Abstract
Studies on an organic extract of a marine fungus, Periconia sp. (strain G1144), led to the isolation of three halogenated cyclopentenes along with the known and recently reported rhytidhyester D; a series of spectrometric and spectroscopic techniques were used to elucidate these structures. Interestingly, two of these compounds represent tri-halogenated cyclopentene derivatives, which have been observed only rarely from Nature. The relative and absolute configurations of the compounds were established via mass spectrometry (MS), nuclear magnetic resonance (NMR) spectroscopy, Mosher’s esters method, optical rotation and GIAO NMR calculations, including correlation coefficient calculations and the use of both DP4+ and dJ DP4 analyses. Several of the isolated compounds were tested for activity in anti-parasitic, antimicrobial, quorum sensing inhibition, and cytotoxicity assays and were shown to be inactive.
Keywords: Periconia sp, marine fungi, GIAO NMR calculations, halogenated cyclopentenes
1. Introduction
Marine and freshwater derived fungi are a rich and diverse source of specialized metabolites (Bills and Gloer, 2016; El-Elimat et al., 2021). Periconia spp. are mitosporic (i.e., asexual) ascomycetes that occur in a variety of ecological niches. For example, they have been isolated as endophytes, pathogens (Romero et al., 2001) and saprobes (Cantrell et al., 2007) from both terrestrial (Chuaseeharonnachai et al., 2016) and aquatic habitats, including both marine (Morrison-Gardiner, 2002) and fresh water (Cai et al., 2002). Members of this genus are well known to biosynthesize compounds with diverse biological properties, including anti-inflammatory, antimicrobial, and anti-human immunodeficiency virus (HIV) activities (Azhari and Supratman, 2021). In addition, a wide range of fungal metabolites have been isolated, which include sesquiterpenes and cytochalasins to diterpenoids and dihydroisocoumarins (Liu et al., 2021; Wu et al., 2015a; Wu et al., 2015b; Zhang et al., 2013; Zhang et al., 2014), suggesting that such fungi have rich biosynthetic capabilities (Knapp et al., 2018).
Halogenated metabolites have been isolated in abundance from marine organisms, likely due to the availability of chloride (~19000 ppm) and bromide (~65 ppm) ions in seawater (Stine, 1929; Butler and Sandy, 2009; Harper and O’Hagan, 1994; Neumann et al., 2008). Examples of halogenated polyketide derivatives include the cyclopentones, cryptosporiopsinol and cryptosporiopsin (Giles and Turner, 1969), or the more recently reported cyclopericodiol (Inose et al., 2019). Of these, cryptosporiopsinol was first described from P. macrospinosa over a half century ago (Giles and Turner, 1969); however, since then, the compound has been under investigated in the literature, leading to some ambiguity in the absolute configuration of stereoisomers and/or closely related analogues (Elsebai et al., 2018; Henderson and Hill, 1982; Hill et al., 1987; Holker and Young, 1975; Höller et al., 1999; Höller et al., 2000; McMullin et al., 2017).
During ongoing studies of fungal metabolites from specimens collected from aquatic habitats throughout North Carolina (El-Elimat et al., 2014a; El-Elimat et al., 2017; El-Elimat et al., 2021; El-Elimat et al., 2014b; Paguigan et al., 2016), an interesting, purple-colored marine derived mitosporic fungus was isolated from decomposing Spartina stems that was identified as a Periconia sp. (strain G1144). In addition to the typical suite of HRMS and 2D-NMR experiments used for structure elucidation of natural products, a series of orthogonal techniques, such as Mosher’s esters methodology (Hoye et al., 2007) and GIAO (gauge-including atomic orbitals) NMR calculations (Barone et al., 2002a; Barone et al., 2002b; Knowles et al., 2021; Neuhaus et al., 2019; Seco et al., 2004; Smith and Goodman, 2009; Willoughby et al., 2014; Willoughby et al., 2020), including correlation coefficient, DP4+ (Smith and Goodman, 2010) and dJ DP4 calculations (Grimblat et al., 2019), were used to ascertain the absolute configuration of these fungal metabolites.
2. Results and Discussion
The fungal culture (strain G1144) displayed a deep purple color when grown on agar media (Fig. S32). This striking characteristic, and the lack of hits when this extract was analyzed via dereplication against an in-house database of over 625 fungal metabolites (El-Elimat et al., 2013; Paguigan et al., 2017), stimulated further studies. Traditional chromatographic protocols led to the isolation of four chlorine containing cyclopentenes (1–4). Compounds 1 and 2 represent previously undescribed tri-halogenated cyclopentene derivatives, while compounds 3 and 4 were stereoisomers of the known compound, cryptosporiopsinol (Giles and Turner, 1969; Holker and Young, 1975; Höller et al., 1999).
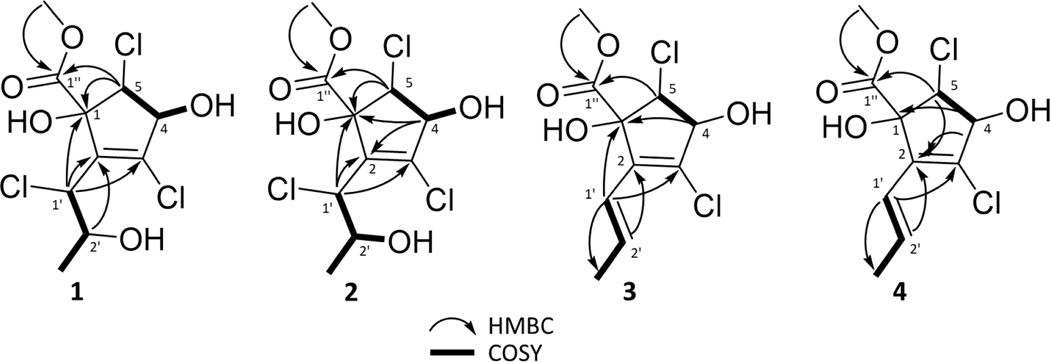
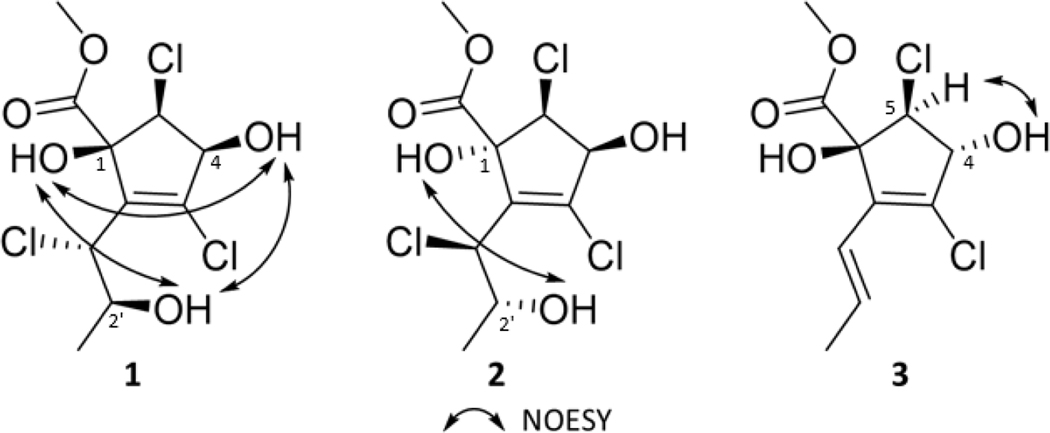
Compound 1 was obtained as an opaque solid material, and the molecular formula was established as C10H13Cl3O5 based on HRESIMS and NMR data, indicating an index of hydrogen deficiency of 3 (Figs. S1 and S2). The molecular ion peak in the HRESIMS data showed the characteristic isotopic pattern for three chlorine atoms in the molecule (Figs. S1). NMR data from 1H, 13C, and HSQC experiments indicated the presence of a methoxy ester (δH/δC 3.91/54.5 and a carbonyl group δC 171.9), plus two more signals (δC 137.7 and 140.4) that were indicative of a fully substituted double bond. COSY correlations between H-4 and H-5, in addition to HMBC correlations between H-5 and both C-1 and C-1’’, were suggestive of a cyclopentene ring (Figs. 2, S4, and S5), with the latter correlation serving to demonstrate the connection point of the methoxy ester side chain. Additionally, the chemical shifts in the 1H and 13C NMR spectra for signals attributable to the cyclopentene core were nearly identical to what was observed in cryptosporiopsinol (Giles and Turner, 1969; Holker and Young, 1975) and in the recently described rhytidhyester D (Zhang et al., 2021), particularly for δC-1 88.2, δC-2 137.7, δH-4/δC-4 4.47/76.0 and δH-5/δC-5 4.43/65.0 (Table S48). The aliphatic side chain of the molecule was verified with the COSY correlations for the spin system between H-1’ to H-2’ to H-3’, and the connection of this chain to the cyclopentene core at position C-2 was supported by HMBC correlations between H-1’ to C-1 and C-3 (Figs. 2, S4, and S5). The position of the hydroxy group at C-4 was noted based on COSY cross peaks between H-4 and 4-OH (Fig. S5). The other two hydroxy groups were assigned based on the significantly different chemical shifts of 1-OH (δ1-OH 4.12), which was more deshielded since it was adjacent to both the ester carbonyl and the vinyl moiety compared to 2’-OH (δ2’-OH 1.95), which was on the aliphatic side chain (Table 1). Since δ2’-OH was a broad singlet when CDCl3 was used as a solvent, NMR data were also collected using DMSO-d6 (Fig. S7–S9). 1H NMR and HSQC data obtained in this aprotic solvent clearly showed the presence of three hydroxy groups with the signals for 2’-OH and 4-OH being doublets and that of 1-OH being a singlet (Fig. S7). The 1D and 2D NMR data (Table 1) were used to elucidate the planar structure of 1, and the relative configuration was suggested based on NOESY data collected in DMSO-d6, which showed correlations between all three hydroxy protons (i.e., 1-OH, 4-OH, and 2’-OH) (Fig. S9).
Figure 2.
Key HMBC and COSY correlations for compounds 1-4.
Table 1.
1H and 13C NMR Data of Compounds 1–4.
| Pos. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | |
| 1 | 88.2, C | - | 86.6, C | - | 80.4, C | - | 87.3, C | - |
| 2 | 137.7, C | - | 137.5, C | - | 134.8, C | - | 133.1, C | - |
| 3 | 140.4, C | - | 142.1, C | - | 134.0, C | - | 137.0, C | - |
| 4 | 76.0, CH | 4.47, bs | 75.8, CH | 4.52, dd (12.06, 6.5) |
80.5, CH | 4.80, d (5.9) |
75.5, CH | 4.47, br s |
| 5 | 65.0, CH | 4.43, d (6.4 | 64.9, CH | 4.47, d (6.5) |
67.9, CH | 4.33, d (6.0) |
66.0, CH | 4.47, br s |
| 1’ | 57.1, CH | 4.51, d (7.3) |
59.5, CH | 4.82, d (6.7) |
121.0, CH | 6.18, m | 120.6, CH | 6.17, m |
| 2’ | 69.5, CH | 4.33, p (6.4) |
70.0, CH | 4.20, m | 132.5, CH | 5.93, dq (15.7, 6.6) |
134.55, CH | 6.17, m |
| 3’ | 20.9, CH3 | 1.34, d (6.2) |
19.8, CH3 | 1.33, d (6.4) |
19.3, CH3 | 1.81, d (7.2) |
19.5, CH3 | 1.82, d (5.1) |
| 1” | 171.9, C | - | 171.9, C | - | 173.0, C | - | 172.1, C | - |
| 2” | 54.5, CH3 | 3.91, s | 54.6, CH3 | 3.92, s | 53.9, CH3 | 3.82, s | 54.6, CH3 | 3.91, s |
| 1-OH | - | 4.12 s | - | 5.11, s | - | - | - | - |
| 4-OH | - | 3.43, d | - | 3.47, d | - | - | - | - |
| 2’-OH | - | 1.95 s | - | 3.10 d | - | - | - | - |
CDCl3 (1H NMR 400 MHz, 13C NMR 100 MHz).
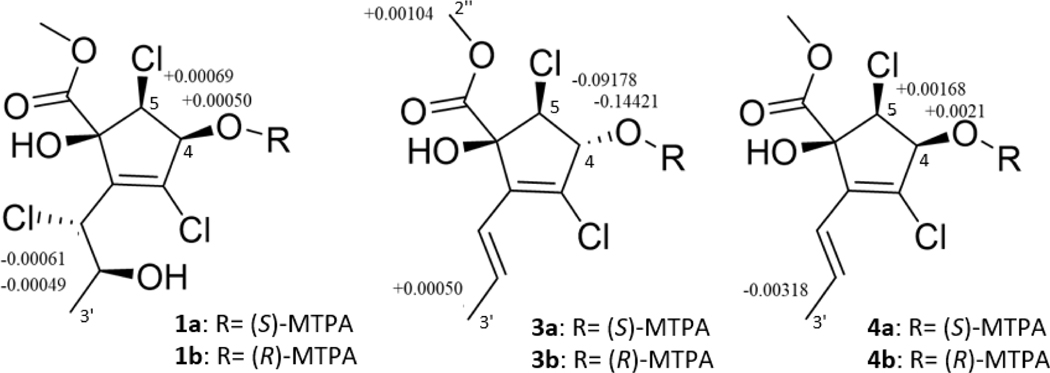
To determine the absolute configuration of 1, both experimental and theoretical approaches were pursued. First, the configuration of the secondary hydroxy moieties (i.e., 4-OH and 2’-OH) were explored via Mosher’s esters analysis. Both positions were highly reactive to Mosher’s reagent, resulting in an excessively complex data set; this observation was also true for the Mosher’s ester analysis of compound 3-4. As such, we used half as much reagent (i.e. 10 μl instead of 20 μl), and for all compounds examined in this study, the reaction favored the 4-OH position. Thus, compound 1 was derivatized at the 4-OH position with both S- and R-3,3,3-trifluoro-2-methoxy-2-phenylpropanoic acid (MTPA), establishing the configuration as 4R (Fig. 4). Pairing this result with the NOESY correlations between 4-OH and 2’-OH (Fig. S9) established the 2’-position as S. In addition, these data were further verified using GIAO (i.e., correlation coefficient and DP4+) calculations (Fig. S36). These calculations were in agreement with each other (Tables S3, S42), suggesting that 1 was either 1S, 4R, 5S, 1’R, 2’S or 1R 4S, 5R, 1’S, 2’R; the former assignments were supported by the Mosher’s esters data (Fig. 4). In addition, optical rotation calculations were used to distinguish between these enantiomers. The calculated and specific rotation of 1 [i.e., [α]D20 +53 vs [α]D20 +158 (c 0.10, CHCl3); Table S41] further verified the absolute configuration as 1S, 4R, 5S, 1’R, 2’S. Compound 1 was ascribed the trivial name cryptosporiopsinol C.
Figure 4.
ΔδH values (Δδ = δS – δR) obtained for (S)- and (R)-MTPA esters of 1, 3 and 4 in pyridine-d5.
Compound 2 was isolated as an opaque solid material and had a molecular formula of C10H13Cl3O5, identical to 1. HRESIMS data of 2 and 1 were also identical (Figs. S10 and S11), but the slight differences in the 1H and 13C NMR chemical shifts (Table 1) indicated a stereoisomeric relationship (Fig. S12). In fact, the HMBC correlations also showed similarities to 1, but additional correlations were observed between H-4 to both C-1 and C-2 (Figs. 2 and S14). COSY correlations between 2’-OH and H-2’ along with 4-OH and H-4 confirmed the positions of the hydroxy groups (Fig. 2). The relative configuration of 2 was suggested based on NOESY correlations between 1-OH and 2’-OH (Figs. 3 and S16).
Figure 3.
Key NOESY correlations for compound 1-3.
To determine the absolute configuration of 2, Mosher’s esters analysis of the compound was not possible due to paucity of sample. Thus, the knowledge base from determining the absolute configuration of 1 was leveraged by using the same approach for 2, including the calculation of both correlation coefficient and DP4+ probabilities. For GIAO NMR calculations based on the NOESY results, 16 conformers were analyzed (Fig. S36), where the correlation coefficient and DP4+ calculations were in agreement, indicating a configuration of either 1S, 4S, 5R, 1’R, 2’S or 1R, 4R, 5S, 1’S, 2’R (Tables S6, S42). These findings were refined by examining the calculated and specific rotation of 2 [i.e., [α]D20 −10 vs [α]D20 −47 (c 0.10, CHCl3); Table S41], thereby deducing the absolute configuration as 1R, 4R, 5S, 1’S, 2’R. Compound 2 was ascribed the trivial name cryptosporiopsinol D.
Compound 3, also an opaque solid material, had an additional point of unsaturation, relative to 1 and 2, as noted by the index of hydrogen deficiency of 4, which was derived from the formula of C10H12Cl2O4 based on HRESIMS and NMR data (Fig. S17). The molecular ion peak in the HRESIMS data showed the characteristic isotopic pattern for two chlorine atoms in the molecule (Fig. S17). The planar structure of 3 (Table 1) was found to be similar to 1 and 2 based on 1H and 13C NMR data (Fig. S18). The difference was that compound 3 had five sp2 carbons (δC-2 134.8, δC-3 134.0, δC-1’ 121.0, δC-2’ 132.5 and δC-1” 173.0) as opposed to three observed in 1 and 2, which was consistent with a double bond between C-1’ and C-2’, possibly via the loss of a chlorine atom and hydroxy group, relative to 1 and 2, as noted in the molecular formula. This double bond was trans as noted by the J value of 15.7 Hz. COSY correlations between H-1’ and H-2’, in combination with the HMBC correlations from H-1’ to C-2 and C-3 and from H-2’ to C-2, confirmed the connection of the sidechain (Figs. 2, S21, S22) to the core. Since 1-OH and 4-OH were observable in neither CDCl3 nor CD3OD, additional NMR data were collected in DMSO-d6 (Figs. S23 and S24). Those 1H NMR and COSY data showed the presence of two hydroxy groups, with 1-OH being a singlet and 4-OH being a doublet (Fig S23). The relative configuration of 3 was suggested based on NOESY data that showed correlations between 4-OH and H-5 (Fig. 3).
To examine the absolute configuration of 3, Mosher’s esters analysis was conducted, as noted for 1, establishing the configuration at position C-4 as S (Fig. 4). Next, to further examine the absolute configuration (i.e., positions C-1, C-4, and C-5), three different NMR calculation methods were tested. First, the four possible conformers were submitted for GIAO NMR calculations (Fig. S36). The correlation coefficient calculations showed that the conformer with the highest probability was 1S, 4S, 5S (99.75%) (Table S9). Then, the DP4+ method was examined, and this also yielded a likely configuration of 1S, 4S, 5S (Table S42). Importantly, this configuration was different than that of, cryptosporiopsinol at position C-5, which was determined by X-ray crystallography to be 1S, 4S, 5R (Giles and Turner, 1969). In addition, a more recent paper was published on what was termed (+)-cryptosporiopsinol, but the absolute configuration was not established in that paper (McMullin et al., 2017). To further strengthen our findings, dJ DP4 calculations were also conducted, which included DP4+ with direct 3JHH couplings, as detailed in recent literature (Grimblat et al., 2019). This method has the added benefit of high performance while being more affordable in terms of computational cost (Grimblat et al., 2019). The absolute configuration as determined by dJ DP4 calculations of 3 were in agreement (Table S44) with those noted above for the DP4+ and correlation coefficient calculations. Finally, optical rotation calculations and the results of Mosher’s esters analysis (i.e., 4S) were used to distinguish between enantiomers. The calculated and specific rotation of 3 agreed with a configuration of 1S, 4S, 5S (i.e., [α]D20 +48 vs [α]D20 +166 (c 0.10, CHCl3); Table S41). Compound 3 was ascribed the trivial name cryptosporiopsinol B.
Compound 4 was isolated as a colorless solid material and had a molecular formula of C10H12Cl2O4, the same as 3. The HRESIMS data of 4 and 3 were identical (Figs. S17 and S25), but differences between their 1H and 13C NMR data suggested a stereoisomeric relationship (Figs. S18 and S26), although the COSY and HMBC correlations of 4 and 3 were nearly identical (Figs. S29 and S30). Additional key HMBC correlations were observed between H-4 and C-2 and between H-5 and C-2 (Fig. 2). To establish the presence of the hydroxy groups, additional 1H NMR data were collected in DMSO-d6. (Fig. S31).
As with 1 and 3, the absolute configuration of 4 was first examined by Mosher’s esters analysis, establishing the configuration of the 4 position as R (Fig. 4). Then, the absolute configuration could be probed at positions 1 and 5 (i.e., four possible conformers) using GIAO NMR calculations (Fig. S36). The results of the correlation coefficient and DP4+ calculations were in agreement, suggesting an absolute configuration of 1S, 4R, 5S (or its enantiomer, 1R, 4S, 5R; Tables S12 and S42). Since δH-4 and δH-5 were overlapping, incorporating the 3JHH coupling constant into the DP4 calculations (i.e., dJ DP4) was not possible. Thus, optical rotation calculations, and the results of Mosher’s esters analysis, were used to distinguish between enantiomers (Fig. 4). The specific rotation of 4 agreed with the calculated values, establishing the absolute configuration of the molecule as 1S 4R 5S (i.e., [α]D20 +55 vs [α]D20 +347 (c 0.10, CHCl3); (Table S41). In conclusion, compound 4 was shown to be identical to rhytidhyester D, a stereoisomer of cryptosporiopsinol, which was described recently from an unrelated endophytic fungus (Rhytidhysteron sp. from Leptospermum brachyandrum) at the same time this study was ongoing (Zhang et al., 2021).
Compounds 1-4 were biosynthesized from a single isolate of strain G1144, which was isolated from decomposing Spartina culms collected from a marine habitat. Thus, we are not entirely certain if G1144 is a true marine species in the strict sense (Overy et al., 2019; Pang et al., 2016). When the strain was cultured in peptone yeast media with 30 ppt sea salts, the fungus displayed healthy growth after three weeks. However, the strain also grew abundantly on distilled water media without sea salts (Fig. S32). For the present study, we refer to this fungus as marine-derived, rather than a true obligate marine fungus, until additional ecological studies are carried out to determine the accurate ecology of Periconia sp. (strain G1144) (Overy et al., 2019; Pang et al., 2016). This conservative approach seems warranted given the fact that other Periconia spp. have been isolated from a variety of terrestrial and aquatic habitats.
These compounds (1-4) were isolated from a culture grown on solid rice media, where 30 ppt sea salts in distilled water were added prior to sterilizing via autoclave (see section 4.3). An obvious question concerned whether sea salts were required to produce these chlorinated analogues. We analyzed retrospectively the extracts of this fungus grown on various media types (Amrine et al., 2018; Graf et al., 2020), so as to examine if 1-4 were present in growths where sea salts were not added. Interestingly, we were not able to detect 1-4 on any other media type except when cultures were grown on rice media with 30 ppt sea salts (Fig. S34). Furthermore, we explored if the dechlorinated versions of 1-4 were present on any media types where salts were not added (Fig S35), and indeed, we were able to detect the accurate mass of the dechlorinated versions of 3 and 4 via extracted ion chromatograms (XIC). This suggests that this fungus has the capacity to produce the dechlorinated versions of these compounds, depending on media types. Regardless, since we were only able to detect 1-4 when 30 ppt sea salts were included in the media, we hypothesize that this salt content was needed for the organism to incorporate chlorine into the molecules, which supports similar observations by (Henderson and Hill, 1982). Biosynthetically, those same authors proposed that the chlorine is incorporated in cryptosporiopsinol (a stereoisomer of 3 and 4) in one of the early steps of the biosynthetic pathway.
Biological activity of 1–4 was not observed in any of the assays employed herein. They were neither cytotoxic against a panel of cancer cell lines (IC50 > 25 μM, Table S45) nor antimicrobial against a broad series of pathogenic microorganisms (MIC > 125μg/mL, Table S46). The compounds were also screened against Naegleria fowleri, a pathogenic amoeba that is responsible for a rapidly progressive central nervous disease called primary amebic meningoencephalitis (Fowler and Carter, 1965), but all were inactive (IC50 > 25 μM) (Table S47). Quorum sensing inhibition of 1 and 4 was evaluated, similar to what was described previously by Figueroa et al. (Figueroa et al., 2014), and no significant inhibition of quorum sensing was observed for these compounds against a clinical isolate of methicillin-resistant Staphylococcus aureus (MRSA) (Figs S37–S41).
3. Conclusion
This paper explored the rich diversity of fungal metabolites obtained from Periconia spp. with the identification of three undescribed and one recently described compounds. The structures and absolute configurations of 1–4 were determined by evaluating 1D and 2D NMR data, mass spectrometry data, optical rotation calculations, Mosher’s esters analysis and GIAO NMR calculations, including correlation coefficient, DP4+ and dJ DP4 calculations. Despite testing the metabolites against a suite of cytotoxicity, antimicrobial, antiparasitic, and quorum sensing inhibition assays, the potential biological activity of these structurally interesting fungal metabolites remains undetermined and is a topic that warrants further study.
4. Experimental
4.1. General Experimental Procedures
UV, and optical rotation data were obtained using a Varian Cary 100 Bio UV−Vis spectrophotometer (Varian Inc.), and a Rudolph Research Autopol (II) polarimeter (Rudolph Research Analytical), respectively. Flash chromatography was performed on a Teledyne ISCO CombiFlash Rf 200 using Silica Gold columns (both from Teledyne ISCO) and monitored by both UV and evaporative light-scattering detectors. Phenomenex Gemini-NX C18 analytical (5 μm; 250 × 4.6 mm), semipreparative (5 μm; 250 × 10.0 mm) and preparative (5 μm; 250 × 21.2 mm), columns (Phenomenex) were used on a Varian Prostar HPLC system equipped with ProStar 210 pumps and a Prostar 335 photodiode array detector (PDA), with data collected and analyzed using Galaxie Chromatography Workstation software (version 1.9.3.2, Varian Inc.). A Waters Acquity UPLC system (Waters Corp.) utilizing a Phenomenex Kinetex C18 column (1.3 μm; 50 × 2.1 mm) was used to evaluate the purity of the isolated compounds with data collected and analyzed using the Thermo Fisher Scientific Xcalibur data acquisition software (Thermo Fisher Scientific). HRMS analysis utilized either a Thermo Fisher Scientific LTQ Orbitrap XL mass spectrometer or a Thermo Fisher Scientific Q Exactive Plus mass spectrometer, both equipped with an electrospray ionization source (Thermo Fisher Scientific). NMR data were collected using either a JEOL ECS-400 MHz NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C, a JEOL ECA-500 MHz NMR spectrometer operating at 500 MHz for 1H and 125 MHz for 13C (JEOL Ltd.), or an Agilent 700 MHz NMR spectrometer (Agilent Technologies) equipped with a cryoprobe, operating at 700 MHz for 1H and 175 MHz for 13C. Residual solvent signals of CDCl3 (δH = 7.260 and δC = 77.160) were utilized for referencing.
4.2. Fungal strain isolation and identification
Strain G1144 was obtained from senescent brown ascospores scattered on the surface of decomposed Spartina culms, which were collected from Holden Beach, North Carolina, USA in July of 2020. In brief, the senescent ascospores were located with a dissecting microscope, picked with a sterile needle, and spread onto antibiotic water agar (Figueroa et al., 2014). Upon germination, the ascospores were transferred aseptically onto peptone yeast glucose with 30 ppt Instant Ocean (hereafter referred to as sea salts). Examination of strain G1144 on different nutrient media from Difco, corn meal agar, Czapek-Dox agar, peptone yeast glucose with 30 ppt sea salts (30g sea salts in 1000 ml of distilled water), oatmeal agar, and potato dextrose agar with autoclaved balsa (Fig. S32) did not reveal any sexual or asexual structures on nutrient media for phenotypic identification. Thus, the fungus was identified via molecular methods coupled with Maximum Likelihood analysis using methods outlined previously (Raja et al., 2017). The internal transcribed spacer region (ITS 1 & 2 and 5.8S nrDNA) was PCR amplified and sequenced using primers ITS1F and ITS4 (Gardes and Bruns, 1993; Raja et al., 2017; White et al., 1990); two sequences of the same strain were obtained for quality control. A BLAST search of the ITS data in NCBI GenBank revealed ≥95% sequence similarity with members of the genus Periconia Tode. Therefore, ITS data were downloaded from several different species of Periconia from recent molecular studies (Cantrell et al., 2007; Crous et al., 2018; Dayarathne et al., 2020; Dong et al., 2020; Hyde et al., 2017; Markovskaja and Kačergius, 2014; Tanaka et al., 2015) and incorporated into a multiple sequence alignment using MUSCLE (Edgar, 2004) in the program Seaview version 4.5.3 (Gouy et al., 2010). The alignment was trimmed to remove ambiguous characters using GBlocks (Talavera and Castresana, 2007). ModelFinder (Kalyaanamoorthy et al., 2017) was used in the program PhyloSuite v.1,2 to select the best-fit model using Akaike Information Criterion. The best fitting substitution model was the general time reversible model with empirical base frequencies, allowing for a proportion of invariable sites, and a discrete Gamma model with four rate categories (GTR+F+I+G4) was selected using Akaike Information Criterion Subsequently, the final alignment was used to infer the Maximum Likelihood of ITS sequence data using IQ-TREE implemented in PhyloSuite (Zhang et al., 2020). Ultrafast bootstrapping was performed with 5000 replicates (Nguyen et al., 2015). Nodes with UFBoot ≥90% are shown on the clades but only nodes ≥95% are considered strongly supported. Based on these results, strain G1144 showed phylogenetic affinities with the genus Periconia, Periconiaceae, Ascomycota (Fig. S33), which was recently emended so that additional modern molecular studies could be possible. (Tanaka et al., 2015) Based on the ITS phylogeny, we could not place stain G1144 into any existing species, as it occurred on an isolated clade (Fig. S33). Some of the aquatic species of Periconia include: Periconia salina (Dayarathne et al., 2020), P. variicolor (Cantrell et al., 2007) P. aquatica, P. prolifica (Kohlmeyer, 1969; Pang et al., 2004; Tanaka et al., 2015), and P. submersa (Hyde et al., 2017). Currently, the genus Periconia is polyphyletic and needs dire taxonomic revisions, especially since the type of strain, P. lichenoides, is unavailable for both morphological and molecular studies (Markovskaja and Kačergius, 2014; Tanaka et al., 2015). Since, our axenic culture did not produce either sexual or asexual micromorphological characters, we herein identify strain G1144 as Periconia sp., Periconiaceae, Pleosporales, Dothideomycetes Ascomycota. The ITS sequences were deposited in GenBank (accession no: ITS: MZ997836, MZ997837).
4.3. Fermentation, Extraction, and Isolation.
The cultures of fungal strain G1144 were maintained on potato dextrose agar (PDA; Difco) as well as peptone yeast glucose agar (1.25 g peptone, 1.25 g yeast extract, 5g D-glucose, 18 g agar + 30 ppt sea salts; 30 g sea salts in 1000 ml of deionized water) (Fig. S32). Since strain G1144 showed good growth on both PDA and PYGA + salt media, the latter media was utilized for further experiments. An agar plug from the leading edge of the PYGA culture was transferred to a sterile tube with 10 ml of liquid PYG + 30 ppt sea salts, and this culture was grown for 12 days on an orbital shaker (100 rpm) at room temp. (~23 °C) and then used to inoculate solid fermentation media, such as rice. Solid-state fermentations (n=4) were carried out in 250-ml Erlenmeyer flasks. To prepare rice medium, 10 g of rice were added to each flask with 20 ml of deionized 30 ppt salt water. After autoclaving these samples at 120 °C for 20 min, the flasks were inoculated with PYG + 30 ppt cultures (described above) and incubated at room temperature for four weeks. Subsequently, each of the four solid-state fermentation cultures were chopped into small pieces using a spatula, and 60 ml of 1:1 MeOH-CHCl3 were added. The cultures were then shaken overnight (~16h) at ~125 rpm at rt. The resulting slurries were filtered in vacuo and then pooled to form a combined filtrate, and the solid residue was rinsed with a small volume of 1:1 MeOH-CHCl3. To the combined filtrate, 270 ml of CHCl3 and 450 ml of H2O were added; the solution was stirred for 20 min and transferred to a separatory funnel. The organic layer was collected and evaporated to dryness under vacuum using a rotary evaporator. The resulting organic extract was then partitioned between 100 ml of 1:1 MeOH-CH3CN and 100 ml of hexanes. The MeOH-CH3CN layer was collected and evaporated to dryness under vacuum. The defatted organic extract (~228 mg) was reconstituted in CHCl3 and absorbed onto celite 545. The extract was then fractionated by flash chromatography using a solvent gradient of hexane-CHCl3-MeOH at a 18 ml/min flow rate and 85.0 column volumes to yield five fractions. Fraction 2 (~97 mg) was fractionated further into 11 subfractions using preparative HPLC with a solvent gradient increasing linearly from 20:80 to 75:25 CH3CN-H2O (acidified with 0.1% formic acid) over 20 min at a flow rate of 21.20 ml/min. Subfractions 4, 6, and 7 yielded compounds 1 (3.94 mg), 3 (5.74), and 4 (30.63 mg) which eluted at ~16 min, 18 min, and 19 min respectively. Subfraction 5 was further purified using semi-preparative HPLC with a solvent gradient of 40:60 to 50:50 CH3CN-H2O (acidified with 0.1% formic acid) over 30 min at a flow rate of 4.60 ml/min to yield compound 2 (0.78 mg), which eluted at 21.5 min.
Cryptosporiopsinol C (1): opaque solid; [α]D20=+53(c 0.10, CHCl3); UV(CHCl3) λmax (log ε) 267.5 (2.70), 241 (3.12) nm; 1H and 13C NMR, Table 1; HRESIMS m/z 318.9917 [M + H]+ (calcd for C10H14Cl3O5, 318.9907), m/z 340.9739 [M + Na]+ (calcd for C10H13Cl3O5Na, 340.9726)
Cryptosporiopsinol D (2): opaque solid; [α]D20=−10(c 0.10, CHCl3); UV(CHCl3) λmax (log ε) 241 (2.29) nm; 1H and 13C NMR, Table 1; m/z 318.9916 [M + H]+ (calcd for C10H14Cl3O5, 318.9907), m/z 340.9738 [M + Na]+ (calcd for C10H13Cl3O5Na, 340.9726)
Cryptosporiopsinol B (3): opaque solid; [α]D20=+48(c 0.10, CHCl3); UV(CHCl3) λmax (log ε) 254 (2.77), 219 (4.15) nm; 1H and 13C NMR, Table 1; HRESIMS m/z 289.0017 [M + Na]+ (calcd for C10H12Cl2O4Na, 289.0010)
Rhytidhyester D (4): colorless solid; [α]D20=+55(c 0.10, CHCl3); UV(CHCl3) λmax (log ε) 257 (1.98), 216 (3.13) nm; 1H and 13C NMR, Table 1; HRESIMS m/z 289.0018 [M + Na]+ (calcd for C10H12Cl2O4Na, 289.0010)
4.4. Cytotoxicity and antimicrobial assays
To evaluate the cytotoxic activity of 1–4 against human melanoma cancer cells (MDA-MB-435), human breast cancer cells (MDA-MB-231), and human ovarian cancer cells (OVCAR3), the assays were performed as detailed recently (Al Subeh et al., 2021). Taxol was used as positive control. Compounds 1–4 were analyzed in four technical replicates and three biological replicates, and all compounds were >95% pure as measured by UPLC (Figs. S1, S10, S17, S25).
Minimal inhibitory concentrations of the compounds were measured by broth microdilution against the following bacteria: Escherichia coli, Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Pseudomonas aeruginosa, and Bacillus anthracis; MICs were measured by broth microdilution of fresh overnight cultures according to the Clinical and Laboratory Standards Institute (CCLI) guidelines with cation-adjusted Mueller–Hinton broth and an inoculum of 105 colony-forming units (CFUs)/ml. Stocks of the compounds were dissolved in Mueller–Hinton broth (Becton-Dickinson, Sparks, MD). The MIC (expressed as μg/ml) was defined as the lowest concentration of compound completely inhibiting the appearance of turbidity by eye and confirmed by absorbance 540 nm. All results represent the average of three independent measurements. Prior to testing, 1-4 were confirmed >95% pure by UPLC (Figs. S1, S10, S17, S25).
4.5. Antiparasitic assay
A clinical isolate of Naegleria fowleri obtained from a 9-year-old boy in Adelaide, Australia, that died of primary amebic meningoencephalitis (PAM) in 1969 was previously purchased from the American Type Culture Collection (ATCC 30215) (Rice et al., 2015). Trophozoites were routinely grown axenically at 34°C in Nelson’s complete medium (NCM) in non-vented 75 cm2 tissue culture flasks (Olympus), until the cells were 80–90% confluent. For sub-culturing, cells were placed on ice to detach the cells from the culture flasks. Detached cells were collected by centrifugation at 4000 rpm at 4°C. Complete NCM media was produced by the addition of 10% FBS and 125 μg of penicillin/streptomycin antibiotics. The trophocidal activity of pure compounds were assessed using the CellTiter-Glo 2.0 luminescent viability assay (Promega, Madison, WI), as previously described (Colon et al., 2018; Rice et al., 2015; Rice et al., 2020a; Rice et al., 2020b). In brief, logarithmic trophozoites of N. fowleri were seeded at 4000 cells/well into white 96-well plates (Costar 3370). All compounds were assessed in 2-fold serial dilutions from the highest concentration of 20 μM for 72 hours. At the 72-hour time point, 25 μl of CellTiter-Glo 2.0 reagent was added to all wells of the plates. The plates were protected from light and contents were mixed using an orbital shaker at 300 rpm at room temperature for 2 min to induce cell lysis. After shaking, the plates were equilibrated at room temperature for 10 min to stabilize the luminescent signal. The ATP luminescent signals (relative light units; RLUs) were measured at 490 nm with a SpectraMax I3X plate reader (Molecular Devices, Sunnyvale, CA). Drug inhibitory concentration (IC50) curves were generated using total ATP RLUs where controls were calculated as the average of replicates using the Levenberg-Marquardt algorithm, using DMSO as the normalization control, as defined in CDD Vault (Burlingame, CA, USA). Values reported are from a minimum of two biological replicates with standard deviations. Prior to testing, 1-4 were confirmed >95% pure by UPLC (Figs. S1, S10, S17, and S25).
4.6. Quorum Sensing Inhibition Assay
A 96-well plate assay was performed to evaluate the inhibition of the production of AIP-I by MRSA, adapted from a published procedure (Todd et al., 2016). Overnight tryptic soy broth (TSB) MRSA cultures were diluted 1:200 with fresh TSB and shaken (200 rpm) at 37°C for 2 hours. A stock solution (5.0 mg/ml) for each sample was prepared in DMSO, which was diluted 10-fold to yield a solution of 0.5 mg/ml and then serially diluted with TSB to yield six concentrations (0.02 mg/ml- 0.50 mg/ml). Aliquots (50 μl) of the diluted solutions were combined in a 96-well plate with 150 μL of TSB and 50 μL of diluted (1:200) MRSA culture. Final sample concentrations in the wells ranged from 3.13 μg/ml to 100 μg/ml. The 96-well plate was shaken (500 rpm) for 5 hours at 37°C. Optical density readings (OD600) were taken hourly at 600 nm to measure growth of the bacteria. After incubation, cells were removed by vacuum filtration. The filtrate was analyzed using positive mode LC-MS to determine relative quantities of AIP-I (m/z 961.3799) and percent of vehicle was calculated as described previously (Todd et al., 2016). Prior to testing, 1, 3 and 4 were confirmed >95% pure by UPLC (Figs. S1, S17, and S25).
4.7. Computational Details
Macromodel (Version 12.6) interface Maestro (Version 12.2) program was used for all molecular mechanics calculations. For all conformational searches, MMFF force field and torsional sampling Monte Carlo Multiple Minimum (MCMM) method were used with extended torsional sampling (Willoughby et al., 2014), The resulting conformers were filtered, checked for duplicity, and minimized using a DFT force field at the M062X/6–31+G (d,p) level of theory. B3LYP/6–311+G (2d,p) level of theory with the IEFPCM model were used at the GIAO method to calculate NMR shielding constants. The obtained shielding constants were eventually converted into chemical shifts (ppm) by referencing TMS to 0 ppm. The final 13C and 1H NMR shifts were calculated for each conformer for each compound based on the total Boltzmann distribution and relative energies. The NMR shifts for each particular species were calculated based on the work of Willoughby et al. (Willoughby et al., 2014; Willoughby et al., 2020) To further calculate the corrected correlation coefficient (r) for the combined 13C and 1H data for compounds 1–4, the calculated 13C and 1H NMR data were first empirically scaled and then the individual correlation coefficients were calculated using excel (=correl(calculated, experimental)). Then, the geometric mean of the correlation coefficients for 13C and 1H was taken (Smith and Goodman, 2009, 2010). The DP4+ and dJ DP4 calculations were carried out as described by the Sarotti Group using their available spreadsheet (Grimblat et al., 2015). For dJ DP4 calculations Boltzmann averaged isotropic shielding values and coupling constants (FC only) were computed using full conformational search (with no constrains) (Grimblat et al., 2019). The conformers generated for the NMR calculations were taken to calculate the optical rotation values. The optical rotations were calculated at the GIAO method at the B3LYP/6–31G (d,p) level of theory in CHCl3 for 1–4 (Srebro et al., 2011; Stephens et al., 2006) based on the total Boltzmann distribution and relative energies using SpecDis software (Bruhn et al., 2013).
Supplementary Material
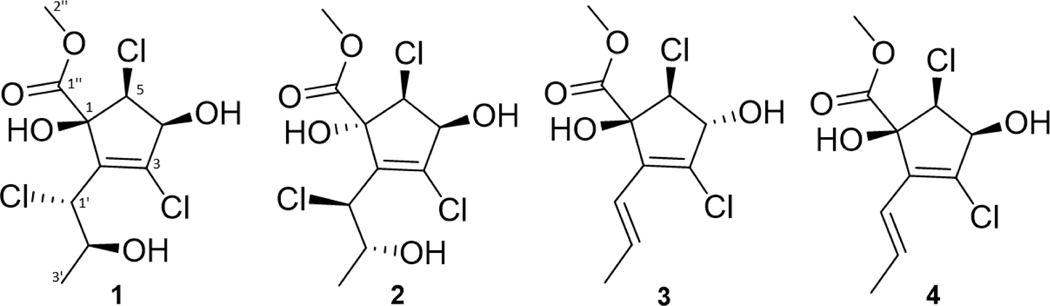
Figure 1.
Structures of Compounds 1–4 isolated from Periconia sp. (strain G1144)
Acknowledgement
This research was supported in part by the National Institutes of Health through both the National Cancer Institute via grant P01 CA125066 and the National Center for Complementary and Integrative Health via grants T32 AT008938 and F31 AT010558. This work was performed in part at the Joint School of Nanoscience and Nanoengineering, a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the National Science Foundation (Grant ECCS-2025462).
Footnotes
Declaration of competing interest
N.H. Oberlies is a member of the Scientific Advisory Board of Mycosynthetix, Inc.
References
- Al Subeh ZY, Raja HA, Maldonado A, Burdette JE, Pearce CJ, Oberlies NH, 2021. Thielavins: tuned biosynthesis and LR-HSQMBC for structure elucidation. J. Antibiot. 74, 300–306. 10.1038/s41429-021-00405-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrine CSM, Raja HA, Darveaux BA, Pearce CJ, Oberlies NH, 2018. Media studies to enhance the production of verticillins facilitated by in situ chemical analysis. J. Ind. Microbiol. Biotechnol. 45, 1053–1065. 10.1007/s10295-018-2083-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhari A, Supratman U, 2021. The Chemistry and Pharmacology of Fungal Genus Periconia: A Review. Sci. Pharm. 89, 34. [Google Scholar]
- Barone G, Duca D, Silvestri A, Gomez-Paloma L, Riccio R, Bifulco G, 2002a. Determination of the Relative Stereochemistry of Flexible Organic Compounds by Ab Initio Methods: Conformational Analysis and Boltzmann-Averaged GIAO 13C NMR Chemical Shifts. Chem. Eur. J. 8, 3240–3245. [DOI] [PubMed] [Google Scholar]
- Barone G, Gomez-Paloma L, Duca D, Silvestri A, Riccio R, Bifulco G, 2002b. Structure validation of natural products by quantum-mechanical GIAO calculations of 13C NMR chemical shifts. Chemistry (Weinheim an der Bergstrasse, Germany) 8, 3233–3239. [DOI] [PubMed] [Google Scholar]
- Bills GF, Gloer JB, 2016. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 4. 10.1128/microbiolspec.FUNK-0009-2016 [DOI] [PubMed] [Google Scholar]
- Bruhn T, Schaumlöffel A, Hemberger Y, Bringmann G, 2013. SpecDis: quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 25, 243–249. 10.1002/chir.22138 [DOI] [PubMed] [Google Scholar]
- Butler A, Sandy M, 2009. Mechanistic considerations of halogenating enzymes. Nature 460, 848–854. 10.1038/nature08303 [DOI] [PubMed] [Google Scholar]
- Cai L, M C, Zhangl K, D K, Cai L, Tsui C, Zhang K, Hyde K, 2002. Aquatic fungi from Lake Fuxian, Yunnan, China. Fungal Divers. 9, 57–70. [Google Scholar]
- Cantrell S, Hanlin R, Emiliano A, 2007. Periconia variicolor sp. nov., a new species from Puerto Rico. Mycologia 99, 482–487. 10.3852/mycologia.99.3.482 [DOI] [PubMed] [Google Scholar]
- Chuaseeharonnachai C, Somrithipol S, Boonyuen N, 2016. Periconia notabilis sp. nov. and a new record and notes on the genus in Thailand. Mycotaxon 131, 491–502. 10.5248/131.491 [DOI] [Google Scholar]
- Colon BL, Rice CA, Guy RK, Kyle DE, 2018. Phenotypic Screens Reveal Posaconazole as a Rapidly Acting Amebicidal Combination Partner for Treatment of Primary Amoebic Meningoencephalitis. J. Infect. Dis. 219, 1095–1103. 10.1093/infdis/jiy622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Luangsa-Ard JJ, Wingfield MJ, Carnegie AJ, Hernández-Restrepo M, Lombard L, Roux J, Barreto RW, Baseia IG, Cano-Lira JF, Martín MP, Morozova OV, Stchigel AM, Summerell BA, Brandrud TE, Dima B, García D, Giraldo A, Guarro J, Gusmão LFP, Khamsuntorn P, Noordeloos ME, Nuankaew S, Pinruan U, Rodríguez-Andrade E, Souza-Motta CM, Thangavel R, van Iperen AL, Abreu VP, Accioly T, Alves JL, Andrade JP, Bahram M, Baral HO, Barbier E, Barnes CW, Bendiksen E, Bernard E, Bezerra JDP, Bezerra JL, Bizio E, Blair JE, Bulyonkova TM, Cabral TS, Caiafa MV, Cantillo T, Colmán AA, Conceição LB, Cruz S, Cunha AOB, Darveaux BA, da Silva AL, da Silva GA, da Silva GM, da Silva RMF, de Oliveira RJV, Oliveira RL, De Souza JT, Dueñas M, Evans HC, Epifani F, Felipe MTC, Fernández-López J, Ferreira BW, Figueiredo CN, Filippova NV, Flores JA, Gené J, Ghorbani G, Gibertoni TB, Glushakova AM, Healy R, Huhndorf SM, Iturrieta-González I, Javan-Nikkhah M, Juciano RF, Jurjević Ž, Kachalkin AV, Keochanpheng K, Krisai-Greilhuber I, Li YC, Lima AA, Machado AR, Madrid H, Magalhães OMC, Marbach PAS, Melanda GCS, Miller AN, Mongkolsamrit S, Nascimento RP, Oliveira TGL, Ordoñez ME, Orzes R, Palma MA, Pearce CJ, Pereira OL, Perrone G, Peterson SW, Pham THG, Piontelli E, Pordel A, Quijada L, Raja HA, Rosas de Paz E, Ryvarden L, Saitta A, Salcedo SS, Sandoval-Denis M, Santos TAB, Seifert KA, Silva BDB, Smith ME, Soares AM, Sommai S, Sousa JO, Suetrong S, Susca A, Tedersoo L, Telleria MT, Thanakitpipattana D, Valenzuela-Lopez N, Visagie CM, Zapata M, Groenewald JZ, 2018. Fungal Planet description sheets: 785–867. Persoonia 41, 238–417. 10.3767/persoonia.2018.41.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayarathne M, Maharachchikumbura S, Hyde K, Devadatha B, Jones G, Chomnunti P, Khongphinitbunjong K, 2020. Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere, 7019. 10.5943/mycosphere/11/1/1 [DOI] [Google Scholar]
- Dong W, Wang B, Hyde KD, McKenzie EHC, Raja HA, Tanaka K, Abdel-Wahab MA, Abdel-Aziz FA, Doilom M, Phookamsak R, Hongsanan S, Wanasinghe DN, Yu X-D, Wang G-N, Yang H, Yang J, Thambugala KM, Tian Q, Luo Z-L, Yang J-B, Miller AN, Fournier J, Boonmee S, Hu D-M, Nalumpang S, Zhang H, 2020. Freshwater Dothideomycetes. Fungal Divers. 105, 319–575. 10.1007/s13225-020-00463-5 [DOI] [Google Scholar]
- Edgar RC, 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Elimat T, Figueroa M, Ehrmann BM, Cech NB, Pearce CJ, Oberlies NH, 2013. High-resolution MS, MS/MS, and UV database of fungal secondary metabolites as a dereplication protocol for bioactive natural products. J. Nat. Prod. 76, 1709–1716. 10.1021/np4004307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Elimat T, Raja HA, Day CS, Chen W-L, Swanson SM, Oberlies NH, 2014a. Greensporones: Resorcylic Acid Lactones from an Aquatic Halenospora sp. J. Nat. Prod. 77, 2088–2098. 10.1021/np500497r [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Elimat T, Raja HA, Day CS, McFeeters H, McFeeters RL, Oberlies NH, 2017. α-Pyrone derivatives, tetra/hexahydroxanthones, and cyclodepsipeptides from two freshwater fungi. Biorg. Med. Chem. 25, 795–804. 10.1016/j.bmc.2016.11.059 [DOI] [PubMed] [Google Scholar]
- El-Elimat T, Raja HA, Figueroa M, Al Sharie AH, Bunch RL, Oberlies NH, 2021. Freshwater Fungi as a Source of Chemical Diversity: A Review. J. Nat. Prod. 84, 898–916. 10.1021/acs.jnatprod.0c01340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Elimat T, Raja HA, Figueroa M, Falkinham JO, Oberlies NH, 2014b. Isochromenones, isobenzofuranone, and tetrahydronaphthalenes produced by Paraphoma radicina, a fungus isolated from a freshwater habitat. Phytochemistry 104, 114–120. 10.1016/j.phytochem.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Elsebai MF, Ghabbour HA, Legrave N, Fontaine-Vive F, Mehiri M, 2018. New bioactive chlorinated cyclopentene derivatives from the marine-derived Fungus Phoma sp. Med. Chem. Res. 27, 1885–1892. 10.1007/s00044-018-2201-1 [DOI] [Google Scholar]
- Figueroa M, Jarmusch AK, Raja HA, El-Elimat T, Kavanaugh JS, Horswill AR, Cooks RG, Cech NB, Oberlies NH, 2014. Polyhydroxyanthraquinones as Quorum Sensing Inhibitors from the Guttates of Penicillium restrictum and Their Analysis by Desorption Electrospray Ionization Mass Spectrometry. J. Nat. Prod. 77, 1351–1358. 10.1021/np5000704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler M, Carter RF, 1965. Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report. Br. Med. J. 2, 740–742. 10.1136/bmj.2.5464.734-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardes M, Bruns TD, 1993. ITS primers with enhanced specificity for basidiomycetes--application to the identification of mycorrhizae and rusts. Mol. Ecol. 2, 113–118. 10.1111/j.1365-294x.1993.tb00005.x [DOI] [PubMed] [Google Scholar]
- Giles D, Turner WB, 1969. Chlorine-containing metabolites of Periconia macrospinosa. J. Chem. Soc. C Org., 2187–2189. 10.1039/J39690002187 [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O, 2010. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224. 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- Graf TN, Kao D, Rivera-Chávez J, Gallagher JM, Raja HA, Oberlies NH, 2020. Drug Leads from Endophytic Fungi: Lessons Learned via Scaled Production. Planta Med. 86, 988–996. 10.1055/a-1130-4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimblat N, Gavín JA, Hernández Daranas A, Sarotti AM, 2019. Combining the Power of J Coupling and DP4 Analysis on Stereochemical Assignments: The J-DP4 Methods. Org. Lett. 21, 4003–4007. 10.1021/acs.orglett.9b01193 [DOI] [PubMed] [Google Scholar]
- Grimblat N, Zanardi MM, Sarotti AM, 2015. Beyond DP4: an Improved Probability for the Stereochemical Assignment of Isomeric Compounds using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 80, 12526–12534. 10.1021/acs.joc.5b02396 [DOI] [PubMed] [Google Scholar]
- Harper DB, O’Hagan D, 1994. The fluorinated natural products. Natural Product Reports 11, 123–133. 10.1039/NP9941100123 [DOI] [PubMed] [Google Scholar]
- Henderson GB, Hill RA, 1982. The biosynthesis of chlorine-containing metabolites of Periconia macrospinosa. J. Chem. Soc., Perkin Trans. 1, 3037–3039. 10.1039/P19820003037 [DOI] [PubMed] [Google Scholar]
- Hill RA, Macaulay GS, MacLachlan WS, 1987. Synthesis of 2,3,5-trihydroxyphenylprop-1-ene and its 4-chloro-, 6-chloro-, and 4,6-dichloro- derivatives. J. Chem. Soc., Perkin Trans. 1, 2209–2215. 10.1039/P19870002209 [DOI] [Google Scholar]
- Holker JSE, Young K, 1975. Biosynthesis of metabolites of Periconia macrospinosa from [1-13C]-, [2-13C]-, and [1,2–13C]-acetate. J. Chem. Soc., Chem. Commun, 525–526. 10.1039/C39750000525 [DOI] [Google Scholar]
- Höller U, König GM, Wright AD, 1999. Three New Metabolites from Marine-Derived Fungi of the Genera Coniothyrium and Microsphaeropsis. J. Nat. Prod. 62, 114–118. 10.1021/np980341e [DOI] [PubMed] [Google Scholar]
- Höller U, Wright AD, Matthee GF, Konig GM, Draeger S, Aust H-J, Schulz B, 2000. Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol. Res. 104, 1354–1365. 10.1017/S0953756200003117 [DOI] [Google Scholar]
- Hoye TR, Jeffrey CS, Shao F, 2007. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2, 2451–2458. 10.1038/nprot.2007.354 [DOI] [PubMed] [Google Scholar]
- Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo A, Thilini Chethana KW, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, He M-Q, Hongsanan S, Huang S-K, Jayasiri SC, Jayawardena RS, Karunarathna A, Konta S, Kušan I, Lee H, Li J, Lin C-G, Liu N-G, Lu Y-Z, Luo Z-L, Manawasinghe IS, Mapook A, Perera RH, Phookamsak R, Phukhamsakda C, Siedlecki I, Soares AM, Tennakoon DS, Tian Q, Tibpromma S, Wanasinghe DN, Xiao Y-P, Yang J, Zeng X-Y, Abdel-Aziz FA, Li W-J, Senanayake IC, Shang Q-J, Daranagama DA, de Silva NI, Thambugala KM, Abdel-Wahab MA, Bahkali AH, Berbee ML, Boonmee S, Bhat DJ, Bulgakov TS, Buyck B, Camporesi E, Castañeda-Ruiz RF, Chomnunti P, Doilom M, Dovana F, Gibertoni TB, Jadan M, Jeewon R, Jones EBG, Kang J-C, Karunarathna SC, Lim YW, Liu J-K, Liu Z-Y, Plautz HL, Lumyong S, Maharachchikumbura SSN, Matočec N, McKenzie EHC, Mešić A, Miller D, Pawłowska J, Pereira OL, Promputtha I, Romero AI, Ryvarden L, Su H-Y, Suetrong S, Tkalčec Z, Vizzini A, Wen T-C, Wisitrassameewong K, Wrzosek M, Xu J-C, Zhao Q, Zhao R-L, Mortimer PE, 2017. Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Divers. 87, 1–235. 10.1007/s13225-017-0391-3 [DOI] [Google Scholar]
- Inose K, Tanaka K, Koshino H, Hashimoto M, 2019. Cyclopericodiol and new chlorinated melleins isolated from Periconia macrospinosa KT3863. Tetrahedron 75, 130470. 10.1016/j.tet.2019.130470 [DOI] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS, 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DG, Németh JB, Barry K, Hainaut M, Henrissat B, Johnson J, Kuo A, Lim JHP, Lipzen A, Nolan M, Ohm RA, Tamás L, Grigoriev IV, Spatafora JW, Nagy LG, Kovács GM, 2018. Comparative genomics provides insights into the lifestyle and reveals functional heterogeneity of dark septate endophytic fungi. Sci. Rep. 8, 6321. 10.1038/s41598-018-24686-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SL, Roberts CD, Augustinović M, Flores-Bocanegra L, Raja HA, Heath-Borrero KN, Burdette JE, Falkinham Iii JO, Pearce CJ, Oberlies NH, 2021. Opportunities and Limitations for Assigning Relative Configurations of Antibacterial Bislactones using GIAO NMR Shift Calculations. J. Nat. Prod. 84, 1254–1260. 10.1021/acs.jnatprod.0c01309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeyer J, 1969. Marine fungi of Hawaii including the new genus Helicascus. Can. J. Bot. 47, 1469–1487. 10.1139/b69-210 [DOI] [Google Scholar]
- Liu J-M, Zhang D-W, Du W-Y, Zhang M, Zhao J-L, Chen R-D, Xie K-B, Dai J-G, 2021. Sesquiterpenes from the endophytic fungus Periconia sp. F-31. J. Asian Nat. Prod. Res, 1–6. 10.1080/10286020.2021.1935892 [DOI] [PubMed] [Google Scholar]
- Markovskaja S, Kačergius A, 2014. Morphological and molecular characterisation of Periconia pseudobyssoides sp. nov. and closely related P. byssoides. Mycol. Prog. 13, 291–302. 10.1007/s11557-013-0914-6 [DOI] [Google Scholar]
- McMullin DR, Green BD, Prince NC, Tanney JB, Miller JD, 2017. Natural Products of Picea Endophytes from the Acadian Forest. J. Nat. Prod. 80, 1475–1483. 10.1021/acs.jnatprod.6b01157 [DOI] [PubMed] [Google Scholar]
- Morrison-Gardiner S, 2002. Dominant fungi from Australian coral reefs. Fungal Divers. 9, 105–121. [Google Scholar]
- Neuhaus GF, Adpressa DA, Bruhn T, Loesgen S, 2019. Polyketides from Marine-Derived Aspergillus porosus: Challenges and Opportunities for Determining Absolute Configuration. J. Nat. Prod. 82, 2780–2789. 10.1021/acs.jnatprod.9b00416 [DOI] [PubMed] [Google Scholar]
- Neumann CS, Fujimori DG, Walsh CT, 2008. Halogenation Strategies In Natural Product Biosynthesis. Chem. Biol. 15, 99–109. 10.1016/j.chembiol.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ, 2015. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 32, 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overy DP, Rämä T, Oosterhuis R, Walker AK, Pang K-L, 2019. The Neglected Marine Fungi, Sensu stricto, and Their Isolation for Natural Products’ Discovery. Mar. Drugs 17. 10.3390/md17010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paguigan ND, El-Elimat T, Kao D, Raja HA, Pearce CJ, Oberlies NH, 2017. Enhanced dereplication of fungal cultures via use of mass defect filtering. J. Antibiot. 70, 553–561. 10.1038/ja.2016.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paguigan ND, Raja HA, Day CS, Oberlies NH, 2016. Acetophenone derivatives from a freshwater fungal isolate of recently described Lindgomyces madisonensis (G416). Phytochemistry 126, 59–65. 10.1016/j.phytochem.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Pang K-L, Jones EBG, Vrijmoed LLP, Vikineswary S, 2004. Okeanomyces, a new genus to accommodate Halosphaeria cucullata (Halosphaeriales, Ascomycota). Bot. J. Linn. Soc. 146, 223–229. 10.1111/j.1095-8339.2004.00314.x [DOI] [Google Scholar]
- Pang K-L, Overy DP, Jones EBG, Calado M.d. L., Burgaud G, Walker AK, Johnson JA, Kerr RG, Cha H-J, Bills GF, 2016. ‘Marine fungi’ and ‘marine-derived fungi’ in natural product chemistry research: Toward a new consensual definition. Fungal Biol. Rev. 30, 163–175. 10.1016/j.fbr.2016.08.001 [DOI] [Google Scholar]
- Raja HA, Miller AN, Pearce CJ, Oberlies NH, 2017. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 80, 756–770. 10.1021/acs.jnatprod.6b01085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CA, Colon BL, Alp M, Göker H, Boykin DW, Kyle DE, 2015. Bis-benzimidazole hits against Naegleria fowleri discovered with new high-throughput screens. Antimicrob. Agents Chemother. 59, 2037–2044. 10.1128/aac.05122-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CA, Colon BL, Chen E, Hull MV, Kyle DE, 2020a. Discovery of repurposing drug candidates for the treatment of diseases caused by pathogenic free-living amoebae. PLOS Negl. Trop. Dis. 14, e0008353. 10.1371/journal.pntd.0008353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CA, Troth EV, Russell AC, Kyle DE, 2020b. Discovery of Anti-Amoebic Inhibitors from Screening the MMV Pandemic Response Box on Balamuthia mandrillaris, Naegleria fowleri, and Acanthamoeba castellanii. Pathogens 9. 10.3390/pathogens9060476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A, Carrion G, Rico-Gray V, 2001. Fungal latent pathogens and endophytes from leaves of Parthenium hysterophorus (Asteraceae). Fungal Divers. 7, 81–87. [Google Scholar]
- Seco JM, Quiñoá E, Riguera R, 2004. The Assignment of Absolute Configuration by NMR. Chemical Reviews 104, 17–118. 10.1021/cr000665j [DOI] [PubMed] [Google Scholar]
- Smith SG, Goodman JM, 2009. Assigning the Stereochemistry of Pairs of Diastereoisomers Using GIAO NMR Shift Calculation. J. Org. Chem. 74, 4597–4607. 10.1021/jo900408d [DOI] [PubMed] [Google Scholar]
- Smith SG, Goodman JM, 2010. Assigning Stereochemistry to Single Diastereoisomers by GIAO NMR Calculation: The DP4 Probability. J. Am. Chem. Soc. 132, 12946–12959. 10.1021/ja105035r [DOI] [PubMed] [Google Scholar]
- Srebro M, Govind N, de Jong WA, Autschbach J, 2011. Optical Rotation Calculated with Time-Dependent Density Functional Theory: The OR45 Benchmark. J. Phys. Chem. A 115, 10930–10949. 10.1021/jp2055409 [DOI] [PubMed] [Google Scholar]
- Stephens PJ, McCann DM, Devlin FJ, Smith AB, 2006. Determination of the Absolute Configurations of Natural Products via Density Functional Theory Calculations of Optical Rotation, Electronic Circular Dichroism, and Vibrational Circular Dichroism: The Cytotoxic Sesquiterpene Natural Products Quadrone, Suberosenone, Suberosanone, and Suberosenol A Acetate. J. Nat. Prod. 69, 1055–1064. 10.1021/np060112p [DOI] [PubMed] [Google Scholar]
- Stine CMA, 1929. Recovery of Bromine from Sea Water. J. Ind. Eng. Chem. 21, 434–442. 10.1021/ie50233a010 [DOI] [Google Scholar]
- Talavera G, Castresana J, 2007. Improvement of Phylogenies after Removing Divergent and Ambiguously Aligned Blocks from Protein Sequence Alignments. Syst. Biol. 56, 564–577. 10.1080/10635150701472164 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T, Hosoya T, 2015. Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud. Mycol. 82, 75–136. 10.1016/j.simyco.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DA, Zich DB, Ettefagh KA, Kavanaugh JS, Horswill AR, Cech NB, 2016. Hybrid Quadrupole-Orbitrap mass spectrometry for quantitative measurement of quorum sensing inhibition. Journal of Microbiological Methods 127, 89–94. 10.1016/j.mimet.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J, 1990. Amplification and direct sequencing of fungal ribosomal RNA genesis for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (Eds.), PCR Protocols. Academic Press, San Diego, pp. 315–322. [Google Scholar]
- Willoughby PH, Jansma MJ, Hoye TR, 2014. A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nat. Protoc. 9, 643–660. 10.1038/nprot.2014.042 [DOI] [PubMed] [Google Scholar]
- Willoughby PH, Jansma MJ, Hoye TR, 2020. Addendum: A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nat. Protoc. 15, 2277. 10.1038/s41596-020-0293-9 [DOI] [PubMed] [Google Scholar]
- Wu Y-H, Chen G-D, He R-R, Wang C-X, Hu D, Wang G-Q, Guo L-D, Yao X-S, Gao H, 2015a. Pericolactines A–C, a New Class of Diterpenoid Alkaloids with Unusual Tetracyclic Skeleton. Sci. Rep. 5, 17082. 10.1038/srep17082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-H, Xiao G-K, Chen G-D, Wang C-X, Hu D, Lian Y-Y, Lin F, Guo L-D, Yao X-S, Gao H, 2015b. Pericocins A–D, New Bioactive Compounds from Periconia sp. Nat. Prod. Commun. 10, 1934578X1501001228. 10.1177/1934578X1501001228 [DOI] [PubMed] [Google Scholar]
- Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT, 2020. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources 20, 348–355. 10.1111/1755-0998.13096 [DOI] [PubMed] [Google Scholar]
- Zhang D, Ge H, Xie D, Chen R, Zou J. h., Tao X, Dai J, 2013. Periconiasins A–C, New Cytotoxic Cytochalasans with an Unprecedented 9/6/5 Tricyclic Ring System from Endophytic Fungus Periconia sp. Org. Lett. 15, 1674–1677. 10.1021/ol400458n [DOI] [PubMed] [Google Scholar]
- Zhang D, Ge H, Zou J-H, Tao X, Chen R, Dai J, 2014. Periconianone A, a new 6/6/6 carbocyclic sesquiterpenoid from endophytic fungus Periconia sp. with neural anti-inflammatory activity. Org. Lett. 16, 1410–1413. 10.1021/ol500197x [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang W, Tan J, Kang F, Chen D, Xu K, Zou Z, 2021. Rhytidhyesters A – D, 4 New Chlorinated Cyclopentene Derivatives from the Endophytic Fungus Rhytidhysteron sp. BZM-9. Planta Med. 87, 489–497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.