Abstract
Morphological descriptions of phyllosoma larvae are essential for correct species identification and investigating the spatiotemporal distribution and recruitment process of spiny and slipper lobsters. Species identification of the phyllosoma larvae in the Scyllarinae subfamily is particularly difficult because of the morphological similarities among species and the scarcity of morphological information describing correct species identity. We extracted mid-to final-stage (V to VIII) phyllosoma larvae (n = 12) belonging to the subfamily Scyllarinae from several plankton samples collected in the Pacific and then performed molecular species identification using mitochondrial DNA COI and 16S rDNA sequence analyses. Three larvae collected around the Ryukyu Archipelago were identified as Chelarctus aureus (stage VI to VIII), and four collected around the Ryukyu Archipelago and Ogasawara Islands were identified as C. virgosus (V to VIII). One larva (V) collected in the central South Pacific was determined to be a subspecies of C. crosnieri. DNA barcodes could not be made for the remaining four larvae (V to VIII) collected around the Ryukyu Archipelago (designated by ?Chelarctus sp-1). Based on the morphological characteristics of the C. virgosus phyllosoma described in this study and the adult distributions reported to date, C. cultrifer phyllosomas previously reported in Japanese and Taiwanese waters are likely to be C. virgosus. This paper also presents a set of diagnostic morphological characteristics that can be used to discriminate among these four species of Chelarctus and from other genera in the subfamily Scyllarinae.
Keywords: Phyllosoma larvae, Slipper lobster, Molecular analysis, Morphology, Identification, Chelarctus
BACKGROUND
Species identification is difficult for spiny and slipper lobster phyllosoma larvae in the Palinuridae and Scyllaridae families because closely related species are morphologically similar and morphological descriptions based on correct species identifications are scarce. There have been numerous morphological descriptions of wild-caught phyllosoma larvae, most of which are not based on confident species identities. Scyllaridae, which comprises 19 extant genera and at least 88 extant species, is more diverse than Palinuridae, which contains 12 extant genera and 58 or more extant species (Yang and Chan 2010; Chan et al. 2013; Chan 2019). Scyllaridae comprises four subfamilies: Arctidinae, Ibacinae, Scyllarinae, and Theninae (Holthuis 1991). While the subfamily Scyllarinae used to contain only one genus, Scyllarus, the Holthuis (2002) revised the genus and divided it into 14 genera, in which more than 52 species have been described (Holthuis 2002; Chan 2010; Yang et al. 2008 2011 2012 2014 2017; Yang and Chan 2012). Scyllarinae is now the most diverse subfamily within the Scyllaridae with an increasing number of descriptions for new species (Yang et al. 2008 2011 2012 2014 2017; Yang and Chan 2012). However, species identification of the phyllosomas within this subfamily may be extremely difficult using morphological keys alone, as most larvae have been given a tentative alphabetic or numeric name such as Scyllarus sp., A, B, I, II, 1, 2 or ? (Prasad and Tampi 1957 1960; Johnson 1971a b; Berry 1974; Phillips et al. 1981; Barnett et al. 1986; McWilliam et al. 1995; Webber and Booth 2001). Nevertheless, complete larval development in the laboratory has been achieved in only six of the species in this subfamily (Robertson 1968 1979; Ito and Lucas 1990; Higa et al. 2005; Kumar et al. 2009; Fernández et al. 2010), leaving researchers to rely on other methods of identification. DNA barcoding has been shown to be useful for linking larvae or juveniles to adults of marine animals (Chen et al. 2013; Wong et al. 2014 2020; Li et al. 2019; Wei et al. 2021) and has been applied to wild-caught phyllosomas of only five species of the subfamily Scyllarinae (Palero et al. 2008 2011 2014; Genis-Armero et al. 2017; Wakabayashi et al. 2017 2020).
In this study, we report the results of our genetic and morphological analyses of mid-to final-stage phyllosoma larvae belonging to the Scyllarinae collected in the Northwest Pacific and central South Pacific Ocean.
MATERIALS AND METHODS
Wild-caught phyllosoma specimens
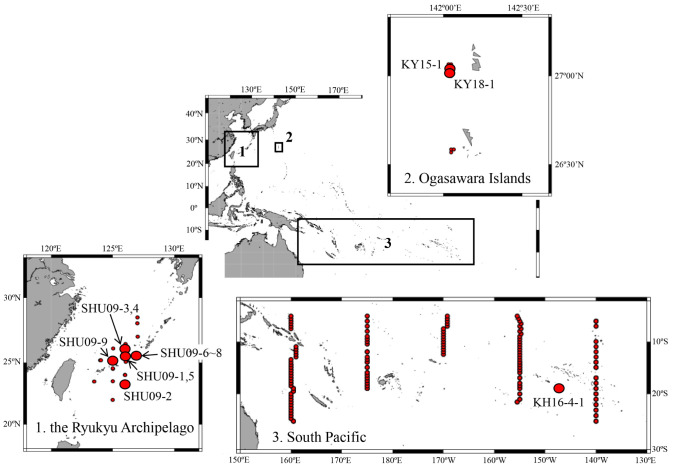
Phyllosomas were collected by three research vessels: [1] the RV Shunyo-Maru (Japan Fisheries Research and Education Agency), which cruised around the Ryukyu Archipelago (22°00'N−29°15'N, 123°30'E−130°00'E) from May 31 to June 26, 2009 (SHU-09); [2] the RV Koyo-Maru (the Tokyo Ogasawara Marine Products Center), which completed two cruises in the coastal waters of the Ogasawara Islands (26°32'N−27°09'N, 142°01'E−142°38'E) in 2015 and 2018 (KY15 and 18); and [3] the RV Hakuho-Maru (the University of Tokyo), which completed a cruise in the South Pacific (5°00'S−25°00'S, 160°00'E−140°00'W) from August 8 to September 28, 2016 (KH 16-4). The survey area and collection location of each phyllosomas used in this study are shown in figure 1. Phyllosomas were sorted on board and fixed in 80–90% ethanol before being transferred to the laboratory. In the laboratory, we first extracted the phyllosomas with round-, oval-, or lemon-shaped cephalic shields and a tapered abdomen that continues from the thorax (see Phillips et al. 1981; Sekiguchi 1986) from each cruise sample. This procedure allowed us to select the phyllosomas from the subfamily Scyllarinae. Subsequently, mid-to final-stage larvae (V–VIII) were selected based on the criteria described by Inoue and Sekiguchi (2006); stage V: 5th pereiopod unsegmented or 2-segmented, pleopods bud, uropods unbiloed or slightly bilobed; stage VI: 5th pereiopod 2 or 3-segmented, pleopods unbilobed, uropods bilobed; stage VII: 5th pereiopod 3-segmented, pleopods bilobed; stage VIII: 1st to 4th pereiopods gilled, 5th pereiopod 4-segmented. We further selected 12 larvae that were as morphologically intact as possible (Table 1). These phyllosoma specimens were deposited into the National Museum of Nature and Science, Tokyo, Japan (NSMT-Cr29022–29033). Body length (BL), cephalic shield length (CL), cephalic shield width (CW), thorax width (TW) and abdominal length (AL) were measured as described by Higa and Shokita (2004) and Palero et al. (2008). Detailed morphological investigations were performed using an Olympus LV100 optical microscope. Mandible and detailed setation were not included in the drawing because they are not useful for species identification. Arrangement of subexopodal spine was not described, since intra-specific and intra-individual variations have been reported in palinurid phyllosoma larvae (Chow et al. 2006a). In the same species, only the earliest developmental stage is completely described in detail, and in subsequent stages only the changes were described.
Fig. 1.
Survey area and collection locations for the phyllosoma larvae sampled by the three research vessels: RV Shunyo-maru (SHU), Koyo-maru (KY), and RV Hakuho-maru (KH). Closed red circles indicate the sampling stations where Scyllarinae phyllosomas were collected. The specimens analyzed in this study were collected from the large circle stations. See table 1 for specimen ID.
Table 1.
Summary of the sampling information, stage and DNA sequence for each of the phyllosoma specimens used in this study
*NWP: Northwest Pacific, CSP: central South Pacific.
Molecular genetic analysis
The selected specimens were rinsed thoroughly with tap water, and one pereiopod was then selected and subjected to dissection. Pereiopods were further rinsed well with tap water, homogenized, and subjected to crude DNA extraction using a blood and tissue kit (Qiagen Inc.). Since we frequently failed to obtain good sequence electropherograms using universal primers for mitochondrial cytochrome oxidase subunit I gene (COI) (Folmer et al. 1994) and for 16S rRNA gene (16S) (Palumbi et al. 1991), we used mixed primers: COI65F1-F4/COI1342R1-R4 (Chow et al. 2006b) for amplifying COI sequence and a newly designed primer pair (16SUF: 5'-TGACTGTGCGAAGGTAGCAT-3'/16SR2: 5'-ACGCCGGTCTGAACTCAAAT-3') for 16S. PCR was performed in 10 μl of total reaction mixture containing 5–10 ng template DNA, 1 μl of 10 × buffer, 1 μl each dNTPs, 2 × 10-3 mol m-3 each primer, and 0.5 U of EX Taq HS DNA polymerase (Takara, Shiga, Japan) on an ABI 9700 Thermal cycler (Applied Biosystems, Foster City, CA, USA). Reaction mixtures were preheated at 94°C for 2 min, followed by 40 amplification cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 50 s), with a final extension at 72°C for 7 min. The PCR product was purified using a PCR purification kit (Qiagen, Hilden, Germany) and was used as the DNA template for cycle sequencing reactions using a Big Dye Terminator Cycle Sequencing Kit (Ver. 3.1, Applied Biosystems) with COI65F1-F4 and 16SR2 primers. Sequencing was conducted using an ABI Prism 3730XL instrument (Applied Biosystems). Nucleotide sequences of one per species or subspecies for each of the 13 genera in the subfamily Scyllarinae, were derived from the database, and those of Scyllarides squammosus (JN701654 and JN701689) were adopted as an outgroup. These sequences were aligned with the sequences obtained in this study using ATGC (Genetix, Osaka, Japan) followed by an eye examination. A neighbor-joining (NJ) tree based on the uncorrected Kimura’s two-parameter distances (K2P) between sequences was constructed using MEGA ver. 6 (Tamura et al. 2013).
RESULTS
DNA barcoding for phyllosoma larvae
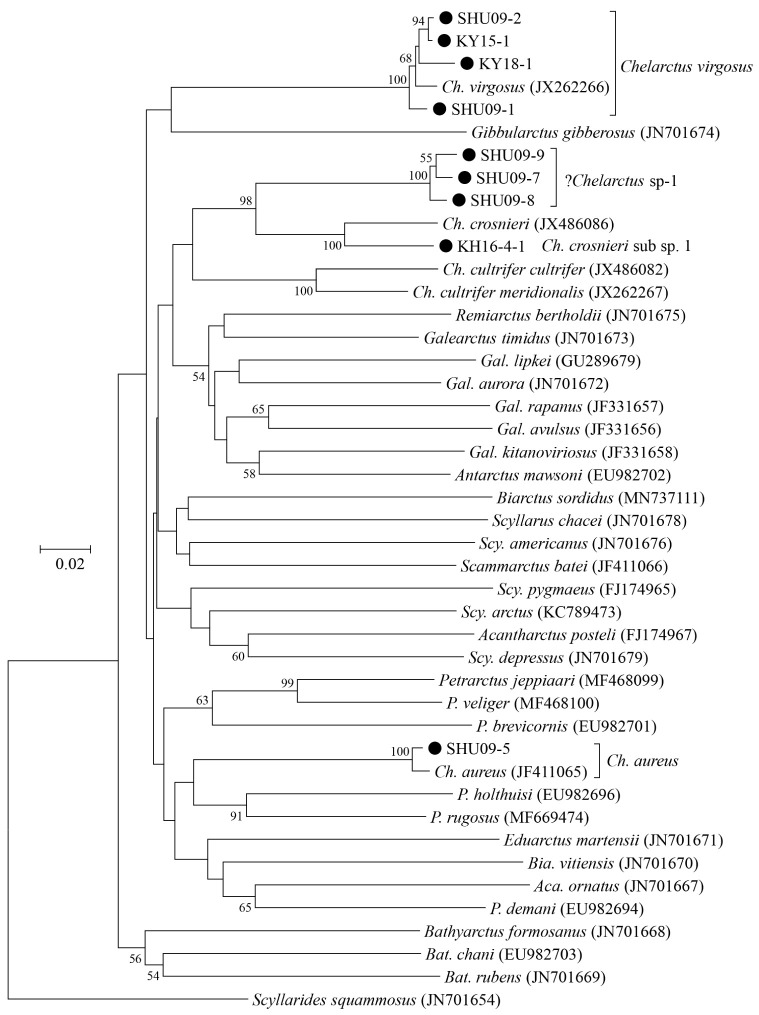
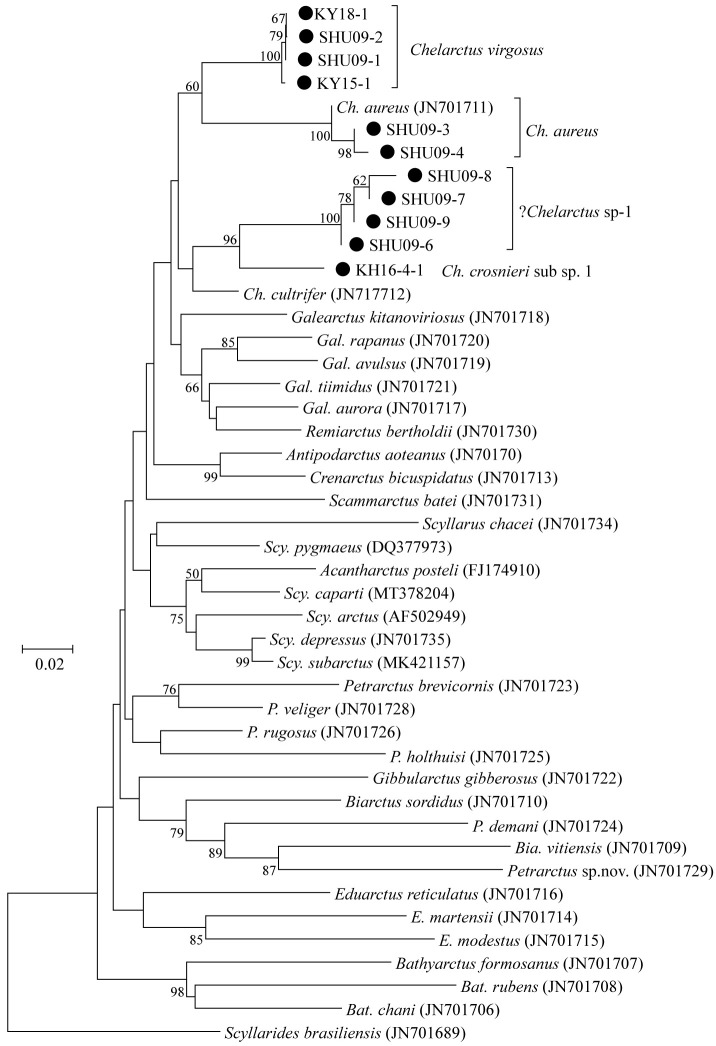
We produced partial COI (564 to 573 bp) and 16S (333 to 404 bp) sequences from nine and 11 phyllosomas, respectively (Table 1). All sequences were deposited into the International Nucleotide Sequence Database Collection (INSDC) under accession numbers LC632672 to LC632682 for 16S and LC632947 to LC632955 for COI. Phylogenetic trees generated using these sequences are shown in figures 2 and 3, and Bathyarctus was the only genus that was commonly monophyletic in both the COI and 16S trees.
Fig. 2.
Neighbor-joining phylogenetic tree drawn using the Kimura-2-parameter distances for each of the partial COI sequences between the subfamily Scyllarinae species. Scyllarides brasiliensis was used as the outgroup. Closed squares indicate the phyllosoma specimens analyzed in this study. Bootstrap values of > 50% (from 1000 replicates) are shown at each node.
Fig. 3.
Neighbor-joining phylogenetic tree drawn using the Kimura-2-parameter distances for each of the partial 16S rDNA sequences for species belonging to the subfamily Scyllarinae. Scyllarides brasiliensis was used as the outgroup. Closed squares indicate the phyllosoma specimens analyzed in this study. Bootstrap values of > 50% (from 1000 replicates) are shown at each node.
These phylogenetic trees unambiguously identified the four larvae (KY15-1, KY18-1, SHU09-1, and SHU09-2) collected around the Ryukyu Archipelago and Ogasawara Islands as Chelarctus virgosus and the three larvae (SHU09-3 to -5) collected around the Ryukyu Archipelago as C. aureus. The K2P distances between the larval and adult reference sequences were 1.0 to 1.9% in COI and 0.3% in 16S (Tables 2 and 3), which were well below the typical values between congeneric species (Hebert et al. 2003; Kochzius et al. 2010).
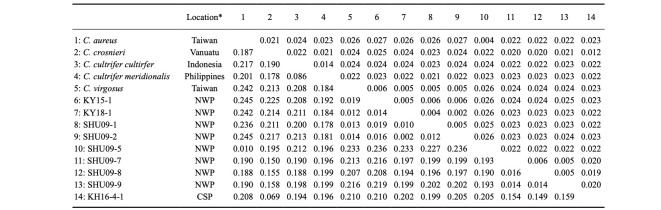
Table 2.
Uncorrecred K2P distance (lower diagonal) and standard error (upper diagonal) for the partial COI sequences from the nine phyllosoma larvae analyzed in this study and the five reference Chelarctus sequences from the database
Sample KH16-4-1 collected in the South Pacific was closely related to C. crosnieri (JX 486086) in the COI tree (Fig. 2). The K2P distance (6.9% in COI) (Table 2) between KH16-4-1 and C. crosnieri was smaller than those between valid species of slipper lobsters (Yang et al. 2008) and comparable to that between the C. cultrifer subspecies (C. cultrifer cultrifer and C. cultrifer meridionalis) (5.6 to 8.3%) (Yang and Chan 2012), so we determined that KH16-4-1 is likely a subspecies of C. crosnieri and designated it as C. crosnieri sub sp.1.
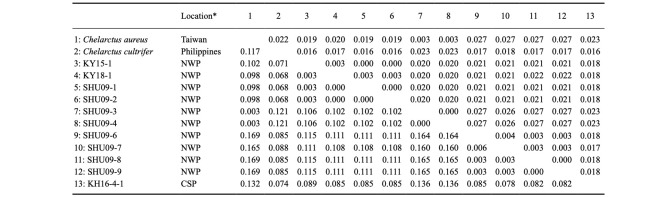
The remaining four larvae (SHU09-6 to -9) collected around the Ryukyu Archipelago could not be assigned to any species, even using a BLAST search. The K2P distances among these larvae were 1.4 to 1.6% in COI and 0.0 to 0.6% in 16S (Tables 2 and 3), indicating that these four specimens were conspecific. These four larvae were nested with other Chelarctus species (Figs. 2 and 3), so we tentatively determined that they belonged to the genus Chelarctus and designated these samples as ?Chelarctus sp-1. The COI and 16S trees (Figs. 2 and 3) suggested that the closest kin for ?Chelarctus sp-1 may be C. crosnieri and C. crosnieri sub sp.1 (KH16-4-1), in which the K2P distances between ?Chelarctus sp-1 and the latter two species ranged from 14.9 to 15.9% in COI and from 7.8 to 8.2% in 16S (Tables 2 and 3).
Table 3.
Uncorrecred K2P distance (lower diagonal) and standard error (upper diagonal) for the partial 16S rDNA sequences from the 11 phyllosoma larvae analyzed in this study and the two Chelarctus reference sequences from the database
Morphological descriptions of phyllosomas
Chelarctus virgosus (Yang and Chan, 2012)
Chelarctus virgosus (Yang and Chan, 2012)
Stage V (KY18-1, Fig. 4)
TL = 8.45 mm; CL = 5.73 mm; CW = 6.71 mm; TW = 3.50 mm; AL = 1.24 mm; CW/CL = 1.17; CW/TW = 1.92. This description was completed for the specimen collected at 27°02'N, 142°05'E (offshore of the Ogasawara Islands) on 31 July 2018.
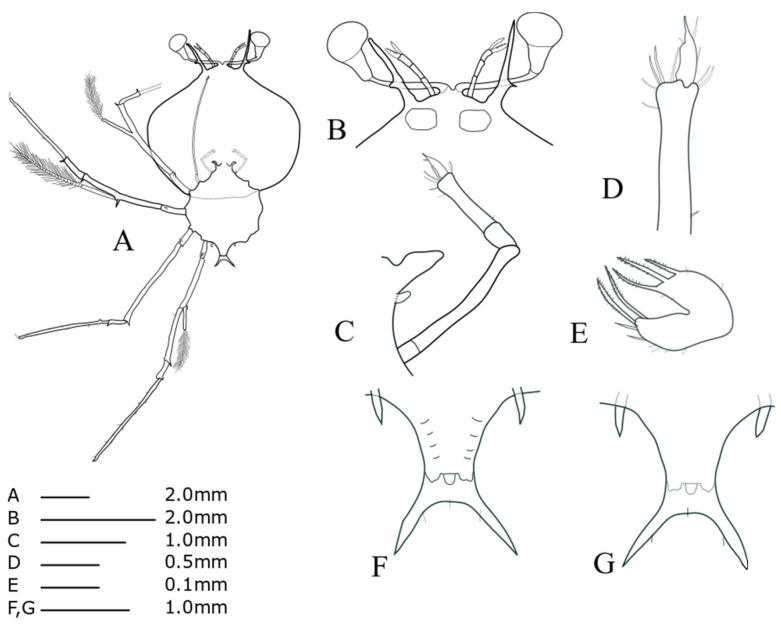
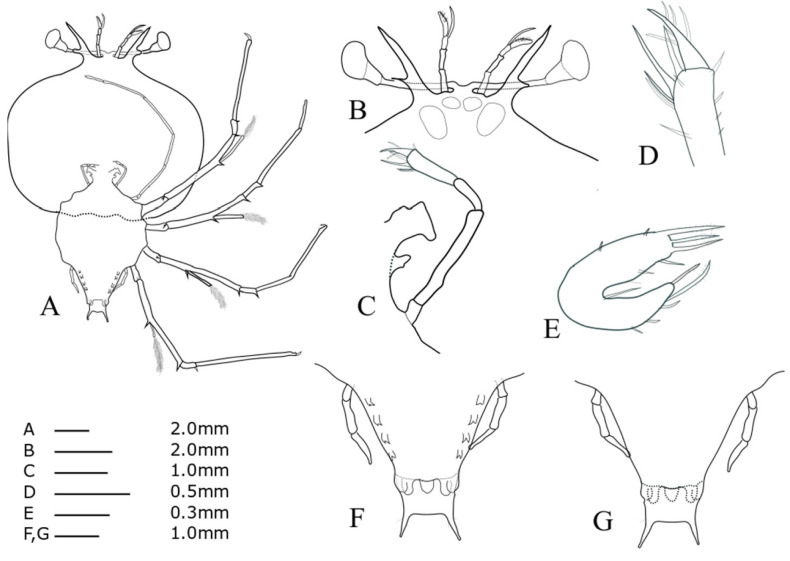
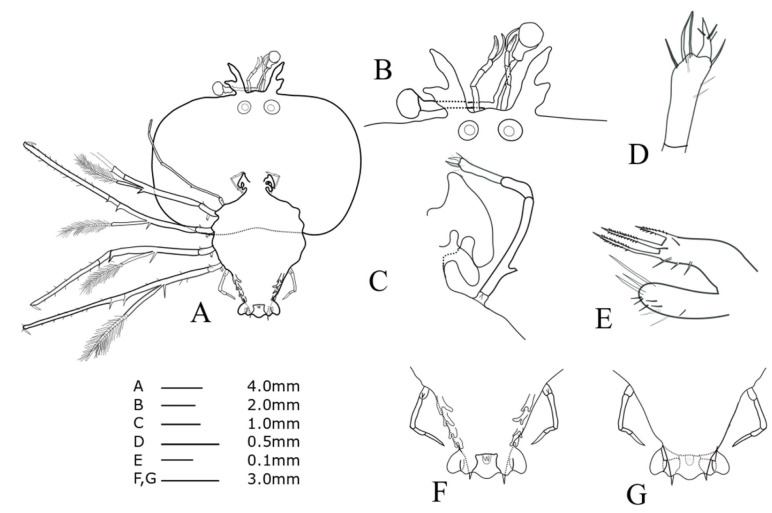
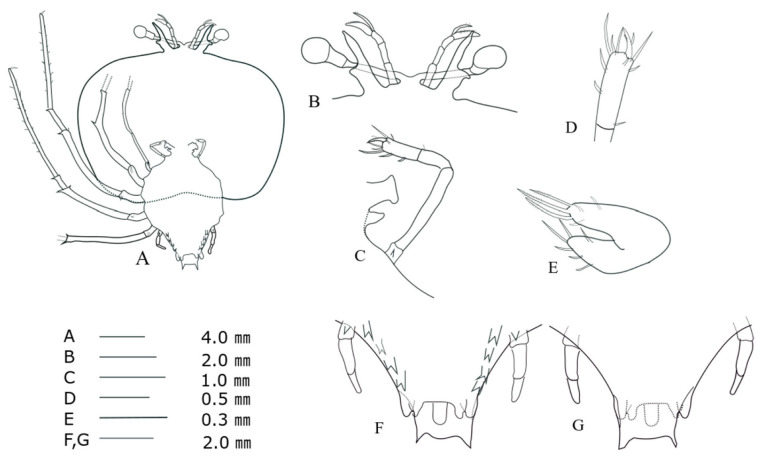
Cephalic shield (Fig. 4A): sub-pentagonal; slightly wider than long, nearly twice as wide as thorax. Antennule (Fig. 4B): biramous, 3-segmented; distal segment with two flagella (primary and accessory); primary flagellum unsegmented with 9‒10 rows of sensory setae, slightly shorter than accessory flagellum. Antenna (Fig. 4B): bifurcated, unsegmented, half H-shaped (├), exceeding antennule; short lateral process projecting horizontally. Maxillule 1 (Fig. 4E): anterior lobe with denticulate three strong setae; posterior lobe with denticulate two strong setae. Maxillule 2 (Fig. 4C): rudimentary flattened. Maxilliped 1 (Fig. 4C): rudimentary bud. Maxilliped 2 (Fig. 4C, D): 5-segmented, coxal spine absent; three short setae on dactylus; at least five long setae on proximal margin of propodus (Fig. 4D). Maxilliped 3 (Fig. 4A): 5-segmented, tiny ventral coxal spine; distal parts of propodus and dactylus setosed densely; exopod absent. Pereiopods 1‒4 (Fig. 4A): biramous, tiny coxal spine and 5-segmented endopod; ischio-merus fused to basis, one stronger and one smaller distal spines on outer and inner sides of ischio-merus; a tiny distal spine on carpus; a dense of setae covering ischio-merus. Pereiopod 5 (Fig. 4F, G): elongated bud, coxal spine absent, much shorter than abdomen. Abdomen (Fig. 4F, G): four pairs of undeveloped pleopod buds; slightly bilobed uropod, rounded posterior margin locating far anterior of central margin of telson; prominent posterolateral spines of telson far beyond posterior central margin of telson, more than three times the length of uropod.
Fig. 4.
Phyllosoma larva of Chelarctus virgosus, stage V. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
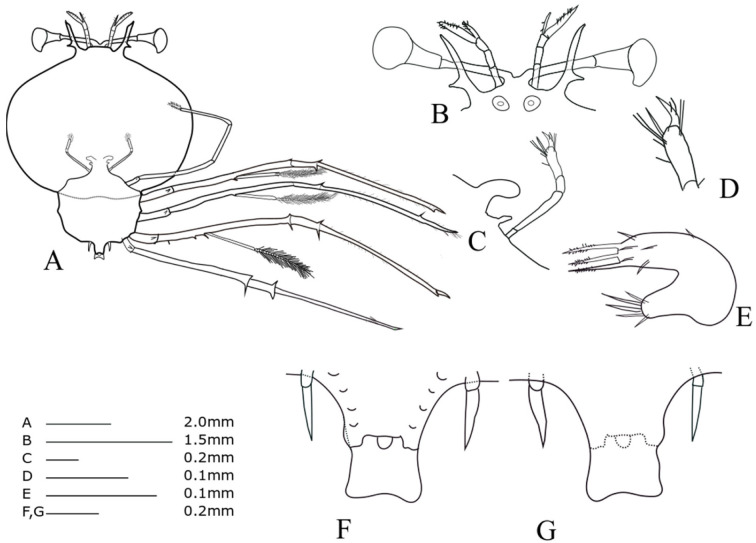
Stage VI (KY15-1, Fig. 5)
TL = 12.66 mm; CL = 7.96 mm; CW = 9.73 mm; TW = 4.58 mm; AL = 2.41 mm; CW/CL = 1.22; CW/TW = 2.12. This description was completed for the specimen collected at 27°07'N, 142°05'E (offshore of Ogasawara Islands), on 30 January 2015.
Cephalic shield (Fig. 5A): more than twice as wide as thorax. Antennule (Fig. 5B): primary flagellum similar in length to accessory flagellum. Antenna (Fig. 5B): similar in length to antennule. Maxilliped 1 (Fig. 5C): cone-shaped buds. Maxilliped 3 (Fig. 5A): propodus more densely setose than stage V (at least 20 setae). Pereiopods 1−4 (Fig. 5A): coxal spines more developed than those during stage V. Pereiopod 5 (Fig. 5F, G): 2-segmented, shorter than half of abdomen. Abdomen (Fig. 5F, G): four pairs of unbilobed pleopods; bilobed uropods; posterolateral spines of telson twice as long as uropod.
Fig. 5.
Phyllosoma larva of Chelarctus virgosus, stage VI. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E, ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
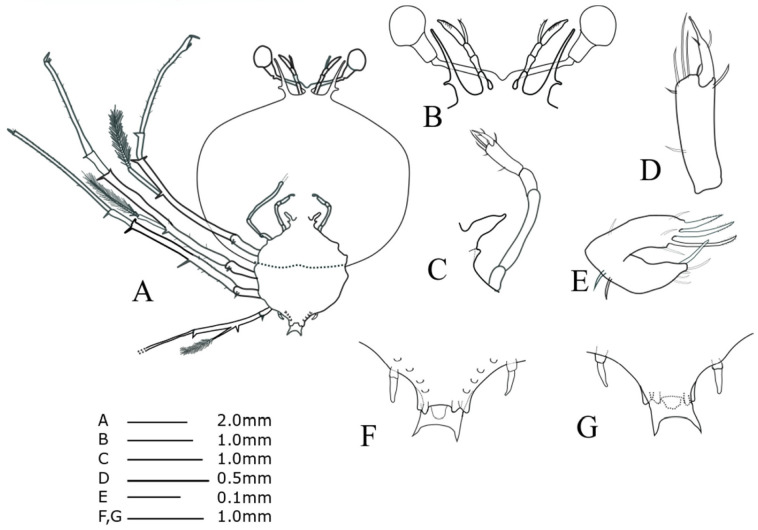
Stage VII (SHU09-1, Fig. 6)
TL = 18.61 mm; CL = 11.89 mm; CW = 14.11 mm; TW = 7.67 mm, AL = 4.66 mm; CW/CL = 1.18; CW/TW = 1.84. This description was completed for the specimen collected at 25°30'N, 126°05'E (around the Ryukyu Archipelago) on 8 June 2009.
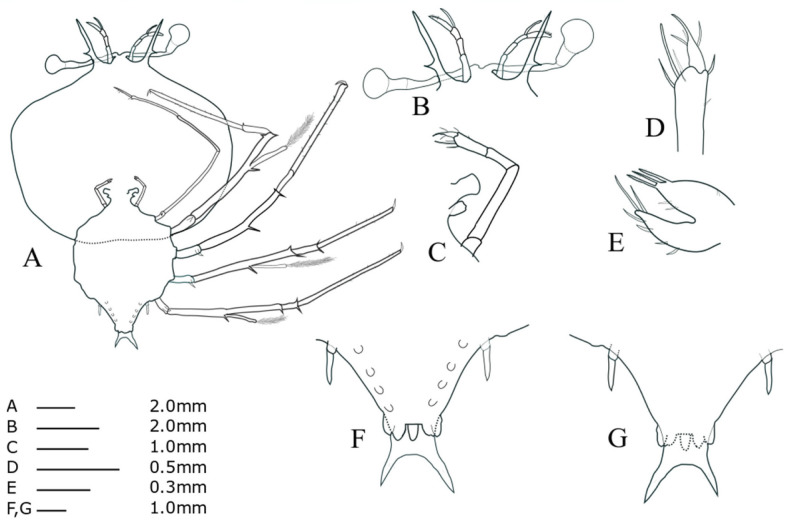
Antennule (Fig. 6B): accessory flagellum longer than primary flagellum; larger number of setae on primary flagella than during stage VI. Antenna (Fig. 6B): lateral process stouter than that of stage VI. Maxillule 2 (Fig. 6C): rudimentary bilobed. Maxilliped 1 (Fig. 6C): slightly biramous. Maxilliped 2 (Fig. 6C, D): four setae on dactylus; two long terminal setae on propodus with four short simple setae. Maxilliped 3 (Fig. 6A): exopod bud, at least 19 setae on propodus. Pereiopods 1−4 (Fig. 6A): more spine-like setae on basis surface than during stage VI; at least 26 setae scattered over the surface of the P1 propodus; P4 longer than other pereiopods. Pereiopod 5 (Fig. 6F, G): 3-segmented, tiny coxal spine, about half of abdomen. Abdomen (Fig. 6F, G): pleopod biramous; posterolateral spines slightly longer than uropod.
Fig. 6.
Phyllosoma larva of Chelarctus virgosus, stage VII. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E, ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
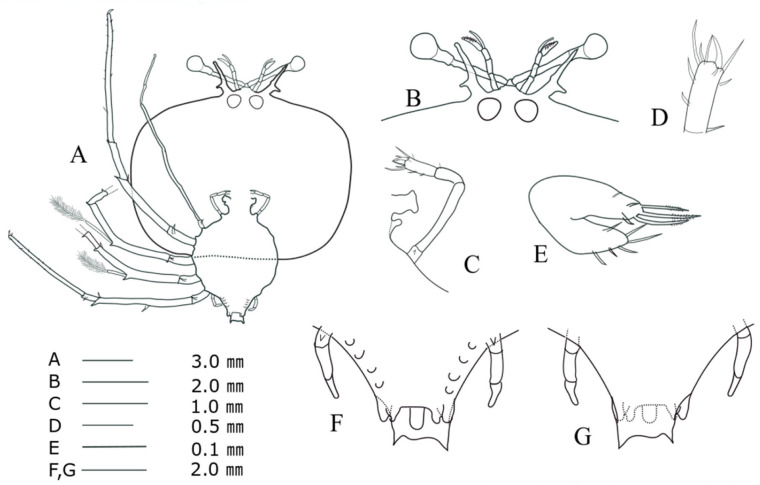
Stage VIII (SHU09-2, Fig. 7)
TL = 20.66 mm; CL = 12.30 mm; CW = 15.97 mm; TW = 7.76 mm; AL = 5.92 mm; CW/CL = 1.25; CW/TW = 2.06. This description was completed for the specimen collected at 23°19'N, 126°09'E (around the Ryukyu Archipelago) on 9 June 2009.
Antenna (Fig. 7B): exceeding antennule. Maxillule 1 (Fig. 7E): six short terminal setae, two long denticulate setae and two normal terminal setae on the posterior lobe and two sub-terminal setae on the outer side of the posterior lobe. Maxillule 2 (Fig. 7C): apparently biramous, more developed posterior lobe than during stage VII. Maxilliped 1 (Fig. 7C): unsegmented and bilobed, outer lobe flattened and rounded. Maxilliped 2 (Fig. 7C, D): an exopod bud. Maxilliped 3: coxal spine absent; propodus more densely setose than stage VII. Pereiopods 1−4 (Fig. 7A): gill buds on base of coxa; at least 33 setae scattered over the surface of the propodus in P1. Pereiopod 5 (Fig. 7F, G): 4-segmented, much longer than half of abdomen. Abdomen (Fig. 7F, G): biramous pleopods larger than stage VII; round uropod margin near reaching but never exceeding posterior central margin of telson; posterolateral spines of telson similar in length of uropod.
Fig. 7.
Phyllosoma larva of Chelarctus virgosus, stage VIII. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
Chelarctus aureus (Holthuis, 1963)
Stage VI (SHU09-3, Fig. 8)
TL = 9.40 mm; CL = 5.81 mm; CW = 8.88 mm; TW = 4.50 mm; AL = 1.26 mm; CW/CL = 1.30; CW/TW = 2.1. This description was completed for the specimen collected at 26°09'N, 126°01'E (around the Ryukyu Archipelago) on 10 June 2009.
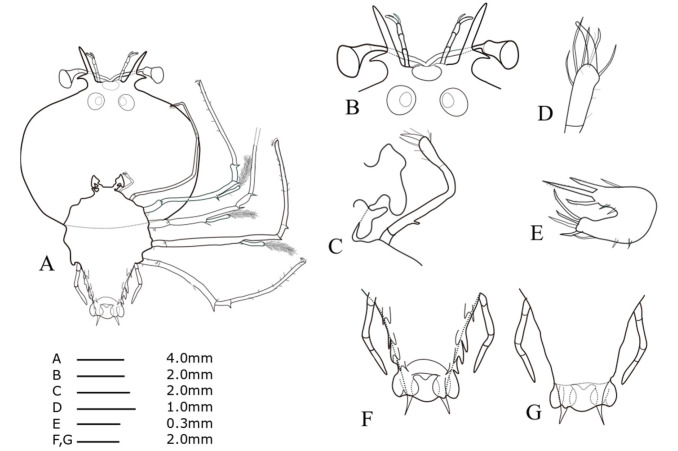
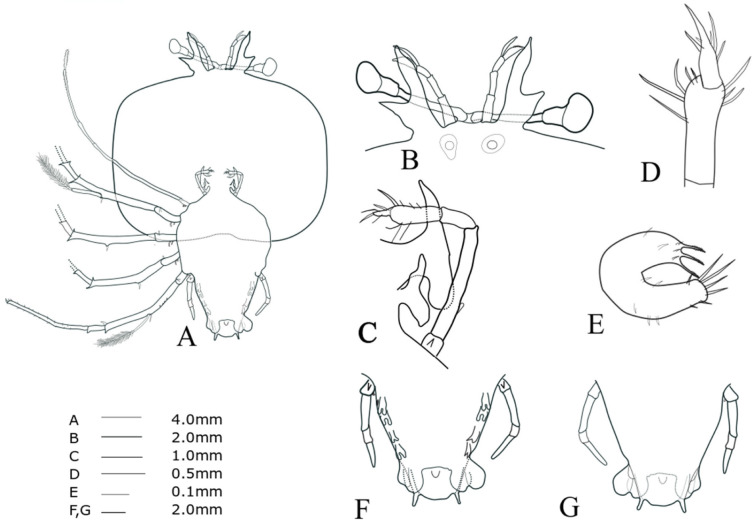
Cephalic shield (Fig. 8A): wide sub-rectangular; wider than long, more than twice as wide as thorax, slightly concaved posterior edge. Antennule (Fig. 8B): biramous, 3-segmented, exceeding antenna; distal segment with two flagella (primary and accessory); primary flagellum unsegmented with at least six rows of sensory setae, slightly longer than accessory flagellum. Antenna (Fig. 8B): bifurcated, unsegmented, half H-shaped (├); short lateral process projecting horizontally. Maxillule 1 (Fig. 8E): anterior lobe with denticulate three strong setae; posterior lobe with denticulate three strong setae. Maxillule 2 (Fig. 8C): rudimentary bilobed. Maxilliped 1 (Fig. 8C): elongated bud. Maxilliped 2 (Fig. 8C, D): 5-segmented, tiny coxal spine; four setae on dactylus; three long setae on distal margin of propodus. Maxilliped 3 (Fig. 8A): damaged propodus; tiny spine on ventral margin of coxa; exopod absent. Pereiopods 1–4 (Fig. 8A): biramous, distinct coxal spine, 5-segmented endopod; ischio-merus fused to basis; two short distal spines on basis; two sharp distal spines on ischio-merus; two distal spines on the carpus; a dense of setae covering ischio-merus. Pereiopod 5 (Fig. 8F, G): 2-segmented, tiny coxal spine, extending to half of abdomen. Abdomen (Fig. 8F, G): four pairs of unbilobed pleopods; bilobed uropods, rounded posterior margin locating far anterior of central margin of telson; posterolateral spines of telson similar in length with uropod exceeding posterior central margin of telson.
Fig. 8.
Phyllosoma larva of Chelarctus aureus, stage VI. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
Stage VII (SHU09-4, Fig. 9)
TL = 17.78 mm; CL = 13.09 mm; CW = 18.19 mm; TW = 7.17 mm; AL= 4.57 mm; CW/CL = 1.34; CW/TW = 2.54. This description was completed for the specimen collected at 26°10'N, 126°01'E (around the Ryukyu Archipelago) on 10 June 2009.
Cephalic shield (Fig. 9A): more than 2.5-times wider than thorax, concaved posterior edge. Antennule (Fig. 9B): primary flagellum with at least 14 rows of sensory setae, similar in length to accessory flagellum. Maxillule 2 (Fig. 9C): slightly bilobed. Maxilliped 1 (Fig. 9C): rudimentary bilobed. Maxilliped 2 (Fig. 9C, D): bearing six short setae on dactylus; exopod bud. Maxilliped 3 (Fig. 9A): 5-segmented, tiny coxal spine; dense of setae covering surface of fourth and fifth segments. Pereiopod 5 (Fig. 9F, G): 3-segmented, distinct coxal spine, exceeding half of abdomen. Abdomen (Fig. 9F, G): four pairs of bilobed pleopods; posterolateral spines of telson slightly shorter than uropod.
Fig. 9.
Phyllosoma larva of Chelarctus aureus, stageVII. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
Stage VIII (SHU09-5, Fig. 10)
TL = 23.38 mm; CL = 16.12 mm; CW = 21.99 mm; TW = 9.85 mm; AL = 8.43 mm; CW/CL = 1.36; CW/TW = 2.23. This description was completed for the specimen collected at 25°30'N, 126°05'E (around the Ryukyu Archipelago) on 8 June 2009.
Antennule (Fig. 10B): primary flagellum with 15 rows of sensory setae, shorter than accessory flagellum. Antenna (Fig. 10B): lateral process slightly projecting antero-diagonally. Maxillule 2 (Fig. 10C): bilobed, more flattened and anterior and posterior lobes extend more than stage VII. Maxilliped 1 (Fig. 10C): bilobed; posterior lobe extending backwards and larger than anterior lobe. Maxilliped 2 (Fig. 10C): distinct coxal spine. Pereiopods 1−4 (Fig. 10A): gill buds present on the base of the coxa, short strong coxal spine, at least 20 pairs of short spine-like setae scattered over the surface of propodus. Pereiopod 5 (Fig. 10F, G): 4-segmented extending more than during stage VII. Abdomen (Fig. 10F, G): round uropod margin near reaching but never exceeding posterior central margin of telson; posterolateral spines of telson shorter than uropod.
Fig. 10.
Phyllosoma larva of Chelarctus aureus, stage VIII. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
Chelarctus crosnieri sub sp. 1
Stage V (KH16-4-1, Fig. 11)
TL = 6.60 mm; CL = 4.70 mm; CW = 5.62 mm; TW = 3.05 mm; AL = 0.61 mm; CW/CL = 1.19; CW/TW = 1.84. This description was completed for the specimen collected from 19°00'S, 147°30'W (offshore of French Polynesia, the south Pacific) on 11 September 2016.
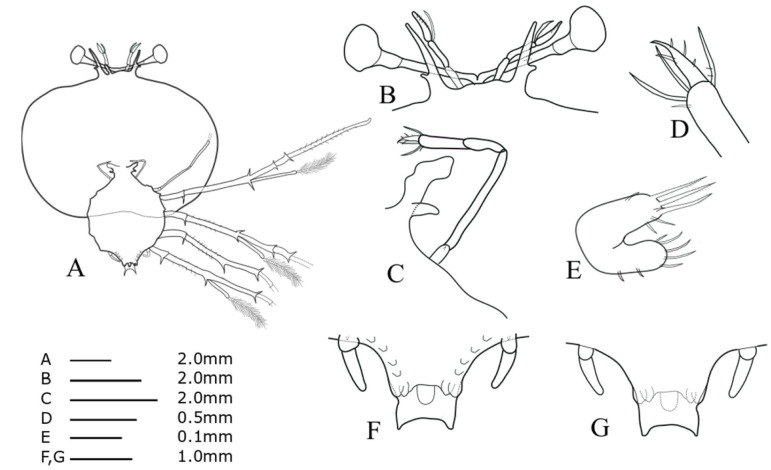
Cephalic shield (Fig. 11A): sub-pentagon, slightly wider than long, nearly twice as wide as thorax. Antennule (Fig. 11B): biramous, 3-segmented, exceeding antenna; distal segment with two flagella (primary and accessory); primary flagellum unsegmented with at least eight rows of sensory setae, much longer than accessory flagellum. Antenna (Fig. 11B): bifurcated, unsegmented, half H-shaped (├), short lateral process projecting horizontally. Maxillule 1 (Fig. 11E): anterior lobe with denticulate three strong setae; posterior lobe with denticulate two strong setae Maxillule 2 (Fig. 11C): rudimentary flattened. Maxilliped 1 (Fig. 11C): rudimentary bud. Maxilliped 2 (Fig. 11C, D): 5-segmented, coxal spine absent, four long terminal setae on propodus, at least four fine setae on dactylus. Maxilliped 3 (Fig. 11A): 5-segmented, ventral coxal spine, distal part of the propodus and dactylus densely setosed; exopod absent. Pereiopods 1–4 (Fig. 11A): biramous, distinct coxal spine and 5-segmented endopod, ischio-merus fused to basis, single long distal spine on basis, two distal spines on ischio-merus and carpus. Pereiopod 5 (Fig. 11F, G): two-segmented, coxal spine absent, extending to half of abdomen. Abdomen (Fig. 11F, G): four pairs of pleopod bud; slightly bilobed uropod, rounded posterior margin locating far anterior of central margin of telson; not well developed posterolateral spines of telson exceeding posterior central margin of telson.
Fig. 11.
Phyllosoma larva of Chelarctus crosnieri sub sp., stage V. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E, ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
?Chelarctus sp-1
Stage V (SHU09-6, Fig. 12)
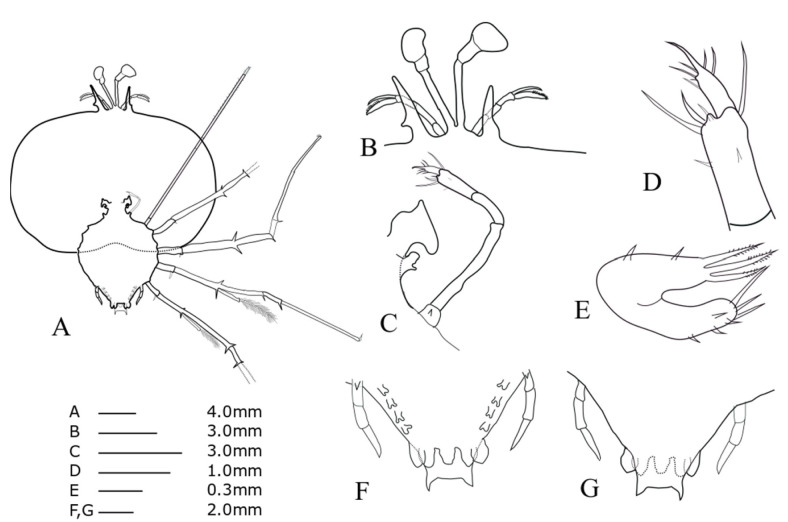
TL = 9.64 mm; CL = 6.09 mm; CW = 7.69 mm; TW = 4.22 mm, AL = 1.24 mm, CW/CL = 1.26, CW/TW = 1.82. This description was completed for the specimen collected at 25°33'N, 127°02'E, Ryukyu Archipelago, on 23 June 2009.
Cephalic shield (Fig. 12A): sub-pentagon; wider than long, nearly twice as wide as thorax. Antennule (Fig. 12B): similar in length to antenna; biramous, 3-segmented, distal segment with two flagella (primary and accessory); primary flagellum unsegmented with at least eight rows of sensory setae, slightly longer than accessory flagellum. Antenna (Fig. 12B): bifurcated, unsegmented, half H-shaped (├), short lateral process projecting horizontally. Maxillule 1 (Fig. 12E): anterior lobe with denticulate three strong setae; posterior lobe with denticulate two strong setae. Maxillule 2 (Fig. 12C): unbilobed. Maxilliped 1 (Fig. 12C): rudimentary bud. Maxilliped 2 (Fig. 12C, D): 5-segmented, coxal spine absent, three short setae on dactylus; six long setae on posterior margin of propodus (Fig. 12D). Maxilliped 3 (Fig. 12A): 5-segmented, distinct coxal spine; distal parts of propodus and dactylus setosed densely; exopod absent. Pereiopods 1–4 (Fig. 12A): biramous, distinct coxal spine and 5-segmented endopod; ischio-merus fused to basis; two distal spines on ischio-merus; two distal spines on the carpus. Pereiopod 5 (Fig. 12F, G): 2-segmented, coxal spine absent, extending to half of abdomen. Abdomen (Fig. 12F, G): four pairs of undeveloped pleopod buds; slightly bilobed uropod, rounded posterior margin locating well anterior of central margin of telson; posterolateral spine of telson well exceeding posterior central margin of telson, similar in length to uropod.
Fig. 12.
Phyllosoma larva of ?Chelarctus sp-1, stage V. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E, ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
Stage VI (SHU09-7, Fig. 13)
TL = 13.71 mm; CL = 10.02 mm; CW= 13.04 mm; TW = 5.66 mm; AL = 2.88 mm; CW/CL = 1.30; CW/TW = 2.30. This description was completed for the specimen collected at 25°33'N, 127°02'E, Ryukyu Archipelago, on 23 June 2009.
Cephalic shield (Fig. 13A): wide sub-rectangular; larger CW/CL and CW/TW than in stage V. Maxillule 2 (Fig. 13C): posterior lobe slightly projecting backward. Maxilliped 1 (Fig. 13C): bud. Maxilliped 2 (Fig. 13C, D): tiny coxal spine; four setae on dactylus. Pereiopod 5 (Fig. 13F, G): 3-segmented, distinct coxal spine, exceeding half of abdomen. Abdomen (Fig. 13F, G): bilobed uropods.
Fig. 13.
Phyllosoma larva of ?Chelarctus sp-1, stage VI. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E, ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
Stage VII (SHU09-8, Fig. 14)
TL = 17.41 mm; CL = 10.95 mm; CW = 16.20 mm; TW = 6.46 mm; AL = 4.69 mm; CW/CL = 1.47; CW/TW = 2.51. This description was completed for the specimen collected at 25°33'N, 127°02'E, Ryukyu Archipelago, on 23 June 2009.
Cephalic shield (Fig. 14A): larger CW/CL and CW/TW than in stage VI. Antennule (Fig. 14B): primary flagellum similar in length to accessory flagellum. Maxilliped 1 (Fig. 14C): rudimentary biramous. Maxilliped 2 (Fig. 14C): distinct coxal spine. Maxilliped 3 (Fig. 14A): damaged. Abdomen (Fig. 14F, G): four pairs of bilobed pleopods.
Fig. 14.
Phyllosoma larva of ?Chelarctus sp-1, stage VII. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
Stage VIII (SHU09-9, Fig. 15)
TL = 23.65 mm; CL = 13.86 mm; CW = 18.84 mm; TW = 8.4 mm; AL = 7.36 mm; CW/CL = 1.35; CW/TW = 2.24. This description was completed for the specimen collected at 25°16'N, 124°58'E, on 19 June 2009.
Cephalic shield (Fig. 15A): smaller CW/CL and CW/TW than in stage VII. Antenna (Fig. 15B): lateral process projecting slightly antero-diagonally. Maxillule 1 (Fig. 15E): posterior lobe with denticulate two strong setae. Maxillule 2 (Fig. 15C): flattened and outer lobe extending backward. Maxilliped 1 (Fig. 15C): biramous; anterior lobe shorter than posterior lobe. Pereiopods 1−4 (Fig. 15A): gill buds. Pereiopod 5 (Fig. 15F, G): 4-segmented. Abdomen (Fig. 15F, G): round uropod margin near reaching but never exceeding posterior central margin of telson; posterolateral spines of telson shorter than uropod.
Fig. 15.
Phyllosoma larva of ?Chelarctus sp-1, stage VIII. A, ventral view; B, ventral view of eye, antennule and antenna; C, 2nd maxillule, 1st and 2nd maxilliped; D, distal end of 2nd maxilliped; E ventral view of 1st maxillule; F, ventral view of 5th pereiopod and abdomen; G, dorsal view of 5th pereiopod and abdomen.
DISCUSSION
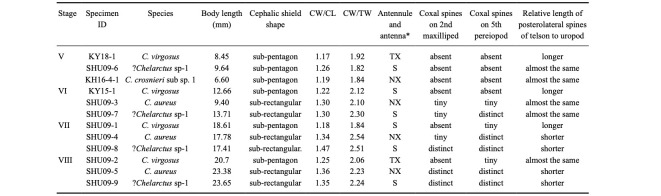
We noticed that the four species investigated in the present study share morphological characteristics among their phyllosomas: sub-pentagon or sub-rectangular shaped cephalic shield, wide cephalic shield (CW/CL = 1.17 to 1.47), half H-shaped antenna, round posterior margin of uropod not exceeding posterior central margin of telson, and eminent posterolateral spines exceeding posterior central margin of telson. This combination of these morphological characteristics is not observed in many species of the other genera of the subfamily Scyllarinae described to date (Robertson 1968 1979; Johnson 1971a b; Ito and Lucas 1990; Higa et al. 2005; Kumar et al. 2009; Palero et al. 2008 2011; Fernández et al. 2010; Genis-Armero et al. 2017; Wakabayashi et al. 2017 2020), except for Scyllarus pygmaeus from the Mediterranean (Palero et al. 2008), Scyllarus depressus from the Atlantic (Robertson 1971, figs. 7–10), and Scyllarus delfini (Acantharctus delfini) from the Southeast Pacific (Johnson 1971c, figs. 1–3; Báez 1973, figs. 5 and 6).
The morphological characteristics of the phyllosomas investigated in this study are summarized in table 4. The phyllosomas were divided into two types of cephalic shield shapes: the phyllosomas of C. virgosus (Figs. 4 to 7) and C. crosnieri sub sp. 1 (only stage V) (Fig. 11), which contain a sub-pentagon-shaped cephalic shield, and those of C. aureus (Figs. 8–10) and ?Chelarctus sp-1 (except for stage V) (Figs. 13–15), which contain a sub-rectangular-shaped cephalic shield. CW/CL ranged from 1.17 to 1.25 in C. virgosus and C. crosnieri sub sp. 1, and 1.26 to 1.47 in C. aureus and ?Chelarctus sp-1. In the sub-pentagon group, stage V phyllosoma of C. crosnieri sub sp. 1 had a more rounded cephalic shield than that of C. virgosus. Phyllosomas of C. virgosus and C. crosnieri sub sp. 1 can be distinguished using the relative length of antennule and antenna. The antenna of C. virgosus phyllosoma was exceeding its antennules during stages V and VIII (Figs. 4 and 7) and almost the same as its antennule during stages VI and VII (Figs. 5, 6). Antennules were exceeding the antenna during stages VI–VIII C. aureus phyllosomas (Figs. 8 to 10) and V phyllosoma of C. crosnieri sub sp. 1 (Fig. 11). Antennule and antenna of ?Chelarctus sp-1 phyllosomas were approximately the same length at all stages (Figs. 12 to 15). Posterolateral spines of the telson in C. virgosus were specifically longer than those of the other species examined.
Table 4.
Morphological characteristics of mid (V) to final (VIII) stage phyllosoma larvae of four Chelarctus species
*antenna exceeding antennule (TX), antennule exceeding antenna (NX), almost the same (S).
It was not challenging to interpret the phylogeny of the slipper lobsters from the subfamily Scyllarinae in this study; however, the results obtained here are not concordant with the taxonomic revisions suggested by Holthuis (2002). Insufficient genetic information and taxon sampling, saturation of the nucleotide substitution, and/or long-branch attraction may cause such discrepancies; alternatively, the taxonomic revisions suggested by Holthuis (2002) may not be an accurate representation of the natural evolution of these animals. The most comprehensive molecular phylogenetic analysis of the slipper lobsters to date (Yang et al. 2012; Palero et al. 2009) and a more recent phylogenetic analysis of Achelata lobsters (Davis et al. 2015) suggest that the Scyllarinae subfamily may be largely subdivided into Bathyarctus and others, which agrees with our phylogenetic results.
Chelarctus cultrifer is known to occur widely in the Indo-western Pacific (Holthuis 2002). Yang and Chan (2012) split this species into two subspecies—C. cultrifer cultrifer from the Indian Ocean and Indonesia and C. cultrifer meridionalis from Indonesia and the Philippines—with relatively large genetic distances (5.6 to 8.3% nucleotide sequence divergence in COI). Yang and Chan (2012) also found many phenotypic differences and large genetic divergence (15.5 to 16.5% in COI) between the adult forms of “C. cultrifer” from Taiwan-Japan and C. cultrifer from the Philippines and described the former as a new species the C. virgosus. Chelarctus virgosus is widely distributed across the Indian Ocean and extends to northeastern Asia, whereas C. cultrifer ssp. is restricted to more southern localities of the Philippines and the Indian Ocean (Yang and Chan 2012), corresponding to the collection area of the C. virgosus phyllosomas identified in this study. Chelarctus virgosus phyllosomas have a sub-pentagon-shaped cephalic shield, antenna exceeding the antennules, and noticeably long posterolateral spines on the telson. Mid-to final-stage phyllosomas of C. cultrifer (Scyllarus cultrifer) from Japanese waters have been reported by Higa and Shokita (2004) and Inoue and Sekiguch (2006), in which their morphological descriptions and body size (stage f-4, f-2, f-1 and final stage in the former and stage IV to VIII in the later) agreed well with those obtained in the present study. Thus, the C. cultrifer phyllosomas previously described in Japanese waters may actually be C. virgosus. The Scyllarus bicuspidatus phyllosoma from Mariana waters (Sekiguchi 1990, fig. 2) and the Indian Ocean (Phillips et al. 1981, fig. 4) also shows great morphological agreement with C. virgosus, with this similarity was already noted by Inoue et al. (2000). True S. bicuspidatus (Crearctus bicuspidatus) phyllosomas reported by Inoue and Sekiguchi (2006) have round cephalic shield, V-shaped antennae, short posterolateral spine of the telson, and much smaller bodies than C. virgosus. Scyllarus sp. A from the South China Sea (Johnson 1971a, figs. 47−53); Scyllarus sp.? from Hawaii (Johnson 1971b, fig. 34); Scyllarus sp. from Hawaii (Johnson 1977, fig. 1); Scyllarus sp. 1, 9, and 13 from off the coast of Japan (Johnson 1979, figs. 5−14); and Scyllarus sp. Z from New Zealand (Webber and Booth 2001, figs. 6−10) may all be C. virgosus, as all of these larvae share half H-shaped antenna, sub-pentagon shaped cephalic shield, and conspicuously long posterolateral spines of telson. However, this inference is still premature because C. cultrifer phyllosomas have not yet been fully catalogued and described in those areas. There have been several reports describing C. cultrifer (Scyllarus cultrifer) phyllosomas identified in the plankton samples collected in the Indian Ocean and off the coast of South Africa (Berry 1974, fig. 49; Prasad et al. 1975, fig. 9; Tampi and George 1975, figs. 29−33). However, all of these larvae had a significantly narrower cephalic shield than the Chelarctus species examined in this study.
Adults of C. aureus have been described in the Philippines, Indonesia, Fiji Islands (Holthuis 2002). More recently, Yang et al. (2014) reported that the distribution of this species extends to Taiwan, corresponding to our collection location of C. aureus phyllosomas (SHU09-3 to SHU09-5) around the Ryukyu Archipelago. A Scyllarus sp. d phyllosoma (designated as stage VI) collected off the north coast of Taiwan (Inoue et al. 2001, fig. 5) showed a great affinity for C. aureus in the present study as it had a wide sub-rectangular-shaped cephalic shield, antennule exceeding antenna, and posterolateral spines of telsons similar in length to the uropod. Chelarctus aureus is not known from Japanese waters (Sekiguchi and Inoue 2002), so the phyllosoma larvae may not be settled in the mainland of Japan.
One phyllosoma specimen (KH16-4-1) collected in the central South Pacific was closely allied with C. crosnieri in the COI tree and designated as C. crosnieri sub sp. 1. Holthuis (2002, figs. 30, 31, 68) described the new species C. crosnieri based on the holotype collected in Tonga, and Yang and Chan (2012) collected C. crosnieri in Vanuatu. Although the collection location of KH16-4-1 is geographically distant from Tonga (c.a. 2500 km away) and Vanuatu (c.a. 4700 km away), the genetic divergence (6.9% in COI) between KH16-4-1 and C. crosnieri (JX486086 by Yang and Chan 2012) is too large to consider them as two extremes of the same species. Therefore, it is likely that there is a distinct population of undescribed species close to C. crosnieri or a subspecies in the tropical to subtropical areas of the central South Pacific. KH16-4-1 (stage V) was the smallest of the phyllosomas examined in this study and had a sub-pentagonal cephalic shield and antennule well exceeding the antenna, which did not match with any phyllosoma larvae reported to date.
Since ?Chelarctus sp-1 was nested with several other Chelarctus species in the phylogenetic tree (Figs. 2, 3), we determined that this species belongs to the genus Chelarctus. A total of five species and subspecies of this genus have been described in the Indo-western Pacific (Holthuis 2002; Yang and Chan 2012; Yang et al. 2012 2014), and COI and 16S sequence data for all these species have been reported (Yang and Chan 2012; Yang et al. 2012 2014). Distinct differences in the COI and 16S sequences between the ?Chelarctus sp-1 and those other Chelarctus species, as well as the morphological uniqueness of ?Chelarctus sp-1 phyllosomas indicate that ?Chelarctus sp-1 may be a unique, previously undescribed species. Nevertheless, the genus to which this species belongs is still uncertain, and we need to obtain the adult specimen to resolve this issue.
Previous studies have suggested that the shape of the antenna, relative arrangement of the tips of the antennule and antenna, relative arrangement of the posterior margins of the uropods and telson, and length of the posterolateral spines on the telson are not very important for species identification at the phyllosoma stage. However, the morphological investigations based on DNA barcoding performed in this study suggest that these characteristics may be useful for species identification. Thus, morphological investigation based on DNA barcoding could allow for the identification of morphological characteristics that are useful and not useful for identifying target species of larval samples. Simple but reliable morphological keys for species identification could facilitate the elucidation of the distribution, transportation, and recruitment processes for various phyllosoma larvae at the species level.
CONCLUSIONS
DNA barcoding analysis using mitochondrial cytochrome oxidase I (COI) and 16S rDNA sequences was performed to identify species of mid to final stage (V to VIII) phyllosoma larvae of the subfamily Scyllarinae collected in the Northwest and central South Pacific. Four larvae collected around the Ryukyu Archipelago and the Ogasawara Islands were identified as Chelarctus virgosus. Three larvae collected around the Ryukyu Archipelago were identified as Chelarctus aureus. DNA barcodes could not be generated for the four larvae collected around the Ryukyu Archipelago, and thus designated as ?Chelarctus sp-1. One larva collected in the central South Pacific was designated as Chelarctus crosnieri sub sp. 1. Half H-shaped antenna, wide sub-pentagon or sub-rectangular shaped cephalic shield, round posterior margin of the uropod not exceeding the central margin of the telson, and long posterolateral spines of the telson were shared among phyllosomas of these four species, and we suggest that a combination of these characteristics can be diagnostic for discriminating these Chelarctus larvae from the other genera of the subfamily Scyllarinae. Species of these four phyllosoma could be distinguished using the shape of the cephalic shield, relative length of the antenna and antennule, and length of the posterolateral spines of the telson.
Acknowledgments
We thank the captains and crew members of the RV Hakuho-Maru, University of Tokyo, RV Shunyo-Maru, Japan Fisheries Research and Education Agency, and RV Koyo-Maru, the Tokyo Ogasawara Marine Products Center, for their invaluable assistance during sampling. We would like to thank Emeritus Professors Katsumi Tsukamoto and Tsuguo Otake, the University of Tokyo, for their generous support in the research cruise. This research was partially supported by JSPS KAKENHI Grant No. 26252030 and the Japan Fisheries Research and Education Agency.
Footnotes
Authors’ contributions: Kenta Ueda and Takashi Yamakawa conceived the study and designed the methodology. Mari Kuroki and Seinen Chow attended the research cruise. Kenta Ueda and Takashi Yanagimoto performed the morphological and molecular analyses. Kenta Ueda and Seinen Chow wrote the first draft of the manuscript, and all authors read, commented on, and approved the manuscript.
Competing interests: There are no conflicts of interest to declare regarding this study.
Availability of data and materials: All nucleotide sequence data are available from the International Nucleotide Sequence Database Collection (INSDC).
Consent for publication: All authors consent to the publication of this manuscript.
Ethics approval consent to participate: Not applicable.
References
- Báez P. 1973. Larvas phyllosoma del Pacifico sur oriental (Crustacea, Macrura, Scyllaridea). Rev Biol Mar Valparaiso 15:115–130.
- Barnett BM, Hartwick RF, Milward NE. 1986. Descriptions of the nisto stage of Scyllarus demani Holthuis, two unidentified Scyllarus species, and the juvenile of Scyllarus martensii Pfeffer (Crustacea: Decapoda: Scyllaridae), reared in the laboratory; and behavioural observations of the nistos of S. demani, S. martensii and Thenus orientalis (Lund). Aust J Mar Freshw Res 37:595–608. doi:10.1071/MF9860595.
- Berry PF. 1974. Palinurid and scyllarid lobster larvae of the Natal coast, South Africa. Invest Rep Oceanogr Res Inst South Africa 34:1‒44.
- Chan TY. 2010. Annotated checklist of the world’s marine lobsters (Crustacea: Decapoda: Astacidea, Glypheidea, Achelata, Polychelida). Raffles Bull Zool Supplement No. 23:153–181.
- Chan TY. 2019. Updated checklist of the world’s marine lobsters. In: E.V. Radhakrishnan, B.F. Phillips and G. Achamveetil (eds). Lobsters: Biology, Fisheries and Aquaculture: 35‒64. Springer, Singapore. doi:10.1007/978-981-32-9094-5_2.
- Chan TY, Ahyong ST, Yang CH. 2013. Priority of the slipper lobster genus Crenarctus Holthuis, 2002, over Antipodarctus Holthuis, 2002 (Crustacea, Decapoda, Scyllaridae). Zootaxa 3701:471‒472. doi:10.11646/zootaxa.3701.4.7. . [DOI] [PubMed]
- Chen HN, Hoeg JT, Chan BKK. 2013. Morphometric and molecular identification of individual barnacle cyprids from wild plankton: an approach to detecting fouling and invasive barnacle species. Biofouling 29:133–145. doi:10.1080/08927014.2012.753061. . [DOI] [PubMed]
- Chow S, Yamada H, Suzuki N. 2006a. Identification of mid-to final stage phyllosoma larvae of the genus Panulirus White, 1847 collected in the Ryukyu Archipelago. Crustaceana 79:745–764. doi:10.1163/156854006778026771.
- Chow S, Suzuki N, Imai H, Yoshimura T. 2006b. Molecular species identification of spiny lobster phyllosoma larvae of the genus Panulirus from the Northwestern Pacific. Mar Biotech 8:260‒267. doi:10.1007/s10126-005-5151-9. . [DOI] [PMC free article] [PubMed]
- Davis KE, Hesketh TH, Delmer C, Wills MA. 2015. Towards a subtree of Arthropoda: a species-level subtree of the spiny, slipper and coral lobsters (Decapoda: Achelate). PLoS ONE 10:e0140110. doi:10.1371/journal.pone.0140110. . [DOI] [PMC free article] [PubMed]
- Fernández L, García C, Alborés I. 2010. Morphological changes in the larval development of the lesser slipper lobster Scyllarus arctus. 9th Larval Biology Symposium, 23−27 August 2010, Wellington, New Zealand.
- Folmer O, Black M, Hoen W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3:294–299. . [PubMed]
- Genis-Armero R, Guerao G, Abelló P, González-Gordillo JI, Cuesta JA, Corbari L, Clark PF, Capaccioni-Azzati R, Palero F. 2017. Possible amphi-Atlantic dispersal of Scyllarus lobsters (Crustacea: Scyllaridae): molecular and larval evidence. Zootaxa 4306:325‒338. doi:10.11646/zootaxa.4306.3.2.
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003. Biological identifications through DNA barcodes. Proc R Soc London B 270:313‒321. doi:10.1098/rspb.2002.2218. . [DOI] [PMC free article] [PubMed]
- Higa T, Fujita Y, Shokita S. 2005. Complete larval development of a scyllarine lobster, Galearctus kitanoviriosus (Harada, 1962) (Decapoda: Scyllaridae: Scyllarinae), reared under laboratory conditions. Crust Res 34:1‒26. doi:10.18353/crustacea.34.0_1.
- Higa T, Shokita S. 2004. Late-stage phyllosoma larvae and metamorphosis of a scyllarid lobster, Chelarctus cultrifer (Crustacea: Decapoda: Scyllaridae), from the northwestern Pacific. Spec Divers 9:221‒249. doi:10.12782/specdiv.9.221.
- Holthuis LB. 1991. Marine lobsters of the world. FAO fisheries synopsis, 13 (125), I.
- Holthuis LB. 2002. The Indo-Pacific scyllarine lobsters (Crustacea, Decapoda, Scyllaridae). Zoosystema 24:499‒683.
- Inoue N, Sekiguchi H, Nagasawa T. 2000. Distribution and identification of phyllosoma larvae in the Tsushima Current region. Bull Jpn Soc Fish Oceanogr 64:129‒137 (in Japanese with English abstract). doi:10.1007/s10872-005-0049-8.
- Inoue N, Sekiguchi H, Yeh SP. 2001. Spatial distributions of phyllosoma larvae (Crustacea: Decapoda: Palinuridae and Scyllaridae) in Taiwanese waters. J Oceanogr 57:535‒548. doi:10.1023/A:1021243301320.
- Inoue N, Sekiguchi H. 2006. Descriptions of phyllosoma larvae of Scyllarus bicuspidatus and S. cultrifer (Decapoda, Scyllaridae) collected in Japanese waters. Plank Benth Res 1:26‒41. doi:10.3800/pbr.1.26.
- Ito M, Lucas JS. 1990. The complete larval development of the scyllarid lobster, Scyllarus demani Holthuis, 1946 (Decapoda, Scyllaridae), in the laboratory. Crustaceana 58:144‒167. doi:10.1163/156854090X00057.
- Johnson MW. 1971a. On palinurid and scyllarid lobster larvae and their distribution in the South China Sea (Decapoda, Palinuridea). Crustaceana 21:247‒282.
- Johnson MW. 1971b. The phyllosoma larvae of slipper lobsters from the Hawaiian Islands and adjacent areas (Decapoda, Scyllaridae). Crustaceana 20:77‒103.
- Johnson MW. 1971c. The phyllosoma larva of Scyllarus delfini (Bouvier) (Decapoda, Palinuridea). Crustaceana 21:161‒164.
- Johnson MW. 1977. On a hitherto unknown phyllosoma larval species of the slipper lobster Scyllarus (Decapoda, Scyllaridae) in the Hawaiian Archipelago. Pac Sci 31:187‒190.
- Johnson MW. 1979. On a North Pacific Scyllarus phyllosoma larva with a forked telson (Decapoda, Scyllaridae). Bull Mar Sci 29:592‒592.
- Kochzius M, Seidel C, Antoniou A, Botla SK, Campo D, Cariani A, Vazquez EG, Hauschild J, Hevet C, Hjörleifsdottir S, Hreggvidsson G, Kappel K, Landi M, Magoulas A, Marteinsson V, Nölte M, Planes S, Tinti F, Turan C, Venugopal MN, Weber H, Blohm D. 2010. Identifying fishes through DNA barcodes and microarrays. PLoS ONE 5:e12620. doi:10.1371/journal.pone. 0012620. . [DOI] [PMC free article] [PubMed]
- Kumar TS, Vijayakumaran M, Murugan TS, Sreeraj G, Muthukumar S 2009. Captive breeding and larval development of the scyllarid lobster Petrarctus rugosus. New Zealand J Mar Freshwat Res 43:101‒112. doi:10.1080/00288330909509985.
- Li JJ, Shih YJ, Ho PH, Jiang GC. 2019. Description of the first zoea of the cavernicolous crab Karstama boholano (Ng, 2002) (Crustacea: Decapoda: Sesarmidae) from Taiwan, with notes on ecology. Zool Stud 58:36. doi:10.6620/ZS.2019.58-36. . [DOI] [PMC free article] [PubMed]
- McWilliam PS, Phillips BF, Kelly S. 1995. Phyllosoma larvae of Scyllarus species (Decapoda, Scyllaridae) from the shelf waters of Australia. Crustaceana 68:537‒566.
- Palero F, Guerao G, Abelló P. 2008. Morphology of the final stage phyllosoma larva of Scyllarus pygmaenus (Crustacea: Decapoda: Syllaridae), identified by DNA analysis. J Plank Res 30:483‒488. doi:10.1093/plankt/fbn012.
- Palero F, Crandall KA, Abelló P, Macpherson E, Pascual M. 2009. Phylogenetic relationships between spiny, slipper and coral lobsters (Crustacea, Decapoda, Achelata). Mol Phyl Evol 50:152‒162. doi:10.1016/j.ympev.2008.10.003. . [DOI] [PubMed]
- Palero F, Guerao G, Clark PF, Abelló P. 2011. Scyllarus arctus (Crustacea: Decapoda: Scyllaridae) final stage phyllosoma identified by DNA analysis, with morphological description. J Mar Biol Assoc UK 91:485‒492. doi:10.1017/S0025315410000 287.
- Palero F, Guerao G, Hall M, Chan T Y, Clark PF. 2014. The ‘giant phyllosoma’ are larval stages of Parribacus antarcticus (Decapoda: Scyllaridae). Inv Syst 28:258‒276. doi:10.1071/IS13037.
- Palumbi SR, Martin AP, Romano S, McMillan WO, Stice L, Grabowski G. 1991. The Simple Fool’s Guide to PCR. Version 2.0. Department of Zoology and Kewalo Marine Laboratory, University of Hawaii, Honolulu, 46 pp.
- Phillips BF, Brown PA, Rimmer DW, Braine SJ. 1981. Description, distribution and abundance of late larval stages of the Scyllaridae (slipper lobsters) in the South-eastern Indian Ocean. Aust J Mar Freshw Res 32:417‒437. doi:10.1071/MF9810417.
- Prasad RP, Tampi PRS. 1957. On the phyllosoma of Mandapam. Proc Nat Inst Sci India 23:48‒67.
- Prasad RP, Tampi PRS. 1960. Phyllosomas of scyllarid lobsters from the Arabian Sea. J Mar Biol Ass India 2:241‒249.
- Prasad RP, Tampi PRS, George MJ. 1975. Phyllosoma larvae from the Indian Ocean collected by the Dana Expedition 1928-1930. J Mar Biol Ass India 17:56‒107.
- Robertson PB. 1968. The complete larval development of the sand lobster, Scyllarus americanus (Smith), (Decapoda, Scyllaridae) in the laboratory, with notes on larvae from the plankton. Bull Mar Sci 18:294‒342.
- Robertson PB. 1971. The larvae and postlarva of the scyllarid lobster Scyllarus depressus (Smith). Bull Mar Sci 21:841‒865.
- Robertson PB. 1979. Larval development of the scyllarid lobster Scyllarus planorbis Holthuis reared in the laboratory. Bull Mar Sci 29:320‒328.
- Sekiguchi H. 1986. Identification of late-stage phyllosoma larvae of the scyllarid and palinurid lobsters in the Japanese waters. Nippon Suisan Gakkaishi 52:1289‒1294. doi:10.2331/suisan. 52.1289.
- Sekiguchi H. 1990. Four species of phyllosoma larvae from the Mariana Waters. Bull Jpn Soc Fish Oceanogr 54:242‒248.
- Sekiguchi H, Inoue N. 2002. Recent advances in larval recruitment processes of scyllarid and palinurid lobsters in Japanese waters. J Oceanogr 58:747‒757. doi:10.1023/A:1022806726150.
- Tampi PRS, George MJ. 1975. Phyllosoma larvae in the IIOE (1960-65) Collections-Systematics. Mahasagar 8:15‒44.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725‒2729. doi:10.1093/molbev/mst197. . [DOI] [PMC free article] [PubMed]
- Wakabayashi K, Yang CH, Chan TY, Phillips BF 2020. The final phyllosoma, nisto, and first juvenile stages of the slipper lobster Petrarctus brevicornis (Holthuis, 1946) (Decapoda: Achelata: Scyllaridae). J Crust Biol 40:237‒246. doi:10.1093/jcbiol/ruaa013.
- Wakabayashi K, Yang CH, Shy JY, He CH, Chan TY. 2017. Correct identification and redescription of the larval stages and early juveniles of the slipper lobster Eduarctus martensii (Pfeffer, 1881) (Decapoda: Scyllaridae). J Crust Biol 37:204‒219. doi:10.1093/jcbiol/rux009.
- Webber WR, Booth JD. 2001. Larval stages, developmental ecology, and distribution of Scyllarus sp. Z (probably Scyllarus aoteanus Powell, 1949) (Decapoda: Scyllaridae). NZ J Mar Freshw Res 35:1025‒1056. doi:10.1080/00288330.2001.9517061.
- WeiJ,GuJ,LiuM,LinB,LeeGY,WaiTC,LamPKS,YanM,Leung PTY. 2021. Littoral water in Hong Kong as a potential transient habitat for juveniles of a temperate deepwater gnomefish, Scombrops boops (Acropomatiformes: Scombropidae). Zool Stud 60:33. doi:10.6620/ZS.2021.60-33. . [DOI] [PMC free article] [PubMed]
- Wong JY, Chen HN, Cha BKK, Tan IKP, Chong VC. 2014. A combined morphological and molecular approach in identifying barnacle cyprids from the Matang Mangrove Forest Reserve in Malaysia: essentials for larval ecology studies. Raff Bull Zool 62:317–329.
- Wong KJH, Tsao YF, Tsai PC, Hsieh WP, Li HR, Machida RJ, Chan BKK. 2020. To the light side: molecular diversity and morphology of stomatopod larvae and juveniles (Crustacea: Malacostraca: Stomatopoda) from crustose coralline algal reefs in Taiwan. Mar Biodivers 51:20. doi:10.1007/s12526-020-01150-z.
- Yang CH, Bracken-Grissom H, Kim D, Crandall KA, Chan TY. 2012. Phylogenetic relationships, character evolution, and taxonomic implications within the slipper lobsters (Crustacea: Decapoda: Scyllaridae). Mol Phyl Evol 62:237‒250. doi:10.1016/j.ympev. 2011.09.019. . [DOI] [PubMed]
- Yang CH, Chan TY. 2010. A new slipper lobster of the genus Galearctus Holthuis, 2002 (Decapoda: cyllaridae) from Taiwan and Japan. In: Fransen et al. (eds). Studies on Malacostraca. Crustaceana Monographs 14: Lipke Bijdeley Holthuis Memorial Volume, pp. 735‒745.
- Yang CH, Chan TY. 2012. On the taxonomy of the slipper lobster Chelarctus cultrifer (Ortmann, 1897) (Crustacea: Decapoda: Scyllaridae), with description of a new species. Raffles Bull Zool 60:449‒460.
- Yang CH, Chen IS, Chan TY. 2008. A new slipper lobster of the genus Petrarctus (Crustacea: Decapoda: Scyllaridae) from the West Pacific. Raffles Bull Zool Suppl 19:71‒81.
- Yang CH, Chen IS, Chan TY. 2011. A new slipper lobster of the genus Galearctus Holthuis, 2002 (Crustacea, Decapoda, Scyllaridae) from New Caledonia. Zoosystema 33:735‒745. doi:10.5252/z2011n2a4.
- Yang CH, Kumar AA, Chan TY. 2017. A new slipper lobster of the genus Petrarctus Holthuis, 2002 (Crustacea, Decapoda, Scyllaridae) from Southwest coast of India. Zootaxa 4329:477‒486. doi:10.11646/zootaxa.4329.5.5. . [DOI] [PubMed]
- Yang CH, Lin CW, Chan TY. 2014. Additional slipper lobsters of the subfamily Scyllarinae Latreille, 1825 (Crustacea, Achelata, Scyllaridae) from Taiwan. Zootaxa 3852:336‒346. doi:10.11646/zootaxa.3852.3.3. . [DOI] [PubMed]