Significance
Why do unrelated poisonous mushrooms (Amanita, Galerina, and Lepiota) make the same deadly toxin, α-amanitin? One of the most effective and fast strategies for organisms to acquire new abilities is through horizontal gene transfer (HGT). With the help of genome sequencing and the finding of two genes for the amanitin biosynthetic pathway, we demonstrate that the pathway distribution resulted from HGT probably through an unknown ancestral fungal donor. In Amanita mushrooms, the pathway evolved, through a series of gene manipulations, to produce very high levels of toxins, generating “the deadliest mushroom known to mankind.”
Keywords: Amanita, Galerina, Lepiota, genome, gene cluster
Abstract
The deadly toxin α-amanitin is a bicyclic octapeptide biosynthesized on ribosomes. A phylogenetically disjunct group of mushrooms in Agaricales (Amanita, Lepiota, and Galerina) synthesizes α-amanitin. This distribution of the toxin biosynthetic pathway is possibly related to the horizontal transfer of metabolic gene clusters among taxonomically unrelated mushrooms with overlapping habitats. Here, our work confirms that two biosynthetic genes, P450-29 and FMO1, are oxygenases important for amanitin biosynthesis. Phylogenetic and genetic analyses of these genes strongly support their origin through horizontal transfer, as is the case for the previously characterized biosynthetic genes MSDIN and POPB. Our analysis of multiple genomes showed that the evolution of the α-amanitin biosynthetic pathways in the poisonous agarics in the Amanita, Lepiota, and Galerina clades entailed distinct evolutionary pathways including gene family expansion, biosynthetic genes, and genomic rearrangements. Unrelated poisonous fungi produce the same deadly amanitin toxins using variations of the same pathway. Furthermore, the evolution of the amanitin biosynthetic pathway(s) in Amanita species generates a much wider range of toxic cyclic peptides. The results reported here expand our understanding of the genetics, diversity, and evolution of the toxin biosynthetic pathway in fungi.
The deadliest mushrooms, such as the Death Cap and the Destroying Angel, belong to the genus Amanita sect. Phalloideae, yet equally poisonous mushrooms are present in the distantly related genera Galerina and Lepiota (1, 2). For centuries, stories and incidents associated with these lethal mushrooms have been widespread (1, 3). These three genera of Agaricales are phylogenetically disjunct, but they produce the same deadly toxin, α-amanitin. This bicyclic octapeptide is a ribosomally encoded posttranslationally modified peptide. The toxin has a human LD50 (the amount fatal to half of a tested population) of 0.1 mg per kg, and one mature Death Cap fruiting body can contain a lethal dose of 10 to 12 mg (3). α-Amanitin and other related cyclic peptides are nonetheless highly valuable, not only as molecular tools (4) but also as potential treatment for diseases like cancers (5), and they have only been chemically synthesized very recently (6, 7). The cytotoxicity found in amanitins is the result of the inhibition of RNA polymerases that precludes mRNA synthesis in the liver (8).
Amanitin-producing fungi rely on the same set of biosynthetic genes for the production of α-amanitin. The proproteins are encoded by a family of genes called the MSDIN family for the first five conserved amino acids in the precursor peptides (9). The initial posttranslational processing step of the α-amanitin precursor peptide is catalyzed by POPB, a specialized member of the prolyl oligopeptidase family of serine proteases (10). The disjunct phylogenetic distribution of α-amanitin in agarics suggests that the MSDIN genes involved in biosynthesis have been dispersed by horizontal gene transfer (HGT) (11, 12). Recent findings in psilocybin-producing mushrooms, including Psilocybe cyanescens, Gymnopilus dilepis, and Panaeolus cyanescens, showed that the biosynthetic pathway for psilocybin is encoded by a gene cluster, which has been transferred throughout phylogenetic-disjunct dung and wood decay mushrooms via HGT (13). The genes coding for psilocybin biosynthesis are located in a gene cluster in Ps. cyanescens, Gy. dilepis, and Pa. Cyanescens, facilitating the HGT process (14, 15). However, such a gene cluster for amanitin biosynthesis has not been found in the Amanita or Lepiota genomes (11, 16).
The α-amanitin–producing Amanita, Galerina, and Lepiota mushrooms are phylogenetically and ecologically disjunct. The amanitin-producing Amanita belongs to the section Phalloideae, and they form a mutualistic ectomycorrhizal symbiosis with trees (17). In contrast, members of the deadly Galerina species, such as Galerina marginata, are white-rot decayers, able to decompose wood by using a large arsenal of ligninolytic oxidoreductases: dye-decolorizing peroxidase and heme-thiolate peroxidase (18). Finally, members of the lethal Lepiota species are soil-saprotrophic species. They have lost genes coding for lignin oxidoreductases, but they have an expanded set of carbohydrate-active enzymes involved in cellulose, hemicellulose, and pectin degradation.
In the present study, we investigated previously unknown amanitin biosynthetic genes and the evolution of the biosynthesis pathway in the Amanita, Galerina, and Lepiota clades. By analyzing independent genome assemblies for the species of these genera, we recaptured the homologous multigene amanitin biosynthesis locus in each poisonous lineage. The enzymatic functions of genes within this locus were confirmed by combined genetic and biochemical approaches. Two genes were confirmed to be important for the production of mature α-amanitin. Based on in-depth phylogenetic, distance, and synteny analyses, the origin of the amanitin biosynthetic pathway was carefully assessed, and three distinct genus-specific evolutionary outcomes were documented. These data support the hypothesis that the amanitin biosynthesis locus follows independent evolutionary pathways in the three deadly mushroom clades and suggest that amanitin production may be part of a larger adaptive strategy to soil niches, which harbor abundant invertebrates that eat or compete with fungi.
Results
Main Features of the Genomes from Amanitin-Producing Mushrooms.
We compared 15 genomes from amanitin-producing mushrooms, including previously unobtained genomes from one Amanita species, two Galerina species, and three Lepiota strains (from two species) (see Materials and Methods and SI Appendix). Whole Genome Shotgun assemblies have been deposited at Data Bank of Japan (DDBJ)/European Nucleotide Archive (ENA)/GenBank under the accession number JAEBUT000000000. We combined these genomes with the genome of G. marginata CBS 339.88, available from the Joint Genome Institute (JGI) MycoCosm database (19), and the Amanita and Lepiota genomes previously published (9, 11, 16, 20, 21). The completeness of the gene repertoires of 13 sequenced genomes, based on Benchmarking Universal Single-Copy Orthologs (BUSCO) and CEGMA (Core Eukaryotic Genes Mapping Approach) analyses, are provided in SI Appendix, Table S1. All the sequenced genomes are dikaryon, except for G. marginata CBS 339.88, which is a monokaryon strain. The high quality of the genomes allowed a detailed analysis of the amanitin biosynthesis loci. In Amanita, the genome size ranges from 45 to 71 Mbp, while in Galerina, it varies from 59 to 101 Mbp and in Lepiota from 37 to 55 Mbp. Notably, Galerina species had on average the largest genome sizes and the lowest content of repetitive sequences (76.89 Mbp and 15.77%, respectively). Further, Galerina genomes have the highest number of predicted genes compared to Lepiota and Amanita, with Amanita having the lowest (SI Appendix, Table S1). The genomes of the Amanita species were the most distinctive, sharing significantly less synteny within the genus and with either Galerina or Lepiota than was shared between the two saprobes (SI Appendix, Fig. S1). This limited genome synteny is possibly due to the high repetitive content of ectomycorrhizal Amanita genomes. In contrast, Galerina and Lepiota species presented significantly higher levels of synteny in the respective genera and between the two groups. Amanitin-producing Amanita and Lepiota species consistently shared more genes than did Galerina and Lepiota, and the Galerina species had a large number of species-specific genes (SI Appendix, Fig. S2).
The Amanitin Biosynthetic Genes and Loci in Galerina.
In contrast to the Amanita species, a single α-amanitin–encoding MSDIN gene, GmAMA1 (with two copies), was identified in the genome of G. marginata CBS 339.88. The functional copy, GmAMA1-1, is located near a POPB gene, GmPOPB (22).
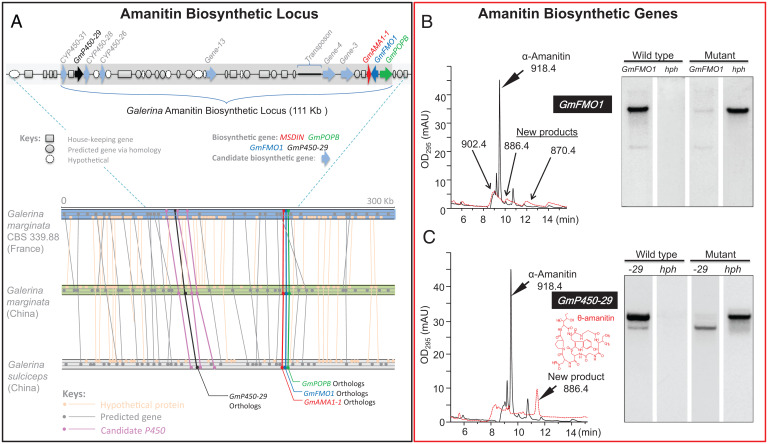
In order to find the genes responsible for amanitin biosynthesis, a search of the functional annotations was performed for each of the relevant types of enzymatic activity (cyclization, hydroxylation, sulfoxidation, formation of the tryptathionine bridge, and epimerization) using specific protein families predicted to act on chemically similar substrates to those found in the amanitin biosynthetic pathway. A set of genes possibly involved in α-amanitin biosynthesis was found in the vicinity of GmAMA1-1 (Fig. 1A). By coupling the gene disruption of these candidate genes to the chemical profiling of amanitin and amanitin-related metabolites, we characterized the cluster of genes involved in amanitin biosynthesis. Two biosynthetic genes, GmFMO1 and GmP450-29, involved in the cyclic peptide biosynthesis were identified. GmFMO1 is predicted to encode a member of the flavin mono-oxygenase (FMO) family of enzymes. Disruption of GmFMO1 abolished the formation of amanitin and gave rise to three compounds with monoisotopic masses of 902.4, 886.4, and 870.4, corresponding to α-amanitin minus one, two, or three oxygen atoms, respectively (Fig. 1B). In addition, there were four genes predicted to encode cytochrome P450s (CYP450s) near GmAMA1-1. Based on Southern blot hybridization, all four genes were found present in three amanitin-producing species and absent from the amanitin-nonproducing species (SI Appendix, Fig. S3). The sequence of one of them, GmP450-29, was successfully disrupted. The GmP450-29 deletion mutant did not biosynthesize α-amanitin, and instead a major product (named θ-amanitin, see below) appeared (Fig. 1C). This compound was isolated and identified by mass spectrometry and nuclear magnetic resonance (NMR) (SI Appendix, Figs. S4 and S5 and Table S4) as an amatoxin lacking hydroxylation at the C-4 position of proline and the C-5 position of isoleucine, indicating that GmP450-29 catalyzes one or both of these hydroxylations. These two positions are major active sites interacting with RNA polymerase II (RNAP II), and the absence of those hydroxylations caused a drastic decrease in the activity by up to 1,000-fold (C-4 position of proline). This particular amatoxin had not previously been observed and has been named θ-amanitin.
Fig. 1.
The amanitin biosynthesis locus and biosynthetic genes in Galerina species. (A) Amanitin biosynthetic genes are color-coded (red, green, blue, and black). Candidate biosynthetic genes are marked as light blue arrowheads. The amanitin biosynthetic loci were aligned for three Galerina genomes. Gene number suffix: position upstream (-) of GmAMA1-1; the two named downstream genes are not numbered based on position. Lines connect the genes (dots) with the highest homology within the genomes. (B) Deletion of GmFMO1 in G. marginata results in termination of α-amanitin production. In liquid chromatography–mass spectrometry (LC-MS) analysis: solid line denotes OD295 for wild type; red dashed line denotes OD295 for GmFMO1 mutant. In the DNA blot analysis, the wild type and the mutant are shown, with hph for the hygromycin B marker. (C) Deletion of GmP450-29 in G. marginata results in termination of α-amanitin production. In the LC-MS analysis: solid line denotes OD295 for wild type; red dashed line denotes OD295 for GmP450-29 mutant. In the DNA blot analysis, the wild type and the mutant are shown, with hph for the hygromycin B marker.
In G. marginata, the two previously known genes (GmAMA1 and GmPOPB) that are required for, and dedicated to, amanitin biosynthesis were separated in the genome by a single gene (Fig. 1A). This suggested that other biosynthetic genes might also be genomically linked to these two genes. Genes upstream and downstream of GmAMA1 and GmPOPB were therefore annotated and searched for their presence or absence in amanitin-producing and nonproducing species of Galerina, and their role was confirmed by targeted gene disruption (SI Appendix, Table S2).
Comparison of Southern blot patterns between poisonous and nonpoisonous species (SI Appendix, Fig. S3) suggested the presence of six additional candidate genes involved in amanitin biosynthesis (Fig. 1, light-blue arrowheads). Consistent with the Southern blotting results, disruption of three of the six genes led to mycelial phenotypes with abolishment or significant reduction of α-amanitin (SI Appendix, Figs. S6–S8). They are located in a gene cluster spanning 111 kbp. To confirm this spatial distribution, a different strain of G. marginata and another species, Galerina sulciceps, both collected from China, were subsequently sequenced. Highly similar gene arrangements were found in all three specimens with the amanitin biosynthetic genes in nearly identical placement (Fig. 1A); the tight arrangement was used throughout this report for locus comparison instead of using the entire harboring scaffold or contig. Based on the above results, gene homologs of AMA1, POPB, FMO1, and P450-29 were selected as markers for the amanitin biosynthetic pathway.
Phylogenetic and Genetic Distance Analyses for rpb2, GmP450-29, and GmFMO1.
With tBLASTn search (GmP450-29 and GmFMO1), the genomic DNAs (gDNAs) of representative species of Amanita, Galerina, and Lepiota were obtained from the respective genomes. Complementary DNAs (cDNAs) were acquired by aligning gDNAs with their BLASTn hits in their respective transcriptome. Gene trees were constructed and showed the same structure as those for MSDIN and POPB (11, 12). Topology conflicts of both gene tree vs. species tree are displayed in SI Appendix, Fig. S9, in which the normal species tree showed Amanita at the base and Lepiota at the terminal, while both gene trees showed that Lepiota was basal and Amanita was terminal. These conflicts, as in our previous reports on MSDIN and POPB genes, provided strong support to the hypothesis of HGT while rejecting the hypothesis of massive gene loss (11, 12). The gene trees were then analyzed using PAML software, which further supported the HGT hypothesis (SI Appendix, Table S5). The species tree, in accordance with that by Matheny et al. (23), represented the phylogeny of the housekeeping gene rpb2, while the gene trees represented the evolutionary relationships of the FMO1 and P450-29 genes. The results showed that there were significant differences in distances, synonymous rates (dS), and nonsynonymous rates (dN) among the three amanitin-producing genera. The distances of rpb2 were significantly higher than those of FMO1 and P450-29 (SI Appendix, Table S5). For MSDIN, POPB, and FMO1, the dN/dS values were below 1, while the value for P450-29 was above 1. In all cases, the dS values of the biosynthetic genes were significantly smaller that of rpb2. These data are consistent with the HGT scenario as smaller distances and lower rates are expected when compared to housekeeping genes.
Amanita Amanitin Biosynthetic Loci.
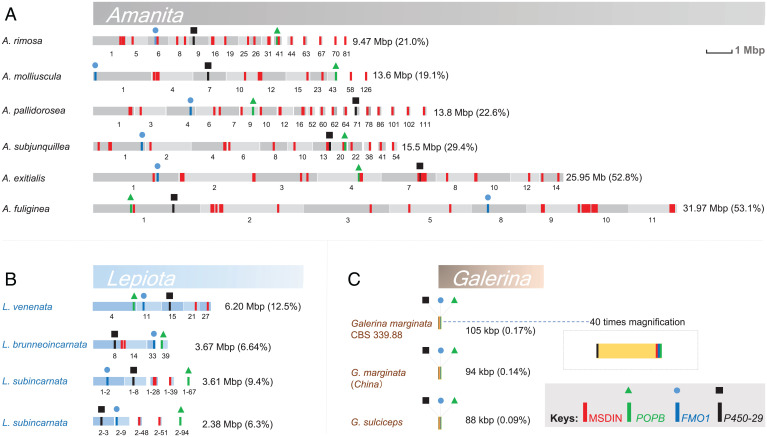
In six Amanita genomes (Amanita phalloides and Amanita bisporigera were left out due to low N50s [the shortest contig length that needs to be included for covering 50% of the genome], indicating small contig size), the amanitin biosynthetic genes were scattered on contigs or scaffolds totaling from 9.47 to 31.97 Mbp (19.1 to 53.1% of the respective genome) (Fig. 2A). These genomes contain a high number of MSDIN gene duplications, whereas the Galerina genomes encode a single MSDIN gene. A summary of MSDIN genes identified in all 15 sequenced amanitin-producing Amanita, Galerina, and Lepiota species is shown in Fig. 3, and the details are shown in SI Appendix, Table S6 and Dataset S1. These genes are distributed in a loose, largely random pattern in deadly Amanita species, but the POPB, FMO1, and P450-29 genes are frequently located near MSDIN genes (Fig. 2A). In the sequenced Amanita genomes, the MSDIN gene distribution is patchy. Duplication of MSDIN genes happens frequently in Amanita species (e.g., Amanita subjunquillea and Amanita rimosa; SI Appendix, Figs. S10 and S11). Copies of identical MSDIN genes are often linked, which partly causes the patchy patterns. Recent studies showed that most of the MSDINs are expressed at the transcription level (16, 20), and through a genome-guided approach we found 14 cyclic peptides in 5 Amanita species (20, 21). Known cyclic peptides are shown in Fig. 3 in bold. Quite noticeably, the α-amanitin–encoding MSDIN is the only gene shared by all amanitin-producing mushrooms (Fig. 3, red letters), and the ubiquitous presence of α-amanitin in these mushrooms was used for the development of a commercial amanitin detection kit (patent number: ZL 2016 1 0991804.1).
Fig. 2.
The amanitin biosynthesis loci. Contigs or scaffolds are indicated as blocks with combined sizes on the right (percentage of genome assemblies in parentheses); the display is in scale, with details in a magnified view for the locus in Galerina species. (A) Distribution of amanitin biosynthesis genes in the genome of lethal Amanita species. (B) Distribution of biosynthesis genes in lethal Lepiota species. (C) Distribution of the biosynthesis genes in lethal Galerina species. Homologs of MSDIN, POPB, P450-29, and FMO1 marked in red, green, blue, and black, respectively.
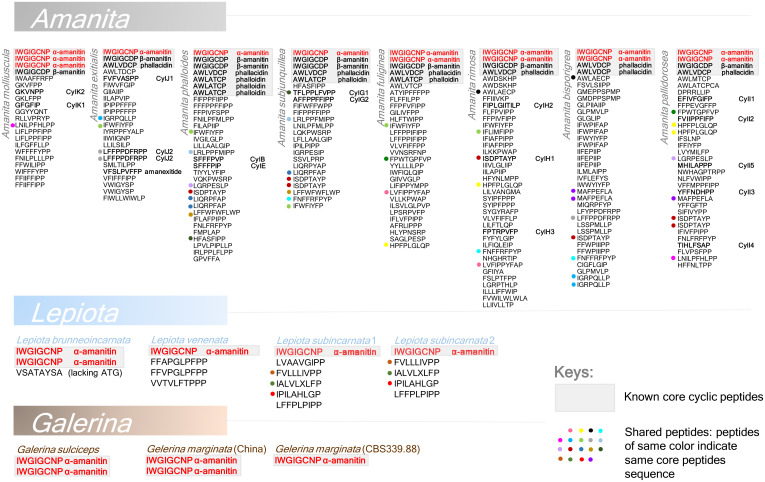
Fig. 3.
MSDIN core peptide sequences and known cyclic peptides in lethal Amanita, Galerina, and Lepiota. Core peptides (the amino acid residues in the cyclic peptide products) of known MSDINs across the three genera are listed on top of each column (shaded in gray). The α-amanitin–forming peptide is marked in red, which is the only one shared across the three genera. Shared core peptides across species are indicated with dots of the same colors in front of the peptides.
Amanitin Biosynthetic Loci in Lepiota.
In the sequenced Lepiota species, the genes involved in amanitin biosynthesis spanned from 2.38 to 6.2 Mbp on the combined contigs or scaffolds (Fig. 2B). Unlike with the Galerina species, the four amanitin biosynthetic genes are distributed in a completely random manner in the genome as none of these genes were found in close vicinity, except for a few MSDIN genes.
Synteny of Amanitin Biosynthetic Loci across Amanita, Galerina, and Lepiota.
With relaxed Synteny Mapping and Analysis Program (SyMAP) settings, the amanitin biosynthetic locus of G. marginata was searched for syntenic blocks against the genomes of A. subjunquillea, Amanita pallidorosea, Lepiota brunneoincarnata, and Lepiota venenata. In all cases, syntenic regions were found at or near the amanitin biosynthetic genes (SI Appendix, Fig. S12), suggesting that the pathways in the three genera possess similar structural regions. The result lends additional support to the HGT origin of the amanitin biosynthesis.
MSDIN Family Expansion in Amanita and Lepiota.
In Galerina, there is no expansion of the MSDIN gene family (12, 22) (Fig. 3 and SI Appendix, Table S6). In contrast, this gene family has undergone a striking expansion in the eight sequenced amanitin-producing Amanita species, with 19 to 40 MSDIN genes found in each of the sequenced genomes (Fig. 3 and Dataset S1). Most of these sequences displayed the canonical MSDIN amino acid signature (SI Appendix, Fig. S13). All known amanitin-producing Amanita species are reported to produce multiple cyclic peptides. For example, at least 12 cyclic peptides have been reported in A. phalloides (1, 3).
In the Lepiota species, we found two to six MSDIN genes (Fig. 3 and SI Appendix, Table S6), indicating a minor expansion. The canonical MSDIN sequence is often replaced by the M_DAN sequence in most of these species.
Toxin Biosynthetic Genes Specific to Amanita.
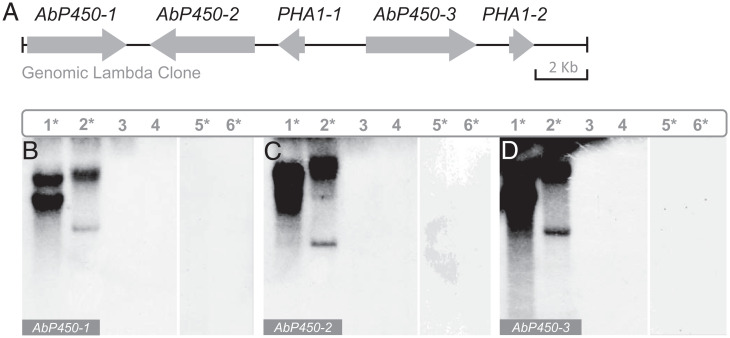
On a lambda gDNA clone of A. bisporigera, three CYP450 genes (AbP450-1, AbP450-2, and AbP450-3) were found near two MSDIN genes encoding phallacidin. These CYP450s were therefore tested for their presence in amanitin-producing Galerina, Lepiota, and amanitin-producing and non–amatin-producing Amanita species using Southern blotting. Hybridization was detected only in amanitin-producing Amanita species (Fig. 4). Consistent with this result, a BLAST search using the three CYP450 genes as queries returned no convincing hits (National Center for Biotechnology Information [NCBI] BLAST+ 2.6.0), except for the amanitin-producing Amanita species (sect. Phalloideae). These results suggest that these three P450s were specific only to the amanitin-producing Amanita species. Structures and sequences of the three genes are shown in SI Appendix, Fig. S14.
Fig. 4.
Southern blotting of amanitin biosynthesis CYP450 genes (AbP450-1, AbP450-2, and AbP450-3) in Amanita, Lepiota, and Galerina species. (A) Map of the genomic lambda clone PA1 from A. bisporigera. DNA blots show taxonomic distribution of AbP450-1 (B), AbP450-2 (C), and AbP450-3 (D). Lanes: 1, A. bisporigera; 2, A. phalloides; 3, A. porphyria; 4, A. franchetii; 5, G. marginata; 6, L. brunneoincarnata. Amanitin-producing species are marked with asterisks. PHA1-1 and PHA1-2 are the two copies of the phallacidin-encoding MSDIN gene.
Discussion
Amanitin Biosynthesis Genes.
Two types of biosynthetic genes were known to participate in the amanitin biosynthesis, i.e., MSDIN and POPB. In this study, we characterized two genes, i.e., FMO1 and P450-29, which are involved in posttranslational modifications of α-amanitin. FMOs and cytochrome CYP450s are two microsomal enzymes involved in metabolizing foreign compounds (xenobiotics) by adding molecular oxygen to their substrates (24). For α-amanitin and the related peptides, oxygenation (hydroxylation and sulfoxidation) is one of the most important posttranslational steps to produce active compounds. Without proper hydroxylation or sulfoxidation, there is a drastic activity decrease (1). The hydroxylation(s) catalyzed by GmP450-29 takes place on C-4 Pro and/or C-5 Ile of α-amanitin, and both are major sites for its affinity to the target RNAP II; e.g., a lack of C-4 Pro hydroxylation causes a 1,000-fold decrease in the activity (1, 25). GmFMO1 is important for amanitin biosynthesis as well, as not only was α-amanitin production abolished in the deletion mutant but the accumulation of the intermediates, probably the toxin minus one to three oxygen atoms, dropped to trace level. This result indicated that the oxygenation carried out by this enzyme is necessary before other posttranslational modifications can readily occur. Due to the difficulty of working with this mutant, identifying the exact site of oxygenation by this enzyme was not possible. The characterization of these two genes greatly improves our understanding of the constitution and function of amanitin biosynthesis.
Diversification of the Amanitin Biosynthetic Pathway.
The amanitin biosynthesis in the Amanita species is more complex and versatile than the amanitin pathways occurring in Galerina and Lepiota mushrooms. The basidiocarps of the Amanita sect. Phalloideae produce the largest set of cyclic peptides as a result of a striking MSDIN gene family expansion; 45 cyclic peptides have been documented in deadly Amanita species so far, although some of these cyclic peptides, such as antamanide and CylA-D, are not known toxins (1). It is widely believed that many more toxic peptides are produced by Amanita mushrooms (1, 16), and our very recent discovery of 14 cyclic peptides in 5 Amanita species through a genome-guided approach confirms this hypothesis. Many MSDIN genes are expressed at the transcriptional level and the corresponding cyclic peptides, with or without posttranslational modifications, have been detected by mass spectrometry (16). In contrast, the three available sequenced Galerina genomes encode a single MSDIN gene and the Galerina mushrooms synthesize a single cyclic peptide, i.e., α-amanitin. The γ-amanitin peptide is also synthesized by Galerina, and α-amanitin is derived from γ-amanitin by posttranslational hydroxylation (26). The presence of a small amount of β-amanitin in G. marginata is likely due to the chemical deamination of α-amanitin. In the four sequenced Lepiota genomes, α-amanitin is the major amanitin metabolite. Other minor amanitins reported in Lepiota (amanin, γ-amanitin, and amaninamide) are likely intermediates of α- or β-amanitin lacking posttranslational hydroxylation(s) (27). The current analysis of the representative genomes of Amanita, Lepiota, and Galerina showed that the α-amanitin–encoding gene is shared by all amatoxin-producing species across the three agaric families. α-Amanitin performed well in our chromogenic test (based on a highly modified Meixner test), becoming the basis of a detection kit for these poisonous mushrooms (http://www.cxbio199.com/sy).
Genes specific to amanitin-producing Amanita species have been recruited into the amanitin biosynthetic pathway in this genus. In this report, we showed that three toxin-related CYP450 genes occur only in amanitin-producing Amanita species (sect. Phalloideae). They are lacking in the genomes of nonpoisonous Amanita species, such as Amanita rubescens or Amanita thiersii (see genome available at JGI), but also in the genomes of amanitin-producing Galerina and Lepiota species. These genes can potentially process a larger range of cyclic peptide substrates. Recruitment of new genes into the amanitin biosynthetic pathway is clearly an evolutionary step leading to a wider repertoire of toxic peptides through additional posttranslational steps.
Genomic arrangement of the amanitin biosynthesis genes in the Amanita, Galerina, and Lepiota genera is genus-specific. A gene cluster–like arrangement (ca. 0.1 Mbp) is found in the three Galerina species, whereas the amanitin biosynthesis genes are randomly scattered over 2 Mbp in the Lepiota species. In Amanita, the amanitin biosynthesis genes are distributed over larger genomic regions, up to 30 Mbp. In comparison to the Lepiota species, genes are not randomly scattered over the genome. Most of the amanitin biosynthesis genes are found in physical proximity; e.g., the POPB and FMO1 genes are frequently found to be linked to MSDIN genes. This approximate gene location likely facilitates the coordinated regulation of their expression. For example, it is well known that the core cyclic peptides, α-amanitin, β-amanitin, phalloidin, and phallacidin, are often expressed in the basidiocarps at similarly high levels (27).
The present genome-wide analysis of the amanitin biosynthesis pathway in three poisonous genera of mushrooms, Galerina, Lepiota, and Amanita, reveals a striking range of genetically encoded biosynthetic capacity in the production of amanitin-related toxins. Galerina has the potential for only one MSDIN-family cyclic peptide, while the Amanita species can potentially biosynthesize hundreds of toxic cyclic peptides, with 45 biochemically confirmed metabolites. The pathway diversification, involving the expansion of the MSDIN gene family and the incorporation of several genes (e.g., CYP450) for posttranslational modifications, dramatically increases the outcome of the amanitin biosynthesis pathway in Amanita mushrooms. To the best of our knowledge, there is no other secondary metabolic pathway in fungi showing so many innovations.
Evolutionary Fates of the Amanitin Biosynthetic Pathway.
In this study, two genes involved in amanitin biosynthesis, P450-29 and FMO1, have been genetically validated through gene disruption and biochemical analysis of the resulting peptides, leading to a better understanding of the function and genomic organization of the amanitin pathway. Our phylogenetic and genetic distance analyses showed topology conflict and shorter genetic distances when compared to the species tree based on the housekeeping gene rpb2. In addition, synteny was observed among the amanitin biosynthetic loci across Amanita, Galerina, and Lepiota. The congruence of these data strongly supports that the distribution of the amanitin biosynthetic pathway is a result of HGT. In addition, the divergence time analysis showed a clear radiation of Lepiota, Galerina, and Amanita from a common ancestor, but the amanitin biosynthetic pathway was only found in three subsets of these lineages (SI Appendix, Fig. S15). Although the evidence for HGT is strong, surprisingly, the evolutionary outcome of the pathway followed distinctive paths in the three genera. In Fig. 5, we propose a putative scenario for the evolutionary fates of the amanitin biosynthesis pathway in the deadly species of Amanita, Galerina, and Lepiota. Using the genome of A. subjunquillea as an example, we showed that the amanitin biosynthesis genes are distributed over a large portion (∼15 Mbp) of the mushroom genome, with POPB, FMO1, and P450-29 each linked to an MSDIN gene. In contrast, the toxin biosynthesis genes are located on a restricted locus of 111 kbp in G. marginata. Notably, the expansion of the MSDIN gene family only takes place in Amanita sect. Phalloideae. All sequenced Galerina species only have a single MSDIN encoding α-amanitin. Finally, the distribution of the amanitin biosynthesis genes in L. brunneoincarnata differs from the Amanita and Galerina species. Unlike the Amanita and Galerina genomes, the Lepiota genome encodes only two MSDIN genes with a random genome location.
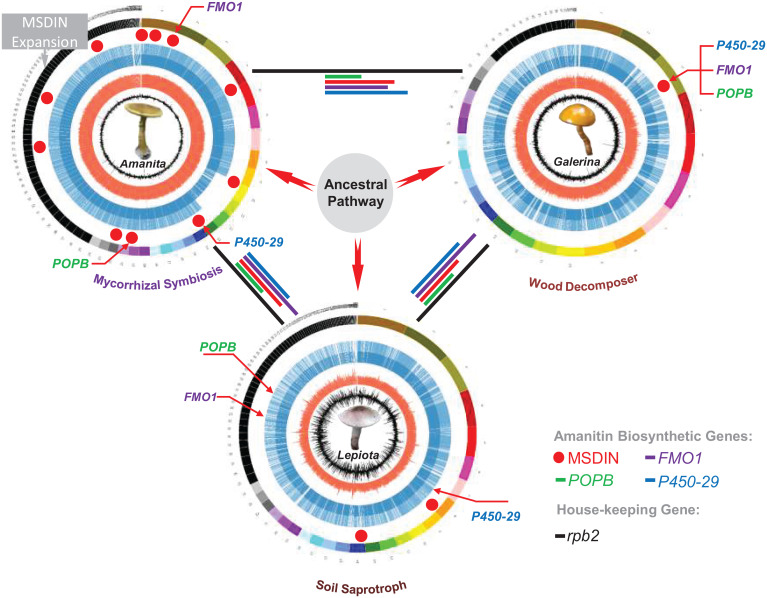
Fig. 5.
Evolutionary outcome of the amanitin biosynthesis pathway in mushrooms. The modes of nutrition, mycorrhizal symbiosis, wood decomposition, and soil saprotroph are indicated below the genomes. Within the representative genomes for Amanita, Galerina, and Lepiota, predicted genes are indicated as blue lines, GC content as red lines, and GC skew as black lines. Four toxin biosynthetic genes are color-labeled to show their respective arrangements in the genomes. Genetic distances of the biosynthetic genes are similarly color-coded as lines connecting genome circles (housekeeping rpb2 indicated as black lines). The thick red arrows represent the best hypothesis from comprehensive evaluations.
Based on the current distribution of the genes involved in amanitin biosynthesis, we initially speculated that the pathway may have originated in the Galerina clade. The gene cluster would have been transferred to other mushroom species by HGT, as observed for many secondary metabolite gene clusters, and the genes would have been physically unlinked over time. However, our recent phylogenetic and genetic distance analyses (11, 12) and the data in this study do not support this hypothesis as the most parsimonious. Another unlikely scenario is the transfer of the amanitin genes from Lepiota to Galerina to Amanita, but the dispersed loci structure and genetic distances of all four biosynthetic genes would be in conflict (the three genera have similar distances to one another). In conclusion, our results based on genomic organization, phylogeny, and genetic distance strongly suggest that direct transfer of the pathway among Amanita, Galerina, and Lepiota is highly unlikely. We therefore posit the hypothesis involving an ancestral donor species (Fig. 5). The genome of this ancestral fungal species likely encoded a gene cluster for the amanitin biosynthesis that would have been transferred to other recipient species. Thereafter, amanitin genes dispersed throughout the genome as a result of genome rearrangements and were subsequently maintained by gene sequestration. Clustering of several genes would have been maintained in the Galerina and Amanita species, retaining some of the traits of the ancestral cluster.
We hypothesize that the amanitin biosynthesis pathway became sequestered within specific lineage genomes because of the loss of the gene cluster organization resulting from transposable element (TE)–driven genome fragmentation and reshuffling. The role of TEs is supported by the fact that TEs are found in the vicinities of most amanitin biosynthesis genes in all sequenced amanitin-producing mushrooms, including Galerina (Fig. 1A).
Amanitins are well known for their bioactive properties in humans, yet their role in the ecology of poisonous mushrooms is still uncertain. While the prevailing hypothesis that these toxins are used as a defense to mycophagy by invertebrates or vertebrates is plausible, this has not yet been confirmed by chemoecological studies. Is it by chance, or do the three distinct nutritional modes of the investigated mushrooms, i.e., wood decay by Galerina, soil saprotrophy by Lepiota, and ectomycorrhizal symbiosis by Amanita, play a role in shaping the pathways into their current fates? This is certainly a fascinating question to tackle in future research.
Materials and Methods
Samples.
Fresh basidiocarps of amanitin-producing mushrooms were collected from the regions of Hunan (Amanita fuliginea, G. marginata, G. sulciceps) and Gansu (L. brunneoincarnata) in China. Two fruiting bodies of Lepiota subincarnata were collected in Italy. All sampled basidiocarps were immediately wrapped in aluminum foil and snap-frozen on dry ice and stored at −80 °C until further analyses. Most mushrooms were collected at or near sites well known for the presence of poisonous mushrooms as reported by the Chinese Center for Disease Control and Prevention. gDNA extraction and genome sequencing were carried out within one month after sampling to ensure quality.
Genome Sequencing.
Morphological identification was done by Zhu L. Yang at the Kunming Institute of Botany. Further, sequencing of the rDNA internal transcribed spacer (Dataset S2) was carried out before genomic sequencing to confirm the taxonomic affiliations. High-molecular-weight gDNA was then extracted from lyophilized fruiting bodies using the Genomic-tip 100/G kit (Qiagen 10243), according to the manufacturer’s instructions. Except for two Lepiota strains (see below), genome sequencing was carried out at the Beijing Genomics Institute (BGI) and NextOmics Biosciences following their standard sequencing procedures.
At BGI, sequencing was done using the Illumina HiSeq 4000 and the Pacific Biosciences (PacBio) RSII with 250 bp, 10 kbp, and 20 kbp DNA libraries. PacBio polymerase reads < 1,000 bp or with a quality score < 80% were removed. Subreads were extracted from polymerase reads and were adapter filtered. Subreads were corrected using Pbdagcon (https://anaconda.org/bioconda/pbdagcon), Falcon (https://github.com/PacificBiosciences/FALCON-integrate), and Proovread (28). The resultant filtered reads were assembled using the Celera Assembler (29) (version 8.3; parameters: doTrim_initialQualityBased = 1, doTrim_finalEvidenceBased = 1, doRemoveSpurReads = 1, doRemoveChimericReads = 1, -d properties -U) or Falcon (version 0.3.0; parameters: -v -dal8 -t32 -h60 -e.96 -l500 -s100 -H3000). Scaffolds were constructed using SSPACE Basic (version 2.0) (30) and gap closing with PBJelly2 (31) (version 15.8.24 with default settings). The GATK (https://gatk.broadinstitute.org/hc/en-us) and SOAP tool packages (SOAP2, SOAPsnp, SOAPindel) (32, 33) were applied for single-base corrections.
At NextOmics Biosciences, high-quality gDNA was extracted as above and used for a 20 kbp library construction. The gDNA was then randomly fragmented using Covaris g-TUBE. Large DNA fragments were enriched and purified by magnetic beads and fragmented DNA was repaired. At the ends of DNA fragments, the stem circular sequencing joints were connected, and unconnected fragments were removed by exonuclease. A 20 kbp library was constructed using a PacBio template prep kit and analyzed using an Agilent 2100 Bioanalyzer for quality control. After the completion of the library, the DNA template and the enzyme complex were transferred to the PacBio Sequel sequencer for real-time single-molecule sequencing. The Illumina HiSeq ×10 platform was used for nucleotide level correction, based on a 350 bp library, and the company’s standard method was applied.
The genomes of L. subincarnata 1 and L. subincarnata 2 strains were sequenced at the Research Technology Support Facility at Michigan State University. The main pipeline included ABySS (34) and ALLPATHS (35). For draft assembly, the two strains were sequenced using the Illumina HiSeq 4000, generating paired-end and mate-pair libraries for both samples. The libraries were cleaned using Trimmomatic (version 0.32; command line options: LEADING:20 TRAILING:20 SLIDINGWINDOW:5:20 MINLEN:85, phred33) (36) and FastQC (version 0.11.3) (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) to remove adaptors and low-quality reads. The cleaned reads were then assembled using ABySS (version 1.5.2; parameters: k = 45). New overlapping paired-end Illumina MiSeq data were then received and cleaned using Trimmomatic and FastQC (same parameters as above). This dataset, along with the mate-pair libraries, was assembled using ALLPATHS-LG. For K-mer analysis, the distribution of read lengths was analyzed using Jellyfish (version 2/2.2.0) (37) to estimate genome size. The distribution graph of the reads showed two peaks. To estimate the size for exclusively homozygous reads, the area under the second curve was calculated.
Besides the above genomes sequenced in this study, those of Amanita molliuscula, A. rimosa, A. pallidorosea, A. subjunquillea, Amanita exitialis, A. phalloides, A. bisporigera, and L. venenata were generated from previous studies (9, 11, 16, 20, 21). In addition, a genome of a monokaryotic strain of G. marginata (France, CBS 339.88) was acquired through the Joint Genome Institute (19).
The quality of the genome assemblies, including the ones previously published by our group, was checked by BUSCO (38) and CEGMA (39) analyses (SI Appendix, Table S1).
Transcriptome sequencing was based on the Illumina RNAseq platform, and clean data were obtained through NextOmics Biosciences via the company’s pipeline. Hisat2 (40) was then used to align transcriptome data with default parameters to produce Sam files. SAMtools (41) was then applied to convert Sam files to binary Bam files. Finally, the assembly was completed using StringTie (42) with default settings.
Genome Synteny Analysis.
The synteny analysis was conducted via the SyMAP 4.2 (43). Alignments were computed using default SyMAP parameters except for the comparison of G. marginata loci to those of the Amanita and Lepiota species (Min Dots = 3, Top n = 2). Genomes were not repeat-masked prior to analysis. MUMmer (44) and BLAT (45) were used to compute the raw anchors. All scaffolds or contigs for all the genomes were loaded in the analysis.
Gene Prediction.
Repeated sequences were identified using RepeatMasker (46), RepeatProteinMasker (47), and Tandem Repeats Finder (48). Repeat sequence prediction was carried out via RepeatModeler (http://www.repeatmasker.org), LTRfinder (49), and PILER (50). Three methods were then applied to predict genes. AUGUSTUS (51) and GENSCAN (52) were used to construct models for de novo prediction. GeneWise (53) was used to annotate homologous protein sequences. Transcriptome data and genome comparison were used to predict gene structure with Exonerate (https://www.ebi.ac.uk/about/vertebrate-genomics/software/exonerate). The predicted results were integrated using EVidenceModeler (54). TransposonPSI (http://transposonpsi.sourceforge.net/) was then applied to remove the genes containing transposons, resulting in the final gene set. The Eukaryotic Orthologous Groups, Gene Ontology, Kyoto Encyclopedia of Genes and Genomes, NCBI nonredundant, and UniProt databases were used for the functional annotation of predicted genes. Amanitin biosynthetic genes were manually annotated via homology comparison using BLAST (55).
BLAST Search.
NCBI BLAST+ 2.6.0. was used for BLAST searches. Nucleotide and amino acid sequences of amanitin biosynthetic genes (MSDIN, POPB, P450-29, and FMO1) from the sequenced genomes of A. molliuscula, A. exitialis, A. phalloides, A. subjunquillea, A. fuliginea, A. rimosa, A. pallidorosea, A. bisporigera, G. marginata, G. sulciceps, L. brunneoincarnata, L. venenata, and L. subincarnata were identified and captured through BLAST (NCBI BLAST+ 2.6.0) alignments. The same search method also applied to all the transcriptomes.
Identification of MSDIN Genes.
The abovementioned search strategy for MSDIN genes can miss sequences with a low percentage similarity. In Lepiota species, the C-terminal amino acid sequences of several MSDIN proteins showed a weak similarity to Amanita sequences. Consequently, we missed three MSDIN genes in L. venenata using this BLAST-based search. To find additional MSDIN sequences in the amanitin-producing mushrooms, we used a modified search strategy. A first round of queries was conducted with the known MSDIN protein and nucleotide sequences from Amanita, Galerina, and Lepiota species. The second search round used the most divergent MSDIN gene sequences resulting from the first search round. When no new MSDIN gene was detected, the query was terminated. When new MSDIN sequences were found, a third search round was performed using the new MSDIN sequences as queries. Three search rounds allowed a thorough identification of MSDIN genes for the present species. We applied this search strategy to the published genomes of A. bisporigera and A. phalloides (16), leading to the identification of additional unpublished MSDIN genes.
Venn Diagram Construction.
Predicted protein sequences of A. subjunquillea, A. molliuscula, L. venenata, G. marginata, and G. sulciceps were clustered in orthogroups using OrthoFinder (56) with default settings. The output dataset was used to construct Venn diagrams using JVENN (http://jvenn.toulouse.inra.fr/app/example.html).
Construction of Amanitin Biosynthetic Loci in Galerina Species.
Nucleotide BLAST was used to locate orthologs to GmAMA1-1—the α-amanitin–encoding MSDIN of G. marginata—in the genome assemblies. Approximately 150-kbp upstream and downstream regions of GmAMA1-1 (300 kbp in total) located in the genomes of G. marginata (from China), G. sulciceps, and G. marginata (CBS 339.88) were chosen for further analysis. The predicted genes situated in the selected regions were then annotated and compared manually. An orthologous gene was determined by finding the best homologous hit for a given gene.
Visualization of Genomes and Amanitin Biosynthetic Genes.
Circos (57) was used to map genomes and amanitin biosynthetic genes. Python scripts for obtaining guanine and cytosine (GC) content and GC skew were generated. Genome annotation files were processed mainly through Excel, and the resulting track files were used for building information tracks in the Biopython environment. The tracks were loaded into Circos to produce a genome overview in the Perl environment. Coordinates of amanitin biosynthetic genes were loaded as a track to show their precise genomic locations. In addition, the coordinates of the biosynthetic genes in each genome were manually labeled in the graph, and the sizes of each contig and scaffold were shown in scale.
Cloning of CYP450 Genes from A. bisporigera.
PCR primers unique to AbP450-1, AbP450-2, and AbP450-3 were designed and used for isolating the genomic clones of each gene. For AbP450-1, primers used were 5′-CTCCAATCCCCCAACCACAAA- 3′ (forward) and 5′-GTCGAACACGGCAACAACAG-3′ (reverse). For AbP450-2, the primers were 5′-GAAAACCGAATCTCCAATCCTC-3′ (forward) and 5′-AGCTCACTCGTTGCCACTAA-3′ (reverse). For AbP450-3, the forward primers were 5′-TTTAGGGCAGTGATTTCGTGACA-3′ and 5′-AACAGGGAGGCGATTATTCAAC-3′.
gDNA sequences were used for primer design to obtain full-length cDNAs by Rapid Amplification of cDNA Ends (RACE) using the SMART RACE kit (Clontech). A cDNA copy of AbP450-1 was obtained using the following primers: 5′-CCAACGACAGGCGGGACACG-3′ (5′-RACE) and 5′-GACCTTTTTGCTTTAACATCTACA-3′ (3′-RACE), and for AbP450-2 with the primers 5′-GTCAACAAGTCCAGGAGACATTCAAC-3′ (5′-RACE) and 5′-ACCGAATCTCCAATCCTCCAACCA-3′0 (3′-RACE). The RACE primers for AbP450-3 were 5′-CGGCGTTCCAAGGCGATGATAATA-3′ (5′-RACE) and 5′-CATCTCCATCGACCCCTTTTTCAGC-3′ (3′-RACE).
Sequences generated from the RACE reactions were used to assemble full-length cDNAs of all three genes. Alignments of genomic and cDNA copies were done using Spidey (https://www.ncbi.nlm.nih.gov/spidey/) and Splign (https://www.ncbi.nlm.nih.gov/sutils/splign/splign.cgi).
Genomic Lambda Library of A. bisporigera.
A lambda gDNA library of A. bisporigera was prepared using the λBlueSTAR Vector System (Novagen 69242) according to the manufacturer’s instructions. Screening of the library was performed using a standard Southern hybridization process, using AMA1 and PHA1 (the phallacidin-encoding MSDIN) as the probes. Positive clones were sequenced using the standard primer walking technique (58).
DNA Isolation from G. marginata.
Mycelium of G. marginata was cultured in liquid medium (HSV-2C) for 15 to 25 d with rotary shaking at 120 rpm at 23 °C. The medium (per liter) contained 1 g yeast extract, 2 g glucose, 0.1 g NH4Cl, 0.1 g CaSO4·5H2O, 1 mg thiamine·HCl, and 0.1 mg biotin, pH 5.2 (59). DNA extraction was performed using lyophilized fruiting bodies or cultures with the DNeasy Plant Mini Kit (Qiagen 69106) (for DNA blot hybridization) and the Genomic-tip 100/G (Qiagen 10243) (for constructing genomic libraries and genome sequencing), following the manufacturer’s protocols.
Gene Knockout in G. marginata.
Agrobacterium-mediated transformation of G. marginata was performed as described earlier (10). For the selected genes, ∼1.5-kbp upstream and downstream sequences were used for homologous recombination (Dataset S3). The fragments were cloned into the pHg vector (60) and transformed into Agrobacterium tumefaciens strain LBA1100 (61). The vectors were linearized before transformation to facilitate crossing over both upstream and downstream of the gene of interest and hence stable gene deletion. Ectopic integration was estimated at ∼10% of all transformants. All the transformants for each gene were analyzed, among which at least four were chemically analyzed. Conclusions about the transformant phenotype were drawn only if all independent deletion transformants displayed the same phenotype.
DNA Blot Hybridization.
Probe labeling, DNA blotting, and filter hybridization followed standard protocols (62). DNA for blotting was cut with PstI and electrophoresed in 0.7% agarose. Hybridizations were performed overnight at 65 °C in 4× SET buffer plus 0.1% sodium pyrophosphate, 0.2% SDS, 10% dextran sulfate, and 625 μg/mL heparin. SET buffer (20×) contains 3 M NaCl, 0.6 M Tris, and 0.04 M EDTA, pH 7.4. For probe preparation, gene fragments (∼500 bp) generated by PCR for each candidate gene in A. bisporigera or G. marginata were used. For G. marginata, genes selected for blotting were located upstream and downstream of GmAMA1-1 and GmPOPB (SI Appendix, Table S2). For the Amanita species, three CYP450 genes were chosen based on the lambda clone results.
Phylogenetic and Genetic Distance Analyses of rpb2, FMO1, and P450-29.
The method adopted in the present study is similar to that in our previous reports (11, 12). Coding sequences (CDS) of rpb2, FMO1, and P450-29 from the genomes used in this study were identified by tBLASTn (NCBI BLAST+ 2.12.0) and amino acid sequences of GmP450-29 and GmFMO1 were applied as query sequences. Accurate CDS sequences were acquired using respective transcriptomes as the reference. The CDSs were aligned by MAFFT v7.304b (63) with default settings. The taxa included representative species from Amanita, Galerina, and Lepiota. For the alignment, GTR + I + G was selected as the best model for the CDSs of rpb2, FMO1, and P450-29 genes, using MrModeltest v2.3 (64) under Akaike Information Criterion. To maintain the correct topology of the rbp2 species tree, more species were selected than the biosynthesis genes (nonorthologous) in the gene trees (FMO1 and P450-29). Maximum likelihood analyses and bootstrapping (1,000 replicates) were performed using RAxML v7 (65). The Codeml program in PAML v4.9 (66) was used for postphylogenetic analysis, including genetic distance and divergence rate calculations.
Characterization of Amanitin Peptides by Liquid Chromatography–Mass Spectrometry.
We analyzed the amanitin-related peptides by using the Agilent Model 1200 high-performance liquid chromatography (HPLC) system coupled to an ultraviolet detector (monitoring at 280, 295, and 305 nm) and an Agilent 6120 mass spectrometer. The HPLC elution solution A was 0.02 M ammonium acetate:acetonitrile (90:10, vol/vol), adjusted to pH 5 with glacial acetic acid, and solution B was 0.02 M aqueous ammonium acetate:acetonitrile (76:24, vol/vol), pH 5 (38).
θ-Amanitin was purified from the P450-29 mutant in two steps. The first separation was performed on a semipreparative C18 column (25 cm × 10 mm, 5 mm; Supelcosil LC-18, Supelco). The flow rate was 2 mL/min with a stepwise gradient profile of 100% A for 3 min, 43% A for 7 min, and 0% A for 9 min. Fractions containing θ-amanitin were pooled, lyophilized, and then redissolved in H2O. The second separation was performed on a 250 × 4.6 mm C18 column (Higgins Analytical, http://www.higanalyt.com). The flow rate was 1 mL/min with a gradient of 100% solution A to 100% solution B in 15 min. The fractions containing θ-amanitin were collected, dried under vacuum, and redissolved in H2O.
NMR.
θ-Amanitin (820.6 μg) was dissolved in 200 μL DMSO-d6 to a final concentration of 4.63 mM. 4,4-Dimethyl-4-silapentane-1-sulfonic acid (10 mM) (Sigma-Aldrich) was included as the chemical shift reference. Then, 1-D and 2-D spectra were recorded at 25 °C on a Bruker Avance 900 MHz spectrometer using a TCI cryoprobe at the Max T. Rogers NMR Facility, Michigan State University.
Divergence Estimation of the Genera Amanita, Galerina, and Lepiota.
Given that fossil records of fungi are limited, it has been difficult to choose a reliable calibration point to estimate the divergence time for any fungal groups. Therefore, an extensive sampling of outgroup species for which fossils were available were selected to estimate the divergence time of those species producing cyclic peptide toxins. The split between Ascomycota and Basidiomycota inferred from the fossil Paleopyrenomycites devonicus was used as the calibration. From that point, a normal distribution with a mean of 582.5 Mya and an SD of 50.15 Mya was applied (67). Three gene fragments, nrLSU, rpb2, and tef1-α, were concatenated for molecular dating using the phylogenetic framework described in James et al. (68). Nucleotide sequences were retrieved from GenBank, the Assembling the Fungal Tree of Life database, JGI (SI Appendix, Table S3), and the genomes of this study. All introns within rpb2 and tef1-α were removed due to the difficulty in alignment when large numbers of less closely related taxa were included. MrModeltest version 2.3 (64) was used to select the best models of evolution using the hierarchical likelihood ratio test. The divergence time was estimated in BEAST version 1.6.1 (69), with the molecular clock and substitutions models unlinked but the trees linked for each gene partition. The BEAST input files were constructed using BEAUti (within BEAST), in which the GTR + G + I model (MrModeltest output) was selected. The lognormal relaxed molecular clock model and the Yule speciation prior set were used to estimate the divergence times and the corresponding credibility intervals. The posterior distributions of parameters were obtained using Markov chain Monte Carlo analysis for 100 million generations with a burn-in percentage of 25%. The convergence of the chains was checked using Tracer version 1.5 (http://tree.bio.ed.ac.uk/software/tracer/2013) to confirm that the analysis reached a stationary distribution. Samples from the posterior distributions were summarized on a maximum clade credibility tree with the maximum sum of posterior probabilities on its internal nodes using TreeAnnotator version 1.8.1 with the posterior probabilities limit set to 0.5 to summarize the mean node heights. FigTree version 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/) was used to visualize the resulting tree and to obtain the means and 95% higher posterior densities.
Supplementary Material
Acknowledgments
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB31010000), the National Natural Science Foundation of China (No. 31972477, 31772377), the International Partnership Program of the Chinese Academy of Sciences (No. 151853KYSB20170026), and the Scientific Research Foundation of the Kunming Institute of Botany, Chinese Academy of Sciences. F.M.M.’s research is supported by the Laboratory of Excellence ARBRE (ANR-11-LABX-0002-01) and the Beijing Advanced Innovation Center for Tree Breeding by Molecular Design. Dr. Jonathan D. Walton has substantially contributed to this work over the past eight years. His saddening passing prevented him from approving the final version of this manuscript. At the request of his family, his name was not associated with the present paper, but we would like to acknowledge his outstanding contribution to this work and thank his family for everlasting support. In addition, we thank Dr. Qing Cai for her kind assistance with the divergence time analysis.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201113119/-/DCSupplemental.
Data Availability
The Whole Genome Shotgun assemblies are available at NCBI (BioProject PRJNA679796). All additional study data are included in the article and/or supporting information.
References
- 1.Walton J. D., The Cyclic Peptide Toxins of Amanita and Other Poisonous Mushrooms (Springer International Publishing AG, Cham, Switzerland, 2018). [Google Scholar]
- 2.Yang Z. L., Atlas of the Chinese Species of Amanitaceae (Science Press, Beijing, China, 2015). [Google Scholar]
- 3.Wieland T., Peptides of Poisonous Amanita Mushrooms (Springer-Verlag New York Inc., New York, NY, 1986). [Google Scholar]
- 4.Buchwalow I. B., Böcker W., Immunohistochemistry: Basics and Methods (Springer-Verlag Berlin Heidelberg, Heidelberg, Germany, 2010). [Google Scholar]
- 5.Liu Y., et al. , TP53 loss creates therapeutic vulnerability in colorectal cancer. Nature 520, 697–701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matinkhoo K., Pryyma A., Todorovic M., Patrick B. O., Perrin D. M., Synthesis of the death-cap mushroom toxin alpha-amanitin. J. Am. Chem. Soc. 140, 6513–6517 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Siegert M. J., Knittel C. H., Süssmuth R. D., A convergent total synthesis of the death cap toxin alpha-amanitin. Angew. Chem. Int. Ed. Engl. 59, 5500–5504 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushnell D. A., Cramer P., Kornberg R. D., Structural basis of transcription: Alpha-amanitin-RNA polymerase II cocrystal at 2.8 A resolution. Proc. Natl. Acad. Sci. U.S.A. 99, 1218–1222 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallen H. E., Luo H., Scott-Craig J. S., Walton J. D., Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. U.S.A. 104, 19097–19101 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo H., et al. , Peptide macrocyclization catalyzed by a prolyl oligopeptidase involved in α-amanitin biosynthesis. Chem. Biol. 21, 1610–1617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lüli Y., et al. , Genome of lethal Lepiota venenata and insights into the evolution of toxin-biosynthetic genes. BMC Genomics 20, 198 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo H., et al. , The MSDIN family in amanitin-producing mushrooms and evolution of the prolyl oligopeptidase genes. IMA Fungus 9, 225–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds H. T., et al. , Horizontal gene cluster transfer increased hallucinogenic mushroom diversity. Evol. Lett. 2, 88–101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gluck-Thaler E., Slot J. C., Dimensions of horizontal gene transfer in eukaryotic microbial pathogens. PLoS Pathog. 11, e1005156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slot J. C., Fungal gene cluster diversity and evolution. Adv. Genet. 100, 141–178 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Pulman J. A., Childs K. L., Sgambelluri R. M., Walton J. D., Expansion and diversification of the MSDIN family of cyclic peptide genes in the poisonous agarics Amanita phalloides and A. bisporigera. BMC Genomics 17, 1038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y. Y., Cai Q., Tang L. P., Liu J. W., Yang Z. L., The family Amanitaceae: Molecular phylogeny, higher-rank taxonomy and the species in China. Fungal Divers. 91, 5–230 (2018). [Google Scholar]
- 18.Martin F., Kohler A., Murat C., Veneault-Fourrey C., Hibbett D. S., Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 14, 760–773 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Riley R., et al. , Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. U.S.A. 111, 9923–9928 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou S., et al. , Novel cyclic peptides from lethal Amanita mushrooms through a genome-guided approach. J. Fungi (Basel) 7, 204 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lüli Y., et al. , Differential expression of amanitin biosynthetic genes and novel cyclic peptides in Amanita molliuscula. J. Fungi (Basel) 7, 384 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo H., Hallen-Adams H. E., Scott-Craig J. S., Walton J. D., Ribosomal biosynthesis of α-amanitin in Galerina marginata. Fungal Genet. Biol. 49, 123–129 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matheny P. B., Curtis J. M., Hofstetter V., Major clades of Agaricales: A multi-locus phylogenetic overview. Mycologia 98, 984–997 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Eswaramoorthy S., Bonanno J. B., Burley S. K., Swaminathan S., Mechanism of action of a flavin-containing monooxygenase. Proc. Natl. Acad. Sci. U.S.A. 103, 9832–9837 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wieland T., Chemical and toxicological studies with cyclopeptides of Amanita phalloides. Pure Appl. Chem. 6, 309–350 (1963). [Google Scholar]
- 26.Muraoka S., Fukamachi N., Mizumoto K., Shinozawa T., Detection and identification of amanitins in the wood-rotting fungi Galerina fasciculata and Galerina helvoliceps. Appl. Environ. Microbiol. 65, 4207–4210 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sgambelluri R. M., et al. , Profiling of amatoxins and phallotoxins in the genus Lepiota by liquid chromatography combined with UV absorbance and mass spectrometry. Toxins (Basel) 6, 2336–2347 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackl T., Hedrich R., Schultz J., Förster F., proovread: Large-scale high-accuracy PacBio correction through iterative short read consensus. Bioinformatics 30, 3004–3011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers E. W., et al. , A whole-genome assembly of Drosophila. Science 287, 2196–2204 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Boetzer M., Pirovano W., SSPACE-longread: Scaffolding bacterial draft genomes using long read sequence information. BMC Bioinformatics 15, 211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.English A. C., et al. , Mind the gap: Upgrading genomes with Pacific Biosciences RS long-read sequencing technology. PLoS One 7, e47768 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R., et al. , SNP detection for massively parallel whole-genome resequencing. Genome Res. 19, 1124–1132 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R., et al. , SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 25, 1966–1967 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Birol I., et al. , De novo transcriptome assembly with ABySS. Bioinformatics 25, 2872–2877 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Butler J., et al. , ALLPATHS: De novo assembly of whole-genome shotgun microreads. Genome Res. 18, 810–820 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolger A. M., Lohse M., Usadel B., Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marçais G., Kingsford C., A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27, 764–770 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seppey M., Manni M., Zdobnov E. M., BUSCO: Assessing genome assembly and annotation completeness. Methods Mol. Biol. 1962, 227–245 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Parra G., Bradnam K., Korf I., CEGMA: A pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics 23, 1061–1067 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Kim D., Langmead B., Salzberg S. L., HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., et al. ; 1000 Genome Project Data Processing Subgroup, The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pertea M., et al. , StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soderlund C., Bomhoff M., Nelson W. M., SyMAP v3.4: A turnkey synteny system with application to plant genomes. Nucleic Acids Res. 39, e68 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delcher A. L., Salzberg S. L., Phillippy A. M., Using MUMmer to identify similar regions in large sequence sets. Curr. Protoc. Bioinformatics Chapter 10, Unit 10.3 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Kent W. J., BLAT—The BLAST-like alignment tool. Genome Res. 12, 656–664 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smit A. F., The origin of interspersed repeats in the human genome. Curr. Opin. Genet. Dev. 6, 743–748 (1996). [DOI] [PubMed] [Google Scholar]
- 47.Tarailo-Graovac M., Chen N., Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinformatics Chapter 4, Unit 4.10 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Benson G., Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 27, 573–580 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z., Wang H., LTR_FINDER: An efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 35, W265-8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgar R. C., PILER-CR: Fast and accurate identification of CRISPR repeats. BMC Bioinformatics 8, 18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanke M., Steinkamp R., Waack S., Morgenstern B., AUGUSTUS: A web server for gene finding in eukaryotes. Nucleic Acids Res. 32, W309-12 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burge C., Karlin S., Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 268, 78–94 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Birney E., Clamp M., Durbin R., GeneWise and genomewise. Genome Res. 14, 988–995 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haas B. J., et al. , Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 9, R7 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altschul S. F., et al. , Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emms D. M., Kelly S., OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16, 157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krzywinski M., et al. , Circos: An information aesthetic for comparative genomics. Genome Res. 19, 1639–1645 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sterky F., Lundeberg J., Sequence analysis of genes and genomes. J. Biotechnol. 76, 1–31 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Muraoka S., Shinozawa T., Effective production of amanitins by two-step cultivation of the basidiomycete, Galerina fasciculata GF-060. J. Biosci. Bioeng. 89, 73–76 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Kemppainen M., Circosta A., Tagu D., Martin F., Pardo A. G., Agrobacterium-mediated transformation of the ectomycorrhizal symbiont Laccaria bicolor S238N. Mycorrhiza 16, 19–22 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Kemppainen M. J., Pardo A. G., pHg/pSILBAγ vector system for efficient gene silencing in homobasidiomycetes: Optimization of ihpRNA-triggering in the mycorrhizal fungus Laccaria bicolor. Microb. Biotechnol. 3, 178–200 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott-Craig J. S., Panaccione D. G., Cervone F., Walton J. D., Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell 2, 1191–1200 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katoh K., Standley D. M., A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 32, 1933–1942 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nylander J. A. A., MrModeltest v2.2 Uppsala: Evolutionary Biology Centre (Uppsala University, 2004). [Google Scholar]
- 65.Stamatakis A., RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Yang Z., PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Lücking R., Huhndorf S., Pfister D. H., Plata E. R., Lumbsch H. T., Fungi evolved right on track. Mycologia 101, 810–822 (2009). [DOI] [PubMed] [Google Scholar]
- 68.James T. Y., et al. , Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature 443, 818–822 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Drummond A. J., Suchard M. A., Xie D., Rambaut A., Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Whole Genome Shotgun assemblies are available at NCBI (BioProject PRJNA679796). All additional study data are included in the article and/or supporting information.