Abstract
Purpose
Globally, concerns have grown regarding the long-term effects of novel coronavirus disease (COVID-19) infection. Therefore, we evaluated the long-term course of persistent symptoms and patient quality of life.
Materials and Methods
This prospective cohort study was conducted at a single tertiary university hospital from August 31, 2020 to March 29, 2021 with adult patients followed at 6 and 12 months after acute COVID-19 symptom onset or diagnosis. Clinical characteristics, self-reported symptoms, EuroQol 5 dimension 5 level (EQ5D-5L) index scores, Korean version of the Patient Health Questionnaire-9 (PHQ-9), Korean version of the Posttraumatic Stress Disorder Checklist-5 (PCL-5-K), and Generalized Anxiety Disorder-7 (GAD-7) were investigated. Symptom persistent or non-persistent groups were defined according to persistency of COVID-19 related symptoms or signs after acute COVID-19 infection, respectively.
Results
Of all 235 patients, 170 (64.6%) patients were eligible for analysis. The median age was 51 (interquartile range, 37–61) years old, and 102 patients were female (60.0%). After 12 months from acute COVID-19 infection, in total, 83 (48.8%) patients still suffered from COVID-19-related symptoms. The most common symptoms included amnesia (24.1%), insomnia (14.7%), fatigue (13.5%), and anxiety (12.9%). Among the five EQ5D-5L categories, the average value of anxiety or depression was the most predominant. PHQ-9 and PCL-5-K scores were statistically higher in the COVID-19–related symptom persistent group than the non-persistent group (p=0.001). However, GAD-7 scores showed no statistical differences between the two groups (p=0.051).
Conclusion
Neuropsychiatric symptoms were the major COVID-19–related symptoms after 12 months from acute COVID-19 infection, reducing quality of life.
Keywords: COVID-19, SARS-CoV-2, persistent symptoms, long-term consequences, long COVID, life quality, health-related quality of life
INTRODUCTION
With the progression of the novel coronavirus disease (COVID-19) pandemic, there have been growing concerns about the long-term impact of COVID-19 infection. Long COVID or an academic literature term post-acute COVID-19 syndrome is the collective term that describes the persistent or new symptoms in a subset of patients who have recovered from acute COVID-19 infection.1 Long COVID comprises various sequelae that affect multiple organ systems, which, in turn, can have a distinct impact on daily functioning and an individual’s ability to work.2 Long COVID can affect recovered COVID-19 patients, regardless of their sex, age, or disease severity, during acute COVID-19 infection.3,4 It has been reported that individuals with COVID-19 experience a range of psychiatric symptoms that persist even several months after acute COVID-19 infection, and the mechanisms of neuropsychiatric sequelae have been associated with neuroinflammation, microvascular thrombosis, or neurodegeneration.5,6 Although the frequency of long COVID diminishes over a period of months after the acute phase of infection,7,8 neurocognitive-related long COVID symptoms have been reported to persist for at least 1 year after acute COVID-19. In fact, the exact period of time for which these symptoms may still be prevalent in these patients remains unknown. In particular, persistent neuropsychiatric symptoms have been reported in both older patients and young adult patients with mild illness during acute COVID-19. Consequently, this can induce a long-term social burden, and research studies pertaining to the clinical progress and improvement of these symptoms is needed. With respect to an individual’s quality of life, cognitive and physical functioning are among the most important factors. Research has shown that COVID-19 affects patients’ quality of life, lifestyle, and mental health.9 However, little is known regarding the long-term consequences of COVID-19 on health-related indices, such as quality of life, changes in lifestyle, and mental health status, at 12 months after acute COVID-19 infection. Daegu is the first city in South Korea to experience a severe COVID-19 outbreak that affected >5000 COVID-19 patients in early 2020. Hence, a significant number of patients have recovered from COVID-19 infection, and this population sample is suitable for analysis of the long-term consequences of COVID-19. Therefore, the purpose of this study was to investigate the impact of long-term consequences of COVID-19 on Korean patients to determine the characteristics of persistent symptoms, quality of life, lifestyle, and mental health. To our knowledge, no other studies have assessed the long-term consequences of COVID-19 after 12 months from acute COVID-19 infection in South Korea.
MATERIALS AND METHODS
Study design and population
This study was conducted at the Kyungpook National University Hospital as a noninterventional prospective cohort study from August 31, 2020 to March 29, 2021. Adult patients diagnosed with COVID-19 by real-time reverse transcription polymerase chain reaction assay from February 17, 2020 to March 24, 2020 were enrolled. We included patients of ages from 20 to 70 years old and those isolated in their home, a therapeutic living center, or a secondary or tertiary hospital during acute COVID-19 infection. We excluded patients who could not complete 12-month follow-up visits for final analysis. All the patients voluntarily agreed to participate in the follow-up study and visited the study hospital at 6 and 12 months after the date of when either the disease symptoms or a positive diagnosis was confirmed. The long-term follow-up protocol was based on the International Severe Acute Respiratory and Emerging Infection Consortium.10 We used surveys translated to Korean, and patients visited the study hospital and completed the individualized questionnaire survey. We collected information on sex, birth year and date, COVID-19 diagnosis date, symptom onset date, isolated place during acute COVID-19 infection, oxygen treatment history, intensive care unit admission history, underlying diseases, symptoms during acute COVID-19 infection, persistent symptoms after acute COVID-19 infection, presence of newly diagnosed diseases after acute COVID-19 infection, exacerbation of underlying diseases after acute COVID-19 infection, and COVID-19 vaccination history. Patients provided their responses to a general symptom questionnaire during acute COVID-19 infection and at 6 and 12 months follow-up visits post–COVID-19 infection. Furthermore, disease severity clinical data were provided by the registry of the Daegu Center for Infectious Diseases Control and Prevention. All data were reviewed by infectious disease physicians.
Disease severity was classified as follows: 1) asymptomatic, individuals who tested positive for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) using a virologic test, but exhibited no symptoms consistent with COVID-19; 2) mild illness, individuals who demonstrated any of the various signs and symptoms of COVID-19, but who did not have shortness of breath, dyspnea, or abnormal chest imaging; 3) moderate illness, individuals who showed evidence of lower respiratory disease during clinical assessment or imaging, but did not require oxygenation other than room air; 4) severe illness, individuals who showed evidence of lower respiratory disease during clinical assessment or imaging and required oxygenation therapy (nasal prong, facial mask, or high-flow oxygen therapy); and 5) critical illness, individuals who experienced respiratory failure, septic shock, and/or multiple organ dysfunction and required mechanical ventilation therapy and/or extracorporeal membrane oxygenation.11,12
Definition
COVID-19–related symptoms were defined as newly identified symptoms that did not exist before acute COVID-19 infection. The duration of symptoms was defined as the date from the start of the first COVID-19–related symptoms identified during acute COVID-19 infection to the date of confirmation of the last COVID-19–related symptoms (last survey date). The symptom persistent group was defined as patients with at least one of 38 categorized persistent symptoms after acute COVID-19 infection. The non-persistent group was defined as patients with no persistent symptoms or signs after acute COVID-19 infection.
Assessment of symptoms
All symptoms were distinguished in the following 38 categories: fever, myalgia, arthralgia, chilly sense, fatigue (a different decline in physical strength compared with pre-disease state), cough, sore throat, rhinorrhea, sputum, dyspnea, chest discomfort, palpitation, arrhythmia, headache, cognitive dysfunction (loss of intellectual functions, such as thinking, remembering, and reasoning, severely enough to interfere with daily functioning), concentration difficulty, amnesia (loss of memory and inability to recall facts, information, and experiences), dizziness, abnormal directional sensibility, insomnia, hallucination, seizure, depression, anxiety, social phobia, obsessive thinking, nausea or vomiting, anorexia, diarrhea, anosmia, ageusia, tinnitus, alopecia, skin rashes, itchy skin, COVID toes (painful red or purple lesions that typically form on the fingers or toes), globus pharyngeus, or paresthesia.
Assessment of life quality and mental health
EuroQol 5 dimension 5 level (EQ5D-5L) index scores were used to assess the quality of life associated with COVID-19–related persistent symptoms. EQ5D-5L index scores were classified into five categories as follows: Mobility, Self-care, Usual activities (e.g., work, study, housework, family or leisure activities), Pain/Discomfort, Anxiety/Depression with five response levels from “no problems” to “extreme problems” or “unable to perform.” Scaled health status on visit day, modified Medical Research Council dyspnea scale, intensity of fatigue, difficulties in performing certain activities after acute COVID-19 infection, and lifestyle changes since COVID-19 infection were evaluated at 12 months after acute COVID-19 infection. Patients who stated that they suffered from psychological symptoms were asked to perform additional mental status examination tests. The Korean version of the Patient Health Questionnaire-9 (PHQ-9) for screening people at risk of depression, the Generalized Anxiety Disorder-7 (GAD-7), and the Korean version of the Posttraumatic Stress Disorder (PTSD) Checklist-5 (PCL-5-K) were used to evaluate mental health more specifically.
Statistical analyses
A descriptive analysis was conducted. Continuous variables are presented as median values with interquartile range (IQR) or mean values with standard deviation, and categorical variables are presented as numbers (%). The frequencies of persistent symptoms were calculated at ≥6 and 12 months after acute COVID-19 infection. Persistent symptoms and quality of life assessment were compared between the symptom persistent and non-persistent groups at 12 months after acute COVID-19 infection. Categorical variables were compared using Fisher’s exact test or the chi-square test, whereas noncategorical variables were analyzed using Student’s t-test or the Mann–Whitney U-test. All tests were considered to be statistically significant at p<0.05. Statistical analyses were performed using R statistics version 4.0.2 (The R Foundation; https://www.r-project.org).
Ethics statement
This study was reviewed and approved by the Institutional Review Board of the Kyungpook National University Hospital (approval no.: 2020-05-004, 2021-02-003). All participants signed informed consent approved by the IRB.
RESULTS
Demographics and characteristics
Of all 235 subjects followed up past the initial 6 months after acute COVID-19 infection, 170 (64.6%, 170/235) included patients who attended all scheduled follow-up visits and were, therefore, eligible for evaluation in our analysis. The 6-month visit from acute COVID-19 infection occurred at 193.0 days (IQR 188.0–206.0), and the 12-month visit from acute COVID-19 infection occurred at 381.6±8.8 days. The majority of patients included in this analysis were females, accounting for 102 (60%) patients in total. The median age of the 170 patients was 51.0 years (IQR, 37.0–60.0). With respect to the age distribution, 29 (17.1%) respondents were aged 20–29 years, 24 (14.1%) were aged 30–39 years, 26 (15.3%) were aged 40–49 years, 44 (25.9%) were aged 50–59 years, and 47 (27.6%) were aged 60–70 years. Regarding disease severity, 6 (3.5%) patients were asymptomatic, 86 (50.6%) had mild disease, 59 (34.7%) had moderate disease, 14 (8.2%) had severe disease, and 5 (2.9%) were in a critical condition.
We compared demographics in the symptom persistent group and non-persistent group, and found that the median age was 52 years old (IQR, 39.0–61.0) in the symptom persistent group, which was significantly higher than the median age of 44 years old (IQR, 30.0–54.0) in the non-persistent group. However, there were no statistical differences between the two groups in terms of age distribution. Furthermore, no statistical differences were identified between the symptom persistent group and non-persistent group up to 12 months in relation to the time of visit, sex, severity, height, body weight, and underlying disease status (p≥0.050) (Table 1).
Table 1. Clinical Characteristics of the 170 Respondents.
| Characteristics | Symptom persistent group (n=129) | Non-persistent group (n=41) | Total (n=170) | p value | |
|---|---|---|---|---|---|
| Duration from COVID-19 diagnosis to 6 months after acute COVID-19 infection (days) | 193.0 [187.0–206.0] | 191.0 [188.0–205.0] | 193.0 [188.0–206.0] | 0.959 | |
| Duration from COVID-19 diagnosis to 12 months after acute COVID-19 infection (days) | 381.6±8.5 | 381.7±9.7 | 381.6±8.8 | 0.957 | |
| Days from COVID-19–related symptom onset to diagnosis | 2.0 [1.0–4.0] | 1.0 [0.0–3.0] | 2.0 [0.0–4.0] | 0.037 | |
| Sex | 0.062 | ||||
| Male | 46 (35.7) | 22 (53.7) | 68 (40.0) | ||
| Female | 83 (64.3) | 19 (46.3) | 102 (60.0) | ||
| Age (yr) | 52.0 [39.0–61.0] | 44.0 [30.0–54.0] | 51.0 [37.0–60.0] | 0.032 | |
| Age distribution (yr) | |||||
| 20–29 | 19 (14.7) | 10 (24.4) | 29 (17.1) | 0.216 | |
| 30–39 | 18 (14.0) | 6 (14.6) | 24 (14.1) | ||
| 40–49 | 17 (13.2) | 9 (22.0) | 26 (15.3) | ||
| 50–59 | 35 (27.1) | 9 (22.0) | 44 (25.9) | ||
| 60–70 | 40 (31.0) | 7 (17.1) | 47 (27.6) | ||
| Age (yr) | 0.050 | ||||
| <50 | 54 (41.9) | 25 (61.0) | 79 (46.5) | ||
| ≥50 | 75 (58.1) | 16 (39.0) | 91 (53.5) | ||
| Height (cm) | 163.2±8.1 | 164.8±10.6 | 163.6±8.8 | 0.399 | |
| Weight (kg) | 63.0 [55.0–70.0] | 68.0 [56.0–76.0] | 63.0 [55.0–72.0] | 0.199 | |
| Current smoker | >0.999 | ||||
| Yes | 2 (1.6) | 1 (2.4) | 3 (1.8) | ||
| No | 127 (98.4) | 40 (97.6) | 167 (98.2) | ||
| Disease severity category | 0.075 | ||||
| Asymptomatic | 2 (1.6) | 4 (9.8) | 6 (3.5) | ||
| Mild | 64 (49.6) | 22 (53.7) | 86 (50.6) | ||
| Moderate | 46 (35.7) | 13 (31.7) | 59 (34.7) | ||
| Severe | 12 (9.3) | 2 (4.9) | 14 (8.2) | ||
| Critical | 5 (3.9) | 0 (0.0) | 5 (2.9) | ||
| Disease severity category | 0.233 | ||||
| <Moderate | 66 (51.2) | 26 (63.4) | 92 (54.1) | ||
| ≥Moderate | 63 (48.8) | 15 (36.6) | 78 (45.9) | ||
| Isolated place during acute COVID-19 | 0.320 | ||||
| Secondary or tertiary hospital | 110 (85.3) | 31 (75.6) | 141 (82.9) | ||
| Therapeutic living center | 16 (12.4) | 9 (22.0) | 25 (14.7) | ||
| Self-home isolation | 3 (2.3) | 1 (2.4) | 4 (2.4) | ||
| Oxygen treatment | 0.283 | ||||
| Yes | 16 (12.4) | 2 (4.9) | 18 (10.6) | ||
| No | 113 (87.6) | 39 (95.1) | 152 (89.4) | ||
| ICU admission history | 0.357 | ||||
| Yes | 6 (4.7) | 0 (0.0) | 6 (3.5) | ||
| No | 123 (95.3) | 41 (100.0) | 164 (96.5) | ||
| MV use | 0.454 | ||||
| Yes | 5 (3.9) | 0 (0.0) | 5 (2.9) | ||
| No | 124 (96.1) | 41 (100.0) | 165 (97.1) | ||
| ECMO use | >0.999 | ||||
| Yes | 1 (0.8) | 0 (0.0) | 1 (0.6) | ||
| No | 128 (99.2) | 41 (100.0) | 169 (99.4) | ||
| Emergent hemodialysis during acute COVID-19 hospitalization | NA | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| No | 129 (100.0) | 41 (100.0) | 170 (100.0) | ||
| Allergic rhinitis | 0.871 | ||||
| Yes | 32 (24.8) | 9 (22.0) | 41 (24.1) | ||
| No | 97 (75.2) | 32 (78.0) | 129 (75.9) | ||
| DM | 0.507 | ||||
| Yes | 20 (15.5) | 4 (9.8) | 24 (14.1) | ||
| No | 109 (84.5) | 37 (90.2) | 146 (85.9) | ||
| HTN | 0.498 | ||||
| Yes | 34 (26.4) | 8 (19.5) | 42 (24.7) | ||
| No | 95 (73.6) | 33 (80.5) | 128 (75.3) | ||
| CKD | 0.761 | ||||
| Yes | 3 (2.3) | 0 (0.0) | 3 (1.8) | ||
| No | 126 (97.7) | 41 (100.0) | 167 (98.2) | ||
| Liver disease | 0.061 | ||||
| Yes | 14 (10.9) | 0 (0.0) | 14 (8.2) | ||
| No | 115 (89.1) | 41 (100.0) | 156 (91.8) | ||
| Hematologic malignancy | NA | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| No | 129 (100.0) | 41 (100.0) | 170 (100.0) | ||
| Solid tumor | >0.999 | ||||
| Yes | 3 (2.3) | 1 (2.4) | 4 (2.4) | ||
| No | 126 (97.7) | 40 (97.6) | 166 (97.6) | ||
| CVA | >0.999 | ||||
| Yes | 2 (1.6) | 0 (0.0) | 2 (1.2) | ||
| No | 127 (98.4) | 41 (100.0) | 168 (98.8) | ||
| COPD | >0.999 | ||||
| Yes | 1 (0.8) | 0 (0.0) | 1 (0.6) | ||
| No | 128 (99.2) | 41 (100.0) | 169 (99.4) | ||
| Heart diseases | 0.583 | ||||
| Yes | 4 (3.1) | 0 (0.0) | 4 (2.4) | ||
| No | 125 (96.9) | 41 (100.0) | 166 (97.6) | ||
COVID-19, coronavirus disease; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation; MV, mechanical ventilation; DM, diabetes mellitus; HTN, hypertension; CKD, chronic kidney disease; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; NA, not available.
Data are presented as median [interquartile range], mean±standard deviation, or n (%).
Evaluation of symptoms or newly diagnosed diseases
After 6 months from acute COVID-19 infection, 85 (50.0%) patients were identified to have at least one COVID-19-related symptom, and 83 (48.8%) patients were found to have at least one COVID-19–related symptom after 12 months from acute COVID-19 infection.
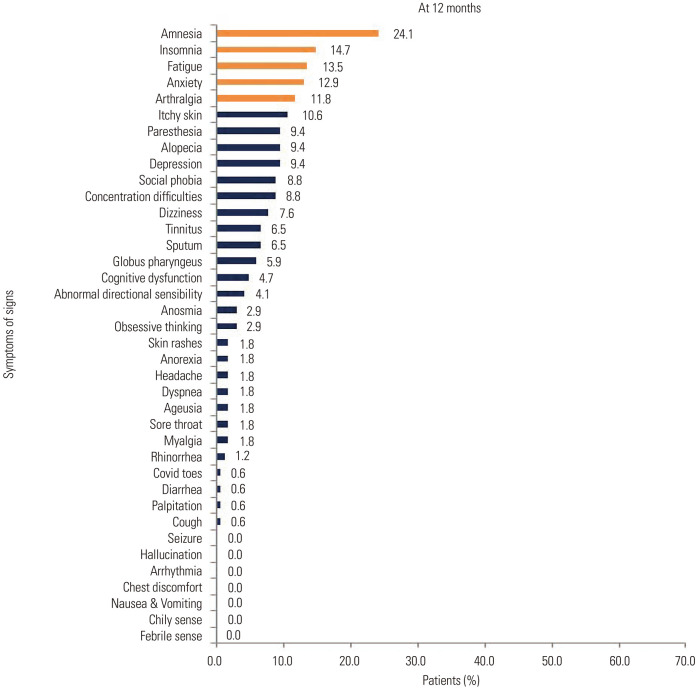
Among the symptoms identified during acute COVID-19 infection, myalgia was the most common symptom, present in 105 (61.8%) patients, followed by cough in 101 (59.4%) patients, fever in 97 (57.1%) patients, sputum in 91 (53.5%) patients, and sore throat in 82 (48.2%) patients (Supplementary Fig. 1A, only online). At 6 months after acute COVID-19 infection, we identified 45 (26.5%) patients with fatigue, followed by 37 patients (21.8%) with amnesia, 35 (20.6%) patients with anxiety, insomnia, and cognitive disorder, and 31 (18.2%) patients with concentration difficulties (Supplementary Fig. 1B, only online). The most frequently identified symptoms at 12 months after acute COVID-19 infection involved amnesia in 41 (24.1%) patients, insomnia in 25 (14.7%) patients, fatigue in 23 (13.5%) patients, anxiety in 22 (12.9%) patients, and arthralgia in 20 (11.8%) patients (Fig. 1).
Fig. 1. Distribution of symptoms or signs after 12 months from acute COVID-19 infection.
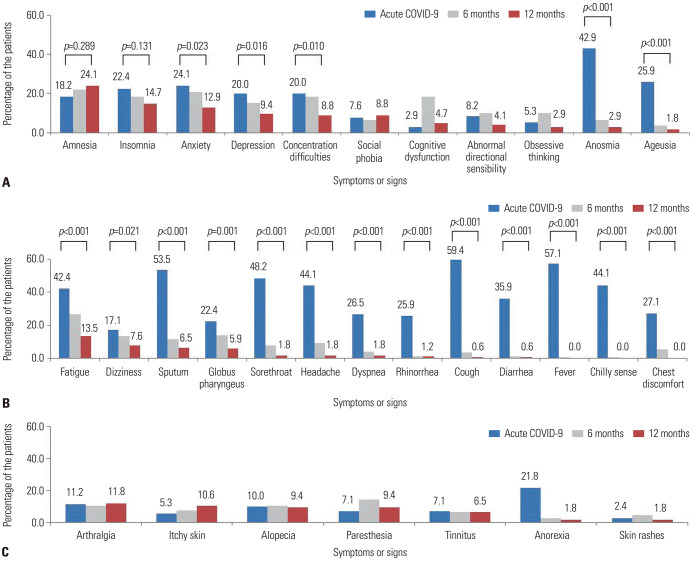
Symptoms identified at the acute COVID-19 infection and at 6 and 12 months after symptom onset or diagnosis were divided into three categories to identify potential changes over time: neuropsychiatric symptoms, constitutional symptoms, and other persistent symptoms. With respect to neuropsychiatric symptoms, the majority of the relevant symptoms improved at 12 months after acute COVID-19 infection. However, a sustained increase in the incidence of amnesia symptoms, the most frequent of all symptoms noted, was confirmed (p=0.289). More specifically, amnesia was identified in 41 (24.1%) patients at 12 months from acute COVID-19 infection, compared to 31 (18.2%) patients during the acute period of COVID-19, which reflects a 5.9% increase (Fig. 2A). Furthermore, 73 (42.9%) patients were diagnosed with anosmia during acute COVID-19 infection, and 5 (2.9%) patients exhibited persistent symptoms after 12 months from acute COVID-19 infection (Fig. 2A). In the case of ageusia, a total of 44 (25.9%) patients was diagnosed with this symptom during acute COVID-19 infection, whereas symptoms persevered for 3 (1.8%) patients after 12 months from acute COVID-19 infection (Fig. 2A). Also, our findings confirmed that, unlike neuropsychiatric symptoms, constitutional symptoms significantly improved over time (p<0.050). In the case of dizziness, it was confirmed that 13 (7.6%) patients continued to have symptoms after 12 months from acute COVID-19 infection (Fig. 2B). Finally, alopecia and tinnitus symptoms were identified in 16 (9.4%) patients and 13 (6.5%) patients, respectively after 12 months from acute COVID-19 infection (Fig. 2C).
Fig. 2. Symptoms identified during acute COVID-19 infection at 6 and 12 months after acute COVID-19 infection. (A) Distribution of neuropsychiatric symptoms. (B) Distribution of constitutional symptoms. (C) Distribution of other symptoms. COVID-19, coronavirus disease.
Our study found that 14 (8.2%) patients were found to have newly diagnosed diseases. Heart disease was found in 1 patient (0.6%), and deep vein thrombosis was found in 2 patients (1.2%). In the non-persistent group, no patient was diagnosed with a new disease since acute COVID-19 infection, while in the symptom persistent group, 14 (10.9%) patients suffered from new diseases including cardiovascular disease and deep vein thrombosis (Supplementary Table 1, only online).
Evaluation of quality of life
Twelve months after acute COVID-19 infection, a total of 39 (23.0%) patients reported that they had not yet fully recovered from COVID-19. In particular, 38 (29.4%) patients felt that they had not fully recovered for up to 12 months, a number which is significantly higher than 1 (2.4%) in the non-persistent group (p<0.001). However, none of the patients required hospitalization due to COVID-19–related complications after acute COVID-19 infection in either the symptom persistent or non-persistent group. The distribution of EQ5D-5L average values for all 170 enrolled patients after 12 months from acute COVID-19 infection is shown in Fig. 3. Among the five EQ5D-5L categories, the average value of anxiety or depression was the most predominant. The EQ5D-5L test conducted in relation to changes in the health conditions of the enrolled patients showed no statistical significant differences between the symptom persistent or non-persistent groups in relation to mobility, self-care, and usual activities (Table 2). However, the assessment of pain/discomfort and anxiety/depression in the symptom persistent group, as opposed to the non-persistent group, showed that there were more individuals affected by these related symptoms (p=0.001 and p=0.015, respectively). Among the five EQ5D-5L categories, anxiety or depression were the most frequent symptoms noted, accounting for 60 (35.4%) patients after 12 months from acute COVID-19 infection. On the day of the survey, when patient health status was assessed on a scale from 0 to 100 (100 representing the best), the symptom persistent group and non-persistent groups reported median values of 80 [70.0–90.0] and 90 [80.0–95.0], respectively, thus indicating that symptom persistent patients perceived their health status as significantly worse than that in patients in the non-persistent group (p<0.001). Regarding the evaluation of the difficulty in breathing in the past day (24 hours), 92 (54.1%) patients responded that they were “out of breath only when they exercised hard,” of whom 63 (48.8%) patients were in the symptom persistent group and 29 (70.7%) patients in the non-persistent group. Therefore, difficulty in breathing in the symptom persistent group could last for a significantly longer period of time (p=0.048). When fatigue was confirmed over the past day, no statistical difference was identified between the symptom persistent and non-persistent groups (p=0.077) (Table 2).
Fig. 3. Distribution of EQ5D-5L average values at 12 months after acute COVID-19 infection in all enrolled patients. Each domain of EQ5D-5L is scored on a 5 point scale: 1, no problem; 2, slight problem; 3, moderate problem; 4, severe problem; and 5, unable to do or extreme problem. EQ5D-5L, EuroQol 5 dimension 5 level.
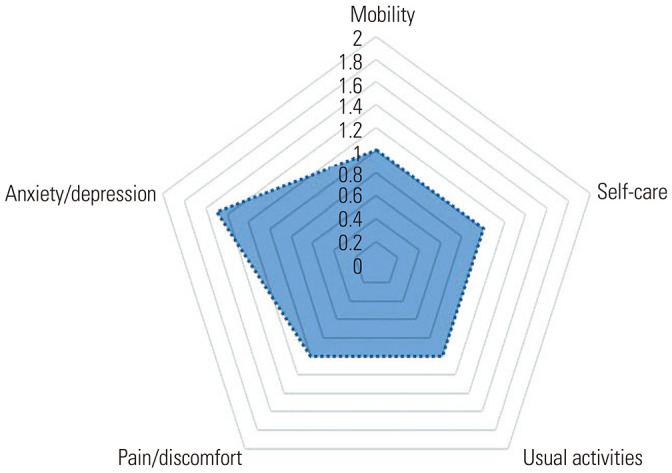
Table 2. Quality of Life Assessment of the 170 Respondents at 12 Months from Acute COVID-19 Infection.
| Characteristics | Symptom persistent group (n=129) | Non-persistent group (n=41) | Total (n=170) | p value | |
|---|---|---|---|---|---|
| Mobility | 0.325 | ||||
| No problems in walking about | 109 (84.5) | 38 (92.7) | 147 (86.5) | ||
| Slight problems in walking about | 18 (14.0) | 2 (4.9) | 20 (11.8) | ||
| Moderate problems in walking about | 1 (0.8) | 0 (0.0) | 1 (0.6) | ||
| Severe problems in walking about | 1 (0.8) | 1 (2.4) | 2 (1.2) | ||
| Unable to walk about | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Self-care | 0.190 | ||||
| No problems washing or dressing | 127 (98.4) | 39 (95.1) | 166 (97.6) | ||
| Slight problems washing or dressing | 2 (1.6) | 1 (2.4) | 3 (1.8) | ||
| Moderate problems washing or dressing | 0 (0.0) | 1 (2.4) | 1 (0.6) | ||
| Severe problems washing or dressing | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Unable to wash or dress | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Usual activities | 0.104 | ||||
| No problems doing usual activities | 105 (81.4) | 37 (90.2) | 142 (83.5) | ||
| Slight problems doing usual activities | 21 (16.3) | 3 (7.3) | 24 (14.1) | ||
| Moderate problems doing usual activities | 3 (2.3) | 0 (0.0) | 3 (1.8) | ||
| Severe problems doing usually activities | 0 (0.0) | 1 (2.4) | 1 (0.6) | ||
| Unable to do usual activities | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Pain/discomfort | 0.001 | ||||
| No pain or discomfort | 82 (63.6) | 35 (85.4) | 117 (68.8) | ||
| Slight pain or discomfort | 47 (36.4) | 4 (9.8) | 51 (30.0) | ||
| Moderate pain or discomfort | 0 (0.0) | 1 (2.4) | 1 (0.6) | ||
| Severe pain or discomfort | 0 (0.0) | 1 (2.4) | 1 (0.6) | ||
| Extreme pain or discomfort | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Anxiety/depression | 0.015 | ||||
| Not anxious or depressed | 75 (58.1) | 35 (85.4) | 110 (64.7) | ||
| Slightly anxious or depressed | 49 (38.0) | 6 (14.6) | 55 (32.4) | ||
| Moderately anxious or depressed | 4 (3.1) | 0 (0.0) | 4 (2.4) | ||
| Severely anxious or depressed | 1 (0.8) | 0 (0.0) | 1 (0.6) | ||
| Extremely anxious or depressed | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| The best health you can imagine on today (the scale is numbered from 0 to 100) | 80.0 [70.0–90.0] | 90.0 [80.0–95.0] | 85.0 [75.0–90.0] | 0.004 | |
| Breathless you feel today (within the last 24 hours) | 0.048 | ||||
| Not troubled by breathlessness except on strenuous exercise | 63 (48.8) | 29 (70.7) | 92 (54.1) | ||
| Short of breath when hurrying or when walking up a slight hill | 62 (48.1) | 11 (26.8) | 73 (42.9) | ||
| Walks slower than most people of my age because of breathlessness, or have to stop for breath when walking at own pace | 4 (3.1) | 1 (2.4) | 5 (2.9) | ||
| Stops for breath after walking 100 yards/ 90–100 meters, or after a few minutes on level ground | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Too breathless to leave the house, or breathless when dressing/undressing | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Intensity of fatigue on average over the last 24 hours, on a scale from 0–10 (0=no fatigue, 10=fatigue as bad as you can imagine)* | 2.0 [2.0–5.0] | 2.0 [2.0–5.0] | 2.0 [2.0–5.0] | 0.077 | |
COVID-19, coronavirus disease.
Data are presented as median [interquartile range] or n (%).
*Intensity of tiredness (0–10): 0: none/2: mild fatigue/5: moderate fatigue/10: severe fatigue.
Assessing discomfort in performing certain activities, we found that, similar to the assessment of difficulty in breathing, 32 (24.9%) patients in the symptom persistent group and 3 (7.3%) patients in the non-persistent group had difficulty in walking including climbing situations (p=0.031). Among all patients included in this study, 70 (41.2%) patients responded that they had difficulties in concentrating or remembering, of whom 64 (49.6%) were in the symptom persistent group and 6 (14.6%) were in the non-persistent group (p<0.001). Furthermore, 16 (9.4%) patients stated they either failed to understand or had prominent difficulties in daily life conversations, but no statistical significance was identified between the symptom persistent and non-persistent groups (p=0.148) (Supplementary Table 2, only online).
Evaluation of lifestyle modification
Following infection with COVID-19, a survey of lifestyle changes revealed that changes in smoking habits occurred in 3 (1.8%) patients. In the case of drinking, 38 (22.3%) patients showed lifestyle changes. More specifically, 3 (2.3%) patients in the symptom persistent group and 2 (4.9%) patients in the non-persistent group responded that they had increased their drinking habits, compared to pre–COVID-19 infection. In contrast, it was confirmed that the symptom persistent group reduced their drinking habits, compared to the non-persistent group. More specifically, 27 (20.9%) patients in the symptom persistent group, and 6 (14.6%) patients in the non-persistent group had their drinking habits reduced. In the case of healthy food ingestion, 72 (42.6%) patients reported changes in their intake behavior. Sixty-two (48.4%) patients in the symptom persistent group and 8 (19.5%) patients in the non-persistent group responded that they adopted a lifestyle of eating healthier food on a more frequent basis, compared to before COVID-19. In particular, this tendency was higher in the symptom persistent group, compared to the non-persistent group (p=0.005). In the case of physical activity (exercise through walking or cycling), lifestyle changes were confirmed in 85 (50.6%) patients. More specifically, 58 (45.7%) patients in the symptom persistent group and 10 (24.4%) patients in the non-persistent group responded that they exercised more frequently than before infection, although no statistical significant differences were identified between the two groups (p=0.117) (Table 3).
Table 3. Lifestyle Change Assessment among the 170 Respondents at 12 Months after Acute COVID-19 Infection.
| Characteristics | Symptom persistent group (n=129) | Non-persistent group (n=41) | Total (n=170) | p value | |
|---|---|---|---|---|---|
| Smoking | 0.245 | ||||
| I do this more often | 0 (0.0) | 1 (2.4) | 1 (0.6) | ||
| I do this less often | 1 (0.8) | 1 (2.4) | 2 (1.2) | ||
| No difference | 10 (7.8) | 4 (9.8) | 14 (8.2) | ||
| I did not do this before COVID-19 | 118 (91.5) | 35 (85.4) | 153 (90.0) | ||
| Drinking alcohol | 0.042 | ||||
| I do this more often | 3 (2.3) | 2 (4.9) | 5 (2.9) | ||
| I do this less often | 27 (20.9) | 6 (14.6) | 33 (19.4) | ||
| No difference | 32 (24.8) | 19 (46.3) | 51 (30.0) | ||
| I did not do this before COVID-19 | 67 (51.9) | 14 (34.1) | 81 (47.6) | ||
| Eating healthy food | 0.005 | ||||
| I do this more often | 62 (48.4) | 8 (19.5) | 70 (41.4) | ||
| I do this less often | 1 (0.8) | 1 (2.4) | 2 (1.2) | ||
| No difference | 43 (33.6) | 17 (41.5) | 60 (35.5) | ||
| I did not do this before COVID-19 | 22 (17.2) | 15 (36.6) | 37 (21.9) | ||
| Physical activity (including walking & cycling) | 0.117 | ||||
| I do this more often | 58 (45.7) | 10 (24.4) | 68 (40.5) | ||
| I do this less often | 12 (9.4) | 5 (12.2) | 17 (10.1) | ||
| No difference | 45 (35.4) | 21 (51.2) | 66 (39.3) | ||
| I did not do this before COVID-19 | 12 (9.4) | 5 (12.2) | 17 (10.1) | ||
COVID-19, coronavirus disease.
Data are presented as n (%).
Evaluation of mental health
A simple depression-specific test (PHQ-9), which was conducted to assess mental health conditions over the past 2 weeks, demonstrated that 19 (11.2%) patients who recovered from COVID-19 showed moderate or higher depression. Moderate or higher depression scores were confirmed only in the symptom persistent group, as opposed to none in the non-persistent group. The PHQ-9 scores were statistically higher in the symptom persistent group than the non-persistent group (p=0.001) (Supplementary Fig. 2, only online). Anxiety screening results identified 8 (4.7%) patients with moderate or higher anxiety levels, but moderate or higher anxiety level was confirmed only in the symptom persistent group, as opposed to none in the non-persistent group. Overall statistical differences in the symptom persistent and non-persistent groups were not identified (p=0.051) (Supplementary Fig. 3, only online). The median PCL-5-K score in the responded patients was 5.0 (2.0–12.0). The PCL-5-K results showed scores of 2 points (0.0–5.0) in the non-persistent group and 6 points (2.0–14.0) in the symptom persistent group. Points were found to be higher in the symptom persistent group, compared to the non-persistent group (p<0.001) (Supplementary Fig. 4, only online).
DISCUSSION
To our knowledge, this is the largest long-term prospective cohort study on persistent COVID-19–related symptoms and health consequences in adult patients after acute COVID-19 infection in Korea. The major findings of this study indicate that 48.8% of the respondents still suffered from COVID-19-related symptoms or signs after 12 months from acute COVID-19 infection. Long-term consequences of COVID-19 can be presented in various symptoms or signs reducing quality of life, but the majority of symptoms tended to improve over time.
COVID-19–related long COVID symptoms have been found to vary in type and frequency depending on the time that assessments were made. After discharge, the most commonly reported symptoms involved fatigue, dyspnea, memory loss, sleep disorders, and concentration difficulty.13,14 In a previous study, the most frequently reported symptoms at 12 months involved reduced exercise capacity, fatigue, dyspnea, concentration problems, problem finding words, and sleeping problems.15 This study, which included patients with a similar female to male ratio to our study, identified that anosmia improved over time, as opposed to reduced exercise capacity and difficulty finding words, which continued to increase up to 12 months post-disease infection.15 Similar to a previous study, our study demonstrated that the most common symptoms at 12 months were amnesia, insomnia, fatigue, and anxiety. Anosmia continued to improve, while amnesia continued to increase until 12 months after acute COVID-19 infection. Similar to a previous 12-month follow-up study, neurological long-term symptoms were the most frequently observed symptoms.16
To date, it is unclear why recovered COVID-19 patients experience long-term symptoms after acute COVID-19 infection. The possible mechanisms of neurological symptoms in COVID-19 survivors are explained by a disruption in the blood–brain barrier caused by inflammation leading to increased permeability for cytokines to enter the central nervous system.17 Neuroinflammation from microglial activation and oxidative stress might contribute to severe long-term cognitive and functional decline. Furthermore, high levels of inflammation, such as cytokine storm in the brain, could impact the long-term consequences of neurodegeneration, thus resulting in Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis decades later because coronaviruses, such as SARS-CoV-2, could remain inside neurons without inducing toxic effects.6,18,19,20 Therefore, continuous follow-up processes in patients recovered from COVID-19 would be needed to identify the true relationship between SARS-CoV-2 infection and neurodegenerative disorders.
Anosmia and ageusia, which are considered to be significant symptoms and clues for the diagnosis of COVID-19, showed that the median time to recovery was 7 days for both symptoms, and most patients with these symptoms recovered within a period of 3 weeks.21 Our findings revealed that anosmia was identified in 42.9% of the included patients during acute COVID-19 infection, and the majority of patients had symptom improvement within a year, as opposed to 2.9% patients who still suffered from anosmia. Furthermore, ageusia was identified in 25.9% of patients during acute COVID-19 infection, and the majority of patients had improved symptoms within a year, as opposed to 1.8% of patients who continued to suffer from this symptom.
In this study, we evaluated newly diagnosed diseases in recovered COVID-19 patients. Heart disease was found in 0.6% and deep vein thrombosis was found in 1.2%, which was lower than 2.4% deep vein thrombosis events within the first year after recovering from acute COVID-19, as shown in a previous study.22 Patients with cardiovascular complications during acute infection or patients experiencing persistent cardiac symptoms may be monitored because cardiovascular sequelae from myocarditis can be persistent, thus causing arrhythmia or cardiomyopathy even in patients with mild or asymptomatic SARS-CoV-2 infection.23,24,25 Various retrospective studies have noted that the prevalence of venous thromboembolisms was < 5% in post-acute COVID-19.26,27
There is accumulating evidence that COVID-19 has a long-term impact on both hospitalized and non-hospitalized patients, which includes not just symptoms, but can have a broader impact on patient quality of life, including mental health.28 Patients with at least one long COVID symptom were previously found to have significantly reduced physical and mental quality of life compared to patients without symptoms.15 Our study showed a similar result in that patients experienced multisystem symptoms for over 12 months, resulting in a significant impact to the patients’ lives, compared to individuals with non-persistent symptoms, after acute COVID-19 infection. After one year, only 95 (55.9%) patients managed to recover completely from COVID-19 infection. Furthermore, when the subjective body condition scale was implemented, lower scores were found in patients with symptoms that lasted for 1 year compared to patients with non-persistent symptom after acute COVID-19 infection.
A telephone survey study focusing on health-related quality of life at 3 months after hospital discharge, with a mean participant age of 53 years old, revealed an overall worsening of EQ5D scores, compared to the time before the onset of COVID-19 symptoms, and participants responded that they were affected by all five domains, especially pain/discomfort and anxiety/depression.29 Although the comparison target group was not the same, our study showed similar results in that patients with persistent symptoms showed a reduced level of pain/discomfort and anxiety/depression, compared to other EQ5D-5L categories and to patients with non-persistent symptoms after acute COVID-19 infection. One year later, 60 (35.4%) patients reported that anxiety or depression affected their quality of life through EQ5D-5L measurements. A total of 70 (41.2%) patients was identified to have difficulties in remembering or concentrating. Our study showed that the adverse impact of COVID-19 on recovered COVID-19 patients is multifactorial and affects aspects of mental health that are related to quality of life in particular.
A previous study conducted among patients who were aged 60 years and older showed that more than half of the patients reported a negative change in their health-related quality of life at 6 months following hospitalization due to COVID-19 and that one out of three patients experienced persistently impaired mobility and ability to perform activities of daily living.30 The risk for severe illness with COVID-19 increases with age, and patients who had already experienced severe COVID-19 had the largest decline in health-related quality of life.30 Prevention of aggravating COVID-19 with vaccination might be an effective measure to prevent a long-term functional decline.
Moreover, a previous study showed that COVID-19 itself can have a significant impact on patients’ lifestyles, not only on their mental health and quality of life.9 Our study revealed that patients with persistent symptoms lasting up to 12 months after acute COVID-19 infection had a higher tendency to eat healthier foods and to drink less, compared to patients with non-persistent symptoms, after acute COVID-19 infection. Although government restrictions or social distancing can facilitate lifestyle modifications, our study shows that long COVID by itself have an impact on patients’ daily lifestyle.
In our study, PHQ-9 tests conducted to verify mental health conditions showed statistical differences between symptom persistent and non-persistent groups. More specifically, we found that depression in recovered COVID-19 patients can be present in varying degrees. In addition to the effects of sociological environmental changes caused by COVID-19, breakdown of social support structures, or the stigmatization of patients,31 long COVID holds the possibility of affecting the mental health of recovered COVID-19 patients.
This study has limitations. First, this was a single center study, and we could not include patients who could not visit a hospital by themselves. Therefore, our results may not be generalized to patients over 70 years old or to patients with immunocompromised state as our study mostly included patients with no underlying diseases. Further research is needed for the impact of these clinical factors with treatment on long COVID. Second, a control group who had not suffered from COVID-19 was not included to completely distinguish the impact of the disease from the social and economic burdens of the SARS-Cov-2 pandemic on overall health-related quality of life and mental health. Third, with only health-related quality of life identified at 1 year after acute COVID-19 infection, we were unable to confirm whether health-related quality of life had improved over time or not. However, previous studies have shown that post-discharge health-related quality of life was poor in all patients but improved during follow up.32 Further research is needed on the progress of continuous improvement in recovered COVID-19 patients with regard to their health-related quality of life.
The main strength of our prospective study is that it highlights the importance of continuous tracking of neuropsychiatric symptoms and the impact of COVID-19 on patient quality of life. The present study assessed patient health-related quality of life with the widely used EQ5D-5L in both non-hospitalized and hospitalized patients of various ages in Korea.
In conclusion, the clinical manifestations of the long-term consequences of COVID-19 occurred in various forms, and neuropsychiatric symptoms were the major COVID-19–related symptoms after 12 months from acute COVID-19 infection, thus reducing patient quality of life.
ACKNOWLEDGEMENTS
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (fund code 2020-ER5334-00, 2021-ER1901-00).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yoonjung Kim and Shin-Woo Kim.
- Data curation: Yoonjung Kim and Shin-Woo Kim.
- Formal analysis: Yoonjung Kim and Shin-Woo Kim.
- Funding acquisition: Shin-Woo Kim.
- Investigation: all authors.
- Methodology: Yoonjung Kim and Shin-Woo Kim.
- Project administration: Yoonjung Kim and Shin-Woo Kim.
- Resources: Yoonjung Kim and Shin-Woo Kim.
- Software: Yoonjung Kim.
- Supervision: Shin-Woo Kim.
- Validation: Yoonjung Kim and Shin-Woo Kim.
- Visualization: Yoonjung Kim and Shin-Woo Kim.
- Writing—original draft: Yoonjung Kim.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Distribution of symptoms or signs during acute COVID-19 (A) and after 6 months from acute COVID-19 infection (B). COVID-19, coronavirus disease.
Distribution of total scores for PHQ-9 in the symptom persistent and non-persistent groups. PHQ-9, Korean version of the Patient Health Questionnaire-9 (depression screening for people at risk).
Distribution of total scores for GAD-7 in the symptom persistent and non-persistent groups. GAD-7, Generalized Anxiety Disorder-7.
Total scores for PCL-5-K in the symptom persistent and non-persistent groups. PCL-5-K, Korean version of Posttraumatic Stress Disorder Checklist-5.
Assessment of Re-Admission, Vaccination, and Newly Diagnosed Diseases among the 170 Respondents at 12 Months after Recovering from Acute COVID-19 Infection
Assessment of Difficulties in Doing Certain Activities among the 170 Respondents at 12 Months after Acute COVID-19 Infection
References
- 1.Korompoki E, Gavriatopoulou M, Hicklen RS, Ntanasis-Stathopoulos I, Kastritis E, Fotiou D, et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J Infect. 2021;83:1–16. doi: 10.1016/j.jinf.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne) 2021;8:653516. doi: 10.3389/fmed.2021.653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuzzo D, Vasto S, Scalisi L, Cottone S, Cambula G, Rizzo M, et al. Post-acute COVID-19 neurological syndrome: a new medical challenge. J Clin Med. 2021;10:1947. doi: 10.3390/jcm10091947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlis RH, Green J, Santillana M, Lazer D, Ognyanova K, Simonson M, et al. Persistence of symptoms up to 10 months following acute COVID-19 illness. [accessed 2021 August 30];medRxiv [Preprint] 2021 doi: 10.1101/2021.03.07.21253072. Available at: [DOI] [Google Scholar]
- 8.Kim Y, Kim SW, Chang HH, Kwon KT, Bae S, Hwang S. Significance and associated factors of long-term sequelae in patients after acute COVID-19 infection in Korea. Infect Chemother. 2021;53:463–476. doi: 10.3947/ic.2021.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park KH, Kim AR, Yang MA, Lim SJ, Park JH. Impact of the COVID-19 pandemic on the lifestyle, mental health, and quality of life of adults in South Korea. PLoS One. 2021;16:e0247970. doi: 10.1371/journal.pone.0247970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ISARIC. Long term follow-up protocol and study [Internet] [accessed on 2021 August 15]. Available at: https://isaric.org/research/covid-19-clinical-research-resources/covid-19-long-term-follow-up-study/
- 11.Kim SW, Kim SM, Kim YK, Kim JY, Lee YM, Kim BO, et al. Clinical characteristics and outcomes of COVID-19 cohort patients in Daegu metropolitan city outbreak in 2020. J Korean Med Sci. 2021;36:e12. doi: 10.3346/jkms.2021.36.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet] [Accessed April 21, 2021]. Available at: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf. [PubMed]
- 13.Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeßle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. 2022;74:1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rank A, Tzortzini A, Kling E, Schmid C, Claus R, Löll E, et al. One year after mild COVID-19: the majority of patients maintain specific immunity, but one in four still suffer from long-term symptoms. J Clin Med. 2021;10:3305. doi: 10.3390/jcm10153305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker HA, Safavynia SA, Evered LA. The ‘third wave’: impending cognitive and functional decline in COVID-19 survivors. Br J Anaesth. 2021;126:44–47. doi: 10.1016/j.bja.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuzzo D, Picone P, Caruana L, Vasto S, Barera A, Caruso C, et al. Inflammatory mediators as biomarkers in brain disorders. Inflammation. 2014;37:639–648. doi: 10.1007/s10753-013-9780-2. [DOI] [PubMed] [Google Scholar]
- 19.Lippi A, Domingues R, Setz C, Outeiro TF, Krisko A. SARS-CoV-2: at the crossroad between aging and neurodegeneration. Mov Disord. 2020;35:716–720. doi: 10.1002/mds.28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020;94:809–810. doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35:e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maestre-Muñiz MM, Arias Á, Mata-Vázquez E, Martín-Toledano M, López-Larramona G, Ruiz-Chicote AM, et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. 2021;10:2945. doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 25.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patell R, Bogue T, Koshy A, Bindal P, Merrill M, Aird WC, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelen MM, Vandenbriele C, Balthazar T, Claeys E, Gunst J, Guler I, et al. Venous thromboembolism in patients discharged after COVID-19 hospitalization. Semin Thromb Hemost. 2021;47:362–371. doi: 10.1055/s-0041-1727284. [DOI] [PubMed] [Google Scholar]
- 28.Garratt AM, Ghanima W, Einvik G, Stavem K. Quality of life after COVID-19 without hospitalisation: good overall, but reduced in some dimensions. J Infect. 2021;82:186–230. doi: 10.1016/j.jinf.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todt BC, Szlejf C, Duim E, Linhares AOM, Kogiso D, Varela G, et al. Clinical outcomes and quality of life of COVID-19 survivors: a follow-up of 3 months post hospital discharge. Respir Med. 2021;184:106453. doi: 10.1016/j.rmed.2021.106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walle-Hansen MM, Ranhoff AH, Mellingsæter M, Wang-Hansen MS, Myrstad M. Health-related quality of life, functional decline, and long-term mortality in older patients following hospitalisation due to COVID-19. BMC Geriatr. 2021;21:199. doi: 10.1186/s12877-021-02140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung SJ, Jun JY. Mental health and psychological intervention amid COVID-19 outbreak: perspectives from South Korea. Yonsei Med J. 2020;61:271–272. doi: 10.3349/ymj.2020.61.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlake JH, Wesselius S, van Genderen ME, van Bommel J, Boxmade Klerk B, Wils EJ. Psychological distress and health-related quality of life in patients after hospitalization during the COVID-19 pandemic: a single-center, observational study. PLoS One. 2021;16:e0255774. doi: 10.1371/journal.pone.0255774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of symptoms or signs during acute COVID-19 (A) and after 6 months from acute COVID-19 infection (B). COVID-19, coronavirus disease.
Distribution of total scores for PHQ-9 in the symptom persistent and non-persistent groups. PHQ-9, Korean version of the Patient Health Questionnaire-9 (depression screening for people at risk).
Distribution of total scores for GAD-7 in the symptom persistent and non-persistent groups. GAD-7, Generalized Anxiety Disorder-7.
Total scores for PCL-5-K in the symptom persistent and non-persistent groups. PCL-5-K, Korean version of Posttraumatic Stress Disorder Checklist-5.
Assessment of Re-Admission, Vaccination, and Newly Diagnosed Diseases among the 170 Respondents at 12 Months after Recovering from Acute COVID-19 Infection
Assessment of Difficulties in Doing Certain Activities among the 170 Respondents at 12 Months after Acute COVID-19 Infection