Abstract
Objective
Obesity has become a worldwide epidemic, with very few long-term successful treatment options for refractory disease. Deep brain stimulation (DBS) of the bilateral lateral hypothalamus (LH) in refractory obesity has been performed safely. However, questions remain regarding the optimal settings and its effects on metabolic rate. The goals of our experiment were to determine the optimal DBS settings and the actual effect of optimal stimulation on energy expenditure.
Methods
After bilateral LH DBS implantation, 2 subjects with treatment refractory obesity underwent 4 days of metabolic testing. The subjects slept overnight in a respiratory chamber to measure their baseline sleep energy expenditure, followed by 4 consecutive days of resting metabolic rate (RMR) testing at different stimulation settings. On day 4, the optimized DBS settings were used, and sleep energy expenditure was measured again overnight in the room calorimeter.
Results
During daily testing, the RMR fluctuated acutely with changes in stimulation settings and returned to baseline immediately after turning off the stimulation. Optimal stimulation settings selected for participants showed a 20% and 16% increase in RMR for the 2 participants. Overnight sleep energy expenditure measurements at these optimized settings on day 4 yielded a 10.4% and 4.8% increase over the baseline measurements for the 2 participants.
Conclusions
These findings have demonstrated the efficacy of optimized DBS of the LH on increasing the RMR acutely and maintaining this increase during overnight sleep. These promising preliminary findings have laid the groundwork for the possible treatment of refractory obesity with DBS.
Keywords: Deep brain stimulation, Functional neurosurgery, Lateral hypothalamus, Neuromodulation, Obesity
INTRODUCTION
Obesity is a worldwide epidemic, increasing in prevalence across all age groups and demographics during the past few decades.1–3 Morbid obesity, or a body mass index of ≥40 kg/m2 or >35 kg/m2 with comorbidities, has been associated with premature death, impaired quality of life, and increased rates of cardiovascular disease, type 2 diabetes, and certain cancers.4–6 Although lifestyle modification is the first-line therapy, nonoperative treatments of morbid obesity have had limited success and successful surgical options, including gastric bypass and sleeve gastrectomy, are associated with significant surgical and nutritional complications and considerable rates of weight relapse and reoperation.7–9 Deep brain stimulation (DBS) is a well-established surgical treatment option for a variety of conditions, including Parkinson’s disease, dystonia, and essential tremor.10–13 With its effectiveness, low rates of complications, adjustability, and reversibility, a bourgeoning interest into new indications and targets for DBS has occurred during the past 2 decades.14,15 A pilot study of DBS for obesity involving bilateral lateral hypothalamus (LH) stimulation in 3 morbidly obese patients was performed in 2013.16 That study demonstrated the safety of DBS of the LH in these patients. However, questions remained regarding the optimal settings for stimulation and the actual effect of stimulation on the metabolic rate. We present the results from a study designed to determine the optimal stimulation settings to induce increases in the resting and sleeping metabolic rates using ventilated-hood and whole-room indirect calorimetry.
METHODS
Subjects
The present trial was conducted under a physician-sponsored Food and Drug Administration-approved investigational device exemption (exemption no. G070067), monitored by the Alleghany-Singer Research Institute West Penn Alleghany Health System and Pennington Biomedical Research Center institutional review boards, and registered at ClinicalTrials.gov (ClinicalTrials.gov identifier, NCT01933113). All participants provided informed consent before the initiation of study procedures. After bilateral LH DBS, electrode implantation (model 3389, Medtronic, Inc., Minneapolis, Minnesota, USA), 2 subjects underwent 4 days of metabolic testing at Pennington Biomedical Research Center (Baton Rouge, Louisiana, USA). The 2 participants had been participants in a previous pilot study for LH DBS for refractory obesity.16 The inclusion criteria for the initial study required that the subjects were morbidly obese for whom bariatric surge had failed according to the modified Reinhold classification system.17 Additional inclusion and exclusion criteria are listed in Table 1. Although 3 subjects had undergone placement of the bilateral DBS leads and underwent testing for the pilot study, 1 of the subjects developed a lead breakage and, because of several years of medical instability pertaining to an unrelated abdominal infection after panniculectomy, was withdrawn from the study. The patient demographics are listed in Table 2.
Table 1.
Inclusion and Exclusion Criteria for Enrollment of Study Participants
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 18 years | Previous brain surgery |
| BMI ≥ 40 kg/m2 or ≥ 35 kg/m2 with a comorbid condition (hypertension, cardiovascular disease, type 2 diabetes, dyslipidemia) |
Dementia or Mini-Mental State Examination score <25 |
| Failure of bariatric surgery (banding or bypass) determined by modified Reinhold classification because patients who are still >50% over an ideal body weight after technically successful surgery |
Unable to fit into MRI or CT scanner (400-lb upper weight limit for CT scanner) |
| Chronic obesity diagnosed by an eating disorder specialist with expertise in treatment of obesity | Psychiatric disorder, including poorly controlled anxiety disorders, psychosis, bipolar disorder, active substance abuse, somatoform disorders, factious disorders, dissociative disorders, and severe personality disorders, excluding depression and binge eating |
| Stable at present body weight for 6-month period | Obesity as part of another medical condition, neurological injury, or lesions related to medication side effect or as part of a genetic syndrome |
| Psychiatric evaluation | Unable to schedule follow-up clinic visits |
| Karnofsky performance scale score >60 | Karnofsky performance scale score <60 |
BMI, body mass index; MRI, magnetic resonance imaging; CT, computed tomography.
Table 2.
Participant Characteristics Before Deep Brain Stimulation Implantation and Before the Present Study
| Participant | Age (years), Sex | Pre-DBS Body Weight (lb); BMI (kg/m2) | Previous Surgical Weight Loss Treatment; Year | Comorbidity | Body Weight (lb)/BMI (kg/m2) Before Present Study | SEE at Baseline (kcal/24 hours) |
|---|---|---|---|---|---|---|
| 1 | 60, F | 278.7; 49.4 | Gastric bypass, 2001 | HTN | 262.8, 46.6 | 1414 |
| 2 | 50, F | 326; 48.1 | Gastric bypass, 2001 | Sleep apnea, DM2, HTN, migraine | 257.7, 38.6 | 1562 |
| 3 | 45, M | 314; 45 | Gastric bypass, 2002 | Lower extremity edema | NA | NA |
DBS, deep brain stimulation; BMI, body mass index; SEE, sleep energy expenditure; F, female; HTN, hypertension; DM2, diabetes mellitus type 2; NA, not applicable.
Experimental Design
In brief, 3 subjects with intractable obesity had had bilateral DBS electrode leads placed bilaterally in the lateral hypothalamic area using frame-based stereotaxy, microelectrode recording, and a standard atlas of the human brain.18,19 The bilateral leads had 4 platinum-iridium contacts, with contact 0 corresponding to the most distal contact on the lead and contact 3 corresponding to the most proximal contact. Postoperative head computed tomography images were merged with preoperative magnetic resonance images, confirming accurate placement of the leads in the lateral hypothalamus, with contact 1 appearing closest to the LH in all 3 patients. In the pilot study, the subjects underwent metabolic testing at Pennington Biomedical Research Center for measurement of energy expenditure under various DBS settings using a whole-body room indirect calorimeter. The surgical technique, safety, and results of that study have been previously reported.16 In the present report, we sought to identify the optimal DBS settings for each subject using bedside indirect calorimetry (ventilated hood) and to determine the effect of this optimal stimulation on energy expenditure using a whole-body room calorimeter. On Day 0, without DBS, the subjects were admitted to the inpatient unit and slept overnight in the metabolic chamber for a baseline measurement of sleep energy expenditure. The subjects exited the chamber the next morning (day 1) and completed w200 minutes of resting metabolic rate (RMR) to test various stimulation settings during a 4-day period.
Body Weight and Composition
The participants’ metabolic weight was measured after a 10-hour fast, after voiding, and wearing a hospital gown. Body composition was measured by dual-energy X-ray absorptiometry using the iDXA (dual-energy X-ray absorptiometry) whole-body scanner (General Electric, Milwaukee, Wisconsin, USA) and analyzed using QDR software, version 11.1 (Hologic, Marlborough, Massachusetts, USA).
Optimal Stimulation Search Testing for Resting Energy Expenditure
The subjects completed 200 minutes of RMR testing for 4 consecutive days to identify the respective “optimal” stimulation setting (i.e., the DBS setting with the greatest RMR; Figure 1). The measurements were conducted using a bedside calorimeter (Max II; AEI Technologies, Pittsburgh, Pennsylvania, USA) under standardized conditions in a private quiet room with controlled ambient conditions, using site-specific standard operating procedures and best practice methods.20 The best practice methods were determined using evidence-based guidelines established by the American Dietetic Association for the most accurate measurements of the RMR using indirect calorimetry.20 Before each test, gas analyzers were calibrated under current ambient conditions (temperature, humidity, pressure) with room air and 2 certified standard gases (Airgas Specialty Gases, Inc., Lenexa, Kansas, USA). The participants rested for a minimum of 30 minutes undisturbed while lying supine and reclining in a hospital bed at a 30° incline. At the completion of the resting period, the ventilated hood was placed over the subject’s head and secured with a plastic sheet around the subject’s pillow. The volunteers were instructed to remain still, with their eyes open, and to refrain from fidgeting as much as possible. Supervised television viewing was permitted during the test. Trained research specialists observed the tests and recorded events such as a cough, sneeze, or yawn that might potentially affect the RMR measurements.
Figure 1.
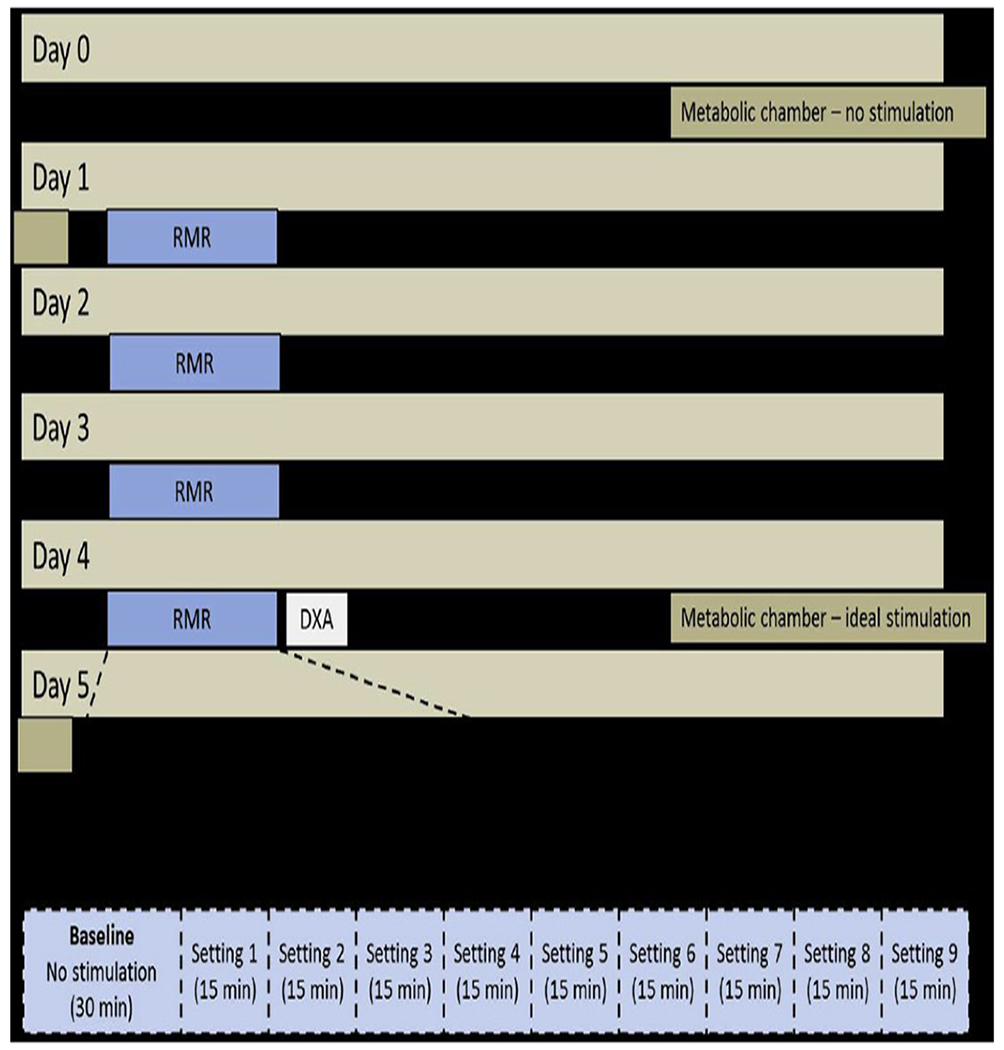
Study design. DXA, bone dual-energy x-ray absorptiometry; RMR, resting metabolic rate.
A 30-minute baseline measurement without DBS was collected, followed by nine 15-minute intervals at DBS settings with alternating voltage, pulse width, and frequency. This protocol (30-minute baseline plus 9 settings for 15minutes each) was completed on days 1, 2, 3, and 4 on each of the 4 DBS electrode contacts, with 1 day dedicated to each contact. Day 1 began with contact 3 (the most proximal contact) and proceeded distally, ending on day 4 with contact 0. We used a single-blind study design; therefore, the participants were unaware of which settings were programmed during testing. A Latin square design was used (Table 3) to determine the order and variation of the pulse width, frequency, and voltage, with a total of 9 variations used each day. The Latin square design was used to maximize the variations in frequency, pulse width, and voltage over the testing period in a standardized manner. This progression of settings was followed each day for each contact in the same order (Table 4).
Table 3.
Latin Square Stimulation Settings Testing Different Combinations of Pulse Width, Frequency, and Voltage
Voltage.
Table 4.
Daily Stimulation Parameter Progression
| Stimulation Setting | Time (minutes) | Pulse Width (msec) | Frequency (Hz) | Goal Voltage (V) |
|---|---|---|---|---|
| 1 | 30 | Off | Off | Off |
| 2 | 15 | 60 | 60 | 1 |
| 3 | 15 | 210 | 185 | 1 |
| 4 | 15 | 90 | 250 | 1 |
| 5 | 15 | 210 | 60 | 3 |
| 6 | 15 | 90 | 185 | 3 |
| 7 | 15 | 60 | 250 | 3 |
| 8 | 15 | 90 | 60 | 5.5 |
| 9 | 15 | 60 | 185 | 5.5 |
| 10 | 15 | 210 | 250 | 4 |
Sleep Energy Expenditure
SEE was measured overnight in a whole-room indirect calorimeter without DBS at baseline (day 0) and with optimal DBS on day 4. The participants entered the chamber at 7:00 PM, dinner was served at 7:30 PM (35% total energy intake as determined by Lam et al.21), and the lights were turned off at 10:30 PM. The subjects were woken at 6:30 AM and exited the chamber at ~7:15 AM. All urine was collected during the chamber stay for calculation of urinary nitrogen excretion.
Analysis
For each DBS interval, the first 5 minutes of RMR data were excluded, and mean RMR was calculated with the volume of oxygen consumption and volume of carbon dioxide production using the Weir equation.22 We selected the “optimal” stimulation setting as the setting that provided the largest increase in RMR that was tolerable to the participants. Several patients were unable to tolerate elevation of the voltage under certain stimulation parameters, because they experienced warmth, nausea, or anxiety. In these situations, the maximum tolerable voltage was used to stimulate and determine the RMR for that designated set of stimulation parameters. SEE was calculated from the volume of oxygen consumption and volume of carbon dioxide production in the whole-room metabolic chamber between 2:00 AM and 5:00 AM, including all the minute periods during which no spontaneous physical activity was detected. The SEE was calculated after correction for nitrogen excretion rates, as previously described.21
RESULTS
Both subjects were postmenopausal, white women with a body mass index >45 kg/m2 before the initial study. The characteristics of the 2 subjects on day 0 of the present study are listed in Table 2. No serious adverse events occurred during study participation. The side effects experienced as a result of stimulation included warmth, nausea, and anxiety. These were all temporally related to increases in voltage and resolved completely as soon as the voltage was decreased.
Energy Expenditure and RMR
The baseline SEE, expressed on a daily basis, was 1414 kcal/24 hours and 1567 kcal/24 hours in subjects 1 and 2, respectively. During the course of days 1-4, 36 different combinations of electrode contact, pulse width, frequency, and voltage were used to measure their effects on RMR. The RMR fluctuated acutely with changes in stimulation settings and returned to baseline immediately after turning off the stimulation. Figure 2 illustrates these acute fluctuations in RMR for the 2 optimal stimulations, and Figure 3 shows the effect of these optimal stimulations on SEE. The optimal settings displayed a 20% elevation in RMR compared with baseline without stimulation (1.32 kcal/min vs. 1.10 kcal/min) in subject 1 and a 16% elevation in RMR compared with baseline without stimulation (1.44 kcal/min vs. 1.24 kcal/min) in subject 2 (Table 5). The measurement of SEE on day 4 using room calorimetry with the subjects stimulated overnight at their optimal settings yielded a 10.5% (1414 kcal/24 hours vs. 1562 kcal/24 hours) and a 4.8% (1567 kcal/24 hours vs. 1643 kcal/24 hours) increase in SEE for participants 1 and 2, respectively, compared with their prestimulation baseline measured on day 0 (Figure 3).
Figure 2.
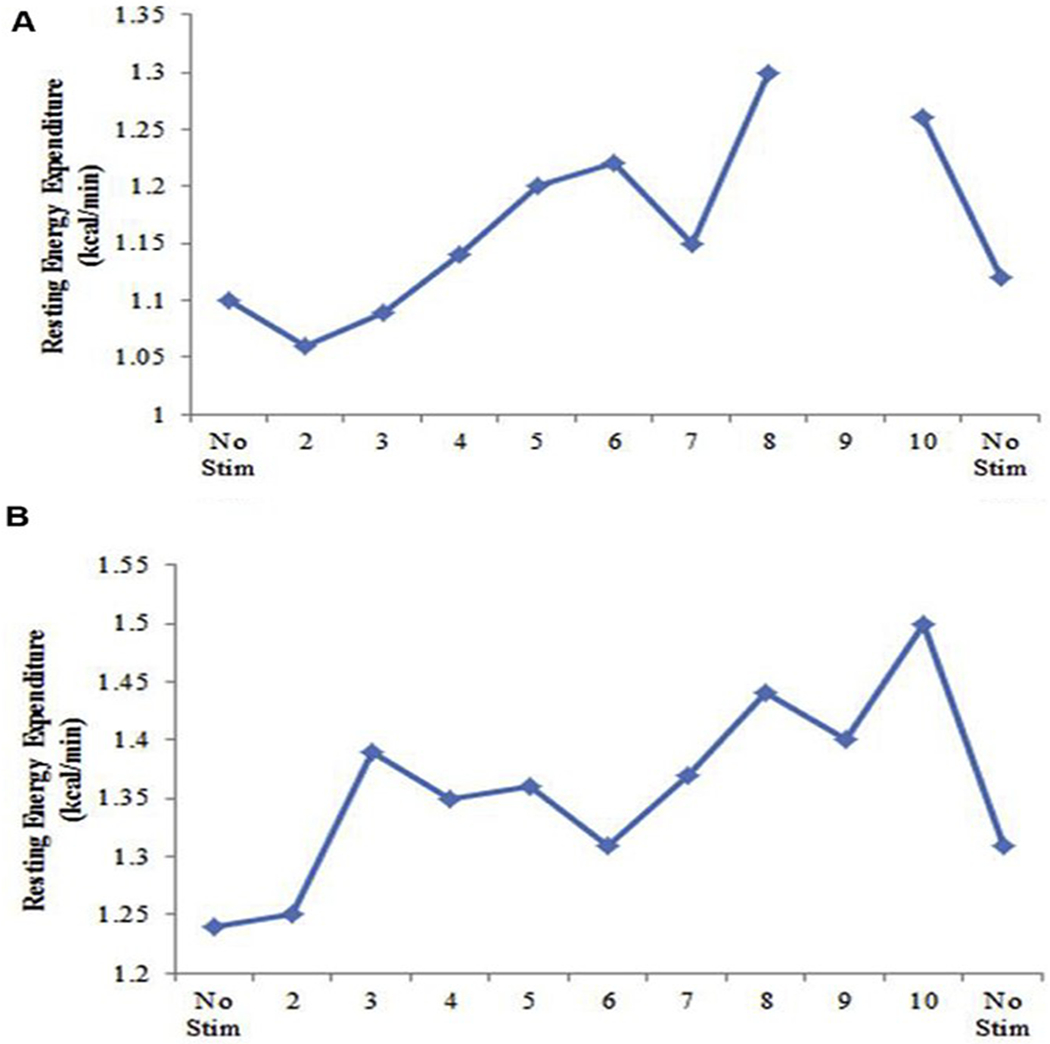
Example day of optimal stimulation search testing (mean resting metabolic rate [RMR] for baseline, stimulation interval, and once stimulation was turned off) for (A) participant 1 and (B) participant 2. Setting 9 for participant 1 on day 2 could not be used because of patient movement at testing.
Figure 3.
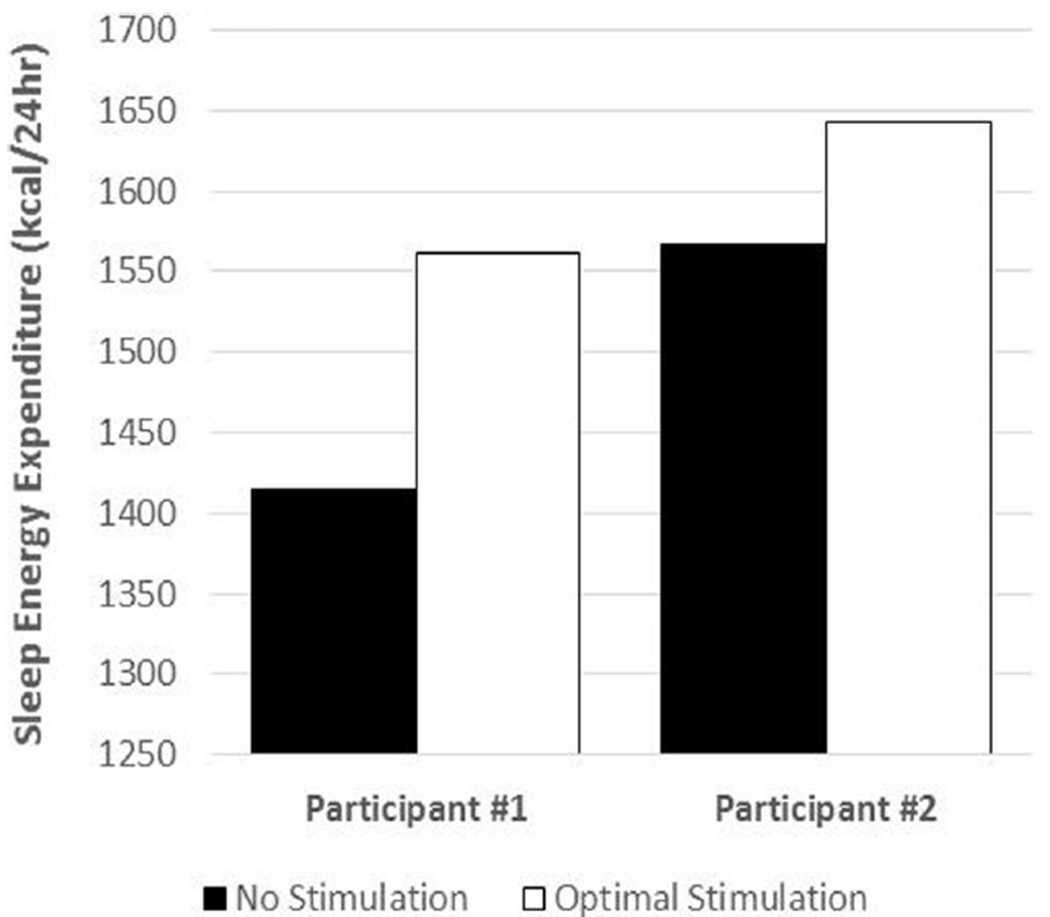
Sleep energy expenditure (kcal/24 hours) without stimulation and at the “optimal” stimulation setting.
Table 5.
Optimally Determined Resting Metabolic Rate
| Optimal Setting | ||||||
|---|---|---|---|---|---|---|
| Participant | ID | Electrode | Pulse Width (msec) | Frequency (Hz) | Voltage (V) | Increase Above Baseline (%) |
| 1 | Day 2, setting 9 | C+, 2− | 60 | 185 | 505 | 20 |
| 2 | Day 4, setting 8 | C+, 0− | 90 | 60 | 3.8 | 16 |
ID, identification; C+, 2−_, Contact 2; C+, 0−_, Contact 0.
DISCUSSION
The goal of the present study was to investigate changes in RMR at different settings during stimulation of the LH with DBS and to find the optimal setting for increasing the RMR in each subject. Both study participants had demonstrable increases in RMR of 16% - 20% when acutely stimulated at their individual optimized settings. Additionally, when these optimized settings were applied throughout the night with the subjects resting and sleeping in a metabolic chamber, these continuous stimulations resulted in a 10.5% and 4.8% increase in SEE, demonstrating that the settings that temporarily increased the RMR also resulted in longer lasting energy expenditure.
Obesity is essentially the result of an imbalance between energy input and expenditure, with much of this control originating from the central nervous system.23 Although the mainstay of the past several decades of obesity treatment has been changes in lifestyle with reduced food intake and increased physical activity, long-term reduction of weight has proved to be difficult, with >90% of patients eventually regaining most, if not all, of the lost weight.24–26 This has left clinicians with few practical options for the treatment of morbid obesity. The most effective long-term treatment of refractory morbid obesity is currently bariatric surgery, involving gastric bypass, sleeve gastrectomy, or gastric banding. Although bariatric surgery has been more effective than other medical treatments in reducing excess body weight and its associated medical comorbidities, it is not without significant morbidity, nutritional complications, and rates of weight relapse.8,27 If DBS, with its adjustability, reversibility, and low rates of complications, can be shown to be effective in the treatment of morbid obesity, it would represent a novel and promising alternative treatment modality. The RMR, independent of physical activity and exercise, is known to constitute well over half of daily energy expenditure, and activity-related thermogenesis (exercise and nonvolitional activities such as maintaining posture) has been found to represent only 20% - 30% of energy expenditure.28 Although long-term weight loss studies for subjects undergoing LH DBS are required, the present study has demonstrated that optimized settings can result in appreciable and promising changes in the RMR and SEE.
In the initial pilot study of LH DBS for the treatment of refractory morbid obesity, these same subjects were observed and monitored for safety and weight loss.16 After an average of 35 months of follow-up, no serious adverse effects from LH DBS were noted, although mild transient (<5 minutes) experiences of nausea, anxiety, and flushed sensations were intermittently observed. After DBS implantation, the subjects were taken to the Pennington Biomedical Research Center, and RMR was measured without and with stimulation. Because the optimal pulse width and frequency for DBS of the LH to increase the RMR were not known, a standard movement disorder programming of 90-msec pulse width and 185-Hz frequency was initially chosen.16 Although not fully optimized, these settings showed increases in RMR during stimulation, which returned to baseline as soon as the stimulation was removed. These findings during stimulation were similar to the findings of the present study, demonstrating the reproducibility of increasing RMR during lateral hypothalamic area stimulation across 2 different studies several years apart.
Multiple potential electrode placement sites have been considered for the use of DBS as a potential modality for obesity treatment, including the LH, ventromedial hypothalamus,29 and nucleus accumbens.30 The LH was chosen for the present study because this region of the brain has been described as the “feeding center” of the central nervous system. Early animal studies have demonstrated that lesioning of the LH resulted in decreased body weight and increased core body temperature.31,32 Additional rat studies have demonstrated that low-frequency (50 Hz) electrical stimulation of the LH resulted in food-seeking behavior,33 and high-frequency (180 - 200) deep brain electrical stimulation of the LH in rats resulted in weight loss compared with the controls.34 In our present study, the optimized settings for our 2 participants demonstrated incongruous and differing results. Participant 1 demonstrated the largest change in RMR using electrode contact 2-, a pulse width of 60 msec, and a frequency of 185 Hz, corroborating the hypothesis from rat studies that high-frequency stimulation of the LH should result in obesity-attenuating effects. In contrast, participant 2 experienced the largest change in RMR using stimulating electrode contact 0-, a pulse width of 90 msec, and a frequency of 60 Hz. Although both participants had notably differing optimal settings, they both experienced demonstrable increases in both RMR and SEE. The LH is a relatively small anatomical target compared with other DBS targets, and it is possible the difference in optimized settings represented minute anatomical differences in electrode placement. Both participants demonstrated lead placement with contact 1 closest to the middle of the target, the lateral hypothalamus; however, participant 2 demonstrated a more lateral trajectory than participant 1 owing to larger ventricles.16 The hypothalamus and surrounding structures comprise a complex and intricate network, and differences in the optimized settings might represent activation of different nuclei and regions. It is possible that the difference in trajectory resulted in activation of different surrounding nuclei when other contacts were stimulated. Additionally, when examining our data, it was notable that although the chosen settings resulted in the largest increases in RMR, when similar voltages were applied to the optimized contact but with different frequencies, these also resulted in significant increases in RMR. It appears that changes in frequency among 60, 185, and 250 Hz are possibly less important regarding the effects on RMR than the optimal electrode contact location and the greatest tolerable voltage. In future optimization of subjects’ settings for increasing the RMR, it might be more efficacious to focus on identifying the optimal electrode contact and maximizing the tolerable voltage rather than exploring the effects of different frequencies and pulse widths.
Additional studies are needed with a larger number of subjects and longer study designs to assess the effect of optimized settings on energy intake, actual weight loss, changes in comorbidities, and long-term metabolic energy expenditure. As an extension of a phase I clinical trial with 2 subjects, the present study was limited by its size and could not allow us to generalize the findings. Additionally, the present study only examined RMR and SEE for short periods (minutes or hours) and could not provide information of the effect of DBS of the LH at longer term (days, months, and years).35,36 Moreover, because the present study did not directly address actual weight loss and the reduction of comorbidities associated with morbid obesity, it is conceivable that the measured increases in energy expenditure did not translate into clinically relevant outcomes.
CONCLUSION
Overall, the present study has demonstrated that DBS of the LH for treatment refractory morbid obesity can be optimized to exhibit demonstrable and reproducible increases in energy expenditure. Long-term investigations of weight loss and associated comorbidities at the optimized settings are necessary; however, the observed increase in energy expenditure suggests that DBS could be a viable alternative surgical option for treatment of refractory morbid obesity.
Conflict of interest statement:
Our research was supported in the form of the donation of devices by Medtronic Corporation.
Abbreviations and Acronyms
- DBS
Deep brain stimulation
- LH
Lateral hypothalamus
- RMR
Resting metabolic rate
- SEE
Sleep energy expenditure
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999; 282:1523–1529. [DOI] [PubMed] [Google Scholar]
- 5.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. [DOI] [PubMed] [Google Scholar]
- 6.Nowicki EM, Billington CJ, Levine AS, Hoover H, Must A, Naumova E. Overweight, obesity, and associated disease burden in the Veterans Affairs ambulatory care population. Mil Med. 2003;168: 252–256. [PubMed] [Google Scholar]
- 7.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004; 292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 8.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomized controlled trials. BMJ. 2013;347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology.2006;31:2384–2393. [DOI] [PubMed] [Google Scholar]
- 11.Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. [DOI] [PubMed] [Google Scholar]
- 12.Rehncrona S, Johnels B, Widner H, Tornqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003;18:163–170. [DOI] [PubMed] [Google Scholar]
- 13.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WH Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T, Mirzadeh Z, Chapple K, Lambert M, Ponce FA. Complication rates, lengths of stay, and readmission rates in “awake” and “asleep” deep brain simulation. J Neurosurg. 2017;127:360–369. [DOI] [PubMed] [Google Scholar]
- 15.Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120:132–139. [DOI] [PubMed] [Google Scholar]
- 16.Whiting DM, Tomycz ND, Bailes J, de Jonge L, Lecoultre V, Wilent B, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg. 2013; 119:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhold RB. Critical analysis of long term weight loss following gastric bypass. Surg Gynecol Obstet. 1982;155:385–394. [PubMed] [Google Scholar]
- 18.Gobbi DG, Peters TM. Generalized 3D nonlinear transformations for medical imaging: an object oriented implementation in VTK. Comput Med Imaging Graph. 2003;27:255–265. [DOI] [PubMed] [Google Scholar]
- 19.Mai K Jr, Assheuer J, Paxinos G. Atlas of the Human Brain. 2nd ed. Amsterdam Elsevier Academic Press; 2004. [Google Scholar]
- 20.Compher C, Frankenfield D, Keim N, Roth-Yousey L. Evidence Analysis Working Group. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881–903. [DOI] [PubMed] [Google Scholar]
- 21.Lam YY, Redman LM, Smith SR, Bray GA, Greenway FL, Johannsen D, et al. Determinants of sedentary 24-h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. Am J Clin Nutr. 2014;99:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rui L. Brain regulation of energy balance and body weight. Rev Endocr Metab Disord. 2013;14:387–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum M, Leibel RL. Pathophysiology of childhood obesity. Adv Pediatr. 1988;35:73–137. [PubMed] [Google Scholar]
- 25.Rosenbaum M, Leibel RL, Hirsch J. Obesity. N Engl J Med. 1997;337:396–407. [DOI] [PubMed] [Google Scholar]
- 26.Wadden TA. Treatment of obesity by moderate and severe caloric restriction: results of clinical research trials. Ann Intern Med. 1993;119(Pt 2):688–693. [DOI] [PubMed] [Google Scholar]
- 27.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam YY, Ravussin E. Indirect calorimetry: an indispensable tool to understand and predict obesity. Eur J Clin Nutr. 2017;71:318–322. [DOI] [PubMed] [Google Scholar]
- 29.Torres N, Chabardes S, Piallat B, Devergnas A, Benabid AL. Body fat and body weight reduction following hypothalamic deep brain stimulation in monkeys: an intraventricular approach. Int J Obesw (Lond). 2012;36:1537–1544. [DOI] [PubMed] [Google Scholar]
- 30.Halpern CH, Tekriwal A, Santollo J, Keating JG, Wolf JA, Daniels D, et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci. 2013;33:7122–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keesey RE, Powley TL. Self-stimulation and body weight in rats with lateral hypothalamic lesions. Am J Physiol. 1973;224:970–978. [DOI] [PubMed] [Google Scholar]
- 32.Harrell LE, Decastro JM, Balagura S. A critical evaluation of body weight loss following lateral hypothalamic lesions. Physiol Behav. 1975;15:133–136. [DOI] [PubMed] [Google Scholar]
- 33.Herberg LJ, Blundell JE. Lateral hypothalamus: hoarding behavior elicited by electrical stimulation. Science. 1967;155:349–350. [DOI] [PubMed] [Google Scholar]
- 34.Sani S, Jobe K, Smith A, Kordower JH, Bakay RA. Deep brain stimulation for treatment of obesity in rats. J Neurosurg. 2007;107:809–813. [DOI] [PubMed] [Google Scholar]
- 35.Bandini LG, Must A, Phillips SM, Naumova EN, Dietz WH. Relation of body mass index and body fatness to energy expenditure: longitudinal changes from preadolescence through adolescence. Am J Clin Nutr. 2004;80:1262–1269. [DOI] [PubMed] [Google Scholar]
- 36.Ravussin E, Swinburn BA. Metabolic predictors of obesity: cross-sectional versus longitudinal data. Int J Obes Relat Metab Disord. 1993;17(suppl 3):S28–S31 [discussion: S41–S22]. [PubMed] [Google Scholar]


