Abstract
Tissue inhibitors of metalloproteinases (TIMPs) are a conserved family of proteins that were originally identified as endogenous inhibitors of matrixin and adamalysin endopeptidase activity. The matrixins and adamalysins are the major mediators of extracellular matrix (ECM) turnover, thus making TIMPs important regulators of ECM structure and composition. Despite their high sequence identity and relative redundancy in inhibitory profiles, each TIMP possesses unique biological characteristics that are independent of their regulation of metalloproteinase activity. As our understanding of TIMP biology has evolved, distinct roles have been assigned to individual TIMPs in cancer progression. In this respect, data regarding TIMP2’s role in cancer have borne conflicting reports of both tumor suppressor and, to a lesser extent, tumor promoter functions. TIMP2 is the most abundant TIMP family member, prevalent in normal and diseased mammalian tissues as a constitutively expressed protein. Despite its apparent stable expression, recent work highlights how TIMP2 is a cell stress-induced gene product and that its biological activity can be dictated by extracellular posttranslational modifications. Hence an understanding of TIMP2 molecular targets, and how its biological functions evolve in the progressing tumor microenvironment may reveal new therapeutic opportunities. In this review, we discuss the continually evolving functions of TIMP proteins, future perspectives in TIMP research, and the therapeutic utility of this family, with a particular focus on TIMP2.
Graphical Abstract
Introduction
Matrix metalloproteinases (MMPs) of the metzincin family are the primary agents responsible for mediating the turnover of the extracellular matrix (ECM). Expression of MMPs and activation of their proteolytic activity, while often associated with many chronic disease states, are also key during embryologic development and for the maintenance of normal tissue structure and function. In the extracellular tissue compartment, the proteolytic functions of these proteases are principally regulated by tissue inhibitors of metalloproteinases (TIMPs). Thus, by definition, TIMPs are pivotal to maintaining tissue homeostasis as highlighted by their role as modulators of proteolytic ECM turnover. However, as is common with newly discovered genes, TIMPs were named with regard to this initially identified function which resulted in a diminution of other potentially relevant biological activities. Irrespectively, since their original discovery TIMPs have been characterized as multifunctional regulators of cellular and ECM biology that can act independently of MMPs to support cardiovascular, immune and cognitive functions, as well as playing roles in chronic disease states such as ischemic disease (myocardial infarction, stroke), degenerative joint disease, cognitive dysfunction (Alzheimer’s and Parkinson’s diseases) as well as cancer progression (1–5).
In this review, we focus on the multifunctional nature of TIMPs in general, with an emphasis on TIMP2, to explore their role in the maintenance of homeostasis under normal conditions and their emerging role in modulating cell behavior during disease progression. Much attention has been focused on the potential therapeutic utility of TIMP3 in cardiovascular disease and the identification of TIMP1 as a poor prognostic indicator for cancer progression, partly mediated through its role in tumor cell survival via CD63 signaling (6). Here, we concentrate on the putative role of TIMP2 in the regulation of metzincin activity, as well as direct cell signaling pathways that modulate cell proliferation, migration, and cell fate. We will focus on the convergence of these multiple TIMP2 functions and the contextual contributions to both normal and disease states. Our purpose is to collect and synthesize data from numerous studies to encourage a clearer understanding of TIMP2 biology and help define new directions for future research.
Brief overview of structure and function of TIMPs
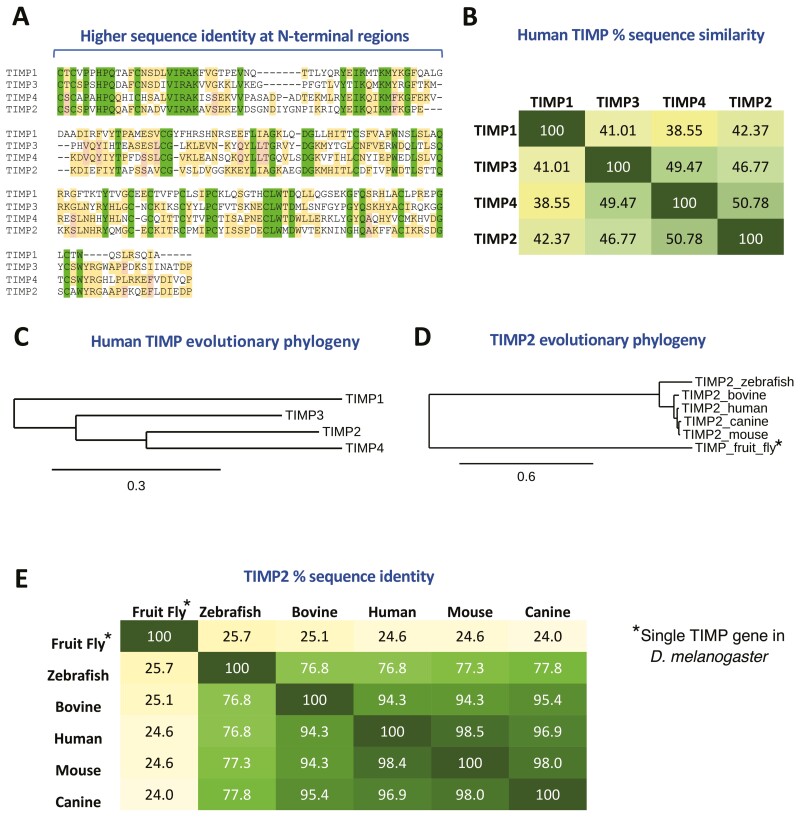
The mammalian TIMP family consists of four genes that display 39–51% identity. TIMP1 is the most distinct member, with the remaining family members having diverged early on to create TIMP2/3/4 that possess slightly higher identities across their primary structures (47–51%) (Figure 1). The proteinase inhibitory activities of TIMPs are mediated by their N-terminal domains and this region displays the greatest sequence similarity (Figure 1A), reflecting their broad-spectrum inhibition of metzincin proteinase activity. It is important to note that TIMP1 displays more restricted targeting due to a low affinity for membrane-type MMPs (MT-MMPs) (7), of which there are six present in humans (8). TIMP3 displays the widest range of targets, effectively inhibiting a host of other metzincin proteinases belonging to the ADAM (A disintegrin and metalloproteinase) and ADAM-TS (ADAM with thrombospondin motifs) families. Similar among TIMPs is the existence of two domains, carboxy- and amino-terminal, that are both stabilized by three internal cysteine bridges, generating six loop structures with a distinct topology that dictate their biological activity, including affinity towards different metzincin targets (9). These two domains give rise to TIMP proteins distinctive OB-fold, wedge shape, as exemplified by TIMP2 in Figure 2A. Unique amongst TIMPs, TIMP3 displays tight binding to the ECM through interaction with heparan sulfate and other sulfated proteoglycans (10). The remaining members are fully soluble and freely diffuse through the ECM, which may be an important consideration in terms of TIMP therapeutic applications. Although the N-terminal domains of TIMPs are responsible for their inhibitory interactions with active MMPs, the C-terminal domains display variable contacts with cognate MMPs that play a role in selectivity, cell-mediated pro-enzyme activation, and affinity towards target MMPs (11, 12). TIMPs are a highly conserved family, the evolution and structure of which are extensively reviewed elsewhere (13,14).
Figure 1.
TIMPs are a highly conserved family of endogenous proteins.
(A) Sequence comparison of the TIMP family identifies a largely conserved family with high sequence identity at their N-terminal regions (fully conserved residues highlighted in green), emphasizing their redundancy in action with regards to metalloproteinase inhibition (TIMP alignments are presented in order of their phylogenetic relationships, see Panel (C)). (B) Percentage sequence identity across the human TIMP family. (C) Evolutionary phylogeny across human TIMP family shows that TIMP1 is the most unique in terms of sequence identity and phylogenetic analysis suggests that it has the most ancient direct ancestor of the human TIMPs. (D and E) TIMP2 sequences are highly conserved, even showing around 25% identity with the single fruit fly (D. melanogaster) timp gene. Phylogenetic analysis was performed through http://www.phylogeny.fr/, using “One-Click” mode (see additional information).
Figure 2.
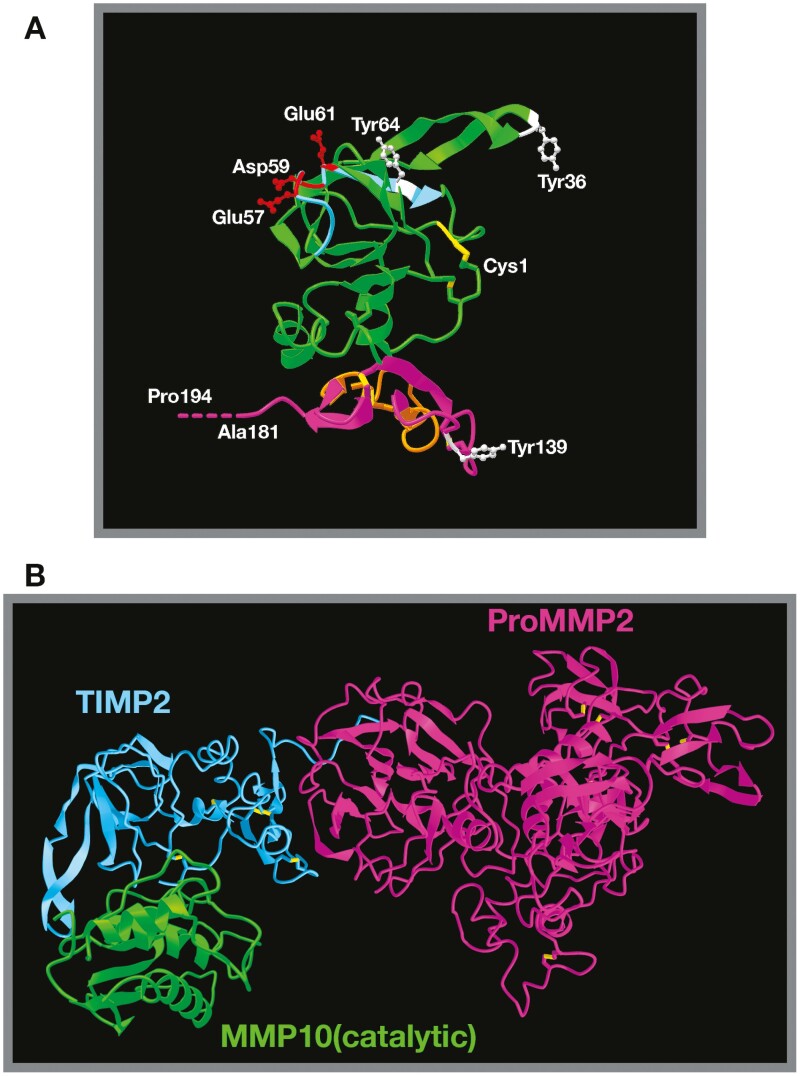
Structure of TIMP2 alone and in complex with pro-MMP2/active MMPs.
(A) TIMP2 3D structure based on X-ray diffraction studies (Protein Data Bank structure 1BR9) (68) demonstrating the classic OB-fold structure. The N-terminal domain is highlighted in green, the C-terminal domain in purple. The six disulfide bonds critical for correct secondary structure are highlighted in yellow and the position of the three phosphorylated tyrosine residues (Tyr36, Tyr64 and Tyr139 of the mature secreted protein) are shown in white, with Tyr64 being critical for regulation of MMP2 and HSP90 interactions. The BC loop (light blue) containing three acidic amino acid residues (Blu57, Asp59 and Glu61, shown in red) is the proposed integrin binding domain. Also shown are the N-terminal cysteine (Cys1) and C-terminal alanine residues (truncated in the analysis). The true C-terminal residue is a proline residue (Pro194), which has been artificially extrapolated and labeled. The orange domain within the C-terminal domain highlights loop 6, which is responsible for direct antagonism at the IGF1-receptor. (B) Structure of TIMP2 in complex with pro-MMP2 (68) superimposed with TIMP2 complexed with the catalytic domain of MMP10 (12) (TIMP2 from this image is hidden) emphasizes the fact that TIMP2 proteinase inhibitory activity is mostly unhindered when in complex with pro-MMP2. The fate of this theoretical heterotrimer is uncertain.
Each member of the TIMP family demonstrates a unique pattern of expression. Canonical regulation is observed at the transcriptional and translational levels, with additional regulation through miRNA expression. In fact, there have been >100 miRNA interactions reported for TIMP2 (15), suggesting that this represents a major mechanism controlling TIMP2 protein levels. In addition, new findings reveal additional levels of functional regulation via posttranscriptional modifications (16–18). TIMP2 is ubiquitously expressed and can be detected in all normal tissues, even showing similar levels of expression across various disease states (19). TIMP3 is also readily found throughout tissues, expression of which is strongly associated with the vascular system (20). TIMP1 expression is less apparent in most normal tissues but is frequently upregulated in inflammatory and disease states (21). TIMP4 displays the most restrictive transcriptional profile (21), although appreciable expression is noted in the heart and cerebellum (22,23). Expression of TIMP1 and 3 are inducible by various stimuli in multiple cell models (24–26) and this regulation is cell-type dependent. On the contrary, TIMP2 transcription is not readily induced by growth factors and cytokines but has recently been characterized as a cell-stress-induced gene product (27). An in-depth, whole-body study of TIMP protein levels and localization in normal tissues is lacking which would provide a clear insight into the regulation and activity of the TIMP family.
TIMP1/3/4 are nested within the introns of a class of genes called synapsins (28), a family of highly abundant neuron-specific synaptic phosphoproteins (29). Despite this association, there is little evidence of a regulatory relationship between TIMPs and synapsins. In contrast, TIMP2 is not nested within a synapsin gene but rather plays host to another gene named CEP295NL (DDC8). This uncharacterized protein-coding gene is described as testis-specific, with RNA sequencing data supporting the idea that its expression is highly restricted (30) (Human Protein Atlas proteinatlas.org). Despite this, reports suggest it may have a wider tissue distribution that mimics TIMP2. Interestingly, both TIMP2 and DDC8 are upregulated in concert following traumatic brain injury in mice (31).
Overview of TIMP2 in cancer biology
As the understanding of TIMP biology has progressed, clear delineations of TIMP roles in tumorigenesis and progression have developed. Although several models initially linked TIMP1 to tumor-suppressive effects (32–34), it has now become widely associated with a worse prognosis in many solid tumors. These effects are largely mediated through interaction with CD63 to support pro-tumorigenic signaling (35–37), reviewed extensively here (6). TIMP4 remains poorly studied, likely due to a largely restricted expression profile that is mostly limited to the brain and heart (22,23). Although a handful of reports exist, there is no consensus on its unique mechanistic activities, if any, in tumors (38). Overlooking a handful of reports, TIMP3 biology is clearly within the realm of tumor suppressor activity (21). Similarly, TIMP2 biological activity leans towards that of a tumor suppressor. Although some questions remain due to various reports linking TIMP2 with tumor cell survival and proliferation during in vitro studies, as well as a poorer prognosis in cancer patients. These reported effects are largely mediated through TIMP2’s interaction with MMP14 and downstream signaling associated with this interaction (39–41). The TIMP2:MMP14 interaction has been described to support mitogenic signaling in cancer cells via PI3K/Akt and MAP kinase signaling activity (39,40,42). Despite its activity through MMP14, TIMP2 can inhibit receptor tyrosine kinase signaling leading to reduced proliferation and/or angiogenesis (43–45). Furthermore, TIMP2-deficient mice display increased tumor growth and angiogenesis, enhanced inflammation (local and systemic), and an increase in the immunosuppressive myeloid-derived suppressor cells in a subcutaneous model of Lewis lung carcinoma (46). The immune regulatory functions of TIMP2 are further enhanced by the observation that TIMP2 (and TIMP1) could reduce TGFβ-induced formation of decidual-like natural killer cells (47). These decidual-like natural killer cells can support and nurture the progressing tumor microenvironment, and TIMP2-mediated reduction of these cells is consistent with other reports of TIMP2-mediated tumor suppressor activity (48). In an orthotopic model of triple-negative breast cancer, daily injection of recombinant human TIMP2 significantly inhibits tumor growth and metastasis (49). In support of its potential utility in reducing metastatic burden, TIMP2 is a potent inhibitor of invadopodia formation using in vitro models of pancreatic and breast carcinomas (50,51). To understand the mechanisms and consequences of TIMP2 bioactivity it is important to explore its interactome. In the following sections, we examine the emerging TIMP2 interactome, regulation of TIMP2 function and therapeutic potential.
TIMP:MMP interactions
The second member of the TIMP family, TIMP2, was initially identified in 1989 as a high-affinity non-covalent binding partner to what was then called type IV procollagenase/72 kDa gelatinase/gelatinase A (now known as pro-MMP2) and was recognized as an endogenous inhibitor of MMP2 collagenolytic activity (52). MMP2 is secreted as a 72kDa zymogen with an 80 amino acid pro-domain that masks the N-terminal catalytic domain, blocking proteinase activity. The TIMP2:pro-MMP2 interaction that facilitated the discovery of TIMP2 is separate from its conserved inhibitory interaction with active MMP catalytic sites. The C-terminal domain of TIMP2 forms a high-affinity interaction with the C-terminal hemopexin domain of pro-MMP2 that produces a stable dimer which likely constitutes the bulk of tissue-resident TIMP2 and MMP2 in the extracellular compartment (53). Similar interactions occur between other members of the TIMP and MMP families, with TIMP1 binding to pro-MMP9, TIMP4 to pro-MMP2, and TIMP3 to both pro-MMP2 and pro-MMP9 (53–56). It should be noted that the TIMP3/4 interactions with pro-MMPs are of slightly lower affinity than those of TIMP1/2 (56,57). The formation of the stable dimer between the C-terminal domains of TIMP2 and pro-MMP2 is crucial for the bulk of pro-MMP2 activation in a well-documented cascade. This pro-MMP2:TIMP2 complex was initially purified from conditioned media generated by the human melanoma cell line A2058 (52). In this system little or no free pro-MMP2 was detected, suggesting that the pro-enzyme-inhibitor complex was the principal form produced by these tumor cells. This finding posed the question; why is proenzyme produced in complex with an endogenous inhibitor? Follow-up studies led to the identification of a cell-mediated activation cascade involving the formation of a trimolecular complex with MMP14 (58), and that TIMP2 binds to pro-MMP2 before cell surface interaction and activation via MMP14, rather than the TIMP2:MMP14 complex acting as an MMP2 receptor. Activation of pro-MMP2, which is in complex with TIMP2, at the cell surface involves the N-terminal of TIMP2 binding to a homodimerized MT MMP14. This is followed by cleavage within the pro-domain of MMP2 by the free MMP14 catalytic site, resulting in a stepwise activation cascade that generates a ~62 kDa proteolytically active MMP2 (aMMP2) (58). The molecular mechanism that governs the separation of TIMP2 from the hemopexin domain of aMMP2, and the fate of TIMP2 following the conclusion of this mechanism, are uncertain. It has been suggested that TIMP2 is internalized, followed by either degradation (59) or dissociation from MMP14 and recycling as an intact TIMP2 molecule (60). The cell-mediated decision of whether TIMP2 is degraded or recycled may rely on other biological input or signals, evidenced by the observation that TIMP2 degradation is enhanced in the presence of collagen IV (61), which may influence broader tissue functions like migration and invasion. New data provides further insights into the TIMP2:pro-MMP2 interaction, revealing regulatory mechanisms dictating their affinity and activity through posttranslational modification and interplay with heat shock protein chaperones, discussed below (18,27). Activated MMP2 localizes to the cell surface via interaction with αVβ3 integrin (62) independent of TIMP2 to mediate cell migration and invasion. Subsequently, this proteinase activation cascade can continue with regards to pro-MMP9 activation, whereby the newly aMMP2 can cleave the pro-domain of MMP9 that is complexed with both TIMP1 and ADAM10 as a separate trimolecular complex at the cell membrane (63,64) (Figure 3). The congregation of membrane-associated complexes requires a level of membrane organization that promotes this cascade, but many of these processes, such as the regulation of MMP14 dimerization remain poorly understood. This pro-MMP2 activation pathway is unique to TIMP2 despite the ability of TIMP3 and TIMP4 to bind to the proenzyme (65–67). Importantly, as depicted by the MMP2 activation mechanism, TIMP2 in complex with pro-MMP2 retains the ability to inhibit the catalytic activity of MMPs. This can be appreciated in Figure 2B by comparing the structures of TIMP2 in complex with pro-MMP2 superimposed with the TIMP2:MMP10(catalytic region) interaction (12,68).
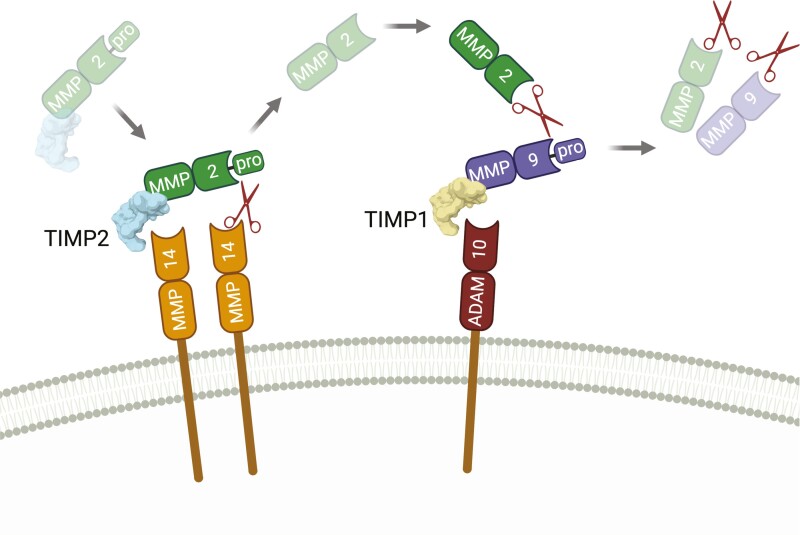
Figure 3.
TIMP-mediated Gelatinase (MMP2/9) activation cascade.
TIMP1 and TIMP2 can mediate a stepwise method of gelatinase activation that also relies on the presence and activity of the metalloproteinases MMP14 (MT1-MMP) and ADAM10. The latent TIMP2:pro-MMP2 complex retains inhibitory activity against MMP14. Binding of TIMP2 to MMP14 allows cleavage of the pro-domain of MMP2 by a free MMP14 in close proximity. Active MMP2, released in the vicinity of the plasma membrane, can cleave pro-MMP9 which is membrane-associated through the TIMP1:ADAM10 inhibitory complex, resulting in dual gelatinase activity at the site of activation. Created with BioRender.com.
MMP14, also referred to as MT1-MMP, has also been described as a TIMP2 receptor that is both structurally and functionally distinct from the pro-MMP2:TIMP2:MMP14 receptor complex mentioned above, initiating mitogenic signaling through the MAP kinase pathway (40) in a manner that is modulated by ECM composition (39). The expression, activity and localization of MMP14 are regulated by multiple mechanisms including dimerization (69) and interactions with other membrane proteins including αVβ3 integrins, CD151 and ADAM12 (70–73). It is unknown whether the cell signaling response downstream of the TIMP2:MMP14 interaction is altered by the presence of pro-MMP2 dimerized with TIMP2. MMP14 expression, localization and activity are captive to ECM composition in that its surface expression is vastly increased in response to excess/aberrant ECM-cell adhesive interactions (74,75). MMP14 is well studied and displays clear associations with pathology. In addition, MMP14 knockout mice display a severe phenotype, including dwarfism, lipodystrophy, osteopenia and arthritis that are associated with deficits in collagen turnover (76,77). MMP14 deficiency is associated with high mortality highlighting its importance in the developmental process (78). The effects of TIMP2:MMP14 induced Erk1/2 and Akt signaling are mediated via Src or LIMK phosphorylation of a unique tyrosine residue (Y573) in the cytoplasmic tail of MMP14 and are independent of MMP14 proteolytic activity (79,80). Recent studies have shown that a phosphomimetic Y573D mutation of MMP14 in mice results in a phenotype similar to MMP14-deficient mice (42). The consequences and fate of TIMP2 interactions with other MT-MMPs are poorly understood. For example, MMP17 (MT4-MMP) has been shown to enhance pro-tumorigenic EGFR signaling using a model of triple-negative breast cancer in a proteinase-independent manner (81), it would be of interest to investigate if/how TIMPs modulate this response.
In addition to targeting all members of the MMP family, TIMP2 also inhibits the function of ADAM12, of the related ADAM family, and ADAMTS8 of the ADAM-TS family (82). ADAM12 exists as a long or short form (ADAM12-L and ADAM12-S, respectively). ADAM12-L is membrane-tethered with the ability to mediate outside-in signaling independent of its proteinase activity, and increased expression is largely associated with cancer (83). No data has been described regarding the outcomes of the TIMP2:ADAM12-L interaction and whether it occurs at appreciable levels in vivo. Although it was initially reported that TIMP2 displayed no inhibitory effects against ADAM10 activity (84), replacement of the TIMP1 C-terminal domain with that of TIMP2 increased affinity for ADAM10 5-fold. The same study also reported that this chimera displayed a >40-fold increase in affinity for ADAM17 (85), suggesting that the inhibitory profiles of the TIMP family are yet to be fully revealed.
Studies have also demonstrated that like TIMPs, MMPs possess proteinase-independent activities (86). For example, MMP3 (Stromelysin-1) maintains adult mammary stem cells through a proteinase-independent manner (87). However, TIMPs likely retain a level of regulation over some of these proteinase-independent processes since the formation of proteinase-inhibitory heterodimers has been shown to increase MMP-affinity for the broad-targeting endocytic receptor low-density lipoprotein receptor-related protein 1 (LRP1) (88,89). LRP1-driven scavenging of extracellular proteinases represents another major regulatory control of matrix composition that works in tandem with TIMPs.
TIMP2:LRP family
The low-density lipoprotein receptor superfamily comprises seven structurally similar transmembrane proteins. Several of these receptors are multi-functional, promiscuous endocytic receptors that include LRP1 and LRP2 (90). TIMP1-3 have been identified as canonical LRP1 ligands, either individually or in complex with pro/active MMPs (91–93); and at least for TIMP1, these interactions can reveal cytokine-like activities (93). Two amino acids, F12 and K47, have been identified in TIMP1 that are crucial for TIMP1 intrinsic dynamics supporting endocytosis and signaling through LRP1. However, mutation of these residues does not disrupt LRP1 binding (94). Interestingly, these two corresponding amino acids are fully conserved in TIMP2 and partially conserved in TIMP3/4 strongly suggesting that similar modes of action occur across the family. LRP2 is known to share numerous LRP1 ligands, although its expression is largely restricted to the apical surfaces within the kidney, thyroid and gall bladder (95–97). Indeed, TIMP2 has been described as an LRP2 ligand when in complex with pro-MMP2, leading to renal reabsorption of the complex into the circulation (98). Although likely, it remains to be seen whether this process translates to other members of the TIMP family.
Although LRP1 is the major mediator of extracellular proteinase recycling, it is itself a target of several proteinases including MMP14, ADAM10 and ADAM12 (99,100). Proteolytic targeting of LRP1 can produce a soluble LRP1 fragment that retains ligand binding capacity and competes with cell surface LRP1 for TIMP3 binding. The resulting TIMP3:soluble LRP1 complex is resistant to LRP1-mediated endocytosis and retains MMP-inhibitory activity (92,101). This observation likely extends to other LRP1 targets, including other members of the TIMP family, impairing the trafficking of key matrisome components.
TIMP2:α3β1 integrin
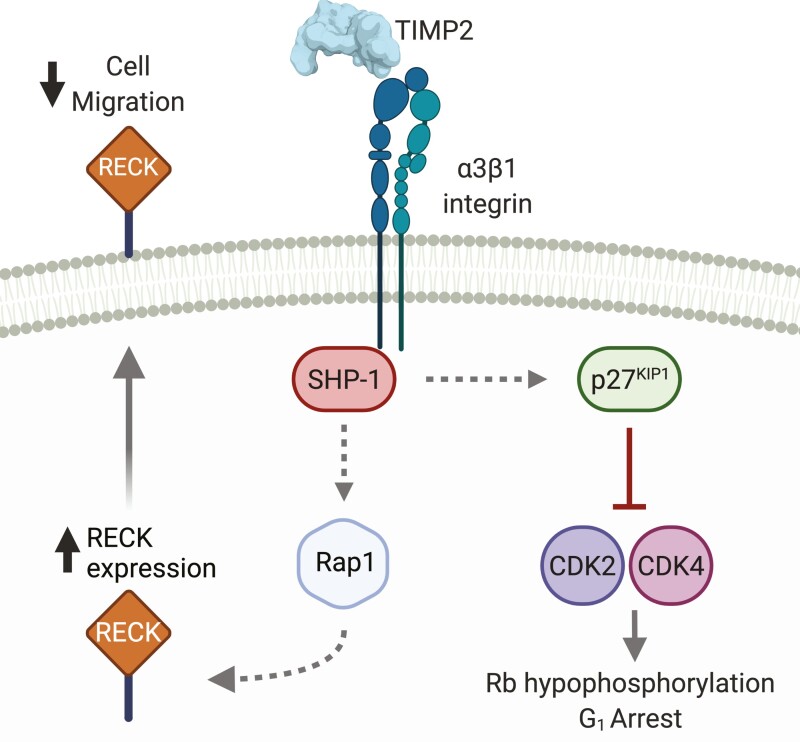
Integrins are transmembrane proteins that act as fundamental bridges (or integrators) between the ECM and the cytoskeleton. They exist as heterodimers with one α and one β subunit that comprise at least 24 unique combinations (102). Not long after its initial discovery, it was identified that TIMP2 could inhibit growth factor-induced endothelial cell and fibroblast proliferation independent of its proteinase inhibitory activity (103–105). Later in-depth characterization of this observation described that TIMP2 could inhibit angiogenesis through direct interaction with α3β1 integrin (α3β1), leading to the enhanced association of Src homology region 2 domain-containing phosphatase-1 (SHP-1) tyrosine phosphatase with vascular endothelial growth factor receptor 2 or fibroblast growth factor receptor 1. This interaction promotes SHP-1 phosphatase activity that directly inhibits the cascade of phosphorylation required to support mitogenic signaling (43). Further studies identified that this interaction could also mediate the upregulation of RECK (Reversion Inducing Cysteine Rich Protein With Kazal Motifs) expression via stimulation of Rap1 signaling. RECK is a membrane-tethered inhibitor of metalloproteinases that acts as a suppressor of angiogenesis, invasion, and metastasis (106) (Figure 4). Later, the crucial peptide sequence required within TIMP2 for the α3β1 interaction was identified as a unique structural region with the N-terminal B-C loop of TIMP-2, and a small synthetic linear peptide sequence derived from this region retained anti-angiogenic activity (107). Not to be limited to cells of the vascular system, the TIMP2 interaction with integrin β1/α3β1 has been shown to regulate neuromuscular junction development and neuronal differentiation, respectively (108,109). In concert with the motor deficits and phenotypic alterations observed in TIMP2-deficient mice (108,110), these findings suggest that TIMP2 may play a regulatory role in nervous system function. The TIMP2:α3β1 interaction may be complicated by tetraspanin CD151, which has been shown to mediate the formation of a ternary complex between itself, α3β1 and MMP14 (72) (Figure 5). Knockdown of CD151 was shown to enhance MMP2 activation, suggesting that CD151 may inhibit the TIMP2:MMP14 interaction that leads to the activation of MMP2 (72). This ternary complex was identified in endothelial cells, suggesting that CD151 and MMP14 may have important regulatory roles in TIMP2s anti-angiogenic activities that occur through α3β1.
Figure 4.
TIMP2 signaling though α3β1 integrin.
In a mechanism that was identified in endothelial cells and fibroblasts, TIMP2 can bind to α3β1 integrin at the cell surface. A key mediator of the downstream effects of TIMP2 binding to α3β1 integrin is the activation of cytosolic tyrosine phosphatase SHP-1. Downstream activity of SHP-1 promotes cell cycle arrest through upregulation and enhanced nuclear localization of p27KIP1. TIMP2 treatment also results in an increase in the expression of the membrane-anchored metalloproteinase inhibitor RECK via Rap1 mediated signaling, leading to reduced cell migration/invasion. Created with BioRender.com.
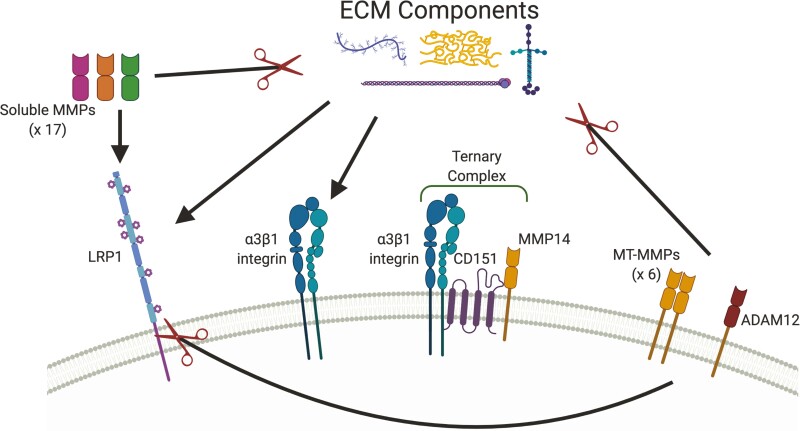
Figure 5.
Interconnectivity within TIMP2 targets.
The TIMP2 interactome displays extensive interactions with each other, largely mediated through components of the extracellular matrix (ECM). MMPs (soluble and membrane-type (MT-MMPs)) and ADAM12 are major mediators of ECM proteolysis, events that modulate all aspects of tissue biology. Cleaved ECM components are endocytosed and degraded largely via LRP1, an event that likely involves co-operation with β1 integrins (not shown). The LRP1 - β1 integrin relationship is complex and incompletely understood, since LRP1 can promote integrin maturation and transport (159) yet also mediate endocytosis of mature β1 integrin (159,160). MT-MMPs and ADAM12 display sheddase activity against LRP1, mediating the release of soluble LRP1 molecules that successfully prevent target endocytosis. Additionally, membrane-type MMP14 has been shown to associated with α3β1 integrin via CD151 that inhibits MMP14s proteinase activity. Integrins are the major membrane proteins that integrate the extracellular matrix and cytoskeleton. Created with BioRender.com.
TIMP1 has also been shown to induce cell signaling events through β1 integrins (6,35), with TIMP1 signaling occurring in conjunction with its cognate receptor, tetraspanin CD63. The consequences of integrin activation by TIMP1 and TIMP2 are vastly different. TIMP1 signaling activity is associated with pro-mitogenic/anti-apoptotic effects, whereas TIMP2 is associated with anti-mitogenic effects (35), supporting the cumulative data that TIMP1 is generally a poor prognostic factor in tumors (21,36,111,112). Interestingly, CD63 also interacts with MMP14 promoting its internalization and degradation (113). Whether the TIMP1:CD63 interaction perturbs MMP14 internalization and degradation remains to be seen.
TIMP2:insulin-like growth factor 1 receptor
Insulin-like growth factor (IGF) signaling is a fundamental regulator of tissue growth and development. Consequently, numerous disease states can be characterized by enhanced IGF pathway activity as a result of IGF-1 receptor (IGF1-R/IGF1R) gene amplification and other genetic alterations associated with the pathway. The IGF-IGF-1R signaling pathway has been implicated in various steps in tumorigenesis including proliferation and metastasis (114). Additionally, IGF-1 signaling through IGF-1R has been shown to promote vascular growth and remodeling (115–117). The anti-angiogenic capabilities of TIMP2 are not strictly limited to the interaction with α3β1 integrin, which is mediated through the N-terminal of TIMP2. It has also been identified that the C-terminal loop 6 of TIMP2 is a direct antagonist for IGF-I at IGF-1R in endothelial cells (Figure 6). This antagonism leads to an inhibition of kinase signaling downstream of IGF-1R, and it is independent of α3β1 and MMP14 interactions (45). These observations highlight a duality in TIMP2 function with regards to its suppressive effects upon angiogenesis/proliferation that make TIMP2 a promising candidate as a biological therapeutic.
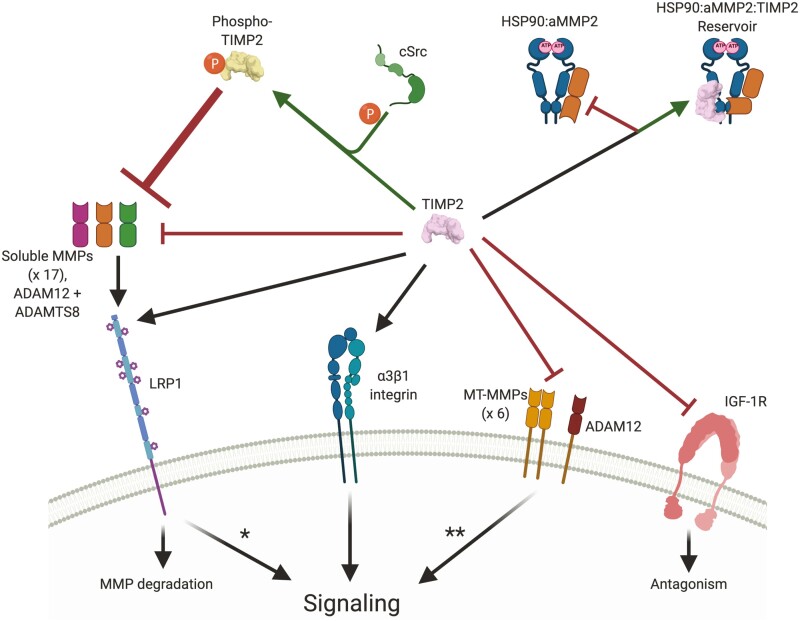
Figure 6.
The simplified TIMP2 interactome.
The major interacting partners for TIMP2 are the Metzincin family of proteinases (specifically, MMPs, ADAM12 and ADAMTS8). This proteinase inhibitory interaction can be positively modified by phosphorylation at tyrosine 90 by extracellular cSrc kinase. Active MMP2 (aMMP2) is stabilized by dimerization with extracellular HSP90, an interaction that can be disrupted by TIMP2. HSP90:aMMP2:TIMP2 can exists in a transient, catalytically inactive state that may act as a reservoir of rapidly available aMMP2. Both MMPs and TIMP2 are endocytosed by LRP1, which occurs with higher affinity when these are in an inhibitory complex. This results in MMP degradation and a mixture of degradation/recycling with regards to TIMP2. In addition, this interaction may result in the activation of intracellular signaling events. Furthermore, membrane-type MMPs and ADAM12 (ADAM12-L isoform) exist in a membrane-associated state and are actively targeted by TIMP2. This interaction can promote downstream signaling and endocytosis of the complex. Separate from TIMP2s proteinase-inhibitory capabilities, TIMP2 has been shown to mediate signaling through α3β1 integrin to promote cell signaling that are largely associated with indirect antagonism of tyrosine kinase receptors (see Figure 2). On the contrary, TIMP2 displays direct antagonism at the IGF-1 receptor (IGF-1R) through a region within its C-terminal domain. It remains to be seen how TIMP2 in complex with pro-MMP2 or specific active MMPs may affect the formation of this multitude of complexes. *Shown for TIMP1:LRP1 interaction only. **Shown for the TIMP2:MMP14 interaction only. Created with BioRender.com.
New directions in the regulation of TIMP2 functions
Recent findings demonstrate that TIMP2 can be tyrosine phosphorylated at three of its seven tyrosine residues (18). Residues Y62, Y90 and Y165 (designated positions include the signal peptide sequence) are phosphorylated by extracellular c-Src kinase. Crucially, phosphorylation at Y90 is essential for the TIMP2:pro-MMP2 interaction and subsequent MMP2 activation via the trimolecular complex formation with MMP14 (Figure 6). Furthermore, Y90 phosphorylation leads to a 5-fold increase in MMP2 active site inhibition by TIMP2, suggesting that this phosphosite could regulate TIMP2 affinity for other MMPs such as MMP14, which could enhance MMP2 activation (18).
Extracellular HSP90 (eHSP90) is an important facilitator of cellular invasion, in part through its interaction with, and stabilization of, 62kDa aMMP2 (118). Although classically seen as a TIMP family member that is constitutively expressed, TIMP2 was recently identified as a stress-inducible protein that is a potent inhibitor of eHSP90 ATPase activity. TIMP2 actively disrupts the aMMP2-stabilizing aMMP2:eHSP90 interaction, maintaining aMMP2 in an inhibitory state. In addition, TIMP2:aMMP2:eHSP90 can exist as a transient, inactive ternary complex that creates a store of stable, proteolytically inactive aMMP2 which, in theory, can be rapidly activated. Secreted AHA1 is an activating HSP90 co-chaperone that directly competes with TIMP2:eHSP90 binding, serving as an activating mechanism promoting aMMP2 enzymatic activity as a new ternary complex of aMMP2:eHSP90:AHA1 (27). The details of the TIMP2:MMP2:eHSP90 interaction are likely linked to the phosphorylation status of TIMP2, which was separately shown to increase TIMP2 affinity for aMMP2 (18).
Assessing the TIMP2 co-expression profile
The expression and activity of TIMP1 and TIMP3 are largely consistent with regards to their roles in tumor progression and association with tumor prognosis. In contrast, alterations in TIMP2 expression are much less pronounced in health versus disease, although TIMP2 physiology seems largely homeostatic and anti-tumorigenic. The limited reports linking TIMP2 with a pro-tumor capacity describe signaling activities mediated through MMP14 (39,40). In one of these studies, it was highlighted that the described anti-apoptotic effects of TIMP2 were reversed when cells were grown in a 3D collagen I matrix, highlighting the context-dependence of TIMP2 activities (39). These observations and the extensive interconnectivity of the TIMP2 interactome (Figures 5 and 6) highlight the importance of assessing how pathology-associated changes in co-expression profiles are important for understanding the consequences of TIMP2 activity. We recently described how TIMP2 displays a consistent and unique co-expression profile in carcinomas. With a focus on breast and lung carcinomas, it was demonstrated that the balance between TIMP2 and inhibitory targets is tipped in the favor of proteinase activity. Additionally, we found that TIMP2 acquires a strong cancer-specific correlation with many genes associated with mesenchymal cell lineages and the matrisome (19). Many of the highly correlating genes exhibit extensive interactions with each other, as well as other TIMPs and TIMP2 targets, supporting the idea that TIMP2 activity can be extensively regulated at a posttranscriptional level in pathologic conditions. Analysis of RNA sequencing data shows that the expression of TIMP2 and MMP2 are highly correlated (19). The activity of these proteins, which likely form high-affinity heterodimers early in their lifespan, is largely assumed to occur in the extracellular space. However, MMP2 activity has been detected in the intracellular compartment (119) and various proteolytic targets have been identified (120). Additionally, oxidative stress can induce the expression of an N-terminal truncated isoform of MMP2 which is the result of activation of an alternate promoter leading to a primary innate immune response (121,122). It remains to be seen if and how TIMPs play a role in this biological response.
Potential therapeutic applications of TIMPs
Following the failure of synthetic MMP inhibitors (MMPis) in multiple clinical trials as cancer therapeutics in the 1990s, due principally to off-target toxicity, further research into the therapeutic targeting of metalloproteinases diminished somewhat. However, the therapeutic potential of TIMPs has garnered interest in recent years due to their stability, multi-functional capabilities, the potential for engineering and their lack of apparent immunogenicity and toxicity. Apprehension regarding the potential immunogenicity of administered recombinant TIMP proteins is dampened since these are abundantly expressed, extracellular endogenous proteins. Furthermore, we recently described that TIMP2 treatment could suppress primary tumor growth and metastasis in a murine model of triple-negative breast cancer. This study reported that there was no evidence of systemic toxicity or weight loss associated with daily TIMP2 treatment over the course of 30 days (49). First-generation MMPis rapidly progressed to clinical trials since they were largely tolerated in rodent models (123–125), and it remains to be determined whether TIMP-based biological therapies will suffer the same fate as MMPis in the future clinical trials. Regardless, many of the factors that contributed to the failure of MMPis are unlikely to emerge in TIMP-based therapies, including but not limited to; broad-spectrum targeting of all metalloenzymes (not just MMPs), poor pharmacokinetics, and metabolic instability, reviewed extensively elsewhere (123–125).
TIMP2 and TIMP3 display exciting potential as therapeutics in several clinical settings such as cancer and myocardial infarction, largely related to their dual functions as metalloproteinase inhibitors and anti-proliferative/anti-angiogenic/anti-invasive agents (43,49,50,126–129). Reported potential therapeutic applications of TIMP1 include its utilization as an MMP-inhibitor post-myocardial infarction or in the attenuation of inflammatory pain (130,131). Additionally, there have been numerous reports regarding the ability of TIMP1 to preserve blood-brain barrier function following various systemic insults (132–134) and that it may provide a neuroprotective function following brain trauma (135). Therapeutic applications of full-length TIMP4 have been explored in a limited capacity, however, TIMP4 knockout mice display cardiovascular defects suggesting an important cardiac tissue homeostatic role for this gene (136).
It is important to note, however, that there are conflicting data regarding the role of TIMPs in cancer progression, with each TIMP harboring reports of pro- and anti-tumor capabilities (39,49,137–142), reviewed as a family here (21). These inconsistencies reflect the context-dependence of TIMP activities, and a more in-depth understanding of these nuanced influences is crucial in the ongoing development of therapeutic applications for this family of proteins. Additionally, although overexpression of MMPs is a consistent feature of many carcinomas, select members of the MMP family play important tumor suppressor functions that may be undesirably affected when therapeutically targeting this system (86). This suggests that the engineering of TIMPs to selectively modify both MMP inhibitory and MMP autonomous functions is a promising future direction currently under development.
In 2017, TIMP2 derived from human umbilical cord plasma was demonstrated to revitalize cognitive function in aged mice (4). However, since this initial report, no further observations have been made to support these findings highlighting the difficulty in obtaining consistency when working with TIMP2. Nonetheless, TIMP2 has the broadest expression of the family in the brain and has been shown to play various regulatory roles concerning neuronal differentiation and neuroinflammation (109,143,144). Additionally, TIMP2 has also been described as a prospective biotherapeutic for the treatment of keloids through the suppression of collagen synthesis (145), emphasizing the potentially broad reach of TIMP-based biotherapeutics.
TIMPs are broad-spectrum MMPis, although there are differences due to discreet variances in the way in which TIMPs interact with different members of the MMP family. As such, TIMP1 is a poor inhibitor of MT-MMPs. However, an individual point mutation in TIMP1 at an area peripheral to the binding interface increased its affinity for MMP14 by two orders of magnitude (146). Interestingly, engineered TIMP variants can be generated that display greatly enhanced or reduced affinities for different MMP targets (7,11,147–149), promoting the potential for engineered TIMPs in therapeutic targeting of different subsets of MMPs and bypassing the gross toxicity associated with synthetic MMPis. Similarly, engineered TIMPs can also be generated with increased affinity for ADAMs, as described for TIMP2 and ADAM12 (150). Of note, the therapeutic utility of TIMP proteins can be restricted by poor serum half-lives, although this can be limited by the use of slow-release methods or modifications such as targeted glycosylation, PEGylation (20 kDa) and albumin/Fc/antibody fusions (128,151). It is expected that therapeutic employment of the TIMP family of proteins, or related analogs, will increase as details of the mechanisms involved in the regulation of critical TIMP interactions and activity are revealed. Although there have been no direct reports of immune responses directed towards exogenous administered TIMP proteins in preclinical models, it is important to consider the heightened potential for immunogenicity when utilizing engineered TIMP mutants. Despite this, the process of retaining or emphasizing desirable TIMP characteristics (including specific MMP inhibition or inhibition of receptor tyrosine kinase signaling), while nullifying potentially negative capabilities (such as cell signaling through MMP14), represents an intriguing prospect that could theoretically lead to engineered disease-specific TIMP therapies.
Conclusion
Although their multifunctionality may be appreciated within the field, the TIMP family of proteins is routinely confined to the ontogeny of proteinase inhibition by researchers. This is most likely due to their nomenclature designated function, but in part may be related to the mild phenotypes in knockout models, reviewed extensively elsewhere (14). The combined knockout of Timp2 and Timp3 resulted in late gestational embryonic lethality, which was rescued by further loss of Timp1 and Timp4 (152). The entire knockout of all four Timp genes (Timp-less mice) to unleash MMP activity results in only ~25% post-natal viability, although these mice are considerably smaller with substantially perturbed skeletal development (152,153). In vitro, Timp-less fibroblasts display a strong myofibroblast phenotype similar to that of cancer-associated fibroblasts (154). The mild phenotypes in single KO models are likely related to the inducible nature of MMPs and a large level of redundancy in the TIMP family with regards to metalloproteinase inhibition, in addition to other means of controlling metalloproteinase activity through alpha-2-macroglobulin, LRP1-mediated recycling, and RECK (155). It should be noted that there are no reports of compensatory increases in the expression of other TIMP family members in individual TIMP KO models. TIMP2/3 are widely expressed, even in tissues with no detectable MMP expression, suggesting that these proteins are important homeostatic mediators. On the contrary, Drosophila melanogaster possesses only a single TIMP gene (timp). Knockout of the single timp gene results in an infertile, grossly deleterious phenotype that leads to premature death (156,157).
Like TIMPs, MMPs are multifunctional proteins with various proteinase-independent activities (86). Collectively, TIMPs promote the endocytosis and degradation of metalloproteinase targets (89), so in this regard, their regulatory effects over metzincins extend further than the direct inhibition of catalytic activity. Even within their unique functions lies redundancy in action, of note being TIMP2 and TIMP3’s distinct modes of inhibiting angiogenesis. Homeostatic control requires a high level of redundancy, acting as a safety net when one system fails in response to pathology. In this regard, the current mutant/knockout TIMP models are vital tools for testing these TIMP-related homeostatic safety nets that may reveal further discrete pathology-associated functions of this intriguing family of proteins.
Glossary
Abbreviations
- α3β1
α3β1 integrin
- ADAM
A disintegrin and metalloproteinase
- aMMP2
active MMP2
- ADAM-TS
ADAM with thrombospondin motifs
- AHA1
activator of HSP90 ATPase
- ECM
extracellular matrix
- eHSP90
extracellular heat shock protein 90
- IGF-1
insulin-like growth factor 1
- IGF-1R
insulin-like growth factor 1 receptor
- LRP
low-density lipoprotein receptor-related protein
- MMP
matrix metalloproteinase
- MMPis
MMP inhibitors
- MT-MMP
membrane-type MMP
- RECK
Reversion Inducing Cysteine Rich Protein With Kazal Motifs
- SHP-1
Src Homology region 2 domain-containing Phosphatase-1
- TIMP
tissue inhibitors of metalloproteinase.
Appendix
Phylogenetic analysis was performed through http://www.phylogeny.fr/, using “One-Click” mode (158). Briefly, Sequences were aligned with MUSCLE (v3.8.31) configured for the highest accuracy (MUSCLE with default settings). After alignment, ambiguous regions (such as those containing gaps and/or poorly aligned sequences) were removed with Gblocks (v0.91b) using the following parameters; minimum length of a block after gap cleaning = 10, no gap positions were allowed in the final alignment, all segments with contiguous non-conserved positions bigger than eight were rejected, minimum number of sequences for a flank position = 85%. The phylogenetic tree was reconstructed using the maximum likelihood method implemented in the PhyML program (v3.1/3.0 aLRT). The WAG substitution model was selected assuming an estimated proportion of invariant sites (of 0.047) and four gamma-distributed rate categories to account for rate heterogeneity across sites. The gamma shape parameter was estimated directly from the data (gamma = 2.686). Reliability for internal branch was assessed using the aLRT test (SH-Like). Graphical representation and edition of the phylogenetic tree were performed with TreeDyn (v198.3).
Contributor Information
David Peeney, Laboratory of Pathology, Center for Cancer Research, NCI, NIH, Bethesda, MD 20892, USA.
Yueqin Liu, Laboratory of Pathology, Center for Cancer Research, NCI, NIH, Bethesda, MD 20892, USA.
Carolyn Lazaroff, Laboratory of Pathology, Center for Cancer Research, NCI, NIH, Bethesda, MD 20892, USA.
Sadeechya Gurung, Laboratory of Pathology, Center for Cancer Research, NCI, NIH, Bethesda, MD 20892, USA.
William G Stetler-Stevenson, Laboratory of Pathology, Center for Cancer Research, NCI, NIH, Bethesda, MD 20892, USA.
Funding
Intramural Research Program of the NIH (W.G.S.-S. Project ID ZIA SC 009179)
Conflict of Interest Statement
None declared.
References
- 1. Stetler-Stevenson, W.G. (2008) Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal, 1, re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vanhoutte, D., et al. (2010) TIMPs and cardiac remodeling: ‘Embracing the MMP-independent-side of the family’. J. Mol. Cell. Cardiol., 48, 445–453. [DOI] [PubMed] [Google Scholar]
- 3. Aoki, T., et al. (2007) Role of TIMP-1 and TIMP-2 in the progression of cerebral aneurysms. Stroke, 38, 2337–2345. [DOI] [PubMed] [Google Scholar]
- 4. Castellano, J.M., et al. (2017) Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature, 544, 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dewing, J.M., et al. (2019) The diverse roles of TIMP-3: insights into degenerative diseases of the senescent retina and brain. Cells, 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grunwald, B., et al. (2019) Recognizing the molecular multifunctionality and interactome of TIMP-1. Trends Cell Biol., 29, 6–19. [DOI] [PubMed] [Google Scholar]
- 7. Lee, M.H., et al. (2003) Unveiling the surface epitopes that render tissue inhibitor of metalloproteinase-1 inactive against membrane type 1-matrix metalloproteinase. J. Biol. Chem., 278, 40224–40230. [DOI] [PubMed] [Google Scholar]
- 8. Itoh, Y. (2015) Membrane-type matrix metalloproteinases: their functions and regulations. Matrix Biol., 44–46, 207–223. [DOI] [PubMed] [Google Scholar]
- 9. Tuuttila, A., et al. (1998) Three-dimensional structure of human tissue inhibitor of metalloproteinases-2 at 2.1 A resolution. J. Mol. Biol., 284, 1133–1140. [DOI] [PubMed] [Google Scholar]
- 10. Yu, W.H., et al. (2000) TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J. Biol. Chem., 275, 31226–31232. [DOI] [PubMed] [Google Scholar]
- 11. Raeeszadeh-Sarmazdeh, M., et al. (2019) Directed evolution of the metalloproteinase inhibitor TIMP-1 reveals that its N- and C-terminal domains cooperate in matrix metalloproteinase recognition. J. Biol. Chem., 294, 9476–9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batra, J., et al. (2013) Matrix metalloproteinase-10/TIMP-2 structure and analyses define conserved core interactions and diverse exosite interactions in MMP/TIMP complexes. PLoS One, 8, e75836e75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brew, K. (2019) Reflections on the evolution of the vertebrate tissue inhibitors of metalloproteinases. FASEB J., 33, 71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brew, K., et al. (2010) The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta, 1803, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karagkouni, D., et al. (2018) DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res., 46, D239–D245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Doren, S.R., et al. (2008) Inactivation of N-TIMP-1 by N-terminal acetylation when expressed in bacteria. Biopolymers, 89, 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yao, H., et al. (2013) SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD. Am. J. Physiol. Lung Cell. Mol. Physiol., 305, L615–L624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sánchez-Pozo, J., et al. (2018) Extracellular phosphorylation of TIMP-2 by secreted c-Src tyrosine kinase controls MMP-2 activity. iScience, 1, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peeney, D., et al. (2019) Matrisome-associated gene expression patterns correlating with TIMP2 in cancer. Sci. Rep., 9, 20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan, D., et al. (2020) Biology of tissue inhibitor of metalloproteinase 3 (TIMP3), and its therapeutic implications in cardiovascular pathology. Front. Physiol., 11, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson, H.W., et al. (2017) TIMPs: versatile extracellular regulators in cancer. Nat. Rev. Cancer, 17, 38–53. [DOI] [PubMed] [Google Scholar]
- 22. Nuttall, R.K., et al. (2004) Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett., 563, 129–134. [DOI] [PubMed] [Google Scholar]
- 23. Karlsson, M., et al. (2021) A single-cell type transcriptomics map of human tissues. Sci. Adv., 7, eabh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwak, H.J., et al. (2006) Transforming growth factor-beta1 induces tissue inhibitor of metalloproteinase-1 expression via activation of extracellular signal-regulated kinase and Sp1 in human fibrosarcoma cells. Mol. Cancer Res., 4, 209–220. [DOI] [PubMed] [Google Scholar]
- 25. Edwards, D.R., et al. (1996) Differential effects of transforming growth factor-beta 1 on the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in young and old human fibroblasts. Exp. Gerontol., 31, 207–223. [DOI] [PubMed] [Google Scholar]
- 26. Jaworski, D.M., et al. (2006) Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression is regulated by multiple neural differentiation signals. J. Neurochem., 98, 234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baker-Williams, A.J., et al. (2019) Co-chaperones TIMP2 and AHA1 competitively regulate extracellular HSP90:Client MMP2 activity and matrix proteolysis. Cell Rep., 28, 1894–1906.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Derry, J.M., et al. (1992) Physical linkage of the A-raf-1, properdin, synapsin I, and TIMP genes on the human and mouse X chromosomes. Genomics, 12, 632–638. [DOI] [PubMed] [Google Scholar]
- 29. Hilfiker, S., et al. (1999) Synapsins as regulators of neurotransmitter release. Philos. Trans. R Soc. Lond. B Biol. Sci., 354, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ponten, F., et al. (2008) The Human Protein Atlas--a tool for pathology. J. Pathol., 216, 387–393. [DOI] [PubMed] [Google Scholar]
- 31. Jaworski, D.M., et al. (2007) Potential regulatory relationship between the nested gene DDC8 and its host gene tissue inhibitor of metalloproteinase-2. Physiol. Genomics, 28, 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khokha, R. (1994) Suppression of the tumorigenic and metastatic abilities of murine B16-F10 melanoma cells in vivo by the overexpression of the tissue inhibitor of the metalloproteinases-1. J. Natl. Cancer Inst., 86, 299–304. [DOI] [PubMed] [Google Scholar]
- 33. Martin, D.C., et al. (1996) Inhibition of SV40 T antigen-induced hepatocellular carcinoma in TIMP-1 transgenic mice. Oncogene, 13, 569–576. [PubMed] [Google Scholar]
- 34. Khokha, R., et al. (1989) Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science, 243, 947–950. [DOI] [PubMed] [Google Scholar]
- 35. Jung, K.K., et al. (2006) Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J., 25, 3934–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grünwald, B., et al. (2019) Recognizing the molecular multifunctionality and interactome of TIMP-1. Trends Cell Biol., 29, 6–19. [DOI] [PubMed] [Google Scholar]
- 37. Grünwald, B., et al. (2016) pancreatic premalignant lesions secrete tissue inhibitor of metalloproteinases-1, which activates hepatic stellate cells via CD63 signaling to create a premetastatic niche in the liver. Gastroenterology, 151, 1011–1024.e7. [DOI] [PubMed] [Google Scholar]
- 38. Melendez-Zajgla, J., et al. (2008) Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol. Cancer, 7, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valacca, C., et al. (2015) TIMP-2 interaction with MT1-MMP activates the AKT pathway and protects tumor cells from apoptosis. PLoS One, 10, e0136797e0136797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sounni, N.E., et al. (2010) Timp-2 binding with cellular MT1-MMP stimulates invasion-promoting MEK/ERK signaling in cancer cells. Int. J. Cancer, 126, 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang, W., et al. (2018) TIMP2 is a poor prognostic factor and predicts metastatic biological behavior in gastric cancer. Sci. Rep., 8, 9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Attur, M., et al. (2020) Membrane-type 1 matrix metalloproteinase modulates tissue homeostasis by a non-proteolytic mechanism. iScience, 23, 101789101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seo, D.W., et al. (2003) TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell, 114, 171–180. [DOI] [PubMed] [Google Scholar]
- 44. Seo, D.W., et al. (2008) TIMP-2 disrupts FGF-2-induced downstream signaling pathways. Microvasc. Res., 76, 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fernandez, C.A., et al. (2010) The anti-angiogenic peptide, loop 6, binds insulin-like growth factor-1 receptor. J. Biol. Chem., 285, 41886–41895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guedez, L., et al. (2012) TIMP-2 targets tumor-associated myeloid suppressor cells with effects in cancer immune dysfunction and angiogenesis. J. Immunother., 35, 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Albini, A., et al. (2021) TIMP1 and TIMP2 downregulate TGFβ induced decidual-like phenotype in natural killer cells. Cancers, 13, 4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Albini, A., et al. (2021) Decidual-like NK cell polarization: from cancer killing to cancer nurturing. Cancer Discov., 11, 28–33. [DOI] [PubMed] [Google Scholar]
- 49. Peeney, D., et al. (2019) TIMP-2 suppresses tumor growth and metastasis in murine model of triple-negative breast cancer. Carcinogenesis, 41, 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benzing, C., et al. (2019) TIMP-2 secreted by monocyte-like cells is a potent suppressor of invadopodia formation in pancreatic cancer cells. BMC Cancer, 19, 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Artym, V.V., et al. (2006) Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res., 66, 3034–3043. [DOI] [PubMed] [Google Scholar]
- 52. Stetler-Stevenson, W.G., et al. (1989) Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J. Biol. Chem., 264, 17374–17378. [PubMed] [Google Scholar]
- 53. Ward, R.V., et al. (1991) The purification of tissue inhibitor of metalloproteinases-2 from its 72 kDa progelatinase complex. Demonstration of the biochemical similarities of tissue inhibitor of metalloproteinases-2 and tissue inhibitor of metalloproteinases-1. Biochem. J., 278, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olson, M.W., et al. (1997) Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J. Biol. Chem., 272, 29975–29983. [DOI] [PubMed] [Google Scholar]
- 55. Troeberg, L., et al. (2002) E. coli expression of TIMP-4 and comparative kinetic studies with TIMP-1 and TIMP-2: insights into the interactions of TIMPs and matrix metalloproteinase 2 (gelatinase A). Biochemistry, 41, 15025–15035. [DOI] [PubMed] [Google Scholar]
- 56. Butler, G.S., et al. (1999) Human tissue inhibitor of metalloproteinases 3 interacts with both the N- and C-terminal domains of gelatinases A and B. Regulation by polyanions. J. Biol. Chem., 274, 10846–10851. [DOI] [PubMed] [Google Scholar]
- 57. Bigg, H.F., et al. (1997) Specific, high affinity binding of tissue inhibitor of metalloproteinases-4 (TIMP-4) to the COOH-terminal hemopexin-like domain of human gelatinase A. TIMP-4 binds progelatinase A and the COOH-terminal domain in a similar manner to TIMP-2. J. Biol. Chem., 272, 15496–15500. [DOI] [PubMed] [Google Scholar]
- 58. Strongin, A.Y., et al. (1995) Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem., 270, 5331–5338. [DOI] [PubMed] [Google Scholar]
- 59. Maquoi, E., et al. (2000) Membrane type 1 matrix metalloproteinase-associated degradation of tissue inhibitor of metalloproteinase 2 in human tumor cell lines. J. Biol. Chem., 275, 11368–11378. [DOI] [PubMed] [Google Scholar]
- 60. Zucker, S., et al. (2004) TIMP-2 is released as an intact molecule following binding to MT1-MMP on the cell surface. Exp. Cell Res., 293, 164–174. [DOI] [PubMed] [Google Scholar]
- 61. Maquoi, E., et al. (2000) Type IV collagen induces matrix metalloproteinase 2 activation in HT1080 fibrosarcoma cells. Exp. Cell Res., 261, 348–359. [DOI] [PubMed] [Google Scholar]
- 62. Brooks, P.C., et al. (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell, 85, 683–693. [DOI] [PubMed] [Google Scholar]
- 63. Li, Z., et al. (2017) Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis. Cancer Sci., 108, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Toth, M., et al. (2003) Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem. Biophys. Res. Commun., 308, 386–395. [DOI] [PubMed] [Google Scholar]
- 65. English, J.L., et al. (2006) Individual Timp deficiencies differentially impact pro-MMP-2 activation. J. Biol. Chem., 281, 10337–10346. [DOI] [PubMed] [Google Scholar]
- 66. Bigg, H.F., et al. (2001) Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res., 61, 3610–3618. [PubMed] [Google Scholar]
- 67. Hernandez-Barrantes, S., et al. (2001) Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem. Biophys. Res. Commun., 281, 126–130. [DOI] [PubMed] [Google Scholar]
- 68. Morgunova, E., et al. (2002) Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc. Natl. Acad. Sci. U.S.A., 99, 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Itoh, Y., et al. (2006) Cell surface collagenolysis requires homodimerization of the membrane-bound collagenase MT1-MMP. Mol. Biol. Cell, 17, 5390–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deryugina, E.I., et al. (2004) Prointegrin maturation follows rapid trafficking and processing of MT1-MMP in furin-negative colon carcinoma LoVo cells. Traffic, 5, 627–641. [DOI] [PubMed] [Google Scholar]
- 71. Itoh, Y., et al. (2004) MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem. Sci., 29, 285–289. [DOI] [PubMed] [Google Scholar]
- 72. Yanez-Mo, M., et al. (2008) MT1-MMP collagenolytic activity is regulated through association with tetraspanin CD151 in primary endothelial cells. Blood, 112, 3217–3226. [DOI] [PubMed] [Google Scholar]
- 73. Albrechtsen, R., et al. (2013) ADAM12 redistributes and activates MMP-14, resulting in gelatin degradation, reduced apoptosis and increased tumor growth. J. Cell Sci., 126, 4707–4720. [DOI] [PubMed] [Google Scholar]
- 74. Galvez, B.G., et al. (2002) ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J. Cell Biol., 159, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ellerbroek, S.M., et al. (2001) Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J. Biol. Chem., 276, 24833–24842. [DOI] [PubMed] [Google Scholar]
- 76. Chun, T.H., et al. (2014) 3-D adipocyte differentiation and peri-adipocyte collagen turnover. Methods Enzymol., 538, 15–34. [DOI] [PubMed] [Google Scholar]
- 77. Chun, T.H., et al. (2010) Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes, 59, 2484–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holmbeck, K., et al. (1999) MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell, 99, 81–92. [DOI] [PubMed] [Google Scholar]
- 79. Nyalendo, C., et al. (2007) Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration. J. Biol. Chem., 282, 15690–15699. [DOI] [PubMed] [Google Scholar]
- 80. Lagoutte, E., et al. (2016) LIMK regulates tumor-cell invasion and matrix degradation through tyrosine phosphorylation of MT1-MMP. Sci. Rep., 6, 24925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Paye, A., et al. (2014) EGFR activation and signaling in cancer cells are enhanced by the membrane-bound metalloprotease MT4-MMP. Cancer Res., 74, 6758–6770. [DOI] [PubMed] [Google Scholar]
- 82. Santamaria, S., et al. (2021) Post-translational regulation and proteolytic activity of the metalloproteinase ADAMTS8. J. Biol. Chem., 297, 101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kveiborg, M., et al. (2008) Cellular roles of ADAM12 in health and disease. Int. J. Biochem. Cell Biol., 40, 1685–1702. [DOI] [PubMed] [Google Scholar]
- 84. Amour, A., et al. (2000) The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett., 473, 275–279. [DOI] [PubMed] [Google Scholar]
- 85. Duan, J.X., et al. (2015) Expanding the activity of tissue inhibitors of metalloproteinase (TIMP)-1 against surface-anchored metalloproteinases by the replacement of its C-terminal domain: implications for anti-cancer effects. PLoS One, 10, e0136384e0136384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kessenbrock, K., et al. (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell, 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kessenbrock, K., et al. (2013) A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell, 13, 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Carreca, A.P., et al. (2020) TIMP-3 facilitates binding of target metalloproteinases to the endocytic receptor LRP-1 and promotes scavenging of MMP-1. Sci. Rep., 10, 12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Arai, A.L., et al. (2020) High-affinity binding of LDL receptor-related protein 1 to matrix metalloprotease 1 requires protease:inhibitor complex formation. Biochemistry, 59, 2922–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. May, P., et al. (2007) The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann. Med., 39, 219–228. [DOI] [PubMed] [Google Scholar]
- 91. Emonard, H., et al. (2004) Low density lipoprotein receptor-related protein mediates endocytic clearance of pro-MMP-2.TIMP-2 complex through a thrombospondin-independent mechanism. J. Biol. Chem., 279, 54944–54951. [DOI] [PubMed] [Google Scholar]
- 92. Scilabra, S.D., et al. (2013) Differential regulation of extracellular tissue inhibitor of metalloproteinases-3 levels by cell membrane-bound and shed low density lipoprotein receptor-related protein 1. J. Biol. Chem., 288, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thevenard, J., et al. (2014) Low-density lipoprotein receptor-related protein-1 mediates endocytic clearance of tissue inhibitor of metalloproteinases-1 and promotes its cytokine-like activities. PLoS One, 9, e103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Verzeaux, L., et al. (2017) Intrinsic dynamics study identifies two amino acids of TIMP-1 critical for its LRP-1-mediated endocytosis in neurons. Sci. Rep., 7, 5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Christensen, E.I., et al. (2007) Role of megalin and cubilin in renal physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol., 158, 1–22. [DOI] [PubMed] [Google Scholar]
- 96. Marinò, M., et al. (2001) Megalin in thyroid physiology and pathology. Thyroid, 11, 47–56. [DOI] [PubMed] [Google Scholar]
- 97. Erranz, B., et al. (2004) Megalin and cubilin expression in gallbladder epithelium and regulation by bile acids. J. Lipid Res., 45, 2185–2198. [DOI] [PubMed] [Google Scholar]
- 98. Johanns, M., et al. (2017) Cellular uptake of proMMP-2:TIMP-2 complexes by the endocytic receptor megalin/LRP-2. Sci. Rep., 7, 4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Selvais, C., et al. (2011) Cell cholesterol modulates metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 (LRP-1) and clearance function. FASEB J., 25, 2770–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu, Q., et al. (2009) LRP1 shedding in human brain: roles of ADAM10 and ADAM17. Mol. Neurodegener., 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Quinn, K.A., et al. (1997) Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J. Biol. Chem., 272, 23946–23951. [DOI] [PubMed] [Google Scholar]
- 102. Kechagia, J.Z., et al. (2019) Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol., 20, 457–473. [DOI] [PubMed] [Google Scholar]
- 103. Murphy, A.N., et al. (1993) Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J. Cell. Physiol., 157, 351–358. [DOI] [PubMed] [Google Scholar]
- 104. Hoegy, S.E., et al. (2001) Tissue inhibitor of metalloproteinases-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J. Biol. Chem., 276, 3203–3214. [DOI] [PubMed] [Google Scholar]
- 105. Seo, D.W., et al. (2006) Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J. Biol. Chem., 281, 3711–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Alexius-Lindgren, M., et al. (2014) The RECK gene and biological malignancy--its significance in angiogenesis and inhibition of matrix metalloproteinases. Anticancer Res., 34, 3867–3873. [PubMed] [Google Scholar]
- 107. Seo, D.W., et al. (2011) An integrin-binding N-terminal peptide region of TIMP-2 retains potent angio-inhibitory and anti-tumorigenic activity in vivo. Peptides, 32, 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lluri, G., et al. (2006) Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates neuromuscular junction development via a beta1 integrin-mediated mechanism. J. Neurobiol., 66, 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pérez-Martínez, L., et al. (2005) Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J. Neurosci., 25, 4917–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jaworski, D.M., et al. (2006) Tissue inhibitor of metalloproteinase-2(TIMP-2)-deficient mice display motor deficits. J. Neurobiol., 66, 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cheng, G., et al. (2016) Higher levels of TIMP-1 expression are associated with a poor prognosis in triple-negative breast cancer. Mol. Cancer, 15, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Aaberg-Jessen, C., et al. (2018) Co-expression of TIMP-1 and its cell surface binding partner CD63 in glioblastomas. BMC Cancer, 18, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Takino, T., et al. (2003) Tetraspanin CD63 promotes targeting and lysosomal proteolysis of membrane-type 1 matrix metalloproteinase. Biochem. Biophys. Res. Commun., 304, 160–166. [DOI] [PubMed] [Google Scholar]
- 114. Osher, E., et al. (2019) Therapeutic targeting of the IGF axis. Cells, 8, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lopez-Lopez, C., et al. (2004) Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. U.S.A., 101, 9833–9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rabinovsky, E.D., et al. (2004) Insulin-like growth factor I plasmid therapy promotes in vivo angiogenesis. Mol. Ther., 9, 46–55. [DOI] [PubMed] [Google Scholar]
- 117. Su, E.J., et al. (2003) Gene therapy vector-mediated expression of insulin-like growth factors protects cardiomyocytes from apoptosis and enhances neovascularization. Am. J. Physiol. Heart Circ. Physiol., 284, H1429–H1440. [DOI] [PubMed] [Google Scholar]
- 118. Eustace, B.K., et al. (2004) Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat. Cell Biol., 6, 507–514. [DOI] [PubMed] [Google Scholar]
- 119. Coker, M.L., et al. (1999) Matrix metalloproteinase synthesis and expression in isolated LV myocyte preparations. Am. J. Physiol., 277, H777–H787. [DOI] [PubMed] [Google Scholar]
- 120. Jobin, P.G., et al. (2017) New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta Mol. Cell Res., 1864, 2043–2055. [DOI] [PubMed] [Google Scholar]
- 121. Lovett, D.H., et al. (2012) A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS One, 7, e34177e34177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim, S.S., et al. (2017) Enhanced expression of two discrete isoforms of matrix metalloproteinase-2 in experimental and human diabetic nephropathy. PLoS One, 12, e0171625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Coussens, L.M., et al. (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science, 295, 2387–2392. [DOI] [PubMed] [Google Scholar]
- 124. Fields, G.B. (2019) The rebirth of matrix metalloproteinase inhibitors: moving beyond the dogma. Cells, 8, 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Winer, A., et al. (2018) Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol. Cancer Ther., 17, 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rai, G.P., et al. (2020) Tissue inhibitor of matrix metalloproteinase-3 has both anti-metastatic and anti-tumourigenic properties. Clin. Exp. Metastasis, 37, 69–76. [DOI] [PubMed] [Google Scholar]
- 127. Kandalam, V., et al. (2010) TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ. Res., 106, 796–808. [DOI] [PubMed] [Google Scholar]
- 128. Chintalgattu, V., et al. (2018) Utility of Glycosylated TIMP3 molecules: inhibition of MMPs and TACE to improve cardiac function in rat myocardial infarct model. Pharmacol. Res. Perspect., 6, e00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Moore, L., et al. (2012) Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail. Rev., 17, 693–706. [DOI] [PubMed] [Google Scholar]
- 130. Ikonomidis, J.S., et al. (2005) Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am. J. Physiol. Heart Circ. Physiol., 288, H149–H158. [DOI] [PubMed] [Google Scholar]
- 131. Knight, B.E., et al. (2019) TIMP-1 attenuates the development of inflammatory pain through MMP-dependent and receptor-mediated cell signaling mechanisms. Front. Mol. Neurosci., 12, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tang, J., et al. (2020) TIMP1 preserves the blood-brain barrier through interacting with CD63/integrin beta 1 complex and regulating downstream FAK/RhoA signaling. Acta Pharm Sin B, 10, 987–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen, F., et al. (2013) TIMP-1 attenuates blood-brain barrier permeability in mice with acute liver failure. J. Cereb. Blood Flow Metab., 33, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Savarin, C., et al. (2013) MMP-independent role of TIMP-1 at the blood brain barrier during viral encephalomyelitis. ASN Neuro, 5, e00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tejima, E., et al. (2009) Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J. Neurotrauma, 26, 1935–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Koskivirta, I., et al. (2010) Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J. Biol. Chem., 285, 24487–24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Albini, A., et al. (1991) Tumor cell invasion inhibited by TIMP-2. J. Natl. Cancer Inst., 83, 775–779. [DOI] [PubMed] [Google Scholar]
- 138. Baker, A.H., et al. (1999) Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br. J. Cancer, 79, 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rojiani, M.V., et al. (2015) TIMP-1 overexpression in lung carcinoma enhances tumor kinetics and angiogenesis in brain metastasis. J. Neuropathol. Exp. Neurol., 74, 293–304. [DOI] [PubMed] [Google Scholar]
- 140. Kornfeld, J.W., et al. (2011) Overexpression of TACE and TIMP3 mRNA in head and neck cancer: association with tumour development and progression. Br. J. Cancer, 104, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Liss, M., et al. (2009) Tissue inhibitor of metalloproteinase-4 is elevated in early-stage breast cancers with accelerated progression and poor clinical course. Am. J. Pathol., 175, 940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ikenaka, Y., et al. (2003) Tissue inhibitor of metalloproteinases-1 (TIMP-1) inhibits tumor growth and angiogenesis in the TIMP-1 transgenic mouse model. Int. J. Cancer, 105, 340–346. [DOI] [PubMed] [Google Scholar]
- 143. Fager, N., et al. (2000) Differential spatial distribution and temporal regulation of tissue inhibitor of metalloproteinase mRNA expression during rat central nervous system development. Mech. Dev., 98, 105–109. [DOI] [PubMed] [Google Scholar]
- 144. Lee, E.J., et al. (2014) The anti-inflammatory role of tissue inhibitor of metalloproteinase-2 in lipopolysaccharide-stimulated microglia. J. Neuroinflammation, 11, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dohi, T., et al. (2015) Tissue inhibitor of metalloproteinase-2 suppresses collagen synthesis in cultured keloid fibroblasts. Plast. Reconstr. Surg. Glob. Open, 3, e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Grossman, M., et al. (2010) The intrinsic protein flexibility of endogenous protease inhibitor TIMP-1 controls its binding interface and affects its function. Biochemistry, 49, 6184–6192. [DOI] [PubMed] [Google Scholar]
- 147. Arkadash, V., et al. (2017) Development of high affinity and high specificity inhibitors of matrix metalloproteinase 14 through computational design and directed evolution. J. Biol. Chem., 292, 3481–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Arkadash, V., et al. (2018) Combinatorial engineering of N-TIMP2 variants that selectively inhibit MMP9 and MMP14 function in the cell. Oncotarget, 9, 32036–32053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lee, M.H., et al. (2005) Total conversion of tissue inhibitor of metalloproteinase (TIMP) for specific metalloproteinase targeting: fine-tuning TIMP-4 for optimal inhibition of tumor necrosis factor-{alpha}-converting enzyme. J. Biol. Chem., 280, 15967–15975. [DOI] [PubMed] [Google Scholar]
- 150. Kveiborg, M., et al. (2010) Selective inhibition of ADAM12 catalytic activity through engineering of tissue inhibitor of metalloproteinase 2 (TIMP-2). Biochem. J., 430, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Batra, J., et al. (2012) PEGylation extends circulation half-life while preserving in vitro and in vivo activity of tissue inhibitor of metalloproteinases-1 (TIMP-1). PLoS One, 7, e50028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Saw, S., et al. (2019) Metalloprotease inhibitor TIMP proteins control FGF-2 bioavailability and regulate skeletal growth. J. Cell Biol., 218, 3134–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Chen, Y., et al. (2019) TIMP loss activates metalloproteinase-TNFα-DKK1 axis to compromise Wnt signaling and bone mass. J. Bone Miner. Res., 34, 182–194. [DOI] [PubMed] [Google Scholar]
- 154. Shimoda, M., et al. (2014) Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat. Cell Biol., 16, 889–901. [DOI] [PubMed] [Google Scholar]
- 155. Oh, J., et al. (2004) Tissue inhibitors of metalloproteinase 2 inhibits endothelial cell migration through increased expression of RECK. Cancer Res., 64, 9062–9069. [DOI] [PubMed] [Google Scholar]
- 156. Godenschwege, T.A., et al. (2000) Inflated wings, tissue autolysis and early death in tissue inhibitor of metalloproteinases mutants of Drosophila. Eur. J. Cell Biol., 79, 495–501. [DOI] [PubMed] [Google Scholar]
- 157. Shilts, J., et al. (2017) Secreted tissue inhibitor of matrix metalloproteinase restricts trans-synaptic signaling to coordinate synaptogenesis. J. Cell Sci., 130, 2344–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dereeper, A., et al. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res., 36, W465–W469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wujak, L., et al. (2018) Low density lipoprotein receptor-related protein 1 couples beta1 integrin activation to degradation. Cell. Mol. Life Sci., 75, 1671–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Rabiej, V.K., et al. (2016) Low density lipoprotein receptor-related protein 1 mediated endocytosis of beta1-integrin influences cell adhesion and cell migration. Exp. Cell Res., 340, 102–115. [DOI] [PubMed] [Google Scholar]