Abstract
Background and Objectives
Mild cognitive impairment (MCI) is an at-risk state for dementia; however, not all individuals with MCI transition to dementia, and some revert to normal cognition (NC). Here, we investigate whether mild behavioral impairment (MBI), the late-life onset of persistent neuropsychiatric symptoms (NPS), improves the prognostic specificity of MCI.
Methods
Participants with MCI from the National Alzheimer's Coordinating Center Uniform Data Set were included. NPS were operationalized with the Neuropsychiatric Inventory Questionnaire to identify participants without NPS and those with MBI (persistent, late-onset NPS). Individuals with late-onset NPS not meeting the MBI persistence criterion (NPS_NOT_MBI) were retained for secondary analyses. Progression to dementia, stable MCI, and reversion to NC after 3 years of follow-up were defined per National Institute on Aging–Alzheimer’s Association and Petersen criteria.
Results
The primary sample consisted of 739 participants (NPS− n = 409 and MBI+ n = 330; 75.16 ± 8.6 years old, 40.5% female). After 3 years, 238 participants (33.6%) progressed to dementia, and 90 (12.2%) reverted to NC. Compared to participants without NPS, participants with MBI were significantly more likely to progress to dementia (adjusted odds ratio [AOR] 2.13, 95% CI 1.52–2.99), with an annual progression rate of 14.7% (vs 8.3% for participants with MCI without NPS). Compared to participants without NPS, participants with MBI were less likely to revert to NC (AOR 0.48, 95% CI 0.28–0.83, 2.5% vs 5.3% annual reversion rate). The NPS_NOT_MBI group (n = 331, 76.5 ± 8.6 years old, 45.9% female) were more likely to progress to dementia (AOR 2.18, 95% CI 1.56–3.03, 14.3% annual progression rate) but not less likely to revert to NC than those without NPS. Accordingly, both NPS_NOT_MBI and MBI+ participants had lower Mini-Mental State Examination scores than NPS− participants after 3 years.
Discussion
Late-onset NPS improve the specificity of MCI as an at-risk state for progression to dementia. However, only persistent late-onset NPS are associated with a lower likelihood of reversion to NC, with transient NPS (i.e., NPS_NOT_MBI) not differing from the NPS− group. Clinical prognostication can be improved by incorporating late-onset NPS, especially those that persist (i.e., MBI), into risk assessments. Clinical trials may benefit from enrichment with these higher-risk participants with MCI.
The global prevalence of dementia is expected to more than triple by 2050.1 Given that neural changes in individuals who go on to develop dementia occur up to 2 decades before the onset of cognitive symptoms,2 the emphasis is on shifting toward markers that permit early detection and treatment.
Mild cognitive impairment (MCI) is a premorbid risk factor for dementia representing a transitional state with objective cognitive impairment but with functional independence maintained.3 However, the annual rate of progression to dementia ranges from only 8% in clinical trials4 to 13% in large registries.5 Furthermore, the objective cognitive impairment that defines MCI can be reversible, with rates of reversion to normal cognition (NC) as high as 16% within 1 year6 and additional reversions thereafter.7-9 Thus, the prognostic utility of MCI as an early marker of dementia may benefit from the incorporation of additional features to improve specificity.
Neuropsychiatric symptoms (NPS) in older adults are associated with cognitive decline in both NC and MCI populations.10,11 As with MCI, NPS alone can lack specificity depending on the natural history. When NPS are characterized by later-life onset, represent a distinct change from long-standing patterns of behavior and personality, are not better accounted for by a psychiatric disorder, and persist for >6 months, they define mild behavioral impairment (MBI).12 These validated criteria, developed by an international consensus group under the rubric of the Alzheimer's Association, describe an important risk state for incident cognitive decline and dementia.12 Moreover, MBI has been associated with Alzheimer disease (AD) risk genes, including the APOE ε4 allele, BIN1, and EPHA1,13 as well as higher AD polygenic risk scores14 and markers for amyloid, tau, and neurodegeneration.15-21
One possibility is that incorporating NPS into case finding in populations without dementia may improve the specificity of MCI. Although MBI can be the index manifestation of dementia and precede mild cognitive changes,22-26 the co-occurrence of MCI and MBI may represent a unique state with a heightened risk of disease progression that is less likely to revert to normal cognitive function. Here, we examined participants with MCI, stratified by MBI status at baseline, and their cognitive status at 3 years. We hypothesized a higher likelihood of progression to dementia and a lower likelihood of reversion back to NC when MCI and MBI are comorbid.
Methods
Study Population: National Alzheimer's Coordinating Center
Data used in this study were obtained from the National Alzheimer's Coordinating Center (NACC) database.27 NACC was established by the National Institute on Aging and consists of multiple National Institute on Aging–funded Alzheimer's Disease Research Centers (ADRCs) that recruit and collect data on participants with cognitive function ranging from normal to dementia. The ADRCs contribute to the NACC Uniform Data Set (UDS) using a prospective, standardized, and longitudinal clinical evaluation administered approximately annually. Sixteen data collection forms are completed by the clinician, covering demographics, neurologic examination findings, and diagnosis. Cognitive diagnosis from the clinician UDS D1 form was used for this study, which included coding for NC, MCI, and dementia, according to the relevant clinical criteria.28-30 Detailed information on the cohort and the UDS is described elsewhere.28-30 APOE genotype is reported by the Centers on the Neuropathology form and the Alzheimer's Disease Genetics Consortium and sent to NACC. The number of ε4 alleles is reported as 0, 1, or 2 and was dichotomized to carrier status for these analyses. NACC-UDS with a November 2020 data freeze was used for this study.
Standard Protocol Approvals, Registrations, and Patient Consents
As determined by the University of Washington Human Subjects Division, use of the NACC database itself is exempt from Institutional Review Board review. However, all contributing ADRCs are required to obtain informed consent from their participants and to maintain their own separate Institutional Review Board review and approval from their institution before submitting data to NACC.
Participant Selection
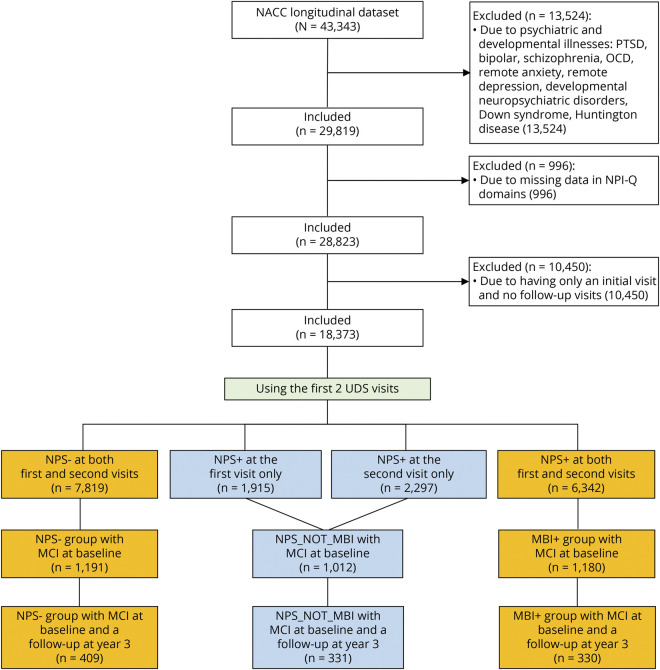
Participant selection is illustrated in Figure 1. NACC participants enrolled between 2005 and 2020 were included. Participants were eligible for this analysis if they were ≥60 years of age, had 2 consecutive visits after enrollment with valid NPI-Q data (to determine NPS and MBI), had follow-up data at 3 years, and had a diagnosis of MCI at the second study visit in accordance with the Petersen31 criteria. Eligible participants were excluded from the analyses if they had a prior diagnosis of a psychiatric condition, including bipolar disorder, depression, anxiety, obsessive compulsive disorder, posttraumatic stress disorder, or schizophrenia, because NPS in this context may not be of late onset and preclude MBI diagnosis. Developmental or neurologic conditions, including neuropsychiatric disorders and genetic conditions such as Down syndrome or Huntington disease, were also exclusion criteria.
Figure 1. Flowchart of Participants From NACC Included for Analysis.
Primary sample is shown in amber, and NPS+ subsample retained for secondary analyses in shown in blue. MBI = mild behavioral impairment; MCI = mild cognitive impairment; NACC = National Alzheimer's Coordinating Center; OCD = obsessive compulsive disorder; NPS = neuropsychiatric symptoms; NPI-Q = Neuropsychiatric Inventory Questionnaire; PTSD = posttraumatic stress disorder; UDS = Uniform Data Set.
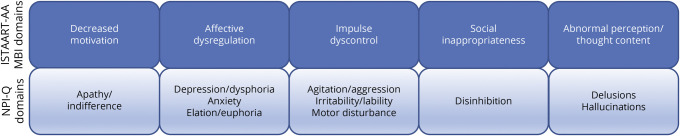
MBI status was derived from the UDS using a published algorithm32,33 to transform the Neuropsychiatric Inventory Questionnaire (NPI-Q)34 items to MBI domains. The NPI-Q is an informant-rated instrument with excellent test-retest validity34 assessing the severity (absent, mild, moderate, and severe) and distress associated with 12 neuropsychiatric domains over the last month. Because the MBI criteria require later-life–emergent NPS to be persistent for ≥6 months, the NPI-Q cannot inform the persistence criterion without repeated administration over an interval of at least 6 months or in the case of NACC in its annual assessments. Ten of the 12 domains assessed by the NPI-Q align with the 5 International Society to Advance Alzheimer's Research and Treatment–Alzheimer’s Association (ISTAART-AA) domains of MBI (Figure 2), namely (1) decreased drive/motivation (apathy/indifference), (2) emotional dysregulation (depression/dysphoria, anxiety, elation/euphoria), (3) impulse dyscontrol (agitation/aggression, irritability/lability, aberrant motor behavior), (4) social inappropriateness (disinhibition), and (5) abnormal perception or thought content (delusions and hallucinations). To derive MBI domain scores, the NPI-Q severity ratings for each of its corresponding MBI domains were used to determine the presence of NPS per domain. The MBI criterion of symptom persistence was operationalized by requiring NPS to be endorsed at 2 consecutive NACC visits, the latter of which was determined as the neurobehavioral baseline (MBI+). MBI− participants had no positive scores. MBI scores were derived from the mean of the 2 consecutive NACC visits (range 0–30), with the cut point for MBI+ being a total score ≥0.
Figure 2. Schematic Overlap of the NPI-Q and ISTAART-AA MBI Domains.
ISTAART-AA = International Society to Advance Alzheimer's Research and Treatment–Alzheimer’s Association; MBI = mild behaviour impairment; NPI-Q = Neuropsychiatric Inventory Questionnaire.
This approach yielded a primary final sample of 409 participants with no past or current NPS (NPS−) and 330 participants who met the NPS emergence and persistence criteria for MBI (MBI+). In addition to these participants, a sample of 331 participants developed late-onset NPS at either the first or second NACC visit (NPS+) but did not meet the MBI persistence criteria because they did not have NPS at both. These individuals with NPS_NOT_MBI were retained for a separate secondary analysis.
Cognitive Status at Follow-up
We defined cognitive status at follow-up using NACC UDS clinician diagnosis at baseline and after 3 years of follow-up. We adopted a conservative definition of reversion to NC, namely a clinician diagnosis of NC after 3 years of follow-up, whereas Impaired-Not-MCI was retained with MCI as stable MCI. Progression to dementia was defined as a clinician diagnosis of dementia.
Statistical Analysis
The primary analysis involved the NPS− and MBI+ comparison, and secondary analyses involved the NPS_NOT_MBI (i.e., transient NPS) group.
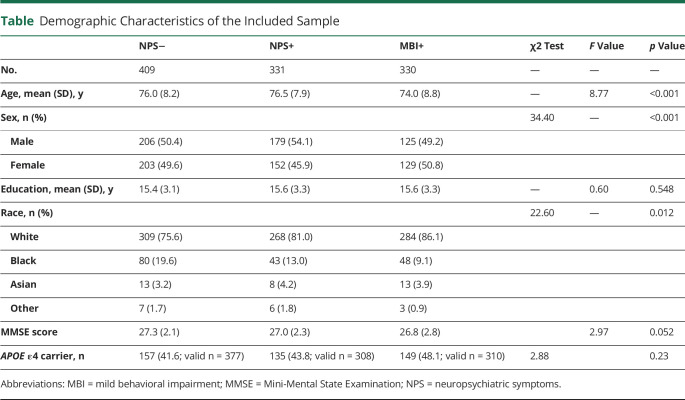
Demographic characteristics were examined for NPS−, NPS_NOT_MBI, and MBI+ participants by χ2 tests for categorical variables and 1-way analysis of variance for continuous variables.
The distribution of cognitive diagnoses at the 3-year follow-up point was assessed with χ2 tests and the likelihood ratio. This distribution was further quantified with multinomial logistic regression adjusted for age, sex, race, years of education (continuous), and APOE ε4 carrier status to assess the progression to dementia or reversion to NC relative to stable MCI at the 3-year UDS visit. Event rates were annualized to determine progression and reversion rates.
Statistical analyses were performed in IBM SPSS Statistics version 26 (Armonk, NY). Statistical significance was set at α ≤ 0.05.
Data Availability
Data access was provided by NACC on the basis of an approved research proposal.
Results
The characteristics of our final sample are described in the Table. The primary sample involved n = 409 NPS− individuals and n = 330 MBI+ individuals. An intermediary group (n = 331) with no history of NPS presenting with late-onset NPS that did not meet MBI duration criteria was retained for secondary analyses (NPS_NOT_MBI). There were group differences in racial (χ2 = 22.6, p = 0.012) and sex (χ2 = 34.4, p < 0.001) distributions, and the MBI+ group was significantly younger (F2, 1,067 = 8.7, p < 0.001). There were no statistically significant differences in years of education, Mini-Mental State Examination (MMSE) score, or APOE ε4 carrier status.
Table.
Demographic Characteristics of the Included Sample
In the primary sample of NPS− and MBI+ participants, 238 participants (33.6%) progressed to dementia, and 90 participants (12.2%) reverted to NC. The UDS definitions were paralleled in MMSE scores, with a diagnosis of dementia at 3 years associated with average scores of 21.5 ± 4.8, stable MCI with average scores of 27.0 ± 2.9, and reversion to NC with average scores of 28.6 ± 1.3 (F2,720 = 213.2, p < 0.001).
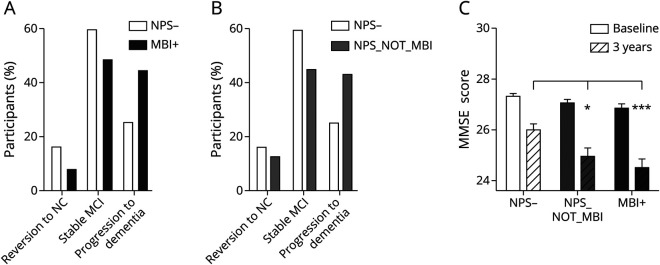
The NPS− and MBI+ groups differed in their likelihood of progressing to AD and reverting to NC (χ2 = 34.7, p < 0.001; Figure 3A). Multinomial logistic regression in which stable MCI was the reference with adjustment for age, sex, education (continuous), race, and APOE ε4 carrier status revealed that MBI+ participants were more likely than NPS− participants to progress to dementia (adjusted odds ratio [AOR] 2.10, 95% CI 1.47–2.99, p < 0.001) and less likely to revert to NC (AOR 0.45, 95% CI 0.26–0.80, p < 0.01). Annual progression rates to dementia were 14.7% for MBI+ participants and 8.3% for NPS− participants, while annual reversion rates were 2.5% for MBI+ participants and 5.3% for NPS− participants.
Figure 3. Progression to AD or Stable MCI and Reversion to NC After 3 Years.
AD = Alzheimer disease; MBI = mild behavioral impairment; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; NC = normal cognition; NPS = neuropsychiatric symptoms.
We repeated analyses using a more liberal definition of reversion from MCI, in which Impaired-Not-MCI was also considered reversion. The number of participants who reverted with this definition increased to 135 (18.3%), and the MBI+ and NPS− groups once again differed in their likelihood of progressing to dementia and reverting to NC (χ2 = 33.8, p < 0.001). Multinomial logistic regression in which stable MCI was the reference with adjustment for age, sex, education (continuous), race, and APOE ε4 carrier status revealed that MBI+ participants were more likely than NPS− participants to progress to dementia (AOR 2.06, 95% CI 1.43–2.96, p < 0.001) and less likely to revert to NC (AOR 0.58, 95% CI 0.36–0.92, p < 0.05).
As a secondary analysis, we examined outcomes in participants with MCI without NPS and the independent group of participants with NPS but for whom symptoms were present for <6 months who therefore did not meet the MBI persistence criterion (NPS_NOT_MBI). After 3 years, 142 participants (42.9%) progressed to dementia, and 52 participants (15.7%) had reverted to NC. This distribution did not differ from those with NPS_NOT_MBI at the first or second visit (χ2 = 0.88, p = 0.64) or from the MBI+ group (χ2 = 1.20, p = 0.54); however, they significantly differed from the NPS− group (χ2 = 27.12, p < 0.001; Figure 3B). Multinomial logistic regressions were repeated in which stable MCI was the reference with adjustment for potential confounders, which revealed that NPS_NOT_MBI participants were more likely than NPS− participants to progress to dementia (AOR 2.25, 95% CI 1.59–3.19, p < 0.001) but not less likely to revert to NC (AOR 0.92, 95% CI 0.57–1.48, p = 0.74). The annual rate of progression to dementia was 14.3% for the late-onset NPS_NOT_MBI participants.
Cognitive testing confirmed the increased risk of cognitive decline with the co-occurrence of MCI and MBI (Figure 3C). After 3 years, participants with MBI+ and NPS_NOT_MBI had significantly lower MMSE scores than NPS− participants.
Discussion
Our longitudinal analyses demonstrate that MBI and late-life–onset NPS improve the specificity of MCI as an at-risk state for incident dementia. Although increased risk for dementia has previously been associated with NPS, this study demonstrates that MCI comorbid with MBI is associated with a lower rate of reversion to NC. Specifically, individuals with comorbid MCI and MBI are at 2-fold greater risk for progression to dementia over 3 years and less than half as likely to revert to NC compared to individuals with MCI but no NPS. Our findings have important clinical implications and indicate that both cognitive function and NPS should be assessed as a matter of routine to improve prognostication.
The importance of MCI conceptually and as a clinical entity cannot be understated. Through identification of premorbid individuals at risk for dementia using a combination of objective cognitive performance and function,3 patients can be identified for early intervention. However, specificity is suboptimal; many individuals with MCI do not progress to dementia, and despite objective signs of cognitive impairment, some individuals revert to a state of NC.
A meta-analysis assessed the progression rate from MCI to dementia across 41 cohort studies stratified by population studies and clinical trials.4 A cumulative proportion of 28.9% and 33.6% of individuals with MCI progressed to AD in population studies and clinical trials, respectively.4 More than half of the participants did not progress to dementia at 10 years with an annual progression rate of ≈7% (AD progression rate 6.8% in population studies and 8.1% in clinical trials). In clinical settings, the rate of progression is higher, estimated at 13.7% person-years (95% CI 13.5%–13.9%).5 The annual progression rate observed in our sample of participants with MCI without NPS therefore aligns with population data, whereas comorbid MCI and MBI, or MCI and late-onset NPS, had a more malignant course.
The reversion from MCI to NC has received less attention than progression to dementia.35 One meta-analysis looking at reversion rates across 25 studies found that 8% of participants in population studies and 25% in the clinical setting reverted from MCI to NC.9 Another longitudinal study of 473 individuals with MCI tracked reversion rates to NC in a community-based cohort and found that 44% of the cohort reverted to NC over a 6-year follow-up.36 Very similar results were recently reported in a community follow-up study wherein 752 individuals with incident MCI were followed up for a mean of 2.4 years, over which time 47% no longer met MCI criteria.37
Several studies in participants with MCI have demonstrated that the presence of NPS, assessed with the NPI-Q, is associated with a greater risk of progression to dementia.38-40 Even subtle NPS in individuals with MCI have been associated with cognitive decline.41 Similarly, a machine learning study compared NPS in 128 participants (78 with NC, 50 with MCI) and progression to dementia. NPS proxies such as the NPI-Q total severity and stress scores were the most important factors predicting progression to dementia from MCI, and the authors concluded that NPS were associated with a higher risk for dementia in MCI.42
Mixed findings have emerged when examining the relationship between NPS and reversion from MCI to NC.43,44 Specifically, a population-based longitudinal study of 622 participants with MCI with 6- and 12-year follow-up analyses44 determined that depression, assessed with the Comprehensive Psychiatric Rating Scale,45 did not explain reversion to NC. Complicating the interpretation of this analysis, however, was an unusually low prevalence of depression46 and a very high reversion rate of 58%. Conversely, in a subanalysis from the NACC cohort, the stability of MCI was examined in 1,121 participants who had previously been determined to have NC and then developed MCI. This study reported that 28.9% of the sample reverted to NC over a mean follow-up of 5.5 years and that reversion to NC was associated with lower burden of mood, anxiety, and hyperactivity symptoms, as well as the initiation of cognitive medications.44 Our findings may reconcile these conflicting findings, indicating that late-onset NPS are enriched in an emergent MCI sample and conversely that low rates of NPS are associated with a greater likelihood of reversion from MCI to NC.
The prognostic utility of MBI arises from 2 criteria: symptoms are later life emergent, and symptoms are persistent. The former distinguishes MBI from psychiatric illness, and the latter eliminates transient symptoms that may manifest in response to life events. These criteria increase the signal-to-noise ratio for identification of behavioral symptoms that represent sequelae of neurodegeneration. Consistent with this, a recent longitudinal study of 2,769 individuals with NC reported that 51.6% of those with MBI demonstrated cognitive and functional decline over 3 years compared to 21.7% of the MBI− group.24 Similarly, a machine learning study of Alzheimer’s Disease Neuroimaging Initiative participants determined that later-life–emergent and persistent NPS (i.e., MBI) and hippocampal atrophy were the features that best predicted dementia diagnosis 40 months hence, with comparable magnitudes of effect.47 Thus, converging data indicate that MBI improves clinical prognostication for cognitive decline and dementia in the older adult population.
To derive MBI status from NACC data, we transformed 2 visits of the NPI-Q data into MBI scores.32 While our data transformation approach has been used in prior studies, the NPI-Q was designed for a dementia population,38,42,48 and we did not use convergent measures to validate the presence of NPS when identified by informants with the NPI-Q. Furthermore, while the NPI-Q has very good psychometric properties, the instrument is completed by informants and therefore is subject to certain limitations, including the informant's neuropsychiatric state, cognitive function, recall bias, and cultural beliefs, among others. Future studies should consider the MBI checklist,49-51 which captures later-life onset of persistent and impactful NPS in accordance with the ISTAART-AA MBI criteria and which was developed specifically for populations without dementia as in this study. The MBI-C can be completed by self or informant. Because we restricted our hypothesis to individuals with MCI, our analyses do not determine whether MBI in NC is associated with greater risk. Recently published data from an NACC sample have determined the 3-year risk for incident MCI/dementia in NC. The 3-year progression rate from NC to dementia was higher in MBI+ (2.3%) vs MBI− (0.2%) individuals.24 However, this is substantially lower than the 3-year progression rates to dementia in MBI+ MCI+ (44.1%) and MCI+ NPS− (24.9%) individuals. Together, these findings indicate that MBI and MCI are not simply additive and may have synergistic effects on the risk of progression to dementia.
Integrating NPS characterizations into dementia risk assessment improves the specificity of MCI as an at-risk state for dementia. When comorbid with MBI, MCI is associated with a greater risk of progression to dementia and a significantly reduced likelihood of reverting to NC. This has implications for prognostication in clinical practice and may be a means of identifying high-risk premorbid individuals for biomarker and treatment studies.
Acknowledgment
The NACC database is funded by National Institute on Aging/NIH grant U24 AG072122. NACC data are contributed by the National Institute on Aging–funded ADRCs: P30 AG019610 (principal investigator [PI] Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Glossary
- AD
Alzheimer disease
- ADRC
Alzheimer's Disease Research Center
- AOR
adjusted odds ratio
- ISTAART-AA
International Society to Advance Alzheimer's Research and Treatment–Alzheimer’s Association
- MBI
mild behavioral impairment
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NACC
National Alzheimer's Coordinating Center
- NC
normal cognition
- NPI-Q
Neuropsychiatric Inventory Questionnaire
- NPS
neuropsychiatric symptoms
- UDS
Uniform Data Set
Appendix. Authors

Study Funding
This work was funded by a research grant to Z. Ismail from the Canadian Institutes of Health Research (BCA 399583).
Disclosure
A. McGirr, S. Nathan, M. Ghahremani, S. Gill, and E.E. Smith have no relevant disclosures. Z. Ismail has received personal fees from Lundbeck/Otsuka. His institution has received funds from Acadia, Biogen, Roche, and Sunovion. Go to Neurology.org/N for full disclosures.
References
- 1.Cova I, Markova A, Campini I, Grande G, Mariani C, Pomati S. Worldwide trends in the prevalence of dementia. J Neurol Sci. 2017;379:259-260. [DOI] [PubMed] [Google Scholar]
- 2.Perl DP. Neuropathology of Alzheimer's disease. Mt Sinai J Med. 2010;77(1):32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985-1992. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AJ, Shiri Feshki M. Rate of progression of mild cognitive impairment to dementia: meta‐analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119(4):252-265. [DOI] [PubMed] [Google Scholar]
- 5.Tifratene K, Robert P, Metelkina A, Pradier C, Dartigues JF. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology. 2015;85(4):331-338. [DOI] [PubMed] [Google Scholar]
- 6.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachdev PS, Lipnicki DM, Crawford J, et al. Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: a population-based study. PLoS One. 2013;8(3):e59649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malek-Ahmadi M. Reversion from mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis Assoc Disord. 2016;30(4):324-330. [DOI] [PubMed] [Google Scholar]
- 9.Canevelli M, Grande G, Lacorte E, et al. Spontaneous reversion of mild cognitive impairment to normal cognition: a systematic review of literature and meta-analysis. J Am Med Dir Assoc. 2016;17(10):943-948. [DOI] [PubMed] [Google Scholar]
- 10.Lo TWB, Karameh WK, Barfett JJ, et al. Association between neuropsychiatric symptom trajectory and conversion to Alzheimer disease. Alzheimer Dis Assoc Disord. 2020;34(2):141-147. [DOI] [PubMed] [Google Scholar]
- 11.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171(5):572-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews SJ, Ismail Z, Anstey KJ, Mortby M. Association of Alzheimer's genetic loci with mild behavioral impairment. Am J Med Genet B Neuropsychiatr Genet. 2018;177(8):727-735. [DOI] [PubMed] [Google Scholar]
- 14.Creese B, Arathimos R, Brooker H, et al. Genetic risk for Alzheimer's disease, cognition, and mild behavioral impairment in healthy older adults. Alzheimers Dement (Amst). 2021;13(1):e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson M, Stomrud E, Insel P, et al. . Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer's disease. Transl Psychiatry 2021;11(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matuskova V, Ismail Z, Nikolai T, et al. . Mild behavioral impairment is associated with atrophy of entorhinal cortex and hippocampus in a memory clinic cohort. Front Aging Neurosci. 2021;13:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao R, Chen H-Y, Gill S, Naude J, Smith EE, Ismail Z. Plasma β-amyloid in mild behavioural impairment-neuropsychiatric symptoms on the Alzheimer's continuum. J Geriatr Psychiatry Neurol. Epub 2021 May 26. [DOI] [PubMed]
- 18.Lussier F, Pascoal T, Therriault J, et al. . Mild behavioral impairment is associated with beta-amyloid and tau across the Alzheimer's disease spectrum. J Cereb Blood Flow Metab. 2019;39:158-159. [Google Scholar]
- 19.Naude JP, Gill S, Hu S, et al. Plasma neurofilament light: a marker of neurodegeneration in mild behavioral impairment. J Alzheimers Dis. 2020;76(3):1017-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill S, Wang M, Mouches P, et al. Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int J Geriatr Psychiatry. 2021;36(9):1398-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruthirakuhan MT, Ismail Z, Herrmann N, Gallagher D, Lanctot K. Mild behavioral impairment is associated with progression to Alzheimer's disease: results from a clinico-pathological study. Alzheimers Dement (in press 2022). DOI: 10.1002/alz.12519 [DOI] [PMC free article] [PubMed]
- 22.Matsuoka T, Ismail Z, Narumoto J. Prevalence of mild behavioral impairment and risk of dementia in a psychiatric outpatient clinic. J Alzheimers Dis. 2019;70(2):505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creese B, Brooker H, Ismail Z, et al. Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. 2019;27(8):823-834. [DOI] [PubMed] [Google Scholar]
- 24.Ismail Z, McGirr A, Gill S, Hu S, Forkert ND, Smith EE. Mild behavioral impairment and subjective cognitive decline predict cognitive and functional decline. J Alzheimers Dis. 2021;80(1):459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsunoda Keiichiro, et al. . “Positive baseline behavioral and psychological symptoms of dementia predict a subsequent cognitive impairment in cognitively normal population”. Neurology and Clinical Neuroscience 9.3 (2021): 218-222. [Google Scholar]
- 26.Taragano FE, Allegri RF, Heisecke SL, et al. Risk of conversion to dementia in a mild behavioral impairment group compared to a psychiatric group and to a mild cognitive impairment group. J Alzheimers Dis. 2018;62(1):227-238. [DOI] [PubMed] [Google Scholar]
- 27.National Alzheimer’s Coordinating Center. Accessed November 8, 2020. naccdata.org.
- 28.Weintraub S, Salmon D, Mercaldo N, et al. . The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychological test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249-258. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis Assoc Disord. 2006;20(4):210-216. [DOI] [PubMed] [Google Scholar]
- 31.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194. [DOI] [PubMed] [Google Scholar]
- 32.Sheikh F, Ismail Z, Mortby ME, et al. Prevalence of mild behavioral impairment in mild cognitive impairment and subjective cognitive decline, and its association with caregiver burden. Int Psychogeriatr. 2018;30(2):233-244. [DOI] [PubMed] [Google Scholar]
- 33.Mortby ME, Ismail Z, Anstey KJ. Prevalence estimates of mild behavioral impairment in a population-based sample of pre-dementia states and cognitively healthy older adults. Int Psychogeriatr. 2018;30(2):221-232. [DOI] [PubMed] [Google Scholar]
- 34.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233-239. [DOI] [PubMed] [Google Scholar]
- 35.Pandya SY, Lacritz LH, Weiner MF, Deschner M, Woon FL. Predictors of reversion from mild cognitive impairment to normal cognition. Dement Geriatr Cogn Disord. 2017;43(3-4):204-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Q, Gwee X, Feng L, et al. Mild cognitive impairment reversion and progression: rates and predictors in community-living older persons in the Singapore Longitudinal Ageing Studies cohort. Dement Geriatr Cogn Dis Extra. 2018;8:226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angevaare MJ, Vonk JMJ, Bertola L, et al. . Predictors of incident mild cognitive impairment and its course in a diverse community-based population. Neurology. 2021;98(1):e15-e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters ME, Rosenberg PB, Steinberg M, et al. Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: the Cache County Study. Am J Geriatr Psychiatry. 2013;21(11):1116-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013;21(7):685-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pink A, Stokin GB, Bartley MM, et al. Neuropsychiatric symptoms, APOE ε4, and the risk of incident dementia: a population-based study. Neurology. 2015;84(9):935-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liew TM. Symptom clusters of neuropsychiatric symptoms in mild cognitive impairment and their comparative risks of dementia: a cohort study of 8530 older persons. J Am Med Dir Assoc. 2019;20(8):1054. e1-1054. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallo SC, Valladares-Rodriguez S, Facal D, Lojo-Seoane C, Fernández-Iglesias MJ, Pereiro AX. Neuropsychiatric symptoms as predictors of conversion from MCI to dementia: a machine learning approach. Int Psychogeriatr. 2020;32(3):381-392. [DOI] [PubMed] [Google Scholar]
- 43.Sugarman MA, Alosco ML, Tripodis Y, Steinberg EG, Stern RA. Neuropsychiatric symptoms and the diagnostic stability of mild cognitive impairment. J Alzheimers Dis. 2018;62(4):1841-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overton M, Pihlsgård M, Elmståhl S. Diagnostic stability of mild cognitive impairment, and predictors of reversion to normal cognitive functioning. Dement Geriatr Cogn Disord. 2019;48(5-6):317-329. [DOI] [PubMed] [Google Scholar]
- 45.Åsberg M., Perris C., Schalling D., & Sedvall G. (Eds.). (1978). CPRS: Development and applications of a psychiatric rating scale. Acta Psychiatrica Scandinavica, Suppl 271, 69. [DOI] [PubMed] [Google Scholar]
- 46.Ismail Z, Elbayoumi H, Fischer CE, et al. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(1):58-67. [DOI] [PubMed] [Google Scholar]
- 47.Gill S, Mouches P, Hu S, et al. Using machine learning to predict dementia from neuropsychiatric symptom and neuroimaging data. J Alzheimers Dis. 2020;75(1):277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liew TM. Neuropsychiatric symptoms in cognitively normal older persons, and the association with Alzheimer's and non-Alzheimer’s dementia. Alzheimers Res Ther. 2020;12:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ismail Z, Agüera-Ortiz L, Brodaty H, et al. The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. 2017;56(3):929-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creese B, Griffiths A, Brooker H, et al. Profile of mild behavioral impairment and factor structure of the Mild Behavioral Impairment Checklist in 5 normal older adults. Int Psychogeriatr 2020;32(6):705-717. [DOI] [PubMed] [Google Scholar]
- 51.Mallo SC, Ismail Z, Pereiro AX, et al. Assessing mild behavioral impairment with the Mild Behavioral Impairment-Checklist in people with mild cognitive impairment. J Alzheimers Dis. 2018;66(1):83-95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data access was provided by NACC on the basis of an approved research proposal.