Abstract
Purpose
To summarize the clinical characteristics, treatment and outcomes of transplant recipients infected with Talaromyces marneffei (TM).
Materials and Methods
A retrospective analysis was performed on 2 patients with Talaromycosis marneffei (TSM) and transplants at the First Affiliated Hospital of Guangxi Medical University, and a systematic literature review was conducted simultaneously.
Results
This article reported two patients after kidney transplantation who developed fever, cough within 3–4 months. Their haemoglobin was decreased. Their chest computed tomography (CT) showed nodules. TM was detected in their blood or bronchoalveolar lavage fluid samples by next-generation sequencing (NGS). After antifungal treatment with voriconazole (VOR), one patient worsened, the other patient died. A total of 21 patients with TSM after transplants were reported in the literature review. Fourteen underwent kidney transplantation, 4 underwent liver transplantation, 2 underwent lung transplantation, and 1 underwent bone marrow transplantation. The median time from initiating the postoperative immunosuppressive therapy to the onset of symptoms or disease changes was 18 (0.5–140) months. Among them, 9 patients developed fever, 7 patients developed cough or expectoration and 4 patients developed dyspnoea. Haemoglobin was decreased in 10 patients. Pulmonary nodules were found in 7 patients. Among the 21 patients, 7 were diagnosed by positive culture, 6 by biopsy, 5 by culture and biopsy. Of the 21 patients, 13 patients improved by antifungal therapy, 8 patients worsened or died. Seven patients who received amphotericin B followed by itraconazole (ITR) therapy all improved. Regarding the use of immunosuppressants in 12 patients, 9 patients had to discontinue or reduce their medications (6 patients improved, 3 patients worsened or died).
Conclusion
Patients with TSM after transplant often have disseminated infections, involving the respiratory, hematopoietic and so on. Fever, cough, decreased haemoglobin and pulmonary nodules often occur approximately 18 months after surgery. The combined applications of culture, biopsy, NGS are helpful for an early diagnosis. Antifungal therapy with amphotericin B followed by itraconazole is recommended, and the dosage of the immunosuppressant should be adjusted timely.
Keywords: Talaromyces marneffei, Talaromycosis marneffei, transplant recipients, immunosuppressants, antifungal drug
Background
TSM is a rare endemic deep fungal disease that is prevalent in China, Thailand and other Southeast Asian regions.1 People with a suppressed immune system, such as patients with acquired immunodeficiency syndrome (AIDS), rheumatic immune system diseases and organ transplantation, are susceptible to TM. Patients who have had an organ transplantation need to take tacrolimus(Tac), mycophenolate mofetil (MMF), glucocorticoids and other antirejection drugs for a long time after the operation, thus causing immune system suppression, which causes the patients to become prone to infection by opportunistic pathogens. Candida and Aspergillus are the first two pathogens causing fungal infection in solid organ transplantation patients.2 Few cases of TM infection after transplantation have been reported. Once disseminated TM infection occurs, the mortality rate is high. Therefore, this paper reported 2 confirmed cases of transplant recipients infected with TM in our hospital, reviewed the systematic literature of 21 transplant recipients infected with TM from January 1990 to July 2021, and summarized and analysed the clinical characteristics, diagnostic methods and treatment plans of transplant recipients infected with TM to improve its clinical understanding.
Methods
A total of 380 patients diagnosed with TSM in the First Affiliated Hospital of Guangxi Medical University from January 2014 to July 2021 were retrospectively analysed, and the 380 patients included 2 patients who were infected with TM after organ transplantation. The clinical characteristics, laboratory and imaging results, and treatment plans of these two patients were analysed. At the same time, the free words “Marneffei” and “transplant” were used to retrieve documents published in the China National Knowledge Infrastructure (CNKI) database, the Wanfang Database, the Weipu Chinese Science and Technology journal database and the Public Medline (PubMed) database from January 1990 to July 2021, and the documents were retrieved and reviewed. The inclusion criteria included posttransplant patients with pathologic/culture/NGS confirmed TM infection. The exclusion criteria included patients who were not infected with TM after organ transplantation.
All data analyses in this report were completed by SPSS Statistics 20.
Result
Case Report
Case 1
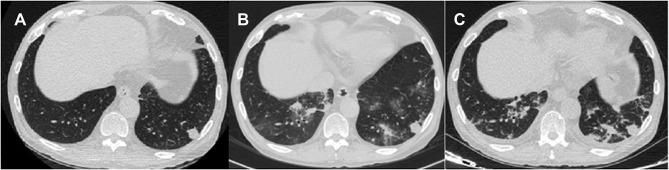
A 61-year-old male patient from Guangxi, China, was admitted to the hospital on October 17, 2019, due to “a repeated cough, expectoration and intermittent fever for more than 5 months”. In late April 2019, the patient developed cough and expectoration without any obvious inciting causes and gradually developed symptoms such as fever and shortness of breath. The patient visited another hospital, the bronchoalveolar lavage fluid (BALF) NGS revealed that the output reading of TM were 11.After antifungal treatment with voriconazole, the above symptoms in the patient improved slightly but still recurred, so the patient came to our hospital. The patient’s previous medical history included hypertension, diabetes, and uraemia; the patient previously had a renal allograft transplantation due to “hypertensive nephropathy in uraemia stage” in December 2018; and the patient took “MMF 720mg every 12 hours+prednisone(PED) 10mg once a day+Tac 25mg every 12 hours” regularly after surgery. The patient’s physical examination on admission showed that his Tmax was 39°C and that moist rhonchus could be heard in his left lower lung. His auxiliary examination was as follows: the white blood cell (WBC) was 9.28×109/L, haemoglobin (Hb) was 113.5 g/L, Plt was 247.8×109/L, the neutrophil ratio was 83.3%, the lymphocyte ratio was 9%, albumin(ALB)was 31.1 g/L, alanine aminotransferase(ALT) was 10U/L, aspartate aminotransferase(AST) was 18U/L, Creatine(Cr) was 128umol/L, procalcitonin(PCT)was 0.079 ng/mL, the erythrocyte sedimentation rate (ESR) was 62 mm, C-reactive protein(CRP)was 97.06 mg/L, plasma 1,3-beta glucan test (G test) was < 10 pg/mL, Galactomannan test (GM test) was 0.598, the CD3+ T lymphocyte count was 893 units/uL, the CD4+ T lymphocyte count was 265 units/uL, immunoglobulin A(IgA) was 1.49 g/L, immunoglobulin M(IgM) was 0.82 g/L, immunoglobulin G(IgG) was 11.73 g/L, immunoglobulin E (IgE) was 1.100IU/mL, complement C3 was 0.792 g/L, complement C4 was 0.191 g/L, and the Tac blood concentration was 14.7 ng/mL. The patient’s sputum culture: Candida and Pseudomonas aeruginosa were cultured from the patient’s BALF. The patient’s chest CT is shown in Figure 1A. The patient’s diagnosis was 1. Talaromyces marneffei combined with Pseudomonas aeruginosa infection. 2. The patient’s status after kidney transplantation.After admission, 0.2 g voriconazole was given as antifungal therapy every 12 hours. The Tac was adjusted according to the Tac concentration. Afterward, the patient had repeated fever, glucocorticoids were stopped, caspofungin was added as an antifungal, and moxifloxacin and cefodizime sodium were given for antibacterial treatment. The chest CT is shown in Figure 1B. The patient was discharged automatically with a voriconazole prescription on October 31 due to aggravation of his illness and due to worsening of his chest CT lesions (Figure 1C).
Figure 1.
(A) (2019–10-18) Multiple nodules, patchy high-density shadows and glass high-density shadows were seen in both lungs. (B) (2019–10-23) Multiple nodules and patchy high-density shadows were present in both lungs. (C) (2019–10-30) Nodules and patchy shadows were seen in both lungs, and bilateral pleural effusion.
Case 2
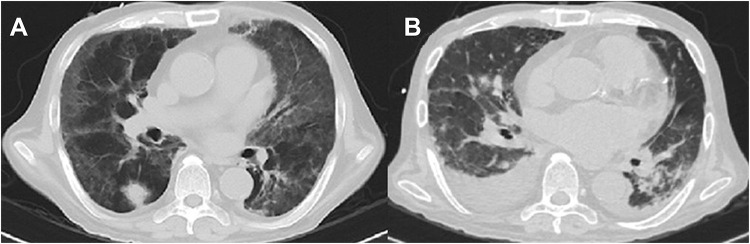
A 55-year-old male patient from Guangxi, China, was admitted to the hospital on June 24, 2019, due to “a repeated fever and shortness of breath after activity for 2 weeks”. The patient’s previous medical history included renal hypertension, uraemia and chronic Type B viral hepatitis, and the patient had undergone an renal allograft transplantation due to “chronic renal insufficiency uraemia stage” on March 6, 2019. The patient took “Tac 3mg every 12 hours + MMF 0.5g every 12 hours+ prednisone 30mg once a day”regularly after surgery for antirejection. The patient’s physical examination on admission included a Tmax 39.5°C, the presence of local moist rales in his left lower lung and multifocal areas of broken skin on the patient’s penis. Laboratory examination showed the following: the WBC count was 1.25×109/L, Hb was 70 g/L, Plt was 30.9×109/L, the neutrophil ratio was 93%, the lymphocyte ratio was 6%, ALB was 24.9 g/L, ALT was 78U/L, AST was 31U/L, Cr was 76umol/L, PCT was 1.86 ng/mL, CRP > 192 mg/L, CD3+ T lymphocyte count was 61 PCS/uL, CD4+ T lymphocyte count was 35 PCS/uL, IgA was 2.55 g/L, IgM was 0.44 g/L, IgG was 12.25 g/L, complement C3 was 0.626 g/L, complement C4 was 0.183 g/L, and the CMV was 9.70×102 copies. The patient’s blood gas analysis (ventilator FiO2 56%) included a pH of 7.42, a PO2 of 76 mmHg, a PCO2 of 23 mmHg, and a BE of 8.0 mmol/L. On the patient’s sputum smear, the fungal fluorescent staining was positive, and hyphae and spores could be seen. The patient’s chest CT scan is shown in Figure 2A. After admission, the patient was treated with cefoperazone sodium and sulbactam sodium, caspofungin, compound sulfamethoxazole, ganciclovir, entecavir in combination with anti-infection therapy and human immunoglobulin to improve the patient’s immunity. On June 26, the original immunomodulating agent discontinued and changed to methylprednisolone 80 mg every day. On June 28, the NGS using the patient’s peripheral blood revealed human polyomavirus Type 1 (BK polyomavirus) of 5098, sporozoan of 378, TM of 20, and human herpesvirus type 5 of 654. Acinetobacter baumannii was cultured from the patient’s sputum. The patient’s diagnosis included: 1. severe pneumonia (bacterial, fungal, Pneumocystis yersinensis, virus) type I respiratory failure; 2. acute respiratory distress syndrome; and 3. status after kidney transplantation. Voriconazole was added to the patient’s treatment regime on 29 June. On July 9, the patient’s clinical symptoms improved, and was changed to oral voriconazole. However, the patient soon developed peripheral hypocytosis, liver function damage, increased B-type natriuretic peptide, and chest lesions (Figure 2B) were more severe than before. On July 26, the patient developed rapid atrial fibrillation and died after unsuccessful resuscitation.
Figure 2.
(A) (2019–06-27) Patchy, flocculent and ground glass-like shadows were seen in both lungs, a nodule was seen in right lung. (B) (2019–07-22) Patchy, nodular and banding shadows had significantly increased, and bilateral pleural effusion.
Literature Review
The 21 cases were all recipients of transplants.A total of 21 cases of TM infection have been reported in the literature. Among the 21 patients, there were 17 males and 4 females, with an average age of 46 (0.58–67) years. The patients involved infections of 14 kidneys, 4 livers, 2 lungs, and 1 grafted bone marrow, which were recruited for this review. The median time from postoperative immunosuppressive therapy to the onset of symptoms or change in the disease status was 18 (0.5–140) months (Table 1).
Table 1.
The Clinical Characteristics, Diagnostic Methods and Other Coinfections of the 21 Patients
| No. | Age (Years) /Sex | Endemic Area | Graft Type | Clinical Manifestations | Involved Systems | Diagnosis Methods | Coinfection |
|---|---|---|---|---|---|---|---|
| P33 | 43/M | Taiwan, China | Renal transplant | Stomach ache | Digestive system, hematopoietic system | Ascitic Fluid culture, Blood culture | Salmonella |
| P44 | 33/M | Taiwan, China | Renal transplant | Melena, shortness of breath | Digestive system, hematopoietic system | Duodenal biopsies, Blood culture | Candida, Acid-fast bacillus |
| P55 | 47/M | Taiwan, China | Renal transplant | Cough, fever, emaciation, skin nodular, skin turgor | Skin, hematopoietic system | Blood Culture, Skin secretion culture | N |
| P66 | 38/M | Hong Kong, China | Renal transplant | Fever, stomach ache, cough | Hematopoietic system, bone, lymph nodes, respiratory system | Blood culture, bone marrow culture, lymph node biopsy | Herpesvirus |
| P77 | 42/F | Taiwan | Renal transplant | Left Buttock pain | Bone | Bone biopsy | N |
| P88 | 67/M | Vietnam | Renal transplant | Stomach ache, diarrhoea | Hematopoietic system, digestive system | Ascitic Fluid culture, blood culture | CMV, Candida |
| P99 | 41/M | China | Renal transplant | Cough, acratia, muscular soreness | Respiratory system | Lung biopsy | Hepatitis C virus (HCV) |
| P1010 | 46/M | Indonesia | Renal transplant | Fever, Face nodular | Skin | Skin biopsy | Candida, Enterobacter faecalis |
| P1111 | 45/F | Guangdong, China | Renal transplant | Oliguria, abdominal distension, cough, fever, emaciation | Respiratory system, digestive system, hematopoietic system | Ascitic Fluid culture, hydrothorax culture, Blood culture | Acinetobacter baumannii, E coli bacteraemia |
| P1212 | 51/M | China | Renal transplant | Fever, stomach ache | Lymph nodes, hematopoietic system | Blood Culture, lymph node biopsy | CMV |
| P1313 | 56/M | India | Renal transplant | Skin turgor | Respiratory system | Lung biopsy | Dematiaceous septate hyphae, Alternaria alternata |
| P1414 | 57/M | China | Bone marrow transplant | Shortness of breath | Hematopoietic system, respiratory system | Blood culture | Candida, Staphylococcus aureus |
| P1515 | 41/F | Taiwan, China | Lung transplant | Shortness of breath, headache | Respiratory system, hematopoietic system, lymph nodes | Blood culture, BALF culture, lymph node culture, lung biopsy | Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus |
| P1616 | 61/M | Myanmar* | Lung transplant | Fever, stomach-ache | Lymph nodes, hematopoietic system | Blood Culture, lymph node biopsy | CMV |
| P1717 | 52/M | China | Liver transplant | Shortness of breath | Urinary system, hematopoietic system, respiratory system | Blood culture, BALF culture, urinary culture, urinary NGS | E coli bacteraemia, CMV |
| P1818 | 52/M | China | Liver transplant | Fever, cough, expectoration | Hematopoietic system, respiratory system | Blood culture | N |
| P1919 | 50/M | China | Renal transplant | Discomfort on micturition | Urinary system, respiratory system | Urinary NGS, BALF NGS | N |
| P2020 | 53/F | China | Renal transplant | Cough, expectoration, activity intolerance | Respiratory system, lymph nodes | Lymph node biopsy, lung biopsy | N |
| P2121 | 0.58/M | China | Liver transplant | Fever | Respiratory system, hematopoietic system, digestive system | Sputum NGS, blood culture, liver biopsy | N |
| P2222 | 34/M | China | Renal transplant | Fever, cough, diarrhoea | Respiratory system, urinary system | Sputum culture, urinary culture | N |
| P2323 | 58/M | China | Liver transplant | Skin vesicles, skin pruritus | Respiratory system, skin | Skin biopsy, lung biopsy | N |
Notes: N is unknown. * means the donor has been to Myanmar.
Among the 21 patients, 9 had fever, 11 had respiratory symptoms, such as cough, expectoration and shortness of breath, and 9 had digestive symptoms, such as abdominal pain, bloating, diarrhoea or black stools. Rashes, nodules or skin swelling occurred in 5 of the patients. In addition, there were 2 patients who had urinary irritation or oliguria, 2 patients had emaciation and 1 patient had bone pain (Table 1).
The 17 patients presented with a disseminated infection involving multiple systems simultaneously (we define involvement of systems as a confirmed TM infection or imaging changes at sites), including the respiratory system in 11 patients, the hematopoietic system in 13 patients, the digestive system and lymph nodes in 5 patients, the urinary system in 3 patients, the skin in 2 patients, and the bone in 1 patient. Among the 21 patients, 7 were confirmed by positive culture, 6 by biopsy, 1 by NGS, 5 by a positive culture and biopsy, 1 by a positive culture and NGS, and 1 by a positive culture, biopsy and NGS. A total of 43 positive specimens were collected, including 13 blood samples, 11 lung samples, 5 digestive system samples, 5 lymph node samples, 4 urine samples, 3 skin samples, and 2 bone samples. Of the 21 patients, 13 had a reported coinfection with other microorganisms, and the remaining 8 had an unknown status. Among the bacterial infections, there were 2 cases of Staphylococcus aureus and E-coli bacteraemia and 1 case of Pseudomonas aeruginosa, Acinetobacter baumannii, acid-fast Bacillus, Enterobacter faecalis and Salmonella. Regarding the viral infections, CMV was present in 4 patients, herpes virus in 1 patient, and hepatitis virus in 1 patient. Among the patients with other fungal infections, 4 patients (57%, 4/7) had Candida,1 patient had respiratory candidiasis, 3 patients had oral candidiasis.1 patient revealed dematiaceous septate hyphae by skin biopsy and revealed Alternaria alternata by culture yielded,1 patient had invasive respiratory aspergillosis.
The WBC counts were recorded in 15 patients, among which 5 patients had increased WBC, 4 patients had decreased WBC, and 6 patients had normal WBC (median 7.19 (1.35–22.60) ×109/L). Anaemia occurred in 83% (10/12) of the patients, with a median Hb of 77.5 (49–155) g/L. Thrombocytopenia occurred in 71% (5/7) of the patients, with a median of 47 (10–156) ×109/L. The lymphocyte ratio was decreased in 50% (3/6) of the patients, with a median of 21 (7–78)%. Creatinine was increased in 86% (12/14) of the patients, including 8 kidney transplantation patients, and the median creatinine level in the remaining 4 patients was 167.50 (109.00–184.76) µmol/L. Albumin was measured in 4 patients and was decreased in all of them. Transaminase was increased in 3 patients. The CD4+ T lymphocyte count was decreased in 2 patients. The CRP was increased in 88% (7/8) of the patients. The PCT levels in 4 patients were increased.
Pulmonary imaging was recorded in 15 patients; 14 showed abnormal signs; 47% (7/15) showed nodule shadows (6 showed multi-node); 40% (6/15) showed hilar and mediastinal lymph node enlargement; 33% (5/15) showed cavitation (4 cases overlapped with tuberculous shadows); and 20% (3/15) showed pleural effusion. There was 1 patient who had a patchy shadow, one patient who had a ground glass shadow, one patient who had consolidation, one patient who had a mosaic sign, and one patient who had airway stenosis or emphysema. One patient was described as having interstitial infiltrates, and one patient was normal.
Of the 21 patients, 7 (33%, 7/21) patients died, including 3 (21%, 3/14) of 14 patients who underwent kidney transplantation and 3 (75%, 3/4) who died of liver transplantation. 1 case of bone marrow transplantation died (100%, 1/1). 18 patients were treated with antifungal drugs, and the median time from appearance of symptoms to antifungal therapy was 30 (10–180) days. Eight patients who were treated with amphotericin B and azole (VOR/itraconazole (ITR)) improved. Single therapy with amphotericin B alone was used in 4 patients, of whom 3 patients died, and 1 patient improved. Six patients were treated with azole alone, and 4 patients improved, 1 patient worsened and 1 patient died. The use of immunosuppressive agents was recorded in 12 patients; 2 died after switching to glucocorticoids; and 1 improved after switching to a new regimen. Nine patients had a dosage reduction or had to discontinue medication; 6 patients improved; 3 patients worsened or died (P1, P11, P23); and they were all treated with glucocorticoids before antifungal therapy (Table 2).
Table 2.
The Treatment Regimens and Outcomes of the 21 Patients
| No. | Immunosuppressive Agents | The Time Interval from Postoperative Immunosuppressive Therapy to the Onset of Symptoms or Disease Changes (Months) | The Time Interval from Invasion to Antifungal Therapy (Days) | The Use of Immunosuppressive Agents During Invasion | Antifungal Drugs | Outcome |
|---|---|---|---|---|---|---|
| P3 | Azathioprine | N | N | N | Amphotericin B 2 days | Died |
| P4 | Azathioprine+CsA/Tac+PED | 48 | N | N | None | Died |
| P5 | Tac+PED5mg qd | 31 | 60 | Reduced Tac dosage | Liposomal amphotericin B 100mg qd, followed by Itraconazole 1 year | Improved |
| P6 | Tac+MMF+PED | 9 | 17 | Reduced Tac dosage, withheld MMF | Amphotericin B 0.5mg/kg/day 30 days, followed by Itraconazole 200mg qd | Improved |
| P7 | Tac3mg bid+ PED5mg qd | 7 | 60 | N | Liposomal amphotericin B 2mg/kg/day 21 days, followed by Itraconazole 200mg qd | Improved |
| P8 | Tac1mg bid+MMF1000 mg bid + PED10mg qd | 48 | 21 | Reduced Tac dosage | Liposomal amphotericin B 3mg/kg/day 21 days, followed by Itraconazole 300mg 3 months | Improved |
| P9 | Tac2-3mg bid+MMF750mg bid | 60 | 30 | N | Voriconazole 200mg qd, followed by Itraconazole 200mg bid 2 weeks | Improved |
| P10 | Tac | 11 | N | N | # | Died |
| P11 | Tac+MMF+PED | 6 | 120 | Withheld Tac | Itraconazole 250mg qd 4 days | Worsened |
| P12 | Tac/CsA+MMF+Methylprednisolone(MP) | 10 | 99 | Withheld Tac and MP, change to CsA, continued MMF | Liposomal amphotericin B 0.4mg/kg/day 14 days, followed by Itraconazole 200mg bid | Improved |
| P13 | Tac 3.5mg twice daily+MMF 360mg bid+ PED 10 mg qd | 24 | 180 | N | Liposomal amphotericin B 3mg/kg/day 1 week, followed by Itraconazole 200mg bid 6 months | Improved |
| P14 | Fludarabine | 48 | 57 | N | Amphotericin B 1mg/kg/day 10 days | Died |
| P15 | Tac4mg bid+MMF 750 mg bid+ PED7.5mg qd | 28 | N | Reduced Tac dosage, Withheld MMF | Voriconazole 200mg bid 12 months | Improved |
| P16 | Tac+MMF+ MP | 4 | 21 | N | Liposomal amphotericin B 5mg/kg/day 6 months | Improved |
| P17 | Tac+MMF+ PED | 36 | 21 | N | Amphotericin B 5mg qd | Died |
| P18 | Tac+MMF | 12 | N | Reduced dosage and Withheld | Voriconazole 47 days change to Amphotericin B 2 weeks, followed by voriconazole 3 months | Improved |
| P19 | * | 36 | 10 | N | Voriconazole 0.2g bid 2 months | Improved |
| P20 | Tac+MMF | 140 | N | Reduced Tac dosage, Withheld MMF | Amphotericin B 2 weeks, followed by Itraconazole 12 months | Improved |
| P21 | * | 0.5 | N | Used MP | # | Died |
| P22 | Tac+MMF+ PED | 12 | 16 | N | Voriconazole 0.2g qd | Improved |
| P23 | MP+Tac+MMF | 3 | 30 | Reduced dosage | Voriconazole 11 days | Died |
Notes: * indicates that immunosuppressants were used in both P19 and P21, but the types are unknown. bid: twice daily, qd: once daily. N means unknown. # means without antifungal therapy.
Discussion
TSM is an opportunistic fungal disease caused by TM infection. TM infection has previously been more common in HIV-positive patients and is now increasingly seen in patients with secondary immunodeficiency diseases such as solid organ and haematopoietic stem cell transplantation. With the development of transplantation technology and the widespread application of immunosuppressive agents, the number of patients in the immunosuppressive state increases, and the probability of opportunistic infection increases. Opportunistic infections can impact immunodeficiency patients, and their fatality rate increases. In previous reports, the increasing number of hosts for TM infection after transplantation is an important cause of death in patients after transplantation. Therefore, it is of great significance to improve the understanding of the clinical characteristics of TM infection in transplant status hosts, the time frame when infection is prone to occur, how to effectively prevent and treat TM, and how to make an early diagnosis to improve the prognosis of patients after transplantation.
In these data, all of the patients had been living in the TM endemic area for a long time or had a sojourner history, which may provide some clues for the suspected diagnosis of TSM. Among the 21 cases reported in the literature, the median time of appearance of symptoms was 18 (0.5–140) months, while the 2 patients who were reported in this report had an earlier median time of infection than the cases in the literature. These patients presented with symptoms or had changes in their disease 3–4 months after surgery while they were taking immunosuppressive drugs.Among the 21 cases reported in the literature, the median time from appearance of symptoms to the start of antifungal treatment was 43.5 (10–180) days. This indicates that the time of infection was different in the host, but the diagnosis was often delayed for a long time.
Among the 23 patients with renal, liver, lung, and bone marrow transplantation, 18 patients had disseminated TSM and presented with systemic symptoms such as fever or emaciation and had corresponding manifestations of the various involved systems, such as cough/expectoration, dyspnoea, stomach ache, ascites, hydrothorax, skin vesicles, lymph node enlargement, etc. The respiratory and hematopoietic systems were the most commonly involved body systems. The patients’ laboratory tests often showed decreased Hb and ALB, decreased Plt, lymphocyte ratio and CD4+ T lymphocyte count, increased inflammatory markers PCT and CRP, and increased transaminase and Cr. The CT signs included nodules and hilar and mediastinal lymph node enlargements, with cavitations and hydrothorax. The patients were mainly diagnosed through culture and pathological tissues to determine the pathogen, among which the blood cultures had the highest positive rate. Cases of confirmed TM infection through NGS have been reported.24 NGS is a genre of technologies that allows for thousands to billions of DNA fragments to be simultaneously and independently sequenced.The applications of NGS in clinical microbiological testing are manifold and include metagenomic NGS (mNGS).25 mNGS enables broad identification of known as well as unexpected pathogens or even the discovery of new organisms.26 NGS can provide quantitative or semiquantitative data regarding the concentration of organisms in the sample via the counting of sequenced reads.27 For clinical mNGS, reporting should include organisms with known or at least suspected pathogenic potential, several agents are opportunistic pathogens, and their presence needs to be evaluated in the context of the patient’s presentation to determine their clinical significance.25 Compared with traditional culture and pathological examination, NGS is particularly important for the diagnosis of immunocompromised hosts, and TM is not a pulmonary colonizing fungus.28 Early diagnosis has a large impact on survival in patients with TSM. We recommended that clinicians initiate antifungal therapy when NGS suggests TM infection. The side effects of antifungal drugs vary from person to person, and close observation is sufficient.
Some patients had the clinical involvement of multiple body systems, but these patients were without fever. However, their blood culture/NGS tests were still positive, suggesting that the fungemia caused by TM was different from bacteraemia.For post-transplant patients, they are susceptible to tuberculosis, non-tuberculosis, fungus, viruses and so on, these pathogens are difficult to culture and take a long time.This will cause delays in diagnosis and treatment, but NGS can detect all of the above pathogens and faster.Therefore, we recommend that for post-transplant patients, when disease progressed, NGS testing should be performed as soon as possible. Except NGS, the novel quantitative polymerase chain reaction(qPCR) can be used as a valuable tool in the diagnosis of T. marneffei infection. The qPCR method is highly sensitive and specific for T. marneffei DNA detection in serum samples.29
The changes in the chest images in TM patients showed a variety of parenchymal and interstitial shadows, which supported a diagnosis of a fungal infection, but these lesions did not have TM specificity. For example, nodules and cavities were also found in the patients with pulmonary aspergillosis and cryptococcosis.30,31 In fungal infections, TM is more likely to invade the lymphatic system, so the patients with hilar or mediastinal lymph node enlargements have classically been associated with TM, air crescent sign has classically been associated with invasive aspergillosis.32,33 Pulmonary CT combined with diffuse ground glass density exudation should be identified as candida and pneumocystis jirovecii infections.31,34 These changes may not only be present in patients with a fungal infection but could also be caused by other bacterial and viral infections. The data in this study indicated that these patients had a high rate of other opportunistic infections, and 65% of the 23 patients had other fungal, bacterial or viral infections. Among the coinfection with other microorganisms, candida was the most common fungal infections, CMV and herpesvirus were the most common viral infections. This suggests that clinicians should take samples from multiple sites when the clinical characteristics, laboratory tests and chest CT abnormalities are abnormal in the transplanted patients who have TM infection. Traditional culture and pathological biopsy combined with NGS detection methods can be used to make early diagnoses and to determine if there is a coinfection involving other pathogens.
For HIV-positive TSM patients, amphotericin B followed by itraconazole therapy is often used as the first-line treatment, and the other treatments include voriconazole and itraconazole.35,36 In this report, the patients who were treated with amphotericin B followed by itraconazole had a 100% improvement rate (7/7). The patients who were initially treated with voriconazole had a 63% improvement rate (5/8). The patient who was treated with itraconazole alone had to discontinue therapy due to progressive respiratory failure. Therefore, when treating a transplanted patient with TSM, the preferred treatment regimen is amphotericin B followed by itraconazole.In these patients who had renal impairment, avoid further damage to kidney function, we recommend that voriconazole can be used for those patients who cannot tolerate amphotericin B.Itraconazole is not recommended for induction therapy, and the antifungal regimen should be changed in time according to the drug efficacy. Azole antifungal drugs are not recommended because they can inhibit Tac metabolism and increase its concentration, leading to renal toxicity.37–39 The results of this report also confirmed that the clinical effect of azole therapy was not satisfactory. This suggests that clinicians should monitor the tacrolimus concentration and should adjust the dose of immunosuppressive drugs in a timely manner if the patient requires the use of azole antifungal drugs.In both case reports in this article, antifungal treatment with caspofungin was used.Caspofungin was effective for treating unclassified invasive fungal infection of immunocompromised patients.40 This may explain why both patients were treated with caspofungin.In a study by Jing Zhang, it can be found that, compared with amphotericin B, itraconazole, and voriconazole, caspofungin showed relatively higher MICs (minimum inhibitory concentrations) among mold and yeast forms.41 This suggests that caspofungin is less effective in patients with TM infection.
Taking immunosuppressive drugs after organ transplantation can lead to low immunity, and the host immune status is an important factor affecting the effect of antifungal therapy. In this report, 9 patients had to reduce immunosuppressive drugs doses or discontinue.Of these patients, 6 (67%) patients improved, 1 patient died, 1 patient worsened, and 1 patient discontinued therapy. Two patients were changed to high dose pulse glucocorticoid therapy and died, and 1 patient improved on the replacement regimen. This suggests that clinicians should not only start antifungal therapy in a timely manner but also should adjust the dosage of immunosuppressants or even stop according to the drug concentration and the patient’s infection. We recommend use high dose pulse glucocorticoid with caution when TM infection occurs in patients in a transplant status.
In 23 patients, the overall mortality was 35% (8/23). The mortality rate of kidney transplant patients was 25% (4/16), liver transplant was 75% (3/4), bone marrow transplant was 100% (1/1), and lung transplant was 0 (0/2). Studying the disease course of these deceased patients found that, of the patients who died after kidney transplantation, 2 patients had increased creatinine on admission, 1 patient had not received antifungal therapy, and 1 patient had high dose pulse glucocorticoid therapy. Among the patients who died after liver transplantation, 1 patient received high dose pulse glucocorticoid therapy without antifungal therapy. This prompts clinicians to assess the patient’s general condition, correctly adjust the immunosuppressive regimen and use antifungal timely when diagnosing and treating post-transplant patients.
Conclusion
Patients with TSM in an organ transplantation status often have disseminated infection involving the respiratory, hematopoietic, digestive, lymphatic, and urinary systems and in their skin and bones. A TM infection should be considered when a patient has a fever, cough, decreased haemoglobin, a decreased lymphocyte ratio or nodules, cavities, and mediastinal hilar lymph node enlargement in their chest CT approximately 18 months after surgery. Also, the epidemic focus can provide some clues. The combination of traditional culture, NGS and pathological biopsy are beneficial to improve the positive rate and early diagnosis, and these diagnostic tests should be performed as early as possible. The antifungal regimen that was used in the patients included amphotericin B followed by itraconazole. If azole antifungal drugs are needed, blood concentrations of the immunosuppressants that the patients are taking, such as tacrolimus, should be dynamically monitored, and the timely adjustment of the proimmunosuppressant dosages can help improve the prognosis of patients.
Acknowledgments
We have not received substantial contributions from non-authors.
Funding Statement
This work was supported by grants from the Natural Science Foundation of China [NSFC 82060364 and 81760010], the Science and Technology Department of Guangxi Zhuang Autonomous Foundation of Guangxi Key Research and Development Program (No. GuikeAB20238025), and Guangxi Natural Science Foundation (NO. 2021GXNSFBA220064).
Abbreviations
TM, Talaromyces marneffei; TSM, Talaromycosis marneffei; AIDS, Acquired Immunodeficiency Syndrome; CNKI, China National Knowledge Infrastructure; PubMed, Public Medline; CT, Computed Tomography; Hb, Haemoglobin; BALF, Bronchoalveolar Lavage Fluid; NGS, Next-Generation Sequencing; HCV, Hepatitis C virus; CMV, Cytomegalovirus; VOR, Voriconazole; ITR, Itraconazole; Tac, Tacrolimus; MMF, Mycophenolate Mofetil; PED, Prednisone; MP, Methylprednisolone; WBC, White Blood Cell; ALB, Albumin; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; Cr, Creatine; ESR, Erythrocyte Sedimentation Rate; PCT, Procalcitonin; CRP, C-reactive protein; G test, 1,3-beta glucan test; GM, Galactomannan; IgA, Immunoglobulin A; IgM, Immunoglobulin M; IgG, Immunoglobulin G; IgE, Immunoglobulin E; MICs, Minimum Inhibitory Concentrations; qPCR, quantitative polymerase chain reaction.
Data Sharing Statement
All the data are fully available without restriction.
Ethics Approval and Informed Consent
This study was approved by the Ethical Review Committee of the First Affiliated Hospital of Guangxi Medical University (2022.KY-E-039).All the sources of transplanted organs have been approved by the ethical review committee.Informed consent was obtained from the donor or their next of kin.All patients or their next of kin provided written informed consent for publication of this case report and any accompanying images. The study was carried out in accordance with the principles of the Declaration of Helsinki. The first author vouches for the completeness and accuracy of the data and for the adherence of the study to the protocol.
Consent for Publication
Signed consent was obtained for the publication of the case details from the patients or their next of kin.
Author Contributions
S. Xing made substantial contributions to the conception and design of the study; acquisition, analysis, and interpretation of the data; and drafting of the manuscript. Y. Qiu, M. Pan, H. Zhang and W.Zeng participated in the analysis and interpretation of the data. J. Zhang made substantial contributions to the conception and design of the study; acquisition, analysis, and interpretation of the data; and critical revision of the manuscript for important intellectual content and was accountable for all aspects of the work to ensure that questions related to the accuracy and integrity of any part of the work were appropriately investigated and resolved. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest related to this work.
References
- 1.Deng Z, Ribas JL, Gibson DW, Connor DH. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis. 1988;10(3):640–652. doi: 10.1093/clinids/10.3.640 [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–1111. [DOI] [PubMed] [Google Scholar]
- 3.Hung CC, Hsueh PR, Chen MY, Hsiao CH, Chang SC, Luh KT. Invasive infection caused by Penicillium marneffei: an emerging pathogen in Taiwan. Clin Infect Dis. 1998;26:202–203. [DOI] [PubMed] [Google Scholar]
- 4.Ko CI, Hung CC, Chen MY, Hsueh PR, Hsiao CH, Wong JM. Endoscopic diagnosis of intestinal Penicilliosis marneffei: report of three cases and review of the literature. Gastrointest Endosc. 1999;50:111–114. [DOI] [PubMed] [Google Scholar]
- 5.Wang JL, Hung CC, Chang SC, Chueh SC, La MK. Disseminated Penicillium marneffei infection in a renal-transplant recipient successfully treated with liposomal amphotericin B. Transplantation. 2003;76:1136–1137. [DOI] [PubMed] [Google Scholar]
- 6.Chan YH, Wong KM, Lee KC, et al. Pneumonia and mesenteric lymphadenopathy caused by disseminated Penicillium marneffei infection in a cadaveric renal transplant recipient. Transpl Infect Dis. 2004;6:28–32. [DOI] [PubMed] [Google Scholar]
- 7.Lin JN, Lin HH, Lai CH, Wang JL, Yu TJ. Renal transplant recipient infected with Penicillium marneffei. Lancet Infect Dis. 2010;10:138. [DOI] [PubMed] [Google Scholar]
- 8.Hart J, Dyer JR, Clark BM, McLellan DG, Perera S, Ferrari P. Travel-related disseminated Penicillium marneffei infection in a renal transplant patient. Transpl Infect Dis. 2012;14:434–439. [DOI] [PubMed] [Google Scholar]
- 9.Zeng X, Tan D, Jiang Y. A case of Talaromyces marneffei infection after kidney transplantation. Chin J Lung Dis. 2017;10:751–753. [Google Scholar]
- 10.Zhou X. A case of Penicilliosis marneffei infection after kidney transplantation. Natl Med J China. 2002;11:44. [Google Scholar]
- 11.Chen T, Kong Y, Luo Y, Lin M, Xu J. A case of renal transplantation complicated with Penicillium marneffei infection. Chin J Tissue Eng Res. 2011;15:10068–10070. [Google Scholar]
- 12.Peng J, Chen Z, Cai R, et al. Recovery from Talaromyces marneffei involving the kidney in a renal transplant recipient: a case report and literature review. Transpl Infect Dis. 2017;19:548. doi: 10.1111/tid.12710 [DOI] [PubMed] [Google Scholar]
- 13.Sethuraman N, Thirunarayan MA, Gopalakrishnan R, Rudramurthy S, Ramasubramanian V, Parameswaran A. Talaromyces marneffei outside endemic areas in India: an emerging infection with atypical clinical presentations and review of published reports from India. Mycopathologia. 2020;185:893–904. [DOI] [PubMed] [Google Scholar]
- 14.Woo PCY, Lau SKP, Lau CCY, et al. Penicillium marneffei fungaemia in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 2005;35:831–833. [DOI] [PubMed] [Google Scholar]
- 15.Stathakis A, Lim KP, Boan P, et al. Penicillium marneffei infection in a lung transplant recipient. Transpl Infect Dis. 2015;17:429–434. [DOI] [PubMed] [Google Scholar]
- 16.Hermans F, Ombelet S, Degezelle K, et al. First-in-man observation of Talaromyces marneffei-transmission by organ transplantation. Mycoses. 2017;60:213–217. [DOI] [PubMed] [Google Scholar]
- 17.Seo JY, Ma YE, Lee JH, Lee ST, Ki CS, Lee NY. A case of disseminated Penicillium marneffei infection in a liver transplant recipient. Korean J Lab Med. 2010;30:400–405. [DOI] [PubMed] [Google Scholar]
- 18.Xu Q, Zhang X. Nursing of a case with Talaromyces marneffei infection after liver transplantation. World Latest Med Inf. 2020;1:27. doi: 10.3969/j.issn.1671-3141.2020.27.133 [DOI] [Google Scholar]
- 19.Xu C, Zhou C, Chen Y, Sun Y, Wu Q. Simultaneous urine and pulmonary alveolar lavage fluid detection in HIV-negative patients with Penicillium marneffei. Zhejiang Med J. 2018;40:2595–6+622. [Google Scholar]
- 20.Vergidis P, Rao A, Moore CB, et al. Talaromycosis in a renal transplant recipient returning from South China. Transpl Infect Dis. 2021;23:e13447. [DOI] [PubMed] [Google Scholar]
- 21.Bai M, Gu Y, Zhou J, et al. A case of disseminated Penicillium marneffei infection. J Diagn Concepts Pract. 2008;17:715–717. [Google Scholar]
- 22.Guo L, Lin Y. Clinical pharmacists participate in the treatment of Penicillium marneffei patients to find the clinical entry point. China J Pharm Econ. 2014;9:116–117. [Google Scholar]
- 23.Wang W, Kang Y, Gao S, Yu L, Liu Y, Shen Z. A case report of fatal Penicilliosis marneffei after liver transplantation. Pract J Organ Transplant. 2018;6:222–225. [Google Scholar]
- 24.Zhu YM, Ai JW, Xu B, et al. Rapid and precise diagnosis of disseminated T. marneffei infection assisted by high-throughput sequencing of multifarious specimens in a HIV-negative patient: a case report. BMC Infect Dis. 2018;18:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol. 2019;14:319–338. doi: 10.1146/annurev-pathmechdis-012418-012751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu CY. Viral pathogen discovery. Curr Opin Microbiol. 2013;16(4):468–478. doi: 10.1016/j.mib.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salipante SJ, Hoogestraat DR, Abbott AN, et al. Coinfection of Fusobacterium nucleatum and Actinomyces israelii in mastoiditis diagnosed by next-generation DNA sequencing. J Clin Microbiol. 2014;52(5):1789–1792. doi: 10.1128/JCM.03133-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson ES, Diamond JM, Bittinger K, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med. 2012;186:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Zheng Y, Wu F, et al. Evaluation of quantitative real-time PCR and Platelia galactomannan assays for the diagnosis of disseminated Talaromyces marneffei infection. Med Mycol. 2020;58(2):181–186. doi: 10.1093/mmy/myz052 [DOI] [PubMed] [Google Scholar]
- 30.Raju S, Ghosh S, Mehta AC. Chest CT signs in pulmonary disease: a pictorial review. Chest. 2017;151:1356–1374. [DOI] [PubMed] [Google Scholar]
- 31.José RJ, Periselneris JN, Brown JS. Opportunistic bacterial, viral and fungal infections of the lung. Medicine. 2020;48:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franquet T, Müller NL, Giménez A, Guembe P, de La Torre J, Bagué S. Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics. 2001;21(4):825–837. doi: 10.1148/radiographics.21.4.g01jl03825 [DOI] [PubMed] [Google Scholar]
- 33.Zhou F, Bi X, Zou X, Xu Z, Zhang T. Retrospective analysis of 15 cases of Penicilliosis marneffei in a southern China hospital. Mycopathologia. 2014;177(5–6):271–279. doi: 10.1007/s11046-014-9737-5 [DOI] [PubMed] [Google Scholar]
- 34.Shen Z, Li S, Yang J, et al. 23 cases clinical study of pneumocystis pneumonia after hematopoietic stem cell transplantation. Chin J Emerg Med. 2021;30:1248–1253. [Google Scholar]
- 35.Le T, Kinh N, Ngo C, et al. A trial of itraconazole or amphotericin B for HIV-associated talaromycosis. N Engl J Med. 2017;376:2329–2340. [DOI] [PubMed] [Google Scholar]
- 36.Huang W, Li T, Zhou C, Wei F, Cao C, Jiang J. Voriconazole versus amphotericin B as induction therapy for talaromycosis in HIV/AIDS patients: a retrospective study. Mycopathologia. 2021;186:269–276. [DOI] [PubMed] [Google Scholar]
- 37.Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805–1808. [DOI] [PubMed] [Google Scholar]
- 38.Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20:753–761. [PubMed] [Google Scholar]
- 39.Vanhove T, Bouwsma H, Hilbrands L, et al. Determinants of the magnitude of interaction between Tacrolimus and Voriconazole/Posaconazole in solid organ recipients. Am J Transplant. 2017;17:2372–2380. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Hu J, Hu Y, et al. Efficacy of Caspofungin in Unclassified Invasive Fungal Infection Cases: a Retrospective Analysis of Patients with Hematological Malignancies in China. Med Sci Monit. 2018;24:5258–5270. doi: 10.12659/MSM.908831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Liu H, Xi L, Chang YC, Kwon-Chung KJ, Seyedmousavi S. Antifungal Susceptibility Profiles of Olorofim (Formerly F901318) and Currently Available Systemic Antifungals against Mold and Yeast Phases of Talaromyces marneffei. Antimicrob Agents Chemother. 2021;65(6):e00256–21. doi: 10.1128/AAC.00256-21 [DOI] [PMC free article] [PubMed] [Google Scholar]