Abstract
The interaction between a tumor and the tumor microenvironment (TME) plays a key role in tumorigenesis and tumor progression. Ubiquitination, a crucial post-translational modification for regulating protein degradation and turnover, plays a role in regulating the crosstalk between a tumor and the TME. Thus, identifying the roles of ubiquitination in the process may assist researchers to investigate the mechanisms underlying tumorigenesis and tumor progression. In the present review article, new insights into the substrates for ubiquitination that are involved in the regulation of hypoxic environments, angiogenesis, chronic inflammation-mediated tumor formation, and the function of cancer-associated fibroblasts and infiltrating immune cells (tumor-associated macrophages, T-cells, myeloid-derived suppressor cells, dendritic cells, and natural killer cells) are summarized. In addition, the potential targets of the ubiquitination proteasome system within the TME for cancer therapy and their therapeutic effects are reviewed and discussed.
Keywords: ubiquitination, deubiquitinases, tumor, tumor microenvironment, crosstalk, cancer therapy
1. Introduction
The tumor microenvironment (TME) refers to the surrounding microenvironment of tumor cells, including surrounding fibroblasts, immune cells, blood vessels, inflammatory cells, various signal molecules and the extracellular matrix (ECM). The TME is a complex environment for the survival and development of tumor cells. The normal functions of non-malignant cells within the TME can be effectively 'hijacked' by malignant cells to facilitate tumor progression. The interaction network between cancer cells and their microenvironment promotes cell proliferation and angiogenesis, inhibits cell apoptosis and immune detection, and activates immune cells to support cell invasion and migration (1). Given the crucial roles of the TME in tumorigenesis and tumor progression, it is evident that the pathways involved serve as potential targets for cancer treatment (2,3).
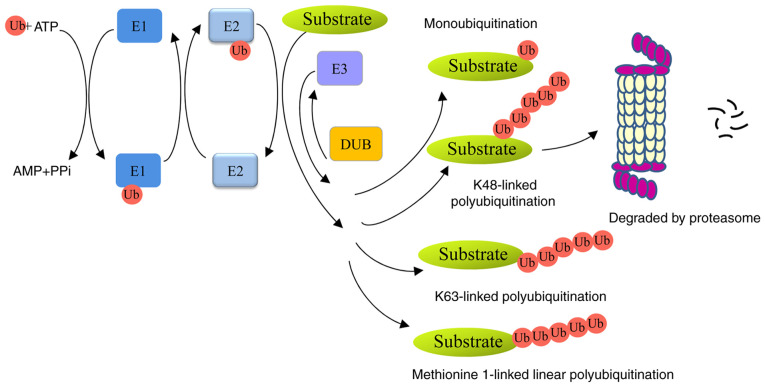
Ubiquitin, a small 76-amino-acid protein, can be covalently tagged to target proteins by monoubiquitination or polyubiquitination. A series of enzymes, including ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s) and ubiquitin ligases (E3s), are involved in this process (4). Polyubiquitin chains, linked at 48 lysine sites (K48) or K11 sites lead to proteolysis in a 26S proteasome-dependent manner. Monoubiquitination or K63-linked polyubiquitin chains are non-proteolytic ubiquitination signals and participate in autophagy, signal transduction and DNA damage repair (5,6). In addition, methionine 1-linked linear ubiquitin chains are assembled by a linear ubiquitin chain assembly complex (LUBAC), and are involved in inflammation, immunity and cell death (7,8). Ubiquitins can be removed from ubiquitinated proteins by deubiquitinases (DUBs) (Fig. 1). Members of the family can be divided into six types: ubiquitin-specific proteases (USPs), ubiquitin carboxyl-terminal hydrolases, ovarian-tumor proteases, Machado-Joseph disease protein proteases, JAB1/MPN/MOV34 metalloenzymes, and monocyte chemotactic protein-induced proteases (9).
Figure 1.
Overview of the ubiquitin proteasome system. The ubiquitin is activated by E1 in an ATP-dependent manner. It is then transferred to E2, recognized by E3 ligase and transferred to the substrate to form a mono- or polyubiquitinated protein. K48-linked polyubiquitin chains lead to 26S proteasome-mediated degradation; monoubiquitination, K63-linked or methionine 1-linked polyubiquitin chains are non-proteolytic ubiquitination signals, which participate in a number of biological processes. ATP, adenosine triphosphate; AMP, adenosine monophosphate; PPi, inorganic pyrophosphate; DUB, deubiquitinase.
Protein ubiquitination is a critical mechanism that modulates the levels and activities of proteins. This process is involved in various activities, including protein degradation, DNA repair activity, gene transcription, and signal transduction (10). The dysregulation of this system is closely associated with various human diseases, including cancers (11). Accumulating evidence demonstrates that E3 ligases and DUBs participate in tumorigenesis and progression through various biological processes, such as the cell cycle, cell proliferation, apoptosis, DNA damage repair and cell signaling (10,12,13). However, the roles of ubiquitination in the crosstalk between a tumor and the TME have rarely been discussed. The present review article comprehensively discusses the roles of ubiquitination in the interaction between tumors and the TME, such as the regulation of the hypoxic environment, angiogenesis and chronic inflammation-mediated tumor formation, as well as the modulation of the function of cancer-associated fibroblasts (CAFs) and infiltrating immune cells (tumor-associated macrophages, T-cells, myeloid-derived suppressor cells, dendritic cells and natural killer cells). In addition, the potential targets of the ubiquitination proteasome system (UPS) within the TME in cancer therapy and their therapeutic effects are reviewed.
2. Roles of the tumor microenvironment in tumorigenesis and progression
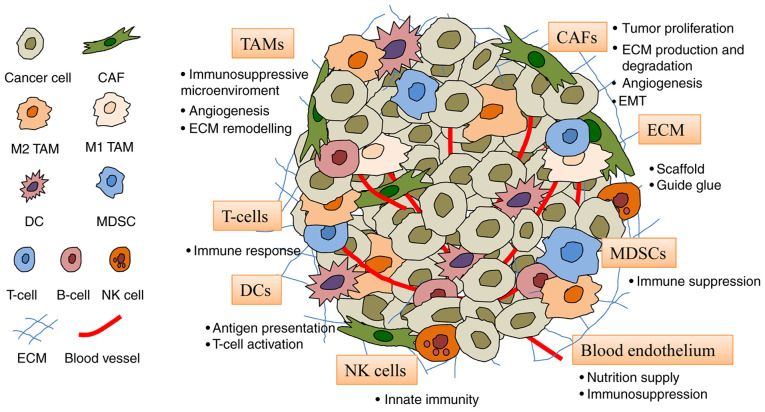
The TME consists of fibroblasts, endothelial cells, immune cells and ECM (Fig. 2). These cells provide support for tumor cells and encourage their proliferation, invasion and migration.
Figure 2.
Constituents of the TME. The TME comprises cells including CAFs, blood endothelial cells, immune cells (TAMs, DCs, T-cells, MDSCs and NK cells) and the ECM. CAF, cancer-associated fibroblast; DC, dendritic cell; ECM, extracellular matrix; MDSC, myeloid-derived suppressor cell; NK, natural killer; TAM, tumor-associated macrophage; TME, tumor microenvironment.
CAFs
CAFs, major components of stromal cells in solid tumors, play pivotal roles in tumor progression via multiple mechanisms, including paracrine and direct interactions, immune response regulation and ECM remodeling (14). CAFs can secrete tumor-promoting growth factors [e.g., transforming growth factor (TGF)-β, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF) and endothelial growth factor (EGF)], inflammatory cytokines [e.g., interleukin (IL)-6 and IL-8] and chemokines (e.g., CXCL8 and CXCL12), subsequently mediating cell proliferation, angiogenesis, migration, immunogenicity and drug resistance. As the main source of collagen-producing cells, CAFs synthesize the ECM and directly communicate with cancer cells, as well as other TME cells, such as endothelial and inflammatory cells. CAFs can also degrade and remodel the ECM by producing matrix metalloproteinases (MMPs) and then contribute to tumor invasion and metastasis in various cancers (15).
Tumor-associated endothelial cells
Angiogenesis plays a crucial role in tumor growth and metastasis, and is regarded as a prognostic marker of cancer (16). Endothelial cells and vascular blood vessel cells not only supply nutrition to tumor tissues, but also promote immune tolerance. For example, endothelial cells reduce the expression of E-selectin, intercellular adhesion molecule 1 and 2, and vascular cell adhesion molecule 1, and also impair the recruitment of cytotoxic T-cells to tumor lesions (17). In addition, the endothelial cells also express inhibitory molecules, such as programmed cell death-ligand1 (PD-L1) and PD-L2, and release soluble factors (prostaglandin E2, IL-6, TGF-β and VEGF) to regulate T-cell responses (18-20). Moreover, endothelial cells allow immunosuppressive myeloid cells to migrate from the blood to the tumor, thus impairing the antitumor immune response (21).
Immune cells
The immune-associated cells in the TME include macrophages, T-cells, myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs) and natural killer (NK) cells (22).
Tumor-associated macrophages (TAMs) are the main immune cell population of the TME in the majority of cancers. Macrophages are polarized by various microenvironments to form heterogeneous populations and can be divided into pro-inflammatory M1 macrophages or anti-inflammatory M2 macrophages. M1 TAMs are activated by Toll-like receptors (TLRs) or Th1 cytokines, such as tumor necrosis factor α (TNF-α), interferon (IFN)-γ, and colony-stimulating factor 2, and exert antitumor effects. M2 TAMs are regulated by IL-4, IL-10, IL-13, TGF-β or prostaglandin E2, and produce anti-inflammatory cytokines, including TGF-β and IL-10, with anti-inflammatory and tumor-promoting effects (23). In tumor tissues, the majority of macrophages are polarized into M2 macrophages, and subsequently promote angiogenesis, inhibit the antitumor immune response, support tumor growth and secrete different factors to regulate ECM remodeling, tumor cell motility and intravasation (24).
T-cells play vital roles in the host defense against cancer. External signals activate immature T-cells and trigger their immune function. Cytotoxic CD8+ T-cells and CD4+ T-helper 1 cells are the main factors of antitumor immunity (25). However, the immunosuppressive TME can induce T-cell dysfunction. Generally, T-cells express programmed cell death protein-1 (PD-1) and tumor cells express PD-L1. Their interaction leads to T-cell suppression and tumor cell survival, that is, cancer cell immunosuppression. In addition, Foxp3+ T-regulatory cells (Tregs) are another T-cell subpopulation involved in the escape of tumor cells.
MDSCs, a heterogeneous population of immature myeloid cells, are the key mediators of immune suppression in the TME. MDSCs inhibit the T-cell immune response mainly by expressing arginase-1, inducible nitric oxide synthase and anti-inflammatory cytokines, such as IL-10 and cyclooxygenase-2 (COX2) (26). MSDCs also express PD-L1 and cytotoxic-T-lymphocyte-antigen-4, and possess the ability to suppress CD8+ T-cell function (27).
DCs are natural immune cells and effective antigen-presenting cells, and participate in immune-mediated cancer elimination via antigen presentation and T-cell activation. In addition, DCs also provide antigens, co-stimulatory molecules, and cytokines for T-cell activation and differentiation, thereby inducing the immune response (28).
NK cells are cytotoxic effectors against cancer in innate immunity that are able to recognize and eliminate tumor cells without antigen presentation. NK cells express a variety of receptors with activated or inhibitory function and NK cell function is determined by the balance of signal input from these receptors. Cytokines (IL-12, IL-15 and IL-18) and transcription factors (e.g., T-bet) promote the activation of NK cells and produce IFN-γ, which plays a critical role in innate and adaptive immune responses (29).
ECM
The ECM is a protein network around tumor cells and TME cells. The main components of the ECM include collagen, proteoglycans, laminin and fibronectin. The ECM not only serves as a scaffold for tissue, but also provides key biochemical and biomechanical cues to guide cell growth, survival, migration and differentiation. In addition, the ECM also plays a role in the regulation of vascular development and immune function (30). Therefore, the ECM also plays a vital role in tumor proliferation, invasion, metastasis and angiogenesis.
3. Roles of ubiquitination in modulating the crosstalk between tumors and the TME
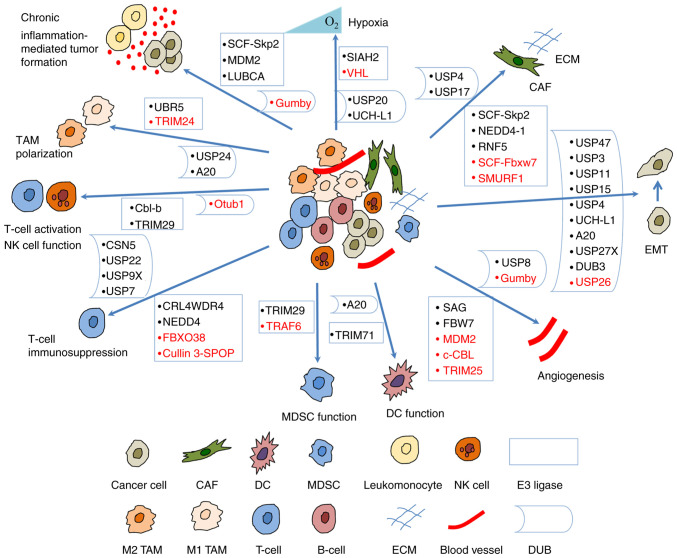
A number of studies have indicated that the UPS regulates tumorigenesis and progression by modulating the crosstalk between a tumor and the TME (Fig. 3). Some of the E3 ligases and their substrates involved are presented in Table I; DUBs are presented in Table II.
Figure 3.
Ubiquitination regulates the crosstalk between tumors and the tumor microenvironment. E3 ligases and DUBs regulate hypoxic microenvironment, angiogenesis, EMT, chronic inflammation-mediated tumor formation, and the crosstalk between CAFs, TAMs, T-cells, MDSCs, DCs, NK cells and tumor cells. Some of them suppress tumorigenesis (shown in red) and others promote tumorigenesis (shown in black). CAF, cancer-associated fibroblast; DC, dendritic cell; DUB, deubiquitinase; EMT, epithelial-mesenchymal transition; MDSC, myeloid-derived suppressor cell; NK, natural killer; TAM, tumor-associated macrophage; UBR5, ubiquitin protein ligase E3 component n-recognin 5; TRIM, tripartite motif containing; USP, ubiquitin-specific protease; Cbl-b, Casitas B-lineage lymphoma proto-oncogene-b; CSN5, COP9 signalosome 5; Otub1, OTU domain-containing ubiquitin aldehyde-binding protein 1; CRL4WDR4, WD repeat 4-containing CUL-RING ubiquitin ligase 4; FBXO38, F-box protein 38; cullin 3-SPOP, cullin 3-speckle-type pox virus and zinc finger protein; TRAF, TNF receptor associated factor; SIAH2, Siah E3 ubiquitin protein ligase 2; VHL, von Hippel Lindau; UCH-L1, ubiquitin C-terminal hydrolase-L1; SCF, Skp-Cul1-F-box; Skp2, S-phase kinase-associated protein-2; RNF5, ring finger protein 5; Fbxw7, F-box and WD repeat domain containing 7; SMURF1, SMAD Specific E3 ubiquitin protein ligase 1.
Table I.
Some E3 ligases involved in the crosstalk between tumors and the TME.
| Biological process | E3 ligase | Target | Category | Cancer/cell type | (Refs.) |
|---|---|---|---|---|---|
| Hypoxia | VHL | HIF-α | Tumor suppressor | (31) | |
| SIAH2 | NRF1 | Oncogene | Breast cancer | (36) | |
| Angiogenesis | MDM2 | HIF-α | Tumor suppressor | (39) | |
| SAG | Oncogene | B16F10 melanoma | (42,110) | ||
| FBW7 | Notch4 | Oncogene | (45) | ||
| c-CBL | β-catenin | Tumor suppressor | (48) | ||
| TRIM25 | SP1 | Tumor suppressor | Gastric cancer | (52) | |
| Chronic inflammation-mediated tumor formation | SCF-Skp2 | FOXO1 | Oncogene | Hepatoma, prostate cancer, lymphoma | (58) |
| MDM2 | FOXOs | Oncogene | (59) | ||
| SCF-Skp2 | E2F1 | HepG2 cells | (65) | ||
| LUBAC | NEMO | Oncogene | B-cell leukemia, breast cancer | (71,72) | |
| CAF | SCF-Fbxw7 | Notch1 | Tumor suppressor | BMSCs, melanoma cells | (90) |
| RNF5 | PTEN | Oncogene | Pancreatic ductal adenocarcinoma cells | (84) | |
| ECM | SCF-Skp2 | SP1 | Oncogene | Oral squamous cell carcinoma, lung cancer cells | (96,97) |
| NEDD4-1 | MT1-MMP | Oncogene | breast cancer cell MCF-7 | (103) | |
| TAM polarization | TRIM24 | CBP | Tumor suppressor | Macrophages | (105) |
| UBR5 | Oncogene | Ovarian cancer | (111) | ||
| T-cell immunosuppression | CRL4WDR4 | PML | Oncogene | Lung cancer | (117) |
| FBXO38 | PD-1 | Tumor suppressor | Colorectal carcinoma, hepatocellular carcinoma | (118) | |
| Cullin 3-SPOP | PD-L1 | Tumor suppressor | Prostate cancer, kidney cancer | (120,121) | |
| NEDD4 | GITR | Oncogene | Melanoma | (132) | |
| MDSC immunosuppression | TRAF6 | STAT3 | Tumor suppressor | Lung cancer | (134) |
| MDSC differentiation | TRIM29 | STING | Oncogene | Nasopharyngeal carcinoma | (137) |
| Myofibroblastic activation | SMURF1 | TGF-βRII | Tumor suppressor | Hepatic stellate cells | (164) |
| Antigen presentation | TRIM71 | Rb, p53 | Oncogene | Breast cancer | (141) |
| NK cell activation | Cbl-b | Tyro3, Mer, Axl | Oncogene | Melanoma, breast cancer | (143) |
| NK cell function | TRIM29 | TAB2 | Oncogene | NK cells | (146) |
CAFs, cancer-associated fibroblasts; Cbl-b, Casitas B-lineage lymphoma proto-oncogene-b; CBP, CREB-binding protein; CRL4WDR4, WD repeat 4-containing CUL-RING ubiquitin ligase 4; cullin 3-SPOP, cullin 3-speckle-type pox virus and zinc finger protein; ECM, extracellular matrix; E2F, E2 promoter binding factor; FBW7, F-box and WD repeat domain-containing 7; FOXO, Forkhead box O; GITR, glucocorticoid induced TNF receptor; LUBAC, a linear ubiquitin chain assembly complex; MDM2, mouse double minute 2 homolog; MDSCs, myeloid-derived suppressor cells; MT1-MMP, membrane type 1-matrix metalloproteinase; NEMO, nuclear factor-κB essential modulator; NK, natural killer; PD-1, programmed cell death protein-1; PD-L1, programmed cell death-ligand 1; PML, promyelocytic leukemia; RNF5, ring finger protein 5; SAG, sensitive to apoptosis gene; SIAH2, Siah E3 ubiquitin protein ligase 2; SCF, Skp-Cul1-F-box; Skp2, S-phase kinase-associated protein-2; SMURF1, SMAD Specific E3 ubiquitin protein ligase 1; TRIM, tripartite motif containing; TAB2, TGF-β activated kinase 1 binding protein 2; TAMs, tumor-associated macrophages; TGF-βRI, TGF-β type I receptor; TGF-βRII, TGF-β type II receptor; UBR5, ubiquitin protein ligase E3 component n-recognin 5; VHL, von Hippel Lindau.
Table II.
Some DUBs involved in the crosstalk between tumors and the TME.
| Biological process | DUB | Target | Category | Cancer/cell type | (Refs.) |
|---|---|---|---|---|---|
| Hypoxia | USP20 | HIF-1α | Oncogene | (33) | |
| UCH-L1 | HIF-1α | Oncogene | Breast cancer, lung cancer | (34) | |
| Chronic inflammation mediated tumor formation | OUTLIN/gumby | NEMO | Tumor suppressor | Hepatocellular carcinoma | (73) |
| Angiogenesis | USP8 | VEGFR2 | Oncogene | Endothelial cells | (37) |
| OUTLIN/gumby | dishevelled2, β-catenin | Tumor suppressor | Endothelial cells | (49,50) | |
| EMT | USP47 | Snail | Oncogene | Colorectal cancer cells | (35) |
| USP3 | SUZ12 | Oncogene | Gastric cancer | (76) | |
| USP11 | Snail 1 | Oncogene | Ovarian cancer | (77) | |
| USP11 | TGF-β RII | Oncogene | Breast cancer | (78) | |
| USP26 | SMAD7 | Tumor suppressor | Glioblastoma | (79) | |
| USP15 | TGF-β RI | Oncogene | Glioblastoma | (80) | |
| USP4 | TGF-β RI | Oncogene | Breast cancer | (81) | |
| UCH-L1 | Oncogene | DU145 prostate cancer cells | (82) | ||
| A20 | Snail 1 | Oncogene | Breast cancer | (83) | |
| USP27X | Snail 1 | Oncogene | Breast and pancreatic cancer | (75) | |
| DUB3 | Snail | Oncogene | Breast cancer | (87) | |
| ECM | USP4, USP17 | 6myc-HAS2 | Oncogene | Breast and lung cells | (91) |
| T-cell activation | Otub1 | AKT | Tumor suppressor | B16F10 melanoma | (113) |
| T-cell immunosuppression | CSN5 | PD-L1 | Oncogene | Breast cancer | (122) |
| USP22 | CSN5, PD-L1 | Oncogene | Non-small cell lung cancer, liver caner | (123,124) | |
| USP9X | PD-L1 | Oncogene | Oral squamous cell carcinoma | (126) | |
| USP7 | Foxp3+, Tip60 | Oncogene | Colorectal cancer HCT116, human prostate cancer PC-3 | (129) | |
| TAM polarization | USP24 | β-TrCP, p300 | Oncogene | Lung cancer | (107) |
| A20 | ERα | Oncogene | Endometrial cancer | (109) | |
| DCs | A20 | TRAF6, TRAF2, cIAP1 | Oncogene | Multiple myeloma | (140) |
CSN5, COP9 signalosome 5; DCs, dendritic cells; DUB, deubiquitinase; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; NEMO, nuclear factor-κB essential modulator; HAS2, Hyaluronan-synthesizing enzyme 2; HIF-1, hypoxia inducible factor-1; PD-L1, programmed cell death-ligand 1; TGF-βRI, TGF-β type I receptor; TGF-βRII, TGF-β type II receptor; TME, tumor microenvironment; TRAF, TNF receptor associated factor; UCH-L1, ubiquitin C-terminal hydrolase-L1; USP, ubiquitin-specific protease.
Roles of ubiquitination in the regulation of hypoxic microenvironments
Hypoxia is a main feature of numerous solid tumors due to rapid tumor growth. To overcome low oxygen levels, tumor cells activate a variety of survival pathways and induce angiogenesis and metastasis. Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator that is related to cellular adaptive responses to hypoxia.
The protein stability of HIFα is mainly regulated by the oxygen-dependent hydroxylation of two proline residues. Under normoxic conditions, hydroxylation is carried out by three proline 4-hydroxylase domain enzymes, i.e., PHD1-3 or EGL nine (Caenorhabditis elegans) homologs (EGLN1-3), that can sense cellular oxygen. The hydroxylation of proline residues is used to label the HIFα subunits for ubiquitination and subsequent proteasome degradation by the von Hippel Lindau (VHL)-containing E3 ligase complex. In addition, HIF-1α and HIF-2α can also be hydroxylated by HIF at a C-terminal asparagine residue. As a result, the recruitment of transcriptional coactivator p300 is blocked, and the transactivation activity of HIF is suppressed. By contrast, HIFs become stable and active under hypoxic conditions due to the inactivation of oxygen-dependent hydroxylases.
In the presence of oxygen, the tumor suppressor protein VHL E3-ubiquitin ligase mediates the degradation of HIFα subunits via ubiquitination (31). Several DUBs can reverse the action of VHL on HIFα subunits (32). For example, USP20 (VDU2) was the first DUB shown to maintain the stability of HIF-1α and upregulate the expression of HIF-1α target genes, such as VEGF (33). Ubiquitin C-terminal hydrolase-L1 (UCH-L1) has been shown to increase the stability of HIF-1α as a deubiquitinating enzyme and promote distant tumor metastases under hypoxic conditions in breast and lung cancers (34).
In the majority of tumors, hypoxia also induces epithelial-mesenchymal transition (EMT) and this enables tumor cells to acquire migratory and invasive capabilities. Ubiquitin-specific protease (USP)47 has been identified in the process of EMT. In colorectal cancer cells, USP47 is activated by the hypoxia-mediated Sox9. USP47 subsequently stabilizes Snail by deubiquitination and promotes EMT and tumor metastasis (35).
Dysfunctional mitochondria are a critical factor for tumorigenesis, and a low mitochondrial gene expression is associated with a poor prognosis of patients with breast cancer. The hypoxia-induced E3 ligase SIAH2 promotes nuclear respiratory factor 1 (NRF1) degradation through the proteasomal pathway under hypoxic conditions, thus leading to the reduction of nuclear-encoded mitochondrial gene expression, metabolic reprogramming, tumor-associated macrophage polarization and pro-tumor immune response. Therefore, the inhibition of NRF1 degradation may represent a potential therapeutic strategy against cancer (36).
Roles of ubiquitination in angiogenesis
Tumor cells produce angiogenic and proliferation factors, such as VEGFA, to stimulate the development of new blood vessels (16). Some transcriptional factors (e.g., HIF) and signaling pathways (e.g., Notch and Wnt) are involved in angiogenesis. Of these, VEGF and its associated receptors are significant players. It has been shown that VEGFR2 ubiquitination is required for degradation through the lysosomes or recycling back to the plasma membrane. Deubiquitinating enzyme USP8 regulates the stability of VEGFR2, thus affecting the endothelial cell response and vascular physiology (37).
As a transcriptional regulator of VEGF, HIF-1α plays a crucial role in angiogenesis. Its stability and functionality are generally affected by ubiquitination. The deubiquitinating enzymes USP20, USP8 and UCHL1 can modulate the HIF-1α level and stability, and regulate VEGF expression (32). Lysine-specific demethylase 1 (LSD1), a flavin adenine dinucleotide dependent demethylase for lysine (K) 4 and 9 of histone H3, has been shown to demethylate non-histone proteins. LSD1 can also demethylate HIF-1α at lysine (K) 391 and suppress PHD2-induced HIF-1α hydroxylation, thereby preventing HIF-1α from ubiquitin-mediated protein degradation (38). The transcription factor TAp73 opposes HIF-1 activity by recruiting mouse double minute 2 homolog (MDM2) and promotes HIF-1α polyubiquitination and degradation, thereby inhibiting angiogenesis and tumor progression (39).
COX-2 also plays a role in angiogenesis and its expression often upregulated in various types of cancer. COX-2 increases the production of VEGF via the extracellular signal-regulated kinase (ERK)/HIF-1α/VEGFA pathway. The centromere protein U, a centromere component for mitosis, is involved in the tumorigenesis of multiple cancer types. This protein has recently been shown to promote angiogenesis by inhibiting the ubiquitin-proteasomal degradation of COX-2 (40).
Cullin-RING ligase is a multi-complex E3 ubiquitin ligase and controls a number of critical biological processes. As a RING protein, sensitive to apoptosis gene (SAG) is critical for the activity of Cullin-RING ligase. SAG is upregulated by the transcription factors AP-1 and HIF-1 in response to reactive oxygen species, mitogen and hypoxia in several cancer types; these factors are associated with a poor prognosis. Stress-inducible SAG can recruit other components of Cullin-RING E3s to promote the ubiquitination and degradation of various substrates (41). Tan et al (42) found that SAG is necessary for endothelial cell migration and tube formation in vitro and tumor angiogenesis in vivo in a murine B16F10 melanoma model. Furthermore, the endothelial deletion of SAG E3 ubiquitin ligase was shown to block tumor angiogenesis (42).
Endothelial Notch signaling limits angiogenesis by preventing budding and catheter formation (43). Tip cells secrete the Notch ligand Dll4 which binds to the Notch receptor on stalk cells. The activation of Notch leads to VEGFR2 transcriptional inhibition and subsequently suppresses endothelial cell proliferation (44). Ubiquitin ligase F-box and WD repeat domain-containing 7 (FBW7) plays a positive role in angiogenesis by inducing Notch ubiquitination and degradation (45).
The Wnt signaling pathway participates in multiple aspects of development and is necessary for appropriate vascular growth in mammals (46). This pathway promotes the accumulation of β-catenin and enhances the transcription of pro-angiogenic genes, such as VEGF and IL-8 (47). The stability of β-catenin can also be regulated by ubiquitination. A previous study revealed that c-CBL induced the ubiquitination of nuclear β-catenin and promoted its degradation, thereby negatively regulating angiogenesis (48). In addition, LUBAC induces linear ubiquitin on β-catenin, facilitates its proteasomal degradation, and DUB OTULIN/gumby interacts with LUBAC to remove linear ubiquitin chains and stabilize β-catenin. Moreover, gumby interacts with disheveled 2 and promotes Wnt signaling during angiogenesis. The loss of the DUB activity of gumby can comprise angiogenesis (49,50).
The process of angiogenesis includes the generation of new vessels, vascular basement membrane degradation, blood vessel ECM remodeling, endothelial cell migration, proliferation and the new generation of matrix components. MMPs play key roles in tumor rupture, tumor neovascularization and subsequent metastasis (51). MMP2, a member of the MMPs family, has been proven to be overexpressed in gastric cancer and promote the proliferation, angiogenesis, and migration of gastric cancer cells. Tripartite motif containing (TRIM)25, an E3 ubiquitin ligase, promotes the ubiquitination of transcription factor SP1 at K610, further suppressing the expression of MMP2 and inhibiting angiogenesis in gastric cancer (52).
Roles of ubiquitination in chronic inflammation-mediated tumor formation
Chronic inflammation is a significant risk factor for cancer development and ~20% of human cancers are related to chronic inflammation (53). Cancer usually occurs in inflammatory tissues, thus indicating the roles of local inflammation in the initiation and progression of cancers. Ubiquitination plays an intrinsic role in the chronic inflammatory TME by regulating transcriptional factors and cytokines (54).
Forkhead box O (FOXO) transcription factors play a crucial role in immune homeostasis, particularly FOXO1 and FOXO3a, which regulate the development and differentiation of lymphocytes, along with the quiescence of primary T-cells. A number of chronic inflammatory processes appear to be related to the loss of activity of these transcription factors (55). There is increasing evidence to suggest that FOXOs function as tumor suppressors and participate in a variety of biological processes, including apoptosis, inflammation regulation, cell cycle checkpoint, oxidative stress resistance and DNA repair (56). The dysregulation of FOXO proteins is usually related to the carcinogenesis, progression and chemoresistance of several human tumors (57). S-phase kinase-associated protein-2 (Skp2), an Skp-Cul1-F-box (SCF) UPS protein subunit, is responsible for the ubiquitination/degradation of FOXO1 in hepatoma and prostate cancer cells, as well as a mouse lymphoma model (58). In addition, MDM2 is a critical universal ubiquitin E3 ligase for the proteasomal degradation of FOXO family members (59).
Cytokines also mediate the crosstalk between malignant cells and surrounding cells in the TME. Pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, TGF-β and the anti-inflammatory cytokine, IL-10, have been shown to be involved in the occurrence and maintenance of cancer (54). The ubiquitin mechanism is triggered under infection-inflammation conditions and promotes the formation of cancer by regulating multiple cytokines. For example, the SAG ubiquitin-proteasome system is significantly upregulated in infected tissues and participates in chronic inflammation-induced cancer via the ubiquitination of key apoptotic factors (pro-apoptotic SARM and Noxa) and by controlling the ratio of pro-and anti-apoptosis factors (60,61).
The melanoma differentiation-associated gene-7 (MDA-7)/IL-24 belongs to the anti-inflammatory IL-10 family and exhibits anticancer effects by inducing apoptosis, inhibiting angiogenesis and stimulating an immune response (62). The MDA-7/IL-24 protein is ubiquitinated, and its degradation is controlled by the 26S proteasome in ovarian and lung cancer cells (63). Inhibiting the degradation of MDA-7/IL-24 can enhance antitumor activity.
The E2 promoter binding factor (E2F) family of transcription factors is an essential mediator of cell proliferation and DNA-damage-induced apoptosis and can exhibit both tumorigenic and tumor suppressor activity. E2F is overexpressed in nasopharyngeal carcinoma (NPC) cells and activates the expression of IL-6, upregulates inflammatory signaling, and promotes malignant proliferation and tumorigenesis in NPC cells (64). The UPS plays a key role in regulating E2F function. In the viral HBx microenvironment, HBx can compete with E2F1 to bind Skp2 E3 ligase, thus resulting in the accumulation of E2F1 and histone methyltransferase mixed-lineage leukemia 1. E2F1 stimulates cell proliferation in early-stage tumors and triggers apoptosis in late-stage tumors. Notably, the differential promoter occupancy of mixed lineage leukemia 1 (with a co-activator or co-repressor) appears to specify the paradoxical functions of E2F1 during the early and late stages of tumor development (65).
Inflammation-related cancers are also characterized by mutagenic DNA lesions (66). Innate immune surveillance systems can recognize cytosolic DNA through the cyclic GMP-AMP synthase (cGAS)/stimulator of IFN genes (STING) pathway and activate downstream non-canonical NF-κB signaling to monitor cell damage and fight against pathogen infection (67). Aberrant DNA fragmentation is common in cancer cells due to abnormal chromosome structure and genome instability (68). Therefore, avoiding this monitoring process is crucial for tumorigenesis. Recently, Wu et al (69) revealed that receptor tyrosine kinase human epidermal growth factor receptor 2 (HER2) effectively inhibited cGAS/STING signaling and prevented cancer cells from undergoing apoptosis. HER2 is closely related to STING and recruits AKT1 (also known as PKB) to directly phosphorylate TANK-binding kinase 1 (TBK1), thus preventing the TBK1-STING association and TBK1 K63-linked ubiquitination, thereby weakening the STING signal (69).
Chronic inflammation and NF-κB activation are related to the development and progression of cancer. The linear ubiquitination of the NF-κB essential modulator mediated by LUBAC is required for NF-κB activation (70). Thus, the dysregulation of linear ubiquitin signaling is associated with cancer development. For example, increased LUBAC expression enhances NF-κB activation, thus accelerating the accumulation of somatic mutations and lymphomagenesis (71). Enhanced linear ubiquitination drives breast cancer development in mice (72). Consistently, DUB OTULIN (gumby) negatively regulates linear ubiquitin signaling and its deficiency in hepatocytes causes the development of hepatocellular carcinoma (73). These results suggest that LUBAC inhibition may be an effective treatment strategy for certain types of cancer.
Roles of ubiquitination in the crosstalk between CAFs and tumor cells
CAFs are the most abundant stromal cells in the TME and regulate tumor cells and other stromal cells via cell-cell contacts and secrete regulatory factors, notably TGF-β, IL-6 and CC-chemokine ligand (CCL)2; they can also synthesize and remodel the ECM. Thus, CAFs are closely related to cancer progression.
TGF-β, a pleiotropic cytokine, plays essential roles in tumor cell EMT, migration and invasion. EMT is a transdifferentiation process from polarized epithelial cells to motile mesenchymal cells; this is associated with the downregulation of E-cadherin and the upregulation of the mesenchymal protein vimentin. TGF-β binding leads to TGF-β type I and type II receptor (TGF-βRI and TGF-βRII) heterocomplex activation. SMAD2/3 are then phosphorylated and shuttled to the nucleus with SMAD4 to regulate the expression of EMT transcription factors (74). EMT is driven by Snail, zinc-finger E-box-binding and basic helix-loop-helix (e.g., Twist) transcription factors; this inhibits epithelial marker genes and activates genes related to the mesenchymal phenotype. Due to the continuous ubiquitination of several F-box-specific E3 ligases, Snail1 has a short lifespan in normal epithelial cells; however, its degradation is prevented in cancer cells and activated fibroblasts. Snail1 is required for the activation of CAFs (75).
Studies have reported that several USP family members are involved in mediating TGF-β-induced EMT. For instance, USP3 expression is upregulated in gastric cancer cells induced by TGF-β. USP3 interacts with and stabilizes the suppressor of zeste 12 protein homolog via deubiquitination, thus enhancing tumor cell EMT and metastasis (76). Snail and Twist are important transcriptional factors in EMT. USP11 is upregulated and promotes EMT by deubiquitinating Snail in ovarian cancer (77). USP11 also regulates TGF-β-induced epithelial-mesenchymal plasticity and promotes human breast cancer metastasis by stabilizing TGF-βRII (78). USP26 has been found to negatively regulate the TGF-β signaling pathway by deubiquitination and stabilizing SMAD7 in glioblastoma (79). USP15 enhances the tumorigenic effects of TGF-β in glioblastoma (80), while USP4 promotes TGF-β-induced EMT and cell migration in breast cancer. Both of these maintain the stability of TGF-βRI (81). Of note, Jang et al (82) found that UCH-L1 was specifically and highly expressed in the metastatic DU145 prostate cancer cell line, but not in the benign or weakly metastatic prostate cancer cells. Furthermore, UCH-L1 was found to promote prostate cancer metastasis via EMT induction (82). Recently, A20 was found to be overexpressed in human basal-like breast cancers and mediates TGFβ1-induced EMT in breast cancers by monoubiquitylating Snail1. The knockdown of A20 reduced lung cancer metastasis in mouse engrafts and orthotopic breast cancer models, thus suggesting roles for A20 and monoubiquitylated Snail1 in metastasis (83). Deubiquitinase USP27X has been shown to be upregulated by TGF-β during EMT and to maintain Snail1 stability in breast and pancreatic cancer. The inhibition of USP27X leads to Snail1 destabilization, suppresses EMT and renders tumor cells sensitive to chemotherapy (75). Collectively, these results indicate that these deubiquitinases regulate EMT and may serve as a target for the inhibition of CMF-induced tumor invasion and chemoresistance.
TGF-α, produced by CAFs, can induce EMT and promote tumor growth and metastasis via EGFR. Recently, a novel E3 ligase, RNF5, has been identified to regulate the crosstalk of pancreatic CAFs and pancreatic ductal adenocarcinoma. The ablation of the Hedgehog signaling gene Smoothened in pancreatic CAFs can activate Glycogen synthase kinase 3β and lead to PTEN phosphorylation. RNF5 has been found to ubiquitinate PTEN and induce PTEN degradation. As a result, AKT and the TGF-α promoter are activated. TGF-α produced by CAFs is known to undergo crosstalk with adjacent tumor cells and to promote tumor growth via the activation of EGFR (84).
Recently, IL-6 was also identified as a potential mediator of the crosstalk between tumor cells and CAFs in esophageal cancer (85). Chronic inflammation leads to the activation of normal fibroblasts which become CAFs. CAFs produce more pro-tumorigenic cytokines, including IL-6. The direct interaction of CAFs enables tumor cells to secrete a higher level of IL-6. IL-6 activates its receptor on tumor cells and CAFs to promote tumor cell proliferation and invasion, and by suppressing apoptosis via the STAT3 and ERK1/2 signaling pathways. Thus, IL-6 signaling has become a therapeutic target for several types of cancer. Wu et al (86) suggested that IL-6 secreted by CAFs promoted EMT and the metastasis of gastric cancer via the JAK2/STAT3 signaling pathway. Recently, DUB3 has been identified as a deubiquitinase of Snail; furthermore, IL-6 promotes tumor cell EMT by inducing DUB3 expression and by stabilizing Snail in breast cancer (87).
Chemokines are secreted proteins and participate in inflammatory and immunoregulatory processes. The increased concentration of chemokines is associated with a poor prognosis (88). Liu et al (89) demonstrated that CAFs produce higher levels of CCL2, CCL5, CCL7 and CXCL16 than peri-tumor fibroblasts in hepatocellular carcinoma. CCL2 and CCL5 promoted cell migration via the Hedgehog pathway; CCL7 and CXCL16 enhanced the migration and invasion of hepatocellular carcinoma cells via the TGF-β pathway (89). Oncosuppressor protein Fbxw7 is a substrate receptor for the SCF ubiquitin ligase complex, and Fbxw7 deficiency has been shown to give rise to various tumors in mice. The loss of Fbxw7 in bone marrow-derived stromal cells promotes cancer metastasis by impairing the degradation of Notch1 and increasing the production of chemokine CCL2. As the precursors of CAFs, bone marrow-derived stromal cells are major components of the TME and represent the sources of CCL2 output (90).
The ECM in tumors changes dynamically and CAFs are the main contributors to ECM stiffness and degradation. The production and degradation of the ECM are regulated by ubiquitination. Hyaluronan, a ubiquitous glycosaminoglycan that is highly expressed in the ECM, accumulates in rapidly remodeling tissues, such as breast cancer. Hyaluronan-synthesizing enzyme 2 (HAS2) catalyzes the production of hyaluronan. USP4 and USP17 have been identified to reduce the ubiquitination of 6myc-HAS2 and modulate the stability and activity of HAS2. USP4 and USP17 are upregulated in malignant lines. USP17 maintains the level of 6myc-HAS2 protein by reducing the polyubiquitination of 6myc-HAS2. USP4 preferentially removes the monoubiquitination of 6myc-HAS2 and regulates the activity of HAS2 (91).
MMPs are a family of zinc-dependent endopeptidases that selectively degrade the components of the ECM (92). MMP-2 is upregulated in a number of cancer types and can reduce cell-cell adhesion, promote tumor invasion and EMT, and can contribute to tumor aggressiveness and a poor prognosis (93-95). Skp2 is usually overexpressed in human cancers and increases the expression of MMP-2 by enhancing transcription factor Sp1 activity (96,97). The expression of MMP-2 can also be regulated by epigenetic mechanisms and aberrant epigenetic regulations of MMP-2 are known to be involved in cancer progression (98). For example, CpG methylation of the MMP-2 promoter is associated with the invasive phenotype. Chromobox 6 is a component of the polycomb repressive complex. As a transcriptional repressor, Chromobox 6 inhibits the expression of MMP-2 in non-invasive cells. The enhanced ubiquitination and degradation of chromo box 6 increases the expression of MMP-2 and enhances the invasion of mesothelioma (99). USP2 is overexpressed in various human cancers and has been regarded as a therapeutic target in cancer (100). Previously, USP2 was revealed to enhance tumor migration and invasion by increasing MMP-2 activity in metastatic triple-negative breast cancer (101).
Membrane type 1-matrix metalloproteinase (MT1-MMP) mediates cancer cell invasion by degrading the basement membrane and ECM, as well as by inducing cell migration. The MT1-MMP-mediated activation of TGF-β signaling enables the autocrine and paracrine-mediated induction of EMT (102). Eisenach et al (103) revealed that the ubiquitin E3 ligase NEDD4-1 mediated monoubiquitination within the MT1-MMP intracellular domain and was involved in MT1-MMP trafficking and modulated cellular invasion through type I collagen matrices.
Roles of ubiquitination in the crosstalk between TAM and tumor cells
During tumorigenesis, TAMs are usually regulated by environmental factors to present the M2 state; this inhibits the cytotoxic function of immune cells and impairs antitumor immunity (104). Macrophage M2 polarization involves the activation of STAT6 and STAT6 acetylation, which is mediated by the acetyltransferase CREB-binding protein (CBP), to suppress macrophage M2 polarization. CBP-associated E3 ligase TRIM24 induces CBP ubiquitination, which facilitates the recruitment of CBP to STAT6, promotes STAT6 acetylation, and inhibits macrophage M2 polarization (105). M2 macrophages promote cancer cell metastasis by secreting growth factors and cytokines, such as IL-6 (106). Recently, Wang et al (107) reported that USP24 was highly expressed in the late stages of lung cancer and promoted lung cancer metastasis by inducing IL-6 expression. USP24 can stabilize β-TrCP, promote IκB degradation in lung cancer cells, and upregulate NF-κB in M2 macrophages, thus leading to the increased expression of IL-6. Furthermore, USP24 stabilizes p300 and reduces the levels of DNMT1, thereby increasing IL-6 transcription in M2 macrophages and lung cancer cells (107).
In estrogen-driven endometrial cancer, the dominant M2 macrophages in endometrial cancer lesions are CD163+ macrophages which play essential roles in carcinogenesis (108). CD163+ macrophages can upregulate the ubiquitin-editing enzyme A20 via cytokines, such as IL-1α, IL-17A and TNF-α in endometrial lesions. A20 maintains estrogen receptor α protein level and enhances endometrial cancer cell proliferation by its deubiquitinase activity (109). Thus, CD163+ macrophages sensitize endometrial cancer cells to estrogen via A20.
Ubiquitin protein ligase E3 component n-recognin 5 (UBR5, also known as EDD) is overexpressed in multiple cancer types, especially breast cancer and ovarian cancer, and is associated with a poor prognosis (110). UBR5 mediates tumor-associated macrophage recruitment and activation via chemokines and cytokines. In addition, UBR5 promotes ovarian tumor cell adhesion and spheroid formation and suppresses apoptosis by regulating p53 levels. Targeting tumor-derived UBR5 impairs TAM recruitment and TME immunosuppression and enhances the therapeutic effects of immunotherapy (111).
Roles of ubiquitination in the crosstalk between T-cells and tumor cells
Ubiquitination is a crucial mechanism that regulates the crosstalk between T-cells and tumor cells, including T-cell immune responses against cancer and tumor immunosuppression. CD8+ T-cells are primary cytotoxic effector cells of the immune system against cancer, which specifically depend on IL-15-mediated AKT signaling for homeostasis (112). DUB Otub1 deubiquitinates AKT and inhibits the IL-15-stimulated activation of AKT, negatively regulating the homeostasis and activation of CD8+ T-cells in immune responses. As previously demonstrated in a mouse model, Otub1 deficiency sensitized CD8+ T-cells and enhanced tumor rejection (113). The linker for activation of T-cells (LAT) is a transmembrane molecule and the key for T-cell activation (114). The ubiquitylation-resistant form of LAT is more stable than that of wild-type LAT in cells and enhances T-cell signaling. Therefore, blocking LAT ubiquitination may be a promising strategy with which to enhance the function of effector T-cells (115).
Promyelocytic leukemia (PML), a pleiotropic tumor suppressor, is usually downregulated in a number of cancer types. Ubiquitin-mediated degradation is a key mechanism for PML downregulation in tumors (116). WD repeat 4-containing CUL-RING ubiquitin ligase 4 is responsible for PML destruction in lung cancer. The WDR4/PML axis induces several oncogenes and promotes lung cancer progression. It also elevates intratumoral Tregs and M2-like macrophages, reduces CD8+ T-cell numbers and induces tumor immunosuppression (117).
Surface PD-1 molecules can be ubiquitinated and degraded by proteasomes in activated T-cells. E3 ubiquitin ligase FBXO38 interacts with PD-1 directly and mediates PD-1 ubiquitination and proteasome degradation. Thus, FBXO38 regulates T-cell antitumor immunity (118). The immunosuppressive activity of PD-L1 can also be modulated by ubiquitination and N-glycosylation. Glycogen synthase kinase (GSK)3β phosphorylates PD-L1 and facilities the proteasome degradation of PD-L1 by β-TrCP. N-glycosylation influences the structure and function of PD-L1 and antagonizes GSK3β binding; thus, only non-glycosylated PD-L1 forms a complex with GSK3β and β-TrCP and undergoes fast protein degradation (119). In human prostate cancer and kidney cancer, cullin 3-speckle-type pox virus and zinc finger protein has been reported to target PD-L1 for ubiquitination and degradation (120,121).
The TME can induce cancer immunosuppression by upregulating PD-L1 protein expression. DUB COP9 signalosome 5 (CSN5) inhibits the ubiquitination and degradation of PD-L1, subsequently enhancing its interaction with PD-L1 to escape T cell immune surveillance (122). USP22 is a novel regulator of PD-L1, which deubiquitinates CSN5 and regulates the protein levels of PD-L1 via the USP22-CSN5-PD-L1 axis. CSN5 can also directly deubiquitinate PD-L1 and maintains its stability in human non-small cell lung cancer and liver cancers (123,124). USP22 is overexpressed and promotes tumor progression in multiple tumor types; the deletion of USP22 in pancreatic tumor cells promotes the infiltration of T-cells and NK cells, thus improving the response to combined immunotherapy (125). Emerging data have demonstrated that USP9X is highly expressed in oral squamous cell carcinoma tissues and stabilizes PD-L1 by deubiquitination. Reducing USP9X expression also blocks oral squamous cell growth (126). Another study demonstrated that USP9X-deficient T-cells were hyperproliferative and caused spontaneous lupus-like autoimmunity and lymphoproliferative diseases in aged mice. Moreover, the mRNA expression of PD-1 was markedly upregulated in cells in which USP9X was knocked down. As such, mice with USP9X depletion have an increased number of PD-1 expressing T-cells (127).
Foxp3+ Tregs play an indispensable role in immunosuppression and lead to tumor immune evasion (128). The Treg lineage-specific transcription factor Foxp3+, and the histone acetyltransferase Tip60, are pivotal to the development and maintenance of the Treg lineage. USP7 maintains Treg functions by stabilizing the expression of Tip60 and Foxp3 (129). Glucocorticoid-induced TNF receptor (GITR), a member of the TNF receptor superfamily, is highly expressed in Tregs, whereas it is expressed at low levels in naive and memory T-cells (130). Its agonist antibody DTA-1 can enhance the CD8+ effector T-cell to Treg ratio by depleting Tregs, as well as activating CD8+ effector T-cells by promoting GITR oligomerization (131). The E3 ligase NEDD4 can also mediate the ubiquitination and degradation of GITR; this protein is overexpressed in metastatic melanoma and inhibits T-cell antitumor immunity. Therefore, NEDD4 can be regarded as a novel prognostic biomarker and therapeutic target for melanoma (132).
Roles of ubiquitination in the crosstalk between MDSCs and tumor cells
MDSCs are crucial immunosuppressive cells and inhibit T-cell proliferation and antitumor responses in the TME. The expansion and function of MDSCs are modulated by the transcription factor, STAT3; the activation of STAT3 is controlled by post-translational modifications, such as ubiquitination and phosphorylation. E3 ligase TNF receptor associated factor (TRAF)6 promotes STAT3 phosphorylation and activation by inducing the K63-linked polyubiquitination of STAT3 (133). Thus, TRAF6 plays a critical role in regulating the function of MDSCs. Song et al (134) reported that TRAF6 was highly expressed in the MDSCs of patients with lung cancer and TRAF6 knockdown impaired the immunosuppressive effects of MDSCs in vitro and in Lewis xenograft mice.
On the other hand, STING has been shown to suppress the differentiation of MDSCs by inhibiting STAT3 activation in the NPC. The underlying mechanism is that STING promotes suppressor of cytokine signaling 1 (SOCS1) expression via the STING/TBK1/IRF3 axis and SOCS1 prevents STAT3 phosphorylation by binding to its SH2 domain in NPC cells and MDSCs (135). Galectins are a family of lectins that mediate a variety of biological functions in tumor progression and immune surveillance (136). Galectin-9 is usually upregulated in tumor tissues and recruits E3 ligase TRIM29 to mediate the ubiquitination and degradation of STING in malignant NPC cells. As a result, galectin-9 activates STAT3, enhances the production of suppressive cytokines and chemokines including IL-1β and IL-6, and promotes MDSC differentiation and expansion in patients with NPC (137).
Roles of ubiquitination in the crosstalk between DCs and tumor cells
DCs are potent antigen-presenting cells with a number of different subtypes, such as monocyte DCs, conventional DCs and plasmacytoid DCs (pDCs) (138). DCs can specifically recognize, process and present cancer antigens, activate CD8+ and CD4+ T-cells, and regulate immune responses. Thus, DCs are key players in tumor immunity (139). Human pDCs selectively express intracellular TLR7 and TLR9 to sense viral stimulation and produce a large number of IFNs. Thus, pDCs play vital roles in antiviral responses. In multiple myeloma, tumor cells educate pDCs to induce tumorigenesis and escape immune surveillance via the E-cadherin (CDH1) pathway. The homophilic interaction between myeloma cells and pDCs activates CDH1 and then upregulates ubiquitin-editing enzyme A20 expression. A20 negatively regulates NF-κB signaling by inhibiting E3 ligases TRAF6, TRAF2 and cIAP1, and suppresses TLR pathways and the production of IFN-α in pDCs (140).
To escape immune surveillance, malignant cells may develop multiple mechanisms, such as reducing antigenicity and establishing an immunosuppressive microenvironment. Long-non-coding RNA long intergenic non-coding RNA for kinase activation (LINK-A) is upregulated in triple-negative breast cancers and is associated with a poor prognosis of patients with breast cancer. LINK-A inhibits protein kinase A (PKA) phosphorylation and the PKA-mediated phosphorylation of the E3 ubiquitin ligase TRIM71. As a result, LINK-A enhances TRIM71-induced ubiquitination and the degradation of peptide-loading complex, thereby downregulating antigen presentation and innate tumor suppression (141).
Roles of ubiquitination in the crosstalk between NK cells and tumor cells
NK cells play key roles in the innate antitumor immune response, and ubiquitination is involved in regulating its function. NK cells express the tyrosine kinase receptors, Tyro3, Mer and Axl, which play essential roles in NK cell functional maturation (142). E3 ligase Casitas B-lineage lymphoma proto oncogene-b (Cbl-b), also expressed in NK cells, participates in controlling NK cell antitumor responses via the ubiquitination of Tyro3, Mer and Axl. The suppression of Cbl-b promotes NK cells to reject melanoma and breast metastases (143,144).
It is well known that IL-15 contributes to NK cell survival and function. It was recently demonstrated by Wang et al (145) that deubiquitination is involved in the regulation of events downstream of IL-15. IL-15 activates AKT, which maintains the stability of X-box binding protein 1 through deubiquitination. Accumulated X-box binding protein 1 in the nucleus recruits the transcription factor T-bet and increases the expression of granzyme B and IFN-γ expression, thus enhancing NK cell function (145). On the other hand, DUB Otub1 induces AKT deubiquitination, attenuating its binding to phosphatidylinositol 3,4,5-trisphosphate, thereby inhibiting AKT activation. Thus, Otub1 controls the NK cell activation induced by IL-15 (113).
IL-12 and IL-18 induce NK cell activation; TGF-β activated kinase 1 binding protein 2 (TAB2) is known to be involved in the process as a key adaptor protein. Dou et al (146) reported that E3 ligase TRIM29 was upregulated in activated NK cells and negatively regulated IFN-γ production by ubiquitinating and degrading TAB2. NK cell functions can be enhanced upon IL-12 and IL-18 stimulation when TRIM29 is deficient. Thus, TRIM29 plays a key role in regulating NK cell activity.
4. Application of UPS modulators in the TME in tumor therapy
Ubiquitination is involved in multiple processes in the crosstalk between a tumor and the TME. Therefore, this process may provide potential targets for cancer treatment (147). Accumulating evidence indicates that modulating the ubiquitination of substrates in the TME may provide a promising strategy for the development of anticancer drugs. Some UPS modulators and their targets are presented in Table III.
Table III.
Anticancer compounds in clinical trials modulating the ubiquitination of substrates in the TME.
| Drug/compound | Substrate | Target | Cancer type | Research stage | (Refs.) |
|---|---|---|---|---|---|
| Bortezomib | Proteasome | Multiple myeloma | Approved | (148) | |
| Delanzomib | Proteasome | Multiple myeloma | Approved | (149) | |
| RA190 | Proteasome | Multiple myeloma, ovarian cancer, hepatocellular carcinoma, intrahepatic cholangiocarcinoma | Research | (150-155) | |
| Thymoquinone | HIF-1α | HIF-1α | Renal cancer | Phase II | (157) |
| Decursin | HIF-1α | HIF-1α | Lung cancer, colon cancer | Approved | (158) |
| JP3 | TRIM25 | MMP-2 | Gastric cancer | Research | (159) |
| MLN4924 | NEDD8-activating enzyme | IκBα | Chronic lymphocytic leukemia B-cells | Phase II | (161) |
| IQGAP1 | SMURF1 | TGF-βRII | Hepatic stellate cell | Research | (164) |
| Gefitinib | EGF signaling | PD-L1 | Breast cancer | Approved | (119) |
| Dinaciclib | CDK1, 2, 5, 9 | PD-L1 | Lung adenocarcinoma | Phase II | (166) |
| Berberine | CSN5 | PD-L1 | Non-small cell lung cancer | Approved | (167) |
| MLN4924 | NEDD8-activiting enzyme | IκBα | Chronic lymphocytic leukemia B-cells | Phase II | (161) |
| P217564 | Tip60, Foxp3 | USP7 | Multiple myeloma, B-cell leukemia, neuroblastoma | Research | (129) |
| HOIPIN-8 | HOIP | NF-κB | Lung carcinoma cells | Research | (163) |
CSN5, COP9 signalosome 5; HIF-1, hypoxia inducible factor-1; IQGAP1, IQ motif containing GTPase activating protein 1; PD-L1, programmed cell death-ligand1; TME, tumor microenvironment; TRIM, tripartite motif containing; SMURF1, SMAD specific E3 ubiquitin protein ligase 1.
Proteasome inhibitors
As a vital component of the UPS, the proteasome has been successfully used as a target for cancer treatment. Bortezomib and Dalanzomib are proteasome inhibitors approved for recurrent refractory multiple myeloma (148,149). These drugs induce the apoptosis of refractory multiple myeloma cells, suppress NF-kB activation and the production of cytokines (e.g., IL-6, insulin-like growth factor 1and VEGF), and inhibit angiogenesis in the TME. The clinical success of existing proteasome inhibitors encourages great efforts to discover more UPS inhibitors.
The bis-benzylidine piperidone RA190 is a novel proteasome inhibitor, targeting 19S proteasome-associated ubiquitin receptor RPN13 [also known as adhesion-regulating molecule-1 (ADRM1)] and exerts anticancer effects in multiple types of cancer (150). For example, RA190 induces the apoptosis and inhibits the proliferation of multiple myeloma cells in vitro and in vivo by exerting anti-myeloma activity (151). STAT3 plays a key role in modulating MDSC immunosuppression and its activation can be inhibited by endoplasmic reticulum stress (152). RA190 induces endoplasmic reticulum stress by accumulating polyubiquitinated proteins. As a result, RA190 reduces STAT3 expression, and reduces the levels of arginase, inducible nitric oxide synthase, and IL-10 in MDSCs, suppresses MDSC immunosuppression, and kills ovarian cancer cells in mice bearing syngeneic ovarian tumors (153). ADRM1 is overexpressed in intrahepatic cholangiocarcinoma and is associated with a poor prognosis of patients with intrahepatic cholangiocarcinoma. RA190 inhibits the cell cycle and induces apoptosis in intrahepatic cholangiocarcinoma by targeting ADRM1 (154). Recently, Soong et al (155) reported that RA190 could kill hepatocellular carcinoma by blocking IκBα degradation and inhibiting NF-κB-mediated inflammation in vitro and in vivo. Moreover, RA190 overcomes bortezomib resistance and exhibits synergistic anticancer activity with bortezomib, lenalidomide or pomalidomide in the treatment of multiple myeloma, or with sorafenib in the treatment of hepatocellular carcinoma (155).
HIF-1α inhibitors
Increased levels of HIF-1α in tumors are associated with angiogenesis, aggressive tumor growth and a poor prognosis (156). Lee et al (157) identified thymoquinone as a HIF-1α inhibitor from a 502 natural compound library. Thymoquinone promoted HIF-1α degradation by suppressing HSP90-mediated stabilization, altering the proteasome-dependent degradation pathway, and blocking anaerobic glycolysis, and selectively induced tumor cell apoptosis in hypoxic renal cancer (157).
Decursin, an active compound extracted from the roots of Angelica gigas, can enhance hydroxylation and ubiquitination and consequently promote HIF-1α degradation under hypoxic conditions. As a result, decursin reduces the expression of HIF-1α target genes, such as VEGF and CXCR4, inhibits cell proliferation and invasion, and induces the apoptosis of A549 human lung cancer and HCT116 human colon cancer cells. Moreover, decursin reduces PD-L1 expression, increases the number of tumor-infiltrating T-lymphocytes (CD3+ and CD8+ cells), suppresses Tregs and MDSCs, and improves immune responses in a Lewis lung carcinoma allograft mouse model (158). Decursin is a novel HIF-1α inhibitor exhibiting anticancer and anti-angiogenic activities.
Targeting MMP-2
MMP-2 is closely related to invasion, angiogenesis and metastasis in several types of cancer. Therefore, MMP-2 is a promising target for tumor treatment. It is known that E3 ubiquitin ligase TRIM25 can promote the ubiquitination and degradation of SP1, further suppressing MMP-2 expression and inhibiting angiogenesis in gastric cancer. Recently, Chen et al (159,160) developed an MMP2-targeted peptide, JP3, based on the functional fragment from JWA protein, a microtubule-binding protein with inhibitory activity against SP1. JP3 maintains TRIM25 stability by phosphorylation at Ser12 and reducing its ubiquitination. Consequently, JP3 suppresses gastric cancer angiogenesis, growth and metastasis in vivo without observable toxic side-effects (159).
Targeting the NF-κB pathway
The activation of the NF-κB pathway leads to cell survival and chemoresistance in chronic lymphocytic leukemia B-cells. The ubiquitination of IκBα may partially account for the constitutive activation of the NF-κB pathway. The small-molecule inhibitor of NEDD8-activating enzyme, MLN4924, blocks Cullin-RING ubiquitin ligase activity, thus leading to the accumulation of IκBα and the inactivation of the NF-κB canonical pathway. Therefore, treatment with MLN4924 could disrupt NF-κB activation, induce the chronic apoptosis of lymphocytic leukemia B-cells and prevent stroma-mediated resistance (161). In addition, the ubiquitin ligase complex LUBAC consists of three subunits: HOIL-1L, HOIP and SHARPIN. Katsuya et al (162) developed a small molecule inhibitor of HOIP, JTP-0819958 (HOIPIN-1), which inhibits LUBAC activity and suppresses NF-κB activation in vitro. These authors also synthesized seven derivatives (HOIPIN-2-8) of HOIPIN-1 and found that HOIPIN-8 could suppress LUBAC- and TNF-α-induced NF-κB activation with high efficiency and without cytotoxicity (163).
Targeting the TGF-β pathway
In the TME, TGF-β induces the activation of pericytes and other mesenchymal stromal cells into tumor-associated myofibroblasts by activating the TGF-β receptor heterocomplex. IQ motif containing GTPase activating protein 1has been shown to suppress this process in the liver by recruiting the E3 ligase SMURF1 to ubiquitinate and induce the degradation of the TGF-β receptor II (TGF-βRII) in hepatic stellate cells (164).
Modulating immunotherapy
The PD-1/PD-L1 axis is a key determinant of physiological immune homeostasis. Inhibition of PD-1/PD-L1 is a promising therapeutic strategy which restores the immune system in cancer patients. EGF stabilizes PD-L1 via GSK3β inactivation in basal-like breast cancer. The inhibition of EGF signaling by gefitinib destabilizes PD-L1, enhances antitumor T-cell immunity, and improves the therapeutic efficacy of PD-1 blockade in syngeneic mouse models (119). Cyclin-dependent kinase 5 (CDK5) plays a critical role in driving tumor formation and development and is highly expressed in various lung cancer cells (165). CDK5 depletion promotes E3 ligase TRIM21-mediated PD-L1 ubiquitination and degradation, and enhances CD8+ T-cell-mediated immune responses in lung adenocarcinoma. A specific inhibitor of CDK5 has yet to be developed. Dinaciclib is a potent inhibitor of CDK1, 2, 5 and 9, and can be downregulated by the PD-L1 protein in lung adenocarcinoma cells (166). Berberine, an anti-inflammatory drug from Chinese medicine, has been found to induce PD-L1 degradation by binding to and inhibiting CSN5 deubiquitination activity in the non-small cell lung cancer lines, H460, H1975, H358 and HCC827. Consequently, berberine activates tumor-infiltrating T-cells, decreases the number of Tregs and MSDCs, and inhibits tumor growth in Lewis xenograft mice. Therefore, berberine can be regarded as a tumor immunotherapeutic agent (167).
The accumulation of Foxp3+ Tregs in the TME is related to tumor immune evasion and a poor prognosis in several solid tumors. The current strategies used to block Treg function are not Treg-specific and exhibit limited and transient efficacy. It has been demonstrated that USP7 is essential for Treg function by stabilizing the expression of Tip60 and Foxp3. Therefore, the pharmacological inhibition of USP7 is a promising strategy which may be used to inhibit Treg function and promote antitumor immunity. The P5091 series of USP7 inhibitors are known to exhibit direct anti-tumor activity in vivo using xenograft models, although the mechanism involved is unclear (168). The second-generation USP7 inhibitor, P217564, selectively modifies the active site of USP7 and exerts a durable inhibitory effect on USP7. As a result, P217564 promotes the ubiquitination and degradation of Foxp3 and Tip60 and impairs Treg functions. Moreover, USP7 regulates a number of other tumor-associated proteins, such as E3 ligase HDM2, FOXO4, PTEN, claspin and UHRF1 (169); thus, inhibiting USP7 has the potential for direct antitumor activity. In this regard, the inhibition of USP7 may provide dual antitumor activities: direct tumoricidal effects and immune-mediated tumor elimination. USP7 selective inhibitors have been found to significantly repress the growth of multiple tumor xenograft models in immunodeficient mice, including multiple myeloma, B-cell leukemia and neuroblastoma (129).
It has been reported that cisplatin can promote the immunotherapeutic effects of NK cells on HCC by reducing the expression of the androgen receptor (AR) and by upregulating the expression of AR-UL16-binding protein 2 (ULBP2). ULBP2 is a major natural killer group 2 member D ligand, which facilitates the activation of NK cells. AR binds to the ULBP2 promoter and negatively regulates the expression of ULBP2. Cisplatin reduces the levels of AR by suppressing AR expression through increased mir-34a-5p expression, or by inducing ubiquitination-mediated AR degradation, thereby increasing ULBP2 expression (170). This finding may provide a potential method for the combination of traditional chemotherapy and immunotherapy to control liver cancer.
5. Conclusions and future perspectives
Ubiquitination plays a vital role in the crosstalk between a tumor and the TME to regulate hypoxic environments, angiogenesis, chronic inflammation-mediated tumor formation, and control the functions of CAFs and infiltrating immune cells. The dysregulation of ubiquitylation is closely associated with the development of various human cancers. Therefore, the UPS has been regarded as a promising therapeutic target for novel anti-cancer drugs. Currently, some ubiquitination modulators in the TME have been identified for anticancer treatment.
It remains a significant challenge to identify specific small molecule modulators for different targets. The development of more specific inhibitors (e.g., CDK5 and USP22 inhibitors) is desirable. Apart from inhibitors, other strategies may also provide opportunities. For example, PROTAC technologies provide a strategy with which to target a number of non-druggable proteins (171). In addition, the crosstalk of other post-translational modifications, such as glycosylation and phosphorylation, can affect the ubiquitination and degradation of proteins (119,159). Thus, ubiquitination can be modulated by altering the glycosylation or phosphorylation of target proteins. Furthermore, epigenetic modulators of target proteins can also be regulated by ubiquitination to control their expression (99,172). In this regard, epigenetic modulators, such as CBX6 and CBX4, can also be regarded as potential targets. A deeper understanding of ubiquitination in the TME may further provide a larger therapeutic window for tumor treatment.
Acknowledgments
The authors would like to thank Professor Fengtang Yang, Shandong University of Technology for providing valuable comments regarding the writing of the manuscript.
Abbreviations
- AR
androgen receptor
- CAFs
cancer-associated fibroblasts
- Cbl-b
Casitas B-lineage lymphoma proto-oncogene-b
- CBP
CREB-binding protein
- CSN5
COP9 signalosome 5
- DUB
deubiquitinase
- E2F
E2 promoter binding factor
- MDA-7
melanoma differentiation-associated gene-7
- MDM2
mouse double minute 2 homolog
- MDSCs
myeloid-derived suppressor cells
- PD-1
programmed cell death protein-1
- PD-L1
programmed cell death-ligand 1
- pDCs
plasmacytoid DCs
- SCF
Skp-Cul1-F-box
- Skp2
S-phase kinase-associated protein-2
- TAMs
tumor-associated macrophages
- TME
tumor microenvironment
- TNF-α
tumor necrosis factor α
- UCH-L1
ubiquitin C-terminal hydrolase-L1
- UPS
ubiquitination proteasome system
- USPs
ubiquitin-specific proteases
Funding Statement
The present study was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2016CM46) and the National Natural Science Foundation of China (grant nos. 82173168 and 81702659).
Availability of data and materials
Not applicable.
Authors' contributions
XZ wrote and drafted the manuscript. TM revised the manuscript critically. SC and DL created the figures, and were involved in the literature search. QP and PW revised and completed the final version of the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR, et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35(Suppl):S199–S223. doi: 10.1016/j.semcancer.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng T, Huang R, Jin J, Gao J, Liu F, Wei Z, Xu X, Chang Z, Lin J, Ta N, et al. A comparative integrated multi-omics analysis identifies CA2 as a novel target for chordoma. Neuro Oncol. 2021;23:1709–1722. doi: 10.1093/neuonc/noab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suryadinata R, Roesley SN, Yang G, Sarcevic B. Mechanisms of generating polyubiquitin chains of different topology. Cells. 2014;3:674–689. doi: 10.3390/cells3030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Erpapazoglou Z, Walker O, Haguenauer-Tsapis R. Versatile roles of k63-linked ubiquitin chains in trafficking. Cells. 2014;3:1027–1088. doi: 10.3390/cells3041027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 8.Jahan AS, Elbæk CR, Damgaard RB. Met1-linked ubiquitin signalling in health and disease: Inflammation, immunity, cancer, and beyond. Cell Death Differ. 2021;28:473–492. doi: 10.1038/s41418-020-00676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya S, Ghosh MK. Cell death and deubiquitinases: Perspectives in cancer. Biomed Res Int. 2014;2014:435197. doi: 10.1155/2014/435197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer. 2018;18:69–88. doi: 10.1038/nrc.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrow JK, Lin HK, Sun SC, Zhang S. Targeting ubiquitination for cancer therapies. Future Med Chem. 2015;7:2333–2350. doi: 10.4155/fmc.15.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Ma L, Wang B, Liu J, Wei W. E3 ubiquitin ligases in cancer and implications for therapies. Cancer Metastasis Rev. 2017;36:683–702. doi: 10.1007/s10555-017-9703-z. [DOI] [PubMed] [Google Scholar]
- 13.Wei R, Liu X, Yu W, Yang T, Cai W, Liu J, Huang X, Xu GT, Zhao S, Yang J, Liu S. Deubiquitinases in cancer. Oncotarget. 2015;6:12872–12889. doi: 10.18632/oncotarget.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99(Pt B):186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Vosseler S, Lederle W, Airola K, Obermueller E, Fusenig NE, Mueller MM. Distinct progression-associated expression of tumor and stromal MMPs in HaCaT skin SCCs correlates with onset of invasion. Int J Cancer. 2009;125:2296–2306. doi: 10.1002/ijc.24589. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Griffioen AW, Damen CA, Blijham GH, Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood. 1996;88:667–673. doi: 10.1182/blood.V88.2.667.bloodjournal882667. [DOI] [PubMed] [Google Scholar]
- 18.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan JK, Day TA, Gillespie MB, Rosenzweig SA, Young MRI. Secretion of vascular endothelial growth factor by oral squamous cell carcinoma cells skews endothelial cells to suppress T-cell functions. Hum Immunol. 2009;70:375–382. doi: 10.1016/j.humimm.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulligan JK, Young MR. Tumors induce the formation of suppressor endothelial cells in vivo. Cancer Immunol Immunother. 2010;59:267–277. doi: 10.1007/s00262-009-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019;8:4709–4721. doi: 10.1002/cam4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch'ng ES. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: Technicalities and challenges in routine clinical practice. Front Oncol. 2020;9:1512. doi: 10.3389/fonc.2019.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laviron M, Boissonnas A. Ontogeny of tumor-associated macrophages. Front Immunol. 2019;10:1799. doi: 10.3389/fimmu.2019.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S, Yan W. T-cell immunometabolism against cancer. Cancer Lett. 2016;382:255–258. doi: 10.1016/j.canlet.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696–2707. doi: 10.1111/cas.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol. 2015;194:2985–2991. doi: 10.4049/jimmunol.1403134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19:120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 31.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 32.Mennerich D, Kubaichuk K, Kietzmann T. DUBs, hypoxia, and cancer. Trends Cancer. 2019;5:632–653. doi: 10.1016/j.trecan.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Wang D, Messing EM, Wu G. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep. 2005;6:373–378. doi: 10.1038/sj.embor.7400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto Y, Zeng L, Yeom CJ, Zhu Y, Morinibu A, Shinomiya K, Kobayashi M, Hirota K, Itasaka S, Yoshimura M, et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1α. Nat Commun. 2015;6:6153. doi: 10.1038/ncomms7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi BJ, Park SA, Lee SY, Cha YN, Surh YJ. Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of Snail: A potential role of Sox9. Sci Rep. 2017;7:15918. doi: 10.1038/s41598-017-15139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma B, Cheng H, Mu C, Geng G, Zhao T, Luo Q, Ma K, Chang R, Liu Q, Gao R, et al. The SIAH2-NRF1 axis spatially regulates tumor microenvironment remodeling for tumor progression. Nat Commun. 2019;10:1034. doi: 10.1038/s41467-019-08618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith GA, Fearnley GW, Abdul-Zani I, Wheatcroft SB, Tomlinson DC, Harrison MA, Ponnambalam S. VEGFR2 trafficking, signaling and proteolysis is regulated by the ubiquitin isopeptidase USP8. Traffic. 2016;17:53–65. doi: 10.1111/tra.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JY, Park JH, Choi HJ, Won HY, Joo HS, Shin DH, Park MK, Han B, Kim KP, Lee TJ, et al. LSD1 demethylates HIF1α to inhibit hydroxylation and ubiquitin-mediated degradation in tumor angiogenesis. Oncogene. 2017;36:5512–5521. doi: 10.1038/onc.2017.158. [DOI] [PubMed] [Google Scholar]
- 39.Amelio I, Inoue S, Markert EK, Levine AJ, Knight RA, Mak TW, Melino G. TAp73 opposes tumor angiogenesis by promoting hypoxia-inducible factor 1α degradation. Proc Natl Acad Sci USA. 2015;112:226–231. doi: 10.1073/pnas.1410609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan T, Zhou D, Shi Z, Qiu Y, Zhou G, Liu J, Yang Q, Cao L, Zhang J. Centromere protein U (CENPU) enhances angiogenesis in triple-negative breast cancer by inhibiting ubiquitin-proteasomal degradation of COX-2. Cancer Lett. 2020;482:102–111. doi: 10.1016/j.canlet.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Li H. Functional characterization of SAG/RBX2/ROC2/RNF7, an antioxidant protein and an E3 ubiquitin ligase. Protein Cell. 2013;4:103–116. doi: 10.1007/s13238-012-2105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan M, Li H, Sun Y. Endothelial deletion of Sag/Rbx2/Roc2 E3 ubiquitin ligase causes embryonic lethality and blocks tumor angiogenesis. Oncogene. 2014;33:5211–5220. doi: 10.1038/onc.2013.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasan SS, Tsaryk R, Lange M, Wisniewski L, Moore JC, Lawson ND, Wojciechowska K, Schnittler H, Siekmann AF. Endothelial Notch signalling limits angiogenesis via control of artery formation. Nat Cell Biol. 2017;19:928–940. doi: 10.1038/ncb3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabellino A, Andreani C, Scaglioni PP. Roles of ubiquitination and SUMOylation in the regulation of angiogenesis. Curr Issues Mol Biol. 2020;35:109–126. doi: 10.21775/cimb.035.109. [DOI] [PubMed] [Google Scholar]
- 45.Tsunematsu R, Nakayama K, Oike Y, Nishiyama M, Ishida N, Hatakeyama S, Bessho Y, Kageyama R, Suda T, Nakayama KI. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J Biol Chem. 2004;279:9417–9423. doi: 10.1074/jbc.M312337200. [DOI] [PubMed] [Google Scholar]
- 46.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11:63–69. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 47.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivanna S, Harrold I, Shashar M, Meyer R, Kiang C, Francis J, Zhao Q, Feng H, Edelman ER, Rahimi N, Chitalia VC. The c-Cbl ubiquitin ligase regulates nuclear β-catenin and angiogenesis by its tyrosine phosphorylation mediated through the Wnt signaling pathway. J Biol Chem. 2015;290:12537–12546. doi: 10.1074/jbc.M114.616623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivkin E, Almeida SM, Ceccarelli DF, Juang YC, MacLean TA, Srikumar T, Huang H, Dunham WH, Fukumura R, Xie G, et al. The linear ubiquitin-specific deubiquitinase gumby regulates angiogenesis. Nature. 2013;498:318–324. doi: 10.1038/nature12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Li M, Ponnusamy S, Chi Y, Xue J, Fahmy B, Fan M, Miranda-Carboni GA, Narayanan R, Wu J, Wu ZH. ABL1-dependent OTULIN phosphorylation promotes genotoxic Wnt/β-catenin activation to enhance drug resistance in breast cancers. Nat Commun. 2020;11:3965. doi: 10.1038/s41467-020-17770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quintero-Fabián S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, Lara-Riegos J, Ramírez-Camacho MA, Alvarez-Sánchez ME. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray GI, Duncan ME, Arbuckle E, Melvin WT, Fothergill JE. Matrix metalloproteinases and their inhibitors in gastric cancer. Gut. 1998;43:791–797. doi: 10.1136/gut.43.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 54.Chang SC, Ding JL. Ubiquitination and SUMOylation in the chronic inflammatory tumor microenvironment. Biochim Biophys Acta Rev Cancer. 2018;1870:165–175. doi: 10.1016/j.bbcan.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Peng SL. Forkhead transcription factors in chronic inflammation. Int J Biochem Cell Biol. 2010;42:482–485. doi: 10.1016/j.biocel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 57.Ramezani A, Nikravesh H, Faghihloo E. The roles of FOX proteins in virus-associated cancers. J Cell Physiol. 2019;234:3347–3361. doi: 10.1002/jcp.27295. [DOI] [PubMed] [Google Scholar]
- 58.Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu W, Ma Q, Chen L, Li P, Zhang M, Ramamoorthy S, Nawaz Z, Shimojima T, Wang H, Yang Y, et al. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem. 2009;284:13987–14000. doi: 10.1074/jbc.M901758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang SC, Ding JL. Ubiquitination by SAG regulates macrophage survival/death and immune response during infection. Cell Death Differ. 2014;21:1388–1398. doi: 10.1038/cdd.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang SC, Choo WQ, Toh HC, Ding JL. SAG-UPS attenuates proapoptotic SARM and Noxa to confer survival advantage to early hepatocellular carcinoma. Cell Death Discov. 2015;1:15032. doi: 10.1038/cddiscovery.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, Yang HY, Sahin AA, Hunt KK, Fuson KL, et al. MDA-7/IL-24 is a unique cytokine-tumor suppressor in the IL-10 family. Int Immunopharmacol. 2004;4:649–667. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Gopalan B, Shanker M, Scott A, Branch CD, Chada S, Ramesh R. MDA-7/IL-24, a novel tumor suppressor/cytokine is ubiquitinated and regulated by the ubiquitin-proteasome system, and inhibition of MDA-7/IL-24 degradation enhances the anti-tumor activity. Cancer Gene Ther. 2008;15:1–8. doi: 10.1038/sj.cgt.7701095. [DOI] [PubMed] [Google Scholar]
- 64.Liu P, Zhang X, Li Z, Wei L, Peng Q, Liu C, Wu Y, Yan Q, Ma J. A significant role of transcription factors E2F in inflammation and tumorigenesis of nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2020;524:816–824. doi: 10.1016/j.bbrc.2020.01.158. [DOI] [PubMed] [Google Scholar]
- 65.Swarnalatha M, Singh AK, Kumar V. Promoter occupancy of MLL1 histone methyltransferase seems to specify the proliferative and apoptotic functions of E2F1 in a tumour microenvironment. Cell Sci. 2013;126(Pt 20):4636–4646. doi: 10.1242/jcs.126235. [DOI] [PubMed] [Google Scholar]
- 66.Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23:50. doi: 10.1186/s12199-018-0740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–472. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu S, Zhang Q, Zhang F, Meng F, Liu S, Zhou R, Wu Q, Li X, Shen L, Huang J, et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat Cell Biol. 2019;21:1027–1040. doi: 10.1038/s41556-019-0352-z. [DOI] [PubMed] [Google Scholar]
- 70.Kensche T, Tokunaga F, Ikeda F, Goto E, Iwai K, Dikic I. Analysis of nuclear factor-κB (NF-κB) essential modulator (NEMO) binding to linear and lysine-linked ubiquitin chains and its role in the activation of NF-κB. J Biol Chem. 2012;287:23626–23634. doi: 10.1074/jbc.M112.347195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jo T, Nishikori M, Kogure Y, Arima H, Sasaki K, Sasaki Y, Nakagawa T, Iwai F, Momose S, Shiraishi A, et al. LUBAC accelerates B-cell lymphomagenesis by conferring resistance to genotoxic stress on B cells. Blood. 2020;136:684–697. doi: 10.1182/blood.2019002654. [DOI] [PubMed] [Google Scholar]
- 72.Song K, Cai X, Dong Y, Wu H, Wei Y, Shankavaram UT, Cui K, Lee Y, Zhu B, Bhattacharjee S, et al. Epsins 1 and 2 promote NEMO linear ubiquitination via LUBAC to drive breast cancer development. J Clin Invest. 2021;131:e129374. doi: 10.1172/JCI129374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Damgaard RB, Jolin HE, Allison MED, Davies SE, Titheradge HL, McKenzie ANJ, Komander D. OTULIN protects the liver against cell death, inflammation, fibrosis, and cancer. Cell Death Differ. 2020;27:1457–1474. doi: 10.1038/s41418-020-0532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lambies G, Miceli M, Martínez-Guillamon C, Olivera-Salguero R, Peña R, Frías CP, Calderó I, Atanassov BS, Dent SYR, Arribas J, et al. TGFβ-Activated USP27X deubiquitinase regulates cell migration and chemoresistance via stabilization of snail1. Cancer Res. 2019;79:33–46. doi: 10.1158/0008-5472.CAN-18-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu X, Liu M, Zhu H, Wang J, Dai W, Li J, Zhu D, Tang W, Xiao Y, Lin J, et al. Ubiquitin-specific protease 3 promotes cell migration and invasion by interacting with and deubiquitinating SUZ12 in gastric cancer. J Exp Clin Cancer Res. 2019;38:277. doi: 10.1186/s13046-019-1270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W, Wang J, Yan H, Zhang K, Liu Y. Upregulation of USP11 promotes epithelial-to-mesenchymal transition by deubiquitinating Snail in ovarian cancer. Oncol Rep. 2019;41:1739–1748. doi: 10.3892/or.2018.6924. [DOI] [PubMed] [Google Scholar]
- 78.Garcia DA, Baek C, Estrada MV, Tysl T, Bennett EJ, Yang J, Chang JT. USP11 enhances TGFβ-Induced epithelial-mesenchymal plasticity and human breast cancer metastasis. Mol Cancer Res. 2018;16:1172–1184. doi: 10.1158/1541-7786.MCR-17-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kit Leng Lui S, Iyengar PV, Jaynes P, Isa ZFBA, Pang B, Tan TZ, Eichhorn PJA. USP26 regulates TGF-β signaling by deubiquitinating and stabilizing SMAD7. EMBO Rep. 2017;18:797–808. doi: 10.15252/embr.201643270. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Eichhorn PJ, Rodó L, Gonzàlez-Juncà A, Dirac A, Gili M, Martínez-Sáez E, Aura C, Barba I, Peg V, Prat A, et al. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat Med. 2012;18:429–435. doi: 10.1038/nm.2619. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu CX, ten Dijke P. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat Cell Biol. 2012;14:717–726. doi: 10.1038/ncb2522. [DOI] [PubMed] [Google Scholar]
- 82.Jang MJ, Baek SH, Kim JH. UCH-L1 promotes cancer metastasis in prostate cancer cells through EMT induction. Cancer Lett. 2011;302:128–135. doi: 10.1016/j.canlet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Lee JH, Jung SM, Yang KM, Bae E, Ahn SG, Park JS, Seo D, Kim M, Ha J, Lee J, et al. A20 promotes metastasis of aggressive basal-like breast cancers through multi-monoubiquitylation of Snail1. Nat Cell Biol. 2017;19:1260–1273. doi: 10.1038/ncb3609. [DOI] [PubMed] [Google Scholar]
- 84.Pitarresi JR, Liu X, Avendano A, Thies KA, Sizemore GM, Hammer AM, Hildreth BE, III, Wang DJ, Steck SA, Donohue S, et al. Disruption of stromal hedgehog signaling initiates RNF5-mediated proteasomal degradation of PTEN and accelerates pancreatic tumor growth. Life Sci Alliance. 2018;1:e201800190. doi: 10.26508/lsa.201800190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, Klein-Szanto AJ, Sahu V, Basu D, Ohashi S, et al. IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res. 2018;78:4957–4970. doi: 10.1158/0008-5472.CAN-17-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, Li J, Li C, Yan M, Zhu Z, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8:20741–20750. doi: 10.18632/oncotarget.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Y, Wang Y, Lin Y, Liu Y, Wang Y, Jia J, Singh P, Chi YI, Wang C, Dong C, et al. Dub3 inhibition suppresses breast cancer invasion and metastasis by promoting Snail1 degradation. Nat Commun. 2017;8:14228. doi: 10.1038/ncomms14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis-tracing the accessory. Oncogene. 2014;33:3217–3224. doi: 10.1038/onc.2013.272. [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Chen S, Wang W, Ning BF, Chen F, Shen W, Ding J, Chen W, Xie WF, Zhang X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer Lett. 2016;379:49–59. doi: 10.1016/j.canlet.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 90.Yumimoto K, Nakayama KI. Fbxw7 suppresses cancer metastasis by inhibiting niche formation. Oncoimmunology. 2015;4:e1022308. doi: 10.1080/2162402X.2015.1022308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mehić M, de Sa VK, Hebestreit S, Heldin CH, Heldin P. The deubiquitinating enzymes USP4 and USP17 target hyaluronan synthase 2 and differentially affect its function. Oncogenesis. 2017;6:e348. doi: 10.1038/oncsis.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stetler-Stevenson WG, Yu AE. Proteases in invasion: Matrix metalloproteinases. Semin Cancer Biol. 2001;11:143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 93.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: Role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108:1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 94.Gontero P, Banisadr S, Frea B, Brausi M. Metastasis markers in bladder cancer: A review of the literature and clinical considerations. Eur Urol. 2004;46:296–311. doi: 10.1016/j.eururo.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Li S, Luo W. Matrix metalloproteinase 2 contributes to aggressive phenotype, epithelial-mesenchymal transition and poor outcome in nasopharyngeal carcinoma. Onco Targets Ther. 2019;12:5701–5711. doi: 10.2147/OTT.S202280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamada S, Yanamoto S, Naruse T, Matsushita Y, Takahashi H, Umeda M, Nemoto TK, Kurita H. Skp2 regulates the expression of MMP-2 and MMP-9, and enhances the invasion potential of oral squamous cell carcinoma. Pathol Oncol Res. 2016;22:625–632. doi: 10.1007/s12253-016-0049-6. [DOI] [PubMed] [Google Scholar]
- 97.Hung WC, Tseng WL, Shiea J, Chang HC. Skp2 overexpression increases the expression of MMP-2 and MMP-9 and invasion of lung cancer cells. Cancer Lett. 2010;288:156–161. doi: 10.1016/j.canlet.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 98.Chernov AV, Sounni NE, Remacle AG, Strongin AY. Epigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells. J Biol Chem. 2009;284:12727–12734. doi: 10.1074/jbc.M900273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sakai K, Nishiuchi T, Tange S, Suzuki Y, Yano S, Terashima M, Suzuki T, Matsumoto K. Proteasomal degradation of polycomb-group protein CBX6 confers MMP-2 expression essential for mesothelioma invasion. Sci Rep. 2020;10:16678. doi: 10.1038/s41598-020-72448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Priolo C, Tang D, Brahamandan M, Benassi B, Sicinska E, Ogino S, Farsetti A, Porrello A, Finn S, Zimmermann J, et al. The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Res. 2006;66:8625–8632. doi: 10.1158/0008-5472.CAN-06-1374. [DOI] [PubMed] [Google Scholar]
- 101.Qu Q, Mao Y, Xiao G, Fei X, Wang J, Zhang Y, Liu J, Cheng G, Chen X, Wang J, Shen K. USP2 promotes cell migration and invasion in triple negative breast cancer cell lines. Tumour Biol. 2015;36:5415–5423. doi: 10.1007/s13277-015-3207-7. [DOI] [PubMed] [Google Scholar]
- 102.Nguyen HL, Kadam P, Helkin A, Cao K, Wu S, Samara GJ, Zhang Q, Zucker S, Cao J. MT1-MMP Activation of TGF-β signaling enables intercellular activation of an epithelial-mesenchymal transition program in cancer. Curr Cancer Drug Targets. 2016;16:618–630. doi: 10.2174/1568009616666160216125634. [DOI] [PubMed] [Google Scholar]
- 103.Eisenach PA, de Sampaio PC, Murphy G, Roghi C. Membrane type 1 matrix metalloproteinase (MT1-MMP) ubiquitination at Lys581 increases cellular invasion through type I collagen. J Biol Chem. 2012;287:11533–11545. doi: 10.1074/jbc.M111.306340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu T, Gan S, Zhu Q, Dai D, Li N, Wang H, Chen X, Hou D, Wang Y, Pan Q, et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat Commun. 2019;10:4353. doi: 10.1038/s41467-019-12384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rőszer T. Understanding the Mysterious M2 Macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang YC, Wu YS, Hung CY, Wang SA, Young MJ, Hsu TI, Hung JJ. USP24 induces IL-6 in tumor-associated microenvironment by stabilizing p300 and β-TrCP and promotes cancer malignancy. Nat Commun. 2018;9:3996. doi: 10.1038/s41467-018-06178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ning C, Xie B, Zhang L, Li C, Shan W, Yang B, Luo X, Gu C, He Q, Jin H, et al. Infiltrating Macrophages Induce ERα Expression through an IL17A-mediated epigenetic mechanism to sensitize endometrial cancer cells to estrogen. Cancer Res. 2016;76:1354–1366. doi: 10.1158/0008-5472.CAN-15-1260. [DOI] [PubMed] [Google Scholar]
- 109.Lv Q, Xie L, Cheng Y, Shi Y, Shan W, Ning C, Xie B, Yang B, Luo X, He Q, et al. A20-mediated deubiquitination of ERα in the microenvironment of CD163+ macrophages sensitizes endometrial cancer cells to estrogen. Cancer Lett. 2019;442:137–147. doi: 10.1016/j.canlet.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 110.Clancy JL, Henderson MJ, Russell AJ, Anderson DW, Bova RJ, Campbell IG, Choong DY, Macdonald GA, Mann GJ, Nolan T, et al. EDD, the human orthologue of the hyperplastic discs tumour suppressor gene, is amplified and overexpressed in cancer. Oncogene. 2003;22:5070–5081. doi: 10.1038/sj.onc.1206775. [DOI] [PubMed] [Google Scholar]
- 111.Song M, Yeku OO, Rafiq S, Purdon T, Dong X, Zhu L, Zhang T, Wang H, Yu Z, Mai J, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun. 2020;11:6298. doi: 10.1038/s41467-020-20140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Zhou X, Yu J, Cheng X, Zhao B, Manyam GC, Zhang L, Schluns K, Li P, Wang J, Sun SC. The deubiquitinase Otub1 controls the activation of CD8+ T cells and NK cells by regulating IL-15-mediated priming. Nat Immunol. 2019;20:879–889. doi: 10.1038/s41590-019-0405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/S0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 115.Kunii N, Zhao Y, Jiang S, Liu X, Scholler J, Balagopalan L, Samelson LE, Milone MC, June CH. Enhanced function of redirected human T cells expressing linker for activation of T cells that is resistant to ubiquitylation. Hum Gene Ther. 2013;24:27–37. doi: 10.1089/hum.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen RH, Lee YR, Yuan WC. The role of PML ubiquitination in human malignancies. J Biomed Sci. 2012;19:81. doi: 10.1186/1423-0127-19-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang YT, Chen J, Chang CW, Jen J, Huang TY, Chen CM, Shen R, Liang SY, Cheng IC, Yang SC, et al. Ubiquitination of tumor suppressor PML regulates prometastatic and immunosuppressive tumor microenvironment. J Clin Invest. 2017;127:2982–2997. doi: 10.1172/JCI89957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meng X, Liu X, Guo X, Jiang S, Chen T, Hu Z, Liu H, Bai Y, Xue M, Hu R, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564:130–135. doi: 10.1038/s41586-018-0756-0. [DOI] [PubMed] [Google Scholar]
- 119.Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, Tan Y, Ci Y, Wu F, Dai X, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–95. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song Y, Xu Y, Pan C, Yan L, Wang ZW, Zhu X. The emerging role of SPOP protein in tumorigenesis and cancer therapy. Mol Cancer. 2020;19:2. doi: 10.1186/s12943-019-1124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lim SO, Li CW, Xia W, Cha JH, Chan LC, Wu Y, Chang SS, Lin WC, Hsu JM, Hsu YH, et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y, Sun Q, Mu N, Sun X, Wang Y, Fan S, Su L, Liu X. The deubiquitinase USP22 regulates PD-L1 degradation in human cancer cells. Cell Commun Signal. 2020;18:112. doi: 10.1186/s12964-020-00612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang X, Zhang Q, Lou Y, Wang J, Zhao X, Wang L, Zhang X, Li S, Zhao Y, Chen Q, et al. USP22 Deubiquitinates CD274 to Suppress Anticancer Immunity. Cancer Immunol Res. 2019;7:1580–1590. doi: 10.1158/2326-6066.CIR-18-0910. [DOI] [PubMed] [Google Scholar]
- 125.Li J, Yuan S, Norgard RJ, Yan F, Yamazoe T, Blanco A, Stanger BZ. Tumor Cell-Intrinsic USP22 suppresses antitumor immunity in pancreatic cancer. Cancer Immunol Res. 2020;8:282–291. doi: 10.1158/2326-6066.CIR-19-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jingjing W, Wenzheng G, Donghua W, Guangyu H, Aiping Z, Wenjuan W. Deubiquitination and stabilization of programmed cell death ligand 1 by ubiquitin-specific peptidase 9, X-linked in oral squamous cell carcinoma. Cancer Med. 2018;7:4004–4011. doi: 10.1002/cam4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Naik E, Webster JD, DeVoss J, Liu J, Suriben R, Dixit VM. Regulation of proximal T cell receptor signaling and tolerance induction by deubiquitinase Usp9X. J Exp Med. 2014;211:1947–1955. doi: 10.1084/jem.20140860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 129.Wang F, Wang L, Wu J, Sokirniy I, Nguyen P, Bregnard T, Weinstock J, Mattern M, Bezsonova I, Hancock WW, Kumar S. Active site-targeted covalent irreversible inhibitors of USP7 impair the functions of Foxp3+ T-regulatory cells by promoting ubiquitination of Tip60. PLoS One. 2017;12:e0189744. doi: 10.1371/journal.pone.0189744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/S1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 131.Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1–10. doi: 10.1016/j.ejca.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 132.Guo Y, Yang L, Lei S, Tan W, Long J. NEDD4 Negatively Regulates GITR via ubiquitination in immune microenvironment of melanoma. Onco Targets Ther. 2019;12:10629–10637. doi: 10.2147/OTT.S212317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Trovato R, Fiore A, Sartori S, Canè S, Giugno R, Cascione L, Paiella S, Salvia R, De Sanctis F, Poffe O, et al. Immunosuppression by monocytic myeloid-derived suppressor cells in patients with pancreatic ductal carcinoma is orchestrated by STAT3. J Immunother Cancer. 2019;7:255. doi: 10.1186/s40425-019-0734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song G, Zhang Y, Tian J, Ma J, Yin K, Xu H, Wang S. TRAF6 regulates the immunosuppressive effects of myeloid-derived suppressor cells in tumor-bearing host. Front Immunol. 2021;12:649020. doi: 10.3389/fimmu.2021.649020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang CX, Ye SB, Ni JJ, Cai TT, Liu YN, Huang DJ, Mai HQ, Chen QY, He J, Zhang XS, et al. STING signaling remodels the tumor microenvironment by antagonizing myeloid-derived suppressor cell expansion. Cell Death Differ. 2019;26:2314–2328. doi: 10.1038/s41418-019-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chou FC, Chen HY, Kuo CC, Sytwu HK. Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci. 2018;19:430. doi: 10.3390/ijms19020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang CX, Huang DJ, Baloche V, Zhang L, Xu JX, Li BW, Zhao XR, He J, Mai HQ, Chen QY, et al. Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation. Oncogenesis. 2020;9:65. doi: 10.1038/s41389-020-00248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fang P, Li X, Dai J, Cole L, Camacho JA, Zhang Y, Ji Y, Wang J, Yang XF, Wang H. Immune cell subset differentiation and tissue inflammation. J Hematol Oncol. 2018;11:97. doi: 10.1186/s13045-018-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC, Liu XQ, Wang D, Li N, Cheng JT, Lyv YN, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13:107. doi: 10.1186/s13045-020-00939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bi E, Li R, Bover LC, Li H, Su P, Ma X, Huang C, Wang Q, Liu L, Yang M, et al. E-cadherin expression on multiple myeloma cells activates tumor-promoting properties in plasmacytoid DCs. J Clin Invest. 2018;128:4821–4831. doi: 10.1172/JCI121421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu Q, Ye Y, Chan LC, Li Y, Liang K, Lin A, Egranov SD, Zhang Y, Xia W, Gong J, et al. Oncogenic lncRNA down-regulates cancer cell antigen presentation and intrinsic tumor suppression. Nat Immunol. 2019;20:835–851. doi: 10.1038/s41590-019-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Caraux A, Lu Q, Fernandez N, Riou S, Di Santo JP, Raulet DH, Lemke G, Roth C. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006;7:747–754. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- 143.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, Jamieson AM, Langdon WY, Ikeda F, Fededa JP, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507:508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci. 2012;125(Pt 2):265–275. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y, Zhang Y, Yi P, Dong W, Nalin AP, Zhang J, Zhu Z, Chen L, Benson DM, Mundy-Bosse BL, et al. The IL-15-AKT-XBP1s signaling pathway contributes to effector functions and survival in human NK cells. Nat Immunol. 2019;20:10–17. doi: 10.1038/s41590-018-0265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dou Y, Xing J, Kong G, Wang G, Lou X, Xiao X, Vivier E, Li XC, Zhang Z. Identification of the E3 Ligase TRIM29 as a critical checkpoint regulator of NK cell functions. J Immunol. 2019;203:873–880. doi: 10.4049/jimmunol.1900171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deng L, Meng T, Chen L, Wei W, Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. 2020;5:11. doi: 10.1038/s41392-020-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): A novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control. 2003;10:361–369. doi: 10.1177/107327480301000502. [DOI] [PubMed] [Google Scholar]
- 149.Piva R, Ruggeri B, Williams M, Costa G, Tamagno I, Ferrero D, Giai V, Coscia M, Peola S, Massaia M, et al. CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood. 2008;111:2765–2775. doi: 10.1182/blood-2007-07-100651. [DOI] [PubMed] [Google Scholar]
- 150.Anchoori RK, Karanam B, Peng S, Wang JW, Jiang R, Tanno T, Orlowski RZ, Matsui W, Zhao M, Rudek MA, et al. A bis-benzylidine piperidone targeting proteasome ubiquitin receptor RPN13/ADRM1 as a therapy for cancer. Cancer Cell. 2013;24:791–805. doi: 10.1016/j.ccr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Song Y, Ray A, Li S, Das DS, Tai YT, Carrasco RD, Chauhan D, Anderson KC. Targeting proteasome ubiquitin receptor Rpn13 in multiple myeloma. Leukemia. 2016;30:1877–1886. doi: 10.1038/leu.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kimura K, Yamada T, Matsumoto M, Kido Y, Hosooka T, Asahara S, Matsuda T, Ota T, Watanabe H, Sai Y, et al. Endoplasmic reticulum stress inhibits STAT3-dependent suppression of hepatic gluconeogenesis via dephosphorylation and deacetylation. Diabetes. 2012;61:61–73. doi: 10.2337/db10-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soong RS, Anchoori RK, Yang B, Yang A, Tseng SH, He L, Tsai YC, Roden RB, Hung CF. RPN13/ADRM1 inhibitor reverses immunosuppression by myeloid-derived suppressor cells. Oncotarget. 2016;7:68489–68502. doi: 10.18632/oncotarget.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu GY, Wang X, Zheng SS, Gao XM, Jia QA, Zhu WW, Lu L, Jia HL, Chen JH, Dong QZ, et al. RA190, a proteasome subunit ADRM1 inhibitor, suppresses intrahepatic cholangiocarcinoma by inducing NF-KB-Mediated cell apoptosis. Cell Physiol Biochem. 2018;47:1152–1166. doi: 10.1159/000490210. [DOI] [PubMed] [Google Scholar]
- 155.Soong RS, Anchoori RK, Roden RBS, Cho RL, Chen YC, Tseng SC, Huang YL, Liao PC, Shyu YC. Bis-benzylidine Piperidone RA190 treatment of hepatocellular carcinoma via binding RPN13 and inhibiting NF-κB signaling. BMC Cancer. 2020;20:386. doi: 10.1186/s12885-020-06896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Powis G, Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol Cancer Ther. 2004;3:647–654. [PubMed] [Google Scholar]
- 157.Lee YM, Kim GH, Park EJ, Oh TI, Lee S, Kan SY, Kang H, Kim BM, Kim JH, Lim JH. Thymoquinone selectively kills hypoxic renal cancer cells by suppressing HIF-1α-mediated glycolysis. Int J Mol Sci. 2019;20:1092. doi: 10.3390/ijms20051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ge Y, Yoon SH, Jang H, Jeong JH, Lee YM. Decursin promotes HIF-1α proteasomal degradation and immune responses in hypoxic tumour microenvironment. Phytomedicine. 2020;78:153318. doi: 10.1016/j.phymed.2020.153318. [DOI] [PubMed] [Google Scholar]
- 159.Chen JJ, Ren YL, Shu CJ, Zhang Y, Chen MJ, Xu J, Li J, Li AP, Chen DY, He JD, et al. JP3, an antiangiogenic peptide, inhibits growth and metastasis of gastric cancer through TRIM25/SP1/MMP2 axis. J Exp Clin Cancer Res. 2020;39:118. doi: 10.1186/s13046-020-01617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen Y, Huang Y, Huang Y, Xia X, Zhang J, Zhou Y, Tan Y, He S, Qiang F, Li A, et al. JWA suppresses tumor angiogenesis via Sp1-activated matrix metalloproteinase-2 and its prognostic significance in human gastric cancer. Carcinogenesis. 2014;35:442–451. doi: 10.1093/carcin/bgt311. [DOI] [PubMed] [Google Scholar]
- 161.Godbersen JC, Humphries LA, Danilova OV, Kebbekus PE, Brown JR, Eastman A, Danilov AV. The Nedd8-activating enzyme inhibitor MLN4924 thwarts microenvironment-driven NF-κB activation and induces apoptosis in chronic lymphocytic leukemia B cells. Clin Cancer Res. 2014;20:1576–1589. doi: 10.1158/1078-0432.CCR-13-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Katsuya K, Hori Y, Oikawa D, Yamamoto T, Umetani K, Urashima T, Kinoshita T, Ayukawa K, Tokunaga F, Tamaru M. High-Throughput screening for linear ubiquitin chain assembly complex (LUBAC) selective inhibitors using homogenous time-resolved fluorescence (HTRF)-based assay system. SLAS Discov. 2018;23:1018–1029. doi: 10.1177/2472555218793066. [DOI] [PubMed] [Google Scholar]
- 163.Katsuya K, Oikawa D, Iio K, Obika S, Hori Y, Urashima T, Ayukawa K, Tokunaga F. Small-molecule inhibitors of linear ubiquitin chain assembly complex (LUBAC), HOIPINs, suppress NF-κB signaling. Biochem Biophys Res Commun. 2019;509:700–706. doi: 10.1016/j.bbrc.2018.12.164. [DOI] [PubMed] [Google Scholar]
- 164.Liu C, Billadeau DD, Abdelhakim H, Leof E, Kaibuchi K, Bernabeu C, Bloom GS, Yang L, Boardman L, Shah VH, Kang N. IQGAP1 suppresses TβRII-mediated myofibroblastic activation and metastatic growth in liver. J Clin Invest. 2013;123:1138–1156. doi: 10.1172/JCI63836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu JL, Wang XY, Huang BX, Zhu F, Zhang RG, Wu G. Expression of CDK5/p35 in resected patients with non-small cell lung cancer: Relation to prognosis. Med Oncol. 2011;28:673–678. doi: 10.1007/s12032-010-9510-7. [DOI] [PubMed] [Google Scholar]
- 166.Gao L, Xia L, Ji W, Zhang Y, Xia W, Lu S. Knockdown of CDK5 down-regulates PD-L1 via the ubiquitination-proteasome pathway and improves antitumor immunity in lung adenocarcinoma. Transl Oncol. 2021;14:101148. doi: 10.1016/j.tranon.2021.101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Y, Liu X, Zhang N, Yin M, Dong J, Zeng Q, Mao G, Song D, Liu L, Deng H. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity inhibiting the deubiquitination activity of CSN5. Acta Pharm Sin B. 2020;10:2299–2312. doi: 10.1016/j.apsb.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R, McDermott JL, Leach CA, Fulcinniti M, Kodrasov MP, et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22:345–358. doi: 10.1016/j.ccr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nicholson B, Suresh Kumar KG. The multifaceted roles of USP7: New therapeutic opportunities. Cell Biochem Biophys. 2011;60:61–68. doi: 10.1007/s12013-011-9185-5. [DOI] [PubMed] [Google Scholar]
- 170.Shi L, Lin H, Li G, Sun Y, Shen J, Xu J, Lin C, Yeh S, Cai X, Chang C. Cisplatin enhances NK cells immunotherapy efficacy to suppress HCC progression via altering the androgen receptor (AR)-ULBP2 signals. Cancer Lett. 2016;373:45–56. doi: 10.1016/j.canlet.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang X, Meng T, Cui S, Feng L, Liu D, Pang Q, Wang P. Ubiquitination of nonhistone proteins in cancer development and treatment. Front Oncol. 2021;10:621294. doi: 10.3389/fonc.2020.621294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ning B, Zhao W, Qian C, Liu P, Li Q, Li W, Wang RF. USP26 functions as a negative regulator of cellular reprogramming by stabilising PRC1 complex components. Nat Commun. 2017;8:349. doi: 10.1038/s41467-017-00301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.