. D.G. and M.S. performed experiments. D.G., M.S. and H.H.N. collected and analyzed data. D.G. prepared the figures. D.G. and H.H.N. wrote the manuscript. H.H.N. supervised the project.
Peer review
Peer review information
Nature Communications thanks Gregory Plano, Benjamin Spiller, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The atomic coordinates and X-ray intensities generated in this study have been deposited in the Worldwide Protein Data Bank (wwPDB) under accession codes 7QIH (YscX50:YscY), 7QII (YscX32:YscY), and 7QIJ (YscVC:YscX32:YscY). Diffraction images are available from the SB Grid Data Bank64 (data.sbgrid.org) with Data ID 905 (YscX50:YscY), Data ID 906 (YscX32:YscY), and Data ID 907 (YscVC:YscX32:YscY). The structure of the YscVC nonamer used as search model to solve the phase problem of the YscVXY structure is available from the wwPDB under accession code 7ALW. The structures used to generate Figs. 8 and 9 are available from the wwPDB. Figure 8a: 2P58 (YscEFG); Fig. 8b: 3WXX (AcrH:AopB); Fig.9a: 7ALW (YscVC nonamer); Fig. 9b: 6CH3 (FlhA:FliS:FliC); Fig. 9c: 6CH2 (FlhA:FliT:FliD); Fig. 9d: 6WA9 (CdsV:CdsO). Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-30487-1.
References
- 1.Dewoody RS, Merritt PM, Marketon MM. Regulation of the Yersinia type III secretion system: traffic control. Front. Cell. Infect. Microbiol. 2013;3:4. doi: 10.3389/fcimb.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubori T, et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 3.Bai F, Li Z, Umezawa A, Terada N, Jin S. Bacterial type III secretion system as a protein delivery tool for a broad range of biomedical applications. Biotechnol. Adv. 2018;36:482–493. doi: 10.1016/j.biotechadv.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portaliou AG, Tsolis KC, Loos MS, Zorzini V, Economou A. Type III secretion: Building and operating a remarkable nanomachine. Trends Biochem. Sci. 2016;41:175–189. doi: 10.1016/j.tibs.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Akeda Y, Galán JE. Chaperone release, and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- 6.Miletic S, et al. Substrate-engaged type III secretion system structures reveal gating mechanism for unfolded protein translocation. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-021-21143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Büttner CR, Sorg I, Cornelis GR, Heinz DW, Niemann HH. Structure of the Yersinia enterocolitica type III secretion translocator chaperone SycD. J. Mol. Biol. 2008;375:997–1012. doi: 10.1016/j.jmb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Schreiner M, Niemann HH. Crystal structure of the Yersinia enterocolitica type III secretion chaperone SycD in complex with a peptide of the minor translocator YopD. BMC Struct. Biol. 2012;12:13. doi: 10.1186/1472-6807-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Discola KF, et al. Membrane and chaperone recognition by the major translocator protein PopB of the type III secretion system of Pseudomonas aeruginosa. J. Biol. Chem. 2014;289:3591–3601. doi: 10.1074/jbc.M113.517920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunelli M, Lokareddy RK, Zychlinsky A, Kolbe M. IpaB-IpgC interaction defines binding motif for type III secretion translocator. Proc. Natl. Acad. Sci. USA. 2009;106:9661–9666. doi: 10.1073/pnas.0812900106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen VS, et al. Structure of AcrH-AopB chaperone-translocator complex reveals a role for membrane hairpins in type III secretion system translocon asssembly. Structure. 2015;23:2022–2031. doi: 10.1016/j.str.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Archuleta TL, Spiller BW. A gatekeeper chaperone complex directs translocator secretion during type three secretion. PLoS Pathog. 2014;10:e1004498. doi: 10.1371/journal.ppat.1004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun P, Tropea JE, Austin BP, Cherry S, Waugh DS. Structural characterization of the Yersinia pestis type III secretion system needle protein YscF in complex with its heterodimeric chaperone YscE/YscG. J. Mol. Biol. 2008;377:819–830. doi: 10.1016/j.jmb.2007.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinaud M, et al. Structure of the heterotrimeric complex that regulates type III secretion needle formation. Proc. Natl. Acad. Sci. USA. 2007;104:7803–7808. doi: 10.1073/pnas.0610098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troisfontaines P, Cornelis GR. Type III secretion: More systems than you think. Physiology. 2005;20:326–339. doi: 10.1152/physiol.00011.2005. [DOI] [PubMed] [Google Scholar]
- 16.Iriarte M, Cornelis GR. Identification of SycN, YscX, and YscY, three new elements of the Yersinia Yop virulon. J. Bacteriol. 1999;181:675–680. doi: 10.1128/JB.181.2.675-680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day JB, Plano GV. The Yersinia pestis YscY protein directly binds YscX, a secreted component of the type III secretion machinery. J. Bacteriol. 2000;182:1834–1843. doi: 10.1128/JB.182.7.1834-1843.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diepold A, Wiesand U, Amstutz M, Cornelis GR. Assembly of the Yersinia injectisome: The missing pieces. Mol. Microbiol. 2012;85:878–892. doi: 10.1111/j.1365-2958.2012.08146.x. [DOI] [PubMed] [Google Scholar]
- 19.Vanden Bergh P, Heller M, Braga-Lagache S, Frey J. The Aeromonas salmonicida subsp. salmonicida exoproteome: Determination of the complete repertoire of Type-Three Secretion System effectors and identification of other virulence factors. Proteome Sci. 2013;11:1–20. doi: 10.1186/1477-5956-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallen MJ, Francis MS, Fütterer K. Tetratricopeptide-like repeats in type-III-secretion chaperones and regulators. FEMS Microbiol. Lett. 2003;223:53–60. doi: 10.1016/S0378-1097(03)00344-6. [DOI] [PubMed] [Google Scholar]
- 21.Gurung JM, et al. Heterologous complementation studies with the YscX and YscY protein families reveals a specificity for Yersinia pseudotuberculosis type III secretion. Front. Cell. Infect. Microbiol. 2018;8:80. doi: 10.3389/fcimb.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis MS, Lloyd SA, Wolf-Watz H. The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis. Mol. Microbiol. 2001;42:1075–1093. doi: 10.1046/j.1365-2958.2001.02702.x. [DOI] [PubMed] [Google Scholar]
- 23.Bröms JE, Edqvist PJ, Carlsson KE, Forsberg A, Francis MS. Mapping of a YscY binding domain within the LcrH chaperone that is required for regulation of Yersinia type III secretion. J. Bacteriol. 2005;187:7738–7752. doi: 10.1128/JB.187.22.7738-7752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lara-Tejero M, Kato J, Wagner S, Liu X, Galán JE. A sorting platform determines the order of protein secretion in bacterial type III systems. Science. 2011;331:1188–1191. doi: 10.1126/science.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diepold A, Kudryashev M, Delalez NJ, Berry RM, Armitage JP. Composition, formation, and regulation of the cytosolic C-ring, a dynamic component of the type III secretion injectisome. PLoS Biol. 2015;13:e1002039. doi: 10.1371/journal.pbio.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saijo-Hamano Y, et al. Structure of the cytoplasmic domain of FlhA and implication for flagellar type III protein export. Mol. Microbiol. 2010;76:260–268. doi: 10.1111/j.1365-2958.2010.07097.x. [DOI] [PubMed] [Google Scholar]
- 27.Moore SA, Jia Y. Structure of the cytoplasmic domain of the flagellar secretion apparatus component FlhA from Helicobacter pylori. J. Biol. Chem. 2010;285:21060–21069. doi: 10.1074/jbc.M110.119412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bange G, et al. FlhA provides the adaptor for coordinated delivery of late flagella building blocks to the type III secretion system. Proc. Natl. Acad. Sci. USA. 2010;107:11295–11300. doi: 10.1073/pnas.1001383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worrall LJ, Vuckovic M, Strynadka NCJ. Crystal structure of the C-terminal domain of the Salmonella type III secretion system export apparatus protein InvA. Protein Sci. 2010;19:1091–1096. doi: 10.1002/pro.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrusci P, et al. Architecture of the major component of the type III secretion system export apparatus. Nat. Struct. Mol. Biol. 2013;20:99–104. doi: 10.1038/nsmb.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majewski DD, Lyons BJE, Atkinson CE, Strynadka NCJ. Cryo-EM analysis of the SctV cytosolic domain from the Enteropathogenic E.coli T3SS injectisome. J. Struct. Biol. 2020;212:107660. doi: 10.1016/j.jsb.2020.107660. [DOI] [PubMed] [Google Scholar]
- 32.Jensen JL, Yamini S, Rietsch A, Spiller BW. The structure of the Type III secretion system export gate with CdsO, an ATPase lever arm. PLoS Pathog. 2020;16:e1008923. doi: 10.1371/journal.ppat.1008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews-Palmer TRS, et al. Structure of the cytoplasmic domain of SctV (SsaV) from the Salmonella SPI-2 injectisome and implications for a pH sensing mechanism. J. Struct. Biol. 2021;213:107729. doi: 10.1016/j.jsb.2021.107729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Wang J, Liu A, Zhang Y, Gao X. Structural and functional analysis of SsaV cytoplasmic domain and variable linker states in the context of the InvA-SsaV chimeric protein. Microbiol. Spectr. 2021;9:e01251-21. doi: 10.1128/SPECTRUM.01251-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhlen L, Johnson S, Cao J, Deme JC, Lea SM. Nonameric structures of the cytoplasmic domain of FlhA and SctV in the context of the full-length protein. PLoS One. 2021;16:e0252800. doi: 10.1371/journal.pone.0252800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diepold A, et al. A dynamic and adaptive network of cytosolic interactions governs protein export by the T3SS injectisome. Nat. Commun. 2017;8:15940. doi: 10.1038/ncomms15940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diepold A, Wiesand U, Cornelis GR. The assembly of the export apparatus (YscR,S,T,U,V) of the Yersinia type III secretion apparatus occurs independently of other structural components and involves the formation of an YscV oligomer. Mol. Microbiol. 2011;82:502–514. doi: 10.1111/j.1365-2958.2011.07830.x. [DOI] [PubMed] [Google Scholar]
- 38.Xing Q, et al. Structures of chaperone-substrate complexes docked onto the export gate in a type III secretion system. Nat. Commun. 2018;9:1773. doi: 10.1038/s41467-018-04137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Walter TS, et al. Lysine methylation as a routine rescue strategy for protein crystallization. Structure. 2006;14:1617–1622. doi: 10.1016/j.str.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, et al. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods. 2014;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baek M, et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science. 2021;373:871–876. doi: 10.1126/science.abj8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souza CDA, Richards KL, Park Y, Schwartz M, Torruellas Garcia J, Schesser Bartra S, Plano GV. The YscE/YscG chaperone and YscF N-terminal sequences target YscF to the Yersinia pestis type III secretion apparatus. Microbiology. 2018;164:338–348. doi: 10.1099/mic.0.000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schubot FD, et al. Three-dimensional structure of a macromolecular assembly that regulates type III secretion in Yersinia pestis. J. Mol. Biol. 2005;346:1147–1161. doi: 10.1016/j.jmb.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 46.Job V, Matteï PJ, Lemaire D, Attree I, Dessen A. Structural basis of chaperone recognition of type III secretion system minor translocator proteins. J. Biol. Chem. 2010;285:23224–23232. doi: 10.1074/jbc.M110.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Portaliou AG, et al. Hierarchical protein targeting and secretion is controlled by an affinity switch in the type III secretion system of enteropathogenic Escherichia coli. EMBO J. 2017;36:3517–3531. doi: 10.15252/embj.201797515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inoue Y, et al. Structural insights into the substrate specificity switch mechanism of the type III protein export apparatus. Structure. 2019;27(6):965–976. doi: 10.1016/j.str.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Terahara, N. et al. Insight into structural remodeling of the FlhA ring responsible for bacterial flagellar type III protein export. Sci. Adv. 4, eaao7054 (2018). [DOI] [PMC free article] [PubMed]
- 50.Mueller U, et al. The macromolecular crystallography beamlines at BESSY II of the Helmholtz-Zentrum Berlin: Current status and perspectives. Eur. Phys. J. 2015;130:1–10. [Google Scholar]
- 51.Gabadinho J, et al. MxCuBE: A synchrotron beamline control environment customized for macromolecular crystallography experiments. J. Synchrotron. Radiat. 2010;17:700–707. doi: 10.1107/S0909049510020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cianci M, et al. P13, the EMBL macromolecular crystallography beamline at the low-emittance PETRA III ring for high- and low-energy phasing with variable beam focusing. J. Synchrotron. Radiat. 2017;24:323–332. doi: 10.1107/S1600577516016465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kabsch W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sparta KM, Krug M, Heinemann U, Mueller U, Weiss MS. XDSAPP2.0. J. Appl. Crystallogr. 2016;49:1085–1092. doi: 10.1107/S1600576716004416. [DOI] [Google Scholar]
- 55.McCoy AJ, et al. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 2006;62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- 57.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liebschner D, et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 2019;75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afonine PV, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karplus PA, Diederichs K. Linking crystallographic model and data quality. Science. 2012;336:1030–1033. doi: 10.1126/science.1218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malý M, Diederichs K, Dohnalek J, Kolenko P. Paired refinement under the control of PAIREF. IUCrJ. 2020;7:681–692. doi: 10.1107/S2052252520005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malý M, Diederichs K, Dohnalek J, Kolenko P. PAIREF: Paired refinement also for Phenix users. Acta Crystallogr. F Struct. Biol. Commun. 2021;77:226–229. doi: 10.1107/S2053230X21006129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morin, A. et al. Cutting Edge: Collaboration gets the most out of software. Elife2, e01456 (2013). [DOI] [PMC free article] [PubMed]
- 65.Eisenberg D, Schwarz E, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 1984;179:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- 66.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates and X-ray intensities generated in this study have been deposited in the Worldwide Protein Data Bank (wwPDB) under accession codes 7QIH (YscX50:YscY), 7QII (YscX32:YscY), and 7QIJ (YscVC:YscX32:YscY). Diffraction images are available from the SB Grid Data Bank64 (data.sbgrid.org) with Data ID 905 (YscX50:YscY), Data ID 906 (YscX32:YscY), and Data ID 907 (YscVC:YscX32:YscY). The structure of the YscVC nonamer used as search model to solve the phase problem of the YscVXY structure is available from the wwPDB under accession code 7ALW. The structures used to generate Figs. 8 and 9 are available from the wwPDB. Figure 8a: 2P58 (YscEFG); Fig. 8b: 3WXX (AcrH:AopB); Fig.9a: 7ALW (YscVC nonamer); Fig. 9b: 6CH3 (FlhA:FliS:FliC); Fig. 9c: 6CH2 (FlhA:FliT:FliD); Fig. 9d: 6WA9 (CdsV:CdsO). Source data are provided with this paper.