Significance
Mutualisms are foundational components of ecosystems and give rise to essential services such as seed dispersal and pollination. Ecologists believe that nearly every species is involved in one or more mutualisms, but it is unknown how consistent behavioral differences among individuals, or personalities, may influence an individual’s role. We scored individuals on a continuum from antagonistic to mutualistic given their contributions to the seed dispersal mutualism and found that personalities affect the extent to which individuals are mutualistic. These findings suggest a novel mechanism generating context dependence in mutualisms and underscore the need to incorporate behavioral diversity into conservation and restoration efforts.
Keywords: intraspecific variation, seed predation, synzoochory, Peromyscus maniculatus, personality
Abstract
Mutualisms are foundational components of ecosystems with the capacity to generate biodiversity through adaptation and coevolution and give rise to essential services such as pollination and seed dispersal. To understand how mutualistic interactions shape communities and ecosystems, we must identify the mechanisms that underlie their functioning. One mechanism that may drive mutualisms to vary in space and time is the unique behavioral types, or personalities, of the individuals involved. Here, our goal was to examine interindividual variation in the seed dispersal mutualism and identify the role that different personalities play. In a field experiment, we observed individual deer mice (Peromyscus maniculatus) with known personality traits predating and dispersing seeds in a natural environment and classified all observed interactions made by individuals as either positive or negative. We then scored mice on a continuum from antagonistic to mutualistic and found that within a population of scatter hoarders, some individuals are more mutualistic than others and that one factor driving this distinction is animal personality. Through this empirical work, we provide a conceptual advancement to the study of mutualism by integrating it with the study of intraspecific behavioral variation. These findings indicate that animal personality is a previously overlooked mechanism generating context dependence in plant–animal interactions and suggest that behavioral diversity may have important consequences for the functioning of mutualisms.
From the ants defending an acacia in exchange for nectar to the plover plucking leftovers from the jaws of a crocodile, it is believed that nearly every organism on earth is involved in at least one mutualistic interaction (1). Mutualisms are relationships between species that are mutually beneficial, are foundational components of ecosystems, are even termed “architectures of biodiversity” given their capacity to generate biodiversity through adaptive evolution and coevolution (2–4), and direct gene flow within populations (5). When a mutualism falls apart, however, there are cascade effects that reach far beyond the players in the interaction (2). Indeed, ecosystem services that we all depend on (such as pollination, seed dispersal, and major biogeochemical cycles) are the products of mutualisms.
The outcome of a mutualism depends on countless interactions between the individuals involved. By extension, there is ample opportunity for variation among individuals to drive these processes (6, 7). Intraspecific variation, such as sexual dimorphism, ontogenetic differences, or resource polymorphism, can foster individual differences in diet, microhabitat preference, foraging behavior, or other forms of resource use (reviewed in ref. 8), and the implications for community dynamics, competition, predation, demographic rates, and evolution have been reviewed comprehensively (6, 9). Empirical investigations examining whether different individuals can affect the outcome of mutualistic interactions are rare, but recent studies have aimed to assess the effects of intraspecific differences related to age, sex, and genetic or morphological variation on mutualistic interactions (6, 10–17). However, we lack empirical studies examining sources of variation that do not fit neatly into these categories, such as consistent intraspecific behavioral differences or personalities (18).
Intraspecific behavioral differences likely play a key role in seed dispersal mutualism via synzoochorous interactions, the intentional transportation of seeds by animals followed by the hoarding of a portion of those seeds (19), which is often referred to as scatter hoarding. For ∼100 My (19), the behavior of individual insects, rodents, and birds has essentially determined which plants will reproduce and where, since dispersers simultaneously consume and kill some seeds while providing an essential service to others (that is, dispersal away from the parent plant where chances of successful recruitment are low) (20). Most synzoochorous species are not wholly mutualistic or antagonistic (19) but instead generate an intermediate outcome, as negative and positive effects are exerted simultaneously by all individuals in a disperser population. However, if we placed each individual in a population of scatter hoarders on a continuum from purely antagonistic to purely mutualistic (19, 21), different individuals would likely vary along this continuum, with some behaving more cooperatively toward plant species than others (such as by caching a greater number of seeds intact). If so, identifying the forces driving this variation would be imperative.
To understand how mutualistic interactions shape communities and ecosystems, we must identify the mechanisms that underlie their functioning. Synzoochory, for example, has been termed a “conditional mutualism” (22, 23) since outcomes vary depending on several biotic and abiotic factors, such as the ratio of seeds to scatter hoarders, the composition of seeds available, soil moisture, fire events, tree density, and seasonal temperature and precipitation (22, 24–27). If individual dispersers differ in their propensities to contribute in mutualistic or antagonistic manners, this would mean that certain individuals may be ultimately more important to the seed dispersal mutualism [i.e., keystone individuals (28)]. It is the culmination of all decisions made by a seed-dispersing animal (e.g., which seed to choose, whether to consume immediately or cache for later, how far to transport, and where to cache) that determine how mutualistic it is on the whole. Interestingly, among all the factors that shape these decisions (29, 30), including intraspecific variation attributable to sexual, ontogenetic, or morphological differences (10), one that is rarely studied is the unique behavioral type of the disperser. A recent conceptual review discusses the capacity for personality traits to influence these processes (31), and a few empirical studies (32–34) show that personality traits (for example, the boldness or risk-taking capacity of an individual) influence decisions made during seed dispersal. Since a single individual makes thousands of these decisions throughout one season, personality type likely affects how mutualistic each disperser behaves on the whole and drives an individual’s position on the antagonism–mutualism continuum.
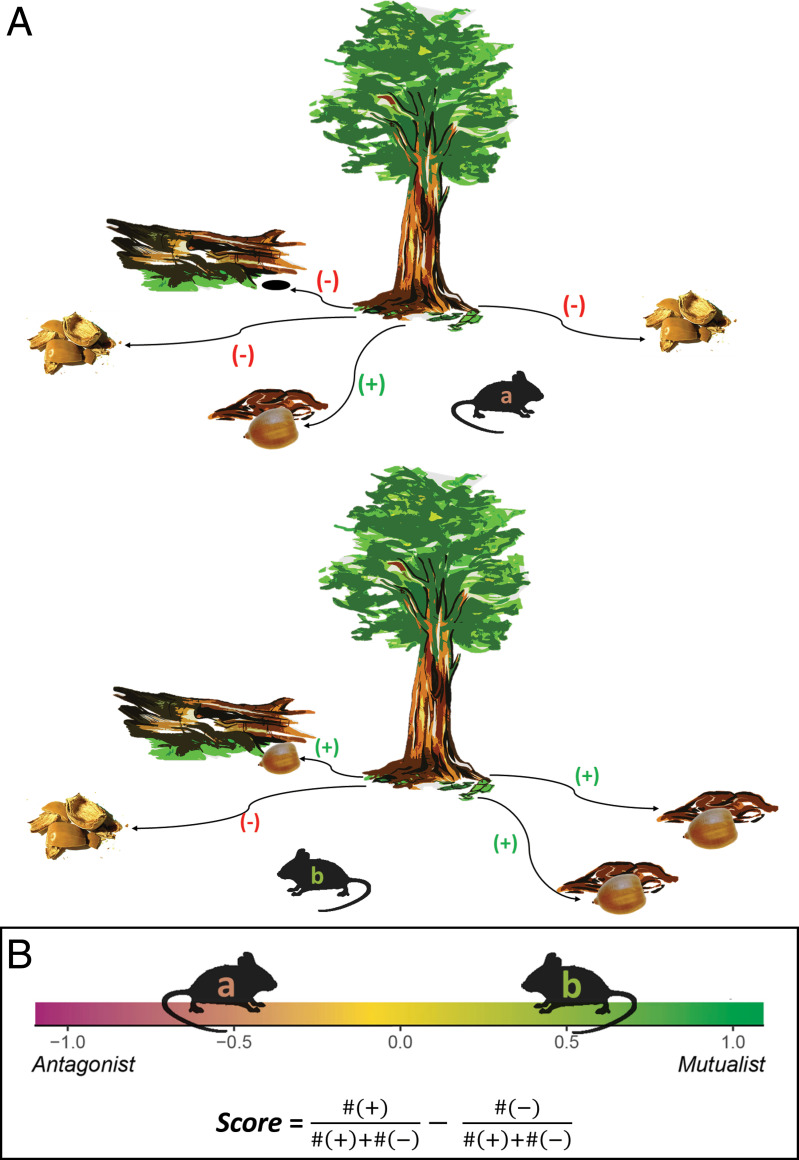
The goal of this study is to examine intraspecific variation in the seed dispersal mutualism and understand the role that personality plays. Our objectives were to observe seed dispersal by known individuals, calculate where they fall along a continuum from antagonist to mutualist (19), and assess whether personality traits affect an individual’s position along the continuum. We implemented a large-scale study wherein we trapped small mammals and measured their personality traits using three standard behavioral assays. We targeted one of the most abundant scatter-hoarding rodents in temperate forests of North America, the deer mouse (Peromyscus maniculatus). We performed a seed experiment and offered seeds of three species and used fluorescent powder (35) to track 792 dispersal routes to assess whether individual interactions were positive or negative (Fig. 1A) and, thus, calculate a score along the antagonism–mutualism continuum (Fig. 1B). Negative interactions included those that immediately precluded a seed from future germination opportunities [i.e., seeds that were consumed at the experiment site, consumed post removal, or taken down a hole where germination is unlikely (36)]. Positive interactions included seeds that were removed from the site and cached intact or left intact at the site after an interaction (SI Appendix, Table S1). Last, we assessed whether personality traits affected each individual’s score along the continuum.
Fig. 1.
(A) Each interaction between a scatter hoarder and a seed can be classified as either positive (seed is dispersed alive and deposited intact) or negative (seed is consumed or taken below ground where germination is unlikely). (B) Using the framework described in ref. 19, all interactions made by an individual can produce an individual’s score along the antagonism–mutualism continuum by subtracting the proportion of negative interactions from the proportion of positive interactions.
Only by identifying the mechanisms underlying this conditional mutualism can we accurately predict outcomes in a changing world. If interindividual differences in behavior is a previously unidentified factor generating context dependence, the loss of certain individuals may drive relationships to the tipping point, poised to shift from mutualism to antagonism. Anthropogenic changes can modify the distribution of personalities within populations (32, 37–39); thus, if personality traits drive an individual’s ecological role (40), altering habitat may impose unexpected consequences on the mutualisms we all depend on. Further, implications of this work could reach far beyond the seed dispersal mutualism; similar mechanisms may shape pollination and plant protection mutualisms where deliberate animal behavior is the driving force.
Results
Repeatability of Behavioral Traits.
From three standardized behavioral assays (an emergence test, an open-field test, and a handling bag test), we examined 819 behavioral observations from 301 individual deer mice with two or more observations and found all behavioral variables to be significantly repeatable (SI Appendix, Table S2). Mean repeatability was 0.330 (range of 0.191 to 0.447), falling in line with similar field studies on deer mice (41) and near the average previously reported for a variety of field and laboratory studies (42). Repeatable traits included the following (SI Appendix, Table S3): mean speed (an indicator of activity), rear rate (activity and exploration), proportion time grooming (anxiety), proportion time center (boldness), handling time (docility), latency to emerge (boldness), and time at end of tunnel (boldness).
Seed Removal Experiments.
At 206 paired seed stations, we observed 1,813 visits by small mammals (1,179 of which were deer mice). Other species to visit included the southern red-backed vole (Myodes gapperi), the American red squirrel (Tamiasciurus hudsonicus), and flying squirrels (Glaucomys spp.). We observed few visits from other species. In total, 1,110 white pine seeds, 0 acorns, and 17 beech seeds were consumed at the sites (or 40%, 0%, and 4.4%, respectively), whereas 1,215 (44.5%), 261 (68%), and 313 (81.5%) were removed from the sites, respectively. We located (or could confidently assume the seed was taken down a hole) 994 removed white pine seeds (82%), 122 acorns (47%), and 201 beech seeds (64%).
Of the 1,179 visits made by deer mice, 955 could be counted as seed interactions (where deliberate contact was made with a seed), and 934 were by tagged deer mice. Of these 934 interactions, 532 were in instances where the cache was located, and the fate of the seed was known or could be confidently inferred (thus, the interaction could be used in further analyses). SI Appendix, Figs. S1–S3 show diagrams detailing the number of each interaction type made by tagged individual deer mice.
Calculating Scores along the Antagonism–Mutualism Continuum.
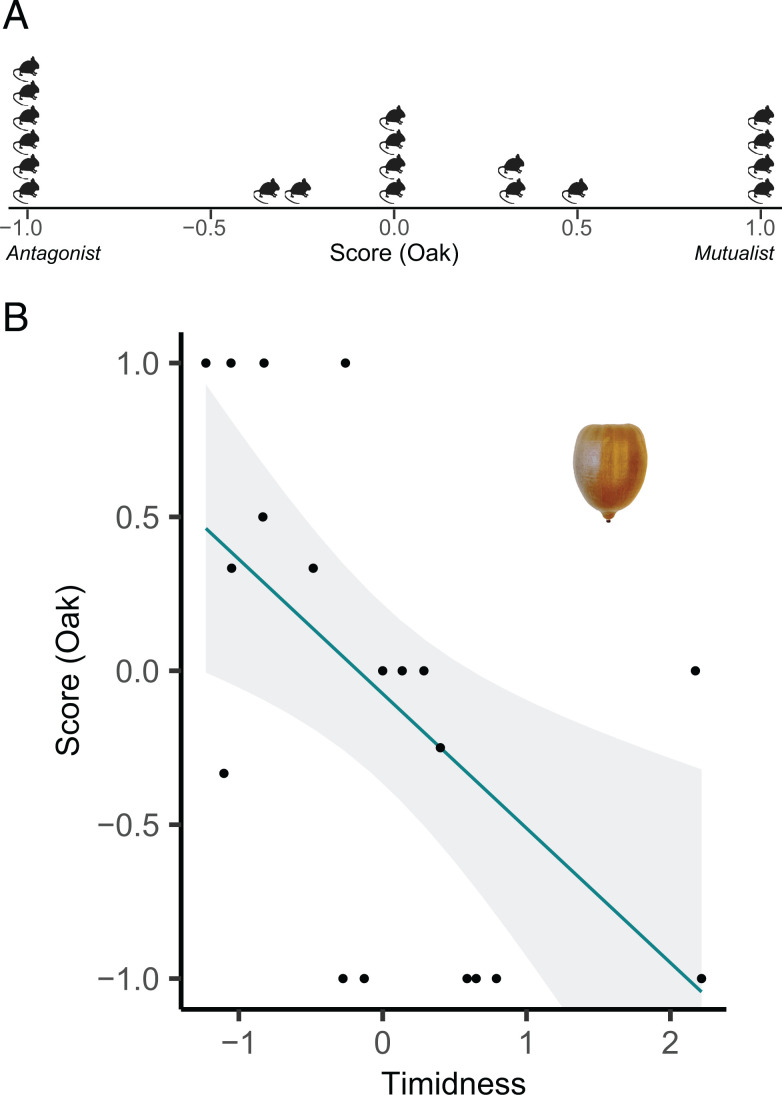
In total, we calculated scores along the antagonism–mutualism continuum for deer mice using 349 observations from 26 individuals interacting with white pine seeds, 135 observations from 21 individuals interacting with beech seeds, and 48 observations from 19 individuals interacting with acorns. On average, mice had negative scores for all three seeds; the mean values for mice interacting with white pine seeds, beech seeds, and acorns were −0.36, −0.37, and −0.07, respectively (SI Appendix, Fig. S4 and Fig. 2A).
Fig. 2.
(A) Individual scores for P. maniculatus along the antagonism–mutualism continuum for red oak (Q. rubra; mean score = −0.07). (B) Predicted relationship (and 95% confidence intervals [95% CIs]) between a personality trait and an individual’s score along the continuum. Timid individuals have lower (more antagonistic) scores for red oak (β = −0.44 ± 0.15 SE) than bolder individuals. Data points represent observed values (one point per individual). Timidness is measured as the time spent at the end of the emergence tunnel before emerging (z standardized), where high values indicate a longer duration at the tunnel end before emerging (high timidness), and low values indicate short durations at the tunnel end before emergence (low timidness, that is, boldness).
Effects of Personality on Scores along the Antagonism–Mutualism Continuum.
For red oak scores (n = 19 individuals), we found that an individual’s degree of boldness/timidness (i.e., time at end of tunnel) affected the position along the antagonism–mutualism continuum. Specifically, timid individuals tended to be more antagonistic (β = −0.44 ± 0.15; SI Appendix, Table S4 and Fig. 2B). We did not find an effect of personality on scores for white pine seeds (n = 26 individuals); instead, the best predictors were body condition index and forest type. Individuals with higher body condition indices fell at a more antagonistic position along the continuum (β = −0.31 ± 0.15; SI Appendix, Table S4 and Fig. S5). The top model predicting the score for American beech (n = 21 individuals) was the null model.
Effects of Personality on Discrete Interactions.
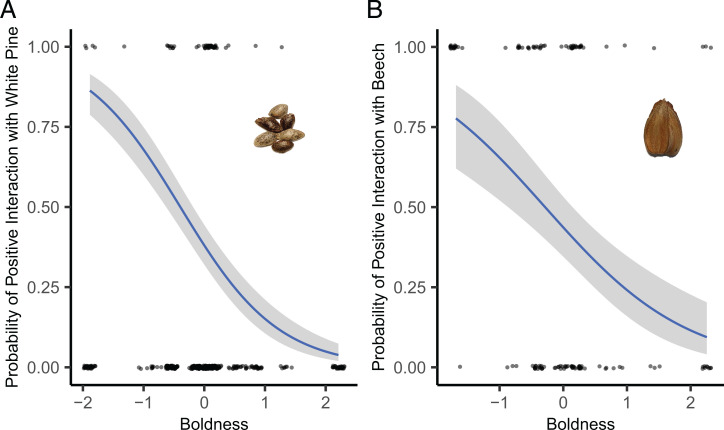
Models predicting each interaction independently showed that repeatable behavioral variables indicating boldness/timidness predicted the probability of interacting positively with white pine and beech seeds (SI Appendix, Table S5). Bolder individuals (key variable was proportion of time in the center) were more likely to interact negatively with both white pine (n = 349 observations from 26 individuals) and beech seeds (n = 135 observations from 21 individuals; β = −1.24 ± 0.41 and β = −0.89 ± 0.33, respectively; Fig. 3 A and B). Other predictors in the top model for white pine interactions were body condition index (β = −0.43 ± 0.20) and forest type.
Fig. 3.
Predicted relationships (and 95% CIs) between a key behavioral variable indicating the degree of boldness/timidness and the probability of interacting positively with seeds. (A and B) Bold P. maniculatus are more likely to have a negative interaction with white pine (P. strobus) (A) and beech (F. grandifolia) (B) than timid individuals (β = −1.24 ± 0.41 SE and β = −0.89 ± 0.33 SE, respectively). Data points represent observed values (one point per individual interaction). Here, boldness is measured as the proportion of time in the center portion of the open-field arena (z standardized), where high values indicate more time in the center (high boldness), and low values indicate less time in the center (low boldness, that is, timidness). The relationship for white pine is shown for the treatment 2 forest type.
Discussion
The seed dispersal mutualism has been termed a conditional mutualism due to the array of biotic and abiotic factors that influence the overall outcome (22). When components, such as the ratio of seeds to dispersers, shift over time or through space, the balance between mutualism and antagonism can be tipped. Until now, knowledge of how individuals may contribute differently to this crucial plant–animal interaction has been limited. We show that personality traits of individual dispersers affect their position along a continuum from antagonist to mutualist. Specifically, the boldness of an individual influenced its tendency to interact positively with seeds. These findings indicate that interindividual differences in behavior, or personalities, are a previously overlooked mechanism driving context dependence in the seed dispersal mutualism. To garner a better mechanistic understanding of the mutualisms that provide essential services such as seed dispersal and pollination, our findings suggest that future research should prioritize understanding the underlying role of intraspecific behavioral diversity in disperser populations.
In this study, deer mice were antagonistic, on average, toward red oak, American beech, and white pine seeds. However, we found that within a population, there is variability in the contributions made by individuals to the seed dispersal mutualism. Specifically, some individuals behave in a far more mutualistic manner than others. In practice, a balance between mutualism and antagonism does not require an equal contribution of predation and successful caching events to break even since just one cache that ends in recruitment has successfully passed on the genetic material of the mother plant. Even if the majority of individuals act as seed predators, a few individuals who cache seeds intact will likely enable the germination rate to exceed 0%, which may translate into a large number of seedlings if seed abundance is high (43). As is true for long-distance dispersal events, the frequency of an event is not necessarily positively correlated with its importance (44). Therefore, positive interactions are disproportionately important to the seed dispersal mutualism since their consequences outweigh those of negative interactions. Here, we show that individuals who provide these positive interactions have personality traits in common. Our previous work has shown that our samples are representative of the greater population (i.e., we are not preferentially sampling certain personality types) (45). Further, the current study was performed among six separate stands within the same experimental forest, and individuals were subject to the same predator communities and weather patterns. By extension, these trends, scaled up to an entire scatter hoarder population over the course of a season, would reflect thousands of seeds cached intact and thousands of others consumed or taken down into underground burrows. Seen in this context, we can begin to understand how heterogeneity in individual contributions can upscale to have ecosystem-level consequences.
When interacting with acorns, bolder mice (i.e., those who emerged from an enclosed space before taking time to assess the safety of surroundings) had more mutualistic scores than timid mice. Bold individuals were more likely to remove acorns from the seed station and cache them intact on or just below the surface (i.e., concealed by a thin layer of detritus, moss, needles, etc.). Timid individuals, instead, were more likely to perform negative interactions such as taking acorns down into an underground burrow (effectively eliminating future chances of germination [50% of negative interactions observed]) or consuming the seed either entirely (8%) or partially (42%) after removal (SI Appendix, Fig. S2). This led to an overall negative relationship between the timidness of an individual and its position on the antagonism–mutualism continuum. This trend may reflect a greater overall perceived risk associated with caching a large seed (a risk that bolder individuals are more willing to take). Larger seeds are generally more conspicuous, and studies have shown that larger seeds are preferentially cached in risky areas (46) to avoid being discovered by competitors. Further, whereas beech and white pine seeds are small enough to be transported in the cheek pouch of a deer mouse, an acorn must be carried externally in the jaws and requires the individual to lift its head to maneuver. Studies have shown that species with cheek pouches tend to disperse smaller seeds (and are likely adapted to do so), whereas pouchless rodents are instead more likely to disperse larger seeds (19, 47). Likely, the mass of the acorn slows the disperser down, and the inability to store the seed in the cheek pouch inhibits the animal’s ability to see optimally and, therefore, remain vigilant. Individuals who are more timid may perform behaviors deemed “safer”, such as moving directly below ground or taking the acorn to a covered area to consume instead of taking the time (and associated risk) to cache the seed on the surface. Examining the relationship between boldness and the tendency to cache large seeds intact in pouchless scatter hoarders, such as red-backed voles, would allow us to understand this relationship more fully, as we would expect this trend to be weaker in a rodent better adapted to disperse large seeds.
Although we observed negative relationships between timidness and antagonistic interactions with both white pine and beech seeds, these are likely driven by different factors. Bold individuals (those who spent more time exploring in a risky area of the open-field arena in the center away from the walls) were far less likely to cache seeds intact after removal. Specifically, bold mice were up to 82% less likely to cache white pine seeds intact and up to 69% less likely to cache beech seeds intact (Fig. 3). The negative interactions observed for white pine seeds were most often instances where individuals remained at the seed site and consumed the seeds immediately (59% of negative interactions observed). Instead, 20% of interactions were seeds consumed after removal, and 21% were seeds taken down into holes. Therefore, the fact that bolder individuals behaved more antagonistically toward white pine is likely because bold individuals were more likely to consume seeds immediately rather than transporting seeds to a safe place prior to consumption. Alternatively, beech seeds were rarely consumed at the seed site (this behavior occurred only ∼4% of the time) but were almost always removed from the site. This finding is consistent with previous research showing the preferential dispersal of larger seeds (3). Negative interactions observed for beech were typically consumption directly after removal (∼54% of negative interactions) or transportation of the seed below ground (39%). The propensity for timid individuals to scatter hoard a beech seed intact may reflect different survival strategies between bold and timid personality types (i.e., prioritizing future energetic reserves over present resource use) and warrants further investigation.
Collectively, deer mice were antagonistic toward red oak, American beech, and white pine seeds. This corroborates previous studies showing that the lower the ratio of seeds to scatter hoarders the greater the proportion of the seed crop that is harvested and predated (22), as this study took place in a nonmast year. It is possible, however, that the trends seen here would be exaggerated in years of seed mast, since, as the scatter hoarder population becomes satiated, the number of cached seeds that would escape predation by competitors (pilferage) and the cache owner (recovery) becomes greater [i.e., the predator satiation hypothesis (48)]. This assumes that the effects of personality on mutualistic behavior are not context dependent (i.e., it assumes that individuals who cache more seeds intact in a nonmast year would also cache more seeds intact in a mast year). Surplus food items typically initiate caching behavior even if the number of cached seeds far outweighs what the animal would need to survive the winter (48), so it is also possible that mast years would dampen the effects of personality type on mutualistic behavior since all individuals would cache more. If the effects of personality on caching probability are consistent among years of high and low resource availability, it is likely in years of seed mast that once the population becomes satiated, the surplus caches made by disproportionately mutualistic individuals would contribute more to recruitment in the plant population. Future studies may focus on years of high resource availability in order to make more general statements about the effect of individuality of the animal mutualist on recruitment rates in the plant partner (21).
Future work should also address other situations under which the effect of personality traits on mutualistic behaviors may be conditional (such as the effects of body condition, ontogenetic effects, or environmental cues such as predator densities or the availability of refuge). It is conceivable that, for example, the degree to which boldness affects an individual’s probability of caching a seed intact depends on whether the individual has a higher or lower body condition index. We note, however, that body condition is not always a good indicator of an individual’s energetic reserves in species that hoard resources (discussed in ref. 37), as these resources are not all stored on the body in the form of fat reserves. In fact, our finding that individuals with lower body condition indices were more likely to cache white pine seeds intact (SI Appendix, Fig. S5) could support the idea that body condition index may be lower in individuals who are caching a greater portion of the resources they find. Finally, one potentially important aspect of an individual’s role as seed predator versus mutualist that was not addressed in this study is the tendency to recover or pilfer caches. In order to fill in the remaining black boxes, studies that allow tracking of a seed through subsequent recaching events [such as using telemetric thread tags (49, 50)] while identifying the individual at each recovery/pilferage are needed. This study should act as a catalyst for future work on this topic.
Mutualistic interactions support much of the earth’s primary production and are, therefore, central pillars of healthy, functioning ecosystems. When one partner in a mutualism is compromised, the other becomes threatened indirectly (51), and maintaining sufficient population sizes of both mutualistic partners is critical to avoid Allee effects (52). Research has shown that bolder and more active individuals are often more likely to be removed from populations via hunting or fishing and may experience differential fitness in response to urbanization (37, 53, 54). In species that act as dispersers in synzoochorous, mycorrhizal, or pollination mutualisms, the removal of certain individuals from populations may, therefore, have unintended consequences if individuals vary in terms of their functional contributions (55). We observed individual deer mice (P. maniculatus) with known personality traits predating and dispersing seeds in a natural environment. We found that within a population of scatter hoarders, there is variability in contributions made to the seed dispersal mutualism. Some individuals are far more mutualistic than others, and one driver of this distinction is animal personality. These findings demonstrate the potential for animal personality to act as a mechanism generating context dependence in the seed dispersal mutualism and provide a conceptual advancement to the study of mutualism, bringing mutualism and intraspecific behavioral variation together in an empirical study.
Materials and Methods
Study Site and Small Mammal Trapping.
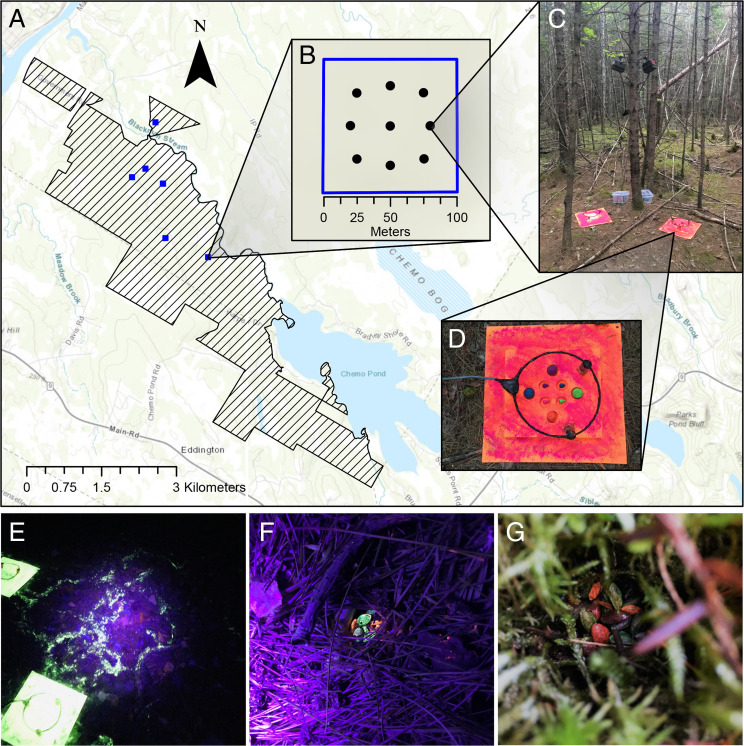
This study was conducted at the Penobscot Experimental Forest (44°51′ N, 68°37′W) in Maine (Fig. 4A). The Penobscot Experimental Forest is a mixed conifer deciduous forest (56) and is dominated by shade-tolerant conifers (57). For more detail about the study area see SI Appendix, Supplementary Methods.
Fig. 4.
(A) Map of the study area at the Penobscot Experimental Forest (44°51′N, 68°37′W) in Maine. (B) Small mammal trapping was performed at six separate trapping grids, and nine seed experiment sites were spaced evenly throughout each grid. (C) Experiment sites contained a set of paired stations monitored by trail cameras. (D) At each station, seed trays were surrounded by antennas attached to permanent radio frequency identification readers to scan and identify tagged individuals. (E) Fluorescent tracking powder allowed cached seeds to be recovered. (F and G) Uniquely marked seeds allowed caches to be attributed to individual dispersers.
From June to October (2016 to 2020), we implemented a large-scale capture–mark–recapture experiment in six separate areas of forest. We trapped small mammals in six trapping grids; each grid was 0.81 ha in area and consisted of 100 flagged points spaced 10 m apart. The mean distance between grids was ∼1.42 km. We placed one Longworth small mammal trap at each flagged point and baited traps with a mixture of sunflower seeds, oats, and freeze-dried mealworms. We bedded traps with cotton stuffing and checked traps twice per day (just after sunrise and in the late afternoon). We trapped at each grid for three consecutive days and nights once per month for 5 mo each year totaling ∼45,000 trap nights (number of active traps × number of nights).
Animal Processing and Behavioral Assays.
All captures were processed at a base area in the grid. Animals were transferred directly from the trap into three standardized behavioral assays to measure behaviors that would later be used to assess personality. An emergence test was used to assess boldness, an open-field test was used to measure activity and exploration in a novel environment, and a handling bag test was used to measure docility and the response to handling by an observer (32, 58). We performed behavioral assays once monthly to ensure that animals would not become habituated. Emergence and open-field tests were videotaped, and behaviors were quantified from videos in the laboratory. For detailed field procedures as well as software and methods used to quantify behavior, see refs. 32 and 59 and SI Appendix, Supplementary Methods. For a complete list of the behaviors measured, their description and interpretation, and supporting sources, see SI Appendix, Table S3 (modified from ref. 10).
After the behavioral assays, we anesthetized animals with isoflurane and inserted PIT tags (passive integrated transponders; Biomark MiniHPT8) subcutaneously at the midback. Animals were marked with a small animal ear tag (National Band, style 1005-1) and a unique haircut. We recorded sex, body mass (using a 100-g Pesola Lightline spring scale), body length, tail length, and age class (juvenile, subadult, or adult based on body size and pelage coloration). Animals were released at the capture site after processing. Previous research in this study system confirms that sampling methods are not biased toward certain personalities and that trapping methods do not impact behavioral measurements in standard assays (45, 59).
Seed Experiments.
In September and October 2020 during natural seed drop, we performed a field experiment to observe individuals with known personality types predating and dispersing seeds in their natural environment. We offered seeds of Northern red oak (Quercus rubra), Eastern white pine (Pinus strobus), and American beech (Fagus grandifolia), and prior to the experiment, seeds were visually inspected for cracks, rot, or weevil holes, and acorns were float tested (SI Appendix, Supplementary Methods).
After trapping was completed in September and October, we positioned seed sites spaced evenly throughout each grid (Fig. 4B). At each site, we placed two seed presentation stations (Fig. 4C). One station consisted of a tray with eight individual wells where we offered four acorns and four beech seeds (Fig. 4D), and the other station had a tray with six wells to each hold five white pine seeds (30 seeds total). Each presentation tray was mounted onto a 30 × 30 cm piece of vinyl. Each seed station was monitored with a trail camera (Reconyx XR6 Ultrafire) and a permanent radio frequency identification (RFID) reader to scan and identify individuals marked with PIT tags. Stations were dusted with ultraviolet (UV) fluorescent tracking powder (TechnoGlow), and seeds were painted a unique color using nontoxic, UV fluorescent paint (Neon Glow, ASTMD-4236 certified) and placed in a known location on the presentation tray. For further information about the seed station setups and methods to ensure that paint did not influence seed selection, cache recovery, or pilferage, see SI Appendix, Supplementary Methods.
Seed stations were set at dusk and visited before dawn so all cache searching could be done in darkness. The observer (A.M.B.) used a UV flashlight (uvBeast) to follow all fluorescent trails from each station (Fig. 4E). The observer recorded whether each trail 1) ended at a seed, 2) ended down a hole, or 3) ended up a tree or faded out. If a seed was found, the observer recorded the identity of the seed and whether the seed was consumed or intact after removal. When trails ended up trees or faded away, these trails could not be linked to a known interaction and were, therefore, not used in further analyses. Fig. 4 F and G depicts images of caches located using these methods. All videos from trail cameras were played back in the laboratory, and identification of each individual visiting the stations was confirmed using the time-stamped RFID reads and the unique haircut seen in the videos (Movie S1). Any instances where a located cache could not be confidently paired with an observed interaction made by a marked individual were not used in further analyses (27 cases for oak, 18 cases for beech, and 6 cases for white pine; SI Appendix, Supplementary Methods).
To minimize visits by nontarget species, stations were removed at dawn and reset at dusk if seeds remained. Seed stations were left active at a site for an average of two nights but were removed after the first night if all seeds were consumed/removed. In total, we offered 412 acorns, 412 beech seeds, and 3,090 white pine seeds at 103 seed sites (206 paired stations).
Statistical Analyses.
First, we performed a repeatability analysis using the rptR package in R (60) to determine which behavioral variables observed during standardized assays could be considered personality traits. We then calculated each individual’s mean best linear unbiased predictor (BLUP) for each behavioral variable over 1,000 simulations with the arm package, and subsequent mentions of personality refer to the mean BLUP value (SI Appendix, Supplementary Methods) (61).
For all Peromyscus who interacted with seeds at presentation stations, we quantified the proportion of interactions that were positive events (the seed was dispersed and cached intact) and the proportion of interactions that were negative events (the seed was consumed at the station, consumed after removal, or could be confirmed to be taken down a hole). In line with ref. 19, we then calculated each individual’s location on the predator–mutualist continuum by subtracting the proportion of negative interactions from the proportion of those that were positive. Individual locations along the continuum, hereafter referred to as scores, were calculated for each seed species separately. SI Appendix, Table S1 shows a breakdown of each interaction type and its classification as positive or negative. We acknowledge that since individuals varied in the number of interactions that went into their calculated score, individuals with a greater number of interactions likely had more precise estimates. While we cannot specifically account for this in the score estimate, we imposed the variable “number of seed interactions” into models predicting the score as detailed below.
Models Predicting Individual Scores along the Antagonism–Mutualism Continuum.
We used a nested hypothesis testing approach (62) to assess whether personality type affects an individual’s location along the continuum (one value per individual). We ran linear models using score as the dependent variable. Throughout our analyses, models within 2.0 ΔAICc (Akaike's information criterion corrected for small sample sizes) of the top model were considered to have equal support (62, 63). We assured noncollinearity between continuous predictor variables before inclusion in models (SI Appendix, Table S6). We first tested covariates suspected to influence individual scores: the number of seed interactions, forest type, trapping grid, sex, an index of body condition [calculated using the scaled-mass index (64)], and body mass (a proxy for age). We compared models to the null, and when more than one model scored higher than the null model, we tested for an additive effect of these variables. We retained the top model from this model set and tested it against seven new models, adding one personality trait measurement to each (SI Appendix, Table S3). We removed one individual Peromyscus from the dataset due to an extreme value (high leverage) in the behavioral variable “proportion of time in the center” so that this trait would not inflate the strength of a regression in which it is included. All continuous predictor variables were scaled (z standardized).
Models Predicting Discrete Interactions with Seeds.
Although both comprehensive and consistent with recent literature, the approach of calculating an individual’s position along the predator–mutualist continuum condenses all interactions by an individual into one single score. To instead assess how personality traits may impact each interaction individually, we performed an additional analysis using mixed-effects models in the R package lme4 (65). We ran logistic models on each seed species separately, using each interaction as a separate observation (a repeated measures design). The dependent variable was a binomial variable with the value 1 if the interaction was classified as positive and 0 if the interaction was classified as negative. We used individual identity as a random intercept in these models and used the same fixed effects and model selection procedures as described above (with the addition of seed mass as a covariate for oak and beech). Running these mixed models predicting the discrete interactions allowed us to incorporate uncertainty due to differing number of interactions among individuals.
Supplementary Material
Acknowledgments
We thank several field assistants and volunteers for their help with data collection, Sara Boone, Ivy Yen, and Margaux Duparcq for support with data collection and project management, Dr. Laura Kenefic and Keith Kanoti for assistance facilitating research in our study area (Penobscot Experimental Forest), Keegan Currier for illustrations, and Malcolm Hunter, Christopher Moore, and two anonymous reviewers for valuable feedback on an earlier version of this manuscript. Rodent silhouettes were obtained from www.phylopic.org. This research was supported by an NSF Career Award to A.M. (IOS number 1940525), a Janet Waldron Doctoral Research Fellowship and a Chase Distinguished Research Assistantship to A.M.B., Penobscot Experimental Forest Research Funds, and the United States Department of Agriculture National Institute of Food and Agriculture McIntire-Stennis Projects ME041620 and ME041913 through the Maine Agricultural & Forest Experiment Station. Maine Agricultural and Forest Experiment Station Publication number 3883.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2113870119/-/DCSupplemental.
Data Availability
All original data and R code used in the main analyses can be accessed on the Figshare repository at https://doi.org/10.6084/m9.figshare.16455570 (66). All other data are included in the article and/or Supporting Information.
References
- 1.Bronstein J. L., Mutualism (Oxford University Press, 2015). [Google Scholar]
- 2.Bascompte J., Jordano P., “Biodiversity and plant-animal coevolution” in Mutualistic Networks (Princeton University Press, 2013), pp. 1–14. [Google Scholar]
- 3.Vander Wall S. B., How plants manipulate the scatter-hoarding behaviour of seed-dispersing animals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 989–997 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bascompte J., Mutualism and biodiversity. Curr. Biol. 29, R467–R470 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Browne L., Ottewell K., Sork V. L., Karubian J., The relative contributions of seed and pollen dispersal to gene flow and genetic diversity in seedlings of a tropical palm. Mol. Ecol. 27, 3159–3173 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Snell R. S., et al. , Consequences of intraspecific variation in seed dispersal for plant demography, communities, evolution and global change. AoB Plants 11, plz016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain S. A., Bronstein J. L., Rudgers J. A., How context dependent are species interactions? Ecol. Lett. 17, 881–890 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Bolnick D. I., et al. , The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 161, 1–28 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Bolnick D. I., et al. , Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwolak R., How intraspecific variation in seed-dispersing animals matters for plants. Biol. Rev. Camb. Philos. Soc. 93, 897–913 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Fuster F., Traveset A., Importance of intraspecific variation in the pollination and seed dispersal functions of a double mutualist animal species. Oikos 129, 106–116 (2020). [Google Scholar]
- 12.Correa S. B., et al. , Overfishing disrupts an ancient mutualism between frugivorous fishes and plants in Neotropical wetlands. Biol. Conserv. 191, 159–167 (2015). [Google Scholar]
- 13.Pérez-Méndez N., Rodríguez A., Nogales M., Intra-specific downsizing of frugivores affects seed germination of fleshy-fruited plant species. Acta Oecol. 86, 38–41 (2018). [Google Scholar]
- 14.Rodríguez-Rodríguez M. C., Jordano P., Valido A., Quantity and quality components of effectiveness in insular pollinator assemblages. Oecologia 173, 179–190 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Tur C., Vigalondo B., Trøjelsgaard K., Olesen J. M., Traveset A., Downscaling pollen-transport networks to the level of individuals. J. Anim. Ecol. 83, 306–317 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Smith G. P., Bronstein J. L., Papaj D. R., Sex differences in pollinator behavior: Patterns across species and consequences for the mutualism. J. Anim. Ecol. 88, 971–985 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Izquierdo L., et al. , Plant intraspecific variation modulates nutrient cycling through its below ground rhizospheric microbiome. J. Ecol. 107, 1594–1605 (2019). [Google Scholar]
- 18.Sih A., Bell A. M., Johnson J. C., Ziemba R. E., Behavioral syndromes: An intergrative overiew. Q. Rev. Biol. 79, 241–277 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Gómez J. M., Schupp E. W., Jordano P., Synzoochory: The ecological and evolutionary relevance of a dual interaction. Biol. Rev. Camb. Philos. Soc. 94, 874–902 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Howe H. F., Smallwood J., Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 13, 201–228 (1982). [Google Scholar]
- 21.Schupp E. W., Jordano P., Gómez J. M., A general framework for effectiveness concepts in mutualisms. Ecol. Lett. 20, 577–590 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Theimer T. C., “Rodent scatterhoarders as conditional mutualists” in Seed Fate: Predation, Dispersal and Seedling Establishment, Forget P. M., Lambert J. E., Hulme P. E., Vander Wall S. B., Eds. (CABI, 2005), pp. 283–295. [Google Scholar]
- 23.Bronstein J. L., Conditional outcomes in mutualistic interactions. Trends Ecol. Evol. 9, 214–217 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Lichti N. I., Steele M. A., Zhang H., Swihart R. K., Mast species composition alters seed fate in North American rodent-dispersed hardwoods. Ecology 95, 1746–1758 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Greenler S. M., Estrada L. A., Kellner K. F., Saunders M. R., Swihart R. K., Prescribed fire and partial overstory removal alter an acorn-rodent conditional mutualism. Ecol. Appl. 29, e01958 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Vander Wall S. B., The influence of environmental conditions on cache recovery and cache pilferage by yellow pine chipmunks (Tamias amoenus) and deer mice (Peromyscus maniculatus). Behav. Ecol. 11, 544–549 (2000). [Google Scholar]
- 27.Moore C. M., Dittel J. W., On mutualism, models, and masting: The effects of seed-dispersing animals on the plants they disperse. J. Ecol. 108, 1775–1783 (2020). [Google Scholar]
- 28.Modlmeier A. P., Keiser C. N., Watters J. V., Sih A., Pruitt J. N., The keystone individual concept: An ecological and evolutionary overview. Anim. Behav. 89, 53–62 (2014). [Google Scholar]
- 29.Lichti N. I., Steele M. A., Swihart R. K., Seed fate and decision-making processes in scatter-hoarding rodents. Biol. Rev. Camb. Philos. Soc. 92, 474–504 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Wang B., Ye C. X., Cannon C. H., Chen J., Dissecting the decision making process of scatter-hoarding rodents. Oikos 122, 1027–1034 (2013). [Google Scholar]
- 31.Zwolak R., Sih A., Animal personalities and seed dispersal: A conceptual review. Funct. Ecol. 34, 1294–1310 (2020). [Google Scholar]
- 32.Brehm A. M., Mortelliti A., Maynard G. A., Zydlewski J., Land-use change and the ecological consequences of personality in small mammals. Ecol. Lett. 22, 1387–1395 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Boone S. R., Brehm A. M., Mortelliti A., Seed predation and dispersal by small mammals in a landscape of fear: Effects of personality, predation risk and land-use change. Oikos 2022, e08232 (2022). [Google Scholar]
- 34.Feldman M., Ferrandiz-Rovira M., Espelta J. M., Muñoz A., Evidence of high individual variability in seed management by scatter-hoarding rodents: Does ‘personality’ matter? Anim. Behav. 150, 167–174 (2019). [Google Scholar]
- 35.Lemke A., Von Der Lippe M., Kowarik I., New opportunities for an old method: Using fluorescent colours to measure seed dispersal. J. Appl. Ecol. 46, 1122–1128 (2009). [Google Scholar]
- 36.Vander Wall S. B., Forget P., Lambert J., Hulme P., Seed Fate: Predation, Dispersal, and Seedling Establishment (CABI, 2005). [Google Scholar]
- 37.Miranda A. C., Schielzeth H., Sonntag T., Partecke J., Urbanization and its effects on personality traits: A result of microevolution or phenotypic plasticity? Glob. Change Biol. 19, 2634–2644 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Lapiedra O., Chejanovski Z., Kolbe J. J., Urbanization and biological invasion shape animal personalities. Glob. Change Biol. 23, 592–603 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Mortelliti A., Brehm A. M., Environmental heterogeneity and population density affect the functional diversity of personality traits in small mammal populations. Proc. Biol. Sci. 287, 20201713 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter M. L., Boone S. R., Brehm A. M., Mortelliti A., Modulation of ecosystem services by animal personalities. Front. Ecol. Environ. 20, 58–63 (2022). [Google Scholar]
- 41.Underhill V., et al. , Personality and behavioral syndromes in two Peromyscus species: Presence, lack of state dependence, and lack of association with home range size. Behav. Ecol. Sociobiol. 75, 9 (2021). [Google Scholar]
- 42.Bell A. M., Hankison S. J., Laskowski K. L., The repeatability of behaviour: A meta-analysis. Anim. Behav. 77, 771–783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hulme P. E., Kollmann J., “Seed predator guilds, spatial variation in post-dispersal seed predation and potential effects on plant demography: A temperate perspective” in Seed Fate Predation, Dispersal and Seedling Establishment, Forget P. M., Lambert J. E., Hulme P. E., Vander Wall S. B., Eds. (CABI, 2005), pp. 9–30. [Google Scholar]
- 44.Nathan R., Long-distance dispersal of plants. Science 313, 786–788 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Brehm A. M., Mortelliti A., Mind the trap: Large-scale field experiment shows that trappability is not a proxy for personality. Anim. Behav. 142, 101–112 (2018). [Google Scholar]
- 46.Steele M. A., et al. , Do scatter hoarders trade off increased predation risks for lower rates of cache pilferage? Behav. Ecol. 25, 206–215 (2014). [Google Scholar]
- 47.Vander Wall S. B., Longland W. S., Cheek pouch capacities and loading rates of deer mice (Peromyscus maniculatus). Gt. Basin Nat. 59, 278–280 (1999). [Google Scholar]
- 48.Vander Wall S. B., The evolutionary ecology of nut dispersal. Bot. Rev. 67, 74–117 (2001). [Google Scholar]
- 49.Hirsch B. T., Kays R., Jansen P. A., A telemetric thread tag for tracking seed dispersal by scatter-hoarding rodents. Plant Ecol. 213, 933–943 (2012). [Google Scholar]
- 50.Jansen P. A., Hirsch B. T., Emsens W., Zamora-Gutierrez V., Wikelski M., Thieving rodents as substitute dispersers of megafaunal seeds. Proc. Natl. Acad. Sci. U.S.A. 109, 12610–12615 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markl J. S., et al. , Meta-analysis of the effects of human disturbance on seed dispersal by animals. Conserv. Biol. 26, 1072–1081 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Winfree R., MacLeod M., Harrison T., Cariveau D. P., “Conserving and restoring mutualisms” in Mutualism, Bronstein J. L., Ed. (Oxford University Press, 2015), pp. 268–282. [Google Scholar]
- 53.Merrick M. J., Koprowski J. L., Should we consider individual behavior differences in applied wildlife conservation studies? Biol. Conserv. 209, 34–44 (2017). [Google Scholar]
- 54.Sih A., Understanding variation in behavioural responses to human-induced rapid environmental change: A conceptual overview. Anim. Behav. 85, 1077–1088 (2013). [Google Scholar]
- 55.McConkey K. R., O’Farrill G., Loss of seed dispersal before the loss of seed dispersers. Biol. Conserv. 201, 38–49 (2016). [Google Scholar]
- 56.Brissette J. C., Kenefic L. S., “History of the Penobscot Experimental Forest, 1950-2010” in Penobscot Experimental Forest: 60 Years of Research and Demonstration in Maine, 1950–2010. GTR-NRS-P-123 United States Department of Agriculture Forest Service, Ed. (USDA Forest Service, 2014), pp. 1–20. [Google Scholar]
- 57.Kimball A. J., “Penobscot Experimental Forest: Resources, administration, and mission” in Penobscot Experimental Forest: 60 Years of Research and Demonstration in Maine, 1950–2010. GTR-NRS-P-123 United States Department of Agriculture Forest Service, Ed. (USDA Forest Service, 2014), pp. 21–30. [Google Scholar]
- 58.Carter A. J., Feeney W. E., Marshall H. H., Cowlishaw G., Heinsohn R., Animal personality: What are behavioural ecologists measuring? Biol. Rev. Camb. Philos. Soc. 88, 465–475 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Brehm A., Tironi S., Mortelliti A., Effects of trap confinement on personality measurements in two terrestrial rodents. PLoS One 15, e0221136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoffel M. A., Nakagawa S., Schielzeth H., rptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644 (2017). [Google Scholar]
- 61.Gelman A., Su Y.-S., arm: Data analysis using regression and multilevel/hierarchical models. R package version 1.10-1 (2018) https://www.academia.edu/37508776/Package_arm_Title_Data_Analysis_Using_Regression_and_Multilevel_Hierarchical_Models. Accessed 1 August 2021.
- 62.Burnham K. P., Anderson D. R., Model Selection and Multimodel Inference: A Practical Information-theoretic Approach (Springer Verlag, ed. 2, 2002). [Google Scholar]
- 63.Buckland S. T., Burnham K. P., Augustin N. H., Model selection: An integral part of inference. Biometrics 53, 603–618 (1997). [Google Scholar]
- 64.Peig J., Green A. J., New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 118, 1883–1891 (2009). [Google Scholar]
- 65.Bates D. M., Maechler M., Bolker B. M., Walker S., lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-8 (2015) cran.r-project.org/package=lme4. Accessed 1 August 2021.
- 66.A. M. Brehm, A. Mortelliti, Animal personalities generate context dependence in the seed dispersal mutualism. Figshare. https://figshare.com/articles/dataset/Animal_personalities_generate_context_dependence_in_the_seed_dispersal_mutualism/16455570. Deposited 18 March 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All original data and R code used in the main analyses can be accessed on the Figshare repository at https://doi.org/10.6084/m9.figshare.16455570 (66). All other data are included in the article and/or Supporting Information.