Abstract
The present and future research efforts in cognitive neuroscience and psychophysiology rely on the measurement, understanding, and interpretation of blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) to effectively investigate brain function. Aging and age-associated pathophysiological processes change the structural and functional integrity of the cerebrovasculature which can significantly alter how the BOLD signal is recorded and interpreted. In order to gain an improved understanding of the benefits, drawbacks, and methodological implications for BOLD fMRI in the context of cognitive neuroscience, it is crucial to understand the cellular and molecular mechanism of age-related vascular pathologies. This review discusses the multifaceted effects of aging and the contributions of age-related pathologies on structural and functional integrity of the cerebral microcirculation as they has been investigated in animal models of aging, including age-related alterations in neurovascular coupling responses, cellular and molecular mechanisms involved in microvascular damage, vascular rarefaction, blood–brain barrier disruption, senescence, humoral deficiencies as they relate to, and potentially introduce confounding factors in the interpretation of BOLD fMRI.
Keywords: aging, microcirculation, neurovascular coupling, senescence
1 |. INTRODUCTION
Basic neuroscience research has established that the brain undergoes extensive changes with advancing age (Wright & Wise, 2018). Basic cognitive aging research has established that psychological function (e.g., episodic and working memory, reasoning, planning, and problem solving) also undergoes extensive age-related changes. The notion that relationships may be found between changes in the aging brain and changes in cognitive performance has led to a generation of research in humans aimed at exploring age-related changes in brain–behavior relationships (see Abdelkarim et al., 2019). Neuroimaging modalities, most prominently functional magnetic resonance imaging (fMRI), have been utilized extensively in this enterprise. FMRI measures blood-oxygen-level-dependent (BOLD) signal that arises from a complex interplay of neural, glial, and vascular elements (Figure 1).
FIGURE 1.
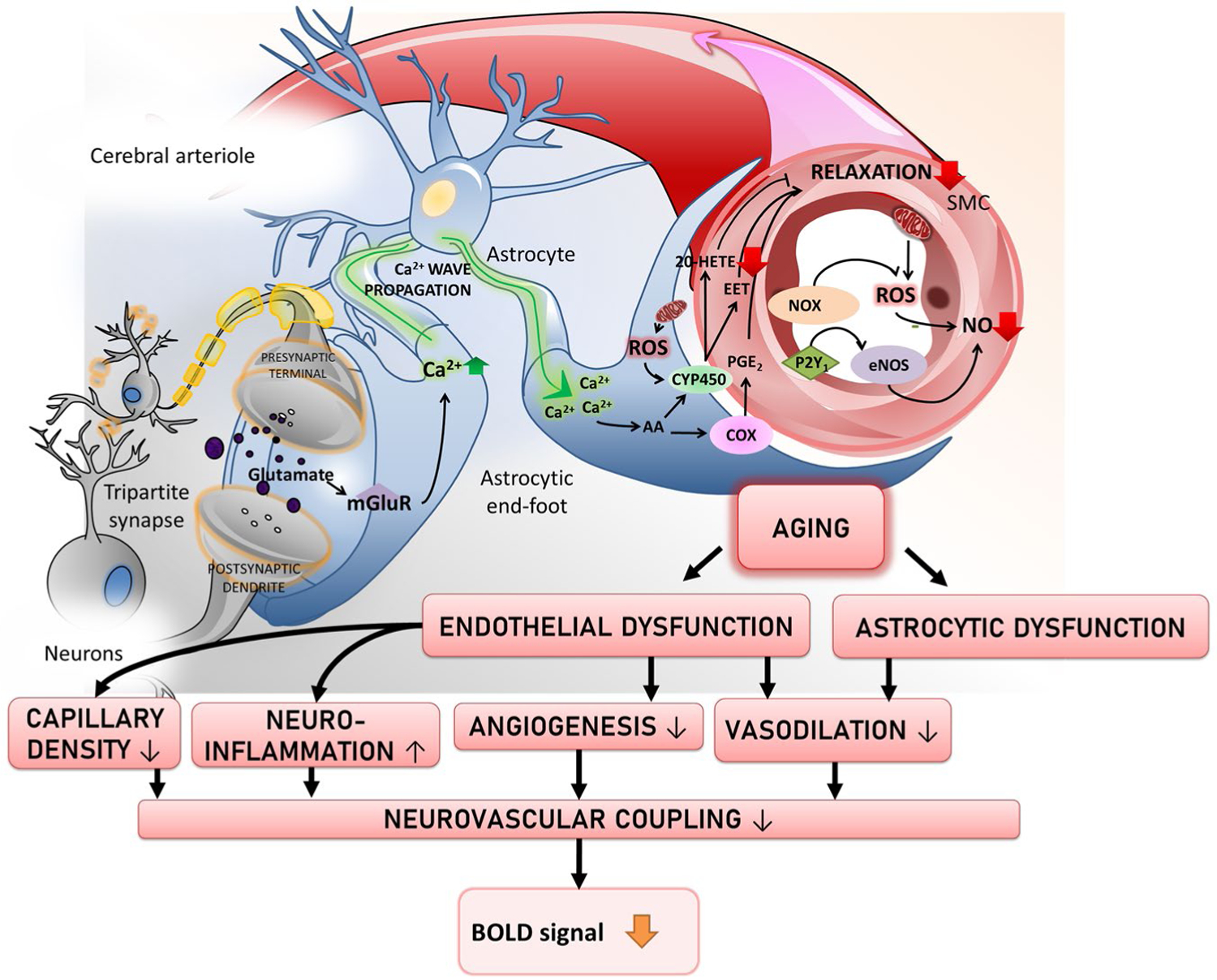
Schematic illustration of how aging and age-related vascular changes affect the neurovascular coupling processes that contribute the BOLD response
Thus, most “neurocognitive aging” studies using fMRI have focused on age-group differences in BOLD signal amplitude. Broadly speaking, this literature has not produced a consistent picture of relationships between age differences in performance and BOLD signal. We argue that reliance on preclinical studies that permit direct measurement of the physiologic components (i.e., vascular, glial, and neural) that together give rise to the BOLD signal can help resolve this confusion and shed light on which of the components in this neural-vascular complex are most closely associated with the pervasive performance changes that characterize the adult aging process.
2 |. CELLULAR MECHANISMS UNDERLYING BOLD
A common misconception is that BOLD directly measures brain regional oxygen consumption, and therefore, neuronal activity. However, this is largely not the case as stimulus-evoked BOLD signals generally represent a decrease in deoxyhemoglobin concentration, and thus, reflect regional increases in blood oxygenation. Early observations from Roy and Sherrington (Roy & Sherrington, 1890) first characterized the intimate relationship between brain function and cerebral blood flow (CBF). They observed task-evoked cerebral vasodilation and attributed it to the increased demand for oxygen and nutrients in response to increased neuronal activity, leading them and other researchers to build on the hypothesis that CBF changes reflect a tight coupling between cellular energy requirements and vascular delivery of glucose and oxygen. Several scientists working in the field of vascular neuroscience have built on these findings to make significant advances in understanding how the underlying vascular, vascular-associated glia, and parenchymal cells contribute to stimulation-evoked hyperemic responses (Attwell et al., 2010), also named neurovascular coupling (NVC). The intuitive idea that changes in CBF are directly controlled by metabolic activity has been revisited over the past decade. A range of cellular mechanisms have been implicated in contributing to NVC responses (Hillman, 2014). In particular, astrocytes (Metea & Newman, 2006; Petzold & Murthy, 2011; Stobart et al., 2013; Takano et al., 2006; Wells et al., 2015), pericytes (Peppiatt et al., 2006), interneurons (Cauli et al., 2004), smooth muscle cells, and endothelial cells (Chen et al., 2014), have been proposed to play a role in the local control of blood through a feedforward mechanism involving neuronal signaling and neurotransmitters (Attwell & Iadecola, 2002; Lauritzen, 2005; Takano et al., 2006). Excellent reviews2, (Hillman, 2014; Tarantini et al., 2017) explore in detail current understanding of the molecular dynamics of neurovascular coupling responses, how they change in aging, and feature schematic illustrations to exemplify candidate mechanisms. Recent evidence supports the idea that astrocytes play an important role in linking neurotransmitter activity to vascular responses, and astrocyte-evoked BOLD signal may associate with oxygen consumption without modulation of neuronal activity (Pellerin & Magistretti, 2004; Takata et al., 2018). In this view, neurovascular coupling responses underlying generation of the BOLD signal would be evoked through activated glial mechanisms modulated by neuronal signaling, rather than by mechanisms that sense energy consumption. However, due to difficulties in reconciling astrocytic distribution, their connectivity, and intracellular Ca2+ transient timings with the spatiotemporal properties of neurovascular coupling, some studies (Chen et al., 2011; McCaslin et al., 2011; Nizar et al., 2013) have challenged the involvement of astrocytes, while others suggest that neurovascular coupling might be mediated by diffusion of products of neuronal activity without the involvement of glial cells. As there is no single mechanism that has been irrefutability demonstrated, the controversy regarding which mechanism, or combination of mechanisms, is responsible for the generation of the BOLD response is open for investigation. Another often-overlooked source of signaling molecules mediating neurovascular coupling is the vasculature itself. Previous work has identified endothelial hyperpolarization as a rapid mechanism by which neurovascular coupling responses may quickly propagate (Bagher & Segal, 2011; Figueroa & Duling, 2009) through gap-junctions within the endothelium itself with little attenuation (Wolfle et al., 2011). An additional endothelial-mediated mechanism involves the slow propagation of endothelial Ca2+ waves (Tallini et al., 2007) that elicit smooth muscle cell relaxation via release of endothelial-derived NO and prostanoids such as prostacyclin (de Wit & Griffith, 2010). In part, the BOLD signal detected by fMRI reflects hemodynamic changes within the brain, which in turn are driven by metabolic alterations and neuronal activity (Keilholz et al., 2017). However, the link between BOLD changes and neuronal activity is indirect, and may be influenced by several nonneuronal processes as well, which include motion and physiological cycles, differences in the physiological baseline which contribute to intra- and intersubject variabilities, and the use of anesthesia alters neural activity, vascular tone, and NVC (Keilholz et al., 2017). Despite the lack of a unified theory, the predominant view is that some combination of the mechanisms listed above must work together to generate the blood flow response and the BOLD signal(Attwell et al., 2010).
3 |. AGE-RELATED IMPAIRMENT OF NEUROVASCULAR COUPLING RESPONSES
The quickly growing field of geroscience has posed itself as an interdisciplinary approach that combines preclinical animals studies and clinical knowledge (Csipo et al., 2019) to understand the relationship between the biology of aging and the pathophysiology of chronic age-related diseases. A range of recent studies both in elderly patients and rodent models shows that aging significantly impairs neurovascular coupling responses (Balbi et al., 2015; D’Esposito et al., 2003; Fabiani et al., 2014; Park et al., 2007; Schroeter et al., 2007; Sorond et al., 2013; Tarantini et al., 2016; Tarantini, Valcarcel-Ares, et al., 2018, 2019; Tarantini et al., 2019; Tong et al., 2012; Zaletel et al., 2005). In aging, changes in the cerebrovascular ultrastructure caused by arteriosclerotic processes, increases in vascular oxidative stress (Logan et al., 2019; Tarantini, Valcarcel-Ares, et al., 2018, 2019; Tarantini et al., 2019), progressively dysfunctional cellular components of the neurovascular unit, and the age-related decline of important humoral vasoprotective factors (IGF-1) (Toth et al., 2015), all are believed to be important contributors to the impairment of neurovascular coupling responses and cognition. Notably, age-related changes in resting-state CBF have been identified in humans (Bertsch et al., 2009; Restom et al., 2007; Zhang et al., 2017). In studies using arterial spin labeling (ASL) perfusion MRI, that were persistent despite efforts to correct for age-related morphological changes (Chen et al., 2011). Measurement of CBF is integral to the measured BOLD signal as studies have suggested an inverse relationship between resting CBF and BOLD fMRI signal (Zebrowitz et al., 2016). The age-related changes in resting CBF have been tentatively attributed to alterations of end-tidal pCO2 (De Vis et al., 2015). In murine models, resting CBF does not appear to change as a function of age (Tarantini et al., 2019) while evidence from isolated rodent cerebral arteries suggest that aging is associated with a decrease in baseline CBF (Faraci & Heistad, 1998). Collectively, age-related changes in BOLD signal have been shown to decrease significantly when lower baseline CBF is accounted for, reinforcing the view that observed age-related BOLD fMRI differences must account for a substantial vascular contribution (Zebrowitz et al., 2016). Although the exact mechanisms of neurovascular coupling are not completely understood, the extant evidence suggests that aging of the cerebrovascular system and age-related cerebrovascular pathologies can influence the BOLD signal independently of neural activity and must be accounted for with appropriate study design, correct analysis, and interpretation of BOLD fMRI data when comparing age groups.
3.1 |. Phenotypic alterations of the vascular structure in aging
With age, the structural integrity of the cerebrovasculature is altered (Farkas et al., 2000; Li et al., 2018), thus, compromising cerebral circulation and leading to potential functional consequences such as increased risk for neurodegeneration and declining cognitive function. Among the age-related phenotypic changes observed in the cerebrovasculature are increased thickness of capillary basement membrane (Alba et al., 2004), age-related rarefaction (Tarantini et al., 2016; Tarantini et al., 2016; Tucsek et al., 2014) in animal models, and decreased cerebral capillary density in aged humans (Brown et al., 2007; Meier-Ruge et al., 1980; Riddle et al., 2003). Vascular rarefaction is of particular interest in the context of BOLD fMRI signal interpretation, as variations in the pulse sequences and magnetic field intensity can bias the BOLD signal to larger vessel sizes (Mueller-Bierl et al., 2007). Arterial stiffness, a feature often present in the larger vasculature of aged individuals (O’Rourke & Hashimoto, 2007) may also be present in the smaller cerebral arterioles and be implicated in experimentally observed CBF pulsatility increases(Springo et al., 2015) despite the overall baseline CBF decreases (Tarumi et al., 2014). These structural alterations may impact vascular elasticity and perfusion capacity possibly influencing CBF and functional changes during stimulus-evoked functional hyperemia which in turn could impact BOLD fMRI signal magnitude and timing (Brown et al., 2003). Additionally, recent evidence has shown that the interaction between increased adiposity and aging can be detrimental for neurovascular function, cerebrovascular integrity, and cognitive function. In their study, Csiszar et al (Tucsek et al., 2014) found that aging exacerbates the obesity-induced decline in microvascular density both in the hippocampus and in the cortex of mice fed a high-fat diet. The extent of hippocampal microvascular rarefaction and the extent of impairment of hippocampal-dependent cognitive function positively correlated, thus, indicating that obesity must be considered as a variable when analyzing BOLD signal in aged individuals.
3.2 |. Oxidative stress, microvascular inflammation, and endothelial dysfunction in aging
Chronic, low-grade inflammation has been identified as a hallmark of aging (Cervellati et al., 2018; Royce et al., 2019), the term “Inflammaging” (Franceschi et al., 2000; Franceschi & Campisi, 2014; Wilhelm et al., 2017) has been used to emphasize this close association between aging and the accompanying age-related low-grade sterile inflammatory processes taking place. Growing evidence suggests that the combination of aging and inflammation accelerates the development of vascular and microvascular pathophysiological processes due to the increased generation of reactive oxygen species (ROS) through increased NAD(P)H oxidase activity (Adler et al., 2003; Donato et al., 2007; Jacobson et al., 2007; van der Loo et al., 2000), and through inefficient mitochondrial oxidative phosphorylation (Balaban et al., 2005; Lesnefsky & Hoppel, 2006; Sure et al., 2018) promoting endothelial dysfunction (Donato et al., 2007). Heightened inflammatory status also associates with microvascular dysfunction in the eye (Lipecz et al., 2019). Direct evidence from animal models of aging has suggested that oxidative stress and endothelial dysfunction are critical players in the etiology of age-related cerebromicrovascular impairment (Fulop et al., 2018) and neurovascular uncoupling (Costea et al., 2019; Park et al., 2007; Tarantini et al., 2016; Toth et al., 2014). In endothelial cells mitochondria and NADPH oxidases are major sources for ROS production (Carvalho & Moreira, 2018; Tarantini, Valcarcel-Ares, et al., 2018, 2019). Endothelium-derived nitric oxide (NO) is a potent vasodilatory gasotransmitter and its production and bioavailability has been found to be an important contributor to NVC responses (Toth et al., 2015). In aging, highly reactive excess superoxide (O2·−) molecules sequester free NO to form peroxynitrate (ONOO−) thus decreasing bioavailability of endothelium-derived NO, impairing the dilatory capability of the cerebromicrovasculature (Csiszar et al., 2002; Park et al., 2007; Tarantini et al., 2016; Tarantini et al., 2017; Tarantini et al., 2018). In support of the role of endothelial dysfunction in impaired NVC responses, experimental evidence suggests that treatments that restore endothelial function (Tarantini et al., 2018; Tarantini et al., 2019), improve NO bioavailability (Wiedenhoeft et al., 2019), inhibit NADPH oxidases (Park et al., 2007), or reduce cerebrovascular oxidative stress (Kiss et al., 2019; Tarantini et al., 2019; Toth et al., 2014) can improve NVC responses in aged animals. Recent experimental studies in mouse models of aging have shown that old mice overexpressing human catalase in the mitochondria (MCAT) also benefit from attenuated age-related oxidative stress, improved microvascular endothelial function and preserved NVC responses (Csiszar et al., 2019). It should be noted that in addition to chronological aging, accelerated cerebromicrovascular aging associated with pathological conditions (e.g., obesity (Csipo et al., 2018), hypertension (Girouard & Iadecola, 2006; Iadecola & Gottesman, 2019)) also impair endothelial function and neurovascular coupling responses and thereby likely modulate the BOLD signal.
3.3 |. Age-related loss of vasodilatory capacity
In addition to impaired response to dilatory stimuli, the intrinsic ability of cerebral vessels to dilate was found to be impaired in aged rodents (Tamaki et al., 1995) and in older humans (Csipo et al., 2019; Fluck et al., 2014) potentially as a consequence of age-related vascular stiffening. Inhalation of higher concentration of CO2 (hypercapnia) has been used to study vascular reactivity in rodent models (Balbi et al., 2015) as well as humans (Fluck et al., 2014; Riecker et al., 2003). Despite the intrinsic limitations of studies involving hypercapnia such as the rate of metabolism and respiratory function as confounding variables, the observed evidence suggests that aged vessels exhibit a lower degree of cerebrovascular reactivity, leading to impaired stimulus-evoked vasodilation. Interestingly, evidence from arteries isolated in aged rodents also show aged vessels display a reduced response to endothelium-dependent vasodilators (Mayhan et al., 1990). Age-related impairment of vascular reactivity may result in decreased BOLD fMRI signal measurements and an overall misrepresentation of stimulus-evoked neural responses in aged individuals compared to younger groups.
3.4 |. Role of astrocytes and aging
Astrocytes are among the most prevalent glial cells in the brain and their ideal positioning at the interface between neurons and microvessels allows for their fine end-feet processes to intimately contact and facilitate communication between neuronal synapses and the cerebral microvasculature (Mishra, 2017). Mounting evidence over the past 15 years has revealed that astrocytes play a crucial role in the mediation of NVC responses (Filosa et al., 2016) by releasing vasodilator metabolites of arachidonic acid and ATP. In recent basic research studies, many astrocyte-derived vasoactive signals have been identified and their role on vessel relaxation or contraction has been confirmed (Filosa et al., 2016). Numerous studies conducted in rodent brain slices and in experimental animals have provided evidence in support of astrocytic participation in the regulation of cerebromicrovascular tone (Attwell et al., 2010; Carmignoto & Gomez-Gonzalo, 2010; Gordon et al., 2007; Iadecola & Nedergaard, 2007) and in modulation of NVC responses in animal models in vivo (Hosford & Gourine, 2019; Tarantini et al., 2015; Xu et al., 2008).
During the stimulus-evoked increase in neuronal activity, many proposed pathways are activated to produce NVC responses, and some evidence has also considered the more direct forms of signaling from activated neurons to vessels (Cauli & Hamel, 2010). A majority of studies performed primarily in brain slices suggest that astrocytes first respond to elevations in extracellular glutamate as the main signal activating neurovascular responses (Fergus & Lee, 1997; Zonta et al., 2003). Mechanistically, the presence of glutamate stimulates a group 1 metabotropic glutamate receptor (mGluR)-dependent elevation of astrocytic intracellular Ca2+ via IP3 signaling resulting in a arteriolar vasodilatory response (Zonta et al., 2003). The increase in end-feet Ca2+ activates calcium sensitive phospholipase A2 which releases arachidonic acid, which is promptly converted by cyclooxygenases into prostaglandins such as PGE2 or PGI2, and by epoxygenases to epoxyeicosatrienoic acids (EETs; Figure 1). Prostaglandins and EETs bind to EP receptors, activate TRPV4 (Nilius et al., 2003) and large conductance BKCa channels. In vivo animal studies and brain slice work support this view by showing that astrocytic Ca2 + transients result in release of arachidonic acid metabolites (Carmignoto & Gomez-Gonzalo, 2010; Koehler et al., 2009; Takano et al., 2006). Interestingly, in specific experimental settings, arachidonic acid metabolites have been shown to elicit vaso-constriction instead of vasodilation (Dabertrand et al., 2013), notably under pathological conditions arachidonic acid can be metabolized into 20-hydroxyeicosatetraenoic acid (20-HETE), a vasoactive constrictor molecule(Toth et al., 2013). This evidence suggests that age-related pathologies such as hypertension must be accounted for in the analysis of BOLD fMRI signals as astrocytic contribution to neurovascular hyperemic responses might become impaired.
Because the manner in which aging affects astrocytes is not completely understood, many studies are interrogating the multifaceted consequences of astrocytic aging, including alterations in Ca2+ wave patterns (Mathiesen et al., 2013) and age-related changes in arachidonic acid metabolite production and release (Keleshian et al., 2013). Recent evidence from rodent studies suggests that, in addition to the newly discovered astrocyte heterogeneity, some astrocytic populations may switch to a “reactive” phenotype during aging (Clarke et al., 2018), resulting in the development of astrogliosis (Rodriguez-Arellano et al., 2016), a pro-inflammatory state characterized by complement activation (Boisvert et al., 2018), synapse elimination, and neuroinflammation. Since astrocyte reactivity and dysfunction are newly discovered key features of age-related pathologies (Matias et al., 2019), a better understanding of age-related changes on astrocytic phenotypes hold the promise of ameliorating potential confounding factors in the interpretation of BOLD fMRI signal in aging populations.
3.5 |. Neurovascular energetics in aging
Evidence from several laboratories indicate that the age-dependent neurovascular uncoupling, or impairment of NVC response magnitude, could be exacerbated by age-related alterations in the cerebral metabolic rate of oxygen (CMRO2) (Lourenco et al., 2018; Peng et al., 2014; Schwarzbauer & Heinke, 1999) or impaired cellular energetics in the neurovascular unit (Tarantini, Valcarcel-Ares, et al., 2018, 2019; Tarantini et al., 2019). The moment-to-moment equilibrium between local O2 supply and consumption after neuronal activation follows a biphasic nature, with an immediate initial increase in regional O2 consumption followed by a positive component follows as the supply overcomes the demand with increased CBF (Thompson et al., 2003). Experiments performed on Wistar rats have shown that aging has no effect on the initial rise of tissue oxygen demand, but older rats showed a significantly higher tissue oxygen supply during the hyperemic response despite an impaired CBF response. These results suggest the presence of an age-related decrease in regional CMRO2 (Lourenco et al., 2018). Other studies have shown both basal and maximal O2 consumption-rate decrease in aged mouse brain slices (Dias et al., 2016) and an age-related reduction in oxidative phosphorylation in F344 rats (Lam et al., 2009), suggesting a critical role for cellular bioenergetics in preserving healthy NVC responses. In vivo murine studies have shown that even short-term treatments aimed at improving cellular energetics are successful in restoring NVC responses (Tarantini, Valcarcel-Ares, et al., 2018, 2019; Tarantini et al., 2019). However, in the context of age-related changes that may affect BOLD fMRI signal interpretation, the relationship between neurovascular uncoupling and CMRO2 may have the strongest confounding effect. The higher O2 demand with diminished supply observed in rodent studies (Lourenco et al., 2018) would result in increased venous deoxy-hemoglobin concentrations (Hutchison et al., 2013; Lu et al., 2011), partially explaining how age-related changes in CMRO2 and CBF would affect the BOLD fMRI response signal.
Another fundamental feature contributing to vascular aging is mitochondrial dysfunction (Chistiakov et al., 2012; Chistiakov et al., 2014; Sobenin et al., 2013; Ungvari et al., 2007, 2008). Age-dependent impairment in mitochondrial function is associated with decline in mitochondrial oxidative phosphorylation, state 3 respiration, and diminished activity of complexes I and IV in the electron transport chain, resulting in increased electron leakage, diminished ATP yield, and generation of mitochondrial ROS (Dai et al., 2012). Recent evidence in animal models demonstrated that ATP and its metabolites (ADP and adenosine) are also important gliotransmitters released by astrocytes upon neuronal stimulation (Heinrich et al., 2012; Toth et al., 2015). In rodents, astrocyte-derived ATP was found to contribute to stimulus-evoked cerebrovascular dilation by increasing NO production (Toth et al., 2015). In humans, ATP synthesis capability has been found to decrease 8% every decade of life (Short et al., 2005) as most cell types experience altered cellular energy homeostasis. Still, little is known about the relationship of aging on purinergic signaling and its contribution to vasomotor responses, thus, introducing confounding factors affecting the interpretation of BOLD fMRI signals.
3.6 |. Senescence
Cellular senescence is a state in which cells no longer go through the stages of the normal cell cycle and do not divide to create new cells. Despite its name, cellular senescence occurs throughout life, and serves to maintain healthy tissue by preventing proliferation of potentially tumorous cells. Under normal, healthy conditions, senescent cells signal for their own removal from tissue. In aging, however, this does not always occur, and senescent cells accumulate in tissue and assume a specific phenotype known as the senescence-associated secretory phenotype (SASP). Cells expressing SASP can secrete inflammatory substances that might harm nearby tissue, and their accumulation has been associated with age-related disease (Campisi et al., 2011; van Deursen, 2014; Ovadya & Krizhanovsky, 2014). The available evidence indicate that endothelial senescence associates with a substantial increase in the release and/or production of pro-inflammatory chemokines and cytokines that exacerbate the heightened inflammatory status of the aged vasculature (Morgan et al., 2013; Ungvari et al., 2019). Also senescent endothelial cells contribute to endothelial dysfunction in aging and worsen the pathophysiological conditions associated with accelerated vascular aging (Brandes et al., 2005; Herrera et al., 2010; Kida & Goligorsky, 2016; Pantsulaia et al., 2016) which may in turn affect negatively neurovascular coupling responses and affect BOLD signal. Due to the crucial importance of astrocytes in mediation of neurovascular coupling, evidence in favor of the importance of astrocyte senescence in the impairment of neurovascular coupling has been growing in recent years (Cohen & Torres, 2019; Csipo et al., 2020; Lye et al., 2019). Senescent astrocytes demonstrate increased levels of intermediate glial fibrillary acidic proteins and vimentin filaments, increased expression of pro-inflammatory cytokines (including interleukin-6 (Bhat et al., 2012)) and increased accumulation of proteotoxic aggregates which are thought to play a role in age-related neuroinflammation and neuronal degeneration (Salminen et al., 2011). Importantly, anticancer treatments, including irradiation (Yabluchanskiy et al., 2020) and chemotherapy (Carlson et al., 2018) can cause neurovascular senescence, which associate with significant impairment of neurovascular coupling responses.
3.7 |. Age-related IGF-1 deficiency
Changes in the systemic milieu in aging is associated with complex changes in the circulating levels of vasoprotective endocrine factors (Kiss et al., 2020; Schafer & LeBrasseur, 2019; Ungvari et al., 2018, 2020; Zhang et al., 2019). Decrease in circulating insulin-like grown factor 1 (IGF-1) as a consequence of aging has recently been shown to exert detrimental effects on various aspects of endothelium-dependent vasodilation (Toth et al., 2015) and contribute to microvascular rarefaction (Norling et al., 2019; Sonntag et al., 1997; Tarantini et al., 2016; Tarantini et al., 2016). Extensive early research has determined that the reduction in IGF-1 is an important component of the age-related decline in cognitive function in multiple species including humans (Frater et al., 2018; Norling et al., 2020; Sonntag et al., 2013). Additionally, genetic IGF-1 deficiency was shown to impair vascular function (Bailey-Downs et al., 2012; Bailey-Downs et al., 2012; Csiszar et al., 2008; Fulop et al., 2018; Tarantini et al., 2016; Tarantini et al., 2016; Tarantini et al., 2016; Tarantini et al., 2017; Toth et al., 2014), including neurovascular coupling responses in rodent models (Toth et al., 2015). A recent study suggested that systemic circulating IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice (Farias Quipildor et al., 2019). Given the close relationship between age-related circulating IGF-1 deficiency and endothelial health and neurovascular coupling responses, it is plausible that IGF-1 levels may play an important role in the modulation of the BOLD signal.
3.8 |. Age-related pathologies
Lipopolysaccharide (LPS)-induced encephalopathy induces neuroinflammation. Long-term neuroinflammation is associated with aging and subsequent cognitive impairment (CI). A recent study used parametric MRI approaches to assess cerebrovascular, blood–brain-barrier (BBB), metabolic and free radical level changes associated with LPS-augmented neuroinflammation, and found that LPS-exposed rat brains had decreased relative cerebral blood flow (rCBF), increased BBB disruption, decreased N-acetyl aspartate (NAA; a neuronal marker), and increased levels of free radicals detected in vivo, compared to saline-treated controls (Figure 2) (Towner et al., 2018), (Towner et al., 2019). These findings imply that increased neuroinflammation results in cerebrovascular, neuronal metabolic and free radical changes, which are similar to the mechanistic alterations underlying age-related BOLD fMRI change.
FIGURE 2.
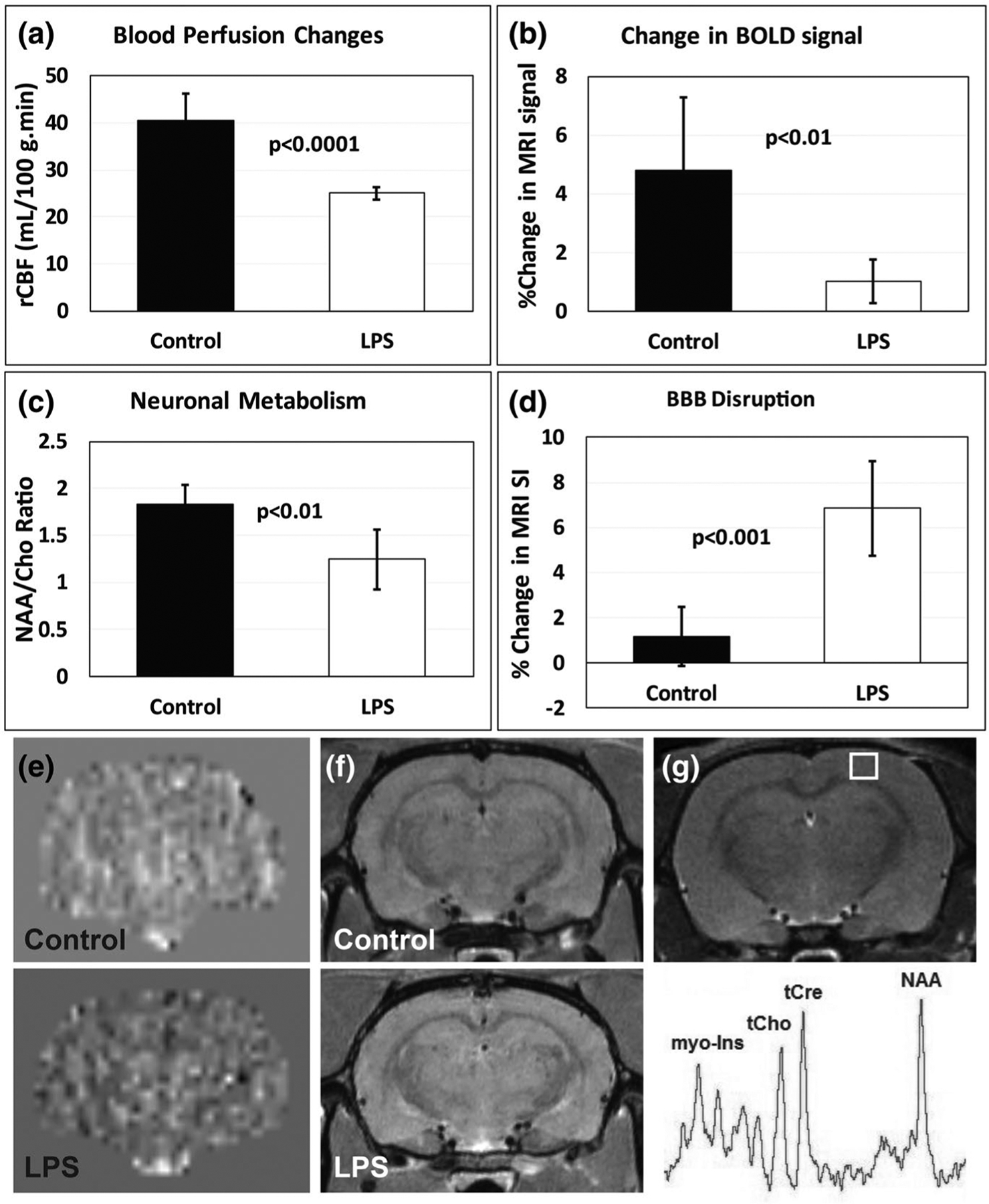
LPS-induced neuroinflammation-associated cerebrovascular and neuronal changes in rat cerebral cortex. (a) Relative cerebral blood flow (rCBV) significantly decreases with LPS-induced neuroinflammation (p < .0001), compared to saline controls (n = 6/group). (b) Blood-oxygen level-dependent (BOLD) contrast in functional MR images (fMRI) from LPS-exposed rat brains are significantly less than controls (p < .01) (n = 6/group). (c) The neuronal metabolite N-acetyl aspartate (NAA) is significantly decreased in LPS-exposed rat cortex (p < .01) (n = 6/group). (d) Blood–brain barrier (BBB) disruption is significantly increased in LPS-exposed rat cerebral cortex compared to controls (p < .001) (n = 6/group). Measured as a percent change in MRI signal intensity obtained post-Gd-DTPA MRI contrast enhancement. (e) Representative rCBF maps obtained from control or LPS-exposed rat brains. (f) Representative contrast-enhanced (CE) MR images post-Gd-DTPA in control or LPS-exposed rat brains. (g) Representative MR image depicting the cortical region for obtaining a MR spectrum. Metabolites: myo-inositol (myo-Ins); total choline (tCho); total creatine (tCr); and N-acetyl aspartate (NAA). All data were obtained 6 weeks post-LPS or saline treatments. Data modified from references (Towner et al., 2018) and (Towner et al., 2019). BOLD fMRI data are unpublished
3.9 |. Aging and exercise
Emerging research demonstrates that exercise is associated with improving several cognitive outcomes (Loprinzi et al., 2018). For example, recent evidence has suggested that physical exercise in aging can play a role in improving neurogenesis, gliogenesis, angiogenesis, cerebral circulation, and growth factor production, as well as parahippocampal function (Loprinzi, 2019a) and modulating episodic memory function (Loprinzi, 2019b). These novel findings imply that incrased physical activinty in aging may promote beneficial microvascular effects that would contribute to a more effective and responsive cerebral circulation to neuronal activity, potentially affecting that relationship between cerebral perfusion and cognitive load. Therefore, exercise may be considered a contributing confounding variable in the interpretation of BOLD fMRI signals in elderly individuals.
4 |. FUNCTIONAL NEAR-INFRARED SPECTROSCOPY IN AGING AND AGE-RELATED DISEASES
Since the original studies in cognitive neuroscience to understand the physiochemical states of brain tissues (Logothetis, 2008), BOLD fMRI has gained currency in neuroimaging and clinical applications (Bauer et al., 2014; Lang et al., 2014). However, the use of BOLD fMRI to date remains somewhat limited mainly due to increasing interest in examining brain activity during exercise, or in populations that are not typical or suitable for the fMRI imaging including infants, those with abnormal aging, or those with limited functional capabilities. The use of BOLD fMRI for research purposes is also limited to large academic centers that are equipped with the expensive technology but compete with the use of the fMRI for clinical purposes. In aging neuroimaging research, fMRI allowed to identify age-related change in hemodynamic pattern in the brain regions responsible for age-related decline in executive function (Wilckens et al., 2017). Physiological modulation of these brain regions demonstrated a less specific cerebral activation and recruitment of additional brain regions in older adults when compared to young controls (DiGirolamo et al., 2001; Milham et al., 2002). However, the cost and ecological burden associated with use of fMRI modalities in aging research have prevented large-scale studies on age-related diseases and development of prospective medical interventions. Therefore, the necessity of new alternative tools with complementary strengths where fMRI is limited led to development of functional near-infrared spectroscopy (fNIRS).
The fNIRS technology originated from Jobsis work (Jobsis, 1977). This work provided the first evidence that near-infrared light can be transmitted through biological tissues. A number of in vivo and human studies allowed for development of the first, although limited, commercially available fNIRS instruments in 1989 (Scarapicchia et al., 2017). Further advancement in fNIRS technology and data analysis allowed for broader application and use in the laboratory and clinic (Huppert et al., 2009; Lagerwaard et al., 2020; Lv et al., 2009). Modern fNIRS tools allow non-invasive and nonionizing methods for measuring functional integrity and hemodynamic brain activity that is spectroscopically extracted from measured concentrations of oxygenated, deoxygenated and total hemoglobin within the brain tissues and, since the original implementation, fNIRS has proven to be a useful tool to study normal and altered brain functions(Yucel et al., 2017). Recent studies that utilized fNIRS to investigate age-related changes in the brain have also used same paradigms applied in fMRI research. In the field of physiopsychology, and in other disciplines, it remains as a topic of intense debate whether the hemodynamic responses measured by fNIRS or fMRI can be correlated to neuronal responses and/or cognitive load. A study from Fabiani et al have discovered the presence of a proportional relationship between neuronal and hemodynamic responses using a combined approach (Fabiani et al., 2014). In this work, young and older individuals were found to exhibit a quadratic relationship between neuronal and hemodynamic effects, with reduced increases of the hemodynamic response at high levels of neuronal activity. Although the relationship between neuronal and vascular measures of brain function departs substantially from linearity between younger and older adults, these finding highlighted that the neurovascular coupling becomes impaired in older adults, meanwhile the neuronal response remains unchanged. These clinical findings further substantiate basic science evidence that aging and other age-related changes (i.e., obesity, lack of exercise, etc.) impair cerebrovascular integrity and its function potentially introducing confounding variables when interpreting vascular outcomes (with fNIRS or fMRI) in elderly patients. Earlier studies compared fNIRS-related brain activation in the prefrontal cortex of healthy young and older adults and provided consistent evidence of the signal observed within the regions responsible for cognitive tasks implemented when compared to BOLD fMRI signal (Habeck, 2010; Vasta et al., 2017; Vermeij et al., 2012, 2014, 2017). In these studies, the authors reported a consistent overactivation of the prefrontal cortex during a relatively low cognitive load in older adults, suggesting a compensatory mechanism. When comparing concentrations of oxygenated hemoglobin in older adults without mild cognitive impairment and in those with nonamnestic and amnestic mild cognitive impairment, Yoon et al. found a progressive decrease in the mean accumulated oxygenated hemoglobin values from normal aging to amnestic cognitive impairment (Yoon et al., 2019). When using fNIRS approach in older adults with mild cognitive impairment and mild Alzheimer’s disease, researchers found a general decline in functional connectivity from normal aging to Alzheimer’s disease with more profound laterality in older adults with Alzheimer’s disease during a verbal fluency task (Tang & Chan, 2018). Another study has also reported abnormal patterns of hemodynamic response across groups of normal aging, mild cognitive impairment, and moderate/severe Alzheimer’s disease in older adults. In this study, the authors demonstrated greater and steeper reductions in oxygenated hemoglobin concentrations as disease severity developed from mild cognitive impairment to Alzheimer’s disease (Li et al., 2018).
Additionally, stimulus intensity and cognitive load have been shown to affect younger and older adults differently. In a human study, participants viewed a single fixation cross at the center of a radial yellow and blue checkerboard flickering at three frequencies (2, 4, and 8 Hz) during the stimulation blocks. Younger participants’ neurovascular response increased as the task became more intense, similar findings were obtained in a study comparing younger and older adults using fNIRS during 1-back and 2-back testing (Figure 3).
FIGURE 3.
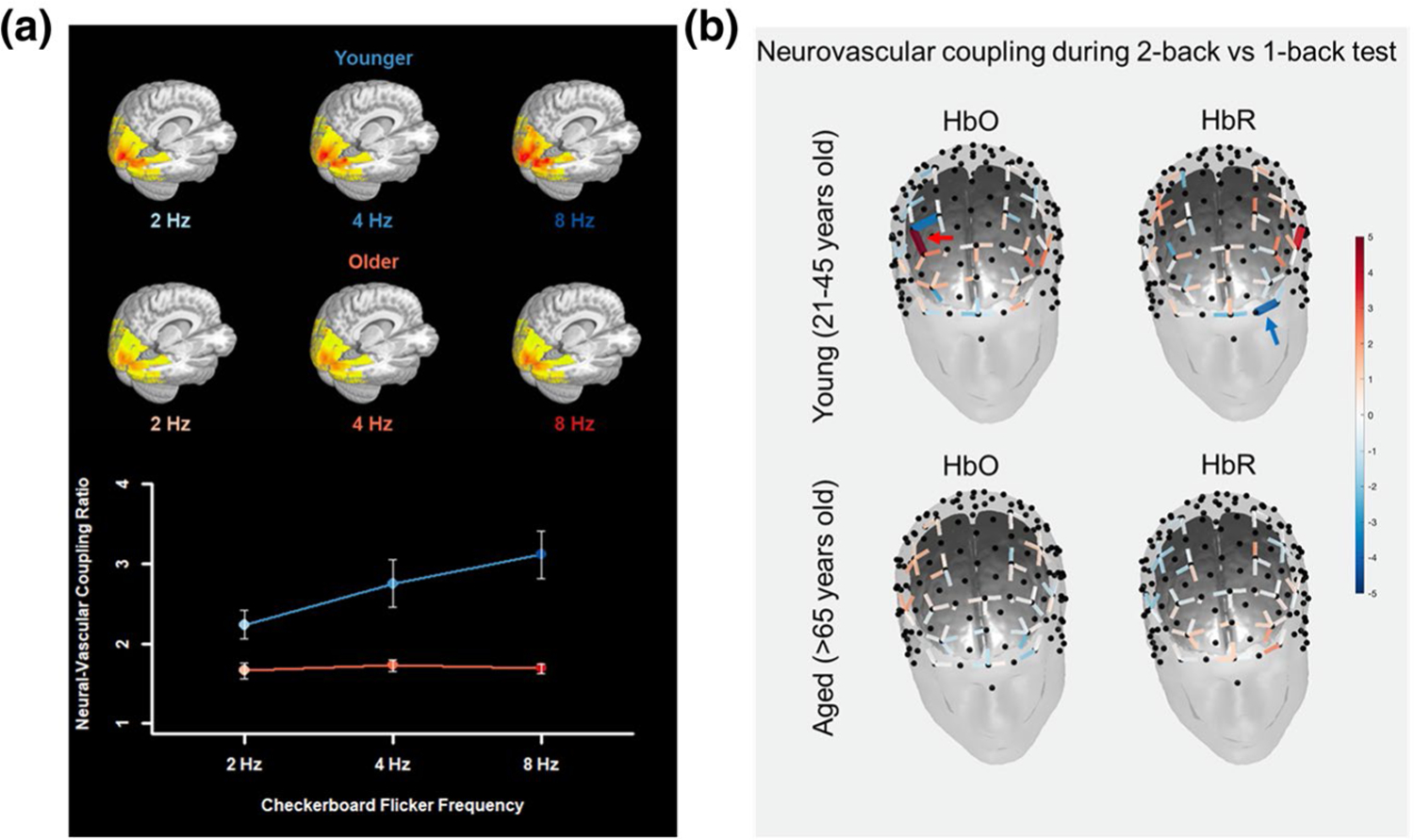
Aging is associated with impaired neurovascular coupling responses during increasing cognitive load. (a) Neural-vascular coupling ratio (ratio of fractional CBF signal change to fractional CMRO2 signal change) in response to visual checkerboard stimulation using MRI. The coupling ratio was greater in younger than older adults across frequencies and increased with increasing frequency in younger adults but older adults. The neural-vascular coupling ratio was calculated from the reciprocal of parameter weights from a 2 (Age-Group) × 3 (Flicker-Frequency) mixed-effects linear model predicting CMRO2, with CBF as a covariate. The coupling ratio was greater in younger adults than older adults. There were significant main effects of both Age-Group in NVC ratio, t(47) = 4.027 (p = 2.044 × 10–4), (linear) main effect of Flicker-Frequency condition in NVC ratio, t(94) = 4.615 (p = 3.056 × 10–5). The ratio increased with increasing frequency more for younger adults than older adults. The Age-Group × Flicker-Frequency interaction effect was significant, F(2, 94) = 9.473 (p = 1.879 × 10–4). (b) fNIRS assessment of neurovascular coupling responses during 1- and 2-back cognitive task in four healthy young (21–45 years of age) and four healthy aged individuals (>65 years of age). Analysis was performed using a pipeline based on General Linear Model (GLM) approach created using the Brain AnalyzIR toolbox. For this comparison, we evaluated neurovascular coupling responses between a more challenging 2-back task and less challenging 1-back task using the contrast [2-back–1-back]. Solid lines represent statistically significant change (q < 0.05, corrected p value) in hemodynamic response between more challenging cognitive task versus less challenging task. We observed an increase in neurovascular coupling responses in young individuals during a more challenging cognitive task, evidenced by a statistically significant increase in the oxy-hemoglobin (HbO) in dorsolateral prefrontal cortex (red arrow) and a statistically significant decrease in the deoxy-hemoglobin (HbR) in left lateral frontopolar cortex (blue arrow). No statistically significant difference in neurovascular coupling responses during increasing cognitive load was observed in older adults. These data suggest an impaired neurovascular coupling response in aged individuals
The fNIRS methodology was further applied in age-related pathologies such as hypertension. In the study by Bu et al., the authors report altered neurovascular coupling measured as a significant decrease in wavelet phase coherence and effective connectivity during resting and standing period in subjects >60 years of age with systolic and diastolic blood pressure over 150 and 90, respectively (Bu et al., 2018). Authors also reported a strong correlation between continuous fNIRS findings and the Montreal Cognitive Assessment cognitive scores in the group of hypertensive older adults, indicating strong contribution of hypertension to cognitive decline in aging. Functional NIRS has also been studied in age-related pathologies such as cancer to measure changes in brain hemodynamics after chemotherapy.
The use of fNIRS technology has also been proposed for longitudinal assessment and monitoring of the cancer treatment related cognitive impairment (Jean-Pierre, 2014). Recent data show that cancer patients after the chemotherapy presented with unbalanced increases in activation in prefrontal cortex regions during cognitive stimulation (Jean-Pierre et al., 2015). These findings were comparable to other that reported similar results in human subjects with cognitive decline. Finally, fNIRS was found useful as a monitoring, therapeutic, and research tool in stroke patients (Yang et al., 2019). The fNIRS has been extensively used for poststroke upper and lower limb function recovery, balance control, and motor learning. In these studies, functional improvements after stroke were associated with improved activation in prefrontal and sensorimotor cortex brain regions (Hara et al., 2013; Hatakenaka et al., 2012; Kato et al., 2002; Mihara et al., 2012; Miyai et al., 2002, 2003; Rea et al., 2014; Takeda et al., 2007). Spontaneous activity in prefrontal cortex oxygenation was also found to be altered in older adults and in patients with cerebral infarction (Li et al., 2010). Several other studies have investigated the dual-task interference between cognitive and physical performance in poststroke subjects. Authors reported that prefrontal cortex activation might prioritize physical demands in poststroke subjects (Mori et al., 2018), unlike in healthy elderly (Holtzer et al., 2016).
Current limitations for fNIRS use are mainly due to technological constraints. Changes in hemoglobin concentrations evoked by neuronal activity can be masked by body physiology related to cardiac cycle, breathing, or blood pressure fluctuations (Csipo et al., 2019). Current approaches to eliminate this interference, that originates from scalp, skull, and brain itself, include frequency-based algorithms and recently emerging time-domain NIRS approach that enables “null-distance” depth resolution (Pifferi et al., 2016). Further, current use of fNIRS technology will significantly benefit from a routine implementation of the diffuse correlation spectroscopy, a technique that is sensitive to red blood cells motion and that may provide a complementary index of blood flow within the measured brain areas (Durduran & Yodh, 2014; Sutin et al., 2016). Additionally, since evidence gathered by analysis of BOLD fMRI may be expensive and studies may often be underpowered as a consequence of high-equipment costs, alternative approaches such as fNIRS are emerging to investigate neurovascular coupling in aged individuals (Csipo et al., 2019). While limited by its inferior spatial resolution and penetration depth, fNIRS has a much higher temporal resolution than fMRI, allowing for measurements of rapid concentration changes in both oxygenated and deoxygenated hemoglobin. Assessment of neurovascular coupling responses by functional near-infrared spectroscopy in humans provides an affordable method to assess hemoglobin concentration changes in cortical brain areas which provides consistent results when compared with fMRI.
5 |. PERSPECTIVES
The basic science studies reviewed here demonstrate how much has been learned in the past decade about the effects of aging on vascular and neurovascular function. The interaction between the age-associated vascular changes and neuronal signals can affect the interpretation and conclusions that can be drawn from BOLD signal analysis. Thus, it is now more evident that BOLD fMRI studies which involve aging as a biological variable must deliver alternative strategies to account for the compromised vascular and neurovascular health in older individuals, as measured differences in BOLD signal may not be attributable only to neural activity. More recent studies seeking to understand the effect of aging on brain function have developed and validated several techniques that aim to separate vascular and neuronal signals when performing BOLD fMRI scans. However, more research is needed to develop and facilitate the adoption of such approaches as a common analysis tool. Further preclinical fMRI studies investigating cerebrovascular outcomes (such as NVC, rarefaction, and BBB permeability) on aged rodents will be of critical importance as the challenges to resolve the components underlying the BOLD signal to better understand the effects of aging on the brain cerebrovasculature remain unanswered.
ACKNOWLEDGMENT
This work was supported by grants from the American Heart Association (ST), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU), the Presbyterian Health Foundation (to ZU), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), and the Cellular and Molecular GeroScience CoBRE (1P20GM125528).
Funding information
National Institute on Aging, Grant/Award Number: 1P20GM125528 and T32AG052363; American Heart Association; Oklahoma Center for the Advancement of Science and Technology; Presbyterian Health Foundation; Geroscience Training Program in Oklahoma, Grant/Award Number: T32AG052363; Cellular and Molecular GeroScience CoBRE, Grant/Award Number: 1P20GM125528
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
REFERENCES
- Abdelkarim D, Zhao Y, Turner MP, Sivakolundu DK, Lu H, & Rypma B (2019). A neural-vascular complex of age-related changes in the human brain: Anatomy, physiology, and implications for neurocognitive aging. Neuroscience and Biobehavioral Reviews, 107, 927–944. 10.1016/j.neubiorev.2019.09.005 [DOI] [PubMed] [Google Scholar]
- Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, & Hintze TH (2003). NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. American Journal of Physiology. Heart and Circulatory Physiology, 285, H1015–H1022. 10.1152/ajpheart.01047.2002 [DOI] [PubMed] [Google Scholar]
- Alba C, Vidal L, Diaz F, Villena A, & de Vargas IP (2004). Ultrastructural and quantitative age-related changes in capillaries of the dorsal lateral geniculate nucleus. Brain Research Bulletin, 64(2), 145–153. 10.1016/j.brainresbull.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, & Newman EA (2010). Glial and neuronal control of brain blood flow. Nature, 468(7321), 232–243. 10.1038/nature09613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, & Iadecola C (2002). The neural basis of functional brain imaging signals. Trends in Neurosciences, 25(12), 621–625. 10.1016/S0166-2236(02)02264-6 [DOI] [PubMed] [Google Scholar]
- Bagher P, & Segal SS (2011). Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Psychologica, 202(3), 271–284. 10.1111/j.1748-1716.2010.02244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, & Csiszar A (2012). Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: A novel model of vascular aging. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 67, 313–329. 10.1093/gerona/glr164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, & Ungvari Z (2012). Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese lewis dwarf rats: Implications for vascular aging. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 67(6), 553–564. 10.1093/gerona/glr197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, & Finkel T (2005). Mitochondria, oxidants, and aging. Cell, 120(4), 483–495. 10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Balbi M, Ghosh M, Longden TA, Jativa Vega M, Gesierich B, Hellal F, Lourbopoulos A, Nelson MT, & Plesnila N (2015). Dysfunction of mouse cerebral arteries during early aging. Journal of Cerebral Blood Flow and Metabolism, 35(9), 1445–1453. 10.1038/jcbfm.2015.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PR, Reitsma JB, Houweling BM, Ferrier CH, & Ramsey NF (2014). Can fMRI safely replace the Wada test for preoperative assessment of language lateralisation? A meta-analysis and systematic review. Journal of Neurology, Neurosurgery and Psychiatry, 85(5), 581–588. 10.1136/jnnp-2013-305659 [DOI] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, & Naumann E (2009). Resting cerebral blood flow, attention, and aging. Brain Research, 1267, 77–88. 10.1016/j.brainres.2009.02.053 [DOI] [PubMed] [Google Scholar]
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, & Torres C (2012). Astrocyte senescence as a component of Alzheimer’s disease. PLoS One, 7(9), e45069. 10.1371/journal.pone.0045069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert MM, Erikson GA, Shokhirev MN, & Allen NJ (2018). The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Reports, 22(1), 269–285. 10.1016/j.celrep.2017.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, & Busse R (2005). Endothelial aging. Cardiovascular Research, 66(2), 286–294. 10.1016/j.cardiores.2004.12.027 [DOI] [PubMed] [Google Scholar]
- Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, & Buxton RB (2003). BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: Differential relationship to global perfusion. Journal of Cerebral Blood Flow and Metabolism, 23(7), 829–837. 10.1097/01.WCB.0000071887.63724.B2 [DOI] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Thore CR, Challa VR, & Anstrom JA (2007). Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. Journal of the Neurological Sciences, 257(1–2), 62–66. 10.1016/j.jns.2007.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu L, Huo C, Xu G, Liu Y, Li Z, Fan Y, & Li J (2018). Alteration in brain functional and effective connectivity in subjects with hypertension. Frontiers in Physiology, 9, 669. 10.3389/fphys.2018.00669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Andersen JK, Kapahi P, & Melov S (2011). Cellular senescence: A link between cancer and age-related degenerative disease? Seminars in Cancer Biology, 21, 354–359. 10.1016/j.semcancer.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BW, Craft MA, Carlson JR, Razaq W, Deardeuff KK, & Benbrook DM (2018). Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. GeroScience, 40(3), 325–336. 10.1007/s11357-018-0025-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, & Gomez-Gonzalo M (2010). The contribution of astrocyte signalling to neurovascular coupling. Brain Research Reviews, 63(1–2), 138–148. 10.1016/j.brainresrev.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Carvalho C, & Moreira PI (2018). Oxidative stress: A major player in cerebrovascular alterations associated to neurodegenerative events. Frontiers in Physiology, 9, 806. 10.3389/fphys.2018.00806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, & Hamel E (2010). Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics, 2, 9. 10.3389/fnene.2010.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, & Hamel E (2004). Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. Journal of Neuroscience, 24(41), 8940–8949. 10.1523/JNEUROSCI.3065-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervellati C, Trentini A, Bosi C, Valacchi G, Morieri ML, Zurlo A, Brombo G, Passaro A, & Zuliani G (2018). Low-grade systemic inflammation is associated with functional disability in elderly people affected by dementia. Geroscience, 40(1), 61–69. 10.1007/s11357-018-0010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Bouchard MB, McCaslin AF, Burgess SA, & Hillman EM (2011). High-speed vascular dynamics of the hemodynamic response. NeuroImage, 54(2), 1021–1030. 10.1016/j.neuroimage.2010.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, & Hillman EM (2014). A critical role for the vascular endothelium in functional neurovascular coupling in the brain. Journal of the American Heart Association, 3, e000787. 10.1161/JAHA.114.000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, & Salat DH (2011). Age-associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage, 55, 468–478. 10.1016/j.neuroimage.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Sobenin IA, Bobryshev YV, & Orekhov AN (2012). Mitochondrial dysfunction and mitochondrial DNA mutations in atherosclerotic complications in diabetes. World Journal of Cardiology, 4(5), 148–156. 10.4330/wjc.v4.i5.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN, & Bobryshev YV (2014). Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Research International, 2014, 238463. 10.1155/2014/238463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, & Barres BA (2018). Normal aging induces A1-like astrocyte reactivity. Proceedings of the National Academy of Sciences, 115(8), E1896–E1905. 10.1073/pnas.1800165115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, & Torres C (2019). Astrocyte senescence: Evidence and significance. Aging Cell, 18, e12937. 10.1111/acel.12937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costea L, Meszaros A, Bauer H, Bauer HC, Traweger A, Wilhelm I, Farkas AE, & Krizbai IA (2019). The blood–brain barrier and its intercellular junctions in age-related brain disorders. International Journal of Molecular Sciences, 20(21), 5472. 10.3390/ijms20215472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, & Yabluchanskiy A (2018). Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. GeroScience, 40(3), 337–346. 10.1007/s11357-018-0028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Lipecz A, Ashpole NM, Balasubramanian P, & Tarantini S (2020). Astrocyte senescence contributes to cognitive decline. Geroscience, 42(1), 51–55. 10.1007/s11357-019-00140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BN, Dzialendzik M, Tarantini S, Balasubramanian P, Kiss T, Yabluchanska V, Silva-Palacios F, Courtney DL, Dasari TW, Sorond F, Sonntag WE, Csiszar A, Ungvari Z, & Yabluchanskiy A (2019). Age-related decline in peripheral vascular health predicts cognitive impairment. GeroScience, 41(2), 125–136. 10.1007/s11357-019-00063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Mukli P, Lipecz A, Tarantini S, Bahadli D, Abdulhussein O, Owens C, Kiss T, Balasubramanian P, Nyul-Toth A, Hand RA, Yabluchanska V, Sorond FA, Csiszar A, Ungvari Z, & Yabluchanskiy A (2019). Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience, 41(5), 495–509. 10.1007/s11357-019-00122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, & Ungvari Z (2008). Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. American Journal of Physiology. Heart and Circulatory Physiology, 295(5), H1882–H1894. 10.1152/ajpheart.412.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, & Kaley G (2002). Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circulation Research, 90(11), 1159–1166. 10.1161/01.RES.0000020401.61826.EA [DOI] [PubMed] [Google Scholar]
- Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z, & Tarantini S (2019). Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience, 41(5), 609–617. 10.1007/s11357-019-00111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabertrand F, Hannah RM, Pearson JM, Hill-Eubanks DC, Brayden JE, & Nelson MT (2013). Prostaglandin E2, a postulated astrocyte-derived neurovascular coupling agent, constricts rather than dilates parenchymal arterioles. Journal of Cerebral Blood Flow and Metabolism, 33, 479–482. 10.1038/jcbfm.2013.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch PS, & Ungvari Z (2012). Mitochondria and cardiovascular aging. Circulation Research, 110(8), 1109–1124. 10.1161/CIRCRESAHA.111.246140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vis JB, Hendrikse J, Bhogal A, Adams A, Kappelle LJ, & Petersen ET (2015). Age-related changes in brain hemodynamics; A calibrated MRI study. Human Brain Mapping, 36(10), 3973–3987. 10.1002/hbm.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C, & Griffith TM (2010). Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Archiv. European Journal of Physiology, 459(6), 897–914. 10.1007/s00424-010-0830-4 [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, & Gazzaley A (2003). Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nature Reviews Neuroscience, 4(11), 863–872. 10.1038/nrn1246 [DOI] [PubMed] [Google Scholar]
- Dias C, Lourenco CF, Ferreiro E, Barbosa RM, Laranjinha J, & Ledo A (2016). Age-dependent changes in the glutamate-nitric oxide pathway in the hippocampus of the triple transgenic model of Alzheimer’s disease: Implications for neurometabolic regulation. Neurobiology of Aging, 46, 84–95. 10.1016/j.neurobiolaging.2016.06.012 [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, & McAuley E (2001). General and task-specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task-switching. NeuroReport, 12(9), 2065–2071. 10.1097/00001756-200107030-00054 [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, & Seals DR (2007). Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circulation Research, 100(11), 1659–1666. 10.1161/01.RES.0000269183.13937.e8 [DOI] [PubMed] [Google Scholar]
- Durduran T, & Yodh AG (2014). Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. NeuroImage, 85, 51–63. 10.1016/j.neuroimage.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, & Gratton G (2014). Neurovascular coupling in normal aging: A combined optical, ERP and fMRI study. NeuroImage, 85, 592–607. 10.1016/j.neuroimage.2013.04.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, & Heistad DD (1998). Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiological Reviews, 78(1), 53–97. 10.1152/physrev.1998.78.1.53 [DOI] [PubMed] [Google Scholar]
- Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, Branch CA, Gubbi S, Patel K, Moellering DR, Tarantini S, Kiss T, Yabluchanskiy A, Ungvari Z, Sonntag WE, & Huffman DM (2019). Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience, 41(2), 185–208. 10.1007/s11357-019-00065-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, De Vos RA, Jansen Steur EN, & Luiten PG (2000). Are Alzheimer’s disease, hypertension, and cerebrocapillary damage related? Neurobiology of Aging, 21(2), 235–243. 10.1016/S0197-4580(00)00122-6 [DOI] [PubMed] [Google Scholar]
- Fergus A, & Lee KS (1997). Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Research, 754(1–2), 35–45. 10.1016/S0006-8993(97)00040-1 [DOI] [PubMed] [Google Scholar]
- Figueroa XF, & Duling BR (2009). Gap junctions in the control of vascular function. Antioxidants & Redox Signaling, 11(2), 251–266. 10.1089/ars.2008.2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Morrison HW, Iddings JA, Du W, & Kim KJ (2016). Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience, 323, 96–109. 10.1016/j.neuroscience.2015.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck D, Beaudin AE, Steinback CD, Kumarpillai G, Shobha N, McCreary CR, Peca S, Smith EE, & Poulin MJ (2014). Effects of aging on the association between cerebrovascular responses to visual stimulation, hypercapnia and arterial stiffness. Frontiers in Physiology, 5, 49. 10.3389/fphys.2014.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, & De Benedictis G (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences, 908(1), 244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- Franceschi C, & Campisi J (2014). Chronic inflammation (inflam-maging) and its potential contribution to age-associated diseases. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(Suppl 1), S4–S9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- Frater J, Lie D, Bartlett P, & McGrath JJ (2018). Insulin-like Growth Factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: A review. Ageing Research Reviews, 42, 14–27. 10.1016/j.arr.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, & Csiszar A (2018). Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. GeroScience, 40(5–6), 513–521. 10.1007/s11357-018-0047-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, Ungvari Z, & Csiszar A (2018). IGF-1 deficiency promotes pathological remodeling of cerebral arteries: A potential mechanism contributing to the pathogenesis of intracerebral hemorrhages in aging. The Journals of Gerontology: Series A, 74(4), 446–454. 10.1093/gerona/gly144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, & Iadecola C (2006). Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. Journal of Applied Physiology, 100(1), 328–335. 10.1152/japplphysiol.00966.2005 [DOI] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, & MacVicar BA (2007). Astrocyte control of the cerebrovasculature. Glia, 55(12), 1214–1221. 10.1002/glia.20543 [DOI] [PubMed] [Google Scholar]
- Habeck CG (2010). Basics of Multivariate Analysis in Neuroimaging Data. Journal of Visualized Experiments, 41, 10.3791/1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Obayashi S, Tsujiuchi K, & Muraoka Y (2013). The effects of electromyography-controlled functional electrical stimulation on upper extremity function and cortical perfusion in stroke patients. Clinical Neurophysiology, 124(10), 2008–2015. 10.1016/j.clinph.2013.03.030 [DOI] [PubMed] [Google Scholar]
- Hatakenaka M, Miyai I, Mihara M, Yagura H, & Hattori N (2012). Impaired motor learning by a pursuit rotor test reduces functional outcomes during rehabilitation of poststroke ataxia. Neurorehabil Neural Repair, 26(3), 293–300. 10.1177/1545968311412053 [DOI] [PubMed] [Google Scholar]
- Heinrich A, Ando RD, Turi G, Rozsa B, & Sperlagh B (2012). K+ depolarization evokes ATP, adenosine and glutamate release from glia in rat hippocampus: A microelectrode biosensor study. British Journal of Pharmacology, 167, 1003–1020. 10.1111/j.1476-5381.2012.01932.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera MD, Mingorance C, Rodriguez-Rodriguez R, & Alvarez de Sotomayor M (2010). Endothelial dysfunction and aging: An update. Ageing Research Reviews, 9(2), 142–152. 10.1016/j.arr.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Hillman EM (2014). Coupling mechanism and significance of the BOLD signal: A status report. Annual Review of Neuroscience, 37(1), 161–181. 10.1146/annurev-neuro-071013-014111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, & Mahoney JR (2016). Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topography, 29(2), 334–343. 10.1007/s10548-015-0465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford PS, & Gourine AV (2019). What is the key mediator of the neurovascular coupling response? Neuroscience and Biobehavioral Reviews, 96, 174–181. 10.1016/j.neubiorev.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, & Boas DA (2009). HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics, 48(10), 280–298. 10.1364/AO.48.00D280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison JL, Lu H, & Rypma B (2013). Neural mechanisms of age-related slowing: The DeltaCBF/DeltaCMRO2 ratio mediates age-differences in BOLD signal and human performance. Cerebral Cortex, 23, 2337–2346. 10.1093/cercor/bhs233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, & Gottesman RF (2019). Neurovascular and cognitive dysfunction in hypertension. Circulation Research, 124(7), 1025–1044. 10.1161/CIRCRESAHA.118.313260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, & Nedergaard M (2007). Glial regulation of the cerebral microvasculature. Nature Neuroscience, 10(11), 1369–1376. 10.1038/nn2003 [DOI] [PubMed] [Google Scholar]
- Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, & Sun D (2007). Aging enhances pressure-induced arterial superoxide formation. American Journal of Physiology. Heart and Circulatory Physiology, 293(3), H1344–H1350. 10.1152/ajpheart.00413.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Pierre P (2014). Integrating functional near-infrared spectroscopy in the characterization, assessment, and monitoring of cancer and treatment-related neurocognitive dysfunction. NeuroImage, 85, 408–414. 10.1016/j.neuroimage.2013.06.075 [DOI] [PubMed] [Google Scholar]
- Jean-Pierre P, Liu H, Bose N, & Tothy PK (2015). Pilot examination of functional Near-Infrared spectroscopy (fNIRS) to quantify chemobrain. Journal of Clinical Oncology, 33, e20680. 10.1200/jco.2015.33.15_suppl.e20680 [DOI] [Google Scholar]
- Jobsis FF (1977). Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science, 198, 1264–1267. 10.1126/science.929199 [DOI] [PubMed] [Google Scholar]
- Kato H, Izumiyama M, Koizumi H, Takahashi A, & Itoyama Y (2002). Near-infrared spectroscopic topography as a tool to monitor motor reorganization after hemiparetic stroke: A comparison with functional MRI. Stroke, 33(8), 2032–2036. 10.1161/01.STR.0000021903.52901.97 [DOI] [PubMed] [Google Scholar]
- Keilholz SD, Pan WJ, Billings J, Nezafati M, & Shakil S (2017). Noise and non-neuronal contributions to the BOLD signal: Applications to and insights from animal studies. NeuroImage, 154, 267–281. 10.1016/j.neuroimage.2016.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleshian VL, Modi HR, Rapoport SI, & Rao JS (2013). Aging is associated with altered inflammatory, arachidonic acid cascade, and synaptic markers, influenced by epigenetic modifications, in the human frontal cortex. Journal of Neurochemistry, 125, 63–73. 10.1111/jnc.12153 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kida Y, & Goligorsky MS (2016). Sirtuins, cell senescence, and vascular aging. Canadian Journal of Cardiology, 32(5), 634–641. 10.1016/j.cjca.2015.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, & Ungvari Z (2019). Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: A potential mechanism for the prevention of vascular cognitive impairment. GeroScience, 41(5), 619–630. 10.1007/s11357-019-00074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, & Ungvari Z (2020). Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. GeroScience, 42(2), 727–748. 10.1007/s11357-020-00180-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, & Harder DR (2009). Astrocytes and the regulation of cerebral blood flow. Trends in Neurosciences, 32(3), 160–169. 10.1016/j.tins.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Lagerwaard B, Nieuwenhuizen AG, de Boer VCJ, & Keijer J (2020). In vivo assessment of mitochondrial capacity using NIRS in locomotor muscles of young and elderly males with similar physical activity levels. GeroScience, 42(1), 299–310. 10.1007/s11357-019-00145-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam PY, Yin F, Hamilton RT, Boveris A, & Cadenas E (2009). Elevated neuronal nitric oxide synthase expression during ageing and mitochondrial energy production. Free Radical Research, 43(5), 431–439. 10.1080/10715760902849813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Duncan N, & Northoff G (2014). Resting-state functional magnetic resonance imaging: Review of neurosurgical applications. Neurosurgery, 74, 453–464; discussion 464–455. 10.1227/NEU.0000000000000307 [DOI] [PubMed] [Google Scholar]
- Lauritzen M (2005). Reading vascular changes in brain imaging: Is dendritic calcium the key? Nature Reviews Neuroscience, 6(1), 77–85. 10.1038/nrn1589 [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, & Hoppel CL (2006). Oxidative phosphorylation and aging. Ageing Research Reviews, 5(4), 402–433. 10.1016/j.arr.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Li R, Rui G, Chen W, Li S, Schulz PE, & Zhang Y (2018). Early detection of Alzheimer’s disease using non-invasive near-infrared spectroscopy. Frontiers in Aging Neuroscience, 10, 366. 10.3389/fnagi.2018.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Choi WJ, Wei W, Song S, Zhang Q, Liu J, & Wang RK (2018). Aging-associated changes in cerebral vasculature and blood flow as determined by quantitative optical coherence tomography angiography. Neurobiology of Aging, 70, 148–159. 10.1016/j.neurobiolaging.2018.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang Y, Li Y, Wang Y, Li J, & Zhang L (2010). Wavelet analysis of cerebral oxygenation signal measured by near infrared spectroscopy in subjects with cerebral infarction. Microvascular Research, 80(1), 142–147. 10.1016/j.mvr.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Lipecz A, Miller L, Kovacs I, Czako C, Csipo T, Baffi J, Csiszar A, Tarantini S, Ungvari Z, Yabluchanskiy A, & Conley S (2019). Microvascular contributions to age-related macular degeneration (AMD): From mechanisms of choriocapillaris aging to novel interventions. GeroScience, 41(6), 813–845. 10.1007/s11357-019-00138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S, Royce GH, Owen D, Farley J, Ranjo-Bishop M, Sonntag WE, & Deepa SS (2019). Accelerated decline in cognition in a mouse model of increased oxidative stress. GeroScience, 41(5), 591–607. 10.1007/s11357-019-00105-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK (2008). What we can do and what we cannot do with fMRI. Nature, 453(7197), 869–878. 10.1038/nature06976 [DOI] [PubMed] [Google Scholar]
- Loprinzi PD (2019a). The effects of physical exercise on parahippocampal function. Physiology International, 106(2), 114–127. 10.1556/2060.106.2019.10 [DOI] [PubMed] [Google Scholar]
- Loprinzi PD (2019b). The role of astrocytes on the effects of exercise on episodic memory function. Physiology International, 106(1), 21–28. 10.1556/2060.106.2019.04 [DOI] [PubMed] [Google Scholar]
- Loprinzi PD, Ponce P, & Frith E (2018). Hypothesized mechanisms through which acute exercise influences episodic memory. Physiology International, 105(4), 285–297. 10.1556/2060.105.2018.4.28 [DOI] [PubMed] [Google Scholar]
- Lourenco CF, Ledo A, Caetano M, Barbosa RM, & Laranjinha J (2018). Age-dependent impairment of neurovascular and neurometabolic coupling in the hippocampus. Frontiers in Physiology, 9, 913. 10.3389/fphys.2018.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, & Park DC (2011). Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cerebral Cortex, 21(6), 1426–1434. 10.1093/cercor/bhq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Zheng Y, Li T, Zhang Z, & Gong H (2009). A portable functional imaging instrument for psychology research based on near-infrared spectroscopy. Frontiers of Optoelectronics in China, 1(3–4), 279–284. 10.1007/s12200-008-0057-6 [DOI] [Google Scholar]
- Lye JJ, Latorre E, Lee BP, Bandinelli S, Holley JE, Gutowski NJ, Ferrucci L, & Harries LW (2019). Astrocyte senescence may drive alterations in GFAPalpha, CDKN2A p14(ARF), and TAU3 transcript expression and contribute to cognitive decline. Geroscience, 41, 561–573. 10.1007/s11357-019-00100-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen C, Brazhe A, Thomsen K, & Lauritzen M (2013). Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. Journal of Cerebral Blood Flow and Metabolism, 33(2), 161–169. 10.1038/jcbfm.2012.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Morgado J, & Gomes FCA (2019). Astrocyte heterogeneity: Impact to brain aging and disease. Frontiers in Aging Neuroscience, 11, 59. 10.3389/fnagi.2019.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG, Faraci FM, Baumbach GL, & Heistad DD (1990). Effects of aging on responses of cerebral arterioles. American Journal of Physiology, 258(4), H1138–H1143. 10.1152/ajpheart.1990.258.4.H1138 [DOI] [PubMed] [Google Scholar]
- McCaslin AF, Chen BR, Radosevich AJ, Cauli B, & Hillman EM (2011). In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: Implications for neurovascular coupling. Journal of Cerebral Blood Flow and Metabolism, 31, 795–806. 10.1038/jcbfm.2010.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ruge W, Hunziker O, Schulz U, Tobler HJ, & Schweizer A (1980). Stereological changes in the capillary network and nerve cells of the aging human brain. Mechanisms of Ageing and Development, 14(1–2), 233–243. 10.1016/0047-6374(80)90123-2 [DOI] [PubMed] [Google Scholar]
- Metea MR, & Newman EA (2006). Glial cells dilate and constrict blood vessels: A mechanism of neurovascular coupling. Journal of Neuroscience, 26(11), 2862–2870. 10.1523/JNEUROSCI.4048-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Miyai I, Hattori N, Hatakenaka M, Yagura H, Kawano T, & Kubota K (2012). Cortical control of postural balance in patients with hemiplegic stroke. NeuroReport, 23(5), 314–319. 10.1097/WNR.0b013e328351757b [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, & Cohen NJ (2002). Attentional control in the aging brain: Insights from an fMRI study of the stroop task. Brain and Cognition, 49, 277–296. 10.1006/brcg.2001.1501 [DOI] [PubMed] [Google Scholar]
- Mishra A (2017). Binaural blood flow control by astrocytes: Listening to synapses and the vasculature. Journal of Physiology, 595(6), 1885–1902. 10.1113/JP270979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, & Kubota K (2003). Longitudinal optical imaging study for locomotor recovery after stroke. Stroke, 34(12), 2866–2870. 10.1161/01.STR.0000100166.81077.8A [DOI] [PubMed] [Google Scholar]
- Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, & Kubota K (2002). Premotor cortex is involved in restoration of gait in stroke. Annals of Neurology, 52(2), 188–194. 10.1002/ana.10274 [DOI] [PubMed] [Google Scholar]
- Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, & Donato AJ (2013). Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. American Journal of Physiology. Heart and Circulatory Physiology, 305(2), H251–H258. 10.1152/ajpheart.00197.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Takeuchi N, & Izumi SI (2018). Prefrontal cortex activation during a dual task in patients with stroke. Gait &Posture, 59, 193–198. 10.1016/j.gaitpost.2017.09.032 [DOI] [PubMed] [Google Scholar]
- Mueller-Bierl BM, Uludag K, Pereira PL, & Schick F (2007). Magnetic field distribution and signal decay in functional MRI in very high fields (up to 9.4 T) using Monte Carlo diffusion modeling. International Journal of Biomedical Imaging, 2007, 70309. 10.1155/2007/70309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Watanabe H, & Vriens J (2003). The TRPV4 channel: Structure-function relationship and promiscuous gating behaviour. Pflugers Archiv. European Journal of Physiology, 446(3), 298–303. 10.1007/s00424-003-1028-9 [DOI] [PubMed] [Google Scholar]
- Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, Cui J, Gratiy SL, Sakadzic S, Boas DA, Beka TI, Einevoll GT, Chen J, Masliah E, Dale AM, … Devor A (2013). In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. Journal of Neuroscience, 33(19), 8411–8422. 10.1523/JNEUROSCI.3285-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling AM, Gerstenecker AT, Buford TW, Khan B, Oparil S, & Lazar RM (2019). The role of exercise in the reversal of IGF-1 deficiencies in microvascular rarefaction and hypertension. GeroScience, 42(1), 141–158. 10.1007/s11357-019-00139-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke MF, & Hashimoto J (2007). Mechanical factors in arterial aging: A clinical perspective. Journal of the American College of Cardiology, 50(1), 1–13. 10.1016/j.jacc.2006.12.050 [DOI] [PubMed] [Google Scholar]
- Ovadya Y, & Krizhanovsky V (2014). Senescent cells: SASPected drivers of age-related pathologies. Biogerontology, 15(6), 627–642. 10.1007/s10522-014-9529-9 [DOI] [PubMed] [Google Scholar]
- Pantsulaia I, Ciszewski WM, & Niewiarowska J (2016). Senescent endothelial cells: Potential modulators of immunosenescence and ageing. Ageing Research Reviews, 29, 13–25. 10.1016/j.arr.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, & Iadecola C (2007). Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. Journal of Cerebral Blood Flow &Metabolism, 27(12), 1908–1918. 10.1038/sj.jcbfm.9600491 [DOI] [PubMed] [Google Scholar]
- Pellerin L, & Magistretti PJ (2004). Neuroenergetics: Calling upon astrocytes to satisfy hungry neurons. The Neuroscientist, 10(1), 53–62. 10.1177/1073858403260159 [DOI] [PubMed] [Google Scholar]
- Peng SL, Dumas JA, Park DC, Liu P, Filbey FM, McAdams CJ, Pinkham AE, Adinoff B, Zhang R, & Lu H (2014). Age-related increase of resting metabolic rate in the human brain. NeuroImage, 98, 176–183. 10.1016/j.neuroimage.2014.04.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, & Attwell D (2006). Bidirectional control of CNS capillary diameter by pericytes. Nature, 443(7112), 700–704. 10.1038/nature05193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, & Murthy VN (2011). Role of astrocytes in neurovascular coupling. Neuron, 71(5), 782–797. 10.1016/j.neuron.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Pifferi A, Contini D, Mora AD, Farina A, Spinelli L, & Torricelli A (2016). New frontiers in time-domain diffuse optics, a review. Journal of Biomedial Optics, 21, 91310. 10.1117/1.JBO.21.9.091310 [DOI] [PubMed] [Google Scholar]
- Rea M, Rana M, Lugato N, Terekhin P, Gizzi L, Brotz D, Fallgatter A, Birbaumer N, Sitaram R, & Caria A (2014). Lower limb movement preparation in chronic stroke: A pilot study toward an fNIRS-BCI for gait rehabilitation. Neurorehabilitation and Neural Repair, 28(6), 564–575. 10.1177/1545968313520410 [DOI] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, & Liu TT (2007). Cerebral blood flow and BOLD responses to a memory encoding task: A comparison between healthy young and elderly adults. NeuroImage, 37(2), 430–439. 10.1016/j.neuroimage.2007.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, & Lichtenwalner RJ (2003). Microvascular plasticity in aging. Ageing Research Reviews, 2(2), 149–168. 10.1016/S1568-1637(02)00064-8 [DOI] [PubMed] [Google Scholar]
- Riecker A, Grodd W, Klose U, Schulz JB, Groschel K, Erb M, Ackermann H, & Kastrup A (2003). Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. Journal of Cerebral Blood Flow &Metabolism, 23(5), 565–573. 10.1097/01.WCB.0000056063.25434.04 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arellano JJ, Parpura V, Zorec R, & Verkhratsky A (2016). Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience, 323, 170–182. 10.1016/j.neuroscience.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Roy CS, & Sherrington CS (1890). On the regulation of the blood-supply of the brain. Journal of Physiology, 11(1–2), 85–158. 10.1113/jphysiol.1890.sp000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce GH, Brown-Borg HM, & Deepa SS (2019). The potential role of necroptosis in inflammaging and aging. GeroScience, 41(6), 795–811. 10.1007/s11357-019-00131-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, & Soininen H (2011). Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. European Journal of Neuroscience, 34(1), 3–11. 10.1111/j.1460-9568.2011.07738.x [DOI] [PubMed] [Google Scholar]
- Scarapicchia V, Brown C, Mayo C, & Gawryluk JR (2017). Functional magnetic resonance imaging and functional near-infrared spectroscopy: Insights from combined recording studies. Frontiers in Human Neuroscience, 11, 419. 10.3389/fnhum.2017.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, & LeBrasseur NK (2019). The influence of GDF11 on brain fate and function. GeroScience, 41(1), 1–11. 10.1007/s11357-019-00054-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Cutini S, Wahl MM, Scheid R, & Yves von Cramon D (2007). Neurovascular coupling is impaired in cerebral microangiopathy—An event-related Stroop study. NeuroImage, 34(1), 26–34. 10.1016/j.neuroimage.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer C, & Heinke W (1999). Investigating the dependence of BOLD contrast on oxidative metabolism. Magnetic Resonance in Medicine, 41, 537–543. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, & Nair KS (2005). Decline in skeletal muscle mitochondrial function with aging in humans. Proceedings of the National Academy of Sciences, 102(15), 5618–5623. 10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobenin IA, Chistiakov DA, Bobryshev YV, Postnov AY, & Orekhov AN (2013). Mitochondrial mutations in atherosclerosis: New solutions in research and possible clinical applications. Current Pharmaceutical Design, 19, 5942–5953. 10.2174/1381612811319330013 [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, & Ungvari Z (2013). Insulin-like growth factor-1 in CNS and cerebrovascular aging. Frontiers in Aging Neuroscience, 5, 27. 10.3389/fnagi.2013.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, & Hutchins PM (1997). Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology, 138, 3515–3520. 10.1210/endo.138.8.5330 [DOI] [PubMed] [Google Scholar]
- Sorond FA, Hurwitz S, Salat DH, Greve DN, & Fisher ND (2013). Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology, 81(10), 904–909. 10.1212/WNL.0b013e3182a351aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, & Ungvari ZI (2015). Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. Journal of Cerebral Blood Flow &Metabolism, 35(4), 527–530. 10.1038/jcbfm.2014.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart JL, Lu L, Anderson HD, Mori H, & Anderson CM (2013). Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences, 110(8), 3149–3154. 10.1073/pnas.1215929110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sure VN, Sakamuri S, Sperling JA, Evans WR, Merdzo I, Mostany R, Murfee WL, Busija DW, & Katakam PVG (2018). A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. GeroScience, 40(4), 365–375. 10.1007/s11357-018-0037-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin J, Zimmerman B, Tyulmankov D, Tamborini D, Wu KC, Selb J, Gulinatti A, Rech I, Tosi A, Boas DA, & Franceschini MA (2016). Time-domain diffuse correlation spectroscopy. Optica, 3(9), 1006–1013. 10.1364/OPTICA.3.001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, & Nedergaard M (2006). Astrocyte-mediated control of cerebral blood flow. Nature Neuroscience, 9(2), 260–267. 10.1038/nn1623 [DOI] [PubMed] [Google Scholar]
- Takata N, Sugiura Y, Yoshida K, Koizumi M, Hiroshi N, Honda K, Yano R, Komaki Y, Matsui K, Suematsu M, Mimura M, Okano H, & Tanaka KF (2018). Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia, 66(9), 2013–2023. 10.1002/glia.23454 [DOI] [PubMed] [Google Scholar]
- Takeda K, Gomi Y, Imai I, Shimoda N, Hiwatari M, & Kato H (2007). Shift of motor activation areas during recovery from hemiparesis after cerebral infarction: A longitudinal study with near-infrared spectroscopy. Neuroscience Research, 59(2), 136–144. 10.1016/j.neures.2007.06.1466 [DOI] [PubMed] [Google Scholar]
- Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, & Kotlikoff MI (2007). Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: Measurements in Cx40BAC GCaMP2 transgenic mice. Circulation Research, 101, 1300–1309. 10.1161/CIRCRESAHA.107.149484 [DOI] [PubMed] [Google Scholar]
- Tamaki K, Nakai M, Yokota T, & Ogata J (1995). Effects of aging and chronic hypertension on cerebral blood flow and cerebrovascular CO2 reactivity in the rat. Gerontology, 41, 11–17. 10.1159/000213657 [DOI] [PubMed] [Google Scholar]
- Tang TB, & Chan YL (2018). Functional connectivity analysis on mild Alzheimer’s disease, mild cognitive impairment and normal aging using fNIRS. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2018, 17–20. 10.1109/EMBC.2018.8512186 [DOI] [PubMed] [Google Scholar]
- Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, & Csiszar A (2016). IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: Implications for the developmental origins of health and disease hypothesis. Age, 38(4), 239–258. 10.1007/s11357-016-9943-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, & Toth P (2015). Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. Journal of Cerebral Blood Flow &Metabolism, 35(11), 1871–1881. 10.1038/jcbfm.2015.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CH, Gordon GR, Ungvari Z, & Csiszar A (2016). Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Experimental Gerontology, 94, 52–58. 10.1016/j.exger.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, & Csiszar A (2016). Circulating IGF-1 deficiency exacerbates hypertension-induced micro-vascular rarefaction in the mouse hippocampus and retrosplenial cortex: Implications for cerebromicrovascular and brain aging. Age, 38(4), 273–289. 10.1007/s11357-016-9931-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, & Ungvari Z (2019). Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biology, 24, 101192. 10.1016/j.redox.2019.101192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, & Ungvari Z (2018). Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell, 17(2), e12731. 10.1111/acel.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, & Ungvari Z (2017). Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell, 16(3), 469–479. 10.1111/acel.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, & Ungvari Z (2017). Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience, 39(5–6), 601–614. 10.1007/s11357-017-0003-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A, & Ungvari Z (2019). Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. GeroScience, 41(5), 533–542. 10.1007/s11357-019-00101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanskiy A, Fulop GA, Hertelendy P, Noa Valcarcel-Ares M, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, & Ungvari Z (2018). Correction to: Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. GeroScience, 40(2), 219. 10.1007/s11357-018-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, & Zhang R (2014). Cerebral hemodynamics in normal aging: Central artery stiffness, wave reflection, and pressure pulsatility. Journal of Cerebral Blood Flow & Metabolism, 34(6), 971–978. 10.1038/jcbfm.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, & Freeman RD (2003). Single-neuron activity and tissue oxygenation in the cerebral cortex. Science, 299, 1070–1072. 10.1126/science.1079220 [DOI] [PubMed] [Google Scholar]
- Tong XK, Lecrux C, Rosa-Neto P, & Hamel E (2012). Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. Journal of Neuroscience, 32(14), 4705–4715. 10.1523/JNEUROSCI.0169-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, & Ungvari Z (2013). Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. American Journal of Physiology. Heart and Circulatory Physiology, 305, H1698–H1708. 10.1152/ajpheart.00377.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, & Ungvari Z (2015). IGF-1 deficiency impairs neurovascular coupling in mice: Implications for cerebromicrovascular aging. Aging Cell, 14, 1034–1044. 10.1111/acel.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, & Ungvari Z (2015). Purinergic glio-endothelial coupling during neuronal activity: Role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. American Journal of Physiology. Heart and Circulatory Physiology, 309, H1837–H1845. 10.1152/ajpheart.00463.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, & Ungvari Z (2014). Resveratrol treatment rescues neurovascular coupling in aged mice: Role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. American Journal of Physiology. Heart and Circulatory Physiology, 306(3), H299–H308. 10.1152/ajpheart.00744.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, & Ungvari Z (2014). IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. Journal of Cerebral Blood Flow and Metabolism, 34(12), 1887–1897. 10.1038/jcbfm.2014.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner RA, Saunders D, Smith N, Gulej R, McKenzie T, Lawrence B, & Morton KA (2019). Anti-inflammatory agent, OKN-007, reverses long-term neuroinflammatory responses in a rat encephalopathy model as assessed by multi-parametric MRI: Implications for aging-associated neuroinflammation. GeroScience, 41(4), 483–494. 10.1007/s11357-019-00094-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner RA, Saunders D, Smith N, Towler W, Cruz M, Do S, Maher JE, Whitaker K, Lerner M, & Morton KA (2018). Assessing long-term neuroinflammatory responses to encephalopathy using MRI approaches in a rat endotoxemia model. GeroScience, 40(1), 49–60. 10.1007/s11357-018-0009-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, & Csiszar A (2014). Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 69(11), 1339–1352. 10.1093/gerona/glu080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, & Csiszar A (2008). Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. American Journal of Physiology. Heart and Circulatory Physiology, 294(5), H2121–H2128. 10.1152/ajpheart.00012.2008 [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, & Csiszar A (2007). Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. American Journal of Physiology. Heart and Circulatory Physiology, 293, H37–H47. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V, & Csiszar A (2018). Mechanisms of vascular aging. Circulation Research, 123(7), 849–867. 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Nyul-Toth A, Kiss T, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Benyo Z, & Csiszar A (2019). Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. GeroScience, 41(6), 727–738. 10.1007/s11357-019-00107-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Sorond F, Merkely B, & Csiszar A (2020). Mechanisms of vascular aging, A geroscience perspective: JACC focus seminar. Journal of the American College of Cardiology, 75(8), 931–941. 10.1016/j.jacc.2019.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, & Lüscher TF (2000). Enhanced peroxynitrite formation is associated with vascular aging. Journal of Experimental Medicine, 192, 1731–1744. 10.1084/jem.192.12.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen JM (2014). The role of senescent cells in ageing. Nature, 509, 439–446. 10.1038/nature13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta R, Cutini S, Cerasa A, Gramigna V, Olivadese G, Arabia G, & Quattrone A (2017). Physiological aging influence on brain hemodynamic activity during task-switching: A fNIRS study. Frontiers in Aging Neuroscience, 9, 433. 10.3389/fnagi.2017.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij A, Kessels RPC, Heskamp L, Simons EMF, Dautzenberg PLJ, & Claassen J (2017). Prefrontal activation may predict working-memory training gain in normal aging and mild cognitive impairment. Brain Imaging and Behavior, 11(1), 141–154. 10.1007/s11682-016-9508-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij A, Meel-van den Abeelen AS, Kessels RP, van Beek AH, & Claassen JA (2014). Very-low-frequency oscillations of cerebral hemodynamics and blood pressure are affected by aging and cognitive load. NeuroImage, 85, 608–615. 10.1016/j.neuroimage.2013.04.107 [DOI] [PubMed] [Google Scholar]
- Vermeij A, van Beek AH, Olde Rikkert MG, Claassen JA, & Kessels RP (2012). Effects of aging on cerebral oxygenation during working-memory performance: A functional near-infrared spectroscopy study. PLoS One, 7, e46210. 10.1371/journal.pone.0046210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JA, Christie IN, Hosford PS, Huckstepp RT, Angelova PR, Vihko P, Cork SC, Abramov AY, Teschemacher AG, Kasparov S, Lythgoe MF, & Gourine AV (2015). A critical role for purinergic signalling in the mechanisms underlying generation of BOLD fMRI responses. Journal of Neuroscience, 35(13), 5284–5292. 10.1523/JNEUROSCI.3787-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenhoeft T, Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Kiss T, Csiszar A, Csiszar A, & Ungvari Z (2019). Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience, 41(6), 711–725. 10.1007/s11357-019-00102-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Hall MH, Erickson KI, Germain A, Nimgaonkar VL, Monk TH, & Buysse DJ (2017). Task switching in older adults with and without insomnia. Sleep Medicine, 30, 113–120. 10.1016/j.sleep.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Nyul-Toth A, Kozma M, Farkas AE, & Krizbai IA (2017). Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. American Journal of Physiology-Heart and Circulatory Physiology, 313(5), H1000–H1012. 10.1152/ajpheart.00106.2017 [DOI] [PubMed] [Google Scholar]
- Wolfle SE, Chaston DJ, Goto K, Sandow SL, Edwards FR, & Hill CE (2011). Non-linear relationship between hyperpolarisation and relaxation enables long distance propagation of vasodilatation. The Journal of Physiology, 589(10), 2607–2623. 10.1113/jphysiol.2010.202580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ME, & Wise RG (2018). Can blood oxygenation level dependent functional magnetic resonance imaging be used accurately to compare older and younger populations? A mini literature review. Frontiers in Aging Neuroscience, 10, 371. 10.3389/fnagi.2018.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, & Pelligrino DA (2008). Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. American Journal of Physiology-Heart and Circulatory Physiology, 294(2), H622–H632. 10.1152/ajpheart.00530.2007 [DOI] [PubMed] [Google Scholar]
- Yabluchanskiy A, Tarantini S, Balasubramanian P, Kiss T, Csipo T, Fulop GA, Lipecz A, Ahire C, DelFavero J, Nyul-Toth A, Sonntag WE, Schwartzman ML, Campisi J, Csiszar A, & Ungvari Z (2020). Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. GeroScience, 42(2), 409–428. 10.1007/s11357-020-00154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Yang Z, Yuan T, Feng W, & Wang P (2019). A systemic review of functional near-infrared spectroscopy for stroke. Current application and future directions. Frontiers in Neurology, 10, 58. 10.3389/fneur.2019.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JA, Kong IJ, Choi J, Baek JY, Kim EJ, Shin YI, Ko MH, Shin YB, & Shin MJ (2019). Neural compensatory response during complex cognitive function tasks in mild cognitive impairment: A near-infrared spectroscopy study. Neural Plasticity, 2019, 1–8. 10.1155/2019/7845104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel MA, Selb JJ, Huppert TJ, Franceschini MA, & Boas DA (2017). Functional near infrared spectroscopy: Enabling routine functional brain imaging. Current Opinion in Biomedical Engineering, 4, 78–86. 10.1016/j.cobme.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel M, Strucl M, Pretnar-Oblak J, & Zvan B (2005). Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Functional Neurology, 20, 115–120. [PubMed] [Google Scholar]
- Zebrowitz L, Ward N, Boshyan J, Gutchess A, & Hadjikhani N (2016). Dedifferentiated face processing in older adults is linked to lower resting state metabolic activity in fusiform face area. Brain Research, 1644, 22–31. 10.1016/j.brainres.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cherian R, & Jin K (2019). Systemic milieu and age-related deterioration. GeroScience, 41(3), 275–284. 10.1007/s11357-019-00075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Gordon ML, & Goldberg TE (2017). Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neuroscience and Biobehavioral Reviews, 72, 168–175. 10.1016/j.neubiorev.2016.11.023 [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, & Carmignoto G (2003). Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neuroscience, 6(1), 43–50. 10.1038/nn980 [DOI] [PubMed] [Google Scholar]


