Abstract
The traditional complications of diabetes mellitus are well known and continue to pose a considerable burden on millions of people living with diabetes mellitus. However, advances in the management of diabetes mellitus and, consequently, longer life expectancies, have resulted in the emergence of evidence of the existence of a different set of lesser-acknowledged diabetes mellitus complications. With declining mortality from vascular disease, which once accounted for more than 50% of deaths amongst people with diabetes mellitus, cancer and dementia now comprise the leading causes of death in people with diabetes mellitus in some countries or regions. Additionally, studies have demonstrated notable links between diabetes mellitus and a broad range of comorbidities, including cognitive decline, functional disability, affective disorders, obstructive sleep apnoea and liver disease, and have refined our understanding of the association between diabetes mellitus and infection. However, no published review currently synthesizes this evidence to provide an in-depth discussion of the burden and risks of these emerging complications. This Review summarizes information from systematic reviews and major cohort studies regarding emerging complications of type 1 and type 2 diabetes mellitus to identify and quantify associations, highlight gaps and discrepancies in the evidence, and consider implications for the future management of diabetes mellitus.
Subject terms: Diabetes complications, Type 1 diabetes, Type 2 diabetes
This article discusses evidence for the emergence of a different set of complications associated with diabetes mellitus from the traditional ones, outlines the risks and burden of these associated complications and considers implications for the future management of diabetes mellitus.
Key points
With advances in the management of diabetes mellitus, evidence is emerging of an increased risk and burden of a different set of lesser-known complications of diabetes mellitus.
As mortality from vascular diseases has declined, cancer and dementia have become leading causes of death amongst people with diabetes mellitus.
Diabetes mellitus is associated with an increased risk of various cancers, especially gastrointestinal cancers and female-specific cancers.
Hospitalization and mortality from various infections, including COVID-19, pneumonia, foot and kidney infections, are increased in people with diabetes mellitus.
Cognitive and functional disability, nonalcoholic fatty liver disease, obstructive sleep apnoea and depression are also common in people with diabetes mellitus.
As new complications of diabetes mellitus continue to emerge, the management of this disorder should be viewed holistically, and screening guidelines should consider conditions such as cancer, liver disease and depression.
Introduction
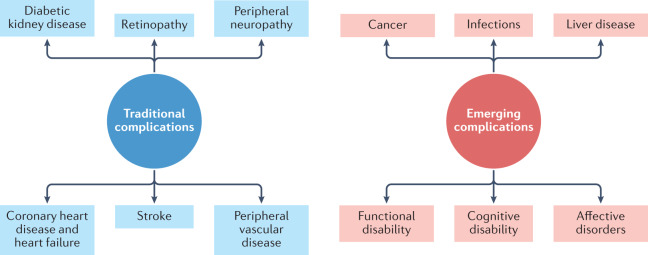
Diabetes mellitus is a common, albeit potentially devastating, medical condition that has increased in prevalence over the past few decades to constitute a major public health challenge of the twenty-first century1. Complications that have traditionally been associated with diabetes mellitus include macrovascular conditions, such as coronary heart disease, stroke and peripheral arterial disease, and microvascular conditions, including diabetic kidney disease, retinopathy and peripheral neuropathy2 (Fig. 1). Heart failure is also a common initial manifestation of cardiovascular disease in patients with type 2 diabetes mellitus (T2DM)3 and confers a high risk of mortality in those with T1DM or T2DM4. Although a great burden of disease associated with these traditional complications of diabetes mellitus still exists, rates of these conditions are declining with improvements in the management of diabetes mellitus5. Instead, as people with diabetes mellitus are living longer, they are becoming susceptible to a different set of complications6. Population-based studies7–9 show that vascular disease no longer accounts for most deaths among people with diabetes mellitus, as was previously the case10. Cancer is now the leading cause of death in people with diabetes mellitus in some countries or regions (hereafter ‘countries/regions’)9, and the proportion of deaths due to dementia has risen since the turn of the century11. In England, traditional complications accounted for more than 50% of hospitalizations in people with diabetes mellitus in 2003, but for only 30% in 2018, highlighting the shift in the nature of complications of this disorder over this corresponding period12.
Fig. 1. Major traditional complications and emerging complications of diabetes mellitus.
The traditional complications of diabetes mellitus include stroke, coronary heart disease and heart failure, peripheral neuropathy, retinopathy, diabetic kidney disease and peripheral vascular disease, as represented on the left-hand side of the diagram. With advances in the management of diabetes mellitus, associations between diabetes mellitus and cancer, infections, functional and cognitive disability, liver disease and affective disorders are instead emerging, as depicted in the right-hand side of the diagram. This is not an exhaustive list of complications associated with diabetes mellitus.
Cohort studies have reported associations of diabetes mellitus with various cancers, functional and cognitive disability, liver disease, affective disorders and sleep disturbance, and have provided new insights into infection-related complications of diabetes mellitus13–17. Although emerging complications have been briefly acknowledged in reviews of diabetes mellitus morbidity and mortality11,17, no comprehensive review currently specifically provides an analysis of the evidence for the association of these complications with diabetes mellitus. In this Review, we synthesize information published since the year 2000 on the risks and burden of emerging complications associated with T1DM and T2DM.
Diabetes mellitus and cancer
The burden of cancer mortality
With the rates of cardiovascular mortality declining amongst people with diabetes mellitus, cancer deaths now constitute a larger proportion of deaths among this population in some countries/regions8,9. Although the proportion of deaths due to cancer appears to be stable, at around 16–20%, in the population with diabetes mellitus in the USA7, in England it increased from 22% to 28% between 2001 and 2018 (ref.9), with a similar increase reported in Australia8. Notably, in England, cancer has overtaken vascular disease as the leading cause of death in people with diabetes mellitus and it is the leading contributor to excess mortality in those with diabetes mellitus compared with those without9. These findings are likely to be due to a substantial decline in the proportion of deaths from vascular diseases, from 44% to 24% between 2001 and 2018, which is thought to reflect the targeting of prevention measures in people with diabetes mellitus18. Over the same time period, cancer mortality rates fell by much less in the population with diabetes mellitus than in that without diabetes9, suggesting that clinical approaches for diabetes mellitus might focus too narrowly on vascular complications and might require revision19. In addition, several studies have reported that female patients with diabetes mellitus receive less-aggressive treatment for breast cancer compared with patients without diabetes mellitus, particularly with regard to chemotherapy20–22, suggesting that this treatment approach might result in increased cancer mortality rates in women with diabetes mellitus compared with those without diabetes mellitus. Although substantial investigation of cancer mortality in people with diabetes mellitus has been undertaken in high-income countries/regions, there is a paucity of evidence from low-income and middle-income countries/regions. It is important to understand the potential effect of diabetes mellitus on cancer mortality in these countries/regions owing to the reduced capacity of health-care systems in these countries/regions to cope with the combination of a rising prevalence of diabetes mellitus and rising cancer mortality rates in those with diabetes mellitus. One study in Mauritius showed a significantly increased risk of all-cause cancer mortality in patients with T2DM23, but this study has yet to be replicated in other low-income and middle-income countries/regions.
Gastrointestinal cancers
Of the reported associations between diabetes mellitus and cancer (Table 1), some of the strongest have been demonstrated for gastrointestinal cancers.
Table 1.
Summary of major systematic reviews and original studies reporting a cancer risk associated with diabetes mellitus
| Study | Diabetes mellitus type | Study type included (n) | Outcome | Risk associated with diabetes mellitus (95% confidence interval) |
|---|---|---|---|---|
| Wang et al.a (2012)24 | All | Cohort (3,626,368b) | Hepatocellular carcinoma | RR 2.01 (1.61–2.51) |
| El-Serag et al.a (2006)25 | All | Cohort, cross-sectional (2,938,889b) | Hepatocellular carcinoma | RR 2.5 (cohort studies) (1.9–3.2) and OR 2.5 (case–control) (1.8–3.5) |
| Huxley et al.a (2005)27 | T2DM | Cohort, cross-sectional (9,220) | Pancreatic cancer | OR 1.82 (1.66–1.89) |
| 1–4 years duration | OR 2.05 (1.87–2.25) | |||
| 5–9 years duration | OR 1.54 (1.31–1.81) | |||
| ≥10 years duration | OR 1.51 (1.16–1.96) | |||
| Carstensen et al.c (2016)30 | T1DM | Cohort (9,149) | Pancreatic cancer | HR 1.53 (males) (1.30–1.79) and HR 1.25 (females) (1.02–1.53) |
| Jiang et al.a (2011)31 | All | Cohort (8,244,732b) | Colorectal cancer | RR 1.27 (1.21–1.34) |
| Deng et al.a (2012)33 | All | Cohort, cross-sectional (3,659,341) | Colorectal cancer | RR 1.26 (1.20–1.31) |
| De Bruijn et al.a (2013)32 | All | Cohort, randomized controlled trials (1,930,309) | Colorectal cancer | HR 1.26 (1.14–1.40) |
| Breast cancer | HR 1.23 (1.12–1.34) | |||
| Liao et al.a (2014)34 | All | Cohort (5,302,259) | Endometrial cancer | RR 1.89 (1.46–2.45) |
| Endometrial cancer disease-specific mortality | RR 1.32 (1.10–1.60) | |||
| Saed et al.a (2019)35 | All | Cohort, cross-sectional (459,167b) | Endometrial cancer | RR 1.72 (1.48–2.01) |
| Friberg et al.a (2007)36 | All | Cohort, cross-sectional (96,003) | Endometrial cancer | RR 2.10 (1.75–2.53) |
| T1DM | RR 3.15 (1.07–9.29) | |||
| Larsson et al.a (2007)38 | T2DM | Cohort, cross-sectional (1,430,122b) | Breast cancer | RR 1.20 (1.12–1.28) |
| Anothaisintawee et al.a (2013)37 | All | Cohort, cross-sectional (1,090,503b) | Breast cancer | OR 1.14 (1.09–1.19) |
| Boyle et al.a (2012)39 | All | Cohort, cross-sectional (21,029b) | Breast cancer (postmenopausal) | RR 1.15 (1.07–1.24) |
| Zhang et al.a (2017)43 | All | Cohort (2,392,245b) | Ovarian cancer | RR 1.32 (1.14–1.52) |
| Weng et al.a (2017)44 | All | Cohort (3,708,313) | Ovarian cancer | RR 1.19 (1.06–1.34) |
| Wang et al.a (2020)45 | All | Cohort, cross-sectional (6,036,434b) | Ovarian cancer | RR 1.20 (1.10–1.31) |
| Lee et al.a (2013)46 | All | Cohort, cross-sectional (1,707,359b) | Ovarian cancer | RR 1.17 (1.02–1.33) |
| Bonovas et al.a (2004)47 | All | Cohort, cross-sectional (890,678b) | Prostate cancer | RR 0.91 (0.86–0.96) |
| Long et al.a (2012)49 | All (Asia only) | Cohort, cross-sectional (1,751,274) | Prostate cancer | RR 1.31 (1.12–1.54) |
HR, hazard ratio; OR, odds ratio; RR, relative risk; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus. aSystematic review. bTotal number of participants obtained through sum of individual study cohort sizes listed in tables or otherwise. cOriginal study.
Hepatocellular carcinoma
In the case of hepatocellular carcinoma, the most rigorous systematic review on the topic — comprising 18 cohort studies with a combined total of more than 3.5 million individuals — reported a summary relative risk (SRR) of 2.01 (95% confidence interval (CI) 1.61–2.51) for an association with diabetes mellitus24. This increased risk of hepatocellular carcinoma with diabetes mellitus is supported by the results of another systematic review that included case–control studies25. Another review also found that diabetes mellitus independently increased the risk of hepatocellular carcinoma in the setting of hepatitis C virus infection26.
Pancreatic cancer
The risk of pancreatic cancer appears to be approximately doubled in patients with T2DM compared with patients without T2DM. A meta-analysis of 36 studies found an adjusted odds ratio (OR) of 1.82 (95% CI 1.66–1.89) for pancreatic cancer among people with T2DM compared with patients without T2DM27 (Table 1). However, it is possible that these findings are influenced by reverse causality — in this scenario, diabetes mellitus is triggered by undiagnosed pancreatic cancer28, with pancreatic cancer subsequently being clinically diagnosed only after the diagnosis of diabetes mellitus. Nevertheless, although the greatest risk (OR 2.05, 95% CI 1.87–2.25) of pancreatic cancer was seen in people diagnosed with T2DM 1–4 years previously compared with people without T2DM, those with a diagnosis of T2DM of more than 10 years remained at increased risk of pancreatic cancer (OR 1.51, 95% CI 1.16–1.96)27, suggesting that reverse causality can explain only part of the association between T2DM and pancreatic cancer. Although T2DM accounts for ~90% of all cases of diabetes mellitus29, a study incorporating data from five nationwide diabetes registries also reported an increased risk of pancreatic cancer amongst both male patients (HR 1.53, 95% CI 1.30–1.79) and female patients (HR 1.25, 95% CI 1.02–1.53) with T1DM30.
Colorectal cancer
For colorectal cancer, three systematic reviews have shown a consistent 20–30% increased risk associated with diabetes mellitus31–33. One systematic review, which included more than eight million people across 30 cohort studies, reported an incidence SRR of 1.27 (95% CI 1.21–1.34) of colorectal cancer31, independent of sex and family history (Table 1). Similar increases in colorectal cancer incidence in patients with diabetes mellitus were reported in a meta-analysis of randomized controlled trials (RCTs) and cohort studies32 and in a systematic review that included cross-sectional studies33.
Female-specific cancers
Endometrial, breast and ovarian cancers all occur more frequently in women with diabetes mellitus than in women without diabetes mellitus.
Endometrial cancer
For endometrial cancer, one systematic review of 29 cohort studies and a combined total of 5,302,259 women reported a SRR of 1.89 (95% CI 1.46–2.45) and summary incidence rate ratio (IRR) of 1.61 (95% CI 1.51–1.71)34 (Table 1). Similar increased risks were found in two systematic reviews incorporating cross-sectional studies35,36, one of which found a particularly strong association of T1DM (relative risk (RR) 3.15, 95% CI 1.07–9.29) with endometrial cancer.
Breast cancer
The best evidence for a link between diabetes mellitus and breast cancer comes from a systematic review of six prospective cohort studies and more than 150,000 women, in which the hazard ratio (HR) for the incidence of breast cancer in women with diabetes mellitus compared with women without diabetes mellitus was 1.23 (95% CI 1.12–1.34)32 (Table 1). Two further systematic reviews have also shown this increased association37,38.
The association of diabetes mellitus with breast cancer appears to vary according to menopausal status. In a meta-analysis of studies of premenopausal women with diabetes mellitus, no significant association with breast cancer was found39, whereas in 11 studies that included only postmenopausal women, the SRR was 1.15 (95% CI 1.07–1.24). The difference in breast cancer risk between premenopausal and postmenopausal women with diabetes mellitus was statistically significant. The increased risk of breast cancer after menopause in women with diabetes mellitus compared with women without diabetes mellitus might result from the elevated concentrations and increased bioavailability of oestrogen that are associated with adiposity40, which is a common comorbidity in those with T2DM; oestrogen synthesis occurs in adipose tissue in postmenopausal women, while it is primarily gonadal in premenopausal women41. Notably, however, there is evidence that hormone-receptor-negative breast cancers, which typically carry a poor prognosis, occur more frequently in women with breast cancer and diabetes mellitus than in women with breast cancer and no diabetes mellitus42, indicating that non-hormonal mechanisms also occur.
Ovarian cancer
Diabetes mellitus also appears to increase the risk of ovarian cancer, with consistent results from across four systematic reviews. A pooled RR of 1.32 (95% CI 1.14–1.52) was reported across 15 cohort studies and a total of more than 2.3 million women43 (Table 1). A SRR of 1.19 (95% CI 1.06–1.34) was found across 14 cohort studies and 3,708,313 women44. Similar risks were reported in meta-analyses that included cross-sectional studies45,46.
Male-specific cancers: prostate cancer
An inverse association between diabetes mellitus and prostate cancer has been observed in a systematic review (RR 0.91, 95% CI 0.86–0.96)47, and is probably due to reduced testosterone levels that occur secondary to the low levels of sex hormone-binding globulin that are commonly seen in men with T2DM and obesity48. Notably, however, the systematic review that showed the inverse association involved mostly white men (Table 1), whereas a systematic review of more than 1.7 million men from Taiwan, Japan, South Korea and India found that diabetes mellitus increased prostate cancer risk49, suggesting that ethnicity might be an effect modifier of the diabetes mellitus–prostate cancer relationship. The mechanisms behind this increased risk in men in regions of Asia such as Taiwan and Japan, where most study participants came from, remain unclear. Perhaps, as Asian men develop diabetes mellitus at lower levels of total adiposity than do white men50, the adiposity associated with diabetes mellitus in Asian men might have a lesser impact on sex hormone-binding globulin and testosterone than it does in white men. Despite the reported inverse association between diabetes mellitus and prostate cancer in white men, however, evidence suggests that prostate cancers that do develop in men with T2DM are typically more aggressive, conferring higher rates of disease-specific mortality than prostate cancers in men without diabetes mellitus51.
An assessment of cancer associations
As outlined above, a wealth of data has shown that diabetes mellitus is associated with an increased risk of various cancers. It has been argued, however, that some of these associations could be due to detection bias resulting from increased surveillance of people with diabetes mellitus in the immediate period after diagnosis52, or reverse causality, particularly in the case of pancreatic cancer53. However, neither phenomenon can account for the excess risks seen in the longer term. An Australian study exploring detection bias and reverse causality found that standardized mortality ratios (SMRs) for several cancer types in people with diabetes mellitus compared with the general population fell over time, but remained elevated beyond 2 years for pancreatic and liver cancers54, suggesting that diabetes mellitus is a genuine risk factor for these cancer types.
A limitation of the evidence that surrounds diabetes mellitus and cancer risk is high clinical and methodological heterogeneity across several of the large systematic reviews, which makes it difficult to be certain of the effect size in different demographic groups. Additionally, many of the studies exploring a potential association between diabetes mellitus and cancer were unable to adjust for BMI, which is a major confounder. However, a modelling study that accounted for BMI found that although 2.1% of cancers worldwide in 2012 were attributable to diabetes mellitus as an independent risk factor, twice as many cancers were attributable to high BMI55, so it is likely that effect sizes for cancer risk associated with diabetes mellitus would be attenuated after adjustment for BMI. Notably, however, low-income and middle-income countries/regions had the largest increase in the numbers of cases of cancer attributable to diabetes mellitus both alone and in combination with BMI55, highlighting the need for public health intervention, given that these countries/regions are less equipped than high-income countries/regions to manage a growing burden of cancer.
As well as the cancer types outlined above, diabetes mellitus has also been linked to various other types of cancer, including kidney cancer56, bladder cancer57 and haematological malignancies; however, the evidence for these associations is not as strong as for the cancers discussed above58. Diabetes mellitus might also be associated with other cancer types such as small intestine cancer, but the rarity of some of these types makes it difficult to obtain sufficient statistical power in analyses of any potential association.
Potential aetiological mechanisms
Several aetiological mechanisms that might be involved in linking diabetes mellitus to cancer have been proposed, including hyperinsulinaemia, hyperglycaemia, inflammation and cellular signalling mechanisms.
Hyperinsulinaemia
Most cancer cells express insulin receptors, through which hyperinsulinaemia is thought to stimulate cancer cell proliferation and metastasis59. Hyperinsulinaemia might also promote carcinogenesis through increased local levels of insulin-like growth factor 1 (IGF1), which has potent mitogenic and anti-apoptotic activities60, owing to decreased levels of insulin-like growth factor binding proteins. As outlined above, people with diabetes mellitus show a strong risk of pancreatic and liver cancers; this increased risk might occur because insulin is produced by pancreatic β-cells and transported to the liver via the portal vein61, thereby exposing the liver and pancreas to high levels of endogenous insulin59.
Hyperglycaemia and inflammation
Hyperglycaemia can induce DNA damage62, increase the generation of reactive oxygen species63 and downregulate antioxidant expression64, all of which are associated with cancer development. Inflammatory markers, including cytokines such as IL-6, appear to have an important role in the association between diabetes and cancer65.
Cellular signalling mechanisms
Several cellular signalling components are common to the pathogenesis of T2DM and cancer. These include the mechanistic target of rapamycin (mTOR), a central controller of cell growth and proliferation; AMP-activated protein kinase, a cellular energy sensor and signal transducer66; and the phosphatidylinositol 3-kinase (PI3K)–AKT pathway, which transduces growth factor signals during organismal growth, glucose homeostasis and cell proliferation67. Dysregulation of any of these cellular signalling components or pathways could contribute to the development of cancer and metabolic disorders, including T2DM, and glucose-lowering drugs such as metformin have been associated with a reduction in cancer cell proliferation through effective inhibition of some of these components68.
Diabetes mellitus and infections
Infection-related complications
Although infection has long been recognized as a complication of diabetes mellitus, an association between diabetes mellitus and infection has not been well documented in epidemiological studies69. Only in the past decade have major studies quantified the burden of infection-related complications in people with diabetes mellitus and explored the specific infections accounting for this burden. In a US cohort of 12,379 participants, diabetes mellitus conferred a significant risk of infection-related hospitalization, with an adjusted HR of 1.67 (95% CI 1.52–1.83) compared with people without diabetes mellitus70 (Table 2). The association was most pronounced for foot infections (HR 5.99, 95% CI 4.38–8.19), with significant associations also observed for respiratory infection, urinary tract infection, sepsis and post-operative infection, but not for gastrointestinal infection, a category that included appendicitis and gastrointestinal abscesses but not viral or bacterial gastroenteritis. Interestingly, a report from Taiwan demonstrated an association between the use of metformin and a lower risk of appendicitis71.
Table 2.
Summary of major systematic reviews and original studies reporting an infection risk associated with diabetes mellitus
| Study | Diabetes mellitus type | Study type included (n) | Outcome | Risk associated with diabetes mellitus (95% confidence interval) |
|---|---|---|---|---|
| Fang et al.a (2021)70 | All | Cohort (12,379) | Infection-related hospitalization | HR 1.67 (1.52–1.83) |
| Hospitalization for foot infections | HR 5.99 (4.38–8.19) | |||
| Luk et al.a (2021)72 | All | Cohort (6,164,082) | Hospitalization for kidney infection (male individuals) | RR 2.50 (1.70–3.50) |
| Hospitalization for kidney infection (female individuals) | RR 2.10 (1.70–2.70) | |||
| Hospitalization for tuberculosis (male individuals) | RR 2.20 (2.00–2.40) | |||
| Hospitalization for tuberculosis (female individuals) | RR 2.10 (1.80–2.40) | |||
| Hospitalization for sepsis (male individuals) | RR 2.30 (2.10–2.50) | |||
| Hospitalization for sepsis (female individuals) | RR 2.30 (2.10–2.50) | |||
| Magliano et al.b (2015)73 | T1DM | Cohort (85,144) | Infection-related mortality | SMR 4.42 (3.68–5.34) |
| Pneumonia-related mortality | SMR 6.23 (4.30–9.00) | |||
| Septicaemia-related mortality | SMR 10.00 (6.70–14.90) | |||
| Osteomyelitis-related mortality | SMR 16.30 (5.20–50.40) | |||
| Magliano et al.a (2015)73 | T2DM | Cohort (1,023,838) | Infection-related mortality | SMR 1.47 (1.42–1.53) |
| Pneumonia-related mortality | SMR 1.20 (1.20–1.30) | |||
| Septicaemia-related mortality | SMR 1.80 (1.70–2.00) | |||
| Osteomyelitis-related mortality | SMR 3.50 (2.90–4.30) | |||
| Martin et al.b (2016)74 | All | RCTs, cohort, cross-sectional (32,067); 90 studies | Surgical site infection | OR 1.77 (adjusted measures; 1.13–2.78); heterogeneity (I2) = 71% |
| McGurnaghan et al.a (2021)79 | All | Cohort (5,463,300) | Fatal or critical care unit-treated COVID-19 | OR 1.40 (1.30–1.49) |
| Rawshani et al.a (2021)80 | T1DM | Cohort (44,639) | COVID-19 hospitalization | HR 2.10 (1.72–2.57) |
| T2DM | Cohort (411,976) | HR 2.22 (2.13–2.32) | ||
| You et al.a (2020)81 | T2DM | Cohort (5,473) | Intensive care unit-treated COVID-19 | OR 1.59 (1.02–2.49) |
| Moon et al.a (2020)82 | All | Cohort (5,307) | Oxygen treatment in COVID-19 | OR 1.35 (1.10–1.66) |
| Ventilator requirement in COVID-19 | OR 1.93 (1.28–2.92) |
COVID-19, coronavirus disease 2019; HR, hazard ratio; OR, odds ratio; RCT, randomized controlled trial; RR, relative risk; SMR, standardized mortality ratio. aOriginal study. bSystematic review.
In an analysis of the entire Hong Kong population over the period 2001–2016, rates of hospitalization for all types of infection remained consistently higher in people with diabetes mellitus than in those without diabetes mellitus72. The strongest association was seen for hospitalization due to kidney infections, for which the adjusted RR was 4.9 (95% CI 3.9–6.2) in men and 3.2 (95% CI 2.8–3.7) in women with diabetes mellitus compared with those without diabetes mellitus in 2016 (Table 2). Diabetes mellitus roughly doubled the risk of hospitalization from tuberculosis or sepsis. The most common cause of infection-related hospitalization was pneumonia, which accounted for 39% of infections across the study period, while no other single cause accounted for more than 25% of infections across the same period. Pneumonia-related hospitalization rates increased substantially from 2001 to 2005, probably as a result of the 2003 severe acute respiratory syndrome (SARS) epidemic and the decreased threshold for pneumonia hospitalization in the immediate post-epidemic period. Rates for hospitalization for influenza increased from 2002 to 2016, possibly because of changes in the virus and increased testing for influenza. Declining rates of hospitalization for tuberculosis, urinary tract infections, foot infections and sepsis could be due to improvements in the management of diabetes mellitus.
Infection-related mortality rates were found to be significantly elevated among 1,108,982 Australians with diabetes mellitus studied over the period 2000–2010 compared with rates in people without diabetes mellitus73. For overall infection-related mortality, SMRs were 4.42 (95% CI 3.68–5.34) for T1DM and 1.47 (95% CI 1.42–1.53) for people with T2DM compared with those without diabetes mellitus (Table 2). Substantially higher infection-related mortality rates were seen in people with T1DM compared with those with T2DM for all infection types, even after accounting for age. Hyperglycaemia is thought to be a driver of infection amongst people with diabetes mellitus (see below)73, which might explain the higher SMRs amongst people with T1DM, in whom hyperglycaemia is typically more severe, than in those with T2DM. The highest SMRs were seen for osteomyelitis, and SMRs for septicaemia and pneumonia were also greater than 1.0 for both types of diabetes mellitus compared with those without diabetes mellitus.
Post-operative infection
Post-operative infection is also an important complication of diabetes mellitus. In a meta-analysis, diabetes mellitus was found to be associated with an OR of 1.77 (95% CI 1.13–2.78) for surgical site infection across studies that adjusted for confounding factors74 (Table 2). The effect size appears to be greatest after cardiac procedures, and one US study of patients undergoing coronary artery bypass grafting found diabetes mellitus to be an independent predictor of surgical site infection, with an OR of 4.71 (95% CI 2.39–9.28) compared with those without diabetes mellitus75. Risks of infection of more than threefold were reported in some studies of gynaecological76 and spinal surgery77 in people with diabetes mellitus compared with those without diabetes mellitus. Increased risks of infection among people with diabetes mellitus were also observed in studies of colorectal and breast surgery and arthroplasty, suggesting that the association between diabetes mellitus and post-operative infection is present across a wide range of types of surgery74.
Respiratory infections
The incidence of hospitalizations due to respiratory infections among people with diabetes mellitus was increasing substantially even before the onset of the coronavirus disease 2019 (COVID-19) pandemic, probably owing to increased life expectancy in these patients as well as an increased likelihood of them being hospitalized for conditions such as respiratory infections, which occur mostly in older age12. This rising burden of respiratory infection, in combination with the rising prevalence of diabetes mellitus, highlights the importance of addressing the emerging complications of diabetes mellitus to minimize impacts on health-care systems in current and future global epidemics.
COVID-19
Although diabetes mellitus does not appear to increase the risk of becoming infected with COVID-19 (ref.78), various population-based studies have reported increased risks of COVID-19 complications among people with diabetes mellitus. In a study of the total Scottish population, people with diabetes mellitus were found to have an increased risk of fatal or critical care unit-treated COVID-19, with an adjusted OR of 1.40 (95% CI 1.30–1.50) compared with those without diabetes mellitus79 (Table 2). The risk was particularly high for those with T1DM (OR 2.40, 95% CI 1.82–3.16)79. Both T1DM and T2DM have been linked to a more than twofold increased risk of hospitalization with COVID-19 in a large Swedish cohort study80. In South Korean studies, T2DM was linked to intensive care unit admission among patients with COVID-19 infection81, and diabetes mellitus (either T1DM or T2DM) was linked to a requirement for ventilation and oxygen therapy82 in patients with COVID-19. Diabetes mellitus appears to be the primary predisposing factor for opportunistic infection with mucormycosis in individuals with COVID-19 (ref.83). The evidence for diabetes mellitus as a risk factor for post-COVID-19 syndrome is inconclusive84,85. Interestingly, an increase in the incidence of T1DM during the COVID-19 pandemic has been reported in several countries/regions86, and some data suggest an increased risk of T1DM after COVID-19 infection87, but the evidence regarding a causal effect is inconclusive.
Pneumonia, MERS, SARS and H1N1 influenza
The data regarding diabetes mellitus and COVID-19 are consistent with the published literature regarding other respiratory infections, such as pneumonia, for which diabetes mellitus has been shown to increase the risk of hospitalization88 and mortality88, with similar effect sizes to those seen for COVID-19, compared with no diabetes mellitus. Diabetes mellitus has also been also linked to adverse outcomes in people with Middle East respiratory syndrome (MERS), SARS and H1N1 influenza89–92, suggesting that mechanisms specific to COVID-19 are unlikely to be responsible for the relationship between diabetes mellitus and COVID-19. Unlike the case for COVID-19, there is evidence that people with diabetes mellitus are at increased risk of developing certain other respiratory infections, namely pneumonia93 and possibly also MERS94.
Potential aetiological mechanisms
The mechanisms that might link diabetes mellitus and infection include a reduced T cell response, reduced neutrophil function and disorders of humoral immunity.
Mononuclear cells and monocytes of individuals with diabetes mellitus secrete less IL-1 and IL-6 than the same cells from people without diabetes mellitus95. The release of IL-1 and IL-6 by T cells and other cell types in response to infection has been implicated in the response to several viral infections96. Thus, the reduced secretion of these cytokines in patients with diabetes mellitus might be associated with the poorer responses to infection observed among these patients compared with people without diabetes mellitus.
In the context of neutrophil function, hyperglycaemic states might give rise to reductions in the mobilization of polymorphonuclear leukocytes, phagocytic activity and chemotaxis97, resulting in a decreased immune response to infection. Additionally, increased levels of glucose in monocytes isolated from patients with obesity and/or diabetes mellitus have been found to promote viral replication in these cells, as well as to enhance the expression of several cytokines, including pro-inflammatory cytokines that are associated with the COVID-19 ‘cytokine storm’; furthermore, glycolysis was found to sustain the SARS coronavirus 2 (SARS-CoV-2)-induced monocyte response and viral replication98.
Elevated glucose levels in people with diabetes mellitus are also associated with an increase in glycation, which, by promoting a change in the structure and/or function of several proteins and lipids, is responsible for many of the complications of diabetes mellitus99. In people with diabetes mellitus, antibodies can become glycated, a process that is thought to impair their biological function100. Although the clinical relevance of this impairment is not clear, it could potentially explain the results of an Israeli study that reported reduced COVID-19 vaccine effectiveness among people with T2DM compared with those without T2DM101.
Diabetes mellitus and liver disease
Nonalcoholic fatty liver disease
The consequences of nonalcoholic fatty liver disease (NAFLD) make it important to recognize the burden of this disease among people with diabetes mellitus. NAFLD and nonalcoholic steatohepatitis (NASH; an advanced form of NAFLD) are major causes of liver transplantation in the general population. In the USA, NASH accounted for 19% of liver transplantations in 2016 — second only to alcoholic liver disease, which was the cause of 24% of transplantations102. In Australia and New Zealand, NAFLD was the primary diagnosis in 9% of liver transplant recipients in 2019, only slightly below the figure for alcoholic cirrhosis of 13%103. In Europe, NASH increased as the reason for transplantations from 1% in 2002 to more than 8% in 2016, in parallel with the rising prevalence of diabetes mellitus104.
NAFLD is highly prevalent among people with T2DM. In a systematic review of 80 studies across 20 countries/regions, the prevalence of NAFLD among 49,419 people with T2DM was 56%105, while the global prevalence of NAFLD in the general population is estimated to be 25%106. In a Chinese cohort study of 512,891 adults, diabetes mellitus was associated with an adjusted HR of 1.76 (95% CI 1.47–2.16) for NAFLD compared with no diabetes mellitus107 (Table 3). Another smaller longitudinal Chinese study also reported an increased risk of developing NAFLD among those with T2DM compared with those without T2DM108. However, most evidence regarding the association between NAFLD and diabetes mellitus is from cross-sectional studies109–111.
Table 3.
Summary of original studies reporting risk of liver disease associated with diabetes mellitus
| Study | Diabetes mellitus type | Study type included (n) | Outcome | Risk associated with diabetes mellitus (95% confidence interval) |
|---|---|---|---|---|
| Pang et al. (2018)107 | All | Cohort (512,891) | NAFLD | HR 1.76 (1.47–2.16) |
| Li et al. (2017)108 | T2DM | Cohort (18,111) | NAFLD | OR 1.40 (1.22–1.62) |
| Loomba et al. (2012)113 | All | Cross-sectional (1,069) | NASH | OR 1.93 (1.37–2.73) |
| Liver fibrosis | OR 3.31 (2.26–4.85) |
HR, hazard ratio; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OR, odds ratio; T2DM, type 2 diabetes mellitus.
NASH and fibrosis
Diabetes mellitus appears to enhance the risk of NAFLD complications, including NASH and fibrosis. An analysis of 892 people with NAFLD and T2DM across 10 studies showed that the prevalence of NASH was 37% (ref.105); figures for the prevalence of NASH in the general population with NAFLD vary greatly across different study populations, ranging from 16% to 68%112. Amongst 439 people with T2DM and NAFLD in seven studies, 17% had advanced fibrosis105. An analysis of 1,069 people with NAFLD in a US study found that diabetes mellitus was an independent predictor for NASH (OR 1.93, 95% CI 1.37–2.73) and fibrosis (3.31, 95% CI 2.26–4.85)113.
Bidirectional relationship between diabetes mellitus and liver disease
The relationship between diabetes mellitus and NAFLD is bidirectional, as NAFLD is associated with an increased risk of developing T2DM114. There is also a notable bidirectional relationship between diabetes mellitus and liver cirrhosis. The prevalence of diabetes mellitus in people with liver cirrhosis has been reported as 20–63%, depending on the severity of liver damage, aetiology and diagnostic criteria115. In an Italian study of 401 participants with cirrhosis, 63% of those with decompensated liver disease had diabetes mellitus compared with 10% of those with well-compensated liver disease116, suggesting that diabetes mellitus is more common in severe cases of liver damage. The association between diabetes mellitus and cirrhosis also varies according to the cause of liver disease. In a US study of 204 people with cirrhosis, the prevalence of diabetes mellitus was 25% among those with cirrhosis caused by hepatitis C virus, 19% among those with cirrhosis from alcoholic liver disease and only 1% among those with cirrhosis due to cholestatic liver disease117. Among the causes of cirrhosis, haemochromatosis has the strongest association with diabetes mellitus, with diabetes mellitus mainly resulting from the iron deposition that is characteristic of haemochromatosis118.
Potential aetiological mechanisms
Several factors have been implicated in the aetiology of liver disease in people with diabetes mellitus, with insulin resistance being the most notable119.
Insulin resistance
Insulin resistance causes lipolysis, thereby increasing the circulating levels of free fatty acids, which are then taken up by the liver as an energy source120. These fatty acids overload the mitochondrial β-oxidation system in the liver, resulting in the accumulation of fatty acids and, consequently, NAFLD121. Of those individuals with NAFLD, 2–3% develop hepatic inflammation, necrosis and fibrosis, which are the hallmarks of NASH122. The exact mechanisms leading to steatohepatitis are unclear, although dysregulated peripheral lipid metabolism appears to be important14.
Ectopic adipose deposition
Excessive or ectopic deposition of adipose tissue around the viscera and in the liver might be an important mechanism underlying both T2DM and liver disease, particularly NAFLD123. Dysfunction of long-term adipose storage in white adipose tissue is known to lead to ectopic adipose deposition in the liver. In this state, increased levels of fatty acyl-coenzyme As, the activated form of fatty acids, might lead to organ dysfunction, including NAFLD124. Ectopic adipose deposition leading to organ-specific insulin resistance has emerged as a major hypothesis for the pathophysiological basis of T2DM, and ectopic adipose in the pancreas could contribute to β-cell dysfunction and, thus, the development of T2DM125.
Diabetes mellitus and affective disorders
Depression
The prevalence of depression appears to be high among people with diabetes mellitus. The strongest evidence for an association comes from a systematic review of 147 studies among people with T2DM, which revealed a mean prevalence of depression of 28%126, while the global prevalence of depression in the general population is estimated at around 13%127. For T1DM, a systematic review reported a pooled prevalence of depression of 12% compared with only 3% in those without T1DM128. The risk of depression among people with diabetes mellitus appears to be roughly 25% greater than the risk in the general population, with consistent findings across several meta-analyses (Table 4). A 2013 study found an adjusted RR of 1.25 (95% CI 1.10–1.44) for incident depression among people with diabetes mellitus compared with those without diabetes mellitus129. Another systematic review of people with T2DM reported a near identical effect size130.
Table 4.
Summary of major systematic reviews reporting risk of affective disorders, cognitive disability and functional disability associated with diabetes mellitus
| Author | Diabetes mellitus type | Study type included (n) | Outcome | Risk associated with diabetes mellitus (95% confidence interval) |
|---|---|---|---|---|
| Rotella et al. (2013)129 | All | Cohort, cross-sectional (497,223) | Depression | HR 1.25 (1.10–1.44) |
| Nouwen et al. (2019)194 | T2DM | Cohort, cross-sectional (48,808) | Depression | RR 1.24 (1.09–1.40) |
| Smith et al. (2013)132 | All | Cohort, cross-sectional (12,626) | Anxiety disorders | OR 1.20 (1.10–1.31) |
| Anxiety symptoms | OR 1.48 (1.02–1.93) | |||
| Lu et al. (2009)154 | All | Cohort (23,257) | Vascular dementia | RR 2.38 (1.79–3.18) |
| Alzheimer disease | RR 1.39 (1.16–1.66) | |||
| Cheng et al. (2012)155 | All | Cohort (44,714) | Vascular dementia | RR 2.48 (2.08–2.96) |
| Alzheimer disease | RR 1.46 (1.20–1.77) | |||
| All-cause dementia | RR 1.51 (1.31–1.74) | |||
| MCI | RR 1.21 (1.02–1.45) | |||
| Li et al. (2019)156 | All | Cohort (1,257,144a) | All-cause dementia | RR 1.69 (1.38–2.07) |
| Xue et al. (2019)157 | All | Cohort, cross-sectional (4,349,111) | All-cause dementia | RR 1.43 (1.33–1.53) |
| Pal et al. (2018)158 | T2DM | Cohort (6,865) | Progression to dementia in MCI | OR 1.53 (1.20–1.97) |
| Wong et al. (2013)173 | All | Cohort, cross-sectional (162,534a) | Mobility disability | OR 1.51 (1.38–1.64) |
| ADL disability | OR 1.82 (1.40–2.36) | |||
| IADL disability | OR 1.65 (1.55–1.74) | |||
| Yang et al. (2016)172 | All age ≥60 years | Cohort (14,685) | Falls | RR 1.64 (1.27–2.11) |
ADL, activities of daily living; HR, hazard ratio; IADL, independent activities of daily living; MCI, mild cognitive impairment; OR, odds ratio; RR, relative risk. aTotal number of participants obtained through sum of individual study cohort sizes listed in tables or otherwise.
Anxiety and eating disorders
Evidence exists for an association of diabetes mellitus with anxiety, and of T1DM with eating disorders. In a systematic review involving 2,584 individuals with diabetes mellitus, a prevalence of 14% was found for generalized anxiety disorder and 40% for anxiety symptoms, whereas the prevalence of generalized anxiety disorder in the general population is estimated as only 3–4%131. People with diabetes mellitus had an increased risk of anxiety disorders (OR 1.20, 95% CI 1.10–1.31) and anxiety symptoms (OR 1.48, 95% CI 1.02–1.93) compared with those without diabetes mellitus in a meta-analysis132 (Table 4), although these findings were based on cross-sectional data. Across 13 studies, 7% of adolescents with T1DM were found to have eating disorders, compared with 3% of peers without diabetes mellitus133.
Broader psychological impacts
There is a substantial literature on a broad range of psychological impacts of diabetes mellitus. Social stigma134 can have profound impacts on the quality of life of not only people with diabetes mellitus, but their families and carers, too135. In a systematic review, diabetes mellitus distress was found to affect around one-third of adolescents with T1DM, which was consistent with the results of studies of adults with diabetes mellitus136. Diabetes mellitus burnout appears to be a distinct concept, and is characterized by exhaustion and detachment, accompanied by the experience of a loss of control over diabetes mellitus137.
Potential aetiological mechanisms
Diabetes mellitus and depression appear to have common biological origins. Activation of the innate immune system and acute-phase inflammation contribute to the pathogenesis of T2DM — increased levels of inflammatory cytokines predict the onset of T2DM138 — and there is growing evidence implicating cytokine-mediated inflammation in people with depression in the absence of diabetes mellitus139. Dysregulation of the hypothalamic–pituitary–adrenal axis is another potential biological mechanism linking depression and diabetes mellitus140. There have been numerous reports of hippocampal atrophy, which might contribute to chronic activation of the hypothalamic–pituitary–adrenal axis, in individuals with T2DM as well as those with depression141,142. A meta-analysis found that, although hypertension modified global cerebral atrophy in those with T2DM, it had no effect on hippocampal atrophy143. This suggests that, although global cerebral atrophy in individuals with T2DM might be driven by atherosclerotic disease, hippocampal atrophy is an independent effect that provides a common neuropathological aetiology for the comorbidity of T2DM with depression. There is a lack of relevant information regarding the potential aetiological mechanisms that link diabetes to other affective disorders.
Diabetes mellitus and sleep disturbance
Obstructive sleep apnoea
Obstructive sleep apnoea (OSA) is highly prevalent among people with diabetes mellitus. In a systematic review of 41 studies of adults with diabetes mellitus, the prevalence of OSA was found to be 60%144, whereas reports for OSA prevalence in the general population range from 9% to 38%145. In a UK study of 1,656,739 participants, T2DM was associated with an IRR for OSA of 1.48 (95% CI 1.42–1.55) compared with no T2DM146. A population-based US study reported a HR of 1.53 (95% CI 1.32–1.77) for OSA in people with T2DM compared with those without diabetes mellitus147. However, the association in this latter report was attenuated after adjustment for BMI and waist circumference (1.08, 95% CI 1.00–1.16), suggesting that the excess risk of OSA among people with diabetes mellitus might be mainly explained by the comorbidity of obesity. Although most studies on OSA have focused on T2DM, a meta-analysis of people with T1DM revealed a similar prevalence of 52%148; however, this meta-analysis was limited to small studies. The association between T2DM and OSA is bidirectional: the severity of OSA was shown to be positively associated with the incidence of T2DM, independent of adiposity, in a large US cohort study149.
Potential aetiological mechanisms
The mechanism by which T2DM might increase the risk of developing OSA is thought to involve dysregulation of the autonomic nervous system leading to sleep-disordered breathing150. Conversely, the specific mechanism behind OSA as a causative factor for T2DM remains poorly understood. It has been suggested that OSA is able to induce insulin resistance151,152 and is a risk factor for the development of glucose intolerance152. However, once T2DM has developed, there is no clear evidence that OSA worsens glycaemic control, as an RCT of people with T2DM found that treating OSA had no effect on glycaemic control153.
Diabetes mellitus and cognitive disability
Dementia and cognitive impairment
Dementia is emerging as a major cause of mortality in both individuals with diabetes mellitus and the general population, and is now the leading cause of death in some countries/regions9. However, compared with the general population, diabetes mellitus increases the risk of dementia, particularly vascular dementia. The association is supported by several systematic reviews, including one of eight population-based studies with more than 23,000 people, which found SRRs of 2.38 (95% CI 1.79–3.18) for vascular dementia and 1.39 (95% CI 1.16–1.66) for Alzheimer disease comparing people with diabetes mellitus with those without diabetes mellitus154 (Table 4). Similar results, as well as a RR of 1.21 (95% CI 1.02–1.45) for mild cognitive impairment (MCI), were reported across 19 population-based studies of 44,714 people, 6,184 of whom had diabetes mellitus155. Two meta-analyses of prospective cohort studies have shown increased risks of all-cause dementia in people with diabetes mellitus compared with those without diabetes mellitus156,157, and T2DM has been shown to increase progression to dementia in people with MCI158.
The boundaries between Alzheimer disease and vascular dementia remain controversial, and these conditions are often difficult to differentiate clinically159. Consequently, vascular dementia might have been misdiagnosed as Alzheimer disease in some studies investigating diabetes mellitus and dementia, resulting in an overestimation of the effect size of the association between diabetes mellitus and Alzheimer disease. Although a cohort study found a significant association between diabetes mellitus and Alzheimer disease using imaging160, autopsy studies have failed to uncover an association between diabetes mellitus and Alzheimer disease pathology161,162, suggesting that vascular mechanisms are the key driver of cognitive decline in people with diabetes mellitus.
Another important finding is a 45% prevalence of MCI among people with T2DM in a meta-analysis, compared with a prevalence of 3–22% reported for the general population163. Notably, however, the prevalence of MCI in individuals with T2DM was similar in people younger than 60 years (46%) and those older than 60 years (44%), which is at odds with previous research suggesting that MCI is most common in older people, particularly those aged more than 65 years164 However, another meta-analysis found cognitive decline in people with T2DM who are younger than 65 years165, suggesting that a burden of cognitive disease exists among younger people with diabetes mellitus.
Potential aetiological mechanisms
Although there is solid evidence that links diabetes mellitus to cognitive disability, our understanding of the underlying mechanisms is incomplete. Mouse models suggest a strong association between hyperglycaemia, the advanced glycation end products glyoxal and methylglyoxal, enhanced blood–brain barrier (BBB) permeability and cognitive dysfunction in both T1DM and T2DM166. The BBB reduces the access of neurotoxic compounds and pathogens to the brain and sustains brain homeostasis, so disruption to the BBB can result in cognitive dysfunction through dysregulation of transport of molecules between the peripheral circulation and the brain167. There appears to be a continuous relationship between glycaemia and cognition, with associations found between even high-normal blood levels of glucose and cognitive decline168. Another hypothetical mechanism involves a key role for impaired insulin signalling in the pathogenesis of Alzheimer disease. Brain tissue obtained post mortem from individuals with Alzheimer disease showed extensive abnormalities in insulin and insulin-like growth factor signalling mechanisms compared with control brain tissue169. Although the synthesis of insulin-like growth factors occurred normally in people with Alzheimer disease, their expression levels were markedly reduced, which led to the subsequent proposal of the term ‘type 3 diabetes’ to characterize Alzheimer disease.
Diabetes mellitus and disability
Functional disability
Disability (defined as a difficulty in functioning in one or more life domains as experienced by an individual with a health condition in interaction with contextual factors)170 is highly prevalent in people with diabetes mellitus. In a systematic review, lower-body functional limitation was found to be the most prevalent disability (47–84%) among people with diabetes mellitus171 The prevalence of difficulties with activities of daily living among people with diabetes mellitus ranged from 12% to 55%, although most studies were conducted exclusively in individuals aged 60 years and above, so the results are not generalizable to younger age groups. A systematic review showed a significant association between diabetes mellitus and falls in adults aged 60 years and above172. A 2013 meta-analysis173 showed an increased risk of mobility disability, activities of daily living disability and independent activities of daily living disability among people with diabetes mellitus compared with those without diabetes mellitus (Table 4). Although this analysis included cross-sectional data, results were consistent across longitudinal and cross-sectional studies, suggesting little effect of reverse causality. However, people with functional disabilities that limit mobility (for example, people with osteoarthritis or who have had a stroke) might be more prone to developing diabetes mellitus owing to physical inactivity174.
Workplace productivity
Decreased productivity while at work, increased time off work and early dropout from the workforce175 are all associated with diabetes mellitus, probably partly due to functional disability, and possibly also to comorbidities such as obesity and physical inactivity176. Given that young-onset diabetes is becoming more common, and most people with diabetes mellitus in middle-income countries/regions are less than 65 years old177, a pandemic of diabetes mellitus-related work disability among a middle-aged population does not bode well for the economies of these regions.
Potential aetiological mechanisms
The mechanisms by which diabetes mellitus leads to functional disability remain unclear. One suggestion is that hyperglycaemia leads to systemic inflammation, which is one component of a multifactorial process that results in disability154. The rapid loss of skeletal muscle strength and quality seen among people with diabetes mellitus might be another cause of functional disability178 (Box 1). In addition, complications of diabetes mellitus, including stroke, peripheral neuropathy and cardiac dysfunction, can obviously directly cause disability179.
Box 1 Diabetes mellitus and skeletal muscle atrophy.
Individuals with diabetes mellitus exhibit skeletal muscle atrophy that is typically mild in middle age and becomes more substantial with increasing age.
This muscle loss leads to reduced strength and functional capacity and, ultimately, increased mortality.
Skeletal muscle atrophy results from a negative balance between the rate of synthesis and degradation of contractile proteins, which occurs in response to disuse, ageing and chronic diseases such as diabetes mellitus.
Degradation of muscle proteins is more rapid in diabetes mellitus, and muscle protein synthesis has also been reported to be decreased.
Proposed mechanisms underlying skeletal muscle atrophy include systemic inflammation (affecting both protein synthesis and degradation), dysregulation of muscle protein anabolism and lipotoxicity.
Mouse models have also revealed a key role for the WWP1/KLF15 pathway, mediated by hyperglycaemia, in the pathogenesis of muscle atrophy.
Diabetes management and control
Although a detailed discussion of the impacts of anti-diabetes mellitus medications and glucose control on emerging complications is beyond the scope of this Review, their potential effect on these complications must be acknowledged.
Medications
Anti-diabetes mellitus medications and cancer
In the case of cancer as an emerging complication, the use of medications for diabetes mellitus was not controlled for in most studies of diabetes mellitus and cancer and might therefore be a confounding factor. People taking metformin have a lower cancer risk than those not taking metformin180. However, this association is mainly accounted for by other factors. For example, metformin is less likely to be administered to people with diabetes mellitus who have kidney disease181, who typically have longer duration diabetes mellitus, which increases cancer risk. A review of observational studies into the association between metformin and cancer found that many studies reporting significant reductions in cancer incidence or mortality associated with metformin were affected by immortal time bias and other time-related biases, casting doubt on the ability of metformin to reduce cancer mortality182. Notably, the use of insulin was associated with an increased risk of several cancers in a meta-analysis183. However, in an RCT of more than 12,000 people with dysglycaemia, randomization to insulin glargine (compared with standard care) did not increase cancer incidence184. Furthermore, cancer rates in people with T1DM and T2DM do not appear to vary greatly, despite substantial differences in insulin use between people with these types of diabetes mellitus.
Anti-diabetes mellitus medications and other emerging complications
Anti-diabetes medications appear to affect the onset and development of some other emerging complications of diabetes mellitus. Results from RCTs suggest that metformin might confer therapeutic effects against depression185, and its use was associated with reduced dementia incidence in a systematic review186. In an RCT investigating a potential association between metformin and NAFLD, no improvement in NAFLD histology was found among people using metformin compared with those given placebo187. An RCT reported benefits of treatment with the glucagon-like peptide 1 receptor agonist dulaglutide on cognitive function in a post hoc analysis188, suggesting that trials designed specifically to test the effects of dulaglutide on cognitive function should be undertaken.
Glucose control
Another important consideration is glycaemic control, which appears to have variable effects on emerging complications. A meta-analysis found no association of glycaemic control with cancer risk among those with diabetes mellitus189, and an RCT found no effect of intensive glucose lowering on cognitive function in people with T2DM190. However, glycaemic control has been associated with improved physical function191, decreased COVID-19 mortality192 and a decreased risk of NAFLD193 in observational studies of patients with diabetes mellitus; notably, no RCTs have yet confirmed these associations.
Conclusions
With advances in the management of diabetes mellitus and associated increased life expectancy, the face of diabetes mellitus complications is changing. As the management of glycaemia and traditional complications of diabetes mellitus is optimized, we are beginning instead to see deleterious effects of diabetes mellitus on the liver, brain and other organs. Given the substantial burden and risk of these emerging complications, future clinical and public health strategies should be updated accordingly. There is a need to increase the awareness of emerging complications among primary care physicians at the frontline of diabetes mellitus care, and a place for screening for conditions such as depression, liver disease and cancers in diabetes mellitus guidelines should be considered. Clinical care for older people with diabetes mellitus should target physical activity, particularly strength-based activity, to reduce the risk of functional disability in ageing populations. Ongoing high-quality surveillance of diabetes mellitus outcomes is imperative to ensure we know where the main burdens lie. Given the growing burden of these emerging complications, the traditional management of diabetes mellitus might need to broaden its horizons.
Acknowledgements
D.T. is supported by an Australian Government Research Training Program (RTP) Scholarship and Monash Graduate Excellence Scholarship. J.E.S. is supported by a National Health and Medical Research Council Investigator Grant. D.J.M. is supported by a National Health and Medical Research Council Senior Research Fellowship.
Glossary
- Activities of daily living
Fundamental skills required to independently care for oneself such as eating, bathing and mobility.
- Independent activities of daily living
Activities that allow an individual to live independently in a community.
- Immortal time bias
The error in estimating the association between an exposure and an outcome that results from misclassification or exclusion of time intervals.
Author contributions
D.T. researched data for the article and wrote the article. J.E.S and D.J.M. contributed substantially to discussion of the content. D.T., J.E.S. and D.J.M reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Emily Gallagher, Norbert Stefan and Assaad Eid for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Jonathan E. Shaw and Dianna J. Magliano.
References
- 1.Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin. Diabetes. 2008;26:77–82. doi: 10.2337/diaclin.26.2.77. [DOI] [Google Scholar]
- 3.Shah AD, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertoni AG, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW, et al. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4:537–547. doi: 10.1016/S2213-8587(16)30010-9. [DOI] [PubMed] [Google Scholar]
- 7.Gregg EW, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391:2430–2440. doi: 10.1016/S0140-6736(18)30314-3. [DOI] [PubMed] [Google Scholar]
- 8.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000–2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care. 2016;39:1018–1026. doi: 10.2337/dc15-2308. [DOI] [PubMed] [Google Scholar]
- 9.Pearson-Stuttard J, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9:165–173. doi: 10.1016/S2213-8587(20)30431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson-Stuttard J, Buckley J, Cicek M, Gregg EW. The changing nature of mortality and morbidity in patients with diabetes. Endocrinol. Metab. Clin. North Am. 2021;50:357–368. doi: 10.1016/j.ecl.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Pearson-Stuttard J, et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2022;10:46–57. doi: 10.1016/S2213-8587(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4:148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 14.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–743. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39:300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 17.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 18.Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–1107. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- 19.Pearson-Stuttard J, Ezzati M, Gregg EW. Multimorbidity — a defining challenge for health systems. Lancet Public. Health. 2019;4:e599–e600. doi: 10.1016/S2468-2667(19)30222-1. [DOI] [PubMed] [Google Scholar]
- 20.Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J. Clin. Oncol. 2011;29:106–117. doi: 10.1200/JCO.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 21.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J. Clin. Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross CP, McAvay GJ, Guo Z, Tinetti ME. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109:2410–2419. doi: 10.1002/cncr.22726. [DOI] [PubMed] [Google Scholar]
- 23.Harding JL, et al. All-cause cancer mortality over 15 years in multi-ethnic Mauritius: the impact of diabetes and intermediate forms of glucose tolerance. Int. J. Cancer. 2012;131:2385–2393. doi: 10.1002/ijc.27503. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int. J. Cancer. 2012;130:1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Desbois AC, Cacoub P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: a contemporary review. World J. Gastroenterol. 2017;23:1697–1711. doi: 10.3748/wjg.v23.i9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huxley R, Ansary-Moghaddam A, Berrington De González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gullo L, et al. Diabetes and the risk of pancreatic cancer. N. Engl. J. Med. 1994;331:81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 29.Gershell L. Type 2 diabetes market. Nat. Rev. Drug Discov. 2005;4:367–368. doi: 10.1038/nrd1723. [DOI] [PubMed] [Google Scholar]
- 30.Carstensen B, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59:980–988. doi: 10.1007/s00125-016-3884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y, et al. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 2011;26:863–876. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 32.De Bruijn KMJ, et al. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br. J. Surg. 2013;100:1421–1429. doi: 10.1002/bjs.9229. [DOI] [PubMed] [Google Scholar]
- 33.Deng L, Gui Z, Zhao L, Wang J, Shen L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig. Dis. Sci. 2012;57:1576–1585. doi: 10.1007/s10620-012-2055-1. [DOI] [PubMed] [Google Scholar]
- 34.Liao C, Zhang D, Mungo C, Andrew Tompkins D, Zeidan AM. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol. Oncol. 2014;135:163–171. doi: 10.1016/j.ygyno.2014.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saed L, et al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer. 2019;19:527. doi: 10.1186/s12885-019-5748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 37.Anothaisintawee T, et al. Risk factors of breast cancer: a systematic review and meta-analysis. Asia-Pac. J. Public Health. 2013;25:368–387. doi: 10.1177/1010539513488795. [DOI] [PubMed] [Google Scholar]
- 38.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int. J. Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 39.Boyle P, et al. Diabetes and breast cancer risk: a meta-analysis. Br. J. Cancer. 2012;107:1608–1617. doi: 10.1038/bjc.2012.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinaldi S, et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int. J. Cancer. 2006;118:2832–2839. doi: 10.1002/ijc.21730. [DOI] [PubMed] [Google Scholar]
- 41.Michels KB, et al. Type 2 diabetes and subsequent incidence of breast cancer in the nurses’ health study. Diabetes Care. 2003;26:1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 42.Bronsveld HK, et al. Diabetes and breast cancer subtypes. PLoS ONE. 2017;12:e0170084. doi: 10.1371/journal.pone.0170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang D, Li N, Xi Y, Zhao Y, Wang T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 2017;130:43–52. doi: 10.1016/j.diabres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Weng L, Wang L, Zhang J, Wang B, Liu H. Association between diabetes mellitus and subsequent ovarian cancer in women: a systematic review and meta-analysis of cohort studies. Medicine. 2017;96:e6396. doi: 10.1097/MD.0000000000006396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Zhong L, Xu B, Chen M, Huang H. Diabetes mellitus and the risk of ovarian cancer: a systematic review and meta-analysis of cohort and case-control studies. BMJ Open. 2020;10:e040137. doi: 10.1136/bmjopen-2020-040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JY, et al. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int. J. Gynecol. Cancer. 2013;23:402–412. doi: 10.1097/IGC.0b013e31828189b2. [DOI] [PubMed] [Google Scholar]
- 47.Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47:1071–1078. doi: 10.1007/s00125-004-1415-6. [DOI] [PubMed] [Google Scholar]
- 48.Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 2013;104:9–14. doi: 10.1111/cas.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long XJ, Lin S, Sun YN, Zheng ZF. Diabetes mellitus and prostate cancer risk in Asian countries: a meta-analysis. Asian Pac. J. Cancer Preven. 2012;13:4097–4100. doi: 10.7314/APJCP.2012.13.8.4097. [DOI] [PubMed] [Google Scholar]
- 50.Rhee EJ. Diabetes in Asians. Endocrinol. Metab. 2015;30:263–269. doi: 10.3803/EnM.2015.30.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bensimon L, Yin H, Suissa S, Pollak MN, Azoulay L. Type 2 diabetes and the risk of mortality among patients with prostate cancer. Cancer Causes Control. 2014;25:329–338. doi: 10.1007/s10552-013-0334-6. [DOI] [PubMed] [Google Scholar]
- 52.Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. 2011;54:2263–2271. doi: 10.1007/s00125-011-2242-1. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JA, et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55:1607–1618. doi: 10.1007/s00125-012-2525-1. [DOI] [PubMed] [Google Scholar]
- 54.Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38:264–270. doi: 10.2337/dc14-1996. [DOI] [PubMed] [Google Scholar]
- 55.Pearson-Stuttard J, et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6:e6–e15. doi: 10.1016/S2213-8587(18)30150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54:1013–1018. doi: 10.1007/s00125-011-2051-6. [DOI] [PubMed] [Google Scholar]
- 57.Xu X, et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS ONE. 2013;8:e58079. doi: 10.1371/journal.pone.0058079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong IY, et al. Association between diabetes and haematological malignancies: a population-based study. Diabetologia. 2021;64:540–551. doi: 10.1007/s00125-020-05338-7. [DOI] [PubMed] [Google Scholar]
- 59.Giovannucci E, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab. Res. Rev. 2009;25:41–49. doi: 10.1002/dmrr.912. [DOI] [PubMed] [Google Scholar]
- 61.Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiology. 2019;34:198–215. doi: 10.1152/physiol.00048.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorenzi M, Montisano DF, Toledo S, Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J. Clin. Invest. 1986;77:322–325. doi: 10.1172/JCI112295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robertson R, Zhou H, Zhang T, Harmon JS. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem. Biophys. 2007;48:139–146. doi: 10.1007/s12013-007-0026-5. [DOI] [PubMed] [Google Scholar]
- 64.Turturro F, Friday E, Welbourne T. Hyperglycemia regulates thioredoxin-ROS activity through induction of thioredoxin-interacting protein (TXNIP) in metastatic breast cancer-derived cells MDA-MB-231. BMC Cancer. 2007;7:96. doi: 10.1186/1471-2407-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y, Liu Y, Dong Y, Vadgama J. Diabetes-associated dysregulated cytokines and cancer. Integr. Cancer Sci. Ther. 2016;3:370–378. doi: 10.15761/ICST.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 67.Huang X, Liu G, Guo J, Su ZQ. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018;14:1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y, et al. Metformin is associated with reduced cell proliferation in human endometrial cancer by inbibiting PI3K/AKT/mTOR signaling. Gynecol. Endocrinol. 2018;34:428–432. doi: 10.1080/09513590.2017.1409714. [DOI] [PubMed] [Google Scholar]
- 69.Knapp S. Diabetes and infection: is there a link?-A mini-review. Gerontology. 2013;59:99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- 70.Fang M, et al. Diabetes and the risk of hospitalisation for infection: the atherosclerosis risk in communities (ARIC) study. Diabetologia. 2021;64:2458–2465. doi: 10.1007/s00125-021-05522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tseng C-H. Metformin use is associated with a reduced risk of acute appendicitis in Taiwanese patients with type 2 diabetes mellitus. Sci. Rep. 2021;11:12400. doi: 10.1038/s41598-021-91902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luk AOY, et al. Temporal trends in rates of infection-related hospitalisations in Hong Kong people with and without diabetes, 2001–2016: a retrospective study. Diabetologia. 2021;64:109–118. doi: 10.1007/s00125-020-05286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magliano DJ, et al. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care. 2015;38:1274–1280. doi: 10.2337/dc14-2820. [DOI] [PubMed] [Google Scholar]
- 74.Martin ET, et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect. Control. Hosp. Epidemiol. 2016;37:88–99. doi: 10.1017/ice.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trussell J, et al. Impact of a patient care pathway protocol on surgical site infection rates in cardiothoracic surgery patients. Am. J. Surg. 2008;196:883–889. doi: 10.1016/j.amjsurg.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 76.Coleman JS, et al. Surgical site infections after hysterectomy among HIV-infected women in the HAART era: a single institution’s experience from 1999–2012. Am. J. Obstet. Gynecol. 2014;210:117.e111–117.e117. doi: 10.1016/j.ajog.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 77.Friedman ND, Sexton DJ, Connelly SM, Kaye KS. Risk factors for surgical site infection complicating laminectomy. Infect. Control. Hosp. Epidemiol. 2007;28:1060–1065. doi: 10.1086/519864. [DOI] [PubMed] [Google Scholar]
- 78.Apicella M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGurnaghan SJ, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9:82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rawshani A, et al. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: a nationwide retrospective cohort study. Lancet Reg. Health Eur. 2021;4:100105. doi: 10.1016/j.lanepe.2021.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.You JH, et al. Clinical outcomes of COVID-19 patients with type 2 diabetes: a population-based study in Korea. Endocrinol. Metab. 2020;35:901–908. doi: 10.3803/EnM.2020.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moon SJ, et al. Independent impact of diabetes on the severity of coronavirus disease 2019 in 5,307 patients in South Korea: a nationwide cohort study. Diabetes Metab. J. 2020;44:737–746. doi: 10.4093/dmj.2020.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aranjani JM, Manuel A, Razack HIA, Mathew ST. Covid-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS. Negl. Trop. Dis. 2021;15:e0009921. doi: 10.1371/journal.pntd.0009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crankson S, Pokhrel S, Anokye NK. Determinants of COVID-19-related length of hospital stays and long COVID in Ghana: a cross-sectional analysis. Int. J. Environ. Res. Public Health. 2022;19:527. doi: 10.3390/ijerph19010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bellan M, et al. Respiratory and psychophysical sequelae among patients with covid-19 four months after hospital discharge. JAMA Netw. Open. 2021;4:e2036142. doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gottesman BL, Yu J, Tanaka C, Longhurst CA, Kim JJ. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 2022;176:414–415. doi: 10.1001/jamapediatrics.2021.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barrett CE, et al. Risk for newly diagnosed diabetes <30 days after SARS-CoV-2 infection among persons aged >18 years-United States, March 1, 2020-June 28, 2021. Morb. Mortal. Wkly. Rep. 2022;71:59–65. doi: 10.15585/mmwr.mm7102e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kornum JB, et al. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care. 2008;31:1541–1545. doi: 10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsuyama R, Nishiura H, Kutsuna S, Hayakawa K, Ohmagari N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health. 2016;16:1203. doi: 10.1186/s12889-016-3881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Badawi A, Ryoo SG. Prevalence of diabetes in the 2009 influenza A (H1N1) and the middle east respiratory syndrome coronavirus: a systematic review and meta-analysis. J. Public. Health Res. 2016;5:130–138. doi: 10.4081/jphr.2016.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang JK, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 93.Ehrlich SF, Quesenberry CP, Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33:55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alraddadi BM, et al. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geerlings SE, Hoepelman AIM. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol. Med. Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 96.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front. Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N. Engl. J. Med. 1999;341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 98.Codo AC, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-dependent axis. Cell Metab. 2020;32:437–446.e435. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miyazawa T, Nakagawa K, Shimasaki S, Nagai R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42:1163–1170. doi: 10.1007/s00726-010-0772-3. [DOI] [PubMed] [Google Scholar]
- 100.Peleg AY, Weerarathna T, McCarthy JS, Davis TME. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab. Res. Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 101.Barda N, Dagan N, Balicer RD. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. reply. N. Engl. J. Med. 2021;384:1970. doi: 10.1056/NEJMc2104281. [DOI] [PubMed] [Google Scholar]
- 102.Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin. Gastroenterol. Hepatol. 2018;16:1356–1358. doi: 10.1016/j.cgh.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fink, M. & Byrne, M. Australia and New Zealand Liver and Intestinal Transplant Registry Annual Report 2019 (Melbourne, Victoria, Australia, 2019).
- 104.Haldar D, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European liver transplant registry study. J. Hepatol. 2019;71:313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Younossi ZM, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 106.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 107.Pang Y, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology. 2018;68:1308–1318. doi: 10.1002/hep.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, et al. Bidirectional association between nonalcoholic fatty liver disease and type 2 diabetes in Chinese population: evidence from the Dongfeng-Tongji cohort study. PLoS ONE. 2017;12:e0174291. doi: 10.1371/journal.pone.0174291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mansour-Ghanaei F, et al. Prevalence of non-alcoholic fatty liver disease in patients with diabetes mellitus, hyperlipidemia, obesity and polycystic ovary syndrome: a cross-sectional study in north of Iran. Diabetes Metab. Syndr. 2019;13:1591–1596. doi: 10.1016/j.dsx.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 110.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 111.Singh SP, et al. Risk factors associated with non-alcoholic fatty liver disease in Indians: a case-control study. J. Clin. Exp. Hepatol. 2015;5:295–302. doi: 10.1016/j.jceh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dufour J-F, et al. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–a targeted literature review. Endocr. Metab. Sci. 2021;3:100089. doi: 10.1016/j.endmts.2021.100089. [DOI] [Google Scholar]
- 113.Loomba R, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 115.Holstein A, Hinze S, Thießen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J. Gastroenterol. Hepatol. 2002;17:677–681. doi: 10.1046/j.1440-1746.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 116.Del Vecchio Blanco C, Gentile S, Marmo R, Carbone L, Coltorti M. Alterations of glucose metabolism in chronic liver disease. Diabetes Res. Clin. Pract. 1990;8:29–36. doi: 10.1016/0168-8227(90)90093-9. [DOI] [PubMed] [Google Scholar]
- 117.Zein NN, Abdulkarim AS, Wiesner RH, Egan KS, Persing DH. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J. Hepatol. 2000;32:209–217. doi: 10.1016/S0168-8278(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 118.Niederau C, et al. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 1985;313:1256–1262. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- 119.Larter CZ, Farrell GC. Insulin resistance, adiponectin, cytokines in NASH: which is the best target to treat? J. Hepatol. 2006;44:253–261. doi: 10.1016/j.jhep.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 120.Marchesini G, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999;107:450–455. doi: 10.1016/S0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 121.Angulo P. Medical progress: nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 122.Porepa L, Ray JG, Sanchez-Romeu P, Booth GL. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182:E526–E531. doi: 10.1503/cmaj.092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616–627. doi: 10.1016/S2213-8587(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 124.Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–324. doi: 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 125.Sattar N, Gill JMR. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:123. doi: 10.1186/s12916-014-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harding KA, et al. Depression prevalence in type 2 diabetes is not related to diabetes–depression symptom overlap but is related to symptom dimensions within patient self-report measures: a meta-analysis. Diabet. Med. 2019;36:1600–1611. doi: 10.1111/dme.14139. [DOI] [PubMed] [Google Scholar]
- 127.Lim GY, et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 2018;8:2861. doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J. Affect. Disord. 2012;142:S8–S21. doi: 10.1016/S0165-0327(12)70004-6. [DOI] [PubMed] [Google Scholar]
- 129.Rotella F, Mannucci E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res. Clin. Pract. 2013;99:98–104. doi: 10.1016/j.diabres.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 130.Nouwen A, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53:2480–2486. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes a systematic review. J. Psychosom. Res. 2002;53:1053–1060. doi: 10.1016/S0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 132.Smith KJ, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J. Psychosom. Res. 2013;74:89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 133.Young V, et al. Eating problems in adolescents with type1 diabetes: a systematic review with meta-analysis. Diabet. Med. 2013;30:189–198. doi: 10.1111/j.1464-5491.2012.03771.x. [DOI] [PubMed] [Google Scholar]
- 134.Schabert J, Browne JL, Mosely K, Speight J. Social stigma in diabetes: a framework to understand a growing problem for an increasing epidemic. Patient. 2013;6:1–10. doi: 10.1007/s40271-012-0001-0. [DOI] [PubMed] [Google Scholar]
- 135.Barnard KD, Speight J, Skinner TC. Quality of life and impact of continuous subcutaneous insulin infusion for children and their parents. Pract. Diabetes Int. 2008;25:278–283. doi: 10.1002/pdi.1280. [DOI] [Google Scholar]
- 136.Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr. Diabetes Rep. 2016;16:1–14. doi: 10.1007/s11892-015-0694-2. [DOI] [PubMed] [Google Scholar]
- 137.Abdoli S, et al. New insights into diabetes burnout and its distinction from diabetes distress and depressive symptoms: a qualitative study. Diabetes Res. Clin. Pract. 2020;169:108446. doi: 10.1016/j.diabres.2020.108446. [DOI] [PubMed] [Google Scholar]
- 138.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 139.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Prestele S, Aldenhoff J, Reiff J. [The HPA-axis as a possible link between depression, diabetes mellitus and cognitive dysfunction] Fortschr. Neurol. Psychiatr. 2003;71:24–36. doi: 10.1055/s-2003-36684. [DOI] [PubMed] [Google Scholar]
- 141.Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 142.Gold SM, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 143.Moulton CD, Costafreda SG, Horton P, Ismail K, Fu CHY. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav. 2015;9:651–662. doi: 10.1007/s11682-014-9348-2. [DOI] [PubMed] [Google Scholar]
- 144.Khalil M, Power N, Graham E, Deschênes SS, Schmitz N. The association between sleep and diabetes outcomes – systematic review. Diabetes Res. Clin. Pract. 2020;161:108035. doi: 10.1016/j.diabres.2020.108035. [DOI] [PubMed] [Google Scholar]
- 145.Senaratna CV, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep. Med. Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 146.Subramanian A, et al. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care. 2019;42:954–963. doi: 10.2337/dc18-2004. [DOI] [PubMed] [Google Scholar]
- 147.Huang T, et al. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. Cohorts. Diabetes Care. 2018;41:2111–2119. doi: 10.2337/dc18-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reutrakul S, et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep. Med. 2016;23:26–45. doi: 10.1016/j.sleep.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagayoshi M, et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep. Med. 2016;25:156–161. doi: 10.1016/j.sleep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ficker JH, et al. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur. Respir. J. 1998;11:14–19. doi: 10.1183/09031936.98.11010014. [DOI] [PubMed] [Google Scholar]
- 151.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J. Appl. Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 152.Ip MSM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 153.Shaw JE, et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am. J. Respir. Crit. Care Med. 2016;194:486–492. doi: 10.1164/rccm.201511-2260OC. [DOI] [PubMed] [Google Scholar]
- 154.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: A systematic review and meta-analysis. PLoS ONE. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern. Med. J. 2012;42:484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 156.Li XY, et al. Midlife modifiable risk factors for dementia: a systematic review and meta-analysis of 34 prospective cohort studies. Curr. Alzheimer Res. 2019;16:1254–1268. doi: 10.2174/1567205017666200103111253. [DOI] [PubMed] [Google Scholar]
- 157.Xue M, et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019;55:100944. doi: 10.1016/j.arr.2019.100944. [DOI] [PubMed] [Google Scholar]
- 158.Pal K, Mukadam N, Petersen I, Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2018;53:1149–1160. doi: 10.1007/s00127-018-1581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 160.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 161.Abner EL, et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimer’s Dement. 2016;12:882–889. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Matioli MNPS, et al. Association between diabetes and causes of dementia: evidence from a clinicopathological study. Dement. Neuropsychol. 2017;11:406–412. doi: 10.1590/1980-57642016dn11-040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.You Y, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 2021;58:671–685. doi: 10.1007/s00592-020-01648-9. [DOI] [PubMed] [Google Scholar]
- 164.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pelimanni E, Jehkonen M. Type 2 diabetes and cognitive functions in middle age: a meta-analysis. J. Int. Neuropsychol. Soc. 2019;25:215–229. doi: 10.1017/S1355617718001042. [DOI] [PubMed] [Google Scholar]
- 166.Rom S, et al. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 2020;10:7274. doi: 10.1038/s41598-020-64349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hussain B, Fang C, Chang J. Blood–brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front. Neurosci. 2021;15:688090. doi: 10.3389/fnins.2021.688090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Anstey KJ, Sargent-Cox K, Eramudugolla R, Magliano DJ, Shaw JE. Association of cognitive function with glucose tolerance and trajectories of glucose tolerance over 12 years in the AusDiab study. Alzheimers Res. Ther. 2015;7:48. doi: 10.1186/s13195-015-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Steen E, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease - Is this type 3 diabetes? J. Alzheimer’s Dis. 2005;7:63–80. doi: 10.3233/JAD-2005-7107. [DOI] [PubMed] [Google Scholar]
- 170.Leonardi M, Bickenbach J, Ustun TB, Kostanjsek N, Chatterji S. The definition of disability: what is in a name? Lancet. 2006;368:1219–1221. doi: 10.1016/S0140-6736(06)69498-1. [DOI] [PubMed] [Google Scholar]
- 171.Lisy K, Campbell JM, Tufanaru C, Moola S, Lockwood C. The prevalence of disability among people with cancer, cardiovascular disease, chronic respiratory disease and/or diabetes: a systematic review. Int. J. Evid. Based Healthc. 2018;16:154–166. doi: 10.1097/XEB.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 172.Yang Y, Hu X, Zhang Q, Zou R. Diabetes mellitus and risk of falls in older adults: a systematic review and meta-analysis. Age Ageing. 2016;45:761–767. doi: 10.1093/ageing/afw140. [DOI] [PubMed] [Google Scholar]
- 173.Wong E, et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1:106–114. doi: 10.1016/S2213-8587(13)70046-9. [DOI] [PubMed] [Google Scholar]
- 174.Havercamp SM, Scandlin D, Roth M. Health disparities among adults with developmental disabilities, adults with other disabilities, and adults not reporting disability in North Carolina. Public. Health Rep. 2004;119:418–426. doi: 10.1016/j.phr.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Herquelot E, Guéguen A, Bonenfant S, Dray-Spira R. Impact of diabetes on work cessation: data from the GAZEL cohort study. Diabetes Care. 2011;34:1344–1349. doi: 10.2337/dc10-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Virtanen M, et al. Work disability among employees with diabetes: latent class analysis of risk factors in three prospective cohort studies. PLoS ONE. 2015;10:e0143184. doi: 10.1371/journal.pone.0143184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cho NH, et al. IDF Diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 178.Seok WP, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 179.Stratton IM, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br. Med. J. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.DeCensi A, et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 181.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patientswith type 2 diabetes and kidney disease a systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35:2665–2673. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Karlstad Ø, et al. Use of insulin and insulin analogs and risk of cancer-systematic review and meta-analysis of observational studies. Curr. Drug. Saf. 2013;8:333–348. doi: 10.2174/15680266113136660067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bordeleau L, et al. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care. 2014;37:1360–1366. doi: 10.2337/dc13-1468. [DOI] [PubMed] [Google Scholar]
- 185.Guo M, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2014;41:650–656. doi: 10.1111/1440-1681.12265. [DOI] [PubMed] [Google Scholar]
- 186.Campbell JM, et al. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J. Alzheimer’s Dis. 2018;65:1225–1236. doi: 10.3233/JAD-180263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haukeland JW, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand. J. Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 188.Cukierman-Yaffe T, et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 2020;19:582–590. doi: 10.1016/S1474-4422(20)30173-3. [DOI] [PubMed] [Google Scholar]
- 189.Johnson JA, Bowker SL. Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia. 2011;54:25–31. doi: 10.1007/s00125-010-1933-3. [DOI] [PubMed] [Google Scholar]
- 190.Launer LJ, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jia Y, et al. Associations of the glycaemic control of diabetes with dementia and physical function in rural-dwelling older Chinese adults: a population-based study. Clin. Interv. Aging. 2021;16:1503–1513. doi: 10.2147/CIA.S319633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lesniak C, et al. Inpatient glycemic control and outcome of COVID-19 patients: a retrospective cohort. SAGE Open. Med. 2021;9:20503121211039105. doi: 10.1177/20503121211039105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Afolabi BI, et al. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. J. Natl Med. Assoc. 2018;110:256–264. doi: 10.1016/j.jnma.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 194.Nouwen A, et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet. Med. 2019;36:1562–1572. doi: 10.1111/dme.14054. [DOI] [PubMed] [Google Scholar]
- 195.Perry BD, et al. Muscle atrophy in patients with Type 2 diabetes mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 2016;22:94–109. [PMC free article] [PubMed] [Google Scholar]
- 196.Hirata Y, et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight. 2019;4:e124952. doi: 10.1172/jci.insight.124952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bassil MS, Gougeon R. Muscle protein anabolism in type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:83–88. doi: 10.1097/MCO.0b013e32835a88ee. [DOI] [PubMed] [Google Scholar]
- 198.Meex RCR, Blaak EE, van Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 2019;20:1205–1217. doi: 10.1111/obr.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]