Abstract
Adult neurogenesis, the process by which neurons are generated in certain areas of the adult brain, declines in an age-dependent manner and is one potential target for extending cognitive healthspan. Aging is a major risk factor for neurodegenerative diseases and, as lifespans are increasing, these health challenges are becoming more prevalent. An age-associated loss in neural stem cell number and/or activity could cause this decline in brain function, so interventions that reverse aging in stem cells might increase the human cognitive healthspan. In this review, we describe the involvement of adult neurogenesis in neurodegenerative diseases and address the molecular mechanistic aspects of neurogenesis that involve some of the key aggregation-prone proteins in the brain (i.e., tau, Aβ, α-synuclein, …). We summarize the research pertaining to interventions that increase neurogenesis and regulate known targets in aging research, such as mTOR and sirtuins. Lastly, we share our outlook on restoring the levels of neurogenesis to physiological levels in elderly individuals and those with neurodegeneration. We suggest that modulating neurogenesis represents a potential target for interventions that could help in the fight against neurodegeneration and cognitive decline.
Keywords: Neurogenesis, neurodegeneration, aging, dentate gyrus, hippocampus, memory
1. Introduction
It is known that aging is a major risk factor for neurodegeneration, and that the most common neurodegenerative diseases are observed in the elderly (Hou et al., 2019). Despite ongoing research and progress in the field, cures for such chronic afflictions have not yet been found. Hence, their burden on society is very high and, as the population ages and lifespans lengthen, it is expected to increase (Dorsey et al., 2013; Zahra et al., 2020). Some researchers have suggested that a decline in the function of the central nervous system (CNS) is one of the main hallmarks of aging in mammals (Hayano et al., 2019). As such, this decline may be susceptible to interventions that attempt to target aging itself, simultaneously delaying or preventing other diseases as well (extending healthspan). One attractive theory is that the decline in brain function with age is caused by a loss in stem cell number and/or activity over time, and that interventions that delay or reverse aging in stem cells might extend human lifespan and healthspan (Schultz and Sinclair, 2016). In the brain, those stem cells are neural stem cells (NSCs), and the process of generating new neurons is called neurogenesis.
1.1. Neurogenesis
Does adult neurogenesis exist in humans?
The discovery of neurogenesis in the adult mammalian brain occurred by serendipity in the 1960s by Joseph Altman and Gopal Das (Altman, 2011, 1962; Altman and Das, 1965). The finding was mostly neglected through the 1970s and 1980s (Altman, 2011), and in 1985, a widely-cited paper denied the possibility of the phenomenon in primates (Rakic, 1985). In the late 1990s it was confirmed that neurogenesis occurs in adult macaques (Gould et al., 1999) and humans (Eriksson et al., 1998), but the controversy remains. For example, recent studies directly contradict each other, with one showing that adult hippocampal neurogenesis (AHN) drops sharply in children to undetectable levels in adults (Sorrells et al., 2018), and the other showing that AHN persists throughout aging (Boldrini et al., 2018). Many questions remain unanswered, and while some studies supported Sorrells et al.’s study (Seki et al., 2019), others claimed that it was not optimized for detecting neurogenesis (due to postmortem delay, long fixation period and other factors). They argued that the findings by Boldrini et al. are more in line with the current body of knowledge that supports the existence of AHN in humans (Kempermann et al., 2018; Lucassen et al., 2020). Moreover, a recent study by Moreno-Jiménez et al. (2019), that was methodically optimized for detecting neurogenesis (through tissue fixation, autofluorescence quenching, epitope retrieval, antibody selection and selection of subjects with a short post-mortem delay), was able to detect immature neurons in the dentate gyrus (DG) of 13 healthy individuals up to the ninth decade of life (Flor-García et al., 2020; Steiner et al., 2019). However, some researchers claim that the number of newborn neurons in those studies is likely to be overestimated, as the expression of putative progenitor cell and immature neuron markers does not present definitive evidence for adult neurogenesis. According to their critique, these markers can be re-expressed in mature adult neurons through the process of “dematuration”, a phenomenon in which mature neurons dedifferentiate to a pseudo-immature status and re-express the molecular markers for neural progenitor cells and immature neurons (Hagihara et al., 2019). A recent pair of “Dual Perspectives” articles argue for and against the existence of AHN, but both agree that further in-depth studies of AHN are extremely important (Moreno-Jiménez et al., 2021; Sorrells et al., 2021). Lastly, a recent study utilizing single-nucleus RNA sequencing to thoroughly profile cells from the hippocampal-entorhinal system showed that AHN is virtually absent from adult human donors (Franjic et al., 2021). Also, it is possible that higher AHN was not detected due of the postmortem delay, the insufficient number of cells profiled (~139k), or other confounding factors. Hence, the controversy still remains and, until neurogenesis has been measured more directly (e.g., after technical advances in magnetic resonance spectroscopic imaging) and until the cells are profiled even more thoroughly, the studies cited above (Boldrini et al., 2018; Elena P. Moreno-Jiménez et al., 2019; Spalding et al., 2013) are the strongest proof to date that neurogenesis persists in humans, even in old age. While a study using magnetic resonance imaging provided evidence for NSCs in vivo (Manganas et al., 2007), others question if the signal is specific to NSCs (Ramm et al., 2009) and note that the method is unable to differentiate hippocampal sub-regions (Boldrini et al., 2018). We also note that even if AHN is virtually absent in aged humans, it may still be possible to reactivate or stimulate it with various interventions.
Where does adult neurogenesis occur?
Neurogenesis is considered to occur in two so-called neurogenic areas of the brain: the subgranular zone (SGZ) of the hippocampal DG and the subventricular zone (SVZ) of the lateral ventricles (Hagg, 2009). While there have been reports of adult neurogenesis in “noncanonical” sites of the mammalian brain, such as the neocortex of primates (Gould et al., 1999), the cerebellum of rabbits (Ponti et al., 2008), the amygdala of mice (Jhaveri et al., 2018) and the striatum of humans (Ernst et al., 2014) (summarized in (Feliciano et al., 2015)), we will mostly focus on the hippocampus, as a consensus hasn’t been reached in regard to neurogenesis occurring in other areas (and in which species), much less the dynamics of the process. For instance, while immature neurons (neuroblasts) migrate from the SVZ to the olfactory bulb (OB) through the distinct rostral migratory stream (RMS) in rodents (Hagg, 2009), the existence and configuration of the adult RMS in humans remains highly debated (Arellano and Rakic, 2011; Bergmann et al., 2015; Sanai et al., 2011), just like the existence and the quantity of postnatal neurogenesis in the human OB (Bergmann et al., 2012; Lledo and Valley, 2016). Unlike those areas, neurogenesis in the hippocampus has been studied to a much greater extent, with rodent studies showing that the exposure to enriched environment has a beneficial effect on both neurogenesis and aging (van Praag et al., 2000), while models of aging-related neurological diseases such as AD and PD show impaired AHN, which could be one of the mediators of their respective pathologies (Toda et al., 2019).
Age- and neurodegeneration-dependent dynamics of neurogenesis
The hippocampus is a major brain region involved in memory, emotional processing, and vulnerability to stress, and is one of the most severely affected areas in AD (Brown, 1999; Dhikav and Anand, 2012). In fact, a defining feature of AD is the accumulation of tau and amyloid-β (Aβ) (Bloom, 2014), which begins in the entorhinal cortex, a major gateway to the hippocampus (Maass et al., 2015), and spreads to the cortex and the hippocampus itself (Khan et al., 2014; Toda et al., 2019). Two recent studies found that the number and maturation of new-born neurons in the hippocampus progressively declined as AD advanced and suggested that this decline might promote cognitive deficits or exacerbate them (Elena P Moreno-Jiménez et al., 2019; Tobin et al., 2019). Therefore, the levels of AHN could be considered as one of the potential biomarkers for neurodegenerative diseases such as AD (Lopez-Toledano et al., 2010). To utilize this biomarker in humans, besides obviously making the measurement less invasive, we would first need to determine the physiological range of AHN in healthy subjects, especially considering how it depends on factors like age, exercise and caloric intake (Levenson and Rich, 2007; Van Praag, 2008). Age-related dynamics of AHN across species have been reviewed elsewhere (Kozareva et al., 2019). We will just mention its dynamics in humans, which has been estimated through 14C levels in the genomic DNA of hippocampal neurons. The model estimated that around 700 new neurons are added in the hippocampus per day (0.004% of DG neurons), which corresponds to an annual turnover of 1.75% of the neurons within the renewing fraction, with a modest age-dependent decline (Spalding et al., 2013). However, it should be noted that the same study reported that the generation of new neurons in the DG does not keep up with the neuronal loss with age, and that the half-life of these newborn neurons in the renewing fraction is 10× shorter (7.1 years) than in the non-renewing fraction. It is not yet known whether just restoring the AHN to physiological levels would confer a therapeutic benefit for cognitive healthspan, or if further increase would be necessary. We will discuss this in more detail in the conclusion.
1.2. Functional relevance of newborn hippocampal neurons
The functional relevance of newborn hippocampal neurons has been implicated in many processes, such as resilience to and remission from stress, pattern separation, memory formation and learning, as well as in neurological disorders such as AD and PD (Anacker et al., 2018; Culig et al., 2017; Gonçalves et al., 2016; Höglinger et al., 2004; Elena P Moreno-Jiménez et al., 2019). As the latter will be described in more detail in section 2 of this review, we will now describe the data supporting the other roles of newborn neurons.
Stress
Animal studies showed that both stress and exposure to stress hormones (glucocorticoids) decrease the generation of hippocampal neurons and increase cell death (Culig and Belzung, 2016; Gould et al., 1998, 1992, 1991, but see Brunson et al., 2005), while chronic treatment with different classes of antidepressants has an opposite effect and increases neurogenesis (Malberg et al., 2000). Early-life stress in rodents has a negative effect on AHN (Criado-Marrero et al., 2020b; Naninck et al., 2015), hippocampal-dependent learning and memory (Rocha et al., 2021; Tzanoulinou et al., 2020) as well as on the later risk for cognitive impairments and AD (Hoeijmakers et al., 2017; Lesuis et al., 2018). In human studies, it has also been shown that smaller hippocampi constitute a risk factor for the development of stress-related psychopathology (Gilbertson et al., 2002). The level of AHN and/or the number neural progenitor cells decrease in patients with MDD and increase after treatment with antidepressants (Boldrini et al., 2012, 2009; Lucassen et al., 2010). A causal relationship between antidepressant treatment and newborn neurons was established, demonstrating that AHN is required for many of the behavioral effects of these drugs (Santarelli et al., 2003). A more nuanced picture emerged with further experiments showing that there are neurogenesis-dependent and -independent effects of antidepressants (David et al., 2009; Surget et al., 2008). Finally, gain-of-function studies where researchers were able to inducibly increase neurogenesis by inhibiting neuronal cell death (apoptosis) in transgenic mice established a role for newborn neurons in both resilience to and remission from stress (Culig et al., 2017; Eliwa et al., 2021; Hill et al., 2015). Another function of the hippocampus related to mood and stress is the regulation of the hypothalamic–pituitary–adrenal (HPA) axis, the main neuroendocrine system in mammals that provides a rapid response and defense against stress (Spiga et al., 2014). The inhibitory hippocampal regulation of the HPA axis is attenuated by exposure to stress (Mizoguchi et al., 2003; Surget et al., 2011), and newborn hippocampal neurons are required for appropriately maintaining this regulation (Schloesser et al., 2009; Snyder et al., 2011), which has implications for stress reactivity and mood disorders such as major depressive disorder (MDD). However, the exact role that newborn neurons play in HPA regulation and vulnerability to stress is not yet resolved (Lucassen et al., 2013), and has been discussed elsewhere (Culig et al., 2017).
Pattern separation
Pattern separation, defined as the process of transforming similar input patterns into less similar output patterns, is suggested to be crucial for discriminating memories that are similar in content and is performed in the DG (Lacy et al., 2010; Treves et al., 2008). Animal studies show that ablation of AHN impairs pattern separation (Clelland et al., 2009; Luu et al., 2012; Tronel et al., 2012), while increasing AHN is sufficient to improve it, regardless of whether the increase is obtained in a specific manner by genetically enhancing the survival of new neurons (Sahay et al., 2011) or through non-specific interventions such as enriched environment (Clemenson et al., 2015b). Behavioral paradigms to study pattern separation in animals, such as the location discrimination task, contextual fear conditioning and the newly developed spontaneous location recognition task, have been described elsewhere (Reichelt et al., 2021) and a meta-analysis of behavioral data supports the conclusion that AHN plays an important role in pattern separation (França et al., 2017).
Human studies that used (high-resolution) (f)MRI have provided compelling evidence for the involvement of DG in pattern separation (Bakker et al., 2008; Berron et al., 2016; Dillon et al., 2017; Hanert et al., 2019). Other indirect evidence has been summarized elsewhere (Lucassen et al., 2020), and includes results from tasks for measurement of pattern separation specifically in human DG (Stark et al., 2019), linking improved performance in it with an fMRI signal in the DG, indirectly supporting the idea that this function of newborn neurons is conserved in humans. DG dysfunction and pattern separation impairments during normal aging have been reported in non-human primates and humans (Small et al., 2004; Toner et al., 2009; Yassa et al., 2010). Further support came from a study that examined the relationship between performance in pattern separation tasks with lifestyle factors correlated with neurogenesis (aerobic exercise and high levels of stress) (Déry et al., 2013). The authors reported opposing effects of aerobic exercise (known to upregulate neurogenesis) and depression (which is associated with reduced DG cell proliferation and/or survival) on memory interference. However, because both of those factors have widespread effects, it is possible that one or more additional variables were affected by exercise and stress, which themselves could have caused or contributed to the observed effects. The exact mechanism through which newborn cells enhance pattern separation is still not known (Gonçalves et al., 2016). There are various difficulties in studying pattern separation, one of them being that to rigorously demonstrate that the DG is involved in pattern separation, it would be necessary to have the knowledge of DG’s inputs and outputs. While a rigorous test of this kind is lacking in vivo, a direct experimental demonstration of multiple forms of temporal pattern separation in DG brain slices has been provided recently (Madar et al., 2019a, 2019b). Likewise, due to the difficulties associated with measuring AHN in vivo, the direct confirmation of the role of newborn neurons in pattern separation is not confirmed in humans.
Learning and memory
Newborn hippocampal neurons have been implicated in many roles associated with memory and learning (Terranova et al., 2019; Zhao et al., 2008), such as the discrimination of temporal contexts (Rangel et al., 2014), cognitive flexibility (Anacker and Hen, 2017; Garthe et al., 2016), and even forgetting (Frankland et al., 2013). Animal studies show that impairments in AHN can result in specific cognitive deficits, for example in spatial relational memory acquisition (Dupret et al., 2008), the retention of long-term spatial memories (Deng et al., 2009) and in contextual fear conditioning (Saxe et al., 2006; Zhang et al., 2021). Conversely, positive regulators of AHN (such as environmental enrichment, astaxanthin supplementation, administration of ginseng, etc.) are all linked to improvements in learning and memory performance in animals, further implicating the role of newborn neurons in these cognitive processes (Sakalem et al., 2017; Yau et al., 2015; Yook et al., 2016). A recent study in which researchers genetically increased NSC cycle activity and numbers by symmetric proliferative divisions in mice found that the resulting increase in neurogenesis compensated the age-dependent decrease in it, rescuing allocentric navigation and contextual memory, hence rejuvenating critical aspects of brain function (Berdugo-Vega et al., 2020). In humans, bilateral surgical lesions of the hippocampal formation result in memory deficits (Scoville and Milner, 1957). While studies relating hippocampal neurogenesis with memory performance in humans are sparse for obvious reasons, one study in patients with chronic drug-resistant temporal lobe epilepsy found that the proliferation and neuronal differentiation capacity of adult human NSCs in vitro (which the authors claim is closely linked to neurogenic potential in vivo) was correlated with each patient’s ability to store and recall memories prior to surgery (Coras et al., 2010). They showed that patients with high proliferation capacity stem cells had a normal memory performance prior to epilepsy surgery, while patients with low proliferation capacity stem cells showed severe learning and memory impairment, which suggests that the encoding of new memories is related to the regenerative (neurogenic) capacity of the hippocampus. Abilities such as encoding new memories of episodes or facts, working memory and processing speed exhibit an age-associated decline in both cross-sectional and longitudinal studies. And cognitive stimulation might protect against these declines by enhancing neurogenesis (Hedden and Gabrieli, 2004).
1.3. Neural stem cells
NSCs are the source of new neurons in the adult mammalian brain and may be a promising therapeutic target. Specifically, targeting neurogenesis through pharmacological or non-pharmacological means may be beneficial for the treatment of a wide array of disorders, ranging from MDD and anxiety disorders to neurodegenerative diseases such as AD and PD (Berger et al., 2020; Coras et al., 2010). However, enhancing the levels of neurogenesis may not always be beneficial, as witnessed by some pathological conditions including epilepsy, where seizures induce neurogenesis and where decreasing its levels may be beneficial (Scharfman and Hen, 2007). Animal models of temporal lobe epilepsy show that prolonged seizures result in an increase of newborn neurons in the DG, but that some of them fail to migrate, differentiate and integrate properly (Scharfman, 2004). This type of aberrant neurogenesis might contribute to recurrent seizures in animals, and a similar process might be at play in some patients with mesial temporal lobe epilepsy (Parent et al., 2006). Since the neurodegenerative diseases we focus on in this review are associated with a reduction, rather than an increase in neurogenesis (Winner and Winkler, 2015), we will describe NSCs and the regulation of their proliferation in the adult mammalian brain in this section. The potential pitfalls of aberrant neurogenesis and/or its increase to supraphysiological levels will be discussed in more detail in the conclusion of this review.
Types of stem cells
Stem cells (and by proxy NSCs) are defined on the basis of two functional properties: a seemingly unlimited capacity for self-renewal and multipotency (Seaberg and van der Kooy, 2003). Self-renewal refers to the ability of these cells to undergo division, maintaining their ability to differentiate into multiple mature cell types - neurons, astrocytes, and oligodendrocytes in the case of NSCs. There are three types of stem cells with the potential to be used in stem cell-based therapies. Two of these are physiological, present at different stages of life: multipotent adult stem cells (ASCs) and pluripotent embryonic stem cells (ESCs), while one type is artificially engineered from a non-pluripotent cell; induced pluripotent stem cells (iPSCs) (Alvarez et al., 2012; Herreros-Villanueva, 2014; Mousavinejad et al., 2016). Although adult NSCs are multipotent, they generate specific cell types depending on the neurogenic region they belong to, resulting in a different outcome of neurogenesis in those areas. SVZ NSCs become fate restricted during embryonic development and produce oligodendrocytes and interneurons of the OB, which are inhibitory in nature (Ghosh, 2019). In contrast, the NSCs in the SGZ generate only excitatory granule neurons of the DG, and normally do not produce oligodendrocytes – their multipotency in vivo is restricted by the RNase III protein Drosha (Rolando et al., 2016). Other notable differences are described elsewhere (Ghosh, 2019; Nakafuku and Águila, 2019; Urbán and Guillemot, 2014) and include the involvement of migratory maturation in SVZ neurogenesis, while SGZ newborn neurons do not require much migration and are restricted to the granule cell layer of the DG.
In the hippocampus, the process of neurogenesis starts with quiescent NSCs. These cells are also called radial glia-like (RGL) cells and they consist of several subpopulations with different properties. In a recent study they were divided into two classes on the basis of their morphology: type α cells and type β cells (Gebara et al., 2016). Type α cells can give rise to neurons, astrocytes and type β cells, while type β cells do not proliferate and may represent an intermediate state in the transformation of type α cells into astrocytes. Once these quiescent cells are activated, they can divide symmetrically to generate additional RGLs (self-renewal), or asymmetrically to produce proliferating intermediate progenitor cells (IPCs, or Type-2 cells). IPCs are lineage-restricted and undergo limited rounds of rapid cell division, giving rise to bipolar neuroblasts (Type 3 cells) and then immature neurons (Berg et al., 2018; Bonaguidi et al., 2012). A study carried out in rats showed that half of these newborn neurons die before they are able to mature and become integrated granule neurons of the DG (Dayer et al., 2003).
Regulation of proliferation
Studies in rodents have shown that the number of NSCs decreases with age, which contributes to reduced neurogenesis (Kuhn et al., 1996; Maslov, 2004). Neurogenesis in rodents also decreases with age as a consequence of several other factors, including decreased proliferation and growth factor signaling (Shetty et al., 2005; Tropepe et al., 1997), increased levels of corticosteroids (Drapeau and Nora Abrous, 2008; Montaron et al., 2006), stem cell senescence (Audesse and Webb, 2020; Cutler and Kokovay, 2020) and epigenetic drift (Chen and Kerr, 2019). However, how aging affects the specific dynamics of processes such as NSC differentiation is not clear. While NSCs are a heterogenous populations, with subsets that may be unevenly affected by aging (Kuhn et al., 2018), they seem to shift from self-renewal during early development towards differentiation via asymmetrical division with aging (Nicaise et al., 2020). Glucocorticoid oscillations have been identified as one of the regulators of NSC proliferation during aging in vivo, possibly through an epigenetic mechanism, but it is not yet known how they affect differentiation (Schouten et al., 2020).
Stem cell frequency and self-renewal potential, as well as overall proliferation rate all decline with age in the mouse forebrain (Molofsky et al., 2006) and hippocampus (Lee et al., 2012). Curiously, despite this age-dependent loss of NSCs and a reduction in neurogenesis, when NSCs are removed from the aged environment (expanded in vitro), they retain their ability for proliferation and multilineage differentiation, generating functional neurons that are similar to that of NSCs in adult mice, albeit with lower efficacy (Ahlenius et al., 2009). This suggests that, with aging, neurogenic niches become unfavorable for neurogenesis. While some authors emphasize that this behavior is dissimilar to other stem cells and identify cell-extrinsic factors in the aged brain as the most relevant aspect that makes NSCs susceptible to aging (Schultz and Sinclair, 2016), it should be noted that age-dependent, intrinsic changes in the NSCs themselves seem to play a role as well (Ahlenius et al., 2009). Intrinsic and extrinsic factors that regulate NSCs have recently been reviewed elsewhere (Matsubara et al., 2021). These intrinsic changes, however, do not seem to affect the potential for functional integration of neurons differentiated from the adult and aged SVZ, in comparison to the neurons differentiated from NSCs of embryonic lateral ganglionic eminence. Taken together, these properties are highly relevant for potential future therapeutic applications, which will be described in more detail in section 4.
2. Neurogenesis in neurodegenerative diseases
Neurodegenerative diseases are a heterogeneous group of brain disorders, including Alzheimer’s disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and others (Cheyuo et al., 2019). Despite the different clinical manifestations and pathological mechanisms, progressive neuron loss/death and structural and functional defects in the neural system are common features of these diseases (Chi et al., 2018). Many studies suggest that dysregulated neurogenesis is a pivotal contributor to neurodegenerative diseases. While several other recent reviews focus on neurogenesis in the context of AD (Essa et al., 2022; Farioli-Vecchioli et al., 2022; Liu et al., 2021), our review explores neurogenesis on a broader level and describes how the most common geroscience interventions affect it. In this section, we mainly focus on the changes of neurogenesis in AD and PD, the two most prevalent neurodegenerative diseases, and briefly discuss other neurodegenerative diseases with reported defects in neurogenesis, such as HD, ataxia telangiectasia (A-T), and Cockayne syndrome (CS).
2.1. Alzheimer’s disease
AD is a progressive neurologic disorder primarily affecting elderly adults and eventually leading to a severe cognitive decline (Alzheimer's Association, 2019). It is the leading cause of dementia, which is predicted to affect 152.8 million people by 2050, highlighting the substantial social and economic burden worldwide (GBD 2019 Dementia Forecasting Collaborators, 2022). Memory impairment is the typical clinical symptom of AD (Jahn, 2013), and other clinical features involved in disease progression include executive dysfunction, language disorder, vision and olfactory impairment, and changes in mood and behavior (Graff-Radford et al., 2021; Kumar et al., 2018). AD patients are classified into early-onset Alzheimer's disease (EOAD) (< age 65), also known as familial AD and late-onset Alzheimer's disease (LOAD) (> age 65), which is more related to highly prevalent sporadic AD (Babcock et al., 2021). Deleterious mutations in amyloid precursor protein (APP), presenilin 1 (PS1), and presenilin 2 (PS2) are risk factors for EOAD. And Apolipoprotein E (APOE) is the major susceptibility gene associated with LOAD (Meyer et al., 1998). They will be discussed in more detail in section 3 of this review.
The progression of AD is associated with aging (Hou et al., 2019). Accumulated DNA damage and attenuated repair could exacerbate AD progression in both humans and mice (Hou et al., 2019). Neurons affected in AD exhibit mitochondrial dysfunction, suggesting a critical role of mitochondria in neuronal degeneration (Kerr et al., 2017). Additionally, the level of nicotinamide adenine dinucleotide (NAD+), a main contributor to mitochondrial dysfunction, decrease with age and in AD (Fang et al., 2017). Inflammation may be another critical neuropathological factor leading to neurodegenerative processes in AD (Fakhoury, 2018; Wang et al., 2019).
AD is more prevalent in females than in males (Alzheimer’s Association, 2019). Females also exhibit greater cognitive decline than males (Rodríguez et al., 2008; Sohn et al., 2018). The negative association between hippocampal volume and memory performance is observed exclusively in older women (Zheng et al., 2017). Female mice also exhibit an earlier age-related reduction of neurogenesis than male mice in the 3xAD animal models, as well as mitochondrial dysfunction (Demarest et al., 2020; Rodríguez et al., 2008). This deviation may be explained by the different levels of circulating sex hormones such as estrogen and testosterone (Clinton et al., 2007).
Except for genetic risk factors, aging and sex, some behaviors, such as sleep disturbance and caloric intake, have also been considered as risk factors for AD. Caloric restriction reduces Aβ and improves memory in AD mice (Hornsby et al., 2016; Schafer et al., 2015). Though many risk factors of AD have been revealed, the underlying mechanisms are still largely unknown, which impedes the discovery of drugs and treatment for the disease. Given the high complexity of AD, focusing on a single gene or pathway might be limiting. Treatments targeting aging, DNA repair, inflammation, and mitochondrial homeostasis as a combined strategy are more promising. And neurogenesis is a crucial modulator connecting them all.
2.1.1. Neurogenesis in AD patients
The hippocampus is one of the most affected brain areas in AD patients (Jahn, 2013). As AD progresses, tangles and plaques develop earlier in the hippocampus, entorhinal cortex, and olfactory bulb before being observed in the cortex (Price et al., 1991). These brain areas involved in neurogenesis might be particularly vulnerable in AD and reflect the disease process. In a genome-wide gene expression association study of AD, neurogenesis-related genes were identified as the top cluster (Talwar et al., 2014). A more recent genetic meta-analysis of LOAD patients also confirmed several novel variants, such as TREM2, ADAM10 and GPRC5B, which were related to immunity, lipid processing, tau and APP pathways (Kunkle et al., 2019). These genes also play roles in the regulation of neurogenesis, further confirming the possible relationship between neurogenesis and AD (Kurabayashi et al., 2013; Raha et al., 2017; Zhuang et al., 2015). Additionally, several genes involved in regulating cell survival and growth, such as CDC42, BDNF, and VEGFA, were reduced in AD patients, which may negatively impact neurogenesis (Baptista and Andrade, 2018; Yan et al., 2019). An epigenetic study on AD patients also found that the hypermethylated genes in AD hippocampus were mostly related to neural differentiation and neurogenesis, supporting neurogenesis-related genes as the main targets of epigenetic changes in AD hippocampus (Altuna et al., 2019). Thus, understanding the mechanisms involved in dysregulation of neurogenesis should provide new opportunities for developing preventive and regenerative therapies for AD.
Although reduced adult neurogenesis during healthy aging has been reported (Morgenstern et al., 2008), its direct effects in AD are still elusive. Earlier studies on AD patients reported increased neurogenesis in the hippocampus (Boekhoorn et al., 2006; Briley et al., 2016; Gomez-Nicola et al., 2014; Jin et al., 2004; Mikkonen et al., 1999) and the increase was associated with higher burdens of Alzheimer-type pathology (Perry et al., 2012; Wharton et al., 2005). The upregulated neurogenesis might be a temporal compensatory mechanism to replenish cells lost through degeneration in AD, which will result in the depletion of the neural progenitor cell (NPC) pool. A similar result was found in an in vitro study, in which NPCs that were derived from fibroblasts of AD patients exhibited accelerated neural differentiation and reduced progenitor cell renewal (Meyer et al., 2019). In contrast, as mentioned before, Moreno-Jimenez et al. (2019) found that AHN persists throughout life and progressively declines as AD progresses in the patients (Elena P Moreno-Jiménez et al., 2019). Furthermore, Tobin et al. found that patients with mild cognitive impairment exhibited fewer NPCs than normal subjects, demonstrating a correlation between cognitive function and neurogenesis in AD pathology (Tobin et al., 2019). Similarly, an increased number of SOX2+ NSCs seems to correlate with normal cognitive capacity in AD (Briley et al., 2016). Indirect evidence associated with neurogenesis, like reduced hippocampal volume and spatial pattern separation impairment in AD patients (Martínez-Pinilla et al., 2016; Parizkova et al., 2020), also show the possibility of declined neurogenesis in AD subjects. Taken together, AHN decreases with the progression of age in AD patients, and this decrease is linked with impaired cognitive function.
2.1.2. Neurogenesis in AD animal models
Decreased neurogenesis has been reported in aged mice (Berdugo-Vega et al., 2020; Kirschen and Ge, 2019), as well as in transgenic animal models of AD(Demars et al., 2010; Li et al., 2009; Rodríguez et al., 2009; Zhang et al., 2007). Strikingly, human NSC transplantation restored cognition in an AD mouse model, suggesting the possibility of boosting neurogenesis as an intervention in AD (McGinley et al., 2018). A summary of neurogenesis studies in AD and other neurodegenerative diseases animal models are presented in Table 1.
Table 1-. Summary of studies on the changes of neurogenesis in animal models of neurodegenerative diseases.
Here we summarized previous studies on the changes of neurogenesis in both SGZ and SVZ in animal models of neurodegenerative disorders, including the type of animal model, investigated area and markers used in studies, as well as the details of phenotypes related to neurogenesis. Altered neurogenesis was found in most of these animal models, which may contribute to the etiology involved in neurodegenerative diseases.
| Disease | Organism | gene | Area | Change in neurogenesis | Markers | Age(month) | Features related to neurogenesis | Refs |
| AD | Mouse | APPS w,Ind | SGZ | ↑ | BrdU, Ki67, PSA-NCAM, β-tubulin III | 3 | Increased BrdU+, Ki67+ cells by increased neuronal differentiation labeled by PSA-NCAM+, β-tubulin III+ cells; | (López-Toledano and Shelanski, 2007) |
| ↓ | 5, 9, 11 | Reduced neurogenesis started at 5-month-old and persisted at 9- and 11 month-old. | ||||||
| APP | SGZ | N.C. | DCX, MCM2, NF68 | 8–9, 18–24 |
No change in DCX+ cells | (Zhang et al., 2007) | ||
| PS1 | SGZ | ↓ | A small decrease in DCX+ cells | |||||
| APP, PS-1 | SGZ | ↓ | Reduced by 60% and in an age dependent way | |||||
| APPswe, PS1-dE9 | SGZ | ↑ | BrdU, DCX | 3, 9 | The memory and hippocampal proliferation were not affected at 3-month-old; Memory impairment, increased Aβ deposits, and | (Yu et al., 2009) | ||
| Disease | Organism | gene | Area | Change in neurogenesis | markers | Age (month) | Features related to neurogenesis | Refs |
| AD | Mouse | APP, PS1, nestin-GFP | SGZ | ↓ | nestin-GFP DCX BrdU GFAP | 7d, 1, 3, 7 | BrdU+-, DCX+- and GFAP+- Nestin-GFP+ cells decreased started from 3 month-old. Abnormal morphologies of dendrites in SGZ; | (Zeng et al., 2016) |
| SVZ | N.C. | The number of nestin-GFP+ cells decrease | ||||||
| PS1HWT | SGZ and SVZ | N.C. | BrdU | 2 | No change in BrdU+ and DCX+ cells | (Demars et al., 2010) | ||
| APPs we, PS1ΔE9 | SGZ | ↓ | BrdU DCX | 2 | Reduced as early as 2 month-old; impaired proliferation and tau hyperphosphorylation exhibited in neurospheres isolated from APPswe/PS1ΔE9 mice | |||
| SVZ | ↓ | |||||||
| AD | Mouse and NPCs | APPswe, PS1ΔE9 | SGZ | - | EdU | 4 | Transplantation of human NPC reduced Aβ load and increased microglia within hippocampal and cortical regions; Improve hippocampal dependent cognition | (McGinley et al., 2018) |
| Disease | Organism | gene | Area | Change in neurogenesis | markers | Age (month) | Features related to neurogenesis | Refs |
| AD | Mouse and NPCs | APPKM670/671NL | SVZ | ↓ | BrdU, DCX, SOX2, GFAP | 1.5 | Decreased OB neurogenesis and fewer Calretinin+ interneurons in OB; Smaller neuron size; More DCX+ neuroblasts and fewer Sox2+ progenitors | (Scopa et al., 2020) |
| Aβ | SGZ | ↓ | GFAP, Ki67, CD44, CD90, CD34, CD45 | 1.5 | Aβ-treated NPCs decreased the expression of Ki67, GFAP, SOX2, and Nestin by suppressing the Wnt signaling pathway | (Oh et al., 2015) | ||
| Rat | Aβ | SGZ | - | - | P5,P7,P15,P25 | Aβ trigger spine loss by partially inhibiting NMDARs | (Shankar et al., 2007) | |
| Mouse | MAPT | SGZ | ↓ | DCX, Ki67 | 2, 6, 12 | Decreased Dcx-NeuN+ cells as early as 2 months of age in both SGZ and SVZ; Decreased Ki67+ cells in SVZ with aging | (Komuro et al., 2015) | |
| SVZ | ↓ | |||||||
| - | - | - | 6 | Aging-dependent short-term memory deficits, hyperactivity and synaptic plasticity defects | (Biundo et al., 2018) | |||
| Disease | Organism | gene | Area | Change in neurogenesis | Markers | Age (month) | Features related to neurogenesis | Refs |
| AD | Mouse | Tau Tg30 | SGZ | ↓ | DCX, Ki67, GFAP | 12 | DCX+ and Ki67+ cells decreased in Tg30 mice but not in Tg30/tau KO mice; GFAP+ cells showed no difference between Tg30 and Tg30/tau KO mice | (Houben et al., 2019) |
| Tg30/TauKO | ||||||||
| APPswe, PS1M146V, MAPTP301L | SGZ | ↓ | HH3 | 2–4, 6, 9, 12 | The age-associated reduction was more significant in female mice; More related to dorsal than ventral hippocampus | (Rodríguez et al., 2008) | ||
| APPswe, PS1M146, MAPTP301L, Polβ | SGZ | ↓ | BrdU | 6, 14 | No change in hippocampal volume and adult neurogenesis at 6 months, but reduced at 14 months; Impaired memory and synaptic plasticity in 3xTg/Pol β+/− mice | (Sykora et al., 2015) | ||
| 5xFAD | SGZ | ↓ | DCX, HH3, calretinin | 2–4, 7 | The number of DCX+, HH3+, and calretinin + cells decreased in 5xFAD hippocampus; | (Moon et al., 2014) | ||
| Disease | Organism | gene | Area | Change in neurogenesis | label | Age (month) | Features related to neurogenesis | Refs |
| AD | Mouse | 5xFAD | SGZ | ↓ | Ki67, DCX, SOX1, SOX2, SOX21 | 2 | SOX1+ and SOX21+ cells decreased in AD mice; DCX+ cells decreased only in male AD mice; SOX2+ cells decreased only in female AD mice; No change in the Ki67+ cells in both gender; The protein levels of BDNF were not affected in the 5xFAD mice | (Zalet el et al., 2018) |
| 5xFAD | SGZ | ↓ | DCX | 10 | Reduced neuron numbers and neurogenesis both in males and females; Restored by overexpression of VGF, a nerve growth factor | (Beckmann et al., 2020) | ||
| PS1 | ventricular zone | ↓ | BrdU | E11.5 | Premature differentiation of NPCs, which leading to early depletion of the neural progenitor population | (Yang et al., 2000) | ||
| PS2 | N.C. | DCX, Ki67 | 1.5–2 | Deletion of PS2 does not affect hippocampal adult neurogenesis | (Dhaliwal et al., 2018) | |||
| Disease | Organism | gene | Area | Change in neurogenesis | label | Age (month) | Features related to neurogenesis | Refs |
| AD | mouse | APOE ε3/APOE ε4 | SGZ | ↓ | Nestin, SOX2, BrdU, GFA P | 3, 6–7, 12–13 | Neurogenesis reduced but astrogenesis increased in APOE-KO Mice; Increased BMP signaling promoted glial differentiation at the expense of neurogenesis in APOE ε4 mice, Presynaptic GABAergic input-mediated maturation of newborn neurons was diminished in APOE ε4 mice | (Li et al., 2009) |
| SVZ | ↓ | |||||||
| GFAP-APOE | SGZ | ↓ | BrdU, GFAP | 2 | Reduced APOE after injury; The injury-induced proliferation of hippocampal neural progenitors is absent in APOE-deficient mice; GFAP-ApoE4 mice decreased neurogenesis after injury. | (Hong et al., 2016) | ||
| nestin-APOE | SGZ | ↓ | DCX | 1, 2, 9 | An overall decrease in type 1 Nestin- and GFAP-expressing neural stem cells | (Yang et al., 2011) | ||
| GFAP-APOE | SGZ | N.C. | synaptophsin, NSE, GFAP | 6, 10, 14 | Impaired learning and working memory; Increased activity and anxiety; no alterations of the expression synaptophysin, NSE, GFAP | (Hartman et al., 2001) | ||
| Disease | Organism | gene | Area | Change in neurogenesis | label | Age (month) | Features related to neurogenesis | Refs |
| A-T | Mouse and NPCs | ATM | - | - | Ki67, GFAP | 3 | No change in the number of proliferating cells; Resistance to apoptosis after irradiation | (Barazzuol et al., 2017) |
| Mouse | SGZ | ↑ | Ki67, EdU, cyclin A, PCNA | 2, 3 | Neurons loss in hippocampus and frontal cortex; Cyclin A+ and PCNA+ cells were significantly elevated | (Shen et al., 2016) | ||
| SGZ | ↓ | BrdU | 1, 2 | ATM down regulated during cells differentiate; Decreased proliferation and survival of NPCs and genomic instability | (Allen et al., 2001) | |||
| PD | C. elegans | SNCA | - | ↓ | - | - | Neuronal and dendritic loss in dopaminergic neurons but not with a motor neuron promoter | (Lakso et al., 2003) |
| Mouse | SVZ | ↓ | PCNA, DCX+ BrdU | 5, 15 | Decreased number of PCNA+, DCX+ and BrdU+ cells and increase in TUNEL+ cells in OB | (Winner et al., 2008) | ||
| Lrrk2 | SGZ | ↓ | DCX, BrdU | 4 | Decreased proliferation both in SGZ and SVZ Neurite outgrowth and spine numbers reduced in new neurons in DG | (Winner et al., 2011) | ||
| SVZ | ↓ | |||||||
| Disease | Organism | gene | Area | Change in neurogenesis | label | Age(month) | Features related to neurogenesis | Refs |
| PD | Mouse and NPCs | PINK1 (PARK6) | SGZ | ↓ | DCX, SOX2 | 3 | Decreased proliferation and TMRE/Mi toTracker Green ratio; Reduced maximum OCR, spare respiratory capacity and growth; Increased apoptosis in PINK1−/− NSCs; Abnorma l morpholo gic features of PINK1−/− DCX+ neurons | (Agnihotri et al., 2017) |
| Parkin (PARK2) | SVZ | ↓ | GFAP | E15 | Arrested neuronal differentiation and abnormal morphology of NPCs; Decreased GFAP+ cells | (Park et al., 2017) | ||
| Rat | SNCA | SGZ | ↓ | BrdU | 4 | Reduced survival of BrdU+ cells in DG, while proliferation not be affected | (Kohl et al., 2016) | |
| CS | Human iPSC | CSB | - | ↓ | PAX6, OCT4, SOX2 | - | Reduced differentiation potential and proliferati on | (Vessoni et al., 2016) |
| Mouse | CSB | SGZ | N.C. | BrdU | E14.5, 4 | Neural progenitors were not affected but showed defective self-renewal | (Sacco et al., 2013) |
Abbreviations: ↑, increase; ↓, decrease; Aβ, amyloid beta; AD, Alzheimer’s disease; APOE, apolipoprotein E; APP, amyloid precursor protein; A-T, Ataxia Telangiectasia; ATM, Ataxia Telangiectasia mutated; BDNF, brain-derived neurotrophic factor; BrdU, Bromodeoxyuridine; DCX, Doublecortin; DG, dentate gyrus; E, embryo; NSC, neural stem cell; OB, olfactory bulb; P, postnatal day; PCNA, proliferating cell nuclear antigen; PD, Parkinson’s disease; Polβ, DNA polymerase β; PS1, presenilin 1; PS2, presenilin 2: SGZ, subgranular zone; SNCA, synuclein alpha; SOX2, sex determining region Y-box 2; SVZ, subventricular zone
The activity of neural precursors may be regulated by risk genes involved in AD such as APP, PS1 and APOE (Li et al., 2009; Smukler et al., 2011; Yang et al., 2011). Interestingly, these AD-associated gene mutations suppress multiple stages of neurogenesis in AD mice (Hamilton et al., 2010). Similar to the finding in humans, the deficits in neurogenesis are observed before the development of amyloid plaques in an APP/PS1 mouse model, supporting the hypothesis that altered neurogenesis might be a potential marker for early development of AD (Unger et al., 2016). Studies in the 5xFAD mouse model showed reduced newborn cells in the SGZ (Moon et al., 2014), while NSC proliferation was not impacted (Zaletel et al., 2018), suggesting that the disruption of neurogenesis occurs during differentiation. Interestingly, Choi et al. found that increasing AHN alone did not improve cognition in the 5xFAD mouse model, whereas increasing both AHN and brain-derived neurotropic factor (BDNF) could simulate exercise-induced improvement in learning and memory, highlighting the importance of the health of the local brain environment (Choi et al., 2018). A recent study on humans also showed the protective role of BDNF on hippocampal connectivity in AD pathology (Franzmeier et al., 2021). Given the brain functions not only rely on the existence of neurons, but also on how effectively the large-scale functional networks are engaged in neuronal activities, it makes sense that a healthier neurogenic niche may better repair the damaged neural network and cognitive function. In both 2xTg AD and 3xTg AD mouse models, differentially methylated genes associated with cognitive improvement in the hippocampus were related to neurogenesis and synaptic function, showing that epigenetic changes targeting in neurogenesis might be related to the functions of learning and memory in AD (Lee et al., 2018; Sandoval-Hernandez et al., 2016). For example, the histone deacetylase inhibitor, valproic acid (VPA), which has been suggested as a potential treatment for AD (Bottero et al., 2021), induced the differentiation of adult hippocampal neural progenitors in vitro (Hsieh et al., 2004). While still an open question, some have argued that strategies aimed at restoring and/or boosting AHN in both normal elderly people and subjects at high risk of AD could emerge as effective strategies to prevent the onset and/or counteracting the progression of the disease (Li Puma et al., 2021).
2.1.3. The effects of DNA damage on neurogenesis in AD
Both degeneration and neurogenesis in AD are tightly connected with DNA damage responses and oxidative stress (Barazzuol et al., 2017; Hou et al., 2018; Shull et al., 2009) (Barazzuol et al., 2017; Hou et al., 2018; J. Li et al., 2020; Shull et al., 2009). Increased DNA damage inhibits neurogenesis and promote cell death both in vivo and in vitro. DNA damage, detected by γH2AX, a marker of DNA double-strand breaks (DSB), accumulates in the brains of AD patients (Lin et al., 2020). Persistent DNA damage by irradiation could compromise hippocampal neurogenesis (Schmal et al., 2019). Reactive oxygen species (ROS) promotes DNA damage and cell death, which contribute to the pathogenesis of AD (Taupin, 2010). Some proteins involved in DNA repair also play a crucial role in neurogenesis. For example, neurons in vulnerable regions of the AD brain, like the hippocampus and frontal cortex, displayed reduced expression of ataxia telangiectasia mutated (ATM) protein and decreased ATM signaling in both humans and mice, which drove abnormal neuronal cell cycle reentry, ultimately causing cell loss (Shen et al., 2016). Also, loss of NEIL1 or NEIL3, the primary DNA glycosylases for base excision repair (BER), leads to the reduction of proliferation capacity of hippocampal NPCs and impaired learning and memory in mice, likely due to the failure in the removal of hydantoin lesions of single-stranded DNA in NPCs (Regnell et al., 2012; Yang et al., 2019). Mitochondrial DNA damage accumulated in the NSC population with knockdown the DNA repair protein, 8-oxoguanine DNA glycosylase (OGG1), and it shifted the differentiation of NSCs toward to astrocytic lineage (Wang et al., 2011). Lower hippocampal volume and a decline in adult neurogenesis were observed in a mouse model with defective DNA repair due to Polβ haploinsufficiency (Hou et al., 2018; Sykora et al., 2015). In conclusion, neurogenesis is particularly susceptible to DNA damage, which further increases the risk of neurodegeneration in AD progression. Boosting DNA repair may be a promising treatment strategy for AD. Potential DNA repair intervention like NAD+-boosting molecules and its effects on neurogenesis will be discussed in section 4.2.1.
2.2. Parkinson’s disease
Parkinson's disease (PD) is the second leading neurodegenerative disease affecting 1–2% of the population age 65 or older, targeting twice as many men as women (Goldman and Fahn, 2020). PD is characterized by neuronal loss in the substantia nigra, which then causes striatal dopamine deficiency and intracellular inclusions, known as Lewy bodies (LBs) (Gibb and Lees, 1988). PD has both motor and non-motor dysfunctions. The motor symptoms are characterized as movement difficulty (slowness and change in gait), muscular rigidity (stiffness), postural instability and tremors in limbs and face (Church, 2021). Nonmotor signs include sleep disturbance, olfactory dysfunction, visual dysfunction, psychiatric symptoms, and cognitive impairment (Obeso et al., 2017).
PD is a heterogeneous and complex disease with multiple genetic, epigenetic, and environmental risk factors. Aging is the leading risk factor (Levi and Michaelson, 2007). Many genes (SNCA, LRRK2, VPS35, PRKN, PINK1, DJ-1, FBXO7, and DNAJC6) are associated with PD pathology (Goldman and Fahn, 2020). Its pathophysiology is related to aggregated α-Synuclein oligomers, defective mitophagy, increased oxidative stress, calcium imbalance, compromised axonal transport, and increased neuroinflammation (Poewe et al., 2017; Grünewald et al, 2019). Currently, there are no effective treatments for PD except for providing relief of symptoms and slowing down the disease progression (Raza and Anjum, 2019). The initial PD managements, which increased the dopamine levels, like deep brain stimulation and dopamine receptor agonist treatments, also increased both adult SVZ and SGZ neurogenesis in humans and animal models of PD, which might further facilitate learning and memory and coping with mood disorders in PD (Chiu et al., 2015; O’Sullivan et al., 2011; Vedam-Mai et al., 2014). Also, the non-motor symptoms in PD may partly related to impaired olfactory and hippocampal function, raising the potential to slow down the neurodegenerative progression in PD through inducing neurogenesis in these areas (Le Grand et al., 2015; Marxreiter et al., 2013).
2.2.1. Neurogenesis in PD patients
PD patients exhibit hippocampal and olfactory dysfunction (Braak et al., 2003; Regensburger et al., 2014). The hippocampal LB density is correlated with the degree of dementia in PD patients, suggesting that alternations of hippocampal connectivity could contribute to the emergence of memory deficits (Carlesimo et al., 2012; Churchyard and Lees, 1997). Memory-related hippocampal atrophy and olfactory defects are exhibited in PD patients (Bohnen et al., 2010; Brück et al., 2004). Dopaminergic neural fibers from substantia nigra and ventral tegmental area could innervate the prefrontal cortex and limbic system including hippocampus, suggesting a functional and an anatomical link between nigrostriatal dopaminergic neurons and hippocampal dependent functions (Deniau et al., 1994; Kahn and Shohamy, 2013). In addition, dopaminergic signaling promoted the proliferation and the survival of newborn cells in hippocampus (Winner et al., 2009). Thus, the decreased neural precursors observed in the OB and DG of PD adults may be associated with impaired dopaminergic innervation in these regions (Höglinger et al., 2004). A reduction in the number of Musashi1-positive cells in the SVZ was observed in PD cases, and the expression of Musashi1 proteins had an inverse relationship with the disease duration (O’Sullivan et al., 2011; Ziabreva et al., 2007). Similarly, SOX2-positive cells declined in the hippocampus of PD patients (Winner et al., 2012). Non-motor symptoms, like olfactory dysfunction, depression, and impaired spatial memory are frequently observed in individuals with PD and often occur before the onset of motor symptoms (Berendse et al., 2001; Lim et al., 2018; Pillon et al., 1997). Importantly, a decline of adult neurogenesis in olfactory structures was noted in the early stages of PD (stage 1) (Braak et al., 2003), which corresponded to the olfactory dysfunction observed in PD patients (Regensburger et al., 2014). A recent study on anosmia (loss of smell) in COVID-19 patients also revealed that SARS CoV-2 infection is a risk factor for PD, and that a potential cause of smelling loss could be the impairment of neurogenesis in the olfactory system (Rethinavel et al., 2021). Overall, these findings strengthen the hypothesis that impairments in neurogenesis may contribute to the non-motor pathogenesis of PD.
2.2.2. Neurogenesis in PD animal models
Genes involved in PD (like SNCA, PINK1, LRRK2, VPS35…) play important roles in the generation and maintenance of the NSC pool as well as the differentiation and survival of NPCs (Le Grand et al., 2015; Lee et al., 2013; Winner et al., 2011, 2008). Interestingly, the deregulated neurogenesis observed in PD animal models appears to regulate various functions related to non-motor symptoms (including hyposmia, depression and anxiety…) observed in PD, strongly suggesting there is a link between neurogenesis deficits and the progression of PD (Bang et al., 2021; Le Grand et al., 2015). Reduced adult neurogenesis has been reported in different PD animal models. For instance, overexpression of α-synuclein (SNCA) compromised neurogenesis in both hippocampus and OB among rats (Kohl et al., 2016), C. elegans (Lakso et al., 2003) and mice (Winner et al., 2004). Another study also found a lower adult neurogenesis and abnormal dendrites of the newborn neurons in SGZ and SVZ areas of an LRRK2-G2019S transgenic mouse model, and the reduction could be partially reversed by enhanced physical activity (Winner et al., 2011). Similar observations were obtained from the neurotoxin-induced PD mouse models, with a significantly declined number of newborn neurons in the rodents’ hippocampus (Singh et al., 2017; Sung, 2015). Regarding cognitive dysfunctions observed in PD animal models, it is often coupled with reduced hippocampal neurogenesis. For example, mice lacking Dorfin, a RING finger E3 ubiquitin ligase implicated in PD, showed reduced AHN and impaired contextual fear conditioning, which is highly hippocampal-dependent (Park et al., 2015). Similarly, impairment of hippocampal neurogenesis-dependent pattern separation was observed after overexpression of a-synuclein in rats, which could be rescued by voluntary running, implicating activating AHN may serve as a neuroprotective treatment to non-motor symptoms in PD (Crowley et al., 2018). There is a strong correlation between dopaminergic degeneration and Parkinsonism (Bernheimer et al., 1973). A promising study to reprogram astrocytes to functional neurons found a therapeutic effect on dopamine levels and motor phenotypes in a PD mouse model (Qian et al., 2020). Dopamine promotes the proliferation and survival of newborn cells in the embryo and adulthood of rodents (Ohtani et al., 2003; Takamura et al., 2014). Depleting dopamine inhibited NPC generation in PD mice (Höglinger et al., 2004). These observations suggested that impaired neurogenesis in PD might be a consequence of dopaminergic denervation. In conclusion, hippocampal neurogenesis associated dysfunctions are common in PD, and likely contribute to cognitive impairment and emotional disorders, which can be relieved by increasing AHN conversely. The profound alterations of neurogenesis in PD have raised attention and may provide a novel strategy for effective therapeutics for it.
2.3. Other neurodegenerative diseases
Next, we will briefly introduce other neurodegenerative diseases, including HD, Ataxia telangiectasia (A-T) and Cockayne syndrome (CS). HD is known as a progressive neurodegenerative brain disease (Ruzo et al., 2018). A-T and CS are the premature aging diseases, and studies of these diseases will contribute to our knowledge of DNA metabolism, cellular senescence, and stem-cell differentiation during aging (Dyer and Sinclair, 1998).
2.3.1. Huntington’s disease
HD is an autosomal dominant neurodegenerative disorder caused by an expansion of the polyglutamine (poly Q) tract in the Huntingtin (HTT) protein (Ruzo et al., 2018). The polyQ repeat length is associated with disease severity, e.g., a person with more than 36 repeats is more likely to develop HD (Rego and de Almeida, 2005). Neuronal loss in the striatum, cortex, and hippocampus, which results in cognitive dysfunction and severe motor impairments, is one typical feature of HD (Ruzo et al., 2018).
The neurons differentiated from iPSCs of HD patients exhibited alterations of growth, metabolism, survival, and death (Lim et al., 2017). In light of this, the mutant HTT gene has been reported to induce the cell cycle re-entry of neurons and impaired neuronal differentiation and further reduce the survival of newborn neurons in rodent striatum and hippocampus (Manickam et al., 2020). In addition, deficits in AHN have been reported in the R6/2 (Gil et al., 2005), the R6/1 (Lazic et al., 2006), and the YAC128 transgenic mouse model (Simpson et al., 2011) of HD, which might underlie the cognitive deficits associated with HD (Gil-Mohapel et al., 2011).
To date, there is no cure for HD, and the treatments available are limited to symptomatic clinical management (Tabrizi et al., 2019). Therapies using stem cell technology have been proposed as a promising treatment of HD (Bachoud-Lévi et al., 2021). Cell therapies aiming to enhance endogenous neurogenesis have shown promising results in HD animal models (Lee et al., 2009; Pollock et al., 2016; Snyder et al., 2010). It was also shown that a combination of stem cell and gene therapy could improve motor functions and extend the lifespan of HD mice (Cho et al., 2019). A recent review also summarizes the preclinical studies using stem cells in HD animal models, highlighting the benefits and promises of stem cells used as a promisor therapeutic strategy for HD (Colpo et al., 2019). Thus, neurogenesis-based cell treatments still offer hope for the future therapies of HD.
2.3.2. Ataxia telangiectasia
A-T is a rare and complex genetic neurodegenerative disorder (affecting ~ 1/40 000–1/100 000 people), caused by mutations in the ATM gene (Taylor et al., 2015). ATM is a sensor of DSBs that is involved in cell cycle checkpoints (Savitsky et al., 1995), and oxidative stress response (Liu et al., 2005). Both A-T patients and ATM-deficient mice exhibit enhanced oxidative damage (Reichenbach et al., 2002; Stern et al., 2002).
ATM is essential in early brain development and adult neurogenesis (Allen et al., 2001; Enriquez-Rios et al., 2017). It is critical for cell proliferation, DNA repair, and apoptosis after DNA damage in both non-cycling and proliferative cells in mice (Enriquez-Rios et al., 2017). Lacking ATM was found to provide resistance to irradiation induced apoptosis and proliferation arrest in mice SVZ, indicating that a failure to activate DNA damage responses disturbs the homeostasis of NSC between quiescence and activation (Barazzuol et al., 2017). NSCs in the hippocampus of ATM−/− mice displayed an abnormally high rate of proliferation and decreased cell survival in vivo, and a weakened ability to differentiate to neurons and oligodendrocyte in vitro (Allen et al., 2001). However, other researchers noted that ATM deficiency did not impair cell proliferation and differentiation, using an immortalized human neural stem cell line (ihNSC) (Carlessi et al., 2009). Conversely, ATM depletion could attenuate the short-term apoptotic response to irradiation-induced DNA damage (Carlessi et al., 2013). Possible explanations of these inconsistent results are that i) the NSCs obtained from different brain regions may have distinct characteristics and developmental patterns; ii) ATM may impact brain functions through distinct mechanisms in different brain regions and diverse cell populations. Indeed, a reduced yield of GABAergic neurons in the ATM-deficient ihNSCs was found, implicating that ATM may not be required for overall neurogenesis, but specific to a GABAergic neuronal differentiation (Carlessi et al., 2013). Notably, GABA signaling regulated NSC proliferation and growth through the ATM/ATR-related phosphorylation of γ-H2AX in mouse ES and NCS cells (Andäng et al., 2008), which is consistent with human clinical findings. Specifically, a lower GABA level has been found in the cerebellum of an A-T patient when compared with control (Perry et al., 1984), and a GABA analog could ameliorate the ataxia manifestation (Gazulla and Benavente, 2006).
2.3.3. Cockayne syndrome
Cockayne syndrome (CS) is a progressive developmental and neurodegenerative disorder resulting in premature death in childhood (Karikkineth et al., 2017). CS patients show severe photosensitivity, growth retardation, accelerated aging, DNA repair and transcription defects, and CNS abnormalities (Ciaffardini et al., 2014). Mutations in CSA (ERCC8) and CSB (ERCC6) cause CS (Laugel et al., 2010; Okur et al., 2020). About 80% of CS cases are caused by mutations in CSB (Vessoni et al., 2016). The CSB protein is essential in various DNA repair processes, including BER, nucleotide excision repair (NER) and double-strand break repair (DSBR) (Tiwari et al., 2021).
Neuronal differentiation and neurogenesis are compromised in human CSB-deficient NSCs and iPSCs (Ciaffardini et al., 2014; Vessoni et al., 2016). Likewise, genes related to neurogenesis were also down-regulated in the fibroblasts of CS patients (Wang et al., 2014). In contrast, this process was not affected in the CSB-deficient mouse model (Sacco et al., 2013). CSA and CSB proteins possess an essential role in the turnover of p53 transcription factors by promoting their ubiquitination and degradation (Latini et al., 2011). Altered p53 activity disrupts the proliferation and differentiation of NPCs in adult neurogenesis (Armesilla-Díaz et al., 2009; Medrano and Scrable, 2005). Accordingly, dysfunction of CSA and CSB may result in defective neurogenesis, further contributing to the dramatic and complex phenotypes in CS patients.
3. The role of the key aggregation-prone proteins in neurogenesis
Two hallmarks of AD brains are the aggregation of Aβ in extracellular plaques and intraneuronal neurofibrillary tangles formation by hyperphosphorylated tau proteins (Alzheimer’s Association, 2019). APP, PS1, PS2 and APOE are identified as the high-penetrant genetic factors contributing to AD (Van Cauwenberghe et al., 2016). The aggregation of α-synuclein in LBs and Lewy neurites is a characteristic feature of PD pathology (Xu and Pu, 2016). Nuclear accumulation, misfolding and abnormal aggregation of the mutant HTT results in selective neuronal neuronal loss predominantly in the striatum and the cortex (McColgan and Tabrizi, 2018). In this section, we will discuss the relationships of those key aggregation-prone proteins with neurogenesis, and an overview on the roles of these proteins in AHN is illustrated in Figure 1.
Figure 1. Key aggregation-prone proteins involved in neurodegenerative diseases and adult hippocampal neurogenesis during aging.
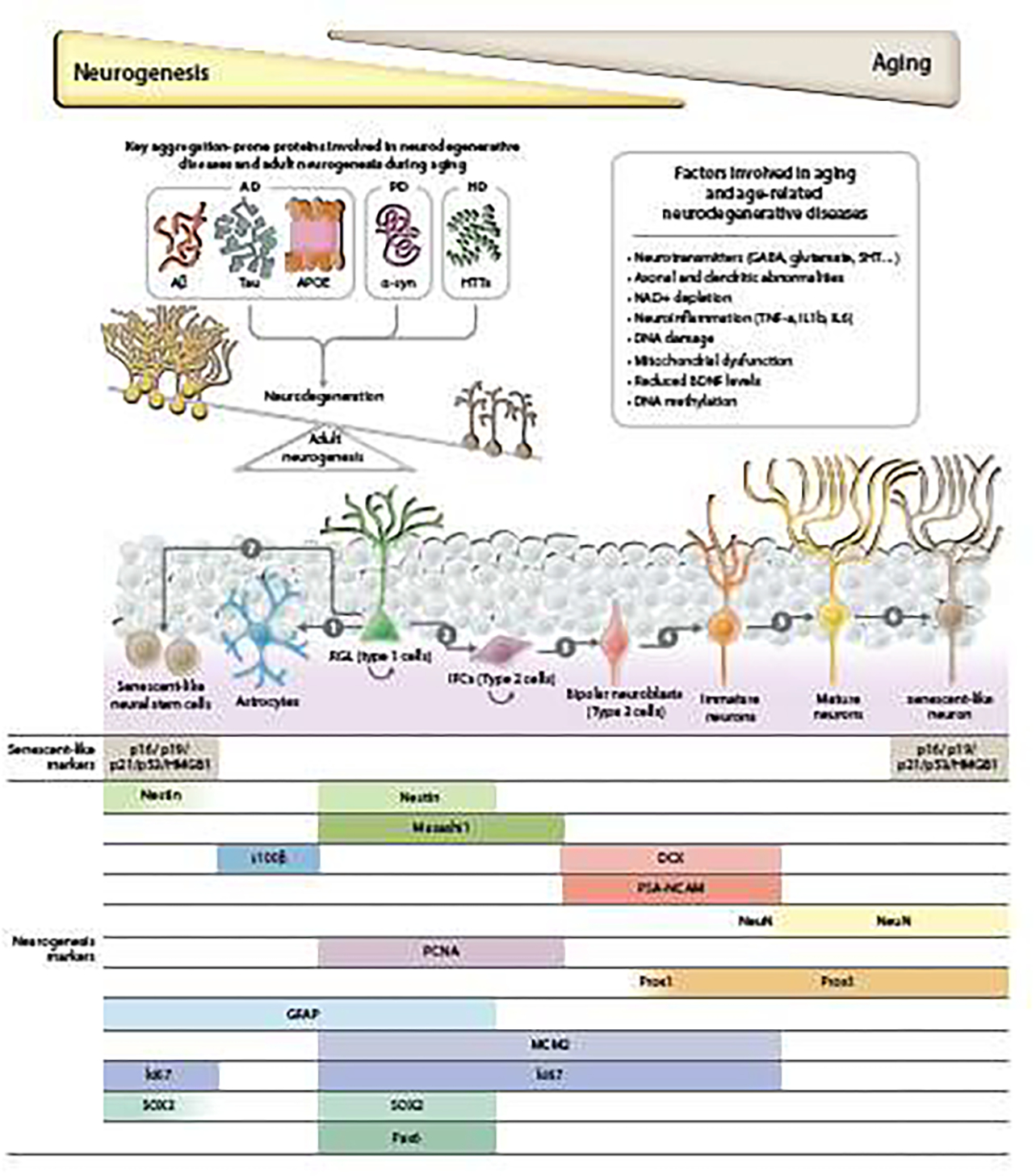
Adult hippocampal neurogenesis decreases with aging. Aggregation-prone proteins, like Aβ, tau and APOE in AD, α-syn in PD and HTTs in HD accumulate during the aging process and induce neurodegeneration as well as impair hippocampal neurogenesis, resulting in the imbalance between these two processes. Various factors that are tightly connected with neurogenesis are involved in the pathologies of aging and neurodegenerative diseases and are discussed in this review. In the hippocampus, the process of neurogenesis starts with radial glia-like (RGL) cells (type 1 cells). The RGL cells keep self-renewing and give rise to astrocytes (1) and intermediate progenitor cells (IPCs or type 2 cells) (2). IPCs proliferate and differentiate into bipolar neuroblasts (3). Those neuroblasts differentiate into immature neurons (4). Then these immature neurons undergo a dynamic maturation process, with some of them dying, and some surviving to become mature neurons and form functional connections to existing neural networks (5). Several studies have demonstrated that markers of senescence, like p16, p19, p53 and HMGB1, are increased in some neurons and neural stem cells (6 &7) (Molofsky et al., 2006; Negredo et al., 2020; Nicaise et al., 2019). This age-associated NSC senescence could result in the depletion of NSCs, ultimately decreasing adult hippocampal neurogenesis and impairing brain function. At the bottom is the schematic of specific markers expressed during hippocampal neurogenesis and senescence.
3.1. Tau
Tau protein is involved in microtubule assembly and stabilization, and commonly found in the cytosol and axons of neurons (Barbier et al., 2019). Hyperphosphorylated tau proteins, which lead to neuritic plaques and neurofibrillary tangles, represent one of the hallmarks of AD (Alzheimer’s Association, 2019). Prominent clinical heterogeneity in the hyperphosphorylated species of soluble, oligomeric, seed-competent tau was found in AD patients, implying that targeting tau is a potential personalized therapeutic approach to slow AD progression (Dujardin et al., 2020).
The relationship between tau and adult neurogenesis has been reviewed elsewhere (Fuster-Matanzo et al., 2012; Houben et al., 2021; Pristera et al., 2013). Tau is able to induce AHN-related deficits, including suppression of proliferation, neuronal atrophy and malfunction, impaired learning and memory, and downregulated GABA signaling, in an age-dependent manner (Dioli et al., 2017). Depletion of tau enhanced neurogenesis and rescued the stress-induced reduction of proliferation in both DG and SVZ of mice (Criado-Marrero et al., 2020a; Dioli et al., 2021, 2017). A recent study revealed that tau impaired AHN by suppressing GABAergic transmission in the hippocampus (Zheng et al., 2020). Strengthening the GABAergic transmission in 3xTg AD mice could efficiently rescue AHN deficits caused by tau accumulation and improve AHN-dependent cognitive functions (Zheng et al., 2020). Tau participates in a variety of cellular cascades regulating cell survival and proliferation. For example, the glycogen synthase kinase-3β (GSK-3β), a crucial tau kinase that plays a role in its hyperphosphorylation, was suggested to be related to the cause of AD and could modulate adult neurogenesis (Liu et al., 2021). The p21-activated kinase 3 (Pak3), regulating synaptic plasticity and neurogenesis, was significantly reduced in the hippocampus and frontal cortex of postmortem brains from AD patients (Fuchsova et al., 2016), but was increased in mice when the tau gene MAPT was deleted (Criado-Marrero et al., 2020a). Activation of the Wnt/β-catenin signaling pathway could also restore neurogenesis reduced by the aggregate tau mutant (Joseph et al., 2017). Finally, neurogenesis requires dynamic control over the cytoskeleton and microtubules, and tau proteins facilitate this process (Morris et al., 2011). Together, targeting tau related pathways (like GSK-3β-PI3K signaling and Wnt/β-catenin signaling) to increase neurogenesis might be a valuable approach against AD. However, the potential molecular mechanisms underlying the relationship between tau and adult neurogenesis still need to be further explored.
3.2. Aβ and APP
Aβ peptides are 36–43 amino acids derived from APP by proteolytic cleavage (Tarasoff-Conway et al., 2015). Aβ accumulation and toxicity can cause neuronal loss and trigger AD pathology (Tillement et al., 2011). Elevated concentrations of Aβ42 and tau in cerebrospinal fluid are biomarkers of AD diagnosis (Fagan and Perrin, 2012), and Aβ42/Aβ40 ratio could further help the separation of AD dementia from other dementia disorders (Hansson et al., 2019). Anti-Aβ drugs had been developed, but many failed in clinical trials, leading to heated debates on the plausibility of the amyloid hypothesis. Recently, FDA approved the first drug, Aduhelm (aducanumab), targeting Aβ plaques removal, but has not been fully clinically demonstrated to be effective for cognition improvement (Mahase, 2021). It is reported that Aβ plaques begin accumulating before AD symptom appears, as early as in 20s (Gonneaud et al., 2017). Cleaning amyloid in AD patients who already have dementia would be too late since the brain has already been severely damaged. Therefore, discovering appropriate early clinical biomarkers of AD should be a future effort to help prevent AD.
APP proteins have a complex relationship with neurogenesis. The soluble APPα (sAPPα) cleaved by α-secretase is neuroprotective, showing the capability to induce NPC proliferation (Chen and Tang, 2006). In contrast, Aβ deposits cleaved by β-secretase or γ-secretase are more toxic to neurogenesis. Depletion of Aβ peptide reduced tau inclusions and induced AHN in the rat hippocampus (Morrone et al., 2020). Furthermore, reducing the accumulation of Aβ plaques after disease progression was accompanied by increased adult neurogenesis in a 2xAD mouse model (Calió et al., 2021). Interestingly, researchers found that the formation and accumulation of intracellular Aβ oligomers could affect the OB neurogenesis in Tg2576 transgenic mice prior to the neurodegenerative progress(Scopa et al., 2020). It is further suggested that impaired neurogenesis is an early marker of AD progression (Price et al., 1991; Unger et al., 2016), emphasizing that targeting the early stages of neurogenesis deficits could be an excellent approach against AD. Combination of drugs targeting Aβ deposits clearance and neurogenesis replenishing may be a promising treatment in AD therapy.
3.3. PS1 and PS2
Presenilins are essential components of the γ-secretase complex, which cleave APP to soluble Aβ peptides (De Strooper et al., 1998). A diminished neural progenitor population was reported in the PS1−/− mouse brain (Yang et al., 2000), resulting in a perinatal lethality (Donoviel et al., 1999; Shen et al., 1997). PS1 mutations produced premature neurogenesis and reduced the number of newborn neurons from iPSCs derived from familial AD patients (Arber et al., 2021). Furthermore, downregulation of PS1 in hippocampal NPCs leads to progressive cognition deficits (Bonds et al., 2015). On the contrary, lacking PS1 or PS2 did not influence cell-intrinsic AHN in mice (Dhaliwal et al., 2018). However, some studies confirmed the critical role of PS1 in neurogenesis related to differentiation and dendritic morphogenesis of NPCs (Hernandez-Sapiens et al., 2022). Thus, presenilins have different roles regarding embryonic development and adult neurogenesis. The premature and abnormal features of newborn neurons mediated by presenilins might contribute to neurodegeneration in AD.
3.4. APOE
Humans have three major APOE alleles: ε2, ε3, and ε4 (van der Lee et al., 2018). APOE ε4 is associated with an increased risk of AD, and approximately 40% of LOAD patients carry this allele (Corder et al., 1993; Liu et al., 2013). APOE ε4 carriers showed high levels of Aβ deposition in the brains of elderly and AD subjects (Serrano-Pozo et al., 2021). APOE variants have also been reported to contribute to the pathogenesis of dementia in PD patients (Brockmann et al., 2017).
APOE is necessary to maintain the DG neural progenitor pool (Yang et al., 2011). Lack of it may temporarily increase the proliferation of early NPCs in the DG, but eventually lead to the depletion of type I NPC pool over time in vitro (Yang et al., 2011). Similarly, lower NPCs and fewer dendritic branches were observed in the hippocampus of APOE KO mice, while cell survival and differentiation were intact (Tensaouti et al., 2018). In addition, an increase in AHN was observed in young adult female APOE2 mice, aged female APOE KO mice, and aged female APOE3 mice compared with control mice (Koutseff et al., 2014; Rijpma et al., 2013). In contrast, both sexes of young adult APOE4 mice and aged female APOE4 mice displayed reduced neurogenesis, highlighting the age- and sex-dependent APOE polymorphisms in adult neurogenesis (Koutseff et al., 2014; Rijpma et al., 2013). APOE4 transgenic mice also exhibited impaired working memory and abnormal neuronal development in the DG (Hartman et al., 2001; Li et al., 2009; Tensaouti et al., 2020). Interestingly, the decreased survival of GABAergic interneurons in APOE4 mice was accompanied by diminished presynaptic GABAergic input-mediated maturation of newborn neurons and elevated tau phosphorylation, suggesting the causable regulation of APOE in neurogenesis (Li et al., 2009). In summary, these data may help to explain the emergence of cognitive decline in humans carrying the APOE ε4 allele and provide a link between the APOE ε4 allele and neurodegenerative diseases.
Whether inducing neurogenesis can ameliorate the deteriorations caused by APOE is still uncertain. Few studies have addressed this question. It seems that some physical manipulations inducing neurogenesis, like environment enrichment (EE) and brain injury, didn’t work on APOE4 mice (Hong et al., 2016; Levi and Michaelson, 2007). Conversely, physical exercise could improve hippocampal-dependent cognition and increased the levels of BDNF and tyrosine kinase B (TrkB) in the APOE4 mice (Nichol et al., 2009). However, the researchers haven’t checked the status of AHN in these mice, which makes it difficult to confirm whether it is caused by enhancement of neurogenesis or not (Nichol et al., 2009). Further studies are needed to explore the effects of other interventions which could increase AHN (e.g., caloric restriction or pharmacological approaches) on APOE4 dysfunction.
3.5. α-synuclein
SNCA/PARK1, the gene that encodes α-synuclein, was the first gene identified to be associated with PD (Polymeropoulos et al., 1997). Evidence showed that α-synuclein regulates the production of dopamine (Yu et al., 2004). It also interacts with tau and drives the formation of pathological inclusions both in vivo and in vitro (Badiola et al., 2011; Giasson et al., 2003; Uemura et al., 2020). A decline of AHN is exhibited in an α-synuclein overexpression rat model (Kohl et al., 2016) and after intranasal administration of α-synuclein in mice (Sherstnev et al., 2021). Moreover, mutant α-synuclein aggravated age-related reduction of neurogenesis in the mouse SVZ and OB (Winner et al., 2008). Conversely, depleting α-synuclein resulted in enhanced neurogenesis in mice DG (Winner et al., 2012). In conclusion, α–synuclein aggregates interfere with the proper regulation of AHN, which mediates PD pathology, highlighting the necessity to further investigate whether stimulation of neurogenesis could mitigate the symptoms caused by α-synuclein in PD patients.
4. Interventions to increase neurogenesis
Here, we group the neurogenesis interventions into distinct categories: (1) non-specific physical/metabolic manipulations, (2) pharmacological approaches and, lastly, (3) genetic and reprogramming strategies. Special emphasis will be given to aging-related interventions and compounds that influence known metabolic pathways in aging, such as AMPK, mTOR and sirtuins. Genetic strategies will be skipped due to space constraints and because they have been only used in animal models so far. Cellular reprogramming approaches have been reviewed elsewhere recently (Rando and Jones, 2021). The summary of how the manipulations affect neurogenesis are shown in Figure 2.
Figure 2. The effects of certain life/healthspan interventions on adult neurogenesis in rodents.
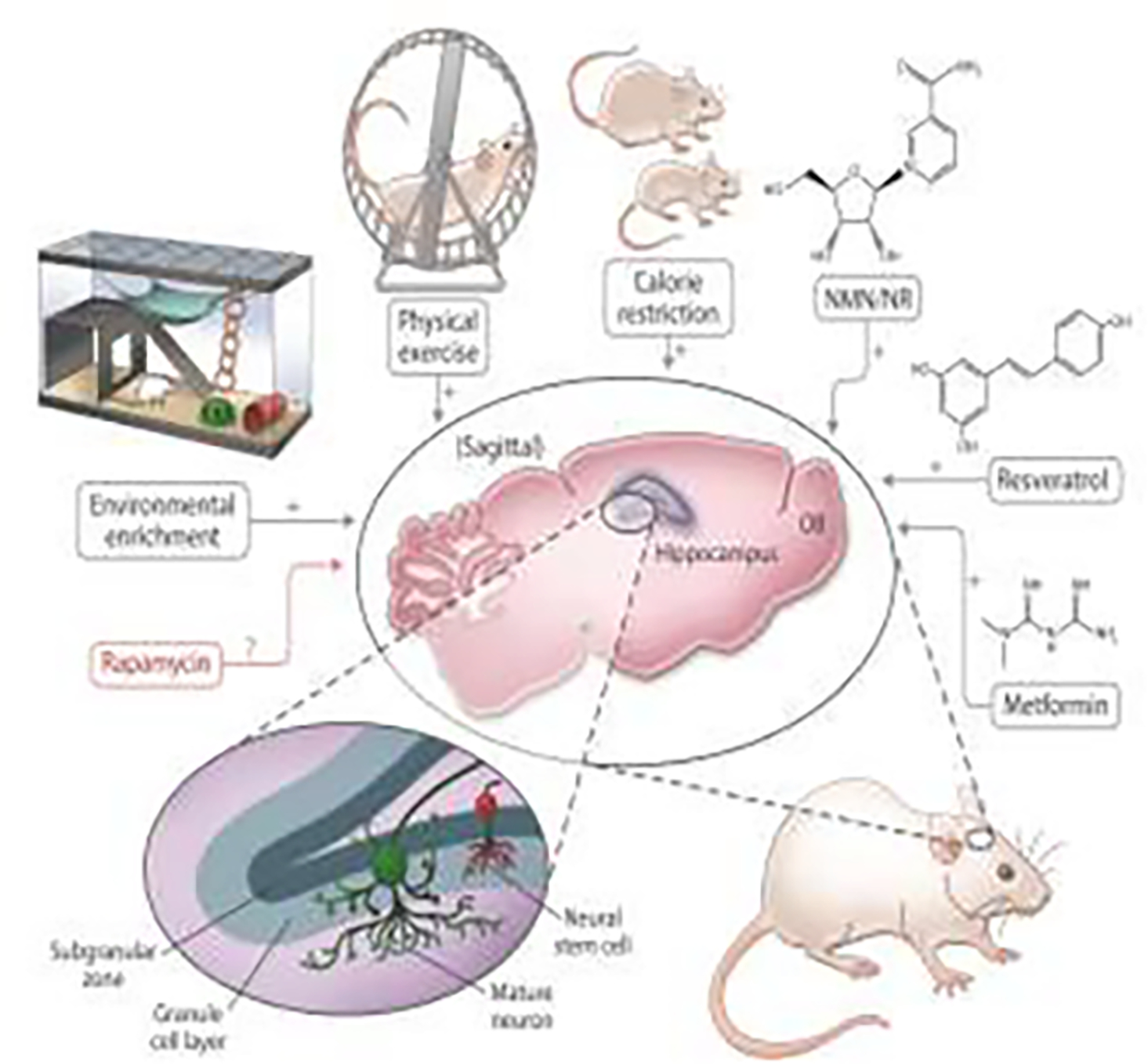
This illustration encapsulates the effects of several common life/healthspan manipulations on adult neurogenesis in rodents, which are described in this review. Environmental enrichment, physical exercise, calorie restriction, NAD-boosting molecules (NMN/NR), resveratrol and metformin all increase neurogenesis in animal models, while the effects of rapamycin have not yet been comprehensively established in that regard.
4.1. Non-specific physical/metabolic manipulations
There are several non-specific physical/metabolic positive regulators of neurogenesis, such as physical exercise (PE), environmental enrichment (EE) and caloric restriction (CR). Interestingly, most of these manipulations have also been associated with increased lifespan and/or healthspan in model organisms. For example, some studies have shown that EE either increases lifespan in mice (Arranz et al., 2010; Thanos et al., 2016; Yamashita et al., 2018) or results in a trend of mean lifespan extension (McMurphy et al., 2018). The effects of EE on healthspan have been reviewed elsewhere (Queen et al., 2020), and the intervention, if translatable, holds promise for extending human healthspan. PE induces similar effects on healthspan in mice (Garcia-Valles et al., 2013), but the effects on lifespan extension have not been clearly established. Some studies showed lifespan extension in rats (Ji et al., 2018), no effect on lifespan in mice (Garcia-Valles et al., 2013) or even reduced lifespan in female rats (Karvinen et al., 2015). While further research is needed to establish a causal relationship between PE and lifespan, current observations point to a beneficial effect on humans, reducing the mortality risk (Lee et al., 2014) and improving multiple health indices (Pasanen et al., 2017), recently summarized elsewhere (Carapeto and Aguayo-Mazzucato, 2021). Lastly, CR is the most robust intervention to increase lifespan and healthspan in species ranging from the budding yeast (Jiang et al., 2000) to rhesus monkeys (Colman et al., 2014). However, some researchers warn that since it is still not completely clear if these benefits result from slowing the aging process or merely avoiding obesity, further studies are needed to ascertain if CR and related interventions (such as intermittent fasting and ketogenic diets) should be recommended for healthy (non-obese) people (Lee et al., 2021).
Environmental enrichment
EE is a somewhat vague concept, commonly defined as “an animal husbandry principle that seeks to enhance the quality of captive animal care by providing the environmental stimuli necessary for optimal psychological and physiological well-being” (Shepherdson et al., 1998). In the case of rodents, it usually means housing them in larger cages that contain a variety of objects that they can interact with, such as plastic tubing, igloos with saucer type wheels, and other various plastic hutch-like toys (Slater and Cao, 2015).
In mice and rats, exposure to EE has long term positive effects on memory and learning (Hymovitch, 1952; Yau et al., 2015), albeit inconsistently (Singhal et al., 2019a, 2019b), and is associated with significantly more new neurons in the DG (Clemenson et al., 2015a). EE is a robust manipulation that is able to induce an increase in AHN across the lifespan of mice, with minor differences between strains (Kempermann et al., 2002, 1998b, 1998a; Leal-Galicia et al., 2008). Housing female 5xFAD transgenic mice in an EE (toys and a running wheel) reduced the Aβ plaque load and vivified AHN (Ziegler-Waldkirch et al., 2018). Similarly, exposure to EE (with toys and a tilted running wheel) restored AHN in a 3xTg-AD mice (J. Rodriguez et al., 2011). EE may also slow the onset and progression of HD (Mo et al., 2015) and restore the deficits in AHN in R6/1 mice (Lazic et al., 2006). A causal link between AHN and EE has been established in a study that exposed GFAP-TK mice to repeated social defeat (a type of psychosocial stress), followed by exposure with EE. The same study showed that repeated social defeat led to a submissive and depressive-like phenotype that was rescued by subsequent exposure to EE, but only if AHN was not disrupted through valganciclovir administration (Schloesser et al., 2010). However, this study (along with many others) suffers from lack of a precise definition of EE. Namely, the cages were enriched not just with variously sized tubes, but running wheels as well. This is important because physical exercise (usually in the form of voluntary running on the wheel) and cognitive stimulation (in the form of EE in the strict sense) have different effects on the neurogenic process. Specifically, EE exerts a survival-promoting effect on newborn cells, while running induces proliferation of precursor cells (Fabel et al., 2009; Olson et al., 2006). It is thought that voluntary running “primes” the DG by increasing the number of progenitors available for selection, which leads to an increase in AHN only if there is a need for these cells. This could have implications for potential therapeutic effects of EE or voluntary running, as it is possible that some neurodegenerative changes might be ameliorated by an increase in NSC proliferation (running), and not through an increased survival of newborn neurons (EE) (or vice versa). For a more thorough discussion about the differences between EE and PE, we point the reader to Rogers et al., 2019.
Physical exercise
PE is usually considered as an “activity and training that causes a substantial increase in heart rate that differs significantly from resting heart rate” (Svensson et al., 2015). In animal studies, voluntary running on a running wheel or a treadmill is usually used, like in some models of EE. Despite this similarity, studies show that they should be treated as distinct interventions and that they, in fact, have additive effects (Fabel et al., 2009; Olson et al., 2006).
It has been shown that PE has profound effects on both learning and memory, shown by studies where late exercise (free access to running wheels) after traumatic brain injury reduced working and retention memory impairments in mice (Piao et al., 2013) and where voluntary wheel running was able to counteract cognitive deficits after chronic corticosterone administration in rats (Yau et al., 2012). It is thought that these positive effects of PE are related to increased levels of neurotrophic factors, elevated expression of anti-inflammatory cytokines, and reduced levels of pro-inflammatory cytokines and activated microglia (Svensson et al., 2015). In regard to neurogenesis itself, running was able to increase cell proliferation in the mouse DG (van Praag et al., 1999), restore hippocampal cell proliferation following chronic administration of corticosterone in rats (Yau et al., 2012) and peripheral administration of lipopolysaccharide in mice (Wu et al., 2007). Aged transgenic mice that exercised on a treadmill displayed improved cognitive function, which was associated with suppressed neuronal cell death in the hippocampus (Um et al., 2011). Similar to EE, PE in the form of voluntary running was able to restore hippocampal neurogenesis in a mouse model of AD (J. Rodriguez et al., 2011). Interestingly, despite the fact that both PE and EE are non-specific manipulations with many other effects, their effect on neurogenesis is highly specific, targeting only the hippocampus and not other neurogenic areas of the brain, such as the OB (Brown et al., 2003). While spatially specific, it has been hypothesized that PE influences AHN in a non-specific way: by activating progenitor cell proliferation and thus increasing the potential for neurogenesis, in a time- and dose-dependent fashion (Holmes et al., 2004). Furthermore, newborn neurons induced by prolonged PE seem to integrate rapidly in the aging brain, elevating the complexity of the network and resulting in a rejuvenated hippocampus of mice (Trinchero et al., 2019). Incidentally, plasma transfer from exercised aged mice to sedentary aged mice ameliorated impaired neurogenesis and cognition in the aged hippocampus, showing that the beneficial effects of exercise on the aged brain can be transferred through administration of blood components (Horowitz et al., 2020). This is supported by a recent cellular parabiosis study that used an in vitro model of neurogenesis where a human hippocampal progenitor cell line was treated with human serum, showing that reduced physical activity can increase hippocampal cell death and the risk for future cognitive decline and dementia (Du Preez et al., 2021).
Caloric restriction
CR is usually defined as a “reduction of caloric intake - typically by 20 – 40% of ad libitum consumption - while maintaining adequate nutrient intake” (Trepanowski et al., 2011). This term is sometimes used interchangeably with dietary restriction, which is an intervention in which specific macro/micronutrients (e.g., proteins, carbohydrates and amino acids) are restricted, with no reduction in total energy intake (Selman, 2014).
CR overall has positive effects on learning and memory. A study carried out in rats showed that their spatial and nonspatial abilities and reference and working memory deteriorate with age – an effect that is antagonized with life-long CR (Pitsikas and Algeri, 1992). Similar studies in mice revealed that chronic CR enhances their learning ability and memory (Hashimoto and Watanabe, 2005; Komatsu et al., 2008; Kuhla et al., 2013; Wahl et al., 2018). One of the mechanisms proposed to explain the beneficial effects of CR on cognitive aging is through changes in neurogenesis. Studies in rodents (reviewed in Arslan-Ergul et al., 2013 and Stangl and Thuret, 2009) found that CR increases AHN (Hornsby et al., 2016). However, it still is not established if this occurs through an increase in cell survival (Lee et al., 2002, 2000), through an increase in NSC proliferation (Kaptan et al., 2015; Park et al., 2013) or both. Furthermore, CR not only enhances proliferation of NSCs in young mice, but also prevents the age-related loss of neurogenesis in the SVZ – an effect associated with an improvement in olfactory memory (Apple et al., 2019).High-fat diet (HFD) has an opposite effect in both mice and rats, impairing hippocampal neurogenesis and proliferation of NSCs (Lindqvist et al., 2006; Park et al., 2010). Interestingly, this effect might be transgenerational, as it was shown that HFD-induced maternal obesity could impair AHN during postnatal development of the offspring (Tozuka et al., 2009). In humans, a randomized clinical trial with long-term CR (25% reduction during 2 years) found positive effects on working memory in healthy individuals (Leclerc et al., 2019). An interventional trial in elderly subjects found that 3 months of CR (reduction of 30% relative to previous habits) had beneficial effects on memory performance (Witte et al., 2009). The same group carried out a study in healthy obese postmenopausal women and found that a CR intervention (12-week low-caloric diet) was able to improve recognition memory, an effect that was specific for the weight loss phase that could not be detected in the subsequent weight maintenance phase (Prehn et al., 2016). This effect was associated with an increase in gray matter volume in the hippocampus (as well as the inferior frontal gyrus) and augmented hippocampal resting-state functional connectivity to parietal areas (Prehn et al., 2016). While these studies suffer from certain limitations (self-reporting being one), their findings support the data from experimental animal studies (mentioned above) and epidemiological studies in humans (Mattson, 1999; Parrott and Greenwood, 2007). It is known that CR can enhance neurogenesis in the animal DG, and a recent study in obese adults has shown that CR may influence memory function through modulating AHN (Kim et al., 2020). Further clinical research is needed to determine if CR has a direct effect on the aging DG, and in which groups of people it has the highest efficiency. For example, CR could work well in overweight people, but have limited benefits, or even adverse effects such as dysmenorrhea (Romashkan et al., 2016), in healthy young people.
4.2. Pharmacological approaches
There are different pharmacological compounds that have been shown to stimulate neurogenesis (set A), to protect against neurodegeneration (set B) and to extend lifespan (set C). In this review, we will focus on those found at the intersection of all three sets. We have further narrowed our criteria to compounds that (1) have been extensively researched and that (2) target different metabolic pathways. With that in mind, the compounds whose effects we will describe in this section are: NAD+ (nicotinamide adenine dinucleotide)-boosting molecules (NBMs), resveratrol, rapamycin and metformin.
The effect of these compounds on lifespan and healthspan is heterogenous, and will be briefly summarized here. NBMs extended the lifespan of mice in some studies (Fang et al., 2016; Zhang et al., 2016), had no effect on it in others (Harrison et al., 2021; Mitchell et al., 2018), and seem to have a positive effect on healthspan (Mitchell et al., 2018), The therapeutic potential of some NBMs has been thoroughly reviewed elsewhere (Yoshino et al., 2018), but long-term clinical studies are needed to establish their safety and physiological outcomes. Resveratrol was able to extend the lifespan of some simpler organisms such as nematodes or fruit flies (Bauer et al., 2004; Wood et al., 2004), but not in mice or rats. While the effects on lifespan are unclear, we decided to include resveratrol in the review because of its beneficial effects on mammal healthspan and because of an excellent safety profile (Bhullar and Hubbard, 2015; Pezzuto, 2019), making it a lower-risk intervention that might have positive effects on certain health indices. The data is much clearer with rapamycin, which can extend lifespan in organisms ranging from yeast and flies to mice, recently summarized in (Selvarani et al., 2021). Additionally, it can also improve healthspan in mice (Bitto et al., 2016; Zhang et al., 2014) and has low toxicity in humans (Ceschi et al., 2015), making it overall a good candidate for geroscience-focused clinical trials. Lastly, the effects of metformin on lifespan are inconclusive and depend on the model organism, sex and dose used. While some studies show a positive effect on lifespan extension (recently reviewed in (Hu et al., 2021)), a study from the Interventions Testing Program found no effect on median or maximum lifespan (Strong et al., 2016). However, it does seem to improve healthspan (recently reviewed in (Mohammed et al., 2021), and clinical trials such as MILES and TAME will help answer questions about the potential prophylactic usage of metformin to counteract certain effects of aging itself.
NAD+-boosting molecules
NBMs that we will focus on are nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR). NR is the precursor to NMN, which is in turn one of the precursors to NAD+ - a molecule that is essential for a myriad of enzymatic processes whose levels decline with aging (Imai and Guarente, 2014). There is a profound connection between NAD+ and sirtuins, which are deacetylase enzymes that regulate numerous fundamental biological processes implicated in both lifespan and neurodegenerative diseases (Alcaín and Villalba, 2009; Fang et al., 2017; Imai and Guarente, 2016).
It has been shown that NMN supplementation improves cognitive function in aged mice (Tarantini et al., 2019) and alleviates aging-induced cognitive impairment in rats while reducing apoptosis in the prefrontal cortex and hippocampus (Hosseini et al., 2019). Systemic NMN administration was able to rescue the aging-associated decline of the NSC pool in mice, but not to drive the proliferation of these aged NSCs (Stein and Imai, 2014). Similarly, in a mouse model of cerebral ischemia, delayed administration of NMN improved regenerative neurogenesis in both the SVZ and DG (Zhao et al., 2015). Similar results were observed with NR: its administration was able to increase neurogenesis in the SVZ and DG in aged mice, while at the same time slightly increasing lifespan (Zhang et al., 2016). The same effect on lifespan wasn’t observed in a transgenic mouse model of amyotrophic lateral sclerosis (ALS) (SOD1G93A mice), but improvements in neurogenesis were observed: NR was able to attenuate the ALS-induced loss of NSCs in the SVZ, SGZ and OB, as well as to enhance the proliferation and migration of NSCs (Zhou et al., 2020). In a model of AD (3xTgAD/Polβ+/− mice), 3 months of NR treatment (in animals that were 16 to 18 months old) was able to reduce neuroinflammation, increase NSC proliferation, decrease tau phosphorylation in the hippocampus as well as improve learning, memory and motor function (Hou et al., 2018). Similar effects were found in the APP/PS1 mouse model of AD, where the same length of NR treatment improved memory and learning in mice 7 to 12 months old, but the effects on neurogenesis were not assessed (Hou et al., 2021). Interestingly, some of these effects seem to be transgenerational, as rats nursed by NR-fed mothers display enhanced hippocampal neurogenesis as adults, as well as advantages in spatial memory and physical performance (Ear et al., 2019).
Resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-stilbene; RSV) is a polyphenolic phytoalexin that is generated in response to stress in specific plants such as grapevines (Bhat et al., 2001). RSV has been classified by some as a sirtuin-activating compound (STAC) and has been suggested to be a caloric restriction mimetic (CRM) (Nikolai et al., 2015). However, we note that some studies suggested that resveratrol does not directly activate SIRT1, a protein which was suggested to be its primary target (Pezzuto, 2019).
RSV administration improves performance in behavioral tests related to memory formation and promotes the survival of newborn hippocampal cells in BALB/c mice at 6 months of age (Torres-Pérez et al., 2015). Similarly, adding RSV to HFD-fed mice positively affected adult hypothalamic neurogenesis, both by its anti-apoptotic effect and through enhancing production of newborn cells in the arcuate nucleus of the hypothalamus (Safahani et al., 2019). In mice exposed to neonatal hypoxic ischemia, RSV could prevent cognitive deficits by promoting AHN (Li et al., 2020). In rats exposed to unpredictable chronic mild stress (UCMS), RSV was able to reverse UCMS-induced impaired cognition function and improve hippocampal expression of BDNF, a key molecule in regulating hippocampal plasticity (Yazir et al., 2015). In late middle-age (21 months) male F344 rats, RSV administration improved learning, memory and mood function, as well as increased neurogenesis in the DG (Kodali et al., 2015). Lastly, RSV administration enhanced AHN in rats exposed to lead early in life, which had a protective effect against learning and memory impairments induced by lead neurotoxicity (Wang et al., 2021).
In humans, a double-blind placebo-controlled study with chronic RSV supplementation (200 mg per day for 6 months; in a formula with quercetin) found positive effects on memory performance in healthy overweight older adults, a finding associated with increased hippocampal functional connectivity (Witte et al., 2014). Moreover, a similar study carried out in patients with mild cognitive impairment (MCI) found that the same dose and duration of RSV supplementation had no significant effects on memory performance, but could preserve hippocampal volume and improve hippocampus resting-state functional connectivity (Köbe et al., 2017). Another study utilizing the same dose and duration of RSV supplementation in healthy elderly individuals failed to find improvements in verbal memory, finding only a trend for positive effects on pattern recognition memory (Huhn et al., 2018). In a shorter study utilizing a lower dose of RSV (150 mg per day for 14 weeks) in postmenopausal women, it improved verbal memory and overall cognitive performance (Evans et al., 2017). In healthy young adults (18–30 years), chronic administration of RSV (500 mg per day for 28 days) was not able to improve cognitive function (Wightman et al., 2015). Finally, a meta-analysis exploring the effect of RSV on cognitive and memory performance concluded that chronic RSV supplementation has no significant impact on those indices (Farzaei et al., 2018). In summary, despite promising effects on neurogenesis in animals, it is difficult to firmly conclude if supplementing humans with RSV increases AHN or if it confers advantages in cognitive and memory performance. Further studies with a longer intervention period and larger sample sizes are needed.
Rapamycin
Rapamycin is a macrolide that directly and specifically inhibits the mechanistic target of rapamycin (mTOR), a nutrient-responsive kinase that is the catalytic subunit of two complexes known as mTOR complex 1 (mTORC1) and as mTOR complex 2 (mTORC2). It has been established that while rapamycin directly inhibits mTORC1, mTORC2 is not sensitive to acute rapamycin treatment and chronic exposure is required to indirectly inhibit mTORC2 (Arriola Apelo and Lamming, 2016). Many of the side effects of prolonged rapamycin treatment, such as immunosuppression and glucose intolerance, are considered due to the inhibition of mTORC2, so inhibitors that are specific to mTORC1 might confer beneficial effects while avoiding the side effects (Saxton and Sabatini, 2017). Rapamycin has been proposed as a CRM (Hughes and Kennedy, 2012), but some researchers believe that it is likely that rapamycin and CR exert their effects through different pathways (Unnikrishnan et al., 2020).
Treatment with rapamycin has been associated with improvements in learning and memory in both young and old animals. In 8-month-old male mice, chronic rapamycin treatment (16 weeks) was able to enhance spatial learning and memory, as well as reduce anxiety- and depression-like behaviors (Halloran et al., 2012). The same study observed that 40 weeks of treatment improved recall of an aversive event in older mice (25 months of age), suggesting that even when started late in life, chronic rapamycin treatment was able to delay cognitive decline associated with aging (Halloran et al., 2012). Similarly, lifelong rapamycin administration in mice (started at 2 months of age) was able to improve learning and memory when tested at 18 months of age, but shorter rapamycin administration in adult mice (12 weeks; started at 15 months of age) wasn’t able to improve cognition in animals with pre-existing, age-dependent learning and memory deficit (Majumder et al., 2012). Finally, chronic treatment (15 months) with rapamycin was able to ameliorate deficits in learning and memory in aged (34-month old) rats (Van Skike et al., 2020). Hence, it is yet to be determined which dosing and duration at which age are optimal for exerting improvements in cognitive function. Interestingly, 12 weeks of rapamycin administration in 22-month old mice resulted in increased abundance of activated NSCs in the SVZ (Leeman et al., 2018). However, different results were obtained in a study where rapamycin (i.p.) significantly reduced the number of proliferating cells in the adult hippocampus of 3-month old mice (Romine et al., 2015). It is difficult to compare these results as (1) the experiments were carried out in different age groups, (2) the method and duration of rapamycin administration was different and (3) as they focus on different neurogenic niches. Hence, further experiments are necessary to determine the effects of rapamycin on neurogenesis - especially in the DG of healthy mice after prolonged administration. In humans, a small study in heart transplant recipients found that short-term (4 weeks) immunosuppression with a rapamycin analogue everolimus was associated with improvements in memory performance and mood (Lang et al., 2009). However, a randomized controlled trial in healthy older adults (25 subjects) revealed no improvements in cognition after 8 weeks of rapamycin treatment, noting that longer trials with larger sample sizes may be warranted (Kraig et al., 2018). A clinical trial exploring the effects of rapamycin in older adults with MCI on cognition is currently active (NCT04200911).
Metformin
Metformin (N,N-dimethylbiguanide) is an anti-diabetic drug that inhibits the mitochondrial respiratory chain complex I, leading to multiple downstream effects such as changing the AMP:ATP and ADP:ATP ratios, which activates AMP-activated protein kinase (AMPK) (Barzilai et al., 2016; Rena et al., 2017). Metformin also inhibits hepatic mTORC1 in a biphasic manner: low dose of metformin requires AMPK and the TSC complex for the inhibition, whereas a high dose inhibits mTORC1 through alternative mechanisms, independently of AMPK and TSC complex (Howell et al., 2017). While it has been argued that metformin acts as a CRM (Kezic et al., 2018), results from other model organisms such as Drosophila and mammals call that into question (Lee and Min, 2013; Slack et al., 2012).
Most (but not all) studies have found a positive effect of metformin on cognitive functions and neurogenesis, both in metabolically compromised and in aged animals. In a mouse model of diabetes induced by streptozotocin, metformin treatment produced an improvement in spatial memory and a decreased loss of neurons in the DG of diabetic mice (De Oliveira et al., 2016). Similar effects were observed in a study by Wang et al. in 2012, who reported that metformin administration enhanced spatial memory formation and promoted neurogenesis in both the SVZ and the SGZ of mice, without depleting the endogenous NPC pool. In a transgenic mouse model of AD (APP/PS1 female mice), 14 days of daily metformin treatment rescued spatial memory deficits, reduced brain Aβ deposition and Aβ levels, prevented neuronal cell death in the hippocampus as well as increased AHN (Ou et al., 2018). Similarly, in 3xTg-AD mice, the same length of metformin treatment rescued impairments in AHN and spatial memory (Syal et al., 2020). It was observed that metformin enhances the proliferation, self-renewal, and neuronal differentiation of adult NPCs through two distinct molecular pathways: a TAp73 pathway mediating self-renewal and proliferation, and an AMPK-aPKC-CBP pathway that is required for metformin-induced neuronal differentiation (Fatt et al., 2015). In HFD-induced insulin resistant rats, metformin restored the learning and memory behaviors that were impaired by long-term HFD consumption (Pintana et al., 2012). Similarly, in HFD-fed obese mice, metformin had a beneficial effect on learning and memory, and also restored impairments in AHN, through the regulation of gut microbiota (Ma et al., 2021). 36 days of metformin administration enhanced the spatial memory of aged rats in the Morris Water Maze (Ashrostaghi et al., 2015). In a D-galactose-induced aging model in mice, metformin administration improved learning and memory ability, assessed by the novel object recognition task (Fatemi et al., 2018). However, one study found no beneficial effect of metformin supplementation on learning in old male mice – in fact, metformin exhibited a deleterious effect on memory retention (Thangthaeng et al., 2017). Metformin use was also associated with impaired cognitive performance in patients with diabetes (Moore et al., 2013), but another study in diabetic individuals showed that metformin treatment was inversely related to cognitive impairment (Ng et al., 2014). In summary, many studies show that metformin improves neurogenesis in various animal models, and is a promising candidate against cognitive impairments in humans. Hence, future research should address the interaction of effects of metformin on cognitive functions with age, sex, dosage, and duration of treatment.
5. Conclusions
We have discussed some alterations of adult neurogenesis in aging and neurodegenerative diseases in this review. The dysfunction of AHN appears to be an early marker of the development of these aging-related diseases and it seems that the regulation of neurogenesis could be an effective intervention against them.
The iPSC technology is widely used to screen anti-neurodegenerative drugs and understand the mechanisms of mutations involved, which could recapitulate the dynamic processes of neurogenesis in vitro (Chen et al., 2020). In addition, iPSCs can be used as the autologous source for stem cell therapy. Transplantation of NSCs is regarded as a prospective therapeutic intervention (De Gioia et al., 2020). In the past few decades, many promising preclinical and early clinical findings of stem cell therapy were obtained from animal models (Ford et al., 2020). However, the risk of teratoma formation and mutations, as well as the optimization of stem cell sources and procedures, continue to be the major issues for further feasible and safe applications, which must be solved (Itakura et al., 2017; Merkle et al., 2017). Therefore, a more in-depth knowledge of the characteristics of NSCs and the related neurotrophic factors and differential stimulations, as well as how to combine genetic engineering like CRISPR/Cas9 and RNAi to modify patient-derived iPSCs for autologous transplantation, will help us tackle the obstacles.
It should also be noted that increasing AHN beyond physiological levels might have harmful effects. While deficits in plasticity are associated with certain disorders and could leave the brain unable to adjust to changing demands, so could a supraphysiological increase in AHN result in maladaptive effects, with structural connections becoming unstable, resulting in compromised cognition and behavior (Pascual-Leone et al., 2011). For example, increasing the number of adult-born cells by blocking cell death in the OB impairs performance in odor discrimination tasks (Mouret et al., 2009). Furthermore, it was shown that adult neurogenesis transiently generates oxidative stress, which has been implicated in a wide variety of CNS disorders (Walton et al., 2012). There is likely a point where the balance between plasticity and stability (neuronal turnover) is at its optimal value for cognition and overall brain health, and further research is needed to determine if there is a threshold at which point too many new neurons become deleterious. Similarly, since AHN can be divided into stages of proliferation, migration and differentiation, strategies targeting different components could be utilized in different conditions. It has been suggested that for MDD and some other conditions that involve the hippocampus, neurogenesis could be induced, while for PD and HD, the optimal strategy would be to induce the local dividing cells to proliferate and then differentiate into small spine neurons (for HD) or dopaminergic neurons (PD) (Gage, 2004).
A similar cost-benefit analysis should be carried out for other manipulations mentioned in the review. For example, NAD+ is a ubiquitous biological molecule that is central to several cellular bioenergetic functions and is used as a cofactor or substrate by hundreds of enzymes (Covarrubias et al., 2021; Lautrup et al., 2019) and, as such, levels of caution should be used in employing system-wide manipulations. Potential risks and benefits of NBMs have been reviewed comprehensively elsewhere (Braidy and Liu, 2020), so we will only briefly mention some of the potential negative consequences. For instance, cancer cells, being highly replicative, have a high energy demand and thus NAD+ supplementation could promote the growth of specific cancers by fueling cell proliferation (Demarest et al., 2019; Lautrup et al., 2019). It has been shown that NMN supplementation can promote the proinflammatory senescence-associated secretory phenotype (SASP), which has tumorigenic properties (Nacarelli et al., 2019). The authors suggest that NAD+ should be supplemented with precision to balance the advantageous “anti-aging” effects with potential detrimental pro-tumorigenic side-effects (Nacarelli et al., 2019). We suggest that the potential negative effects of the SASP might also be offset by combinational therapy – for example, administering NBMs after clearing senescent cells with senolytics has the potential to synergize. More recently, a study which utilized an NR washout phase in its experimental design showed that the beneficial effects of NR are not sustained after its removal in aged animals, and that removing NR might have undesirable consequences (Zong et al., 2021). The authors conclude that the supplementation regimen may need to be sustained long-term to maintain its benefits, and we argue that further studies with washout periods in aged animals are necessary to ascertain organism-wide effects of cessation of NR supplementation.
The potential shortcomings of utilizing system-wide (mTOR, AMPK, sirtuins) manipulations should be explored in detail. Optimal levels of these manipulations should be determined, their interactions in a form of combinatorial therapy, as well as methods to precisely target dysfunctional systems. Despite potential shortcomings, the currently available data suggests that modulating neurogenesis represents an important target for manipulations that could help in the fight against neurodegenerative disorders and cognitive decline, ultimately leading to improvements in both lifespan and healthspan.
Highlights.
Formation of new neurons (neurogenesis) plays a significant role throughout lifespan
The incidence of neurodegeneration increases with aging, while neurogenesis decreases
Age-associated decline in brain function may be caused by neural stem cell defects
Geroscience interventions that target the aging process mostly enhance neurogenesis
Enhancement of neurogenesis is beneficial in aging and neurodegeneration
Optimal levels of neurogenesis induction and adverse effects should be determined
Abbreviations
- Aβ
amyloid beta
- AD
Alzheimer’s disease
- AHN
adult hippocampal neurogenesis
- APOE
apolipoprotein E
- A-T
Ataxia Telangiectasia
- ATM
Ataxia Telangiectasia mutated
- BDNF
brain-derived neurotrophic factor
- BER
Base excision repair
- BrdU
Bromodeoxyuridine
- CDC42
Cell division control protein 42 homolog
- CNS
central nervous system
- CR
caloric restriction
- CS
Cockayne syndrome
- DCX
Doublecortin
- DG
dentate gyrus
- DNAJC6
DnaJ Heat Shock Protein Family (Hsp40) Member C6
- DSB
DNA double-strand break
- EE
Environmental enrichment
- EOAD
early-onset Alzheimer's disease
- FBXO7,
F-Box Protein 7
- GABA
gamma-aminobutyric acid
- γH2AX
gamma H2A histone family member X
- HD
Huntington’s disease
- ihNSC
immortalized human neural stem cell line
- LB
Lewy body
- LOAD
late-onset Alzheimer's disease
- LRRK2
Leucine Rich Repeat Kinase 2
- MCI
mild cognitive impairment
- NAD+
nicotinamide adenine dinucleotide
- NEIL1
Nei Like DNA Glycosylase 1
- NEIL3
Nei Like DNA Glycosylase 3
- NER
Nucleotide excision repair
- NPC
Neural progenitor cell
- NSC
neural stem cell
- OB
olfactory bulb
- PCNA
proliferating cell nuclear antigen
- PD
Parkinson’s disease
- PE
Physical exercise
- PINK1
PTEN Induced Kinase 1
- Polβ
DNA polymerase β
- poly Q
polyglutamine
- PRKN
parkin RBR E3 ubiquitin protein ligase
- PS1
presenilin 1
- PS2
presenilin 2
- RMS
rostral migratory stream
- RSV
Resveratrol
- SGZ
subgranular zone
- SNCA
synuclein alpha
- SOX2
sex determining region Y-box 2
- SVZ
subventricular zone
- VEGFA
vascular Endothelial Growth Factor A
- VPS35
VPS35 Retromer Complex Component
Footnotes
Declaration of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z, 2009. Neural Stem and Progenitor Cells Retain Their Potential for Proliferation and Differentiation into Functional Neurons Despite Lower Number in Aged Brain. J. Neurosci. 29, 4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaín FJ, Villalba JM, 2009. Sirtuin activators. Expert Opin. Ther. Pat. 19, 403–414. doi: 10.1517/13543770902762893 [DOI] [PubMed] [Google Scholar]
- Allen DM, van Praag H, Ray J, Weaver Z, Winrow CJ, Carter TA, Braquet R, Harrington E, Ried T, Brown KD, 2001. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 15, 554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, 2011. The Discovery of Adult Mammalian Neurogenesis, in: Seki T, Sawamoto K, Parent JM, Alvarez-Buylla A (Eds.), Neurogenesis in the Adult Brain I. Springer; Japan, Tokyo, pp. 3–46. doi: 10.1007/978-4-431-53933-9_1 [DOI] [Google Scholar]
- Altman J, 1962. Are New Neurons Formed in the Brains of Adult Mammals? Science (80-. ). 135, 1127–1128. doi: 10.1126/science.135.3509.1127 [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD, 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335. doi: 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- Altuna M, Urdánoz-Casado A, de Gordoa JS-R, Zelaya MV, Labarga A, Lepesant JMJ, Roldán M, Blanco-Luquin I, Perdones Á, Larumbe R, 2019. DNA methylation signature of human hippocampus in Alzheimer’s disease is linked to neurogenesis. Clin. Epigenetics 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez CV, Garcia-Lavandeira M, Garcia-Rendueles MER, Diaz-Rodriguez E, Garcia-Rendueles AR, Perez-Romero S, Vila TV, Rodrigues JS, Lear PV, Bravo SB, 2012. Defining stem cell types: understanding the therapeutic potential of ESCs, ASCs, and iPS cells. J. Mol. Endocrinol. 49, R89–R111. doi: 10.1530/JME-12-0072 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2019. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 15, 321–387. doi: 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- Anacker C, Hen R, 2017. Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood. Nat. Rev. Neurosci. 18, 335–346. doi: 10.1038/nrn.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R, 2018. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559, 98–102. doi: 10.1038/s41586-018-0262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andäng M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, 2008. Histone H2AX-dependent GABA A receptor regulation of stem cell proliferation. Nature 451, 460–464. [DOI] [PubMed] [Google Scholar]
- Apple DM, Mahesula S, Fonseca RS, Zhu C, Kokovay E, 2019. Calorie restriction protects neural stem cells from age-related deficits in the subventricular zone. Aging (Albany. NY). 11, 115–126. doi: 10.18632/aging.101731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Rakic P, 2011. Neuroscience: Gone with the wean. Nature 478, 333–4. doi: 10.1038/478333a [DOI] [PubMed] [Google Scholar]
- Armesilla-Díaz A, Bragado P, Del Valle I, Cuevas E, Lázaro I, Martin C, Cigudosa JC, Silva A, 2009. p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience 158, 1378–1389. [DOI] [PubMed] [Google Scholar]
- Arranz L, De Castro NM, Baeza I, Maté I, Viveros MP, De La Fuente M, 2010. Environmental enrichment improves age-related immune system impairment: Long-term exposure since adulthood increases life span in mice. Rejuvenation Res. 13, 415–428. doi: 10.1089/rej.2009.0989 [DOI] [PubMed] [Google Scholar]
- Arriola Apelo SI, Lamming DW, 2016. Rapamycin: An InhibiTOR of aging emerges from the soil of Easter island. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 71, 841–849. doi: 10.1093/gerona/glw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan-Ergul A, Ozdemir AT, Adams MM, 2013. Aging, neurogenesis, and caloric restriction in different model organisms. Aging Dis. 4, 221–32. [PMC free article] [PubMed] [Google Scholar]
- Ashrostaghi Z, Ganji F, Sepehri H, 2015. Effect of metformin on the spatial memory in aged rats. Natl. J. Physiol. Pharm. Pharmacol. 5, 416–420. doi: 10.5455/njppp.2015.5.1208201564 [DOI] [Google Scholar]
- Audesse AJ, Webb AE, 2020. Mechanisms of enhanced quiescence in neural stem cell aging. Mech. Ageing Dev. 191, 111323. doi: 10.1016/j.mad.2020.111323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoud-Lévi A, Massart R, Rosser A, 2021. Cell therapy in Huntington’s disease: Taking stock of past studies to move the field forward. Stem Cells 39, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiola N, de Oliveira RM, Herrera F, Guardia-Laguarta C, Gonçalves SA, Pera M, Suárez-Calvet M, Clarimon J, Outeiro TF, Lleó A, 2011. Tau enhances α-synuclein aggregation and toxicity in cellular models of synucleinopathy. PLoS One 6, e26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CELL, 2008. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 319, 1640–2. doi: 10.1126/science.1152882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista P, Andrade JP, 2018. Adult hippocampal neurogenesis: regulation and possible functional and clinical correlates. Front. Neuroanat. 12, 44. doi: 10.3389/fnana.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzuol L, Ju L, Jeggo PA, 2017. A coordinated DNA damage response promotes adult quiescent neural stem cell activation. PLoS Biol. 15, e2001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier P, Zejneli O, Martinho M, Lasorsa A, Belle V, Smet-Nocca C, Tsvetkov PO, Devred F, Landrieu I, 2019. Role of tau as a microtubule-associated protein: structural and functional aspects. Front. Aging Neurosci. 11, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA, 2016. Metformin as a Tool to Target Aging. Cell Metab. 23, 1060–1065. doi: 10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL, 2004. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc. Natl. Acad. Sci. 101, 12980–12985. doi: 10.1073/pnas.0403493101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B, 2012. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci. 15, 349–357. [DOI] [PubMed] [Google Scholar]
- Berdugo-Vega G, Arias-Gil G, López-Fernández A, Artegiani B, Wasielewska JM, Lee C-C, Lippert MT, Kempermann G, Takagaki K, Calegari F, 2020. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat. Commun. 11, 1–12. doi: 10.1038/s41467-019-14026-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Booij J, Francot CMJE, Bergmans PLM, Hijman R, Stoof JC, Wolters EC, 2001. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 50, 34–41. [DOI] [PubMed] [Google Scholar]
- Berg DA, Bond AM, Ming G, Song H, 2018. Radial glial cells in the adult dentate gyrus: what are they and where do they come from? F1000Research 7, 277. doi: 10.12688/f1000research.12684.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Lee H, Young AH, Aarsland D, Thuret S, 2020. Adult Hippocampal Neurogenesis in Major Depressive Disorder and Alzheimer’s Disease. Trends Mol. Med. 26, 803–818. doi: 10.1016/J.MOLMED.2020.03.010 [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MSY, Steier P, Kutschera W, Johnson L, Landén M, Druid H, Spalding KL, Frisén J, 2012. The Age of Olfactory Bulb Neurons in Humans. Neuron 74, 634–639. doi: 10.1016/j.neuron.2012.03.030 [DOI] [PubMed] [Google Scholar]
- Bergmann O, Spalding KL, Frisén J, 2015. Adult Neurogenesis in Humans. Cold Spring Harb. Perspect. Biol. 7, a018994. doi: 10.1101/cshperspect.a018994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F, 1973. Brain dopamine and the syndromes of Parkinson and Huntington Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 20, 415–455. [DOI] [PubMed] [Google Scholar]
- Berron D, Schütze H, Maass A, Cardenas-Blanco A, Kuijf HJ, Kumaran D, Düzel E, 2016. Strong Evidence for Pattern Separation in Human Dentate Gyrus. J. Neurosci. 36, 7569. doi: 10.1523/JNEUROSCI.0518-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KPL, Kosmeder JW, Pezzuto JM, 2001. Biological Effects of Resveratrol. Antioxid. Redox Signal. 3, 1041–1064. doi: 10.1089/152308601317203567 [DOI] [PubMed] [Google Scholar]
- Bhullar KS, Hubbard BP, 2015. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta - Mol. Basis Dis 1852, 1209–1218. doi: 10.1016/j.bbadis.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Bitto A, Ito TK, Pineda VV, Letexier NJ, Huang HZ, Sutlief E, Tung H, Vizzini N, Chen B, Smith K, Meza D, Yajima M, Beyer RP, Kerr KF, Davis DJ, Gillespie CH, Snyder JM, Treuting PM, Kaeberlein M, 2016. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife 5, 1–17. doi: 10.7554/eLife.16351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biundo F, Del Prete D, Zhang H, Arancio O, D’Adamio L, 2018. A role for tau in learning, memory and synaptic plasticity. Sci Rep 8, 3184. doi: 10.1038/s41598-018-21596-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom GS, 2014. Amyloid-β and Tau. JAMA Neurol. 71, 505. doi: 10.1001/jamaneurol.2013.5847 [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ, 2006. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol. Dis. 24, 1–14. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Müller MLTM, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA, 2010. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain 133, 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ, 2018. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22, 589–599.e5. doi: 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V, 2012. Hippocampal Angiogenesis and Progenitor Cell Proliferation Are Increased with Antidepressant Use in Major Depression. Biol. Psychiatry 72, 562–571. doi: 10.1016/j.biopsych.2012.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V, 2009. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34, 2376–89. doi: 10.1038/npp.2009.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Song J, Ming G, Song H, 2012. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr. Opin. Neurobiol. 22, 754–761. doi: 10.1016/j.conb.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds JA, Kuttner-Hirshler Y, Bartolotti N, Tobin MK, Pizzi M, Marr R, Lazarov O, 2015. Presenilin-1 dependent neurogenesis regulates hippocampal learning and memory. PLoS One 10, e0131266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottero V, Powers D, Yalamanchi A, Quinn JP, Potashkin JA, 2021. Key Disease Mechanisms Linked to Alzheimer’s Disease in the Entorhinal Cortex. Int. J. Mol. Sci. 22, 3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos RAI, Steur ENHJ, Braak E, 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Braidy N, Liu Y, 2020. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp. Gerontol. 132, 110831. doi: 10.1016/j.exger.2020.110831 [DOI] [PubMed] [Google Scholar]
- Briley D, Ghirardi V, Woltjer R, Renck A, Zolochevska O, Taglialatela G, Micci M-A, 2016. Preserved neurogenesis in non-demented individuals with AD neuropathology. Sci. Rep. 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann K, Lerche S, Dilger SS, Stirnkorb JG, Apel A, Hauser A-K, Liepelt-Scarfone I, Berg D, Gasser T, Schulte C, 2017. SNPs in Aβ clearance proteins: Lower CSF Aβ1–42 levels and earlier onset of dementia in PD. Neurology 89, 2335–2340. [DOI] [PubMed] [Google Scholar]
- Brown E, 1999. Hippocampal Remodeling and Damage by Corticosteroids Implications for Mood Disorders. Neuropsychopharmacology 21, 474–484. doi: 10.1016/S0893-133X(99)00054-8 [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG, 2003. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17, 2042–6. doi: 10.1046/j.1460-9568.2003.02647.x [DOI] [PubMed] [Google Scholar]
- Brunson KL, Baram TZ, Bender RA, 2005. Hippocampal neurogenesis is not enhanced by lifelong reduction of glucocorticoid levels. Hippocampus 15, 491–501. doi: 10.1002/hipo.20074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO, 2004. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J. Neurol. Neurosurg. Psychiatry 75, 1467–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace R, Sleegers K, Van Broeckhoven C, 2016. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimer’s Dement. 12, 733–748. [DOI] [PubMed] [Google Scholar]
- Calió ML, Mosini AC, Marinho DS, Salles GN, Massinhani FH, Ko GM, Porcionatto MA, 2021. Leptin enhances adult neurogenesis and reduces pathological features in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 148, 105219. [DOI] [PubMed] [Google Scholar]
- Carapeto PV, Aguayo-Mazzucato C, 2021. Effects of exercise on cellular and tissue aging. Aging (Albany. NY). 13, 14522–14543. doi: 10.18632/aging.203051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G, 2012. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology 78, 1939–1945. [DOI] [PubMed] [Google Scholar]
- Carlessi L, De Filippis L, Lecis D, Vescovi A, Delia D, 2009. DNA-damage response, survival and differentiation in vitro of a human neural stem cell line in relation to ATM expression. Cell Death Differ. 16, 795–806. [DOI] [PubMed] [Google Scholar]
- Carlessi L, Poli EF, De Filippis L, Delia D, 2013. ATM-deficient human neural stem cells as an in vitro model system to study neurodegeneration. DNA Repair (Amst). 12, 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceschi A, Heistermann E, Gros S, Reichert C, Kupferschmidt H, Banner NR, Krähenbühl S, Taegtmeyer AB, 2015. Acute sirolimus overdose: a multicenter case series. PLoS One 10, e0128033. doi: 10.1371/journal.pone.0128033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Kerr C, 2019. The Epigenetics of Stem Cell Aging Comes of Age. Trends Cell Biol. 29, 563–568. doi: 10.1016/j.tcb.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-D, Li H-Q, Cui M, Dong Q, Yu J-T, 2020. Pluripotent stem cells for neurodegenerative disease modeling: an expert view on their value to drug discovery. Expert Opin. Drug Discov. 15, 1081–1094. [DOI] [PubMed] [Google Scholar]
- Chen Y, Tang BL, 2006. The amyloid precursor protein and postnatal neurogenesis/neuroregeneration. Biochem. Biophys. Res. Commun. 341, 1–5. [DOI] [PubMed] [Google Scholar]
- Cheyuo C, Aziz M, Wang P, 2019. Neurogenesis in neurodegenerative diseases: Role of MFG-E8. Front. Neurosci. 13, 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W-H, Depboylu C, Hermanns G, Maurer L, Windolph A, Oertel WH, Ries V, Höglinger GU, 2015. Long-term treatment with L-DOPA or pramipexole affects adult neurogenesis and corresponding non-motor behavior in a mouse model of Parkinson’s disease. Neuropharmacology 95, 367–376. [DOI] [PubMed] [Google Scholar]
- Cho IK, Hunter CE, Ye S, Pongos AL, Chan AWS, 2019. Combination of stem cell and gene therapy ameliorates symptoms in Huntington’s disease mice. NPJ Regen. Med. 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, 2018. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science (80-. ). 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchyard A, Lees AJ, 1997. The relationship between dementia and direct involvement of the hippocampus and amygdala in Parkinson’s disease. Neurology 49, 1570–1576. [DOI] [PubMed] [Google Scholar]
- Ciaffardini F, Nicolai S, Caputo M, Canu G, Paccosi E, Costantino M, Frontini M, Balajee AS, Proietti-De-Santis L, 2014. The cockayne syndrome B protein is essential for neuronal differentiation and neuritogenesis. Cell Death Dis. 5, e1268–e1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ, 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–3. doi: 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemenson GD, Deng W, Gage FH, 2015a. Environmental enrichment and neurogenesis: From mice to humans. Curr. Opin. Behav. Sci. 4, 56–62. doi: 10.1016/j.cobeha.2015.02.005 [DOI] [Google Scholar]
- Clemenson GD, Lee SW, Deng W, Barrera VR, Iwamoto KS, Fanselow MS, Gage FH, 2015b. Enrichment rescues contextual discrimination deficit associated with immediate shock. Hippocampus 25, 385–392. doi: 10.1002/hipo.22380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, McGaugh JL, LaFerla FM, 2007. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol. Dis. 28, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM, 2014. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 5, 1–5. doi: 10.1038/ncomms4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, Villmann C, Hahnen E, Neuhuber W, Weigel D, Buchfelder M, Stefan H, Beck H, Steindler DA, Blumcke I, 2010. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain 133, 3359–3372. doi: 10.1093/brain/awq215 [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small Gw., Roses AD, Haines JL, Pericak-Vance MA, 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science (80-. ). 261, 921–923. [DOI] [PubMed] [Google Scholar]
- Covarrubias AJ, Perrone R, Grozio A, Verdin E, 2021. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22, 119. doi: 10.1038/S41580-020-00313-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, Winner B, Winkler J, Masliah E, 2008. α-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J. Neurosci. 28, 4250–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado-Marrero M, Sabbagh JJ, Jones MR, Chaput D, Dickey CA, Blair LJ, 2020a. Hippocampal neurogenesis is enhanced in adult tau deficient mice. Cells 9, 210. doi: 10.3390/cells9010210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado-Marrero M, Smith TM, Gould LA, Kim S, Penny HJ, Sun Z, Gulick D, Dickey CA, Blair LJ, 2020b. FKBP5 and early life stress affect the hippocampus by an age-dependent mechanism. Brain, Behav. Immun. - Heal 9, 100143. doi: 10.1016/j.bbih.2020.100143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig L, Belzung C, 2016. Acute Stress and Anxiety, in: Adult Neurogenesis in the Hippocampus. Elsevier, pp. 207–228. doi: 10.1016/B978-0-12-801977-1.00009-X [DOI] [Google Scholar]
- Culig L, Surget A, Bourdey M, Khemissi W, Le Guisquet A-M, Vogel E, Sahay A, Hen R, Belzung C, 2017. Increasing adult hippocampal neurogenesis in mice after exposure to unpredictable chronic mild stress may counteract some of the effects of stress. Neuropharmacology 126, 179–189. doi: 10.1016/j.neuropharm.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Cutler RR, Kokovay E, 2020. Rejuvenating subventricular zone neurogenesis in the aging brain. Curr. Opin. Pharmacol. 50, 1–8. doi: 10.1016/j.coph.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R, 2009. Neurogenesis-Dependent and - Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron 62, 479–493. doi: 10.1016/j.neuron.2009.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford A. a, Cleaver KM, Yassaee M, Cameron H. a, 2003. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 460, 563–72. doi: 10.1002/cne.10675 [DOI] [PubMed] [Google Scholar]
- De Gioia R, Biella F, Citterio G, Rizzo F, Abati E, Nizzardo M, Bresolin N, Comi G. Pietro, Corti S, 2020. Neural stem cell transplantation for neurodegenerative diseases. Int. J. Mol. Sci. 21, 3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira WH, De Santana Nunes AK, De França MER, Dos Santos LA, Lós DB, Rocha SWS, De Sousa Barbosa KP, Rodrigues GB, Peixoto CA, 2016. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 1644, 149–160. doi: 10.1016/j.brainres.2016.05.013 [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F, 1998. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390. [DOI] [PubMed] [Google Scholar]
- Demarest TG, Babbar M, Okur MN, Dan X, Croteau DL, Fakouri NB, Mattson MP, Bohr VA, 2019. NAD + Metabolism in Aging and Cancer. Annu. Rev. Cancer Biol. 3, 105–130. doi: 10.1146/annurev-cancerbio-030518-055905 [DOI] [Google Scholar]
- Demarest TG, Varma VR, Estrada D, Babbar M, Basu S, Mahajan UV, Moaddel R, Croteau DL, Thambisetty M, Mattson MP, 2020. Biological sex and DNA repair deficiency drive Alzheimer’s disease via systemic metabolic remodeling and brain mitochondrial dysfunction. Acta Neuropathol. 140, 25–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars M, Hu Y, Gadadhar A, Lazarov O, 2010. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J. Neurosci. Res. 88, 2103–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH, 2009. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–42. doi: 10.1523/JNEUROSCI.3362-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déry N, Pilgrim M, Gibala M, Gillen J, Wojtowicz JM, MacQueen G, Becker S, 2013. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front. Neurosci. 7, 1–15. doi: 10.3389/fnins.2013.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal J, Kannangara TS, Vaculik M, Xue Y, Kumar KL, Maione A, Béïque J-C, Shen J, Lagace DC, 2018. Adult hippocampal neurogenesis occurs in the absence of Presenilin 1 and Presenilin 2. Sci. Rep. 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhikav V, Anand K, 2012. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 15, 239. doi: 10.4103/0972-2327.104323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SE, Tsivos D, Knight M, McCann B, Pennington C, Shiel AI, Conway ME, Newson MA, Kauppinen RA, Coulthard EJ, 2017. The impact of ageing reveals distinct roles for human dentate gyrus and CA3 in pattern separation and object recognition memory. Sci. Reports 2017 71 7, 1–13. doi: 10.1038/s41598-017-13853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioli C, Patrício P, Pinto Lucilia-Goreti, Marie C, Morais M, Vyas S, Bessa JM, Pinto Luisa, Sotiropoulos I, 2021. Adult neurogenic process in the subventricular zone-olfactory bulb system is regulated by Tau protein under prolonged stress. Cell Prolif. 54. doi: 10.1111/CPR.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioli C, Patrício P, Trindade R, Pinto LG, Silva JM, Morais M, Ferreiro E, Borges S, Mateus-Pinheiro A, Rodrigues AJ, 2017. Tau-dependent suppression of adult neurogenesis in the stressed hippocampus. Mol. Psychiatry 22, 1110–1118. [DOI] [PubMed] [Google Scholar]
- Donoviel DB, Hadjantonakis A-KK, Ikeda M, Zheng H, Hyslop PSG, Bernstein A, 1999. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 13, 2801–10. doi: 10.1101/gad.13.21.2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey ER, George BP, Leff B, Willis AW, 2013. The coming crisis: Obtaining care for the growing burden of neurodegenerative conditions. Neurology 80, 1989–1996. doi: 10.1212/WNL.0b013e318293e2ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Nora Abrous D, 2008. Stem cell review series: role of neurogenesis in age-related memory disorders. Aging Cell 7, 569–89. doi: 10.1111/j.1474-9726.2008.00369.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Preez A, Lefèvre-Arbogast S, Houghton V, de Lucia C, Low DY, Helmer C, Féart C, Delcourt C, Proust-Lima C, Pallàs M, Ruigrok SR, Altendorfer B, González-Domínguez R, Sánchez-Pla A, Urpi-Sardà M, Andres-Lacueva C, Aigner L, Lucassen PJ, Korosi A, Manach C, Samieri C, Thuret S, 2021. The serum metabolome mediates the concert of diet, exercise, and neurogenesis, determining the risk for cognitive decline and dementia. Alzheimers. Dement. doi: 10.1002/ALZ.12428 [DOI] [PubMed] [Google Scholar]
- Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, Mark B, Klickstein N, Corjuc DL, Corjuc BT, 2020. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 26, 1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest J-M, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV, 2008. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3, e1959. doi: 10.1371/journal.pone.0001959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer CA, & Sinclair AJ, 1998. The premature ageing syndromes: insights into the ageing process. Age and Ageing, 27(1), 73–81. doi: 10.1093/ageing/27.1.73 [DOI] [PubMed] [Google Scholar]
- Ear PH, Chadda A, Gumusoglu SB, Schmidt MS, Vogeler S, Malicoat J, Kadel J, Moore MM, Migaud ME, Stevens HE, Brenner C, 2019. Maternal Nicotinamide Riboside Enhances Postpartum Weight Loss, Juvenile Offspring Development, and Neurogenesis of Adult Offspring. Cell Rep. 26, 969–983.e4. doi: 10.1016/j.celrep.2019.01.007 [DOI] [PubMed] [Google Scholar]
- Eliwa H, Brizard B, Le Guisquet AM, Hen R, Belzung C, Surget A, 2021. Adult neurogenesis augmentation attenuates anhedonia and HPA axis dysregulation in a mouse model of chronic stress and depression. Psychoneuroendocrinology 124, 105097. doi: 10.1016/J.PSYNEUEN.2020.105097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Rios V, Dumitrache LC, Downing SM, Li Y, Brown EJ, Russell HR, McKinnon PJ, 2017. DNA-PKcs, ATM, and ATR interplay maintains genome integrity during neurogenesis. J. Neurosci. 37, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH, 1998. Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–7. doi: 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J, 2014. Neurogenesis in the striatum of the adult human brain. Cell 156, 1072–83. doi: 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- Essa H, Peyton L, Hasan W, León BE, Choi D-S, 2022. Implication of Adult Hippocampal Neurogenesis in Alzheimer’s Disease and Potential Therapeutic Approaches. Cells 11, 286. doi: 10.3390/cells11020286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HM, Howe PRC, Wong RHX, 2017. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 9, 27. doi: 10.3390/nu9010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G, 2009. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front. Neurosci. 3, 1–7. doi: 10.3389/neuro.22.002.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Perrin RJ, 2012. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark. Med. 6, 455–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhoury M, 2018. Microglia and astrocytes in Alzheimer’s disease: Implications for therapy. Curr. Neuropharmacol. 16, 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Kassahun H, Croteau DL, Scheibye-Knudsen M, Marosi K, Lu H, Shamanna RA, Kalyanasundaram S, Bollineni RC, Wilson MA, Iser WB, Wollman BN, Morevati M, Li J, Kerr JS, Lu Q, Waltz TB, Tian J, Sinclair DA, Mattson MP, Nilsen H, Bohr VA, 2016. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 24, 566–581. doi: 10.1016/j.cmet.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, Bohr VA, 2017. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol. Med. 23, 899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Ricci V, Middei S, 2022. Adult Hippocampal Neurogenesis in Alzheimer’s Disease: An Overview of Human and Animal Studies with Implications for Therapeutic Perspectives Aimed at Memory Recovery. Neural Plast. 2022, 9959044. doi: 10.1155/2022/9959044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaei MH, Rahimi R, Nikfar S, Abdollahi M, 2018. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients. Pharmacol. Res. 128, 338–344. doi: 10.1016/j.phrs.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Fatemi I, Khaluoi A, Kaeidi A, Shamsizadeh A, Heydari S, Allahtavakoli M, 2018. Protective effect of metformin on D-galactose-induced aging model in mice. Iran. J. Basic Med. Sci. 21, 19–25. doi: 10.22038/ijbms.2017.24331.6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt M, Hsu K, He L, Wondisford F, Miller FD, Kaplan DR, Wang J, 2015. Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation. Stem Cell Reports 5, 988–995. doi: 10.1016/j.stemcr.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano DM, Bordey A, Bonfanti L, 2015. Noncanonical Sites of Adult Neurogenesis in the Mammalian Brain. Cold Spring Harb. Perspect. Biol. 7, a018846. doi: 10.1101/cshperspect.a018846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor-García M, Terreros-Roncal J, Moreno-Jiménez EP, Ávila J, Rábano A, Llorens-Martín M, 2020. Unraveling human adult hippocampal neurogenesis. Nat. Protoc. doi: 10.1038/s41596-019-0267-y [DOI] [PubMed] [Google Scholar]
- Ford E, Pearlman J, Ruan T, Manion J, Waller M, Neely GG, Caron L, 2020. Human pluripotent stem cells-based therapies for neurodegenerative diseases: Current status and challenges. Cells 9, 2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França TFA, Bitencourt AM, Maximilla NR, Barros DM, Monserrat JM, 2017. Hippocampal neurogenesis and pattern separation: A meta-analysis of behavioral data. Hippocampus 27, 937–950. doi: 10.1002/hipo.22746 [DOI] [PubMed] [Google Scholar]
- Franjic D, Skarica M, Ma S, Arellano JI, Tebbenkamp ATN, Choi J, Xu C, Li Q, Morozov YM, Andrijevic D, Vrselja Z, Spajic A, Santpere G, Li M, Zhang S, Liu Y, Spurrier J, Zhang L, Gudelj I, Rapan L, Takahashi H, Huttner A, Fan R, Strittmatter SM, Sousa AMM, Rakic P, Sestan N, 2021. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron 1–18. doi: 10.1016/j.neuron.2021.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Köhler S, Josselyn SA, 2013. Hippocampal neurogenesis and forgetting. Trends Neurosci. 36, 497–503. doi: 10.1016/j.tins.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Franzmeier N, Ren J, Damm A, Monté-Rubio G, Boada M, Ruiz A, Ramirez A, Jessen F, Düzel E, Gómez OR, 2021. The BDNF Val66Met SNP modulates the association between beta-amyloid and hippocampal disconnection in Alzheimer’s disease. Mol. Psychiatry 26, 614–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsova B, Juliá AA, Rizavi HS, Frasch AC, Pandey GN, 2016. Expression of p21-activated kinases 1 and 3 is altered in the brain of subjects with depression. Neuroscience 333, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster-Matanzo A, Llorens-Martín M, Jurado-Arjona J, Avila J, Hernández F, 2012. Tau protein and adult hippocampal neurogenesis. Front. Neurosci. 6, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, 2004. Structural plasticity of the adult brain. Dialogues Clin. Neurosci. 6, 135–141. doi: 10.31887/DCNS.2004.6.2/fgage [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Valles R, Gomez-Cabrera M, Rodriguez-Mañas L, Garcia-Garcia FJ, Diaz A, Noguera I, Olaso-Gonzalez G, Viña J, 2013. Life-long spontaneous exercise does not prolong lifespan but improves health span in mice. Longev. Heal. 2, 14. doi: 10.1186/2046-2395-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Roeder I, Kempermann G, 2016. Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus 26, 261–71. doi: 10.1002/hipo.22520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazulla J, Benavente I, 2006. Adult-onset ataxia-telangiectasia. A clinical and therapeutic observation. Neurologia 21, 447–451. [PubMed] [Google Scholar]
- GBD 2019 Dementia Forecasting Collaborators, 2022. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet. Public Heal. 7, e105–e125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara E, Bonaguidi MA, Beckervordersandforth R, Sultan S, Udry F, Gijs P-J, Lie DC, Ming G-L, Song H, Toni N, 2016. Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells 34, 997–1010. doi: 10.1002/stem.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM, Jones DT, Murray ME, 2021. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20, 222–234. doi: 10.1016/S1474-4422(20)30440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald A, Kumar KR, Sue CM., 2019. New insights into the complex role of mitochondria in Parkinson’s disease[J]. Progress in neurobiology, 177: 73–93 [DOI] [PubMed] [Google Scholar]
- Ghosh HS, 2019. Adult Neurogenesis and the Promise of Adult Neural Stem Cells. J. Exp. Neurosci 13. doi: 10.1177/1179069519856876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM-Y, 2003. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science (80-. ). 300, 636–640. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees A, 1988. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 51, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Simpson JM, Ghilan M, Christie BR, 2011. Neurogenesis in Huntington’s disease: can studying adult neurogenesis lead to the development of new therapeutic strategies? Brain Res. 1406, 84–105. [DOI] [PubMed] [Google Scholar]
- Gil JMAC, Mohapel P, Araújo IM, Popovic N, Li J-Y, Brundin P, Petersén Å, 2005. Reduced hippocampal neurogenesis in R6/2 transgenic Huntington’s disease mice. Neurobiol. Dis. 20, 744–751. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK, 2002. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 5, 1242–7. doi: 10.1038/nn958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J, Fahn S, 2020. Parkinson disease and related disorders. Rosenberg’s Mol. Genet. Basis Neurol. Psychiatr. Dis 19–30. [Google Scholar]
- Gomez-Nicola D, Suzzi S, Vargas-Caballero M, Fransen NL, Al-Malki H, Cebrian-Silla A, Garcia-Verdugo JM, Riecken K, Fehse B, Perry VH, 2014. Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain 137, 2312–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves JT, Schafer ST, Gage FH, 2016. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167, 897–914. doi: 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Gonneaud J, Arenaza-Urquijo EM, Mézenge F, Landeau B, Gaubert M, Bejanin A, de Flores R, Wirth M, Tomadesso C, Poisnel G, Abbas A, Desgranges B, Chételat G, 2017. Increased florbetapir binding in the temporal neocortex from age 20 to 60 years. Neurology 89, 2438–2446. doi: 10.1212/wnl.0000000000004733 [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS, 1992. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 12, 3642–50. doi: 10.1007/s11576-006-0057-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG, 1999. Neurogenesis in the neocortex of adult primates. Science 286, 548–52. doi: 10.1126/science.286.5439.548 [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E, 1998. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. U. S. A. 95, 3168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS, 1991. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J. Comp. Neurol. 313, 479–485. doi: 10.1002/cne.903130308 [DOI] [PubMed] [Google Scholar]
- Hagg T, 2009. From Neurotransmitters to Neurotrophic Factors to Neurogenesis. Neurosci. 15, 20–27. doi: 10.1177/1073858408324789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara H, Murano T, Ohira K, Miwa M, Nakamura K, Miyakawa T, 2019. Expression of progenitor cell/immature neuron markers does not present definitive evidence for adult neurogenesis. Mol. Brain 12, 1–6. doi: 10.1186/s13041-019-0522-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R, Richardson A, Hart MJ, Galvan V, 2012. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 223, 102–113. doi: 10.1016/j.neuroscience.2012.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LK, Aumont A, Julien C, Vadnais A, Calon F, Fernandes KJL, 2010. Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer’s disease. Eur. J. Neurosci. 32, 905–920. [DOI] [PubMed] [Google Scholar]
- Hampel H, Bürger K, Teipel SJ, Bokde ALW, Zetterberg H, Blennow K, 2008. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimer’s Dement. 4, 38–48. [DOI] [PubMed] [Google Scholar]
- Hanert A, Rave J, Granert O, Ziegler M, Pedersen A, Born J, Finke C, Bartsch T, 2019. Hippocampal Dentate Gyrus Atrophy Predicts Pattern Separation Impairment in Patients with LGI1 Encephalitis. Neuroscience 400, 120–131. doi: 10.1016/J.NEUROSCIENCE.2018.12.046 [DOI] [PubMed] [Google Scholar]
- Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P, 2019. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers. Res. Ther. 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L, 2006. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 5, 228–234. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Reifsnyder P, Kumar N, Fernandez E, Flurkey K, Javors MA, Lopez-Cruzan M, Macchiarini F, Nelson JF, Bitto A, Sindler AL, Cortopassi G, Kavanagh K, Leng L, Bucala R, Rosenthal N, Salmon A, Stearns TM, Bogue M, Miller RA, 2021. 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell 20. doi: 10.1111/acel.13328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM, 2001. Behavioral phenotyping of GFAP-apoE3 and-apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer’s-like neuropathology. Exp. Neurol. 170, 326–344. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Watanabe S, 2005. Chronic food restriction enhances memory in mice - Analysis with matched drive levels. Neuroreport 16, 1129–1133. doi: 10.1097/00001756-200507130-00019 [DOI] [PubMed] [Google Scholar]
- Hayano M, Yang J-H, Bonkowski MS, Amorim JA, Ross JM, Coppotelli G, Griffin P, Chew YC, Guo W, Yang X, Vera DL, Salfati EL, Das A, Thakur S, Kane AE, Mitchell SJ, Mohri Y, Nishimura EK, Schaevitz L, Garg N, Balta A-M, Rego MA, Gregory-Ksander M, Jakobs TC, Zhong L, Wakimoto H, Mostoslavsky R, Wagers AJ, Tsubota K, Bonasera SJ, Palmeira CM, Seidman JG, Seidman C, Wolf NS, Kreiling JA, Sedivy JM, Murphy GF, Oberdoerffer P, Ksander BR, Rajman LA, Sinclair DA, 2019. DNA Break-Induced Epigenetic Drift as a Cause of Mammalian Aging. SSRN Electron. J. doi: 10.2139/ssrn.3466338 [DOI] [Google Scholar]
- Hedden T, Gabrieli JDE, 2004. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 5, 87–96. doi: 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- Hernandez-Sapiens MA, Reza-Zaldívar EE, Márquez-Aguirre AL, Gómez-Pinedo U, Matias-Guiu J, Cevallos RR, Mateos-Díaz JC, Sánchez-González VJ, Canales-Aguirre AA, 2022. Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease. Neural Regen. Res. 17, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros-Villanueva M, 2014. Embryonic stem cell factors and pancreatic cancer. World J. Gastroenterol. 20, 2247. doi: 10.3748/wjg.v20.i9.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R, 2015. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology 40, 2368–2378. doi: 10.1038/npp.2015.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC, 2004. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 7, 726–735. doi: 10.1038/nn1265 [DOI] [PubMed] [Google Scholar]
- Hoeijmakers L, Ruigrok SR, Amelianchik A, Ivan D, van Dam AM, Lucassen PJ, Korosi A, 2017. Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain. Behav. Immun. 63, 160–175. doi: 10.1016/J.BBI.2016.12.023 [DOI] [PubMed] [Google Scholar]
- Holmes MM, Galea LAM, Mistlberger RE, Kempermann G, 2004. Adult hippocampal neurogenesis and voluntary running activity: Circadian and dose-dependent effects. J. Neurosci. Res. 76, 216–222. doi: 10.1002/jnr.20039 [DOI] [PubMed] [Google Scholar]
- Hong S, Washington PM, Kim A, Yang C-P, Yu T-S, Kernie SG, 2016. Apolipoprotein E regulates injury-induced activation of hippocampal neural stem and progenitor cells. J. Neurotrauma 33, 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby AKEE, Redhead YT, Rees DJ, Ratcliff MSGG, Reichenbach A, Wells T, Francis L, Amstalden K, Andrews ZB, Davies JS, 2016. Short-term calorie restriction enhances adult hippocampal neurogenesis and remote fear memory in a Ghsr-dependent manner. Psychoneuroendocrinology 63, 198–207. doi: 10.1016/j.psyneuen.2015.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz AM, Fan X, Bieri G, Smith LK, Sanchez-Diaz CI, Schroer AB, Gontier G, Casaletto KB, Kramer JH, Williams KE, Villeda SA, 2020. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science (80-. ). 369, 167–173. doi: 10.1126/science.aaw2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini L, Farokhi-Sisakht F, Badalzadeh R, Khabbaz A, Mahmoudi J, Sadigh-Eteghad S, 2019. Nicotinamide Mononucleotide and Melatonin Alleviate Aging-induced Cognitive Impairment via Modulation of Mitochondrial Function and Apoptosis in the Prefrontal Cortex and Hippocampus. Neuroscience 423, 29–37. doi: 10.1016/j.neuroscience.2019.09.037 [DOI] [PubMed] [Google Scholar]
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA, 2019. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. doi: 10.1038/s41582-019-0244-7 [DOI] [PubMed] [Google Scholar]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, Stevnsner TV, Mattson MP, Bohr VA, 2018. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. 115, E1876–E1885. doi: 10.1073/pnas.1718819115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, Mattson MP, Croteau DL, Bohr VA, 2021. NAD+ supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc. Natl. Acad. Sci. U. S. A. 118. doi: 10.1073/pnas.2011226118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben S, Homa M, Yilmaz Z, Leroy K, Brion J-PP, Ando K, 2021. Tau Pathology and Adult Hippocampal Neurogenesis: What Tau Mouse Models Tell us? Front. Neurol. 12, 610330. doi: 10.3389/fneur.2021.610330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, Hoxhaj G, Saghatelian A, Shaw RJ, Manning BD, 2017. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 25, 463–471. doi: 10.1016/j.cmet.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH, 2004. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. U. S. A. 101, 16659–16664. doi: 10.1073/pnas.0407643101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Xie F, Xiao Y, Lu C, Zhong J, Huang D, Chen J, Wei J, Jiang Y, Zhong T, 2021. Metformin: A Potential Candidate for Targeting Aging Mechanisms. Aging Dis. 12, 480. doi: 10.14336/AD.2020.0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KJ, Kennedy BK, 2012. Rapamycin paradox resolved. Science (80-. ). 335, 1578–1579. doi: 10.1126/science.1221365 [DOI] [PubMed] [Google Scholar]
- Huhn S, Beyer F, Zhang R, Lampe L, Grothe J, Kratzsch J, Willenberg A, Breitfeld J, Kovacs P, Stumvoll M, Trampel R, Bazin P-LL, Villringer A, Witte AV, 2018. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults – A randomized controlled trial. Neuroimage 174, 177–190. doi: 10.1016/j.neuroimage.2018.03.023 [DOI] [PubMed] [Google Scholar]
- Hymovitch B, 1952. The effects of experimental variations in problem solving in the rat. J. Comp. Psychol. 45, 313–321. [DOI] [PubMed] [Google Scholar]
- Imai S. ichiro, Guarente L, 2014. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24, 464–471. doi: 10.1016/j.tcb.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai SI, Guarente L, 2016. It takes two to tango: Nad+ and sirtuins in aging/longevity control. npj Aging Mech. Dis. 2, 1–6. doi: 10.1038/npjamd.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura G, Kawabata S, Ando M, Nishiyama Y, Sugai K, Ozaki M, Iida T, Ookubo T, Kojima K, Kashiwagi R, 2017. Fail-safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem cell reports 8, 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, J., Noristani N, Olabarria H, M., Fletcher J, Somerville DD, T., Yeh Y, C., Verkhratsky A, 2011. Voluntary Running and Environmental Enrichment Restores Impaired Hippocampal Neurogenesis in a Triple Transgenic Mouse Model of Alzheimers Disease. Curr. Alzheimer Res. 8, 707–717. doi: 10.2174/156720511797633214 [DOI] [PubMed] [Google Scholar]
- Jahn H, 2013. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 15, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri DJ, Tedoldi A, Hunt S, Sullivan R, Watts NR, Power JM, Bartlett PF, Sah P, 2018. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol. Psychiatry 23, 521–532. doi: 10.1038/mp.2017.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji N, Luan J, Hu F, Zhao Y, Lv B, Wang W, Xia M, Zhao X, Lao K, 2018. Aerobic exercise‑stimulated Klotho upregulation extends life span by attenuating the excess production of reactive oxygen species in the brain and kidney. Exp. Ther. Med. 16, 3511–3517. doi: 10.3892/etm.2018.6597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM, 2000. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 14, 2135–2137. doi: 10.1096/fj.00-0242fje [DOI] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA, 2004. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. 101, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M, Anglada-Huguet M, Paesler K, Mandelkow E, Mandelkow E-M, 2017. Anti-aggregant tau mutant promotes neurogenesis. Mol. Neurodegener. 12, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptan Z, Akgün-Dar K, Kapucu A, Dedeakayoʇullari H, Batu Ş, Üzüm G, 2015. Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; Hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain Res. 1618, 194–204. doi: 10.1016/j.brainres.2015.05.041 [DOI] [PubMed] [Google Scholar]
- Karikkineth AC, Scheibye-Knudsen M, Fivenson E, Croteau DL, Bohr VA, 2017. Cockayne syndrome: Clinical features, model systems and pathways. Ageing Res. Rev. 33, 3–17. doi: 10.1016/j.arr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvinen S, Waller K, Silvennoinen M, Koch LG, Britton SL, Kaprio J, Kainulainen H, Kujala UM, 2015. Physical activity in adulthood: Genes and mortality, Scientific Reports. Nature Publishing Group. doi: 10.1038/srep18259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH, 1998a. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr. Biol. 8, 939–944. doi: 10.1016/S0960-9822(07)00377-6 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, Gould E, Hen R, Abrous DN, Toni N, Schinder AF, Zhao X, Lucassen PJ, Frisén J, 2018. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 23, 25–30. doi: 10.1016/j.stem.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH, 2002. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 52, 135–143. doi: 10.1002/ana.10262 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1998b. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 18, 3206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–5. doi: 10.1038/386493a0 [DOI] [PubMed] [Google Scholar]
- Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF, 2017. Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci. 40, 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezic A, Popovic L, Lalic K, 2018. mTOR Inhibitor Therapy and Metabolic Consequences: Where Do We Stand? Oxid. Med. Cell. Longev. 2018, 1–8. doi: 10.1155/2018/2640342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA, 2014. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat. Neurosci. 17, 304–311. doi: 10.1038/nn.3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Pinto AM, Bordoli C, Buckner LP, Kaplan PC, Arenal I.M. del, Jeffcock EJ, Hall WL, Thuret S, 2020. Energy Restriction Enhances Adult Hippocampal Neurogenesis-Associated Memory after Four Weeks in an Adult Human Population with Central Obesity; a Randomized Controlled Trial. Nutrients 12. doi: 10.3390/NU12030638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen GW, Ge S, 2019. Young at heart: Insights into hippocampal neurogenesis in the aged brain. Behav. Brain Res. 369, 111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köbe T, Witte AV, Schnelle A, Tesky VA, Pantel J, Schuchardt JP, Hahn A, Bohlken J, Grittner U, Flöel A, 2017. Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front. Neurosci. 11, 1–11. doi: 10.3389/fnins.2017.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK, 2015. Resveratrol Prevents Age-Related Memory and Mood Dysfunction with Increased Hippocampal Neurogenesis and Microvasculature, and Reduced Glial Activation. Sci. Rep. 5, 1–16. doi: 10.1038/srep08075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl Z, Abdallah N. Ben, Vogelgsang J, Tischer L, Deusser J, Amato D, Anderson S, Müller CP, Riess O, Masliah E, 2016. Severely impaired hippocampal neurogenesis associates with an early serotonergic deficit in a BAC α-synuclein transgenic rat model of Parkinson’s disease. Neurobiol. Dis. 85, 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl Z, Regensburger M, Aigner R, Kandasamy M, Winner B, Aigner L, Winkler J, 2010. Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington’s disease. BMC Neurosci. 11, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Chiba T, Yamaza H, Yamashita K, Shimada A, Hoshiyama Y, Henmi T, Ohtani H, Higami Y, de Cabo R, Ingram DK, Shimokawa I, 2008. Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescence-accelerated mouse strain P8. Exp. Gerontol. 43, 339–346. doi: 10.1016/j.exger.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Koutseff A, Mittelhaeuser C, Essabri K, Auwerx J, Meziane H, 2014. Impact of the apolipoprotein E polymorphism, age and sex on neurogenesis in mice: Pathophysiological relevance for Alzheimer’s disease? Brain Res. 1542, 32–40. [DOI] [PubMed] [Google Scholar]
- Kozareva DA, Cryan JF, Nolan YM, 2019. Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell 18, 1–18. doi: 10.1111/acel.13007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig E, Linehan LA, Liang H, Romo TQ, Liu Q, Wu Y, Benavides AD, Curiel TJ, Javors MA, Musi N, Chiodo L, Koek W, Gelfond JAL, Kellogg DL, 2018. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp. Gerontol. 105, 53–69. doi: 10.1016/j.exger.2017.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhla A, Lange S, Holzmann C, Maass F, Petersen J, Vollmar B, Wree A, 2013. Lifelong Caloric Restriction Increases Working Memory in Mice. PLoS One 8. doi: 10.1371/journal.pone.0068778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H, Dickinson-Anson H, Gage F, 1996. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16, 2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Toda T, Gage FH, 2018. Adult Hippocampal Neurogenesis: A Coming-of-Age Story. J. Neurosci. 38, 10401–10410. doi: 10.1523/JNEUROSCI.2144-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Sidhu J, Goyal A, Tsao JW, 2022. Alzheimer Disease, StatPearls. StatPearls Publishing. [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CEL, 2010. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem. 18, 15–18. doi: 10.1101/lm.1971111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Vartiainen S, Moilanen A, Sirviö J, Thomas JH, Nass R, Blakely RD, Wong G, 2003. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human α-synuclein. J. Neurochem. 86, 165–172. [DOI] [PubMed] [Google Scholar]
- Lang UE, Heger J, Willbring M, Domula M, Matschke K, Tugtekin SM, 2009. Immunosuppression Using the Mammalian Target of Rapamycin (mTOR) Inhibitor Everolimus: Pilot Study Shows Significant Cognitive and Affective Improvement. Transplant. Proc. 41, 4285–4288. doi: 10.1016/j.transproceed.2009.08.050 [DOI] [PubMed] [Google Scholar]
- Lanke V, Moolamalla STR, Roy D, Vinod PK, 2018. Integrative analysis of hippocampus gene expression profiles identifies network alterations in aging and Alzheimer’s disease. Front. Aging Neurosci. 10, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini P, Frontini M, Caputo M, Gregan J, Cipak L, Filippi S, Kumar V, Vélez-Cruz R, Stefanini M, Proietti-De-Santis L, 2011. CSA and CSB proteins interact with p53 and regulate its Mdm2-dependent ubiquitination. Cell Cycle 10, 3719–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugel V, Dalloz C, Durand M, Sauvanaud F, Kristensen U, Vincent M-C, Pasquier L, Odent S, Cormier-Daire V, Gener B, 2010. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum. Mutat. 31, 113–126. [DOI] [PubMed] [Google Scholar]
- Lautrup S, Sinclair DA, Mattson MP, Fang EF, 2019. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 30, 630–655. doi: 10.1016/j.cmet.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE, Grote HE, Blakemore C, Hannan AJ, Van Dellen A, Phillips W, Barker RA, 2006. Neurogenesis in the R6/1 transgenic mouse model of Huntington’s disease: effects of environmental enrichment. Eur. J. Neurosci. 23, 1829–1838. [DOI] [PubMed] [Google Scholar]
- Le Grand JN, Gonzalez-Cano L, Pavlou MA, Schwamborn JC, 2015. Neural stem cells in Parkinson’s disease: a role for neurogenesis defects in onset and progression. Cell. Mol. Life Sci. 72, 773–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Galicia P, Castañeda-Bueno M, Quiroz-Baez R, Arias C, 2008. Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol. Learn. Mem. 90, 511–518. doi: 10.1016/j.nlm.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Leclerc E, Trevizol AP, Grigolon RB, Subramaniapillai M, McIntyre RS, Brietzke E, Mansur RB, 2019. The effect of caloric restriction on working memory in healthy non-obese adults. CNS Spectr. 2, 1–7. doi: 10.1017/S1092852918001566 [DOI] [PubMed] [Google Scholar]
- Lee D-C, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN, 2014. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 64, 472–81. doi: 10.1016/j.jacc.2014.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP, 2000. Dietary restriction increases the number of newly generated neural cells, and BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 15, 99–108. doi: 10.1385/JMN:15:2:99 [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP, 2002. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 80, 539–547. doi: 10.1046/j.0022-3042.2001.00747.x [DOI] [PubMed] [Google Scholar]
- Lee K-S, Wu Z, Song Y, Mitra SS, Feroze AH, Cheshier SH, Lu B, 2013. Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical Notch signaling pathway. Genes Dev. 27, 2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MB, Hill CM, Bitto A, Kaeberlein M, 2021. Antiaging diets: Separating fact from fiction. Science 374, eabe7365. doi: 10.1126/science.abe7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chu K, Jung K, Im W, Park J, Lim H, Won C, Shin S, Lee SK, Kim M, 2009. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 66, 671–681. [DOI] [PubMed] [Google Scholar]
- Lee SH, Min KJ, 2013. Caloric restriction and its mimetics. BMB Rep. 46, 181–187. doi: 10.5483/BMBRep.2013.46.4.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Clemenson GD, Gage FH, 2012. New neurons in an aged brain. Behav. Brain Res. 227, 497–507. doi: 10.1016/j.bbr.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman DS, Hebestreit K, Ruetz T, Webb AE, McKay A, Pollina EA, Dulken BW, Zhao X, Yeo RW, Ho TT, Mahmoudi S, Devarajan K, Passegué E, Rando TA, Frydman J, Brunet A, 2018. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science (80-. ) 359, 1277–1283. doi: 10.1126/science.aag3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuis SL, Hoeijmakers L, Korosi A, de Rooij SR, Swaab DF, Kessels HW, Lucassen PJ, Krugers HJ, 2018. Vulnerability and resilience to Alzheimer’s disease: early life conditions modulate neuropathology and determine cognitive reserve. Alzheimers. Res. Ther. 10, 95. doi: 10.1186/s13195-018-0422-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson CW, Rich NJ, 2007. Eat less, live longer? New insights into the role of caloric restriction in the brain. Nutr. Rev. 65, 412–415. doi: 10.1301/nr.2007.sept.412-415 [DOI] [PubMed] [Google Scholar]
- Levi O, Michaelson DM, 2007. Environmental enrichment stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J. Neurochem. 100, 202–210. [DOI] [PubMed] [Google Scholar]
- Levy G, 2007. The Relationship of Parkinson Disease With Aging. Arch. Neurol. 64, 1242–1246. doi: 10.1001/ARCHNEUR.64.9.1242 [DOI] [PubMed] [Google Scholar]
- Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y, 2009. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 5, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li X, Liu Z, Wu S, Guo J, Shi R, Sun Y, Wang Y, Yin H, 2020. Resveratrol reserved hypoxia-ischemia induced childhood hippocampal dysfunction and neurogenesis via improving mitochondrial dynamics. Neurosci. Res. 161, 51–58. doi: 10.1016/j.neures.2019.11.012 [DOI] [PubMed] [Google Scholar]
- Li J, Shang Y, Wang L, Zhao B, Sun C, Li Jiali, Liu S, Li C, Tang M, Meng F-L, 2020. Genome integrity and neurogenesis of postnatal hippocampal neural stem/progenitor cells require a unique regulator Filia. Sci. Adv. 6, eaba0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Puma DD, Piacentini R, Grassi C, 2021. Does Impairment of Adult Neurogenesis Contribute to Pathophysiology of Alzheimer’s Disease? A Still Open Question. Front. Mol. Neurosci. 0, 258. doi: 10.3389/FNMOL.2020.578211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bao X, Wang R, 2016. Neurogenesis-based epigenetic therapeutics for Alzheimer’s disease. Mol. Med. Rep. 14, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Lim J, Bang Y, Choi HJ, 2018. Abnormal hippocampal neurogenesis in Parkinson’s disease: relevance to a new therapeutic target for depression with Parkinson’s disease. Arch. Pharm. Res. 41, 943–954. [DOI] [PubMed] [Google Scholar]
- Lim RG, Salazar LL, Wilton DK, King AR, Stocksdale JT, Sharifabad D, Lau AL, Stevens B, Reidling JC, Winokur ST, 2017. Developmental alterations in Huntington’s disease neural cells and pharmacological rescue in cells and mice. Nat. Neurosci. 20, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Kapoor A, Gu Y, Chow MJ, Peng J, Zhao K, Tang D, 2020. Contributions of DNA damage to Alzheimer’s disease. Int. J. Mol. Sci. 21, 1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C, 2006. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 13, 1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x [DOI] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, Bu G, 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang H, Ma Y, 2021. Molecular mechanisms of altered adult hippocampal neurogenesis in Alzheimer’s disease. Mech. Ageing Dev. 111452. [DOI] [PubMed] [Google Scholar]
- Liu N, Stoica G, Yan M, Scofield VL, Qiang W, Lynn WS, Wong PKY, 2005. ATM deficiency induces oxidative stress and endoplasmic reticulum stress in astrocytes. Lab. Investig. 85, 1471–1480. [DOI] [PubMed] [Google Scholar]
- Lledo P-M, Valley M, 2016. Adult Olfactory Bulb Neurogenesis. Cold Spring Harb. Perspect. Biol. 8, a018945. doi: 10.1101/cshperspect.a018945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Toledano MA, Ali Faghihi M, Patel NS, Wahlestedt C, 2010. Adult Neurogenesis: A Potential Tool for Early Diagnosis in Alzheimer’s Disease? J. Alzheimer’s Dis. 20, 395–408. doi: 10.3233/JAD-2010-1388 [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Fitzsimons CP, Korosi A, Joels M, Belzung C, Abrous DN, 2013. Stressing new neurons into depression? Mol. Psychiatry 18, 396–397. doi: 10.1038/mp.2012.39 [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Fitzsimons CP, Salta E, Maletic-Savatic M, 2020. Adult neurogenesis, human after all (again): classic, optimized, and future approaches. Behav. Brain Res. 381, 112458. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Stumpel MW, Wang Q, Aronica E, 2010. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 58, 940–949. doi: 10.1016/j.neuropharm.2010.01.012 [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Toni N, Kempermann G, Frisen J, Gage FH, Swaab DF, 2020. Limits to human neurogenesis—really?. Molecular psychiatry, 25(10), 2207–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Sill OC, Gao L, Becker S, Wojtowicz JM, Smith DM, 2012. The role of adult hippocampal neurogenesis in reducing interference. Behav. Neurosci. 126, 381–391. doi: 10.1037/a0028252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Xiao W, Li H, Pang P, Xue F, Wan L, Pei L, Yan H, 2021. Metformin restores hippocampal neurogenesis and learning and memory via regulating gut microbiota in the obese mouse model. Brain. Behav. Immun 95, 68–83. doi: 10.1016/J.BBI.2021.02.011 [DOI] [PubMed] [Google Scholar]
- Maass A, Berron D, Libby LA, Ranganath C, Düzel E, 2015. Functional subregions of the human entorhinal cortex. Elife 4, 1–20. doi: 10.7554/eLife.06426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madar AD, Ewell LA, Jones MV, 2019a. Pattern separation of spiketrains in hippocampal neurons. Sci. Rep. 9, 5282. doi: 10.1038/s41598-019-41503-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madar AD, Ewell LA, Jones MV, 2019b. Temporal pattern separation in hippocampal neurons through multiplexed neural codes. PLOS Comput. Biol. 15, e1006932. doi: 10.1371/journal.pcbi.1006932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E, 2021. FDA approves controversial Alzheimer’s drug despite uncertainty over effectiveness. [DOI] [PubMed]
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S, 2012. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell 11, 326–335. doi: 10.1111/j.1474-9726.2011.00791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS, 2000. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M, 2007. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science 318, 980–5. doi: 10.1126/science.1147851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Pinilla E, Ordóñez C, del Valle E, Navarro A, Tolivia J, 2016. Regional and Gender Study of Neuronal Density in Brain during Aging and in Alzheimer’s Disease. Front. Aging Neurosci 8. doi: 10.3389/fnagi.2016.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, 2004. Neural Stem Cell Detection, Characterization, and Age-Related Changes in the Subventricular Zone of Mice. J. Neurosci. 24, 1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Matsuda T, Nakashima K, 2021. Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors. Cells 10, 1145. doi: 10.3390/cells10051145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, 1999. Impact of dietary restriction on brain aging and neurodegenerative disorders: Emerging findings from experimental and epidemiological studies. J. Anti. Aging. Med. 2, 331–336. doi: 10.1089/rej.1.1999.2.331 [DOI] [Google Scholar]
- McGinley LM, Kashlan ON, Bruno ES, Chen KS, Hayes JM, Kashlan SR, Raykin J, Johe K, Murphy GG, Feldman EL, 2018. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer’s disease. Sci. Rep. 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurphy T, Huang W, Queen NJ, Ali S, Widstrom KJ, Liu X, Xiao R, Siu JJ, Cao L, 2018. Implementation of environmental enrichment after middle age promotes healthy aging. Aging (Albany. NY). 10, 1698–1721. doi: 10.18632/aging.101502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano S, Scrable H, 2005. Maintaining appearances—the role of p53 in adult neurogenesis. Biochem. Biophys. Res. Commun. 331, 828–833. [DOI] [PubMed] [Google Scholar]
- Melrose HL, Kent CB, Taylor JP, Dachsel JC, Hinkle KM, Lincoln SJ, Mok SS, Culvenor JG, Masters CL, Tyndall GM, 2007. A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience 147, 1047–1058. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Ghosh S, Kamitaki N, Mitchell J, Avior Y, Mello C, Kashin S, Mekhoubad S, Ilic D, Charlton M, 2017. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 545, 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Feldman HM, Lu T, Drake D, Lim ET, Ling K-H, Bishop NA, Pan Y, Seo J, Lin Y-T, 2019. REST and neural gene network dysregulation in iPSC models of Alzheimer’s disease. Cell Rep. 26, 1112–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Tschanz JT, Norton MC, Welsh-Bohmer KA, Steffens DC, Wyse BW, Breitner JCS, 1998. APOE genotype predicts when—not whether—one is predisposed to develop Alzheimer disease. Nat. Genet. 19, 321–322. [DOI] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Tapiola T, Alafuzoff I, Miettinen R, 1999. Hippocampal plasticity in Alzheimer’s disease: changes in highly polysialylated NCAM immunoreactivity in the hippocampal formation. Eur. J. Neurosci. 11, 1754–1764. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, Palacios HH, Ali A, Navas-Enamorado I, Di Francesco A, Kaiser TA, Waltz TB, Zhang N, Ellis JL, Elliott PJ, Frederick DW, Bohr VA, Schmidt MS, Brenner C, Sinclair DA, Sauve AA, Baur JA, de Cabo R, 2018. Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab. 27, 667–676.e4. doi: 10.1016/j.cmet.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T, 2003. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience 119, 887–897. doi: 10.1016/S0306-4522(03)00105-2 [DOI] [PubMed] [Google Scholar]
- Mo C, Hannan AJ, Renoir T, 2015. Environmental factors as modulators of neurodegeneration: insights from gene-environment interactions in Huntington’s disease. Neurosci. Biobehav. Rev. 52, 178–92. doi: 10.1016/j.neubiorev.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Mohammed I, Hollenberg MD, Ding H, Triggle CR, 2021. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front. Endocrinol. (Lausanne). 12. doi: 10.3389/fendo.2021.718942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ, 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452. doi: 10.1038/nature05091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, Piazza PV, Abrous DN, 2006. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol. Aging 27, 645–654. doi: 10.1016/j.neurobiolaging.2005.02.014 [DOI] [PubMed] [Google Scholar]
- Moon M, Cha M-Y, Mook-Jung I, 2014. Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J. Alzheimer’s Dis. 41, 233–241. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, Faux NG, Martins R, Szoeke C, Rowe C, Watters DA, 2013. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 36, 2981–2987. doi: 10.2337/dc13-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes L, de Moraes Mello LEA, Shimabukuro MK, de Castro Batista CM, Mendez-Otero R, 2009. Lack of association between PSA-NCAM expression and migration in the rostral migratory stream of a Huntington’s disease transgenic mouse model. Neuropathology 29, 140–147. [DOI] [PubMed] [Google Scholar]
- Moreno-Jiménez Elena P., Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M, 2019. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560. doi: 10.1038/s41591-019-0375-9 [DOI] [PubMed] [Google Scholar]
- Moreno-Jiménez Elena P, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M, 2019. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560. [DOI] [PubMed] [Google Scholar]
- Moreno-Jiménez EP, Terreros-Roncal J, Flor-García M, Rábano A, Llorens-Martín M, 2021. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 41, 2541–2553. doi: 10.1523/JNEUROSCI.0675-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern NA, Lombardi G, Schinder AF, 2008. Newborn granule cells in the ageing dentate gyrus. J. Physiol. 586, 3751–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L, 2011. The many faces of tau. Neuron 70, 410–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone CD, Bazzigaluppi P, Beckett TL, Hill ME, Koletar MM, Stefanovic B, McLaurin J, 2020. Regional differences in Alzheimer’s disease pathology confound behavioural rescue after amyloid-β attenuation. Brain 143, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret A, Lepousez G, Gras J, Gabellec M-M, Lledo P-M, 2009. Turnover of Newborn Olfactory Bulb Neurons Optimizes Olfaction. J. Neurosci. 29, 12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavinejad M, Andrews PW, Shoraki EK, 2016. Current Biosafety Considerations in Stem Cell Therapy. Cell J. 18, 281–7. doi: 10.22074/cellj.2016.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, Noma K, Baur JA, Schug Z, Tang H-Y, Speicher DW, David G, Zhang R, 2019. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat. Cell Biol. 2019 213 21, 397–407. doi: 10.1038/s41556-019-0287-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakafuku M, Águila Á, 2019. Developmental dynamics of neurogenesis and gliogenesis in the postnatal mammalian brain in health and disease: Historical and future perspectives. WIREs Dev. Biol 1–20. doi: 10.1002/wdev.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck EFG, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, Korosi A, 2015. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus 25, 309–28. doi: 10.1002/hipo.22374 [DOI] [PubMed] [Google Scholar]
- Negi SK, Guda C, 2017. Global gene expression profiling of healthy human brain and its application in studying neurological disorders. Sci. Rep. 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B, 2014. Long-term metformin usage and cognitive function among older adults with diabetes. J. Alzheimer’s Dis. 41, 61–68. doi: 10.3233/JAD-131901 [DOI] [PubMed] [Google Scholar]
- Nicaise AM, Willis CM, Crocker SJ, Pluchino S, 2020. Stem Cells of the Aging Brain. Front. Aging Neurosci. 12, 1–23. doi: 10.3389/fnagi.2020.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW, 2009. Exercise improves cognition and hippocampal plasticity in APOE ε4 mice. Alzheimer’s Dement. 5, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolai S, Pallauf K, Huebbe P, Rimbach G, 2015. Energy restriction and potential energy restriction mimetics. Nutr. Res. Rev. 28, 100–120. doi: 10.1017/S0954422415000062 [DOI] [PubMed] [Google Scholar]
- O’Sullivan SS, Johnson M, Williams DR, Revesz T, Holton JL, Lees AJ, Perry EK, 2011. The effect of drug treatment on neurogenesis in Parkinson’s disease. Mov. Disord. 26, 45–50. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Goto T, Waeber C, Bhide PG, 2003. Dopamine modulates cell cycle in the lateral ganglionic eminence. J. Neurosci. 23, 2840–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okur MN, Lee J-H, Osmani W, Kimura R, Demarest TG, Croteau DL, Bohr VA, 2020. Cockayne syndrome group A and B proteins function in rRNA transcription through nucleolin regulation. Nucleic Acids Res. 48, 2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR, 2006. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus 16, 250–260. doi: 10.1002/hipo.20157 [DOI] [PubMed] [Google Scholar]
- Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, Huang J, Xu B, Long D, Li J, Li Q, Xu L, Xuan A, 2018. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain. Behav. Immun 69, 351–363. doi: 10.1016/j.bbi.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH, 2006. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann. Neurol. 59, 81–91. doi: 10.1002/ana.20699 [DOI] [PubMed] [Google Scholar]
- Parizkova M, Lerch O, Andel R, Kalinova J, Markova H, Vyhnalek M, Hort J, Laczó J, 2020. Spatial Pattern Separation in Early Alzheimer’s Disease. J. Alzheimer’s Dis. 76, 121–138. [DOI] [PubMed] [Google Scholar]
- Park H, Chung KM, An H-K, Gim J-E, Hong J, Woo H, Cho B, Moon C, Yu S-W, 2019. Parkin promotes mitophagic cell death in adult hippocampal neural stem cells following insulin withdrawal. Front. Mol. Neurosci. 12, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Park M, Choi J, Park KY, Chung HY, Lee J, 2010. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci. Lett. 482, 235–239. doi: 10.1016/j.neulet.2010.07.046 [DOI] [PubMed] [Google Scholar]
- Park JH, Glass Z, Sayed K, Michurina TV, Lazutkin A, Mineyeva O, Velmeshev D, Ward WF, Richardson A, Enikolopov G, 2013. Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur. J. Neurosci. 37, 1987–1993. doi: 10.1111/ejn.12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Lee H-J, Lee HL, Son DJ, Ju JH, Hyun BK, Jung SH, Song J-K, Lee DH, Hwang CJ, 2017. Parkin knockout inhibits neuronal development via regulation of proteasomal degradation of p21. Theranostics 7, 2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott MD, Greenwood CE, 2007. Dietary influences on cognitive function with aging: From high-fat diets to healthful eating. Ann. N. Y. Acad. Sci. 1114, 389–397. doi: 10.1196/annals.1396.028 [DOI] [PubMed] [Google Scholar]
- Pasanen T, Tolvanen S, Heinonen A, Kujala UM, 2017. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomised controlled trials. Br. J. Sports Med. 51, 1459–1465. doi: 10.1136/bjsports-2016-097132 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, Rotenberg A, 2011. Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TMS-EEG and TMS-fMRI. Brain Topogr 24, 302–315. doi: 10.1007/s10548-011-0196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Johnson M, Ekonomou A, Perry RH, Ballard C, Attems J, 2012. Neurogenic abnormalities in Alzheimer’s disease differ between stages of neurogenesis and are partly related to cholinergic pathology. Neurobiol. Dis. 47, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TL, Kish SJ, Hinton D, Hansen S, Becker LE, Gelfand EW, 1984. Neurochemical abnorrnalities in a patient with ataxia-telangiectasia. Neurology 34, 187. [DOI] [PubMed] [Google Scholar]
- Pezzuto JM, 2019. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 27, 1–14. doi: 10.4062/biomolther.2018.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao CS, Stoica BA, Wu J, Sabirzhanov B, Zhao Z, Cabatbat R, Loane DJ, Faden AI, 2013. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol. Dis. 54, 252–263. doi: 10.1016/j.nbd.2012.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon B, Ertle S, Deweer B, Bonnet A-M, Vidailhet M, Dubois B, 1997. Memory for spatial location in ‘de novo’parkinsonian patients. Neuropsychologia 35, 221–228. [DOI] [PubMed] [Google Scholar]
- Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC, 2012. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 91, 409–414. doi: 10.1016/j.lfs.2012.08.017 [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Algeri S, 1992. Deterioration of spatial and nonspatial reference and working memory in aged rats: Protective effect of life-long calorie restriction. Neurobiol. Aging 13, 369–373. doi: 10.1016/0197-4580(92)90110-J [DOI] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE, 2017. Parkinson disease. Nat. Rev. Dis. Prim. 3, 1–21. [DOI] [PubMed] [Google Scholar]
- Pollock K, Dahlenburg H, Nelson H, Fink KD, Cary W, Hendrix K, Annett G, Torrest A, Deng P, Gutierrez J, 2016. Human mesenchymal stem cells genetically engineered to overexpress brain-derived neurotrophic factor improve outcomes in Huntington’s disease mouse models. Mol. Ther. 24, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, 1997. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science (80-. ). 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Ponti G, Peretto P, Bonfanti L, 2008. Genesis of Neuronal and Glial Progenitors in the Cerebellar Cortex of Peripuberal and Adult Rabbits. PLoS One 3, e2366. doi: 10.1371/journal.pone.0002366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, Szela A-M, Fabian S, Grittner U, Spranger J, Flöel A, 2016. Caloric Restriction in Older Adults—Differential Effects of Weight Loss and Reduced Weight on Brain Structure and Function. Cereb. Cortex 27, bhw008. doi: 10.1093/cercor/bhw008 [DOI] [PubMed] [Google Scholar]
- Pristera A, Saraulli D, Farioli-Vecchioli S, Strimpakos G, Costanzi M, di Certo MG, Cannas S, Ciotti MT, Tirone F, Mattei E, Cestari V, Canu N, 2013. Impact of N-tau on adult hippocampal neurogenesis, anxiety, and memory. Neurobiol Aging 34, 2551–2563. doi: 10.1016/j.neurobiolaging.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Qian H, Kang X, Hu J, Zhang D, Liang Z, Meng F, Zhang X, Xue Y, Maimon R, Dowdy SF, 2020. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 582, 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen NJ, Hassan QN, Cao L, 2020. Improvements to Healthspan Through Environmental Enrichment and Lifestyle Interventions: Where Are We Now? Front. Neurosci. 14, 1–17. doi: 10.3389/fnins.2020.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm P, Couillard-Despres S, Plötz S, Rivera FJ, Krampert M, Lehner B, Kremer W, Bogdahn U, Kalbitzer HR, Aigner L, 2009. A nuclear magnetic resonance biomarker for neural progenitor cells: is it all neurogenesis? Stem Cells 27, 420–3. doi: 10.1634/stemcells.2008-0816 [DOI] [PubMed] [Google Scholar]
- Rakic P, 1985. Limits of neurogenesis in primates. Science (80-. ). 227, 1054–1056. doi: 10.1126/science.3975601 [DOI] [PubMed] [Google Scholar]
- Rando TA, Jones DL, 2021. Regeneration, Rejuvenation, and Replacement: Turning Back the Clock on Tissue Aging. Cold Spring Harb. Perspect. Biol. a040907. doi: 10.1101/cshperspect.a040907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel LM, Alexander AS, Aimone JB, Wiles J, Gage FH, Chiba AA, Quinn LK, 2014. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nat. Commun. 5. doi: 10.1038/ncomms4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensburger M, Prots I, Winner B, 2014. Adult hippocampal neurogenesis in Parkinson’s disease: impact on neuronal survival and plasticity. Neural Plast. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnell CE, Hildrestrand GA, Sejersted Y, Medin T, Moldestad O, Rolseth V, Krokeide SZ, Suganthan R, Luna L, Bjørås M, 2012. Hippocampal adult neurogenesis is maintained by Neil3-dependent repair of oxidative DNA lesions in neural progenitor cells. Cell Rep. 2, 503–510. [DOI] [PubMed] [Google Scholar]
- Rego AC, de Almeida LP, 2005. Molecular targets and therapeutic strategies in Huntington’s disease. Curr. Drug Targets-CNS Neurol. Disord. 4, 361–381. [DOI] [PubMed] [Google Scholar]
- Reichelt AC, Kramar CP, Ghosh-Swaby OR, Sheppard PAS, Kent BA, Bekinschtein P, Saksida LM, Bussey TJ, 2021. The spontaneous location recognition task for assessing spatial pattern separation and memory across a delay in rats and mice. Nat. Protoc. 16, 5616–5633. doi: 10.1038/s41596-021-00627-w [DOI] [PubMed] [Google Scholar]
- Reichenbach J, Schubert R, Schindler D, Müller K, Böhles H, Zielen S, 2002. Elevated oxidative stress in patients with ataxia telangiectasia. Antioxidants redox Signal. 4, 465–469. [DOI] [PubMed] [Google Scholar]
- Rena G, Hardie DG, Pearson ER, 2017. The mechanisms of action of metformin. Diabetologia 60, 1577–1585. doi: 10.1007/s00125-017-4342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethinavel HS, Ravichandran S, Radhakrishnan RK, Kandasamy M, 2021. COVID-19 and Parkinson’s disease: Defects in neurogenesis as the potential cause of olfactory system impairments and anosmia. J. Chem. Neuroanat. 101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpma A, Jansen D, Arnoldussen IAC, Fang XT, Wiesmann M, Mutsaers MPC, Dederen PJ, Janssen CIF, Kiliaan AJ, 2013. Sex differences in presynaptic density and neurogenesis in middle-aged ApoE4 and ApoE knockout mice. J. Neurodegener. Dis. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M, Wang D, Avila-Quintero V, Bloch MH, Kaffman A, 2021. Deficits in hippocampal-dependent memory across different rodent models of early life stress: systematic review and meta-analysis. Transl. Psychiatry 11, 231. doi: 10.1038/s41398-021-01352-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A, 2008. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One 3, e2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JJ, Jones VC, Verkhratsky A, 2009. Impaired cell proliferation in the subventricular zone in an Alzheimer’s disease model. Neuroreport 20, 907–912. [DOI] [PubMed] [Google Scholar]
- Rogers J, Renoir T, Hannan AJ, 2019. Gene-environment interactions informing therapeutic approaches to cognitive and affective disorders. Neuropharmacology 145, 37–48. doi: 10.1016/j.neuropharm.2017.12.038 [DOI] [PubMed] [Google Scholar]
- Rolando C, Erni A, Grison A, Beattie R, Engler A, Gokhale PJ, Milo M, Wegleiter T, Jessberger S, Taylor V, 2016. Multipotency of Adult Hippocampal NSCs In Vivo Is Restricted by Drosha/NFIB. Cell Stem Cell 19, 653–662. doi: 10.1016/j.stem.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Romashkan SV, Das SK, Villareal DT, Ravussin E, Redman LM, Rochon J, Bhapkar M, Kraus WE, Group, for the C.S., 2016. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget 7, 19124. doi: 10.18632/ONCOTARGET.8093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romine J, Gao X, Xu X-M, So KF, Chen J, 2015. The proliferation of amplifying neural progenitor cells is impaired in the aging brain and restored by the mTOR pathway activation. Neurobiol. Aging 36, 1716–1726. doi: 10.1016/j.neurobiolaging.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Ruzo A, Croft GF, Metzger JJ, Galgoczi S, Gerber LJ, Pellegrini C, Wang H, Fenner M, Tse S, Marks A, 2018. Chromosomal instability during neurogenesis in Huntington’s disease. Development 145. [DOI] [PubMed] [Google Scholar]
- Sacco R, Tamblyn L, Rajakulendran N, Bralha FN, Tropepe V, Laposa RR, 2013. Cockayne syndrome b maintains neural precursor function. DNA Repair (Amst). 12, 110–120. [DOI] [PubMed] [Google Scholar]
- Safahani M, Aligholi H, Noorbakhsh F, Djalali M, Pishva H, Modarres Mousavi SM, Alizadeh L, Gorji A, Koohdani F, 2019. Switching from high-fat diet to foods containing resveratrol as a calorie restriction mimetic changes the architecture of arcuate nucleus to produce more newborn anorexigenic neurons. Eur. J. Nutr. 58, 1687–1701. doi: 10.1007/s00394-018-1715-0 [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R, 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470. doi: 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakalem ME, Seidenbecher T, Zhang M, Saffari R, Kravchenko M, Wördemann S, Diederich K, Schwamborn JC, Zhang W, Ambrée O, 2017. Environmental enrichment and physical exercise revert behavioral and electrophysiological impairments caused by reduced adult neurogenesis. Hippocampus 27, 36–51. doi: 10.1002/hipo.22669 [DOI] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai H-H, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo J-M, Rowitch DH, Alvarez-Buylla A, 2011. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478, 382–6. doi: 10.1038/nature10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Hernandez AG, Hernández HG, Restrepo A, Munoz JI, Bayon GF, Fernandez AF, Fraga MF, Cardona-Gomez GP, Arboleda H, Arboleda GH, 2016. Liver X receptor agonist modifies the DNA methylation profile of synapse and neurogenesis-related genes in the triple transgenic mouse model of Alzheimer’s disease. J. Mol. Neurosci. 58, 243–253. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R, 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–9. doi: 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science (80-. ). 268, 1749–1753. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang J, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR, 2006. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 103, 17501–6. doi: 10.1073/pnas.0607207103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM, 2017. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. doi: 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, Alldred MJ, Lee SH, Calhoun ME, Petkova E, Mathews PM, Ginsberg SD, 2015. Reduction of β-amyloid and γ-secretase by calorie restriction in female Tg2576 mice. Neurobiol. Aging 36, 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, 2004. Functional Implications of Seizure-Induced Neurogenesis, in: Advances in Experimental Medicine and Biology. pp. 192–212. doi: 10.1007/978-1-4757-6376-8_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hen R, 2007. Neuroscience. Is more neurogenesis always better? Science 315, 336–8. doi: 10.1126/science.1138711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M, 2010. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry 15, 1152–1163. doi: 10.1038/mp.2010.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K, 2009. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport 20, 553–7. doi: 10.1097/WNR.0b013e3283293e59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten M, Bielefeld P, Garcia-Corzo L, Passchier EMJ, Gradari S, Jungenitz T, Pons-Espinal M, Gebara E, Martín-Suárez S, Lucassen PJ, De Vries HE, Trejo JL, Schwarzacher SW, De Pietri Tonelli D, Toni N, Mira H, Encinas JM, Fitzsimons CP, 2020. Circadian glucocorticoid oscillations preserve a population of adult hippocampal neural stem cells in the aging brain. Mol. Psychiatry 2020 25 (7), 1382–1405. doi: 10.1038/s41380-019-0440-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmal Z, Isermann A, Hladik D, von Toerne C, Tapio S, Rübe CE, 2019. DNA damage accumulation during fractionated low-dose radiation compromises hippocampal neurogenesis. Radiother. Oncol. 137, 45–54. [DOI] [PubMed] [Google Scholar]
- Schultz MB, Sinclair DA, 2016. When stem cells grow old: Phenotypes and mechanisms of stem cell aging. Dev. 143, 3–14. doi: 10.1242/dev.130633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopa C, Marrocco F, Latina V, Ruggeri F, Corvaglia V, La Regina F, Ammassari-Teule M, Middei S, Amadoro G, Meli G, 2020. Impaired adult neurogenesis is an early event in Alzheimer’s disease neurodegeneration, mediated by intracellular Aβ oligomers. Cell Death Differ. 27, 934–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B, 1957. LOSS OF RECENT MEMORY AFTER BILATERAL HIPPOCAMPAL LESIONS. J. Neurol. Neurosurg. Psychiatry 20, 11–21. doi: 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D, 2003. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 26, 125–131. doi: 10.1016/S0166-2236(03)00031-6 [DOI] [PubMed] [Google Scholar]
- Seki T, Hori T, Miyata H, Maehara M, Namba T, 2019. Analysis of proliferating neuronal progenitors and immature neurons in the human hippocampus surgically removed from control and epileptic patients. Sci. Rep. 9, 18194. doi: 10.1038/s41598-019-54684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, 2014. Dietary restriction and the pursuit of effective mimetics. Proc. Nutr. Soc. 73, 260–270. doi: 10.1017/S0029665113003832 [DOI] [PubMed] [Google Scholar]
- Selvarani R, Mohammed S, Richardson A, 2021. Effect of rapamycin on aging and age-related diseases-past and future. GeroScience 43, 1135–1158. doi: 10.1007/s11357-020-00274-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Das S, Hyman BT, 2021. APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 20, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S, 1997. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89, 629–639. [DOI] [PubMed] [Google Scholar]
- Shen X, Chen J, Li J, Kofler J, Herrup K, 2016. Neurons in vulnerable regions of the Alzheimer’s disease brain display reduced ATM signaling. Eneuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherdson DJ, Mellen JD, Hutchins M, 1998. Tracing the path of environmental enrichment in zoos. Second Nat. Environ. Enrich. Captiv. Anim. doi: 10.1086/420493 [DOI] [Google Scholar]
- Sherstnev VV, Solov’eva OA, Gruden’ MA, Ratmirov AM, Konovalova EV, 2021. Hippocampal Neurogenesis, Dopaminergic Neurons of the Substantia Nigra, and Behavior after Intranasal Administration of Native α-Synuclein Protein to Ageing Mice. Neurochem. J. 15, 71–78. doi: 10.1134/S181971242101013X [DOI] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA, 2005. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: Role of astrocytes. Glia 51, 173–186. doi: 10.1002/glia.20187 [DOI] [PubMed] [Google Scholar]
- Shull ERP, Lee Y, Nakane H, Stracker TH, Zhao J, Russell HR, Petrini JHJ, McKinnon PJ, 2009. Differential DNA damage signaling accounts for distinct neural apoptotic responses in ATLD and NBS. Genes Dev. 23, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JM, Gil-Mohapel J, Pouladi MA, Ghilan M, Xie Y, Hayden MR, Christie BR, 2011. Altered adult hippocampal neurogenesis in the YAC128 transgenic mouse model of Huntington disease. Neurobiol. Dis. 41, 249–260. [DOI] [PubMed] [Google Scholar]
- Singh S, Mishra A, Mishra SK, Shukla S, 2017. ALCAR promote adult hippocampal neurogenesis by regulating cell-survival and cell death-related signals in rat model of Parkinson’s disease like-phenotypes. Neurochem. Int. 108, 388–396. [DOI] [PubMed] [Google Scholar]
- Singhal G, Morgan J, Jawahar MC, Corrigan F, Jaehne EJ, Toben C, Breen J, Pederson SM, Hannan AJ, Baune BT, 2019a. The effects of short-term and long-term environmental enrichment on locomotion, mood-like behavior, cognition and hippocampal gene expression. Behav. Brain Res. 368, 111917. doi: 10.1016/j.bbr.2019.111917 [DOI] [PubMed] [Google Scholar]
- Singhal G, Morgan J, Jawahar MC, Corrigan F, Jaehne EJ, Toben C, Breen J, Pederson SM, Hannan AJ, Baune BT, 2019b. Short-term environmental enrichment, and not physical exercise, alleviate cognitive decline and anxiety from middle age onwards without affecting hippocampal gene expression. Cogn. Affect. Behav. Neurosci. 19, 1143–1169. doi: 10.3758/s13415-019-00743-x [DOI] [PubMed] [Google Scholar]
- Slack C, Foley A, Partridge L, 2012. Activation of AMPK by the Putative Dietary Restriction Mimetic Metformin Is Insufficient to Extend Lifespan in Drosophila. PLoS One 7, 1–7. doi: 10.1371/journal.pone.0047699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater AM, Cao L, 2015. A Protocol for Housing Mice in an Enriched Environment. J. Vis. Exp. 2015. doi: 10.3791/52874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA, 2004. From The Cover: Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc. Natl. Acad. Sci. 101, 7181–7186. doi: 10.1073/pnas.0400285101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE, 2011. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8, 281–293. [DOI] [PubMed] [Google Scholar]
- Snyder BR, Chiu AM, Prockop DJ, Chan AWS, 2010. Human multipotent stromal cells (MSCs) increase neurogenesis and decrease atrophy of the striatum in a transgenic mouse model for Huntington’s disease. PLoS One 5, e9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron H a, 2011. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–61. doi: 10.1038/nature10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn D, Shpanskaya K, Lucas JE, Petrella JR, Saykin AJ, Tanzi RE, Samatova NF, Doraiswamy PM, 2018. Sex differences in cognitive decline in subjects with high likelihood of mild cognitive impairment due to Alzheimer’s disease. Sci. Rep. 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A, 2018. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. doi: 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Zhang Z, Kang G, Pastor-Alonso O, Biagiotti S, Page CE, Sandoval K, Knox A, Connolly A, Huang EJ, Garcia-Verdugo JM, Oldham MC, Yang Z, Alvarez-Buylla A, 2021. Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus. J. Neurosci. 41, 2554–2565. doi: 10.1523/JNEUROSCI.0676-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Buchholz BA, Westerlund I, Mash DC, Boström E, Vial C, Possnert G, Druid H, Frisén J, 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–27. doi: 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Walker JJ, Terry JR, Lightman SL, 2014. HPA axis-rhythms. Compr. Physiol. 4, 1273–1298. doi: 10.1002/cphy.c140003 [DOI] [PubMed] [Google Scholar]
- Stangl D, Thuret S, 2009. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 4, 271–282. doi: 10.1007/s12263-009-0134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Kirwan CB, Stark CEL, 2019. Mnemonic Similarity Task: A Tool for Assessing Hippocampal Integrity. Trends Cogn. Sci. 23, 938–951. doi: 10.1016/j.tics.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Imai SI, 2014. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 33, 1321–1340. doi: 10.1002/embj.201386917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner E, Tata M, Frisén J, 2019. A fresh look at adult neurogenesis. Nat. Med. 25, 542–543. doi: 10.1038/s41591-019-0408-4 [DOI] [PubMed] [Google Scholar]
- Stern N, Hochman A, Zemach N, Weizman N, Hammel I, Shiloh Y, Rotman G, Barzilai A, 2002. Accumulation of DNA damage and reduced levels of nicotine adenine dinucleotide in the brains of Atm-deficient mice. J. Biol. Chem. 277, 602–608. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE, 2016. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884. doi: 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y-H, 2015. Effects of treadmill exercise on hippocampal neurogenesis in an MPTP/probenecid-induced Parkinson’s disease mouse model. J. Phys. Ther. Sci. 27, 3203–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C, 2008. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry 64, 293–301. doi: 10.1016/j.biopsych.2008.02.022 [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C, 2011. Antidepressants recruit new neurons to improve stress response regulation. Mol. Psychiatry 16, 1177–1188. doi: 10.1038/mp.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Lexell J, Deierborg T, 2015. Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior. Neurorehabil. Neural Repair 29, 577–589. doi: 10.1177/1545968314562108 [DOI] [PubMed] [Google Scholar]
- Syal C, Kosaraju J, Hamilton L, Aumont A, Chu A, Sarma SN, Thomas J, Seegobin M, Jeffrey Dilworth F, He L, Wondisford FE, Zimmermann R, Parent M, Fernandes K, Wang J, 2020. Dysregulated expression of monoacylglycerol lipase is a marker for anti-diabetic drug metformin-targeted therapy to correct impaired neurogenesis and spatial memory in Alzheimer’s disease. Theranostics 10, 6337. doi: 10.7150/THNO.44962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora P, Misiak M, Wang Y, Ghosh S, Leandro GS, Liu D, Tian J, Baptiste BA, Cong W-N, Brenerman BM, 2015. DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res. 43, 943–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, Blair NF, Craufurd D, Priller J, Rickards H, 2019. Targeting huntingtin expression in patients with Huntington’s disease. N. Engl. J. Med. 380, 2307–2316. [DOI] [PubMed] [Google Scholar]
- Takamura N, Nakagawa S, Masuda T, Boku S, Kato A, Song N, An Y, Kitaichi Y, Inoue T, Koyama T, 2014. The effect of dopamine on adult hippocampal neurogenesis. Prog. Neuro-Psychopharmacology Biol. Psychiatry 50, 116–124. [DOI] [PubMed] [Google Scholar]
- Talwar P, Silla Y, Grover S, Gupta M, Agarwal R, Kushwaha S, Kukreti R, 2014. Genomic convergence and network analysis approach to identify candidate genes in Alzheimer’s disease. BMC Genomics 15, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Süle Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z, 2019. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 24, 101192. doi: 10.1016/j.redox.2019.101192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, 2015. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol 11, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P, 2010. A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. (Formerly Curr. Med. Chem. Nerv. Syst. Agents) 10, 16–21. [DOI] [PubMed] [Google Scholar]
- Taylor AMR, Lam Z, Last JI, Byrd PJ, 2015. Ataxia telangiectasia: more variation at clinical and cellular levels. Clin. Genet. 87, 199–208. [DOI] [PubMed] [Google Scholar]
- Tensaouti Y, Stephanz EP, Yu T-S, Kernie SG, 2018. ApoE regulates the development of adult newborn hippocampal neurons. Eneuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tensaouti Y, Yu T-S, Kernie SG, 2020. Apolipoprotein E regulates the maturation of injury-induced adult-born hippocampal neurons following traumatic brain injury. PLoS One 15, e0229240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JI, Ogawa SK, Kitamura T, 2019. Adult hippocampal neurogenesis for systems consolidation of memory. Behav. Brain Res. 372. doi: 10.1016/J.BBR.2019.112035 [DOI] [PubMed] [Google Scholar]
- Thangthaeng N, Rutledge M, Wong JM, Vann PH, Forster MJ, Sumien N, 2017. Metformin impairs spatial memory and visual acuity in old male mice. Aging Dis. 8, 17–30. doi: 10.14336/AD.2016.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Hamilton J, O’Rourke JR, Napoli A, Febo M, Volkow ND, Blum K, Gold M, 2016. Dopamine D2 gene expression interacts with environmental enrichment to impact lifespan and behavior. Oncotarget 7, 19111–19123. doi: 10.18632/oncotarget.8088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillement L, Lecanu L, Papadopoulos V, 2011. Alzheimer’s disease: effects of β-amyloid on mitochondria. Mitochondrion 11, 13–21. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Baptiste BA, Okur MN, Bohr VA, 2021. Current and emerging roles of Cockayne syndrome group B (CSB) protein. Nucleic Acids Res. 49, 2418–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, Kim N, Dawe RJ, Bennett DA, Arfanakis K, 2019. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 24, 974–982. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Parylak SL, Linker SB, Gage FH, 2019. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 24, 67–87. doi: 10.1038/s41380-018-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE, 2009. Visual object pattern separation deficits in nondemented older adults. Learn. Mem. 16, 338–342. doi: 10.1101/lm.1315109 [DOI] [PubMed] [Google Scholar]
- Torres-Pérez M, Tellez-Ballesteros RI, Ortiz-López L, Ichwan M, Vega-Rivera NM, Castro-García M, Gómez-Sánchez A, Kempermann G, Ramirez-Rodriguez GB, 2015. Resveratrol enhances neuroplastic changes, including hippocampal neurogenesis, and memory in Balb/C mice at six months of age. PLoS One 10, 1–21. doi: 10.1371/journal.pone.0145687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Wada E, Wada K, 2009. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 23, 1920–1934. doi: 10.1096/fj.08-124784 [DOI] [PubMed] [Google Scholar]
- Trepanowski JF, Canale RE, Marshall KE, Kabir MM, Bloomer RJ, 2011. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: A summary of available findings. Nutr. J. doi: 10.1186/1475-2891-10-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Tashiro A, Witter MP, Moser EI, 2008. What is the mammalian dentate gyrus good for? Neuroscience 154, 1155–1172. doi: 10.1016/j.neuroscience.2008.04.073 [DOI] [PubMed] [Google Scholar]
- Trinchero MF, Herrero M, Schinder AF, 2019. Rejuvenating the Brain With Chronic Exercise Through Adult Neurogenesis. Front. Neurosci. 13, 1–8. doi: 10.3389/fnins.2019.01000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest J-MM, Piazza P-VV, Koehl M, Abrous DN, 2012. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus 22, 292–8. doi: 10.1002/hipo.20895 [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D, 1997. Transforming Growth Factor-α Null and Senescent Mice Show Decreased Neural Progenitor Cell Proliferation in the Forebrain Subependyma. J. Neurosci. 17, 7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S, Gantelet E, Sandi C, Márquez C, 2020. Programming effects of peripubertal stress on spatial learning. Neurobiol. Stress 13, 100282. doi: 10.1016/j.ynstr.2020.100282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura N, Uemura MT, Luk KC, Lee VM-Y, Trojanowski JQ, 2020. Cell-to-cell transmission of tau and α-synuclein. Trends Mol. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um HS, Kang EB, Koo JH, Kim HT, Jin-Lee, Kim EJ, Yang CH, An GY, Cho IH, Cho JY, 2011. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci. Res. 69, 161–173. doi: 10.1016/j.neures.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Unger MS, Marschallinger J, Kaindl J, Höfling C, Rossner S, Heneka MT, Van der Linden A, Aigner L, 2016. Early changes in hippocampal neurogenesis in transgenic mouse models for Alzheimer’s disease. Mol. Neurobiol. 53, 5796–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnikrishnan A, Kurup K, Salmon AB, Richardson A, 2020. Is rapamycin a dietary restriction mimetic? Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 75, 4–13. doi: 10.1093/gerona/glz060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbán N, Guillemot F, 2014. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front. Cell. Neurosci 8, 396. doi: 10.3389/fncel.2014.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee SJ, Wolters FJ, Ikram MK, Hofman A, Ikram MA, Amin N, van Duijn CM, 2018. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 17, 434–444. [DOI] [PubMed] [Google Scholar]
- Van Praag H, 2008. Neurogenesis and exercise: Past and future directions. NeuroMolecular Med. 10, 128–140. doi: 10.1007/s12017-008-8028-z [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 2000. Neural consequences of enviromental enrichment. Nat. Rev. Neurosci. 1, 191–198. doi: 10.1038/35044558 [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270. doi: 10.1038/6368 [DOI] [PubMed] [Google Scholar]
- Van Skike CE, Lin AL, Roberts Burbank R, Halloran JJ, Hernandez SF, Cuvillier J, Soto VY, Hussong SA, Jahrling JB, Javors MA, Hart MJ, Fischer KE, Austad SN, Galvan V, 2020. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell 19, 1–11. doi: 10.1111/acel.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedam-Mai V, Gardner B, Okun MS, Siebzehnrubl FA, Kam M, Aponso P, Steindler DA, Yachnis AT, Neal D, Oliver BU, 2014. Increased precursor cell proliferation after deep brain stimulation for Parkinson’s disease: a human study. PLoS One 9, e88770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessoni AT, Herai RH, Karpiak JV, Leal AMS, Trujillo CA, Quinet A, Agnez Lima LF, Menck CFM, Muotri AR, 2016. Cockayne syndrome-derived neurons display reduced synapse density and altered neural network synchrony. Hum. Mol. Genet. 25, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl D, Solon-Biet SM, Wang QP, Wali JA, Pulpitel T, Clark X, Raubenheimer D, Senior AM, Sinclair DA, Cooney GJ, de Cabo R, Cogger VC, Simpson SJ, Le Couteur DG, 2018. Comparing the Effects of Low-Protein and High-Carbohydrate Diets and Caloric Restriction on Brain Aging in Mice. Cell Rep. 25, 2234–2243.e6. doi: 10.1016/j.celrep.2018.10.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Shin R, Tajinda K, Heusner CL, Kogan JH, Miyake S, Chen Q, Tamura K, Matsumoto M, 2012. Adult neurogenesis transiently generates oxidative stress. PLoS One 7. doi: 10.1371/journal.pone.0035264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Shen Y, Chuang H, Chiu C, Ye Y, Zhao L, 2019. Neuroinflammation in Alzheimer’s disease: microglia, molecular participants and therapeutic choices. Curr. Alzheimer Res. 16, 659–674. [DOI] [PubMed] [Google Scholar]
- Wang J, Gallagher D, Devito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD, 2012. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11, 23–35. doi: 10.1016/j.stem.2012.03.016 [DOI] [PubMed] [Google Scholar]
- Wang R, Wu Z, Bai L, Liu R, Ba Y, Zhang H, Cheng X, Zhou G, Huang H, 2021. Resveratrol improved hippocampal neurogenesis following lead exposure in rats through activation of SIRT1 signaling. Environ. Toxicol. 36, 1664–1673. doi: 10.1002/TOX.23162 [DOI] [PubMed] [Google Scholar]
- Wang W, Esbensen Y, Kunke D, Suganthan R, Rachek L, Bjørås M, Eide L, 2011. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J. Neurosci. 31, 9746–9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chakravarty P, Ranes M, Kelly G, Brooks PJ, Neilan E, Stewart A, Schiavo G, Svejstrup JQ, 2014. Dysregulation of gene expression as a cause of Cockayne syndrome neurological disease. Proc. Natl. Acad. Sci. 111, 14454–14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton SB, Williams GH, Stoeber K, Gelsthorpe CH, Baxter L, Johnson AL, Ince PG, 2005. Expression of Ki67, PCNA and the chromosome replication licensing protein Mcm2 in glial cells of the ageing human hippocampus increases with the burden of Alzheimer-type pathology. Neurosci. Lett. 383, 33–38. [DOI] [PubMed] [Google Scholar]
- Wightman EL, Haskell-Ramsay CF, Reay JL, Williamson G, Dew T, Zhang W, Kennedy DO, 2015. The effects of chronic trans -resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 114, 1427–1437. doi: 10.1017/S0007114515003037 [DOI] [PubMed] [Google Scholar]
- Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J, 2004. Human wild-type α-synuclein impairs neurogenesis. J. Neuropathol. Exp. Neurol. 63, 1155–1166. [DOI] [PubMed] [Google Scholar]
- Winner B, Melrose HL, Zhao C, Hinkle KM, Yue M, Kent C, Braithwaite AT, Ogholikhan S, Aigner R, Winkler J, 2011. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol. Dis. 41, 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Regensburger M, Schreglmann S, Boyer L, Prots I, Rockenstein E, Mante M, Zhao C, Winkler J, Masliah E, 2012. Role of α-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J. Neurosci. 32, 16906–16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, Couillard-Despres S, Masliah E, Winkler J, 2008. Mutant α-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol. Aging 29, 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Winkler J, 2015. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 7, a021287. doi: 10.1101/cshperspect.a021287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Flöel A, 2009. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. U. S. A. 106, 1255–1260. doi: 10.1073/pnas.0808587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Margulies DS, Floel A, 2014. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 34, 7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D, 2004. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689. doi: 10.1038/nature02789 [DOI] [PubMed] [Google Scholar]
- Wu C-W, Chen Y-C, Yu L, Chen H, Jen CJ, Huang A-M, Tsai H-J, Chang Y-T, Kuo Y-M, 2007. Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J. Neurochem. 103, 2471–2481. doi: 10.1111/j.1471-4159.2007.04987.x [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Kawai N, Ueno O, Matsumoto Y, Oohashi T, Honda M, 2018. Induction of prolonged natural lifespans in mice exposed to acoustic environmental enrichment. Sci. Rep. 8, 1–8. doi: 10.1038/s41598-018-26302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Ding F, Zhao Y, 2019. Integrated identification of key genes and pathways in Alzheimer’s disease via comprehensive bioinformatical analyses. Hereditas 156, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Figueroa DM, Hou Y, Babbar M, Baringer SL, Croteau DL, Bohr VA, 2019. NEIL1 stimulates neurogenesis and suppresses neuroinflammation after stress. Free Radic. Biol. Med. 141, 47–58. doi: 10.1016/j.freeradbiomed.2019.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-P, Gilley JA, Zhang G, Kernie SG, 2011. ApoE is required for maintenance of the dentate gyrus neural progenitor pool. Development 138, 4351–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Handler M, Shen J, 2000. Role of Presenilin-1 in Murine Neural Development. Ann. N. Y. Acad. Sci. 920, 165–170. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL, 2010. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus 21, n/a–n/a. doi: 10.1002/hipo.20808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau S-Y, Lau BW-M, Zhang E-D, Lee JC-D, Li A, Lee TMC, Ching Y-P, Xu A. -m., So K-F, 2012. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience 222, 289–301. doi: 10.1016/j.neuroscience.2012.07.019 [DOI] [PubMed] [Google Scholar]
- Yau SY, Li A, So K-FF, 2015. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast. 2015, 1–13. doi: 10.1155/2015/717958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazir Y, Utkan T, Gacar N, Aricioglu F, 2015. Resveratrol exerts anti-inflammatory and neuroprotective effects to prevent memory deficits in rats exposed to chronic unpredictable mild stress. Physiol. Behav. 138, 297–304. doi: 10.1016/j.physbeh.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Yook JS, Okamoto M, Rakwal R, Shibato J, Lee MC, Matsui T, Chang H, Cho JY, Soya H, 2016. Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice. Mol. Nutr. Food Res. 60, 589–599. doi: 10.1002/mnfr.201500634 [DOI] [PubMed] [Google Scholar]
- Yoshino J, Baur JA, Imai S. ichiro, 2018. NAD + Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 27, 513–528. doi: 10.1016/j.cmet.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Zuo X, Li Y, Zhang C, Zhou M, Zhang YA, Uéda K, Chan P, 2004. Inhibition of tyrosine hydroxylase expression in α-synuclein-transfected dopaminergic neuronal cells. Neurosci. Lett. 367, 34–39. [DOI] [PubMed] [Google Scholar]
- Zahra W, Rai SN, Birla H, Singh S. Sen, Dilnashin H, Rathore AS, Singh SP, 2020. The Global Economic Impact of Neurodegenerative Diseases: Opportunities and Challenges. Bioeconomy Sustain. Dev 333–345. doi: 10.1007/978-981-13-9431-7_17 [DOI] [Google Scholar]
- Zaletel I, Schwirtlich M, Perović M, Jovanović M, Stevanović M, Kanazir S, Puškaš N, 2018. Early Impairments of hippocampal neurogenesis in 5xFAD mouse model of alzheimer’s disease are associated with altered expression of SOXB transcription factors. J. Alzheimer’s Dis. 65, 963–976. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang L, Zhan A, Wang M, Tian L, Guo W, Pan Y, 2021. Long-term exposure to a hypomagnetic field attenuates adult hippocampal neurogenesis and cognition. Nat. Commun. 12, 1174. doi: 10.1038/s41467-021-21468-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R, 2007. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp. Neurol. 204, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J, 2016. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (80-. ). 352, 1436–1443. doi: 10.1126/science.aaf2693 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Réndon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K, 2014. Rapamycin Extends Life and Health in C57BL/6 Mice. Journals Gerontol. Ser. A 69A, 119–130. doi: 10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH, 2008. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–60. doi: 10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Guan Y-F, Zhou X-M, Li G-Q, Li Z-Y, Zhou C-C, Wang P, Miao C-Y, 2015. Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase–Nicotinamide Adenine Dinucleotide Cascade. Stroke 46, 1966–1974. doi: 10.1161/STROKEAHA.115.009216 [DOI] [PubMed] [Google Scholar]
- Zheng J, Li H-L, Tian N, Liu F, Wang L, Yin Y, Yue L, Ma L, Wan Y, Wang J-Z, 2020. Interneuron accumulation of phosphorylated tau impairs adult hippocampal neurogenesis by suppressing GABAergic transmission. Cell Stem Cell 26, 331–345. e6. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Li R, Xiao F, He R, Zhang S, Li J, 2017. Sex Matters: Hippocampal Volume Predicts Individual Differences in Associative Memory in Cognitively Normal Older Women but Not Men. Front. Hum. Neurosci 11. doi: 10.3389/fnhum.2017.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Zhu L, Qiu W, Liu Y, Yang F, Chen W, Xu R, 2020. Nicotinamide riboside enhances mitochondrial proteostasis and adult neurogenesis through activation of mitochondrial unfolded protein response signaling in the brain of ALS SOD1g93a mice. Int. J. Biol. Sci. 16, 284–297. doi: 10.7150/ijbs.38487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziabreva I, Ballard C, Johnson M, Larsen JP, McKeith I, Perry R, Aarsland D, Perry E, 2007. Loss of Musashi1 in Lewy body dementia associated with cholinergic deficit. Neuropathol. Appl. Neurobiol. 33, 586–590. [DOI] [PubMed] [Google Scholar]
- Ziegler-Waldkirch S, Marksteiner K, Stoll J, DÉrrico P, Friesen M, Eiler D, Neudel L, Sturn V, Opper I, Datta M, Prinz M, Meyer-Luehmann M, 2018. Environmental enrichment reverses Aβ pathology during pregnancy in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 6, 44. doi: 10.1186/s40478-018-0549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong L, Tanaka-Yano M, Park B, Yanai H, Turhan FT, Croteau DL, Tian J, Fang EF, Bohr VA, Beerman I, 2021. NAD+ augmentation with nicotinamide riboside improves lymphoid potential of Atm−/− and old mice HSCs. npj Aging Mech. Dis. 7, 25. doi: 10.1038/s41514-021-00078-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


