Abstract
Electronic cigarettes or vaping products have been marketed as a safer alternative to smoking, but very little is known about the health effects in the human lung, particularly in the distal airways, a key site of airway obstruction and destruction in chronic obstructive pulmonary disease that is often exacerbated by viral infections. The aim of this study was to investigate the effects of electronic cigarette vapor (e-vapor) on human distal airway epithelial responses to influenza A virus (IAV) infection. We isolated primary small airway epithelial cells (SAECs) from donor lungs free of lung disease, and cultured them at air–liquid interface (ALI). To measure markers of epithelial injury such as integrity of epithelial barrier structure and function, we selected a regimen of non-toxic, barrier preserving e-vapor exposure of cultured cells to 15 puffs of e-vapor from a commercially available e-cigarette once per day for 3 days, prior to IAV infection. After 72 h of infection, media and cell lysates were collected to measure cytokines involved in inflammatory and antiviral responses. Pre-exposure to e-vapor with IAV infection, compared to IAV infection alone, significantly increased inflammatory and antiviral mediators including IL-8, CXCL10, IFN-beta, and MX1. Our results suggest that e-vapor exposure amplifies human distal airway pro-inflammatory response to IAV infection, independently of the severity of cell injury during viral infection.
Keywords: e-vapor, Influenza A virus, Inflammation, Small airway epithelium
Introduction
Electronic cigarettes (e-cigs) are handheld, battery-operated devices with growing popularity in the U.S. market as an alternative to smoking, or as an aid in smoking cessation (U.S. Department of Health and Human Services 2014, 2016). However, while it is increasingly evident that e-cig use is unsafe, their adverse effects on lung health are not fully elucidated. Experimental exposure models established in mice and in cultured human lung cells, including bronchial epithelial cells determined that e-cigs increase lung inflammatory cytokines, mucins, and oxidative stress, and impair epithelial cell function (Garcia-Arcos et al. 2016; Glynos et al. 2018; Haswell et al. 2017; Madison et al. 2019; Scott et al. 2018; Wu et al. 2014). Whereas most studies focused on effects on large airways, there is a paucity of studies on the effects of e-cigs on human distal airways such as small airway epithelial cells (SAEC). The repetitive injury of small airways, defined as <2 mm diameter, is a key contributor to the loss of distal airways and alveoli and tissue remodeling characteristic of chronic obstructive pulmonary disease (COPD) (Garcia-Arcos et al. 2016; U.S. Department of Health and Human Services 2014). Inhalation of the small molecules contained in e-cig vapors (e-vapor) escapes the filtration system of the large airways, reaching small airways and alveoli, potentially altering gas exchange (Denney and Ho 2018; Garcia-Arcos et al. 2016; Glynos et al. 2018; Scott et al. 2018). Our group has demonstrated that the distal airway epithelium shares a similar response as the upper airway epithelium when exposed to e-vapor, by increasing the inflammatory cytokine IL-6 (Gellatly et al. 2020; Haswell et al. 2017; Norton et al. 2014; Scott et al. 2018; U.S. Department of Health and Human Services 2016). However, little is known about how e-cig exposure may affect distal airway defense against viral infections.
Viral infections such as influenza A virus (IAV) significantly contribute to COPD exacerbations (de Miguel-Diez et al. 2012; Gerke et al. 2013; Santa-Olalla Peralta et al. 2010). While cigarette smokers have been shown to have impaired nasal epithelium antiviral response to IAV infection (Jaspers et al. 2010), the antiviral response in e-cig users has not been defined. IAV infection in upper airway cells and in mouse models shows an appropriate production of antiviral cytokines including interferon (IFN)-beta and CXCL10 which are important in driving an effective innate viral response (Andreakos et al. 2017; Chan et al. 2010; Denney and Ho 2018; Haswell et al. 2017; Jaspers et al. 2010; Liu et al. 2011; Mogensen and Paludan 2001; Mordstein et al. 2010; Oslund et al. 2014; Sukhanova 1988). Appropriate levels of antiviral mediators during infection will aid in viral clearance by recruiting immune cells and inhibiting viral replication interference, but dysregulated or excessive expression may harm the lung. It has been shown that overexpression of interferons can cause further damage to lung epithelium and result in cell death (Chen et al. 2017; Major et al. 2020).
The overall goal of this study was to determine how e-vapor exposure impacts small airway response after IAV infection. By performing air–liquid interface culture of SAECs, we found that e-vapor exposure, even at sub-toxic concentrations, that do not directly cause epithelial cell barrier disruption, amplifies the pro-inflammatory response (e.g., IL-8 and CXCL10) to IAV.
Materials and methods
Influenza A virus preparation
The pandemic Influenza A/California/07/2009 virus H1N1 was kindly provided by Dr. Kevin Harrod from University of Alabama at Birmingham. The virus was passaged in Madin-Darby canine kidney (MDCK, ATCC, Manassas, VA) cells, as previously published (Chan et al. 2010; Daly et al. 1995; Hartshorn et al. 2006; Kunisaki and Janoff 2009). MDCK cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM, Fisher Scientific, Waltham, MA) supplemented with 10% fetal calf serum (MilliporeSigma, Burlington, MA), L-glutamine, and antibiotics. Virus was propagated in MDCK cells in DMEM supplemented with L-glutamine, antibiotics, and 1.5 µg/ml of N-tosyl-l-phenylalanine chloromethyl ketone (TPCK) treated trypsin (ThermoFisher Scientific, Waltham, MA) and harvested at 72 h post infection and tittered by plaque assay using MDCK cells (Numata et al. 2020).
Small airway epithelial cell isolation and culturing
Human lungs from de-identified organ donors whose lungs were not suitable for transplantation were donated for medical research through the International Institute for the Advancement of Medicine (Philadelphia, PA) or the Donor Alliance of Colorado (Denver, CO). Six different donors were selected based on non-smoking status (Table 1), no history of clinical lung disease, a clear chest radiograph that indicates no active infection, and time on a ventilator for less than five days, as described previously (Gellatly et al. 2020).
Table 1.
Demographic information of small airway epithelial cell donors
| Subject # | Gender | Age (years) | Smoking status | Cause of death |
|---|---|---|---|---|
| 1 | Male | 18 | Never | Blunt Injury |
| 2 | Female | 19 | Never | Blunt Injury |
| 3 | Male | 36 | Never | Anoxia |
| 4 | Male | 40 |
Non-smoker (>12 years) |
Blunt Injury |
| 5 | Male | 44 | Never | Head Trauma |
| 6 | Male | 57 | Never | Blunt Injury |
Small airway epithelial cells (SAECs) characterized by being less than 2 mm in diameter, were collected from dissected pieces of the distal lung using a 2 mm bronchoscopy brush (Medline Industries, Northfield, IL). The flexible bristles of the brush allowed penetration of airways < 2 mm, after which the brush was placed in sterile PBS. The cells were isolated by centrifugation, re-suspended in PBS, counted, and plated onto an irradiated NIH 3T3 fibroblast feeder layer in F-media (Gellatly et al. 2020). Once large visible colonies had formed (7–10 days), they were removed with 0.25% trypsin (Corning Inc., Corning, NY) and plated onto double collagen-coated 12-well transwells (Corning Inc., Corning, NY) for air–liquid interface (ALI) culture in Pneumacult ALI medium (Stemcell Technologies, Vancouver, Canada). Briefly, cells on the transwells were under submerged culture for 7–10 days to form a monolayer, and then cultured at ALI for 21 days to promote mucociliary differentiation. To switch from submerged to ALI culture, medium in the upper chamber was reduced from 250 µl for submerged culture to 50 µl for ALI culture to keep cells hydrated and promote cell mucociliary differentiation.
E-vapor exposure
JUUL Labs (Washington D.C.) Virginia tobacco flavor at 3% (35 mg/ml) nicotine strength was used for this study. According to the manufacturer, the e-liquid pods consist of up to 90% propylene glycol and glycerol in a 30:60 ratio, benzoic acid, purified/USP grade/pharmaceutical grade nicotine, and proprietary flavorings, which does not include diacetyl, acetylproprionyl, or 2,3-pentanedione as flavoring ingredients.
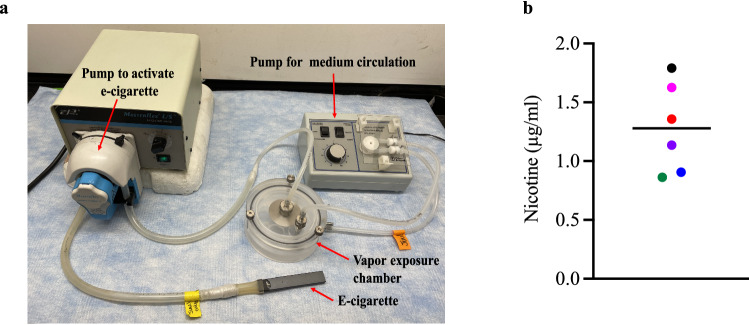
JUUL e-vapor was generated using a JUULbattery and JUULpod attached to tubing on a MasterFlex L/S Economy Variable Speed Drive (Fig. 1a) (Gellatly et al. 2020). After 21 days of ALI culture, the apical surfaces of SAECs were exposed directly to either 15 puffs of air as control, or 15 puffs of e-vapor over 15 min. Each puff is defined as 55 ml over 8 s draw every minute in a British American Tobacco (BAT) exposure chamber (Thorne and Adamson 2013) containing warm DMEM circulated by a Fisher Scientific Variable Flow Minipump, for a total of 15 min (Gellatly et al. 2020). Duration was determined from behavioral studies of patients using an e-cigarette (Garcia-Arcos et al. 2016) and nicotine serum levels after use (Chiadmi and Schlatter 2014; Marsot and Simon 2016; Yingst et al. 2019), as well as modified from International Organization for Standardization (ISO) standards for cigarette smoking experiments.
Fig. 1.
E-vapor exposure model. a A British American Tobacco (BAT) smoke exposure chamber connected to a Fisher Scientific variable flow mini pump, for cell culture medium circulation, and a MasterFlex L/S Economy Variable Speed Drive, for airflow or e-cigarette vapor activation. b Nicotine concentrations in apical supernatants following a single e-vapor exposure (24 h). Each data point represents a separate experiment with horizontal line indicating the average
Nicotine measurements in the media e-vapor-exposed SAEC
Nicotine concentrations in the apical media of e-vapor-exposed SAEC were measured by GC/MS based on previously described methods (Chiadmi and Schlatter 2014; Gellatly et al. 2020). Nicotine in the apical surface of SAEC was detected in a range of 0.86–1.79 µg/ml 24 h after one exposure to e-vape (Fig. 1b). This concentration is relevant to levels of exposure of humans in vivo, where serum nicotine concentrations were determined to be about 1000 times lower than those in the airway epithelial lining fluid (Chiadmi and Schlatter 2014; Marsot and Simon 2016; Yingst et al. 2019).
Infection of SAEC with IAV
Immediately after the last vaping exposure, 100 µl of Pneumacult ALI medium with 1.5 µg/ml of TPCK-treated trypsin and 3 × 103 PFU/ml of IAV was applied to the apical surface for two hours, incubated at 37 °C and 5% CO2. After two hours of incubation, the virus-containing medium was removed, and apical surface washed with warmed PBS to remove unbound virus. The apical medium was then replaced with 50 µl of Pneumacult ALI medium without TPCK-treated trypsin or IAV to continue ALI culture for 72 h, after which apical and basolateral supernatants were collected, and cells were lysed in RLT for RNA extraction to determine mRNA quantification by RT-qPCR.
Bulk RNA sequencing of SAECs
Bulk RNA sequencing (RNA seq) was performed by Novogene (Sacramento, CA) from total RNA samples of ALI cultured SAECs to confirm the nature of cells derived from small airways. Illumina NovaSeq 6000 was used for sequencing with paired-end 150, 20 million read pair per sample with reference genome, Homo sapiens (GRCh38/hg38).
Enzyme-linked immunosorbent assay (ELISA)
Human CXCL10 levels were measured using an ABST enzyme-linked immunosorbent assay (ELISA) (PeproTech, Rocky Hill, NJ). Human IL-8, and IFN-β levels were measured by respective DuoSet ELISA kits (R&D Systems, Minneapolis, MN). All cytokines were measured in basolateral supernatants, according to manufacturers’ instructions.
Quantitative real-time PCR (RT-qPCR)
mRNA expression of IFN-beta, CXCL10, MX1, and intracellular IAV was measured by quantitative real-time PCR (RT-PCR). Total RNA was extracted using Mini Spin Columns for RNA Extraction (Epoch Life Science Inc., Missouri City, TX) following manufacturer’s instructions. qPCR was performed using a probe-based method with 18s rRNA as a reference gene (ThermoFisher, Waltham, MA). Target gene mRNA analyses for Mx1 were performed using commercially available primer and probe sets (Taqman Gene Expression Assays, ThermoFisher, Waltham, MA). The custom-made (Integrated DNA Technologies, Coralville, IA) specific primers and probes for IFN-β were 5′-GACGGA-GAAGATGCAGAAGAG-3′ (forward), 5′-CCACCCAGTGCTGGAGAA-3′ (reverse), and 5′-TGCCTTTGCCAT-CCAAGAGAT-3″; and for IAV were 5′-GACCRATCCTGTCACCTCTGAC-3′ (forward), 5′-AGGGCATTYTGGACAAAKCGTCTA-3′ (reverse), and 5′-TGCAGTCCTCGCTCACTGGGCACG-3′ (probe).
SAEC barrier function measurements
Transepithelial electrical resistance (TEER) measurements were performed using Epithelial Voltohmmeter (EVOM2; World Precision Instruments, Sarasota, FL) to assess cell barrier integrity, as described previously (Gellatly et al. 2020; Wang et al. 2018). The resistance across the cell layer was measured (Rtotal) for each of the treatment samples, and the cell layer resistance (Rcells) was measured by subtracting the blank measurement of the membrane from the total resistance.
Statistics
Normally distributed data were analyzed using Analysis of variance (ANOVA) followed by Tukey’s test for multiple group comparisons. Due to the large variation of some data seen between subjects, but consistent data changes in the same subject cells exposed to the combination treatment of e-vapor exposure and IAV infection versus IAV infection alone, we used a paired t test to compare these two treatments. A p value of < 0.05 was considered statistically significant.
Results
Confirmation of SAECs by morphology and bulk RNA sequencing analysis
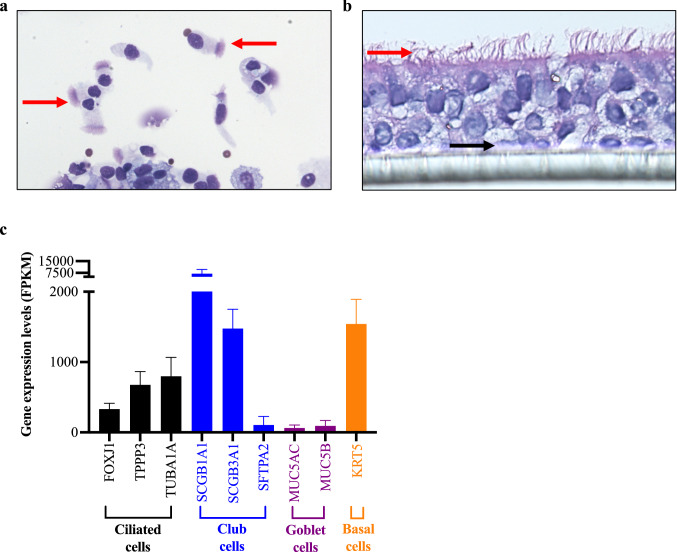
SAECs isolated from donor lungs via airway brushing were confirmed by Hema 3 differential staining in cytospin slides by visualization of ciliated cells (Fig. 2a). Once SAEC isolation was confirmed, cells were cultured in ALI. As shown in Fig. 2b, SAECs grown on transwells were able to differentiate into ciliated cells and secretory cells. To further confirm the nature of distal airway epithelium, bulk RNA sequencing (RNA-seq) was performed to identify specific distal small airway epithelial markers (Fig. 2c). SAEC-specific markers identified included club-cell secretory proteins (SCGB1A1 and SCGB3A1) and surfactant protein (SFTPA) (Crystal et al. 2008). Ciliated cell differentiation was confirmed by cilia specific markers including forkhead box protein (FOXJ1), tubulin polymerization-promoting protein (TPPP3), and acetylated tubulin (TUBA1A). Airway mucus differentiation was confirmed by expression of mucin 5AC (MUC5AC) and mucin 5B (MUC5B). These data support our SAEC ALI culture as a physiologically relevant model to study the role of vaping in human small airway response to viral infection.
Fig. 2.
Morphology of small airway ciliated epithelial cells (SAEC) isolated from a healthy non-smoker human donor lung. a Image of cytospin of distal bronchial brushings from a non-smoker donor. Red arrows indicate ciliated cells. b SAEC grown on transwells demonstrate ciliary differentiation after 21 days of air–liquid interface (ALI) culture. Red arrows indicated ciliated cells and black arrows indicate basal cells. c Bulk RNA sequencing data showing mucociliary markers of SAEC as well as specific markers of club cells in SAEC. FPKM stands for fragments per kilobase of transcript per million mapped reads indicating a relative expression of a gene proportional to the number of cDNA fragments of origin. N = 5 donors. Data were expressed as means ± SEM
Maintenance of SAEC ALI barrier integrity during e-vapor exposure
Transepithelial electrical resistance (TEER) was used to assess epithelial barrier function/integrity. The regimen selected for e-vapor exposure alone did not reduce baseline SAEC TEER, in contrast to the expected effect of IAV infection, which significantly reduced TEER levels (Fig. 3). Furthermore, e-vapor exposure of IAV-infected SAEC did not worsen TEER levels compared to IAV infection alone.
Fig. 3.
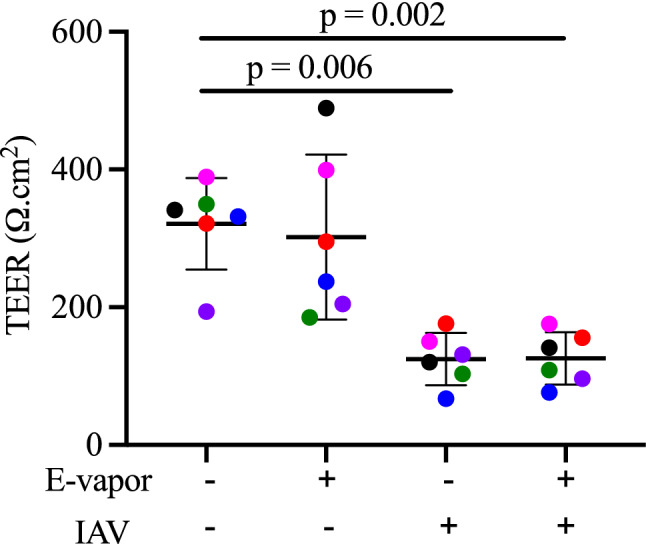
Integrity of air–liquid interface (ALI) cultured small airway ciliated epithelial cells (SAEC) during e-vapor and IAV exposures. Transepithelial electrical resistance (TEER) indicates lack of e-vapor effect on barrier integrity at baseline or following IAV infection (72 h). Each data point (colored dot) represents the average of 3 replicates from each respective individual donor (n = 6 donors); horizontal lines indicate averages and standard deviation; ANOVA with post hoc Tukey’s test
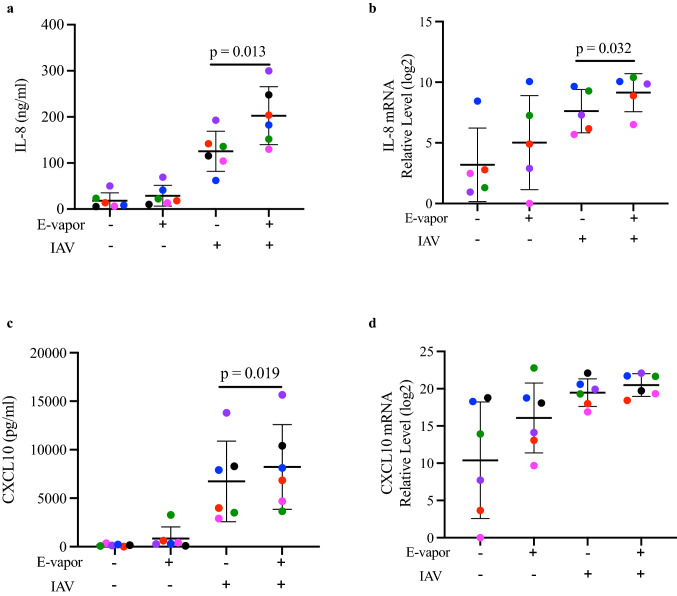
E-vapor amplified inflammatory responses in SAECs infected with IAV
To determine the pro-inflammatory effect of IAV infection in e-vapor exposed SAECs, we measured pro-inflammatory cytokines secreted into the basolateral supernatant of the ALI culture, and intracellular mRNA expression. IL-8, a neutrophilic chemoattractant cytokine, was significantly increased in SAECs exposed to both e-vapor and IAV compared to IAV alone (Fig. 4a). A similar effect was noted for the IL-8 mRNA expression (Fig. 4b). CXCL10, also known as interferon-inducible protein, was also significantly increased in the e-vapor and IAV exposed SAECs compared to IAV alone (Fig. 4c). IAV infection alone markedly increased CXCL10 mRNA, with no additional effect noted in the IAV infection group also exposed to e-vapor (Fig. 4d).
Fig. 4.
Inflammatory response by e-vapor exposed SAEC following influenza A virus (IAV) infection. E-vapor exposure followed by IAV infection significantly increased IL-8 protein (a), IL-8 mRNA (b), and CXCL10 protein (c) compared to IAV alone measured (at 72 h) by ELISA in basolateral supernatants or by qRT-PCR in cell lysates of SAEC grown at ALI. CXCL10 mRNA expression (d) did not further increase compared to IAV infection alone. Each data point (colored dot) represents the average of 3 replicates from each respective individual donor (n = 6 donors); horizontal lines indicate averages and standard deviation; paired t test
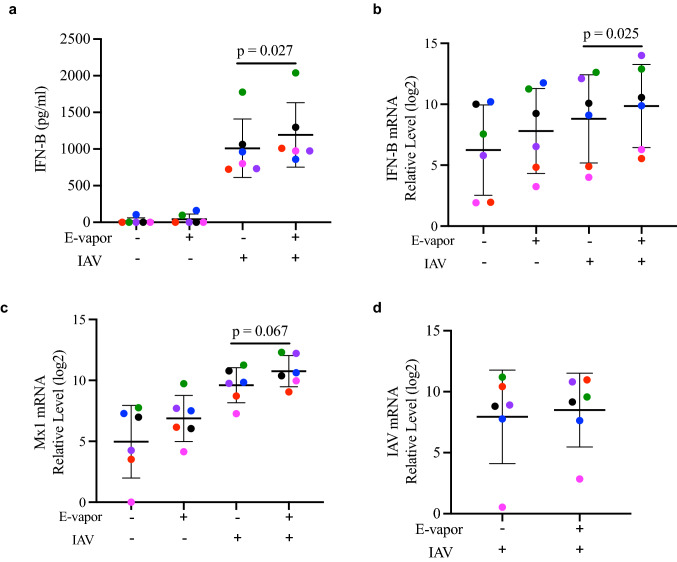
E-vapor increased antiviral gene expression, but did not affect intracellular viral load
We next sought to determine the impact of e-vapor on the antiviral responses to IAV. Antiviral genes IFN-beta and MX1 were analyzed as they have been shown to be involved in both antiviral and pro-inflammatory processes (Andreakos et al. 2017; Centers for Disease Control and Prevention 2020; Chan et al. 2010; Denney and Ho 2018; Huang et al. 2013; Mogensen and Paludan 2001; Mordstein et al. 2010; Oslund et al. 2014). A significant increase of IFN-beta protein and mRNA expression was induced by e-vapor in IAV-infected SAECs compared to IAV infection alone (Fig. 5a, b). E-vapor also tended to increase the IAV-infected SAEC expression of MX1, when compared to IAV infection alone (Fig. 5c). These effects were unlikely to be due to a direct effect of e-vapor on viral load, since IAV RNA levels were similar in infected e-vapor exposed or unexposed SAEC (Fig. 5d).
Fig. 5.
Viral immune response by e-vapor exposed SAEC following IAV infection. E-vapor exposure followed by IAV infection significantly increased IFN-β protein (a) and IFN-β mRNA expression (b) and tended to increase MX1 mRNA expression (c) compared to IAV alone, measured (at 72 h) by ELISA in basolateral supernatants or by qRT-PCR in cell lysates of SAEC grown at ALI (d). Intracellular IAV at 72 h post infection indicates no significant effect of e-vapor exposure on viral load. Each data point (colored dot) represents the average of 3 replicates from each respective individual donor (n = 6 donors); horizontal lines indicate averages and standard deviation; paired t test
Discussion
While e-cigarettes have been marketed as a safer alternative to conventional cigarette smoking, e-cigs have been a major area of study following the 2019 outbreak of e-cigarette or vaping product use-associated lung injury (EVALI), that resulted in over 2800 hospitalizations and 73 deaths (Centers for Disease Control and Prevention 2020). In this study, we report to our knowledge for the first time the effects of vaping on viral infection of epithelial cells collected from human small airways, a critical site for lung disease development. Our data demonstrate the amplifying effects of vaping on inflammation induced by IAV infection.
Several studies using large (tracheal or bronchial) airway epithelial culture systems showed detrimental effects of e-cig or tobacco smoke on the upper airways in defending against pathogens and other foreign material entering the lungs, via inhibition of mucociliary clearance (Knowles and Boucher 2002; Kulkarni et al. 2010). Small airways vulnerability to highly virulent pathogens, such as IAV (Denney and Ho 2018) is a concern, since injury or inflammation of small airway epithelium more severely affects gas exchange and airflow obstruction. Given the structural and functional differences of small airway epithelial cells compared to large airway epithelial cells, (i.e., higher ratio of club cells to goblet cells), it is imperative to understand how vaping impacts the response of SAEC to IAV infection. Our study is the first to directly fill this critical gap of knowledge.
Previous studies comparing e-vapor and cigarette smoke in patient sputum and bronchoalveolar lavage have shown that there is significantly more neutrophilic inflammation induced by e-vapor (Reidel et al. 2018). However, these studies were not able to reveal the contribution of distal airways to exaggerated neutrophilic inflammation in vapors. Using an air–liquid interface culture model, we succeeded in generating well-differentiated small airway epithelium, which mimics human small airways, made up of mainly ciliated cells and club cells. As shown in this study, e-vapor pre-exposure significantly amplified IL-8 and CXCL10 production in SAEC infected with IAV. IL-8 is a well-known neutrophilic chemoattractant, giving our model of human SAECs important clinical implications. Increased neutrophil infiltration in the airways has been shown to lead to significant damage and can be a marker of acute lung disease, as was seen with the 2019 outbreak of EVALI (Centers for Disease Control and Prevention 2020). Notably, CXCL10 has also been shown to enhance lung neutrophilic inflammation in patients or mice with acute respiratory distress syndrome (ARDS) associated with IAV infection (Ichikawa et al. 2013). Together, our IL-8 and CXCL10 data suggest the detrimental pro-inflammatory effects of vaping during the flu season.
To demonstrate the functional implication of excessive production of pro-inflammatory mediators, we measured cell injury in SAEC treated with e-vapor and IAV. Previous studies have shown that in lung cell cultures including human bronchial epithelial cell lines, human nasal brushings, human alveolar macrophages, and in vivo murine models, cell injury can occur from exposure to e-cigs, which were comparable to the effects seen with cigarette smoke exposure (Chen et al. 2019; Garcia-Arcos et al. 2016; Glynos et al. 2018; Haswell et al. 2017; Reidel et al. 2018). Tissue barrier function is important to the maintenance of a functional airway passage, which is compromised during aggressive IAV infection and could potentially be damaged by the use of e-cigs alone (Chen et al. 2019; Garcia-Arcos et al. 2016). However, whether e-cigs further damage IAV-infected epithelial cells has not been investigated. Our work has extended the previous research in that IAV infection alone exerts the injurious effect on SAEC. E-vapor did not exaggerate the injurious effect of IAV infection on small airway epithelium during the 72 h of IAV infection. The “disconnection” of amplified pro-inflammatory response and lack of exaggerated cell injury by e-vapor in IAV-infected cells suggests that the pro-inflammatory event may occur without the expense of cell injury. Moreover, our findings also support the concept that e-vapor, unlike tobacco smoke, is usually less toxic to the cells (Glynos et al. 2018; Haswell et al. 2017). Nonetheless, future studies are warranted to test if e-vapor during the prolonged IAV infection will amplify the damaging effect of IAV on epithelial integrity. As cilial dysfunction and deficiency of club-cell secretory proteins have been reported in the airways of patients with COPD (Gamez et al. 2015; Leopold et al. 2009), we utilized the bulk RNA sequencing data to compare the expression levels of genes involved in ciliogenesis (e.g., FOXJ1) and club-cell secretory proteins (e.g., SCGB1A1 and SCGB3A1) in SAEC exposed to e-vapor with or without IAV infection. We found that e-vapor alone consistently reduced (about twofold) the expression of FOXJ1, SCGB1A1 and SCGB3A1, which was further reduced (about tenfold) by IAV infection. Our data suggest, like tobacco smoke, e-vapor not only modulates the inflammatory response, but also may impair other functions of distal airway epithelium.
Previous studies generally suggest impaired antiviral immunity in tobacco smokers or tobacco smoke-exposed upper airway epithelium (Strzelak et al. 2018). It remains unclear about the role of e-vapor in distal airway antiviral responses. However, we saw an increase in antiviral gene expression in e-vapor exposed and IAV-infected SAEC. IAV has been shown to induce a significant response of IFNs (Chan et al. 2010; Huang et al. 2013), but the enhancing effect of e-vapor on IFNs and IFN-stimulating genes is unexpected. Moreover, such an increase of antiviral genes was not coupled with a decrease of viral load in SAECs. We speculate that in severely damaged SAECs infected with IAV, excessive IFNs may not be able to significantly inhibit viral replication. Our novel finding suggests that IFN supplemental therapies may not be beneficial for vapers with an acute IAV infection. Instead, reducing the excessive IFN signaling may be necessary.
The limitations of this study include that it only measured effects of the relatively short-term (3 days) exposures of SAECs to e-vapor, which may not be similar to those of chronic or lifelong e-vapor exposure. Besides the experimental limitations of long-term culture of primary lung cells, given their relatively recent use, the long-term consequences of e-cigarette use on the human lung health remain unknown. However, our finding of pro-inflammatory effects of short-term e-vapor exposures can inform on the design of future long-term studies examining the prevalence or severity of respiratory viral infections in habitual vapers. Further, since commercial e-cigarettes have proprietary formulations with unknown ratios of propylene glycol, glycerin, benzoic acid, and flavorings our study did not clarify which components of e-vapor might be responsible for the effects noted, or whether additives, such as vitamin E or tetrahydro cannabinoids, implicated in the EVALI epidemic worsen these effects. These investigations, as well as inquiry into the mechanisms responsible for the enhancing effect of pro-inflammatory lung epithelial response to IAV infection remain to be explored in future.
Conclusion
Using a clinically relevant human primary small airway epithelial cell culture model, this study has provided the first evidence that exposure to e-vapor is sufficient to exaggerate distal airway pro-inflammatory responses to IAV infection.
Acknowledgements
The authors thank Nicole Pavelka, Christina Lisk, Nastaran Mues, and Dennis R. Voelker for their technical assistance.
Funding
This work was supported by the NIH grant R01 HL144396.
Declarations
Conflict of interest
The authors state that they have no conflicts of interest to declare.
Ethics approval
The Institutional Review Board (IRB) at National Jewish Health, Denver, Colorado deemed this research as nonhuman subject research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Niccolette Schaunaman and Taylor Crue have contributed equally to this work.
Contributor Information
Irina Petrache, Email: PetracheI@NJHealth.org.
Hong Wei Chu, Email: ChuHW@NJHealth.org.
References
- Andreakos E, Salagianni M, Galani IE, Koltsida O. Interferon-lambdas: front-line guardians of immunity and homeostasis in the respiratory tract. Front Immunol. 2017;8:1232. doi: 10.3389/fimmu.2017.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2020) Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html Accessed 25 January 2022
- Chan RW, Yuen KM, Yu WC, et al. Influenza H5N1 and H1N1 virus replication and innate immune responses in bronchial epithelial cells are influenced by the state of differentiation. PLoS ONE. 2010;5(1):e8713. doi: 10.1371/journal.pone.0008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Liu J, Cao X. Regulation of type I interferon signaling in immunity and inflammation: a comprehensive review. J Autoimmun. 2017;83:1–11. doi: 10.1016/j.jaut.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Chen IL, Todd I, Fairclough LC. Immunological and pathological effects of electronic cigarettes. Basic Clin Pharmacol Toxicol. 2019;125(3):237–252. doi: 10.1111/bcpt.13225. [DOI] [PubMed] [Google Scholar]
- Chiadmi F, Schlatter J. Simultaneous determination of cotinine and trans-3-hydroxycotinine in urine by automated solid-phase extraction using gas chromatography-mass spectrometry. Biomed Chromatogr. 2014;28(4):453–458. doi: 10.1002/bmc.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5(7):772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly K, Nguyen P, Woodland DL, Blackman MA. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J Virol. 1995;69(12):7416–7422. doi: 10.1128/JVI.69.12.7416-7422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel-Diez J, Carrasco-Garrido P, Hernandez-Barrera V, et al. Hospitalizations from pandemic influenza (pH1N1) infections among patients with asthma or COPD in Spain. J Infect. 2012;65(1):95–98. doi: 10.1016/j.jinf.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Denney L, Ho LP. The role of respiratory epithelium in host defence against influenza virus infection. Biomed J. 2018;41(4):218–233. doi: 10.1016/j.bj.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamez AS, Gras D, Petit A, et al. Supplementing defect in club cell secretory protein attenuates airway inflammation in COPD. Chest. 2015;147(6):1467–1476. doi: 10.1378/chest.14-1174. [DOI] [PubMed] [Google Scholar]
- Garcia-Arcos I, Geraghty P, Baumlin N, et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 2016;71(12):1119–1129. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellatly S, Pavelka N, Crue T, et al. Nicotine-free e-cigarette vapor exposure stimulates IL6 and mucin production in human primary small airway epithelial cells. J Inflamm Res. 2020;13:175–185. doi: 10.2147/JIR.S244434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke AK, Tang F, Yang M, Foster ED, Cavanaugh JE, Polgreen PM. Predicting chronic obstructive pulmonary disease hospitalizations based on concurrent influenza activity. COPD. 2013;10(5):573–580. doi: 10.3109/15412555.2013.777400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynos C, Bibli SI, Katsaounou P, et al. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2018;315(5):L662–L672. doi: 10.1152/ajplung.00389.2017. [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, White MR, Tecle T, Holmskov U, Crouch EC. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J Immunol. 2006;176(11):6962–6972. doi: 10.4049/jimmunol.176.11.6962. [DOI] [PubMed] [Google Scholar]
- Haswell LE, Baxter A, Banerjee A, et al. Reduced biological effect of e-cigarette aerosol compared to cigarette smoke evaluated in vitro using normalized nicotine dose and RNA-seq-based toxicogenomics. Sci Rep. 2017;7(1):888. doi: 10.1038/s41598-017-00852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhu W, Zeng X, Li S, Li X, Lu C. Innate and adaptive immune responses in patients with pandemic influenza A(H1N1)pdm09. Arch Virol. 2013;158(11):2267–2272. doi: 10.1007/s00705-013-1692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa A, Kuba K, Morita M, et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am J Respir Crit Care Med. 2013;187(1):65–77. doi: 10.1164/rccm.201203-0508OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers I, Horvath KM, Zhang W, Brighton LE, Carson JL, Noah TL. Reduced expression of IRF7 in nasal epithelial cells from smokers after infection with influenza. Am J Respir Cell Mol Biol. 2010;43(3):368–375. doi: 10.1165/rcmb.2009-0254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R, Rampersaud R, Aguilar JL, Randis TM, Kreindler JL, Ratner AJ. Cigarette smoke inhibits airway epithelial cell innate immune responses to bacteria. Infect Immun. 2010;78(5):2146–2152. doi: 10.1128/IAI.01410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold PL, O'Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PLoS ONE. 2009;4(12):e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Guo S, Hibbert JM, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22(3):121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129(10):4290–4304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369(6504):712–717. doi: 10.1126/science.abc2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsot A, Simon N. Nicotine and cotinine levels with electronic cigarette: a review. Int J Toxicol. 2016;35(2):179–185. doi: 10.1177/1091581815618935. [DOI] [PubMed] [Google Scholar]
- Mogensen TH, Paludan SR. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001;65(1):131–150. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordstein M, Neugebauer E, Ditt V, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84(11):5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton KJ, June KM, O'Connor RJ. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob Induc Dis. 2014;12(1):17. doi: 10.1186/1617-9625-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata M, Mitchell JR, Tipper JL, et al. Pulmonary surfactant lipids inhibit infections with the pandemic H1N1 influenza virus in several animal models. J Biol Chem. 2020;295(6):1704–1715. doi: 10.1074/jbc.RA119.012053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslund KL, Zhou X, Lee B, et al. Synergistic up-regulation of CXCL10 by virus and IFN gamma in human airway epithelial cells. PLoS ONE. 2014;9(7):e100978. doi: 10.1371/journal.pone.0100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel B, Radicioni G, Clapp PW, et al. E-Cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197(4):492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Olalla Peralta P, Cortes-Garcia M, Vicente-Herrero M, et al. Risk factors for disease severity among hospitalised patients with 2009 pandemic influenza A (H1N1) in Spain, April - December 2009. Euro Surveill. 2010;15(38):19667. doi: 10.2807/ese.15.38.19667-en. [DOI] [PubMed] [Google Scholar]
- Scott A, Lugg ST, Aldridge K, et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018;73(12):1161–1169. doi: 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15(5):1033. doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhanova LP. [Instant postnatal adaptation of newborn infants and preclinical diagnosis of its disorders] Akush Ginekol (mosk) 1988;7:42–46. [PubMed] [Google Scholar]
- Thorne D, Adamson J. A review of in vitro cigarette smoke exposure systems. Exp Toxicol Pathol. 2013;65(7–8):1183–1193. doi: 10.1016/j.etp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . E-Cigarette use among youth and young adults: a report of the surgeon general. Atlanta (GA): Publications and Reports of the Surgeon General; 2016. [Google Scholar]
- U.S. Department of Health and Human Services (2014) The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Reports of the Surgeon General, Atlanta (GA)
- Wang H, He L, Liu B, et al. Establishment and comparison of air-liquid interface culture systems for primary and immortalized swine tracheal epithelial cells. BMC Cell Biol. 2018;19(1):10. doi: 10.1186/s12860-018-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS ONE. 2014;9(9):e108342. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst JM, Foulds J, Veldheer S, et al. Nicotine absorption during electronic cigarette use among regular users. PLoS ONE. 2019;14(7):e0220300. doi: 10.1371/journal.pone.0220300. [DOI] [PMC free article] [PubMed] [Google Scholar]