Abstract
Calcineurin (CaN) is a unique calcium (Ca2+) and calmodulin (CaM)-dependent serine/threonine phosphatase that becomes activated in the presence of increased intracellular Ca2+ level. CaN then functions to dephosphorylate target substrates including various transcription factors, receptors, and channels. Once activated, the CaN signaling pathway participates in the development of multiple organs as well as the onset and progression of various diseases via regulation of different cellular processes. Here, we review current literature regarding the structural and functional properties of CaN, highlighting its crucial role in the development and pathogenesis of immune system disorders, neurodegenerative diseases, kidney disease, cardiomyopathy and cancer.
Keywords: Calcineurin, Cancer, Cardiomyopathy, Development, Immune system disease, Kidney disease, Neurodegeneration
Introduction
Calcineurin (CaN, alternate name protein phosphatase 2B) is best characterized as a unique Ca2+ and calmodulin (CaM)-dependent serine/threonine phosphatase that activates substrates important in various cellular processes ranging from activation of T cells to regulation of cell apoptosis.1, 2, 3 In the canonical CaN pathway, which was first described in immune cells, CaN becomes activated following an increase in calcium (Ca2+) concentration. Following activation, CaN dephosphorylates nuclear factor of activated T-cells (NFAT) allowing its nuclear translocation to regulate the expression of interleukin (IL)-2 and IL-4.3,4 Meanwhile, within the noncanonical CaN pathway, CaN activation leads to the dephosphorylation of other substrates, including dynamin-related protein 1 (Drp1),5 Na+/H+-exchanger 1 (NHE1),6 TWIK-related spinal cord K+ channel (TRESK),7 calcineurin response zinc finger (CRZ1),3 and kinase suppressor of ras 2 (KSR2).8 In this manner, CaN regulates the function of various organ systems, including the immune system, heart, kidney, neurons.9, 10, 11, 12 Meanwhile, CaN dysfunction leads to developmental defects, contributing to the pathogenesis of many common disorders. Accordingly, CaN inhibitors (CNIs), such as cyclosporine A (CsA) and tacrolimus (FK506), have been developed to inhibit CaN functions for the treatment of clinical diseases.4,13,14
In the 40 years since Claude Klee first described the structure and regulation of Ca2+-activated CaN, accumulating studies have revealed that CaN plays an essential role in a myriad of physiological and pathological processes. Published reviews on this topic have been presented in Table S13,4,13, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, however, these articles largely focus on the structure and functions of CaN. In particular, CaN alterations are reportedly associated with the development and the onset and progression of immune system disease, neurodegenerative diseases, kidney disease, cardiomyopathy, and cancer. Here, we provide a comprehensive description of the pivotal roles of CaN in development and disease.
Calcineurin: structure and activation insights
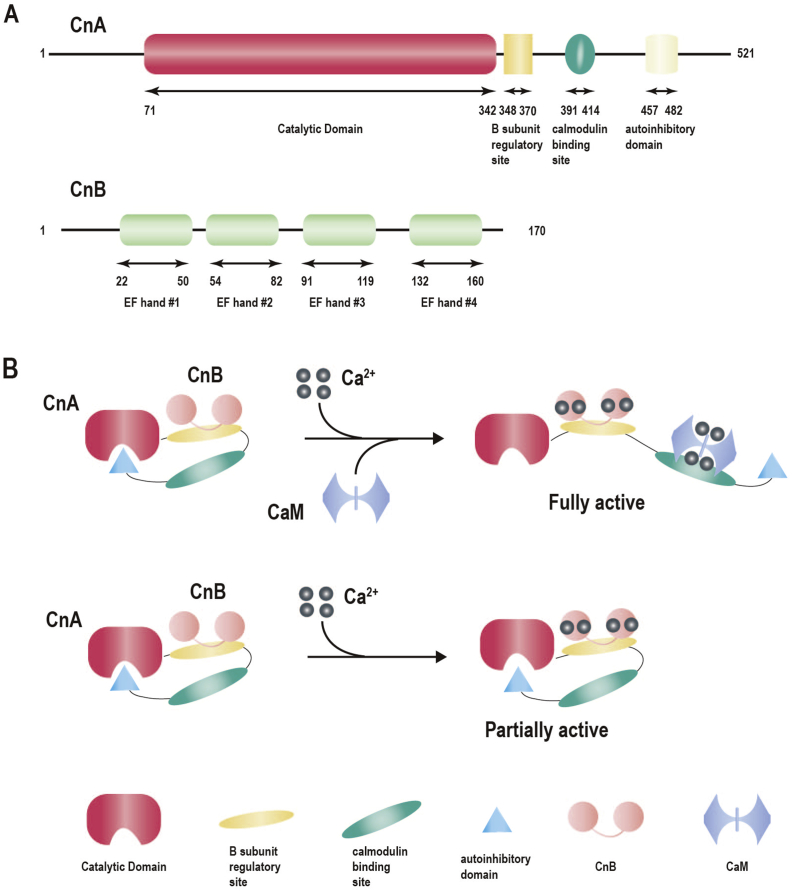
CaN is broadly distributed throughout the cytoplasm of lymphocytes, as well as nerve, cardiac muscle, skeletal muscle, lung, spleen, and liver cells. Its structure is highly conserved from yeast to humans with high homology observed at the nucleotide and amino acid levels.60 CaN is a heterodimer, comprising a 60 kDa catalytic A subunit (CnA) and a 19 kDa regulatory B subunit (CnB). Further, it contains a catalytic domain, B subunit regulatory domain, calmodulin-binding domain, and an autoinhibitory domain3,61,62 (Fig. 1A). The latter three regions together constitute the regulatory region of CnA. Constitutive activation of CnA occurs via removal of its last two regions, which occurs independently of Ca2+ signaling.63 Meanwhile, CnB, the regulatory subunit, is structurally homologous to CaM, both of which are Ca2+ dependent. CnB contains two Ca2+-binding lobes connected by a short linker comprising four Ca2+-binding EF-hand motifs (EF hands), which participate in the activation of CnA61,63 (Fig. 1A). Three isoforms exist for CnA: CnAα (PPP3CA), CnAβ (PPP3CB) and CnAγ (PPP3CC), while CnB exists in two forms (CnB1 and CnB2).63 CnAγ expression is restricted to the testis and the brain, while CnB1 is found only in testis.63,64 All other CaN isoforms are ubiquitously expressed.63 Moreover, CnAα and CnAβ share 81% similarity,65 with the primary difference being the unique repeating proline sequence present in the N-terminus of CnAβ, which is highly associated with substrate recognition. Similarly, CnB1 and CnB2 have 83% sequence homology and appear to exhibit redundant functions.61
Figure 1.
The structure of CaN and mechanism of activation. (A) Structure of calcineurin, with boxes indicating functional domains and lines indicating intervening amino acid sequence. (B) The proposed mechanism of activation of calcineurin.
The native form of CaN is enzymatically inactive with crystal structure analysis revealing blockade of the catalytic center by the autoinhibitory domain. Meanwhile, Ca2+ signal and CaM induce the necessary conformational changes to allow for complete activation of CaN. Specifically, internal and external signals trigger Ca2+ transport across the cell membrane by Ca2+ pumps and subsequent release of Ca2+ from endoplasmic reticulum (ER) stores.66 The increased cellular cytosolic Ca2+ binds CnB, causing dissociation of the calmodulin-binding domain from the B subunit regulatory domain. Thereafter, CaM binds to the calmodulin-binding domain, resulting in displacement of an autoinhibitory domain from the catalytic center, effectively activating the protein3 (Fig. 1B). However, in the absence of CaM, the increased cytosolic Ca2+ binds to the four Ca2+-binding EF-hand motifs of CaN, leading to a partially-activated state (Fig. 1B). Activation of CaN results in the dephosphorylation of substrates responsible for regulating various cellular processes.
Calcineurin and development
CaN signaling is required for a broad spectrum of developmental processes in a variety of organ systems, including immune, kidney, heart, nervous, and musculoskeletal systems, as well as the differentiation of bone, cartilage, muscle, skin, and fat.9,10,34,54 Therefore, inhibition of genes associated with CaN pathway contribute to defects or alterations in kidney maturation, cardiomyocyte maturation, heart valve formation, vascular development, skeletal muscle differentiation and T cell development. In fact, a study found that CnAα–deficient mice exhibited significantly impaired development of T helper type 2 cells (Th2).67 Meanwhile, another study reported defects in nephrogenic zone (NZ) and superficial glomeruli development, altered cell cycle in the NZ, and impaired kidney function, leading to progressive kidney failure and a shortened lifespan in CnAα knockout mice.10,68 Further, CnAβ-deficient mice demonstrated a significant reduction in CD3 positive cells, as well as CD4 and CD8 single positive cells.9 The proliferative capacity of T cells in CnAβ−/−mice as well as IL-2 production was reportedly decreased in response to PMA ionomycin stimulation and T cell receptor cross-linking.9 Moreover, CnB1 mutant embryos do not develop beyond E9.5 and display defects in angiogenesis and axonal outgrowth, as evidenced by the lack of fusion and remodeling of the vascular plexus into larger vessels.69 Nfatc1 knockout-mice exhibited abnormal heart valves resulting in embryonic lethality.70 Still further, Nfatc3 and Nfatc4 deficient embryos displayed cardiovascular abnormalities and heart failure at E10.5 with distinct skeletal muscle defects.54 Meanwhile, pharmacological inhibition of CaN signaling affects embryogenesis. In mice, treatment with CsA from E7.5 to E8.5 resulted in defective vascular remodeling.69 Meanwhile, FK506 treatment contributed to the development of edema, gut coiling disruption, and teratogenesis in the kidney, heart, gut, liver, and somatic tissue during Xenopus development.71 Cumulatively, these results indicate that CaN is a necessary component for normal organ development. However, the impact of CaN signaling in development is far more complex than what we yet known and requires further exploration to elucidate precisely how CaN-NFAT signaling regulates the development of different organs.
Calcineurin and disease
CaN is a multifunctional protein that participates in nearly all aspects of cellular functioning. Specifically, it is actively involved in various biological processes under both physiological and pathological conditions, including T cell activation, cell apoptosis, cell cycling, cell proliferation, cell migration, cell invasion, stem cell generation, as well as cell transformation and fate. As such, CaN dysfunction has been found to contribute to the pathogenies of many common disorders, as summarized in Figure S1. The sheer number of pathological conditions associated with CaN implies its central role in the regulation of cell physiology. Below, we summarize the main findings reporting a link between alterations in CaN and several diseases.
Immune system disorders
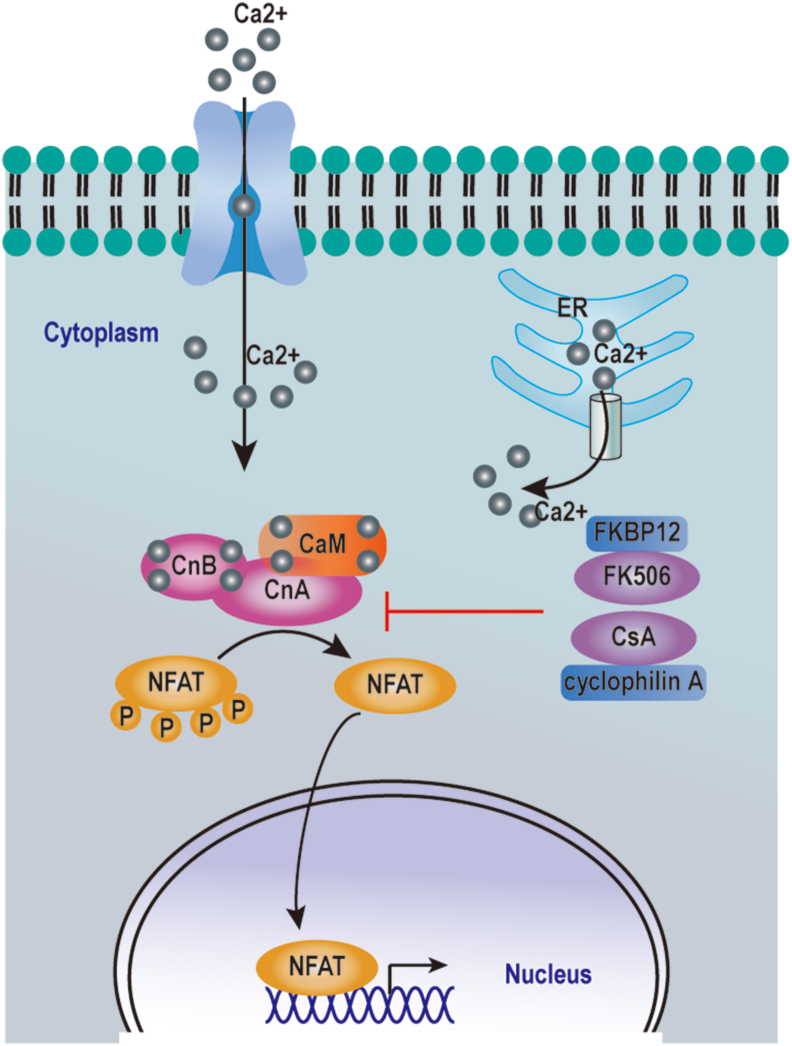
CaN is considered to be a key enzyme in the immune response with its function originally described in T cells. Increasing intracellular Ca2+ concentration in T lymphocytes leads to CaM binding with CaN, which then dephosphorylates NFAT1, NFAT2, NFAT3, and NFAT4, leading to their nuclear translocation. Activation of NFAT upregulates cytokines responsible for T cell activation, including IL-2, IL-4, IL-17, interferon-γ (IFNγ), and tumor necrosis factor-α (TNF-α)3,72 (Fig. 2). Meanwhile, accumulation of activated T cells in host tissues and organs leads to initiation of the inflammatory process, inducing the onset and progression of chronic inflammatory disease and autoimmune diseases.
Figure 2.
The CaN-NFAT pathway in T cells. Intracellular and extracellular signals trigger an initial cytoplasmic Ca2+ increase. Elevated cytoplasmic Ca2+ activates CaN, which dephosphorylates the NFAT transcription factors, causing NFATs to translocate into the nucleus and initiate gene transcription. CsA and FK506 inhibit the activity of CaN through their interaction with the immunophilins called cyclophilin A and FK-binding protein 12 (FKBP12) respectively.
Autoimmune diseases are characterized by T cell activation and an increase in interleukin turnover, while the Ca2+-CaN-NFAT pathway is reportedly dysregulated in autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Indeed expression of CaN has been discovered in monocytes/macrophages and vascular endothelial cells in RA synovia, in which it promotes the expression of IL-2, IL-17, and TNF-α, thereby contributing to the development of chronic inflammation.13 Moreover, Ca2+ influx and nuclear NFAT levels are abnormally increased in activated T cells of SLE patients.73 This aberrant activation of the Ca2+-CaN-NFAT pathway upregulates CD40 ligand expression, which subsequently induces antibody production and dendritic cell (DC) activation in SLE patients.74 Meanwhile, inhibition of the CaN-NFAT pathway suppresses production of inflammatory cytokines and co-stimulatory molecules by T cells. Hence, CaN-NFAT signaling is believed to represent an attractive target for therapeutic approaches to control autoimmune responses. CNIs, including CsA and FK506, inhibit CaN activity through their interaction with immunophilins, namely cyclophilin A, and FK-binding protein 12 (FKBP12), respectively4 (Fig. 2), and are widely administered for the treatment of autoimmune diseases. In fact, CNIs effectively improve patient's clinical symptoms and elevate survival rates. However, the use of CsA and FK506 is associated with certain adverse effects, including hypertension, nephrotoxicity, neurotoxicity and metabolic disorders.4,75 It is, therefore, necessary to conduct further research to identify novel CNIs capable of not only inhibiting CaN activity but also doing so without eliciting serious adverse effects.
While CaN has long been considered a unique regulator of T-cell activity,36 it is also expressed in other immune cells, including B cells,76,77 macrophages,78,79 and DCs.80,81 The CaN-NFAT pathway in myeloid cells participates in mounting immune responses to bacteria,82, 83, 84 fungi,85 and viruses.86 The molecular mechanism underpinning activation of the CaN-NFAT pathway involves ligand binding of multiple different pattern recognition receptors (PRRs), such as TLRs and C-type lectin receptors, which subsequently activate spleen tyrosine kinase (Syk)/phospholipase (PLC). This signaling cascade promotes an increase in the Ca2+ concentration in monocytes, macrophages, and DCs, triggering CaN-NFAT-IL2 signaling upon recognition of complex particulate antigens.36 In fact, a recent study showed that CR3 engagement with Mycobacterium leprae (pathogen responsible for leprosy) activates Syk, inducing CaN-dependent nuclear translocation of the transcription factor NFAT, which selectively augmented the production of IL-2, IL-10, and IL-1β.84 Meanwhile, the addition of CsA significantly reduced the levels of these cytokines. Furthermore, human DCs treated with CsA exhibited reduced IFN-γ responses to Sendai virus infection.87 Similarly, when bone marrow-derived dendritic cells (BMDCs), depleted of CaN were stimulated with Aspergillus fumigatus, they exhibited reduced expression of Ptx3, a molecule of particular importance in antifungal activity.88 Furthermore, the Ca2+-CaN-NFAT-IL-2 pathway in DCs modulates Th17 cell expansion in response to Aspergillus-germinated particles; whereas conditional knockout of IL-2 in CD11c+ cells impairs fungus recognition, represses phagocytosis, and disrupts Th17 responses to live conidia.80 These results indicate that CaN inhibition increases the risk of associated with a myriad of infections. In accordance with these experimental results, transplant patients treated with CNIs exhibited an increased risk of bacterial and fungal infections. Therefore, novel specific CaN inhibitors are required for transplant patients capable of reducing the risk of infection.
Kidney disease
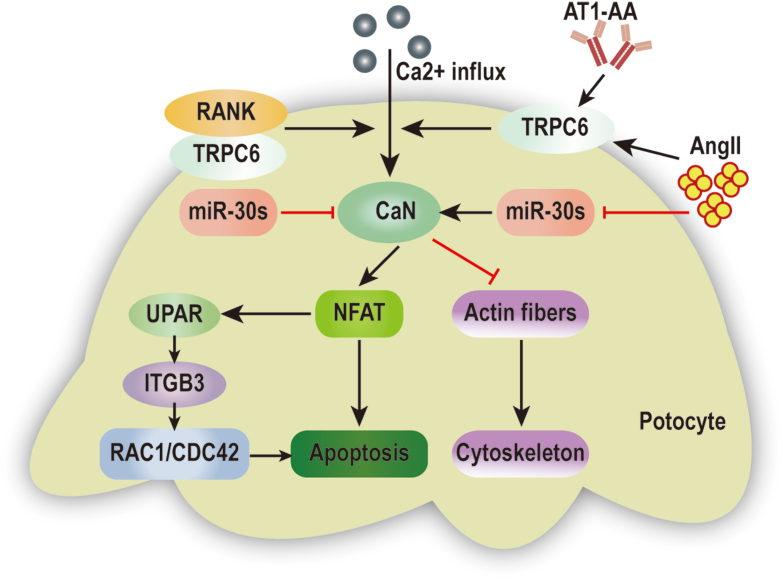
CaN is also an important element in the pathogenesis of glomerular hypertrophy, injury, and sclerosis. Specifically, Gooch and colleagues observed an increase in CaN expression in the glomeruli, and activation of CaN in the renal cortex of rats following induction of diabetes by hyperglycemia.89 Meanwhile, inhibition of CaN with CsA reduces whole kidney hypertrophy and completely blocks both glomerular hypertrophy and extracellular matrix (ECM) accumulation,90 suggesting that CaN mediates the latter symptoms in diabetic nephropathy in vivo. Compelling evidence has also shown that podocyte injury is a common and typical feature of glomerular injury and sclerosis, and is an initiating factor of the pathogenic process. The disruption of podocyte actin fibers, cytoskeletal damage, and podocyte apoptosis contributes to foot process effacement, glomerular filtration barrier damage, and proteinuria, which ultimately leads to kidney dysfunction.91 Many studies have been performed to investigate how the activation of Ca2+/CaN signaling induces podocyte loss and cytoskeletal damage. Their results suggest that angiotensin II (AngII), or angiotensin II type I receptor agonistic autoantibody (AT1-AA), enhance the expression of transient receptor potential channel 6 (TRPC6), which stimulates Ca2+/CaN signaling, ultimately leading to actin fiber damage and podocyte injury via several pathways, as shown in Figure 3.92,93 One mechanism involves the binding of receptor activator of NF-κB (RANK) to TRPC6, which promotes podocyte loss and impairs glomerular function by stimulating CaN activity and increasing nuclear NFAT accumulation.94 AngII also downregulates microRNA-30 (miR-30) family members, resulting in CaN activation, and facilitating dephosphorylation and degradation of the actin-binding protein, synaptopodin (SYNPO), which induces cytoskeletal injury and apoptosis in podocytes.95,96 Moreover, a recent study revealed that miR-30 deficiency leads to CaN-NFAT signaling activation, which in turn activates the urokinase plasminogen activator receptor-integrin β3 (uPAR-ITGB3) pathway, ultimately altering Rac family small GTPase 1 (RAC1) and cell division cycle 42 (CDC42) activity, and inducing podocyte injury91 (Fig. 3).
Figure 3.
Signal molecules regulating CaN activity to mediate podocyte injury. (1) AT1-AA induces podocyte injury via activation of the TRPC6- Ca2+/CaN pathway. (2) Ang II contributes to podocyte injury by increasing TRPC6 expression, which activates CaN/NFAT signaling. (3) Ang II induces Ca2+/CaN signaling and podocyte injury by downregulating miR-30 family members. (4) RANK promotes podocyte injury by activating Ca2+/CaN/NFAT signaling. (5) MiR-30 family members inhibit uPAR-ITGB3 signaling activation through the CaN/NFAT pathway.
Cardiomyopathy
Cardiac hypertrophy (CH) occurs in a number of disease states in response to increased cardiac workload and can readily progress to ventricular dilatation, contractile dysfunction, and heart failure. As such, many studies have focused on the molecular mechanisms of cardiac myocyte hypertrophy and have found that myocyte hypertrophy is activated by multiple intracellular signaling pathways including Ca2+-dependent signalings.97 Upon exposure to hypertrophic stimuli, the increase in intracellular Ca2+ activates CaN signaling pathways which promotes activation of hypertrophic gene transcription and subsequently induces the onset and development of pathological hypertrophy. As a critical mediator of CH, CaN is involved in the development of cardiac hypertrophy via regulation of downstream targets including NFAT, myocyte enhancer factor2 (MEF2), GATA binding protein4 (GATA4), nuclear factor-κ-gene binding (NF-κB), and Drp1, as well as interaction with other pathways such as protein kinase C (PKC), mitogen-activated protein kinase (MAPK), calmodulin-dependent protein kinase II (CaMKII), phosphatidylinositol 3-kinase (PI3-K), and Wnt pathways.56,97,98 Moreover, enhanced CaN activity may positively correlate with CH. For instance, a study reported an increase in serum CaN activity in hypertensive and hypertrophic patients.99 Moreover, significantly increased concentration of intracellular Ca2+ was found to enhance CaN and NFATc4 expression in cardiomyocyte hypertrophy.100 Additionally, transgenic mice expressing activated forms of CaN, or downstream targets exhibit CH and heart failure. Specifically, constitutive activation of CnA was reported to induce strong CH resulting in heart failure within the first weeks of life. This result is consistent with previous reports showing enhanced CH in response to overexpression of a constitutively active form of NFAT.101 In contrast, genetic or pharmacological inhibition of CaN signaling significantly attenuates the pathogenesis of CH and dysfunction in response to various stresses.100 Indeed, a recent study demonstrated that specific deletion of CnB1 in cardiomyocyte reduces AngII-induced increases in ventricular mass, cardiomyocyte cross-sectional area, and left ventricular wall thickness, preventing AngII-induced CH.12 Previous reports also found that constitutive deficiency of the catalytic subunit CnAβ, or NFATc3 or NFATc2 diminish AngII-mediated heart weight gain.12 Similarly, overexpression of the endogenous CaN inhibitors (regulator of calcineurin 1, carabin) represses cardiac growth and attenuates heart function.102
Neurodegenerative disease
Expression of CaN is particularly high in neurons, accounting for 1% of total neural protein, in which it is localized in the cytosol, presynaptic and postsynaptic terminals. Besides, CaN is also expressed in astrocytes and microglia.45 It has been reported that CaN plays a critical role in the maintenance and plasticity of spines, as well as the acquisition of learning, memory, and long-term potentiation (LTP), among other functions.103 Alterations in Ca2+ homeostasis are related to the accumulation of misfolded protein aggregates in different neurodegenerative disorders, including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), transmissible spongiform encephalopathies (TSEs), and amyotrophic lateral sclerosis (ALS).44,104 Meanwhile, accumulation of misfolded/unfolded aggregated proteins leads to sustained ER stress, which dysregulates Ca2+ homeostasis in various neurodegenerative diseases and results in activation of CaN.105 Aberrantly activated CaN is increasingly linked to a variety of pathologic features associated with neurodegenerative disorders, including synaptic dysfunction and loss, neuroinflammation, and neuronal cell death.106,107
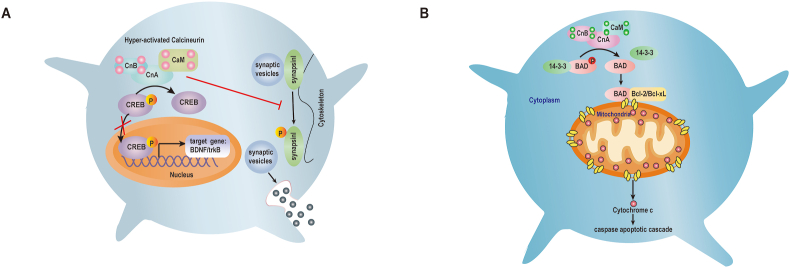
The outcomes associated with aberrant Ca2+ dynamics particularly impairs neurotransmission in synaptic spines and hyperactivation of CaN. Both the release and uptake of neurotransmitters including synaptobrevin, synapsin, rabphilin2A, synaptotagmin, and dephosphins via exocytosis and endocytosis rely on CaN activity.108 Hence, CaN is an important regulator of synaptic transmission in both the pre- and post-synaptic compartments. In presynaptic terminals, hyperactivated CaN dephosphorylates synapsin I, a phosphoprotein that tethers neurotransmitter-containing vesicles to the cytoskeleton109 (Fig. 4A). Once phosphorylated, synapsin I detaches from the vesicles, which become exocytosed from the cell, thereby releasing neurotransmitters into the synapse.110 Meanwhile, in postsynaptic terminals, CaN has been shown to dephosphorylate and thus inactivate, the N-methyl-d-aspartate receptor (NMDA-R), thereby reducing the amount of time that the ion channel remains open.111,112 CaN has also been reported to enhance or prolong the desensitization period of other ligand-gated channels, including γ-aminobutyric acid type A receptors (GABAARs)113 and serotonin.114 Besides, it was observed that CnB1-deficiency in mice slows synaptic vesicle secretion in the hippocampal glutamatergic synapses, which may be related to Ca2+ entry via neurotransmitter release triggering N-type Ca2+ channels enhanced by CaN.115
Figure 4.
Role of activated CaN in synaptic dysfunction and neuronal death. (A) (1) Activated CaN dephosphorylates CREB, inhibiting its translocation to the nucleus and reducing CREB target gene expression required for neuronal growth and synaptic plasticity. (2) Activated CaN dephosphorylates synapsin I, inhibiting neurotransmitter release by abrogating synaptic vesicle transport. (B) CaN dephosphorylates BAD, which dissociates from scaffolding proteins and forms a dimer with Bcl-2 or Bcl-xL, leading to the release of cytochrome c and the apoptotic cascade that results in neuronal cell death.
Changes in Ca2+ regulation during neurodegenerative disease also leads to inhibition of LTP, synaptic dysfunction and memory loss through disruption of CaN signaling cascades. Meanwhile, hyperactivated CaN-mediated endocytosis of the a-amino-3-hydroxy-5- methyl-4-isoxazole propionic acid (AMPA) receptors is associated with Aβ oligomer-induced synaptic dysfunction and memory loss related to LTP repression.116 Furthermore, CaN activates protein phosphatases 1 (PP1) which functions to phosphatase AMPA receptors.117 However, activation of CaN inhibits the activity of NMDA receptors and the enhanced AMPA receptor internalization.118 Meanwhile, CaN is also involved in regulating synaptic plasticity by controlling the expression of target genes via cAMP response element-binding (CREB), which is a major target of CaN.119 Under normal conditions, CREB becomes activated by phosphorylation and nuclear translocation. Phosphorylated CREB (pCREB) then modulates the expression of target genes necessary for neuronal growth and survival, including brain-derived neurotrophic factor (BDNF) and its receptor, tropomyosin-related kinase B (trkB)120, 121, 122 (Fig. 4A). However, hyperactivated CaN induces the dephosphorylation and inactivation of CREB, thereby inhibiting CREB-target gene expression, and causing synaptic dysfunction and memory loss.119 CaN also interacts with and activates PP1, thereby indirectly promoting PP1-dependent CREB dephosphorylation.46 Interestingly, a study showed that pCREB levels are significantly reduced in the hippocampi of AD patients,123 while another group reported that hippocampal pCREB immunoreactivity was decreased in the Tg2576 murine model of AD, however, was restored following FK506 treatment.124 Taken together, these data suggest that aberrant CaN activity promotes synaptic dysfunction, highlighting the importance of regulating CaN activity at an appropriate level.
Many forms of injury or disease in the central nervous system (CNS) activate astrocytes and microglia. Astrocytes form a critical part of the neurovascular unit, which support and maintain an appropriate neuronal environment. Activated astrocytes secrete numerous pro-inflammatory cytokines and other factors involved in neuroinflammation45 and therefore, serve as a hallmark of this process. Meanwhile, CaN plays a critical role in the neuroinflammatory signaling inherent to astrocytes during neural damage and dysfunction.45,112 Although it is only weakly expressed in the astrocytes of healthy adult neural tissue, CaN is strongly expressed in activated astrocytes during aging, injury, and/or disease.45,125 For example, Christopher and colleagues observed that intense CaN expression is localized in activated astrocytes surrounding amyloid plaques in an AD murine model.45 Moreover, in vitro, amyloid β-protein (Aβ) stimulates CaN activation in primary rat astrocyte cultures.126 In addition, CaN expression is upregulated in astrocytes from gerbil hippocampi subjected to bilateral carotid artery occlusion.45 Similarly, numerous CN-positive astrocytes are present in human hippocampi during the very early stages of cognitive decline. Once activated in astrocytes, CaN dephosphorylates NFAT, which translocates from the cytoplasm to the nucleus. In the nucleus, NFAT interacts with distinct DNA binding elements to drive the expression of numerous immune/inflammatory factors.45,127 A study by Hafiz and colleagues shows that nuclear NFAT1 levels in astrocytes increase in patients with mild cognitive impairment and nuclear localization of NFAT3 in astrocytes is apparent in those with AD.127 CN also interacts with NFκB, peroxisome proliferator-activated receptor γ (PPARγ), and/or forkhead box O3 (FOXO3) transcription factors to differentially affect neuroinflammation in astrocytes.45 These results suggest that CaN signaling in astrocytes is involved in the neuroinflammatory processes that lead to injury, disease, and aging.
Over-activated CaN has also been implicated in reversible neuronal apoptosis. Hyperactivated CaN dephosphorylates Bcl2-associated death protein (BAD), which is normally bound to the 14-3-3 protein in the cytosol and which is phosphorylated on certain serine residues.128 Following dephosphorylation of BAD by CaN, BAD dissociates from its scaffolding proteins and translocates from the cytosol to the mitochondria. In the mitochondrial outer cell membrane, BAD forms a dimer with another pro-apoptotic protein, Bcl-2/Bcl-xL, triggering the release of cytochrome c, which contributes to the activation of the post mitochondrial caspase apoptotic cascade46,129 (Fig. 4B). Recently, a study reported that prion protein increases CaN activity, resulting in decreased AMPK phosphorylation at threonine residue 172 and increased autophagy activation, which induces neuronal cell death; wherease FK506 may prevent this effect.107 Taken together, inhibition of CaN may represent a novel therapeutic approach for preventing neurodegenerative diseases.
Cancer
In recent years, emerging evidence has shown that CaN may play an important role in the development and progression of human cancers.130 Indeed, activation of CaN and its downstream targets has been defined as having oncogenic potential in colorectal, breast, prostate, ovarian, pancreatic, and liver cancers, as well as glioblastoma, lung cancer, and leukemia.77,131, 132, 133, 134, 135, 136, 137, 138 Specifically, activated CaN reportedly regulates cancer stem cell survival and proliferation, cell migration, invasion, and metastasis in response to hypoxic conditions, inflammation, and vascular endothelial growth factor signaling.
The molecular mechanisms mediating these effects are based on the ability of CaN to dephosphorylate and activate NFAT and other target genes. Importantly, NFAT is constitutively activated or overexpressed in numerous cancers and can contribute to cancer development and progression.130 Meanwhile, the CaN/NFATc1 signaling pathway is critically involved in the pathogenesis of solid tumors. Recently, nuclear NFATc1 was identified in human colon cancer specimens (stage II and stage III), as well as in human colon cancer cell lines, while being described as being strongly associated with poor survival rates. Studies have also shown that this transcription factor could promote migration capacity of colorectal cancer cells (CRC) via modulating runt-related transcription factor 2 (RUNX2) and gelsolin (GSN).52 Meanwhile, nuclear NFATc1 was detected in 50.6% of triple negative breast tumors, in 69.5% of pancreatic carcinomas and activated in hepatocellular carcinoma cells.133,139 NFATc1 enhances proliferation and migration of breast cancer, pancreatic cancer, and hepatocellular carcinoma cells through regulating certain oncogenes such as c-myc. Loss-of-function studies show that NFATc1 silencing in 4T1cells (breast cancer cells) inhibits their migratoty and proliferative capacity. Besides, treatment with CsA in pancreatic carcinoma cell lines inhibits the nuclear localization of transcriptionally active NFATc1 and cell cycle progression. In addition, Xu and colleagues found that NFATc1 is significantly higher in ovarian cancer tissues than in paired normal control tissues and activates the extracellular regulated protein kinases1/2 (ERK1/2)/p38/MAPK signal pathway,140 which leads to promotion of cell growth and tumorigenesis. Meanwhile, the involvement of CaN and NFATc1 has also been reported in hematologic malignancies. In fact, nuclear localization of NFATc1 was detectable in 72% of Burkitt's lymphoma (BL) cases and 28% of diffuse large B cell lymphoma (DLBCL) cases.141 Nuclear accumulation of NFATc1 was also discovered in aggressive T cell lymphoma.141
Additional CaN targets include cyclin D1, glycogen synthase kinase-3b (GSK-3b), nuclear factor I (NFI), kinase suppressor of ras 2 (KSR2), and c-Jun, which have all been shown to have pro-tumorigenic roles.137,142, 143, 144, 145 For example, CaN dephosphorylates cyclin D1 at residue T286, inhibiting its degradation, thereby facilitating cell cycle progression and robust cell growth in invasive breast cancer cells.142 Similarly, CaN enhances transcription of nuclear factor I (NFI) via dephosphorylation, altering the migratory properties of malignant glioma (MG) cells.137 Additionally, CaN stabilizes c-Jun by dephosphorylating it at Ser-243 to enhance its tumorigenic ability in COS-7 cells.145
Activation of CaN and its dephosphorylated substrates regulate various genes critical for proliferation, apoptosis, migration, and survival in both solid tumors and lymphoid malignancies. Hence, efficient inhibition of CaN and its critical effectors, may be useful as a therapeutic strategy for cancer. In fact, a report showed that pancreatic cancer cells treated with CsA or FK506 exhibited a dramatic time- and dose-dependent reduction in proliferation.139 Moreover, FK506 treatment of a breast cancer mouse model decreased tumor growth and angiogenesis in vivo and reduced migration of breast cancer cells in vitro.146 Both CsA and FK506 treatment markedly increase the number of apoptotic cells in lymphoma and leukemia cell lines, and induces regression of T-cell acute lymphoblastic leukemia (T-ALL) in mice, prolonging their survival.147 It appears that combination therapy comprising CNIs and other anti-cancer drugs may represent a promising approach for targeted treatment. A recent study revealed that when non-small cell lung cancer (NSCLC) cells were treated with crizotinib and CsA, apoptosis was promoted, and G2/M arrest was induced compared with crizotinib-only treatment.148 In contrast, CsA and FK506 increased the risk of cancers in organ-transplant patients due to suppression of tumor immunosurveillance mechanisms.49 For example, CsA treatment promotes the rapid growth and survival of renal cancer cells via activating Ras and inducing the expression of cytoprotective molecule heme oxygenase-1 (HO-1).149 CsA increases expression of activating transcription factor 3 (ATF3) which enhances keratinocyte tumor formation and suppresses cancer cell senescence.150 Therefore, further investigations are required to explore the downstream effectors of activated CaN in different malignancies to develop more specific inhibitors targeting these effectors for the treatment of cancers, which might overcome limitations linked to direct inhibition of CaN activity.
Concluding remarks and future perspectives
CaN plays a central role in a number of physiological and pathological processes, owing to its ability to control a network of transcriptional regulators coupled to posttranscriptional and posttranslational modifications that amplify the initial signals. CaN is a versatile protein able to modulate several fundamental pathways within the cell including activation, apoptosis, cycle, proliferation, migration, and invasion, as well as stem cell generation, transformation and fate. Inhibiting CaN activity may serve as a promising therapeutic strategy for several diseases. CNIs such as CsA and FK506 have been tested as therapeutic drugs in mouse models of disease and are currently used in clinical settings.124 For example, CsA and FK506 have been widely used to prevent organ rejection in transplant patients, as well as for the treatment of aggressive forms of RA and SLE.4,13 However, their use is associated with certain side effects, including hypertension, nephrotoxicity, neurotoxicity and metabolic disorders.4 It is therefore important that further research be conducted to investigate newly discovered molecular mechanisms of CaN activity and regulation, to enable more specific targeting of signaling pathways downstream of CaN activation. Specifically, new inhibitors should be assessed for their ability to interact with specific substrates or sets of substrates to inhibit CaN signal transmission. Thus, more efficient therapies may be designed to prevent excessive intracellular Ca2+ and correctly regulate CaN signaling. Importantly, drugs designed to affect CaN function can be applied to many prevalent diseases and pathological processes, with important social, medical and economic impacts.
Author contributions
Writing and original draft preparation: Lei Chen; review and editing: Chunyan Yao; Min Song. All authors have read and agreed to the published version of the manuscript.
Conflict of interests
The authors declare no conflict of interests.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2021.03.002.
Contributor Information
Lei Chen, Email: chenleisxk@gmail.com.
Min Song, Email: songmin85@163.com.
Chunyan Yao, Email: yaochunyan@tmmu.edu.cn.
Abbreviations
- CaN
calcineurin
- Ca2+
calcium
- CaM
calmodulin
- NFAT
nuclear factor of activated T-cells
- IL-2
interleukin 2
- IL-4
interleukin 4
- Drp1
dynamin-related protein 1
- NHE1
Na+/H+-exchanger 1
- TRESK
TWIK-related spinal cord K+ channel
- CRZ1
calcineurin response zinc finger
- KSR2
kinase suppressor of ras 2
- CNIs
CaN inhibitors
- CsA
cyclosporine A
- FK506
tacrolimus
- CnA
catalytic A subunit
- CnB
regulatory B subunit
- EF hands
EF-hand motifs
- ER
endoplasmic reticulum
- Th2
T helper type 2
- NZ
nephrogenic zone
- IL-17
interleukin 17
- IFNγ
interferon-γ
- TNF-α
tumor necrosis factor-α
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- DC
dendritic cell
- FKBP12
FK-binding protein 12
- DCs
dendritic cells
- PRRs
pattern recognition receptors
- CLR
C-type lectin receptors
- Syk
spleen tyrosine kinase
- PLC
phospholipase
- BMDCs
bone marrow-derived dendritic cells
- COX-2
cyclooxygenase-2
- ECM
extracellular matrix
- AngII
angiotensin II
- AT1-AA
angiotensin II type I receptor agonistic autoantibody
- TRPC6
transient receptor potential channel 6
- RANK
receptor activator of NF-κB
- SYNPO
synaptopodin
- uPAR-ITGB3
urokinase plasminogen activator receptor-integrin β3
- RAC1
Rac family small GTPase 1
- CDC42
cell division cycle 42
- CH
cardiac hypertrophy
- MEF2
myocyte enhancer factor2
- GATA4
GATA binding protein4
- NF-κB
nuclear factor-κ-gene binding
- PKC
protein kinase C
- MAPK
mitogen-activated protein kinases
- CaMKII
calmodulin-dependent protein kinase II
- PI3–K
phosphatidylinositol 3-kinase
- RCAN1
regulator of calcineurin 1
- LTP
long-term potentiation
- AD
Alzheimer's disease
- PD
Parkinson's disease
- HD
Huntington's disease
- TSEs
transmissible spongiform encephalopathies
- ALS
amyotrophic lateral sclerosis
- NMDA-R
N-methyl-d-aspartate receptor
- GABAARs
γ-aminobutyric acid type A receptors
- CREB
cAMP response element-binding
- AMPA
amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- pCREB
phosphorylated CREB
- BDNF
brain-derived neurotrophic factor
- trkB
tropomyosin-related kinase B
- CNS
central nervous system
- Aβ
amyloid β-protein
- MCI
mild cognitive impairment
- PPARγ
peroxisome proliferator-activated receptor γ
- FOXO3
forkhead box O3
- BAD
Bcl2-associated death protein
- CRC
colorectal cancer cells
- GSN
gelsolin
- ERK1/2
extracellular regulated protein kinases1/2
- BL
Burkitt's lymphoma
- DLBCL
diffuse large B cell lymphoma
- GSK-3b
glycogen synthase kinase-3b
- NFI
nuclear factor I
- KSR2
kinase suppressor of ras 2
- MG
malignant glioma
- T-ALL
T-cell acute lymphoblastic leukemia
- NSCLC
non-small cell lung cancer
- SCC
skin squamous cell carcinoma
- HO-1
heme oxygenase-1
- ATF3
activating transcription factor 3
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Calcineurin dysfunctions result in many common disorders.
References
- 1.Klee C.B., Ren H., Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273(22):13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 2.Hogan P.G., Li H. Calcineurin. Curr Biol. 2005;15(12):R442–R443. doi: 10.1016/j.cub.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Li H., Rao A., Hogan P.G. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21(2):91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy J., Cyert M.S. Identifying new substrates and functions for an old enzyme: calcineurin. Cold Spring Harb Perspect Biol. 2020;12(3):a035436. doi: 10.1101/cshperspect.a035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X., Jia L., Yu W., Du H. Dephosphorylation by calcineurin regulates translocation of dynamin-related protein 1 to mitochondria in hepatic ischemia reperfusion induced hippocampus injury in young mice. Brain Res. 2019;1711:68–76. doi: 10.1016/j.brainres.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Hendus-Altenburger R., Wang X., Sjøgaard-Frich L.M., et al. Molecular basis for the binding and selective dephosphorylation of Na+/H+ exchanger 1 by calcineurin. Nat Commun. 2019;10(1):3489. doi: 10.1038/s41467-019-11391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czirják G., Tóth Z.E., Enyedi P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J Biol Chem. 2004;279(18):18550–18558. doi: 10.1074/jbc.M312229200. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty M.K., Ritt D.A., Zhou M., et al. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol Cell. 2009;34(6):652–662. doi: 10.1016/j.molcel.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bueno O.F., Brandt E.B., Rothenberg M.E., Molkentin J.D. Defective T cell development and function in calcineurin A beta -deficient mice. Proc Natl Acad Sci U S A. 2002;99:9398–9403. doi: 10.1073/pnas.152665399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooch J.L., Toro J.J., Guler R.L., Barnes J.L. Calcineurin A-alpha but not A-beta is required for normal kidney development and function. Am J Pathol. 2004;165(5):1755–1765. doi: 10.1016/s0002-9440(10)63430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norris C.M. Calcineurin: directing the damage in Alzheimer disease: an Editorial for 'Neuronal calcineurin transcriptional targets parallel changes observed in Alzheimer disease brain' on page 24. J Neurochem. 2018;147(1):8–11. doi: 10.1111/jnc.14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Martinez S., Lozano-Vidal N., Lopez-Maderuelo M.D., Jiménez-Borreguero L.J., Armesilla Á.L., Redondo J.M. Cardiomyocyte calcineurin is required for the onset and progression of cardiac hypertrophy and fibrosis in adult mice. FEBS J. 2019;286(1):46–65. doi: 10.1111/febs.14718. [DOI] [PubMed] [Google Scholar]
- 13.Park Y.J., Yoo S.A., Kim M., Kim W.U. The role of calcium-calcineurin-NFAT signaling pathway in health and autoimmune diseases. Front Immunol. 2020;11:195. doi: 10.3389/fimmu.2020.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu R., Tajima S., Suetsugu K., Watanabe H., Egashira N., Masuda S. Biomarkers for individualized dosage adjustments in immunosuppressive therapy using calcineurin inhibitors after organ transplantation. Acta Pharmacol Sin. 2019;40(2):151–159. doi: 10.1038/s41401-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klee C.B., Draetta G.F., Hubbard M.J. Calcineurin. Adv Enzymol Relat Areas Mol Bio. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- 16.Goto S., Yamamoto H., Hamasaki T., et al. Calcineurin: structure and regulation of the cellular functions. Tanpakushitsu kakusan koso. Protein, nucleic acid, enzyme. 1998;43(12 Suppl):1706–1714. [PubMed] [Google Scholar]
- 17.Hemenway C.S., Heitman J. Calcineurin. Structure, function, and inhibition. Cell Biochem Biophys. 1999;30(1):115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- 18.Aramburu J., Rao A., Klee C.B. Calcineurin: from structure to function. Curr Top Cell Regul. 2000;36:237–295. doi: 10.1016/s0070-2137(01)80011-x. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree G.R. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276(4):2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- 20.Ke H., Huai Q. Structures of calcineurin and its complexes with immunophilins-immunosuppressants. Biochem Biophys Res Commun. 2003;311(4):1095–1102. doi: 10.1016/s0006-291x(03)01537-7. [DOI] [PubMed] [Google Scholar]
- 21.Smith H.S. Calcineurin as a nociceptor modulator. Pain Physician. 2009;12(4):E309–E318. [PubMed] [Google Scholar]
- 22.Musson R.E., Smit N.P. Regulatory mechanisms of calcineurin phosphatase activity. Curr Med Chem. 2011;18(2):301–315. doi: 10.2174/092986711794088407. [DOI] [PubMed] [Google Scholar]
- 23.Hogan P.G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17(8):2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 24.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 25.Amasaki Y. [Calcineurin inhibitors and calcineurin-NFAT system] Nihon Rinsho Meneki Gakkai Kaishi. 2010;33(5):249–261. doi: 10.2177/jsci.33.249. [DOI] [PubMed] [Google Scholar]
- 26.Stoddard B.L., Flick K.E. Calcineurin-immunosuppressor complexes. Curr Opin Struct Biol. 1996;6(6):770–775. doi: 10.1016/s0959-440x(96)80006-6. [DOI] [PubMed] [Google Scholar]
- 27.Halloran P.F., Kung L., Noujaim J. Calcineurin and the biological effect of cyclosporine and tacrolimus. Transplant Proc. 1998;30(5):2167–2170. doi: 10.1016/s0041-1345(98)00577-6. [DOI] [PubMed] [Google Scholar]
- 28.Dumont F.J. FK506, an immunosuppressant targeting calcineurin function. Curr Med Chem. 2000;7(7):731–748. doi: 10.2174/0929867003374723. [DOI] [PubMed] [Google Scholar]
- 29.Hamawy M.M. Molecular actions of calcineurin inhibitors. Drug News Perspect. 2003;16(5):277–282. doi: 10.1358/dnp.2003.16.5.829315. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Martínez S., Redondo J.M. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem. 2004;11(8):997–1007. doi: 10.2174/0929867043455576. [DOI] [PubMed] [Google Scholar]
- 31.Luger T., Paul C. Potential new indications of topical calcineurin inhibitors. Dermatology. 2007;215(Suppl 1):45–54. doi: 10.1159/000102119. [DOI] [PubMed] [Google Scholar]
- 32.Chakkera H.A., Kudva Y., Kaplan B. Calcineurin inhibitors: pharmacologic mechanisms impacting both insulin resistance and insulin secretion leading to glucose dysregulation and diabetes mellitus. Clin Pharmacol Ther. 2017;101(1):114–120. doi: 10.1002/cpt.546. [DOI] [PubMed] [Google Scholar]
- 33.Stankunas K., Graef I.A., Neilson J.R., Park S.H., Crabtree G.R. Signaling through calcium, calcineurin, and NF-AT in lymphocyte activation and development. Cold Spring Harbor Symp Quant Biol. 1999;64:505–516. doi: 10.1101/sqb.1999.64.505. [DOI] [PubMed] [Google Scholar]
- 34.Aramburu J., Heitman J., Crabtree G.R. Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep. 2004;5(4):343–348. doi: 10.1038/sj.embor.7400133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandewalle A., Tourneur E., Bens M., Chassin C., Werts C. Calcineurin/NFAT signaling and innate host defence: a role for NOD1-mediated phagocytic functions. Cell Commun Signal. 2014;12:8. doi: 10.1186/1478-811X-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bendickova K., Tidu F., Fric J. Calcineurin-NFAT signalling in myeloid leucocytes: new prospects and pitfalls in immunosuppressive therapy. EMBO Mol Med. 2017;9(8):990–999. doi: 10.15252/emmm.201707698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogan P.G. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium. 2017;63:66–69. doi: 10.1016/j.ceca.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaeth M., Feske S. NFAT control of immune function: New Frontiers for an Abiding Trooper. F1000Research. 2018;7:260. doi: 10.12688/f1000research.13426.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J.U., Kim L.K., Choi J.M. Revisiting the concept of targeting NFAT to control T cell immunity and autoimmune diseases. Front Immunol. 2018;9:2747. doi: 10.3389/fimmu.2018.02747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peleg Y., Bomback A.S., Radhakrishnan J. The evolving role of calcineurin inhibitors in treating lupus nephritis. Clin J Am Soc Nephrol. 2020;15(7):1066–1072. doi: 10.2215/CJN.13761119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui H., Lu Y.F., Moriwaki A. Physiological role of calcineurin in central nervous system. Tanpakushitsu kakusan koso. 1998;43(8 Suppl):1039–1046. [PubMed] [Google Scholar]
- 42.Kato K. The role of calcineurin on the induction of synaptic plasticity. Nihon shinkei seishin yakurigaku zasshi. 2000;20:189–198. [PubMed] [Google Scholar]
- 43.Groth R.D., Dunbar R.L., Mermelstein P.G. Calcineurin regulation of neuronal plasticity. Biochem Biophys Res Commun. 2003;311(4):1159–1171. doi: 10.1016/j.bbrc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee A., Soto C. Role of calcineurin in neurodegeneration produced by misfolded proteins and endoplasmic reticulum stress. Curr Opin Cell Biol. 2011;23(2):223–230. doi: 10.1016/j.ceb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furman J.L., Norris C.M. Calcineurin and glial signaling: neuroinflammation and beyond. J Neuroinflammation. 2014;11:158. doi: 10.1186/s12974-014-0158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah S.Z., Hussain T., Zhao D., Yang L. A central role for calcineurin in protein misfolding neurodegenerative diseases. Cell Mol Life Sci. 2017;74(6):1061–1074. doi: 10.1007/s00018-016-2379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarasova E.O., Gaydukov A.E., Balezina O.P. Calcineurin and its role in synaptic transmission. Biochemistry (Mosc) 2018;83(6):674–689. doi: 10.1134/S0006297918060056. [DOI] [PubMed] [Google Scholar]
- 48.Buchholz M., Ellenrieder V. An emerging role for Ca2+/calcineurin/NFAT signaling in cancerogenesis. Cell Cycle. 2007;6(1):16–19. doi: 10.4161/cc.6.1.3650. [DOI] [PubMed] [Google Scholar]
- 49.Medyouf H., Ghysdael J. The calcineurin/NFAT signaling pathway: a novel therapeutic target in leukemia and solid tumors. Cell Cycle. 2008;7(3):297–303. doi: 10.4161/cc.7.3.5357. [DOI] [PubMed] [Google Scholar]
- 50.Gachet S., Ghysdael J. Calcineurin/NFAT signaling in lymphoid malignancies. Gen Physiol Biophys. 2009;28:F47–F54. [PubMed] [Google Scholar]
- 51.Dotto G.P. Calcineurin signaling as a negative determinant of keratinocyte cancer stem cell potential and carcinogenesis. Cancer Res. 2011;71(6):2029–2033. doi: 10.1158/0008-5472.CAN-10-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gang W., Yu-Zhu W., Yang Y., Shi F., Fu X.L., Zhang H. The critical role of calcineurin/NFAT (C/N) pathways and effective antitumor prospect for colorectal cancers. J Cell Biochem. 2019;120(12):19254–19273. doi: 10.1002/jcb.29243. [DOI] [PubMed] [Google Scholar]
- 53.Gooch J.L. An emerging role for calcineurin Aalpha in the development and function of the kidney. Am J Physiol Renal Physiol. 2006;290(4):F769–F776. doi: 10.1152/ajprenal.00281.2005. [DOI] [PubMed] [Google Scholar]
- 54.Schulz R.A., Yutzey K.E. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004;266(1):1–16. doi: 10.1016/j.ydbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Molkentin J.D. Calcineurin and beyond: cardiac hypertrophic signaling. Circ Res. 2000;87(9):731–738. doi: 10.1161/01.res.87.9.731. [DOI] [PubMed] [Google Scholar]
- 56.Fiedler B., Wollert K.C. Interference of antihypertrophic molecules and signaling pathways with the Ca2+-calcineurin-NFAT cascade in cardiac myocytes. Cardiovasc Res. 2004;63(3):450–457. doi: 10.1016/j.cardiores.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Molkentin J.D. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63(3):467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 58.Fiedler B., Wollert K.C. Targeting calcineurin and associated pathways in cardiac hypertrophy and failure. Expert Opin Ther Targets. 2005;9(5):963–973. doi: 10.1517/14728222.9.5.963. [DOI] [PubMed] [Google Scholar]
- 59.Yi X., Li X., Jiang X. Carabin. Endogenous calcineurin inhibitor, a potential diagnostic and therapeutic target for cardiac hypertrophy in heart failure. Int J Cardiol. 2016;212:57–58. doi: 10.1016/j.ijcard.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 60.Rusnak F., Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80(4):1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 61.Creamer T.P. Calcineurin. Cell Commun Signal. 2020;18(1):137. doi: 10.1186/s12964-020-00636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brauer B.L., Moon T.M., Sheftic S.R., et al. Leveraging new definitions of the LxVP SLiM to discover novel calcineurin regulators and substrates. ACS Chem Biol. 2019;14(12):2672–2682. doi: 10.1021/acschembio.9b00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J., Balakrishnan-Renuka A., Hagemann N., et al. A novel interaction between ATOH8 and PPP3CB. Histochem Cell Biol. 2016;145(1):5–16. doi: 10.1007/s00418-015-1368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francis C.E., Bai Y. Differential expression of cyclosporine A-Induced calcineurin isoform-specific matrix metalloproteinase 9 (MMP-9) in renal fibroblasts. Biochem Biophys Res Commun. 2018;503(1):2549–2554. doi: 10.1016/j.bbrc.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y., Holstein D.M., Aime S., Bollo M., Lechleiter J.D. Calcineurin beta protects brain after injury by activating the unfolded protein response. Neurobiol Dis. 2016;94:139–156. doi: 10.1016/j.nbd.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Z., Li H., He L., et al. Discovery of small-molecule inhibitors of the HSP90-calcineurin-NFAT pathway against glioblastoma. Cell Chem Biol. 2019;26(3):352–365. doi: 10.1016/j.chembiol.2018.11.009. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamashita M., Katsumata M., Iwashima M., et al. T cell receptor-induced calcineurin activation regulates T helper type 2 cell development by modifying the interleukin 4 receptor signaling complex. J Exp Med. 2000;191(11):1869–1879. doi: 10.1084/jem.191.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H., Ye W., Guan G., et al. Developmental regulation of calcineurin isoforms in the rodent kidney: association with COX-2. Am J Physiol Renal Physiol. 2007;293(3):F1898–F1904. doi: 10.1152/ajprenal.00360.2007. [DOI] [PubMed] [Google Scholar]
- 69.Graef I.A., Chen F., Chen L., et al. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105(7):863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 70.Mass E., Wachten D., Aschenbrenner A.C., et al. Murine Creld1 controls cardiac development through activation of calcineurin/NFATc1 signaling. Dev Cell. 2014;28(6):711–726. doi: 10.1016/j.devcel.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Yoshida Y., Kim S., Chiba K., Kawai S., Tachikawa H., Takahashi N. Calcineurin inhibitors block dorsal-side signaling that affect late-stage development of the heart, kidney, liver, gut and somitic tissue during Xenopus embryogenesis. Dev Growth Differ. 2004;46(2):139–152. doi: 10.1111/j.1440-169X.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 72.Ishizawa K., Wang Q., Li J., et al. Calcineurin dephosphorylates Kelch-like 3, reversing phosphorylation by angiotensin II and regulating renal electrolyte handling. Proc Natl Acad Sci U S A. 2019;116(8):3155–3160. doi: 10.1073/pnas.1817281116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kyttaris V.C., Wang Y., Juang Y.T., Weinstein A., Tsokos G.C. Increased levels of NF-ATc2 differentially regulate CD154 and IL-2 genes in T cells from patients with systemic lupus erythematosus. J Immunol. 2007;178(3):1960–1966. doi: 10.4049/jimmunol.178.3.1960. [DOI] [PubMed] [Google Scholar]
- 74.Desai-Mehta A., Lu L., Ramsey-Goldman R., Datta S.K. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97(9):2063–2073. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moes A.D., Hesselink D.A., Zietse R., et al. Calcineurin inhibitors and hypertension: a role for pharmacogenetics? Pharmacogenomics. 2014;15(9):1243–1251. doi: 10.2217/pgs.14.87. [DOI] [PubMed] [Google Scholar]
- 76.Muller M.R., Rao A.N.F.A.T. Immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10(9):645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 77.Bucher P., Erdmann T., Grondona P., et al. Targeting chronic NFAT activation with calcineurin inhibitors in diffuse large B-cell lymphoma. Blood. 2020;135(2):121–132. doi: 10.1182/blood.2019001866. [DOI] [PubMed] [Google Scholar]
- 78.Miskin J.E., Abrams C.C., Goatley L.C., Dixon L.K. A viral mechanism for inhibition of the cellular phosphatase calcineurin. Science. 1998;281(5376):562–565. doi: 10.1126/science.281.5376.562. [DOI] [PubMed] [Google Scholar]
- 79.Lin C.C., Law B.F., Hettick J.M. Acute 4,4'-methylene diphenyl diisocyanate exposure-mediated downregulation of miR-206-3p and miR-381-3p activates inducible nitric oxide synthase transcription by targeting calcineurin/NFAT signaling in macrophages. Toxicol Sci. 2020;173(1):100–113. doi: 10.1093/toxsci/kfz215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zelante T., Wong A.Y., Ping T.J., et al. CD103(+) dendritic cells control Th17 cell function in the lung. Cell Rep. 2015;12(11):1789–1801. doi: 10.1016/j.celrep.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 81.Mencarelli A., Khameneh H.J., Fric J., et al. Calcineurin-mediated IL-2 production by CD11c(high)MHCII(+) myeloid cells is crucial for intestinal immune homeostasis. Nat Commun. 2018;9(1):1102. doi: 10.1038/s41467-018-03495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zanoni I., Ostuni R., Capuano G., et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460(7252):264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 83.Ranjan R., Deng J., Chung S., et al. The transcription factor nuclear factor of activated T cells c3 modulates the function of macrophages in sepsis. J Innate Immun. 2014;6(6):754–764. doi: 10.1159/000362647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doz-Deblauwe E., Carreras F., Arbues A., et al. CR3 engaged by PGL-I triggers syk-calcineurin-NFATc to rewire the innate immune response in leprosy. Front Immunol. 2019;10:2913. doi: 10.3389/fimmu.2019.02913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Juvvadi P.R., Fox D., 3rd, Bobay B.G., et al. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nat Commun. 2019;10:4275. doi: 10.1038/s41467-019-12199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miskin J.E., Abrams C.C., Dixon L.K. African swine fever virus protein A238L interacts with the cellular phosphatase calcineurin via a binding domain similar to that of NFAT. J Virol. 2000;74(20):9412–9420. doi: 10.1128/jvi.74.20.9412-9420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tajima K., Amakawa R., Ito T., et al. Immunomodulatory effects of cyclosporin A on human peripheral blood dendritic cell subsets. Immunology. 2003;108(3):321–328. doi: 10.1046/j.1365-2567.2003.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zelante T., Wong A.Y., Mencarelli A., et al. Impaired calcineurin signaling in myeloid cells results in downregulation of pentraxin-3 and increased susceptibility to aspergillosis. Mucosal Immunol. 2017;10(2):470–480. doi: 10.1038/mi.2016.52. [DOI] [PubMed] [Google Scholar]
- 89.Gooch J.L., Tang Y., Ricono J.M., Abboud H.E. Insulin-like growth factor-I induces renal cell hypertrophy via a calcineurin-dependent mechanism. J Biol Chem. 2001;276(45):42492–42500. doi: 10.1074/jbc.M102994200. [DOI] [PubMed] [Google Scholar]
- 90.Gooch J.L., Barnes J.L., Garcia S., Abboud H.E. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003;284(1):F144–F154. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]
- 91.Lang Y., Zhao Y., Zheng C., et al. miR-30 family prevents uPAR-ITGB3 signaling activation through calcineurin-NFATC pathway to protect podocytes. Cell Death Dis. 2019;10(6):401. doi: 10.1038/s41419-019-1625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nijenhuis T., Sloan A.J., Hoenderop J.G., et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011;179(4):1719–1732. doi: 10.1016/j.ajpath.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Y., Zhang L., Xu G., et al. Angiotensin II type I receptor agonistic autoantibody induces podocyte injury via activation of the TRPC6- calcium/calcineurin pathway in pre-eclampsia. Kidney Blood Press Res. 2018;43(5):1666–1676. doi: 10.1159/000494744. [DOI] [PubMed] [Google Scholar]
- 94.Liang S., Zhang H., Du Y., et al. RANK deficiency ameliorates podocyte injury by suppressing calcium/calcineurin/NFATc1 signaling. Kidney Blood Press Res. 2018;43(4):1149–1159. doi: 10.1159/000492049. [DOI] [PubMed] [Google Scholar]
- 95.Wu J., Zheng C., Wang X., et al. MicroRNA-30 family members regulate calcium/calcineurin signaling in podocytes. J Clin Invest. 2015;125(11):4091–4106. doi: 10.1172/JCI81061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Y., Wu J., Zhang M., et al. Angiotensin II induces calcium/calcineurin signaling and podocyte injury by downregulating microRNA-30 family members. J Mol Med (Berl). 2017;95(8):887–898. doi: 10.1007/s00109-017-1547-z. [DOI] [PubMed] [Google Scholar]
- 97.Lim H.W., New L., Han J., Molkentin J.D. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol. 2001;276(19):15913–15919. doi: 10.1074/jbc.M100452200. [DOI] [PubMed] [Google Scholar]
- 98.Chen C.H., Lin J.W., Huang C.Y., et al. The combined inhibition of the CaMKIIδ and calcineurin signaling cascade attenuates IGF-IIR-induced cardiac hypertrophy. J Cell Physiol. 2020;235(4):3539–3547. doi: 10.1002/jcp.29242. [DOI] [PubMed] [Google Scholar]
- 99.Cao J.L., Yang Y.Q., Nabeel D.M., et al. Correlation between serum calcineurin activity and left ventricular hypertrophy in hypertensive patients and its clinical significance. Cardiology. 2018;139(2):124–131. doi: 10.1159/000481280. [DOI] [PubMed] [Google Scholar]
- 100.Parra V., Rothermel B.A. Calcineurin signaling in the heart: the importance of time and place. J Mol Cell Cardiol. 2017;103:121–136. doi: 10.1016/j.yjmcc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Felkin L.E., Narita T., Germack R., et al. Calcineurin splicing variant calcineurin Aβ1 improves cardiac function after myocardial infarction without inducing hypertrophy. Circulation. 2011;123(24):2838–2847. doi: 10.1161/CIRCULATIONAHA.110.012211. [DOI] [PubMed] [Google Scholar]
- 102.Gao C., Wang Y. Positive role for a negative calcineurin regulator in cardiac hypertrophy. Hypertension. 2016;67(5):841–842. doi: 10.1161/HYPERTENSIONAHA.116.07140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.O'Neal M.A., Stallings N.R., Malter J.S. Alzheimer's disease, dendritic spines, and calcineurin inhibitors: a new approach? ACS Chem Neurosci. 2018;9(6):1233–1234. doi: 10.1021/acschemneuro.8b00213. [DOI] [PubMed] [Google Scholar]
- 104.Hopp S.C., Bihlmeyer N.A., Corradi J.P., et al. Neuronal calcineurin transcriptional targets parallel changes observed in Alzheimer disease brain. J Neurochem. 2018;147(1):24–39. doi: 10.1111/jnc.14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shah S.Z., Zhao D., Khan S.H., Yang L. Unfolded protein response pathways in neurodegenerative diseases. J Mol Neurosci. 2015;57(4):529–537. doi: 10.1007/s12031-015-0633-3. [DOI] [PubMed] [Google Scholar]
- 106.Tu S., Okamoto S., Lipton S.A., Yang L., Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease. Mol Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong J.M., Moon J.H., Park S.Y. Human prion protein-mediated calcineurin activation induces neuron cell death via AMPK and autophagy pathway. Int J Biochem Cell Biol. 2020;119:105680. doi: 10.1016/j.biocel.2019.105680. [DOI] [PubMed] [Google Scholar]
- 108.Sun T., Wu X.S., Xu J., et al. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 2010;30(35):11838–11847. doi: 10.1523/JNEUROSCI.1481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosaka M., Hammer R.E., Südhof T.C. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron. 1999;24(2):377–387. doi: 10.1016/s0896-6273(00)80851-x. [DOI] [PubMed] [Google Scholar]
- 110.Orlando M., Lignani G., Maragliano L., et al. Functional role of ATP binding to synapsin I in synaptic vesicle trafficking and release dynamics. J Neurosci. 2014;34(44):14752–14768. doi: 10.1523/JNEUROSCI.1093-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reese L.C., Taglialatela G. Neuroimmunomodulation by calcineurin in aging and Alzheimer's disease. Aging Dis. 2010;1(3):245–253. [PMC free article] [PubMed] [Google Scholar]
- 112.Lim D., Mapelli L., Canonico P.L., Moccia F., Genazzani A.A. Neuronal activity-dependent activation of astroglial calcineurin in mouse primary hippocampal cultures. Int J Mol Sci. 2018;19(10):2997. doi: 10.3390/ijms19102997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nicholson M.W., Sweeney A., Pekle E., et al. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/Ca2+/calcineurin signalling downstream of GABAA receptors. Mol Psychiatry. 2018;23(9):1851–1867. doi: 10.1038/s41380-018-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boddeke H.W., Meigel I., Boeijinga P., Arbuckle J., Docherty R.J. Modulation by calcineurin of 5-HT3 receptor function in NG108-15 neuroblastoma x glioma cells. Br J Pharmacol. 1996;118(7):1836–1840. doi: 10.1111/j.1476-5381.1996.tb15611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim S.H., Ryan T.A. Balance of calcineurin Aα and CDK5 activities sets release probability at nerve terminals. J Neurosci. 2013;33(21):8937–8950. doi: 10.1523/JNEUROSCI.4288-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao W.Q., Santini F., Breese R., et al. Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem. 2010;285(10):7619–7632. doi: 10.1074/jbc.M109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee H.K., Barbarosie M., Kameyama K., Bear M.F., Huganir R.L. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405(6789):955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 118.Jurado S., Biou V., Malenka R.C. A calcineurin/AKAP complex is required for NMDA receptor-dependent long-term depression. Nat Neurosci. 2010;13(9):1053–1055. doi: 10.1038/nn.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reese L.C., Taglialatela G. A role for calcineurin in Alzheimer's disease. Curr Neuropharmacol. 2011;9(4):685–692. doi: 10.2174/157015911798376316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lonze B.E., Ginty D.D. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 121.Tao X., Finkbeiner S., Shaywitz A.J., Greenberg M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 122.Huang E.J., Reichardt L.F. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto-Sasaki M., Ozawa H., Saito T., Rösler M., Riederer P. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res. 1999;824(2):300–303. doi: 10.1016/s0006-8993(99)01220-2. [DOI] [PubMed] [Google Scholar]
- 124.Dineley K.T., Kayed R., Neugebauer V., et al. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res. 2010;88(13):2923–2932. doi: 10.1002/jnr.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sompol P., Furman J.L., Pleiss M.M., et al. Calcineurin/NFAT signaling in activated astrocytes drives network hyperexcitability in Aβ-bearing mice. J Neurosci. 2017;37(25):6132–6148. doi: 10.1523/JNEUROSCI.0877-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abdul H.M., Sama M.A., Furman J.L., et al. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29(41):12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abdul H.M., Furman J.L., Sama M.A., Mathis D.M., Norris C.M. NFATs and alzheimer's disease. Mol Cell Pharmacol. 2010;2(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- 128.Springer J.E., Azbill R.D., Nottingham S.A., Kennedy S.E. Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J Neurosci. 2000;20(19):7246–7251. doi: 10.1523/JNEUROSCI.20-19-07246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Agostinho P., Lopes J.P., Velez Z., Oliveira C.R. Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem Int. 2008;52(6):1226–1233. doi: 10.1016/j.neuint.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 130.Brun M., Godbout R. Activation of calcineurin in cancer: many paths, one hub. Transl Cancer Res. 2016;5:S497–S506. [Google Scholar]
- 131.Wen L., Javed T.A., Dobbs A.K., et al. The protective effects of calcineurin on pancreatitis in mice depend on the cellular source. Gastroenterology. 2020;159(3):1036–1050. doi: 10.1053/j.gastro.2020.05.051. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang G., Wang Y.Z., Yu Y., Wang J.J. Inhibitory ASIC2-mediated calcineurin/NFAT against colorectal cancer by triterpenoids extracted from Rhus chinensis Mill. J Ethnopharmacol. 2019;235:255–267. doi: 10.1016/j.jep.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 133.Quang C.T., Leboucher S., Passaro D., et al. The calcineurin/NFAT pathway is activated in diagnostic breast cancer cases and is essential to survival and metastasis of mammary cancer cells. Cell Death Dis. 2015;6:e1658. doi: 10.1038/cddis.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Manda K.R., Tripathi P., Hsi A.C., et al. NFATc1 promotes prostate tumorigenesis and overcomes PTEN loss-induced senescence. Oncogene. 2016;35(25):3282–3292. doi: 10.1038/onc.2015.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xin B., Ji K.Q., Liu Y.S., et al. Higher expression of calcineurin predicts poor prognosis in unique subtype of ovarian cancer. J Ovarian Res. 2019;12(1):75. doi: 10.1186/s13048-019-0550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang S., Kang X., Cao S., Cheng H., Wang D., Geng J. Calcineurin/NFATc1 pathway contributes to cell proliferation in hepatocellular carcinoma. Dig Dis Sci. 2012;57(12):3184–3188. doi: 10.1007/s10620-012-2255-8. [DOI] [PubMed] [Google Scholar]
- 137.Brun M., Glubrecht D.D., Baksh S., Godbout R. Calcineurin regulates nuclear factor I dephosphorylation and activity in malignant glioma cell lines. J Biol Chem. 2013;288(33):24104–24115. doi: 10.1074/jbc.M113.455832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ma N.Q., Liu L.L., Min J., et al. The effect of down regulation of calcineurin Aα by lentiviral vector-mediated RNAi on the biological behavior of small-cell lung cancer and its bone metastasis. Clin Exp Metastasis. 2011;28(8):765–778. doi: 10.1007/s10585-011-9408-6. [DOI] [PubMed] [Google Scholar]
- 139.Buchholz M., Schatz A., Wagner M., et al. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25(15):3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu W., Gu J., Ren Q., et al. NFATC1 promotes cell growth and tumorigenesis in ovarian cancer up-regulating c-Myc through ERK1/2/p38 MAPK signal pathway. Tumour Biol. 2016;37(4):4493–4500. doi: 10.1007/s13277-015-4245-x. [DOI] [PubMed] [Google Scholar]
- 141.Marafioti T., Pozzobon M., Hansmann M.L., et al. The NFATc1 transcription factor is widely expressed in white cells and translocates from the cytoplasm to the nucleus in a subset of human lymphomas. Br J Haematol. 2005;128(3):333–342. doi: 10.1111/j.1365-2141.2004.05313.x. [DOI] [PubMed] [Google Scholar]
- 142.Goshima T., Habara M., Maeda K., Hanaki S., Kato Y., Shimada M. Calcineurin regulates cyclin D1 stability through dephosphorylation at T286. Sci Rep. 2019;9(1):12779. doi: 10.1038/s41598-019-48976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim Y., Lee Y.I., Seo M., et al. Calcineurin dephosphorylates glycogen synthase kinase-3 beta at serine-9 in neuroblast-derived cells. J Neurochem. 2009;111(2):344–354. doi: 10.1111/j.1471-4159.2009.06318.x. [DOI] [PubMed] [Google Scholar]
- 144.Fernandez M.R., Henry M.D., Lewis R.E. Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK. Mol Cell Biol. 2012;32(18):3718–3731. doi: 10.1128/MCB.06754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang C.C., Wang J.M., Kikkawa U., et al. Calcineurin-mediated dephosphorylation of c-Jun Ser-243 is required for c-Jun protein stability and cell transformation. Oncogene. 2008;27(17):2422–2429. doi: 10.1038/sj.onc.1210888. [DOI] [PubMed] [Google Scholar]
- 146.Siamakpour-Reihani S., Caster J., Bandhu Nepal D., et al. The role of calcineurin/NFAT in SFRP2 induced angiogenesis--a rationale for breast cancer treatment with the calcineurin inhibitor tacrolimus. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0020412. e20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Medyouf H., Alcalde H., Berthier C., et al. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007;13(6):736–741. doi: 10.1038/nm1588. [DOI] [PubMed] [Google Scholar]
- 148.Liu Z., Jiang L., Li Y., et al. Cyclosporine A sensitizes lung cancer cells to crizotinib through inhibition of the Ca2 +/calcineurin/Erk pathway. EBioMedicine. 2019;42:326–339. doi: 10.1016/j.ebiom.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Balan M., Chakraborty S., Flynn E., et al. Honokiol inhibits c-Met-HO-1 tumor-promoting pathway and its cross-talk with calcineurin inhibitor-mediated renal cancer growth. Sci Rep. 2017;7(1):5900. doi: 10.1038/s41598-017-05455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu X., Nguyen B.C., Dziunycz P., et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465(7296):368–372. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.