Abstract
During adolescence, heavy binge-like ethanol consumption can lead to frontocortical structural and functional impairments. These impairments are likely driven by adolescence being a critical time point for maturation of brain regions associated with higher-order cognitive functioning. Rodent models of heavy binge-like ethanol exposure show consistent disruptions to the typical development of the prefrontal cortex (PFC). All deep cortical layers receive cholinergic projections that originate from the Nucleus basalis of Meynert (NbM) complex. These cholinergic projections are highly involved in learning, memory, and attention. Adolescent intermittent ethanol exposure (AIE) induces cholinergic dysfunction as a result of an epigenetic suppression of the genes that drive the cholinergic phenotype. The current study used a model of AIE to assess structural and functional changes to the frontal cortex and NbM following binge-like ethanol exposure in adolescence. Western blot analysis revealed long-term disruptions of the cholinergic circuit following AIE: choline acetyltransferase (ChAT) was suppressed in the NbM and vesicular acetylcholine transporter (VAChT) was suppressed in the orbitofrontal cortex (OFC). In vivo microdialysis for acetylcholine efflux during a spatial memory task determined changes in cholinergic modulation within the PFC following AIE. However, AIE spared performance on the spatial memory task and on an operant reversal task. In a second study, Golgi-Cox staining determined that AIE increased apical dendritic complexity in the OFC, with sex influencing whether the increase in branching occurred near or away from the soma. Spine density or maturity was not affected, likely compensating for a disruption in neurotransmitter function following AIE.
Keywords: acetylcholine, ethanol, adolescence, orbital frontal cortex
Introduction
Heavy binge-like alcohol exposure during adolescence, in humans and animal models, can manifest in frontocortical structural and functional impairments.1-4 Such disruptions are likely driven by the maturation of brain regions associated with higher-order cognitive functions that occurs in adolescence.5-8 Subsequently, it is not surprising that heavy binge drinking during adolescence has been associated with impairments in executive functions.9,10 In rodent models, heavy binge-like ethanol exposure impacts the typical development of the prefrontal cortex (PFC). There are persistent suppressions of PFC cortical volume and myelination, whereas markers of neuroinflammation and neurodegeneration are increased.11-15 Such models also revealed that heavy binge-type ethanol exposure produces deficits in set-shifting16,17, working memory18,19, behavioral inhibition20, and reversal learning.12,13,21 These aberrant behaviors are classified under the domains of cognitive and behavioral flexibility and result in an inability to readily adapt to changes in the environment22,23
A key neural alteration that occurs after heavy alcohol exposure during adolescence, is the disruption of the forebrain cholinergic circuits, which are involved in learning, memory and attention. Specifically, following adolescent intermittent ethanol exposure in rodents (AIE), there is a significant suppression of the cholinergic phenotype in neurons within the medial septum/diagonal band (MS/DB), which projects to the hippocampus, and the Nucleus basalis of Meynert complex (NbM) that projects to the cortex.20,24,25,26 This AIE-induced dysfunction is a result of an epigenetic suppression of the genes that drive the cholinergic phenotype.27 The damages to forebrain cholinergic populations are age-specific; there is no loss seen when the binge ethanol exposure occurs in adult rodents.26 However, despite the AIE-induced loss of cholinergic projection neurons to the hippocampus, behaviorally-evoked acetylcholine (ACh) efflux can be spared in the hippocampus following AIE17, and therefore we did not include hippocampal assessment in this series of experiments. In contrast, following AIE there is a significant suppression of ACh efflux activated by spatial exploration in the medial PFC.17 Subsequently, the frontal cortical cholinergic system may be particularly susceptible to AIE-induced toxicity, so the focus of the current study is another PFC region, the OFC. The OFC is critical for behavioral inhibition20, and reversal learning12,13,21 and pathology in this region likely contributes to the cognitive impairment associated with heavy alcohol exposure during adolescence.
All deep cortical layers receive cholinergic projections that originate from the NbM complex, which includes the NbM, horizontal diagonal band (HDB) and substantia innominata (SI).28,29 ACh release in the cortex drives neuronal excitability through altering the presynaptic release of other neurotransmitters (glutamate, GABA) and coordinating the dynamics of local circuits.30 There are two modes of ACh transmission, tonic and phasic (transient), and the roles of these two patterns appear to be dissociable. The faster kinetics of cholinergic signaling in the PFC mediates cue detection, including cue-triggered changes in goal-directed behavior.31,32 In contrast, the slower and more spatially broader ACh fluctuations are important for arousal, encoding, increasing the signal-to-noise ratio.33 It has been suggested that the slower cholinergic neuromodulation in the PFC favors staying on task, over switching to an alternative action.34
In the current study, we assessed changes in key cholinergic markers in the NbM and OFC, and measured tonic ACh efflux in the orbital frontal cortex (OFC) during a spatial navigation task to determine whether AIE broadly changes cholinergic modulation within the PFC. We assessed behavior in a reversal task to determine if AIE disrupts the ability to change discrete action plans. Finally, we examined whether AIE leads to long-term changes in dendritic architecture within the OFC.
General Experimental Procedures
Subjects
Pre-adolescent male and female Sprague-Dawley rats were obtained from litters bred at Binghamton University animal facility. Only one pup of each sex (both sexes equally distributed in all treatment conditions) from a litter was randomly assigned to a given treatment condition (see below). All rats were maintained in a temperature (20 degrees Celsius) and humidity-controlled colony room in a light/dark cycle (7:00-19:00) and were housed in pairs or, if needed, a triad by sex. Rats in both Treatment conditions (see below) had free access to water and standard rat chow (Purina Lab Diet 5012), except in Experiment 2, where the rats were initially food restricted two weeks prior to the start of behavioral tasks in order to maintain weights at 85% of their free-feeding body weight values.
All procedures were in accordance with the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee (IACUC) at the State University of New York at Binghamton. The protocols used in the present experiment were approved by the Binghamton University Institutional Animal Care and Use Committee.
Adolescent Intermittent Ethanol Exposure
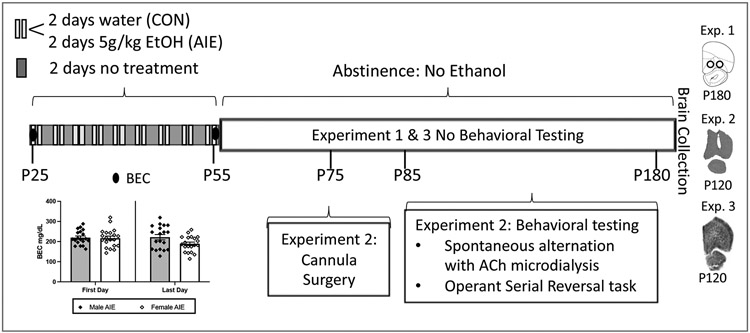
Pre-adolescent rats (PD 25) were subject to 16 intragastric gavages of either 20% ethanol (v/v; AIE; Experiment 1= 7 males, 7 females; Experiment 2= 11 males, 9 females; Experiment 3 =6 males and 6 females) or water control (CON; Experiment 1= 6 males, 6 females; Experiment 2= 9 males, 9 females; Experiment 3 = 6 males and 5 females), administered at a dose of 5 g/kg. The dosing schedule followed a 2-day on/off cycle, where rats were dosed once per day for 2 days, followed by a 2-day recovery period. Blood samples were collected an hour following the first and eighth gavage, and blood ethanol content (BEC) levels were determined using an AM1 Alcohol Analyzer (Analox Instruments, Ltd, UK). Figure 1 illustrates the timeline and BECs for all experiments.
Figure 1. Adolescent Intermittent Ethanol (AIE) exposure protocol and experiment timeline.
Schematic outlining the AIE exposure protocol, and the timeline for cannula implantation surgery (Exp 2), behavioral testing (Exp 2) and tissue collection (Exp 1, 2, 3). Blood ethanol concentrations (BECs) following the first and last intraoral gastric gavage are illustrated in the chart insert (N = 19 males, 20 females). BECs did not significantly differ between the sexes.
Experiment 1:
Choline acetyltransferase (ChAT) is responsible for the biosynthesis of acetylcholine and is an indicator of the functional state of cholinergic neurons. Vesicular acetylcholine transporter (VAChT) tightly regulates the release of ACh and is found predominantly in synaptic vesicles at nerve terminals. Western blot procedures were used to assess the persistent loss of ChAT in the NbM and VAChT in the OFC following AIE.
Rats were decapitated on approximately PD 180 (due to Covid-19 restrictions), and the brains were flash frozen by brief submersion in cold Methyl Butane, that was maintained on dry ice, and then stored at −80 °C until further use. Brain regions of interest, the NBM and OFC were bilaterally micropunched (1.0–2.0 mm; EMS-Core Sampling Tools, Electron Microscopy Sciences, Hatfield, PA, USA) during the cryostat collection procedure (−20 °C). NbM punches were collected through a range of coordinates [AP (−1.1mm : −2.6mm), ML (+/− 2.5mm : +/− 4.0mm), and DV (−5.0mm : 8.0 mm)]. OFC tissue punches were collected in a similar manner [AP( +4.7mm : +3.0mm), ML (+/− 2mm), and DV ( −5.0mm : −8.0mm)]. Brain punches were stored at −80 °C until tissue lysis.
Tissues were homogenized in lysis buffer (1% SDS, 1 mM EDTA, 10 mM Tris) containing protease inhibitors (Halt™ Protease Inhibitor Cocktail, Thermo Scientific, Waltham, MA, USA) and centrifuged at 4°C, 12000g for 30 minutes. Protein concentrations were determined using a bicinchoninic acid method (Pierce, Rockford, IL, USA) and compared to bovine serum albumin standards. 30 μg total protein samples of the OFC and NbM samples were denatured and separated by electrophoresis on Novex™ 8–16% Tris-Glycine sodium dodecyl sulfate polyacrylamide gels (Invitrogen, Carlsbad, CA, USA), transferred to a polyvinylidene difluoride membranes (Invitrogen, Carlsbad, CA, USA), and blocked for one hour in 5% BSA, 0.01% Tween-20 in TBS. Following this, membranes were incubated overnight with a goat antibody against ChAT (70 kDa, NbM=1:500 dilution; Millipore, Burlington, MA, USA; AB144P) and mouse antibody raised against VAChT (55 kDa OFC= 1:1000 dilution; Millipore, Burlington, MA, USA; SAB4200559). Blots were then exposed to a peroxidase-conjugated secondary antibody (NbM = mouse anti-goat HRP 1:2000 dilution, Santa Cruz, Dallas, TX, USA; OFC = goat anti-mouse HRP 1:10000 dilution, Thermo Scientific, Waltham, MA, USA) for 1 h and protein levels were detected with enhanced chemiluminescence (Pierce™ ECL Western Blotting Substrate, Thermo Scientific, Waltham, MA, USA). β-actin (Chicken anti β-actin −1:2000 dilution (primary antibody), Origene, Rockville, MD, USA, TA349013; Goat anti-chicken HRP – 1:10000 dilution (Secondary antibody), Abcam, Cambridge, UK; Ab97135) was used for normalization.
Statistical analysis
For Experiment 1, a two-factor ANOVA (Treatment: AIE vs. CON; Sex: Females vs. Males) was used to assess suppression of the cholinergic markers (ChAT, VAChT) in the NbM and OFC. When significance in the omnibus F test was found, Fisher's LSD post hoc tests were used to assess the differences between Treatment (AIE vs. CON) and between Sex (Females vs. Males). The SPSS statistical package was used for all analyses and values of p < 0.05 was considered significant. Homogeneity of variance was assessed with Levene’s test. Any violations in homogeneity of variance across sex or treatment conditions are noted. There were no significant sex differences in cholinergic markers, but studies were not powered to detect difference in hormonal status in male or female rats.
Experiment 2:
Cannula Implantation Surgery
For Experiment 3, after AIE, rats underwent cannula implantation surgery and recovery for a 3-week period. A single cannula was placed in the OFC, counterbalanced across hemisphere, 2-3 weeks following the cessation of AIE treatment. Prior to surgery, administration of a ketamine (87.5mg/mL)/xylazine (12.5mg/mL) mixture at a dosage of 1.0 mL/kg was administered (intraperitoneal) as anesthesia. Rodents were placed into a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). A guide cannula (5 mm; Synaptech Technology Inc., Marquette, MI, USA) was placed into the OFC coordinates relative to Bregma (Fig 2C) = AP: +4.7, ML: +/−2.0, DV: −4.0 (Paxinos & Watson, 2013). Dental acrylic cement with anchor bone screws secured guide cannula to the skull. Carprofen (5 g/kg; Zoetis, Kalamazoo, MI, USA) was administered prior to surgery, as well as 24-hours and 48-hours post-surgery, as an analgesic. After surgery, rats recovered for 10 days before behavioral testing. Spontaneous alternation testing (with ACh microdialysis) occurred first, followed by operant serial reversal learning.
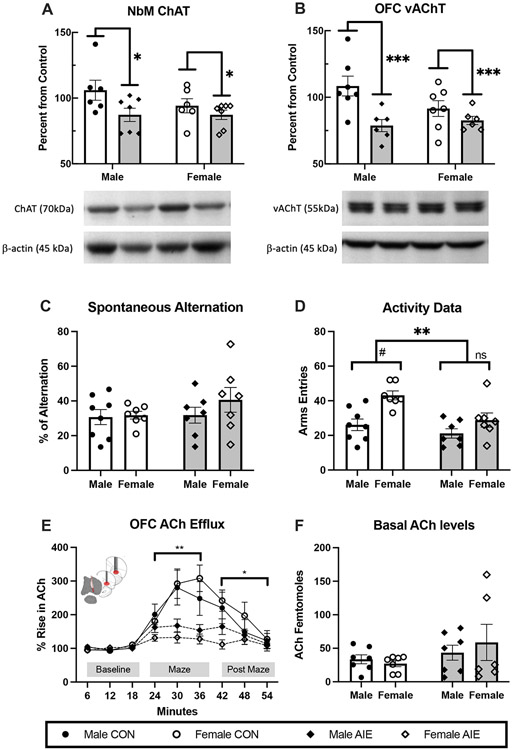
Figure 2. Adolescent Intermittent Ethanol (AIE) leads to a suppression of cholinergic markers in the Nucleus basalis Magnocellularis (NbM)-Orbital Frontal Cortical (OFC) circuit and blunts acetylcholine (ACh) efflux within the OFC during spontaneous alternation but does not impair spatial exploration.
(A) Western blot analyses revealed that AIE decreased the choline acetyltransferase (ChAT) protein levels in the NbM and (B) vesicular acetylcholine transporter (VAChT) protein levels in the OFC, compared to the control condition. Data are presented as the mean ± SEM percent of control. (C) Average percent alternation scores by AIE treatment. There was no effect of AIE on alternation scores. Data are presented as the mean ± SEM. (D) Arm entries during spontaneous alternation. AIE rats were less active than control rats. In the Control condition, females were more active than males. Data are presented as the mean ± SEM percent. (E) Orbital frontal ACh efflux before (baseline), during (maze) and after spontaneous alternation behavior (after maze). There was a main effect of phase, with ACh levels increasing during maze behavior. There was also a significant interaction between AIE treatment and phase: Regardless of sex, AIE blunted the rise in OFC ACh efflux during maze exploration and into the post maze period. Data are presented as the mean ± SEM percent of baseline. (F) There were no Treatment nor Sex differences in the basal levels of ACh. Data are presented as the mean ± SEM. Treatment effects*= p<0.05, **= p<0.01, # represents a significant Sex effect, p< 0.05
Spontaneous alternation testing with in vivo microdialysis
Each rat was food restricted overnight and tested on a single spontaneous alternation session. Both ethanol and water pretreated animals were at similar weights prior to food restriction, and all animals lost weight at similar rates. In vivo microdialysis protocols were followed as previously described.35 On the day of testing, a microdialysis probe (S-5020, 2mm; Synaptech Technology Inc., Marquette, MI, USA) was inserted into OFC guide cannula and the rat was placed into an opaque habituation chamber to acclimate for a period of 60-min prior to maze testing. The probe was connected to a CMA microinfusion pump (CMA/400 pump) and an artificial cerebrospinal fluid solution (7.4 pH solution: 127.6 mM NaCl, 0.9 mM NaH2PO4, 2 mM Na2HPO4, 4 mM KCl, 1.3 mM CaCl2 dihydrate, 1.0 mM glucose, and 0.9 mM MgCl2) with 500 nM neostigmine hydrobromide (Sigma-Aldrich Corp., St. Louis, MO, USA) was perfused continuously at a rate of 2.0 μL/min.36 Baseline dialysate collection began after the 60-minute acclimation period, and samples were collected for three 6-minute intervals.
Spontaneous alternation was conducted in a plus maze (105.5 cm x 14.4 cm x 15 cm). The rat was placed into the center of the apparatus and allowed to explore the maze for 18-minutes of testing, during which arm entries (all four paws within an arm) were recorded. Dialysate was continuously collected during maze behavior. An alternation was defined as entry into four different arms in overlapping successive sequences of 4 arm entries (for example, in the successive arm entries of B, A, D, C, A, D, C, A, D, B, C, D, B, A; the first sequence of BADC was an alternation, but the next 4- arm sequence ADCA was not). The percent alternation score is equal to the ratio of actual alternations to possible alternations (total alternations/[trial number-3] X 100). Following maze testing, the rat was returned to the opaque habituation chamber for an 18-minute collection of post maze dialysate.
High-performance liquid chromatography
High-performance liquid chromatography (HPLC) with electrochemical detection (Amuza, San Diego, CA, USA) was used to assay ACh from dialysate samples. Chromatographs obtained were analyzed using the software program Envision (provided by Amuza, San Diego, CA, USA). ACh peaks were quantified by comparison to peak heights of standard solutions (100 nM, 20 nM, and 4 nM standards).
Operant Serial Reversal Methods
Operant chambers (30 cm x 33 cm x 23 cm; Med Associates Inc., St. Albans, VT, USA) were enclosed within sound attenuating boxes (59 cm x 55 cm x 36 cm) equipped with a running fan. Each operant chamber contained two retractable levers separated by a magazine which dispensed food reward (Rodent Purified Dustless Precision Pellet; Bio-Serve, Flemington, NJ, USA). Above each retractable lever were stimulus lights, and a house light located on the opposite side of the chamber from the magazine provided the main source of illumination during operant training. Operant chambers were interfaced with MED-PC (Med Associates Inc. St Albans, VT, USA).
Rats were gradually food restricted to 85% of their free feed weight over the course of one week before the start of pre-training, and this level of food restriction was maintained throughout operant testing. Each of the 2 days prior to the start of pre-training, rats were exposed to the food reward used during operant training. During operant pre-training and training, rats were assigned to operant chambers and underwent testing on one task per day, for a maximum of 1-hour duration of training.
During pre-training, rats were placed in the operant chamber and randomly assigned to be shaped in pressing the left or right lever until 50 lever presses were recorded within a 30-minute period. Only one lever was extended during this task while both cue lights were illuminated. Following mastery of the left or right lever shaping, rats were trained to press the opposite lever under the previously mentioned conditions until criterion was met. Each response was rewarded on a Fixed-Ratio-1 reward schedule. After shaping left and right lever presses, rats underwent 90 trials of retractable lever training. During each trial of the retractable lever training either the left or right retractable levers randomly extended for 10 seconds, while both cue lights were illuminated. Rats were rewarded following a lever press within this 10 second window, otherwise the trial was considered an omission. In order to master the retractable lever training task, rats were required to make fewer than 5 omissions in 90 trials over two consecutive days.
The spatial reversal task had an initial acquisition phase which was followed by three rule shifts. For the acquisition task, the rewarded (left or right) lever was opposite of the rats predetermined side bias. A randomly determined cue light above a lever was illuminated for 3 seconds prior to lever extension, remained illuminated until the end of the trial, and was not predictive of the rewarded lever. Both levers were presented and the rat was required to make a choice within 10 seconds. A correct response dispensed a single food pellet, while incorrect responses and omissions (not making a choice within 10 seconds) were not rewarded. After each response, or omission, a 20 second inter-trial-interval preceded the start of the next trial. For all tasks, 10 consecutive correct responses were required to complete the task. If the rat did not complete the task in 200 trials over the course of 1 hour, the animal would be retested on the same task the next day and responses on the task would be combined. The next three reversal tasks required the rat press the opposite lever from the previous phase. The number of trials required to reach criterion (10 consecutive correct lever presses), the number of errors (unrewarded lever presses), the latency to lever press, and the latency to collect food reward were recorded for each phase.
Tissue Collection
One week after completion of the operant set shifting test, rats were euthanized (Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MO, USA) by rapid decapitation. The whole brain was removed, cut sagittal and placed in a 4% paraformaldehyde solution for 1 week followed by submersion into a 30% sucrose solution in 0.1 M PBS at 4 C for 4-8 days.
Cresyl violet staining
To determine the location of cannula implantation and probe insertion, the hemispheres brains were sliced at 40 microns using a microtome (Sm2000r Leica Biosystems, Wetzler, Germany) and stored in cyroprotectant solution (62.8 mg Na2HPO4, 160 mL dH2O, 120 mL 120 mL glycerol) at −20 C.
Brain sections from the OFC were mounted onto slides immediately after slicing and stained using a cresyl violet protocol.25,37 Slides were placed in a series of ethanol (95%, 70%, 50%) and water (distilled H20) treatments to rehydrate the material before staining with the cresyl violet stain solution (FD Cresyl Violet Solution; PS102-2; FD Neurotechnologies, Columbia, MD, USA). The tissue was stained in cresyl violet for 5 min and dehydrated again in reverse order with placing the tissue in distilled water, followed by 50%, 70% and 95% ethanol and finally Citrisolv (04-355-121; Fisher Scientific, Waltham, MA, USA). Slides were cover slipped using Permount mounting medium (SP15- 500; Fisher Scientific, Waltham, MA, USA) in preparation to be microscopically analyzed.
Statistical analysis
A repeated-measures analysis of variance (ANOVA), with Sex (AIE female x AIE male) as the between-subjects factor, was used to analyze BEC (across time). For Experiment 2, a two-factor ANOVA (Treatment: AIE vs. CON; Sex: Females vs. Males) was used to assess the spontaneous alternation data such as percentage of alternation and number of arms entries. To identify changes in ACh efflux from baseline, during maze, and after behavioral testing phases, the microdialysis data were analyzed using a two-factor ANOVA with repeated measures (Phase x Sample). Fisher's LSD post hoc tests were used to assess the differences between Treatment (AIE vs. CON) and between Sex (Females vs. Males). Operant set shifting data were analyzed using two-factor ANOVA for each dependent variable in each task. The SPSS statistical package was used for all analyses and values of p < 0.05 were considered significant. Homogeneity of variance was assessed with Levene’s test. Any violations in homogeneity of variance across sex or treatment conditions are noted. There were no significant sex differences in cholinergic function or behavior, but studies were not powered to detect difference in hormonal status in male or female rats.
Experiment 3:
Golgi-Cox staining
Approximately 60 days after treatment (PD115-125), rats (AIE, CON) were euthanized (Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MO, USA) by rapid decapitation under light. The whole brain was removed, divided with a sharp blade into coronal blocks of approximately 5 mm thickness to allow for successful impregnation and placed in Golgi-Cox solution A + B (FD Rapid Golgi Stain Kit; Cat.#PK401, FD Neuro Technologies, Inc. Columbia, MD, USA) for 2 weeks, followed by Solution C for one month at room temperature in the dark. The block containing the orbital frontal cortex was sliced coronally at 200 μm-thick using a sliding microtome (Sm2000r Leica Biosystems, Wentzler, Germany). Slices (Bregma 5.16 to 3.72)38 were immediately transferred sequentially to double gelatinized slides at 6 slices per slide using Solution C. After being kept in the dark for maximum 72-hours, the slices were stained under red light with solution D+E and underwent a dehydration process according with the protocol as outlined by FD Rapid Golgi Stain Kit User Manual. Slides were cover slipped using Permount immediately after development. Following cover slipping, the slides were left untouched in a dark room for one month until completely dry.
Dendritic Analysis
Prior to analysis, the slides were coded randomly by a secondary experimenter to blind the experimenter to condition. Using a computer-based neuron tracing system (NeuroLucida; MicroBrightField, Williston, VT, USA), eight neurons for each animal in the OFC were identified under the 5x objective. Using a 20x objective, the experimenter ensured that the neuron fit the following criteria: pyramidal-shaped cell body, located in layer III in the OFC, apical dendrite with depths between 200 and 500 μm from the cortical surface, have branch orders 3 and 5 with at least 60 μm each on the apical dendrite and neurons was not broken. Once the neuron had been determined to meet the aforementioned criteria, the experimenter traced the neuron’s cell body and apical dendrites, under the 40x objective. In order to determine dendritic complexity, the total length and a Sholl analysis were performed for each neuron to analyze dendritic complexity. The average number of intersections with each radii 20μm of concentric sphere surface was analyzed for each group
For all neurons, spine analysis was performed on the same dendritic branches for Order 3 and Order 5 previously traced using the 100x magnification with an oil immersion lens. The spines were assessed and marked in a total of 60μm in length of branches in orders 3 and 5 of the apical dendrite and the spine density was calculated per 10μm. All spines identified were also classified as mature and immature following the protocol outlined by Risher and collegues.39 The percentage of mature and immature spines were calculated for each animal.
Statistical analysis
The effect of Treatment and Sex on apical dendritic length were analyzed using a two-factor analysis of variance (ANOVA). A two-factor ANOVA with repeated measures for Distance from the soma was used in Sholl analysis, followed by Fisher's LSD post hoc tests to assess the differences in dendritic complexity between Treatment (CON vs. AIE) and Sex (Males vs. Females). A repeated measures ANOVA was also used to evaluate within-subject effects (Phenotype [mature, immature] and branch Order [3rd , 5th]) and between-subject effects (Treatment, Sex) for spine density. Two-factor repeated-measures analysis of variance (ANOVA) with branch Order as the within-subject variable and Treatment and Sex as the between-subject variables was used to analyze the spine density of each spine phenotype separate and the percentage of mature or immature spines. The percentage of mature spines versus immature spines was analyzed using a repeated-measures ANOVA with Phenotype as the within-subject variable. The SPSS statistical package was used for all analyses and values of p < 0.05 was considered significant. Any violations in homogeneity of variance across sex or treatment conditions are noted. There were significant sex differences, but studies were not powered to detect difference in hormonal status in male or female rats.
Results
AIE rats reached heavy binge-like levels of intoxication
Rats exposed to AIE had high BEC that well exceeded binge EtOH benchmarks of 80 mg/dL40 and are in the range of extreme binge levels, over 200 mg/dl.41 There was a significant Experiment X Time interaction (F [2, 34] = 3.99, p < 0.05, η2 = 0.45). In Experiment 2 (first day = 203.73 mg/dl, SEM = 10.85; final day =180.14 mg/dl, SEM = 9.44) and Experiment 3 (first day = 216.31 mg/dl, SEM =12.29; final day = 180.74 mg/dl, SEM = 10.69) the BEC was higher on the first day of gavage compared to the last day of gavage. However, in Experiment 1 there was no difference in BEC as a function of sampling time (First day = 230.46 mg/dl, SEM = 10.51; final day = 250.63 mg/dl, SEM = 9.14). Male and females did not have significantly different BEC levels (F [1,34] = 3.45, p = 0.72, η2 = 0.09), and there were no Sex x Experiment interactions detected (F [2,34] = 2.44, p > 0.103, η2 = 0.13).
Experiment 1
Cholinergic markers are persistently down regulated in the nucleus basalis magnocellularis and the orbitofrontal cortex.
As shown in Figure 2A, ChAT expression within the in the NbM was significantly lower in AIE treated rats (F [1,22] = 5.39, p < 0.05, η2 = 0.19), compared to control rats, and no Sex or Treatment x Sex interactions were significant. Additionally, VAChT expression in the OFC (Figure 2B) was significantly reduced in AIE treated rats (F [1, 22] = 9.57, p = 0.005, η2 =0.31), compared to control rats. No Sex, or Treatment x Sex interactions were detected.
Experiment 2
AIE spared spatial memory assessed using a spontaneous alternation task
Analysis of the percentage alternation did not reveal and differences as a function of ethanol exposure (Treatment) or Sex (both: F [1,29] ≤ 1.04, p = 0.32, η2 = 0.40; Fig 2C). Thus, this type of spatial memory is spared following AIE. However, the number of arm entries during testing were affected by Treatment (F [1,25] =8.74, p = 0.007, η2 =0.26) and by Sex (F [1,25] = 14.40, p = 0.001, η2 = 0.37; Fig 2D). Fisher's LSD post hoc tests showed that activity of CON animals on the maze were higher than AIE rats (p=0.007), and females were more active than male rats (p=0.001) during the testing. T-tests showed that CON female rats made significantly more arm entries than CON male rats (t [13] = 4.03; p = 0.001) and more than AIE female rats (t [10] = 2.95; p = 0.014). AIE male rats did not differ from CON male rats (t[13]= 1.15; p = 0.26) or AIE female rats (t[10]= −1.57; p = 0.14).
AIE reduced ACh efflux in the orbital frontal cortex
Overall, AIE treatment did not affect baseline ACh concentration in the OFC, but blunted activity-dependent ACh efflux during navigation of the plus maze. The analysis of ACh efflux in the OFC indicated a main effect of Treatment (F [1,23] = 12.26, p = 0.002, η2 = 0.24; Fig. 2E). However, there was no effect of Sex (F[1,23] =0.17, p = 0.69, η2 = 0.01; or interaction between Treatment and Sex (F [1,23] = 1.23, p = 0.28, η2 = 0.01). While CON rats showed a large increase in efflux of ACh during maze testing, this effect was notably blunted in AIE rats as there were significant interactions between Treatment x Phase (F [2,50] = 5.598, p = 0.006, η2 = 0.18), and Treatment x phase x samples (F [4,100] = 4.52, p = 0.002, η2 = 0.15). Due to the significant interactions, the ACh efflux data were analyzed separately during behavioral testing (maze) and after maze phases. During maze and after maze phase, AIE-treated rats had blunted OFC ACh efflux compared to CON rats (maze: F[1,23] = 9.73; p = 0.005, η2 = 0.28; after maze: F[1,23] = 4.53; p = 0.044, η2 = 0.14). Homogeneity of variance was found to have been violated when analyzing basal femtomoles ACh levels in the OFC (Fig. 2F) using Levene's test (3,25) = 25.39, p <0.001; therefore, the F statistic could not reliably predict significant differences between groups. A Kruskal-Wallis non-parametric test was used instead, revealing no significant difference in baseline femtomoles between groups (H[3] = 1.08, p > 0.05).
AIE did not disrupt operant reversal learning
AIE did not affect the number of trials to criterion or number of errors in any phase of the operant testing (see Figure 3). All groups demonstrated progressive learning of the serial reversal task marked by significant reductions in the number of trials to reach criterion (F[3,105] = 7.02, p < 0.001, η2 = 0.16); and fewer errors made (F[3,105] = 11.27, p < 0.001, η2 = 0.24), however no treatment, or sex effects were detected in performance across reversals. Across successive reversals, all subjects collected the food reward more quickly (F[3,105] = 9.69, p < 0.001, η2 = 0.21), but did not differ in their response time to lever press (F[3,105) = 0.52, p > 0.05), and no Treatment or Sex effects were observed.
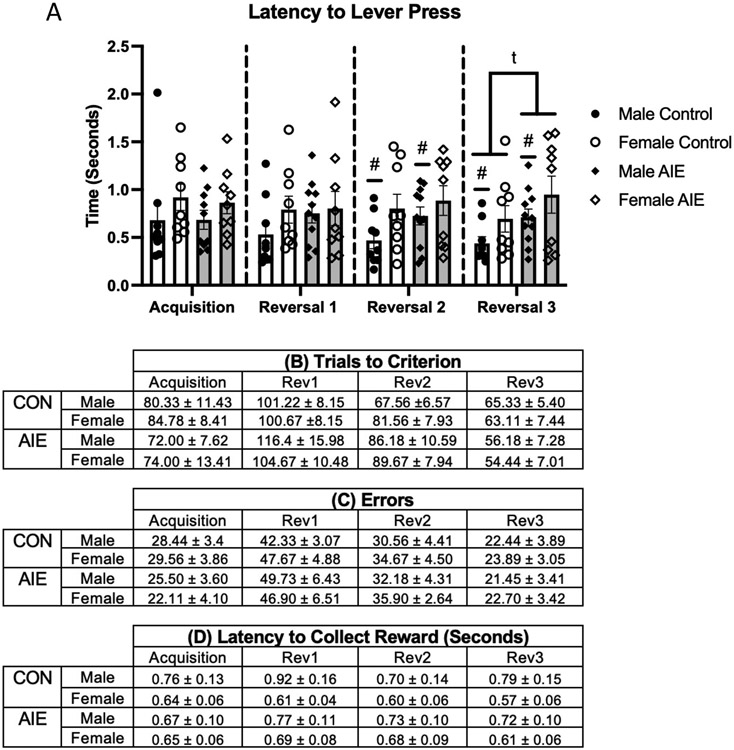
Figure 3. Adolescent intermittent ethanol exposure (AIE) did not affect acquisition, reversal learning nor response times in an operant two-lever paradigm.
The latency to lever press on any task was not affected by AIE (A). However, during the second and third reversal female rats took longer to make a decision than male rats. In addition, there was a trend for male AIE rats to take longer to press the lever than male control rats. The number trials to reach criteria (B) and the number of errors made (C) by AIE- and CON-treated female and male rats during acquisition and several serial reversals were not different. Latencies to collect reward (D) were not different as a function of task, sex or AIE treatment. All data are presented as the mean ± SEM. # represents a significant Sex effect, p< 0.05; t represents a trend towards a significant treatment effect, p=0.06.
During the acquisition, there was no effect of Treatment or Sex (both F’s[1,34] < 1.0, p > 0.15). There were no group differences in terms of errors on this task as a function of Treatment or Sex (both F’s [1,33] < 1.93, p > 0.15). Latency to lever press was also analyzed and identified no Treatment or Sex effects (all F’s [1,33] < 2.47, p > 0.10). Additionally, no Treatment or Sex effects were present in latency to collect reward (both F’s [1,33] < 2.82, p > 0.10). AIE treatment did not produce any differences in measured behavior on the acquisition of the serial reversal task.
For trials to criterion on the first reversal, no Treatment or Sex differences were observed (both F’s [1,35] <1.0, p > 0.15). Similarly, analyzing the number of errors revealed no Treatment or Sex effects (F’s [1,35) <1.0, p>0.15. Latency to lever press did not differ as a function of Treatment or Sex (both F’s [1,35] <1.59, p > 0.15). Additionally, there were no group differences in latency to collect reward based on Treatment or Sex (both F’s [1,35] < 2.82, p > 0.10). Similar to the acquisition, AIE treated animals performed similarly to controls on the first reversal.
On the second reversal, no Treatment or Sex differences were observed in the number of trials required to reach criterion (both F’s [1,35] < 2.71, p > 0.10). Additionally, there were no Treatment or Sex differences on the number of errors made (F’s [1,35] < 1.0, p > 0.15). While no Treatment (F [1,35] = 1.62, p > 0.15), differences were observed in latency to lever press, a significant effect of Sex was observed (F [1,35] = 4.67, p < 0.05, η2 = 0.12): Male rats (M = 0.59 seconds; SEM = 0.08) responded more quickly to lever presentation than female rats (M =0.84 seconds; SEM = 0.09). Lastly, no Treatment or Sex differences were identified in latency to collect food reward (F’s [1,35] < 1.0, p > 0.10). While AIE did not produce significant changes in performance on the second reversal, an emerging sex difference was identified where male rats responded more quickly to lever presentation than female rats.
On the third, and final, reversal no Treatment or Sex effects were observed in trials or errors to reach criterion (both F’s [1,35] < 1.67, p > 0.10). Analyzing latency to lever press on the third reversal revealed a trending Treatment effect (F [1,35] = 3.74, p = 0.06, η2 = 0.10), where CON rats (M = 0.56 seconds, SEM = 0.09) responded quicker to lever presentation than AIE rats (M = 0.81 seconds, SEM = 0.10). There was also a significant main effect of Sex F (1,35) = 4.34, p < 0.05, η2 = 0.11, with female rats (M = 0.82 seconds, SEM = 0.10) responding slower than male rats (M = 0.57 seconds, SEM = 0.05). Lastly, when examining latency to collect food reward, there were no differences as a function of Treatment or Sex (both F’s [1,35] < 2.31, p > 0.10). On the final reversal, the observed sex difference of males responding faster to lever presentation continued, while CON rats initiated the lever press faster than AIE rats.
Experiment 3:
Representative images and tracing of a neuron cell body, apical dendrite and dendritic spines in OFC are provided in Figure 4A.
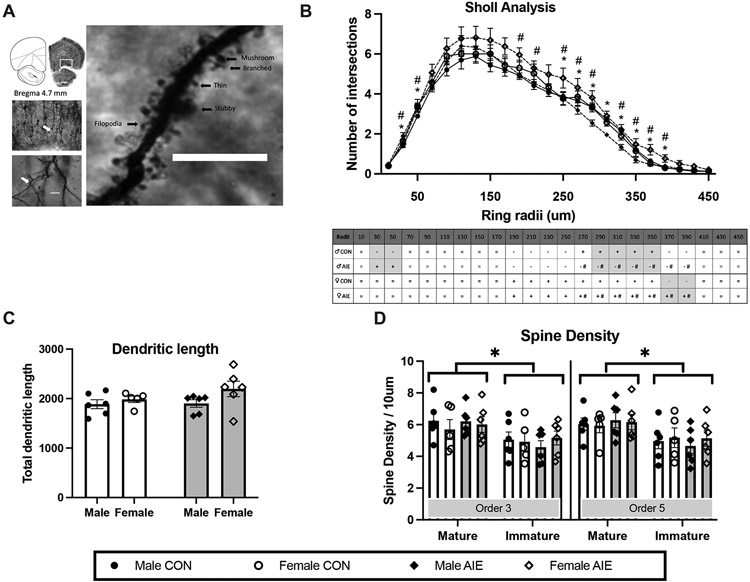
Figure 4. Adolescent intermittent ethanol exposure increased the apical dendritic complexity in pyramidal neurons within the orbital frontal cortex (OFC).
(A) Representative image of the apical dendrites of pyramidal neurons in the OFC (middle left: 5x objective; bottom left: 20x objective; Scale bar = 25 μm) in OFC. The tracing demonstrates the ordered branches and the arrow shows the order 5. The right image is then magnified to a 100x objective (oil lens) in order to determine the spine density and phenotype (example spine phenotype classifications; Scale bar = 10 μm). (B) Sholl analysis revealed that AIE increased the number of dendritic branches, close to the soma for males and away from the soma for females. (C) Dendritic length was not affected by AIE. (D) Spine density was affected by AIE. However, there were more spines of the mature phenotype, relative to the immature phenotype, in both 3 and 5 order branches. All data are presented as the mean ± SEM. # represents a significant Sex effect, p< 0.05; * represents significant Treatment (CON vs. AIE) effect, p< 0.05; #* together represents a Sex X Treatment interaction p< 0.05; + represents increase in number of branch intersection; - represents decrease in number of branch interactions; = represents no significant difference.
AIE and Sex influence apical dendritic complexity in the OFC
AIE changed apical dendritic branching in a sex-dependent fashion that was dependent on the dendritic domain or distance from the soma. A repeated measure ANOVA (Treatment x Sex x Distance from soma) of apical dendritic intersections in OFC revealed main effects of Treatment (F [1, 16] = 8.35, p = 0.011, η2 = 0.34), Sex (F [1, 16] = 17.67, p = 0.001, η2 = 0.52) and Distance from soma (F [23, 368] = 35.86, p < 0.001, η2 = 0.95). Also, the analysis revealed several significant interactions: Treatment X Sex (F [1, 16] = 5.48, p = 0.032, η2 = 0.25), Treatment X Distance (F [23, 368] = 1.73, p < 0.021, η2 = 0.09) and Distance X Sex (F [23, 368] = 1.86, p = 0.01, η2 = 0.10). Fisher's LSD post hoc tests showed that female rats have significantly more intersections than male rats, particularly at the later intersections (190 μm [p = 0.028], 210 μm [p = 0.041], 270 μm [p = 0.049], 290 μm [p = 0.016]).
A detailed analysis of the influence of AIE on dendritic complexity as a function of sex revealed that the dendritic complexity of CON male rats started with less intersections than AIE male rats at the early intersections (30 μm (t [10] = 2.32, p = 0.043) and 50 μm (t [10] = 2.57, p = 0.028); however, at the later intersections (290 μm through 350 μm; t’s [10] > 4.62, p’s < 0.01), the CON male rats have significantly more intersections than the AIE male rats. There was a trend for CON female rats to have fewer intersections compared with AIE female rats at the later intersections (370 μm (t [9] = 2.16, p = 0.058) and at 390 μm (t (9) = 2.19, p = 0.055). AIE female rats had more intersections than AIE male rats at 270 μm through 390 μm (t [10] < 3.50, p < 0.05).
AIE did not affect the length of OFC dendrites
Total dendritic length is another dendritic complexity parameter that can be used to examine the effects of AIE as a function of sex (Fig 4C). A two-way ANOVA showed only a trend for female rats to have a longer dendritic length in apical dendrites than male rats (F [1, 22] = 3.43, p = 0.079, η2 = 0.53), regardless of treatment condition. There was no effect of Treatment on the total dendritic length of apical dendrites in the OFC.
AIE exposure did not alter the total spine density or alter the density of mature or immature phenotypes, but mature dendritic spines were the most prevalent in the OFC
The ANOVA revealed no effects of Order of branches (3 vs 5; F[1,19] = 0.40, p = 0.53), Treatment (F[1,19] = 0.012, p = 0.91) or Sex (F[1,19] = 0.013, p = 0.91) in total spine density. However, the analysis revealed a main effect of Phenotype (F [1, 19] = 7.067, p = 0.016, η2 = 0.27, see Fig 4D). There was a significantly higher percentage of mature spines, relative to immature spines, regardless of Treatment or Sex.
In a separate analysis, we analyzed the density of immature and mature dendritic spines. Mushroom and stubby phenotypes were classified as mature spines, whereas filopodia and thin phenotypes were classified as immature spines. There was a trend for a significant interaction between Order, Treatment and Sex on the density of stubby spines (F [1, 19] = 3.849, p = 0.065, η2 = 0.16). Overall, this interaction is driven by a small decrease in the density of stubby spines in Order 5 branches (average of spine density in Order 5 = 1.21 and Order 3 = 1.29), in female rats (average of spine density in females = 1.09 and males = 1.41) and AIE rats (average of spine density in AIE = 1.20 and CON=1.30). Furthermore, there were no main effects of Order (F [1,19] = 0.40, p = 0.83), Treatment (F [1,19] = 0.15, p = 0.69) or Sex (F [1,19] = 0.43, p = 0.51) on the percentage of mature or immature spines.
Discussion
The key findings of these experiments are the following: (1) Regardless of sex, there is persistent loss of ChAT protein expression in the NbM, and a loss of VAChT protein expression in the OFC, demonstrating long-term disruption of this cholinergic circuit following adolescent ethanol exposure; (2) Independent of sex, behaviorally-evoked cholinergic tone in the OFC is dramatically and persistently disrupted by binge-type ethanol exposure during adolescence; (3) The branching of apical dendric trees in the OFC is persistently increased following binge-type ethanol exposure during adolescence, but sex influences whether the increase in branching occurs near or away from the soma; (4) Not all types of reversal learning are impaired by adolescent binge-type ethanol exposure.
Previous work demonstrated that there is about a 30% suppression of the cholinergic phenotype in the nucleus basalis magnocellularis complex.25,26 This nucleus innervates the cortical mantle, including the OFC. 28,29 The results from Experiment 1 demonstrate the loss of the expression of ChAT protein in the NbM and revealed a related loss of VAChT in the OFC. A decrease in VAChT expression influences the amount of ACh loaded in synaptic vesicles and therefore its release.43 In adult mice, genetic elimination of VAChT from the forebrain caused deficits in reversal learning assessed by the Morris water maze and operant two-choice touch screen.43,44 Furthermore, performance on a 5-choice serial reaction time task is impaired by forebrain VAChT deletion, while impulsive and perseveration behaviors are spared.43
It should be noted that even small to moderate changes in the expression of VAChT has the potential to change synaptic transmission.42 Thus, although we do not see a general suppression of ACh levels, as basal femtomole values of ACh are not changed by AIE, the behaviorally-evoked changes in cholinergic tone are dramatically suppressed in the OFC following AIE. Interesting, hippocampal ACh efflux during maze mapping is not affected by AIE, but medial PFC ACh levels are also suppressed.25 Thus, despite the reduction in neurons expressing the cholinergic phenotype in all basal forebrain nuclei following AIE26, only the cortical projection regions display a significant reduction of activity-dependent cholinergic tone. Thus, the forebrain cholinergic projection to PFC is particularly vulnerable to binge-type adolescent ethanol exposure.45
We did not see impairment of spatial mapping on the spontaneous alternation task and this is supported by past work.17 However, we did see that AIE rats were less active on the maze, potentially due to their known increase in anxiety.45,46 Whether cholinergic dysfunction in the amygdala contributes to anxiety like behaviors following AIE needs further investigation. There were no sex-driven effects, nor interactions with AIE, on cholinergic parameters or spatial maze performance. However, the studies were not designed to access hormonal status in females (estrus cycle) or male (dominance), but differences in variance across many measurements were not observed. Therefore, it is unknown whether hormonal status interacts with cholinergic function following adolescent ethanol exposure.
An assessment of reversal learning was included as performance on such tasks can be dependent on the OFC47,48 and reversal learning can be disrupted by AIE.12,27,49 However, the serial reversal paradigm used did not reveal AIE-induced deficits. The operant two-choice lever paradigm, without modulating reinforcement values, may have not been complex or difficult enough to evoke the OFC as rats may have adapted a simple win-shift strategy rather than a complex stimulus, response, reward contingency map, which is OFC dependent.46,47
The persistent microstructural changes to the OFC induced by intermittent ethanol exposure throughout adolescence are somewhat subtle: an increase in dendric branch complexity at different distances from the soma that is modulated by sex. Male rats exposed to AIE had an increase in dendritic branching close to the soma, whereas female rats exposed to AIE had an increase in dendritic branching away from the soma. Abnormal dendritic branching can lead to decreased or excessive synaptic connectivity and can contribute to alterations in cognitive functions.
Dendritic arborization is dynamic and determines the synaptic input field, but itself is influenced by synaptic activity and pathological conditions can lead to dendritic remodeling.50 As a morphogen, ACh has powerful effects on dendritic arborization in adulthood and development.51-53 Release of ACh can reconfigure cortical microcircuitry. However, various dendritic changes are region specific in response to cholinergic modulation.51-52 The AIE-induced altered ACh tone is unlikely to be the sole contributor to dendritic remodeling following chronic ethanol exposure during adolescence or adulthood. Other studies have examined PFC dendritic remodeling after ethanol exposure on a shorter time scale and found that withdrawal is a critical component. A recent review demonstrates that cycles of alcohol intoxication and withdrawal lead to poorer behavioral and brain outcomes, especially if the ethanol exposure occurs during development.54
In rodents, ethanol exposure, followed by withdrawal, changes dendritic arborization and dendritic spine density in the PFC and hippocampus.39,55-57 Excitotoxic events, such as withdrawal from ethanol, can cause dendritic remodeling; For example 3-hours following Chronic Intermittent Ethanol (CIE) in adult mice, but not during intoxication, there was an increase the number of dendritic intersections at shorter distances from the soma in both basal and apical dendrites within the mPFC.56 This study also found CIE increased the spine density of pyramidal neurons in the mPFC, but CIE did not alter the total combined length of the apical and basal dendritic trees.
Similarly, in the lateral OFC, CIE in the absence of withdrawal in adult mice, had no effect on spine morphology or spine density in layer II/III pyramidal cells. However, when mice were allowed to undergo a 7-day withdrawal period, there were significant increases in spine density and long thin spines.58 The mature spine phenotype, mushroom-shaped or short stubby spines, were unchanged by CIE.
Different PFC regions show differential reactions to ethanol exposure and withdrawal. The effects of adult chronic ethanol exposure on the length of basal and apical dendrites of lateral OFC and medial PFC neurons were examined 3-days following ethanol withdrawal.59 It was found that following 3-days withdrawal from CIE there was hypertrophy of distal apical non-terminal dendrites and dendritic retraction of proximal apical dendrites of prelimbic (PrL) pyramidal neurons, but no effect on the arborization of pyramidal neurons in the OFC or infralimbic (Il) cortex. Adult CIE increases dendritic arborization and spine densities within basal and apical dendrites of pyramidal neurons represents an aberrant reorganization.56
Intermittent ethanol exposure during the adolescent period, relative to the adult period, suppresses spine density in a region-specific manner within the mPFC. The spine density within IL was significantly reduced 3 days following intermittent ethanol exposure, relative to air, in the adolescent exposed group, but not in adult-exposed mice.55 In contrast, in the PrL, overall spine density was not affected by AIE exposure, but was significantly lower in the adult mice than the adolescent mice. However, rats exposed to intermittent ethanol during early-mid adolescence (PD28–42), with brains assessed in adulthood, displayed a persistent increase in the density of long/thin dendritic spines, with no change in stubby or mushroom spines of layer 5 pyramidal neurons in the PrL cortex.57 Thus, the cortical microstructure recovery after protracted abstinence may also be region specific.
The increase in dendritic branching near the soma of the apical section of OFC neurons in adult male rats exposed to AIE is consistent with the observed abnormal structure of pyramidal neurons in the mPFC of male rats after adult long-term ethanol consumption56,60 However, our study included both male and female rats and we did observe alterations in dendritic complexity following AIE, but the dendritic domain affected differed as a function of sex. During the adolescence period, there are changes in dendritic arborization in the cortex of rats that are sex-specific. Specifically, the dendritic ramification of PFC neurons occurs earlier in females, relative to males, which is consistent with an earlier onset of puberty.61 Furthermore, cortical neurons of P28 female mice, compared with male mice, display greater dendritic complexity across the apical arbor.62 We found that female rats exposed to AIE have an increase in OFC neuronal apical dendritic arbors at the distal domain and this may be a “locking in” of an adolescent neuroanatomical characteristic in females.63 However, AIE-treated male rats have an increase in ramifications proximal to the soma, and then a decrease in the distal ramifications in OFC neurons. The increase of proximal ramifications in male rats and enhanced distal ramifications in female rats observed in OFC neurons after AIE suggests that developmental ethanol exposure may have protracted effects on the integration of various synaptic inputs, which are dependent on the distance or location on the dendrite. Synapses close to the soma produce greater excitatory postsynaptic potentials, over synapses that are a considerable distance from the soma.64 Furthermore, changes in complexity or density at different dendritic domains can also influence synaptic integration from various regions: Proximal apical dendrites receive inputs local-circuit neurons, whereas the distal apical tuft receives more diverse inputs from the thalamus and other cortical regions.65 Thus, the sex difference in dendritic density at different domains following AIE could produce different functional consequences that are dependent on local or distant circuit integration. However, the interpretation of the Sholl analysis is largely qualitative descriptive, as a comprehensive understanding of the functional factors of differences in dendritic density at different domains is currently limited66. Further investigation is required to understand how AIE-induced changes in dendritic complexity affects the integration of synaptic inputs to OFC neurons.
In summary, repeated extreme binge-type alcohol exposure leads to a long-term persistent change in the OFC cholinergic profile and microstructural organization. The dramatic suppression of behavioral-evoked ACh may contribute to the increase in dendritic complexity, as ACh is a known morphogen. The slight increase in apical dendritic branching within the OFC may be a compensatory response to the reduced cholinergic tone and altered cortical dopamine levels57, both reported following AIE. Data from human imaging and neuropsychological profile assessment suggests that there are persistent effects of adolescent binge drinking on specific cognitive domains and frontocortical measures that extend well into adulthood, and this has been replicated in rodents.67 However, the nature and degree of persistent brain changes and cognitive dysfunction is still being determined. Behavioral tests that have a more complex integration of changes in environmental and reward contingencies, with the need to modify response strategies, are likely to be more sensitive to AIE-induced brain damage within the OFC.
Supplementary Material
Funding Sources:
This work was supported by U01 AA028710 (LMS), P50 AA017823 (LMS), T32 AA025606 (JDJ)
Abbreviation List
- Ach
Acetylcholine
- AIE
Adolescent intermittent ethanol
- ChAT
Choline acetyltransferase
- CON
Control gavage treatment
- HDB
horizontal diagonal band
- MS/DB
medial septum/diagonal band
- NbM
Nucleus basalis of Meynert
- OFC
Orbital frontal cortex
- PFC
prefrontal cortex
- SI
substantia innominata
- VAChT
Vesicular acetylcholine transporter
References
- 1.Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL, Shih YI. Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addict Biol. 2018;23:810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172:531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neurosci Biobehav Rev. 2016;70:244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci. 2018;19:197–214. [DOI] [PubMed] [Google Scholar]
- 5.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron. 2010;67:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol. 2017;44:122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–22. [DOI] [PubMed] [Google Scholar]
- 8.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadhav KS, Boutrel B. Prefrontal cortex development and emergence of self-regulatory competence: The two cardinal features of adolescence disrupted in context of alcohol abuse. Eur J Neurosci. 2019;50:2274–2281. [DOI] [PubMed] [Google Scholar]
- 10.Sanhueza C, García-Moreno LM, Expósito J. Weekend alcoholism in youth and neurocognitive aging. Psicothema. 2011;23:209–214. [PubMed] [Google Scholar]
- 11.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol. 2009;44:115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman LG, Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014;116:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas WM, Bengston L, Gilpin NW, Whitcomb BW, Richardson HN. Alcohol binge drinking during adolescence or dependence during adulthood reduces prefrontal myelin in male rats. J Neurosci. 2014;34:14777–14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Crews FT. Adolescent intermittent ethanol exposure enhances ethanol activation of the nucleus accumbens while blunting the prefrontal cortex responses in adult rat. Neuroscience. 2015:293;92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gass JT, Glen WB, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology. 2014;39: 2570–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez GM, Lew BJ, Vedder LC, Savage LM. Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience. 2017;348:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbia C, Cadaveira F, Lopez-Caneda E, Caamano-Isorna F, Rodriguez Holguin S, Corral M. Working memory over a six-year period in young binge drinkers. Alcohol. 2017;61:17–23. [DOI] [PubMed] [Google Scholar]
- 19.Salling MC, Skelly MJ, Avegno E, Regan S, Zeric T, Nichols E, Harrison NL. Alcohol consumption during adolescence in a mouse model of binge drinking alters the intrinsic excitability and function of the prefrontal cortex through a reduction in the hyperpolarization-activated cation current. J Neurosci. 2018;38:6207–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013;244:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badanich KA, Fakih ME, Gurina TS, Roy EK, Hoffman JL, Uruena-Agnes AR, Kirstein CL. Reversal learning and experimenter-administered chronic intermittent ethanol exposure in male rats. Psychopharmacology. 2016;233:3615–3626. [DOI] [PubMed] [Google Scholar]
- 22.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. [DOI] [PubMed] [Google Scholar]
- 23.Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol Rev. 2016;68:1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boutros N, Semenova S, Liu W, Crews FT, Markou A. Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int J Neuropsychopharmacol. 2015;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez GM, Savage LM. Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience. 2017;361:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS One. 2014;9:e113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vetreno RP, Bohnsack JP, Kusumo H, Liu W, Pandey SC, Crews FT. Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: Restoration with voluntary exercise. Addict Biol. 2020;25:e12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolf NJ, Hernit MC, Butcher LL. Cholinergic and non-cholinergic projections from the rat basal forebrain revealed by combined choline acetyltransferase and Phaseolus vulgaris leucoaglutinin immunohistochemistry. Neurosci Lett. 1986;66:281–286. [DOI] [PubMed] [Google Scholar]
- 29.Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, et al. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: An experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex. 2015;25:118–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter MJ. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: Converging electrochemical and fMRI evidence from rats and humans. J Neurosci. 2013;33:8742–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Runfeldt MJ, Sadovsky AJ, MacLean JN. Acetylcholine functionally reorganizes neocortical microcircuits. J Neurophysiol. 2014;112:1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarter M, Lustig C, Howe WM, Gritton H, Berry, AS. Deterministic functions of cortical acetylcholine. Eur J Neurosci. 2014;39:1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage LM, Chang Q, Gold PE. Diencephalic damage decreases hippocampal acetylcholine release during spontaneous alternation testing. Learn Mem. 2003;10:242–246. [DOI] [PubMed] [Google Scholar]
- 36.Vinson PN, Justice JB Jr. Effect of neostigmine on concentration and extraction fraction of acetylcholine using quantitative microdialysis. J Neurosci Methods. 1997;73(1):61–67. [DOI] [PubMed] [Google Scholar]
- 37.Paul CA, Beltz B, Berger-Sweeney J. The nissl stain: A stain for cell bodies in brain sections. CSH Protoc. 2008;pdb.prot4805. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson G. The rat brain in stereotaxic coordinates. Cambridge, MA: Academic Press; 2013. [Google Scholar]
- 39.Risher ML, Fleming RL, Risher WC, et al. Adolescent intermittent alcohol exposure: Persistence of structural and functional hippocampal abnormalities into adulthood. Alcohol Clin Exp Res. 2015;39(6):989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen-Louie TT, Tracas A, Squeglia LM, Matt GE, Eberson-Shumate S, Tapert SF. Learning and memory in adolescent moderate, binge, and extreme-binge drinkers. Alcohol Clin Exp Res. 2016;40:1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prado VF, Roy A, Kolisnyk B, Gros R, Prado MA. Regulation of cholinergic activity by the vesicular acetylcholine transporter. Biochem J. 2013;450:265–274. [DOI] [PubMed] [Google Scholar]
- 43.Kolisnyk B, Al-Onaizi MA, Hirata PH, Guzman MS, Nikolova S, Barbash S, Soreq H, Bartha R, Prado MA, Prado VF. Forebrain deletion of the vesicular acetylcholine transporter results in deficits in executive function, metabolic, and RNA splicing abnormalities in the prefrontal cortex. J Neurosci. 2013;33:14908–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martyn AC, De Jaeger X, Magalhães AC, Kesarwani R, Gonçalves DF, Raulic S, Guzman MS, Jackson MF, Izquierdo I, Macdonald JF, Prado MA, Prado VF. Elimination of the vesicular acetylcholine transporter in the forebrain causes hyperactivity and deficits in spatial memory and long-term potentiation. Proc Natl Acad Sci. U.S.A. 2021;109:17651–17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyzar EJ, Zhang H, Pandey SC. Adolescent alcohol exposure epigenetically suppresses amygdala arc enhancer RNA expression to confer adult anxiety susceptibility. Biol Psychiatry. 2019;85:904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varlinskaya EI, Kim EU, Spear LP. Chronic intermittent ethanol exposure during adolescence: Effects on stress-induced social alterations and social drinking in adulthood. Brain Res. 2017;1654(Pt B):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: An updated perspective. Neuroscience. 2017;345:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galaj E, Kipp BT, Floresco SB, Savage LM. Persistent alterations of accumbal cholinergic interneurons and cognitive dysfunction after adolescent intermittent ethanol exposure. Neuroscience. 2019;404:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanoue V, Copper HM. Branching mechanisms shaping dendrite architecture. Dev Bio. 2019;451:16–25. [DOI] [PubMed] [Google Scholar]
- 51.Ballesteros-Yáñez I, Benavides-Piccione R, Bourgeois JP, Changeux JP, DeFelipe J. Alterations of cortical pyramidal neurons in mice lacking high-affinity nicotinic receptors. Proc Natl Acad Sci. U.S.A 2010;107:11567–11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang L, Tian MK, Bailry CDC, Lambe EK. Dendritic spine density of prefrontal layer 6 pyramidal neurons in relation to apical dendrite sculpting by nicotinic acetylcholine receptors. Front Cell Neurosci. 2015; 9:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mychasiuk R, Muhammad A, Gibb R, Kolb B Long-term alterations to dendritic morphology and spine density associated with prenatal exposure to nicotine. Brain Res. 2013;1499:53–60. [DOI] [PubMed] [Google Scholar]
- 54.Spear LP. Timing eclipses amount: The critical importance of intermittency in alcohol exposure effects. Alcohol Clin Exp Res. 2020;44:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jury NJ, Pollack GA, Ward MJ, Bezek JL, Ng AJ, Pinard CR, Bergstrom HC, Holmes A. Chronic Ethanol During Adolescence Impacts Corticolimbic Dendritic Spines and Behavior. Alcohol Clin Exp Res. 2017;41(7):1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct. 2015;220:1705–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, Glover EJ, Floresco SB, Crews FT, Krishnan HR, Pandey SC, Chandler LJ. Binge-like alcohol exposure during adolescence disrupts dopaminergic neurotransmission in the adult prelimbic cortex. Neuropsychopharmacology. 2017;42:1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGuier NS, Padula AE, Lopez MF, Woodward JJ, Mulholland PJ. Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol. 2015;49(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klenowski PM, Fogarty MJ, Shariff M, Belmer A, Bellingham MC, Bartlett SE. Increased synaptic excitation and abnormal dendritic structure of prefrontal cortex Layer V pyramidal neurons following prolonged binge-like consumption of ethanol. eNeuro. 2016;3(6):ENEURO.0248-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markham JA, Mullins SE, Koenig JI. Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress. J Comp Neurol. 2013;521(8):1828–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keil KP, Sethi S, Wilson MD, Chen H, Lein PJ. In vivo and in vitro sex differences in the dendritic morphology of developing murine hippocampal and cortical neurons. Sci Rep. 2017;7(1):8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, Rodd ZA, Spear LP, Swartzwelder HS, Vetreno RP. Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcohol Clin Exp Res. 2019;43(9):1806–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spruston N Pyramidal neurons: Dendritic structure and synaptic integration. Nature Rev. Neurosci 2008;9:206–221. [DOI] [PubMed] [Google Scholar]
- 65.Luebke JI, Weaver CM, Rocher AB, Rodriguez A, Crimins JL, Dickstein DL, Wearne SL, Hof PR. Dendritic vulnerability in neurodegenerative disease: insights from analyses of cortical pyramidal neurons in transgenic mouse models. Brain Struct Funct. 2010;214:181–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bird AD, Cuntz H. Dissecting sholl analysis into its functional components. Cell Rep. 2019;27:3081–3096.e5. [DOI] [PubMed] [Google Scholar]
- 67.Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav. 2020;192:172906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.