Key Points
Question
Compared with estimated glomerular filtration rate (eGFR) equations that include Black vs non-Black race or cystatin C, do eGFR equations without race or cystatin C alter estimates of racial differences in risk of kidney failure requiring replacement therapy and mortality?
Findings
In this retrospective individual-level data analysis of 62 011 participants, decreased eGFR was related to higher risk for all equations and within both racial groups. Compared with alternative equations, the creatinine-based eGFR equation without race attenuated racial differences in the risk of kidney failure requiring replacement therapy and mortality at normal eGFR and showed no significant racial differences in the risk at reduced eGFR.
Meaning
Among eGFR equations without race, the equation including both creatinine and cystatin C, but not the equation including creatinine without cystatin C, demonstrated racial differences in the risk of kidney failure requiring replacement therapy and mortality.
Abstract
Importance
At a given estimated glomerular filtration rate (eGFR), individuals who are Black have higher rates of mortality and kidney failure with replacement therapy (KFRT) compared with those who are non-Black. Whether the recently adopted eGFR equations without race preserve racial differences in risk of mortality and KFRT at a given eGFR is unknown.
Objective
To assess whether eGFR equations with and without race and cystatin C document racial differences in risk of KFRT and mortality in populations including Black and non-Black participants.
Design, Setting, and Participants
Retrospective individual-level data analysis of 62 011 participants from 5 general population and 3 chronic kidney disease (CKD) US-based cohorts with serum creatinine, cystatin C, and follow-up for KFRT and mortality from 1988 to 2018.
Exposures
Chronic Kidney Disease Epidemiology Collaboration equation with serum creatinine (eGFRcr with and without race), cystatin C (eGFRcys without race), or both markers (eGFRcr-cys without race).
Main Outcomes and Measures
The prevalence of decreased eGFR at baseline and hazard ratios of KFRT and mortality in Black vs non-Black participants were calculated, adjusted for age and sex. Analyses were performed within each cohort and with random-effect meta-analyses of the models.
Results
Among 62 011 participants (20 773 Black and 41 238 non-Black; mean age, 63 years; 53% women), the prevalence ratio (95% CI; percent prevalences) of eGFR less than 60 mL/min/1.73 m2 comparing Black with non-Black participants was 0.98 (95% CI, 0.93-1.03; 11% vs 12%) for eGFRcr with race, 0.95 (95% CI, 0.91-0.98; 17% vs 18%) for eGFRcys, and 1.2 (95% CI, 1.2-1.3; 13% vs 11%) for eGFRcr-cys but was 1.8 (95% CI, 1.7-1.8; 15% vs 9%) for eGFRcr without race. During a mean follow-up of 13 years, 8% and 4% of Black and non-Black participants experienced KFRT and 34% and 39% died, respectively. Decreased eGFR was associated with significantly greater risk of both outcomes for all equations. At an eGFR of 60 mL/min/1.73 m2, the hazard ratios for KFRT comparing Black with non-Black participants were 2.8 (95% CI, 1.6-4.9) for eGFRcr with race, 3.0 (95% CI, 1.5-5.8) for eGFRcys, and 2.8 (95% CI, 1.4-5.4) for eGFRcr-cys vs 1.3 (95% CI, 0.8-2.1) for eGFRcr without race. The 5-year absolute risk differences for KFRT comparing Black with non-Black participants were 1.4% (95% CI, 0.2%-2.6%) for eGFRcr with race, 1.1% (95% CI, 0.2%-1.9%) for eGFRcys, and 1.3% (95% CI, 0%-2.6%) for eGFRcr-cys vs 0.37% (95% CI, −0.32% to 1.05%) for eGFRcr without race. Similar patterns were observed for mortality.
Conclusions and Relevance
In this retrospective analysis of 8 US cohorts including Black and non-Black individuals, the eGFR equation without race that included creatinine and cystatin C, but not the eGFR equation without race that included creatinine without cystatin C, demonstrated racial differences in the risk of KFRT and mortality throughout the range of eGFR. The eGFRcr-cys equation may be preferable to the eGFRcr equation without race for assessing racial differences in the risk of KFRT and mortality associated with low eGFR.
This individual-level data analysis assesses whether estimated glomerular filtration rate (eGFR) equations with and without race and cystatin C document racial differences in the risk of kidney failure with replacement therapy (KFRT) and mortality in populations including Black and non-Black participants.
Introduction
Studies that used Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations with creatinine (alone or with cystatin C) and included a coefficient for race (Black or non-Black) to account for higher mean serum creatinine concentrations in Black vs non-Black individuals documented racial differences in the association of a low estimated glomerular filtration rate (eGFR) with risk of kidney failure with replacement therapy (KFRT) and mortality.1 However, the use of race in GFR estimation and other clinical algorithms has been challenged because race is a social and not a biologic construct and does not fully capture the diversity within vs between racial groups.2
A creatinine-based eGFR equation that does not incorporate race was developed by CKD-EPI in 20213 and recommended for implementation in clinical laboratories by the National Kidney Foundation–American Society of Nephrology (NKF-ASN) Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease.4 This equation had small biases compared with directly measured GFR in Black and non-Black individuals, with a median of up to 4 mL/min/1.73 m2 lower and higher values than measured GFR, respectively. As a result of eliminating race from eGFR, Black individuals are estimated to have a 2.0% higher prevalence of chronic kidney disease (CKD), and non-Black individuals a 1.5% lower prevalence, compared with older equations that included race. Whether these changes alter the association of decreased eGFR values with higher rates of KFRT and mortality within and between racial groups is unknown. This is important for assessing efforts to reduce the higher risk of KFRT in Black compared with non-Black individuals and tailoring risk-reduction strategies across the full spectrum of eGFR.
This study examined whether GFR estimating equations based on different filtration markers (creatinine [eGFRcr] vs cystatin C [eGFRcys] vs both [eGFRcr-cys]) and equations that included or excluded race altered risk estimates for KFRT and mortality for Black compared with non-Black participants.
Methods
Chronic Kidney Disease Prognosis Consortium
This study was approved for use of deidentified data by the institutional review board at the Johns Hopkins Bloomberg School of Public Health. The need for informed consent was waived by the institutional review board. The Chronic Kidney Disease Prognosis Consortium (CKD-PC) is a research group consisting of investigators representing cohorts from around the world. The consortium has been described elsewhere.5 Investigators shared data for the purpose of collaborative meta-analyses to study prognosis in CKD. This study focused on cohorts that included both Black and non-Black individuals, had available creatinine and cystatin C (11 065 participants were excluded due to missing cystatin C data), and participated in analyses relating eGFRcr and eGFRcys to the outcomes of KFRT and mortality. The study included 5 general population cohorts (Atherosclerosis Risk in Communities [ARIC], Cardiovascular Health Study [CHS], Multi-Ethnic Study of Atherosclerosis [MESA], National Health and Nutrition Examination Survey [NHANES] III, and Reasons for Geographic and Racial Differences in Stroke [REGARDS]) and 3 CKD cohorts (African American Study of Kidney Disease and Hypertension [AASK], Modification of Diet in Renal Disease [MDRD], and Chronic Renal Insufficiency Cohort [CRIC]).6,7,8,9,10,11 Data were collected from 1988 to 2018. Information on race was provided in the original cohort data and was self-reported by participants in most cohorts using fixed categories (eAppendix 1 in Supplement 1).
eGFR Equations
Measurement of creatinine and cystatin C followed previously reported methods.12,13,14,15 Values from each cohort were calibrated to the extent possible using the available information. Serum creatinine assays were calibrated to the Roche enzymatic method (Roche-Hitachi P-Module instrument with Roche Creatinine Plus assay; Hoffman-La Roche Ltd), traceable to National Institute of Standards and Technology creatinine standard reference material 967.16 Serum cystatin C assays were calibrated to the Siemens Dade Behring Nephelometer, traceable to International Federation of Clinical Chemistry and Laboratory Medicine Working Group for the Standardization of Serum Cystatin C and the Institute for Reference Materials and Measurements certified reference materials.17,18
Seven GFR estimating equations developed by the CKD-EPI collaboration and recommended or discussed by the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines or the 2021 NKF-ASN Task Force were analyzed.1,4 The primary focus was on 4 equations (2009 eGFRcr[ASR], 2021 eGFRcr[AS], 2012 eGFRcys[AS], and 2021 eGFRcr-cys[AS]), including those which calculate eGFR after removing race (AS [age and sex] vs ASR [age, sex, and race]) and using cystatin C (cr-cys vs cr) as recommended in 2021. Because the race coefficient for eGFRcr-cys(ASR) is small, results are presented in eTables 4 and 6 and eFigure 1 in Supplement 1, as are those for 2 additional equations that were not recommended by the NKF-ASN Task Force (eGFRcr[ASR-NB] and eGFRcr-cys[ASR-NB]). The modifier non-Black (NB) in eGFRcr(ASR-NB) and eGFRcr-cys(ASR-NB) denotes omitting the Black race coefficient from the 2009 equations without refitting other coefficients.2 The abbreviations indicate the year of equation development (2009,12 2012,13 or 20213), the filtration markers used (creatinine [cr] and/or cystatin C [cys]), and the demographic variables included (age, sex, and/or race [ASR]), consistent with previous studies.3,4 All of the equations evaluated were developed by CKD-EPI. Therefore, the abbreviations do not include CKD-EPI as a prefix. eGFRcr(ASR) was defined as the reference because it is the most widely used of these equations.12,13 eTable 1 in Supplement 1 summarizes the nomenclature, variables, recommended use, and performance of eGFR relative to measured GFR for each equation. eTable 2 in Supplement 1 shows the formulas used for each equation.3,4
Outcomes
The primary outcomes were KFRT, all-cause mortality, and cardiovascular mortality. Six cohorts had data on KFRT, defined as start of kidney replacement therapy or death due to kidney disease other than acute kidney injury. Mortality and cause of death were available for each cohort. Cardiovascular mortality was defined as death due to myocardial infarction, heart failure, sudden cardiac death, or stroke.
Statistical Analyses
Analyses were conducted retrospectively within each cohort, and meta-analyses were performed across cohorts using multivariable random-effects models (eAppendix 1.2 in Supplement 1). eGFR distribution analyses (density plots and prevalence estimates) were limited to the general population cohorts, and confidence intervals were estimated by bootstrap. For the association of eGFR with outcomes, eGFR from each equation was modeled as piece-wise linear splines with knots at 60 mL/min/1.73 m2 and 90 mL/min/1.73 m2. Analyses were performed within each cohort using Cox proportional hazards regression models adjusted for age, sex, and interaction terms for eGFR splines and race (non-Black vs Black). eGFR spline coefficients were then combined across cohorts using multivariate meta-analysis and hazard ratios with 95% CIs, estimated for each 1 mL/min/1.73 m2 of eGFR from 15 to 120 for Black and non-Black participants separately, compared with a reference of 80 mL/min/1.73 m2 in non-Black participants. Log-log plots (ie, visual inspection) confirmed validity of the proportional hazards assumption using eGFR categories by different equations. Differences between Black and non-Black individuals for a given equation and eGFR level were expressed as hazard ratios and differences in estimated 5-year risk. Point estimates were displayed at eGFR values of 60 mL/min/1.73 m2 and 30 mL/min/1.73 m2, because they define CKD stages. Difference in estimated 5-year risk was calculated by combining the meta-analyzed hazard ratio with the 5-year risk for the reference point estimated from the REGARDS cohort. For analyses of KFRT, death was a censoring event. To examine consistency across cohorts, relative hazard ratios were calculated as the ratio of the hazard ratios at specific values of eGFR for each eGFR equation compared with eGFRcr(ASR) within each cohort (eAppendix 1.2 in Supplement 1).
In exploratory analysis, the predictive discrimination performance of each eGFR equation with demographic variables was examined using 5-fold cross-validation C statistics, separately within each cohort. C statistics were summarized across cohorts as the median and interquartile interval. Change in C statistics between different eGFR equations within each cohort was estimated with the user-written “somersd” command19 and then with meta-analysis across cohorts. The significance threshold of each hypothesis test was set at 2-sided P < .05. Because of the potential for type I error due to multiple comparisons, findings of the analyses should be interpreted as exploratory. All analyses were conducted using Stata MP version 16 (StataCorp).
Results
Study Populations
For these analyses, the 5 population-based cohorts included 18 073 Black participants and 38 017 non-Black participants (2% Asian, 8% Hispanic, 89% non-Hispanic White, and 1% other race and ethnicity [listed for each individual cohort in eAppendix 1.4 in Supplement 1]) and the 3 CKD cohorts included 2700 Black participants and 3221 non-Black participants (15% Hispanic, 78% non-Hispanic White, and 7% other race and ethnicity) for a total of 62 011 participants. The mean (SD) age was 63 (12) years, and 53% were women, with no missing data for age, sex, or race (Table 1; eTable 3 in Supplement 1).
Table 1. Baseline Characteristics and Outcomes Among Study Participants by Cohort and Self-reported Race Group.
| Source | Participant race | No. | Age, mean (SD), y | Female, % | Mean (SD), mL | Kidney failure requiring treatment | All-cause mortality | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFRcr | eGFRcys | eGFRcr-cys | ||||||||||
| Age, sex, and race | Age and sex | Age and sex | Age and sex | Follow-up, mean (SD), y | 5-y risk, % | Follow-up, mean (SD), y | 5-y risk, % | |||||
| General population | ||||||||||||
| ARIC | Black | 2484 | 62 (6) | 64 | 92 (21) | 83 (18) | 89 (19) | 89 (18) | 21.0 (2.7) | 0.65 (0.40-1.05) | 17.2 (6.0) | 6.1 (5.2-7.1) |
| Non-Black | 8731 | 63 (6) | 53 | 85 (14) | 89 (14) | 83 (18) | 89 (14) | 21.3 (1.7) | 0.31 (0.21-0.45) | 17.8 (5.6) | 5.1 (4.6-5.6) | |
| CHS | Black | 495 | 77 (5) | 65 | 71 (19) | 66 (17) | 69 (20) | 70 (19) | 10.7 (5.4) | 19.4 (16.2-23.2) | ||
| Non-Black | 2489 | 78 (5) | 58 | 65 (16) | 69 (16) | 65 (18) | 69 (17) | 10.0 (5.4) | 22.5 (20.9-24.2) | |||
| MESA | Black | 1846 | 62 (10) | 55 | 86 (19) | 79 (16) | 90 (20) | 88 (18) | 12.7 (3.3) | 4.9 (4.0-6.0) | ||
| Non-Black | 4848 | 62 (10) | 52 | 82 (15) | 86 (15) | 89 (20) | 91 (17) | 13.2 (3.0) | 3.4 (2.9-3.9) | |||
| NHANES III | Black | 1742 | 51 (20) | 52 | 96 (29) | 86 (25) | 98 (27) | 95 (26) | 18.0 (7.8) | 9.9 (8.6-11.4) | ||
| Non-Black | 5280 | 59 (20) | 53 | 86 (24) | 90 (23) | 88 (28) | 92 (26) | 16.5 (8.1) | 12.7 (11.8-13.6) | |||
| REGARDS | Black | 11 506 | 65 (9) | 61 | 88 (24) | 80 (21) | 79 (24) | 82 (23) | 11.3 (3.6) | 1.62 (1.40-1.88) | 11.9 (3.8) | 9.0 (8.5-9.5) |
| Non-Black | 16 669 | 66 (9) | 50 | 82 (17) | 86 (17) | 77 (22) | 84 (20) | 11.4 (3.4) | 0.36 (0.27-0.46) | 12.0 (3.6) | 8.0 (7.6-8.4) | |
| Overall | Black | 18 073 | 63 (11) | 60 | 89 (24) | 81 (21) | 83 (24) | 85 (22) | 12.7 (5.2) | 1.47 (1.28-1.68) | 13.2 (5.2) | 8.6 (8.2-9.0) |
| Non-Black | 38 017 | 65 (12) | 52 | 82 (18) | 86 (18) | 81 (23) | 86 (20) | 14.0 (5.9) | 0.41 (0.34-0.50) | 14.0 (5.7) | 8.3 (8.1-8.6) | |
| CKD population | ||||||||||||
| AASK | Black | 949 | 55 (11) | 39 | 46 (15) | 42 (13) | 48 (18) | 45 (15) | 7.4 (3.4) | 16.5 (14.3-19.1) | 7.7 (3.4) | 9.3 (7.5-11.4) |
| CRIC | Black | 1650 | 58 (11) | 51 | 44 (15) | 40 (13) | 51 (23) | 46 (18) | 8.0 (4.5) | 22.7 (20.7-24.9) | 9.8 (4.1) | 14.4 (12.8-16.2) |
| Non-Black | 2277 | 58 (11) | 41 | 45 (15) | 47 (16) | 54 (24) | 51 (20) | 8.8 (4.4) | 15.7 (14.2-17.3) | 10.1 (3.7) | 11.2 (10.0-12.6) | |
| MDRD | Black | 101 | 50 (12) | 44 | 36 (17) | 33 (15) | 35 (16) | 33 (15) | 7.7 (6.0) | 37.0 (28.2-47.4) | 13.4 (5.4) | 9.9 (5.5-17.6) |
| Non-Black | 944 | 52 (13) | 39 | 35 (15) | 36 (16) | 32 (14) | 33 (15) | 7.8 (5.7) | 39.3 (36.2-42.5) | 14.1 (5.0) | 8.1 (6.5-10.0) | |
| Overall | Black | 2700 | 56 (11) | 47 | 44 (15) | 38 (13) | 49 (21) | 45 (17) | 7.8 (4.2) | 21.1 (19.5-22.7) | 9.2 (4.1) | 12.5 (11.3-13.9) |
| Non-Black | 3221 | 56 (12) | 40 | 42 (16) | 44 (16) | 47 (24) | 46 (20) | 8.5 (4.8) | 22.8 (21.3-24.3) | 11.3 (4.5) | 10.3 (9.3-11.4) | |
| Populations combined | ||||||||||||
| Overall | Black | 20 773 | 62 (11) | 59 | 83 (27) | 72 (23) | 79 (26) | 80 (26) | 12.0 (5.3) | 7.3 (6.9-7.7) | 12.7 (5.3) | 9.1 (8.7-9.5) |
| Non-Black | 41 238 | 64 (12) | 51 | 79 (21) | 83 (21) | 78 (24) | 83 (23) | 13.4 (6.0) | 3.9 (3.7-4.1) | 13.8 (5.6) | 8.5 (8.2-8.8) | |
Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; CKD, chronic kidney disease; CRIC, Chronic Renal Insufficiency Cohort; cr, creatinine; cys, cystatin; eGFR, estimated glomerular filtration rate; MESA, Multi-Ethnic Study of Atherosclerosis; MDRD, Modification of Diet in Renal Disease; NHANES, National Health and Nutrition Examination Survey III; REGARDS, Reasons for Geographic and Racial Differences in Stroke.
Prevalences of Decreased eGFR by Race and Their Ratios Across Equations
The prevalences of eGFR less than 60 mL/min/1.73 m2 (CKD stage G3+) in Black vs non-Black individuals were 11% vs 12% for eGFRcr(ASR), 17% vs 18% for eGFRcys(AS), and 13% vs 11% for eGFRcr-cys(AS) compared with 15% vs 9% among Black vs non-Black individuals for eGFRcr(AS) (Table 2, Figure 1; eTable 4 in Supplement 1). As a result, the Black relative to non-Black prevalence ratios of eGFR less than 60 mL/min/1.73 m2 were 0.98 (95% CI, 0.93-1.03) for eGFRcr(ASR), 0.95 (95% CI, 0.91-0.98) for eGFRcys(AS), 1.2 (95% CI, 1.2-1.3) for eGFRcr-cys(AS), and 1.8 (95% CI, 1.7-1.8) for eGFRcr(AS).
Table 2. Prevalence of Decreased eGFR and Age- and Sex-Adjusted Hazard Ratios of KFRT for Different GFR Estimating Equations, by Racea.
| Equation | Participant race | Prevalence in general population cohorts and their ratio by race, % (95% CI) | Hazard ratios of KFRT vs eGFR = 80 mL/min/1.73 m2 and their ratio by race (95% CI) | |||
|---|---|---|---|---|---|---|
| eGFR <30 mL/min/1.73 m2 | eGFR <60 mL/min/1.73 m2 | eGFR = 30 mL/min/1.73 m2 | eGFR = 60 mL/min/1.73 m2 | eGFR = 80 mL/min/1.73 m2 | ||
| 2009 eGFRcr (age, sex, and race) | Black | 1.7 (1.5-1.9) | 11 (11-12) | 119 (41-351) | 9.3 (4.9-17.9) | 3.7 (2.0-6.7) |
| Non-Black | 0.70 (0.61-0.78) | 12 (11-12) | 74 (35-160) | 3.3 (2.5-4.4) | 1 [Reference] | |
| Ratio, Black vs non-Black | 2.5 (2.0-2.9) | 0.98 (0.93-1.03) | 1.6 (1.1-2.3) | 2.8 (1.6-4.9) | 3.7 (2.0-6.7) | |
| 2021 eGFRcr (age and sex) | Black | 1.9 (1.7-2.2) | 15 (15-16) | 78 (27-226) | 5.1 (2.6-9.9) | 2.3 (1.2-4.2) |
| Non-Black | 0.52 (0.44-0.59) | 8.7 (8.4-9.0) | 74 (32-172) | 3.8 (2.6-5.7) | 1 [Reference] | |
| Ratio, Black vs non-Black | 3.8 (3.1-4.4) | 1.8 (1.7-1.8) | 1.0 (0.8-1.4) | 1.3 (0.8-2.1) | 2.3 (1.2-4.2) | |
| 2012 eGFRcys (age and sex) | Black | 2.7 (2.5-2.9) | 17 (16-17) | 84 (24-299) | 8.0 (3.6-17.8) | 3.2 (2.0-5.0) |
| Non-Black | 1.8 (1.7-1.9) | 18 (17-18) | 41 (14-126) | 2.7 (1.7-4.3) | 1 [Reference] | |
| Ratio, Black vs non-Black | 1.5 (1.3-1.7) | 0.95 (0.91-0.98) | 2.0 (1.0-4.0) | 3.0 (1.5-5.8) | 3.2 (2.0-5.0) | |
| 2021 eGFRcr-cys (age and sex) | Black | 2.2 (1.9-2.4) | 13 (13-14) | 119 (37-377) | 9.7 (5.0-18.9) | 2.9 (1.6-5.3) |
| Non-Black | 0.81 (0.72-0.90) | 11 (10-11) | 74 (32-170) | 3.5 (2.6-4.8) | 1 [Reference] | |
| Ratio, Black vs non-Black | 2.7 (2.3-3.1) | 1.2 (1.2-1.3) | 1.6 (1.1-2.4) | 2.8 (1.4-5.4) | 2.9 (1.6-5.3) | |
Abbreviations: cr, creatinine; cys, cystatin; eGFR, estimated glomerular filtration rate; KFRT, kidney failure with replacement therapy.
Total sample size of 56 090 for prevalence estimates in general population cohorts, and 45 311 in hazard ratio estimation of KFRT. Ratios in Black compared with non-Black participants are prevalence ratios for the first 2 data columns and hazard ratios for the last 3 columns, and calculated by dividing the estimate in Black participants by the estimate in non-Black participants. Note that the ratio of 2 hazard ratios with the same reference group, eg, non-Black participants at eGFR of 80 mL/min/1.73 m2, is itself a hazard ratio. For example, 1.6 is the hazard ratio of KFRT in Black compared with non-Black participants at an eGFRcr(ASR) of 30 mL/min/1.73 m2.
Figure 1. Risk of Kidney Failure With Replacement Therapy (KFRT) Associated With Level of Estimated Glomerular Filtration Rate (eGFR) in Black and Non-Black Participants for Different GFR Estimating Equations.
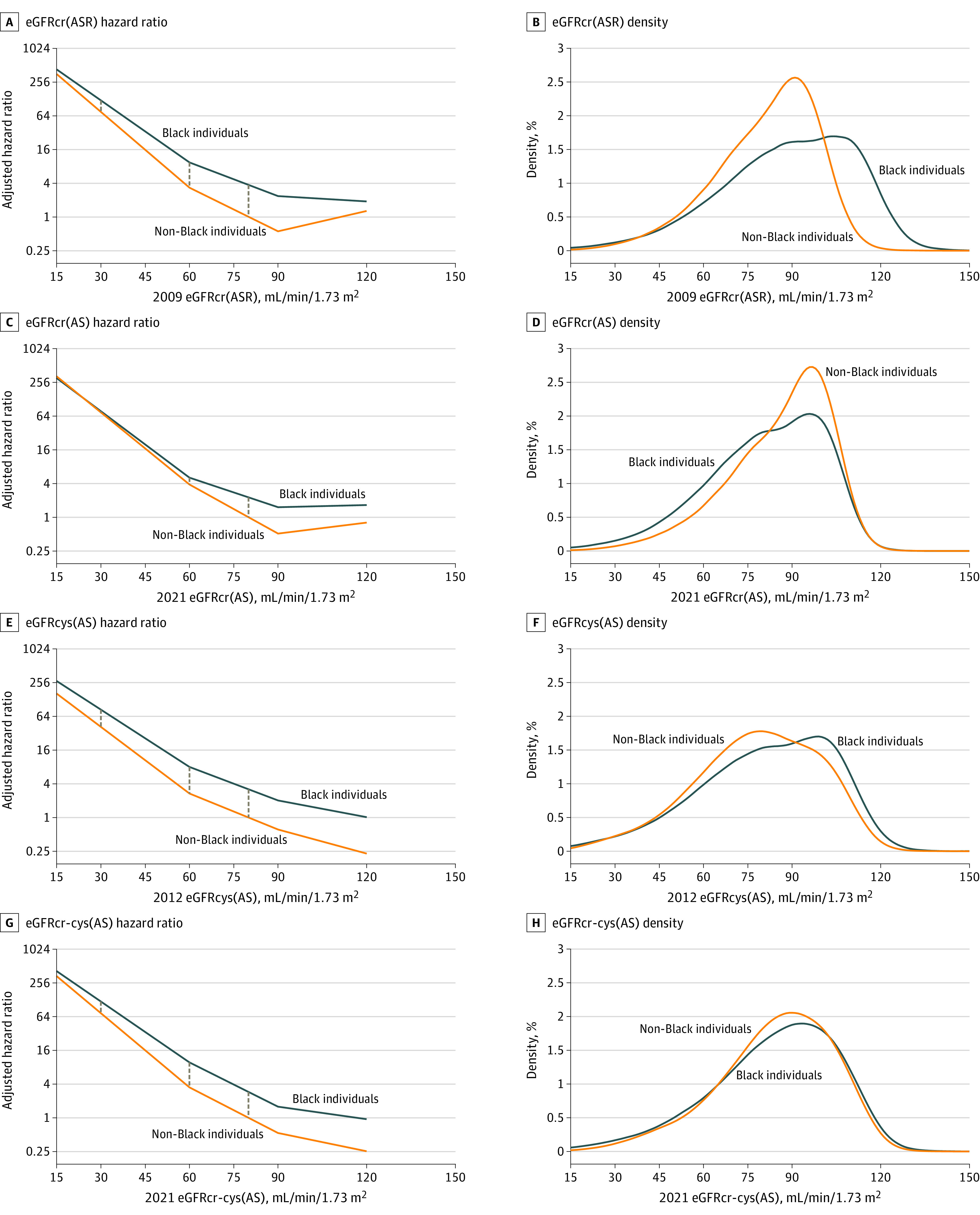
Vertical lines show the difference in log hazard ratios between Black and non-Black participants for a given eGFR, which are smaller for 2021 eGFRcr(AS) than for the other equations. The hazard ratio is adjusted for age and sex and calculated vs the reference point (80 mL/min/1.73 m2 in the non-Black population). Point estimates at eGFRs of 30, 60, and 80 mL/min/1.73 m2 are reported in Table 2. The KFRT meta-analysis included participants from AASK, ARIC, CRIC, MDRD, and REGARDS. Density plots show the distribution of eGFR in the population-based cohorts (ARIC and REGARDS). AS indicates age and sex and ASR, age, sex, and race.
Comparison of Risk Associations for KFRT
A total of 3265 KFRT events occurred over a mean (SD) follow-up of 12 (5) years in Black participants and 13 (6) years in non-Black participants. The 5-year estimated absolute risks of KFRT were 7.3% (95% CI, 6.9%-7.7%) in Black and 3.9% (95% CI, 3.7%-4.1%) in non-Black participants overall. For all equations, in both Black and non-Black populations, the risk of KFRT was significantly greater at lower vs higher eGFR (Figure 1; eFigure 1 in Supplement 1). Age- and sex-adjusted risks of KFRT were significantly higher in Black compared with non-Black participants for eGFRcr(ASR), eGFRcys(AS), and eGFRcr-cys(AS) across all eGFR levels (Figure 1). For example, the age- and sex-adjusted hazard ratio of KFRT at an eGFR of 60 mL/min/1.73 m2 (compared with the referent of non-Black persons at an eGFR of 80 mL/min/1.73 m2) was 9.3 (95% CI, 4.9-17.9) in Black vs 3.3 (95% CI, 2.5-4.4) in non-Black individuals using eGFRcr(ASR), resulting in a significantly higher risk of KFRT in Black relative to non-Black participants (hazard ratio, 2.8 [95% CI, 1.6-4.9], Table 2; 5-year absolute estimated risk difference, 1.4% [95% CI, 0.2%-2.6%], eTable 4 in Supplement 1). Similar hazard ratios were observed for eGFRcys(AS) and eGFRcr-cys(AS): the Black relative to non-Black participant hazard ratios of KFRT at an eGFR of 60 mL/min/1.73 m2 compared with the reference of eGFR of 80 mL/min/1.73 m2 were 3.0 (95% CI, 1.5-5.8) and 2.8 (95% CI, 1.4-5.4), respectively (5-year risk differences of 1.1% [95% CI, 0.2%-1.9%] and 1.3% [95% CI, 0%-2.6%]). In contrast, racial differences in KFRT risk were smaller for eGFRcr(AS) at values of 60 mL/min/1.73 m2 or lower (Figure 1), with the Black relative to non-Black participant hazard ratio of KFRT at an eGFR of 60 mL/min/1.73 m2 no longer significantly different (hazard ratio, 1.3 [95% CI, 0.8-2.1]; 5-year risk difference, 0.37% [95% CI, −0.32% to 1.05%]). Results were similar at an eGFR of 30 mL/min/1.73 m2, with absence of statistically significant racial differences in risk of KFRT for eGFRcr(AS) in contrast to the other 3 equations (Table 2; eFigure 2 in Supplement 1). Similar patterns of significantly higher estimated risk of KFRT in Black relative to non-Black participants were observed for eGFRcr-cys(ASR) and eGFRcr-cys(ASR-NB), whereas eGFRcr(ASR-NB) was similar to eGFRcr(AS), which showed attenuation of racial differences in risk (eTable 4 in Supplement 1).
Comparisons of equations applied within each cohort showed that compared with eGFRcr(ASR), eGFRcys(AS) and eGFRcr-cys(AS) resulted in similar estimates (7 of the 8 relative hazard ratios were close to 1.0 with confidence intervals including 1.0; Figure 2; eTable 5 in Supplement 1). In contrast, the comparison of eGFRcr(AS) with eGFRcr(ASR) resulted in relative hazard ratios significantly less than 1.0 in all 4 cohorts with data, indicating lower Black relative to non-Black participant hazard ratio estimates with eGFRcr(AS).
Figure 2. Relative Hazard Ratios for Black vs Non-Black Participants Using Different Glomerular Filtration Rate (GFR) Estimating Equations Compared With 2009 eGFRcr(ASR) in Each Participating Cohort.
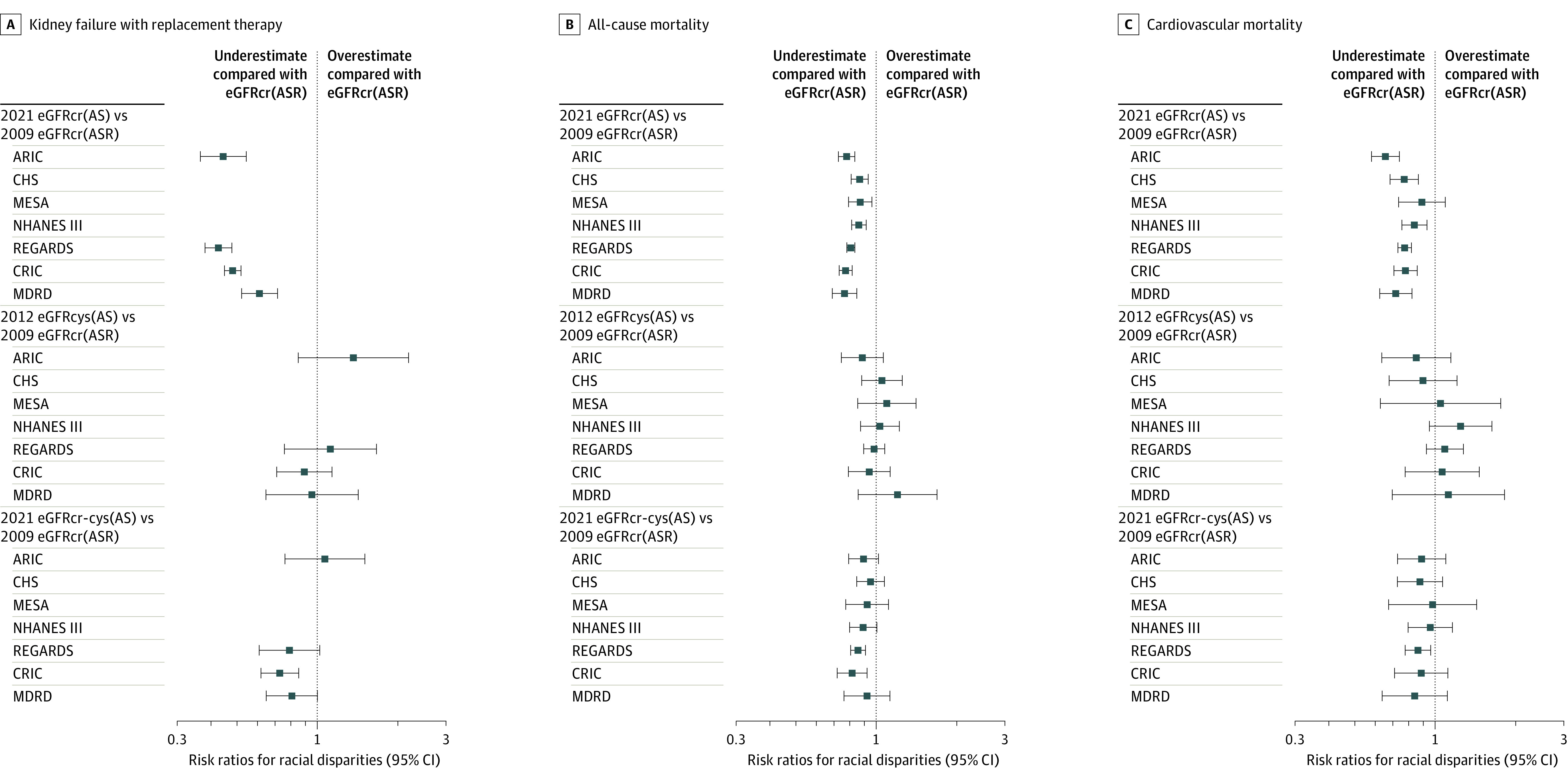
Estimates are the ratio of Black vs non-Black participant hazard ratio at eGFR of 60 mL/min/1.73 m2 using each equation compared with the corresponding hazard ratio for the 2009 eGFRcr(ASR) adjusted for age and sex. The 2021 eGFRcr(AS) results in a significant attenuation of racial differences compared with the 2009 eGFRcr(ASR) in 17 of 18 relative hazard ratios (95% CIs did not include 1.0). In contrast, for the 2012 eGFRcys(AS) and 2021 eGFRcr-cys(AS), 32 of 36 95% CIs for the relative hazard ratios contain 1.0. Risk ratios less than 1 represent underestimation compared with eGFRcr (age, sex, and race). The data are available in eTable 5 in Supplement 1. ARIC indicates Atherosclerosis Risk in Communities; AS, age and sex; ASR, age, sex, and race; CHS, Cardiovascular Health Study; CRIC, Chronic Renal Insufficiency Cohort; MDRD, Modification of Diet in Renal Disease; MESA, Multi-Ethnic Study of Atherosclerosis; NHANES, National Health and Nutrition Examination Survey; and REGARDS, Reasons for Geographic and Racial Differences in Stroke.
Comparison of Risk Associations for All-Cause and Cardiovascular Mortality
Decreased eGFR was associated with significantly higher age- and sex-adjusted risk for all-cause and cardiovascular mortality for all equations (Table 3; eFigure 1 in Supplement 1). Compared with non-Black participants, mortality risk at an eGFR of 60 mL/min/1.73 m2 was significantly higher in Black participants for nearly all estimates for eGFRcr(ASR), eGFRcys(AS), and eGFRcr-cys(AS). For example, eGFRcys(AS) showed Black compared with non-Black participant hazard ratios of 1.2 (95% CI, 1.1-1.4) for all-cause mortality and 1.4 (95% CI, 1.2-1.7) for cardiovascular mortality, with 5-year risk differences of 1.0% (95% CI, 0.4%-1.7%) and 0.71% (95% CI, 0.38%-1.03%), respectively. In contrast, when the eGFRcr(AS) equation was used, risks were not significantly different in Black compared with non-Black participants, with hazard ratios of 1.0 (95% CI, 0.9-1.1) for all-cause mortality and 1.1 (95% CI, 0.9-1.3) for cardiovascular mortality, with 5-year risk differences of −0.04% (95% CI, −0.49% to 0.42%) and 0.08% (95% CI, −0.19% to 0.35%), respectively (Table 3; eTable 6 in Supplement 1).
Table 3. Age- and Sex-Adjusted Hazard Ratios of All-Cause and Cardiovascular Mortality Across Different GFR Estimating Equations by Racea.
| Equations | Participant race | Hazard ratio vs eGFR = 80 mL/min/1.73 m2 and their ratio by race (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| All-cause mortality | Cardiovascular mortality | ||||||
| eGFR = 30 mL/min/1.73 m2 | eGFR = 60 mL/min/1.73 m2 | eGFR = 80 mL/min/1.73 m2 | eGFR = 30 mL/min/1.73 m2 | eGFR = 60 mL/min/1.73 m2 | eGFR = 80 mL/min/1.73 m2 | ||
| 2009 eGFRcr (age, sex, and race) | Black | 3.3 (2.4-4.6) | 1.3 (1.2-1.5) | 1.1 (1.1-1.2) | 4.3 (2.4-7.7) | 1.8 (1.4-2.2) | 1.3 (1.2-1.6) |
| Non-Black | 3.0 (2.6-3.4) | 1.1 (1.0-1.2) | 1 [Reference] | 3.7 (2.9-4.7) | 1.3 (1.1-1.5) | 1 [Reference] | |
| Ratio, Black vs non-Black | 1.1 (0.9-1.4) | 1.2 (1.1-1.4) | 1.1 (1.1-1.2) | 1.2 (0.8-1.7) | 1.4 (1.1-1.7) | 1.3 (1.2-1.6) | |
| 2021 eGFRcr (age and sex) | Black | 3.0 (2.2-4.1) | 1.2 (1.1-1.3) | 1.1 (1.0-1.2) | 3.9 (2.3-6.6) | 1.5 (1.2-1.8) | 1.3 (1.1-1.4) |
| Non-Black | 3.3 (2.8-3.8) | 1.2 (1.1-1.3) | 1 [Reference] | 3.8 (3.0-4.8) | 1.4 (1.2-1.6) | 1 [Reference] | |
| Ratio, Black vs non-Black | 0.9 (0.7-1.2) | 1.0 (0.9-1.1) | 1.1 (1.0-1.2) | 1.0 (0.7-1.5) | 1.1 (0.9-1.3) | 1.3 (1.1-1.4) | |
| 2012 eGFRcys (age and sex) | Black | 4.4 (3.2-6.1) | 1.8 (1.5-2.1) | 1.4 (1.2-1.6) | 5.7 (3.5-9.2) | 2.4 (1.9-3.1) | 1.6 (1.4-1.9) |
| Non-Black | 3.8 (3.1-4.8) | 1.4 (1.3-1.6) | 1 [Reference] | 4.6 (3.5-6.0) | 1.7 (1.5-2.0) | 1 [Reference] | |
| Ratio, Black vs non-Black | 1.2 (0.8-1.6) | 1.2 (1.1-1.4) | 1.4 (1.2-1.6) | 1.2 (0.8-1.9) | 1.4 (1.2-1.7) | 1.6 (1.4-1.9) | |
| 2021 eGFRcr-cys (age and sex) | Black | 4.0 (2.9-5.5) | 1.6 (1.4-1.8) | 1.2 (1.1-1.3) | 5.1 (3.0-8.6) | 2.1 (1.6-2.6) | 1.4 (1.2-1.5) |
| Non-Black | 3.9 (3.3-4.5) | 1.4 (1.3-1.5) | 1 [Reference] | 4.5 (3.7-5.5) | 1.6 (1.4-1.9) | 1 [Reference] | |
| Ratio, Black vs non-Black | 1.0 (0.8-1.4) | 1.1 (1.0-1.2) | 1.2 (1.1-1.3) | 1.1 (0.7-1.7) | 1.3 (1.0-1.6) | 1.4 (1.2-1.5) | |
Abbreviations: cr, creatinine; cys, cystatin; eGFR, estimated glomerular filtration rate.
Total sample of 62 011 for hazard ratio estimation for all-cause mortality, and 61 062 for cardiovascular mortality. Ratios in Black compared with non-Black participants are hazard ratios, and calculated by dividing the estimate in Black participants by the estimate in non-Black participants. Note that the ratio of 2 hazard ratios with the same reference group, eg, non-Black participants at eGFR of 80 mL/min/1.73 m2, is itself a hazard ratio. For example, 1.2 is the hazard ratio of all-cause mortality in Black compared with non-Black participants at an eGFRcr(ASR) of 60 mL/min/1.73 m2.
Within each cohort, comparing equations for all-cause and cardiovascular mortality showed that, similar to KFRT, eGFRcr(AS) resulted in a smaller estimate of the Black to non-Black participant hazard ratio (relative hazard ratios significantly <1.0 for 13 of 14 estimates in Figure 2). In contrast, eGFRcys(AS) and eGFRcr-cys(AS) were similar to eGFRcr(ASR) (25 of 28 relative hazard ratios not significantly different from 1.0).
Comparison of Risk Discrimination Across Equations
When included in a model with age and sex, the 5-fold cross-validated C statistics for KFRT had a median value of greater than 0.78 for all eGFR equations within race groups. Compared with eGFRcr(ASR), equations that included cystatin C in addition to creatinine had significantly higher cross-validated discrimination in race-stratified analyses or overall in race-adjusted analyses (0.013 to 0.018 increase in the C statistic, all P values < .03, eTable 4 in Supplement 1). Similarly, equations that included cystatin C in addition to creatinine had higher discrimination for mortality and cardiovascular mortality compared with eGFRcr(ASR) (0.008 to 0.013 increase in the C statistic, all P values < .05 except P = .06 for eGFRcr-cys[ASR-NB]; eTable 6 in Supplement 1). Within race groups, or overall when adjusting for race, the discrimination of eGFRcr(ASR), eGFRcr(AS), and eGFRcr(ASR-NB) were not significantly different (0.000 with confidence intervals of < ±0.001).
Discussion
This retrospective analysis of Black and non-Black adults participating in 8 US cohort studies showed that decreased eGFR values, calculated with the eGFRcr(AS) equation, were significantly associated with higher KFRT risk within each race group. However, this equation attenuated racial differences in risk of KFRT and mortality at decreased eGFR values compared with the eGFRcr(ASR), eGFRcys(AS), and eGFRcr-cys(AS) equations. The eGFRcr-cys(AS) equation, which provides a more accurate estimate of measured GFR than these other equations,3 was significantly associated with both KFRT and mortality risk in both Black and non-Black participants, and demonstrated a higher risk of these outcomes in Black relative to non-Black individuals. These data suggested that eGFRcr-cys(AS) may be preferred for measuring the higher risk of KFRT and mortality among Black individuals at both normal and moderately decreased eGFR because this equation was an accurate estimate of measured GFR and also documented the higher risk of KFRT and mortality compared with alternative eGFR equations.
Valid measures of the racial differences in the risk of KFRT and mortality are important for tailoring intervention strategies and documenting progress toward racial equity in kidney health. Overall, the findings of this study support the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease recommendations that the new 2021 CKD-EPI creatinine equation without race (the eGFRcr[AS] equation)3,4 be implemented in clinical laboratories, because creatinine remains the dominant filtration marker for estimating GFR and this equation discriminates risk well within each racial group. However, data presented here showed that adding cystatin C to the eGFR equation without race is necessary to estimate racial difference in risk and improve accuracy at the individual patient level.
Black individuals have higher rates of incident KFRT and mortality than non-Black individuals.20,21 eGFR has been used to detect, stage, and treat CKD and to examine disparities in CKD prevalence and risk for adverse outcomes.1,22 Most of the US literature has used eGFRcr equations that include a race coefficient (predominantly the MDRD Study and 2009 CKD-EPI eGFRcr[ASR]). These equations showed a similar or slightly lower prevalence of moderately reduced eGFR in Black compared with non-Black individuals, in contrast to the higher KFRT incidence in Black than in non-Black adults.23,24 Possible causes for the discrepancy in racial differences in prevalence of reduced eGFR vs incidence of KFRT have been documented, including faster decline in measured GFR, more severe albuminuria, and other risk factors for CKD progression, underdiagnosis and undertreatment of CKD, as well as adverse social determinants of health, racial discrimination, and structural racism disproportionately experienced by Black compared with White individuals in the US.4,24,25,26
Consistent with findings in NHANES,3 the current study showed that the eGFRcr(AS) equation resulted in a higher estimated prevalence of reduced eGFR among Black individuals and lower estimated prevalence in non-Black individuals as compared with eGFRcr(ASR). This is likely due to eGFRcr(AS) having an approximate 4-mL/min/1.73 m2 systematic underestimation of measured GFR in Black individuals and similar overestimation of measured GFR in non-Black individuals.3 The higher prevalence of decreased eGFR among Black individuals based on eGFRcr(AS) has the potential benefit of increasing awareness of CKD and promoting earlier interventions to reduce disease progression in Black individuals, but also the potential harm of overdiagnosis. However, the lower estimation of racial differences in KFRT and mortality risk by eGFR(AS) could have unintended consequences for research and public health policies, such as reduced emphasis on understanding existing risk factors for racial disparities in KFRT and mortality risk.
The present analysis focused on eGFR, because eGFR is routinely used in clinical practice to assess the presence and severity of kidney disease and to assess the risk of progression to kidney failure. Risk tools, such as the kidney failure risk equation, are increasingly used in clinical decision-making and benefit from the incorporation of other risk factors in addition to eGFR.27 The current study built on this work to show that including cystatin C in the eGFR equation may improve the predictive capability of eGFRcr: the eGFRcr-cys(AS) equation had significantly greater discrimination of KFRT relative to eGFRcr(ASR) and eGFRcr(AS). These findings support the NKF-ASN Task Force recommendations to increase cystatin C testing, because it is less affected by muscle mass and race group than serum creatinine. This recommendation is further supported by its recommendation for confirmation of CKD in the KDIGO 2012 guidelines.28 Cystatin C has been standardized since 2010,18 improves risk prediction compared with equations that include creatinine alone,6,29 and is now integrated into early CKD detection guidelines30 and CKD curricula.31
Limitations
This study has several limitations. First, the study lacked specific information on factors that may differ between racial groups, such as measures of social determinants of health, and therefore could not statistically adjust for all potential causes for observed racial differences. Second, there may be unmeasured heterogeneity in methods of measurement across cohorts, although the overall results were consistent across all cohorts. Third, insufficient numbers of individuals from racial and ethnic groups other than Black and White preclude comparing outcomes across other race and ethnic groups.32 Fourth, other outcomes of interest, such as cardiovascular disease events and heart failure, were not examined. Fifth, physicians’ decisions to start KFRT may have been influenced by serum creatinine and eGFRcr reporting or other factors, whereas cystatin C is not widely available in clinical practice. Sixth, data on measured GFR were not available in all cohorts. Seventh, none of the models underwent external validation outside the cohorts in which they were developed. Eighth, this study assessed discrimination, but not calibration, which is important in assessing how accurately an estimated probability of an outcome can be applied to an individual patient.
Conclusions
In this retrospective analysis of 8 US cohorts including Black and non-Black individuals, the eGFR equation without race that included creatinine and cystatin C, but not the eGFR equation without race that included creatinine without cystatin C, demonstrated racial differences in the risk of KFRT and mortality throughout the range of eGFR. The eGFRcr-cys equation may be preferable to the eGFRcr equation without race for assessing racial differences in the risk of KFRT and mortality associated with low eGFR.
eAppendix 1. Data analysis overview and analytic notes for some individual cohorts
eAppendix 2. Acknowledgements and funding for collaborating cohorts
eTable 1. CKD-EPI equations, nomenclature, variables, recommended use, and performance of eGFR relative to mGFR
eTable 2. Current and new CKD-EPI equations for estimating GFR on the natural scale expressed for specified sex, serum creatinine or serum cystatin C – from Inker et al
eTable 3. Baseline characteristics and event numbers (A) and follow-up time (B) among study participants by cohort and self-reported race group
eTable 4. Age- and sex-adjusted hazard ratios of KFRT (A), 5-year risks (B), prevalence (C), and c-statistics (D) across different GFR estimating equations, by race
eTable 5. Relative hazard ratios for Black vs. Non-Black participants using different GFR estimating equations compared to 2009 eGFRcr (ASR) in each participating cohort
eTable 6. Age- and sex-adjusted hazard ratios (A), 5-year risks (B), and c-statistics (C) of all-cause and cardiovascular mortality across different GFR estimating equations, by race
eFigure 1. Age- and sex-adjusted hazard ratios of Kidney Failure with Replacement Therapy (KFRT, panel A), mortality (panel B), and cardiovascular mortality (panel C) associated with level of eGFR in Black and non-Black participants for different GFR estimating equations calculated versus the reference point 80 ml/min per 1.73 m2 in the non-Black population
eFigure 2. Hazard ratios of Kidney Failure with Replacement Therapy (KFRT) in Black vs. non-Black participants at an eGFR of 30 for different GFR estimation equations meta-analyzed and within each cohort
eReferences
Nonauthor Collaborators. Chronic Kidney Disease Prognosis Consortium
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-150. [Google Scholar]
- 2.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019;322(2):113-114. doi: 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 3.Inker LA, Eneanya ND, Coresh J, et al. ; Chronic Kidney Disease Epidemiology Collaboration . New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. J Am Soc Nephrol. 2021;32(12):2994-3015. doi: 10.1681/ASN.2021070988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, Ballew SH, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium . Cohort profile: the Chronic Kidney Disease Prognosis Consortium. Int J Epidemiol. 2013;42(6):1660-1668. doi: 10.1093/ije/dys173 [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Matsushita K, Ärnlöv J, et al. ; CKD Prognosis Consortium . Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi: 10.1056/NEJMoa1214234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20(12):2617-2624. doi: 10.1681/ASN.2009010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlipak MG, Katz R, Kestenbaum B, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30(3):171-178. doi: 10.1159/000212381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bui AL, Katz R, Kestenbaum B, et al. Cystatin C and carotid intima-media thickness in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2009;53(3):389-398. doi: 10.1053/j.ajkd.2008.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics . National Health and Nutrition Examination Survey. Accessed June 1, 2020. https://www.cdc.gov/nchs/nhanes/index.htm
- 11.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135-143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inker LA, Schmid CH, Tighiouart H, et al. ; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Couture SJ, Tighiouart H, et al. ; CKD-EPI GFR Collaborators . A new panel-estimated GFR, including β2-microglobulin and β-trace protein and not including race, developed in a diverse population. Am J Kidney Dis. 2021;77(5):673-683.e1. doi: 10.1053/j.ajkd.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karger AB, Eckfeldt JH, Rynders GP, et al. Long-term longitudinal stability of kidney filtration marker measurements: implications for epidemiological studies and clinical care. Clin Chem. 2021;67(2):425-433. doi: 10.1093/clinchem/hvaa237 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration . Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766-772. doi: 10.1373/clinchem.2006.077180 [DOI] [PubMed] [Google Scholar]
- 17.Grubb A, Blirup-Jensen S, Lindström V, Schmidt C, Althaus H, Zegers I; IFCC Working Group on Standardisation of Cystatin C (WG-SCC) . First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619-1621. doi: 10.1515/CCLM.2010.318 [DOI] [PubMed] [Google Scholar]
- 18.Blirup-Jensen S, Grubb A, Lindstrom V, Schmidt C, Althaus H. Standardization of cystatin C: development of primary and secondary reference preparations. Scand J Clin Lab Invest Suppl. 2008;241:67-70. doi: 10.1080/00365510802150067 [DOI] [PubMed] [Google Scholar]
- 19.Newson RB. Comparing the predictive powers of survival models using Harrell’s C or Somers’ D. Stata J. 2010;10(3):339-358. doi: 10.1177/1536867X1001000303 [DOI] [Google Scholar]
- 20.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med. 1982;306(21):1276-1279. doi: 10.1056/NEJM198205273062106 [DOI] [PubMed] [Google Scholar]
- 21.Krop JS, Coresh J, Chambless LE, et al. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159(15):1777-1783. doi: 10.1001/archinte.159.15.1777 [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2)(suppl 1):S1-S266. [PubMed] [Google Scholar]
- 23.United States Renal Data System . 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2019. [Google Scholar]
- 24.McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17(6):1710-1715. doi: 10.1681/ASN.2005111200 [DOI] [PubMed] [Google Scholar]
- 25.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51(6):1908-1919. doi: 10.1038/ki.1997.260 [DOI] [PubMed] [Google Scholar]
- 26.Norton JM, Moxey-Mims MM, Eggers PW, et al. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27(9):2576-2595. doi: 10.1681/ASN.2016010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green JA, Ephraim PL, Hill-Briggs F, et al. Integrated digital health system tools to support decision making and treatment preparation in CKD: the PREPARE NOW Study. Kidney Med. 2021;3(4):565-575.e1. doi: 10.1016/j.xkme.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825-830. doi: 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 29.Potok OA, Ix JH, Shlipak MG, et al. The difference between cystatin C- and creatinine-based estimated GFR and associations with frailty and adverse outcomes: a cohort analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. 2020;76(6):765-774. doi: 10.1053/j.ajkd.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shlipak MG, Tummalapalli SL, Boulware LE, et al. ; Conference Participants . The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34-47. doi: 10.1016/j.kint.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Inker LA, Titan S. Measurement and estimation of GFR for use in clinical practice: core curriculum 2021. Am J Kidney Dis. 2021;78(5):736-749. doi: 10.1053/j.ajkd.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 32.Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555-562. doi: 10.1038/ki.2010.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Data analysis overview and analytic notes for some individual cohorts
eAppendix 2. Acknowledgements and funding for collaborating cohorts
eTable 1. CKD-EPI equations, nomenclature, variables, recommended use, and performance of eGFR relative to mGFR
eTable 2. Current and new CKD-EPI equations for estimating GFR on the natural scale expressed for specified sex, serum creatinine or serum cystatin C – from Inker et al
eTable 3. Baseline characteristics and event numbers (A) and follow-up time (B) among study participants by cohort and self-reported race group
eTable 4. Age- and sex-adjusted hazard ratios of KFRT (A), 5-year risks (B), prevalence (C), and c-statistics (D) across different GFR estimating equations, by race
eTable 5. Relative hazard ratios for Black vs. Non-Black participants using different GFR estimating equations compared to 2009 eGFRcr (ASR) in each participating cohort
eTable 6. Age- and sex-adjusted hazard ratios (A), 5-year risks (B), and c-statistics (C) of all-cause and cardiovascular mortality across different GFR estimating equations, by race
eFigure 1. Age- and sex-adjusted hazard ratios of Kidney Failure with Replacement Therapy (KFRT, panel A), mortality (panel B), and cardiovascular mortality (panel C) associated with level of eGFR in Black and non-Black participants for different GFR estimating equations calculated versus the reference point 80 ml/min per 1.73 m2 in the non-Black population
eFigure 2. Hazard ratios of Kidney Failure with Replacement Therapy (KFRT) in Black vs. non-Black participants at an eGFR of 30 for different GFR estimation equations meta-analyzed and within each cohort
eReferences
Nonauthor Collaborators. Chronic Kidney Disease Prognosis Consortium


