Abstract
Spinal cord injury is a serious damage to the spinal cord that can lead to life-long disability. Based on its etiology, spinal cord injury can be classified as traumatic or non-traumatic spinal cord injury. Furthermore, the pathology of spinal cord injury can be divided into two phases, a primary injury phase, and a secondary injury phase. The primary spinal cord injury phase involves the initial mechanical injury in which the physical force of impact is directly imparted to the spinal cord, disrupting blood vessels, axons, and neural cell membranes. After the primary injury, a cascade of secondary events begins, expanding the zone of neural tissue damage, and exacerbating neurological deficits. Secondary injury is a progressive condition characterized by pro-inflammatory cytokines, reactive oxygen species, oxidative damage, excitatory amino acids such as glutamate, loss of ionic homeostasis, mitochondrial dysfunction, and cell death. This secondary phase lasts for several weeks or months and can be further subdivided into acute, subacute, and chronic. One of the most frequent and devastating complications developed among the spinal cord injury population is cognitive impairment. The risk of cognitive decline after spinal cord injury has been reported to be 13 times higher than in healthy individuals. The exact etiology of this neurological complication remains unclear, however, many factors have been proposed as potential contributors to the development of this disorder, such as concomitant traumatic brain injury, hypoxia, anoxia, autonomic dysfunction, sleep disorders such as obstructive sleep apnea, body temperature dysregulation, alcohol abuse, and certain drugs. This review focuses on a deep understanding of the pathophysiology of spinal cord injury and its relationship to cognitive impairment. We highlight the main mechanisms that lead to the development of this neurological complication in patients with spinal cord injury.
Key Words: autonomic dysfunction, cognitive impairment, spinal cord injury, traumatic brain injury
Introduction
Spinal cord injury (SCI) is a serious damage to the spinal cord that can lead to lifelong disability. To define the etiology of SCI it is first necessary to describe the underlying mechanisms that lead to the injury itself, which can be classified as traumatic SCI or non-traumatic SCI. Non-traumatic SCI can occur due to an underlying pathology, such as autoimmune, infectious, vascular, or oncologic diseases (Venkatesh et al., 2019). Traumatic SCI causes include fractures, motor vehicle accidents, acts of violence, and recreational activities. Regardless of the cause, the injury leads to motor, sensory and autonomic dysfunction below the level of the lesion, with paralysis and multiple system dysfunction being one of the most severe consequences (Craig et al., 2015). This event represents serious consequences for the affected population, both physically and psychologically. SCI predisposes individuals to the development of psychological alterations such as excessive fatigue, feelings of tiredness, depressive states, poor-self efficacy, and anxiety (Craig et al., 2012, 2015), which, together with the severe conditions caused by the direct aggression on the spinal cord, do not only compromise the patient’s health but also their quality of life (Craig et al., 2012).
For decades, SCI has been a major health concern since affected individuals have shown a drastic decrease in life expectancy. Life expectancy after SCI is 3.7 years and is determined by several factors, such as the spinal cord affected level, the etiology of the injury, ethnicity, and gender, among others (2012). However, thanks to improved health services and technological advances, the survival rate is currently over 90% of individuals in the first year and close to 50% in the 40 years post-injury (Middleton et al., 2012). According to the National Spinal Cord Injury Statistical Center, the incidence of SCI is approximately 54 cases per one million people in the United States, which is equivalent to about 17,900 SCI cases per year (2012). Furlan et al. (2013) reported that the incidence varies between 8.0 to 246.0 cases per million inhabitants per year, whereas Jazayeri et al. (2015) reported that the incidence ranges from 3.6 to 195.4 individuals per million around the world. The most frequently affected group includes people between the ages of 29 and 43, with a higher frequency in males when compared to females (Devivo, 2012; No authors listed, 2012). In Mexico, the annual incidence of SCI is estimated to be 18.1 per million inhabitants and occurs more frequently in men between 16 to 35 years (Estrada-Mondaca et al., 2007). The absence of updated statistical data makes it impossible to provide more information on the epidemiology of SCI in Mexico.
One of the most frequent and worrisome alterations developed among the SCI population is cognitive impairment, as it has been reported that the risk of suffering any form of cognitive dysfunction after SCI is 13 times greater than in healthy individuals (Craig et al., 2017). A study by Wilmot et al. (1985) showed that up to 64% of SCI individuals suffered from some degree of cognitive dysfunction after injury. A recent study by Mahmoudi et al. (2021) reported that both middle-aged and older adults with SCI had a higher incidence of Alzheimer’s disease when compared to those without SCI. This same study reported that SCI approximately doubles the risk of early-onset Alzheimer’s disease (among people 45–64 years old). In addition, it has been shown that people of any age can develop cognitive impairment after SCI, but evidence suggests that adults are at a higher risk (Craig et al., 2017). Many studies have focused on delineating the mechanisms through which SCI patients develop cognitive impairment. A longitudinal study conducted by Richards et al. (1988) shows concomitant traumatic brain injury (TBI) as a potential cause of the development of cognitive impairment seen in SCI patients. In addition, numerous studies (Tolonen et al., 2007; Craig et al., 2017) support this theory, including a systematic review by Sachdeva et al. (2018), which states that between 16–59% of SCI patients present with a concomitant TBI, which in turn generates a cascade of neurological complications such as problems with attention, concentration, memory, judgment, irritability, emotional lability and acting in a socially inappropriate way, among others (Tolonen et al., 2007). Given the high incidence of cognitive impairment observed in SCI patients and the difficulty of reaching its diagnosis in early stages (Tolonen et al., 2007), it is important to elucidate its etiology, pathophysiological mechanisms, and the most current rehabilitation and treatment strategies to achieve an adequate recovery and social reintegration of patients.
Search Strategy and Eligibility Criteria
The search and selection processes were carried out between May and June 2021. The search was posteriorly extended in November of the same year. The following databases were used: PubMed, Research Gate, and NCBI.
A keyword literature search was performed for all the published literature between 1985 and the present. For a more targeted search, the following MeSH terms were used: spinal cord injury, cognitive impairment, spinal cord injury pathophysiology, spinal cord injury-induced cognitive impairment, spinal cord injury-induced autonomic dysfunction, traumatic brain injury-induced cognitive impairment. A manual search was also carried out within the references referring to important articles.
A total of 130 papers were retrieved through electronic research, of which 92 were included in this review. Only studies published in the last 2 decades were considered for the retrieval of information, with the exception of some pioneer publications in the field. There were no limitations in terms of the type of study consulted, since systematic reviews, clinical trials, meta-analyses, among others, were used.
Spinal Cord Injury Pathophysiology
The pathology of SCI can be divided into two phases, primary and secondary injury (Norenberg et al., 2004; Oyinbo, 2011; Alizadeh et al., 2019; Anjum et al., 2020). The primary phase occurs immediately after the aggression and involves the initial mechanical injury in which the physical force of impact is directly imparted to the spinal cord, disrupting blood vessels, axons, and neural-cell membranes (Oyinbo, 2011; Venkatesh et al., 2019; Anjum et al., 2020). According to Bunge et al., four macroscopic morphological types of injury can be defined in this initial phase: solid cord injury, contusion/cavity, laceration, and massive compression (Bunge et al., 1993; Norenberg et al., 2004). In addition to the neuronal death that occurs at the immediate moment of injury, a series of biochemical, molecular, and physiological processes occur in a cascade fashion. Some of these events include vascular alterations (Norenberg et al., 2004; Alizadeh et al., 2019), such as vasospasm, hyperemia, hemodynamic abnormalities, multiple hemorrhages, and systemic hypotension (Norenberg et al., 2004; Oyinbo, 2011), which in turn can lead to spinal shock and eventually ischemia (Norenberg et al., 2004; O’Shea et al., 2017), aggravating the neuronal cell death. Additional consequences of vascular damage include extravasation of immune cells into the damaged spinal cord tissue, especially monocytes, neutrophils, T and B lymphocytes, and macrophages, which in turn secrete several inflammatory cytokines, promoting neuronal inflammation (Anjum et al., 2020). Other alterations seen during this phase include plasma membrane compromise, disturbances in calcium homeostasis, and the beginning of other alterations such as accumulation of neurotransmitters, free radical formation, edema, lipid peroxidation, demyelination, Wallerian degeneration, and cyst and fibroglial scar formation that will predominate during the phase of secondary injury (Rowland et al., 2008; Oyinbo, 2011; Alizadeh et al., 2019; Anjum et al., 2020).
After the primary injury, a cascade of secondary events is initiated, expanding the zone of neural tissue damage, and exacerbating neurological deficits. Secondary injury is a progressive condition characterized by pro-inflammatory cytokines, reactive oxygen species, oxidative damage, excitatory amino acids such as glutamate, loss of ionic homeostasis, mitochondrial dysfunction, and cell death (Venkatesh et al., 2019). The secondary phase is triggered by the primary stage and begins just minutes after the initial injury; it lasts for several weeks or months. During this phase there is a progressive deterioration in the spinal cord tissue which affects not only the area involved in the primary injury but also areas close to this site, therefore becoming a critical therapeutic target for the prevention of injury progression (Venkatesh et al., 2019). As part of this stage, recent studies suggest that extracellular vesicles (EVs) participate in the progression of secondary injury by transporting parent-cell specific signaling cargoes (e.g., signal lipids, genetic information, cytokines, receptors) that alter the function of recipient cells within the central nervous system (CNS) and beyond. Interestingly, local EVs release may also contribute to recovery and repair mechanisms relevant to SCI (Dutta et al., 2021). This secondary phase can be further subdivided into three stages: acute (seconds to a few minutes after injury), subacute (minutes to weeks after injury), and chronic (months to years after injury) (Bunge et al., 1993; Tator and Koyanagi, 1997; Dumont et al., 2001; Norenberg et al., 2004; Rowland et al., 2008; Oyinbo, 2011; O’Shea et al., 2017; Alizadeh et al., 2019; Anjum et al., 2020).
Acute stage
Although many of the disturbances present in the primary phase can still be seen at this stage of injury, its main characteristic is the interruption of the spinal cord vascular supply (Anjum et al., 2020), causing variable levels of ischemia and hypoxia. Impairment of spinal cord vascular supply, accompanied by hypotension and diminished perfusion into the spinal cord, due to edema and inflammation, is one of the prompt consequences of the primary injury (Alizadeh et al., 2019). Vascular alterations ultimately lead to cell death and tissue destruction through the following mechanisms: ionic imbalance, adenosine triphosphate deficiency, oxygen depletion, free radical formation, necrosis, and glutamate excitotoxicity (Alizadeh et al., 2019).
Sub-acute stage
This stage is characterized by axonal demyelination, Wallerian degeneration, axonal remodeling, and initiation of glial scar formation (gliosis) (Anjum et al., 2020). Compared to the acute stage, the sub-acute stage presents a major tissue damage promoted by apoptosis. After the injury, glutamate is widely released into the extracellular space (Dumont et al., 2001; Oyinbo, 2011), causing both an impairment of Na+/K+ ATPase function and intracellular calcium accumulation (Dumont et al., 2001), ultimately causing apoptosis through excitotoxic cell death (Xu et al., 2005). The demyelination of the remaining axons at the injury site is the result of the apoptotic process that oligodendrocytes undergo (Oyinbo, 2011; Venkatesh et al., 2019; Anjum et al., 2020). Wallerian degeneration, an event related to the demyelination process, represents the anterograde degeneration of axons and their myelin sheaths that have been damaged after the injury (Norenberg et al., 2004).
Chronic stage
This stage is mainly characterized by the formation of a cystic cavity, axonal dieback, and maturation of glial scar (O’Shea et al., 2017; Anjum et al., 2020). Few days after the initial injury, astrocytes undergo hypertrophy and hyperplasia, a process known as reactive astrocytosis. These cells, called gemistocytes, present a highly active metabolic state (Norenberg et al., 2004). As a result of hypertrophy, gemistocytes develop longer and thicker cytoplasmic processes that form an interlocking network, promoting the formation of an astroglial scar. The formation of the glial scar favors the formation of an isolated deleterious microenvironment, therefore preventing an adequate regeneration at the injury site (Ibarra et al., 2019). Some of the systemic complications that SCI patients develop are shown in Table 1 (Sezer et al., 2015; Stricsek et al., 2017; Peterson et al., 2021).
Table 1.
Systemic complications after spinal cord injury
| Organ/system affected | Complications |
|---|---|
| Pulmonary | Acute lung injury |
| Acute respiratory distress syndrome | |
| Respiratory failure | |
| Pulmonary embolism | |
| Pleural effusion | |
| Lobar collapse | |
| Pneumonia | |
| Pneumothorax and hemothorax | |
| Hematologic | Deep venous thrombosis |
| Anemia | |
| Thrombocytopenia | |
| Coagulopathies | |
| Renal | Acute kidney disease |
| Hematuria | |
| Urinary tract infections | |
| Chronic kidney disease | |
| Neuropsychiatric | Depression |
| Cognitive disorders | |
| Anxiety | |
| Autonomic dysreflexia | |
| Cardiovascular | Arrhythmia |
| Bradycardia | |
| Myocardial infarction | |
| Heart failure | |
| Pulmonary edema | |
| Hypertension | |
| Atherosclerosis | |
| Metabolic | Type 2 diabetes mellitus |
| Hypercholesterolemia | |
| Non-alcoholic fatty liver disease | |
| Metabolic syndrome | |
| Skin | Pressure ulcers |
Cognitive Impairment in Spinal Cord Injury Patients
Cognitive impairment represents a well-known complication among the SCI population and is manifested by the presence of different alterations in cognitive function, such as a decreased attention and concentration capacity, impaired visuospatial perception, decreased ability to resolve problems, impaired processing speed, memory, and learning ability (Murray et al., 2007; Molina et al., 2018; Chiaravalloti et al., 2020a, b). These cognitive alterations result in a decreased quality of life (Murray et al., 2007; Chiaravalloti et al., 2020a, b) and may favor the appearance of aggressive behaviors in patients as well as a higher risk of hospital readmission (Chiaravalloti et al., 2020a, b). The exact etiology of these cognitive disturbances remains unclear. As it appears to be a multifactorial condition, many factors have been proposed as potential contributors to the development of this disorder. Studies have suggested concomitant traumatic brain injury, hypoxia, and anoxia, cardiovascular and cerebrovascular dysfunction (Murray et al., 2007), sleep disorders such as obstructive sleep apnea (Bonekat et al., 1990), body temperature dysregulation, substance, and alcohol abuse (Grant, 1987), as possible inductors of cognitive impairment in SCI patients.
The severity and incidence of this pathology have also been investigated. With this respect, Molina and colleagues reported the main differences in cognitive function between the acute and chronic stages of SCI. In their study, they stated that cognitive impairment presents a higher incidence and severity in the chronic than in the acute stage (Molina et al., 2018). However, cognitive function deficits are present in both stages, with some of the patients being impaired since the acute stage and worsening over time. These data suggest the progressive nature of cognitive impairment and the maximum peak that it can reach during the later stages of the injury (Molina et al., 2018). Another interesting finding of this study was the higher prevalence of cognitive impairment in the group of patients under treatment with neuroactive drugs to treat mood and pain disorders, suggesting that these drugs could have a negative impact on cognition among people with SCI (Molina et al., 2018).
The impact of the anatomic level of injury in the spinal cord on cognitive function has also been investigated. In a study carried out by Wecht et al. (2018), it was shown that patients with SCI at or above the T1 level have a lower performance on cognitive tasks (Wecht et al., 2018). On the other hand, given the fundamental role of the spinal cord in the functions of the autonomic nervous system, it has been suggested that hemodynamic events after SCI (chronic hypotension and orthostatic hypotension), particularly in individuals with high spinal cord lesions (i.e., above T6), may contribute to the development of distinct patterns of cognitive impairment (Chiaravalloti et al., 2020a). In line with these findings, Chiaravalloti et al. (2020a, b) also identified a relationship between some cognitive functions and hemodynamic changes, concluding that, an increase in cerebral vascular resistance leads to the worsened performance of the individual in tasks that involve cognitive activity.
It is worth mentioning that the presence of pain after SCI represents an adverse impact not only on cognitive function but also on the quality of life and physical functions (Murray et al., 2007), as it has been demonstrated that adults with cognitive dysfunction are at increased risk for the development of mental health problems such as depression and anxiety (Craig et al., 2017). Due to the lack of capacity of some studies to identify the more subtle cognitive alterations, Chiaravalloti et al. (2020b) highlighted the importance of carrying out a correct assessment of cognitive function after SCI (Barbetta et al., 2014). They proposed the use of a detailed neuropsychological assessment that evaluates all domains of cognition, rather than using general screening measures that assess global cognition (Chiaravalloti et al., 2020b).
Pathophysiology
SCI induces chronic neuroinflammation and neurodegeneration in brain regions linked to memory, emotions, and pain regulation. These pathological findings in preclinical models demonstrate that secondary injury phase processes extend far beyond the initial site of injury and may explain the cognitive deficits observed in patients with SCI (Khan et al., 2021) (Figure 1). In fact, several studies have reported that SCI can cause varying degrees of cognitive impairment, which can negatively impact an individual’s quality of life. Thus, elucidating the mechanisms that lead to the development of this disorder is of great importance to make an accurate diagnosis and provide prompt treatment.
Figure 1.
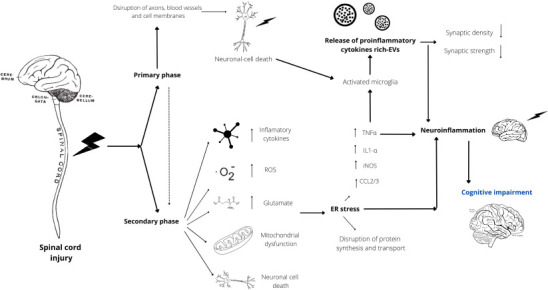
Diagram schematizing the pathophysiology of spinal cord injury (SCI)-mediated cognitive impairment.
After an aggression to the spinal cord, there is a primary response phase, characterized by alterations in the cellular microenvironment and neuronal death, followed by a late secondary response phase, where a series of events such as the release of proinflammatory cytokines, reactive oxygen species (ROS), glutamate excitotoxicity, mitochondrial dysfunction, and neuronal cell death aggravate the initial state of injury. This phase triggers a process known as “endoplasmic reticulum (ER) stress”, which is characterized by promoting an increase in several proinflammatory cytokines, resulting in the activation of local microglia and the release of extracellular vesicles (EVs) containing proinflammatory cytokines, which in turn travel through the bloodstream to critical areas of the brain. This event coupled with neuronal death and ER stress leads to a state of neuroinflammation that ultimately leads to the development of cognitive impairment. CCL2/3: Chemokine (C-C motif) ligand 2/3; ER: endoplasmic reticulum; EVs: extracellular vesicles; IL1-α: interleukin 1α; iNOS: inducible nitric oxide synthase; ROS: reactive oxygen species; TNFα: tumor necrosis factor α.
A study conducted by Wu et al. (2016) postulated a relationship between the development of cognitive impairment and a phenomenon known as “endoplasmic reticulum (ER) stress”. The ER is a large and dynamic cellular organelle that performs several vital functions contributing to normal homeostases, such as protein synthesis, folding, modification and transport, calcium storage, and lipid metabolism (Schwarz and Blower, 2016; Wu et al., 2016). Insults to this organelle lead to alterations in its function, disturbing the normal pathways for synthesis and transport of proteins and leading to an accumulation of misfolded proteins within this structure (Oakes and Papa, 2015). This results in the activation of another phenomenon called “unfolded protein response”, designed to restore protein homeostasis and prevent cell death (Wu et al., 2016). ER stress is implicated in the development of several neurodegenerative diseases, including ischemia, diabetes, bipolar disorder, and can lead to cell death (Hossain et al., 2015; Wu et al., 2016; Sefiani and Geoffroy, 2021). This phenomenon is related to neuroinflammation through proinflammatory cytokines and reactive oxygen species cellular pathways, as it has been demonstrated that tumor necrosis factor alpha (TNF-α), interleukin (IL)-1α, inducible nitric oxide synthase, and CCL2/3 show significant elevations in the hippocampi after SCI. GRP78, a marker for ER stress, is still elevated in the hippocampus 4 months after SCI in a linear relationship to the severity of the SCI, implicating that SCI causes chronic ER stress (Sefiani and Geoffroy, 2021). Furthermore, evidence from Wu et al. (2016) showed that the number of GRP78+ cells was elevated in key areas of the brain that contribute to memory formation and maintenance, such as the hippocampus, cortex, and thalamus. Chronic ER stress has been shown to elevate inflammatory markers, induce neuronal death, reduce neural stem cell proliferation, and impair autophagy (Sefiani and Geoffroy, 2021). Therefore, activation of ER stress in the brain may contribute to diminished neuronal survival in key brain regions for cognition, which in turn is associated with the development of cognitive impairment.
Inflammation occurs immediately following SCI, peripheral macrophages infiltrate into the spinal cord, and resident microglia transform into activated microglia to remove dead cells and promote healing. However, while acute inflammation can have beneficial effects, the chronic inflammation that develops after SCI has serious negative outcomes. Inflammation is not confined to the primary site of injury or even the spinal cord. After SCI, the brain also expresses proinflammatory cytokines associated with activated microglia, producing neurotoxicity in their surroundings. Activated microglia have been identified in the thalamus, hippocampus, and frontal cortex in rodent models of SCI, becoming activated as soon as 7 days post-SCI and remaining for at least 10 weeks. These changes are associated with cognitive impairment (Brakel and Hook, 2019).
The dentate gyrus (DG) of the hippocampus is a region of the brain that is believed to have a key role in the process of learning and the formation of new memory. Wu et al. (2016) reported a disruption in neurogenesis in the DG of the hippocampus by causing a reduction in the number of neurons within the hippocampi and disrupting the ability of immature neurons to differentiate into the mature neuronal lineage, thus contributing to impaired memory formation. A study reported by Jure et al. (2017) supported the latter as they found that decreased neurogenesis in the mouse hippocampi was associated with microglial activation and astrogliosis within the DG after SCI. Of note, they demonstrated that hippocampal neurogenesis and glial responses in the DG were highly dependent on SCI severity. Evidence strongly suggests that SCI promotes microglial activation in the brain by altering gene expression of the M1 and M2 microglia phenotypes in the hippocampus. The M1 microglia phenotype, also known as inflammatory microglia, is known to promote neurotoxic effects by releasing proinflammatory cytokines, which in turn inhibit neurogenesis, neuronal differentiation, and neuronal survival. SCI rats showed an increase in proinflammatory mediators, such as TNF α, inducible nitric oxide synthase, IL-6, and reactive oxygen species in hippocampal tissue. In addition, CCL21, an effective microglia-activating chemokine, is only expressed in injured neurons in the CNS and can quickly cause neuroinflammation at local and remote sites of injury. Levels of CCL21 are increased in numerous brain areas after SCI, thus indirectly contributing to microglia activation and neuroinflammation. These changes are associated with neuronal loss in the hippocampus, cortex, and thalamus (Wu et al., 2014a, b; Jure et al., 2017; Chen et al., 2020; Sefiani and Geoffroy, 2021).
EVs are membrane-delimited particles that are secreted by nearly all cell types and can be detected in all body fluids, including blood, urine, and cerebrospinal fluid. As carriers of diverse bioactive cargoes (e.g., proteins, lipids, and nucleic acids) that can be modified in response to external stimuli, EVs have emerged as pathological mediators following neurotrauma such as SCI. Upon proinflammatory stimulation (e.g., IL-1β, TNF-α, IFN-γ, or lipopolysaccharide), microglia release EVs enriched with proinflammatory cytokines (e.g., IL-1β) and miRNAs (e.g., miR-146a-5p) that are transferred to neurons, leading to downregulation of key pre- and post-synaptic proteins (e.g., synaptotagmin 1 and neuroligin 1), thus reducing synaptic density and strength. EVs from lipopolysaccharide-stimulated microglia also carry the enzyme glutaminase that may contribute to neurotoxicity through excessive glutamate production. Importantly, microglial EVs released under these stimuli may also contribute to inflammation by activating surrounding microglia and astrocytes. Furthermore, in response to similar proinflammatory stimuli, astrocytes undergo a phenotypic transformation known as reactive astrogliosis. EVs derived from reactive astrogliosis are enriched in small GTPases, cytokines, proteins, and miRNAs that inhibit neuronal function by decreasing neurite outgrowth and spike firing rates. EVs derived from reactive astrogliosis may also lead to neuronal apoptosis. These findings support a role for EVs produced by activated glia in mediating secondary injury following SCI. Moreover, emerging evidence suggests that circulating EVs play an important role in both CNS injury progression and systemic inflammatory complications (e.g., pulmonary dysfunction) following neurotrauma (Dutta et al., 2021).
Circulating EVs following SCI could promote inflammation in recipient target organs, particularly the brain. A recent study performed by Khan et al evaluated the latter by intracerebroventricular injection of plasma EVs from either SCI or control mice into healthy mice. At 24 hours post-injection, they found an increased expression of several inflammatory genes in the brain cortex associated with injection of SCI plasma EVs, including markers related to astrocyte reactivity, thus demonstrating that SCI alters plasma EVs and may contribute to remote inflammatory effects in the brain. Furthermore, increased intracellular IL-1β and IL-1α levels in brain astrocytes were observed (Khan et al., 2021). These data suggest that EVs-containing proinflammatory cargo are released by cells directly from the injury site and travel through the blood circulation to seed brain inflammation, which may contribute to long-term neurodegeneration and associated cognitive deficits following SCI (Dutta et al., 2021; Khan et al., 2021).
Main contributors
The altered cognitive patterns observed after SCI could also be the result of different comorbidities, the most important being TBI.
Traumatic brain injury
Traumatic SCI often occurs in association with TBI, as the incidence of concomitant TBI in patients with SCI has been reported to be as high as 60%. Other reports suggest that up to 74% of individuals suffer from concomitant TBI after traumatic SCI (Cohen et al., 2017), suggesting that the simultaneous occurrence of TBI and SCI is more common than it may seem. Evidence shows that TBI plays an important role in the appearance of cognitive impairment in SCI patients (Davidoff et al., 1988; Richards et al., 1988; Tolonen et al., 2007; Craig et al., 2017). A series of two studies conducted by Macciocchi et al. (2004, 2008) demonstrated the relationship between TBI and cognitive impairment, concluding that TBI is a frequent injury that concurs with traumatic SCI and that the low performance of SCI patients in the Cognitive FIM test score reveals lower functional gains during rehabilitation in patients suffering from concomitant TBI after SCI. Memory impairment is one of the most frequent neurological manifestations after suffering from TBI (Paterno et al., 2017; Calvillo and Irimia, 2020). Many studies have sought to elucidate the pathophysiological mechanisms responsible for the development of cognitive impairment after TBI. According to a systematic review conducted by Paterno et al. (2017), alterations in the DG and the CA3 and CA1 areas of the hippocampus following an episode of TBI appear to be the main events responsible for the onset of memory impairment. This hypothesis supports the fact that the hippocampus is a fundamental structure, if not the most important, in the development of memory, and any insult to it can contribute to the development of varying degrees of cognitive impairment. Despite this evidence, some authors do not find a direct relationship between TBI and the appearance of cognitive impairment (Craig et al., 2017), so future research is needed to address this issue.
Unfortunately, TBI is often underdiagnosed in patients with SCI, and up to 58.5% of individuals with traumatic SCI have a missed diagnosis of TBI and it should also be noticed that a previous history of TBI can lead to impulsivity, risk behaviors, and cognitive impairment. Therefore, more research is needed to investigate which individuals with SCI and TBI are at risk of developing cognitive impairment and how TBI may contribute to the development of cognitive impairment in the chronic phase of SCI (Distel et al., 2020).
Autonomic nervous system dysfunction
Autonomic dysfunction (AD) is one of the most frequent and devastating complications of SCI. It occurs in up to 90% of individuals with upper thoracic (at or above the T6 level) and cervical SCI (Furlan et al., 2003; Elliott and Krassioukov, 2006; Groothuis et al., 2010; Krassioukov, 2012). Some manifestations seen during an AD episode are bradycardia or tachycardia and dangerous fluctuations in blood pressure, from 50 mmHg or less during an orthostatic challenge to 300 mmHg (Elliott and Krassioukov, 2006; Hubli et al., 2015; Sachdeva et al., 2019); alterations in the circadian blood pressure oscillations might occur (Krassioukov, 2012). Autonomic dysreflexia –the main alteration of AD– can occur during the acute phase of SCI (Krassioukov, 2012). This phenomenon is defined as an extreme hypertensive state (systolic blood pressure up to 300 mmHg) and is caused by exacerbated sympathetic activity; it mainly occurs in patients with SCI at or above the T6 level and is triggered by either noxious or non-noxious stimuli (Krassioukov, 2012; Sachdeva et al., 2019). Notably, an increase of more than 20-30 mmHg in systolic blood pressure during the resting state can be considered an AD episode (Elliott and Krassioukov, 2006; Groothuis et al., 2010). In general, the blood pressure at rest of patients with SCI is lower than in healthy individuals, with disabling episodes of orthostatic hypotension. However, as mentioned before, life-threatening episodes of autonomic dysreflexia may occur, where systolic blood pressure can reach up to 300 mmHg potentially leading to stroke and death. These cardiovascular abnormalities are attributed to autonomic instability caused by the destruction of the descending vasomotor pathways, which results in the loss of excitatory supraspinal input to the spinal sympathetic preganglionic neurons. The destruction of the descending vasomotor pathways is considered the major factor that contributes to the persistent sympathetic atony after SCI (Teasell et al., 2000; Furlan et al., 2003). Dramatic fluctuations in blood pressure can have dangerous consequences for the cerebrovascular system, increasing the risk of strokes, intracranial hemorrhages, and seizures (Furlan et al., 2003; Elliott and Krassioukov, 2006; Groothuis et al., 2010). Interestingly, certain brain areas, such as the cortex, hippocampus, and basal ganglia are more prone to ischemic damage (Sachdeva et al., 2019). Therefore, blood pressure dysregulation and the consequent sustained hypotensive state, which can last for more than 24 hours, may contribute to the development of the cognitive impairment seen in SCI patients by reducing cerebral blood flow to these areas of the brain (Jegede et al., 2010). A study aimed at demonstrating the contribution of changes in blood pressure and cerebral blood flow velocity in the development of cognitive impairment in SCI patients reported that alterations in blood pressure reflected an inability to maintain and adjust the cerebral blood flow velocity to achieve an adequate metabolic supply to the brain during the performance of tasks involving cognitive functions (Wecht et al., 2018).
Psychiatric disorders
A history of premorbid neuropsychological conditions may be associated with cognitive impairment after SCI, and possibly led to the initial injury. Multiple studies have identified psychiatric diseases such as attention-deficit/hyperactivity disorder as predisposing adolescents to injuries, including SCI. In fact, the rate of “severe injury” (fracture of skull, neck, and trunk; intracranial injury; injuries to nerves and spinal cord) is three times higher in the population with attention-deficit/hyperactivity disorder when compared with a population without attention-deficit/hyperactivity disorder. Moreover, individuals with impulsivity/risk-taking behavior are more likely to experience an SCI event. In addition to these premorbid psychiatric conditions, there are increased rates of psychological and psychiatric disorders following SCI. Up to 17% of SCI patients are diagnosed with a psychological disorder at discharge from rehabilitation and 25% at 6 months following discharge. Depression is the most common psychiatric diagnosis (14.1%) in SCI patients. Less common disorders include bipolar disorder (4.2%), general anxiety (4.2%), and posttraumatic stress disorder (1.4%) (Distel et al., 2020). Marvel and Paradiso (2004) found that depression, bipolar disorder, and anxiety are associated with cognitive impairment. Furthermore, mood disorders were found to be associated with both functional and structural abnormalities in the prefrontal cortex, hippocampus, and anterior cingulate cortex (Marvel and Paradiso, 2004). In contrast, Davidoff et al reported no effect of depression on cognition in SCI individuals and stated that depression and cognitive impairment are two separated and causally unrelated problems after SCI (Davidoff et al., 1990; Distel et al., 2020). In addition, Craig et al. (2017) found that levels of depression and anxiety were not different between SCI patients with and without cognitive impairment in the inpatient rehabilitation stage. However, both psychiatric disorders increased significantly after living 6 months in the community in patients with cognitive impairment when compared to those without cognitive impairment. Therefore, psychiatric disorders may be a consequence of cognitive impairment rather than a cause (Craig et al., 2017; Distel et al., 2020).
Substance abuse
Up to 50% of the population with substance abuse and alcohol ingestion has been reported to be at increased risk of cognitive impairment (Davidoff et al., 1992). Substance abuse is common both before and after an SCI event and is often a contributor to the initial SCI. In fact, alcohol contributes to 35% to 40% of traumatic SCI and more than 30% of SCI patients have a positive test for illicit drugs. In addition, up to 8.6% of SCI patients have evidence of alcohol abuse 6 months after injury and up to 32% have evidence of overall substance abuse (Distel et al., 2020).
Alcohol and drug abuse have a variety of cognitive effects, with dysfunction being most noticed in attention, memory, planning, behavior, and decision making. Marijuana is becoming widely accepted and used for the treatment of pain and spasticity. However, evidence suggests that cannabis intake is harmful to cognitive function, including verbal memory, processing speed, and executive functions (Distel et al., 2020).
Polypharmacy
Many pharmacologic agents interfere with neurotransmission in brain areas that control cognitive function, and this effect is greater the more agents are prescribed to a patient (Salimzade et al., 2017). Kitzman et al. (2017) found that 56% of SCI patients were prescribed 5 or more medications, compared to 27% of those without SCI. Of the SCI population, 23% were prescribed 10 or more medications, compared to only 7% of those without SCI. Moreover, high-risk medications (defined as sedative-hypnotics, anxiolytics, antispasmodics, serotoninergic agents, narcotics, antiepileptics, tricyclic antidepressants, and skeletal muscle relaxants) were prescribed in up to 92% of SCI patients, compared to 44% of those without SCI (Kitzman et al., 2017).
Benzodiazepines and nonbenzodiazepine hypnotics act by enhancing gamma-aminobutyric acid-A, the primary inhibitory neurotransmitter in the CNS. Both medications are commonly associated with cognitive impairment and have been shown to have amnestic and non-amnestic cognitive effects in patients with SCI (Tannenbaum et al., 2012; Distel et al., 2020). Baclofen acts as an agonist at GABA-B receptors and is often the first choice of treatment for spasticity. Studies performed in mouse models have shown evidence that baclofen leads to cognitive impairment. However, there have not been studies examining these effects in humans (Levin et al., 2004; Pujol et al., 2018; Distel et al., 2020). Opioids work by binding to opioid receptors in the CNS and are still used for analgesia in SCI patients. These medications have been shown to cause both amnestic and non-amnestic impairments, including difficulties with sustained attention, reaction time, executive functioning, response inhibition, and set-shifting. In addition, only a small amount of opioid is required to cause cognitive impairment (Tannenbaum et al., 2012; Berryman et al., 2014; Distel et al., 2020). Antipsychotics act by increasing dopamine in the CNS and are often used in hospitalized patients with SCI who develop delirium and for sleep. However, evidence suggests that these medications may impair recall and reaction time (Tannenbaum et al., 2012; Distel et al., 2020). Antiepileptics can act in a variety of ways to enhance inhibitory neurotransmitters or suppress neuronal excitability. These medications are often used in SCI patients to treat seizures or neuropathic pain. However, potential adverse cognitive effects include impaired memory, concentration, processing speed, and verbal fluency (Eddy et al., 2011). Two antiepileptic drugs that are commonly used in SCI patients are gabapentin and pregabalin, which act as calcium channel alpha 2-delta blockers and affect the release of neurotransmitters. Both agents have been shown to have negative cognitive effects in humans (Chavant et al., 2011; Shem et al., 2018). Lastly, anticholinergic agents are also frequently prescribed to SCI patients to treat bladder overactivity (e.g., bladder antimuscarinics) and for mood/pain management (e.g., tricyclic antidepressants). The cholinergic system projects into the cortex and hippocampus and is involved in memory storage and retrieval, perception, and attention. Krebs and colleagues have postulated that the intake of antimuscarinic drugs for bladder dysfunction after SCI may act as possible contributors to the development of cognitive impairment (Krebs et al., 2018). Among antimuscarinic drugs, oxybutynin shows the greatest negative effect on cognition. On the other hand, tricyclic antidepressants are also related to cognitive impairment, particularly with attention and reaction time (Tannenbaum et al., 2012; Distel et al., 2020).
Chronic pain and chronic fatigue
Pain has been shown in the literature to interfere with cognitive processing, most commonly affecting attention and both working and short-term memory. This effect on cognition may be due to the brain plasticity associated with chronic pain, resulting in structural and functional changes in different cortical regions that are associated with learning, memory, fear, and emotional responses. Moreover, chronic pain can lead to a very complex functional reorganization of the default mode network, a region that governs numerous elements of cognitive function such as attention allocation, working memory, and decision-making (Mazza et al., 2018).
On the other hand, chronic fatigue may also contribute to cognitive impairment following SCI since patients with chronic SCI have higher levels of chronic fatigue when compared with controls. In fact, this same study also reported that SCI patients experienced an increased feeling of “tiredness” after completing a 2-hour cognitive task (Craig et al., 2012).
Respiratory disorders
Respiratory disorders are common in both the acute and chronic stages of SCI (Berlowitz et al., 2016). Individuals with high cervical tetraplegia are at risk of chronic respiratory failure with hypercapnia and hypoxemia (Brown et al., 2006), which are both associated with cognitive impairment in other pulmonary disorders (Cleutjens et al., 2014). However, the relationship between SCI-induced respiratory failure and cognition remains unknown.
Obstructive sleep apnea (OSA) is the most common form of sleep breathing disorder among individuals with tetraplegia (Sachdeva et al., 2018), approaching almost 60% of patients (Distel et al., 2020). Arterial oxygen desaturation and sleep disruption cause patients with OSA to present with daytime sleepiness. In able-bodied individuals, hypoxia (e.g., in hypoxemic lung disease), as well as untreated cases of OSA, has been associated with neuropsychological disorders. Individuals with tetraplegia and oxygen desaturation < 80% are associated with impaired attention, concentration, immediate and short-term memory, cognitive flexibility, and working memory. Moreover, individuals with tetraplegia and OSA are associated with impaired attention, information processing, and immediate memory (Sachdeva et al., 2018).
Post-intensive care unit syndrome
In the setting of an acute SCI event treatment begins in the intensive care unit. Individuals who are hospitalized with critical disorders and are in the intensive care unit are at increased risk of developing significant cognitive impairment both acutely and chronically. This cognitive impairment is known as post-intensive care syndrome and constitutes the sequel related to injuries upon discharge from critical care (Distel et al., 2020). Acute brain dysfunction occurs in 20% to 80% of patients admitted to the intensive care unit (Girard et al., 2008), and even after discharge, cognitive impairment occurs in 30% to 80% of patients. The risk factors that have been associated with post-intensive care syndrome are the same factors that are often present in SCI patients during the acute stage; include hypotension, sedation, hypoxemia, prolonged mechanical ventilation, multiorgan failure, systemic administration of corticosteroids, and younger ager (Colbenson et al., 2019; Distel et al., 2020).
Cortical reorganization
SCI produces an extensive brain reorganization due to cortical circuit deafferentation, leading to the atrophy not only of the spinal cord but also of the sensorimotor cortex and corticospinal tract. The decrease of corticospinal tract integrity and cortical grey matter volume is directly correlated with spinal cord atrophy in humans, suggesting that trauma-induced spinal degenerative process spread towards the brain (Jure and Labombarda, 2017). Possible mechanisms of cortical reorganization following SCI include the slowing of spontaneous cortical activity, cortical atrophy, and neuronal loss through apoptosis in the sensorimotor cortex (Nardone et al., 2013). Although most studies focus on atrophy/plasticity of the sensorimotor cortex, other regions of the brain that are not directly associated with the injury site have been reported to be affected after SCI, such as the anterior cingulate cortex, periaqueductal gray matter, superior cerebellar cortex, hippocampus, and medial prefrontal cortex (involved with emotional responses, memory, decision making) (Nicotra et al., 2006; Wrigley et al., 2009; Distel et al., 2020; Li et al., 2020b).
Neuroinflammation
SCI leads to chronic inflammation of the brain through glial activation and progressive neurodegeneration in animal models (Faden et al., 2016; Li et al., 2020a). SCI induces the upregulation of the neurotoxic reactive phenotype M1 of microglia, which increases the expression of proinflammatory cytokines (such as IL-6 and TNF α), reactive oxygen species, and nitric oxide, which leads to tissue inflammation. This SCI-induced neuroinflammation in the cortex, thalamus, and hippocampus leads to neuronal loss, a pathway similar to models with TBI (Wu et al., 2014a; Li et al., 2020a). Therefore, SCI causes progressive chronic neuroinflammation, leading to neurodegeneration in key brain regions associated with cognitive impairment. However, the precise molecular mechanisms underlying these changes have not been completely elucidated (Li et al., 2020a).
Regarding neurogenesis, glial activation through SCI-induced neuroinflammation has been shown to reduce neurogenesis. In fact, several studies have reported decreased neurogenesis in the brain at chronic stages of SCI. These findings, combined with the theory of impaired neurogenesis being one of the underlying mechanisms of cognitive impairment, may provide an explanation of why SCI patients have a significantly higher risk of cognitive impairment (Li et al., 2020a).
Other contributors
It is well known that the elderly population is at greater risk of developing a decline in cognition and is even considered the most important risk factor for the development of abnormalities in the domains of memory, language, processing speed and executive function (Mollayeva et al., 2017). Recent studies showed significant cognitive impairment in elderly participants with SCI (> 60 years) compared to younger ones (< 40 years) across cognitive tests. Elderly adults with SCI had decreased short-term capacity and working memory capacity when compared to young patients with SCI. An exception to the latter was the population with higher verbal ability and more years of education, as these two variables were associated with a better memory span (Sachdeva et al., 2018). Moreover, the evidence suggests that the development of cognitive impairment is observed mainly in men compared to women (Wood, 2017). Lastly, it is important to mention that sociodemographic factors, education level, and self-reported preinjury history of learning disability are predictive factors of performance on cognitive tests. Up to 13.7% of SCI patients had a self-reported history of learning problems. Therefore, learning difficulties prior to the injury may play an important role in the development of cognitive impairment in SCI patients (Distel et al., 2020). It is, therefore, necessary to consider not only age but also other factors when trying to predict the risk that an SCI patient has of developing cognitive impairment.
Conclusion
This review has focused on recent evidence indicating that SCI patients are at increased risk of developing cognitive impairment. TBI represents the main contributor to cognitive impairment in SCI patients. However, other factors may play an important role in its development, such as hypoxia and anoxia, autonomic nervous system dysfunction, sleep disorders such as obstructive sleep apnea, body temperature dysregulation, substance and alcohol abuse, and advanced age. Although significant advances in recent decades have decreased morbidity and mortality after SCI, the relationship between SCI and cognitive impairment continues to be a contributing factor to the decline in the quality of life of these patients. Therefore, it is important that the therapeutic approach of patients with SCI includes a personalized neuropsychological assessment that evaluates all domains of cognition.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury:an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y. Spinal cord injury:pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbetta DC, Cassemiro LC, Assis MR. The experience of using the scale of functional independence measure in individuals undergoing spinal cord injury rehabilitation in Brazil. Spinal Cord. 2014;52:276–281. doi: 10.1038/sc.2013.179. [DOI] [PubMed] [Google Scholar]
- 4.Berlowitz DJ, Wadsworth B, Ross J. Respiratory problems and management in people with spinal cord injury. Breathe (Sheff) 2016;12:328–340. doi: 10.1183/20734735.012616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Do people with chronic pain have impaired executive function?A meta-analytical review. Clin Psychol Rev. 2014;34:563–579. doi: 10.1016/j.cpr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Bonekat HW, Andersen G, Squires J. Obstructive disordered breathing during sleep in patients with spinal cord injury. Paraplegia. 1990;28:392–398. doi: 10.1038/sc.1990.52. [DOI] [PubMed] [Google Scholar]
- 7.Brakel K, Hook MA. SCI and depression:Does inflammation commandeer the brain? Exp Neurol. 2019;320:112977. doi: 10.1016/j.expneurol.2019.112977. [DOI] [PubMed] [Google Scholar]
- 8.Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–868. [PMC free article] [PubMed] [Google Scholar]
- 9.Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 10.Calvillo M, Irimia A. Neuroimaging and psychometric assessment of mild cognitive impairment after traumatic brain injury. Front Psychol. 2020;11:1423. doi: 10.3389/fpsyg.2020.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavant F, Favreliere S, Lafay-Chebassier C, Plazanet C, Perault-Pochat MC. Memory disorders associated with consumption of drugs:updating through a case/noncase study in the French PharmacoVigilance Database. Br J Clin Pharmacol. 2011;72:898–904. doi: 10.1111/j.1365-2125.2011.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Liang L, Cao S, Hou G, Zhang Q, Ma H, Shi B. Serum CCL21 as a potential biomarker for cognitive impairment in spinal cord injury. Biomed Res Int. 2020;2020:6692802. doi: 10.1155/2020/6692802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiaravalloti ND, Weber E, Wylie G, Dyson-Hudson T, Wecht JM. Patterns of cognitive deficits in persons with spinal cord injury as compared with both age-matched and older individuals without spinal cord injury. J Spinal Cord Med. 2020a;43:88–97. doi: 10.1080/10790268.2018.1543103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiaravalloti ND, Weber E, Wylie G, Dyson-Hudson T, Wecht JM. The impact of level of injury on patterns of cognitive dysfunction in individuals with spinal cord injury. J Spinal Cord Med. 2020b;43:633–641. doi: 10.1080/10790268.2019.1696076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleutjens FA, Janssen DJ, Ponds RW, Dijkstra JB, Wouters EF. Cognitive-pulmonary disease. Biomed Res Int. 2014;2014:697825. doi: 10.1155/2014/697825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen ML, Tulsky DS, Holdnack JA, Carlozzi NE, Wong A, Magasi S, Heaton RK, Heinemann AW. Cognition among community-dwelling individuals with spinal cord injury. Rehabil Psychol. 2017;62:425–434. doi: 10.1037/rep0000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colbenson GA, Johnson A, Wilson ME. Post-intensive care syndrome:impact, prevention, and management. Breathe (Sheff) 2019;15:98–101. doi: 10.1183/20734735.0013-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig A, Tran Y, Wijesuriya N, Middleton J. Fatigue and tiredness in people with spinal cord injury. J Psychosom Res. 2012;73:205–210. doi: 10.1016/j.jpsychores.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Craig A, Nicholson Perry K, Guest R, Tran Y, Dezarnaulds A, Hales A, Ephraums C, Middleton J. Prospective study of the occurrence of psychological disorders and comorbidities after spinal cord injury. Arch Phys Med Rehabil. 2015;96:1426–1434. doi: 10.1016/j.apmr.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Craig A, Guest R, Tran Y, Middleton J. Cognitive impairment and mood states after spinal cord injury. J Neurotrauma. 2017;34:1156–1163. doi: 10.1089/neu.2016.4632. [DOI] [PubMed] [Google Scholar]
- 21.Davidoff G, Thomas P, Johnson M, Berent S, Dijkers M, Doljanac R. Closed head injury in acute traumatic spinal cord injury:incidence and risk factors. Arch Phys Med Rehabil. 1988;69:869–872. [PubMed] [Google Scholar]
- 22.Davidoff G, Roth E, Thomas P, Doljanac R, Dijkers M, Berent S, Morris J, Yarkony G. Depression and neuropsychological test performance in acute spinal cord injury patients:lack of correlation. Arch Clin Neuropsychol. 1990;5:77–88. [PubMed] [Google Scholar]
- 23.Davidoff GN, Roth EJ, Richards JS. Cognitive deficits in spinal cord injury:epidemiology and outcome. Arch Phys Med Rehabil. 1992;73:275–284. [PubMed] [Google Scholar]
- 24.Devivo MJ. Epidemiology of traumatic spinal cord injury:trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 25.Distel DF, Amodeo M, Joshi S, Abramoff BA. Cognitive dysfunction in persons with chronic spinal cord injuries. Phys Med Rehabil Clin N Am. 2020;31:345–368. doi: 10.1016/j.pmr.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part I:pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Dutta D, Khan N, Wu J, Jay SM. Extracellular vesicles as an emerging frontier in spinal cord injury pathobiology and therapy. Trends Neurosci. 2021;44:492–506. doi: 10.1016/j.tins.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. 2011;4:385–407. doi: 10.1177/1756285611417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott S, Krassioukov A. Malignant autonomic dysreflexia in spinal cord injured men. Spinal Cord. 2006;44:386–392. doi: 10.1038/sj.sc.3101847. [DOI] [PubMed] [Google Scholar]
- 30.Estrada-Mondaca S, Carreon-Rodriguez A, Parra-Cid Mdel C, Leon CI, Velasquillo-Martinez C, Vacanti CA, Belkind-Gerson J. Spinal cord injury and regenerative medicine. Salud Publica Mex. 2007;49:437–444. doi: 10.1590/s0036-36342007000600011. [DOI] [PubMed] [Google Scholar]
- 31.Faden AI, Wu J, Stoica BA, Loane DJ. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol. 2016;173:681–691. doi: 10.1111/bph.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furlan JC, Fehlings MG, Shannon P, Norenberg MD, Krassioukov AV. Descending vasomotor pathways in humans:correlation between axonal preservation and cardiovascular dysfunction after spinal cord injury. J Neurotrauma. 2003;20:1351–1363. doi: 10.1089/089771503322686148. [DOI] [PubMed] [Google Scholar]
- 33.Furlan JC, Sakakibara BM, Miller WC, Krassioukov AV. Global incidence and prevalence of traumatic spinal cord injury. Can J Neurol Sci. 2013;40:456–464. doi: 10.1017/s0317167100014530. [DOI] [PubMed] [Google Scholar]
- 34.Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care 12 Suppl. 2008;3:S3. doi: 10.1186/cc6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant I. Alcohol and the brain:neuropsychological correlates. J Consult Clin Psychol. 1987;55:310–324. doi: 10.1037//0022-006x.55.3.310. [DOI] [PubMed] [Google Scholar]
- 36.Groothuis JT, Rongen GA, Deinum J, Pickkers P, Danser AH, Geurts AC, Smits P, Hopman MT. Sympathetic nonadrenergic transmission contributes to autonomic dysreflexia in spinal cord-injured individuals. Hypertension. 2010;55:636–643. doi: 10.1161/HYPERTENSIONAHA.109.147330. [DOI] [PubMed] [Google Scholar]
- 37.Hossain MM, DiCicco-Bloom E, Richardson JR. Hippocampal ER stress and learning deficits following repeated pyrethroid exposure. Toxicol Sci. 2015;143:220–228. doi: 10.1093/toxsci/kfu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubli M, Gee CM, Krassioukov AV. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertens. 2015;28:173–181. doi: 10.1093/ajh/hpu122. [DOI] [PubMed] [Google Scholar]
- 39.Ibarra A, Mendieta-Arbesu E, Suarez-Meade P, Garcia-Vences E, Martinon S, Rodriguez-Barrera R, Lomeli J, Flores-Romero A, Silva-Garcia R, Buzoianu-Anguiano V, Borlongan CV, Frydman TD. Motor recovery after chronic spinal cord transection in rats:a proof-of-concept study evaluating a combined strategy. CNS Neurol Disord Drug Targets. 2019;18:52–62. doi: 10.2174/1871527317666181105101756. [DOI] [PubMed] [Google Scholar]
- 40.Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide:a systematic review. Eur Spine J. 2015;24:905–918. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- 41.Jegede AB, Rosado-Rivera D, Bauman WA, Cardozo CP, Sano M, Moyer JM, Brooks M, Wecht JM. Cognitive performance in hypotensive persons with spinal cord injury. Clin Auton Res. 2010;20:3–9. doi: 10.1007/s10286-009-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jure I, Labombarda F. Spinal cord injury drives chronic brain changes. Neural Regen Res. 2017;12:1044–1047. doi: 10.4103/1673-5374.211177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jure I, Pietranera L, De Nicola AF, Labombarda F. Spinal cord injury impairs neurogenesis and induces glial reactivity in the hippocampus. Neurochem Res. 2017;42:2178–2190. doi: 10.1007/s11064-017-2225-9. [DOI] [PubMed] [Google Scholar]
- 44.Khan NZ, Cao T, He J, Ritzel RM, Li Y, Henry RJ, Colson C, Stoica BA, Faden AI, Wu J. Spinal cord injury alters microRNA and CD81+exosome levels in plasma extracellular nanoparticles with neuroinflammatory potential. Brain Behav Immun. 2021;92:165–183. doi: 10.1016/j.bbi.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitzman P, Cecil D, Kolpek JH. The risks of polypharmacy following spinal cord injury. J Spinal Cord Med. 2017;40:147–153. doi: 10.1179/2045772314Y.0000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krassioukov A. Autonomic dysreflexia:current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med. 2012;22:39–45. doi: 10.1097/JSM.0b013e3182420699. [DOI] [PubMed] [Google Scholar]
- 47.Krebs J, Scheel-Sailer A, Oertli R, Pannek J. The effects of antimuscarinic treatment on the cognition of spinal cord injured individuals with neurogenic lower urinary tract dysfunction:a prospective controlled before-and-after study. Spinal Cord. 2018;56:22–27. doi: 10.1038/sc.2017.94. [DOI] [PubMed] [Google Scholar]
- 48.Levin ED, Weber E, Icenogle L. Baclofen interactions with nicotine in rats:effects on memory. Pharmacol Biochem Behav. 2004;79:343–348. doi: 10.1016/j.pbb.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Cao T, Ritzel RM, He J, Faden AI, Wu J. Dementia, depression, and associated brain inflammatory mechanisms after spinal cord injury. Cells. 2020a;9:1420. doi: 10.3390/cells9061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Ritzel RM, Khan N, Cao T, He J, Lei Z, Matyas JJ, Sabirzhanov B, Liu S, Li H, Stoica BA, Loane DJ, Faden AI, Wu J. Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice. Theranostics. 2020b;10:11376–11403. doi: 10.7150/thno.49199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macciocchi S, Seel RT, Thompson N, Byams R, Bowman B. Spinal cord injury and co-occurring traumatic brain injury:assessment and incidence. Arch Phys Med Rehabil. 2008;89:1350–1357. doi: 10.1016/j.apmr.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 52.Macciocchi SN, Bowman B, Coker J, Apple D, Leslie D. Effect of co-morbid traumatic brain injury on functional outcome of persons with spinal cord injuries. Am J Phys Med Rehabil. 2004;83:22–26. doi: 10.1097/01.PHM.0000104661.86307.91. [DOI] [PubMed] [Google Scholar]
- 53.Mahmoudi E, Lin P, Peterson MD, Meade MA, Tate DG, Kamdar N. Traumatic spinal cord injury and risk of early and late onset Alzheimer's disease and related dementia:large longitudinal study. Arch Phys Med Rehabil. 2021;102:1147–1154. doi: 10.1016/j.apmr.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am. 2004;27:19–36. doi: 10.1016/S0193-953X(03)00106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazza S, Frot M, Rey AE. A comprehensive literature review of chronic pain and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:183–192. doi: 10.1016/j.pnpbp.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Middleton JW, Dayton A, Walsh J, Rutkowski SB, Leong G, Duong S. Life expectancy after spinal cord injury:a 50-year study. Spinal Cord. 2012;50:803–811. doi: 10.1038/sc.2012.55. [DOI] [PubMed] [Google Scholar]
- 57.Molina B, Segura A, Serrano JP, Alonso FJ, Molina L, Perez-Borrego YA, Ugarte MI, Oliviero A. Cognitive performance of people with traumatic spinal cord injury:a cross-sectional study comparing people with subacute and chronic injuries. Spinal Cord. 2018;56:796–805. doi: 10.1038/s41393-018-0076-0. [DOI] [PubMed] [Google Scholar]
- 58.Mollayeva T, Pacheco N, D'Souza A, Colantonio A. The course and prognostic factors of cognitive status after central nervous system trauma:a systematic review protocol. BMJ Open. 2017;7:e017165. doi: 10.1136/bmjopen-2017-017165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray RF, Asghari A, Egorov DD, Rutkowski SB, Siddall PJ, Soden RJ, Ruff R. Impact of spinal cord injury on self-perceived pre- and postmorbid cognitive, emotional and physical functioning. Spinal Cord. 2007;45:429–436. doi: 10.1038/sj.sc.3102022. [DOI] [PubMed] [Google Scholar]
- 60.Nardone R, Holler Y, Brigo F, Seidl M, Christova M, Bergmann J, Golaszewski S, Trinka E. Functional brain reorganization after spinal cord injury:systematic review of animal and human studies. Brain Res. 2013;1504:58–73. doi: 10.1016/j.brainres.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 61.Nicotra A, Critchley HD, Mathias CJ, Dolan RJ. Emotional and autonomic consequences of spinal cord injury explored using functional brain imaging. Brain. 2006;129:718–728. doi: 10.1093/brain/awh699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.No authors listed. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2012;35:197–198. doi: 10.1179/1079026812Z.00000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury:defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 64.O'Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127:3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury:a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 67.Paterno R, Folweiler KA, Cohen AS. Pathophysiology and treatment of memory dysfunction after traumatic brain injury. Curr Neurol Neurosci Rep. 2017;17:52. doi: 10.1007/s11910-017-0762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peterson MD, Berri M, Lin P, Kamdar N, Rodriguez G, Mahmoudi E, Tate D. Cardiovascular and metabolic morbidity following spinal cord injury. Spine J. 2021;21:1520–1527. doi: 10.1016/j.spinee.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pujol CN, Paasche C, Laprevote V, Trojak B, Vidailhet P, Bacon E, Lalanne L. Cognitive effects of labeled addictolytic medications. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:306–332. doi: 10.1016/j.pnpbp.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Richards JS, Brown L, Hagglund K, Bua G, Reeder K. Spinal cord injury and concomitant traumatic brain injury. Results of a longitudinal investigation. Am J Phys Med Rehabil. 1988;67:211–216. doi: 10.1097/00002060-198810000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies:promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 72.Sachdeva R, Gao F, Chan CCH, Krassioukov AV. Cognitive function after spinal cord injury:a systematic review. Neurology. 2018;91:611–621. doi: 10.1212/WNL.0000000000006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sachdeva R, Nightingale TE, Krassioukov AV. The blood pressure pendulum following spinal cord injury:implications for vascular cognitive impairment. Int J Mol Sci. 2019;20:2464. doi: 10.3390/ijms20102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salimzade A, Hosseini-Sharifabad A, Rabbani M. Comparative effects of chronic administrations of gabapentin, pregabalin and baclofen on rat memory using object recognition test. Res Pharm Sci. 2017;12:204–210. doi: 10.4103/1735-5362.207201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwarz DS, Blower MD. The endoplasmic reticulum:structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sefiani A, Geoffroy CG. The potential role of inflammation in modulating endogenous hippocampal neurogenesis after spinal cord injury. Front Neurosci. 2021;15:682259. doi: 10.3389/fnins.2021.682259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sezer N, Akkus S, Ugurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015;6:24–33. doi: 10.5312/wjo.v6.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shem K, Barncord S, Flavin K, Mohan M. Adverse cognitive effect of gabapentin in individuals with spinal cord injury:preliminary findings. Spinal Cord Ser Cases. 2018;4:9. doi: 10.1038/s41394-018-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stricsek G, Ghobrial G, Wilson J, Theofanis T, Harrop JS. Complications in the management of patients with spine trauma. Neurosurg Clin N Am. 2017;28:147–155. doi: 10.1016/j.nec.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29:639–658. doi: 10.1007/BF03262280. [DOI] [PubMed] [Google Scholar]
- 81.Tator CH, Koyanagi I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg. 1997;86:483–492. doi: 10.3171/jns.1997.86.3.0483. [DOI] [PubMed] [Google Scholar]
- 82.Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- 83.Tolonen A, Turkka J, Salonen O, Ahoniemi E, Alaranta H. Traumatic brain injury is under-diagnosed in patients with spinal cord injury. J Rehabil Med. 2007;39:622–626. doi: 10.2340/16501977-0101. [DOI] [PubMed] [Google Scholar]
- 84.Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D. Spinal cord injury:pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019;377:125–151. doi: 10.1007/s00441-019-03039-1. [DOI] [PubMed] [Google Scholar]
- 85.Wecht JM, Weir JP, Katzelnick CG, Wylie G, Eraifej M, Nguyen N, Dyson-Hudson T, Bauman WA, Chiaravalloti N. Systemic and cerebral hemodynamic contribution to cognitive performance in spinal cord injury. J Neurotrauma. 2018;35:2957–2964. doi: 10.1089/neu.2018.5760. [DOI] [PubMed] [Google Scholar]
- 86.Wilmot CB, Cope DN, Hall KM, Acker M. Occult head injury:its incidence in spinal cord injury. Arch Phys Med Rehabil. 1985;66:227–231. doi: 10.1016/0003-9993(85)90148-0. [DOI] [PubMed] [Google Scholar]
- 87.Wood RL. Accelerated cognitive aging following severe traumatic brain injury:a review. Brain Inj. 2017;31:1270–1278. doi: 10.1080/02699052.2017.1332387. [DOI] [PubMed] [Google Scholar]
- 88.Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- 89.Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI. Spinal cord injury causes brain inflammation associated with cognitive and affective changes:role of cell cycle pathways. J Neurosci. 2014a;34:10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu J, Stoica BA, Luo T, Sabirzhanov B, Zhao Z, Guanciale K, Nayar SK, Foss CA, Pomper MG, Faden AI. Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment. Involvement of cell cycle activation. Cell Cycle. 2014b;13:2446–2458. doi: 10.4161/cc.29420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu J, Zhao Z, Kumar A, Lipinski MM, Loane DJ, Stoica BA, Faden AI. Endoplasmic reticulum stress and disrupted neurogenesis in the brain are associated with cognitive impairment and depressive-like behavior after spinal cord injury. J Neurotrauma. 2016;33:1919–1935. doi: 10.1089/neu.2015.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu GY, Hughes MG, Zhang L, Cain L, McAdoo DJ. Administration of glutamate into the spinal cord at extracellular concentrations reached post-injury causes functional impairments. Neurosci Lett. 2005;384:271–276. doi: 10.1016/j.neulet.2005.04.100. [DOI] [PubMed] [Google Scholar]


