Abstract
Study Objectives:
Obstructive sleep apnea (OSA) in children is associated with acute metabolic, cardiovascular, and neurocognitive abnormalities. The long-term outcomes of childhood OSA into adulthood have not been established. We performed a 20-year follow-up of patients with polysomnography-documented OSA in childhood compared to a healthy control group to evaluate the long-term anthropometric, sleep, cognitive, and cardiovascular outcomes.
Methods:
Children diagnosed with severe OSA between ages 1 and 17 years (mean, 4.87 ± 2.77) were prospectively contacted by telephone as young adults after approximately 20 years. Data collected included reported anthropometric information, educational level, health history, and Berlin questionnaire scores.
Results:
Young adults with confirmed severe OSA in childhood had significantly higher adulthood body mass index (P = .038), fewer academic degrees (P < .001), and more snoring (P = .045) compared to control patients. The apnea-hypopnea index during childhood trended toward predicting cardiovascular outcomes and the results of the Berlin questionnaire in adulthood.
Conclusions:
Adults with a history of severe childhood OSA have a high risk of snoring, elevated body mass index, and lower academic achievement in adulthood. Thus, children with severe OSA may be at increased risk of chronic diseases later in life. The intervening coronavirus disease 2019 (COVID-19) pandemic has introduced considerable additional neurobehavioral morbidity complicating the identification of the full long-term consequences of childhood OSA.
Citation:
Nosetti L, Zaffanello M, Katz ES, et al. Twenty-year follow-up of children with obstructive sleep apnea. J Clin Sleep Med. 2022;18(6):1573–1581.
Keywords: children, obstructive sleep apnea, sleep-disordered breathing, COVID-19
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is a common cause of acute metabolic, cardiovascular, and neurocognitive sequelae in children. The long-term consequences of childhood obstructive sleep apnea into adulthood are unknown.
Study Impact: Adults with a history of severe childhood obstructive sleep apnea have a high risk of snoring, elevated body mass index, and lower academic achievement in adulthood. The apnea-hypopnea index during childhood trended toward predicting cardiovascular outcomes and the results of the Berlin questionnaire in adulthood.
INTRODUCTION
Obstructive sleep apnea (OSA) consists of a spectrum of abnormal breathing patterns during sleep characterized by a combination of snoring, increased upper airway resistance, electrocortical arousals, and/or gas exchange abnormalities.1 The prevalence of OSA in children is 1%–4%.2–4 The main risk factors for OSA in children are adenotonsillar hypertrophy,5 obesity, craniofacial abnormalities,6 and neuromuscular disease. OSA-associated morbidity includes elevated blood pressure, daytime sleepiness, learning problems, and growth failure.1,7,8 There is a correlation between the severity of OSA and hypertension9,10 and neurocognitive dysfunction.11
If untreated, pediatric OSA may lead to substantial morbidity that may not be completely reversible with available treatment.12 Thus, the adverse consequences may not simply be confined to the child’s immediate well-being and development but may continue to be detrimental to the patient’s long-term health into adulthood.13 Nevertheless, there is very little long-term outcome data on adults who experienced OSA in childhood. Therefore, the aim of the current study was to assess the predictive performance of the apnea-hypopnea index (AHI) evaluated during childhood in predicting health consequences in these children as adults after a 20-year follow-up.
METHODS
Study design and population
This was a case-control study with a prospective questionnaire to evaluate the clinical characteristics of adult patients who were diagnosed with severe OSA (AHI > 10 events/h) in childhood between 1996 and 2006. The study was conducted from January 2020–July 2020.
Data collection
Data collection was carried out in 3 phases. The first phase was to identify children with severe OSA diagnosed at the Sleep Disorders Laboratory, Department of Pediatrics, Insubria University, F. Del Ponte Hospital, Varese (Italy), between 1996 and 2006 (time zero; T0). The patients with OSA included in this study were otherwise typically developing children with no known genetic conditions, neurodevelopmental disorders, psychiatric disorders, or cardiac disease. The second phase was to administer a questionnaire to these patients as young adults in 2020 if they could be contacted (adult follow-up; T1). Finally, the third phase was to enroll young adults without a history of pediatric OSA as control patients.
Phase 1
At T0, the clinical data were collected from medical records including sex, age, height, weight, symptoms, parental history of OSA, exposure to passive smoke, and results of polysomnography (PSG).
Phase 2
At T1, an attempt was made to contact patients identified as having OSA as children using a telephone interview (∼20-year follow-up) to obtain age, height, weight, Berlin questionnaire results,14 educational level (academic achievement), treatment (surgery), and cardiovascular complications (myocardial infarction or hypertension). The complete telephone questionnaire is available in Appendices 1 and 2 in the supplemental material.
Phase 3
The control group participants were recruited from young adults in a family practice clinic in Lombardy, Italy. The inclusion criteria included the absence of symptoms of OSA in childhood and no previous PSG.
PSG
Overnight PSG was performed at T0 using a Healthdyne Technologies instrument (Alice 3, Marietta, GA). The channels recorded included nasal pressure (nasal cannulas), nasal flow (thermistor), chest/abdominal movement (inductive bands), pulse oximetry, electrocardiogram, and transcutaneous carbon dioxide. Sleep staging was based on data from electroencephalogram (channels: C4-M1, C3-M2, O1-M2, O2-M1, F4-M1, F3-M2), electrooculogram (ROC/M1, LOC/M2), and submental electromyogram. PSG also included audio/video recordings and a body position sensor. The sleep stage scoring criteria were derived from Kales and Rechtshaffen.15 An obstructive apnea was defined as a decrease in the thermistor of at least 90% lasting at least 2 breaths. An obstructive hypopnea was defined as a reduction in the thermistor of 50%–90% associated with either a 3% oxygen desaturation or an electroencephalogram arousal. An oxygen desaturation was defined as a 3% drop in the baseline oxygen saturation. The AHI was defined as the total number of obstructive apneas and hypopneas divided by the total sleep time.
The Berlin questionnaire is divided into questions regarding snoring, daytime somnolence, hypertension, and body mass index (BMI). Scores from the snoring and daytime somnolence categories were considered positive if the responses indicated frequent symptoms (> 3–4 times/week), whereas hypertension was considered present if there was a history of hypertension, and the BMI was considered elevated if it was > 30 kg/m2. Patients were scored as being at high risk for OSA if they had a positive score in 2 or more categories, and those who did not were scored as being at low risk.16 Obesity was defined as a BMI > 2 standard deviations from the mean for age.
Statistical analysis
Data were presented as n (%) or mean (standard deviation). Differences of categorical variables were analyzed using the chi-square test. Quantitative variables were compared using the Kruskal-Wallis test. No missing values were observed. AHI was categorized based on tertiles as follows: first tertile 10 < AHI ≤ 13 events/h, second tertile 13 < AHI ≤ 20 events/h, third tertile AHI > 20 events/h.
The performance of AHI in predicting cardiovascular complications, Berlin questionnaire score ≥ 2, and obesity was assessed using a receiver operating characteristic (ROC) analysis.17 Estimation of the area under the curve was performed using nonparametric ROC analysis and significance was tested using the method described by DeLong et al.18 Moreover, to avoid overrating the test performance in the ROC analysis, we performed a 5-fold cross validation.19 Analyses were performed using R 3.6.2 software (GNU General Public License, Boston, MA). A P value < .05 was considered statistically significant. In addition, a multiple-variable logistic mixed model was fitted to predict 2 positive values on the Berlin questionnaire using the predictor variables of BMI, sex, and AHI. Another multiple-variable logistic mixed model was fitted to predict hypertension using the predictor variables of BMI, sex, and AHI.
RESULTS
Characteristics of study population
There were 180 children from our hospital identified to have had severe OSA between 1996 and 2006, and 100 (55.5%) were successfully contacted and enrolled in the study. No differences were found between patients lost to follow-up and those who completed the study (Table S1 (538KB, pdf) in the supplemental material). At T0, the mean age at OSA diagnosis was 4.87 (± 2.77) years. At T1, the mean age at follow-up was 23.58 (± 4.04) years. Table 1 presents the T0 baseline demographic, clinical, and polysomnographic information.
Table 1.
Baseline characteristics of children with severe OSA at baseline (T0).
| Characteristics | Number |
|---|---|
| Physical Characteristics | |
| n | 100 |
| Males, n (%) | 51 (51.00) |
| Age, y, mean (SD) | 4.87 (2.77) |
| Height, cm, mean (SD) | 105.95 (16.70) |
| Weight, kg, mean (SD) | 20.28 (14.69) |
| BMI, kg/m2, mean (SD) | 16.64 (4.59) |
| Obesity, n (%) | 16 (16.00) |
| AHI, events/h, mean (SD) | 19.91 (11.37) |
| Parental/caregiver risk and treatment | |
| Parental history of OSA, n (%) | 26 (26.00) |
| Cigarette smoke exposure, n (%) | 32 (32.00) |
| Tonsil and/or adenoid surgery, n (%) | 90 (90.00) |
| Reported symptoms | |
| Nocturnal snoring, n (%) | 96 (96.00) |
| Oral or mixed breath, n (%) | 93 (93.00) |
| Apneas, n (%) | 90 (90.00) |
| Nasal voice, n (%) | 76 (76.00) |
| Rhinitis, n (%) | 68 (68.00) |
| Nocturnal movements, n (%) | 67 (67.00) |
| URI, n (%) | 64 (64.00) |
| Nocturnal cough, n (%) | 44 (44.00) |
| Nocturnal anxiety, n (%) | 43 (43.00) |
| Irritability, n (%) | 41 (41.00) |
| Feeling of suffocation, n (%) | 39 (39.00) |
| Wheezing, n (%) | 34 (34.34) |
| Enuresis, n (%) | 30 (30.00) |
| Drowsiness, n (%) | 25 (25.00) |
| Insomnia, n (%) | 24 (24.00) |
| Diurnal headache, n (%) | 22 (22.00) |
| Growth delay (height ≤ fifth percentile), n (%) | 16 (16.00) |
| School problems, n (%) | 7 (7.00) |
| Cyanosis, n (%) | 5 (5.00) |
AHI = apnea-hypopnea index, BMI = body mass index, OSA = obstructive sleep apnea, SD = standard deviation, T0 = baseline (time zero), URI = upper respiratory infection.
Table 2 reports the characteristics of the children with severe OSA at T0 categorized according the AHI tertiles. At T0, obesity was statistically significant higher in the third tertile (P = .023). No baseline symptoms were statistically significantly different between the AHI tertiles. Drowsiness and nocturnal anxiety trended higher in children with more severe OSA but did not reach statistical significance (P = .058 and P = .062, respectively).
Table 2.
Characteristics of the study sample at baseline (T0) stratified by AHI severity tertiles.
| Characteristics | First Tertile AHI (10–13 events/h) | Second Tertile AHI (13–20 events/h) | Third Tertile AHI (> 20 events/h) | P |
|---|---|---|---|---|
| n | 35 | 31 | 34 | |
| Sex, male, n (%) | 15 (42.86) | 15 (48.39) | 21 (61.76) | .274 |
| Age, y, mean (SD) | 4.60 (2.45) | 4.87 (2.68) | 5.15 (3.19) | .773 |
| Height, cm, mean (SD) | 105.46 (16.30) | 105.61 (13.79) | 106.76 (19.73) | .946 |
| Weight, kg, mean (SD) | 18.50 (10.16) | 19.08 (10.66) | 23.21 (20.59) | .771 |
| BMI z score | –0.16 (1.40) | –0.13 (1.77) | 0.66 (2.02) | .248 |
| Obesity, n (%) | 2 (5.71) | 4 (12.90) | 10 (29.41) | .023 |
| Polysomnography | ||||
| Average SpO2, mean (SD) | 96.38 (1.50) | 95.75 (1.76) | 95.20 (2.41) | .016 |
| Minimum SpO2, mean (SD) | 76.60 (7.77) | 75.74 (7.24) | 66.65 (10.74) | < .001 |
| ODI, events/h, mean (SD) | 105.63 (42.41) | 127.77 (40.24) | 222.85 (93.96) | < .001 |
| AHI, events/h, mean (SD) | 11.49 (1.13) | 16.27 (1.88) | 31.91 (12.14) | < .001 |
| Parent/caregiver risk | ||||
| Parental OSA history, n (%) | 11 (31.43) | 10 (32.26) | 5 (14.71) | .181 |
| Parental smoking, n (%) | 10 (28.57) | 12 (38.71) | 10 (29.41) | .627 |
| Children’s symptoms | ||||
| Oral/mixed breathing, n (%) | 32 (91.43) | 27 (87.10) | 34 (100.00) | .114 |
| Nocturnal snoring, n (%) | 34 (97.14) | 28 (90.32) | 34 (100.00) | .126 |
| Obstructive apneas, n (%) | 34 (97.14) | 27 (87.10) | 29 (85.29) | .211 |
| Nasal voice, n (%) | 27 (77.14) | 24 (77.42) | 25 (73.53) | .917 |
| Nocturnal movements, n (%) | 22 (62.86) | 19 (61.29) | 26 (76.47) | .349 |
| Rhinitis, n (%) | 26 (74.29) | 18 (58.06) | 24 (70.59) | .342 |
| URI, n (%) | 24 (68.57) | 19 (61.29) | 21 (61.76) | .783 |
| Nocturnal anxiety, n (%) | 17 (48.57) | 8 (25.81) | 18 (52.94) | .062 |
| Irritability, n (%) | 11 (31.43) | 14 (45.16) | 16 (47.06) | .356 |
| Wheezing, n (%) | 13 (37.14) | 8 (25.81) | 13 (39.39) | .473 |
| Drowsiness, n (%) | 8 (22.86) | 4 (12.90) | 13 (38.24) | .058 |
| Feeling of suffocation, n (%) | 13 (37.14) | 14 (45.16) | 12 (35.29) | .690 |
| Diurnal headache, n (%) | 7 (20.00) | 5 (16.13) | 10 (29.41) | .408 |
| Enuresis, n (%) | 9 (25.71) | 11 (35.48) | 10 (29.41) | .685 |
| Insomnia, n (%) | 12 (34.29) | 5 (16.13) | 7 (20.59) | .192 |
| Height ≤ fifth percentile, n (%) | 5 (14.29) | 5 (16.13) | 6 (17.65) | .930 |
| School problems, n (%) | 1 (2.86) | 2 (6.45) | 4 (11.76) | .346 |
| Cyanosis, n (%) | 3 (8.57) | 1 (3.23) | 1 (2.94) | .485 |
AHI = apnea-hypopnea index, BMI = body mass index, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, SD = standard deviation, SpO2 = oxygen saturation, T0 = baseline (time zero), URI = upper respiratory infection.
Table 3 reports the characteristics of enrolled patients with OSA recruited by telephone interview at follow-up (T1). The Berlin questionnaire score (score ≥ 2; %) at T1 was significantly higher in patients categorized in the third childhood AHI tertile (20.6%; P = .003). The cardiovascular complications trended higher with more severe OSA but did not reach statistical significance (14.7%; P = .095). All other follow-up variables at T1 were comparable among childhood AHI tertiles. There were no differences in outcomes based on treatment received. Of the 8 young adults with childhood OSA who had obesity at T1, only 1 was also obese at T0. Of the 17 patients with AHI ≥ 13 events/h who had drowsiness at T0, only 4 had drowsiness at T1.
Table 3.
Characteristics of the study population after 20-year follow-up categorized according to AHI tertiles obtained at T0.
| Characteristics | First Tertile AHI (10–13 events/h) | Second Tertile AHI (13–20 events/h) | Third Tertile AHI (> 20 events/h) | P |
|---|---|---|---|---|
| n | 35 | 31 | 34 | |
| Age, y, mean (SD) | 23.63 (3.61) | 23.45 (3.94) | 23.65 (4.63) | .978 |
| Height, cm, mean (SD) | 169.54 (10.83) | 170.77 (9.85) | 171.3 (10.01) | .756 |
| Weight, kg, mean (SD) | 65.74 (10.83) | 66.10 (15.20) | 72.09 (16.42) | .128 |
| BMI, kg/m2, mean (SD) | 22.93 (3.76) | 22.88 (4.90) | 24.39 (3.85) | .248 |
| Obesity, n (%) | 1 (2.86) | 4 (12.90) | 3 (8.82) | .316 |
| Education | 24 (68.57) | 22 (70.97) | 22 (64.71) | .861 |
| Snoring, n (%) | 11 (31.43) | 9 (29.03) | 17 (50.00) | .151 |
| Your snoring is: | .256 | |||
| Slightly louder than breathing, n (%) | 8 (66.67) | 3 (33.33) | 6 (35.29) | |
| Louder than talking, n (%) | 2 (16.67) | 6 (66.67) | 8 (47.06) | |
| As loud as talking, n (%) | 2 (16.67) | 0 (0.00) | 3 (17.76) | |
| How often do you snore? | .124 | |||
| 1–2 times/wk, n (%) | 3 (25.00) | 4 (44.44) | 4 (23.53) | |
| 3–4 times/wk, n (%) | 8 (66.67) | 1 (11.11) | 8 (47.06) | |
| Almost every day, n (%) | 1 (8.33) | 4 (44.44) | 5 (29.41) | |
| Has your snoring ever bothered other people? | 4 (33.33) | 6 (66.67) | 9 (52.94) | .302 |
| Has anyone noticed that you stop breathing during sleep? | .558 | |||
| 1–2 times/wk, n (%) | 2 (5.71) | 3 (9.68) | 4 (11.76) | |
| 1–2 times/mo, n (%) | 0 (0.00) | 0 (0.00) | 1 (2.94) | |
| 3–4 times/wk, n (%) | 0 (0.00) | 0 (0.00) | 1 (2.94) | |
| Rarely or never, n (%) | 33 (94.29) | 28 (90.32) | 28 (82.35) | |
| Berlin questionnaire score ≥ 2, n (%) | 0 (0.00) | 1 (3.23) | 7 (20.59) | .003 |
| Treatments | ||||
| Adenotonsillectomy, n (%) | 23 (65.71) | 20 (64.52) | 21 (61.76) | .941 |
| Tonsillectomy, n (%) | 1 (2.86) | 1 (3.23) | 4 (11.76) | .219 |
| Adenoidectomy, n (%) | 7 (20.00) | 7 (22.58) | 6 (17.65) | .884 |
| No treatment, n (%) | 4 (11.43) | 3 (9.68) | 3 (8.82) | .935 |
| Cardiovascular sequelae, n (%) | 1 (2.86) | 1 (3.23) | 5 (14.71) | .095 |
AHI = apnea-hypopnea index, BMI = body mass index, SD = standard deviation, T0 = baseline (time zero).
Table 4 presents the demographic, anthropometric, symptom, treatment, and health outcomes of patients and control patients in adulthood at T1. Patients with confirmed severe OSA in childhood had significantly higher adulthood BMI (P = .038) and fewer academic degrees (P < .001). Among reported symptoms, nocturnal snoring was more frequent in adult patients (P = .045), particularly both slightly and louder snoring (P < .001) than in control patients. Regarding treatment, history of childhood surgery (adenotonsillectomy or adenoidectomy) was more frequent in patients than in control patients (P < .005). Surprisingly, nocturnal anxiety (P < .001), insomnia (P < .025), drowsiness (P < .001), and irritability (P < .001) were more commonly reported in control patients. Of the 16 patients who had growth delay at T0, only 1 developed obesity at T1. The academic achievements of these 16 children were significantly different from that of the remaining 84 children; in particular, children with growth delay more frequently had less academic experience (ie, less than 8 years of education; 68.75% vs 25.00%; P = .002).
Table 4.
Demographics, physical characteristics, symptoms during sleep and daytime, treatment, and health complications of adult patients and control patients.
| Characteristics | Patients | Control Patients | P |
|---|---|---|---|
| n | 100 | 100 | — |
| Age, y, mean (SD) | 23.58 (4.04) | 24.17 (3.88) | .337 |
| Sex, male, n (%) | 53 (53.00) | 44 (44.00) | .258 |
| Weight, kg, mean (SD) | 68.01 (14.45) | 65.22 (14.35) | .161 |
| Height, cm, mean (SD) | 170.54 (10.18) | 171.00 (10.06) | .983 |
| BMI, kg/m2, mean (SD) | 23.33 (4.19) | 22.10 (3.28) | .038 |
| Academic degree, n (%) | < .001 | ||
| Primary school | 1 (1.00) | 0 (0.00) | |
| Middle school | 31 (31.00) | 8 (8.00) | |
| High school | 47 (47.00) | 60 (60.00) | |
| College degree | 21 (21.00) | 32 (32.00) | |
| Snoring, n (%) | 37 (37.00) | 23 (23.00) | .045 |
| Your snoring is: | .013 | ||
| Slightly louder than breathing | 17 (44.74) | 15 (65.22) | |
| As loud as talking | 4 (10.53) | 6 (26.09) | |
| Louder than talking | 17 (44.74) | 2 (8.70) | |
| How often do you snore? | < .001 | ||
| Almost every day | 10 (26.32) | 4 (15.38) | |
| 3–4 times/wk | 17 (44.74) | 6 (23.08) | |
| 1–2 times/wk | 11 (28.95) | 5 (19.23) | |
| 1–2 times/mo | 0 (0.00) | 6 (23.08) | |
| Never | 0 (0.00) | 5 (19.23) | |
| Snoring bothered other people? | 19 (50.00) | 13 (50.00) | > .99 |
| Stop breathing during sleep?, n (%) | .048 | ||
| 1–2 times/wk | 9 (9.00) | 1 (1.00) | |
| 1–2 times/mo | 1 (1.00) | 3 (3.00) | |
| 3–4 times/wk | 1 (1.00) | 0 (0.00) | |
| Rarely or never | 89 (89.00) | 96 (96.00) | |
| Berlin questionnaire score ≥ 2, n (%) | 8 (8.00) | 2 (2.00) | .101 |
| Nocturnal anxiety, n (%) | 2 (2.00) | 29 (29.00) | < .001 |
| Insomnia, n (%) | 19 (19.00) | 34 (34.00) | .025 |
| Drowsiness, n (%) | 18 (18.00) | 53 (53.00) | < .001 |
| Irritability, n (%) | 19 (19.00) | 43 (43.00) | < .001 |
| Adenotonsillectomy, n (%) | 64 (64.00) | 2 (2.00) | < .001 |
| Tonsillectomy, n (%) | 6 (6.00) | 1 (1.00) | .124 |
| Adenoidectomy, n (%) | 20 (20.00) | 5 (5.00) | .003 |
| Hypercholesterolemia, n (%) | 2 (2.00) | 10 (10.00) | .037 |
| Cardiovascular sequelae, n (%) | 7 (7.00) | 5 (5.00) | .766 |
BMI = body mass index, SD = standard deviation.
ROC/regression analysis
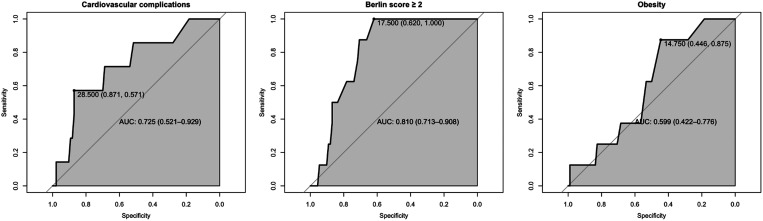
The area under the curve and 95% confidence interval (CI) for the T0 AHI in predicting T1 cardiovascular complications, obesity, and Berlin questionnaire scores ≥ 2 are presented in Figure 1. Good predictive performances of AHI at T0 were found for Berlin questionnaire scores ≥ 2 (0.81; 95% CI, 0.71–0.91) and cardiovascular complications (0.725; 95% CI, 0.52–0.93) at the T1 20-year follow-up. The multiple-variable logistic mixed model to predict 2 positive values on Berlin questionnaire scores, accounting for BMI and sex, showed that the AHI yielded an odds ratio of 1.185 with a 95% CI of 1.029–1.365. The multiple-variable logistic mixed model to predict hypertension, accounting for BMI and sex, showed that the AHI yielded an odds ratio of 1.152 with a 95% CI of 0.999–1.333.
Figure 1. Receiver operating characteristic curves for AHI.
Performance of AHI severity during childhood in predicting cardiovascular complications, Berlin questionnaire score ≥ 2, and obesity at 20-year follow-up. AHI = apnea-hypopnea index, AUC = area under the curve.
DISCUSSION
We observed that obesity was more common in children with more severe OSA at baseline (T0), as expected. At T1 follow-up (approximately 20 years later), children who had had the most severe OSA no longer had a higher BMI relative to children with more mild OSA, but did have a significantly higher Berlin questionnaire score, indicating more sleep-disordered breathing symptoms. Compared to control patients, patients with childhood OSA at follow-up had higher BMI, more snoring, and lower academic achievements. However, most children with OSA did not have obesity as young adults. The AHI at T0 trended toward predicting the T1 long-term follow-up report for both the Berlin questionnaire (score ≥ 2) and cardiovascular complications. Interestingly, control patients had more nocturnal anxiety, insomnia, drowsiness, and irritability, which we hypothesize to have occurred because they were recruited during the coronavirus disease 2019 (COVID-19) pandemic, whereas patients with childhood OSA were interviewed mostly prepandemic.
In our sample of enrolled children with OSA, a family history of sleep-disordered breathing was present in 26%, which did not differ among AHI tertiles. Family studies have shown that relatives of patients with OSA have a 2- to 4-fold increased risk of developing OSA compared with control patients because of genetic risk factors including obesity.20 The prevalence of OSA ranges from 13%–55% in children with obesity.21,22 In our study of children with severe OSA, 16% were obese, with a significantly higher number at 29.4% for patients in the third AHI tertile (AHI > 20 events/h). At follow-up, the percentage of patients with OSA with obesity decreased to 8%, but the average BMI at T1 was greater than that of the control patients. The incidence of snoring also decreased over time from 96% during childhood to 36% after a 20-year follow-up. It is not surprising to see such improvement; the AHI has also been reported to decrease after a 5%–10% weight loss.23 However, despite the reduction in snoring and obesity in the highest AHI tertile over the study period, there was a persistence in increased sleep-related breathing disorders, as evidenced by the higher Berlin questionnaire scores.
The Berlin questionnaire is a widely used questionnaire for OSA and has been reported to have a high sensitivity and specificity for OSA in adults.24 Interestingly, young adults with a history of very severe childhood OSA (third tertile; AHI > 20 events/h) had a significantly higher Berlin questionnaire score (20.6%, P = .003) compared with children with less severe OSA, along with control patients.
Childhood OSA is a strong risk factor for neurobehavioral problems, such as sleepiness, impaired attention, hyperactivity, learning disorders, memory impairment, poor academic performance, and depression.11,25,26 Although excessive daytime sleepiness is frequently reported by adults with OSA, it occurs less frequently in children.27 Calhoun et al28 reported that excessive daytime sleepiness was mainly associated with obesity, asthma, anxiety/depression, and difficulty falling asleep, but not OSA. On the other hand, Liu et al29 reported that daytime sleepiness mediated the relationship between OSA symptoms and depression, loneliness, and poor school performance in Chinese children. Children with parent-reported OSA symptoms may be at high risk for poor progress in reading, writing, and math30,31 and experience unsatisfactory progress/learning problems.31 In our data, children with OSA achieved a lower level of academic achievement compared to control patients.
In total, 90% of young adult patients reported having airway surgery after a diagnosis of OSA in childhood. Nevertheless, young adults with childhood OSA had worse sleep respiratory symptoms at night than adult control patients. Specifically, young adults with childhood OSA had more snoring (37% vs 23%; P = .045), more snoring that was louder than talking (44.7% vs 8.7%; P = .013), and more apneas (P = .048) compared to the control group. Previous work has also shown that children likely to manifest persistent OSA after surgical removal of enlarged upper airway lymphoid tissues include those with severe baseline OSA.32
Our finding that adult control patients experienced more nocturnal anxiety (29% vs 2%; P < .001), insomnia (34% vs 19%; P = .025), drowsiness (53% vs 18%; P < .001), and irritability (43% vs 19%; P < .001) compared to adults with childhood OSA was initially surprising. However, the patients with OSA were surveyed before the COVID-19 pandemic, whereas the control patients were interviewed during the pandemic, which severely affected Lombardy, Italy.33 The consequences of the COVID-19 pandemic have included social isolation, reduced sleep quality, increased anxiety and mental stress, weight gain, and sleep deprivation.34,35 Thus, there may be an overlap between the psychological consequences of OSA36–38 and mental health problems secondary to the COVID-19 pandemic (depression and anxiety).39 Similarly, a survey conducted among French adults showed an increased rate of sleep problems from 49% in 2017 to 74% after the lockdown (May 7–10, 2020), suggesting that the pandemic may have a long-lasting psychological impact.40 During the first period of the COVID-19 pandemic, young people reported mental distress, particularly among females (78.09%) and students (66.82%).41 Furthermore, a high percentage of respondents to a survey conducted during the pandemic presented with anxiety and anxiety-depressive disorders.42
Little long-term follow-up data have been presented regarding cardiovascular outcomes after pediatric OSA. Walter et al43 reported that the parasympathetic activity derived from heart rate variability analysis decreased in children with OSA after resolution of their OSA but increased in children with persistent OSA after 3 years. Similarly, Chan et al44 found that children with moderate-severe OSA early in life had an elevated nocturnal systolic blood pressure and less blood pressure dipping overnight after a 10-year follow-up. In the present study, the prognostic performance of AHI severity during childhood in predicting cardiovascular complications in adulthood was confirmed by ROC analysis. However, we found no differences in the frequency of cardiovascular complications between young adults with childhood OSA and control patients. Perhaps control patients recruited directly from a medical clinic are more likely than the general population to have had cardiovascular complications.
Limitations
This study was limited by the unfortunate occurrence of the COVID-19 pandemic during data collection. As a result of the pandemic lockdown, participants noted decreased physical activity (reported by 53% of the participants), increased sedentary time (reported by 63%), increased sleeping hours,45 and increased snacking.46 We cannot exclude the possibility that the increased incidence of hypercholesterolemia present in the control patients resulted from dysregulated nutrition and/or reduced physical activity during the initial period of the pandemic. Other limitations include the requirement that control patients accurately report their childhood OSA symptom status, that control patients were recruited from a family medicine practice and therefore may have been biased toward people with medical illnesses, and the lack of objective testing obtained at follow-up. We do not have specific socioeconomic or parental education data on the parents of the control group participants, and therefore the interpretation of academic performance between the groups is limited. The strength of the study is that it is the first study to document the long-term young-adult outcomes of severe childhood OSA.
CONCLUSIONS
Adults with a history of severe childhood OSA have a higher risk of having snoring, elevated BMI, and lower academic achievement in adulthood. An elevated childhood AHI is associated with both cardiovascular complications and a Berlin questionnaire score ≥ 2 in young adulthood. Thus, children with severe OSA may be at increased risk of chronic diseases later in life. The intervening COVID-19 pandemic has introduced considerable additional neurobehavioral morbidity, limiting the identification of the full long-term consequences of childhood OSA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Pediatric Sleep Disorders Center, Department of Pediatrics, F. Del Ponte Hospital, Insubria University, Varese, Italy. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- OSA
obstructive sleep apnea
- PSG
polysomnography
- ROC
receiver operating characteristic
- T0
baseline (time zero)
- T1
20-year follow-up
REFERENCES
- 1. Kaditis A , Kheirandish-Gozal L , Gozal D . Algorithm for the diagnosis and treatment of pediatric OSA: a proposal of two pediatric sleep centers . Sleep Med. 2012. ; 13 ( 3 ): 217 – 227 . [DOI] [PubMed] [Google Scholar]
- 2. Lumeng JC , Chervin RD . Epidemiology of pediatric obstructive sleep apnea . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 242 – 252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spilsbury JC , Storfer-Isser A , Rosen CL , Redline S . Remission and incidence of obstructive sleep apnea from middle childhood to late adolescence . Sleep. 2015. ; 38 ( 1 ): 23 – 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunetti L , Rana S , Lospalluti ML , et al . Prevalence of obstructive sleep apnea syndrome in a cohort of 1,207 children of southern Italy . Chest. 2001. ; 120 ( 6 ): 1930 – 1935 . [DOI] [PubMed] [Google Scholar]
- 5. Nosetti L , Zaffanello M , De Bernardi F , et al . Age and upper airway obstruction: a challenge to the clinical approach in pediatric patients . Int J Environ Res Public Health. 2020. ; 17 ( 10 ): 3531 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaffanello M , Antoniazzi F , Tenero L , Nosetti L , Piazza M , Piacentini G . Sleep-disordered breathing in paediatric setting: existing and upcoming of the genetic disorders . Ann Transl Med. 2018. ; 6 ( 17 ): 343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaffanello M , Piacentini G , La Grutta S . Beyond the growth delay in children with sleep-related breathing disorders: a systematic review . Panminerva Med. 2020. ; 62 ( 3 ): 164 – 175 . [DOI] [PubMed] [Google Scholar]
- 8. Zaffanello M , Piacentini G , La Grutta S . The cardiovascular risk in paediatrics: the paradigm of the obstructive sleep apnoea syndrome . Blood Transfus. 2020. ; 18 ( 3 ): 217 – 225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang K-T , Chiu S-N , Weng W-C , Lee P-L , Hsu W-C . Analysis of 24-hour ambulatory blood pressure monitoring in children with obstructive sleep apnea: a hospital-based study . Medicine (Baltimore). 2015. ; 94 ( 40 ): e1568 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhattacharjee R , Kheirandish-Gozal L , Pillar G , Gozal D . Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children . Prog Cardiovasc Dis. 2009. ; 51 ( 5 ): 416 – 433 . [DOI] [PubMed] [Google Scholar]
- 11. Hunter SJ , Gozal D , Smith DL , Philby MF , Kaylegian J , Kheirandish-Gozal L . Effect of sleep-disordered breathing severity on cognitive performance measures in a large community cohort of young school-aged children . Am J Respir Crit Care Med. 2016. ; 194 ( 6 ): 739 – 747 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tauman R , Gozal D . Obstructive sleep apnea syndrome in children . Expert Rev Respir Med. 2011. ; 5 ( 3 ): 425 – 440 . [DOI] [PubMed] [Google Scholar]
- 13. Dehlink E , Tan HL . Update on paediatric obstructive sleep apnoea . J Thorac Dis. 2016. ; 8 ( 2 ): 224 – 235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan A , Yin JDC , Tan LWL , van Dam RM , Cheung YY , Lee C-H . Using the Berlin questionnaire to predict obstructive sleep apnea in the general population . J Clin Sleep Med. 2017. ; 13 ( 3 ): 427 – 432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kales AR , Rechtshaffen A . A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington, DC: : US Government Printing Office; ; 1968. . [Google Scholar]
- 16. Netzer NC , Stoohs RA , Netzer CM , Clark K , Strohl KP . Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome . Ann Intern Med. 1999. ; 131 ( 7 ): 485 – 491 . [DOI] [PubMed] [Google Scholar]
- 17. Hajian-Tilaki K . Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation . Caspian J Intern Med. 2013. ; 4 ( 2 ): 627 – 635 . [PMC free article] [PubMed] [Google Scholar]
- 18. DeLong ER , DeLong DM , Clarke-Pearson DL . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach . Biometrics. 1988. ; 44 ( 3 ): 837 – 845 . [PubMed] [Google Scholar]
- 19. Cilluffo G , Fasola S , Ferrante G , et al . Overrating classifier performance in ROC analysis in the absence of a test set: evidence from simulation and Italian CARATkids validation . Methods Inf Med. 2019. ; 58 ( S 02 ): e27 – 42 . [DOI] [PubMed] [Google Scholar]
- 20. McNamara F , Sullivan CE . Pediatric origins of adult lung diseases. 3: the genesis of adult sleep apnoea in childhood . Thorax. 2000. ; 55 ( 11 ): 964 – 969 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sánchez-López AM , Noack-Segovia JP , Núñez-Negrillo AM , Latorre-García J , Aguilar-Cordero MJ . Childhood obesity and its influence on sleep disorders: Kids-Play Study . Int J Environ Res Public Health. 2020. ; 17 ( 21 ): 7948 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verhulst SL , Van Gaal L , De Backer W , Desager K . The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents . Sleep Med Rev. 2008. ; 12 ( 5 ): 339 – 346 . [DOI] [PubMed] [Google Scholar]
- 23. Canapari CA , Hoppin AG , Kinane TB , Thomas BJ , Torriani M , Katz ES . Relationship between sleep apnea, fat distribution, and insulin resistance in obese children . J Clin Sleep Med. 2011. ; 7 ( 3 ): 268 – 273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung F , Yegneswaran B , Liao P , et al . Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients . Anesthesiology. 2008. ; 108 ( 5 ): 822 – 830 . [DOI] [PubMed] [Google Scholar]
- 25. Budhiraja R , Quan SF . Outcomes from the Tucson Children’s Assessment of Sleep Apnea Study (TuCASA) . Sleep Med Clin. 2009. ; 4 ( 1 ): 9 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsukada E , Kitamura S , Enomoto M , et al . Prevalence of childhood obstructive sleep apnea syndrome and its role in daytime sleepiness . PLoS One. 2018. ; 13 ( 10 ): e0204409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gozal D , Wang M , Pope DW Jr . Objective sleepiness measures in pediatric obstructive sleep apnea . Pediatrics. 2001. ; 108 ( 3 ): 693 – 697 . [DOI] [PubMed] [Google Scholar]
- 28. Calhoun SL , Vgontzas AN , Fernandez-Mendoza J , et al . Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: the role of obesity, asthma, anxiety/depression, and sleep . Sleep. 2011. ; 34 ( 4 ): 503 – 507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu J , Liu X , Ji X , Wang Y , Zhou G , Chen X . Sleep disordered breathing symptoms and daytime sleepiness are associated with emotional problems and poor school performance in children . Psychiatry Res. 2016. ; 242 : 218 – 225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harding R , Haszard JJ , Schaughency E , Drummond B , Galland B . Parent report of children’s sleep disordered breathing symptoms and limited academic progress in reading, writing, and math . Sleep Med. 2020. ; 65 : 105 – 112 . [DOI] [PubMed] [Google Scholar]
- 31. Galland B , Spruyt K , Dawes P , McDowall PS , Elder D , Schaughency E . Sleep disordered breathing and academic performance: a meta-analysis . Pediatrics. 2015. ; 136 ( 4 ): e934 – e946 . [DOI] [PubMed] [Google Scholar]
- 32. Gozal D , Tan H-L , Kheirandish-Gozal L . Treatment of obstructive sleep apnea in children: handling the unknown with precision . J Clin Med. 2020. ; 9 ( 3 ): 888 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alicandro G , Remuzzi G , La Vecchia C . Italy’s first wave of the COVID-19 pandemic has ended: no excess mortality in May, 2020 . Lancet. 2020. ; 396 ( 10253 ): e27 – e28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mello MTD , Silva A , Guerreiro RC , et al . Sleep and COVID-19: considerations about immunity, pathophysiology, and treatment . Sleep Sci. 2020. ; 13 ( 3 ): 199 – 209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mazza C , Ricci E , Biondi S , et al . A nationwide survey of psychological distress among Italian people during the COVID-19 pandemic: immediate psychological responses and associated factors . Int J Environ Res Public Health. 2020. ; 17 ( 9 ): 3165 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rezaeitalab F , Moharrari F , Saberi S , Asadpour H , Rezaeetalab F . The correlation of anxiety and depression with obstructive sleep apnea syndrome . J Res Med Sci. 2014. ; 19 ( 3 ): 205 – 210 . [PMC free article] [PubMed] [Google Scholar]
- 37. Frangopoulos F , Zannetos S , Nicolaou I , et al . The complex interaction between the major sleep symptoms, the severity of obstructive sleep apnea, and sleep quality . Front Psychiatry. 2021. ; 12 : 630162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akberzie W , Hesselbacher S , Aiyer I , Surani S , Surani ZS . The prevalence of anxiety and depression symptoms in obstructive sleep apnea . Cureus. 2020. ; 12 ( 10 ): e11203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nasserie T , Hittle M , Goodman SN . Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review . JAMA Netw Open. 2021. ; 4 ( 5 ): e2111417 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peretti-Watel P , Alleaume C , Léger D , Beck F , Verger P ; COCONEL Group . Anxiety, depression and sleep problems: a second wave of COVID-19 . Gen Psychiatr. 2020. ; 33 ( 5 ): e100299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rens E , Smith P , Nicaise P , Lorant V , Van den Broeck K . Mental distress and its contributing factors among young people during the first wave of COVID-19: a Belgian survey study . Front Psychiatry. 2021. ; 12 : 575553 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Htun YM , Thiha K , Aung A , et al . Assessment of depressive symptoms in patients with COVID-19 during the second wave of epidemic in Myanmar: a cross-sectional single-center study . PLoS One. 2021. ; 16 ( 6 ): e0252189 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walter LM , Biggs SN , Nisbet LC , et al . Improved long-term autonomic function following resolution of sleep-disordered breathing in preschool-aged children . Sleep Breath. 2016. ; 20 ( 1 ): 309 – 319 . [DOI] [PubMed] [Google Scholar]
- 44. Chan KC , Au CT , Hui LL , Wing YK , Li AM . Childhood OSA is an independent determinant of blood pressure in adulthood: longitudinal follow-up study . Thorax. 2020. ; 75 ( 5 ): 422 – 431 . [DOI] [PubMed] [Google Scholar]
- 45. Galali Y . The impact of COVID-19 confinement on the eating habits and lifestyle changes: a cross sectional study . Food Sci Nutr. 2021. ; 9 ( 4 ): 2105 – 2113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deschasaux-Tanguy M , Druesne-Pecollo N , Esseddik Y , et al . Diet and physical activity during the coronavirus disease 2019 (COVID-19) lockdown (March-May 2020): results from the French NutriNet-Santé cohort study . Am J Clin Nutr. 2021. ; 113 ( 4 ): 924 – 938 . [DOI] [PMC free article] [PubMed] [Google Scholar]