Abstract
OBJECTIVES
Elderly patients show a higher incidence of ischemic and bleeding events after percutaneous transluminal coronary intervention (PCI). We sought to investigate outcomes in elderly patients treated with antithrombotic strategy guided by bleeding and ischemic risks after revascularization with last generation everolimus-eluting stent (EES).
METHODS
Prospective multicenter registry including patients over 75 years revascularized with EES and antithrombotic therapy guided by clinical presentation, PCI complexity and PRECISE DAPT score. Co-primary safety endpoints were: (1) composite of cardiac death, myocardial infarction and stent thrombosis and; (2) bleeding (BARC 2-5). Primary efficacy endpoint was target lesion revascularization. A matched group of patients revascularized with current drug-eluting stents and no such tailored antithrombotic therapy was used as control.
RESULTS
Finally, 1064 patients were included in SIERRA-75 cohort, 80.8 ± 4.2 years, 36.6% women, 71% acute coronary syndromes (ACS) and 53.6% complex PCI. Co-primary safety endpoint of major adverse cardiovascular events was met in 6.2%, co-primary safety endpoint of bleeding in 7.8% and primary efficacy endpoint of TKLR in 1.5%. The multivariable adjusted model showed no significant association of the prescribed short/long dual antiplatelet therapy (DAPT) durations with any endpoint suggesting a well tailored therapy. No stent thrombosis reported in the subgroup with 1-3 months DAPT duration. As compared to control group, bleeding BARC 2-5 was significantly lower in SIERRA-75 group (7.4% vs. 10.2%, P = 0.04) as well as the net safety-efficacy endpoint (14.3% vs. 18.5%, P = 0.02).
CONCLUSIONS
In elderly population, the application of this risks-adjusted antithrombotic protocol after revascularization with last generation EES seems to be associated with an improved prognosis in terms of ischemic and bleeding outcomes.
Keywords: BARC, =, Bleeding, Academic, Research, Consortium
Elderly patients represent a rapidly growing cohort in percutaneous coronary interventions (PCI). They undergo a more complex PCI because of associated comorbidities and challenging anatomies. In addition, advanced age imposes a higher risk of bleeding and cardiovascular events in follow up.[1]
Dual antiplatelet therapy (DAPT) is generally indicated for 6 to 12 months to prevent ischemic events after PCI. Nonetheless, because DAPT is associated with a significant increase in the risk of bleeding complications, shortening the duration of DAPT has been proposed among those patients deemed at high risk of bleeding, such as the elderly.[2,3] However, advanced age itself is not a major criteria of high bleeding risk as defined by the Academic Research Consortium (HBR-ARC).[4]
We strongly believe that elderly patients do not have to be systematically treated with very short DAPT periods, but rather a consideration of both, ischemic and bleeding risks, should be pursued in order to select the most adequate DAPT regimen. The ischemic risks can be estimated by considering clinical presentation and PCI complexity. With the purpose of bleeding risk assessment the 2017 ESC guidelines for DAPT included the use of the PRECISE DAPT score to guide decisions.[3,5] To the best of our knowledge, no protocols assessing both bleeding and ischemic risks have been designed and prospectively evaluated in this challenging population.
The XIENCE® Sierra everolimus-eluting coronary stent, EES (Abbott, Santa Clara, US) carries all the positive features of the precursor stents of the XIENCE® family and brings a very low crossing profile which further increases deliverability and flexibility, which could facilitate complex PCI.[6-8] Multiple clinical trials have reported a low risk of stent thrombosis and major cardiovascular events with EES.[6,7]
The SIERRA-75 registry was designed to evaluate clinical outcomes in elderly patients who are prescribed an antithrombotic regimen guided by assessement of ischemic and bleeding risks after PCI with the last generation of EES.
METHODS
Population in Prospective SIERRA-75 Cohort
This is a prospective multicenter registry (35 centers in Spain and 7 in Portugal) including patients aged over 75 years with an indication for percutaneous revascularization treated with the last generation of EES.
The inclusion criteria were the following: (1) patients older than 75 years; (2) clinical indication of percutaneous revascularization; (3) presence of significant de novo lesions in native coronary arteries with no limits in the number of vessels/lesions to treat; and (4) having provided signed informed consent.
The exclusion criteria were: (1) cardiogenic shock at the time of PCI; (2) cardiac arrest prior to PCI; (3) patient life expectancy < 1 year; and (4) participation in another investigational drug or device study; and (5) clinical decision precluding the use of DES; and (6) confirmed allergy to aspirin and/or thienopyridines.
All patient data, baseline and follow-up, were collected in an electronic database designed specifically for the study in which a complete anonymization of the data was guaranteed.
Clinical follow up after the index procedure included outpatient clinical visits and telephone contact at 1, 6 and 12 months. All events were evaluated and adjudicated after reported by the coordinating study center (two interventional cardiologists) which requested clinical information to the investigators when needed. A Contract Research Organization was in charge of the monitorization process.
The study was approved by the clinical research committees of each participating institution. All patients provided informed consent for participation. The study was registered with the identifier: NCT03567733.
Population in Retrospective Control Group
For the purpose of the control group, a retrospective registry of consecutive patients treated with currently used DES other than the EES was conducted applying the same inclusion-exclusion criteria as in the prospective registry. In general, DAPT was indicated for 6 months in stable patients and for 12 months in patients with ACS following the guidelines currently available,[2,3] but a shorter period was prescribed in high risk bleeding settings (additional bleeding criteria other than advanced age). The recruitment period was immediately prior to the enrollment period of the prospective registry avoiding any time overlap between both registries. A propensity score matching was conducted to pair cases from both registries. All clinical, angiographic and procedural variables were used in the process except for the duration of the antithrombotic strategies, since this comparative analysis was aimed to assess the effect of the risk-based antithrombotic treatment adjustment model used in the SIERRA-75 cohort.
The data was obtained from hospital records and databases and after adequate anonymization was uploaded into a specifically designed database with standardized criteria for clinical, angiographic and procedural variables. Adverse events were centrally adjudicated according to the same criteria applied in the prospective registry in a blinded fashion. The study was approved by the clinical research committees of each participating institution.
Endpoints and Definitions
There were two co-primary safety endpoints at 12 months, one was a composite of cardiac death, myocardial infarction and definitive/probable stent thrombosis and the other one was bleeding BARC 2-5 (Bleeding Academic Research Consortium 2-5). The primary efficacy endpoint at 12 months was the incidence of clinically driven target lesion revascularization (TLR).
The secondary endpoints at 12 months included: all cause death, cardiac death, myocardial infarction, revascularization, definite or probable stent thrombosis and stroke. Other secondary endpoints were a composite of cardiac death, myocardial infarction, definitive/probable stent thrombosis and TLR and finally the net clinical effect was a composite of cardiac death, myocardial infarction, definitive/probable stent thrombosis, TLR and bleeding BARC 2-5.
Death was regarded as cardiac in origin unless an obvious non-cardiac cause could be identified. Myocardial infarction was defined according to the fourth Universal Definition by the European Society of Cardiology and the American College of Cardiology Foundation. TLR was defined as either repeat percutaneous or surgical revascularization for a lesion anywhere within the stent or the 5-mm borders proximal or distal to the stent. Stent thrombosis was defined according to the ARC criteria. Bleeding events were categorized according to BARC criteria. Device success was defined as the attainment at the target site of a final residual diameter stenosis of less than 25% with a TIMI III flow.
Complex PCI was defined as the presence of at least one of the following: 3 vessels treated, ≥ 3 lesions treated, total stent length implanted > 60 mm, bifurcation with ≥ 2 stents implanted, use of atherectomy devices, left main coronary artery stenting, PCI on a chronic total occlusion and PCI on a bypass graft.
Procedures in the Prospective SIERRA-75 Cohort
All lesions were treated with the last generation EES. Plaque preparation techniques, use of intravascular imaging and postdilatation were all at the discretion of the operator. Only patients with a single index revascularization procedure were included, in which all lesions the operator considered significant would be treated.
The patients were treated before the procedure with antiplatelet therapy according to clinical indication and at the discretion of the cardiologist in charge. There were no restrictions on the choice of the P2Y12 inhibitor but clopidogrel was strongly recommended in patients showing higher bleeding risks. In patients requiring chronic oral anticoagulation, the use of direct anticoagulants was encouraged as well.
The recommended risks-adjusted stretagies for antithrombotic therapy were as follows:
(A) No indication for chronic oral anticoagulation: (1) Stable condition and no complex PCI: PRECISE DAPT score ≥ 25 Short therapy (1-3 months); PRECISE DAPT score < 25 Long therapy (6 months). (2) Acute coronary syndrome or complex PCI: PRECISE DAPT score ≥ 25 Short therapy (3-6 months); PRECISE DAPT score < 25 Long therapy (12 months).
(B) Indication for chronic oral anticoagulation: (1) Stable condition and no complex PCI: PRECISE DAPT score ≥ 25 Short therapy (triple therapy NO or < 1 month/dual therapy 6 months); PRECISE DAPT score < 25 Long therapy (triple therapy 1 month/dual therapy > 6 months). (2) acute coronary syndrome or complex PCI: PRECISE DAPT score ≥ 25 Short therapy (triple therapy ≤ 1 month/dual therapy 6 months); PRECISE DAPT score < 25 Long therapy (triple therapy 1-3 months/dual therapy 12 months). Investigators were encouraged to adhere to these guidelines, but these were not mandatory and final decisions were left ultimately to the discretion of the clinicians in charge.
Statistics
The calculation of the sample size was made with the following considerations. In the subgroup of elderly patients of the LEADERS FREE trial (789 patients), the primary safety endpoint (composite of cardiac death, myocardial infarction and definite/probable stent thrombosis) at 12 months was 10.7% with the Biofreedom® stent (Biosensors, Singapore).[9] In the XIMA trial, the same composite endpoint was met in 7% of the 399 octogenarians treated with EES.[10] Using this estimate, 1844 patients (922 per group) are required to have a 80% chance of detecting, as significant at the 5% level, a superiority of EES over Biofreedom®. Incidence of TLR at 12 months was 5.8% in the Biofreedom® arm of the elderly subgroup of LEADERS FREE trial. In the XIMA trial TLR at 12 months was detected in 2% of patients treated with EES. Using this estimate, 808 patients (404 per group) are required to have a 80% chance of detecting, as significant at the 5% level, a superiority of EES over Biofreedom®. Given these results, and an expected attrition rate up to 3% a minimum of 1000 patients are required to evaluate the superiority of both safety and efficacy endpoints.
As a secondary analysis, a control group of patients treated with current DES was considered in which the type and duration of antithrombotic therapy was at the discretion of the physicians in charge. The selection criteria for this retrospective registry were the same as those applied in the prospective SIERRA-75 registry. Additionally, the patients from both groups were matched by propensity score index. The propensity score matching was aimed to pair each patient in control group with a patient in SIERRA-75 group. This procedure involved two stages: (1) the propensity scores were estimated using logistic regression in which treatment with SIERRA-75 was used as the outcome variable and all the clinical, angiographic and procedural covariates as predictors except for the duration of the antithrombotic strategies, since this comparative analysis was aimed to assess the effect of the risk-based antithrombotic treatment adjustment model used in the SIERRA-75 cohort. (2) Patients were matched using simple 1: 1 nearest neighbor matching that is based on a “greedy” matching algorithm that sorts the observations in the group by their estimated propensity score. It then matches sequentially each unit in the control group to a unit in the SIERRA-75 group that has the closest propensity score, using calipers of width equal to 0.1 the standard deviation of the logit of the propensity score.
Continuous variables are presented as mean ± SD or median (interquartile range) and categorical variables as percentages. Distribution was assessed for each variable with the Kolmogorov-Smirnov test. Accordingly, continuous variables were compared with the Student t test if they followed a normal distribution and by Wilcoxon tests when this was not the case. The categorical variables were compared with the chi-square test or Fisher exact test, as required. Kaplan-Meier curves for event-free survival were obtained for each group and compared using the log-rank test. A Cox proportional-hazards multivariable stepwise regression model was used to determine hazard ratios for primary outcomes and to identify independent predictors. All clinical, angiographic and procedural variables showing an association with the dependent variable in the univariate analysis (P < 0.2) were included in the multivariable models. The rate of missing values for the analyzed variables was extremely low, but in any case, these were not imputed in the categorical classification. A P value < 0.05 was considered as statistically significant. All statistical analyses were performed using SPSS version 19 for windows and Medcalc version 19.
RESULTS
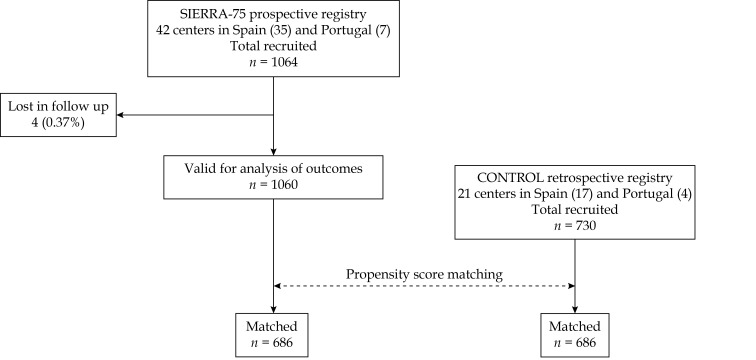
Finally, 1,064 patients were included in the prospective registry SIERRA75 in the period June-2018 to August-2019 and 730 in the retrospective registry (February-2016 to May-2018). Figure 1 shows the flow chart of the study.
Figure 1.
Flow chart of the study.
The baseline clinical characteristics of the unmatched groups are shown in supplementary Table 1. The angiographic and procedural characteristics of unmatched groups are listed in supplementary Table 2.
Table 1-1.
| Continued | ||||
| Unmatched | SIERRA-75 | Control | P | 95% CI |
| Data are presented as mean ± SD or n (%). Anemia was defined as a hemoglobin value of less than 13 g/dL in a man or less than 12 g/dL in a woman. ACS: acute coronary syndrome; BMI: body mass index; CABG: coronary artery bypass grafting; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction: MI: myocardial infarction; NSTEMI: non ST elevated myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST elevated myocardial infarction. | ||||
| Previous major bleeding | 51 (4.7%) | 50 (6.8%) | 0.06 | −0.06% to 4.4% |
| Peripheral vascular disease | 96 (9%) | 66 (9%) | 1 | −2.6% to 2.8% |
| GFR < 30 mL/min | 71 (6.7%) | 39 (5.3%) | 0.2 | −0.91% to 3.6% |
| GFR 30-60 mL/min | 418 (39.3%) | 260 (35.6%) | 0.12 | −0.9% to 8.2% |
| Anemia | 530 (49.8%) | 292 (40%) | 0.0001 | 5.1% to 14.4% |
| PRECISE DAPT | 30.5 ± 11.4 | 30.9 ± 11.6 | 0.52 | −0.68% to 1.54% |
| CHA2DS2−VASc | 4.2 ± 1.2 | 4.04 ± 1.3 | 0.007 | −0.27% to −0.039% |
| HAS BLED | 2.7 ± 0.8 | 2.51 ± 0.83 | 0.0001 | −0.27% to −0.11% |
| GRACE score | 133 ± 26 | 136 ± 23.5 | 0.01 | 0.64% to 5.36% |
| LVEF, % | 53 ± 12 | 52.6 ± 11.4 | 0.45 | −1.5% to 0.71% |
| NSTEMI | 421 (39.6%) | 255 (34.9%) | 0.04 | 0.14% to 9.2% |
| STEMI | 187 (17.6%) | 146 (20%) | 0.23 | −1.2% to 6.1% |
| Unstable angina | 160 (15%) | 118 (16.1%) | 0.50 | −2.2% to 4.59% |
| ACS | 757 (71.1%) | 515 (70.5%) | 0.72 | −3.58% to 4.97% |
After matching, two groups of 686 patients each were obtained. The baseline clinical, angiographic and procedural characteristics of matched groups are shown in Tables 1 and 2. No significant differences were observed between groups for any of the variables. The groups only differed, as expected by definition, in the corresponding times on DAPT, on triple therapy and on dual therapy, all of them being longer in the control group (time on DAPT 8.8 ± 3.7 vs. 11 ± 3 months (P < 0.001), time on triple therapy 2.1 ± 2 vs. 2.8 ± 2.2 months (P < 0.001) and time on dual therapy 8.1 ± 4 vs. 8.6 ± 4.6 months (P = 0.02).
Table 1. Angiographic and procedural characteristics in matched groups.
| Matched | SIERRA-75
N = 686 |
Control
N = 686 |
P | 95% CI |
| Age | 80.6 ± 4.3 | 80.8 ± 4.4 | 0.38 | −0.26% to 0.66% |
| Women | 242 (35.2%) | 233 (34%) | 0.64 | −3.83% to 6.22% |
| BMI | 27.3 ± 3.9 | 27.1 ± 3.9 | 0.34 | −0.61% to 0.21% |
| Diabetes | 300 (43.7%) | 273 (39.8%) | 0.14 | −1.32% to 9.09% |
| Diabetes insulin-treated | 61 (8.8%) | 58 (8.4%) | 0.79 | −2.59% to 3.4% |
| Hypertension | 562 (82%) | 542 (79%) | 0.16 | −1.2% to 7.2% |
| Dyslipidemia | 415 (60.5%) | 395 (57.5%) | 0.26 | −2.2% to 8.18% |
| Smoker | 41 (6%) | 48 (7%) | 0.45 | −1.6% to 3.7% |
| Previous MI | 142 (20.7%) | 146 (21.2%) | 0.82 | −3.81% to 4.81% |
| Previous stroke | 65 (9.5%) | 77 (11.2%) | 0.30 | −1.54% to 4.95% |
| Previous PCI | 137 (20%) | 127 (18.5%) | 0.48 | −2.68% to 5.67% |
| Previous CABG | 20 (2.9%) | 26 (3.8%) | 0.35 | −1.05% to 2.89% |
| Previous heart failure | 75 (11%) | 66 (9.6%) | 0.40 | −1.83% to 4.64% |
| Previous major bleeding | 41 (6%) | 45 (6.5%) | 0.70 | −2.1% to 3.1% |
| Peripheral vasc. disease | 60 (8.7%) | 61 (8.9%) | 0.89 | −2.82% to 3.23% |
| GFR < 30 mL/min | 40 (5.8%) | 39 (5.6%) | 0.87 | −2.29% to 2.7% |
| GFR 30-60 mL/min | 261 (38%) | 245 (35.7%) | 0.37 | −2.8% to 7.38% |
| Anemia | 306 (44.6%) | 278 (40.5%) | 0.12 | −1.13% to 9.3% |
| PRECISE DAPT | 30.3 ± 11.4 | 30.8 ± 12 | 0.43 | −0.74% to 1.74% |
| CHA2DS2−VASc | 4.15 ± 1.2 | 4.06 ± 1.2 | 0.16 | −0.22% to 0.037% |
| HAS BLED | 2.6 ± 0.83 | 2.53 ± 0.85 | 0.13 | −0.16% to −0.02% |
| GRACE score | 134 ± 25 | 135.5 ± 23 | 0.25 | −1.04% to 4.04% |
| LVEF, % | 53.4 ± 11.7 | 52.8 ± 11.5 | 0.30 | −1.83% to 0.63% |
| NSTEMI | 263 (38.3%) | 242 (35.3%) | 0.25 | −2.1% to 8.08% |
| STEMI | 126 (18.3%) | 134 (19.5%) | 0.57 | −2.95% to 5.34% |
| Unstable angina | 104 (15.2%) | 110 (16%) | 0.68 | −3.05% to 4.65% |
| ACS | 493 (72%) | 486 (70.8%) | 0.62 | −3.58% to 5.97% |
| Unmatched | N = 1064 | N = 730 | ||
| Age | 80.8 ± 4.2 | 80.75 ± 4.5 | 0.8 | −0.45% to 0.35% |
| Women | 389 (36.6%) | 245 (33.5%) | 0.18 | −1.4 to 7.5% |
| BMI | 27.4 ± 4 | 27.1 ± 4 | 0.12 | −0.68% to 0.08% |
| Diabetes | 462 (43.4%) | 288 (39.4%) | 0.11 | −0.65% to 8.6% |
| Diabetes insulin-treated | 106 (10%) | 60 (8.2%) | 0.2 | −0.97% to 4.4% |
| Hypertension | 885 (83.2%) | 584 (80%) | 0.08 | −0.42% to 6.9% |
| Dyslipidemia | 664 (62.4%) | 415 (56.8%) | 0.02 | 0.99% to 10.2% |
| Smoker | 71 (6.6%) | 51 (7%) | 0.7 | −1.9% to 2.9% |
| Previous MI | 213 (20%) | 153 (20.9%) | 0.6 | −0.29% to 4.7% |
| Previous stroke | 90 (8.4%) | 80 (11%) | 0.06 | −0.16% to 5.5% |
| Previous PCI | 234 (22%) | 133 (18.2%) | 0.05 | −0.004% to 7.57% |
| Previous CABG | 31 (2.9%) | 29 (4%) | 0.21 | −0.6% to 2.99% |
| Previous heart failure | 110 (10.3%) | 72 (9.8%) | 0.7 | −2.4% to 3.3% |
Table 2. Angiographic and procedural characteristics in matched groups.
| Matched | IERRA-75
N = 686 |
Control
N = 686 |
P | 95% CI |
| Data are presented as mean ± SD or n (%). | ||||
| Radial access | 598 (87.1%) | 609 (88.7%) | 0.36 | −1.86% to 5.07% |
| Complex PCI | 389 (56.7%) | 408 (59.5%) | 0.25 | −2.42% to 7.99% |
| Number of diseased vessels | 1.76 ± 0.8 | 1.72 ± 0.8 | 0.35 | −0.12% to 0.045% |
| 1 vessels | 324 (47.2%) | 343 (50%) | 0.29 | −2.48% to 8.06% |
| 2 vessels | 206 (30%) | 200 (29.1%) | 0.70 | −3.92% to 5.72% |
| 3 vessels | 156 (22.7%) | 143 (20.8%) | 0.40 | −2.47% to 6.26% |
| Number of lesions treated | 1.43 ± 0.7 | 1.42 ± 0.72 | 0.79 | −0.085% to 0,065% |
| Intravascular imaging | 49 (7.1%) | 45 (6.5%) | 0.65 | −2.1% to 3.3% |
| Clopidogrel | 522 (76%) | 497 (72.5%) | 0.34 | −1.13% to 8.11% |
| Ticagrelor | 164 (24%) | 189 (27.5%) | 0.14 | −1.13% to 8.11% |
| Oral anticoagulants | 120 (17.5%) | 113 (16.4%) | 0.58 | −2.88% to 5.08% |
| Lesions treated | N = 982 | N = 974 | P | 95% CI |
| Minimum lumen diameter, | 0.91 ± 0.8 | 0.93 ± 0.8 | 0.50 | −0.051% to 0.091% |
| Reference vessel diameter, | 3 ± 0.55 | 3.01 ± 0.52 | 0.70 | −0.037% to 0.057% |
| Angiographic stenosis, % | 87.6 ± 11 | 88 ± 11 | 0.49 | −0.58% to 1.38% |
| B2/C lesion type | 412 (60%) | 434 (63.2%) | 0.22 | −1.11% to 7.49% |
| Calcium (moderate/severe) | 212 (30.9%) | 225 (32.8%) | 0.40 | −2.23% to 6.02% |
| Bifurcation | 119 (17.3%) | 125 (18.2%) | 0.66 | −2.49% to 4.29% |
| Chronic total occlusion | 65 (9.5%) | 80 (11.6%) | 0.20 | −0.63% to 4.84% |
| Stent length, mm | 21.5 ± 8 | 22 ± 8 | 0.24 | −0.21% to 1.21% |
| Stent diameter, mm | 2.96 ± 0.54 | 2.97 ± 0.55 | 0.73 | −0.038% to 0.058% |
| Angiographic success | 677 (98.7%) | 671 (97.8%) | 0.28 | −0.29% to 2.15% |
The demographic and clinical profile of these patients corresponded to real practice, with a high burden of vascular risk factors and frequent antecedents of cardiovascular disease. The value of the risks scores indicated an overall high risk for both bleeding and thromboembolic events. The most common indication for PCI was the acute coronary syndrome (ACS). The radial access site was predominant, half of patients showed multivessel disease and a slightly over half of patients underwent complex PCI.
Clinical Follow Up
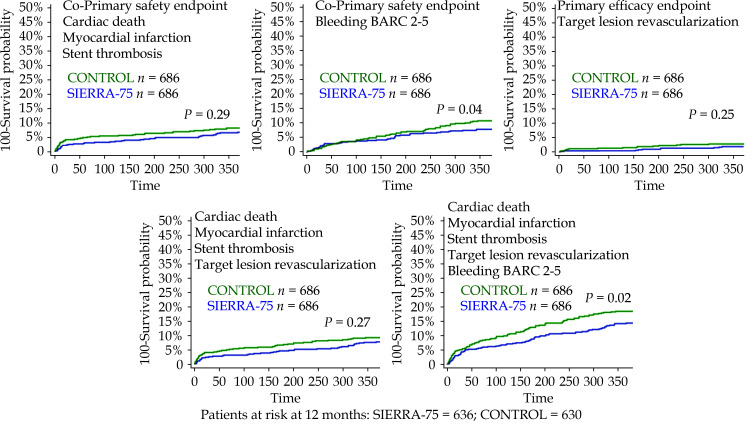
Patients were followed up for 12 months with only four patients being lost in SIERRA 75 (0.37%). The cumulative incidences for all primary and secondary endpoints in unmatched groups are described in supplementary Table 3. The cumulative incidences for all primary and secondary endpoints in matched groups are described in Table 3. A numerically lower incidence was observed in SIERRA75 group for all cardiac events. The incidence of bleeding BARC 2-5 was significantly lower in the SIERRA-75 group (7.4% vs. 10.2%, P = 0.04) as well as the net clinical effect (14.3% vs. 18.5%, P = 0.02).
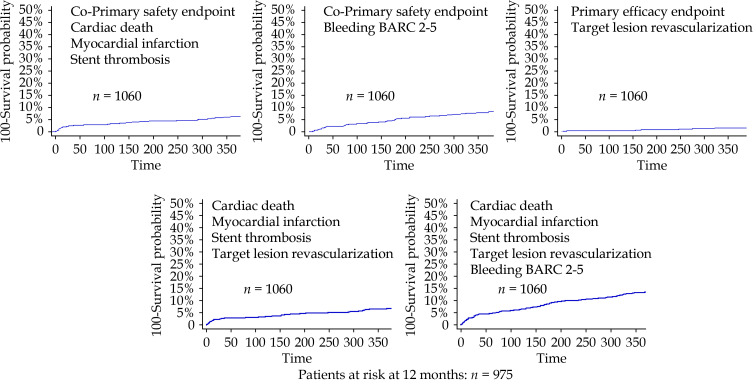
The survival curves for the primary and secondary endpoints for the SIERRA 75 registry are shown in Figure 2 and for the matched groups in Figure 3. The main rsults of the comparative analysis between matched groups is presented in Supplementary Figure 1.
Figure 2.
Kaplan-Meier survival curves for the primary and secondary endpoints in the SIERRA-75 prospective cohort.
Figure 3.
Kaplan-Meier survival curves for the primary and secondary endpoints in the matched SIERRA-75 and CONTROL groups.
ACS: acute coronary syndrome; DES: drug-eluting stents; EES: everolimus-eluting stents; MI: myocardial infarction; PCI: percutaneous coronary intervention; ST: stent thrombosis; TLR: target lesion revascularization.
Specific Analysis for the Prospective SIERRA-75 Registry
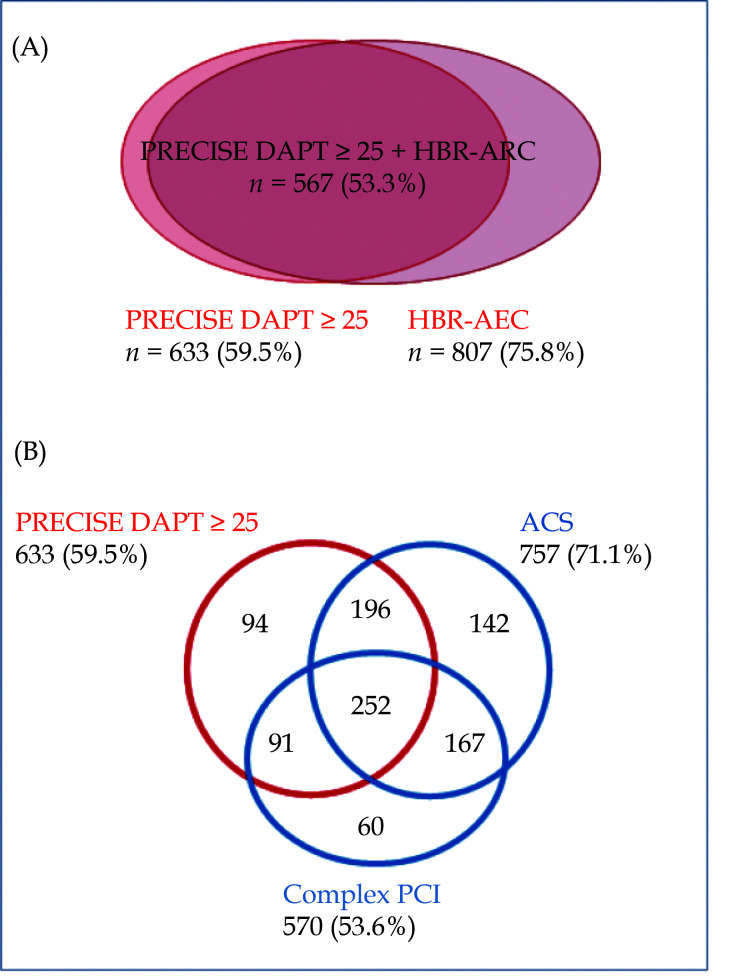
The distributions of patients according to different high bleeding risk criteria and according to bleeding and ischemic risks are shown in Figure 4. There was a highly frequent overlap between conditions. Among the patients deeemed at high risk of bleeding (PRECISE DAPT ≥ 25), 85% showed a high ischemic risk (ACS or complex PCI) and 40% a very high ischemic risk (ACS plus complex PCI). On the other hand, among patients with high ischemic risk (ACS or complex PCI), 59% showed a high risk of bleeding. Thus, despite the overlap, it is still possible to discriminate patients with different risk balances and to provide a more tailored therapy.
Figure 4.
Distribution of patients according to different high bleeding risk criteria (A) and bleeding and ischemic risks criteria (B).
ACS: acute coronary syndrome; HBR-ARC: high bleeding risk based on academic research consortium; PCI: percutaneous coronary intervention.
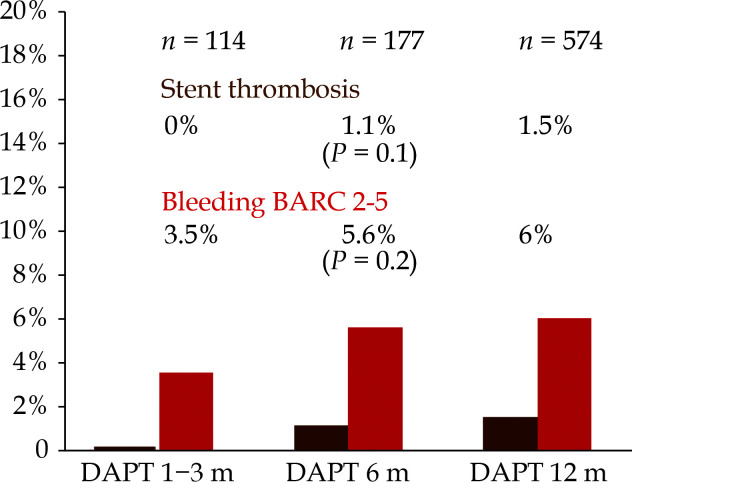
The adjusted hazard ratios for the primary safety endpoints in the short and long antithrombotic therapy subgroups are depicted in Table 3 with no significant effect on outcomes, in overall and highest risk groups. The observation of a slight tendency to more bleeding with a short therapy and to less with a long one shows that only patients with the highest risk of bleeding were referred to short therapy, particularly among those with ACS or complex PCI. All these findings suggest a fairly well individualized approach based on both, ischemic and bleeding risks. The rates of stent thrombosis and major bleeding according to the final duration of DAPT in patients not requiring oral anticoagulation are shown in Figure 5. No stent thrombosis were reported in the subgroup with the shorter DAPT duration (1-3 months).
Table 3. Clinical events at 12 months follow up in matched groups.
| SIERRA-75 | Control | P | |
| N= 686 | N= 686 | ||
| Data are presented as n (%). BL: bleeding BARC 2-5; MI: myocardial infarction; ST: stent thrombosis; TLR: target lesion revascularization. | |||
| Co-Primary safety endpoints | |||
| Cardiac death/MI/ST | 46 (6.7%) | 56 (8.1%) | 0.29 |
| Bleeding BARC 2-5 | 51 (7.4%) | 70 (10.2%) | 0.04 |
| Primary efficacy endpoint | |||
| Target lesion revascularization | 12 (1.7%) | 18 (2.6%) | 0.25 |
| Secondary endpoints | |||
| All cause death | 50 (7.3%) | 56 (8.2%) | 0.53 |
| Cardiac death | 26 (3.8%) | 30 (4.4%) | 0.57 |
| Myocardial infarction | 25 (3.6%) | 32 (4.6%) | 0.35 |
| Stent thrombosis definite/probable | 10 (1.5%) | 16 (2.3%) | 0.23 |
| All revascularizations | 24 (3.5%) | 32 (4.7%) | 0.26 |
| Stroke | 7 (1%) | 6 (0.9%) | 0.84 |
| Cardiac death/MI/ST/TLR | 53 (7.7%) | 64 (9.3%) | 0.27 |
| Cardiac death/MI/ST/TLR/BL | 98 (14.3%) | 127 (18.5%) | 0.02 |
Figure 5.
Rates of stent thrombosis and bleeding BARC 2-5 according to the duration of DAPT in patients without chronic oral anticoagulation.
BARC 2-5: bleeding academic research consortium 2-5; DAPT: dual antiplatelet therapy.
The independent predictors for the co-primary safety endpoint of MACE were diabetes (HR = 1.4, 95% CI: 1.08-1.85; P = 0.01) and glomerular filtration rate < 30 mL/min (HR = 3, 95% CI: 1.27-7.39; P = 0.01) and for the co-primary safety endpoint of bleeding BARC 2-5 were complex PCI (HR = 1.9, 95% CI: 1.05-3.47; P = 0.03) and glomerular filtration rate < 30 mL/min (HR = 2.82, 95% CI: 1.19-6.70; P = 0.02).
DISCUSSION
The main findings of this study are that in the elderly population undergoing PCI with last generation, EES the use of a risks-adjusted antithrombotic strategy based on clinical presentation, PCI complexity and PRECISE DAPT score, could be associated with an improved prognosis in terms of ischemic and bleeding outcomes. The duration of antithrombotic therapy did not have an independent predictive effect on ischemic or hemorrhagic events, which could be attributed to the adjustment of these therapies according to individual ischemic and hemorrhagic risks. Finally, the use of late generation EES in this complex setting was associated with low rate of device related events.
Older patients are more likely to present risky coronary anatomy, for this reason the procedures are often technically more complex. Added to this is the clinical complexity, showing a higher prevalence of comorbidities. On the other hand, antithrombotic treatment after PCI, necessary to reduce the incidence of thrombotic events, is associated with an increased risk of bleeding. Although being elderly per se does not necessarily imply a high risk of bleeding, this does occur when age is associated with other factors such as chronic oral anticoagulation, anemia, gastrointestinal disease, cancer or biological frailty.[1]
Given all these circumstances, antithrombotic treatment in the elderly should be as adjusted as possible to the ischemic and hemorrhagic risk levels of each particular case, avoiding a systematic short DAPT approach.
Because of the high prevalence of complex PCI in this population, we decided to evaluate the performance of a latest generation EES model. EES are supported by an extensive medical literature that consistently shows the low incidence of stent thrombosis.[6] In fact, it is the only DES that has been evaluated in a trial focused on octogenarian population.[10]
Regarding ischemic risk, the clinical indication (ACS versus stable) and the type of procedure (complex versus simple PCI) can be very informative. Regarding hemorrhagic risk, the ARC-HBR classification may be useful, and so the PRECISE DAPT score.[4,5] The latter has been validated in large cohorts, including patients with oral anticoagulation, showing a reasonably good level of prediction,[5,11,12] and was incorporated into the 2017 ESC guidelines on DAPT.[3] To our knowledge, this is the first study evaluating the prospective application of this score in the elderly population undergoing PCI in clinical practice.
There was a highly frequent overlap between high ischemic/bleeding risk conditions, but despite this, it was still possible to prescribe a more tailored therapy. In SIERRA-75 the mean time on antithrombotic combination therapies was significantly shorter than in the retrospective control registry, but it is noteworthy that short and long patterns of antithrombotic therapy were not independent prognostic predictors, nor was the short one associated with more MACE nor the long one associated with a higher incidence of bleeding. This was also true in the groups with a higher level of ischemic risks.
In the comparison with the paired control group, a significant difference is observed in the incidence of major bleeding, most likely related to the more frequent use of short therapies in the SIERRA-75. However, this was not associated with more ischemic events, but rather there was a trend towards a lower incidence of all MACE in the SIERRA-75 group, especially TLR and stent thrombosis, which are more related to the type of stent. This would be in line with studies that have shown a high safety profile for EES with very short DAPT durations.[13]
The primary safety endpoint of MACE was lower as compared to the Biofreedom® arm in the elderly subgroup of LEADERS FREE trial (6.2% vs. 10.7%, P < 0.001) mostly driven by differences in myocardial infarction and stent thrombosis rates. The primary efficacy endpoint of TLR was lower in SIERRA-75 as well (1.5% vs. 5.8%, P < 0.001). [9] In the same context of patients at high risk of bleeding with a short duration of DAPT, the zotarolimus eluting stent Onyx® showed a somewhat higher rate of TLR per year (2.8%) and a similar rate of definitive or probable stent thrombosis (1.3%) and the bioabsorbable polymer drug-eluting stent Synergy® associated a TLR of 2% and a stent thrombosis rate of 1%.[14,15]
Regarding bleeding, it is interesting to note that the systematic application of a very short duration of DAPT in the elderly, one month in LEADERS FREE, associated a higher incidence of BARC 2-5 bleeding (14.3%).[9] However, it is plausible that the elderly patients in this trial presented more additional features of hemorrhagic risk.
In the SENIOR trial, which included patients older than 75 years treated with DAPT for one month in stable condition and 6 months in ACS, the incidence of BARC 2-5 bleeding was more comparable (5%), although the patient profile was less complex than in our real practice SIERRA-75 registry.[15] In SENIOR trial, despite this less complex clinical and PCI profile, the incidence of cardiac death (4%) and MI (4%) at one year was similar or slightly higher than in SIERRA-75.
These findings support the hypothesis that, in the elderly population undergoing PCI, the use of an antithrombotic treatment strategy guided by individual risk levels, estimated through objective variables such as clinical presentation, the complexity of the PCI and the PRECISE DAPT score, may be more convenient in prognostic terms.
LIMITATIONS
The non-randomized design of the study is a major limitation. This is an observational registry and so is affected by the inherent limitations to all observational studies. Even though we applied the same inclusion-exclusion criteria in both retrospective and prospective registries, and despite pairing of patients by propensity score matching, the potential effect of bias is not eliminated and consequently the comparison of results between registries should be taken very cautiously. The study was funded by a company with an important conflict of interest in the study. Nonetheless, the execution of the study was totally in charge of the investigators without any kind of intervention by the funding company. The adherence of the cardiologists in charge of the patients to the proposed risk-based antithrombotic treatment protocol was not totally exhaustive and patient adherence to prescribed therapies was not assessed during follow-up. Noteworthy, an underutilization of intravascular imaging was noted despite the high rate of complex PCI, and this practice could have had an adverse effect on outomes.
CONCLUSIONS
In elderly population undergoing PCI with late generation EES, the implementation of a risks-adjusted antithrombotic protocol based on clinical presentation, PCI complexity and PRECISE DAPT score, seems to be associated with an improved prognosis in terms of both ischemic and bleeding outcomes.
AUTHORSHIP STATEMENT
All individuals listed as authors qualify for authorship and have participated sufficiently in the work to take public responsibility for appropriate portions of the content: (1) substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published.
FUNDING STATEMENT
The study was funded by an unrestricted grant from Abbott Laboratories (COR10620). Study design, selection of participating centers, collection, management and interpretation of data and manuscript preparation was an exclusive responsibility of the investigators in EPIC foundation and association, with no involvement/interference of the funding company.
CLINICAL TRIAL REGISTRATION
CONFLICTS OF INTEREST
Jose M de la Torre Hernandez: Receipt of grants / research supports: Abbott Medical, Biosensors, Bristol Myers Squibb, Amgen. Receipt of honoraria or consultation fees: Boston Scientific, Medtronic, Biotronik, Astra Zeneca, Daiichi-Sankyo. Juan Sanchis: Fees for talks from Abbott Vascular
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
ACKNOWLEDGEMENT
I certify that persons who have made substantial contributions to the research and/or manuscript but who do not fulfill authorship criteria, are named with their specific contributions in this Acknowledgments section of the manuscript; and all persons named in this Acknowledgments section have provided the corresponding author with permission to be named in the manuscript. Mario Sadaba Sagredo (Hospital de Galdakao, Bilbao, Spain); Geoffrey Yanes Bowden (Hospital Clinico Universitario de Canarias, Santa Cruz de Tenerife, Spain); Vicente Peral (Hospital Universitario Son Espases, Palma de Mallorca, Spain); Eduardo Molina (Hospital Universitario Virgen de las Nieves, Granada, Spain); Fernando Sarnago (Hospital Universitario 12 de Octubre, Madrid, Spain); Imanol Otaegui (Hospital de Vall d´Hebron, Barcelona, Spain); Miguel Artaiz Urdaci (Clinica Universitaria de Navarra, Pamplona, Spain); Dinis Martins (Hospital do Divino Espirito Santo, Ponta Delgada, Portugal); Jose Moreu (Hospital Virgen de la Salud, Toledo, Spain); Jose Luis Díez Gil (Hospital La Fe, Valencia, Spain); Francisco J Morales (Hospital Puerto Real, Cadiz, Spain); Víctor Aragón Extremera (Complejo Hospitalario de Jaen, Jaen, Spain); Ignacio Cruz (Hospital Clinico Universitario de Salamanca, Salamanca, Spain); Alfonso Torres Bosco (Hospital Txagorritxu-Araba, Vitoria, Spain); Javier Robles (Hospital de Burgos, Burgos, Spain); Julio Hernandez (Hospital Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain).
References
- 1.Andreotti F, Rocca B, Husted S, et al Antithrombotic therapy in the elderly: Expert position paper of the European society of cardiology working group on thrombosis. Eur Heart J. 2015;36:3238–3249. doi: 10.1093/eurheartj/ehv304. [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Bittl JA, et al 2016 ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 3.Valgimigli M, Bueno H, Byrne RA, et al 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 4.Urban P, Mehran R, Colleran R, et al Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140:240–261. doi: 10.1161/CIRCULATIONAHA.119.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa F, van Klaveren D, James S, et al Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 6.Palmerini T, Biondi-Zoccai G, Della Riva D, et al Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 7.Valgimigli M, Sabaté M, Kaiser C, et al Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. BMJ. 2014;349:g6427. doi: 10.1136/bmj.g6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsis A, Valgimigli M Device profile of the XIENCE V and XIENCE Sierra stents for the treatment of coronary artery disease: an overview of safety and efficacy. Expert Rev Med Devices. 2020;17:383–390. doi: 10.1080/17434440.2020.1747434. [DOI] [PubMed] [Google Scholar]
- 9.Morice MC, Talwar S, Gaemperli O, et al Drug-coated versus bare-metal stents for elderly patients: a predefined sub-study of the LEADERS FREE trial. Int J Cardiol. 2017;243:110–115. doi: 10.1016/j.ijcard.2017.04.079. [DOI] [PubMed] [Google Scholar]
- 10.de Belder A, de la Torre Hernandez JM, Lopez-Palop R, et al. A prospective randomized trial of everolimus-eluting stents versus bare-metal stents in octogenarians: the XIMA Trial (Xience or Vision Stents for the Management of Angina in the Elderly) J Am Coll Cardiol 2014; 63: 1371–1375.
- 11.Costa F, Van Klaveren D, Feres F, et al PRECISE-DAPT Study investigators. dual antiplatelet therapy duration based on ischemic and bleeding risks after coronary stenting. J Am Coll Cardiol. 2019;73:741–754. doi: 10.1016/j.jacc.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 12.Costa F, Valgimigli M, Steg PG, et al Antithrombotic therapy according to baseline bleeding risk in patients with atrial fibrillation undergoing percutaneous coronary intervention: applying the PRECISE-DAPT score in RE-DUAL PCI. Eur Heart J Cardiovasc Pharmacother. 2022;8:216–222. doi: 10.1093/ehjcvp/pvaa135. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe H, Domei T, Morimoto T, et al STOPDAPT-2 Investigators. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 Randomized Clinical Trial. JAMA. 2019;321:2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windecker S, Latib A, Kedhi E, et al ONYX ONE Investigators. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med. 2020;382:1208–1218. doi: 10.1056/NEJMoa1910021. [DOI] [PubMed] [Google Scholar]
- 15.Varenne O, Cook S, Sideris G, et al SENIOR investigators. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR):a randomised single-blind trial. Lancet. 2018;391:41–50. doi: 10.1016/S0140-6736(17)32713-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.