Significance
Ctenophore cydippid larvae are not larvae at all and begin adult reproduction at an early age (∼14 vs. ∼60 d) and small size (∼1 vs. ∼100 mm) relative to attainment of what has been considered the adult stage. This overturns the previous understanding of the ctenophore life cycle, which was believed to be a unique form of biphasic life cycle with two separate sexually reproductive periods. Practically, these results clarify ecological controls regulating ctenophore reproduction and will aid management of this invasive species. Additionally, the 2-wk egg-to-egg generation time will open new avenues of research in this understudied but informative taxon.
Keywords: larval reproduction, dissogeny, ctenophore, life history evolution, Mnemiopsis leidyi
Abstract
A substantial body of literature reports that ctenophores exhibit an apparently unique life history characterized by biphasic sexual reproduction, the first phase of which is called larval reproduction or dissogeny. Whether this strategy is plastically deployed or a typical part of these species’ life history was unknown. In contrast to previous reports, we show that the ctenophore Mnemiopsis leidyi does not have separate phases of early and adult reproduction, regardless of the morphological transition to what has been considered the adult form. Rather, these ctenophores begin to reproduce at a small body size and spawn continuously from this point onward under adequate environmental conditions. They do not display a gap in productivity for metamorphosis or other physiological transition at a certain body size. Furthermore, nutritional and environmental constraints on fecundity are similar in both small and large animals. Our results provide critical parameters for understanding resource partitioning between growth and reproduction in this taxon, with implications for management of this species in its invaded range. Finally, we report an observation of similarly small-size spawning in a beroid ctenophore, which is morphologically, ecologically, and phylogenetically distinct from other ctenophores reported to spawn at small sizes. We conclude that spawning at small body size should be considered as the default, on-time developmental trajectory rather than as precocious, stress-induced, or otherwise unusual for ctenophores. The ancestral ctenophore was likely a direct developer, consistent with the hypothesis that multiphasic life cycles were introduced after the divergence of the ctenophore lineage.
Whether indirect development has a single or multiple origins, when it arose, and whether the adult or larval body plan is ancestral are classic and ongoing debates in zoology (1–4). Competing hypotheses include adult-first hypotheses with either a single intercalation of larvae (5) or multiple gains of a larval form (4, 6) and a larva-first hypothesis suggesting a common origin of an ancestral larva with later diversification of adult forms (7). Broad definitions of indirect development include some combination of morphological, physiological, or ecological differences between the larval and adult forms, the magnitude of which is at least partly a matter of philosophy (8). However, most working definitions include a major postembryonic morphological or ecological change before which the animal is not sexually reproductive. Asexual reproduction at preadult stages is widespread in marine invertebrates and includes diverse strategies such as budding, fission, or parthenogenesis (9–11) but almost never the production and fertilization of mature gametes. Thus, spawning does not necessarily preclude identification of a life stage as larval, but a true sexually reproductive phase in larval life followed by a normal adult phase is an extraordinary claim. A lineage of marine animals called ctenophores has been cited as a rare—and potentially sole—example of true sexual reproduction in larvae, involving maturation of both male and female gametes and fertilization. This phenomenon has been noted in literature as far back as 1892 (12); it has been explicitly called larval reproduction or dissogeny and observed in several phylogenetically distant species of both tentaculate cydippid and lobate ctenophores (13–20). In favor of this view, it appears from published literature that small larval and large adult spawning are discontinuous (SI Appendix, Table S1) in distantly related species and under diverse experimental conditions. The reported pause between reproductive phases correlates with temporary degeneration of the gonads, as well as a period of overall morphological change in at least some species. Various secondary morphologies appear to have arisen multiple times in ctenophores’ evolution (21, 22). However, species that change little as they grow (lifelong cydippids) are also understood to exhibit larval reproduction (19, 20).
Phylogenomic studies often place ctenophores as the sister group to all other animals (23–28), although this placement remains controversial, as other studies place sponges in this position (29–32). Either reconstruction requires losses or multiple independent origins of complex traits; a multiphasic life history is one complex trait that would require multiple origins or a loss in ctenophores under the latter hypothesis if ctenophores lack larvae. Thus, whether ctenophores are direct developers is a key consideration in the debate over when, how, and how many times multiphasic life history evolved, regardless of which tree topology is used. Furthermore, while it is conventional for the ctenophore literature to refer to larval ctenophores or a cydippid larva stage, if ctenophores lack a larval stage, then this terminology should be amended to be more precise. Finally, if both cydippid and lobate stages represent adults, then the introduction of this morphological transition represents a type of postembryonic development distinct from a larva-to-adult transition.
Ctenophore life history thus has implications for broader research on animal origins, life history evolution, and ctenophore ecology. If spawning at a small size is physiologically distinct from later spawning, it might imply that ctenophores have cryptic larvae, which would provide evidence for the hypothesis that the last common ancestor of animals had separate larval and adult stages. Moreover, ctenophores would be a unique representative of true sexual reproduction during a larval life stage. Available alternative hypotheses, such as that size at sexual maturation in ctenophores is a plastic trait that responds to environmental factors or epigenetic programming or that small size at maturation is a genetic trait favored by selection in certain environments (such as a bottleneck during invasion), are of interest in their own right. Finally, if the observed small size of reproducing individuals in pioneer populations is a product of resource limitation, predation pressure, or some other pressure specific to low-density populations, then successful colonization should be signaled by the reliable appearance of large-size individuals, making the size distribution of animals a key indicator of invasion success.
Our results in the ctenophore Mnemiopsis leidyi are consistent with the hypothesis that reproduction at small and large sizes in ctenophores reflects a single, continuous process of maturation and resource acquisition, contradicting prior studies of small-size reproduction in ctenophores. Despite their morphological transition at larger sizes, there is no pause in reproduction. The observation of exclusively small-size populations of ctenophores in certain contexts, particularly as new invaders, likely reflects their invasion ecology rather than an adaptation to the novel environment. The mechanism and meaning of secondary morphologies that appear in some ctenophores bear further investigation, but morphological change alone is not metamorphosis.
Results
Progeny of Cydippids Develop Normally and Become Fertile around the Same Time as Parentals.
The parental cydippids’ gonads are visibly enlarged (Fig. 1 A–F). As previously reported (13, 18), only four of the eight pairs of gonads become visibly enlarged, suggesting that only half the full complement of gonads becomes fully mature at this stage. However, it is unknown whether all gonads are equally active in larger individuals as well.
Fig. 1.
Appearance of reproductive cydippids’ gonads and progeny. (A) Apical (aboral) view of a reproductively mature cydippid. Four of the eight gonads are visibly enlarged (large arrowheads); the adtentacular (closer to the plane of the tentacles) four gonads are visibly smaller (small arrows). (B) Lateral view of a reproductively mature cydippid. Oral pole faces up. Enlarged adesophageal (orthogonal to the plane of the tentacles) gonads face the viewer. (C) Unenlarged adtentacular gonad under higher magnification (side view). (D) Enlarged adesophageal gonad in the same animal (side view). Arrowhead highlights a visible immature oocyte. (E) Face-on view of a gonad in a live, reproductively active cydippid. (F) High-magnification view of an ovary in a reproductively active cydippid. Arrowheads highlight individual large cells, likely nurse cells. (G) Apical (aboral) view of a live offspring of a cydippid ∼24 hpf. (H) Live offspring of a cydippid ∼24 hpf. Oral pole faces up. Scale bar A, B = 1 mm; scale bar C, D, E, G, and H = 100 µm; scale bar F = 20 µm.
These cydippid-stage M. leidyi produce normal embryos that begin as one-cell zygotes enclosed in a fertilization envelope (Fig. 1 G and H). The embryos develop into morphologically normal cydippids on a similar timeline to embryos produced by large lobate-stage M. leidyi, although the percentage of embryos that develop normally is lower: mean 19.8% of cydippids’ self-fertilized progeny (n = 746 embryos from four biological replicates) vs. 90.9% of lobates’ self-fertilized progeny develop into morphologically normal, free-swimming cydippids by 24 h postfertilization (hpf) (n = 129 embryos from four biological replicates). These F1 offspring cydippids themselves become fertile around the same developmental stage as their parents. We have observed embryo production through the F3 generation.
Cydippids’ Reproductive Output Is Best Modeled with a Time-Explicit Negative Binomial Model.
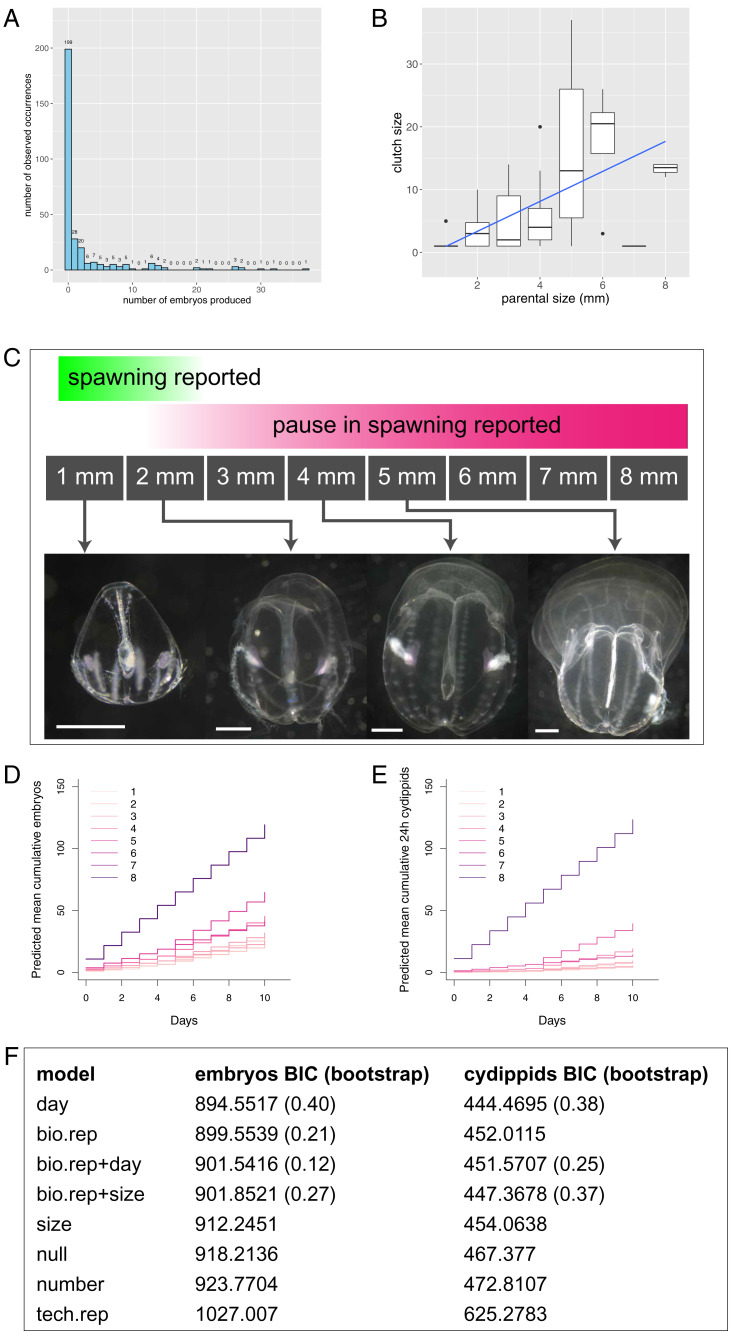
While previous reports suggested a minority of individuals are capable of small-size spawning, most isolated individuals > 1 mm spawned at least once during our observation period (34/56 from four biological replicates over 4 to 11 d of observation), and almost every individual observed for 6 or more days produced at least one embryo. However, daily spawning is rare; among all isolated individuals observed on at least 4 d that spawned at least once (32 individuals from four biological replicates), only a single individual produced an embryo every day of the observation period. When actively spawning cydippids (∼1 mm and up) spawn, they usually produce only one to two embryos per day (Fig. 2A) under this culture regime. This pattern of irregular spawning persists; however, as they grow past ∼5 mm, they may occasionally produce several dozen embryos in a 24-h period.
Fig. 2.
Daily reproductive output of individual M. leidyi at body size 1 to 8 mm. (A) Histogram of embryos counted in daily observations of 56 individuals from four biological replicates. Numbers above each bar indicate the number of times a given clutch size was produced. (B) Clutch size (embryos per clutch on days when any embryos are produced) increases with parental body size (n = 98 clutches produced by 32 individuals from four biological replicates) when zero spawn days are excluded; the blue line shows the mean, and shading around the line shows the 95% CI. The 7-mm individuals were relatively undersampled, so we believe this to be an outlier. (C) The transition from cydippid to lobate morphology begins ∼2 mm and is apparent by ∼5 mm, which overlaps with the appearance of lobate morphology. Bars in each panel represent 1 mm. (D and E) Staircase graphs showing predicted mean cumulative reproductive output of isolated individuals at body size 1 to 8 mm. (D) Embryos produced. (E) Daily normal hatched offspring produced by 24 hpf. Each colored line represents reproductive output at a different parental body size in millimeters; lines do not represent specific individuals. Specific individuals’ outputs across different body sizes can be seen in SI Appendix, Fig. S1C. The hypothesized gap period of 2 to 4 mm is highly sampled in the dataset, but no such pause is apparent. Repeated measures of 68 isolated individuals at various body sizes of 1- to 8-mm diameter, totaling 307 daily observations, from seven biological replicates. (F) The two columns represent the BIC results for all models evaluated in the size experiments shown in D and E; bootstrapping results are in parentheses. The model with the lowest BIC should be understood as the model that most closely represents the generating process given the data; the nonparametric bootstrapping results indicate the uncertainty around selection of that model (SI Appendix for model derivation). Abbreviations: bio.rep = biological replicate; tech.rep = technical replicate.
Following the approach of a recent analysis of small-size spawning in another ctenophore (19), we plotted parental body size vs. clutch size when zero reproductive output days are dropped and confirmed that our species also exhibits a similar positive correlation between size and fecundity (Fig. 2B). However, moving forward, we wanted to use a model that accommodates these highly skewed, zero-inflated data. We thus formulated a de novo, hierarchical stochastic model of births randomly occurring over time according to a set of biological hypotheses regarding the drivers of offspring production. This hierarchical model is a modification of a pure birth process (33) that incorporates individual heterogeneity in birth rates. Using this model has the dual advantage that by modeling the birth process as a temporal biological process, it 1) explicitly incorporates the embedded time dependency in the counts done over time and 2) admits the inclusion of birth rate heterogeneity as a random effect that ultimately recapitulates naturally an excess of zeros in the observed counts. Traditional generalized linear models to test the effect of different experimental and biological factors on the accumulation of births should not be used here because they erroneously ignore the biological time dependency in the counts and therefore may result in excessive type I errors in hypothesis testing, as well as severe model choice errors (33). The full hierarchical model derivation, our maximum likelihood parameter estimation, and our nonparametric bootstrap model selection approaches using evidential statistics and the Bayesian information criterion (BIC) (34–38) are detailed in the SI Appendix. SI Appendix, Fig. S1 shows a visual representation of the modeling process, and SI Appendix, Fig. S1C shows embryo production and parental body size over time for three real individuals. Our R code permits similar visualizations for any individual in our dataset.
We found that the model using parental body size was slightly outperformed by models using other parameters, notably days in the experiment for the raw data. However, the median bootstrap difference between the lowest and the second-lowest BICs suggested much stronger evidence for biological replicate + body size (median ΔBIC1 = 7.742626, ΔBIC2 = 5.493028) than experimental day (median ΔBIC1 = 3.846758, ΔBIC2 = 12.474642) as a driver of 24 hpf offspring production (SI Appendix, Data S1).
Crucially, there is no pause in spawning as previously hypothesized, as illustrated by plotting embryos or cydippids produced by parental body size category (Fig. 2 C and D). Previous studies (detailed in SI Appendix, Table S1) reported a pause in reproductive effort beginning at a certain size threshold (beginning by 3.0 mm in all cases and ranging from an ∼0.6- to 2.8-mm body width or a 0.75- to 2.8-mm body length). However, by following single individuals, we observed that spawning continues across sizes 1.0 to 8.0 mm; we documented concurrent spawning and growth during the observation period, suggesting that there is not a binary switch between growth and reproduction.
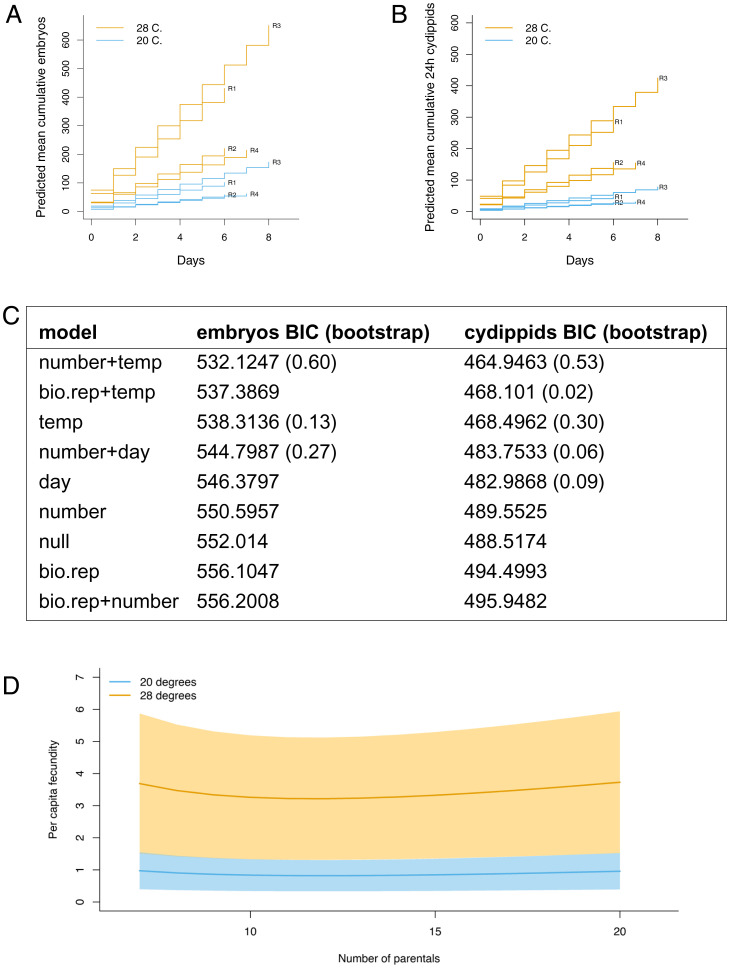
Incubation Temperature Is Positively Correlated with Reproductive Output.
We used two temperatures near the extremes of typical temperatures used to culture M. leidyi and known to permit normal growth (39). We found that rearing temperature is positively associated with both the number of eggs produced per parental cydippid and the number of eggs that develop into normal offspring by 24 hpf (Fig. 3). The best supported model for embryo production includes both temperature and number of parentals (embryo median ΔBIC1 = 3.608418, median ΔBIC2 = 6.382366, vs. all other selected models’ median ΔBIC1 = 2.615262, ΔBIC2 = 6.779690). The same model was also best supported for cydippid production (cydippid median ΔBIC1 = 2.637563, median ΔBIC2 = 4.1911, vs. all other selected models’ ΔBIC1 = 1.948039, ΔBIC2 = 6.665559).
Fig. 3.
Environmental temperature affects fecundity, as shown by predicted mean cumulative reproductive output at two temperatures, 20 and 28 °C, indicating a daily increase from ∼0.88 embryos/parent to ∼2.05 embryos/parent between the two temperatures. Repeated measures of 133 parental cydippids cultured in four groups of 7 to 20 animals from four biological replicates, totaling 63 total daily observations. (A) Reproductive M. leidyi cydippids produce more embryos and (B) more normal 24 hpf offspring when incubated at the warmer temperature. (C) The two columns represent the BIC results for all models evaluated in the temperature experiments shown in A and B; bootstrapping results are in parentheses. The model with the lowest BIC should be understood as the model that most closely represents the generating process given the data; the nonparametric bootstrapping results indicate the uncertainty around selection of that model (SI Appendix for model derivation). (D) Per capita fecundity at 20 and 28 °C. Shaded area (transparent) shows the 95% CI; by chance, the CIs of the samples are very closely apposed. Abbreviations: bio.rep = biological replicate; temp = temperature.
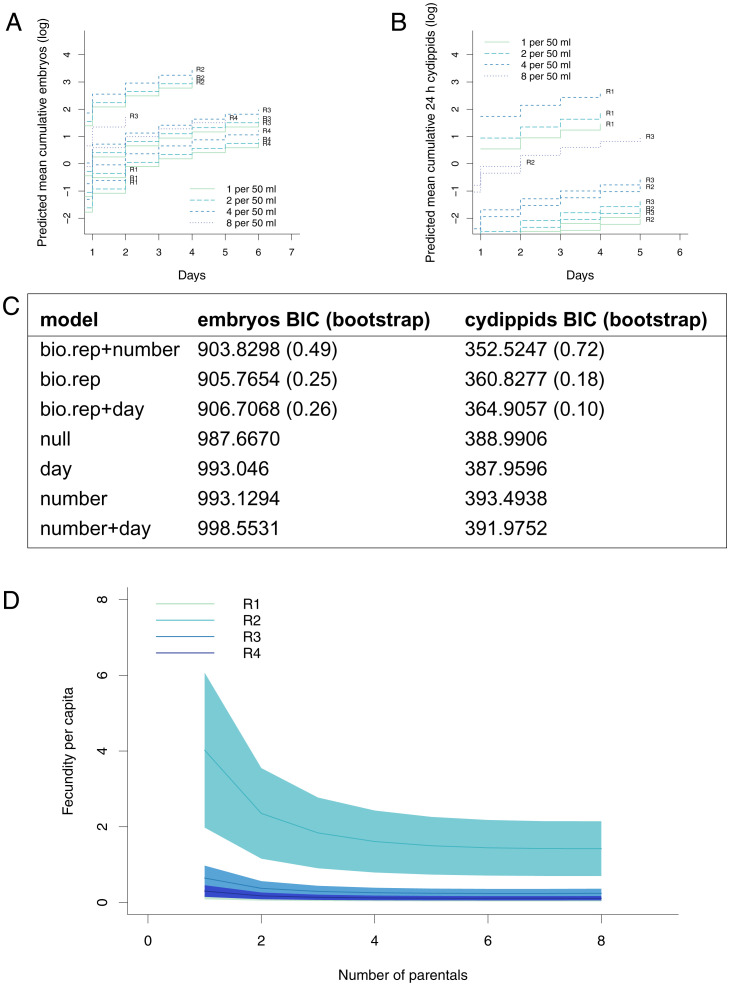
Fecundity Is Relatively Insensitive to Culture Density.
We hypothesized that culture density might play a role in inducing small-size spawning, whether as a response to stress or a mechanism such as community spawning. We tested cultures of one, two, four, and eight individuals in a constant volume of 50 mL (Fig. 4). The per capita fecundity slightly decreased when the parental density in the culture increased; this density-dependent effect was most apparent in a single biological replicate that had higher overall fecundity. Most of the effect is driven by the transition from one to two individuals per culture. Importantly, animals cultured singly spawn, so there is not a requirement for presence of a conspecific to spawn at a small size. The best performing model for both embryo and normal cydippid production incorporates biological replicate and number of parentals into its parameters, indicating extremely strong support for this model (embryo median ΔBIC1 = 8.553733, median ΔBIC2 = 11.56055, vs. all other selected models’ median ΔBIC1 = 2.527544, ΔBIC2 = 5.399919; cydippid median ΔBIC1 = 9.143399, ΔBIC2 = 11.699243, vs. all other selected models’ median ΔBIC1 = 3.381177, ΔBIC2 = 4.849772).
Fig. 4.
Fecundity increases with culture density. (A and B) Predicted mean cumulative reproductive output at four culture densities (one to eight animals per 50-mL culture). There were 297 individual observations of n = 60 treatment replicates totaling 174 animals from four biological replicates. (C) The two columns represent the BIC results for all models evaluated in the density experiments shown in A and B; bootstrapping results are in parentheses. The model with the lowest BIC should be understood as the model that most closely represents the generating process given the data; the nonparametric bootstrapping results indicate the uncertainty around selection of that model (SI Appendix for model derivation). (D) Per capita fecundity for each biological replicate. Shaded area shows SE.
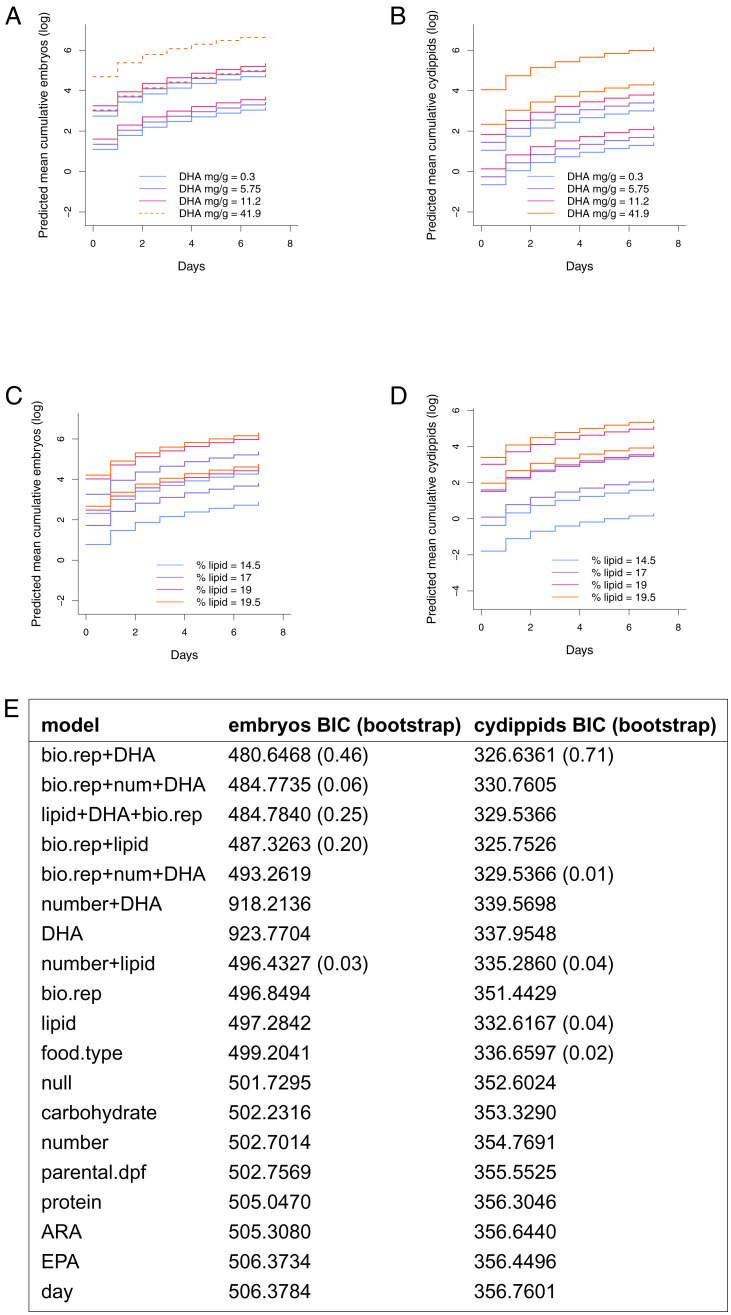
Fecundity Is Conditional on Nutrition.
Several preliminary observations indicated that spawning at small sizes is affected by diet. First, we found that cydippids transitioned onto artemia rather than rotifer-based diets entirely ceased spawning after ∼3 d. We also observed that when our standard cultures (35 to 50 individuals per ∼200-mL culture in standard finger bowls) were fed with rotifers raised on complex commercial algal feed vs. nonenriched feedstock, the bowls of cydippids fed the nonenriched rotifers ceased spawning entirely after ∼5 d. These preliminary experiments suggested the hypothesis that diet is an important factor in cydippid spawning, especially as starvation for a similar duration (2 to 4 d) has been shown previously to dramatically reduce spawning in lobate-stage M. leidyi at similar temperatures (40, 41).
To identify the nutritional requirements of cydippids to spawn, we purchased commercial single- and multispecies algal feeds with different nutritional composition to enrich their prey rotifers’ diets. Diets based on these feeds or similar commercial options have been used in similar experiments with different focal taxa [e.g., (42, 43)]. To identify macronutrients required for cydippid-stage spawning, we tested four prey rotifer diets with varying macronutrient (protein, carbohydrate, and lipid) and essential fatty acid (docosahexaenoic acid [DHA], eicosapentaenoic acid [EPA], and arachidonic acid [ARA]) contents. Using manufacturer-reported nutritional data (SI Appendix, Table S2), we analyzed the relationship of prey animals’ dietary macronutrients and essential fatty acids to predator ctenophore fecundity. We found evidence that lipids and particularly the fatty acid DHA positively correlate with fecundity (Fig. 5). The protein content of these diets is relatively similar, and thus carbohydrate and lipid content are inversely related. Broad ranges of EPA, DHA, and ARA were provided by these diets. The general results were similar whether scoring embryos produced or number of normal 24 hpf offspring produced and for DHA or total lipids (Fig. 5). The best supported model incorporated biological replicate and feed DHA concentration (embryo median ΔBIC1 = 2.069955, median ΔBIC2 = 3.772602, median ΔBIC3 = 6.110557, vs. all other selected models’ ΔBIC1 = 3.302318, ΔBIC2 = 4.145272, ΔBIC3 = 12.803460; cydippid median ΔBIC1 = 3.36046, median ΔBIC2 = 5.75557, vs. all other selected models’ ΔBIC1 = 5.170143, ΔBIC2 = 6.36331).
Fig. 5.
Rotifer (prey) nutrition affects M. leidyi capacity to spawn at small sizes (1 to 3 mm). Repeated measures for 8 d of n = 148 animals in cultures of 12 to 20 individuals per treatment from two biological replicates, totaling 64 daily observations. (A) Predicted mean cumulative reproductive output of embryos at four dietary levels of DHA. (B) Predicted mean cumulative reproductive output normal 24 hpf offspring cydippids at four dietary levels of DHA. Dashed line is used where two treatment lines overlap (19.5% in B and D) to facilitate visualization. (C) Predicted mean cumulative reproductive output of embryos at four dietary levels of total lipids. (D) Predicted mean cumulative reproductive output normal 24 hpf offspring cydippids at four dietary levels of total lipids. (E) The two columns represent the BIC results for all models evaluated in the diet experiments shown in A–D; bootstrapping results are in parentheses. The model with the lowest BIC should be understood as the model that most closely represents the generating process given the data; the nonparametric bootstrapping results indicate the uncertainty around selection of that model (SI Appendix for model derivation). Abbreviations: bio.rep = biological replicate; num = number; DHA = docosahexaenoic acid; parental.dpf = parental days post-fertilization (parental age).
The Ctenophore B. ovata Also Spawns at a Small Size.
We also observed small-size spawning in another ctenophore from a lineage that does not undergo a morphological transition as it grows, Beroe ovata. This species is thought to preferentially outcross, so we did not isolate individuals, but three dishes containing two, three, or four animals as small as 0.3 × 0.7 mm and as large as 2 × 3 mm spawned over several days and produced normal 24 hpf offspring. We counted as many as 33 normal 24 hpf offspring produced by a single pair in 1 d.
Discussion
With adequate nutrition, M. leidyi will spawn reliably and continuously, beginning at small sizes (∼1 mm and up, compared to maximum sizes reached by adults of >60 to 100 mm), regardless of their overall morphology, i.e., cydippid vs. lobate body shape. Warm temperatures and high-quality nutrition, especially higher content of the essential fatty acid DHA, further increase their fecundity (Fig. 6). Where these conditions occur in the wild, small-size spawning may contribute to M. leidyi recruitment—perhaps considerably, given the potential for geometric population growth. Since temperature and food availability are two major factors that control reproductive output in a range of marine invertebrates, and have been amply demonstrated in the literature as key factors in M. leidyi spawning at a larger size (17, 40, 41, 44, 45), their apparent sufficiency to induce spawning at a small body size in M. leidyi suggests that small-size spawning is a typical part of this animal’s life cycle rather than a rare, inducible phenomenon or otherwise physiologically distinct from later reproduction. Experiments reported here were carried out with animals collected throughout more than a calendar year and from several locations; thus, we are confident that we did not sample only individuals sharing a unique genetic predisposition to small-size spawning and that seasonality is not an important factor.
Fig. 6.
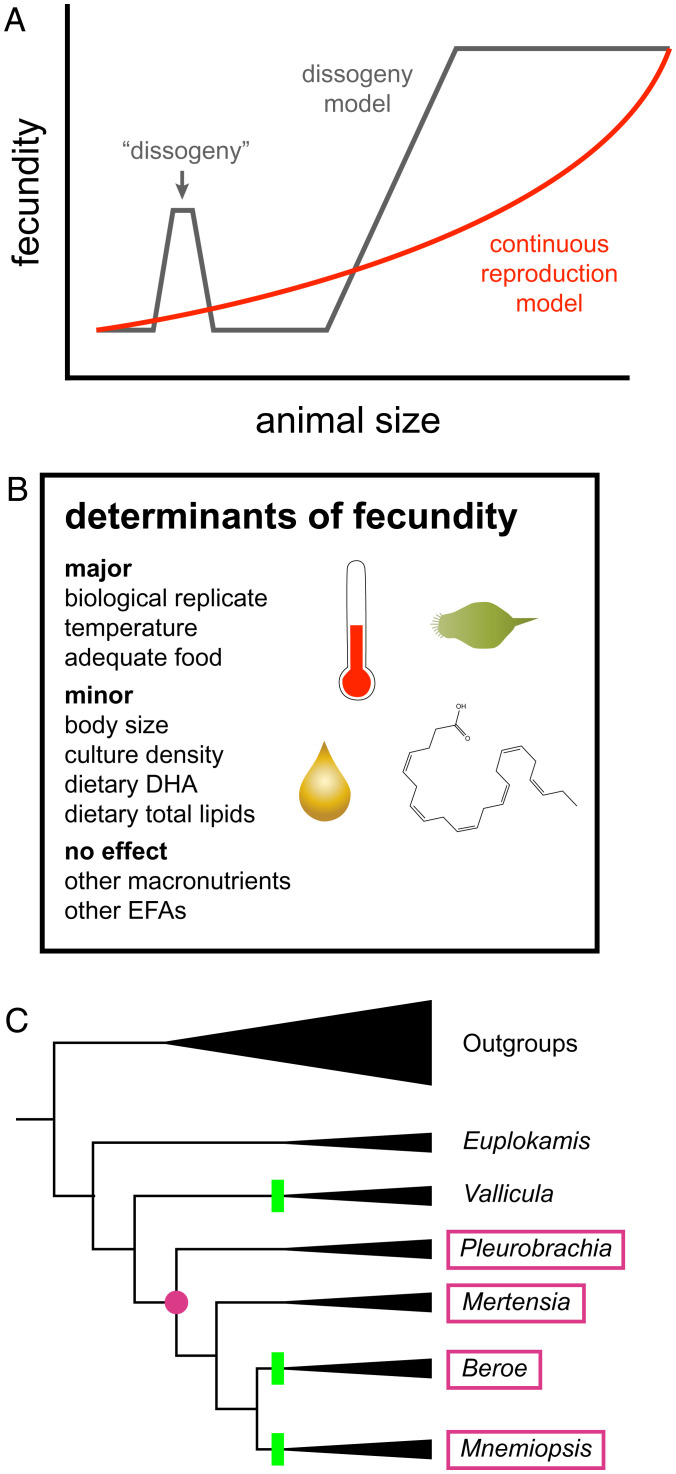
Revised model for ctenophore sexual maturation. (A) Ctenophores do not have two separate phases of reproduction as described in previous literature (dissogeny model). Rather, hatched ctenophores become fertile early and slowly increase their reproductive output (continuous reproduction model). (B) Several variables tested affect fecundity similarly in small-size M. leidyi as in published reports on large-size animals. These reproductive characteristics—responsiveness to nutrition, temperature, culture density, continuous and gradually increasing reproductive capacity, and the commonness of small-size spawning—all clearly suggest that M. leidyi are functionally adult from a body size ⪆ 1 mm, regardless of morphology. (C) Small-size reproduction mapped onto a cladogram of the Ctenophora. Tree topology based on Whelan et al. (22). Pink boxes containing a genus name indicate a branch with one or more species that have been shown to reproduce at a small size (∼0.5 to 1 mm) (16, 19, 20, 46); the pink circle indicates the most recent common ancestor of these taxa. Green bars indicate lineages with at least some species exhibiting morphologies other than cydippid at some developmental stage, as reconstructed in Whelan et al. (22). Beroids do not have a cydippid form at any stage. Reproduction at similarly small sizes has not been ruled out in the other ctenophore lineages. Abbreviations: DHA = docosahexaenoic acid; EFAs = essential fatty acids.
Moreover, small-size spawning appears to be continuous with, and physiologically indistinguishable from, spawning at larger sizes. Our bootstrap simulations agree with prior work that clutch size generally scales with parental body size, consistent with the observation that body size determines ∼20% of the egg production rate in larger M. leidyi (46). However, in contrast to prior reports of small-size spawning, we show clearly that there is no stereotyped pause in spawning in the previously reported size range. We highly sampled animals in the hypothesized size range for this pause of ∼2.2 to 8+ mm (SI Appendix, Table S1), which overlaps with the morphological transition from cydippid to lobate in this species of ∼2 to 5 mm (Fig. 2C), and found that spawning continues at these sizes, conclusively ruling out a pause. The morphological differences between cydippid and lobate stages primarily affect feeding structures and are known to affect feeding behavior (47–49), but the continuity of reproduction suggests that this morphological and ecological transition is not homologous with, or even analogous to, metamorphosis, despite its apparent irreversibility (40), and is unrelated to adulthood per se. Rather, these successive forms may be better understood as serial ecomorphs, perhaps even arising plastically at some point in the past.
It remains possible that other ctenophore species pause spawning in this size range. However, we believe that technical differences in experimental design adequately explain why we did not observe a pause in spawning where others have. First, we fed all experiments such that at least some food remained after 24 h. Anecdotally, we observed that even heavier feeding might increase fecundity but at the expense of higher mortality due to fouling in closed cultures. At least one previous report of such a gap was shown under controlled experimental feeding conditions (16) where a constant amount of prey was provided for all individuals regardless of size; intermediate-size animals simply may have been inadequately nourished for reproduction. Second, we made more and longer-term daily observations of both individuals and groups. The robustness of this dataset overcomes limitations imposed by the inherent stochasticity in reproduction rates. Nearly all individuals > 1 mm we observed over 6 or more days spawned at least once in that time. Most published reports using wild-caught small-size animals observed the number of embryos produced in a short (24 to 48 h) period after collection (19, 20). In our observation, it is extremely rare for M. leidyi cydippids to spawn daily, with gaps of several days between spawns being common, so shorter sampling periods would create the impression that not all small individuals spawn.
This reproductive strategy was likely present early in the ctenophore lineage. The phylogenetic distribution of species in which spawning at an extremely small size relative to its potential maximum has been reported, including our observation in B. ovata, is consistent with small-size spawning in the stem lineage leading to the last common ancestor of Mnemiopsis, Pleurobrachia, Mertensia, and Beroe (Fig. 6C). We thus propose elimination of the terms dissogeny, larva, and metamorphosis from the ctenophore literature, as these reproductively mature animals are clearly adults despite their size. We recommend that ctenophores be described by their body size, type, and reproductive status as needed for clarity (e.g., prereproductive cydippids).
However, this is not to say that differing environments do not differentially favor reproduction at smaller vs. larger sizes or that small-size spawning is not an important driver of their invasion biology. On the contrary, evidence from the field suggests that local conditions may dramatically favor reproduction at small body size in other species of ctenophores. In a Mertensia ovum population newly colonizing a warmer region, 95% of animals found in zooplankton sampling nets were under 1.1 mm (20), suggesting that this population is maintained entirely by small-size spawning. Similarly, temporospatial variation was observed in whether small-size or large-size individuals contribute to recruitment in a species colonizing cooler waters, Pleurobrachia globosa (19). The relative abundance of cydippid-stage M. leidyi increases with water temperature and dramatically so above 25 °C in its invaded region (50). Thus, spawning at small sizes may be an important driver of ctenophore ecology, particularly during invasion, as one introduced individual’s offspring can grow exponentially after only ∼2 wk postfertilization.
High juvenile attrition (51) or heterogenous environmental conditions (48) selectively favoring early reproduction are both plausible mechanisms for the observed importance of early reproduction to recruitment of newly established ctenophore populations. However, many ctenophores, especially the focal species of this study, are well adapted for survival under varying abiotic conditions (39, 50, 52–54). Furthermore, their reproductive output at large body sizes is enormous at thousands of eggs per day, accounting for as much as 10% of their total carbon (55). Thus, the life history theory that low-density populations are generally advantaged by faster time to reproduction (10) seems to better explain why pioneer populations disproportionately recruit through early spawns. As a population approaches the carrying capacity, intraspecific competition becomes relatively more important compared to sparse populations. However, the prey concentration required for egg production in large animals is typically exceeded in M. leidyi’s introduced range (40), so the drivers of this observed effect in the wild will be a fruitful area for future study.
While precise consensus definitions for direct vs. indirect animal development remain elusive, we propose that absence of sexual reproduction is likely one universal feature of larvae, as dissogeny or larval reproduction in ctenophores was the only specific example of such proposed in the scientific literature. Definitive absence of a larval life stage should reassure researchers who use ctenophores to study aspects of their biology such as regeneration and development, since small-size individuals represent an adult life stage and embryos produced by parents of any size represent normal development. On the other hand, the adult morphological transition from cydippid to lobate exhibited in some ctenophores represents a new study system for postembryonic development. We also found an effect of dietary DHA on ctenophore fecundity, which has been observed in many bilaterian animals but our results suggest may be a panmetazoan trait. Furthermore, since juvenile cydippids can be spawned reliably with improved nutrition, ctenophores will become a more accessible developmental model system even to researchers located inland or otherwise far from ctenophore species of interest. We have seen that M. leidyi can be raised reliably to reproductive age in 2 wk rather than months and that it is possible to keep reproductively active, overlapping generations of animals in a compact footprint and without complex culturing systems. M. leidyi may become a viable candidate for a ctenophore genetic model system, permitting establishment of transgenic lines and direct investigation of previously inaccessible developmental phenomena such as maternal effects and development of the primordial germ cells.
Materials and Methods
Animals.
Lobate-stage M. leidyi and similarly sized B. ovata were collected in the waters surrounding the Whitney Laboratory for Marine Bioscience in Marineland, FL, from floating docks on site and near Flagler Beach, Anastasia Island, or Indian River Lagoon. Each biological replicate reported is the product of a captive spawn of two or more unique wild-caught individuals; no parental lobate was used in more than one replicate. Animals spawned overnight based on their endogenous circadian cycle. Hatchlings were reared in 2-L glass beakers or ∼18-L High Density Polyethylene (HDPE) containers in ultraviolet-sterilized filtered seawater. All experiments reported here were from the offspring of animals collected between December 2019 and March 2021.
Cultures.
Depending upon the experiment, cydippids were reared in 2- to 5-gallon HDPE buckets, 1- to 4-L glass beakers, 50-mL glass beakers, glass finger bowls, 6-well plates, or 24-well plates. Constant-volume density experiments were performed in 50-mL glass beakers with one to eight animals. Other isolation experiments were performed in 24-well plates. Bulk culture experiments such as food type were performed in glass finger bowls with ∼10 to 40 animals.
Experiments were kept on a 12-h light cycle at 28 °C unless otherwise noted. In all cases, cydippids were fed daily such that a slight excess of prey animals remained in the culture container between feedings, and water was changed completely each day before feeding. For the experiments shown in SI Appendix, Data S6 (replicates AA and AC) and as shown in SI Appendix, Fig. S1C, some animals were fed more heavily to speed body growth but at a cost of greatly increased mortality in culture. Animals in these growth-specific experiments were measured daily and thus exhibit slight fluctuations in size. The reproductive output vs. growth experiments shown in Fig. 2 C and D were measured twice weekly, so their growth patterns appear more stable, but this reflects lack of measurement.
A biological replicate is defined as the progeny of one or more unique wild-caught lobates (in practice, typically ∼5 to 10) spawned in the same container on a single day and never reused for any other replicate. These bulk cultures were kept at ambient room temperature and light conditions and fed daily with rotifers fed RGcomplete. To set up an experiment, animals were collected from the bulk culture into a single container, measured, and transferred to one of the experimental containers at random (alternating or rotating between each treatment container until either the goal number was reached or there were no more animals left).
Whether body length or width is used varies across studies, but we found body width easier to measure. We measured approximate body diameter in the equatorial tentacular plane, similarly to Wang et al. (19). The conversion factor to body length, which has been used in other studies, is ∼0.8 (16, 19). However, since our method to repeatedly estimate body size allowed us to track many more measurements, we recommend it to others.
To measure M. leidyi cydippid size, we used known-diameter polypropylene disposable pipettes. We cut gradually tapering plastic transfer pipettes at different points along their length with razor blades to obtain the desired internal diameter as measured with a miniruler (Ted Pella, Inc., Redding, CA) in 0.5-mm steps below 4 mm and 1-mm steps above 4 mm. Body sizes reported are rounded up to the nearest size category. Animals were transferred under a dissecting microscope to estimate body size. B. ovata body size was measured as width multiplied by length using a miniruler.
Rotifer Care and Feeding.
Cydippids were fed primarily rotifers (Brachionus plicatilis, L type, Reed Mariculture, Campbell, CA) fed with RGcomplete (Reed Mariculture) unless otherwise noted; alternative rotifer feeds were purchased from the same supplier. Rotifers were maintained as described in Ramon-Mateu et al. (56) and fed daily with the appropriate microalgal feed.
To identify macronutrients required for cydippid-stage spawning, we tested four prey rotifer diets: 1) B. plicatilis (L type) rotifers fed a commercial, complex microalgal diet (RGcomplete); 2) B. plicatilis fed a high-EPA, single-algae commercial feed, Nannochloropsis concentrate; 3) B. plicatilis fed a high-DHA, single-algae commercial feed, Isochrysis concentrate; 4) B. plicatilis fed a mixture of equal parts of the two single-algae diets.
Manufacturer-reported nutritional content of the algal feeds is available in SI Appendix, Table S2.
Imaging.
Live images were captured with either Zeiss Stemi 508 microscope equipped with a Ximea 12.4 megapixel camera system (MC124CG-SY-UB) for the whole-animal view of parental cydippids or a Zeiss Axio Imager M2 coupled with an AxioCam (HRc) digital camera, using ZEN software for all other images.
Data Analysis.
Data analysis was performed in R (57). All raw data, as .csv files, are available in SI Appendix, Data S2–S6. All R code used to generate the results is available, in standard R generalized linear model notation for ease of use, on GitHub (https://github.com/jmponciano/ctenophores). Details of the statistical models are available in SI Appendix.
For each experiment, we fitted a different set of models for the mean number of births of embryos occurring over time. We then carried out model selection using the BIC to compare the relative fit of these models to the data to choose among possible explanatory models, including a null model for each. To test the reliability and support in the resulting chosen best models for every experiment, we adopted the recent nonparametric bootstrap model selection methodology of Taper et al. (34). To do that, we performed sampling with replacement of each of the datasets while preserving the structure of the data, generated a set of 100 nonparametric bootstrap samples each time to generate nonparametric bootstrap (n = 100) results, and compared the difference in the first two BICs (delta-ICs) under the best supported model vs. all other models (see SI Appendix, Data S1 for results of all models tested, including bootstrap results and first two delta-ICs for best model vs. all other models).
Supplementary Material
Acknowledgments
We thank Leonardo Ibarra-Castro for advice on rotifer culture and feeds and providing rotifers for pilot experiments, discussions on nutrition, and feedback on a previous version of this manuscript; Joseph Ryan for use of lab space and equipment, discussions, and feedback on a previous version of the manuscript; Dorothy G. Mitchell for animal care and collection assistance; Maria Cuellar for helpful conversations regarding the data structure; and Fredrik Hugosson and Mark L. Taper for feedback on previous versions of the manuscript. Our conclusions and any errors are our own and do not necessarily represent the views of those acknowledged. Funding: National Science Foundation Postdoctoral Research Fellowship in Biology under Grant 2010755 (A.E.); National Science Foundation IOS-1755364 (M.Q.M.); National Aeronautics and Space Administration Grants SC37607-01/P0153802 and 80NSSC18K1067 (M.Q.M.); and NSF Grant No. 2052372 to University of Florida supporting J.M.P.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122052119/-/DCSupplemental.
Data Availability
R code data have been deposited in GitHub (https://github.com/jmponciano/ctenophores) (58). All study data are included in the article and/or supporting information.
References
- 1.Hall B. K., Wake M. H., “Introduction: Larval development, evolution, and ecology” in The Origin and Evolution of Larval Forms, Hall B. K., Wake M. H., Eds. (Academic Press, 1999), pp. 1–20. [Google Scholar]
- 2.Nielsen C., “Origin and diversity of marine larvae” in Evolutionary Ecology of Marine Invertebrate Larvae, Carrier T., Reitzel A., Heyland A., Eds. (Oxford University Press, 2018), pp. 3–15. [Google Scholar]
- 3.Marlow H., “Evolutionary development of marine larvae” in Evolutionary Ecology of Marine Invertebrate Larvae, Carrier T., Reitzel A., Heyland A., Eds. (Oxford University Press, 2018), pp. 16–33. [Google Scholar]
- 4.Sly B. J., Snoke M. S., Raff R. A., Who came first-larvae or adults? Origins of bilaterian metazoan larvae Life history modes and metazoan phylogeny. J. Dev. Biol. 47, 623–632 (2003). [PubMed] [Google Scholar]
- 5.Wang J., et al. , Evolutionary transcriptomics of metazoan biphasic life cycle supports a single intercalation origin of metazoan larvae. Nat. Ecol. Evol. 4, 725–736 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Raff R. A., Origins of the other metazoan body plans: The evolution of larval forms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1473–1479 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen C., Life cycle evolution: Was the eumetazoan ancestor a holopelagic, planktotrophic gastraea? BMC Evol. Biol. 13, 171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop C. D., et al. , What is metamorphosis? Integr. Comp. Biol. 46, 655–661 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Vaughn D., Why run and hide when you can divide? Evidence for larval cloning and reduced larval size as an adaptive inducible defense. Mar. Biol. 157, 1301–1312 (2010). [Google Scholar]
- 10.Allen J. D., Reitzel A. M., Jaeckle W., “Asexual reproduction of marine invertebrate embryos and larvae” in Evolutionary Ecology of Marine Invertebrate Larvae, Carrier T., Reitzel A., Heyland A., Eds. (Oxford University Press, 2018), pp. 67–81. [Google Scholar]
- 11.Poulin R., Progenesis and reduced virulence as an alternative transmission strategy in a parasitic trematode. Parasitology 123, 623–630 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Chun C., “Die Dissogonie, eine neue Form der geschlechtlichen Zeugung” in Festschrift Zum Siebzigsten Geburtstage Rudorf Leuckarts, R. Engelmann, Ed. (Leipzig, Germany, 1892), pp. 77–108. [Google Scholar]
- 13.Garbe A., Untersuchungen über die Entstehung der Geschlechtsorgane bei den Ctenophoren. Z. Wiss. Zool. 69, 472–491 (1901). [Google Scholar]
- 14.Totton A. K., Egg-laying in Ctenophora. Nature 174, 360 (1954). [Google Scholar]
- 15.Greve W., Cultivation experiments on North Sea ctenophores. Helgol. Wiss. Meeresunters. 20, 304–317 (1970). [Google Scholar]
- 16.Hirota J., “Laboratory culture and metabolism of the planktonic Ctenophore, Pleurobrachia bachei A. Agassiz” in Biological Oceanography of the Northern North Pacific Ocean, Motoda S., Takenouti Y. A., Eds. (Idemitsu Shoten, 1972), pp. 465–484. [Google Scholar]
- 17.Baker L. D., Reeve M. R., Laboratory culture of the lobate ctenophore Mnemiopsis mccradyi with notes on feeding and fecundity. Mar. Biol. 26, 57–62 (1974). [Google Scholar]
- 18.Martindale M. Q., Larval reproduction in the ctenophore Mnemiopsis mccradyi. Mar. Biol. 94, 409–414 (1987). [Google Scholar]
- 19.Wang S., Zhang G., Zhou K., Sun S., Long-term population variability and reproductive strategy of a northward expanded ctenophore Pleurobrachia globosa Moser, 1903 in a temperate bay, China. J. Exp. Mar. Biol. Ecol. 533, 151457 (2020). [Google Scholar]
- 20.Jaspers C., et al. , Ctenophore population recruits entirely through larval reproduction in the central Baltic Sea. Biol. Lett. 8, 809–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podar M., Haddock S. H. D., Sogin M. L., Harbison G. R., A molecular phylogenetic framework for the phylum Ctenophora using 18S rRNA genes. Mol. Phylogenet. Evol. 21, 218–230 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Whelan N. V., et al. , Ctenophore relationships and their placement as the sister group to all other animals. Nat. Ecol. Evol. 1, 1737–1746 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Shen X.-X., Evans B., Dunn C. W., Rokas A., Rooting the animal tree of life. Mol. Biol. Evol. 38, 4322–4333 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn C. W., et al. , Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Lemer S., et al. , Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc. Biol. Sci. 286, 20190811–20190831 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan J. F., et al. ; NISC Comparative Sequencing Program, The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science 342, 1242592 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hejnol A., et al. , Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc. Biol. Sci. 276, 4261–4270 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borowiec M. L., Lee E. K., Chiu J. C., Plachetzki D. C., Extracting phylogenetic signal and accounting for bias in whole-genome data sets supports the Ctenophora as sister to remaining Metazoa. BMC Genomics 16, 987 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redmond A. K., McLysaght A., Evidence for sponges as sister to all other animals from partitioned phylogenomics with mixture models and recoding. Nat. Commun. 12, 1783 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simion P., et al. , A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr. Biol. 27, 958–967 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Pisani D., et al. , Genomic data do not support comb jellies as the sister group to all other animals. Proc. Natl. Acad. Sci. U.S.A. 112, 15402–15407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapli P., Telford M. J., Topology-dependent asymmetry in systematic errors affects phylogenetic placement of Ctenophora and Xenacoelomorpha. Sci. Adv. 6, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennis B., Allee effects: Population growth, critical density, and the chance of extinction. Nat. Resour. Model. 3, 481–538 (1989). [Google Scholar]
- 34.Taper M. L., Lele S. R., Ponciano J. M., Dennis B., Jerde C. L., Assessing the global and local uncertainty of scientific evidence in the presence of model misspecification. Front. Ecol. Evol. 6, 679155 (2021). [Google Scholar]
- 35.Jerde C. L., et al. , Strong evidence for an intraspecific metabolic scaling coefficient near 0.89 in fish. Front. Physiol. 10, 1166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taper M. L., Ponciano J. M., Evidential statistics as a statistical modern synthesis to support 21st century science. Popul. Ecol. 58, 9–29 (2016). [Google Scholar]
- 37.Dennis B., Ponciano J. M., Taper M. L., Lele S. R., Errors in statistical inference under model misspecification: Evidence, hypothesis testing, and AIC. Front. Ecol. Evol. 7, 372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponciano J. M., Taper M. L., Model projections in model space: A geometric interpretation of the AIC allows estimating the distance between truth and approximating models. Front. Ecol. Evol. 7, 413 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gambill M., Møller L. F., Peck M. A., Effects of temperature on the feeding and growth of the larvae of the invasive ctenophore Mnemiopsis leidyi. J. Plankton Res. 37, 1001–1005 (2015). [Google Scholar]
- 40.Jaspers C., Møller L. F., Kiørboe T., Reproduction rates under variable food conditions and starvation in Mnemiopsis leidyi: Significance for the invasion success of a ctenophore. J. Plankton Res. 37, 1011–1018 (2014). [Google Scholar]
- 41.Reeve M. R., Syms M. A., Kremer P., Growth dynamics of a ctenophore (Mnemiopsis) in relation to variable food supply. I. Cabon biomass, feeding, egg production, growth and assimilation efficiency. J. Plankton Res. 11, 535–552 (1989). [Google Scholar]
- 42.Watanabe W. O., et al. , Live prey enrichment and artificial microdiets for larviculture of Atlantic red porgy Pagrus pagrus. Aquacult. Rep. 3, 93–107 (2016). [Google Scholar]
- 43.Camargo-Cely A., Collin R., Combined effects of temperature, salinity, and diet simulating upwelling and nonupwelling seasons alter life-history characteristics of a tropical invertebrate. Ecol. Evol. 9, 14368–14378 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehtiniemi M., Lehmann A., Javidpour J., Myrberg K., Spreading and physico-biological reproduction limitations of the invasive American comb jelly Mnemiopsis leidyi in the Baltic Sea. Biol. Invasions 14, 341–354 (2012). [Google Scholar]
- 45.van Walraven L., Langenberg V. T., van der Veer H. W., Seasonal occurrence of the invasive ctenophore Mnemiopsis leidyi in the western Dutch Wadden Sea. J. Sea Res. 82, 86–92 (2013). [Google Scholar]
- 46.Kasuya T., Ishimaru T., Murano M., Reproductive characteristics of the lobate ctenophore Bolinopsis mikado (Moser). Plankton Benthos Res. 3, 72–77 (2008). [Google Scholar]
- 47.Haddock S. H. D., Comparative feeding behavior of planktonic ctenophores. Integr. Comp. Biol. 47, 847–853 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Rapoza R., Novak D., Costello J. H., Life-stage dependent, in situ dietary patterns of the lobate ctenophore Mnemiopsis leidyi Agassiz 1865. J. Plankton Res. 27, 951–956 (2005). [Google Scholar]
- 49.Sutherland K. R., Costello J. H., Colin S. P., Dabiri J. O., Ambient fluid motions influence swimming and feeding by the ctenophore Mnemiopsis leidyi. J. Plankton Res. 36, 1310–1322 (2014). [Google Scholar]
- 50.Mahmoudi N., Babanezhad M., Seyfabadi J., Ahmadi M. R., Spatiotemporal relationships between life stages of the invasive ctenophore, Mnemiopsis leidyi, and environmental parameters in the southern Caspian Sea. J. Great Lakes Res. 46, 1262–1276 (2020). [Google Scholar]
- 51.Wright J., Bolstad G. H., Araya-Ajoy Y. G., Dingemanse N. J., Life-history evolution under fluctuating density-dependent selection and the adaptive alignment of pace-of-life syndromes. Biol. Rev. Camb. Philos. Soc. 27, 818–857 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Jaspers C., Møller L. F., Kiørboe T., Salinity gradient of the Baltic Sea limits the reproduction and population expansion of the newly invaded comb jelly Mnemiopsis leidyi. PLoS One 6, e24065 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lilley M. K. S., Thibault-Botha D., Lombard F., Respiration demands increase significantly with both temperature and mass in the invasive ctenophore Mnemiopsis leidyi. J. Plankton Res. 36, 831–837 (2014). [Google Scholar]
- 54.Haraldsson M., et al. , Environmental constraints of the invasive Mnemiopsis leidyi in Scandinavian waters. Limnol. Oceanogr. 58, 37–48 (2013). [Google Scholar]
- 55.Jaspers C., Costello J. H., Colin S. P., Carbon content of Mnemiopsis leidyi eggs and specific egg production rates in northern Europe. J. Plankton Res. 37, 11–15 (2015). [Google Scholar]
- 56.Ramon-Mateu J., Edgar A., Mitchell D., Martindale M. Q., “Experimental methods to study ctenophore whole-body regeneration” in Methods in Molecular Biology: Whole Body Regeneration, Blanchoud S., Galliot B., Eds. (Springer, 2022), pp. 95–119. [Google Scholar]
- 57.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2018). [Google Scholar]
- 58.J. M. Ponciano, A. Edgar. Ctenophores. GitHub. https://github.com/jmponciano/ctenophores. Deposited 21 February 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
R code data have been deposited in GitHub (https://github.com/jmponciano/ctenophores) (58). All study data are included in the article and/or supporting information.