Abstract
Traumatic injury is a major cause of morbidity and mortality worldwide, despite significant advances in treatments. Most deaths occur either very early, through massive head trauma/CNS injury or exsanguination (despite advances in transfusion medicine), or later after injury often through multiple organ failure and secondary infection. Extracellular vesicles (EVs) are known to increase in the circulation after trauma and have been used to limited extent as diagnostic and prognostic markers. More intriguingly, EVs are now being investigated as both causes of pathologies post trauma, such as trauma-induced coagulopathy, and as potential treatments. In this review, we highlight what is currently known about the role and effects of EVs in various aspects of trauma, as well as exploring current literature from investigators who have begun to use EVs therapeutically to alter the physiology and pathology of traumatic insults. The potential effectiveness of using EVs therapeutically in trauma is supported by a large number of experimental studies, but there is still some way to go before we understand the complex effects of EVs in what is already a complex disease process.
Keywords: exosomes, injury, microparticles, microvesicles, treatment
1 |. INTRODUCTION
Trauma remains one of the leading causes of death around the globe and is the leading cause of death in those between 1 and 45 years of age in the United States.1 The overall most common cause of death among patients with traumatic injuries is CNS injury, followed by exsanguination and organ failure, respectively.2 Traditionally, the temporal distribution of mortality due to trauma was trimodal, with immediate/acute, early, and late peaks.3 Taken together, neurologic injuries and circulatory collapse compromise 80% of both immediate and early deaths. Most deaths occur at the scene or within a few hours–days from transport to the hospital and were deemed unavoidable.3,4 Late deaths, which are considered possibly preventable, are due to organ failure, including acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF), and infections/sepsis, and are important targets of ongoing studies. With advancements in research leading to enhanced care, the late peak is nearly eliminated, and the distribution is now chiefly bimodal.5 However, unchanged are the immediate and early deaths, with the peak of early deaths only occurring earlier, accentuating the enormity of injuries to the central nervous system and circulation. Most deaths still occur promptly following trauma, and the “golden hour” is still of pertinence and constitutes a domain in which advances in treatment can result in considerable improvement in trauma survival and outcomes. For this reason, there is considerable interest in finding novel innovative and effective therapeutic strategies to alter mortality both early and late after trauma.
Extracellular vesicles (EVs), phospholipid bilayer membrane-enclosed spherical extracellular structures,6 serve as means of cell-to-cell communication, carrying their messages in their cargo of proteins, lipids, and nucleotides.7 Moreover, EVs often carry surface markers, receptors, and proteins characteristic of their cell of origin.8 All cells release EVs under both physiologic and pathologic conditions, including after trauma and hemorrhagic shock, and so are often considered as good biomarker candidates given their regular detection in all bodily fluids.9 The growing body of research indicates that these vesicles have distinct roles and carry out definitive functions.10 Consequently, there is an emerging appeal to apply this knowledge clinically and use EVs in the diagnosis, prognosis, and treatment of various diseases.11,12
Based on their mechanism of biogenesis and size, EVs can be classified into 3 main groups: (i) exosomes, (ii) microvesicles (MVs)/microparticles (MPs), and (iii) apoptotic bodies.13 Considerable variation also exists between the multiple isolation methods for EVs, and this inevitably has led to disparities in the size, yield, stability, functionality, and purity of the resultant samples being described and analyzed within the literature. Because of this lack of uniformity in the isolation and characterization methods used in the reported studies, and so as to abolish any equivocation about which type of EVs are being referred to, we use the term EVs throughout this review as an umbrella term encompassing exosomes, MPs, and MVs.
In this narrative review, we discuss the emerging potential of EVs in the treatment of trauma, summarizing the current literature on EV-based therapeutics in animal models for hemostasis, inflammation, acute lung injury (ALI), traumatic brain injury (TBI), and spinal cord injury (SCI) in the setting of trauma. A literature search was conducted using PubMed for all articles published prior to February 2021 using various search terms for EVs (EV, MV, MP, exosome, and ectosome) and “trauma” or “injury”. Summarized are the ones that dealt with the treatment of traumatic injury using EVs.
2 |. EFFECT OF TRAUMA ON EV RELEASE
Several studies have shown that the levels of EVs are elevated after injury.14–17 Kuravi et al.16 examined total circulating EV numbers in severely injured trauma patients over a period of 4 weeks and investigated EV cell of origin by assessing cell-specific markers on the isolated EVs by flow cytometry. They were able to show that patients with injury severity scores (ISSs) > 20 (i.e., major trauma) had elevated circulating EV numbers compared with healthy controls, and that the EVs isolated were primarily derived from endothelial cells, platelets, and leukocytes.16 This elevation in the number of circulating EVs following traumatic injury could be the result of cellular activation and active release of EVs, or cellular damage to tissues, blood cells, and vessel walls.16 Interestingly, EV concentration was independent of total platelet and leukocyte cell numbers, suggesting a process of activation specific to trauma that increases EV release. EV levels remained elevated for up to 28 days after injury,16 which may suggest ongoing activation of cells and release of EVs, as well as a potential role of EV in pathologic processes post trauma. Another study by Fröhlich et al.17 also showed evidence of a positive correlation between injury severity and the levels of platelet-derived EVs (PDEVs) and endothelial-derived EVs (EDEVs), which were characterized using flow cytometry. Polytrauma patients (ISSs ≥ 16) showed EDEVs levels that were 13.1- and 8.5-times higher than those found in healthy patients and single-system trauma patients (ISSs < 16), respectively. Similarly, when compared with those of healthy individuals, PDEVs values were, respectively, 3.5 folds and 10 folds higher in single-system trauma patients and polytrauma patients. It is still unknown whether EV number is related to outcome independently of trauma severity, which represents one of the gaps in current knowledge in the field. Studies are also needed to explore the effect, or lack of one therefore, sex has on EVs or their contents in trauma.
3 |. EVS IN HEMOSTASIS
Following major traumatic injury, hemorrhage is responsible for more than 80% of deaths in the operating room, the majority of first-hour mortality and nearly 50% of 24-h mortality following the initiation of care.18 Trauma-induced coagulopathy (TIC), a condition consisting of impaired clotting cascade with profound, continuous bleeding, affects 1 in 4 trauma patients and puts them at risk for higher transfusion requirements, prolonged hospital stay, increased complications, and quintuple the 24-h mortality rate.19,20
The pathogenesis of TIC is multifactorial, with the endothelium (lining of blood vessels) being intimately related to the dysfunction of coagulation. Subsequent to critical injury and the resultant hemorrhagic shock (decreased oxygen perfusion to tissues), the glycocalyx on the endothelium breaks down, resulting in syndecan-1 shedding and evidence of other pathways of endothelial cell activation. These effects precipitate disruption of coagulation cascade regulation, leakage of endothelial barrier, increased inflammation, tissue damage, and organ failure, and altogether poorer outcomes in trauma patients. This syndrome has been termed the endotheliopathy of trauma (EoT),20–22 which may result either from TIC or contribute to the genesis of TIC.
EV shedding from various cells is intensified as a result of cellular activation and injury, and EVs are now being investigated as both culprits in the pathogenesis of complications of trauma such as TIC and EoT, and also as a potential savior for these conditions if used therapeutically. As part of the PROMMTT trial (Prospective Observational Multicenter Major Trauma Transfusion), the Cohen group evaluated the cell of origin of EVs using flow cytometry in 180 trauma patients compared with 65 relatively uninjured patients.15 PROMMTT patients were found to have elevated RBC-derived EVs (RDEVs), EDEVs, and leukocyte-derived EVs (LDEVs). The number of tissue factor (TF)-bearing EVs (TFBEVs) increased in trauma patients compared with control, uninjured patients, as well as increased thrombin generation, an important indicator of clotting and coagulation efficiency. The percentages of EVs from different cell origins was also altered significantly in trauma patients.15 Platelet EVs percentage dropped from 87% in the control group to 64%, RDEVs percentage increased from 6% to 10%, LDEVs percentage went from 6% to 12%, and EDEVs increased from 1% to 14% in trauma patients. Absolute numbers of EVs are hard to define, but percentages in this study are certainly revealing of a potential role for endothelial cell EVs in response to trauma. A subset of the trauma patients was found to be coagulopathic (defined as having abnormal clotting profiles with an INR [international normalization ratio—an indicator of prothrombin time {PT}] ≥ 3 or aPTT [activated partial thromboplastin time] ≥ 35 s) and had higher bleeding, as defined by the need for blood/RBC transfusion, and also increased mortality.15 Interestingly, this subset had significantly lower PDEVs, TFBEVs, and decreased thrombin. As thrombin generation is necessary for hemostasis, these patients had increased rates of clinically significant bleeding and mortality. It is hard to know whether the decrease represents reduced release in these patients, or a consumptive deficit with microthrombi trapping EVs in organs leaving systemic defects.
Trauma patients often receive large amount of blood products as part of their resuscitation, and recent research has focused in identifying the presence of EVs in these blood products, and the role they may play in coagulation and TIC after severe hemorrhage and injury. Matijevic et al., with a focus on EVs, studied the content and functionality of thawed fresh frozen plasma (FFP)23 from the initial thaw (FFP-0) until day 5 of storage (FFP-5). Functionality studies included plasma thrombin generation, thromboelastography for clotting properties, and prothrombinase assay to measure procoagulant activity of EVs. Platelets formed the majority of residual cells in FFP-0 and high levels of platelet EVs were noted (4408 × 103/L). In comparison, FFP-5 revealed a 50% decline in EV numbers, a 29% decline in procoagulant activity, and a 56% decline in potential thrombin generation. Their studies also showed FFP-5 had reduced clotting speed and a longer time to reach maximum clot size on thromboelastography, a measurement of hemostasis ability.23 Filtration of EVs decreased thrombin generation, which was restored upon replacement of the removed EVs.23 They concluded that reduced prohemostatic ability of FFP-5 can be partially explained by the decrease in EV concentration with storage, and hence speculation that EV presence in thawed plasma can be advantageous and protective in the initial management of hemorrhagic shock.23 This provides evidence of the potential for EVs in a hemorrhage setting to be beneficial and the possibility of effective use as a therapeutic.
The presence of phosphatidylserine (PS) on EVs has also been implicated in their ability to induce clotting in trauma patients. PS, which is asymmetrically scattered on the inner surface of the platelet cell membrane, loses its asymmetry and is expressed on the cell surface of EVs released by activated platelets.24–26 As a result of this exposure, PS acts as a catalyst facilitating the congregation of coagulation factors into complexes, thereby promoting coagulation and thrombin generation. Windeløv et al.26 examined the relationship between PS-positive (PS+) PDEV levels in trauma patients and clot formation and transfusion requirements in these patients. Low PS+PDEVs levels were associated with reduced clot formation and higher need for RBCs transfusion over 24 h. However, interestingly there was no association between platelet aggregation and levels of PS+PDEVs, which suggests that platelet function is not altered by the levels of PS+PDEVs, and that their effects may be on platelet interaction with other cells, for example, polymorphonuclear leukocyte (PMNs), or perhaps on coagulation in another way that remains to be elucidated. These findings also indicate that PDEVs may play a vital role in regulating development of TIC, especially the early phase, and that giving patients PS+PDEVs may be beneficial early after severe hemorrhage or trauma. Other studies have also correlated levels of EVs with survival after trauma. One study investigated if a correlation between the levels of EVs and 24-h mortality and 24-h transfusion requirements exists.27 They found that lower EV levels at admission were correlated with higher transfusion requirements, and that, over time, increased or maintained EV levels were found in survivors, whereas decreased levels were found in nonsurvivors. Similarly, a separate study showed that markedly lower levels of PDEVs and reduced thrombin generation potential of EVs were found in nonsurvivors of traumatic injury in comparison to patients who survived.14 EVs could potentially provide a nidus to hemostasis as illustrated in Figure 1.
FIGURE 1. Extracellular vesicles, a potential nidus to hemostasis.
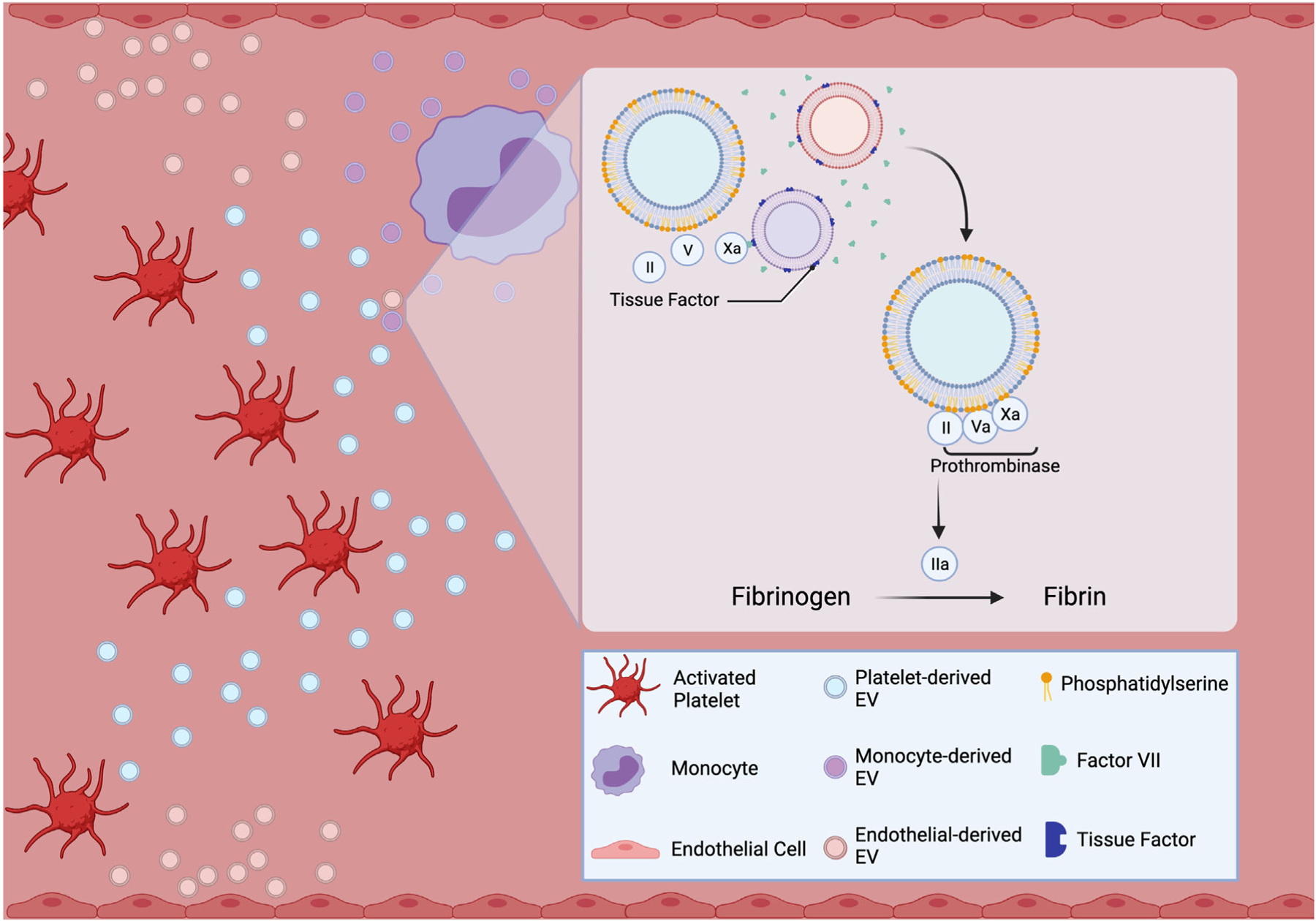
Subsequent to traumatic injury, tissue-factor-bearing extracellular vesicles trigger the clotting cascade via the extrinsic pathway. The loss of phosphatidylserine asymmetry allows it to act as a catalyst for activated factors Xa and Va, ultimately leading to thrombin generation and fibrin formation. “Created with BioRender.com.”
The role of EVs in inducing coagulation is not necessarily straight-forward and may involve complex cell activation and interactions. To investigate some of these mechanisms, Caspers et al.28 pooled the plasma of 20 trauma patients from which EVs were extracted. Using flow cytometry, they measured the surface markers to create a phenotype profile of the EVs secreted after injury. Then, the isolated EVs were added in low and high concentration to an in vitro assay of lethal triad (acidosis, coagulopathy, and hypothermia), and coagulation analysis of EVs was done to investigate their procoagulative activity. Results showed that the most abundant phenotype was PDEV, followed by EDEV and monocyte-derived EVs, all of which expressed TF.28 The addition of EVs in either low or high concentration did not result in significant changes to standard coagulation assays, initiation of coagulation, clot stability, or kinetics. However, the addition of EVs in high concentration caused a significant increase in P-selectin expression and platelet activation, which enhanced their contribution to clot formation.28 These data suggest that use of EVs as a therapeutic strategy to alter coagulation may need some more investigation associated with understanding mechanisms of their effects on multiple cell types in hemorrhage and trauma.
In an attempt to employ the prohemostatic properties of EVs, 2 groups have designed and carried out experiments in which EVs were used as treatments. Our group has been interested in understanding the hemostatic and thrombotic properties of trauma-derived PDEVs. Our group found increased numbers of circulating PDEVs isolated following human and murine trauma, and this was associated with increased thrombin generation and platelet aggregation.29 We also carried out adoptive transfer experiments in age and sex-matched mice using our established model of polytrauma.29 This severe model has been shown to induce TIC, and includes induced hemorrhagic shock, ongoing bleeding via a liver laceration, and bilateral femoral pseudofracture.29 Isolated PDEVs from mice that underwent polytrauma (1–4 × 108 per mouse) or control (vehicle injection alone) were injected via the penile vein into healthy mice 30 min before bleeding time was assessed using a tail-tip bleeding assay. Bleeding time in PDEV-injected mice was significantly decreased. Similarly, in a mouse model of uncontrolled hemorrhage after a liver laceration, there was marked reduction in blood loss in the PDEV-treated group (4 × 108 PDEV given 30 min before resection of the left middle lobe of the liver) compared with the PBS control group.29 Lopez et al.30 also used a similar model of liver laceration in rats to show that human PDEVs exhibited prohemostatic properties in vivo. In their study, they also sought to draw comparison between the effects of PDEV administration compared with fresh platelets (FPLT), commonly given to trauma patients as part of the resuscitation protocol for severe hemorrhage.30 They resected roughly 50% of the middle liver lobe and then administered either PDEVs, FPLT, or vehicle control after 60 s via the jugular vein. The PDEV-treated rats had significantly decreased blood loss and significant improvement in mean arterial blood pressure, plasma protein concentration, lactate level, and base excess, compared with either control or FPLT-treated rats. Once again, these findings suggest PDEVs are at least partly responsible for the therapeutic advantages of platelet transfusion in the treatment of hemorrhage, and that, additionally, they attenuate ischemia and metabolic acidosis and maintain hemodynamic stability. These studies highlight the potential of giving PDEVs in acute hemorrhage to improve outcomes.
4 |. EVS IN HYPERCOAGULATION AND THROMBOSIS
Not only can severe trauma cause hypocoagulation, but particularly in hemorrhage survivors, a late, hypercoagulable state of TIC can also follow major trauma.20 Hypercoagulation is often aggravated by microthrombotic events, including an end stage, disseminated intravascular coagulation like phenotype—in this case, a consumptive coagulopathy—systemic inflammatory response syndrome (SIRS), and ARDS, and macrothrombotic events such as venous thromboembolism, including deep vein thrombosis (DVT) and pulmonary embolism.31
One of the risk factors for thrombotic complications posttrauma is having prolonged procoagulation in phospholipid assays.14,16 Elevated levels of EVs from trauma patients were found to similarly affect phospholipid assays compared with healthy controls.16 Kuravi et al.16 also correlated the quantity of procoagulant phospholipid (PPL) in the plasma of patients and controls with clotting time (CT), using an automated assay. CTs were proportional to the amount of PPL present in the sample, as well as a positive correlation between CT and EV counts. This was further correlated in patients where coagulation was hastened on days 1 and 3 following injury when EVs were also elevated. Little overlap was detected in responses to trauma of control EVs suggesting procoagulant EVs do indeed promote hypercoagulability in trauma patients. These findings suggest that EVs may affect hypercoagulation after trauma, which has implications for potential use of EVs as a therapeutic to regulate coagulation in trauma patients.
The relationship between EVs, thrombin generation, and injury severity was examined by Park et al.32 in blunt trauma patients.32 They observed that the concentration of plasma procoagulant EVs and peak thrombin generation in blunt trauma patients correlated positively with injury severity.32 Interestingly, the standard clotting assays (PT, aPTT) for these patients were within the normal range. In their prospective study of 443 trauma patients with average ISS 13, they noted that thrombin peak height (337.6 vs. 320.6, p 0.0081), as well as time to peak thrombin generation (4.73 vs. 5.11 min, p < 0.001) were significantly greater and shorter, respectively, compared with healthy volunteers.33 Subgroup analysis confirmed that decreased thrombin peak height and time to peak height were positively associated with greater injury severity. Trauma patients also had a significantly greater total number of procoagulant PS+EVs and PDEVs by 12 h after the acute injury period.33 These findings suggest that blunt trauma predisposes individuals to accelerated thrombin generation, and increased procoagulant EVs, which corresponds with hypercoagulable states found post injury in trauma. Our group further investigated the role of trauma-induced PDEVs using a mouse model of inferior vena cava ligation and induced DVT.29 Animals were given 10 × 108 posttrauma-isolated PDEVs via the tail vein 30 min before inferior vena cava ligation. Clots were collected and analyzed at 24 h after ligation and we found that PDEVs localized to the clot site and also enhanced clot formation.29 Similar analysis of EDEVs by Fröhlich et al.17 found that EDEV levels were negatively correlated with aPTT, INR, and rotational thromboelastometry—intrinsic temogram CT, suggesting enhanced clot formation and a procoagulative effect. Interestingly, they also shed light on effects of EDEVs in destruction of clots via fibrinolysis, as they also showed evidence of a negative correlation with fibrinogen temogram values.17 All these studies have major implications when determining whether EVs could and should be used as a therapeutic for hemorrhage, given the known potential complications associated with hypercoagulation after trauma.
5 |. EVS AND TRAUMA-INDUCED INFLAMMATION
Major trauma leads to a pronounced immunologic dysfunction and an altered host defense system.34 In an attempt to remove injured cells and tissues and reestablish hemostasis, an immune response is evoked and is followed by the initiation of repair and healing processes.35 It was previously postulated that trauma induced innate immune hyperinflammatory responses that preceded hypoinflammatory responses modulated by adaptive immunity.34 However, it has now been demonstrated that pro- and anti-inflammatory responses are induced concurrently rather than sequentially, and both commence promptly after injury.36 Many cell types and signaling mechanisms have been associated with both hyper-and hypoinflammation, and recently, it has been appreciated that much of the signaling may involve EVs. This is an obvious important area of research as immune dysregulation has been implicated in the onset of MOF and posttrauma infection, both of which are major factors in posttrauma late morbidity and mortality.
Mesenteric lymph (ML) has been implicated as an important conduit used by inflammatory mediators from hypoxic/damaged intestine to activate systemic immune responses after trauma/hemorrhage.37 Several studies have focused on analysis of MLEVs in trauma and hemorrhage models. Kojima et al.38 gathered MLEVs at 3 time-points (preshock, shock, during resuscitation) in a rat model of trauma/hemorrhagic shock with resuscitation. In comparison with baseline (preshock), the concentration of EVs increased 2-fold during shock and decreased 4-fold following resuscitation.38 Interestingly, EVs seem to be the component of ML having the most inflammatory signaling capacity, as MLEVs isolated during resuscitation increased dose-dependent monocyte NFκB expression 8-fold compared with EV-depleted ML.38 MLEVs isolated during resuscitation also markedly increased TNF-α production in macrophages compared with baseline or shock MLEVs or corresponding EV-lacking ML.38 The same group also investigated cell surface marker expression on MLEVs obtained either pre- or postshock.39 Their data suggest high levels of epithelial cell-specific marker (EpCAM) and CD63 in both pre- and postshock samples, suggesting MLEVs originate primarily in intestinal epithelial cells, which is not surprising. In addition, postshock MLEVs expressed high levels of MHC-II and Fas ligand (FasL) on their surface. Importantly, this study also showed postshock MLEVs induced a 2-fold increase in apoptosis in cultured DCs and reduced LPS-mediated dendritic cell (DC) expression of CD80/CD86 and DC antigen-presenting capacity and ability to stimulate lymphocyte proliferation.39 It therefore seems that intestinal epithelial-derived MLEVs can induce proinflammatory responses and help blunt adaptive immune responses, and this may occur by affecting DC survival and activation. Worth mentioning here is that bacteria have been shown to produce EVs, but, to date, we found no published research to identify if their contribution increases following trauma or intestinal ischemia secondary to shock. This could be an important future area of investigation to determine if bacterial EVs affect outcome.
Blood collected from trauma patients on admission to the emergency department showed lower levels of circulating EVs when compared with controls.40 This contradicts what was shown by many previous studies and can be possibly due to early sample collection or development of EV–cell complexes that would not be detected. Flow cytometry showed that the majority of EVs from trauma patients were of platelet origin, suggesting an important role for PDEV in host immunity after trauma. LPS was used to stimulate whole blood obtained from healthy donors, to which either EV-depleted or EV-rich plasma was added. When compared with plasma of healthy controls, the resultant immune response, as measured by levels of IL-6, IL-10, and TNF- α, was dampened when trauma patient plasma was used. Furthermore, a significant decrease in IL-6 and TNF-α was observed when EV-depleted plasma of either healthy or trauma patients was used. Similarly, Ogura et al.41 showed that increased PDEVs are associated with SIRS after trauma. Activated platelets augmented EV generation and intensified P-selectin-dependent platelet–leukocyte (both monocyte and PMN) interaction.41 Blood samples from trauma patients and healthy controls showed that following severe injury PMNs are activated and increased production of PMN-EVs as well as their oxidative activity on days 2–5 after trauma.42 CD11b expression was increased on PMN-EVs with a diameter < 1.0 μm, whereas CD62L was increased on all sizes of EVs from 1 day to up to 10 days after trauma.42 Variables representing systemic vascular endothelial damage, blood thrombomodulin and soluble e-selectin levels, were measured and showed no significant change between the time points.42 Circulating EVs after trauma were also shown by others to be proinflammatory.16 Trauma patient plasma containing EVs increased adhesion of PBMC, but not PMN, to HUVEC monolayers when compared with plasma of healthy controls. These data suggest that EVs induced by trauma are generally proinflammatory, potentially increase leukocyte migration across endothelium and increase proinflammatory signaling in multiple cell types.
6 |. TRAUMATIC ALI
Often, lungs are the first to be impacted in MOF after trauma, followed by the kidneys and the liver.43 Other organs typically involved are the brain and heart.44 The causes of ALI after trauma are numerous, including: lung contusion and tissue damage from the trauma itself; systemic hemorrhagic shock, hypoperfusion and systemic inflammation; posttrauma sepsis or infection; or even after resuscitation with blood products (transfusion-associated ALI) and volume overload.45 At the very end of the spectrum that ALI comprises is ARDS, which is a major cause of acute respiratory failure after trauma.46,47 ARDS is induced primarily via a dysregulated immune response following systemic or local trauma, which can activate and injure alveolar endothelium and epithelial type I and II pneumocytes leading to infiltration of inflammatory cells.46 Injury to type I alveolar cells and endothelium directly impairs the alveolar–capillary barrier, increasing the permeability to fluid and inflammatory cell migration. Injury to type II alveolar cells results in diminished surfactant secretion and diminished clearance of alveolar fluid. Altogether this results in pulmonary edema, which severely impairs gaseous exchange in the lung and gives rise to hypoxemia.46 This inflammatory exudative phase can further advance to a proliferative phase during which fibrin deposits are seen throughout the lung, and this decreases the pulmonary compliance and ability to bring air into the lungs.47 Remodeling is induced, and this can result in lung fibrosis where lungs become laden with scarring.47 The current treatment of ARDS is predominantly supportive, consisting of fluid management, supplemental oxygen, which is often followed by intubation and mechanical ventilation, neuromuscular blockade, and prone positioning.48 ARDS is experienced by 30% of trauma patients, and trauma patients with ARDS have a 3-fold higher mortality rate than those without.45 Hence, there is intense research investigating novel treatment strategies that are highly necessary.
Dysregulated immune responses after trauma are thought to be induced by inflammatory mediators that are gut-derived and transported in the ML,38 and evidence suggests that inflammatory responses by immune cells are regulated by biologic actions of ML.49 Deitch et al.37 provided seminal work in animal models of trauma and hemorrhage to show that ML diversion or ligation of the lymphatic duct reduces priming of systemic neutrophils, reduces vascular permeability, and reduces resultant lung injury, thus highlighting the crucial role of the gut and ML in SIRS, and in particular the gut–lung axis in ALI. MLEVs from trauma/hemorrhagic shock plasma can activate NF-κB activation in alveolar macrophages similarly to monocytes, and this also results in increased production of proinflammatory cytokines from macrophages.49 In vivo, intravenous injection of MLEVs isolated after trauma/hemorrhage induced inflammatory cell infiltration, altered pulmonary vascular permeability and histologic evidence of ALI compared with ML lacking EVs or MLEVs from nontrauma animals.49 Figure 2 summarizes the effects of MLEVs isolated after resuscitation on inflammation and lung injury.
FIGURE 2. Summary of the in vivo and in vitro effects of postshock mesenteric lymph extracellular vesicles.
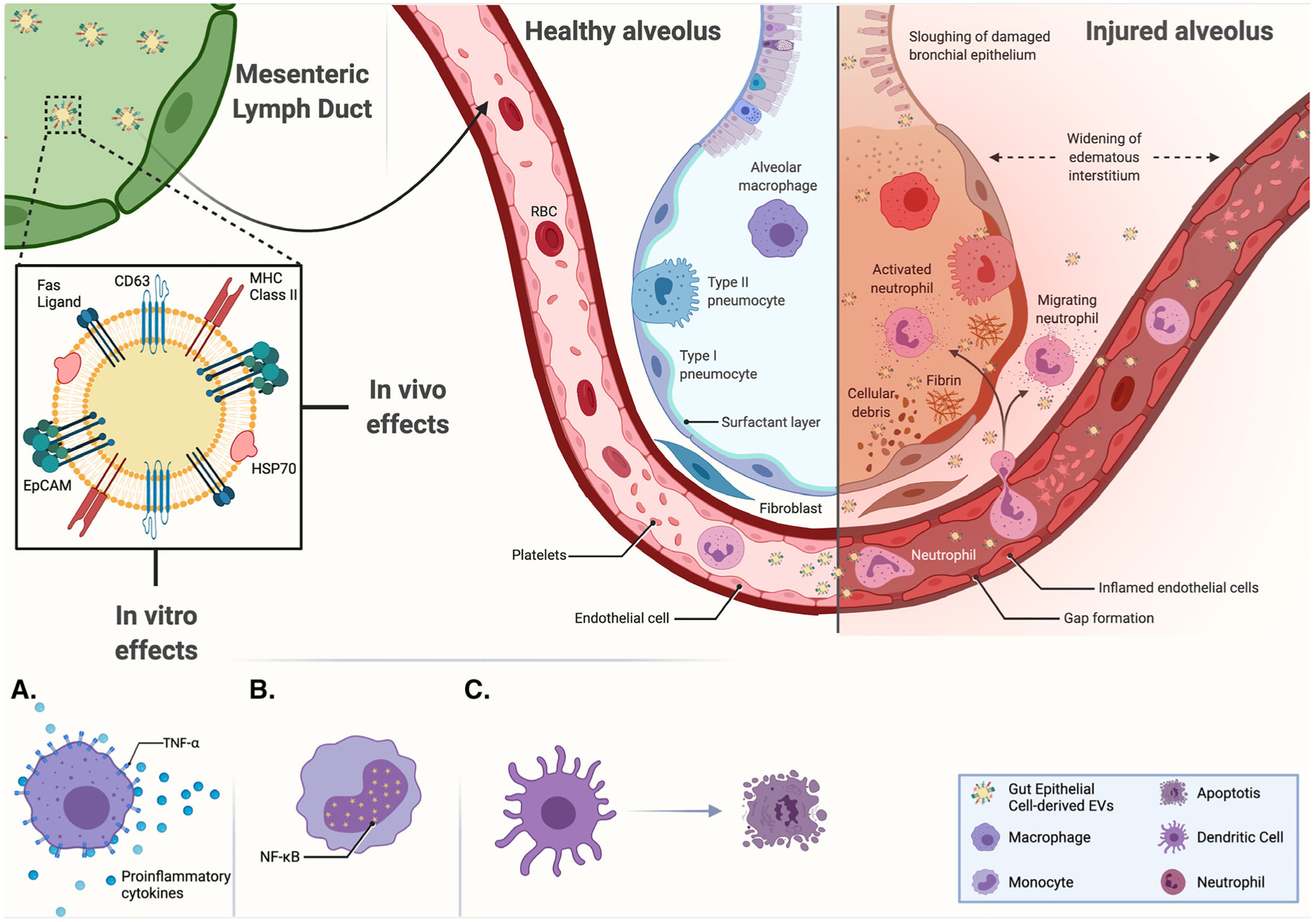
Following hemorrhagic shock, MLEVs isolated during resuscitation were found to display CD63, EpCAM, HSP70, FasL, and MHC class II on their surface. In vitro, they were shown to increase TNF- α and proinflammatory cytokines production from macrophages (A), NFκB expression in monocytes (B), and the apoptosis of cultured dendritic cells (C). While in vivo, their collective proinflammatory and endothelial effects result in a histologic image of acute lung injury. Adapted from “Acute Respiratory Distress Syndrome (ARDS),” by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates
In the lung, EVs can also originate from alveolar epithelial cells, pulmonary vascular endothelial cells, and alveolar macrophages under both physiologic and pathologic conditions.47 ARDS increases EVs production by different types of cells, as can be identified in both the vascular and alveolar compartments.47 As detailed above in this review, ample evidence suggests that circulating EVs have proinflammatory and procoagulant properties, and also the ability to cause endothelial dysfunction and vascular leakage after trauma, which in the lung contributes to the pathophysiology of ARDS.47 At the same time, there is abundant research demonstrating that certain types of EVs, can elicit anti-inflammatory, anticoagulatory, and barrier-improving activity.47 Although EVs have largely been viewed in the context of lung as prospective diagnostic and prognostic markers,47 we should not dismiss potential therapeutic benefits. Considerable effort is underway to define more clearly the role of EVs in both the pathogenesis and resolution of ARDS, and with improved understanding may come novel therapeutic opportunities.
Many cell types have been described for derivation of EVs for use in treatment of animal experimental models. At present, the most studied cell type is the mesenchymal stem cell (MSC) as it has been described numerous times how EVs exhibit and exert actions close to those of their cell of origin.50 MSCs are multipotential stem cells that can develop into numerous nonhematopoietic cell types including chondrocytes, adipocytes, myoblasts, or osteoblasts, and are a cell of great interest in regenerative medicine and tissue engineering.51
Several groups have shown promising data for the use of EVs in treating traumatic ALI. Li et al.52 showed that pretreatment with bone marrow (BM)-derived MSC-EVs can increase survival in a rat model of traumatic ALI. One potential mechanism of MSC-EV effect was shown to be through the inhibition of purinergic type 2 receptor, P2X7, which is up-regulated in rats with traumatic ALI. This group showed that MSC-EVs contained inhibitory micro-RNAs (miRs), including miR-124–3p. Loss-of-function/gain-of-function studies involving both miR-124–3p and P2X7 showed improved survival in the rats where either P2X7 was silenced, or MSC-EVs contained miR-124–3p,52 with multiple markers of reduced lung injury, inflammation and immune cell activation and migration. Potter et al.53 showed that MSC-EVs have the potential to be used in the treatment of hemorrhagic shock-induced ALI. In their in vivo mouse model, they examined the impact of human MSC versus human MSC-EVs on the endothelial permeability of lung vasculature in a fixed-pressure hemorrhagic shock model. They found that, when given intravenously, both MSC and MSC-EVs markedly reduced lung endothelial permeability, as well as reduced cytoskeletal rearrangement.53 Interestingly, analysis of phosphorylated-protein expression profiles in lung tissues from their in vivo model, showed that MSC and MSC-EVs induced different patterns of protein phosphorylation compared with no treatment, with MSC-EVs treatment resulting in a heatmap closer to sham (uninjured) animals. In vitro, human lung microvascular endothelial cells treated with MSC-conditioned media impeded thrombin from affecting endothelial cell permeability, and this did not occur when cells were treated with MSC-EVs.53 Taken together, their findings suggest that different signaling pathways and mechanisms are activated by MSC-EVs in comparison with MSC, and further studies are clearly required to strengthen our understanding of the part EVs play in the bigger picture of ARDS and to utilize their therapeutic potential in its treatment.
6.1 |. EVs in TBI
TBI occurs in 2 stages: a primary phase and a secondary phase.54–57 The primary phase is caused by the direct physical force and its resultant injury to the brain, with intracranial hemorrhage, parenchymal damage, and axonal shearing.56 Although medical therapies cannot prevent this primary phase from occurring, better understanding of the biochemical consequences of this phase of TBI could result in improved diagnostic tests to understand the extent of such an injury for prognostic reasons. The secondary phase, however, is characterized by neuroinflammation related to the host’s attempt to restore structural integrity and improve function in the injured tissue, leading to excitotoxicity, blood–brain barrier disruption, oxidative stress, and mitochondrial dysfunction.56 Although this response is adaptive as an attempt to heal from the injury, hyperinflammation and coagulopathy can lead to worsened outcomes for TBI patients.55,57 A mechanistic understanding of the components of this response is of great interest as they could be potential therapeutic targets where intervention could lead to better functional outcomes in TBI patients.
As will be discussed in this section, EVs are of interest in TBI for numerous reasons. First, they are an early product of injury and could therefore be used for diagnostic purposes, to quantify the extent of damage after a traumatic injury. Furthermore, EVs have been implicated as causes of pathology after TBI, with studies identifying potential targets for intervention that could mitigate the damage caused. Finally, EVs derived from multiple sources are being investigated as a possible biologic therapy for TBI, promoting healing and return to function while avoiding hypercoagulability or hyperinflammation that can cause long-term damage in TBI patients.
A field of study that has become of more popular interest in the last several years is how protein Tau is related to TBI, especially in the setting of media interest in chronic traumatic encephalopathy as it relates to professional athletes and other patients who undergo repeated TBI. Tau is well known to be implicated in the development of Alzheimer’s disease, and it has been found that TBI can accelerate the development of Alzheimer’s pathology.58 Wang et al.59 found that after TBI, there was an increase in both total and phosphorylated Tau in brain EVs. Furthermore, treatment with EVs derived from mice with wild-type Tau after TBI worsened already-poor motor function impairment and cognitive function impairment in TBI mice, whereas treatment with EVs derived from Tau-knockout mice after TBI did not.59 This implies that Tau-loaded EVs may have a dose-dependent effect on worsening neurologic outcomes after TBI. More work needs to be done to better delineate the function of Tau in TBI and the role of EVs in tauopathy.
Although EVs may be implicated in multiple forms of pathology after TBI,54,55 they are also being investigated as the source of potential therapeutics after TBI as well.60–65 The first method by which EVs are being studied for such use is as a vector for the delivery of oligonucleotides, small molecules, or proteins. Wang et al.60 found that EVs could be used as therapeutic vectors when modified to contain plasmids expressing proteins to decrease apoptosis. In this study, when these EVs were given intraventricularly to mice after TBI, the mice had improved excitatory postsynaptic currents in the hippocampus, as well as improved cognitive and motor function. While intraventricular delivery of EVs would be feasible in those patients with severe enough TBI to warrant external ventricular drain placement, use of intravenously delivered EVs (as discussed further in this section) may be more useful across the spectrum of TBI populations. By creating designer EVs based on new developments in research, they may be used as vectors for gene therapy in TBI, similar to the way in which EVs are already being investigated or are approved for drug delivery in other disease processes including cancer and neurodegenerative diseases.66–68
Another therapeutic avenue being explored by other investigators is the use of naturally derived EVs, without artificial modification, as potential therapies for TBI. Naturally derived EVs are an attractive potential therapeutic as they are known to have low immunogenicity and toxicity, and as they are known to be tolerated as they are endogenously produced, regulatory approval may be easier to obtain than for those EVs that are synthesized or modified.69,70 Furthermore, as the therapies would be specifically EV based as opposed to being true cell therapy, there is even more interest as there is no concern for uncontrolled cell division.71 Gao et al.61 found that human umbilical cord endothelial colony-forming cell (ECFC)-derived EVs could be given intravenously to mice after TBI, with multiple beneficial effects. Mice treated with these EVs had decreased brain edema, improved neurologic functional recovery as evidenced by beam-walking and corner tests, and reduced injury-induced blood–brain barrier permeability through reduced degradation of tight junctions.
MSCs with their potential for differentiation promote repair in the injury bed, and secrete a variety of neuroprotective trophic factors (such as vascular endothelial growth factor [VEGF], brain-derived neurotrophic factor, and others) shown to promote neuronal regeneration and repair in animal studies.72 Zhang et al.62 found that rat-derived MSC EVs given intravenously 24 h after injury improved functional recovery in a rat model of TBI, with better spatial learning and sensorimotor functional recovery as evaluated by the modified neurologic severity score (mNSS) test. In a subsequent study, the same group showed that human-derived MSC-EVs given to rats starting day 1 after injury did not actually decrease the cortical lesion volume caused by the TBI.63 Despite this, they again showed improvement in spatial learning and mNSS scores, and also identified increased angiogenesis and reduced neuroinflammation in MSC-EV-treated animals. Similarly, Kim et al.64 found that human-derived MSC-EVs given to mice shortly after TBI decreased inflammatory cytokines in the brain 12 h after the injury. Furthermore, they also found preservation of pattern separation function and spatial learning ability in these mice 1 month after the TBI. Ni et al.65 found that rat-derived MSC-EVs given immediately after TBI reduced brain tissue loss at 3 and 14 days and improved functional recovery as well. These findings were related to decreased inflammatory cytokine production, as well as a shift from the inflammatory M1 to the anti-inflammatory, reparative M2 phenotype in microglia. The mechanisms by which MSC-derived EVs have these numerous effects after TBI require further study, but the work so far clearly demonstrates that this is a potentially valuable therapeutic avenue.
Both MSC- and ECSC-derived EVs are of particular interest because of the underlying cell line’s pluripotency and the ability to drive the cell’s production of the EV of interest. However, the ability to produce these targeted EVs on a scale to allow for generalized medical use is not a trivial matter. One rate-limiting factor for the production of these naturally derived EVs is the ability to create large-scale cultures in the face of limited number of passages before there could be a change in the biologic properties of the cells.71,73 Oncogenic immortalization of these cell lines could potentially mitigate this issue, as well as the use of bioreactors to be able to increase the scale of EVs isolated from MSCs.73 These limitations will need to be probed further on a systems level to be able to make this promising research widely applicable.
The studies discussed represent only a small number of the studies undertaken to investigate the use of EVs in TBI treatment which are summarized in Table 1.
TABLE 1.
Experimental studies assessing the benefit of using extracellular vesicles in traumatic brain injury treatment
| Study Type | Animal Model | EV Type | Source of EVs | Route of Administration | EV Dose | Treatment Timing | Follow-Up Time | Outcome |
|---|---|---|---|---|---|---|---|---|
| In vivo and in vitro102 | Mouse | Exosomes | Mouse astrocytes | N/A | N/A | 20 min after injury | 1, 3, 7, and 14 days after injury |
|
| In vivo and in vitro103 | Rat | Exosomes | Rat astrocytes+normal/damaged neurons |
|
2 μl Of 2400x-enriched exosomes (~3 ± 0.75 μg protein) |
|
1 week | Astrocytal exosomes bearing GJA1-20 k can stimulate neuronal recovery by decreasing the phosphorylation of Cx43, restoring mitochondrial function, and reducing the rate of apoptosis. |
| In vivo64 | Mouse | EVs | Human BM MSC | Intravenous via tail vein | About 30 μg of chromatographically concentrated CM or 1 × 106 hMSCs (donors 6015 or 7074) | 1 h after injury | 28–35 days |
|
| In vivo104 | Yorkshire Swine | Exosomes | Human BM MSC | Intravenously via the left external jugular vein | 1 × 1012 particles/5 ml of LR | 1 h after hemorrhage | 6 h after resuscitation |
|
| In vivo105 | Yorkshire Swine | Exosomes | Human BM MSC | Intravenously via the left external jugular vein | 1 × 1013 particles/5 ml of LR | 1 h after hemorrhage | 6 h after resuscitation | Triggered transcriptomic changes indicative of neuroprotection by:
|
| In vivo62 | Wistar Rat | Exosomes | Rat BM MSC | Intravenously via tail vein | 100 μg total protein of exosome precipitate in 0.5 ml PBS per rat | 24 h after TBI | 1, 4, 7, 14, 21, 28, and 31–35 days after TBI |
|
| In vivo and in vitro61 | Mouse | Exosomes | Human umbilical cord blood-derived ECFCs | Intravenously via tail vein | Exosomes isolated from 4 × 106 cells | 2 h after TBI | 1, 3, 7 and 14 days post TBI |
|
| In vivo65 | Mouse | Exosomes | Rat BM MSCs | Retro-orbital injection | 30 μg protein of BMSCs exosomes suspended in 150 μl PBS | 15 min after CCI-induced TBI | 1, 3, 7, and 14 days post TBI |
|
| In vitro106 | Mouse | Exosomes | Mouse HT-22 neurons | N/A | 100 μl of exosome solutions | N/A | N/A | Exosomal miR-21-5p, whose level rises from the acute to the chronic phase of TBI, diminished neuronal autophagy by targeting Rab11a. |
| In vivo and in vitro107 | Wistar Rat | Exosomes | Human exfoliated deciduous teeth MSC | Locally into the brain | 500 μg/ml and 1000 μg/ml | N/A | 1, 3, 7, 14, and 21 days post TBI |
|
| In vivo and in vitro108 | Rat | Extracellular vesicles | Human neural stem cells | Intravenously via tail vein | 3 injections of 4e10 EVs/kg in 1.6 ml of isotonic PBS | 4–6 h, 24–26 h, and 48–50 h post-CCI | 4, 7, 14, 21, and 28 days post-CCI | NSC-EVs in male rats:
|
| In vivo and in vitro109 | Mouse | Exosome | BV2 microglial cells | Intravenously via tail vein | 30 μg total protein of exosome precipitate in 200, μl PBS/mice | 1 h after first injury | 28–33 days post injury | Microglial exosomes rich in miR-124-3p ameliorated neuroinflammation and stimulated neurite outgrowth, resulting in better neurologic outcomes by inhibiting PDE4B and thus hindering the mTOR signaling pathway. |
| In vivo and in vitro110 | Mouse | Exosome | BV2 microglial cells | Intravenously via tail vein | 30 μg total protein of exosome precipitate in 200 μl PBS/mouse) | 1 h after first impact | 1, 3, 7, 14, and 21–26 days post injury | Microglial exosomes enriched with miR-124-3p reduced modified neurologic severity score (mNSS) values and increased Morris water maze (MWM) test results by protecting neurons and inhibiting neuronal autophagy. |
| In vivo111 | Fisher 344 Rat | Exosomes +/− long noncoding RNA MALAT1 | Human adipose MSC | Intravenously via jugular vein | 100 μg in 500 μl of sterile saline | 3 h after TBI | 1, 3, 7, and 11 days | Treatment with exosomes rich in MALAT1:
|
| In vivo112 | Rats | Exosomes | Human MSCs | Intravenously via tail vein | 50, 100, 200 μg/rat For the therapeutic window study: 100 μg | 1 day after For the therapeutic window study: 1, 4, or 7 days after TBI | Weekly 1–5 weeks, and 31–35 days post TBI | Treatment with exosomes at all the investigated doses and treatment windows resulted in markedly:
|
| In vivo113 | Yorkshire Swine | Exosomes | Human BM MSCs | Intravenously | 1 × 1013/4 ml of NS | 9 h after injury (6 h after resuscitation) and on days 1, 5, 9, and 13 post injury | 1–30 days |
|
| In vivo and in vitro114 | Rat | Exosomes enriched with miR-124 | Rat BM MSCs | Intravenously via tail vein | 3 × 109 particles/each rat | 24 h after | 7, 14, 21, and 24–28 days post TBI |
|
| In vivo60 | Mouse | Exosomes | Human adipose MSCs | Intracerebroventricular injection | 20 μg total protein per rat, 2.0 × 1010 particles/ml | 24 h after | 1, 3, 7, 14, 21, 28, and 35 days postinjury |
|
| In vivo115 | Rat | Exosome | Human adipose MSCs | Intracerebroventricular injection | 20 μg total protein per rat, 2.0 × 1010 particles/ml | 24 h after | 1, 3, 7, 14, 21, 28, and 35 days postinjury |
|
| In vivo and ex vivo63 | Rat | Exosomes | Human MSCs | Intravenous via tail vein | 3 × 109 particles | 24 h after | 14–35 days after injury |
|
6.2 |. EVs in SCI
In many ways the pathophysiology of SCI shares features with that of TBI.74 Injury is divided into a primary and secondary phases, with the former comprising the initial physical tissue insult that results in mechanical damage to the spinal cord tracts, and the latter encompassing the consequences of the resultant immune response and neuroinflammation, which can bring about its own deleterious effects.74 As surgical treatment with urgent decompression and stabilization remains the only means to treat primary SCI aside from injury prevention and avoidance,74 research into SCI therapies have predominantly focused on the mitigation of secondary injury and modulation of the immune milieu following SCI in order to promote healing.
It is within this context that EV-based therapies have been studied. As indicated above in studies investigating treatments for TBI, much of the extant literature involves study of the use of EVs generated from MSCs. However, MSCs themselves are significantly limited in their ability to traverse the brain–spinal cord barrier, and therefore lose some degree of therapeutic potential in their inability to reach the injury site unless administered intrathecally or directly to the site of SCI.75
Accordingly, it had been conjectured that some of the benefit of intravenously administered MSCs derives from EV-related paracrine signaling, and this spurred a body of literature demonstrating that administration of MSC-derived EVs can influence the cellular microenvironment of the injured spinal cord and correlate with improved outcomes. The major theme of these microenvironment changes seems to be toning down the acute inflammatory response and creation of conditions more conducive to neuronal recovery.76 Zhao et al.77 evaluated the use of BM MSC-EVs in a rat model of transection-type SCI, and noted an improvement in functional outcomes (as measured by the Basso, Beattie, Bresnahan locomotor rating scale [BBBS], a common validated metric of motor function following rodent SCI) in injured rats that received MSC-EVs compared with those receiving vehicle only. Notably, this coincided with attenuation of expected elevations in complement protein levels and complement mRNA synthesis within the injured spinal cord, and an overall decrease in injured cord phosphorylated NF-κB in the group treated with MSC-EVs, prompting the conclusion that some aspect of EV-based paracrine signaling mediated these findings.77 Sun et al.78 found similar results in a murine model of blunt SCI where the intervention, human umbilical cord MSC-derived EVs, produced down-regulation of inflammatory cytokines such as TNF- α, IL-6, and IFN- γ, while also producing improved functional BBBS outcomes. Notably, a decrease in acute inflammation has also been demonstrated with the use of neural stem cell (NSC)-derived EVs in a murine model of SCI, where the EV group had decreased levels of TNF- α, IL-6, and proapoptotic markers like Bax and caspase-3. This conclusion in this study was that NSCEVs produce decreased inflammation via an increase in autophagy, as inhibition of autophagosome formation with 3-methyladenine in an in vitro experiment where primary neurons were treated with NSCEVs functionally negated the previously observed anti-inflammatory effects of the EVs.79
The end result of these alterations of the injury site cytokine milieu seems to be a shift in immune cell phenotypes and the induction of a proresolution environment. One established mediator of secondary injury is the A1 astrocyte, a proinflammatory phenotype adopted by astrocytes in the setting of acute neuroinflammation, largely stimulated by NF-κB pathway activation.74 Their privileged position as necessary components of the blood–CNS barrier necessitates their involvement in the margination and extravasation of neutrophils and the initiation of the acute inflammatory response. A1 astrocytes also contribute to the formation of glial scar within the injury bed, which in part serves to limit inflammation to the injury bed itself, but also impedes recovery and regeneration within this space. Furthermore, A1 astrocytes are independently neurotoxic, promoting death of mature neurons, neuronal precursors, and mature oligodendrocytes, and are posited to be the driving force behind neuronal death following axonal disruption.80 With this in mind, several groups have elucidated the effects of MSC-EVs on glial phenotypes, finding a general suppression of A1 astrocytes which have correlated to more limited injury. Wang et al.81 conducted a series of in vitro experiments wherein they cocultured astrocytes obtained from rats subjected to blunt SCI with MSCs or MSC-EVs, finding a reduction in A1 astrocyte phenotypes in groups exposed to MSCs or MSC-EVs compared with controls. They subsequently redemonstrated these findings in vivo by administering MSC-EVs to rats subjected to SCI, observing improved motor outcomes and decreased injury bed A1 astrocytes in the MSC-EV group. Based on in vitro analysis, they concluded that the mechanism of this effect was likely through inhibition of p65 phosphorylation and nuclear transport.81 Liu et al.82 noted similar findings in a similar model of rat blunt SCI, showing a decrease in A1 reactive astrocytes in animals treated with BM MSC-EVs. They also noted improved motor outcomes, decreased glial scar formation, reduced apoptosis, and resulted in smaller lesion size in these animals at 28 days after SCI, implying that suppression of A1 reactive astrocytes encourages a proresolution environment within the injury bed.82 Finally, the Romanelli group83 detected a decrease in reactive astrogliosis following administration of MSC-EV in a rat SCI model, which correlated with decreased glial scarring and lesion size. They further noted in vitro and in vivo evidence of direct interaction between the MSC-EVs and microglia, which reduced IL-1β and IL-6 induction (normally a very early biochemical hallmark of SCI).83 There is also evidence that MSC-EVs colocalize with M2-phenotype macrophages after intravenous administration, providing more context for a therapeutic mechanism that relies on altering the immunologic milieu of the injury bed to better allow for neuronal regeneration.84
Another mechanism of EV action in the therapy of SCI appears to involve the stimulation of angiogenesis and the strengthening of the brain–CNS barrier. It is known that the brain–CNS barrier is disrupted in the course of SCI due partly to the inciting trauma, but also due to pericyte migration away from damaged vascular units in the course of the acute immune response. This results in increased permeability of the brain–CNS barrier and may even serve to enlarge the lesion by virtue of the altered circulatory dynamics.85 It is thought that the pericyte migration is mediated by NF-κB signaling, and Lu et al.75 have demonstrated that the decrease in NF-κB signaling associated with MSC-EV use in rat SCI produced a resultant decrease in the degree of pericyte migration observed as well as a decrease in the permeability of the brain–CNS barrier.
Several groups have used EVs from cell types other than MSC to produce similar effects. For example, Zhong et al.86 used EVs derived from NSC as a therapeutic intervention in a mouse SCI model, finding that these EVs contained a high concentration of VEGF-A, which was associated with decreased injury lesion size, improved microvascular regeneration, and improved functional motor outcomes. Notably, all of these findings were negated when using EVs derived from NSC in which VEGF-A had been transcriptionally silenced.86 One group used EVs derived from cultured pericytes to demonstrate decreased lesion size and a decrease in injury site edema following SCI in a mouse model.87 Following an analysis of protein expression, they concluded that this decrease in edema and lesions size occurred in the context of EV-mediated normalization of expressed tight junction-associated claudin-5 protein levels. The authors concluded that the pericyte-derived EVs contributed to vascular endothelial stability through this regulation of tight junctions, though it is not clear whether this effect is unique to pericyte-derived EVs (in the context of the pericyte’s role in the neurovascular unit) or a nonspecific effect of EVs derived from other lineages.87
Given this extensive evidence of EV-mediated paracrine signaling, some groups have focused on studying miRNA, which have been demonstrated to be abundant within EVs and serve as a major means of cell–cell communication between the EV-spawning cell and other cell types.88 Zhou et al.89 focused on miR-21-5p, a specific miRNA known to induce antiapoptotic effects. In a rat model of SCI, MSC-EV produced results consistent with the studies described before: decreased lesion size, improved functional outcomes, and decreased neuronal apoptosis.89 However, the authors also noted an abundance of miR-21-5p present within MSC-EVs. They subsequently computationally determined (and experimentally confirmed using inhibitors against miR-21-5p) that this specific miRNA was likely to influence expression of FasL, an important component of proapoptotic signaling via the death-ligand pathway.89 Similar results were obtained by another group that additionally identified miR-19b as a potent effector of PTEN suppression, a mechanism suspected to encourage neuronal regeneration within the context of SCI.90
Other research into the therapeutic properties of EVs in SCI has focused on the modification of EVs prior to their use as SCI therapies. In several cases, the modification has involved targeting miRNA signaling by enriching EVs with specific miRNAs. After prior studies in a zebrafish SCI model determined a role for miR-133b in promoting neuronal regeneration via a RhoA-dependent mechanism, Li et al.91 sought to evaluate the hypothesis that miR-133b modified MSC-EVs would improve functional recovery and attenuate neuronal loss in a rat SCI model via RhoA down-regulation. Their findings supported this hypothesis, though interestingly the control MSC-EVs failed to demonstrate any meaningful improvement in functional outcome or lesion size, in contrast to much of the published data detailed earlier.91 Huang et al.92 targeted a different miRNA, miR-126, finding that MSC transfected with this miRNA produced EVs with high miR-126 content, and their use in rats subjected to SCI was associated with improvements in lesion size and functional BBBS status, which they attributed to angiogenesis and inhibition of programmed cell death mediated by down-regulation of SPRED1 and PIK3R2. Another group performed a similar experiment using small interfering RNA (siRNA) directed against PTEN, capitalizing on a base of literature demonstrating PTEN down-regulation as a key target in allowing CNS neuronal regeneration.93 Their study in a rat SCI model found an improvement in axonal growth, improved lesion bed vascularity, and a decrease in reactive astrogliosis—all consistent with a proresolution, proregenerative cellular milieu.93
Aside from transfecting MSCs with small RNA components, groups have also explored different culture conditions for EV-generating cells, which can alter EV contents on the basis of the cellular response induced by the specific context of the culture modification. Ruppert et al.94 performed a study similar to others described previously, using MSC-EV in a rat model of SCI, but also had a separate treatment cohort where the MSCs were cultured with inflammatory stimulation from TNF-α and IFN-γ. Overall, there were similar results seen between the inflammatory group and the noninflammatory MSC-EVs with respect to motor recovery (both significantly increased compared with controls) though the former were associated with improved sensory recovery as well.94 All MSC-EV groups produced decreased inflammation at the injury site, and the authors demonstrated that the MSC response to preconditioning with inflammatory stimulus allowed for creation of EVs with a higher proportion of immunomodulating cytokines and miRNA.94 Ma et al.95 cultured NSC with insulin-like growth factor 1 and observed the subsequent production of EVs rich in miR-219a-2–3p. In rats with SCI, use of these EVs were associated with reduced lesion size and improved BBBS functional outcomes in the context of miRNA-mediated Yin Yang 1 down-regulation and resultant decreased activation of the NF-κB pathway.95
Overall, there is strong evidence suggesting an EV-based approach to treatment of SCI is promising. This could be a huge advance for SCI patients as there is currently little available to specific induce good outcomes after SCI.
7 |. CONCLUDING REMARKS
Although the nascent EV-based therapies provide a promising era of novel treatment modalities that can potentially cause a substantial decrease in the burden of trauma morbidity and mortality, our understanding and experience of the field are still in their infancy. Numerous studies established the stability of EVs in the circulation and during storage, and demonstrated their ability to be lyophilized and still retain their activity and functionality.96–98 These characteristics of EVs, in addition to their low immunogenicity, excellent biocompatibility, and ability to cross biologic barriers make EVs a superior therapeutic option.99–101 Still, transitioning into translational studies requires us to obtain answers for extensive questions relating to: finding the optimal isolation and characterization methods for EVs; determining the optimal dosing strategies for EVs that may be dependent on injury site and desired outcome; ideal route of administration; importance of therapeutic windows; understanding and identifying EV contents that may have beneficial and desired effects, while limiting adverse effects; ideal cell source for EVs; side effects of EV treatments both short and long term; potential antidotes and ways to mitigate potential adverse effects; effect of donor age and sex on EV contents and function; potential for modification of contents to optimize therapeutic effect; and developing artificial EV-like nanoparticles. One thing that would clearly benefit the progression of therapeutic use of EVs is introducing stringent guidelines and scientific standards designed to allow better reproducibility of studies and ensure safety and therapeutic potential of any suggested treatments. There are still a multitude of obstacles to over-come before we are able to implement EV usage clinically, but the potential of using EVs in a range of disease processes, as well as in trauma, is clearly both intriguing and exciting.
ACKNOWLEDGMENTS
Funding support for this review was from NIH R01-HL141080 to M. J. S. and M. D. N., from NIH R01-GM102146, and from NIH R35GM119526.
Abbreviations:
- AKT
sprotein kinase B
- ALI
acute lung injury
- aPTT
activated partial thromboplastin time
- ARDS
acute respiratory distress syndrome
- BAX
BCL2-associated X Protein
- BBB
blood–brain barrier
- BBBS
Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale
- BCL2
B-cell lymphoma 2 gene
- BM
bone marrow
- BMSCs
bone marrow stromal cells
- CCI
controlled cortical impact
- CT
clotting time
- DC
dendritic cell
- DVT
deep vein thrombosis
- ECFCs
endothelial colony-forming cells
- ED
emergency department
- EDEVs
endothelial-derived extracellular vesicles
- EoT
endotheliopathy of trauma
- EpCAM
epithelial cell adhesion molecule
- EVs
extracellular vesicles
- FasL
Fas ligand
- FFP
fresh frozen plasma
- FPLT
fresh platelets
- INR
international normalized ratio
- ISS
injury severity score
- LDEVs
leukocyte-derived EVs
- LR
lactated ringer
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- MiRs
microRNA (miRNA)
- ML
mesenteric lymph
- MLEVs
mesenteric lymph extracellular vesicles
- mNSS
modified neurologic severity score
- MOF
multiple organ failure
- MPs
microparticles
- MSC
mesenchymal stem cells
- mTOR
mechanistic target of rapamycin kinase
- MVs
microvesicles
- MWM
Morris water maze
- NS
normal saline
- NSC
neural stem cells
- P2X7
purinergic type 2 receptor X7
- PDE4B
phosphodiesterase 4B
- PDEVs
platelet-derived extracellular vesicles
- PIK3R2
phosphoinositide-3-kinase regulatory subunit 2
- PMN
polymorphonuclear leukocytes
- PPL
procoagulant phospholipid
- PS
phosphatidylserine
- PT
prothrombin time
- PTEN
phosphate and Tensin homolog
- RDEVs
RBC (erythrocyte)-derived Extracellular Vesicles
- RhoA
Ras homolog family member A
- SCI
spinal cord injury
- siRNA
small interfering RNA
- SIRS
systemic inflammatory response syndrome
- SPRED1
Sprouty-related EVH1 Domain-containing Protein 1
- TBI
traumatic brain injury
- TF
tissue factor
- TFBEVs
tissue factor-bearing extracellular vesicles
- TIC
trauma-induced coagulopathy
- VEGF
vascular endothelial growth factor
- VEGF-A
vascular endothelial growth factor As.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS), Leading Causes of Death, United States, national and regional, 1999–2019. [online] http://www.cdc.gov/injury/wisqars (Accessed April 21, 2021). [PubMed]
- 2.Epidemiology of trauma deaths: a reassessment. J Trauma Acute Care Surg. [online] https://journals.lww.com/jtrauma/Fulltext/1995/02000/Epidemiology_of_Trauma_Deaths__A_Reassessment.6.aspx (Accessed March 22, 2021). [Google Scholar]
- 3.Sobrino J, Shafi S. Timing and causes of death after injuries. Proc (Bayl Univ Med Cent). 2013;26:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tien H, Chu PTY, Brenneman F. Causes of death following multiple trauma. Curr Orthop. 2004;18:304–310. [Google Scholar]
- 5.Gunst M, Ghaemmaghami V, Gruszecki A, Urban J, Frankel H, Shafi S. Changing epidemiology of trauma deaths leads to a bimodal distribution. Proc (Bayl Univ Med Cent). 2010;23:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. [DOI] [PubMed] [Google Scholar]
- 7.Ali M, Pham A, Wang X, Wolfram J, Pham S. Extracellular vesicles for treatment of solid organ ischemia-reperfusion injury. Am J Transplant. 2020;20:3294–3307. [DOI] [PubMed] [Google Scholar]
- 8.Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228:R57–71. [DOI] [PubMed] [Google Scholar]
- 9.Šibíková M, Živný J, Janota J. Cell membrane-derived microvesicles in systemic inflammatory response. Folia Biol (Praha). 2018;64:113–124. [DOI] [PubMed] [Google Scholar]
- 10.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. [DOI] [PubMed] [Google Scholar]
- 11.Mustonen A-M, Nieminen P Extracellular vesicles and their potential significance in the pathogenesis and treatment of osteoarthritis. Pharmaceuticals (Basel). 2021:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, He L, Huang X, et al. Recent progress of exosomes in multiple myeloma: pathogenesis, diagnosis, prognosis and therapeutic strategies. Cancers (Basel). 2021:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curry N, Raja A, Beavis J, Stanworth S, Harrison P. Levels of procoagulant microvesicles are elevated after traumatic injury and platelet microvesicles are negatively correlated with mortality. J Extracell Vesicles. 2014;3:25625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matijevic N, Wang Y-WW, Wade CE, et al. , PROMMTT Study Group. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thromb Res. 2014;134:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuravi SJ, Yates CM, Foster M, et al. Changes in the pattern of plasma extracellular vesicles after severe trauma. PLoS One. 2017;12:e0183640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fröhlich M, Schäfer N, Caspers M, et al. Temporal phenotyping of circulating microparticles after trauma: a prospective cohort study. Scand J Trauma Resusc Emerg Med. 2018;26:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–11. [DOI] [PubMed] [Google Scholar]
- 19.Cardenas JC, Wade CE, Holcomb JB. Mechanisms of trauma-induced coagulopathy. Curr Opin Hematol. 2014;21:404–409. [DOI] [PubMed] [Google Scholar]
- 20.Moore EE, Moore HB, Kornblith LZ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, et al. Syndecan-1: a quantitative marker for the endotheliopathy of trauma. J Am Coll Surg. 2017;225:419–427. [DOI] [PubMed] [Google Scholar]
- 22.Nair AB, Schreiber MA, Pati S. Defining and assessing the endotheliopathy of trauma and its implications on trauma-induced coagulopathy and trauma-related outcomes. In: Moore HB, Neal MD and Moore EE, eds. Trauma Induced Coagulopathy. Cham: Springer International Publishing; 2021:117–133. [Google Scholar]
- 23.Matijevic N, Wang Y-WW, Kostousov V, Wade CE, Vijayan KV, Holcomb JB. Decline in platelet microparticles contributes to reduced hemostatic potential of stored plasma. Thromb Res. 2011;128:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42:423–438. [DOI] [PubMed] [Google Scholar]
- 25.Suades R, Padró T, Vilahur G, Badimon L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb Haemost. 2012;108:1208–1219. [DOI] [PubMed] [Google Scholar]
- 26.Windeløv NA, Johansson PI, Sørensen AM, et al. Low level of procoagulant platelet microparticles is associated with impaired coagulation and transfusion requirements in trauma patients. J Trauma Acute Care Surg. 2014;77:692–700. [DOI] [PubMed] [Google Scholar]
- 27.Matijevic N, Wang Y-WW, Holcomb JB, Kozar R, Cardenas JC, Wade CE. Microvesicle phenotypes are associated with transfusion requirements and mortality in subjects with severe injuries. J Extracell Vesicles. 2015;4:29338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caspers M, Schäfer N, Fröhlich M, et al. Microparticles profiling in trauma patients: high level of microparticles induce activation of platelets in vitro. Eur J Trauma Emerg Surg. 2020;46:43–51. [DOI] [PubMed] [Google Scholar]
- 29.Dyer MR, Alexander W, Hassoune A, et al. Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice. J Thromb Haemost. 2019;17:1733–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez E, Srivastava AK, Burchfield J, et al. Platelet-derived-extracellular vesicles promote hemostasis and prevent the development of hemorrhagic shock. Sci Rep. 2019;9:17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selby R, Geerts W, Ofosu FA, et al. Hypercoagulability after trauma: hemostatic changes and relationship to venous thromboembolism. Thromb Res. 2009;124:281–287. [DOI] [PubMed] [Google Scholar]
- 32.Park MS, Owen BAL, Ballinger BA, et al. Quantification of hypercoagulable state after blunt trauma: microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151:831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park MS, Xue A, Spears GM, et al. Thrombin generation and procoagulant microparticle profiles after acute trauma: a prospective cohort study. J Trauma Acute Care Surg. 2015;79:726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahel PF, Smith WR, Moore EE. Role of biological modifiers regulating the immune response after trauma. Injury. 2007;38:1409–1422. [DOI] [PubMed] [Google Scholar]
- 35.Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. 2018;19:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce A, Pittet J-F. Inflammatory response to trauma: implications for coagulation and resuscitation. Curr Opin Anaesthesiol. 2014;27:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129:39–47. [DOI] [PubMed] [Google Scholar]
- 38.Kojima M, Gimenes-Junior JA, Langness S, et al. Exosomes, not protein or lipids, in mesenteric lymph activate inflammation: unlocking the mystery of post-shock multiple organ failure. J Trauma Acute Care Surg. 2017;82:42–50. [DOI] [PubMed] [Google Scholar]
- 39.Kojima M, Costantini TW, Eliceiri BP, Chan TW, Baird A, Coimbra R. Gut epithelial cell-derived exosomes trigger posttrauma immune dysfunction. J Trauma Acute Care Surg. 2018;84:257–264. [DOI] [PubMed] [Google Scholar]
- 40.Balvers K, Curry N, Kleinveld DJB, et al. Endogenous microparticles drive the proinflammatory host immune response in severely injured trauma patients. Shock. 2015;43:317–321. [DOI] [PubMed] [Google Scholar]
- 41.Ogura H, Kawasaki T, Tanaka H, et al. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma. 2001;50:801–809. [DOI] [PubMed] [Google Scholar]
- 42.Fujimi S, Ogura H, Tanaka H, et al. Increased production of leukocyte microparticles with enhanced expression of adhesion molecules from activated polymorphonuclear leukocytes in severely injured patients. J Trauma. 2003;54:114–9. discussion 119. [DOI] [PubMed] [Google Scholar]
- 43.Moore EE. Organ Claude H. Jr. Memorial Lecture: splanchnic hypoperfusion provokes acute lung injury via a 5-lipoxygenase–dependent mechanism. Am J Surg. 2010;200:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossetti E, Di Nardo M, Ricci Z. Multiple organ dysfunction in the pediatric intensive care unit. Critical Care Nephrology. Elsevier; 2019:1215–1218.e1. [Google Scholar]
- 45.Matthay MA, Pati S, Lee J-W. Concise review: mesenchymal stem (stromal) cells: biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells. 2017;35:316–324. [DOI] [PubMed] [Google Scholar]
- 46.Lee JH, Park J, Lee J-W. Therapeutic use of mesenchymal stem cell-derived extracellular vesicles in acute lung injury. Transfusion. 2019;59:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L364–81. [DOI] [PubMed] [Google Scholar]
- 48.Monsel A, Zhu Y-G, Gudapati V, Lim H, Lee JW. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther. 2016;16:859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kojima M, Gimenes-Junior JA, Chan TW, et al. Exosomes in post-shock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 2018;32:97–110. [DOI] [PubMed] [Google Scholar]
- 50.Lanyu Z, Feilong H. Emerging role of extracellular vesicles in lung injury and inflammation. Biomed Pharmacother. 2019;113:108748. [DOI] [PubMed] [Google Scholar]
- 51.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. [DOI] [PubMed] [Google Scholar]
- 52.Li Q-C, Liang Y, Su Z-B. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2019;316:L1107–L1117. [DOI] [PubMed] [Google Scholar]
- 53.Potter DR, Miyazawa BY, Gibb SL, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J Trauma Acute Care Surg. 2018;84:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiong Y, Mahmood A, Chopp M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin J Traumatol. 2018;21:137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Z, Wang M, Tian Y, et al. Cardiolipin-mediated procoagulant activity of mitochondria contributes to traumatic brain injury-associated coagulopathy in mice. Blood. 2016;127:2763–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agoston DV, Shutes-David A, Peskind ER. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017;31:1195–1203. [DOI] [PubMed] [Google Scholar]
- 57.Crowley MG, Liska MG, Borlongan CV. Stem cell therapy for sequestering neuroinflammation in traumatic brain injury: an update on exosome-targeting to the spleen. J Neurosurg Sci. 2017;61:291–302. [DOI] [PubMed] [Google Scholar]
- 58.Nemetz PN, Leibson C, Naessens JM, et al. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol. 1999;149:32–40. [DOI] [PubMed] [Google Scholar]
- 59.Wang B, Han S. Exosome-associated tau exacerbates brain functional impairments induced by traumatic brain injury in mice. Mol Cell Neurosci. 2018;88:158–166. [DOI] [PubMed] [Google Scholar]
- 60.Wang B, Han S. Modified exosomes reduce apoptosis and ameliorate neural deficits induced by traumatic brain injury. ASAIO J. 2019;65:285–292. [DOI] [PubMed] [Google Scholar]
- 61.Gao W, Li F, Liu L, et al. Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp Neurol. 2018;307:99–108. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Chopp M, Zhang ZG, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim D, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci USA. 2016;113:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni H, Yang S, Siaw-Debrah F, et al. Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front Neurosci. 2019;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Armstrong JPK, Stevens MM. Strategic design of extracellular vesicle drug delivery systems. Adv Drug Deliv Rev. 2018;130:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker S, Busatto S, Pham A, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witwer KW, Wolfram J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sil S, Dagur RS, Liao K, et al. Strategies for the use of extracellular vesicles for the delivery of therapeutics. J Neuroimmune Pharmacol. 2020;15:422–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park K-S, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu P, Yang X. The efficacy and safety of mesenchymal stem cell transplantation for spinal cord injury patients: a meta-analysis and systematic review. Cell Transplant. 2019;28:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23:812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Y, Zhou Y, Zhang R, et al. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front Neurosci. 2019;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15:541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao C, Zhou X, Qiu J, et al. Exosomes derived from bone marrow mesenchymal stem cells inhibit complement activation in rats with spinal cord injury. Drug Des Dev Ther. 2019;13:3693–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun G, Li G, Li D, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. [DOI] [PubMed] [Google Scholar]
- 79.Rong Y, Liu W, Wang J, et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L, Pei S, Han L, et al. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cell Physiol Biochem. 2018;50:1535–1559. [DOI] [PubMed] [Google Scholar]
- 82.Liu W, Wang Y, Gong F, et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36:469–484. [DOI] [PubMed] [Google Scholar]
- 83.Romanelli P, Bieler L, Scharler C, et al. Extracellular vesicles can deliver anti-inflammatory and anti-scarring activities of mesenchymal stromal cells after spinal cord injury. Front Neurol. 2019;10:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lankford KL, Arroyo EJ, Nazimek K, Bryniarski K, Askenase PW, Kocsis JD. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018;13:e0190358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang J-H, Yin X-M, Xu Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J Neurotrauma. 2017;34:3388–3396. [DOI] [PubMed] [Google Scholar]
- 86.Zhong D, Cao Y, Li C-J, et al. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp Biol Med. 2020;245:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan X, Wu Q, Wang P, et al. Exosomes derived from pericytes improve microcirculation and protect blood-spinal cord barrier after spinal cord injury in mice. Front Neurosci. 2019;13:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. [DOI] [PubMed] [Google Scholar]
- 89.Zhou X, Chu X, Yuan H, et al. Mesenchymal stem cell derived EVs mediate neuroprotection after spinal cord injury in rats via the microRNA-21–5p/FasL gene axis. Biomed Pharmacother. 2019;115:108818. [DOI] [PubMed] [Google Scholar]
- 90.Xu G, Ao R, Zhi Z, Jia J, Yu B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J Cell Physiol. 2019;234:10205–10217. [DOI] [PubMed] [Google Scholar]
- 91.Li D, Zhang P, Yao X, et al. Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front Neurosci. 2018;12:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J-H, Xu Y, Yin X-M, Lin F-Y. Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience. 2020;424:133–145. [DOI] [PubMed] [Google Scholar]
- 93.Guo S, Perets N, Betzer O, et al. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13:10015–10028. [DOI] [PubMed] [Google Scholar]
- 94.Ruppert KA, Nguyen TT, Prabhakara KS, et al. Human mesenchymal stromal cell-derived extracellular vesicles modify microglial response and improve clinical outcomes in experimental spinal cord injury. Sci Rep. 2018;8:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma K, Xu H, Zhang J, et al. Insulin-like growth factor-1 enhances neuroprotective effects of neural stem cell exosomes after spinal cord injury via an miR-219a-2–3p/YY1 mechanism. Aging (Albany, NY). 2019;11:12278–12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.US20160158291A1 - Processes for producing stable exosome formulations - Google Patents [online] https://patents.google.com/patent/US20160158291A1/en (Accessed August 18, 2021).
- 97.Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017;20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chao FC, Kim BK, Houranieh AM, et al. Infusible platelet membrane microvesicles: a potential transfusion substitute for platelets. Transfusion. 1996;36:536–542. [DOI] [PubMed] [Google Scholar]
- 100.Shi A, Li J, Qiu X, et al. TGF-β loaded exosome enhances ischemic wound healing in vitro and in vivo. Theranostics. 2021;11:6616–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pofali P, Mondal A, Londhe V. Exosome as a natural gene delivery vector for cancer treatment. Curr Cancer Drug Targets. 2020;20:821–830. [DOI] [PubMed] [Google Scholar]
- 102.Long X, Yao X, Jiang Q, et al. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflamm. 2020;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen W, Zheng P, Hong T, et al. Astrocytes-derived exosomes induce neuronal recovery after traumatic brain injury via delivering gap junction alpha 1–20 k. J Tissue Eng Regen Med. 2020;14:412–423. [DOI] [PubMed] [Google Scholar]
- 104.Williams AM, Bhatti UF, Brown JF, et al. Early single-dose treatment with exosomes provides neuroprotection and improves blood-brain barrier integrity in swine model of traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg. 2020;88:207–218. [DOI] [PubMed] [Google Scholar]
- 105.Williams AM, Higgins GA, Bhatti UF, et al. Early treatment with exosomes following traumatic brain injury and hemorrhagic shock in a swine model promotes transcriptional changes associated with neuroprotection. J Trauma Acute Care Surg. 2020;89:536–543. [DOI] [PubMed] [Google Scholar]
- 106.Li D, Huang S, Zhu J, et al. Exosomes from MiR-21–5p-increased neurons play a role in neuroprotection by suppressing Rab11a-mediated neuronal autophagy in vitro after traumatic brain injury. Med Sci Monit. 2019;25:1871–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y, Yang Y-Y, Ren J-L, Xu F, Chen F-M, Li A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. 2017;8:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun MK, Passaro AP, Latchoumane C-F, et al. Extracellular vesicles mediate neuroprotection and functional recovery after traumatic brain injury. J Neurotrauma. 2020;37:1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang S, Ge X, Yu J, et al. Increased miR-124–3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32:512–528. [DOI] [PubMed] [Google Scholar]
- 110.Li D, Huang S, Yin Z, et al. Increases in miR-124–3p in microglial exosomes confer neuroprotective effects by targeting FIP200-mediated neuronal autophagy following traumatic brain injury. Neurochem Res. 2019;44:1903–1923. [DOI] [PubMed] [Google Scholar]
- 111.Patel NA, Moss LD, Lee J-Y, et al. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J Neuroinflamm. 2018;15:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y, Zhang Y, Chopp M, Zhang ZG, Mahmood A, Xiong Y. Mesenchymal stem cell-derived exosomes improve functional recovery in rats after traumatic brain injury: a dose-response and therapeutic window study. Neurorehabil Neural Repair. 2020;34:616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Williams AM, Dennahy IS, Bhatti UF, et al. Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J Neurotrauma. 2019;36:54–60. [DOI] [PubMed] [Google Scholar]
- 114.Yang Y, Ye Y, Kong C, et al. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res. 2019;44:811–828. [DOI] [PubMed] [Google Scholar]
- 115.Cheng Y-Y, Zhao H-K, Chen L-W, et al. Reactive astrocytes increase expression of proNGF in the mouse model of contused spinal cord injury. Neurosci Res. 2020;157:34–43. [DOI] [PubMed] [Google Scholar]


