Significance
Here, with single-molecule fluorescence microscopy, we study the catalytic behavior of individual Pt atoms at single-turnover resolution, and then reveal the unique catalytic properties of Pt single-atom catalyst and the difference in catalytic properties between individual Pt atoms and Pt nanoparticles. Further density functional theory calculation indicates that unique catalytic properties of Pt single-atom catalyst could be attributed intrinsically to the unique surface properties of Pt1-based active sites.
Keywords: single-molecule fluorescence microscopy, single-atom catalysis, catalytic kinetics and dynamics, individual atom, surface restructuring
Abstract
Due to the importance of single-atom catalysts (SAC), here, the catalysis of Pt SAC was studied at the single-molecule single-atom level. Both static and dynamic activity heterogeneity are observed in Pt SAC. It reveals that the intrinsic catalytic activity of Pt SAC is higher than that of Pt nanoparticles (NPs), although they follow the same bimolecular competition mechanism. Significantly, Pt SAC presents no catalysis-induced surface restructuring, meaning that the dynamic activity fluctuation of Pt SAC can only be attributed to the spontaneous surface restructuring, and the catalysis process does not affect much of the structure of Pt1-based active sites, all different from Pt NP catalysis, in which the surface restructuring and the catalysis can affect each other. Further, density functional theory (DFT) calculation indicates that the unique catalytic properties of Pt SAC or the different catalytic properties between Pt SAC and NPs could be attributed to the strong adsorptions of both reactant and product on Pt SAC, large surface energy of Pt SAC, and strong binding of Pt1 on support. Knowledge revealed here provides fundamental insights into the catalysis of atomically dispersed catalyst.
Atomically dispersed metals or metal single-atom catalysts (SACs) have recently attracted a large amount of attention due to their extremely high atom efficiency or metal utilization (∼100%) (1–4). For precious metals, such as Pt (3, 5, 6), Au (7, 8), Pd (9–11), and Ir (12, 13), their SACs as the most cost-effective catalysts are very desirable for practical applications.
For metal SACs, the individual metal atoms are anchored tightly on supports (such as carbon materials, metals, or metal oxides) via a strong interaction between individual metal atoms and supports (6, 10, 14, 15). Such strong anchoring usually occurs via the vacancy or defects on supports (5, 11, 13, 16). The catalytic activity or selectivity of a SAC usually depends strongly on the choice of support (3, 17–21), which directly determines the structure of the single-metal atom–based active sites. To deeply understand the unique catalytic properties of SACs and reveal the property differences between traditional metal nanoparticle (NP)-based catalysts and SAC (6, 9, 13, 15, 22–24), here, based on single-molecule fluorescence microscopy (SMFM) (25–29), we study the nanocatalysis of Ceria (CeO2)-supported (30) Pt SAC for a fluoregenic reaction (31, 32) at the single-molecule single-atom level in real time with single-turnover resolution. It reveals both static and dynamic activity heterogeneity in the catalysis of Pt SAC. Significantly, no catalysis-induced surface restructuring can be observed on Pt SAC, which means that the dynamic activity fluctuation of Pt SAC can only be attributed to the spontaneous surface restructuring, and the catalysis process does not affect the surface restructuring of Pt SAC on CeO2. Further, density functional theory (DFT) calculation indicates that the observed unique catalytic properties of Pt SAC and the difference of catalytic properties between Pt SAC and NPs could be attributed to the stronger adsorptions of both reactant and product on Pt SAC, larger surface energy of Pt SAC, and stronger binding of Pt1 on CeO2 than those on Pt NPs. Such results deepen our understanding of the intrinsic catalytic properties of SAC and may help in the design of highly efficient SACs.
Results and Discussion
Synthesis and Characterization of Atomically Dispersed Pt1@CeO2.
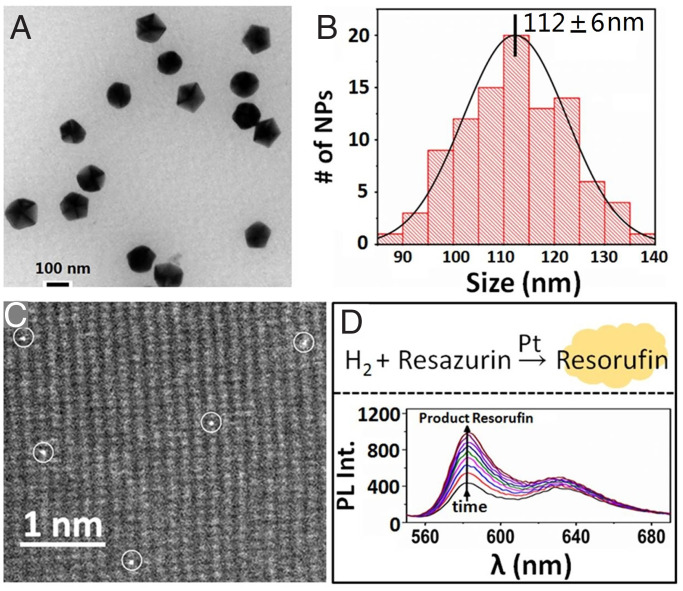
CeO2 nanocrystals, the support of Pt SACs adopted here, were synthesized based on literature (33), with Ce(NO3)3 as precursor via hydrothermal treatment at 160 °C and then pyrolysis at 1,000 °C sequentially. As shown in Fig. 1 A and B, the obtained CeO2 nanocrystals with an average size of about 112 ± 6 nm could be dispersed individually without aggregation. After the sparse deposition of individual Pt atoms on CeO2 nanocrystals (SI Appendix), the obtained sample, named as Pt1@CeO2, was characterized by high-angle annular dark-field scanning transmission electron micros (HAADF-STEM) to monitor the dispersion of Pt atoms on CeO2 support. Typically, for the sample of Pt1@CeO2 with Pt loading of 0.05 wt.% (Fig. 1C), as expected, the individual Pt atoms are dispersed sparsely on the surface of CeO2 nanocrystals. To study the catalytic activity of Pt1@CeO2, the Pt-catalyzed fluoregenic reaction (the reduction reaction of nonfluorescent resazurin to highly fluorescent resorufin by H2; Fig. 1D) was adopted as the model reaction (31, 32). The control experiment shows that the pure CeO2 nanocrystals are inert to such reaction (SI Appendix, Fig. S1); only with the addition of Pt1@CeO2, the reduction reaction of resazurin by hydrogen can occur rapidly as indicated by the time-dependent increase of fluorescence signal of the product resorufin at 583 nm (Fig. 1D and SI Appendix, Fig. S1), confirming that the individual Pt atoms (Pt1@CeO2), just like traditional Pt NPs (31), indeed, can effectively catalyze such fluoregenic reduction reaction.
Fig. 1.
Characterization of atomically dispersed Pt1@CeO2. (A) Typical TEM image of CeO2 nanocrystals as support for Pt SACs. (B) Size statistical analysis of CeO2 nanocrystals. (C) Typical HAADF-STEM image of the Pt1@CeO2 catalyst (with Pt 0.05 wt %) to show the dispersion of individual Pt atoms on surface of CeO2. (D) (Top) The Pt catalyzed reduction reaction of resazurin by hydrogen to produce fluorescent product resorufin; (Bottom) the in situ fluorescence spectra of the Pt1@CeO2(0.05 wt % of Pt)-catalyzed reduction reaction of resazurin by H2 to produce highly fluorescent resorufin in aqueous solution (λex = 532 nm). The arrow indicates the gradual formation of product resorufin with time.
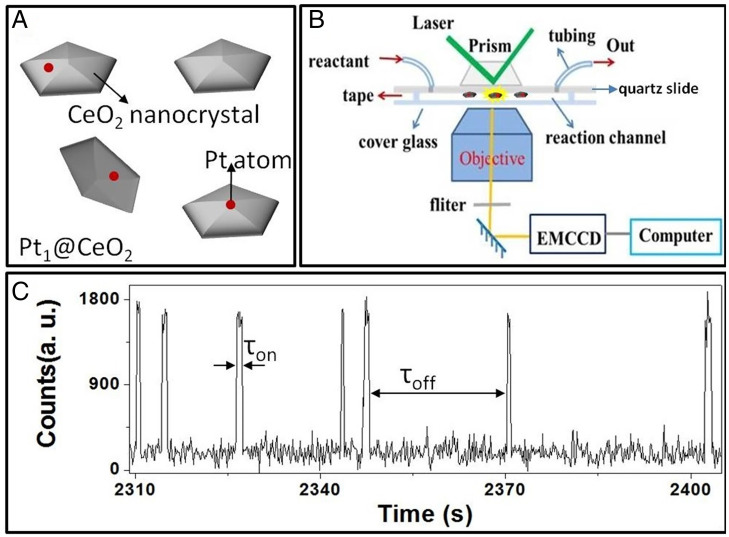
The single-molecule nanocatalysis of Pt1@CeO2 was then done based on the above fluorogenic reaction. To study the nanocatalysis of such Pt SAC at the single-molecule single-atom level, based on the mass density (1.9 g/mL) of obtained powder of CeO2 nanocrystals with an average size of 112 nm (Fig. 1 A and B), a Pt1@CeO2 catalyst with an extremely low Pt loading of 0.00001 wt.% (or 0.1 ppm) (SI Appendix, Scheme S1) was synthesized for the fluoregenic reaction to make sure that the averaged number (n) of Pt atoms on a single CeO2 NP is much smaller than one (n < 1). It means, for such Pt1@CeO2, there is statistically only one or zero Pt atoms on the surface of a single CeO2 nanocrystal. By sparsely dispersing such individual CeO2 NPs with one or zero Pt atoms on a quartz slide surface (SI Appendix, Fig. S2), one can make sure that the fluorescence signal obtained from each location is from the product molecules formed on a single Pt atom sitting on a CeO2 nanocrystal (Fig. 2A). Such single-molecule nanocatalysis was conducted in a microfluidic channel, as shown in Fig. 2B. By flowing the solution containing both nonfluorescent resazurin and saturated hydrogen into the channel, the reduction of resazurin by hydrogen was then catalyzed by Pt1@CeO2 to produce rapidly fluorescent product resorufin, which was then excited by a green (532 nm) laser to produce fluorescence and detected via an electron-multiplying charge coupled device (EMCCD) camera at an operating rate of 100 ms per frame. The stochastic fluorescence bursts at many localized spots with individual Pt1@CeO2 were recorded in movies by total internal reflection fluorescence (TIRF) microscope (34, 35); each spot gives out a stochastic trajectory with fluorescence bursts (Fig. 2C) to indicate the in situ catalysis process occurring on a single Pt atom–based active site (26). Each fluorescence burst could be attributed to the formation and the subsequent dissociation of a fluorescent product resorufin molecule on a single Pt atom–based active site. The observed stochastic fluorescence off–on signal on turnover trajectory of fluorescence contains two waiting times, τoff and τon (Fig. 2C), by dividing a catalytic cycle into two parts. τoff is the waiting time before the formation of a product molecule; τon represents the time that one product molecule spends before it dissociates from catalyst surface (26).
Fig. 2.
Scheme of Pt1@CeO2 and single-molecule nanocatalysis. (A) Scheme to show that the number of Pt atoms on a single CeO2 nanocrystal is one or zero for Pt1@CeO2 with Pt loading of 0.1 ppm. (B) Experimental optical setup for TIRF microscopy and the single-molecule nanocatalysis in a microflow cell based on the reduction reaction of resazurin by H2 to produce resorufin catalyzed by atomically dispersed Pt1@CeO2. (C) Typical fluorescence intensity turnover trajectory of a single atomically dispersed Pt1@CeO2 in H2-saturated resazurin (10 nM) solution at 100-ms time resolution.
The Catalytic Kinetics of Atomically Dispersed Pt1@CeO2.
It has been known that the above τoff contains the kinetic information of the catalytic product formation process, and τon contains that of product dissociation process (26). Therefore, the whole catalytic kinetics on a single Pt atom can be probed by resolving these two waiting times. Here, the reaction kinetics can be defined by the statistical properties. The statistical properties of <τoff>−1 and <τon>−1 obtained from a single trajectory represent the time-averaged product formation rate and product desorption rate on a Pt atom, respectively (26). When averaging the turnover trajectories from many individual Pt atoms, the relationship between reaction rate and substrate concentrations can be obtained reliably.
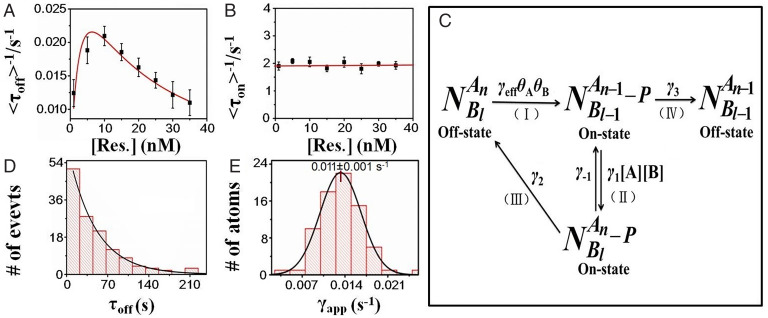
Interestingly, as shown in Fig. 3A, in the H2-saturated ([H2] = 0.8 mM) solution, the average product formation rate (<τoff>−1) on a single Pt atom initially increases with substrate resazurin concentration and then decreases inversely after a maximum, similar to the catalytic product formation process on a single Pt NP for the same reaction (SI Appendix, Fig. S3A) (31). Obviously, such decay of the product formation rate at high substrate concentration could be attributed to the bimolecular competition mechanism between two different substrate molecules (resazurin and hydrogen) (25, 29) rather than the deactivation (SI Appendix, Fig. S4). Moreover, as shown in SI Appendix, Fig. S5, at the same substrate concentration and reaction temperature (298 K), the product formation rates obtained here on a single Pt atom (Pt1@CeO2) are all smaller than that on a single Pt NP with an average size of 4.9 nm (31), mainly due to the fact that the product formation rate on a single Pt NP is the sum of the product formation rates of multiple Pt active sites on the surface of a Pt NP. As for the product desorption process on a single Pt1@CeO2, Fig. 3B shows that the product dissociation rate (<τon>−1) is independent of the substrate concentration, which is also the same as that observed from traditional single Pt NP for the same product dissociation process (SI Appendix, Fig. S3B) (31).
Fig. 3.
Catalytic kinetics of atomically dispersed Pt1@CeO2. (A and B) Single-molecule catalytic kinetics of atomically dispersed Pt1@CeO2. In H2-saturated solution, the product formation rate (<τoff>−1) (A) is dependent on resazurin concentration, and the product desorption rate (<τon>−1) (B) is independent of it. Each datum is obtained from the average of more than 80 individual turnover trajectories or particles, with the error bar showing SE. Solid lines are fitted with Eq. 1 with γeff = 0.12 s−1, αA = 1.07 mM−1, and αB = 0.32 nM−1. (C) Kinetic mechanism of the reduction reaction catalyzed by Pt NP, including the product formation and dissociation process. The meaning of each symbol is described in detail in the SI Appendix. (D) τoff distribution from a single trajectory of atomically dispersed Pt1@CeO2 with [resazurin] = 30 nM in H2-saturated solution; it is fitted by a single exponential with the constant γapp = 0.011 ± 0.001 s−1. (E) The distribution of γapp from multiple trajectories or individual atomically dispersed Pt1@CeO2; solid line is Gaussian fit.
The above analysis indicates that the total catalytic reaction follows the bimolecular reaction mechanism (25, 29), as shown in Fig. 3C, in which the substrate and product molecules maintain fast adsorption/desorption equilibrium on the catalyst. Based on previous knowledge, the product formation rate per particle can be expressed as (26)
| [1] |
and the product dissociation rate is
| [2] |
Here, [A] and [B] represent the concentrations of H2 and resazurin, respectively; γeff is the effective rate constant for the catalytic product formation process on a single particle (Fig. 3C); γ is the effective rate constant per active site; nT is the total number of active site on a single particle; αA and αB are the adsorption equilibrium constants of H2 and resazurin; γ2 and γ3 are the rate constants for the indirect and direct dissociation process of product; and . For this case, since there is only one or zero atom on a single CeO2 NP (Pt1@CeO2), then, here, nT =1, so γeff = γ.
By fitting the experimental data of the product formation rates and dissociation rates using the above equations (Fig. 3 A and B), the corresponding kinetic parameters for the product formation process (γeff or γ, αA, and αB) and product direct dissociation process (γ3) were obtained as shown in Table 1. To reveal the difference of the catalytic properties between single Pt atoms and single Pt NPs, the kinetic parameters obtained before for single Pt NPs (∼4.9 nm) (31) for the same reduction reaction are also listed in Table 1 for comparison. It clearly shows that the effective rate constant γeff per Pt NO is about 4 times that per Pt atom obtained here, mainly due to the fact that the number of active sites (nT) on a single Pt NP is much larger than one (Table 1). While, due to the fact that the value of nT on a single Pt NP (∼4.9 nm) is much larger than one or five, one can expect that the value of “apparent” γ (= γeff/nT) per active site on Pt NP is smaller than that per active site on Pt SAC (Pt1@CeO2), such a difference could be mainly attributed to the different structure of active sites and the steric hindrance effect induced by the crowding of active sites on the surface of traditional Pt NPs (SI Appendix, Fig. S6); it is also part of the reason for the higher atom efficiency or metal utilization of SAC than traditional metal NPs. As for the adsorption ability of substrate molecules, as shown in Table 1, H2 adsorption on Pt1@CeO2 is stronger than that on Pt NPs, while the resazurin adsorption on Pt1@CeO2 shows no big difference from that on Pt NPs; as for the desorption of product resorufin, γ3 in Table 1 shows that the direct dissociation pathway on Pt NPs is faster than that on Pt1@CeO2, indicating that the adsorption of product resorufin on Pt1@CeO2 is stronger than that on Pt NPs.
Table 1.
Comparison of the catalytic kinetics and dynamics between Pt SAC and Pt NPs (31)
| n T | <γeff> | <γ> | αA | αB | <γ3> | v spon-off | v spon-on | |
|---|---|---|---|---|---|---|---|---|
| (s−1) | (s−1) | (mM−1) | (nM−1) | (s−1) | (s−1) | (s−1) | ||
| Pt NPs | >>1 | 0.51 ± 0.12 | (0.51/nT) | 0.31 ± 0.20 | 0.28 ± 0.15 | 2.35 ± 0.02 | 0.003 ± 0.001 | 0.002 ± 0.001 |
| <<0.12 | ||||||||
| Pt1@CeO2 | 1 | 0.12 ± 0.02 | 0.12 | 1.07 ± 0.10 | 0.32 ± 0.05 | 1.91 ± 0.10 | 0.007 ± 0.001 | 0.011 ± 0.002 |
The Static Heterogeneity of Catalytic Activity of Atomically Dispersed Pt1@CeO2.
In order to further quantify the difference of catalytic activity of individual atomically dispersed Pt1@CeO2, the probability density function foff(τ) (26, 36) of τoff for product formation process was adopted to evaluate the static heterogeneity of the catalytic activity among different single Pt atoms,
| [3] |
Here, γapp is the apparent catalytic rate constant for product formation process on a single Pt atom (Pt1@CeO2). As shown in Fig. 3D, a typical distribution of τoff from one turnover trajectory was fitted with Eq. 3 to obtain the value of apparent catalytic rate constant γapp of a single Pt atom on CeO2. Fig. 3E shows the distribution of γapp from multiple individual Pt atoms. The broad distribution of γapp, corresponding to a large value (90%) of the heterogeneity index (defined as the full width at half maximum/(ln4)1/2 [full width at half maximum from the Gaussian distribution] divided by the average (<γapp>)) (31), indicates a huge static activity heterogeneity among different individual Pt atoms. Such static heterogeneity could be attributed to the different microenvironments among different individual Pt atoms mainly induced by the support of CeO2 nanocrystals, such as the different facets with different oxygen vacancies or defects for the anchoring of individual Pt atoms (37–39).
The Catalytic Dynamics of Atomically Dispersed Pt1@CeO2.
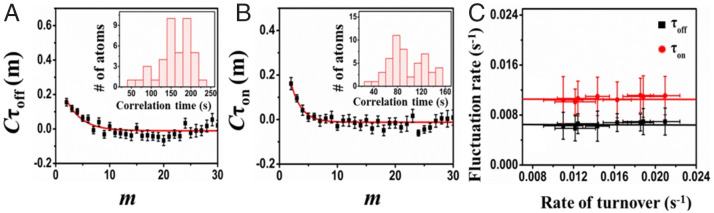
To further study the catalytic dynamics of individual atomically dispersed Pt1@CeO2, we determined the activity fluctuations of multiple individual atomically dispersed Pt1@CeO2. The activity fluctuations are reflected by the variation of reaction rates in both the product formation process (τoff) and product dissociation process (τon). Here, to analyze the activity fluctuations of individual Pt atoms on CeO2, the individual τoff and τon were extracted from multiple individual turnover trajectories, and then calculated with the autocorrelation function (26). Here, τ represents τoff or τon, m is the turnover index number from the sequence, and . If Pt1@CeO2 possesses catalytic dynamic heterogeneity, Cτ(m) will be positive and show a decay behavior with a decay time constant; such a time constant is the activity fluctuation correlation time (26).
As shown in Fig. 4 A and B, both the typical Cτoff and Cτon present an exponential decay tendency, indicating the existence of activity fluctuations in the catalytic product formation (τoff) and dissociation process (τon), respectively, occurring on the active sites of single Pt1@CeO2. For the single-atom Pt corresponding to the data shown in Fig. 4 A and B, the decay constants of Cτ at a certain substrate concentration are moff = 1.9 ± 0.5 turnovers and mon = 1.2 ± 0.4 turnovers. Based on the average turnover time (83 s) of this turnover trajectory, the fluctuation correlation times for the τoff and τon reactions are ∼159 and 96 s, respectively. Here, the fluctuation timescales of the dynamic surface restructuring can be reflected by these two correlation times (26). Moreover, the activity fluctuations could be attributed to the small-scale dynamic conformation restructuring or distortion. That is to say that the correlation times of fluctuation activity are the timescales of the surface restructuring dynamics. For an SAC of Pt1@CeO2, the activity fluctuation of individual atomically dispersed Pt1@CeO2 could be attributed to small-scale dynamic conformation restructuring or distortion around the individual active sites, similar to that of NPs (26, 27). Here, the distributions (Fig. 4 A and B, Insets) of the fluctuation correlation times of atomically dispersed Pt1@CeO2 are wide (the width of the time distribution is about 200 s), probably due to the different microenvironment around each Pt1@CeO2, resulting in the diverse interaction between Pt atoms and support CeO2.
Fig. 4.
(A and B) Exemplary autocorrelation function Cτ(t) of τoff (A) and τon (B) from turnover trajectories of individual atomically dispersed Pt1@CeO2 at 35 nM resazurin. The x axis is the turnover index. The solid line is the fit with a single exponential for decay constants of moff = 1.9 ± 0.5 turnovers and mon = 1.2 ± 0.4 turnovers. (Insets) The distribution of the fluctuation time for τoff and τon processes, respectively. (C) Dependences of the rates of activity fluctuation (the inverse of fluctuation correlation time) of τoff and τon processes on the turnover rates. Each data point is an average from >80 trajectories here. Error bars are SE.
To further reveal the relevance between activity fluctuations and surface restructuring, we plotted the activity fluctuation rates (voff = vspon-off + vcata-off, von = vspon-on + vcata-on; vspon is the spontaneous fluctuation rate, and vcata is the catalysis-induced fluctuation rate) against the turnover rates at various reactant concentrations for both τoff and τon processes (26). Here, the fluctuation rates are the inverses of the correlation times. As shown in Fig. 4C, for atomically dispersed Pt1@CeO2, the activity fluctuation rates for both the τoff reaction and the τon reaction are almost independent of the turnover rates (Fig. 4C), indicating that the fluctuation rate does not change with the substrate concentration, and the values of both vcata-off and vcata-on are zero at any substrate concentration. The substrate concentration–independent activity fluctuation observed here on Pt SCA reflects that the underlying dynamic conformation restructuring existing among atomically dispersed Pt1@CeO2 is mainly due to the spontaneous surface restructuring of the single-atom Pt–based active site (Pt1@CeO2) (26); the catalytic process or the substrate binding/product unbinding approximately has no effect on structure of the Pt SAC-based active sites. As for the Pt NPs, interestingly, as shown in SI Appendix, Fig. S7 and reported before (31), the catalytic process or the substrate binding/product unbinding can affect hugely the surface restructuring of the Pt NP surface, which is also called “catalysis-induced surface restructuring.” Such a huge difference between Pt SAC and the Pt NPs revealed here probably indicates that the binding of Pt1 to CeO2 is much stronger than the binding of Pt1 to Ptn, or the structure of Pt1–CeO2 is much more stable than that of Pt1–Ptn. Furthermore, as shown in Fig. 4C and Table 1, the rate of spontaneous surface restructuring can be obtained (vspon-off =0.007 ± 0.001 s−1 for the product formation process [τoff] and vspon-on =0.011 ± 0.002 s−1 for product desorption [τon] process), corresponding to a timescale of about 90 s to 160 s of the spontaneous surface reconstruction. The revealing of two different rates of spontaneous surface restructuring indicates that there are at least two different models of spontaneous surface restructuring on the same surface; the slow one (0.007 ± 0.001 s−1) can affect the product formation process or the substrate binding, and the faster one (0.011 ± 0.002 s−1) can affect the product dissociation process or the product unbinding. Interestingly, as shown in Table 1, for both the product formation and dissociation process on Pt NPs, the rates of spontaneous surface restructuring are smaller than that on Pt SAC (Pt1@CeO2), indicating that the structure of the individual Pt active site on Pt NP is much more stable than that of the single Pt atom–based active site on CeO2 surface, and the reactant binding/product unbinding can activate the surface of Pt NPs and speed up or enhance its surface restructuring.
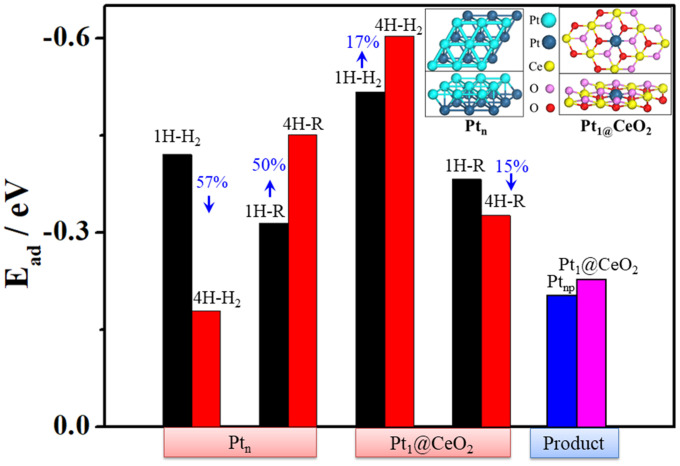
Furthermore, we did a DFT calculation to deeply understand the above observation about the unique properties of Pt SAC and the property differences between Pt SAC and Pt NPs(Ptn) shown above in Table 1. Based on the HAADF-STEM image (Fig. 1C) of Pt1@CeO2 and previous reports about the structure of Pt1@CeO2 (33, 38), DFT calculations about Pt1@CeO2 were done here based on a model with individual Pt atoms trapped by surface Ce vacancies on CeO2 crystals (Fig. 5 and SI Appendix, Table S1). Firstly, we calculated the adsorption energies (Ead) of both reactants and products on different surfaces. For the case with the coexistence of one H2 molecule and one resazurin molecule around the active site (SI Appendix, Fig. S8A) (40), results in Fig. 5 show clearly that adsorption energies (Ead) of both H2 (Ead1H-H2 = −0.517 eV) and resazurin (Ead1H-R = −0.382 eV) on Pt1@CeO2 are larger than those (Ead1H-H2 = −0.420 eV; Ead1H-R = −0.314 eV) on Ptn, indicating stronger adsorptions of both H2 and resazurin on Pt1@CeO2 than on Ptn, consistent with the obtained values of αA and αB shown in Table 1. As for the adsorption of product resorufin, its Ead on Pt1@CeO2 (−0.228 eV) is also larger than that (−0.204 eV) on Ptn, indicating a stronger adsorption of resorufin on Pt1@CeO2 than on Ptn, further confirming the slower dissociation (smaller γ3) of product resorufin from Pt1@CeO2 than that from Ptn shown in Table 1. To understand the bimolecular competition mechanism for this redox reaction catalyzed by both Pt1@CeO2 and Pt NPs (Fig. 3C and SI Appendix, Fig. S5), we further calculated Ead of reactants (H2 and resazurin) by increasing the number of H2 molecules or [H2] around the active sites (41, 42). For the case with the coexistence of four H2 molecules and one resazurin molecule around the active site (SI Appendix, Fig. S8B), Fig. 5 shows the variations of Ead with the increase of [H2]: For the adsorption on Pt1/CeO2, the adsorption energy of H2 increases about 17%, while the adsorption energy of resazurin decreases 15% inversely; as for the adsorption on Pt NPs, the adsorption energy of H2 decreases 57%, while the adsorption energy of resazurin increases inversely about 50%. Obviously, all these results confirm the bimolecular competition mechanism observed on both Pt1@CeO2 and Pt NPs.
Fig. 5.
Adsorption energies (Ead) of H2, resazurin (R) and product resorufin (Product) on different surfaces (Ptn, Pt1@CeO2). Insert shows local structures of Pt(111) surface on Pt NPs (Ptn) and Pt1@CeO2(111). The 1H and 4H represent the cases with one and four H2 molecules around different surfaces, respectively.
Moreover, we also found that the surface energy (Esurf; Table 2) of Pt1@CeO2 (0.190 eV/Å2, in SI Appendix) is larger than that (0.090 eV/Å2) of Ptn, confirming the observed higher activity (γ) and faster spontaneous surface restructurings (vspon-off and vspon-on) of Pt1@CeO2 than that of Pt NPs (Table 1) (43). Further results (Table 2) show that the binding of a Pt single atom on substrate CeO2 (Eb = −12.560 eV) is much stronger than that (Eb = −6.972 eV) on Pt NPs, explaining the above observations on Pt1@CeO2 without catalysis-induced surface restructuring (Fig. 4C) and the observations on Pt NPs with catalysis-induced surface restructuring (SI Appendix, Fig. S7) (31). To further confirm such difference, HAADF-STEM was adopted to analyze the possible effect of the catalysis process on the microstructure of the active sites on both Pt1@CeO2 and Pt NPs. As shown in SI Appendix, Fig. S9 A and B, after a long-term (10 h) catalytic process for the fluoregenic reaction shown in Fig. 1D, the microstructures of Pt1-based active sites on CeO2 show almost no variation; such a fact indicates that the restructuring during catalysis is too gentle to much affect the structure of a Pt1-based active site on CeO2, confirming that the underlying dynamic conformation restructuring existing among atomically dispersed Pt1@CeO2 is mainly due to the spontaneous surface restructuring of the single-atom Pt–based active site (Pt1@CeO2), while the surface structure of traditional Pt NPs shows tremendous variation after the same catalytic process as shown in SI Appendix, Fig. S9 C and D, indicating that the catalysis process on Pt NPs is relatively violent and then leads to serious surface restructuring. Such facts confirm the above catalytic property differences between Pt1@CeO2 and Pt NPs. Significantly, the stronger binding (large Eb) of the Pt atom on CeO2 and the faster spontaneous restructuring of Pt1@CeO2 than Pt NPs indicate that the spontaneous surface restructuring observed on Pt1@CeO2 mainly originates from the surface restructuring of CeO2 around the Pt1 center; it also implies that the reactants/products adsorption/desorption or catalysis process can intensify the restructuring amplitude of a surface with a slow spontaneous restructuring, while, if the initial spontaneous restructuring of a surface is fast enough, then the reactants/products adsorption/desorption or catalysis process cannot much affect its surface restructuring. So, as shown in Table 2, for Pt NPs, the catalytic process and surface reconstruction can affect each other, like the previous observation on Au NPs (26), while, for the unique SAC Pt1/CeO2 studied here, its surface reconstruction can affect the catalytic process; inversely, the catalytic process cannot affect its surface reconstruction. In other words, the fast spontaneous reconstruction of a surface can weaken the effect from its environment.
Table 2.
Comparison of surface energy (Esurf), binding energy (Eb), and effect of the surface reconstruction on catalysis between Pt NPs and Pt1@CeO2
| Esurf | Eb | Effect between catalytic process and surface reconstruction of different surfaces | |
|---|---|---|---|
| (eV/Å2) | (eV) | ||
| Pt NPs | 0.09 | −6.972 | Catalytic process  surface reconstruction surface reconstruction |
| Pt1@CeO2 | 0.19 | −12.560 | Catalytic process  surface reconstruction surface reconstruction |
Discussion
In summary, the catalytic behavior of Pt SAC on CeO2 (Pt1@CeO2) was studied via SMFM at the single-molecule single-atom level. Both static and dynamic activity heterogeneity were observed in the catalysis of Pt1@CeO2. It was found that Pt SAC follows the same bimolecular competition mechanism as Pt NP does for the same catalytic reaction, while the intrinsic catalytic activity per active site on Pt SAC is much higher than that on Pt NPs. Significantly, it was also found that the dynamic activity fluctuation of Pt SAC can only be attributed to the spontaneous surface restructuring, and no catalysis-induced surface restructuring can be observed due to the fast spontaneous surface restructuring, indicating that the catalysis process does not much affect the surface restructuring around the Pt1-based active site on CeO2, different from Pt NP catalysis in which the surface restructuring and the catalysis can affect each other. Further DFT calculation indicates that all these unique catalytic properties of Pt SAC or the difference from Pt NPs could be attributed to the stronger adsorption of both reactant and product on Pt1@CeO2, larger surface energy of Pt1@CeO2, and stronger binding of Pt1 on CeO2 than those on Pt NPs. The knowledge revealed here provides fundamental insights into the catalytic behaviors of atomically dispersed catalyst.
Experimental Section
Materials and Methods.
Platinum(IV) chloride, Cerium(III) nitrate hexahydrate, Propionic acid, and Ethylene glycol. These chemicals were used as received without further purification. Ultrapure Millipore water (18.2 MΩ cm) was used as the solvent throughout. Reactant resazurin was purchased from Sigma-Aldrich.
Synthesis of single-atom Pt1@CeO2 catalyst.
The synthesis of SAC Pt1@CeO2 with different amount of Pt loading was based on literature (33), with Ce(NO3)3 and PtCl4 as precursors via sequential hydrothermal treatment at 160 °C and pyrolysis at 1,000 °C. The specific reaction process is introduced in SI Appendix.
Single-molecule experiments.
Single-molecule fluorescence measurements were performed on a home-built prism-type TIRF microscope based on an Olympus IX71 inverted microscope (SI Appendix). A continuous wave circularly polarized 532-nm laser beam was focused onto a small region on the sample. Then the fluorescence signal of the product was collected by a water immersion objective, and projected onto a camera controlled by Andor IQ software. The home-written interactive data language program was used to analyze the movies, which allows us to extract a time trajectory of fluorescence intensity from individual fluorescence spots on the sample.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 21925205, 22072145, 21733004, and 21721003), National Key R&D Program of China (grants 2017YFE9127900 and 2018YFB1502302) and K. C. Wong Education Foundation and Science.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2114639119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Liu P., et al. , Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–801 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Jones J., et al. , Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Qiao B., et al. , Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Li X., et al. , Single-atom Pt as Co-catalyst for enhanced photocatalytic H2 evolution. Adv. Mater. 28, 2427–2431 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Kwak J. H., et al. , Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on γ-Al2O3. Science 325, 1670–1673 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Zhai Y., et al. , Alkali-stabilized Pt-OHx species catalyze low-temperature water-gas shift reactions. Science 329, 1633–1636 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Comas-Vives A., et al. , Single-site homogeneous and heterogeneized gold(III) hydrogenation catalysts: Mechanistic implications. J. Am. Chem. Soc. 128, 4756–4765 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Shi H., Xu B. Q., Catalysis by gold: Isolated surface Au3+ ions are active sites for selective hydrogenation of 1,3-butadiene over Au/ZrO2 catalysts. Angew. Chem. Int. Ed. Engl. 44, 7132–7135 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Abbet S., et al. , Acetylene cyclotrimerization on supported size-selected Pdn clusters (1 ≤ n ≤ 30): One atom is enough! J. Am. Chem. Soc. 122, 3453–3457 (2000). [Google Scholar]
- 10.Vilé G., et al. , A stable single-site palladium catalyst for hydrogenations. Angew. Chem. Int. Ed. Engl. 54, 11265–11269 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Yan H., et al. , Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 137, 10484–10487 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Lin J., et al. , Remarkable performance of Ir1/FeO(x) single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 135, 15314–15317 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Ortalan V., Uzun A., Gates B. C., Browning N. D., Direct imaging of single metal atoms and clusters in the pores of dealuminated HY zeolite. Nat. Nanotechnol. 5, 506–510 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Yang M., et al. , Catalytically active Au-O(OH)x-species stabilized by alkali ions on zeolites and mesoporous oxides. Science 346, 1498–1501 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Yang X., Yang L., Lin S., Zhou R., New insight into the doping effect of Pr2O3 on the structure-activity relationship of Pd/CeO2-ZrO2 catalysts by Raman and XRD Rietveld analysis. J. Phys. Chem. C 119, 6065–6074 (2015). [Google Scholar]
- 16.Sun S., et al. , Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 3, 1775–1785 (2013). [Google Scholar]
- 17.Liu X., Korotkikh O., Farrauto R., Selective catalytic oxidation of CO in H2: Structural study of Fe oxide-promoted Pt/alumina catalyst. Appl. Catal. A 226, 293–303 (2002). [Google Scholar]
- 18.Kalakkad D., Datye A. K., Robota H., Interaction of platinum and ceria probed by transmission electron microscopy and catalytic reactivity. Appl. Catal. B 1, 191–219 (1992). [Google Scholar]
- 19.Oh S. H., Mitchell P., Siewert R., Methane oxidation over alumina-supported noble metal catalysts with and without cerium additives. J. Catal. 132, 287–301 (1991). [Google Scholar]
- 20.Schwartz J. M., Schmidt L. D., Microstructures of Pt-Ce and Rh-Ce particles on alumina and silica. J. Catal. 138, 283–293 (1992). [Google Scholar]
- 21.Abdel-Mageed A., Widmann D., Olesen S., Chorkendorff I., Behm R., Selective CO methanation on highly active Ru/TiO2 catalysts: Identifying the physical origin of the observed activation/deactivation and loss in selectivity. ACS Catal. 8, 5399–5414 (2018). [Google Scholar]
- 22.Kaden W. E., Wu T., Kunkel W. A., Anderson S. L., Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science 326, 826–829 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Wei H., et al. , FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 5, 5634 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Yang M., et al. , A common single-site Pt(II)-O(OH)x- species stabilized by sodium on “active” and “inert” supports catalyzes the water-gas shift reaction. J. Am. Chem. Soc. 137, 3470–3473 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Chen T., et al. , Catalytic kinetics of different types of surface atoms on shaped Pd nanocrystals. Angew. Chem. Int. Ed. Engl. 55, 1839–1843 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Xu W., Kong J. S., Yeh Y. T., Chen P., Single-molecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics. Nat. Mater. 7, 992–996 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., et al. , Unique size-dependent nanocatalysis revealed at the single atomically precise gold cluster level. Proc. Natl. Acad. Sci. U.S.A. 115, 10588–10593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X., Xu W., Liu G., Panda D., Chen P., Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level. J. Am. Chem. Soc. 132, 138–146 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Liu X., Chen T., Song P., Zhang Y., Xu W., Single-molecule nanocatalysis of Pt nanoparticles. J. Phys. Chem. C 122, 1746–1752 (2018). [Google Scholar]
- 30.Nagai Y., et al. , Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide-support interaction. J. Catal. 242, 103–109 (2006). [Google Scholar]
- 31.Liu X., Chen T., Xu W., Revealing the thermodynamics of individual catalytic steps based on temperature-dependent single-particle nanocatalysis. Phys. Chem. Chem. Phys. 21, 21806–21813 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Han K. S., Liu G., Zhou X., Medina R. E., Chen P., How does a single Pt nanocatalyst behave in two different reactions? A single-molecule study. Nano Lett. 12, 1253–1259 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Xie P., et al. , Nanoceria-supported single-atom platinum catalysts for direct methane conversion. ACS Catal. 8, 4044–4048 (2018). [Google Scholar]
- 34.Zhang Y., Song P., Fu Q., Ruan M., Xu W., Single-molecule chemical reaction reveals molecular reaction kinetics and dynamics. Nat. Commun. 5, 4238 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., et al. , Superresolution fluorescence mapping of single-nanoparticle catalysts reveals spatiotemporal variations in surface reactivity. Proc. Natl. Acad. Sci. U.S.A. 112, 8959–8964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu W., Shen H., Liu G., Chen P., Single-molecule kinetics of nanoparticle catalysis. Nano Res. 2, 911–922 (2009). [Google Scholar]
- 37.Nie L., et al. , Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Ye X., et al. , Insight of the stability and activity of platinum single atoms on ceria. Nano Res. 12, 1401–1409 (2019). [Google Scholar]
- 39.Tang Y., Wang Y.-G., Li J., Theoretical investigations of Pt1@CeO2 single-atom catalyst for CO oxidation. J. Phys. Chem. C 121, 11281–11289 (2017). [Google Scholar]
- 40.Kuai L., et al. , Titania supported synergistic palladium single atoms and nanoparticles for room temperature ketone and aldehydes hydrogenation. Nat. Commun. 11, 48 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao W., et al. , Single Mo1(Cr1) atom on nitrogen-doped graphene enables highly selective electroreduction of nitrogen into ammonia. ACS Catal. 9, 3419–3425 (2019). [Google Scholar]
- 42.Yang Y., Zhao Z., Cui R., Wu H., Cheng D., Structures, thermal stability, and chemical activity of crown-jewel-structured Pd–Pt nanoalloys. J. Phys. Chem. C 119, 10888–10895 (2015). [Google Scholar]
- 43.Zhuang H., Tkalych A. J., Carter E. A., Surface energy as a descriptor of catalytic activity. J. Phys. Chem. C 120, 23698–23706 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.