Video
Difficult scenarios encountered during colorectal full thickness resection and their management: (1) Inability to advance device to target lesion; (2) injury to extraluminal structures; (3) anal trauma; (4) anal stenosis; (5) luminal edema after resection; (6) difficulty in grasping lesion; (7) recommendation for “mini time-out”; (8) Summary.
Abbreviations: EFTR, endoscopic full-thickness resection; ESD, endoscopic submucosal dissection; FTRD, full-thickness resection device
Introduction
Endoscopic resection is a well-established modality for the minimally invasive treatment of superficial lesions throughout the colon and rectum. Although EMR and endoscopic submucosal dissection (ESD) provide excellent results, they have limited efficacy in certain situations, such as deeper lesions and lesions with dense submucosal fibrosis.1,2 In these situations, endoscopic full-thickness resection (EFTR) provides an alternative endoscopic resection modality, potentially sparing patients from surgical resection.
Colorectal EFTR
There are multiple suitable indications for colorectal EFTR: (1) residual/recurrent fibrotic polyps not amenable to EMR/ESD, (2) large adenomas for hybrid EMR/EFTR or ESD/EFTR, (3) postpolypectomy scars from incompletely resected malignant polyps or NETs, (4) small subepithelial tumors, and (5) lesions of indeterminate nature for which diagnosis can inform management strategy. Various techniques have been described for EFTR; however, at present, the most commonly performed technique involves the use of a dedicated over-the-scope full-thickness resection device (FTRD) (Ovesco Endoscopy AG, Tubingen, Germany). EFTR with the FTRD system is generally performed in the following fashion:
-
1.
The target lesion is identified and assessed for suitability for EFTR. Adequate bowel preparation is emphasized to the patient, and if necessary, a complete colonoscopy is repeated before attempting resection. For subepithelial lesions, EUS can prove useful in estimating the size of the lesion (Table 1). A prOVE Cap (Ovesco Endoscopy) can be used to test for the possible application of the respective EFTR system before assembly of the kit. It can ensure that the endoscope will be able to reach the targeted lesion with the mounted device and check mobilization of the lesion into the cap with the grasper device. The lesion is marked using the marking probe included with the EFTR device (Erbe USA, VIO300D: forcedCOAG, effect 1, Pmax 20W).
-
2.
The FTRD is prepared. The device consists of an applicator cap mounted on the colonoscope, with an over-the-scope clip at the tip of the cap and a snare running alongside the scope via a plastic sleeve. A hand wheel mounted onto the scope handle controls the deployment of the over-the-scope clip.
-
3.
The FTRD is introduced via the anus and advanced to the marked lesion. The lesion is grasped with forceps that are supplied with the EFTR device and pulled into the cap. With few exceptions, suctioning should be generally avoided to avoid entrapment of adjacent organs.
-
4.
Once the lesion is fully retracted into the cap (with marking dots visible inside the cap), the hand wheel is turned to deploy the clip with continued tension on the lesion. During this step, all endoscope wheels should be unlocked to ensure smooth deployment of the EFTR hand wheel. An assistant immediately closes the attached snare, and applied cautery cuts the tissue approximately 2 mm above the clip (Erbe USA, VIO300D: highCUT, effect 4, Pmax 200W).
-
5.
The entire scope with the lesion is then withdrawn, and the specimen is pinned for specimen processing.
-
6.
A standard colonoscope is readvanced to examine the resection site and assess for completeness of the resection and for any adverse events.
Table 1.
Endoscopic full-thickness resection (EFTR) compatibility
| EFTR kit | Size | Endoscope size requirement | Endoscope compatibility |
|---|---|---|---|
| Colonic | Diameter: 21 mm Depth: 23 mm |
11.5-13.2 mm | CF190/CFH190, PCF, 2T GIF |
| Diagnostic | Diameter: 19.5 mm Depth: 23 mm |
10.5-12 mm | PCFH190, GIF2TH180, GIF2T160 |
| Gastroduodenal | Diameter: 19.5 mm Depth: 23 mm |
10.5-12 mm | 1TH190, 1TQ160, 2TH180 |
Multiple studies have demonstrated excellent technical and clinical success from the procedure.3, 4, 5 Adverse events include perforation, bleeding, postpolypectomy syndrome, fistula formation, appendicitis, and recurrence and can necessitate further endoscopic management and/or surgical intervention.4,6 Adverse events may be the result of mechanical or operator failure, most often because of improper clip deployment or issues with snare resection.4,7,8
At our center, we have performed each EFTR procedure in a standardized fashion with at least 2 experienced endoscopy technicians and a nurse present during the procedure. Procedures are generally booked in the middle of the week, with sufficient time allotment to accommodate any potential adverse events. Given that grasping the lesion is considered the “point of no return” in EFTR, a “mini time-out” is performed immediately before this step to ensure the endoscopist and assistants are aware of their roles and to check the status and readiness of both the FTRD and electrosurgical generator (Table 2). This process has proved critically important to minimizing adverse events in our EFTR experience.
Table 2.
Mini time-out
|
|
|
Unique technical challenges of colorectal EFTR
Although there are many similarities between FTRD systems and endoscopic band ligation or multiband mucosectomy systems (with which endoscopists tend to be familiar), there are also many considerations and potential pitfalls that are unique to the FTRD system and can affect a successful EFTR. Given the potential severity of adverse events, endoscopists should be aware of possible hurdles. Here, we present multiple scenarios we have experienced during colorectal EFTR, which we began incorporating in 2020 in our tertiary care practice, and we highlight key considerations for success (Video 1, available online at www.giejournal.org) (Fig. 1).
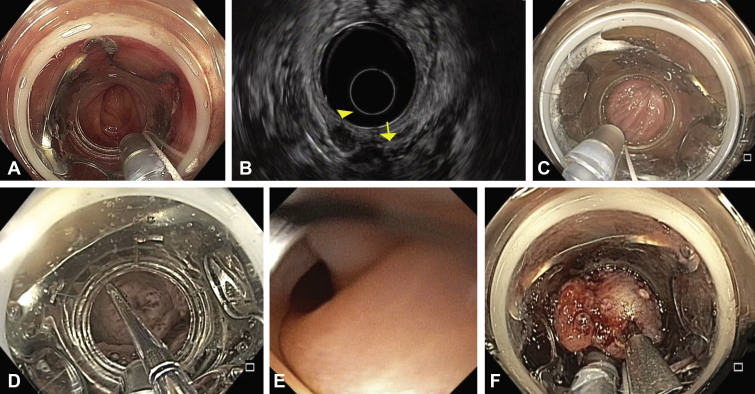
Figure 1.
Difficult scenarios encountered during colorectal full-thickness resection. A, Difficulty in navigating the sigmoid colon because of diverticulosis. B, Rectal subepithelial lesion (arrowhead) adjacent to the vagina (arrow) on endosonographic examination. C, Anal trauma upon insertion of the full-thickness resection device. D, Balloon dilation of anal stenosis. E, Luminal edema post resection. F, Use of an anchor device to facilitate grasping of lesion into cap.
Inability to reach target lesion
Diverticulosis, adhesions, and angulations can pose a challenge in any colonoscopy, but given the added bulk of the FTRD set, this can preclude resection. The colonoscope cap diameter is 21 mm, and the cap depth adds 23 mm onto the colonoscope. This adds difficulty in scope handling, and it is important to ensure the cap is well seated on the tip of the colonoscope to avoid dislodgement during insertion. There are multiple options to aid in the insertion process, including using a different endoscope (Table 1). For example, switching to a gastroduodenal FTRD set is an option because it has a smaller diameter (19.5 mm), but it also has a cap depth of 23 mm and is not confirmed to be able to traverse difficult segments. Other options include abdominal pressure, changing patient position, dilation (with a balloon dilator to facilitate passage, separate from the EFTR kit), or underwater advancement.
Injury to extraluminal structures
Particularly in the rectum in female patients, there may be adjacent extraluminal structures. Given the nature of the FTRD mechanism, avoiding entrapment or injury of external structures is critical. Although endoscopic ultrasound can be useful, the proximity of gynecological structures adjacent to the anterior rectal wall may result in EFTR being contraindicated. Our practice is to always perform an EUS before rectal EFTR. Although there are no clear criteria for distance from neighboring structures that would preclude EFTR, it is essential that consideration be given to adjacent structures that could be damaged in the course of EFTR. This remains important even outside the rectum, as fistulization with neighboring organs can occur.6
Anal trauma
The FTRD set contains a 21-mm-diameter cap, with a large (and very sharp) over-the-scope clip. Device insertion carries the possibility of marked trauma, particularly in the presence of hemorrhoids or anal fissures. The use of 2% lidocaine jelly applied directly to the anal canal, in conjunction with copious lubrication of the FTRD set and the colonoscope, can minimize potential insertion trauma.
Anal stenosis
Insertion of the FTRD device should be done with extreme care. In addition to lidocaine jelly and copious lubrication, the device should be inserted carefully and slowly. Despite this, there may be difficulty in device passage in patients with anal stenosis. In these cases, a balloon dilator inflated in front of the device can be used to facilitate device passage.
Luminal edema after resection
Even after successful EFTR, there can be marked luminal edema, with loss of clear lumen. This has been described in multiple series.4,8,9 The etiology of this is not clear, although postpolypectomy syndrome may be implicated. Attempts should be made to identify the lumen. A smaller-diameter endoscope, such as a gastroscope or ultra-slim pediatric endoscope, can be used to identify the lumen and pass the endoscope proximally if the lumen is not readily identified. In cases where the lumen cannot be identified or accessed, the patient can be observed (either admitted or observed at home), with slow advancement of diet and careful attention to clinical symptoms of colonic obstruction.
Difficulty in grasping lesion
A common indication for EFTR is nonlifting of a target lesion. However, successful EFTR still requires the lesion to be brought into the FTRD cap. At times, the supplied grasping forceps will not grasp sufficiently and can tear the lesion as it is being withdrawn into the cap. In these cases, we suggest grasping a different portion of the lesion (usually at the base or along the right/bottom edge), pulling in a consistent, slow yet firm manner, with very light suctioning and gentle maneuvering of the endoscope. Suctioning should be considered as a last resort, given the risk of entrapment of adjacent organs. Assistance from a second endoscopist may be helpful in helping to deploy the FTRD clip in cases where the primary endoscopist is holding the endoscope and holding tension on the grasper simultaneously. Particularly for subepithelial lesions, an alternative option is to use either the OTSC Anchor device (Ovesco AG) or a tissue helix device (Apollo Endosurgery, Austin, Tex, USA) to achieve a deeper grasp.10
Discussion
Colorectal EFTR is useful in multiple scenarios where superficial methods such as EMR and ESD are unfavorable. EFTR has been demonstrated to be effective in providing complete resection of challenging lesions such as nonlifting lesions, previously incomplete resections, subepithelial tumors, and difficult locations such as adjacent to diverticula or appendiceal lesions.8,9,11,12 Technical success is reported to be very favorable and adverse events are rare, although they can be severe.3, 4, 5 Nevertheless, as we have demonstrated, there are multiple nuances and pitfalls that can make EFTR difficult or unsuccessful, especially for the unprepared or unsuspecting endoscopist.
Conclusion
Colorectal EFTR can offer complete en bloc resection of challenging, deep, and fibrotic lesions not amenable to existing superficial endoscopic resection techniques. Despite favorable technical and clinical success, endoscopists should be aware of the potential pitfalls and nuances of EFTR to maximize the chances of a successful endoscopic resection.
Disclosure
Dr Coronel is a consultant for Boston Scientific. All other authors disclosed no financial relationships.
Supplementary data
Difficult scenarios encountered during colorectal full thickness resection and their management: (1) Inability to advance device to target lesion; (2) injury to extraluminal structures; (3) anal trauma; (4) anal stenosis; (5) luminal edema after resection; (6) difficulty in grasping lesion; (7) recommendation for “mini time-out”; (8) Summary.
References
- 1.Aslanian H.R., Sethi A., Bhutani M.S., et al. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343–350. doi: 10.1016/j.vgie.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajan E., Wong Kee, Song L.M. Endoscopic full thickness resection. Gastroenterology. 2018;154:1925–1937.e2. doi: 10.1053/j.gastro.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht H., Raithel M., Braun A., et al. Endoscopic full-thickness resection (EFTR) in the lower gastrointestinal tract. Tech Coloproctol. 2019;23:957–963. doi: 10.1007/s10151-019-02043-5. [DOI] [PubMed] [Google Scholar]
- 4.Meier B., Stritzke B., Kuellmer A., et al. Efficacy and safety of endoscopic full-thickness resection in the colorectum: results from the German colonic FTRD registry. Am J Gastroenterol. 2020;115:1998–2006. doi: 10.14309/ajg.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 5.Andrisani G., Soriani P., Manno M., et al. Colo-rectal endoscopic full-thickness resection (EFTR) with the over-the-scope device (FTRD®): a multicenter Italian experience. Dig Liver Dis. 2019;51:375–381. doi: 10.1016/j.dld.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Vargas J.I., Rowsell C., Mosko J.D. Enterocolonic fistula after endoscopic full-thickness resection of a peri-appendiceal orifice adenoma. Gastrointest Endosc. 2020;91:1405–1406. doi: 10.1016/j.gie.2020.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Ichkhanian Y., Vosoughi K., Diehl D.L., et al. A large multicenter cohort on the use of full-thickness resection device for difficult colonic lesions. Surg Endosc. 2021;35:1296–1306. doi: 10.1007/s00464-020-07504-9. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A., Bauerfeind P., Gubler C., et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719–725. doi: 10.1055/s-0034-1391781. [DOI] [PubMed] [Google Scholar]
- 9.Tang S.J., Naga Y.M., Wu R., et al. Over-the-scope clip-assisted endoscopic full thickness resection: a video-based case series. Surg Endosc. 2020;34:2780–2788. doi: 10.1007/s00464-020-07481-z. [DOI] [PubMed] [Google Scholar]
- 10.Bauder M., Schmidt A., Caca K. Endoscopic full-thickness resection of duodenal lesions-a retrospective analysis of 20 FTRD cases. United European Gastroenterol J. 2018;6:1015–1021. doi: 10.1177/2050640618773517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt A., Beyna T., Schumacher B., et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018;67:1280–1289. doi: 10.1136/gutjnl-2016-313677. [DOI] [PubMed] [Google Scholar]
- 12.Andrisani G., Pizzicannella M., Martino M., et al. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: a single-centre study. Dig Liver Dis. 2017;49:1009–1013. doi: 10.1016/j.dld.2017.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Difficult scenarios encountered during colorectal full thickness resection and their management: (1) Inability to advance device to target lesion; (2) injury to extraluminal structures; (3) anal trauma; (4) anal stenosis; (5) luminal edema after resection; (6) difficulty in grasping lesion; (7) recommendation for “mini time-out”; (8) Summary.
Difficult scenarios encountered during colorectal full thickness resection and their management: (1) Inability to advance device to target lesion; (2) injury to extraluminal structures; (3) anal trauma; (4) anal stenosis; (5) luminal edema after resection; (6) difficulty in grasping lesion; (7) recommendation for “mini time-out”; (8) Summary.