Abstract
Lupus nephritis (LN) develops in more than a third of all systemic lupus erythematosus (SLE) patients and is the strongest predictor of morbidity and mortality. Increased circulating levels of type I interferon (IFN I) and anti-double stranded DNA (anti-dsDNA) and anti-RNA binding protein (anti-RNP) antibodies lead to increased glomerular injury via leukocyte activation and glomerular infiltration. Uncontrolled Toll-like receptor (TLR) signaling in leukocytes results in increased production of IFN I and anti-dsDNA antibodies. ITGAM gene codes for integrin CD11b, the α-chain of integrin heterodimer CD11b/CD18, that is highly expressed in leukocytes and modulates TLR-dependent pro-inflammatory signaling. Three nonsynonymous SNPs in the ITGAM gene strongly correlate with increased risk for SLE and LN and with IFN I levels. Here we review the literature on the role of CD11b on leukocytes in LN. We also incorporate conclusions from several recent studies that show that these ITGAM SNPs result in a CD11b protein that is less able to suppress TLR-dependent pro-inflammatory pathways in leukocytes, that activation of CD11b via novel small molecule agonists suppresses TLR-dependent pathways, including reductions in circulating levels of IFN I and anti-dsDNA antibodies, and that CD11b activation reduces LN in model systems. Recent data strongly suggests that integrin CD11b is an exciting new therapeutic target in SLE and LN and that allosteric activation of CD11b is a novel therapeutic paradigm for effectively treating such autoimmune diseases.
Overview
Lupus nephritis (LN) is a debilitating complication of systemic lupus erythematosus (SLE) with poorly defined etiology and ineffective treatment options. LN is characterized by infiltration of immune cells to the kidneys, leading to damaging inflammation and proteinuria. CD11b, the α-chain of the integrin CD11b/CD18 (αMβ2, CR3, Mac-1) is expressed on the surface of infiltrating macrophages and neutrophils. The ITGAM gene, which encodes for CD11b, has single nucleotide polymorphisms (SNPs) which reduce integrin activation and are strongly associated with SLE and LN susceptibility. CD11b modulates several biological functions including cell adhesion, migration, and signaling. Toll-like receptor (TLR) signaling in leukocytes mediates several pro-inflammatory cytokines and type 1 interferon (IFN I), a circulating biomarker for SLE and LN. Recent studies show that CD11b activation suppresses TLR-dependent pro-inflammatory signaling reducing inflammatory damage and LN in experimental systems. Here, we give an overview of CD11b, its associations in SLE and LN, and suggest that allosteric activation of CD11b is potential novel therapeutic strategy for SLE and LN.
Lupus nephritis and systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a debilitating autoimmune disease that affects multiple end organs. It is also highly heterogeneous in its clinical manifestations, with mild-to-moderate presentations in some patients, versus significantly debilitating symptoms in many others. The disease etiology is multifactorial, and the exact disease mechanism remains unclear (1). SLE affects >2 million Americans and >5 million people worldwide (2,3). More than 40% of all adult SLE patients and >80% of pediatric SLE patients develop glomerular injury and cardiovascular complications, resulting in kidney impairment and the development of lupus nephritis (LN), which remains the strongest predictor of morbidity and mortality (4–6). Young women of childbearing age are disproportionately more susceptible to SLE and LN, with a ratio of 9:1 (2,7,8). LN is a severe end-organ manifestation of lupus which often develops within the first 5 years of SLE diagnosis, though it is also often the presenting manifestation of underlying SLE (9). LN is characterized by immune complex deposition in the glomerular capillaries and glomerulonephritis, which can rapidly progress from chronic kidney disease (CKD) to end stage renal disease (ESRD) (5). LN is currently managed via glucocorticoids and immunosuppressive aggressive treatments, remission is achieved in only a fraction of patients, and a majority develop kidney complications resulting in end-stage renal disease (ESRD) within 5–10 years of disease diagnosis. Thus, targeted and improved LN treatments are urgently needed and an unmet medical need. A key to development of improved therapeutics is a better understanding of the underlying mechanisms at a molecular and cellular level.
Immune complex deposition in the glomerular capillaries, in conjunction with elevated levels of circulating pro-inflammatory factors, is often the initiating cause of LN and drives tissue damage (10). The immune complexes comprise of anti-nuclear or anti-double stranded DNA (dsDNA) antibodies, anti-RNP antibodies, anti-glomerular antibodies, activated complement fragments, neutrophil extracellular traps (NETs), DNA fragments and apoptotic cell debris. The complexes are deposited in the glomerular capillaries due the presence of autoreactive antigens in the matrix, the inability of the glomerular filter to clear these complexes or both. The deposited complexes induce influx of innate immune cells from circulation, where the recruited cells initiate a pro-inflammatory program that results in recruitment and activation of leukocytes and lymphocytes, loss of glomerular integrity and a progressive glomerular damage. The recruited cells also secrete pro-inflammatory mediators that initiate such signaling in nearby resident cells in the glomeruli, including podocytes, mesangial cells and the resident macrophages. Furthermore, the deposited pro-inflammatory milieu contains damage-associated molecular patterns (DAMPs) that are recognized by various cellular receptors, such as the family of toll-like receptors (TLRs) (11), and initiate pro-inflammatory signaling, which produces a feed-forward loop that causes further cellular injury, apoptosis, glomerulosclerosis and kidney damage. Macrophages are increased in LN and are associated with more severe disease and poorer outcomes (12).
Systemic inflammation due to aberrant activation of the immune compartment, including leukocytes, B-cells and lymphocytes, drives the formation of immune complexes and increased production of pro-inflammatory mediator in SLE. Defective clearance of apoptotic materials and an unrestrained immune response towards it creates a feed-forward loop that results in systemic inflammation and autoimmunity (13). Overactive neutrophils release NETs during a unique form of cell death, called “NETosis,” which also serves as a source of autoantigen upon B cell activation (5,14,15). SLE patients display higher NET levels compared to healthy controls (14). These levels have been associated with disease severity and failure of NET removal is associated with LN development. Failure to remove apoptotic debris and NETing neutrophils leads to accumulation of these debris and immune complexes in the kidneys, promoting the damaging inflammation seen in LN (16).
Among the circulating pro-inflammatory mediators, levels of a major family of immunomodulatory cytokines, type I interferon (IFN I), are elevated in the sera of SLE patients, correlate with disease activity and are diagnostic (17–20). Increased IFN I activity is also a heritable risk factor and is pathogenic (21). Although type I interferons are key mediators of host response against viral and bacterial pathogens, their activation has also been recognized as being central in the development of SLE/LN (22). Increased IFN I levels are correlated with increased severity of LN (23) and are associated with accelerated glomerulonephritis in mice (24,25). Elevated IFN I levels result in increased expression of several downstream targets, that can be measured as an IFN I signature (26). Elevation in IFN I levels also activates T cells, dendritic cells and B cells, that lead to secretion of autoantibodies and IC generation, resulting kidney IC deposition and in tissue scarring in LN (21,27,28). The plasmacytoid DCs (pDCs) and myeloid cells are among the main cellular sources of IFN I production, via stimulation of their TLRs and other molecular sensors of pathogen-associated molecular patterns (PAMPs) (29,30). Given the central role of IFN I and its receptor (type I interferon receptor, IFNAR), an antibody blocking this interaction, anifrolumab, was recently approved for treatment in SLE (31,32).
CD11b is encoded by the ITGAM gene
Integrins are heterodimeric receptors with an α- and a β-subunit that are non-covalently associated with each other (for an excellent recent review, please see (33)). They have a large extracellular domain that binds to its ligands, single-pass transmembrane helices and short cytoplasmic tails, that bind to a variety of intracellular proteins to link integrins to the cytoskeleton and induce signaling. The integrin CD11b/CD18 comprises of α-chain CD11b (also known as αM, encoded by the gene ITGAM) and β-chain CD18 (also known as β2, encoded by gene ITGB2) and is highly expressed on leukocytes, such as neutrophils, monocytes and macrophages (34–37). The dimeric integrin receptor CD11b/CD18 is also known as αMβ2, Mac-1 and CR3 and mediates cell adhesion, migration and signaling in leukocytes to modulate many biological functions of these cells, including phagocytosis, inflammatory damage and tissue repair (27,38–41).
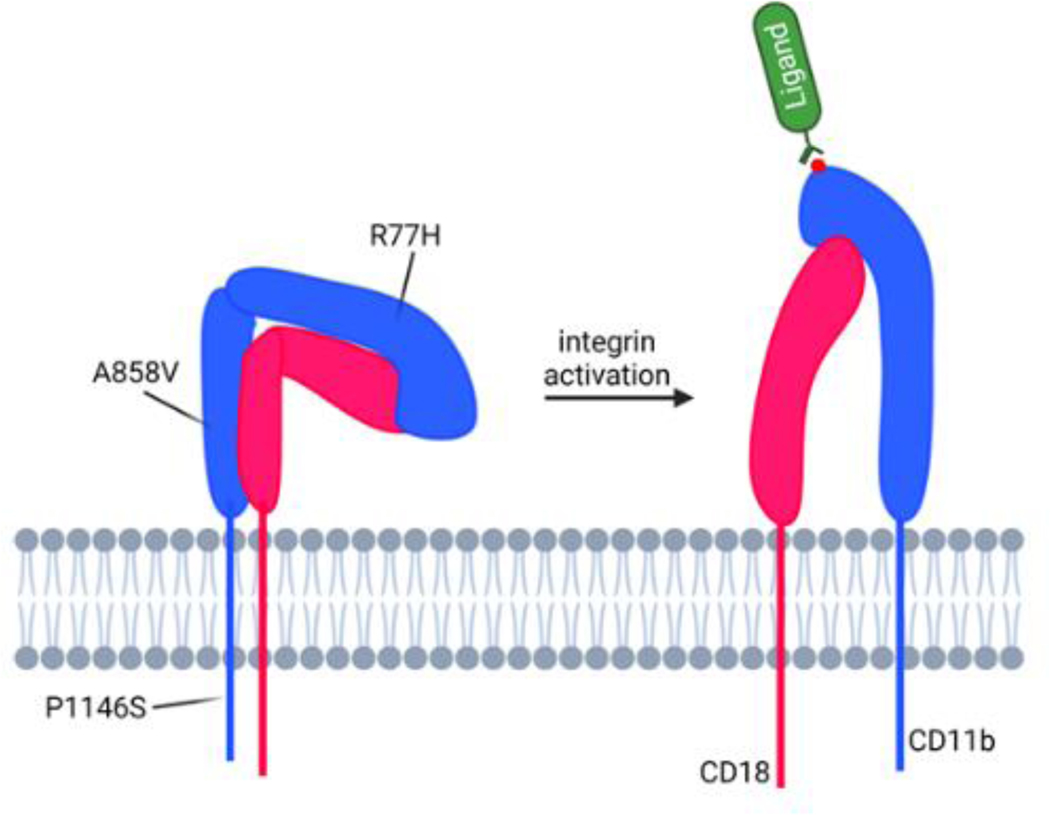
The function of CD11b/CD18 is dynamically regulated on the cell membrane by large conformational changes. In circulating leukocytes, CD11b/CD18 is expressed in a low affinity, closed conformation. Structural studies with the recombinant ectodomain of homologous integrin CD11c/CD18 showed that its large extracellular domain adopts a bent “V” shaped structure in its low affinity conformation, with a “head” where ligands can bind and two “legs” which further span the plasma membrane connecting the ectodomain to the intracellular proteins and cytoskeleton (Figure 1A) (42,43). The various domains of the CD11b and CD18 are organized in a way to allow for conformational switching of the receptor (Figure 1B) and the short cytoplasmic tails in each of the two sub-units bind to cytoplasmic proteins, that help convey signaling changes from inside of the cell to the extracellular domains (inside-out signaling) or from extracellular ligands to the inside of the cells (outside-in signaling) by these receptors. The binding of cytoplasmic proteins to the integrin cytoplasmic tails or engagement of extracellular ligands by the integrin head domain induces large conformational changes in the integrin structure that drives the intracellular signaling pathways. In CD11b/CD18, the ligand binding is mediated by a compact domain in CD11b called the αA-domain (also called αI-domain) and is mediated via a metal ion dependent adhesion site (MIDAS) (44–46). Previous structural studies showed that the αA-domain also exists in two major conformations, with its low affinity, closed state predominating in circulating cells that converts into a high-affinity, ligand competent open state upon activation (Figure 1C) (47,48).
Figure 1. Structure of integrin CD11b/CD18.
A. A structural model of full-length integrin CD11b/CD18 in its low-affinity conformation based on published structure of the ectodomain of a homologous β2 integrin CD11c/CD18 (also known as αXβ2) (149). The model also depicts the relative location of the ligand binding αA-domain and the ligand binding site (metal ion dependent adhesion site, MIDAS). B. Domain organization in the individual chains of the integrin CD11b/CD18. C. Overlay model of the αA-domain of CD11b in two conformational states – low-affinity, inactive conformation (blue, from 1jlm.pdb (61)) and high-affinity active conformation (pink, from 1m1u.pdb (150)). The activation-induced changes in an allosteric pocket near the F-strand and the α7 helix, which shows the largest change between the two structures (arrow) are also shown. A metal ion at the MIDAS site is shown as a red sphere.
Coding SNPs in ITGAM are a risk factor for LN
The increased incidence of SLE in individuals with specific genetic backgrounds, such as of African, Hispanic or Asian ancestry, as compared to those of European ancestry suggests a role for genetic predisposition for this autoimmune disease. Indeed, genome-wide association studies (GWAS) over the last two decades have identified over 100 genetic risk loci that are linked to the increased risk for SLE and LN (49). Fine mapping of these loci identified single-nucleotide polymorphisms (SNPs) in a number of immune response genes. Studies also showed that SNPs in ITGAM, including three coding region SNPs (rs1143678, rs1143679 and rs1143683, which result in mis-sense mutations P1146S (a C>T substitution), R77H (G>A) and A858V (C>T) respectively in the protein, significantly correlate with incidence of SLE and other complications of SLE such as LN, discoid rash and cardiovascular disease (1,9,14,40,50–53). This association of ITGAM SNPs with SLE and LN holds among diverse ancestral backgrounds, suggesting it to be a common disease linked pathway (7,52,54–56). Recent studies have also shown that two of the three coding SNPs in ITGAM (rs1143678 and rs1143683) are in complete linkage disequilibrium (LD), forming a haplotype (27,51,54) and the SNPs rs1143678 and rs1143679 are often in LD (27,52,56). Finally, the prevalence of LN among SLE patients increases in patients with the ITGAM SNPs rs1143679 or rs1143683 as compared to SLE patients without renal nephritis (54,57).
How these ITGAM SNPs confer risk for SLE and LN is not completely clear, although recent studies have shed some new light. These SNPs in ITGAM are present in up to 20% of patients and only occur in 1–2% of the general population, suggesting a strong mechanistic link between these SNPs and SLE/LN (27,40,51–53). They appear to reduce function of the encoded protein CD11b, including integrin activation, ligand binding and cell adhesion, phagocytosis and catch-bond formation (42,58–60) without change in surface expression (51,61). Mapping of the SNPs on integrin structure shows that they are dispersed in three different domains, with rs1143679 (resulting in the R77H mutation) in the propeller domain, rs1143683 (resulting in the A858V mutation) in the calf-2 domain and rs1143678 (resulting in the P1146S mutation) in the cytoplasmic tail, suggesting their role in modulating CD11b-dependent signaling (Figure 2).
Figure 2. Integrin activation and LN associated mutations.
A diagram showing large conformational change in the integrin CD11b/CD18 that is expected upon integrin activation. The integrin exists primarily in a bent, low-affinity conformation on the surface of circulating leukocytes under basal conditions. Upon activation, the dimer changes conformation and adopts a more upright, active, high-affinity form that is bound to large, extracellular ligands with high affinity at the MIDAS site (shown with a red sphere representing a metal ion at the MIDAS site). Relative locations of each of the three CD11b mutations encoded by the comment ITGAM variants are also shown on the diagram.
CD11b is a key modulator of pathogenic mechanisms
The integrin CD11b/CD18 plays important roles in leukocyte biology (62). It is expressed on monocytes, neutrophils, dendritic cells, natural killer cells, macrophages, and a subset of B and T cells (63–68). As a receptor, CD11b/CD18 dimer binds over 40 ligands, including intercellular adhesion molecule (ICAM) family members, complement protein iC3b and fibrinogen (69–73), and modulates multiple leukocyte functions, including cell adhesion, transmigration, phagocytosis, pro-inflammatory signaling, tissue recruitment, oxidative burst and apoptosis. Given these roles, CD11b/CD18 has long been considered to promote host defense pathways as a pro-inflammatory receptor (74), although recent studies have also shown it to have an anti-inflammatory role by modulating intracellular signaling of other pro-inflammatory receptors (21,27,53,75). This suggests that CD11b acts in a highly contextual manner to promote or regulate inflammation.
CD11b, the α-chain of CD11b/CD18, was initially identified in patients with leukocyte adhesion deficiency-1 (LAD1) (76–79). LAD1 is an autosomal recessive disorder that is characterized by recurrent bacterial infections, impaired wound healing and aberrant granulocyte and leukocyte functions. Leukocytes in LAD1 patients show a lack of β2 integrin surface expression, due to mutations in either the β- or the α-chains of CD11b/CD18. LAD1 patients present with recurrent, life-threatening infections and an inability to fight off and destroy invading pathogens. LAD1 neutrophils show markedly reduced adhesion, chemotaxis and extravasation, and an inability to kill bacteria. This suggested that a main role of CD11b was to promote inflammation to control infections. Mice deficient in CD11b (CD11b−/−) mimic many, although not all, of the characteristics observed in LAD1 patients (80). CD11b−/− neutrophils display impaired adhesion, spreading, phagocytosis and oxidative burst and show defective transmigration and tissue recruitment (their extravasation is paradoxically increased) and delayed apoptosis. CD11b deficient mice also show increased susceptibility to inflammatory and autoimmune diseases (81–86), although they also show increased tissue infiltration of leukocytes, elevated IC deposition and immune-mediated glomerular injury in lupus-prone mice (84), suggesting additional anti-inflammatory roles for CD11b. CD11b deficiency results in increased levels of pro-inflammatory cytokines in circulation in models of sepsis and SLE (21,27,87). Thus, this propensity of genetically or functionally CD11b deficient animals to display overly increased immune responses and tissue injury is not unexpected as CD11b has a key functional role in leukocyte migration and recruitment and in phagocytosis mediated clearing of opsonized particles, apoptotic debris and ICs, and the lack of CD11b would affect control of exuberant immune responses. Conversely, some studies have also shown that CD11b promotes inflammatory injury and that blocking CD11b (or CD18) or the deficiency of CD11b reduced inflammation and tissue injury in other animal models (88–91). Therefore, the published literature is not completely clear as to whether blocking CD11b with antagonists or its absence (via knockouts or knock downs) has therapeutic applicability in inflammatory and autoimmune diseases, and that the approach may be highly experimental model system and tissue context dependent.
Aberrant TLR-dependent pro-inflammatory signaling plays a key role in driving systemic inflammation in SLE and LN. Over-abundance or ineffective clearance of apoptotic cells and cellular debris, such as single-stranded RNA (ssRNA), double-stranded DNA (dsDNA) and non-methylated endogenous DNA fragments (CpG DNA) stimulate TLRs 3, 7/8 and 9. Stimulated TLRs recruit adaptor proteins MyD88, TRIF, TRAF3 or TRAM, which as subsequently sequentially detected by a set of kinases, such as TBK1, and ubiquitinases, such as Cbl-b, to activate transcription factors NFκB, AP-1 and IRF3/5/7. This results in activation of pro-inflammatory pathways generating IL-1β, IL-6, TNFα and IFN I (92,93) (Figure 3). The resulting signaling produces increased levels of pro-inflammatory mediators in circulation, including IFN I, and a feed-forward loop that results in activation of immune cells, further production of ICs and end-organ injury. CD11b cross-talks with a number of cellular receptors on immune cells, including TLRs, to regulate intracellular signaling. Recent data suggests that CD11b acts to negatively regulate TLR-signaling in leukocytes (21,27,94,95). Stimulation with TLR4 agonist LPS increases secretion of proinflammatory cytokines IL-6, TNFα, and IL-1β through activation of TLR-dependent canonical NFκB pathways, leading to end-organ damage over time, and significantly higher levels of these cytokines are produced in CD11b-deficient cells. Basally, circulating leukocytes express CD11b/CD18 on cell surface in a low ligand-competent (inactive) conformation, where it rapidly changes its conformation to ligand-competent (active) conformation, in response to various inside-out or outside-in signals, such as increased chemokine and cytokine gradients near sites of inflammation and stimulation of other receptors, such as TLRs (96–98). TLR stimulation, in addition to activating NFκB and IFN I pathways, also results in inside-out signaling mediated activation of CD11b that occurs via the activation of phosphatidylinositol-3-OH kinase (PI3K), the second messengers diacylglycerol (DAG) and Ca2+ activating protein kinase C and small GTPase Rap1, that subsequently engages interacting proteins RIAM and RapL that un-restrain cytoplasmic protein talin-1 to bind to the integrin tail and stabilize its active conformation (99,100). Subsequently, active CD11b induces phosphorylation of kinases Src and Syk, which further phosphorylate MyD88 and TRIF leading to their ubiquitin-mediated degradation via Cbl-b (21). Consequently, there is reduction in activation of transcription factor NFκB, which correlates with reduced production of IL-6 and other pro-inflammatory proteins. Macrophages deficient in CD11b have reduced TLR-induced degradation of MyD88 and TRIF resulting in enhanced activation of NFκB and other TLR-dependent pathways and inflammatory cytokine production (21,27). This suggests that CD11b suppresses pro-inflammatory signaling pathways downstream of TLR stimulation, that this break on inflammatory signaling is missing in the CD11b−/− cells, and that defective regulation of this pathway underlies LN and SLE pathobiology. CD11b also helps maintain autoreactivity by negatively regulating BCR signaling (101), T-cell activation (34), Th17 cell development (83) and DC maturation. Collectively, functional CD11b is more than a pro-inflammatory receptor and likely maintains homeostasis and tolerance by modulating pro-inflammatory pathways. Moreover, recent studies also suggest that CD11b also engages specific cell surface expressed ligands on the same cells, in cis, thereby regulating biological functions of such ligands. These include ICAM-1, FcγRIIA, and SIRPα, suggesting the multiple additional mechanisms CD11b uses to limit pro-inflammatory signaling by other proteins and ligands (100,102,103).
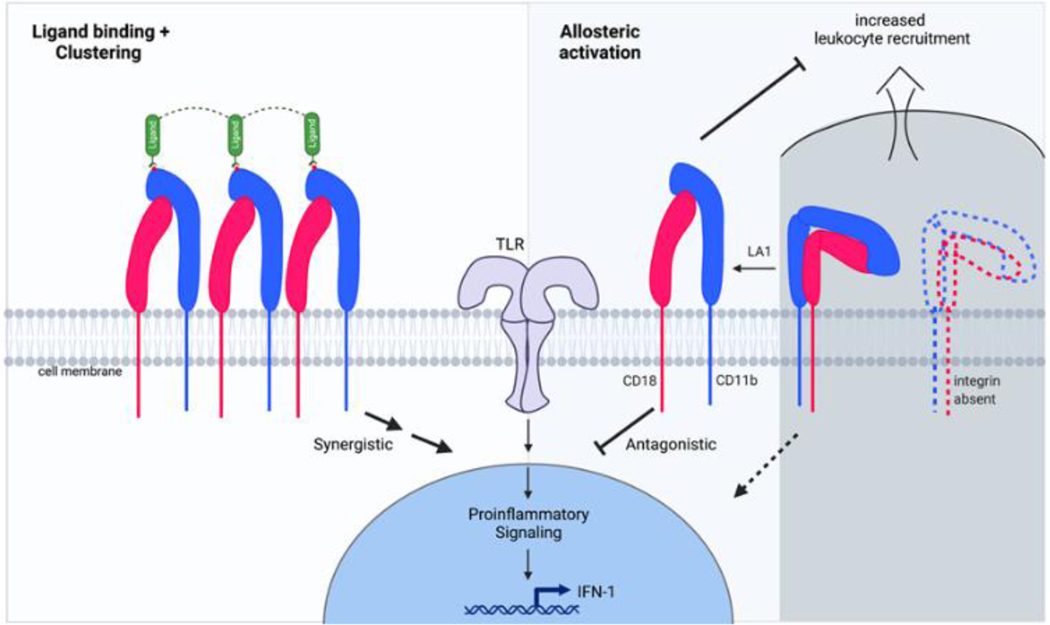
Figure 3. TLR-dependent signaling pathways are modulated by allosteric activation of CD11b.
The conventional TLR-dependent signaling is mediated via membrane recruited MyD88/TRIF proteins, that result in phosphorylation and nuclear import for NFκB complex, thereby transcriptionally upregulating pro-inflammatory molecules, such as IL-6, IL-1β and TNFα. A second arm of TLR-dependent signaling results in AKT-dependent phosphorylation and degradation of FOXO3, that results in de-repression of IFN I pathway, resulting in transcriptional upregulation of IFN I. Novel small molecule allosteric agonists, such as LA1, activate CD11b that results in recruitment of kinases Src and Syk, leading to phosphorylation and subsequent degradation of MyD88/TRIF downstream of TLRs. This leads to suppression of p65 phosphorylation and reduced nuclear translocation of NFκB, suppressing generation of pro-inflammatory cytokines IL-6, IL-1α and TNFα. Allosteric activation of CD11b also suppresses AKT phosphorylation, decreasing pFOXO3 levels, that allows import of FOXO3 to repress IRF3/IRF7 mediated expression of IFNα/β.
Conversely, CD11b can also positively induce pro-inflammatory signaling (87) (Figure 4). CD11b/CD18 engagement with soluble or immobilized ligands, such as fibrinogen and ICAM-1, induces integrin clustering that activates pro-inflammatory NFκB signaling, delays neutrophil apoptosis, releases proteolytic granules and induces cytotoxic activity (104–110). Ligation of CD11b/CD18 with ligands, such as fibrinogen, and clustering also induces increased production of pro-inflammatory cytokines, superoxide and proteases in neutrophils that result in increased tissue damage (111–115), although studies have also shown that high avidity ligation of integrins produced rapid but transient expression of inflammatory cytokines IL6 and TNFα, and that longer term engagement resulted in a suppression of pro-inflammatory pathways (116). CD11b can similarly positively regulate TLR signaling in DCs (117). Studies have also shown that integrins cluster only upon engagement with multimeric or immobilized ligands and that integrin conformational change per se, such as with an activating mutation I316G in the αA-domain, that converts it into a ligand-competent conformation, does not lead to induction of the pro-inflammatory pathways or the reduced neutrophil apoptosis as is observed with ligand engaged, clustered integrins (118,119). Collectively, these studies suggest that CD11b has a regulatory role in controlling run-away TLR signaling, that absence of CD11b fails to suppress the TLR-signaling pathways and that although integrin engagement with high avidity ligands is synergistic with inflammatory pathways and is pro-inflammatory, integrin conformational change to a ligand-competent conformation alone does not induce pro-inflammatory signaling (Figure 4).
Figure 4. The many roles of CD11b in promoting or suppressing inflammatory pathways.
Ligand binding and clustering of CD11b/CD18 integrins on cell surface typically result in enhancing TLR-dependent pro-inflammatory signals and are involved in pathogen clearance, oxidative burst and other immune surveillance functions of the innate immune cells (left half). Absence of CD11b results in loss of this integrin on cell surface, that also promotes pro-inflammatory pathways, increased tissue recruitment of leukocytes, reduced ability to clear pathogens, yet increased expression of pro-inflammatory mediators, such as IL-6, IL-1βand TNFα, due to over-active TLR-dependent signaling pathways in CD11b−/− cells. Conversely, the presence of CD11b limits TLR-pathways and its allosteric activation, either pharmacologically or genetically, significantly dampens the exuberant TLR-dependent pro-inflammatory pathways in leukocytes, reduces leukocyte infiltration and tissue damage. Such activation is also able to rescue some of the functional deficits in cells carrying LN associated ITGAM SNPs, suggesting allosteric agonism of integrins as a novel therapeutic strategy.
Recent studies with the LN associated three ITGAM coding region SNPs (rs1143679 (R77H), rs1143678 (P1146S), and rs1143683 (A858V)) describe the effects of mutation-induced functional deficiency in the integrin receptor. CD11bR77H mutant expressing cells display a reduced ability to bind to ligands, including ICAM1 and iC3b (61,120,121). Similarly, firm adhesion of CD11b expressing neutrophils was significantly reduced in cells from individuals carrying any of the three ITGAM SNPs (51). Neutrophils from donors carrying any of the three ITGAM variant alleles showed significantly reduced phagocytosis (51,121,122) and that the effect for pronounced even in cells heterozygous for the mutant allele, suggesting the importance of functionally competent integrin (51,61,121,122). Furthermore, monocytes carrying the R77H substitution failed to suppress the expression of pro-inflammatory mediators IL-6, TNF-α, and IL-1β, suggesting that the mutation impaired the ability of expressed CD11b to restrain the TLR-mediated NFκB pathways (95,120,121). The regulatory effect of CD11b on BCR signaling was also significantly diminished in the cells carrying the R77H mutation (101). Finally, we recently showed that IFN-I serum activity is significantly increased in SLE patients carrying these three ITGAM SNPs (27). Given that IFN-I is transcriptionally induced via activation of transcription factors IRF3 and IRF7, downstream of TLR stimulation, where AKT phosphorylates repressor FOXO3 to mark it for degradation, freeing up IRF3/7 mediated transcription of IFN I (123,124) (Figure 3). Donor cells carrying the above three ITGAM SNPs showed increased IRF3/7 and decreased FOXO3 in the nucleus, and consequently, increased levels of IFN I, due to the mutant CD11b having reduced functionality that failed to suppress TLR-dependent pro-inflammatory signaling (27).
Together, these studies firmly establish that integrin CD11b plays a major role in modulating exuberant pro-inflammatory signaling pathways. The genetic deletion or functional deficits in CD11b, associated with the LN linked mutations, fail to suppress the overactive signaling pathways, indicating that restoration of CD11b function could be a therapeutic opportunity in LN.
Allosteric activation of CD11b is a novel therapeutic paradigm in LN
Recent publications establish that CD11b plays a largely protective role in regulating systemic inflammation and autoimmunity in SLE and LN. Thus, agents that would promote the anti-inflammatory functions of CD11b without impacting its ligand binding and other biological functions, allosteric agonists of CD11b, have the potential to further strengthen the immune-protective activities of CD11b. Towards that, we and others utilized novel small molecule allosteric agonists of CD11b, such as molecule LA1, that we previously discovered (41) to determine their applicability in controlling pro-inflammatory signaling in cells and inflammation and tissue damage in vivo (41,125,126). LA1 does not block the ligand binding pocket of CD11b and promotes CD11b-dependent cell adhesion and reduces cell migration, although, like the activating I316G mutation, does not induce clustering (41,127). LA1 binding to CD11b in human monocytes and macrophages down-regulated TLR7/8-dependent pro-inflammatory signaling (125), including TLR-dependent NFκB signaling and generation of proinflammatory mediators IL-1β, and IL-6 (27). CD11b activation via LA1 also suppressed TLR dependent IRF3/7 pathway by repressing the phosphorylation mediated degradation of FOXO3 leading to significant reductions in IFN I production. In two different in vivo models of LN, CD11b activation with LA1 significantly reduced levels of pro-inflammatory mediators in circulation, renal IC deposition, kidney infiltration of activated leukocytes and significantly decreased glomerular injury and proteinuria (27,37). Importantly, in vivo effects of LA1 were dependent on CD11b and were not observed in CD11b−/− animals. Studies also showed that TLR-stimulated increased expression of pro-inflammatory IL1β, IL6 and IFN I in donor cells carrying the above three ITGAM SNPs was significantly reduced by treatment with the CD11b agonist. Similar studies with other β2 integrin agonist antibodies showed that integrin activation can rescue functional deficits in cells carrying the LN ITGAM mutations (61).
To further confirm this mechanism using an orthogonal system, we recently generated a novel knock-in model of constitutive active CD11b in mice (39). Here, the knock-in mice constitutively express the I332G mutation in the activation-sensitive allosteric pocket of ligand-binding αA-domain of CD11b that promotes high-affinity conformation of the integrin, mimicking binding of the allosteric agonist LA1. CD11bI332G knock-in mice showed significant reduction in recruitment of neutrophils and macrophages upon induction of acute inflammation. The mutation also protected animals against development of hyperlipidemia induced atherosclerosis, suggesting that allosteric activation of CD11b is protective in the setting of inflammatory diseases. Future studies will test its role in the setting of SLE and LN.
Collectively, the studies with pharmacologic or genetic activation of CD11b show that allosteric activation of CD11b can suppress the dysfunctional TLR-dependent pro-inflammatory pathways in primary leukocytes and in vivo, reduce proteinuria and protect kidney from injury, suggesting it to be a highly promising novel therapeutic strategy for LN.d
CD11b agonism is therapeutic in other inflammatory diseases
Leukocytes extravasate from circulation and into infected, injured or inflamed tissues to better control the damage and help heal the tissues (128–130). However, excessive leukocyte infiltration results in increased tissue damage, reduced healing and fibrotic scarring. Similarly, tumors recruit leukocytes from circulation and an increased abundance of CD11b+ leukocytes, such as tumor associated macrophages (TAMs), in the tumor microenvironment correlates with poorer outcomes in multiple types of cancer (131–133). As CD11b/CD18 dimer is a key adhesion receptor that mediates such leukocyte trafficking, it has been extensively targeted as a therapeutic strategy in experimental models of inflammation and cancer. Antibodies that block CD11b or its various ligands, thus interfering with leukocyte adhesion, blocked leukocyte influx in models of acute injury, chronic injury and cancer, and reduced disease (134–140).
Counterintuitively, although CD11b agonists transiently increase cell adhesion it also reduces leukocyte extravasation in a CD11b-dependent fashion (41). Administration of CD11b agonists into models of acute inflammation, chronic injury and transplantation showed significant reduction in recruitment of CD11b+ leukocytes, resulting in significant disease amelioration (41,53,126,127,141–143)
Similarly, administration of CD11b agonists in experimental models of breast cancer, lung cancer, melanoma and pancreatic cancer showed a significant reduction in the infiltration of CD11b+ cells into tumor microenvironment, and a significant suppression of tumor growth (38,144,145). Similarly, CD11b KI mice show significant reduction in tumor growth (38). Collectively, these data confirm the validity of CD11b as a therapeutic target in inflammatory diseases and cancer, and suggest CD11b agonism as a promising therapeutic strategy. Indeed, a novel CD11b agonist, GB1275, is currently completing Phase I clinical trials in oncology (146– 148).
Conclusions
SNPs in ITGAM are associated with risk for SLE and LN in approx. 20% of SLE and LN patients and are also associated with increased IFN I levels in patients. Recent studies have shown that integrin CD11b plays a vital role in modulating pro-inflammatory TLR-signaling pathways in leukocytes. Studies have also shown that the coding ITGAM SNPs associated with SLE and LN produce a defective integrin that shows reduced biological functions in leukocytes, including reduced binding to known ligands, reduced cell adhesion, phagocytosis, and catch-bond formation, and inability to reduce pro-inflammatory cytokine production downstream of TLR-pathways, suggesting that exogenous activation of CD11b could be a potential therapeutic strategy. Indeed, allosteric activation of CD11b, via pharmacologic or genetic approaches, results in suppression of TLR-dependent inflammatory pathways in leukocytes, including production of IFN I, as well as rescues the functional deficits in human donor cells expressing the mutant integrin. These studies show that integrin CD11b is an important therapeutic target in SLE and LN and firmly establish allosteric activation of CD11b as a novel, key therapeutic paradigm for effectively treating such autoimmune diseases.
Acknowledgements
We thank members of the Gupta laboratory for helpful discussions. This work was supported in part by grants from the NIH (R01DK084195 and R01CA244938 to VG), the BearsCare, the Aligning Science Across Parkinson’s (ASAP) Initiative of The Michael J. Fox Foundation (MJFF) (ASAP-000523) and with resources from the Rush University Medical Center. All authors have read the COI disclosure policy for Translational Research and the authorship agreement.
Abbreviations
- SLE
Systemic Lupus Eryethmatosus
- LN
Lupus Nephritis
- ITGAM
Integrin Alpha
- IFN
Interferon
- TLR
Toll-like Receptor
Footnotes
Financial
VG is an inventor of issued and pending patents related to CD11b agonists and has the potential for financial benefit from their future commercialization. VG is also a co-founder of and holds equity interests in Adhaere Pharmaceuticals, Inc (now part of Gossamer Bio, Inc) and 149 Bio, LLC.
Conflicts of Interest
The authors have no additional financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Practice and Research: Clinical Rheumatology.2017;31(3):306–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ugarte-Gil MF, González LA, Alarcón GS. Lupus: the new epidemic. Lupus.2019;28(9):1031–50. [DOI] [PubMed] [Google Scholar]
- 3.Mu Q, Zhang H, Luo XM. SLE: Another autoimmune disorder influenced by microbes and diet? Frontiers in Immunology. 2015;6:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maidhof W, Hilas O. Lupus: An Overview of the Disease And Management Options. 2012;37(4):240–9 [PMC free article] [PubMed] [Google Scholar]
- 5.Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clinical Journal of the American Society of Nephrology. 2017;12(5):825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsokos GC. Systemic Lupus Erythematosus. New England Journal of Medicine. 2011. Dec;365(22):2110–21. [DOI] [PubMed] [Google Scholar]
- 7.Harley JB, Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nature Genetics. 2008. Feb;40(2):204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uramoto KM, Michet CJ Jr., Thumboo J, Sunku J, O’Fallon WM, Gabriel SE Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis & Rheumatism. 1999. Jan;42(1):46–50. [DOI] [PubMed] [Google Scholar]
- 9.Anders HJ, Saxena R, Zhao M, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nature Reviews Disease Primers. 2020. Jan 23;6(1):7. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MM. The Pathology of Lupus Nephritis. Seminars in Nephrology. 2007. Jan;27(1):22–34. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald KA, Kagan JC. Toll-like Receptors and the Control of Immunity. Cell. 2020. Mar;180(6):1044–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson A. Renal Mononuclear Phagocytes in Lupus Nephritis. ACR Open Rheumatology. 2021. Jul;3(7):442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrivastav M, Niewold TB. Nucleic Acid Sensors and Type I Interferon Production in Systemic Lupus Erythematosus. Frontiers in Immunology. 2013;4:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruschi M, Bonanni A, Petretto A, Vaglio A, Pratesi F, Santucci L, et al. Neutrophil extracellular traps profiles in patients with incident systemic lupus erythematosus and lupus nephritis. Journal of Rheumatology. 2020. Mar 1;47(3):377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lech M, Anders H-J. The Pathogenesis of Lupus Nephritis. Journal of the American Society of Nephrology. 2013. Sep;24(9):1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting Neutrophils Induce Endothelial Damage, Infiltrate Tissues, and Expose Immunostimulatory Molecules in Systemic Lupus Erythematosus. The Journal of Immunology. 2011. Jul 1;187(1):538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune Interferon in the Circulation of Patients with Autoimmune Disease. New England Journal of Medicine. 1979. Jul 5;301(1):5–8. [DOI] [PubMed] [Google Scholar]
- 18.Kirou KA, Lee C, George S, Louca K, Peterson MGE, Crow MK. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis & Rheumatism. 2005. May;52(5):1491–503. [DOI] [PubMed] [Google Scholar]
- 19.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes & Immunity. 2007. Sep 21;8(6):492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kariuki SN, Franek BS, Kumar AA, Arrington J, Mikolaitis RA, Utset TO, et al. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Research & Therapy. 2010;12(4):R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nature Immunology. 2010. Aug;11(8):734–42. [DOI] [PubMed] [Google Scholar]
- 22.Crow YJ, Stetson DB. The type I interferonopathies: 10 years on. Nature Reviews Immunology. 2021. Oct 20;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellano G, Cafiero C, Divella C, Sallustio F, Gigante M, Pontrelli P, et al. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Research & Therapy. 2015. Dec 22;17(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairhurst A, Mathian A, Connolly JE, Wang A, Gray HF, George TA, et al. Systemic IFN-α drives kidney nephritis in B6. Sle123 mice. European Journal of Immunology. 2008. Jul;38(7):1948–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairhurst A-M, Xie C, Fu Y, Wang A, Boudreaux C, Zhou XJ, et al. Type I Interferons Produced by Resident Renal Cells May Promote End-Organ Disease in Autoantibody-Mediated Glomerulonephritis. The Journal of Immunology. 2009. Nov 15;183(10):6831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences. 2003. Mar 4;100(5):2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faridi MH, Khan SQ, Zhao W, Lee HW, Altintas MM, Zhang K, et al. CD11b activation suppresses TLR-dependent inflammation and autoimmunity in systemic lupus erythematosus. Journal of Clinical Investigation. 2017. Apr 3;127(4):1271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catalina MD, Bachali P, Geraci NS, Grammer AC, Lipsky PE. Gene expression analysis delineates the potential roles of multiple interferons in systemic lupus erythematosus. Communications Biology. 2019. 23;2:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLRindependent pathways of type I interferon induction in systemic autoimmunity. Nature Medicine. 2007. May 3;13(5):543–51. [DOI] [PubMed] [Google Scholar]
- 30.Kontaki E, Boumpas DT. Innate immunity in systemic lupus erythematosus: Sensing endogenous nucleic acids. Journal of Autoimmunity. 2010. Nov;35(3):206–11. [DOI] [PubMed] [Google Scholar]
- 31.Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. New England Journal of Medicine. 2020. Jan 16;382(3):211–21. [DOI] [PubMed] [Google Scholar]
- 32.Burki TK. FDA approval for anifrolumab in patients with lupus. The Lancet Rheumatology. 2021. Oct;3(10):e689. [Google Scholar]
- 33.Bachmann M, Kukkurainen S, Hytönen VP, Wehrle-Haller B. Cell Adhesion by Integrins. Physiological Reviews. 2019. Oct 1;99(4):1655–99. [DOI] [PubMed] [Google Scholar]
- 34.McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. Journal of immunology. 1992. Aug 15;149(4):1326–33. [PubMed] [Google Scholar]
- 35.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proceedings of the National Academy of Sciences. 1992. Apr 15;89(8):3320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hynes R. Integrins: A family of cell surface receptors. Cell. 1987. Feb;48(4):549–54. [DOI] [PubMed] [Google Scholar]
- 37.Springer T, Galfré G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. European Journal of Immunology. 1979. Apr;9(4):301–6. [DOI] [PubMed] [Google Scholar]
- 38.Geraghty T, Rajagopalan A, Aslam R, Pohlman A, Venkatesh I, Zloza A, et al. Positive Allosteric Modulation of CD11b as a Novel Therapeutic Strategy Against Lung Cancer. Frontiers in Oncology. 2020. May 21;10:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez L, Li X, Ramos-Echazabal G, Faridi H, Zigmond ZM, Santos Falcon N, et al. A Genetic Model of Constitutively Active Integrin CD11b/CD18. The Journal of Immunology. 2020. Nov 1;205(9):2545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagerholm SC, Macpherson M, James MJ, Sevier-Guy C, Lau CS. The CD11b-integrin (ITGAM) and systemic lupus erythematosus. Lupus. 2013;22(7):657–63. [DOI] [PubMed] [Google Scholar]
- 41.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, et al. Small moleculemediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Science Signaling. 2011. Sep 6;4(189):ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. Activation of Leukocyte β2 Integrins by Conversion from Bent to Extended Conformations. Immunity. 2006. Oct;25(4):583–94. [DOI] [PubMed] [Google Scholar]
- 43.Sen M, Springer TA. Leukocyte integrin αL β2 headpiece structures: The αI domain, the pocket for the internal ligand, and concerted movements of its loops. Proceedings of the National Academy of Sciences. 2016. Mar 15;113(11):2940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamers C, Plüss CJ, Ricklin D. The Promiscuous Profile of Complement Receptor 3 in Ligand Binding, Immune Modulation, and Pathophysiology. Frontiers in Immunology. 2021. Apr; 12:662164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li R, Rieu P, Griffith DL, Scott D, Amin Arnaout M. Two Functional States of the CD11b A-Domain: Correlations with Key Features of Two Mn 2-complexed Crystal Structures The Journal of Cell Biology. 1998. Dec;143(6):1523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahalingam B, Ajroud K, Alonso JL, Anand S, Adair BD, Horenstein AL, et al. Stable Coordination of the Inhibitory Ca 2+ Ion at the Metal Ion-Dependent Adhesion Site in Integrin CD11b/CD18 by an Antibody-Derived Ligand Aspartate: Implications for Integrin Regulation and Structure-Based Drug Design. The Journal of Immunology. 2011. Dec 15;187(12):6393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J-O, Bankston LA, Liddington R, Arnaout A. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995. Dec;3(12):1333–40. [DOI] [PubMed] [Google Scholar]
- 48.Morgan J, Saleem M, Ng R, Armstrong C, Wong SS, Caulton SG, et al. Structural basis of the leukocyte integrin Mac-1 I-domain interactions with the platelet glycoprotein Ib. Blood Advances. 2019. May 14;3(9):1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nature Immunology. 2020. Jun 4;21(6):605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ponticelli C. New therapies for lupus nephritis. Clinical journal of the American Society of Nephrology. 2006. Jul;1(4):863–8. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y, Wu J, Kucik DF, White NB, Redden DT, Szalai AJ, et al. Multiple lupusassociated ITGAM variants alter mac-1 functions on neutrophils. Arthritis and Rheumatism. 2013. Nov;65(11):2907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nath SK, Han S, Kim-Howard X, Kelly JA, Viswanathan P, Gilkeson GS, et al. A nonsynonymous functional variant in integrin-αM (encoded by ITGAM) is associated with systemic lupus erythematosus. Nature Genetics. 2008. Feb 20;40(2):152–4. [DOI] [PubMed] [Google Scholar]
- 53.Khan SQ, Khan I, Gupta V. CD11b activity modulates pathogenesis of lupus nephritis. Frontiers in Medicine. 2018. Mar; 5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W, Zhao M, Hirankarn N, Lau CS, Mok CC, Chan TM, et al. ITGAM is associated with disease susceptibility and renal nephritis of systemic lupus erythematosus in Hong Kong Chinese and Thai. Human Molecular Genetics. 2009;18(11):2063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, et al. Association of Systemic Lupus Erythematosus with C8orf13–BLK and ITGAM–ITGAX. New England Journal of Medicine. 2008. Feb 28;358(9):900–9. [DOI] [PubMed] [Google Scholar]
- 56.Han S, Kim-Howard X, Deshmukh H, Kamatani Y, Viswanathan P, Guthridge JM, et al. Evaluation of imputation-based association in and around the integrin-α-M (ITGAM) gene and replication of robust association between a non-synonymous functional variant within ITGAM and systemic lupus erythematosus (SLE). Human Molecular Genetics. 2009;18(6):1171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim-Howard X, Maiti AK, Anaya J-M, Bruner GR, Brown E, Merrill JT, et al. ITGAM coding variant (rs1143679) influences the risk of renal disease, discoid rash and immunological manifestations in patients with systemic lupus erythematosus with European ancestry. Annals of the Rheumatic Diseases. 2010. Jul 1;69(7):1329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munoz LE, van Bavel C, Franz S, Berden J, Herrmann M, van der Vlag J. Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus. 2008; 17(5):371–5 [DOI] [PubMed] [Google Scholar]
- 59.Toong C, Adelstein S, Phan TG. Clearing the complexity: Immune complexes and their treatment in lupus nephritis. International Journal of Nephrology and Renovascular Disease. 2011;4;17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harbor Perspectives in Biology. 2011. Mar;3(3):a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosetti F, Chen Y, Sen M, Thayer E, Azcutia V, Herter JM, et al. A Lupus-Associated Mac-1 Variant Has Defects in Integrin Allostery and Interaction with Ligands under Force. Cell Reports. 2015. Mar 17;10(10):1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnaout MA. Biology and structure of leukocyte β2 integrins and their role in inflammation. F1000Research. 2016. Oct 4;5:2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Springer T, Galfré G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. European Journal of Immunology. 1979. Apr;9(4):301–6. [DOI] [PubMed] [Google Scholar]
- 64.Anderson DC, Miller LJ, Schmalstieg FC, Rothlein R, Springer TA. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. Journal of immunology. 1986. Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- 65.Hynes R. Integrins: A family of cell surface receptors. Cell. 1987. Feb;48(4):549–54. [DOI] [PubMed] [Google Scholar]
- 66.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proceedings of the National Academy of Sciences. 1992. Apr 15;89(8):3320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plow EF, Zhang L. A MAC-1 attack: integrin functions directly challenged in knockout mice. Journal of Clinical Investigation. 1997. Mar 15;99(6):1145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. Journal of immunology. 1992. Aug 15;149(4):1326–33. [PubMed] [Google Scholar]
- 69.Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia-Aguilar J, Hibbs ML, et al. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). Journal of Cell Biology. 1990. Dec 1;111(6):3129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gahmberg CG. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Current Opinion in Cell Biology. 1997. Oct;9(5):643–50. [DOI] [PubMed] [Google Scholar]
- 71.Beller DI, Springer TA, Schreiber RD. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. Journal of Experimental Medicine. 1982. Oct 1;156(4):1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altieri DC, Bader R, Mannucci PM, Edgington TS. Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. Journal of Cell Biology. 1988. Nov 1;107(5):1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Podolnikova NP, Podolnikov A v., Haas TA, Lishko VK, Ugarova TP Ligand recognition specificity of leukocyte integrin αmβ2 (Mac-1, CD11b/CD18) and its functional consequences. Biochemistry. 2015. Feb 17;54(6):1408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosetti F, Mayadas TN. The many faces of Mac-1 in autoimmune disease. Immunological reviews. 2016. Jan;269(1):175–93. [DOI] [PubMed] [Google Scholar]
- 75.Wang L, Gordon RA, Huynh L, Su X, Min K-HP, Han J, et al. Indirect Inhibition of Toll-like Receptor and Type I Interferon Responses by ITAM-Coupled Receptors and Integrins. Immunity. 2010. Apr;32(4):518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dana N, Todd RF, Pitt J, Springer TA, Arnaout MA. Deficiency of a surface membrane glycoprotein (Mo1) in man. The Journal of clinical investigation. 1984. Jan;73(1):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arnaout MA, Pitt J, Cohen HJ, Melamed J, Rosen FS, Colten HR. Deficiency of a Granulocyte-Membrane Glycoprotein (gp150) in a Boy with Recurrent Bacterial Infections. New England Journal of Medicine. 1982. Mar 25;306(12):693–9. [DOI] [PubMed] [Google Scholar]
- 78.Springer T, Galfré G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. European Journal of Immunology. 1979. Apr;9(4):301–6. [DOI] [PubMed] [Google Scholar]
- 79.Springer TA, Thompson WS, Miller LJ, Schmalstieg FC, Anderson DC. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. Journal of Experimental Medicine. 1984. Dec 1;160(6):1901–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, et al. A Novel Role for the β2 Integrin CD11b/CD18 in Neutrophil Apoptosis: A Homeostatic Mechanism in Inflammation. Immunity. 1996. Dec;5(6):653–66. [DOI] [PubMed] [Google Scholar]
- 81.Alexander JJ, Chaves LD, Chang A, Jacob A, Ritchie M, Quigg RJ. CD11b is protective in complement-mediated immune complex glomerulonephritis. Kidney International. 2015. May;87(5):930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosetti F, Tsuboi N, Chen K, Nishi H, Ernandez T, Sethi S, et al. Human Lupus Serum Induces Neutrophil-Mediated Organ Damage in Mice That Is Enabled by Mac-1 Deficiency. The Journal of Immunology. 2012. Oct 1;189(7):3714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ehirchiou D, Xiong Y, Xu G, Chen W, Shi Y, Zhang L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. Journal of Experimental Medicine. 2007. Jul 9;204(7):1519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kevil CG, Hicks MJ, He X, Zhang J, Ballantyne CM, Raman C, et al. Loss of LFA-1, but not Mac-1, Protects MRL/MpJ-Faslpr Mice from Autoimmune Disease. The American Journal of Pathology. 2004. Aug;165(2):609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaves LD, Bao L, Wang Y, Chang A, Haas M, Quigg RJ. Loss of CD11b Exacerbates Murine Complement-Mediated Tubulointerstitial Nephritis. PLoS ONE. 2014. Mar 14;9(3):e92051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou M, Wang X, Shi Y, Ding Y, Li X, Xie T, et al. Deficiency of ITGAM Attenuates Experimental Abdominal Aortic Aneurysm in Mice. Journal of the American Heart Association. 2021. Apr 6;10(7):e019900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmid MC, Khan SQ, Kaneda MM, Pathria P, Shepard R, Louis TL, et al. Integrin CD11b activation drives anti-tumor innate immunity. Nature Communications. 2018. Dec 19;9(1):5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimizu K, Libby P, Shubiki R, Sakuma M, Wang Y, Asano K, et al. Leukocyte Integrin Mac-1 Promotes Acute Cardiac Allograft Rejection. Circulation. 2008. Apr 15;117(15):1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou H, Li Y, Gui H, Zhao H, Wu M, Li G, et al. Antagonism of Integrin CD11b Affords Protection against Endotoxin Shock and Polymicrobial Sepsis via Attenuation of HMGB1 Nucleocytoplasmic Translocation and Extracellular Release. The Journal of Immunology. 2018. Jan 17;200(5):1771–80. [DOI] [PubMed] [Google Scholar]
- 90.Tang T, Rosenkranz A, Assmann KJ, Goodman MJ, Gutierrez-Ramos J-C, Carroll MC, et al. A Role for Mac-1 (CDIIb/CD18) in Immune Complex–stimulated Neutrophil Function In Vivo: Mac-1 Deficiency Abrogates Sustained Fcγ Receptor–dependent Neutrophil Adhesion and Complement-dependent Proteinuria in Acute Glomerulonephritis. Journal of Experimental Medicine. 1997. Dec 1;186(11):1853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahn G-O, Tseng D, Liao C-H, Dorie MJ, Czechowicz A, Brown JM . Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proceedings of the National Academy of Sciences. 2010. May 4;107(18):8363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fitzgerald KA, Kagan JC. Toll-like Receptors and the Control of Immunity. Cell. 2020. Mar;180(6):1044–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piccinini AM, Midwood KS. DAMPening Inflammation by Modulating TLR Signalling. Mediators of Inflammation. 2010;2010:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Means TK, Luster AD. Integrins limit the Toll. Nature Immunology. 2010. Aug;11(8):691–3. [DOI] [PubMed] [Google Scholar]
- 95.Roberts AL, Fürnrohr BG, Vyse TJ, Rhodes B. The complement receptor 3 (CD11b/CD18) agonist Leukadherin-1 suppresses human innate inflammatory signalling. Clinical and Experimental Immunology. 2016. Aug 19;185(3):361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hynes RO. Integrins: bidirecional, allosteric signaling machines. Cell. 2002. Sep;110(6):673–87. [DOI] [PubMed] [Google Scholar]
- 97.Ginsberg MH, Du X, Plow EF. Inside-out integrin signalling. Current Opinion in Cell Biology. 1992. Oct;4(5):766–71. [DOI] [PubMed] [Google Scholar]
- 98.Altieri DC, Edgington TS. The saturable high affinity association of factor X to ADPstimulated monocytes defines a novel function of the Mac-1 receptor. The Journal of biological chemistry. 1988. May 25;263(15):7007–15. [PubMed] [Google Scholar]
- 99.Lagarrigue F, Kim C, Ginsberg MH. The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood. 2016. Jul 28;128(4):479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nolte MA, Margadant C. Activation and suppression of hematopoietic integrins in hemostasis and immunity. Blood. 2020. Jan 2;135(1):7–16. [DOI] [PubMed] [Google Scholar]
- 101.Ding C, Ma Y, Chen X, Liu M, Cai Y, Hu X, et al. Integrin CD11b negatively regulates BCR signalling to maintain autoreactive B cell tolerance. Nature Communications. 2013. Dec 22;4(1):2813. [DOI] [PubMed] [Google Scholar]
- 102.Morrissey MA, Kern N, Vale RD. CD47 Ligation Repositions the Inhibitory Receptor SIRPA to Suppress Integrin Activation and Phagocytosis. Immunity. 2020. Aug;53(2):290302.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fan Z, McArdle S, Marki A, Mikulski Z, Gutierrez E, Engelhardt B, et al. Neutrophil recruitment limited by high-affinity bent β2 integrin binding ligand in cis. Nature Communications. 2016. Nov 31;7(1):12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential Roles for M 2 Integrin Clustering or Activation in the Control of Apoptosis Via Regulation of Akt and ERK Survival Mechanisms. The Journal of Cell Biology. 2000. Dec 11;151(6):1305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Watson RW, Rotstein OD, Nathens AB, Parodo J, Marshall JC. Neutrophil apoptosis is modulated by endothelial transmigration and adhesion molecule engagement. Journal of immunology. 1997. Jan 15;158(2):945–53. [PubMed] [Google Scholar]
- 106.Rubel C, Gómez S, Fernández GC, Isturiz MA, Caamaño J, Palermo MS. FibrinogenCD11b/CD18 interaction activates the NF-κB pathway and delays apoptosis in human neutrophils. European Journal of Immunology. 2003. May;33(5):1429–38. [DOI] [PubMed] [Google Scholar]
- 107.Pluskota E, Soloviev DA, Szpak D, Weber C, Plow EF. Neutrophil Apoptosis: Selective Regulation by Different Ligands of Integrin α M β 2. The Journal of Immunology. 2008. Sep 1;181(5):3609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mócsai A, Ligeti E, Lowell CA, Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. Journal of immunology. 1999. Jan 15;162(2):1120–6. [PubMed] [Google Scholar]
- 109.Rubel C, Fernández GC, Dran G, Bompadre MB, Isturiz MA, Palermo MS. Fibrinogen Promotes Neutrophil Activation and Delays Apoptosis. The Journal of Immunology. 2001. Feb 1;166(3):2002–10. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Q, Lee W-B, Kang J-S, Kim LK, Kim Y-J. Integrin CD11b negatively regulates Mincle-induced signaling via the Lyn–SIRPα–SHP1 complex. Experimental & Molecular Medicine. 2018. Feb 5;50(2):e439–e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3bopsonized target cells. Journal of Clinical Investigation. 1996. Jul 1;98(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Au BT, Williams TJ, Collins PD. Zymosan-induced IL-8 release from human neutrophils involves activation via the CD11b/CD18 receptor and endogenous platelet-activating factor as an autocrine modulator. Journal of immunology. 1994. Jun 1;152(11):5411–9. [PubMed] [Google Scholar]
- 113.Xiong Y-M, Chen J, Zhang L. Modulation of CD11b/CD18 Adhesive Activity by Its Extracellular, Membrane-Proximal Regions. The Journal of Immunology. 2003. Jul 15;171(2):1042–50. [DOI] [PubMed] [Google Scholar]
- 114.von Asmuth EJ, van der Linden CJ, Leeuwenberg JF, Buurman WA. Involvement of the CD11b/CD18 integrin, but not of the endothelial cell adhesion molecules ELAM-1 and ICAM-1 in tumor necrosis factor-alpha-induced neutrophil toxicity. Journal of immunology. 1991. Dec 1;147(11):3869–75. [PubMed] [Google Scholar]
- 115.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB journal. 1994. May;8(8):504–12. [PubMed] [Google Scholar]
- 116.Wang L, Gordon RA, Huynh L, Su X, Park Min KH, Han J, et al. Indirect Inhibition of Toll-like Receptor and Type I Interferon Responses by ITAM-Coupled Receptors and Integrins. Immunity. 2010. Apr;32(4):518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ling GS, Bennett J, Woollard KJ, Szajna M, Fossati-Jimack L, Taylor PR, et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nature Communications. 2014. May 15;5(1):3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim M, Carman CV., Yang W, Salas A, Springer TA The primacy of affinity over clustering in regulation of adhesiveness of the integrin αLβ2. Journal of Cell Biology. 2004. Dec 20;167(6):1241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pluskota E, Soloviev DA, Szpak D, Weber C, Plow EF. Neutrophil Apoptosis: Selective Regulation by Different Ligands of Integrin αM β2. The Journal of Immunology. 2008. Sep 1;181(5):3609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.MacPherson M, Lek HS, Prescott A, Fagerholm SC. A Systemic Lupus Erythematosus-associated R77H Substitution in the CD11b Chain of the Mac-1 Integrin Compromises Leukocyte Adhesion and Phagocytosis. Journal of Biological Chemistry. 2011. May;286(19):17303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rhodes B, Fürnrohr BG, Roberts AL, Tzircotis G, Schett G, Spector TD, et al. The rs1143679 (R77H) lupus associated variant of ITGAM (CD11b) impairs complement receptor 3 mediated functions in human monocytes. Annals of the Rheumatic Diseases. 2012. Dec;71(12):2028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fossati-Jimack L, Ling GS, Cortini A, Szajna M, Malik TH, McDonald JU, et al. Phagocytosis Is the Main CR3-Mediated Function Affected by the Lupus-Associated Variant of CD11b in Human Myeloid Cells. PLoS ONE. 2013. Feb 22;8(2):e57082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Litvak V, Ratushny AV., Lampano AE, Schmitz F, Huang AC, Raman A, et al. A FOXO3–IRF7 gene regulatory circuit limits inflammatory sequelae of antiviral responses. Nature. 2012. Oct 16;490(7420):421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin R, Heylbroeck C, Pitha PM, Hiscott J. Virus-Dependent Phosphorylation of the IRF-3 Transcription Factor Regulates Nuclear Translocation, Transactivation Potential, and Proteasome-Mediated Degradation. Molecular and Cellular Biology. 1998. May;18(5):2986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reed JH, Jain M, Lee K, Kandimalla ER, Faridi MH, Buyon JP, et al. Complement Receptor 3 Influences Toll-like Receptor 7/8-Dependent Inflammation: Implications for Autoimmune Diseases Characterixed by Antibody Reactivity to Ribonucleoproteins. Journal of Biological Chemistry. 2013. Mar;288(13):9077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roberts AL, Fürnrohr BG, Vyse TJ, Rhodes B. The complement receptor 3 (CD11b/CD18) agonist Leukadherin-1 suppresses human innate inflammatory signalling. Clinical and experimental immunology. 2016;185(3):361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Faridi MH, Altintas MM, Gomez C, Duque JC, Vazquez-Padron RI, Gupta V. Small molecule agonists of integrin CD11b/CD18 do not induce global conformational changes and are significantly better than activating antibodies in reducing vascular injury. Biochimica et Biophysica Acta - General Subjects. 2013. Jun;1830(6):3696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nourshargh S, Alon R. Leukocyte Migration into Inflamed Tissues. Immunity. 2014. Nov;41(5):694–707. [DOI] [PubMed] [Google Scholar]
- 129.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007. Sep;7(9):678–89. [DOI] [PubMed] [Google Scholar]
- 130.Fagerholm SC, Guenther C, Llort Asens M, Savinko T, Uotila LM. Beta2-Integrins and Interacting Proteins in Leukocyte Trafficking, Immune Suppression, and Immunodeficiency Disease. Frontiers in Immunology. 2019. Feb 19;10:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discovery. 2011. Jun;1(1):54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, et al. Molecular Profiling Reveals a Tumor-Promoting Phenotype of Monocytes and Macrophages in Human Cancer Progression. Immunity. 2014. Nov;41(5):815–29. [DOI] [PubMed] [Google Scholar]
- 133.Gentles AJ, Newman AM, Liu CL, Bratman S v, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nature Medicine. 2015. Aug 20;21(8):938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ahn G-O, Tseng D, Liao C-H, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proceedings of the National Academy of Sciences. 2010. May 4;107(18):8363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rogers C, Edelman ER, Simon DI. A mAb to the beta2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proceedings of the National Academy of Sciences. 1998. Aug 18;95(17):10134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simon DI, Chen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1–/– mice reveals a role for inflammation in vascular repair after angioplasty. Journal of Clinical Investigation. 2000. Feb 1;105(3):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang T, Rosenkranz A, Assmann KJM, Goodman MJ, Gutierrez-Ramos J-C, Carroll MC, et al. A Role for Mac-1 (CDIIb/CD18) in Immune Complex–stimulated Neutrophil Function In Vivo: Mac-1 Deficiency Abrogates Sustained Fcγ Receptor–dependent Neutrophil Adhesion and Complement-dependent Proteinuria in Acute Glomerulonephritis. Journal of Experimental Medicine. 1997. Dec 1;186(11):1853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y, Gao H, Shi C, Erhardt PW, Pavlovsky A, A. Soloviev D, et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nature Communications. 2017. Aug 11;8(1):15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flick MJ, Du X, Witte DP, Jiroušková M, Soloviev DA, Busuttil SJ, et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. Journal of Clinical Investigation. 2004. Jun 1;113(11):1596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wolf D, Hohmann J-D, Wiedemann A, Bledzka K, Blankenbach H, Marchini T, et al. Binding of CD40L to Mac-1’s I-Domain Involves the EQLKKSKTL Motif and Mediates Leukocyte Recruitment and Atherosclerosis—But Does Not Affect Immunity and Thrombosis in Mice. Circulation Research. 2011. Nov 11;109(11):1269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yao X, Dong G, Zhu Y, Yan F, Zhang H, Ma Q, et al. Leukadherin-1-Mediated activation of CD11b Inhibits LPS-Induced pro-inflammatory response in macrophages and protects mice against endotoxic shock by blocking LPS-TLR4 interaction. Frontiers in Immunology. 2019;10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jagarapu J, Kelchtermans J, Rong M, Chen S, Hehre D, Hummler S, et al. Efficacy of Leukadherin-1 in the Prevention of Hyperoxia-Induced Lung Injury in Neonatal Rats. American Journal of Respiratory Cell and Molecular Biology. 2015. Dec;53(6):793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ehirchiou D, Bernabei I, Chobaz V, Castelblanco M, Hügle T, So A, et al. CD11b Signaling Prevents Chondrocyte Mineralization and Attenuates the Severity of Osteoarthritis. Frontiers in Cell and Developmental Biology. 2020. Dec 18;8:611757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schmid MC, Khan SQ, Kaneda MM, Pathria P, Shepard R, Louis TL, et al. Integrin CD11b activation drives anti-tumor innate immunity. Nature Communications. 2018. Dec 19;9(1):5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, Knolhoff BL, et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Science Translational Medicine. 2019. Jul 3;11(499). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rasco DW, Bendell JC, Wang-Gillam A, Park W, O’Reilly EM, Zhou L, et al. A phase I/II study of GB1275, a first-in-class oral CD11b modulator, alone, and combined with pembrolizumab in specified advanced solid tumors or with chemotherapy in metastatic pancreatic cancer (KEYNOTE-A36). Journal of Clinical Oncology. 2020. May 20;38(15_suppl):3085–3085.32667832 [Google Scholar]
- 147.Park H, Bendell JC, Messersmith WA, Rasco DW, de Bono JS, Strickler JH, et al. Preliminary clinical and biologic results of GB1275, a first-in-class oral CD11b modulator, alone and with pembrolizumab, in advanced solid tumors (KEYNOTE A36). Journal of Clinical Oncology. 2021. May 20;39(15_suppl):2505–2505. [Google Scholar]
- 148.Wang-Gillam A, Rasco DW, Park W, O’Reilly E, Messersmith W, DeNardo DG, et al. Abstract CT247: A phase 1/2 study of GB1275, a first-in-class CD11b modulator, as monotherapy and with an anti-PD-1 antibody in specified advanced solid tumors or with chemotherapy in metastatic pancreatic cancer (KEYNOTE-A36). In: Tumor Biology. American Association for Cancer Research; 2020. p. CT247–CT247. [Google Scholar]
- 149.Vorup-Jensen T, Ostermeier C, Shimaoka M, Hommel U, Springer TA. Structure and allosteric regulation of the X 2 integrin I domain. Proceedings of the National Academy of Sciences. 2003. Feb 18;100(4):1873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xiong J-P, Li R, Essafi M, Stehle T, Arnaout MA. An Isoleucine-based Allosteric Switch Controls Affinity and Shape Shifting in Integrin CD11b A-domain. Journal of Biological Chemistry. 2000. Dec;275(49):38762–7. [DOI] [PubMed] [Google Scholar]