This epidemiologic follow-up study investigates the long-term findings of the Age-Related Macular Degeneration Study 2 (AREDS2) trial regarding lung cancer development and progression to age-related macular degeneration.
Key Points
Question
What were the long-term findings of Age-Related Eye Disease Study 2 (AREDS2) supplements regarding development of lung cancer or progression to late age-related macular degeneration (AMD)?
Findings
In this epidemiologic follow-up study of the AREDS2 cohort of 3882 participants and 6351 eyes, 10-year follow-up results showed that development of lung cancer nearly doubled in participants assigned to beta carotene among former smokers but not those assigned to lutein/zeaxanthin. Lutein/zeaxanthin was associated with a reduction in the risk of progression to late AMD when compared with beta carotene.
Meaning
These findings suggest that the AREDS2 supplement with lutein/zeaxanthin instead of beta carotene was safe, with no association with developing lung cancer and a potential beneficial association with further reduction in progression to late AMD.
Abstract
Importance
After the Age-Related Eye Disease Study 2 (AREDS2) study, the beta carotene component was replaced by lutein/zeaxanthin for the development of the revised AREDS supplement. However, it is unknown if the increased risk of lung cancer observed in those assigned beta carotene persists beyond the conclusion of the AREDS2 trial and if there is a benefit of adding lutein/zeaxanthin to the original AREDS supplement that can be observed with long-term follow-up.
Objective
To assess 10-year risk of developing lung cancer and late age-related macular degeneration (AMD).
Design, Setting, and Participants
This was a multicenter epidemiologic follow-up study of the AREDS2 clinical trial, conducted from December 1, 2012, to December 31, 2018. Included in the analysis were participants with bilateral or unilateral intermediate AMD for an additional 5 years after clinical trial. Eyes/participants were censored at the time of late AMD development, death, or loss to follow-up. Data were analyzed from November 2019 to March 2022.
Interventions
During the clinical trial, participants were randomly assigned primarily to lutein/zeaxanthin and/or ω-3 fatty acids or placebo and secondarily to no beta carotene vs beta carotene and low vs high doses of zinc. In the epidemiologic follow-up study, all participants received AREDS2 supplements with lutein/zeaxanthin, vitamins C and E, and zinc plus copper. Outcomes were assessed at 6-month telephone calls. Analyses of AMD progression and lung cancer development were conducted using proportional hazards regression and logistic regression, respectively.
Main Outcomes and Measures
Self-reported lung cancer and late AMD validated with medical records.
Results
This study included 3882 participants (mean [SD] baseline age, 72.0 [7.7] years; 2240 women [57.7%]) and 6351 eyes. At 10 years, the odds ratio (OR) of having lung cancer was 1.82 (95% CI, 1.06-3.12; P = .02) for those randomly assigned to beta carotene and 1.15 (95% CI, 0.79-1.66; P = .46) for lutein/zeaxanthin. The hazard ratio (HR) for progression to late AMD comparing lutein/zeaxanthin with no lutein/zeaxanthin was 0.91 (95% CI, 0.84-0.99; P = .02) and comparing ω-3 fatty acids with no ω-3 fatty acids was 1.01 (95% CI, 0.93-1.09; P = .91). When the lutein/zeaxanthin main effects analysis was restricted to those randomly assigned to beta carotene, the HR was 0.80 (95% CI, 0.68-0.92; P = .002). A direct analysis of lutein/zeaxanthin vs beta carotene showed the HR for late AMD was 0.85 (95% CI, 0.73-0.98; P = .02). The HR for low vs high zinc was 1.04 (95% CI, 0.94-1.14; P = .49), and the HR for no beta carotene vs beta carotene was 1.04 (95% CI, 0.94-1.15; P = .48).
Conclusions and Relevance
Results of this long-term epidemiologic follow-up study of the AREDS2 cohort suggest that lutein/zeaxanthin was an appropriate replacement for beta carotene in AREDS2 supplements. Beta carotene usage nearly doubled the risk of lung cancer, whereas there was no statistically significant increased risk with lutein/zeaxanthin. When compared with beta carotene, lutein/zeaxanthin had a potential beneficial association with late AMD progression.
Introduction
In 2001, the Age-Related Eye Diseases Study (AREDS) Research Group demonstrated the effectiveness of the AREDS supplement of antioxidant vitamins and zinc with copper in reducing the risk of progression to late age-related macular degeneration (AMD) by 25% over 5 years in eyes with intermediate AMD.1 During the course of the AREDS trial, 2 randomized controlled clinical trials of beta carotene for the prevention of lung cancer, other cancers, and cardiovascular disease demonstrated that beta carotene increased the risk of lung cancer in cigarette smokers.2,3 In 2013, the AREDS2 Research Group reported the findings of a 5-year randomized controlled clinical trial that assessed the effects of adding lutein/zeaxanthin and/or ω-3 fatty acids to the original AREDS supplement in persons with bilateral large drusen or unilateral large drusen with late AMD in the fellow eye.4 An important secondary goal was to examine the effect of removing beta carotene from the original AREDS formulation. Participants assigned to beta carotene experienced a 2-fold increased risk of lung cancer, with the majority of cases in former smokers. No statistically significant increased risk of lung cancer was observed in participants randomly assigned to lutein/zeaxanthin, despite an earlier report that suggested that lutein supplementation might be associated with an increased risk of lung cancer.5
Based on the results of the AREDS2 study, the beta carotene component was replaced by lutein/zeaxanthin for the development of the revised AREDS supplement, which is now labeled and marketed as the AREDS2 supplement. However, important questions remain. For example, does the apparent increased risk of lung cancer observed in those assigned beta carotene, but not in those assigned lutein/zeaxanthin, persist beyond the conclusion of the AREDS2 trial? Is there a benefit of adding lutein/zeaxanthin to the original AREDS supplement that can be observed with long-term follow-up? To address these and other questions, AREDS2 participants were invited to participate in an epidemiologic follow-up study reported here.
Methods
This was a 5-year epidemiologic follow-up study of the AREDS2 cohort, conducted from December 1, 2012, to December 31, 2018. The study design of the original AREDS2 has been described elsewhere6 but a brief summary is provided in eFigure 1 in Supplement 1. Institutional review board approval was obtained at each clinic and all participants provided written informed consent. During the clinical trial phase, the participants were offered funds for their transportation. The research was conducted under the tenets of the Declaration of Helsinki and was compliant with the Health Insurance Portability and Accountability Act. The rationale for this study design is also found in the eMethods in Supplement 1. The relevant Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies have been followed.
At the end of the 5-year AREDS2 clinical trial, surviving participants were invited to participate in a follow-up study via telephone contact that was conducted every 6 months until December 2018 (eFigure 2 in Supplement 1). This study, supported by the National Institutes of Health (NIH), was required to gather information on race and ethnicity. Using guidelines from the NIH Health Policy on Reporting Race and Ethnicity Data: Subjects in Clinical Research, self-reported race and ethnicity of the AREDS2 participants were collected with 2 ethnic categories (ie, Hispanic or Latino and not Hispanic or Latino) and 6 racial categories (ie, American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, White, or other). Participants were able to select more than 1 racial category. A subset of AREDS2 participants were also invited for an in-clinic study visit to obtain imaging and to document the progression of diseases at year 10 (ie, 5 years after the end of the 5-year randomized trial). This article focused on the follow-up study via telephone contact, whereas the details of the in-clinic evaluation will be presented in another article. The new AREDS2 supplements (lutein, 10 mg/zeaxanthin, 2 mg; zinc oxide, 80 mg; cupric oxide, 2 mg; vitamin C, 500 mg; and vitamin E, 400 international units), which were recommended daily, were provided by mail by Bausch and Lomb for the follow-up study participants.
Outcome Assessment
All follow-up study participants were contacted by telephone at 6-month intervals to query the participants for new diagnosis of lung cancer, occurrence of an eye examination, and new diagnosis of late AMD, including number of intravitreal injections for neovascular AMD. If a participant reported a diagnosis of lung cancer or a diagnosis or treatment of late AMD, the participant was asked to provide a signed consent for medical record(s) release to allow for the retrieval of information from the treating physician. When possible, pathological reports were obtained to further verify the diagnosis of cancer. Mortality was evaluated by treatment assignment.
Progression to Late AMD
For the current analyses, the classification that includes any geographic atrophy (central and noncentral) rather than just central geographic atrophy in the definition of late AMD was adopted when ocular images were evaluated. For eyes that did not progress to late AMD by the 5-year trial closeout, progression to late AMD in the 10-year follow-up study was considered positive from (1) centralized grading of fundus photographs obtained at the 10-year follow-up in-person study visit; (2) clinician grading from dilated fundus examination or the spectral domain optical coherence tomography, color fundus photographs, and treatment history; (3) structured telephone interview ascertainment of history of anti–vascular endothelial growth factor injections; or (4) verified report of late AMD from ophthalmic medical records.
Statistical Analysis
The unit of analysis for the ophthalmic outcomes was the eye. The primary outcomes included the development of lung cancer and time to progression to late AMD (defined as any geographic atrophy or neovascular AMD when possible). A logistic regression model was used to evaluate the risk of development lung cancer as the time of development is not always known. Lung cancer analyses focused on the secondary randomization to beta carotene vs no beta carotene and primary randomization of lutein/zeaxanthin vs no lutein/zeaxanthin. For the progression to late AMD, a Cox proportional hazards model incorporated the method by Wei et al7 for obtaining robust variance estimates that allows for the dependence among multiple event times and the use of 1 or both eyes. These analyses used all available data from the clinical trial and the follow-up period. The models were adjusted for baseline AREDS AMD severity level. The analyses censored eyes at the time of late AMD development and participants who died or were lost to follow-up during the entire 10 years. All analyses were conducted using SAS, version 9.4 (SAS Institute). Given that this was an exploratory analysis of a completed clinic trial, 95% CIs are reported as guidelines for clinical importance. P values are 2-sided, and not adjusted for multiple analyses. Because these are post hoc analyses, no level of statistical significance was specified. All analyses were conducted under the intention-to-treat principle. The current analyses considered the entire study, from the beginning of the 5-year clinical trial through to the end of the follow-up study. This approach is consistent with that used in the previous studies described in our section on rationale for the study (eMethods in Supplement 1), even though the randomized intervention was no longer in place. Data were analyzed from November 2019 to March 2022.
Results
A total of 4203 participants were originally enrolled in the AREDS2. The mean (SD) age at baseline was 73.1 (7.7) years; 2396 (57%) were women. This follow-up study included 3882 participants (mean [SD] baseline age, 72.0 [7.7] years; 2240 women [57.7%]; 1642 men [42.3%]) and 6351 eyes. Participant self-reported race and ethnicity in the AREDS2 10-year follow-up were as follows: 1 American Indian or Alaska Native (0%), 14 Asian (0.5%), 39 Black or African American (1.3%), 73 Hispanic (1.9%), 3 Native Hawaiian or Other Pacific Islander (0.1%), 3809 non-Hispanic (98.1%), 2838 White (97.1%), and 28 other race and ethnicity (1%).
At the completion of the 5-year randomized clinical trial and before the start of the AREDS2 follow-up study, 424 participants were known to be deceased or subsequently found in a search of the 2019 National Death Index (US Centers for Disease Control and Prevention). The participants comprised the following, related to their randomization during the 5 years of the randomized trial: control group (n = 937), lutein/zeaxanthin–only group (n = 975), docosahexaenoic acid (DHA)/eicosapentaenoic acid (EPA)–only group (n = 989), and the lutein/zeaxanthin plus DHA/EPA group (n = 981) (eFigure 2 in Supplement 1).
The numbers of participants who were evaluated during the entire study and those who specifically contributed to the epidemiologic follow-up study (ie, the latter 5 years) are provided in the Supplement (eFigure 3, eTable, and eResults in Supplement 1). The study began with 4203 participants and 424 died before the start of the follow-up study. A total of 2923 participants (77% of the surviving enrolled AREDS2 participants [n = 3779]) were evaluated in the follow-up study: 2877 of the 2923 (98.4%) received telephone calls only whereas 673 (23%) of those participants who received telephone calls and 36 (1.2%) who did not participate in the telephone calls also had in-person examinations at the clinical sites at year 10.
Analyses by Treatment Groups
During the full 10-year study period, a total of 117 of all study participants developed lung cancer and 3040 of 6351 study eyes (48%) developed late AMD (subtypes unknown). The lung cancer analyses were focused on the secondary randomizations to beta carotene vs no beta carotene (eFigures 4 and 5 in Supplement 1) and the main effects of the primary randomization of lutein/zeaxanthin vs no lutein/zeaxanthin (eFigure 6 in Supplement 1). Because this was a 2 × 2 factorial design in the primary randomization, we conducted analyses with the comparisons of each of the 4 treatment groups as originally planned for AMD outcomes (lutein/zeaxanthin only, ω-3 fatty acids only, both treatments or placebo). We also analyzed the data using the main effects, in which we included all participants who were randomly assigned to lutein/zeaxanthin vs not randomly assigned to lutein/zeaxanthin because we did not find an ω-3 fatty acid treatment effect and combining all participants would maximize our power for the late AMD comparisons of lutein/zeaxanthin. Although these analyses were conducted as intent-to-treat analyses, it is important to recognize that all participants were offered the AREDS2 supplements in the follow-up study. Ninety percent of the 3882 participants were estimated to have received the AREDS2 supplements provided by mail. At the halfway point of the second 5-year follow-up, when questioned at each telephone visit whether the participant was taking AREDS or AREDS2 supplements, 2664 of 2923 participants (91%) responded to be taking AREDS2 supplement, and 30 of 2923 were taking AREDS supplements. Because lutein/zeaxanthin were added to the AREDS supplements and beta carotene was removed, essentially all participants, regardless of their randomized assignment, were taking lutein/zeaxanthin and not beta carotene during the follow-up study.
Lung Cancer Development
By the end of the 5-year clinical trial portion of AREDS2, 39 study participants had reported having lung cancer (28 of 1348 [2.1%] in the beta carotene group and 11 of 1341 [0.9%] in the no beta carotene group; nominal P = .04).4,8 Current smokers, at the start of AREDS2, were not included in the secondary beta carotene randomization.
By the end of the AREDS2 follow-up study, 117 participants (2.7%) were known to have developed lung cancer. The rates of development of lung cancer are displayed by randomized treatment assignment and by smoking status (Table). As expected, those who were current smokers and former smokers had higher rates of development of lung cancer than nonsmokers. Of those randomly assigned to beta carotene, 6 of 637 nonsmokers (0.94%) and 32 of 711 former smokers (4.5%) developed lung cancer, whereas among those randomly assigned to no beta carotene, 4 of 606 nonsmokers (0.66%) and 17 of 735 former smokers (2.3%) developed lung cancer. In this analysis of lung cancer development in those participating in the primary randomization, the main outcomes of those randomly assigned to lutein vs no lutein (N total of 4203 participants) was 1.15 (95% CI, 0.79-1.66; P = .46), in the secondary randomization that excluded smokers, the odds ratio (OR) was 1.82 (95% CI, 1.06-3.12; P = .03) for those randomly assigned to beta carotene (eFigures 4 and 5 in Supplement 1). The OR for the risk of developing lung cancer in former smokers vs nonsmokers was 1.84 (95% CI, 1.07-3.16; P = .02).
Table. Lung Cancer Development by Nutrient Factor and Smoking.
| Treatment group | No./total No. (%) | |||
|---|---|---|---|---|
| Smoker | Total events | |||
| Never | Former | Current | ||
| Overall | 19/1824 (1.04) | 74/2097 (3.5) | 24/282 (8.5) | 117/4203 (2.7) |
| Lutein/zeaxanthin onlya | 5/469 (1.07) | 20/501 (3.99) | 7/74 (9.46) | 32/1044 (3.0) |
| DHA/EPA onlya | 6/451 (1.33) | 18/548 (3.28) | 8/69 (11.59) | 32/1068 (2.9) |
| Lutein/zeaxanthin and DHA/EPAa | 4/494 (0.81) | 21/519 (4.05) | 6/66 (9.09) | 31/1079 (2.8) |
| Controla | 4/410 (0.98) | 15/529 (2.84) | 3/73 (4.11) | 22/1012 (2.2) |
| Secondary randomization | ||||
| Beta carotene | 6/637 (0.94) | 32/711 (4.5) | NAb | 38/1348 (2.8) |
| No beta carotene | 4/606 (0.66) | 17/735 (2.3) | NAb | 21/1341 (1.5) |
| Main outcomes: randomization | ||||
| Lutein/zeaxanthin | 9/963 (0.93) | 41/1020 (4.02) | 13/140 (9.29) | 63/2123 (2.9) |
| No lutein | 10/861 (1.16) | 33/1077 (3.06) | 11/142 (7.75) | 54/2080 (2.5) |
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; NA, not applicable.
All participants in the Age-Related Eye Disease Study (AREDS) were offered the AREDS supplement, which contained 15 mg of beta carotene. However, those participants who were current smokers or former smokers who stopped smoking within the year before enrollment were not given the AREDS supplements. They were enrolled if they could be randomized to the secondary randomization that included no beta carotene.
Participants who were current smokers or former smokers who stopped smoking within the past year before enrollment were not randomly assigned to beta carotene.
Progression to Late AMD—Lutein/Zeaxanthin
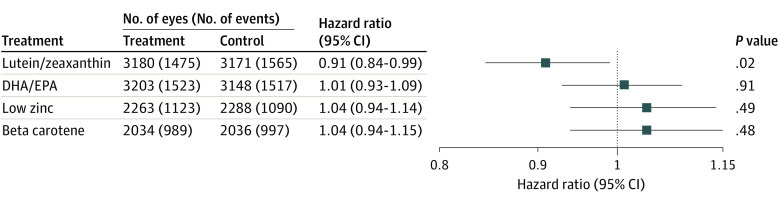
When the main effects in this 2 × 2 factorial design were evaluated for lutein/zeaxanthin (the comparison of those assigned to lutein/zeaxanthin vs not assigned to lutein/zeaxanthin), the hazard ratio (HR) for progression to late AMD by 10 years was 0.91 (95% CI, 0.84-0.99; P = .02) (Figure 1). The Kaplan-Meier probability of progressing to late AMD by 10 years was 47.9% for those taking lutein/zeaxanthin and 49.0% for those not taking lutein/zeaxanthin (eFigure 7 in Supplement 1). When the comparison of lutein/zeaxanthin vs no lutein/zeaxanthin was restricted to only those assigned in the secondary randomizations to beta carotene, the HR at 10 years was 0.80 (95% CI, 0.68-0.92; P = .002). In a subgroup analysis, a direct comparison of those randomly assigned to lutein/zeaxanthin only vs those randomly assigned to beta-carotene only in 2057 eyes (1260 participants) demonstrated an HR of 0.85 (95% CI, 0.73-0.98; P = .02), favoring lutein/zeaxanthin over beta carotene. A test of interaction between lutein/zeaxanthin with beta carotene was not statistically significant. The Kaplan-Meier probability of progressing to late AMD by 10 years was 52.2% for beta carotene and 49.5% for lutein/zeaxanthin (eFigure 8 in Supplement 1).
Figure 1. Progression to Late Age-Related Macular Degeneration: Hazard Ratios of Main Outcomes of Each of the Nutrients in Primary and Secondary Randomization.
DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid.
Progression to Late AMD—DHA/EPA
In comparing those assigned to DHA/EPA with those not assigned to DHA/EPA, the HR by 10 years was 1.01 (95% CI, 0.93-1.09; P = .91) (Figure 1). The Kaplan-Meier probability of progression to late AMD by 10 years was 48.6% for DHA/EPA and 48.3% for no DHA/EPA (eFigure 9 in Supplement 1). Because DHA/EPA components were not added to the AREDS2 supplement, the DHA/EPA combination was not provided during the follow-up study.
Analyses by the 4 Treatment Groups
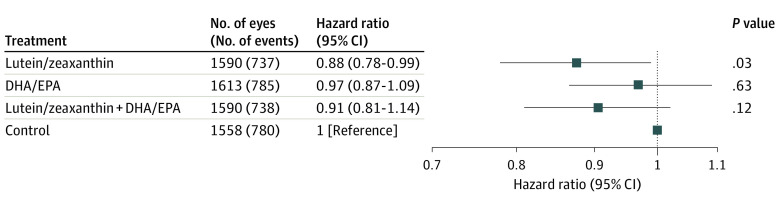
Analyses were also conducted comparing all 4 treatment groups. Compared with the control (placebo) group, HRs for progression to late AMD at 10 years were as follows: lutein/zeaxanthin, 0.88 (95% CI, 0.78-0.99; P = .03); DHA/EPA, 0.97 (95% CI, 0.87-1.09; P = .63); and the combination of lutein/zeaxanthin and DHA/EPA, 0.91 (95% CI, 0.81-1.02; P = .12) (Figure 2). The Kaplan-Meier probabilities of progression to late AMD by 10 years were 49.2% for placebo, 47.3% for lutein/zeaxanthin, 48.8% for DHA/EPA, and 48.4% for the combination (eFigure 10 in Supplement 1).
Figure 2. Progression to Late Age-Related Macular Degeneration: Hazard Ratios of Each of the Individual Treatment Groups vs Controla .
aPlacebo for lutein and zeaxanthin. DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid.
Secondary Outcomes of Beta Carotene and Zinc
For the comparison of the secondary randomization of low (25 mg) vs high (80 mg) zinc oxide, the HR at 10 years was 1.04 (95% CI, 0.94-1.14; P = .49) (Figure 1). Similar analyses for the secondary randomization of beta carotene (25 mg vs none) demonstrated an HR of 1.04 (95% CI, 0.94-1.15; P = .48) (Figure 1).
Mortality
The probability of death did not differ significantly by treatment groups: lutein/zeaxanthin vs no lutein/zeaxanthin or DHA/EPA vs no DHA/EPA (eFigures 11 and 12 in Supplement 1, respectively). The Kaplan-Meier probabilities of death for those randomly assigned to lutein/zeaxanthin and no lutein/zeaxanthin by 10 years were 28.6% and 28.7%, respectively. Similarly, the Kaplan-Meier probabilities of death for those randomly assigned to DHA/EPA and no DHA/EPA by 10 years were 29.7% and 27.5%, respectively. The Kaplan-Meier probabilities of death at 10 years for those randomly assigned to beta carotene and no beta carotene were 28.9% and 27.8% and for those to low zinc (25 mg) vs high zinc (80 mg) were 30.9% vs 28.4% (eFigures 13 and 14 in Supplement 1, respectively).
Discussion
The results of this epidemiologic follow-up study of the AREDS2 clinical trial suggest further support for the safety of lutein/zeaxanthin as a replacement for beta carotene in the original AREDS supplement. We observed no statistically significant increased risk of lung cancer with the use of lutein/zeaxanthin, whereas beta carotene maintained its almost doubling of the risk of lung cancer. Many of the lung cancer cases were in former smokers, and the risk of lung cancer was higher in former smokers than never smokers (OR, 1.84; 95% CI, 1.07-3.16). These data, combined with data from previous studies, suggest that beta carotene should not be included in the AREDS2 formulation. In addition, lutein/zeaxanthin may not only be safer in terms of lung cancer risk but may also provide an incremental increase in prevention of progression to late AMD.
It should also be noted that the placebo group in AREDS2 is not a true placebo group as this study tested the addition of lutein/zeaxanthin to the original AREDS supplement, which was essentially given to all the AREDS2 cohort. Despite the cessation of the randomization of treatment and the provision of the AREDS2 supplements, which provided lutein/zeaxanthin potentially to all the participants during the follow-up study, we found a persistent long-term beneficial association of lutein/zeaxanthin with progression to late AMD in both the primary analyses of the 4 separate treatment groups (HR, 0.88; 95% CI, 0.78-0.99) and the main outcome analyses (HR, 0.91; 95% CI, 0.81-1.02) of the entire cohort. In the analyses restricted to participants randomly assigned to beta carotene (comparison groups demonstrated in eFigure 4 in Supplement 1), there was also an associated reduction (HR, 0.80; 95% CI, 0.68-0.92) in progression to late AMD at 10 years. This subgroup analysis showed a stronger protective association of lutein/zeaxanthin with progression to late AMD when compared directly with beta carotene (eFigure 8 in Supplement 1). The rationale for evaluating lutein/zeaxanthin vs beta carotene is explained in the eDiscussion in Supplement 1. The totality of information, both clinical (lung cancer and AMD outcomes) and laboratory, provides evidence for using lutein/zeaxanthin rather than beta carotene in a supplement designed to slow the progression of AMD in patients with intermediate AMD or late AMD in 1 eye.
Strengths and Limitations
A strength of this study was the large number of participants who were committed to the follow-up portion of this study, considering that the mean age of this population was 73.1 years at baseline. The limitations of this study include the self-reported outcome measures of the ocular outcomes and the outcome of lung cancer, although this limitation was mitigated to the extent possible by medical records validation. This method of ascertainment of ocular and cancer outcomes has been demonstrated to be valid in studies such as a recently completed randomized trial, the Vitamin D and Omega-3 Trial (VITAL),9 which evaluated the incidence of AMD using similar methodology, and additional studies of AMD including the Physicians’ Health Study10 and the Women's Antioxidant and Folic Acid Cardiovascular Study.11 Another limitation was the lack of ophthalmic images on all the AREDS2 participants during the follow-up study, resulting in the inability to differentiate the 2 subtypes of late AMD. We were unable to determine the long-term association of the AREDS2 supplements with the 2 subtypes of late AMD separately.
Potential lack of generalizability was another limitation, because the AREDS2 population was generally highly educated and well nourished, with above-average intake of dietary nutrients. Another potential limitation was that these were post hoc analyses, and no adjustments were made for multiple comparisons. Further, the control (placebo) group was also offered the AREDS2 supplements during the extended follow-up; the association of this treatment with the study results cannot be determined. Nonetheless, the point estimates of all these analyses involving lutein/zeaxanthin were uniformly in the direction of a protective association.
How important clinically is this association? The AREDS2 was designed to evaluate the addition of lutein/zeaxanthin to the original AREDS supplement, which already was shown to have an absolute 7.6% beneficial effect or relative 25% beneficial effect of reducing the risk of progression to late AMD over a period of 5 years. Potentially, there may be an additional absolute 2.7% beneficial effect or additional relative 10% to 20% additional beneficial effect of lutein/zeaxanthin, although the actual treatment effect may be difficult to assess because it would not be possible to randomly assign study participants to placebo at this point. Even a modest increase in the treatment effect to prevent late AMD would be clinically important because of the exceedingly large number (288 million) of patients anticipated to be affected globally in 2040.12
Conclusions
Results of this epidemiologic follow-up study of the AREDS2 clinical trial suggest a beneficial association of lutein/zeaxanthin that persisted throughout the 10-year follow-up study. In addition, the results indicate that this is a safe supplement, with no increased risk of developing lung cancer, potentially providing reassurance to eye care professionals who are treating patients with intermediate AMD or late AMD in 1 eye that the AREDS2 supplement is safe and effective, even for long-term use.
eFigure 1. Original AREDS2 Study Design
eFigure 2. AREDS2 10-Year Follow-up Study Design
eFigure 3. Number of AREDS2 Participants in 10-Year Follow-up
eFigure 4. Main Effects of Beta carotene Analysis
eFigure 5. Detailed Evaluation of Beta carotene and Zinc in Secondary Randomization by the Primary Randomization Group
eFigure 6. AREDS2 10-Year Follow-up Study—Main Effects of Lutein/Zeaxanthin and ω-3 Fatty Acids
eFigure 7. Progression to Late AMD by Main Effects: Lutein/Zeaxanthin vs No Lutein/Zeaxanthin
eFigure 8. Progression to Late AMD by Comparing Directly Lutein/Zeaxanthin vs Beta carotene
eFigure 9. Progression to Late AMD by Treatment With and Without ω-3 Fatty Acids
eFigure 10. Progression to Late AMD by the 4 Primary Treatment Groups, Lutein/Zeaxanthin, DHA/EPA, Lutein/Zeaxanthin and DHA + EPA, and Controls
eFigure 11. Mortality by Lutein/Zeaxanthin Assignment
eFigure 12. Mortality by DHA/EPA Assignment
eFigure 13. Mortality by Beta carotene
eFigure 14. Mortality by Zinc Level
eTable. Characteristics of AREDS2 Participants by Participation Status in the 10-Year Follow-up
eMethods
eResults
eDiscussion
eReferences
Nonauthor Collaborators
References
- 1.Age-Related Eye Disease Study Research Group . A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-1436. doi: 10.1001/archopht.119.10.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150-1155. doi: 10.1056/NEJM199605023341802 [DOI] [PubMed] [Google Scholar]
- 3.Albanes D, Heinonen OP, Taylor PR, et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88(21):1560-1570. doi: 10.1093/jnci/88.21.1560 [DOI] [PubMed] [Google Scholar]
- 4.Age-Related Eye Disease Study 2 Research Group . Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005-2015. doi: 10.1001/jama.2013.4997 [DOI] [PubMed] [Google Scholar]
- 5.Satia JA, Littman A, Slatore CG, Galanko JA, White E. Long-term use of beta-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: results from the Vitamins and Lifestyle (VITAL) study. Am J Epidemiol. 2009;169(7):815-828. doi: 10.1093/aje/kwn409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew EY, Clemons T, SanGiovanni JP, et al. ; AREDS2 Research Group . The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012;119(11):2282-2289. doi: 10.1016/j.ophtha.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84(408):1065-1073. [Google Scholar]
- 8.Chew EY, Clemons TE, Sangiovanni JP, et al. ; Age-Related Eye Disease Study 2 (AREDS2) Research Group . Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142-149. doi: 10.1001/jamaophthalmol.2013.7376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christen WG, Cook NR, Manson JE, et al. ; VITAL Research Group . Effect of vitamin D and ω-3 fatty acid supplementation on risk of age-related macular degeneration: an ancillary study of the VITAL randomized clinical trial. JAMA Ophthalmol. 2020;138(12):1280-1289. doi: 10.1001/jamaophthalmol.2020.4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121(2):525-534. doi: 10.1016/j.ophtha.2013.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christen WG, Glynn RJ, Chew EY, Albert CM, Manson JE. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med. 2009;169(4):335-341. doi: 10.1001/archinternmed.2008.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. doi: 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Original AREDS2 Study Design
eFigure 2. AREDS2 10-Year Follow-up Study Design
eFigure 3. Number of AREDS2 Participants in 10-Year Follow-up
eFigure 4. Main Effects of Beta carotene Analysis
eFigure 5. Detailed Evaluation of Beta carotene and Zinc in Secondary Randomization by the Primary Randomization Group
eFigure 6. AREDS2 10-Year Follow-up Study—Main Effects of Lutein/Zeaxanthin and ω-3 Fatty Acids
eFigure 7. Progression to Late AMD by Main Effects: Lutein/Zeaxanthin vs No Lutein/Zeaxanthin
eFigure 8. Progression to Late AMD by Comparing Directly Lutein/Zeaxanthin vs Beta carotene
eFigure 9. Progression to Late AMD by Treatment With and Without ω-3 Fatty Acids
eFigure 10. Progression to Late AMD by the 4 Primary Treatment Groups, Lutein/Zeaxanthin, DHA/EPA, Lutein/Zeaxanthin and DHA + EPA, and Controls
eFigure 11. Mortality by Lutein/Zeaxanthin Assignment
eFigure 12. Mortality by DHA/EPA Assignment
eFigure 13. Mortality by Beta carotene
eFigure 14. Mortality by Zinc Level
eTable. Characteristics of AREDS2 Participants by Participation Status in the 10-Year Follow-up
eMethods
eResults
eDiscussion
eReferences
Nonauthor Collaborators