Abstract
Intraductal cribriform (IDC) and invasive cribriform morphologies are associated with worse prostate cancer outcomes. Limited retrospective studies have associated IDC and cribriform morphology with germline mutations in DNA repair genes, particularly BRCA2. These findings, which prompted the National Comprehensive Cancer Network® (NCCN) Guidelines for Prostate Cancer and Genetic/Familial High- Risk Assessment to consider germline testing for individuals with IDC/cribriform histology, have been questioned in a recent prospective study. A deepened understanding of the molecular mechanisms driving disease aggressiveness in cribriform morphology is critical to provide more clarity in clinical decision making. This review summarizes the current understanding of IDC and cribriform prostate cancer, with an emphasis on clinical outcomes and molecular alterations.
Keywords: cribriform, intraductal cribriform, prostate cancer, outcomes, Grade Groups, BRCA2, germline testing, PTEN, SChLAP1, Cancer Associated Fibroblasts, ASPN, FAP, Tumor microenvironment
Introduction
Cribriform growth is a specific morphologic pattern seen across different types of neoplasms, defined as cohesive tumor cells with the presence of circular spaces, creating a sieve-like or Swiss cheese appearance. Cribriform histology can occur in invasive prostate cancer as well as intraductal carcinoma (IDC) of the prostate. Recent attention has been paid to cribriform prostate cancer and its association with clinical outcomes and genetic alterations. Herein we review the current literature on cribriform prostate cancer, including invasive tumor and IDC, with a focus on clinical outcomes, genetic implications, molecular findings, and imaging.
Gleason Grading, Grade Groups, Cribriform Morphology, and Intraductal Carcinoma
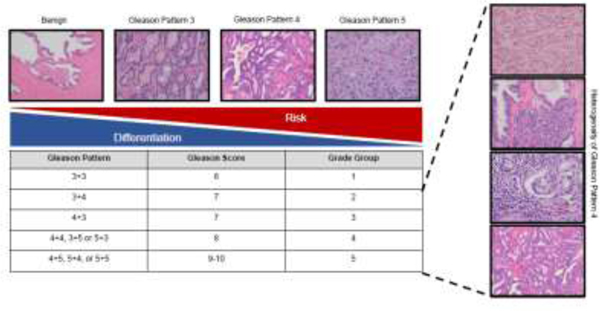
Prostate cancer is a heterogeneous disease that varies in morphology, genetics, and outcome, thereby, making treatment decisions challenging. Men with indolent disease are often effectively managed with active surveillance while some men with aggressive cancer progress to metastatic disease despite curative-intent therapy. Grade Groups, based on the original Gleason grading system, remain one of the strongest prognostic indicators of clinical outcome 1. This system stratifies individuals into one of five Grade Groups based on the overall Gleason Score (GS) 2, 3. Increased GS is associated with worse cancer outcomes. Individuals with GS ≤ 6 are more likely to have favorable outcomes while individuals with GS 8–10 have a high risk of adverse outcomes. Men with GS 7 show varied outcomes, and their risk stratification has been improved by the Grade Group system (Figure 1) 4.
Figure 1. Overview of the Gleason Grading and Grade Group System.
Representative images for each Gleason Pattern, including the respective Gleason Scores and corresponding Grade Group number. The Gleason Pattern 4 spans Grade Groups 2–5. Cancer risk and gland differentiation are inversely related as Grade Group increases.
Despite grading improvements, risk stratification remains challenging for some men due to the morphological heterogeneity present in Gleason pattern 4 cancer. Gleason pattern 4 consists of four architectural subtypes: ill-formed, fused, glomeruloid and cribriform 5. Specifically, cribriform morphology has been associated with adverse outcomes compared to other Gleason pattern 4 morphologies 6–10. Originating from the Latin word ‘cribrum,’ meaning sieve, cribriform describes the appearance of perforations within sheets of epithelial cells 2, 11. Chua et al., proposed that cribriform patterning contributes to the buildup of multiple unfavorable events (termed ‘nimbosus’, for the gathering of stormy clouds) 12 potentially leading to more adverse outcomes 7, 8, 13.
The Gleason Grading system has evolved to reflect the aggressive phenotype observed with cribriform morphology. Originally, cribriform patterns were assigned to Gleason pattern 3. In 2005, most cribriform patterns were re-assigned to Gleason pattern 4, with only small, rounded, and well-circumscribed cribriform patterns remaining as Gleason pattern 3 3. The 2014 revisions by the International Society of Urological Pathology (ISUP) recommended that all cribriform patterns be recognized as Gleason pattern 4 regardless of size or morphology 1. The 2014 revisions also introduced Grade Groups, which further stratified men with GS 7. Men with GS 7 (3+4) were assigned to Group 2 and GS 7 (4+3) assigned to Group 3, suggesting increased aggressiveness in men with more prominent pattern 4 prostate cancer 1, 3. Despite these advancements, cribriform architecture still spans groups 2–5 14, leading to variability in patient outcome and treatment decisions 1. In addition, there is still ambiguity in how to categorize the invasive cribriform morphological mimicker, prostate IDC. IDC features the proliferation of malignant epithelial cells with similar morphology as invasive cribriform cancer but within prostatic ducts 15. Histological staining of basal markers is the definitive assay to distinguish IDC from invasive cribriform tumors, which lack basal cell markers 5, 16. Co- occurrence between these morphological features is common, with 47% of cribriform prostate cancer containing areas of IDC and 68% of IDC containing cribriform patterns 6. Other morphologic patterns of IDC include comedo necrosis, solid growth, and extreme nuclear atypia. Both invasive cribriform tumors and IDC in prostate adenocarcinoma are associated with worse outcomes 6, 15, 17, 18. Due to the strong morphological and biological overlap, many studies combine these subtypes into a single prognostic group for analysis. The current ISUP guidelines recommend incorporating the grade of IDC into the GS when invasive cancer is present 19, while the GUPS recommends against including IDC in the final GS 20. However, both societies recommend reporting the presence of cribriform morphology and IDC and hold that cribriform morphology should be an exclusion criterium for active surveillance.
Clinical Outcomes
A growing body of evidence suggests that cribriform morphology contributes to adverse outcomes and has potential to impact clinical decisions. Several studies have investigated the clinical implication of cribriform tumor found on prostate biopsy. Cribriform growth detected at initial biopsy corresponds with higher Gleason Grade and tumor stage at radical prostatectomy 21. In addition, Kweldam et al. showed that in men with Grade Group 2 (GS 3+4) prostate cancer at biopsy, cribriform morphology or IDC, and not percent Gleason pattern 4 was an independent predictor of biochemical recurrence 22. Few studies have reported the impact of cribriform tumor on therapy resistance, however, a recent study of neoadjuvant androgen blockade in men with locally high-risk prostate cancer identified an association of cribriform morphology at biopsy with a worse response to therapy, suggesting that cribriform tumor may contribute to hormone ablation therapy resistance 23.
Additional studies have also investigated the clinical impact of cribriform morphology detected at radical prostatectomy. Increased lymph node positive status at prostatectomy has been reported in men with IDC or cribriform morphology 24. Additionally, cribriform morphology detected at radical prostatectomy is associated with an increased risk of biochemical recurrence 6–9 and a shorter time to biochemical recurrence, with an average time of 34 months compared to 120 months for men without cribriform morphology 7. The association of cribriform morphology at radical prostatectomy with worse biochemical and metastasis-free survival is independent of Grade Group in patients with Grade Group 4 (Gleason grade 8) prostate cancer 25. Consistent with this, studies have reported that men with cribriform morphology at the time of prostatectomy are more likely to develop metastases 9 and experience a shorter time to metastasis 7. Studies have also shown that men with cribriform morphology at biopsy or radical prostatectomy have decreased overall survival and increased disease- specific death 7, 10, 15, 26.
Due to the association of cribriform morphology and IDC with adverse outcomes, Van Leenders et al. proposed a new grading system, cGrade, which incorporates both cribriform and IDC into the Grade Group system to increase prognostic value 18. According to this modified system, for patients with Grade Group 2–5 prostate cancers, the cGrade is the same as the current Grade Group if cribriform morphology and IDC are present, however, the absence of these morphologies results in a cGrade of the current Grade Group minus 1. In the European Randomized Study of Screening for Prostate Cancer cohort, cGrade improved prognostication of both disease-specific survival and metastasis-free survival compared to the current Grade Group system. To date, cGrade has not been incorporated into ISUP or GUPS prostate cancer grading consensus statements.
Inconsistencies Diagnosing and Reporting Cribriform Prostate Cancer and IDC
Although studies suggest that inclusion of cribriform morphology improves risk stratification, reporting inconsistencies limit the clinical utility of cribriform morphology to identify at-risk individuals. Specifically, a 2019 pre-meeting survey for the Genitourinary Pathology Society (GUPS) revealed only 40% of U.S. pathologists reported the presence of cribriform glands on patient biopsies 20. Amongst pathologists who do report the presence of cribriform morphology, inconsistencies remain, including differences in size cutoffs and reporting style (i.e., presence/absence versus overall tumor percent) 20. Currently there is not a strong consensus on sizing criteria when distinguishing small (simple) and large (expansile) cribriform glands. Some groups define large cribriform glands as having more than 12 luminal spaces 27–29, with other groups using benign glands as a reference 6, 30. It is unclear if cribriform gland size leads to outcome differences. Hollemans et al. reported that only large cribriform patterning was an independent predictor of biochemical recurrence and metastasis in patients with Grade 2 prostate cancer 30 while other studies suggest both small and large cribriform glands lead to adverse outcomes and increased PSA failure 27, 31. Ambrosini et al. proposed the use of an automated reporting system for patient biopsies to reduce inter-observer variability and to increase the detection of cribriform 32. Taken together, these data suggest that interobserver variability could lead to inconsistences in cribriform morphology reporting, emphasizing the importance of refining current definitions to reduce reporting discrepancies.
Biopsy under sampling also contributes to differences in cribriform morphology reporting between biopsy and radical prostatectomy. Hollemans et al. assessed under sampling in patient biopsies through matched radical prostatectomy sections, revealing that 40% of men were false negative for cribriform at initial biopsy 33. Recent studies support these findings, suggesting biopsies have high specificity (87.2–94.9%) but low sensitivity (47.2–56.6%) in detecting cribriform morphology (Table 1) 34, 35. Discordant results between biopsy and prostatectomy led to significant tumor upstaging following radical prostatectomy. These findings emphasize the importance of both improving detection methods and considering the implementation of multimodal detection methods for men negative for cribriform morphology at biopsy to better assess treatment decisions.
Table 1. Cribriform-specific Sensitivity and Specificity for Various Detection Methods.
Sensitivity and specificity of various prostate cancer detection methods in identifying cribriform morphology are outlined. In general, techniques have high specificity and low sensitivity.
| Detection Method | Sensitivity (cribriform specific) | Specificity (cribriform specific) |
|---|---|---|
| Systematic Biopsy (SB) | 20.7% 37
43% 33 44.7% 35 56.5% 34 |
29.2% 37
97% 33 93% 35 87.2% 34 |
| Targeted biopsy (TB) (Fusion Biopsy/MRI-TBx) | 28.6% 37
53.5% 34 |
32.4% 37
76.5% 34 |
| 68Ga-PSMA PET/CT | 76% 43 | 86% 43 |
| Nomogram | 79.2% 44 | 84% 44 |
| Mp-MRI | 90.5% 41 | *Specificity not calculated due to all MRIs being performed in men with prostate cancer (no control) |
Imaging Cribriform Prostate Cancer
Improved imaging modalities to identify cribriform morphology at disease onset are needed. Currently, multiparametric magnetic resonance imaging (mpMRI), a non- invasive 3D imaging technique, has risen to prominence in prostate cancer detection. Small retrospective studies suggested cribriform morphology is invisible to mpMRI 36–38 with only 17% of cribriform patterning detected upon analysis 37. However, recent studies contradict these findings. Van Houdt et. al. determined cribriform was not an indicator of mpMRI invisible regions 39. Instead, invisible tumors were more likely to contain lower grade (GG1–2) prostate cancer and heterogeneous morphology compared to visible tumors 39. Other studies suggest mpMRI improves detection of cribriform morphology compared to standard biopsy methods 40, 41 with a cribriform-specific sensitivity of 90.5% 41. Consistent with these findings, cribriform morphology was identified as an independent factor associated with increased PI-RADS score derived from MRI analysis 42. Cumulatively, these studies suggest that mpMRI may serve as a promising diagnostic tool for detection of cribriform morphology.
Other novel detection methods include positron emission tomography (PET) scans with nuclear labeled prostate-specific membrane antigen ligands (PSMA), termed 68Ga- PSMA PET/CT 43. Cribriform morphology had increased uptake, supporting the utility of 68Ga-PSMA PET/CT as a less invasive prediction tool, especially when combined with other detection techniques such as MRI. Lastly, Gao et al. 2020 developed of a novel risk-nomogram that combines clinical characteristics with mpMRI analysis to serve as an improved prognostic indicator of cribriform morphology 44. The three independent predictors that increased accurate detection of cribriform morphology were prostate specific antigen density (PSAD), PI-RADS score, and overall biopsy GS. Although further validation is needed to assess clinical utility, these results emphasize the importance of assessing multiple factors when predicting risk of cribriform morphology and highlight promising future developments to prostate cancer imaging techniques.
Genetic, Genomic, and Molecular Alterations
Much research has focused on identifying the genetic, genomic, and molecular underpinnings of cribriform prostate cancer and IDC aggressiveness. Retrospective studies have associated IDC with germline mutations in DNA repair genes, particularly BRCA2 45–47. Pathogenic germline alterations in BRCA1/2 correspond with higher risk of metastases and worse survival 48, 49, and additionally have implications for both familial testing and therapeutic selection. This association of BRCA2 and IDC in these studies prompted the National Comprehensive Cancer Network® (NCCN) guidelines for prostate cancer IDC 50 and Genetic/Familial High-Risk Assessment 51 to include a consideration for germline testing in men who have prostate cancer with cribriform morphology or IDC. Recently, a prospective study by Lozano et al., found no association between germline BRCA2 mutations and cribriform/IDC histology when analyzing primary prostate tumor samples 52. These new findings call into question the current guidelines and demonstrate the need for further investigation into the role of germline BRCA2 alterations in cribriform morphology prostate cancer.
In contrast to germline mutations, Lozano et al. reported an association of biallelic somatic loss of BRCA2 with both cribriform and IDC histological subtypes 52, thereby, supporting a role for BRCA2 in the development of cribriform morphology and IDC. Additionally, cribriform morphology has been reported to have increased genomic instability and copy number alterations when compared to other Gleason pattern 4 morphologies 10 and non-cribriform prostate cancers 16. Cribriform morphology is also associated with deletions in common tumor suppressor genes including CHD1, TP53, NKX3–1, MAP3K7, RB1 and PTEN 10, 16. PTEN, TP53, and RB1 loss are increased in metastatic castration-resistant prostate cancer (mCRPC) and are associated with therapy resistance 53–57. Genetic amplifications in cribriform prostate cancer are less common, with gain of MYC and LY6 being reported 16. Genetic rearrangements of the ETS-related gene (ERG) have also been reported 58; however, Elfandy et al. found that ERG fusions were not significantly different in men with cribriform morphology compared to other non- cribriform patterns when analyzed in the prostate TCGA cohort 10. In addition to chromosomal abnormalities, point mutations have also been reported, impacting genes involved in DNA repair (TP53, ATM), ubiquitination (SPOP), and transcription (FOXA1)10.
Few studies have assessed the association of cribriform morphology with epigenetic alterations. DNA methylation signatures are of interest as altered DNA methylation is common in aggressive prostate cancers, including mCRPC 53. Elfandy et al. found that patients in the TCGA prostate cohort with cribriform morphology also showed increased methylation, resulting in a molecular phenotype that closely resembled metastatic prostate cancer 10. Increased methylation of several genes corresponded to decreased expression of some genes including those associated with aggressive prostate cancer (EVX1, EPHX3, ABDH9, IRAK3), while hypermethylation of EZH2 led to increased expression. Oklhov-Mitsel et al., previously reported the hypermethylation of APC, RASSF1 and TBX15 in samples with cribriform morphology 59; however, Elfandy et al. did not detect a difference in the methylation profile of these genes in the TCGA cohort. Thus, future studies will be informative in further elucidating the epigenetic signature of cribriform prostate cancer and to determine if these alterations serve as useful biomarkers for the identification of at-risk patients.
Genomic instability likely contributes to the dysregulation of important regulatory pathways enriched in prostate cancers with cribriform morphology including mTORC1, MYC, MAPK, KRAS and JAK-STAT 10. Aberrations in RNA have also been described in prostate cancer with cribriform morphology. Increased expression in the long noncoding RNA SChLAP1 was recently reported 12. SChLAP1 overexpression is strongly associated with increased metastases 60, suggesting it could serve as a valuable marker of aggressive disease. Patients with cribriform prostate cancer also score worse in RNA- based screening tests including the OncotypeDx Genomic Prostate Score® (GPS) assay 31 and the Decipher test 17. As the NCCN guidelines recommend these assays for men with low or intermediate-risk disease 50, their association with cribriform morphology could serve to further assess patient risk and improve prognostic decisions.
Protein alterations have also been reported in cribriform morphology. IHC analysis of primary prostate cancers demonstrated increased phosphorylation of the proto- oncogene MET 61, which has been associated with resistance to androgen deprivation therapy in preclinical models 62. Other enriched proteins include EGFR 63 and Ki-67 64, suggesting cribriform patterning has a higher number of actively proliferating cells compared to other morphologies. Downregulated proteins include PTEN 65, 66, p27 65, and CD44 63. Chen et al., also reported the dysregulation of important AR-interacting proteins, NRIP and DDB2 67. NRIP and AR expression positively correlated while DDB2 showed decreased expression in prostate cancer tissues. This pattern occurred more frequently in men with cribriform morphology, suggesting that cribriform could alter AR signaling to drive cancer aggressiveness.
Collectively, these data suggest that cribriform prostate cancer has a unique molecular repertoire compared to other prostate cancer morphologies that may contribute to worse outcomes. Although many of these molecular alterations have expression profiles associated with cribriform morphology, the biomarkers are not solely cribriform- specific. The discovery of new biomarkers that are unique to cribriform morphology would be valuable in increasing the detection and treatment options for cribriform prostate cancer (Figure 2).
Figure 2. Summary of Factors Altered in Cribriform Morphology Prostate Cancer.
Factors associated with cribriform morphology prostate cancer and IDC. Figure created with BioRender.com.
Tumor Microenvironment
Less is known about how the tumor microenvironment impacts cribriform prostate cancer. The tumor microenvironment consists of a heterogeneous cellular population and a diverse connective tissue composition, both of which can influence tumor progression and metastasis. Alterations within integrin signaling have been observed in patients with cribriform prostate cancer 68. Connell et al., revealed cribriform patterning had aberrant and increased expression of the Integrin αv (ITGAV). ITGAV expression formed a dense stromal ring around cribriform glands, leading to the hypothesis that ITGAV serves as a mediator of crosstalk between stromal cells and cancer cells, resulting in signaling regulation, stem-cell maintenance, and tumor survival 68. Increased integrin expression has been suggested to increase cancer cell migration, as increased expression has been reported in bone metastases in prostate cancer patients 68.
Hypoxia is thought to occur in cribriform cancer due to the 3D architecture of cribriform glands. And indeed, central comedo necrosis is often seen with cribriform IDC. Most cribriform glands do not directly contact the surrounding stroma and contain limited intervening stroma, thus restricting the amount of oxygen able to penetrate the dense gland architecture 69. The architecture of most other prostate morphologies contain tumor cells in direct contact with the stroma, thereby, limiting this hypoxic effect 69. In support of this, Chua et al., reported increased hypoxia in patients with cribriform patterning, which corresponded with increased disease recurrence and resistance to therapy 12.
The stroma surrounding cribriform glands was recently reported to be highly heterogenous and enriched in cancer associated fibroblasts (CAFs) subtypes expressing markers associated with worse outcomes 70. Specifically, ASPN+ and FAP+ CAFs were elevated in cribriform morphology prostate cancer compared to other Gleason pattern 4 morphologies and lower Gleason patterns 70. Altogether, these findings suggest cribriform prostate cancer contains a heterogenous and reactive stroma that may contribute to worse cancer outcomes. Further studies are needed to determine mechanistically how specific fibroblast subtypes contribute to cancer progression and disease aggressiveness (Figure 2).
Concluding Remarks
Cribriform prostate cancer remains an aggressive form of prostate cancer. Although current research has improved our understanding of the nature of cribriform prostate cancer, the specific mechanisms driving cribriform aggressiveness are unknown. Future studies should focus on discovering cribriform specific biomarkers, enhancing the current model systems, and increasing early detection methods. Combined, these findings will provide increased understanding to the specific mechanisms driving cancer aggressiveness in men with cribriform morphology as well as unveil novel biomarkers that could lead to earlier disease detection and therapeutic targets. By improving disease detection, reporting, and enhancing therapeutic outcome, this could not only lead to increased risk stratification but also improve quality of life in men impacted with cribriform prostate cancer.
Acknowledgements:
This work was supported by the American Cancer Society 131356-RSG-17-160-01-CSM (PJH, JG), the National Cancer Institute/National Institute of Health RO1CA211695-01A1 (PJH, JG), the Eckstein Foundation (PJH), Microenvironmental Influences in Cancer Training Program T32CA009592 (ABH).
Footnotes
Conflict of interest: Paula Hurley receives royalties from Horizon Discovery for the generation of targeted cell lines. No potential conflicts of interest were disclosed by the other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen N, Zhou Q. The evolving Gleason grading system. Chin J Cancer Res. 2016;28:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50:125–128. [PubMed] [Google Scholar]
- 3.Epstein JI. Prostate cancer grading: a decade after the 2005 modified system. Mod Pathol. 2018;31:S47–63. [DOI] [PubMed] [Google Scholar]
- 4.Wright JL, Salinas CA, Lin DW, et al. Prostate cancer specific mortality and Gleason 7 disease differences in prostate cancer outcomes between cases with Gleason 4 + 3 and Gleason 3 + 4 tumors in a population based cohort. J Urol. 2009;182:2702–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kweldam CF, van der Kwast T, van Leenders GJ. On cribriform prostate cancer. Transl Androl Urol. 2018;7:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trudel D, Downes MR, Sykes J, Kron KJ, Trachtenberg J, van der Kwast TH. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur J Cancer. 2014;50:1610–1616. [DOI] [PubMed] [Google Scholar]
- 7.Kweldam CF, Wildhagen MF, Steyerberg EW, Bangma CH, van der Kwast TH, van Leenders GJ. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol. 2015;28:457–464. [DOI] [PubMed] [Google Scholar]
- 8.Kir G, Sarbay BC, Gumus E, Topal CS. The association of the cribriform pattern with outcome for prostatic adenocarcinomas. Pathol Res Pract. 2014;210:640–644. [DOI] [PubMed] [Google Scholar]
- 9.Dong F, Yang P, Wang C, et al. Architectural heterogeneity and cribriform pattern predict adverse clinical outcome for Gleason grade 4 prostatic adenocarcinoma. Am J Surg Pathol. 2013;37:1855–1861. [DOI] [PubMed] [Google Scholar]
- 10.Elfandy H, Armenia J, Pederzoli F, et al. Genetic and Epigenetic Determinants of Aggressiveness in Cribriform Carcinoma of the Prostate. Mol Cancer Res. 2019;17:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branca G, Ieni A, Barresi V, Tuccari G, Caruso RA. An Updated Review of Cribriform Carcinomas with Emphasis on Histopathological Diagnosis and Prognostic Significance. Oncol Rev. 2017;11:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua MLK, Lo W, Pintilie M, et al. A Prostate Cancer “Nimbosus”: Genomic Instability and SChLAP1 Dysregulation Underpin Aggression of Intraductal and Cribriform Subpathologies. Eur Urol. 2017;72:665–674. [DOI] [PubMed] [Google Scholar]
- 13.Tom MC, Nguyen JK, Luciano R, et al. Impact of Cribriform Pattern and Intraductal Carcinoma on Gleason 7 Prostate Cancer Treated with External Beam Radiotherapy. J Urol. 2019;202:710–716. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Hirsch MS. A Comparison of Genitourinary Pathology Society (GUPS) and International Society of Urological Pathology (ISUP) Prostate Cancer Grading Guidelines. Am J Surg Pathol. 2021. [DOI] [PubMed]
- 15.Van der Kwast T, Al Daoud N, Collette L, et al. Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur J Cancer. 2012;48:1318–1325. [DOI] [PubMed] [Google Scholar]
- 16.Bottcher R, Kweldam CF, Livingstone J, et al. Cribriform and intraductal prostate cancer are associated with increased genomic instability and distinct genomic alterations. BMC Cancer. 2018;18:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor AS, Morgan TM, Wallington DG, Chinnaiyan AM, Spratt DE, Mehra R. Correlation between cribriform/intraductal prostatic adenocarcinoma and percent Gleason pattern 4 to a 22-gene genomic classifier. Prostate. 2020;80:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Leenders G, Kweldam CF, Hollemans E, et al. Improved Prostate Cancer Biopsy Grading by Incorporation of Invasive Cribriform and Intraductal Carcinoma in the 2014 Grade Groups. Eur Urol. 2020;77:191–198. [DOI] [PubMed] [Google Scholar]
- 19.van Leenders G, van der Kwast TH, Grignon DJ, et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am J Surg Pathol. 2020;44:e87–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein JI, Amin MB, Fine SW, et al. The 2019 Genitourinary Pathology Society (GUPS) White Paper on Contemporary Grading of Prostate Cancer. Arch Pathol Lab Med. 2020. [DOI] [PubMed]
- 21.Haffner MC, Salles DC, Gao G, Epstein JI. Gleason pattern 4 with cribriform morphology on biopsy is associated with adverse clinicopathological findings in a prospective radical prostatectomy cohort. Hum Pathol. 2020;98:74–80. [DOI] [PubMed] [Google Scholar]
- 22.Kweldam CF, Kummerlin IP, Nieboer D, et al. Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3+4=7 prostate cancer. Mod Pathol. 2017;30:1126–1132. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Qi M, Zhang J, et al. Differential response to neoadjuvant hormonal therapy in prostate cancer: Predictive morphological parameters and molecular markers. Prostate. 2019;79:709–719. [DOI] [PubMed] [Google Scholar]
- 24.Downes MR, Xu B, van der Kwast TH. Gleason grade patterns in nodal metastasis and corresponding prostatectomy specimens: impact on patient outcome. Histopathology. 2019;75:715–722. [DOI] [PubMed] [Google Scholar]
- 25.Hollemans E, Verhoef EI, Bangma CH, et al. Cribriform architecture in radical prostatectomies predicts oncological outcome in Gleason score 8 prostate cancer patients. Mod Pathol. 2021;34:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kweldam CF, Kummerlin IP, Nieboer D, et al. Disease-specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod Pathol. 2016;29:630–636. [DOI] [PubMed] [Google Scholar]
- 27.Iczkowski KA, Torkko KC, Kotnis GR, et al. Digital quantification of five high- grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am J Clin Pathol. 2011;136:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenney JK, Wei W, Hawley S, et al. Histologic Grading of Prostatic Adenocarcinoma Can Be Further Optimized: Analysis of the Relative Prognostic Strength of Individual Architectural Patterns in 1275 Patients From the Canary Retrospective Cohort. Am J Surg Pathol. 2016;40:1439–1456. [DOI] [PubMed] [Google Scholar]
- 29.Keefe DT, Schieda N, El Hallani S, et al. Cribriform morphology predicts upstaging after radical prostatectomy in patients with Gleason score 3 + 4 = 7 prostate cancer at transrectal ultrasound (TRUS)-guided needle biopsy. Virchows Arch. 2015;467:437–442. [DOI] [PubMed] [Google Scholar]
- 30.Hollemans E, Verhoef EI, Bangma CH, et al. Large cribriform growth pattern identifies ISUP grade 2 prostate cancer at high risk for recurrence and metastasis. Mod Pathol. 2019;32:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenland NY, Zhang L, Cowan JE, Carroll PR, Stohr BA, Simko JP. Correlation of a Commercial Genomic Risk Classifier with Histological Patterns in Prostate Cancer. J Urol. 2019;202:90–95. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosini P, Hollemans E, Kweldam CF, Leenders G, Stallinga S, Vos F. Automated detection of cribriform growth patterns in prostate histology images. Sci Rep. 2020;10:14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollemans E, Verhoef EI, Bangma CH, et al. Concordance of cribriform architecture in matched prostate cancer biopsy and radical prostatectomy specimens. Histopathology. 2019;75:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ericson KJ, Wu SS, Lundy SD, Thomas LJ, Klein EA, McKenney JK. Diagnostic Accuracy of Prostate Biopsy for Detecting Cribriform Gleason Pattern 4 Carcinoma and Intraductal Carcinoma in Paired Radical Prostatectomy Specimens: Implications for Active Surveillance. J Urol. 2020;203:311–319. [DOI] [PubMed] [Google Scholar]
- 35.Masoomian M, Downes MR, Sweet J, et al. Concordance of biopsy and prostatectomy diagnosis of intraductal and cribriform carcinoma in a prospectively collected data set. Histopathology. 2019;74:474–482. [DOI] [PubMed] [Google Scholar]
- 36.Truong M, Hollenberg G, Weinberg E, Messing EM, Miyamoto H, Frye TP. Impact of Gleason Subtype on Prostate Cancer Detection Using Multiparametric Magnetic Resonance Imaging: Correlation with Final Histopathology. J Urol. 2017;198:316–321. [DOI] [PubMed] [Google Scholar]
- 37.Truong M, Feng C, Hollenberg G, et al. A Comprehensive Analysis of Cribriform Morphology on Magnetic Resonance Imaging/Ultrasound Fusion Biopsy Correlated with Radical Prostatectomy Specimens. J Urol. 2018;199:106–113. [DOI] [PubMed] [Google Scholar]
- 38.Houlahan KE, Salmasi A, Sadun TY, et al. Molecular Hallmarks of Multiparametric Magnetic Resonance Imaging Visibility in Prostate Cancer. Eur Urol. 2019;76:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Houdt PJ, Ghobadi G, Schoots IG, et al. Histopathological Features of MRI- Invisible Regions of Prostate Cancer Lesions. J Magn Reson Imaging. 2020;51:1235–1246. [DOI] [PubMed] [Google Scholar]
- 40.Prendeville S, Gertner M, Maganti M, et al. Role of Magnetic Resonance Imaging Targeted Biopsy in Detection of Prostate Cancer Harboring Adverse Pathological Features of Intraductal Carcinoma and Invasive Cribriform Carcinoma. J Urol. 2018;200:104–113. [DOI] [PubMed] [Google Scholar]
- 41.Tonttila PP, Ahtikoski A, Kuisma M, Paakko E, Hirvikoski P, Vaarala MH. Multiparametric MRI prior to radical prostatectomy identifies intraductal and cribriform growth patterns in prostate cancer. BJU Int. 2019;124:992–998. [DOI] [PubMed] [Google Scholar]
- 42.Naito H, Kato T, Ishikawa R, et al. The Impact of Histopathological Features of Prostate Cancerous Lesions on Multiparametric Magnetic Resonance Imaging Findings using PI-RADS Version 2. Urology. 2021;149:174–180. [DOI] [PubMed] [Google Scholar]
- 43.Gao J, Zhang C, Zhang Q, et al. Diagnostic performance of (68)Ga-PSMA PET/CT for identification of aggressive cribriform morphology in prostate cancer with whole-mount sections. Eur J Nucl Med Mol Imaging. 2019;46:1531–1541. [DOI] [PubMed] [Google Scholar]
- 44.Gao J, Zhang Q, Fu Y, et al. Combined clinical characteristics and multiparametric MRI parameters for prediction of cribriform morphology in intermediate-risk prostate cancer patients. Urol Oncol. 2020;38:216–224. [DOI] [PubMed] [Google Scholar]
- 45.Risbridger GP, Taylor RA, Clouston D, et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur Urol. 2015;67:496–503. [DOI] [PubMed] [Google Scholar]
- 46.Taylor RA, Fraser M, Livingstone J, et al. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat Commun. 2017;8:13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schweizer MT, Antonarakis ES, Bismar TA, et al. Genomic Characterization of Prostatic Ductal Adenocarcinoma Identifies a High Prevalence of DNA Repair Gene Mutations. JCO Precis Oncol. 2019;3. [DOI] [PMC free article] [PubMed]
- 48.Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Na R, Zheng SL, Han M, et al. Germline Mutations in ATM and BRCA1/2 Distinguish Risk for Lethal and Indolent Prostate Cancer and are Associated with Early Age at Death. Eur Urol. 2017;71:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prostate NCCN-. 2.2021. [Google Scholar]
- 51.National Comprehensive Cancer Network - Genetic/Familial High-Risk Assessment: Breast O, and Pancreatic 2.2021. [Google Scholar]
- 52.Lozano R, Salles DC, Sandhu S, et al. Association between BRCA2 alterations and intraductal and cribriform histologies in prostate cancer. European Journal of Cancer. 2021;147:74–83. [DOI] [PubMed] [Google Scholar]
- 53.Friedlander TW, Roy R, Tomlins SA, et al. Common structural and epigenetic changes in the genome of castration-resistant prostate cancer. Cancer Res. 2012;72:616–625. [DOI] [PubMed] [Google Scholar]
- 54.Torquato S, Pallavajjala A, Goldstein A, et al. Genetic Alterations Detected in Cell-Free DNA Are Associated With Enzalutamide and Abiraterone Resistance in Castration-Resistant Prostate Cancer. JCO Precis Oncol. 2019;3. [DOI] [PMC free article] [PubMed]
- 55.Abida W, Armenia J, Gopalan A, et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis Oncol. 2017;2017. [DOI] [PMC free article] [PubMed]
- 56.Annala M, Vandekerkhove G, Khalaf D, et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov. 2018;8:444–457. [DOI] [PubMed] [Google Scholar]
- 57.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosquera JM, Perner S, Demichelis F, et al. Morphological features of TMPRSS2-ERG gene fusion prostate cancer. J Pathol. 2007;212:91–101. [DOI] [PubMed] [Google Scholar]
- 59.Olkhov-Mitsel E, Siadat F, Kron K, et al. Distinct DNA methylation alterations are associated with cribriform architecture and intraductal carcinoma in Gleason pattern 4 prostate tumors. Oncol Lett. 2017;14:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prensner JR, Zhao S, Erho N, et al. RNA biomarkers associated with metastatic progression in prostate cancer: a multi-institutional high-throughput analysis of SChLAP1. Lancet Oncol. 2014;15:1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukai S, Yorita K, Yamasaki K, et al. Expression of human kallikrein 1-related peptidase 4 (KLK4) and MET phosphorylation in prostate cancer tissue: immunohistochemical analysis. Hum Cell. 2015;28:133–142. [DOI] [PubMed] [Google Scholar]
- 62.Cannistraci A, Federici G, Addario A, et al. C-Met/miR-130b axis as novel mechanism and biomarker for castration resistance state acquisition. Oncogene. 2017;36:3718–3728. [DOI] [PubMed] [Google Scholar]
- 63.Xiao GQ, Nguyen E, Unger PD, Sherrod AE. Comparative expression of immunohistochemical biomarkers in cribriform and pattern 4 non-cribriform prostatic adenocarcinoma. Exp Mol Pathol. 2020;114:104400. [DOI] [PubMed] [Google Scholar]
- 64.Fu L, Hwang M, Adeniran AJ, Humphrey PA. Proliferation index of different Gleason pattern 4 histomorphologies and associated pattern 3 adenocarcinoma of the prostate. Hum Pathol. 2017;70:1–5. [DOI] [PubMed] [Google Scholar]
- 65.Ronen S, Abbott DW, Kravtsov O, et al. PTEN loss and p27 loss differ among morphologic patterns of prostate cancer, including cribriform. Hum Pathol. 2017;65:85–91. [DOI] [PubMed] [Google Scholar]
- 66.Shah RB, Shore KT, Yoon J, Mendrinos S, McKenney JK, Tian W. PTEN loss in prostatic adenocarcinoma correlates with specific adverse histologic features (intraductal carcinoma, cribriform Gleason pattern 4 and stromogenic carcinoma). Prostate. 2019;79:1267–1273. [DOI] [PubMed] [Google Scholar]
- 67.Chen HH, Fan P, Chang SW, Tsao YP, Huang HP, Chen SL. NRIP/DCAF6 stabilizes the androgen receptor protein by displacing DDB2 from the CUL4A- DDB1 E3 ligase complex in prostate cancer. Oncotarget. 2017;8:21501–21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Connell B, Kopach P, Ren W, et al. Aberrant integrin alphav and alpha5 expression in prostate adenocarcinomas and bone-metastases is consistent with a bone-colonizing phenotype. Transl Androl Urol. 2020;9:1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verhoef EI, van Cappellen WA, Slotman JA, et al. Three-dimensional analysis reveals two major architectural subgroups of prostate cancer growth patterns. Mod Pathol.2019;32:1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hesterberg AB, Rios BL, Wolf EM, et al. A distinct repertoire of cancer- associated fibroblasts is enriched in cribriform prostate cancer. J Pathol Clin Res. 2021. [DOI] [PMC free article] [PubMed]