Introduction
As much as our understanding of human health and disease is predicated on behaviors and exposures during wakefulness, several decades of research have begun to uncover the importance of sleep and its impacts on physiology and pathology. From an evolutionary perspective, sleep places humans in a state of vulnerability, and yet this behavior is shared universally by mammals.1 However, we now understand that sleep duration and timing are essential for optimal cognitive and physiologic functioning.2–4 Sleep and wakefulness are impacted by several external and internal factors, but none are more important than the endogenous circadian system.
The term circadian is derived from Latin meaning “about a day” and the circadian “clock” is an endogenous, self-sustaining, biological time-keeping system with a period of approximately 24 hours in humans.5 This system is both extremely precise, with a standard deviation of 12 minutes within mice models and 8 minutes in humans, yet entrainable to external and behavioral stimuli known as ‘zeitgeibers’ (i.e. ‘time-givers’).6,5,7 Mechanistically, the precision of the circadian clock is established by an autonomous, transcriptional auto-regulatory feedback loop that exists both centrally within the suprachiasmatic nucleus of the hypothalamus, as well as in peripheral tissues.8, 9 The suprachiasmatic nucleus further supports the system’s pliability by modulating the pineal gland’s secretion of the hormone melatonin in response to changes in light-timing exposure.7–10 In the laboratory, the circadian clock is measured via oscillations in physiological processes (i.e., core body temperature, cortisol, or melatonin concentrations).11 Circadian “rhythms” are the physiological outputs of the circadian clock, such as the circadian-based variations in hormone secretion, enzyme activity, or organ function, among others.12,11
Much of what is known about the circadian clock and its impacts on physiology has come from studies in which the clock is desynchronized from confounding behaviors like sleep, wakefulness, eating and physical activity, as well as from external stimuli such as light, all of which having the potential to influence physiological rhythms. This desynchronization is also understood as circadian misalignment, a behavioral pattern in which sleep and activity occur during conflicting circadian phases (e.g., sleeping during the day, eating and activity during the night). Circadian misalignment can also be observed naturally in individuals experiencing irregular sleep either resulting from endogenous factors, such as sleep disorders, or from exogenous factors such as work and social schedules (Figure 1). We will review the fundamental relationships between the circadian timing system and biological processes, considering how common practices, such as shift work and variations in sleep timing on work/school versus work/school free days (i.e., social jetlag) disrupt circadian alignment and predispose individuals to cardiovascular, metabolic, and pulmonary disease.
Figure 1. Relationships between light exposure, activity/rest patterns, and the melatonin rhythm during circadian alignment, shift work, and social jetlag.
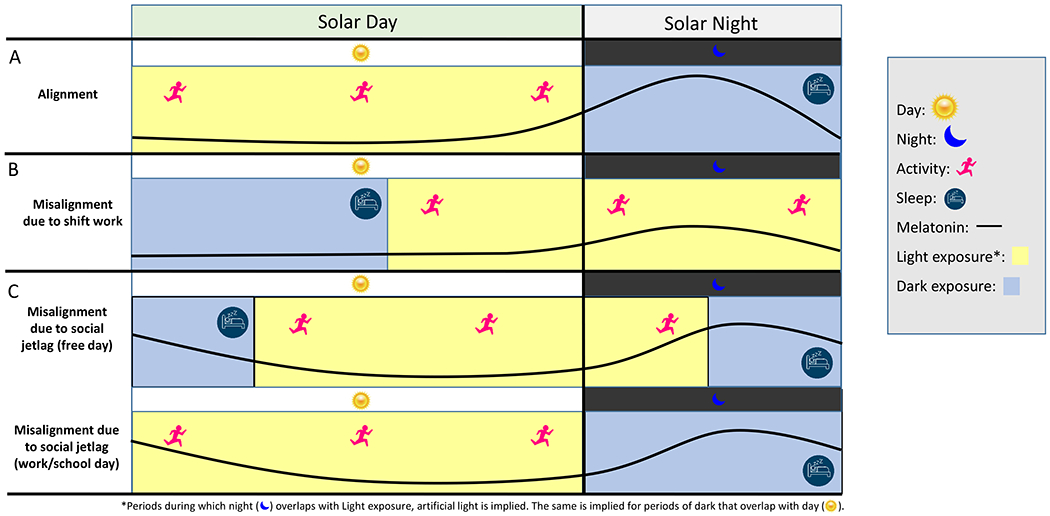
The top row (A) displays an alignment in which activity/rest, the internal biological circadian day/night (represented by the circadian melatonin rhythm) and light exposure are synchronized, the middle row (B) demonstrates how work hours (i.e., shift work) create a misalignment by altering light exposure and activity patterns and dampens the melatonin peak at night, and the bottom row (C) represents misalignment caused from social jetlag on the weekend. Specifically, social jetlag is a phenomenon in which individuals shift bedtime later, and thereby are exposed to light later, on free days resulting in a free-day delay in the circadian clock (C, top row) which subsequently causes a misalignment when an individual must return to a work/school schedules (C, bottom row).
Circadian Impacts on Physiology
The self-regulatory genes that define the circadian clock can themselves directly impact the transcription of genes involved in biologically relevant pathways. Research into mouse models have shown that nearly half of all transcripts in the genome are under circadian control.13 This is true even within complex tissues such as the liver and heart, which both demonstrate circadian variations in gene expression, though the expression profile varies significantly between the two.14 This variation in gene expression ultimately manifests in circadian dynamics of macroscopic physiology.
Cardiovascular System
Within the cardiovascular system, many processes are influenced by circadian timing. Under tightly-controlled in-laboratory environments, blood pressure displays circadian rhythmicity with a predictable zenith and nadir in the absence of sleep.15 However, sleep further causes a “dip” in blood pressure in most healthy individuals.16 While, in the heart, this is likely the result of cortisol and vagal modulation, variation in blood pressure can also be attributed to the influence of circadian timing in both the kidney–mediated by the Renin-Angiotensin-Aldosterone system–and via blood-flow dynamics within the vasculature.17–19 Circadian timing, in addition to diurnal variation, may also play a role in cardiovascular dysfunction, as evidenced by the observed increase in major cardiovascular events during the morning.20, 21 Several factors have been implicated in this epidemiological phenomena and evidence suggests that the circadian-based variation in cortisol–a stress hormone that is elevated during the morning and impacts cardiovascular and metabolic functioning–may play a role.22 Thus, these factors place a pre-disposed heart at increased risk.
Metabolism
Many rate-limiting enzymes involved in metabolic pathways, such as in fatty acid and sterol biosynthesis, are under circadian control.23, 24 Moreover, many metabolic hormones and subsequent metabolites demonstrate circadian rhythmicity.25 Glucose homeostasis also appears to depend on circadian influences, with one study finding that ablation of circadian genes in the pancreas triggers poor glucose control and the onset of diabetes mellitus in mice.26 In a seminal study investigating the impact of circadian timing on postprandial glucose, insulin, and cortisol, healthy participants were given iso-caloric meals every 6 or 12 hours over a 36 hour period, with both scenarios involving a “morning” (0600h) and “evening” (2000h) meal. Results showed that postprandial glucose response was greater following evening meals but, interestingly, was not associated with a commensurate increase in insulin secretion.12 This latter finding suggests possible circadian variation in beta-cell function in the pancreas, i.e., variation in insulin production in response to increased blood glucose. The circadian variation in insulin sensitivity and beta-cell function, specifically as it relates to circadian misalignment, will be discussed later. The Dawn Phenomena, a symptom of diabetes in which an increased dosage of insulin in type 2 diabetics is required in the morning for what is believed to be the result of decreased insulin sensitivity, may also exemplify how circadian timing impacts physiology in the diseased state.27
The process of respiration displays circadian rhythmicity during controlled in-laboratory settings, with increased and decreased respiratory rates observed in the morning and evening, respectively.28 Moreover, this variation observed in the laboratory persists even as the circadian clock is dissociated from behaviors such as sleep, wakefulness, eating and activity.28, 29 The decrease in apparent respiratory drive at night is believed to play a major role in the pathophysiology of sleep disorders as well as contributing to increased rates of respiratory failure at night.30 As seen with diabetes, chronic diseases like asthma are also affected by circadian timing. Most notably, it has long been observed that asthma symptoms increase at night, likely reflecting the injurious affects that circadian variation can have on predisposed individuals.31–34
Shift work and Social Jetlag
Sleep deficiency and dysregulation can result from several mechanisms involving endogenous and/or exogenous factors. We will consider how human behaviors, such as shift work and social jet lag (Figure 1), affect sleep behavior, induce circadian misalignment, and impact health.35–38
Shift Work
An estimated 17-20% of the US workforce engages is some form of shift work, which typically involves periods of work that occur during normal sleep time.39 In this way shift work can precipitate circadian misalignment which manifests most directly in irregular sleep schedules, less overall sleep, and poorer sleep hygiene when compared to a daytime work schedule.40 The American Academy of Sleep Medicine has developed the Shift-Work Sleep Disorder diagnosis in response to this robust finding of sleep deficiency among shift workers.41 Contrary to what may be commonly believed, adjustment to night-shift work, as measured via melatonin phase, appears minimal even among chronic night-shift workers with one report finding that less than a quarter of these individuals demonstrate “substantial” circadian adjustment.42, 43 Without the capacity to sufficiently shift the circadian clock, many shift workers are exposed to extended episodes of circadian misalignment, which is linked to a multitude of adverse health outcomes.40, 43–48
Regarding chronic disease, shift workers have up to a 40% increase risk of cardiovascular disease, a 25-45% increase risk in obesity, a 10-16% increased risk of diabetes, and up to a 55% increase in odds of having asthma.44, 45, 47, 49 Given these epidemiological findings, recent work has been done in laboratory settings to understand how much of these adverse health trends can be explained by circadian misalignment versus other factors such as food intake, activity level, sleep and light exposure.
Cardiovascular System
In investigating the impacts of shift work on cardiovascular biomarkers, one study exposed chronic shift workers to two 3-day protocols, one of which simulated night work (12-hour inverted behavioral and environmental schedule) and the other simulated day work. Circadian misalignment, as represented by a simulated night shift, was significantly correlated with increased blood pressure and increased C-reactive protein, an inflammatory marker implicated in the progression of heart disease.50, 51 Exposure to similar protocols have revealed that circadian misalignment significantly increases the serum concentration of other inflammatory markers, including IL-6 and TNFα, which are themselves risk factors for cardiovascular disease.52 Other risk factors for disease have also been found to change with exposure to shift work. Blood pressure “dipping” (≥10% reduction in blood pressure as compared to daytime levels) during sleep is a typical phenomenon in healthy individuals, while “non-dippers” (<10% reduction in blood pressure as compared to daytime levels) have been found to have increased mortality and cardiovascular disease risk.53, 54 A study investigating how shift work impacts “dipping” status found that among newly hired transit operators who began working the early-morning shift and who were considered “dippers” prior to employment, 62% were converted to a “non-dipper” status at 90 days of working early-morning shifts.55 This is in comparison to those who began working the day shift, all of whom were found to have healthy blood pressure dipping at the 90-day mark; 50% of whom had converted to a dipping status during the study monitoring period.55 Similar studies have been conducted to understand how shift work predisposes individuals to metabolic disease.
Metabolism
As stated earlier, shift work is associated with increased rates of obesity and metabolic disease.46, 49, 56 To elucidate probable mechanisms, researchers using rodent models have shown that restricting feeding to only the rest phase—as is similar with night-shift workers who eat a significant portion of their calories during the circadian rest phase—results in significant increases in body weight, fat deposits and body mass index.57, 58 This was true even though there was no statistical difference with control mice regarding total caloric intake and physical activity. Similar findings in human studies suggest that the mechanism of weight gain secondary to circadian misalignment is partly due to changes in energy expenditure that are not explained by variations in physical activity and caloric intake.59 In one study, researchers used an 8-day crossover protocol in which participants were exposed to either 5-days of simulated night shifts or day shifts, which was then repeated later using the same 8-day protocol with the alternative simulated schedule (night vs day shift). The study found that diet-induced thermogenesis (DIT), which represents a major component of daily energy expenditure, was 44% lower following evening as compared to morning meals and was primarily explained by circadian influence rather than behavioral pattern.60 Surprisingly, circadian misalignment did not significantly impact DIT in this study although a similar study using a 6-day protocol with 3-days of inverted sleep and wakefulness (circadian misalignment) found a 3-4% decrease in overall energy expenditure, with a 4% decrease in DIT following meals consumed later in the evening.61 This research suggests that food intake during the evening hours (i.e., during the circadian rest-phase), is metabolized differently than during the circadian daytime. For shift workers, food intake at night is often a necessity, which, considering this research, may help explain the increased body weight seen in epidemiological studies.
Underlying these links between adverse changes in body composition and simulated shift work is the change observed in insulin sensitivity and glucose tolerance under circadian misalignment. In a direct experiment looking into the impacts of melatonin on glucose tolerance, participants given an oral dose of melatonin in either the evening or morning had subsequent impairment in glucose tolerance.62 This is relevant as mealtimes for shift workers often coincide with episodes of increased melatonin (i.e., at night during the circadian rest phase). In a more thorough in-laboratory examination of shift work pattern and glucose control, participants in an extended circadian disruption study were subjected to 3 weeks of both sleep restriction and extended days (i.e., >24 hours).63 The study found that there was an increase in fasting glucose and post-prandial glucose levels of 8% and 14%, respectively, with corresponding decreases in insulin levels of 12-27% compared to baseline.63 The study also found that a 9-day recovery phase following misalignment restored insulin and glucose ranges to baseline levels in most participants, implying that the system is rectifiable.
Insulin dysfunction seen in circadian misalignment highlights the interplay between the function and/or reactivity of pancreatic beta cells and insulin sensitivity in peripheral tissues. In normal circadian alignment, beta-cell function is decreased during evening hours, resulting in decreased serum insulin relative to blood glucose levels.12 This may explain why those who eat later tend to have poorer metabolic profiles.35, 64, 65 However, the mechanism changes when the circadian system is misaligned.64 In a study of chronic shift-workers exposed to a 12-hour inverted behavioral schedule to mimic shift work, researchers found misalignment to result in a 10% increase in late phase post-prandial insulin even while postprandial glucose remained elevated.43 These results suggest that glucose tolerance diminishes secondarily to decreased insulin sensitivity in peripheral tissues. This may help explain the increased incidence in type 2 diabetes among shift-workers as this disease is partly defined by insulin insensitivity.66
Shift work is not the only behavioral pattern to impact sleep, circadian rhythms, and health. Social jetlag is a similar phenomenon with a less drastic effect on sleep patterns but with the potential to have equally deleterious health consequences.
Social Jetlag
Social jetlag is defined as a sleep pattern that varies between work/school days and free-days (Figure 2). During consecutive work or school days, individuals are likely unable to obtain sufficient sleep, or sleep at un-preferred times, and thus resort to “sleeping-in” on free-days to recover. Typically, sleep is increased by 2-3hours during this ‘weekend recovery’ sleep, which subsequently shifts the individual’s wakefulness schedule in a manner similar to travel-related jetlag.67 Social jetlag is most common among late chronotypes–colloquially known as “night owls”–whose biological waking time is at odds with work hours.68 Late chronotypes, in comparison to early chronotypes who fall asleep early and wake early, are more common and represent a growing demographic in industrialized nations due to societal-selection for prolonged social/work schedules with increased light exposure at night.68, 69
Figure 2. Example of a social jetlag schedule.

Delayed sleep onset on weekends due to individually preferred bedtimes results in later sleep and light exposure timing. Return to work/school-determined sleep pattern abruptly advances sleep timing in a way similar to air-travel associated jetlag.
Poor health is more commonly observed in late chronotypes, who are 2.5 times more likely than early chronotypes to report their general health to be poor or fair.70 Late chronotypes who are more likely to experience severe social jetlag are also more likely to smoke, participate in sleep-interfering behaviors, are less physically active and report increased rates of sleep disturbance.71 Objectively speaking, late chronotypes have a 2.5 fold increase in type 2 diabetes mellitus, 1.2 fold increase in obesity, and more broadly a 1.3 fold increase in metabolic syndrome, the odds of which increasing by 30% for every additional hour of “oversleep” on free-days.72, 73 Late chronotypes are also at increased risk of developing asthma.72 In terms of cardiovascular health, late chronotypes have 1.3 fold increase in hypertension and those experiencing social jetlag have a 20% increased cardiovascular risk which also increases by 30% for each additional hour of “oversleep”.72, 74 Some have linked increased cardiovascular-related deaths on Mondays to the impacts of social jetlag, though others cite a lack of sufficient evidence for the epidemiological phenomenon.75, 76 Lab research into social jetlag has found similar underlying pathological processes to that of shift work. Regarding insulin sensitivity changes, a study using healthy participants found there to be a 20% reduction in early-morning oral and intravenous insulin sensitivity following 5-days of sleep restriction (5 hours per night), with the magnitude of insulin sensitivity directly correlated with the magnitude of circadian misalignment.77 An investigation into the chronic impacts of social jetlag in mice found that shifting the 12-hour light exposure schedule by 6-hours every seven days resulted in decreased lifespan and survival rates.38
Though shift work is a more severe form of circadian misalignment, social jetlag is linked to a degree of misalignment sufficient to generate similar adverse trends in cardiovascular, metabolic and respiratory health. From a population health standpoint, social jetlag is more widely experienced in industrialized nations and its consequences more broadly felt, as in one study that found social jetlag induced by time zones to associate not only with poorer health trends, but also poorer economic performance.78 The adverse impacts of social jetlag are not experienced exclusively by working adults. In fact, social jetlag appears to be an epidemic among adolescent populations. 79
The physiologic changes occurring during adolescence, in conjunction with the psychosocial factors related to this phase of early life represents a “perfect storm” that predisposes this population to social jetlag.79, 80 Melatonin release is delayed during adolescence in part due to normal physiological changes, but also due to increased light sensitivity.81, 82 Furthermore, adolescence represents a phase of increased social responsibility and independence (i.e., sleep-time autonomy, academic scheduling and pressure, and increased screen time).83 These factors place adolescents at increased risk of experiencing social jetlag, which is positively associating with anxiety symptoms, poorer eating habits, and body mass index percentile.69, 83–85 However, sleep deprivation and social jetlag among adolescents can be mitigated by delaying school start times, though such singular measures come with their own socio-political hurdles.86
Countermeasures
The most salient physiological symptoms of shift work and social jetlag are sleep disruption and decreased alertness while awake. Symptom management has largely centered around the latter via the use of stimulants. Caffeine has for centuries been used to increase awareness and has been shown to improve psychomotor vigilance and wakefulness, particularly during times of circadian misalignment.87, 88 Periodic naps, in conjunction with caffeine, have been found to further augment wakefulness, alertness and psychomotor vigilance testing on simulated night shifts.87 However caffeine can diminish the quality of daytime sleep recovery, thus making the balance between increasing alertness and maintaining sleep quality difficult to achieve.89 Modafinil, a psychostimulant used to treat excessive daytime sleepiness in patients with narcolepsy and obstructive sleep apnea, has been shown to decrease sleepiness in laboratory settings, as well as improve tests of memory and attention when compared to placebo.90–92 However, there is no improvement in duration or quality of daytime recovery sleep, indicating that long-term use may likewise be less effective.90 Though capable of improving performance measures during the short-term, stimulants diminish the duration and quality of recovery sleep, which is essential to restoring and/or adjusting the circadian clock while also being protective against disease. Therefore, approaches to improve sleep recovery have also been tested, including the use of melatonin which has been shown to improve sleep duration during daytime sleep.93 However, there is minimal effect on performance measures assessed during subsequent evening shifts.93
Alternatively, changes in shift-scheduling and other environmental measures may help in diminishing the degree of circadian misalignment. Human circadian rhythms have been shown to respond more quickly to phase delay, (i.e., going to bed and waking up later), than phase advance, (i.e., going to bed and waking up earlier).94 Thus, establishing shift schedules to rotate clockwise (day to afternoon, afternoon to night) and also to involve smaller magnitudes in transition (<6-hours) may mitigate misalignment.94, 95 Controlling for other variables like light exposure–e.g. wearing sunglasses prior to day-recovery sleep, or “pulses” of bright light during work–, room temperature during sleep and work, as well as food intake can also improve overall perception of sleepiness and be protective against adverse health effects of shift work.94, 96, 97
For those working permanent night shifts, the goal is to allow for both alertness and productivity during the night shift, while also preserving some degree of daytime wakefulness to be utilized socially on free-days. Without a medicinal quick-fix, a “compromised” shift-schedule in the circadian clock has been proposed, (i.e., permanent shift in the circadian-phase positions).98 By using bright light pulses during simulated shift work, dark sunglasses for use outside to block blue-enriched light, adherence to scheduled sleep times in dark rooms, and outdoor afternoon light exposures to mitigate extreme rhythm delay, researchers have been able to delay participants’ circadian clock to have a nadir in alertness at ~10:00.99 In comparison to controls who had a circadian nadir of ~06:00, the treatment group was found to have increased overall sleep–sufficient sleep during daytime following night shifts and late nighttime on days off–, as well as improved performance during night shifts.” As with all attempts at circadian entrainment, the challenge with a compromised circadian-phase shift is adherence to the sleep-behavior regimen.
Summary
The circadian timing system is a powerful biological system that, when in sync with our daily habits, aids in optimal physiological functioning. However, when exposed to environments that induce misalignment, research demonstrates not only acute, but also long-term impacts on cardiovascular, metabolic, and respiratory health (Figure 3). This is not restricted to those working night shifts, as the adoption of prolonged work and social schedules, and increased light exposure at night have led many in industrialized countries to suffer from varying degrees of circadian misalignment. This social jetlag has also been shown to contribute to worsening health trends. Several modalities have been researched to address the symptoms of sleep deficiency induced by shift work and social jetlag, yet it seems conservative measures, most notably that of consistent sleep schedules in addition to proper sleep hygiene (light exposure, room temperatures, food intake), are the most protective and cost effective.
Figure 3. Cardiometabolic impacts when the circadian system is disrupted by social jetlag and shift work.
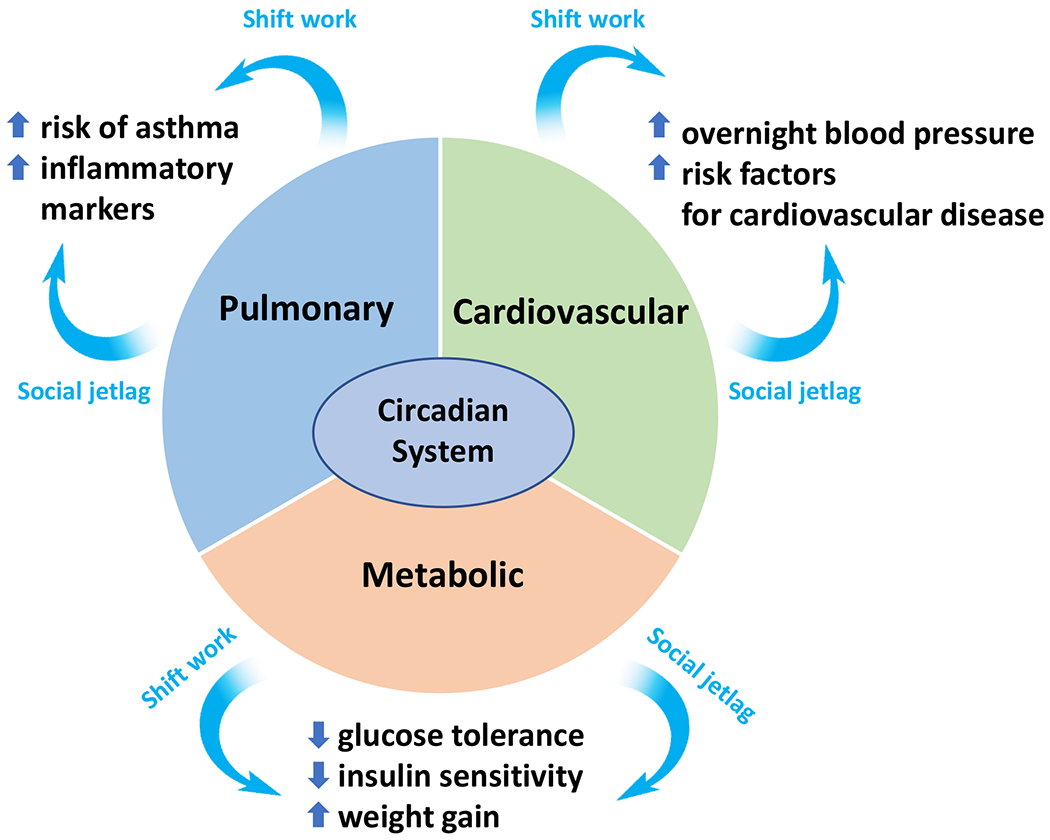
A typically well-functioning network between the circadian system and major physiological systems is disrupted by social jetlag and shift work, resulting in adverse health patterns that predispose to disease development and progression. Blue arrows point to examples of the disruptive sequelae of social jetlag and shift work.
Synopsis:
A growing body of evidence has placed an increasing emphasis on how sleep affects health. Not only does insufficient sleep make us subjectively feel worse, but is associated with chronic diseases that are considered epidemics in industrialized nations. This is in part due to the growing need for prolonged work and social schedules, exemplified by shift work, late-night weekends, and early morning work/school start-times (i.e., social jetlag). Here, we will consider fundamental relationships between the circadian clock and biological processes and discuss how common practices like shift work and social jetlag contribute to sleep disruption, circadian misalignment, and adverse health outcomes.
Clinics Care Points.
Assessment of sleep patterns, behaviors, and hygiene is an essential component of a thorough medical history.
Understand that chronic diseases are significantly impacted by and often develop within a context of poor sleep hygiene, therefore sleep health should be addressed in care planning and disease management.
- Encourage proper sleep hygiene beyond the usual “try to sleep more”:
- Establishing and adhering to consistent sleep schedule on work and off days.
- Control light exposure leading up to and during sleep, i.e. no cellphones in bed.
- Moderate use of stimulants.
- Recovery days following periods of sleep deficiency are effective at rectifying the circadian system.
Minimize large meals during evening periods when one is typically sleeping.
Advocate for your patients with their employers, highlighting not just the health benefits of consistent work and sleep schedules, but also the improved productivity.
Ensure you yourself practice proper sleep hygiene; before you can take care of others, you must take care of yourself.
Key Points:
The endogenous circadian time-keeping system influences human physiology both in healthy and in diseased states.
Misalignment between behaviors and the circadian clock (i.e., circadian misalignment) disrupts optimal functioning of physiologic processes, predisposing individuals to cardiovascular, metabolic, and respiratory disease.
Working during biological nighttime hours (i.e., shift work) and weekly changes in sleep and subsequent circadian timing (i.e., social jetlag) are common causes of circadian misalignment and are associated with impaired health profiles.
Countermeasures, such as improving sleep hygiene, maintaining consistent sleep schedules, and scheduling work timing to match endogenous circadian timing, have been shown to be effective in combating circadian misalignment.
Support:
This work was supported by National Institutes of Health (NIH) grants K01HL146992, R56 HL156948, R35 HL155681, U19OH010154, and by the Oregon Institute of Occupational Health Sciences at Oregon Health & Science University via funds from the Division of Consumer and Business Services of the State of Oregon (ORS 656.630).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The Authors have nothing to disclose.
Bibliography
- 1.Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. Jul 2008;62(7):1764–76. doi: 10.1111/j.1558-5646.2008.00392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besedovsky L, Lange T, Haack M. The Sleep-Immune Crosstalk in Health and Disease. Physiol Rev. Jul 1 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. Mar 2009;1156:168–97. doi: 10.1111/j.1749-6632.2009.04416.x [DOI] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Miller MA. Sleep and Cardio-Metabolic Disease. Curr Cardiol Rep. Sep 19 2017;19(11):110. doi: 10.1007/s11886-017-0916-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. Jun 25 1999;284(5423):2177–81. doi: 10.1126/science.284.5423.2177 [DOI] [PubMed] [Google Scholar]
- 6.Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. Feb 2004;19(1):35–46. doi: 10.1177/0748730403260776 [DOI] [PubMed] [Google Scholar]
- 7.VanderLeest HT, Houben T, Michel S, et al. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. Mar 6 2007;17(5):468–73. doi: 10.1016/j.cub.2007.01.048 [DOI] [PubMed] [Google Scholar]
- 8.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. Dec 2006;21(6):494–506. doi: 10.1177/0748730406293889 [DOI] [PubMed] [Google Scholar]
- 10.Roenneberg T, Daan S, Merrow M. The art of entrainment. J Biol Rhythms. Jun 2003;18(3):183–94. doi: 10.1177/0748730403018003001 [DOI] [PubMed] [Google Scholar]
- 11.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Balériaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. Apr 2005;20(2):178–88. doi: 10.1177/0748730404273983 [DOI] [PubMed] [Google Scholar]
- 12.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. Apr 1992;262(4 Pt 1):E467–75. doi: 10.1152/ajpendo.1992.262.4.E467 [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proceedings of the National Academy of Sciences. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storch KF, Lipan O, Leykin I, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. May 2 2002;417(6884):78–83. doi: 10.1038/nature744 [DOI] [PubMed] [Google Scholar]
- 15.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. Apr 15 2011;108(8):980–4. doi: 10.1161/circresaha.110.233668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo K, Woo B, Wong M, Tam W. Subjective sleep quality, blood pressure, and hypertension: a meta-analysis. J Clin Hypertens (Greenwich). Mar 2018;20(3):592–605. doi: 10.1111/jch.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheer FAJL, Hu K, Evoniuk H, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proceedings of the National Academy of Sciences. 2010;107(47):20541–20546. doi: 10.1073/pnas.1006749107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thosar SS, Rueda JF, Berman AM, et al. Separate and interacting effects of the endogenous circadian system and behaviors on plasma aldosterone in humans. Am J Physiol Regul Integr Comp Physiol. Feb 1 2019;316(2):R157–r164. doi: 10.1152/ajpregu.00314.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thosar SS, Berman AM, Herzig MX, et al. Circadian Rhythm of Vascular Function in Midlife Adults. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(6):1203–1211. doi: 10.1161/atvbaha.119.312682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. Oct 1 1987;60(10):801–6. doi: 10.1016/0002-9149(87)91027-7 [DOI] [PubMed] [Google Scholar]
- 21.Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. Nov 21 1985;313(21):1315–22. doi: 10.1056/nejm198511213132103 [DOI] [PubMed] [Google Scholar]
- 22.Crawford AA, Soderberg S, Kirschbaum C, et al. Morning plasma cortisol as a cardiovascular risk factor: findings from prospective cohort and Mendelian randomization studies. Eur J Endocrinol. Oct 2019;181(4):429–438. doi: 10.1530/eje-19-0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. Dec 3 2010;330(6009):1349–54. doi: 10.1126/science.1195027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Martelot G, Claudel T, Gatfield D, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. Sep 2009;7(9):e1000181. doi: 10.1371/journal.pbio.1000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. May 2005;90(5):2537–44. doi: 10.1210/jc.2004-2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. Jul 29 2010;466(7306):627–31. doi: 10.1038/nature09253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Pathogenesis of the Dawn Phenomenon in Patients with Insulin-Dependent Diabetes Mellitus. New England Journal of Medicine. 1985;312(23):1473–1479. doi: 10.1056/nejm198506063122302 [DOI] [PubMed] [Google Scholar]
- 28.Zitting KM, Vujovic N, Yuan RK, et al. Human Resting Energy Expenditure Varies with Circadian Phase. Curr Biol. Nov 19 2018;28(22):3685–3690 e3. doi: 10.1016/j.cub.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol. Aug 1 2000;526 Pt 3(Pt 3):683–94. doi: 10.mi/j.1469-7793.2000.00683.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schäfer T Respiratory pathophysiology: sleep-related breathing disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2006;5:Doc01. [PMC free article] [PubMed] [Google Scholar]
- 31.Turner-Warwick M Epidemiology of nocturnal asthma. Am J Med. Jul 29 1988;85(1b):6–8. doi: 10.1016/0002-9343(88)90231-8 [DOI] [PubMed] [Google Scholar]
- 32.Hetzel MR, Clark TJ. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax. Oct 1980;35(10):732–8. doi: 10.1136/thx.35.10.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiro SG. Nocturnal asthma and sudden death. Am Fam Physician. Dec 1976;14(6):62–71 [PubMed] [Google Scholar]
- 34.Scheer F, Hilton MF, Evoniuk HL, et al. The endogenous circadian system worsens asthma at night independent of sleep and other daily behavioral or environmental cycles. Proc Natl Acad Sci U S A. Sep 14 2021;118(37)doi: 10.1073/pnas.2018486118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHill AW, Czeisler CA, Phillips AJK, et al. Caloric and Macronutrient Intake Differ with Circadian Phase and between Lean and Overweight Young Adults. Nutrients. Mar 11 2019;11(3)doi: 10.3390/nu11030587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Research. 2009/08/15/ 2009;168(3):259–261. doi: 10.1016/j.psychres.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 37.Merikanto I, Lahti T, Kronholm E, et al. Evening types are prone to depression. Chronobiol Int. Jun 2013;30(5):719–25. doi: 10.3109/07420528.2013.784770 [DOI] [PubMed] [Google Scholar]
- 38.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006:R914–6. vol. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMenamin TM. A Time to Work: recent trends in shift work and flexible schedules. Monthly LabRev. 2007;130(3) [Google Scholar]
- 40.Costa G Sleep deprivation due to shift work. Handb Clin Neurol. 2015;131:437–46. doi: 10.1016/b978-0-444-62627-1.00023-8 [DOI] [PubMed] [Google Scholar]
- 41.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. Nov 2014; 146(5):1387–1394. doi: 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 42.Folkard S Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int. Apr 2008;25(2):215–24. doi: 10.1080/07420520802106835 [DOI] [PubMed] [Google Scholar]
- 43.Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J Clin Endocrinol Metab. Mar 2016;101(3):1066–74. doi: 10.1210/jc.2015-3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vetter C, Dashti HS, Lane JM, et al. Night Shift Work, Genetic Risk, and Type 2 Diabetes in the UK Biobank. Diabetes Care. Apr 2018;41(4):762–769. doi: 10.2337/dc17-1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maidstone RJ, Turner J, Vetter C, et al. Night shift work is associated with an increased risk of asthma. Thorax. Jan 2021;76(1):53–60. doi: 10.1136/thoraxjnl-2020-215218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vetter C, Devore EE, Wegrzyn LR, et al. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. Jama. Apr 26 2016;315(16):1726–34. doi: 10.1001/jama.2016.4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown DL, Feskanich D, Sánchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. American journal of epidemiology. Jun 1 2009;169(11):1370–7. doi: 10.1093/aje/kwp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akerstedt T, Wright KP Jr. Sleep Loss and Fatigue in Shift Work and Shift Work Disorder. Sleep Med Clin. Jun 1 2009;4(2):257–271. doi: 10.1016/j.jsmc.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. Nov 2001;58(11):747–52. doi: 10.1136/oem.58.11.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer F. Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. J Biol Rhythms. Apr 2017;32(2):154–164. doi: 10.1177/0748730417697537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castro AR, Silva SO, Soares SC. The Use of High Sensitivity C-Reactive Protein in Cardiovascular Disease Detection. J Pharm Pharm Sci. 2018;21(1):496–503. doi: 10.18433/jpps29872 [DOI] [PubMed] [Google Scholar]
- 52.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. Mar 8 2016;113(10):E1402–11. doi: 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. Jan 2008;51(1):55–61. doi: 10.1161/hypertensionaha.107.100727 [DOI] [PubMed] [Google Scholar]
- 54.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. Nov 2002;20(11):2183–9. doi: 10.1097/00004872-200211000-00017 [DOI] [PubMed] [Google Scholar]
- 55.McHill AW, Velasco J, Bodner T, Shea SA, Olson R. Rapid changes in overnight blood pressure after transitioning to early-morning shiftwork. Sleep. Aug 9 2021;doi: 10.1093/sleep/zsab203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mason IC, Qian J, Adler GK, Scheer F. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. Mar 2020;63(3):462–472. doi: 10.1007/s00125-019-05059-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). Nov 2009;17(11):2100–2. doi: 10.1038/oby.2009.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. Mar 2010;151(3):1019–29. doi: 10.1210/en.2009-0864 [DOI] [PubMed] [Google Scholar]
- 59.Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond). Aug 18 2004;1(1):5. doi: 10.1186/1743-7075-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FA. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring). Oct 2015;23(10):2053–8. doi: 10.1002/oby.21189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McHill AW, Melanson EL, Higgins J, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A. Dec 2 2014;111(48):17302–7. doi: 10.1073/pnas.1412021111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubio-Sastre P, Scheer FA, Gómez-Abellán P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. Oct 1 2014;37(10):1715–9. doi: 10.5665/sleep.4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. Apr 11 2012;4(129):129ra43. doi: 10.1126/scitranslmed.3003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qian J, Dalla Man C, Morris CJ, Cobelli C, Scheer F. Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes Metab. Oct 2018;20(10):2481–2485. doi: 10.1111/dom.13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mattson MP, Allison DB, Fontana L, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. Nov 25 2014;111(47):16647–53. doi: 10.1073/pnas.1413965111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. Apr 9-15 2005;365(9467):1333–46. doi: 10.1016/s0140-6736(05)61032-x [DOI] [PubMed] [Google Scholar]
- 67.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1-2):497–509. doi: 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- 68.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. Feb 2003;18(1):80–90. doi: 10.1177/0748730402239679 [DOI] [PubMed] [Google Scholar]
- 69.Hena M, Garmy P. Social Jetlag and Its Association With Screen Time and Nighttime Texting Among Adolescents in Sweden: A Cross-Sectional Study. Front Neurosci. 2020;14:122. doi: 10.3389/fnins.2020.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30-49 years). J Biol Rhythms. Feb 2006;21(1):68–76. doi: 10.1177/0748730405283154 [DOI] [PubMed] [Google Scholar]
- 71.Suh S, Yang HC, Kim N, et al. Chronotype Differences in Health Behaviors and Health-Related Quality of Life: A Population-Based Study Among Aged and Older Adults. Behav Sleep Med. Sep-Oct 2017;15(5):361–376. doi: 10.1080/15402002.2016.1141768 [DOI] [PubMed] [Google Scholar]
- 72.Merikanto I, Lahti T, Puolijoki H, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. May 2013;30(4):470–7. doi: 10.3109/07420528.2012.741171 [DOI] [PubMed] [Google Scholar]
- 73.Parsons MJ, Moffitt TE, Gregory AM, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond). May 2015;39(5):842–8. doi: 10.1038/ijo.2014.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gamboa Madeira S, Reis C, Paiva T, Moreira CS, Nogueira P, Roenneberg T. Social jetlag, a novel predictor for high cardiovascular risk in blue-collar workers following permanent atypical work schedules. J Sleep Res. May 4 2021:e13380. doi: 10.1111/jsr.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arntz HR, Müller-Nordhorn J, Willich SN. Cold Monday mornings prove dangerous: epidemiology of sudden cardiac death. Curr Opin Crit Care. Jun 2001;7(3):139–44. doi: 10.1097/00075198-200106000-00001 [DOI] [PubMed] [Google Scholar]
- 76.Barnett AG, Dobson AJ. Excess in cardiovascular events on Mondays: a meta-analysis and prospective study. J Epidemiol Community Health. Feb 2005;59(2):109–14. doi: 10.1136/jech.2003.019489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eckel RH, Depner CM, Perreault L, et al. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr Biol. Nov 16 2015;25(22):3004–10. doi: 10.1016/j.cub.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 78.Giuntella O, Mazzonna F. Sunset time and the economic effects of social jetlag: evidence from US time zone borders. Journal of Health Economics. 2019/05/01/ 2019;65:210–226. doi: 10.1016/j.jhealeco.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 79.Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA. An update on adolescent sleep: New evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. doi: 10.1016/j.adolescence.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. Jun 2011;58(3):637–47. doi: 10.1016/j.pcl.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. Dec 2006;29(12):1632–41. doi: 10.1093/sleep/29.12.1632 [DOI] [PubMed] [Google Scholar]
- 82.Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased Sensitivity of the Circadian System to Light in Early/Mid-Puberty. J Clin Endocrinol Metab. Nov 2015;100(11):4067–73. doi: 10.1210/jc.2015-2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Touitou Y, Touitou D, Reinberg A. Disruption of adolescents’ circadian clock: The vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J Physiol Paris. Nov 2016;110(4 Pt B):467–479. doi: 10.1016/j.jphysparis.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 84.Mathew GM, Li X, Hale L, Chang AM. Sleep duration and social jetlag are independently associated with anxious symptoms in adolescents. Chronobiol Int. Apr 2019;36(4):461–469. doi: 10.1080/07420528.2018.1509079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mathew GM, Hale L, Chang AM. Social jetlag, eating behaviours and BMI among adolescents in the USA. Br J Nutr. Nov 14 2020;124(9):979–987. doi: 10.1017/s0007114520001804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carvalho-Mendes RP, Dunster GP, de la Iglesia HO, Menna-Barreto L. Afternoon School Start Times Are Associated with a Lack of Both Social Jetlag and Sleep Deprivation in Adolescents. J Biol Rhythms. Aug 2020;35(4):377–390. doi: 10.1177/0748730420927603 [DOI] [PubMed] [Google Scholar]
- 87.Schweitzer PK, Randazzo AC, Stone K, Erman M, Walsh JK. Laboratory and field studies of naps and caffeine as practical countermeasures for sleep-wake problems associated with night work. Sleep. Jan 2006;29(1):39–50. doi: 10.1093/sleep/29.1.39 [DOI] [PubMed] [Google Scholar]
- 88.McHill AW, Smith BJ, Wright KP Jr., Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythms. Apr 2014;29(2):131–43. doi: 10.1177/0748730414523078 [DOI] [PubMed] [Google Scholar]
- 89.Carrier J, Fernandez-Bolanos M, Robillard R, et al. Effects of caffeine are more marked on daytime recovery sleep than on nocturnal sleep. Neuropsychopharmacology. Apr 2007;32(4):964–72. doi: 10.1038/sj.npp.1301198 [DOI] [PubMed] [Google Scholar]
- 90.Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. Aug 4 2005;353(5):476–86. doi: 10.1056/NEJMoa041292 [DOI] [PubMed] [Google Scholar]
- 91.Broughton RJ, Fleming JA, George CF, et al. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology. Aug 1997;49(2):444–51. doi: 10.1212/wnl.49.2.444 [DOI] [PubMed] [Google Scholar]
- 92.Kuan YC, Wu D, Huang KW, et al. Effects of Modafinil and Armodafinil in Patients With Obstructive Sleep Apnea: A Meta-analysis of Randomized Controlled Trials. Clin Ther. Apr 2016;38(4):874–88. doi: 10.1016/j.clinthera.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 93.Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. J Sleep Res. Sep 2001;10(3):181–92. doi: 10.1046/j.1365-2869.2001.00256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wallace PJ, Haber JJ. Top 10 evidence-based countermeasures for night shift workers. Emergency Medicine Journal. 2020;37(9):562–564. doi: 10.1136/emermed-2019-209134 [DOI] [PubMed] [Google Scholar]
- 95.Harrison EM, Walbeek TJ, Maggio DG, Herring AA, Gorman MR. Circadian profile of an emergency medicine department: scheduling practices and their effects on sleep and performance. The Journal of emergency medicine. 2020;58(1):130–140. [DOI] [PubMed] [Google Scholar]
- 96.Gupta CC, Centofanti S, Dorrian J, et al. Altering meal timing to improve cognitive performance during simulated nightshifts. Chronobiology international. 2019;36(12):1691–1713. [DOI] [PubMed] [Google Scholar]
- 97.Smith MR, Eastman CI. Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep. 2012;4:111–32. doi: 10.2147/nss.S10372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. Apr 1999;31(2):87–98. doi: 10.3109/07853899908998783 [DOI] [PubMed] [Google Scholar]
- 99.Smith MR, Fogg LF, Eastman CI. Practical Interventions to Promote Circadian Adaptation to Permanent Night Shift Work: Study 4. Journal of Biological Rhythms. 2009;24(2):161–172. doi: 10.1177/0748730409332068 [DOI] [PubMed] [Google Scholar]


