Significance
Viruses are strong sources of natural selection pressure during human evolutionary history. Investigating genetic diversity and detecting signatures of natural selection at host genes related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection help to identify functionally important variation. We conducted a large study of global genomic variation at host genes that play a role in SARS-CoV-2 infection with a focus on underrepresented African populations. We identified nonsynonymous and regulatory variants at ACE2 that appear to be targets of recent natural selection in some African populations. We detected evidence of ancient adaptive evolution at TMPRSS2 in the human lineage. Genetic variants that are targets of natural selection are associated with clinical phenotypes common in patients with coronavirus disease 2019.
Keywords: SARS-CoV-2/COVID-19, genetic variation, phenotype association, natural selection, African diversity
Abstract
Human genomic diversity has been shaped by both ancient and ongoing challenges from viruses. The current coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a devastating impact on population health. However, genetic diversity and evolutionary forces impacting host genes related to SARS-CoV-2 infection are not well understood. We investigated global patterns of genetic variation and signatures of natural selection at host genes relevant to SARS-CoV-2 infection (angiotensin converting enzyme 2 [ACE2], transmembrane protease serine 2 [TMPRSS2], dipeptidyl peptidase 4 [DPP4], and lymphocyte antigen 6 complex locus E [LY6E]). We analyzed data from 2,012 ethnically diverse Africans and 15,977 individuals of European and African ancestry with electronic health records and integrated with global data from the 1000 Genomes Project. At ACE2, we identified 41 nonsynonymous variants that were rare in most populations, several of which impact protein function. However, three nonsynonymous variants (rs138390800, rs147311723, and rs145437639) were common among central African hunter-gatherers from Cameroon (minor allele frequency 0.083 to 0.164) and are on haplotypes that exhibit signatures of positive selection. We identify signatures of selection impacting variation at regulatory regions influencing ACE2 expression in multiple African populations. At TMPRSS2, we identified 13 amino acid changes that are adaptive and specific to the human lineage compared with the chimpanzee genome. Genetic variants that are targets of natural selection are associated with clinical phenotypes common in patients with COVID-19. Our study provides insights into global variation at host genes related to SARS-CoV-2 infection, which have been shaped by natural selection in some populations, possibly due to prior viral infections.
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Coronaviruses are enveloped, positive-sense, and single-stranded RNA viruses, many of which are zoonotic pathogens that crossed over into humans. Seven coronavirus species, including SARS-CoV-2, have been discovered that, depending on the virus and host physiological condition, may cause mild or lethal respiratory disease. There is considerable variation in disease prevalence and severity across populations and communities. Importantly, minority populations in the United States appear to have been disproportionally affected by COVID-19 (1, 2). For example, in Chicago, more than 50% of COVID-19 cases and nearly 70% of COVID-19 deaths are in African Americans (who make up 30% of the population of Chicago) (1). While social and economic factors are largely responsible for driving COVID-19 health disparities, investigating genetic diversity at host genes related to SARS-CoV-2 infection could help identify functionally important variation, which may play a role in individual risk for severe COVID-19 infection.
In this study, we focused on four key genes playing a role in SARS-CoV-2 infection (3). The ACE2 gene, encoding the angiotensin-converting enzyme-2 protein, was reported to be a main binding site for severe acute respiratory syndrome coronavirus (SARS-CoV) during an outbreak in 2003, and evidence showed stronger binding affinity to SARS-CoV-2, which enters the target cells via ACE2 receptors (3, 4). The ACE2 gene is located on the X chromosome (chrX); its expression level varies among populations (5); and it is ubiquitously expressed in the lung, blood vessels, gut, kidney, testis, and brain, all organs that appear to be affected as part of the COVID-19 clinical spectrum (6). SARS-CoV-2 infects cells through a membrane fusion mechanism, which in the case of SARS-CoV, is known to induce down-regulation of ACE2 (7). Such down-regulation has been shown to cause inefficient counteraction of angiotensin II effects, leading to enhanced pulmonary inflammation and intravascular coagulation (7). Additionally, altered expression of ACE2 has been associated with cardiovascular and cerebrovascular disease, which is highly relevant to COVID-19 as several cardiovascular conditions are associated with severe disease. TMPRSS2, located on the outer membrane of host target cells, binds to and cleaves ACE2, resulting in activation of spike proteins on the viral envelope and facilitating membrane fusion and endocytosis (8). Two additional genes, DPP4 and LY6E, have been shown to play an important role in the entry of SARS-CoV-2 virus into host cells. DPP4 is a known functional receptor for the Middle East respiratory syndrome coronavirus (MERS-CoV), causing a severe respiratory illness with high mortality (9, 10). LY6E encodes a glycosylphosphatidylinositol-anchored cell surface protein, which is a critical antiviral immune effector that controls coronavirus infection and pathogenesis (11). Mice lacking LY6E in hematopoietic cells were susceptible to murine coronavirus infection (11).
Previous studies of genetic diversity at ACE2 and TMPRSS2 in global human populations did not include an extensive set of African populations (5, 12–14). No common coding variants (defined here as minor allele frequency [MAF] > 0.05) at ACE2 were identified in any prior population studies. However, few studies included diverse indigenous African populations whose genomes harbor the greatest diversity among humans. This leads to a substantial disparity in the representation of African ancestries in human genetic studies of COVID-19, impeding health equity as the transferability of findings based on non-African ancestries to African populations can be low (15). Including more African populations in studying the genetic diversity of genes involved in SARS-CoV-2 infection is extremely necessary. Additionally, the evolutionary forces underlying global patterns of genetic diversity at host genes related to SARS-CoV-2 infection are not well understood. Using methods to detect natural selection signatures at host genes related to viral infections helps identify putatively functional variants that could play a role in disease risk.
We characterized genetic variation and studied natural selection signatures at ACE2, TMPRSS2, DPP4, and LY6E in ethnically diverse human populations by analyzing 2,012 genomes from ethnically diverse Africans (referred to as the “African diversity” dataset), 2,504 genomes from the 1000 Genomes Project (1KG), and whole-exome sequencing of 15,977 individuals of European ancestry (EA) and African ancestry from the Penn Medicine BioBank (PMBB) dataset (SI Appendix, Fig. S1). The African diversity dataset includes populations with diverse subsistence patterns (hunter-gatherers, pastoralists, agriculturalists) and speaking languages belonging to the four major language families in Africa (Khoesan; Niger–Congo, of which Bantu is the largest subfamily; Afroasiatic; and Nilo-Saharan). We identify functionally relevant variation, compare the patterns of variation across global populations, and provide insight into the evolutionary forces underlying these patterns of genetic variation. In addition, we perform an association study using the variants identified from whole-exome sequencing at the four genes and clinical traits derived from electronic health record (EHR) data linked to the subjects enrolled in the PMBB. The EHR data include diseases related to organ dysfunctions associated with severe COVID-19, such as respiratory, cardiovascular, liver, and renal complications. Our study of genetic variation in genes involved in SARS-CoV-2 infection provides data to investigate infection susceptibility within and between populations and indicates that variants in these genes may play a role in comorbidities relevant to COVID-19 severity.
Results
Coding Variation at ACE2 among Global Populations.
In total, we identified 41 amino acid changing variants (Fig. 1A and Dataset S1). Twenty-eight (69%), 20 (49%), 18 (44%), and 16 (40%) of the nonsynonymous variants were predicted to be deleterious or likely deleterious based on scores generated from Combined Annotation Dependent Depletion (CADD) (16), Sorting Intolerant From Tolerant (SIFT) (17), Polymorphism Phenotyping v2 (PolyPhen2) (18), and Consensus Deleteriousness Score of Missense Mutations (Condel) (19), respectively (Dataset S1).
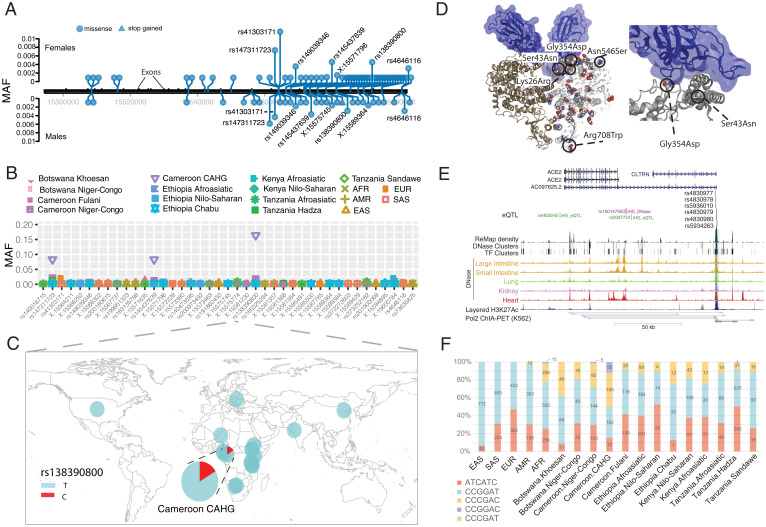
Fig. 1.
Genetic variation at ACE2. (A) Location of coding variants and their MAF at ACE2 identified from the pooled dataset. (B) MAF of coding variants in diverse global ethnic groups. (C) The geographic distribution of the MAF for rs138390800 at ACE2 in diverse global ethnic groups is highlighted. Each pie denotes frequencies of alleles in the corresponding population. (D) Locations of identified nonsynonymous variants within the secondary structure of the ACE2 protein. (E) Six regulatory eQTLs located in an upstream enhancer of ACE2. RNA Pol2 ChIA-PET data and DNase-seq data of the large intestine, small intestine, lung, kidney, and heart are from ENCODE (68). (F) Haplotype frequencies of the six eQTLs in global populations. The six eQTLs were ordered by their genomic position on the chromosome (i.e., with the following order: rs4830977, rs4830978, rs5936010, rs4830979, rs4830980, and rs5934263). Haplotypes with frequency <0.01 are not shown.
Among the 41 coding variants identified at ACE2, all are rare (MAF < 0.05) in the pooled global population dataset (Fig. 1A and Dataset S1). However, there are variants that are common (MAFs ≥ 0.05) in the central African hunter-gatherer (CAHG) population from Cameroon (often referred to as “pygmies”) (Fig. 1B). One of these variants, rs138390800 (Lys341Arg), is a deleterious nonsynonymous variant based on SIFT and CADD and present at high frequency (MAF = 0.164) in the CAHG, while it is rare in other African populations and absent in non-African populations (Fig. 1C). Two other nonsynonymous variants, rs147311723 (Leu731Phe; MAF = 0.083) and rs145437639 (Asp597Glu; MAF = 0.083), are also common only in the CAHG population (Fig. 1B and Dataset S1). These three nonsynonymous variants are the only common coding variants found at ACE2 in any of the populations examined.
We then investigated the potential role of these 41 coding variants in the conformation of the ACE2 protein. The 41 coding variants are distributed across the entire ACE2 protein (Fig. 1D and Dataset S1), including its receptor binding domain (RBD) region, which binds to the SARS-CoV-2 spike protein, dimerization interface, and transmembrane helix. In particular, two nonsynonymous variants Gly354Asp (chrX:15581230_C_T) and Ser43Asn (chrX:15600784_C_T) are both found directly in the RBD binding region of ACE2 (Fig. 1D and Dataset S1); the former is only found in low frequency in one population, the Fulani from Cameroon (MAF = 0.004), and the latter is also an African-specific variant that is at low frequency in only three East African populations, two of which are Afroasiatic-speaking populations from Kenya (MAF = 0.018) and Ethiopia (MAF = 0.008) (Dataset S1). The variant Arg708Trp (rs776995986) occurs in the region identified as the TMPRSS2 cleavage site in ACE2 (20) and is found only in the Afroasiatic-speaking populations from Ethiopia (MAF = 0.002). The presence of arginine residues has been shown to be important in “multibasic” cleavage sites (3). Therefore, due to the drastic change in physiochemical properties of the residue, this variation could be expected to interfere in TMPRSS2 cleavage efficiency, although it warrants experimental validation. Finally, two variants are located at glycosylation sites. Variant Asn546Ser (rs756905974), which causes the loss of a conserved glycosylation site on the ACE2 protein, is found only in the South Asian (SAS) populations (MAF = 0.001). Variant Lys26Arg (rs4646116), found in individuals from the European (EUR; MAF = 0.005), African (AFR; MAF = 0.001), and SAS (MAF = 0.002) populations from the 1KG dataset (Dataset S1), occurs near both the conserved ACE2 glycosylation site Asn90 and the RBD binding site. The modification to a similarly positively charged positive residue could suggest a role for electrostatic interactions, although no direct interference with RBD binding could be deduced without further studies.
Regulatory Variation at ACE2 among Global Populations.
In contrast to coding variants, which have direct effects on protein structure in all cells expressing a gene, the effects of regulatory genetic variants are relatively difficult to determine (21). We first extracted 2,053 expression quantitative trait loci (eQTLs) significantly associated with ACE2 gene expression (P < 0.001) from the Genotype-Tissue Expression (GTEx) project database (6) (Dataset S1). To narrow down candidate functional variants, we focus on the eQTLs located in the promoter regions of target genes or in enhancers supported by chromatin interaction data (22) (Dataset S1).
We identified six eQTLs (rs4830977, rs4830978, rs5936010, rs4830979, rs4830980, and rs5934263) located in a strong deoxyribonuclease (DNase) peak 73.3 kb upstream of ACE2 that have direct interactions with ACE2 based on RNA Pol2 chromatin interaction analysis by paired-end tag sequencing (ChIA-PET) data (Fig. 1E, Dataset S1, and SI Appendix, Fig. S2). All six single nucleotide polymorphisms (SNPs) are eQTLs of ACE2, and all of them have positive normalized effect sizes (NESs; NES > 0.2) and significant P values (P < 0.00008) in brain, tibial nerve, tibial artery, pituitary, and prostate cells (Dataset S1 and SI Appendix, Fig. S3). In non-African populations, these six eQTLs are in high linkage disequilibrium (LD; R2 = 0.91 to 1.0) (SI Appendix, Fig. S4), and thus, there are two common haplotypes: “CCGGAT” and “ATCATC.” The frequency for the ATCATC haplotype ranges from 0.31 to 0.47 in all populations except the East Asian population, which has a frequency of 0.068 at all six SNPs (Fig. 1F). In African populations, LD is lower (R2 > 0.5) (SI Appendix, Fig. S4), and there are three common haplotypes: CCGGAT (0.564), ATCATC (0.308), and “CCCGAC” (0.116). Of note, every allele in the haplotype CCGGAT is correlated with higher expression of ACE2 in the cortex of the brain, while alleles in haplotype ATCATC are correlated with lower expression of ACE2; other haplotypes have alleles with both positive and negative effect sizes in different tissues (Dataset S1). Haplotype CCCGAC is only present in populations with African ancestry, and its frequency is highest in the Botswana Khoesan (0.38) and Cameroon CAHG (0.38) hunter-gatherer populations. We also identified one variant (rs186029035) located in a strong transcription factor (TF) binding and DNase region (based on the Encyclopedia of DNA Elements, ENCODE) in the 16th intron of ACE2. This variant is only common in the Cameroon CAHG population, and therefore, there are no eQTL data for this SNP in the GTEx database (MAF = 0.153) (Dataset S1).
Signatures of Natural Selection at ACE2.
As indicated above, most of the nonsynonymous variants at ACE2 are rare in global populations, and many of them are predicted to be deleterious, indicating that this gene is under strong purifying selection. To formally test for signatures of natural selection at ACE2, we first examined the ratio of nonsynonymous and synonymous variants at each gene using the the ratio of nonsynonymous to synonymous substitutions (dN/dS) test (23) (Materials and Methods). The dN/dS for all pooled samples was 0.77, indicating that ACE2 is under moderate purifying selection globally (Dataset S2 and SI Appendix, Fig. S5). However, in the East Asian population, we observed seven nonsynonymous variants (all of them are rare) and only one synonymous variant, and the dN/dS value is 1.85, indicating an excess of nonsynonymous variation. In other populations, the dN/dS ratio ranges from 0 to 0.79 (Dataset S2 and SI Appendix, Fig. S5). Thus, ACE2 appears to be under strong purifying selection in most populations but may be under weak purifying selection in the East Asian population. We next applied the McDonald–Kreitman (MK) test (24), which compares the ratio of fixed nonsynonymous sites between humans and chimpanzee (Dn = 8) and fixed synonymous sites (Ds = 6) with the ratio of polymorphic nonsynonymous sites among populations (Pn = 41) relative to polymorphic synonymous sites (Ps =14), and found that it is not significant (odds ratio [OR] = 0.45, P = 0.94, two-sided Fisher’s exact test) (Dataset S3 and SI Appendix, Figs. S6 and S7).
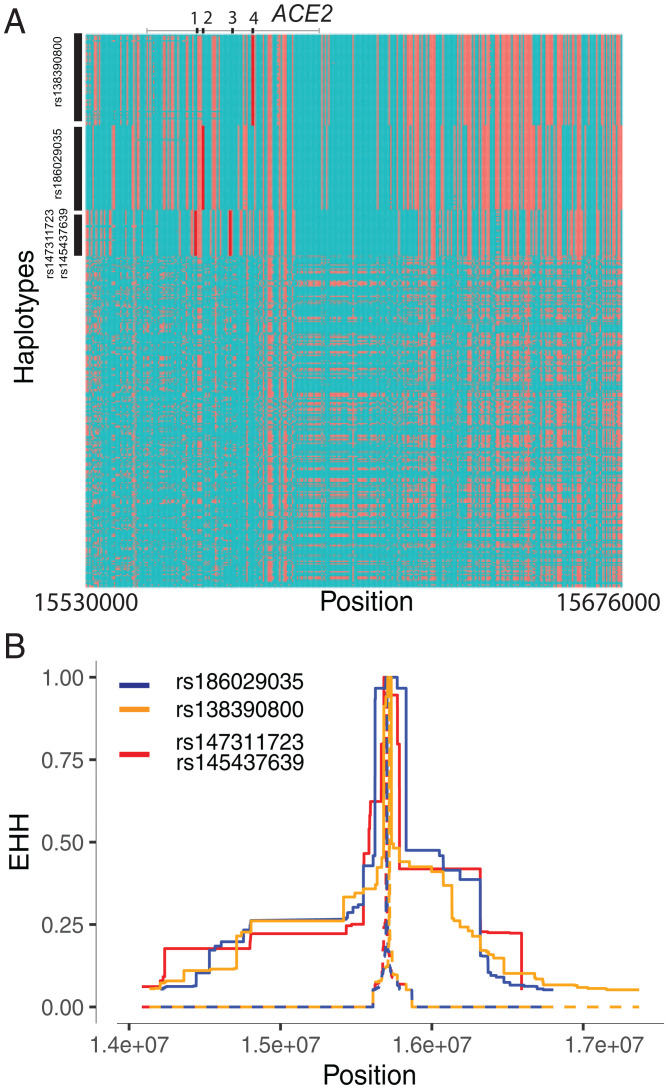
Because the above-mentioned methods are more suitable for detecting signals of natural selection acting over long timescales (25, 26), we then tested for signatures of recent positive selection at ACE2 in global populations using the integrated haplotype score (iHS) test (27) to detect extended haplotype homozygosity (EHH) (28), which identifies regions of extended LD surrounding a positively selected locus. We first focused on the three common nonsynonymous variants in the CAHG population from Cameroon (rs138390800, rs147311723, and rs145437639; MAF = 0.083 to 0.164) and a common putative regulatory variant (rs186029035, located in TF and DNase regions in the 16th intron of ACE2; MAF = 0.153). The derived alleles of these variants exist on three different haplotype backgrounds; rs147311723 and rs145437639 are on the same haplotype backgrounds, while rs138390800 and rs186029035 are on similar but distinct haplotype backgrounds (Fig. 2A). The derived alleles of the corresponding SNPs on each haplotype background show EHH extending longer than 2 Mb, while the ancestral alleles of these SNPs harbor haplotypes extending less than 0.3 Mb (Fig. 2B). We then calculated the iHS of each of these variants to determine whether these extended haplotypes are unusually long compared with other SNPs with a similar allele frequency; the iHS values were not significant for any of these variants (iHS values for rs138390800, rs147311723, and rs145437639 in CAHG were 0.5, −0.08, and −0.55, respectively) (Dataset S4 and SI Appendix, Fig. S8). However, if selection was acting on multiple haplotypes simultaneously, the EHH and iHS tests would not be well powered to detect selection (29). We also used the di statistic (30) to measure if allele frequencies at these candidate SNPs were significantly differentiated between Cameroon CAHG and other populations. The di values of SNPs rs138390800 and rs186029035 were in the top 1.4% and 1.7%, respectively, of di values for all SNPs examined, indicating that that allele frequencies at these variants are among the most highly differentiated in the CAHG population, consistent with local adaptation. However, it should be noted that in the CAHG, these four variants (rs138390800, rs147311723, rs145437639, and rs186029035) are in complete LD based on D’ (D’ = 1) with the six eQTLs described above (SI Appendix, Figs. S9 and S10), indicating that the alleles are on the same haplotype background. Thus, it is not possible to distinguish if the nonsynonymous variants are targets of selection or if they are “hitchhiking” to high frequency due to selection on flanking regulatory variants. Given the high LD in the region, it is possible that multiple functional variants on the same haplotype backgrounds have been under selection.
Fig. 2.
Natural selection signatures at ACE2 in the Cameroon CAHG populations. (A) Haplotypes over 150 kb flanking ACE2 in CAHG populations. The x axis denotes the genetic variant position, and the y axis represents haplotypes. Each haplotype (one horizontal line) is composed of the genetic variants (columns). Red dots indicate the derived allele, while green dots indicate the ancestral allele. Haplotypes surrounded by the upper left vertical black lines suggest that these haplotypes carry derived allele(s) of the labeled variant near the corresponding black line. For example, the first black line denotes all the haplotypes that have the derived allele at rs138390800 (dark red line). Haplotypes carrying rs138390800, rs147311723, rs145437639, and rs186029035 show more homozygosity than other haplotypes; 1, 2, 3, and 4 along the top of the plot denote positions for rs147311723, rs186029035, rs145437639, and rs138390800, respectively. (B) EHH of rs138390800, rs186029035, and rs147311723 (rs145437639 is in strong LD with rs147311723) at ACE2 in CAHG populations.
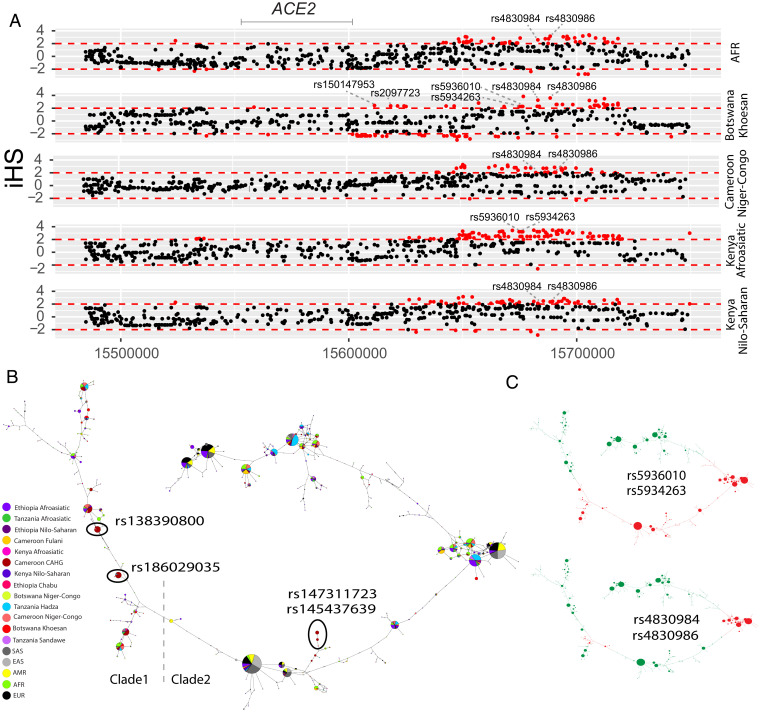
We then investigated signatures of recent positive selection at candidate regulatory variants near ACE2 in the global datasets. In total, there are 234 variants that had high iHS scores (|iHS| > 2) in at least one population extending over an ∼200-kb region (Dataset S4), and 48% (n = 113) of these variants are either eQTLs or located at DNase hypersensitive regions, which are in high LD based on D’ (Dataset S4 and SI Appendix, Figs. S9 and S10). Among the region near the transcription start site (TSS) (<10 kb from ACE2), there are two variants in high LD (D’ = 1) that had high iHS scores in the San population from Botswana (Fig. 3A); rs150147953 is located in a DNase peak in multiple tissues, including lung, intestine, and heart, and rs2097723 is an eQTL of ACE2 in the brain (Fig. 1E, Dataset S4, and SI Appendix, Fig. S11). We also identified high iHS signals at the region 50 to 120 kb upstream of ACE2 (chrX:15650000-15720000) in the AFR 1KG population, the San from Botswana, and Niger–Congo-speaking populations from Cameroon as well as Afroasiatic- and Nilo-Saharan–speaking populations from Kenya (Fig. 3A and SI Appendix, Fig. S7). Two SNPs in this region (rs5936010 and rs5934263) have elevated iHS scores (|iHS| > 2) in the San population from Botswana and the Afroasiatic population from Kenya (Fig. 3A) and are part of the six eQTLs described above, located within a strong enhancer interacting with the promoter of ACE2 (Fig. 1E). Two additional eQTLs that are in complete LD with the six eQTLs (D’ = 1) (SI Appendix, Fig. S10), rs4830984 and rs4830986, had high iHS scores in four of the five African populations listed above (all but Kenya Afroasiatic) (Fig. 3A).
Fig. 3.
Natural selection signatures at the upstream region of ACE2 in African populations. (A) iHS signals at the upstream region of ACE2 (chrX:15650000-15720000) in African populations. Each dot represents a SNP. Red dots denote SNPs that are significant (|iHS| > 2). The gray solid lines denote the gene body region of ACE2. Putatively causal tag SNPs are annotated in the plots. (B) Haplotype network over 150 kb flanking ACE2 in diverse ethnic populations. The network was constructed with SNPs that showed iHS signals in all populations and overlapped with DNase regions or eQTLs. The four functional candidates identified in Cameroon CAHG were also included in the networks. Each pie represents a haplotype, each color represents a geographical population, and the size of the pie is proportional to that haplotype frequency. The dashed line denotes the boundary of clade 1 and clade 2. Black ovals denote haplotypes containing the corresponding variants. (C) Haplotypes containing variants rs5936010, rs5934263, rs4830984, and rs4830986 are highlighted. Red pies denote haplotypes containing the derived allele of the corresponding variants, while green pies denote haplotypes containing the ancestral allele of the corresponding variants.
We performed haplotype network analysis to examine phylogenetic relationships among haplotypes at ACE2 in global populations for SNPs showing signatures of natural selection (Fig. 3 B and C). We identified two haplotype clades; one (clade 1) is nearly specific to Africans, and the other (clade 2) encompasses global populations (Fig. 3B). In the CAHG, haplotypes containing the rs138390800 (Lys341Arg) nonsynonymous variant and the rs186029035 regulatory variant are in clade 1, whereas haplotypes containing the rs147311723 (Leu731Phe) and rs145437639 (Asp597Glu) nonsynonymous variants are located in clade 2 (Fig. 3B). Haplotypes containing the two regulatory variants (rs5936010 and rs5934263) located 50 to 120 kb upstream of ACE2 are shared in global populations, and the nearby regulatory variants rs4830984 and rs4830986 are sublineages on those haplotype backgrounds (Fig. 3 B and C).
Genetic Variation at TMPRSS2 among Global Populations.
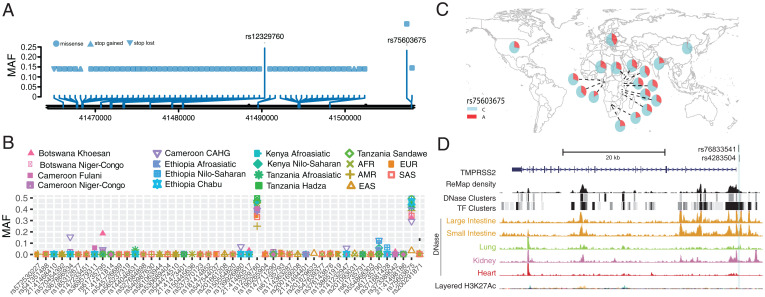
The TMPRSS2 protein enhances the spike protein–driven viral entry of SARS-CoV-2 into cells (3). At this gene, we identified 48 nonsynonymous variants. Among the nonsynonymous variants, only two (rs12329760 [Val197Met] and rs75603675 [Gly8Val]) have high MAF (>0.05) in the pooled global dataset (Fig. 4A and Dataset S1). While rs75603675 is highly variable in non–East Asian populations (AFR = 0.3, AMR = 0.27, EUR = 0.4, and SAS = 0.2), it is not highly variable in East Asians (MAF = 0.02) (Fig. 4 B and C and Dataset S1). In addition, some nonsynonymous variants were common and specific to African populations. Notably, the nonsynonymous variant rs61735795 (Pro375Ser) had a high MAF in the Khoesan-speaking population from Botswana (MAF = 0.18). This variant is present at low frequency in populations from Cameroon (MAF < 0.01) and Ethiopia (MAF < 0.03) and was absent in non-African populations. The nonsynonymous variant rs367866934 (Leu403Phe) is common in the Cameroonian CAHG population (MAF = 0.15) and has low frequency (MAF = 0.02) in other populations from Cameroon, but it is absent from non-Cameroonian populations (Fig. 4B and Dataset S1). Another nonsynonymous variant rs61735790 (His18Arg) is common in the CAHG populations from Cameroon (MAF = 0.12) and the Nilo-Saharan populations from Ethiopia (MAF = 0.12) but is rare in other populations (Fig. 4B and Dataset S1).
Fig. 4.
Genetic variation at TMPRSS2. (A) Location of coding variants and their MAF at TMPRSS2 identified from the pooled dataset. (B) MAF of coding variants in diverse global ethnic groups. (C) The geographic distribution of the MAF for rs75603675 at TMPRSS2 in diverse global ethnic groups is highlighted. Each pie denotes frequencies of alleles in the corresponding population. (D) Two regulatory eQTLs located in the promoter region of the TMPRSS2 gene. DNase-seq data of the large intestine, small intestine, lung, kidney, and heart are from ENCODE (68).
We identified two regulatory SNPs (rs76833541 and rs4283504) in the promoter region of the TMPRSS2 gene that have been identified as eQTLs of TMPRSS2 in testis (Fig. 4D, Dataset S1, and SI Appendix, Fig. S12). The MAF of rs76833541 is higher in EUR (MAF = 0.16) than other populations (EAS = 0.002, AFR = 0.006, AMR = 0.06, and SAS = 0.05), and the MAF of rs4283504 is more common in EAS (MAF = 0.21) than other populations (EUR = 0.11, AFR = 0.04, AMR = 0.12, and SAS = 0.14) (Dataset S1 and SI Appendix, Fig. S13).
Signatures of Natural Selection at TMPRSS2.
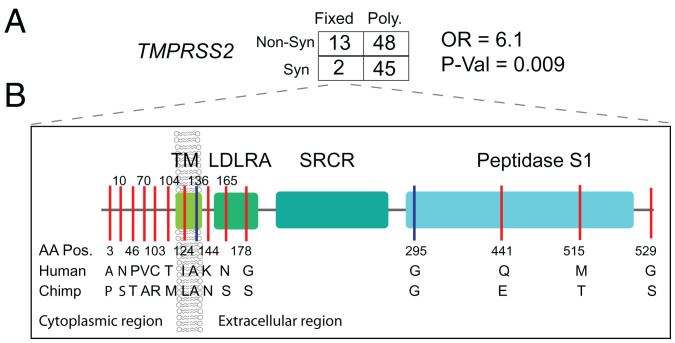
We applied the MK test at TMPRSS2 and observed that Dn/Ds (13/2) is significantly larger than Pn/Ps (48/45) among pooled human samples (OR = 6.1, P value = 0.009, Fisher’s exact test) (Fig. 5A and Dataset S3) as well as in individual ethnic groups (OR ranged from 5.0 to 17), indicating positive selection in the hominin lineage after divergence from chimpanzee. Notably, there are 13 nonsynonymous and 2 synonymous variants at TMPRSS2 (ENST00000398585.7) (SI Appendix, Fig. S14 shows ENST00000332149.10) that were fixed in human populations. The nonsynonymous variants are located in different structural domains of TMPRSS2. Amino acids A3P, N10S, T46P, A70V, R103C, and M104T are located in the cytoplasmic region, which may function in intracellular signal transduction (31). L124I is located in the transmembrane region, N144K is located in the extracellular region, S165N and S178G are located in the low-density lipoprotein (LDL) receptor class A domain, E441Q and T515M are located in the serine peptidase (Peptidase S1) domain that is involved in the interaction with the SARS-CoV-2 spike protein (3), and S529G is located in the last amino acid position of the protein (Fig. 5B). In contrast to the MK test, the dN/dS ratio test was not significant in any population, indicating no excess of nonsynonymous to synonymous variation within populations (Dataset S2 and SI Appendix, Fig. S5).
Fig. 5.
Natural selection signatures at TMPRSS2. (A) The result of the MK test for TMPRSS2 in the pooled dataset. Nonsyn indicates nonsynonymous variants; Syn indicates synonymous variants. “Fixed” denotes variants that were fixed between the human and the chimpanzee; “Poly” represents polymorphic variants within human populations. The transcript ENST00000398585.7 was used for the calculation. (B) Illustration of locations of variants that are divergent between the human and chimpanzee lineages on the TMPRSS2 protein domains. Boxes denote the protein domains of TMPRSS2. Red lines represent nonsynonymous variants that occurred in the corresponding domains of TMPRSS2, with the amino acids and positions of the human and the chimpanzee annotated at the bottom of the lines. Blue lines denote synonymous variants. LDLRA, LDL receptor class A; SRCR, scavenger receptor cysteine-rich domain 2; TM, transmembrane domain.
We also tested for recent positive selection at TMPRSS2 in all ethnic groups using iHS (Dataset S4 and SI Appendix, Fig. S15). We found many SNPs (n = 153) with high iHS scores (|iHS| > 2) in different ethnic groups in a 78-kb region encompassing the TMPRSS2 gene that show high levels of LD (chrX:41454000-41541000) (SI Appendix, Figs. S16 and S17). We identified a nonsynonymous variant (rs150969307) that shows a signature of positive selection (iHS = 2.01) and is common only in the Chabu hunter-gatherer population from Ethiopia (MAF = 0.079) (Dataset S4). We found that more than one-third of SNPs with |iHS| scores >2.0 (62 of 153) are located in putative regulatory regions (Dataset S4 and SI Appendix, Fig. S18).
Genetic Variation and Signatures of Natural Selection at DPP4 and LY6E.
DPP4 is a receptor for MERS-CoV and was reported to interact with SARS-CoV-2 (10). At this gene, we identified 47 nonsynonymous variants and 1 loss-of-function variant (Dataset S1). There were no common nonsynonymous variants in the pooled global dataset (SI Appendix, Fig. S19A), suggesting that this gene is extremely conserved during human evolutionary history. Only one nonsynonymous variant (rs1129599, Ser437Thr) was common in the Fulani pastoralists from Cameroon (MAF = 0.081), was present at low frequency in other African populations, and was absent in non-African populations (SI Appendix, Fig. S19 B and C). In addition to the nonsynonymous variants, one loss-of-function variant was identified at DPP4. The variant rs149291595 (Q170*) has low MAF in some African populations (MAF < 0.05) but is absent in non-African populations.
We identified four eQTLs (rs1861978, rs35128070, rs17574, and rs13015258) in the promoter region of the DPP4 gene (SI Appendix, Figs. S19D and S20). Three of the variants (rs1861978, rs35128070, and rs17574) are significant eQTLs in the transverse colon, and rs13015258 is an eQTL in the lung (P < 5.9e-6) (Dataset S1 and SI Appendix, Fig. S19). The minor alleles of these three variants are rare in EAS (MAF < 0.05) but common in all other populations (MAF > 0.15) (Dataset S1 and SI Appendix, Fig. S21). The fourth SNP, rs13015258, resides in the center of a cluster of DNase peaks identified in ENCODE (SI Appendix, Fig. S19D), with MAF ranging from 0.38 in the AMR population to 0.6 in other populations (Dataset S1 and SI Appendix, Fig. S21).
The MK test result of DPP4 was not significant in either the pooled samples (Dn = 3, Ds = 5, Pn = 45, Ps = 33; OR = 0.44, P = 0.9, two-sided Fisher’s exact test) or each population separately (Dataset S3 and SI Appendix, Fig. S7). For the dN/dS test, we observed ratios ranging from 0 to 0.52 in individual populations, indicating that DPP4 is highly conserved (Dataset S2 and SI Appendix, Fig. S5) within human populations. Using the iHS test, we identified eight SNPs that had extremely high iHS scores (|iHS| > 2) in the Khoesan populations from Botswana (Dataset S4 and SI Appendix, Fig. S22). Five of these SNPs (rs10166124, rs2284872, rs2284870, rs7608798, and rs2160927) are in LD (D’ > 0.95) with each other (SI Appendix, Fig. S23). The SNP rs2284870 is located in a strong DNase peak in heart tissue (Dataset S4 and SI Appendix, Fig. S24).
Studies show that mice lacking LY6E were highly susceptible to a usually nonlethal mouse coronavirus (11). At LY6E, we observed 28 nonsynonymous variants, and all of them, except rs11547127 (MAF = 0.057), have MAFs that are rare in the pooled global dataset (Dataset S1 and SI Appendix, Fig. S25A). However, some nonsynonymous variants are common in specific populations (SI Appendix, Fig. S25B). For instance, the nonsynonymous variant rs111560737 (Asp104Asn) was common in the southern African Khoesan population from Botswana (MAF = 0.36) and the Chabu population from Ethiopia (MAF = 0.17) (SI Appendix, Fig. S25C). Three loss-of-function variants (rs200177123 [stop gained, Ser59*], chr8:143020941, and chr8:143020946) were also identified at LY6E, and all of them are rare.
We identified three regulatory eQTLs (rs13252864, rs17061979, and rs114909654) located within 2 kb of the transcription start site of LY6E (SI Appendix, Fig. S25D), all of which are significant in esophageal mucosa (P < 1e-5) (Dataset S1 and SI Appendix, Fig. S26), which has a high expression level of LY6E (transcript per million, TPM = 108, GTEx). The minor alleles of rs13252864 and rs114909654 are common in African populations (MAF > 0.15) while very rare in other populations (MAF < 0.02) (SI Appendix, Fig. S27), whereas the MAF of rs17061979 is relatively high in EAS (0.18) and SAS (0.13) and rare in other populations (MAF < 0.05) (SI Appendix, Fig. S27).
The MK test for LY6E was not significant in either the pooled samples (Dn = 0, Ds = 4, Pn = 9, Ps = 9; OR = 0, P = 0.9, two-sided Fisher’s exact test) or each population separately (OR ranging from 0 to 0.52) (Dataset S3 and SI Appendix, Fig. S7). For the dN/dS test, we observed ratios ranging from 0 to 0.68 in individual populations, indicating that LY6E is highly conserved (Dataset S2 and SI Appendix, Fig. S5). We identified 19 variants that had extreme high iHS scores (|iHS| > 2) (Dataset S4 and SI Appendix, Fig. S28), some of which are in LD in specific populations (SI Appendix, Fig. S29). One variant (rs867069115) shows an extreme iHS score in the Hadza hunter-gatherer population from Tanzania (iHS = −2.94). This variant is located in a regulatory region ∼1.9 kb downstream of LY6E within DNase and TF peaks in the lung, intestine, kidney, heart, stomach, pancreas, and skeletal muscle from ENCODE (SI Appendix, Fig. S30); is common only in the Hadza population (MAF = 0.14); is rare in other African populations (MAF < 0.05); and is absent in all non-African populations (Dataset S1). SNP rs10283236, which shows an extreme iHS value in the CEU population, is an eQTL of LY6E located within DNase and TF clusters identified in ENCODE (∼4.14 kb downstream of LY6E) active in many tissues, including lung, kidney, and small intestine.
Associations between Genetic Variation in Host Genes and Clinical Disease Phenotypes.
We examined associations of genetic variation at four host genes relevant to SARS-CoV-2 infection with clinical phenotypes using the PMBB cohort that consists of exome-sequencing data from 15,977 participants between the ages of 19 and 89 y (52% female) with extensive clinical data available through their EHRs. Of these, 7,061 individuals were of European ancestry (EA) (42%), and 8,916 were of African ancestry (AA) (55%) (SI Appendix, Table S1).
Gene burden phenome-wide association study with clinical phenotypes.
To test for the association between rare coding variants and clinical phenotypes, we applied a gene-based approach (32, 33) as well as single-variant analysis. First, we performed a gene-based analysis by collapsing the coding region variants with MAF < 0.01 that are annotated as nonsynonymous or putative loss-of-function (pLOF) variants. We examined ∼1,800 phecodes derived from the EHR and performed a phenome-wide association study (PheWAS) with individual SNPs (32–34). After multiple testing correction, we identified one association in the AA population and five associations in the EA population reaching study-wide significance (P = 6.6 × 10−6 [0.05/(1,866 codes × 4 genes)]) (Dataset S5). Myocarditis, a rare cardiovascular disease caused by viral infection, was the top PheWAS association with ACE2 in the AA population but was not significant in the EA population. Although the population difference for this specific association is unclear, recent studies have reported that COVID-19 patients have a 16 times higher risk of myocarditis (35). Furthermore, ACE2 is expressed in heart tissue and its upregulation in cardiomyocites has an important role in both dilated cardiomyopathy and hypertrophic cardiomyopathy (36–38).
Gene burden association analyses with 12 COVID-19–relevant organ dysfunctions.
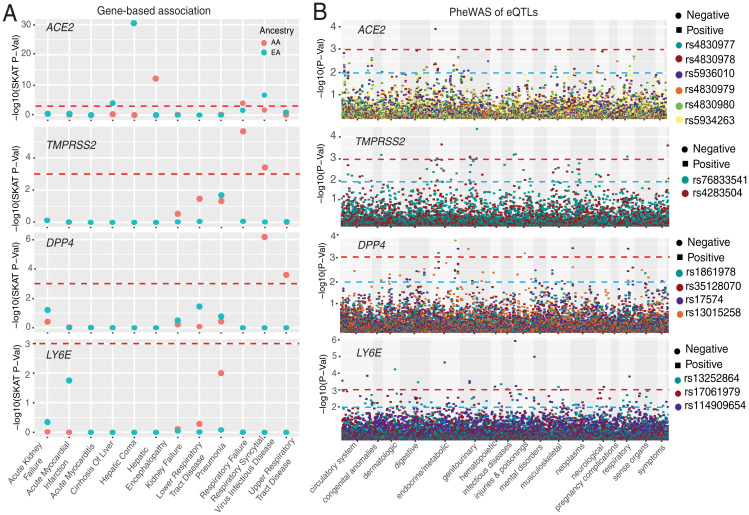
We tested for the association of rare coding variants with 12 phenotypes, encompassing COVID-19–relevant disease classes affecting different organ systems, defined by EHR-based diagnosis codes (Dataset S5). In the AA population, the phenotypes with the most significant associations with ACE2 were hepatic encephalopathy and respiratory failure (Fig. 6A and Table 1). The association with respiratory failure is interesting as it is one of the key severe clinical features reported for COVID-19 (39–43). However, the same association was not significant in the EA population, which could be explained by lack of power due to a lower number of coding variants at ACE2 in EA. Within the EA population, the most significant associations with ACE2 included hepatic coma, respiratory syncytial virus infectious disease, and cirrhosis of the liver (Table 1). In the gene-based analysis of variants in DPP4, we identified significant associations (only in the sequence kernel association test [SKAT] model) with respiratory syncytial virus infectious disease and upper respiratory tract disease in the AA population (Fig. 6A, Table 1, and Dataset S5); this observation was not observed in the EA population. None of the associations with DPP4, TMPRSS2, and LY6E reach statistical significance in gene burden analysis.
Fig. 6.
Associations between genetic variations at four genes and clinical disease phenotypes. (A) Gene-based association result between coding variants at four genes and 12 disease classes. The disease classes are shown on the x axis, and the y axis represents the P values. (B) PheWAS plot of the eQTLs associated with four genes and ∼1,800 disease codes across 17 disease categories. The disease categories are shown on the x axis, and the y axis represents the −log10 of the P values. The colored dots represent eQTLs and the direction of effect of the association. The red dashed lines denote the 0.0001 cutoff, and the blue dashed lines represent the 0.001 cutoff.
Table 1.
Associations of ACE2, DPP4, TMPRSS2, and LY6E with 12 disease classes derived from EHR data
| Disease phenotype | Gene | Cases | Controls | Carrier controls | Carrier cases | SKAT P | Burden P | Burden OR | Burden SE | 95% CI | Dataset |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatic encephalopathy | ACE2 | 97 | 8,045 | 441 | 5 | 1.1E-12 | 0.0043 | 5.73 | 0.61 | 0.55–2.94 | AA |
| Respiratory syncytial virus infectious disease | DPP4 | 56 | 6,392 | 85 | 1 | 6.8E-07 | 0.1221 | 6.06 | 1.17 | −0.48–4.09 | AA |
| Respiratory failure | TMPRSS2 | 199 | 6,392 | 11 | 2 | 2.3E-06 | 0.0124 | 7.31 | 0.80 | 0.43–3.55 | AA |
| Respiratory failure | ACE2 | 199 | 6,392 | 351 | 12 | 9.0E-05 | 0.0509 | 3.10 | 0.58 | 0–2.26 | AA |
| Upper respiratory tract disease | DPP4 | 144 | 6,392 | 85 | 3 | 2.5E-04 | 0.0978 | 4.16 | 0.86 | −0.26–3.11 | AA |
| Respiratory syncytial virus infectious disease | TMPRSS2 | 56 | 6,392 | 11 | 1 | 3.9E-04 | 0.0217 | 11.63 | 1.07 | 0.36–4.55 | AA |
| Hepatic coma | ACE2 | 16 | 6,817 | 318 | 1 | 4.3E-31 | 0.0019 | 10.45 | 0.76 | 0.87–3.83 | EA |
| Respiratory syncytial virus infectious disease | ACE2 | 40 | 5,859 | 274 | 3 | 2.3E-07 | 0.1650 | 3.61 | 0.92 | −0.53–3.1 | EA |
| Cirrhosis of liver | ACE2 | 10 | 6,817 | 43 | 1 | 1.8E-04 | 0.0837 | 9.40 | 1.30 | −0.3–4.78 | EA |
PheWAS of eQTLs near COVID-19 host immunity-related genes.
Lastly, we conducted PheWAS of eQTLs identified near the host genes. For the six eQTLs identified near ACE2, rs5936010 and rs5934263 (targets of positive selection in both Afroasiatic populations from Kenya and Khoesan populations from Botswana) were significantly associated with type 2 diabetes (P = 1.23 × 10−4, OR = 1.1) and hypertension (P = 8.8 × 10−4, OR = 1.13), respectively. The association was only observed in the AA population (Fig. 6B and Dataset S6). The PheWAS of the two regulatory eQTLs (rs76833541 and rs4283504) near TMPRSS2 described above identified association of rs76833541 with abnormal glucose (P = 8.9 × 10−4, OR = 1.5) in EA and rs4283504 with glucocorticoid deficiency (P = 0.001, OR = 2.7) in AA (Fig. 6B). The PheWAS of four regulatory eQTLs near DPP4 identified the association with malignant neoplasm of the rectum (commonly referred as colon cancer). The SNP rs17574 was associated with increased risk (P = 4.49 × 10−04, OR = 1.8) of colon cancer; however, the observation was only observed among AA individuals. The association analysis of regulatory variants near LY6E identified significant associations with “severe protein-calorie malnutrition” (rs114909654, P = 2.35 × 10−05, OR = 1.9) and “acute post hemorrhagic anemia” (rs114909654, P = 6.4 × 10−04, OR = 1.6) in the AA population. In the EA population, “chronic ulcer of skin” with rs13252864 (P = 0.001, OR = 2.2) was the most significant association (Fig. 6B and Dataset S6). Among EHR-derived phenotypes for respiratory disorders, the rs35128070 eQTL near DPP4 was associated with “abnormal results of function study of pulmonary system” (P = 0.002, OR = 1.6) in the AA population (Fig. 6B and Dataset S6).
Discussion
Investigating global patterns of genetic variation at genes that play a role in SARS-CoV-2 infection could provide insights into potential differences in susceptibility to COVID-19 among diverse human populations. However, African populations are underrepresented in the majority of current genetic studies of COVID-19 susceptibility and severity, despite the fact that they have the highest genetic diversity among human populations (44, 45) and have high burdens of infectious disease (44, 45).
Three Nonsynonymous Variants That Are Common and Specific to CAHG at ACE2 Are on Haplotypes with Signatures of Positive Selection.
Several studies have investigated patterns of genetic variation at ACE2, a receptor of SARS-CoV-2 entry (5, 12–14). None of these studies identified any common coding variation at ACE2, suggesting that ACE2 is evolutionarily conserved. However, these studies did not include an extensive set of African populations. At ACE2, we identified 41 nonsynonymous variants, most of which are rare, suggesting that they are under purifying selection. Tests based on dN/dS indicate that East Asians have an excess of nonsynonymous variation at ACE2, indicating that weak purifying selection has influenced patterns of variation in that population. However, we identified three common nonsynonymous variants (rs138390800, rs147311723, and rs145437639) at ACE2 with MAF ranging from 0.083 to 0.164 in CAHGs, which were the only common coding variants (defined here as MAF > 0.05) found in global populations studied here and by others (5, 12–14). We observed that the derived alleles of the common nonsynonymous SNPs (rs138390800, rs147311723, rs145437639) and one putative regulatory variant (rs186029035) at ACE2 in CAHG show evidence of EHH, with the extended haplotypes extending longer than 2 Mb, although they did not show deviation from neutrality based on the iHS test. However, we do not have much power to detect a selection signal using this test because the SNPs are on three different haplotype backgrounds in CAHG, possibly due to selection on existing variation (e.g., “soft selection”), which decreases the power to detect significant iHS scores. Moreover, each haplotype is at a relatively low frequency (0.083 to 0.164), which further reduces the power of the iHS test. Allele frequencies at two of the putative functional variants are among the most highly differentiated between the CAHG population and other populations, consistent with local adaptation, as indicated by the di values of SNPs rs138390800 and rs186029035, which were in the top 1.4 and 1.7%, respectively, of di values for all SNPs examined. The CAHGs are traditionally hunter-gatherers living in a rainforest ecosystem who consume wild animals. They have high exposure to animal viruses and were reported to have relative resistance to viral infection (46). Thus, it is possible that this locus is adaptive for protection from infectious diseases in this population. Future in vitro or in vivo studies will be needed to determine the functional significance of these variants.
TMPRSS2 Shows Adaptive Evolution in the Human Lineage after Divergence from Chimpanzee.
At TMPRSS2, we identified 48 nonsynonymous variants, only 2 of which had a high MAF (>0.05) in the pooled global dataset (rs12329760 and rs75603675). However, some variants have high MAF in two African hunter-gatherer populations. Notably, the nonsynonymous variant rs61735795 (Pro375Se) is only common in the Khoesan-speaking San population from Botswana (MAF = 0.18), and the nonsynonymous variant rs367866934 (Leu403Phe) is only common in the Cameroonian CAHG populations (MAF = 0.15). At TMPRSS2, we observed a signature of adaptive evolution in the human lineage after divergence from chimpanzee ∼6 Mya (47). In total, 13 nonsynonymous variants located on different structural domains of TMPRSS2 were fixed in human populations. Among them, E441Q and T515M are located in the Peptidase S1 domain that plays an important role in acute respiratory syndrome–like (SARS) coronavirus (SARS-CoV-2) infection (48), and six (A3P, N10S, T46P, A70V, R103C, and M104T) are at the cytoplasmic amino terminal domains of TMPRSS2, which plays an important role in signal transduction. By contrast, the coding regions of DPP4 and LY6E are evolutionarily conserved.
eQTLs for Four Genes Vary in Frequency among Populations and Show Signatures of Natural Selection and Associations with Clinical Phenotypes.
SARS-CoV replication is significantly reduced in ACE2 knockout mice (49), and cells with low expression of ACE2 were resistant to SARS-CoV-2 infection (50). It has also been shown that both SARS-CoV and SARS-CoV-2 infection could down-regulate ACE2 expression (8, 49, 51). The expression of ACE2 and TMPRSS2 in nasal and bronchial epithelial cells is higher in adults than children and in healthy individuals compared with smokers or patients with chronic obstructive pulmonary disease (51, 52). Therefore, differences in expression levels of ACE2 and TMPRSS2 could influence the susceptibility and host reactions to SARS-CoV-2. Previous studies identified eQTLs influencing ACE2 gene expression, showing differences in allele frequency among different populations (5, 53). For instance, the C allele at the eQTL rs1978124 (53) and the G allele at the eQTL rs4646127 (5), both associated with high ACE2 expression, are close to 100% frequency in East Asians but are <80% frequency in other populations. Recently, a rare eQTL (rs190509934, MAF = 0.3%) has been identified that is associated with decreased ACE2 expression and reduced risk of severe COVID-19 disease (54).
We systematically identified regulatory eQTLs associated with ACE2, TMPRSS2, DPP4, and LY6E gene expression and highlighted the eQTLs showing highly differentiated MAF among populations and/or signatures of natural selection. Regulatory eQTLs that differ in frequency across ethnically diverse populations may play a role in local adaptation and disease susceptibility (55). These eQTLs are located in chromatin immunoprecipitation sequencing (ChIP-seq) and DNase peaks, and they have the potential to influence transcription factor binding and thus, change the promoter or enhancer activities in specific tissues (56, 57). Notably, some of the eQTLs in the upstream regions of ACE2 were under selection in African populations. For example, rs5936010 and rs5934263, located within a strong enhancer interacting with the promoter of ACE2 as suggested by ChIA-PET, harbored significant iHS scores (|iHS| > 2) in both Afroasiatic populations from Kenya and the San population from Botswana. Further, PheWAS of these eQTLs in the PMBB populations identified association of eQTLs at ACE2 with type 2 diabetes (rs5936010) and hypertension (rs5934263). These are known preexisting conditions that increase risk of severe illness due to COVID-19 (58–60). Among respiratory diseases, only one eQTL at ACE2 had nominal association (rs4830977) with acute sinusitis. The association was only identified in the AA population and had a protective effect (OR = 0.78 [0.66 to 0.95]). The eQTLs we analyzed are from the GTEx V8 database (61), and 84.6% of the donors are people of European and western Eurasian descent. Therefore, it is possible that we are missing some regulatory variants that are only present in specific ancestry groups due to the lack of sample diversity. Further experimental testing of predicted regulatory variants will provide insights into differences in gene expression regulation at ACE2, TMPRSS2, DPP4, and LY6E among different populations.
ACE2 and TMPRSS2 Are Significantly Associated with Respiratory, Cardiac, and Blood Phenotypes in the PMBB Dataset.
The gene-based genetic association analyses of nonsynonymous variants at ACE2, TMPRSS2, DPP4, and LY6E identified several associations with clinical phenotypes. We observed that respiratory failure has significant association with ACE2 and TMPRSS2 among the PMBB AA population. That is a particularly interesting finding as respiratory failure is one of the clinical outcomes observed in some patients with COVID-19 (39–43). However, this association was not significant in the EA population. This observation could be explained by the low number of coding variants and carriers at ACE2 and TMPRSS2 among EA and hence, low power to detect an association. An association with myocarditis, a rare cardiovascular disease caused by viral infection, was also observed in the AA population. Recent studies have reported a link between SARS-CoV-2–induced cardiac injury, such as myocarditis, among COVID-19 patients (62). Further, ACE2 has known expression in heart tissue, and it plays an important role in transcriptional dysregulation in cardiomyocytes—cells that make up cardiac muscles (36–38). We observed an association between ACE2 and myocarditis only in the AA population, but as noted above, we may not have as much power to detect an association in EA. Blood clotting abnormalities in lungs and other organs in COVID-19 patients have been reported by several studies (63). In autopsies of COVID-19 patients, thrombosis was found to be a prominent finding across multiple organs, even despite extensive anticoagulation treatment and regardless of the timing of clinical progression, indicating that thrombosis might be at play in the early stages of disease (63). One hypothesis to explain this observation is that the dysfunction of endothelial cells may play an important role in increased risk of thrombosis (64). We observed associations between the internationalized normalized ratio (INR) derived from the prothrombin time test with ACE2 and LY6E in a gene-based association test. The INR test measures the time it takes blood to clot and is an important measure for individuals with blood clotting disorders or on blood thinners.
Characterizing the genetic variation and clinical phenotype associations at these four genes that play a key role in SARS-CoV-2 infection could be relevant for understanding individual differences in infection susceptibility. We performed evolutionary analyses to dissect the forces underlying global patterns of genetic variation and identified variants that may be targets of selection. It will be important to determine the functional effects of these candidate adaptive variants using in vitro and in vivo approaches in future studies. Additional studies will be needed to investigate the impact of genetic variation in modulating susceptibility/resistance to SARS-CoV-2 infection and other coronaviruses across ethnically diverse populations.
Materials and Methods
Genomic Data and Populations.
The genomic data used in this study were from three sources: the Africa 6K project (referred to as the African diversity dataset), which is part of the Trans-Omics in Precision Medicine consortium (65); the 1KG (66); and the PMBB. From the Africa 6K project, a subset of 2,012 high-coverage (>30×) whole-genome sequences of ethnically diverse African populations (SI Appendix, Fig. S1) was included (SI Appendix). Institutional review board (IRB) approval was obtained from the University of Maryland and the University of Pennsylvania. Written informed consent was obtained from all participants, and research/ethics approval and permits were obtained from the following institutions prior to sample collection: Tanzania Commission for Science and Technology, National Institute for Medical Research, and Muhimbili University of Health and Allied Sciences in Dar es Salaam, Tanzania; the University of Botswana and the Ministry of Health in Gaborone, Botswana; the University of Addis Ababa and the Federal Democratic Republic of Ethiopia Ministry of Science and Technology National Health Research Ethics Review Committee; and the Cameroonian National Ethics Committee and the Cameroonian Ministry of Public Health. The PMBB participants were recruited through the University of Pennsylvania Health System by enrolling at the time of clinic visit. Patients participated by donating either blood or a tissue sample and allowing researchers access to their EHR information, and all participants provided written informed consent. The PMBB is approved under IRB protocol 813913.
Variant Annotation.
We used Ensembl Variant Effect Predictor (VEP) for variant annotations (67) (SI Appendix). For gene-based association analysis using the PMBB dataset, we collapsed all the predicted nonsynonymous variants with rare exome variant ensemble learner (REVEL) score > 0.5 and pLOFs with MAF < 0.01. We assigned variants as pLoFs if the variant was annotated by VEP as start_lost, splice_donor_variant, splice_acceptor_variant, frameshift_variant, stop_gained, and stop_lost. All genome coordinates followed the GRCh38 assembly.
Characterization of Putative Regulatory Variation.
We extracted variants located within a ±10-kb distance to their TSS as well as enhancers supported by RNA Pol2 ChIA-PET data from ENCODE (68). These variants were further filtered by overlapping with DNase I hypersensitive sites sequencing (DNase-seq) and ChIP-seq peaks from Roadmap (69), ENCODE (68), and Remap2 (70) or overlapping with significant single-tissue eQTLs (P value < 0.001) from the GTEx V8 database (6).
EHR Phenotypes and Association Testing.
We focused on the phenotypes characterized as primary organ dysfunctions in the early studies on COVID-19 (Dataset S5 and SI Appendix). Broadly, we centered our analyses on these four broad clinical conditions/phenotypes: respiratory injury/failure, acute liver injury/failure, acute cardiac injury/failure, and acute kidney injury/failure. These disease classes are well characterized in human disease ontologies, such as Monarch Disease Ontology (SI Appendix).
We used the R SKAT package for conducting a gene-based dispersion test and Biobin for gene burden analysis (32, 71, 72). Here, multiple genetic variations in a gene region were collapsed to generate a gene burden/dispersion score, and regression methods were used to test for association between the genetic score and a phenotype or trait (SI Appendix).
Structural Analysis of Nonsynonymous Variations on the ACE2-S Protein Binding Interface.
We determined the three-dimensional protein location of all nonsynonymous coding variants identified in this study using experimentally determined structures of the ACE2 protein complexed with the RBD of SARS-CoV-2 spike glycoprotein based on cryoelectron microscopy available in the Protein Data Bank [ID code 6M17 (73)]. All structural analysis and figures were prepared using VMD (74) (SI Appendix)
Detecting Signatures of Natural Selection.
We used two methods [the McDonald–Kreitman test (24) and the dN/dS test (23)] to test for signals of selection acting on the four candidate genes over long timescales and two methods [EHH (28) and iHS (27)] to detect recent (e.g., last ∼10,000 y before present) signatures of positive selection (SI Appendix). We used di statistics to identify SNPs that are highly differentiated in allele frequency between populations based on unbiased estimates of pairwise FST (30) (SI Appendix). Haplotype networks were constructed by PopART (75) using the built-in minimum spanning algorithm.
Description of Supplemental Data.
The supplemental data include SI Appendix, SI Methods, Figs. S1–S30, and Table S1, the legends of Datasets S1–S6 and the Regeneron Genetic Center authors and contribution statements.
Supplementary Material
Acknowledgments
Molecular data for the Trans-Omics in Precision Medicine (TOPMed) program were supported by the National Heart, Lung and Blood Institute (NHLBI). Core support, including centralized genomic read mapping and genotype calling, along with variant quality metrics and filtering were provided by TOPMed Informatics Research Center Grant 3R01HL-117626-02S1 (Contract HHSN268201800002I). Core support, including phenotype harmonization, data management, sample-identity quality control, and general program coordination, were provided by NIH funded TOPMed Data Coordinating Center Grants R01HL-120393 and U01HL-120393 (Contract HHSN268201800001I). Funding for this study was provided by ADA 1-19-VSN-02 (to S.A.T.) and by NIH grants R01LM010098 (to S.M.W.), X01HL139409 (to S.A.T. ), 1R35GM134957 (to S.A.T.), R01GM113657 (to S.A.T.), R01DK104339 (to S.A.T.), and R01AR076241 (to S.A.T.). A.V. is supported by NIH grant R01GM138597. C.F.-N. holds a Presidential Professorship at the University of Pennsylvania, is a recipient of the Langer Prize by the American Institute of Chemical Engineers Foundation and acknowledges funding from NIH (R35GM138201), the Defense Threat Reduction Agency (DTRA; HDTRA11810041 and HDTRA1-21-1-0014) and from a Health-Tech Accelerator Award, the Nemirovsky Prize, the Institute for Diabetes, Obesity, and Metabolism, the Mental Health AIDS Research Center and the Dean’s Innovation Fund from the Perelman School of Medicine at the University of Pennsylvania. The PMBB is approved under IRB protocol# 813913 and supported by Perelman School of Medicine at University of Pennsylvania, a gift from the Smilow family, and the National Center for Advancing Translational Sciences of the NIH under CTSA award number UL1TR001878. Genome Sequencing for “NHLBI TOPMed: Integrative Genomic Studies of Heart and Blood Related Traits in Africans (Africa6K)” was performed at Broad Genomics (hhsn268201600034i). We acknowledge the studies and participants who provided biological samples and data for TOPMed and PMBB. We thank Alex Harris, Alexander Platt, and Srilakshmi Raj for discussing the evolutionary analysis in the paper. We thank the following individuals and organizations for their assistance in collecting samples for this project: Kenya: Lilian A. Nyndodo, Eva Aluvalla, Daniel Kariuki, Fathya Abdo, and Hussein Musa; Ethiopia: Solomon Taye, Birhanu Mekaunintie, and Alemayehu Moges; Tanzania: Kweli Powell, Holly Mortensen, Mariki Euphrasia, Ruth Matiyas, John G. Memra, Holliness Santa, Emanuel Kimario, and Reginald Kavishe; Botswana: Michael Campbell, Ari Ho-Foster, Maitseo M. M. Bolane, Maungo Moswang, Gaolape Mpoloka, Kingsley Motshegwe, and Mothusi Molatlhegi; and Cameroon: Eric Mbunwe, Sali Django, Dickson Ndizi, Valentine Ngum Ndze, Julius Fonsah, Eric Ngwang, Grace N. Tenjei, Meagan Rubel, Peter Kfu, Association Culturelle pour le Dévelopment Bagyeli/Bakola de l'Ocean, Centre d'Action pour le Dévelopment Durable des Autochtones Pygmées, and Mbororo Social and Cultural Development Association. We especially thank all African participants for their important contributions to this study.
Footnotes
Reviewers: R.K., Temple University; and X.L., Chinese Academy of Sciences Shanghai Institute of Nutrition and Health.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2123000119/-/DCSupplemental.
Contributor Information
Collaborators: Abecasis Goncalo, Baras Aris, Cantor Michael, Coppola Giovanni, Economides Aris, Lotta Luca A., Overton John D., Reid Jeffrey G., Shuldiner Alan, Beechert Christina, Forsythe Caitlin, Fuller Erin D., Gu Zhenhua, Lattari Michael, Lopez Alexander, Overton John D., Schleicher Thomas D., Padilla Maria Sotiropoulos, Widom Louis, Wolf Sarah E., Pradhan Manasi, Manoochehri Kia, Ulloa Ricardo H., Banerjee Nilanjana, Cantor Michael, Li Dadong, Sharma Deepika, Bai Xiaodong, Balasubramanian Suganthi, Blumenfeld Andrew, Boutkov Boris, Eom Gisu, Habegger Lukas, Hawes Alicia, Khalid Shareef, Krasheninina Olga, Lanche Rouel, Mansfield Adam J., Maxwell Evan K., Nafde Mrunali, O’Keeffe Sean, Orelus Max, Panea Razvan, Polanco Tommy, Rasool Ayesha, Reid Jeffrey G., Salerno William, Staples Jeffrey C., Jones Marcus B., LeBlanc Michelle, and Mitnaul Lyndon J.
Data Availability
All data are included in the manuscript and/or supporting information.
References
- 1.Yancy C. W., COVID-19 and African Americans. JAMA 323, 1891–1892 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Alcendor D. J., Racial disparities-associated COVID-19 mortality among minority populations in the US. J. Clin. Med. 9, 2442 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M., et al. , SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walls A. C., et al. , Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281–292.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y., et al. , Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 6, 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GTEx Consortium, Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 348, 648–660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silhol F., Sarlon G., Deharo J. C., Vaïsse B., Downregulation of ACE2 induces overstimulation of the renin-angiotensin system in COVID-19: Should we block the renin-angiotensin system? Hypertens. Res. 43, 854–856 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glowacka I., et al. , Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 85, 4122–4134 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wit E., van Doremalen N., Falzarano D., Munster V. J., SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14, 523–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vankadari N., Wilce J. A., Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 9, 601–604 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaender S., et al. , LY6E impairs coronavirus fusion and confers immune control of viral disease. Nat. Microbiol. 5, 1330–1339 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou Y., et al. , New insights into genetic susceptibility of COVID-19: An ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 18, 216 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benetti E., et al. , ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur. J. Hum. Genet. 28, 1602–1614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirulli E. T., Riffle S., Bolze A., Washington N. L., Revealing variants in SARS-CoV-2 interaction domain of ACE2 and loss of function intolerance through analysis of >200,000 exomes. bioRxiv [Preprint] (2020). 10.1101/2020.04.07.030544 (Accessed 4 May 2020). [DOI]
- 15.Zhang C., Hansen M. E. B., Tishkoff S. A., Advances in integrative African genomics. Trends Genet. 38, 152–168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rentzsch P., Witten D., Cooper G. M., Shendure J., Kircher M., CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 47, D886–D894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng P. C., Henikoff S., SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adzhubei I., Jordan D. M., Sunyaev S. R., Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 7, 7.20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González-Pérez A., López-Bigas N., Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, Condel. Am. J. Hum. Genet. 88, 440–449 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heurich A., et al. , TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 88, 1293–1307 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloss B. S., Dinger M. E., Realizing the significance of noncoding functionality in clinical genomics. Exp. Mol. Med. 50, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duggal G., Wang H., Kingsford C., Higher-order chromatin domains link eQTLs with the expression of far-away genes. Nucleic Acids Res. 42, 87–96 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nei M., Gojobori T., Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3, 418–426 (1986). [DOI] [PubMed] [Google Scholar]
- 24.McDonald J. H., Kreitman M., Adaptive protein evolution at the Adh locus in Drosophila. Nature 351, 652–654 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Sabeti P. C., et al. , Positive natural selection in the human lineage. Science 312, 1614–1620 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Kryazhimskiy S., Plotkin J. B., The population genetics of dN/dS. PLoS Genet. 4, e1000304 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voight B. F., Kudaravalli S., Wen X., Pritchard J. K., A map of recent positive selection in the human genome. PLoS Biol. 4, e72 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabeti P. C., et al. ; International HapMap Consortium, Genome-wide detection and characterization of positive selection in human populations. Nature 449, 913–918 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheinfeldt L. B., Tishkoff S. A., Recent human adaptation: Genomic approaches, interpretation and insights. Nat. Rev. Genet. 14, 692–702 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akey J. M., et al. , Tracking footprints of artificial selection in the dog genome. Proc. Natl. Acad. Sci. U.S.A. 107, 1160–1165 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper J. D., Clements J. A., Quigley J. P., Antalis T. M., Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J. Biol. Chem. 276, 857–860 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Moore C. B., Wallace J. R., Frase A. T., Pendergrass S. A., Ritchie M. D., BioBin: A bioinformatics tool for automating the binning of rare variants using publicly available biological knowledge. BMC Med. Genomics 6 (suppl. 2), S6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S., et al. ; NHLBI GO Exome Sequencing Project—ESP Lung Project Team, Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am. J. Hum. Genet. 91, 224–237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma S. S., et al. , Rare variants in drug target genes contributing to complex diseases, phenome-wide. Sci. Rep. 8, 4624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boehmer T. K., et al. , Association between COVID-19 and myocarditis using hospital-based administrative data – United States, March "369"/>2020-January 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 1228–1232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siripanthong B., et al. , Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 17, 1463–1471 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker N. R., et al. ; Human Cell Atlas Lung Biological Network; Human Cell Atlas Lung Biological Network Consortium Members, Myocyte-specific upregulation of ACE2 in cardiovascular disease: Implications for SARS-CoV-2-mediated myocarditis. Circulation 142, 708–710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi S., et al. , Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 5, 802–810 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D., et al. , Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen N., et al. , Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395, 507–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grasselli G., et al. , Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 323, 1574–1581 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arentz M., et al. , Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 323, 1612–1614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C., et al. , Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tishkoff S. A., et al. , The genetic structure and history of Africans and African Americans. Science 324, 1035–1044 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sirugo G., Williams S. M., Tishkoff S. A., The missing diversity in human genetic studies. Cell 177, 26–31 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry G. H., et al. , Adaptive, convergent origins of the pygmy phenotype in African rainforest hunter-gatherers. Proc. Natl. Acad. Sci. U.S.A. 111, E3596–E3603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glazko G. V., Nei M., Estimation of divergence times for major lineages of primate species. Mol. Biol. Evol. 20, 424–434 (2003). [DOI] [PubMed] [Google Scholar]
- 48.David A., Khanna T., Beykou M., Hanna G., Sternberg M. J. E., Structure, function and variants analysis of the androgen-regulated TMPRSS2, a drug target candidate for COVID-19 infection. bioRxiv [Preprint] (2020) 10.1101/2020.05.26.116608 (Accessed 26 May 2020). [DOI]
- 49.Kuba K., et al. , A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 11, 875–879 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Letko M., Marzi A., Munster V., Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 5, 562–569 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verdecchia P., Cavallini C., Spanevello A., Angeli F., The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 76, 14–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunyavanich S., Do A., Vicencio A., Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323, 2427–2429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J., et al. , Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 19, e13168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horowitz J. E., et al. ; Regeneron Genetics Center, Genome-wide analysis provides genetic evidence that ACE2 influences COVID-19 risk and yields risk scores associated with severe disease. Nat. Genet., 10.1038/s41588-021-01006-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mogil L. S., et al. , Genetic architecture of gene expression traits across diverse populations. PLoS Genet. 14, e1007586 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Degner J. F., et al. , DNase I sensitivity QTLs are a major determinant of human expression variation. Nature 482, 390–394 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown C. D., Mangravite L. M., Engelhardt B. E., Integrative modeling of eQTLs and cis-regulatory elements suggests mechanisms underlying cell type specificity of eQTLs. PLoS Genet. 9, e1003649 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Z., McGoogan J. M., Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y., et al. ; medical team from Xiangya Hospital to support Hubei, China, Association of diabetes mellitus with disease severity and prognosis in COVID-19: A retrospective cohort study. Diabetes Res. Clin. Pract. 165, 108227 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apicella M., et al. , COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 8, 782–792 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.GTEx Consortium; Laboratory, Data Analysis &Coordinating Center (LDACC)—Analysis Working Group; Statistical Methods groups—Analysis Working Group; Enhancing GTEx (eGTEx) groups; NIH Common Fund; NIH/NCI; NIH/NHGRI; NIH/NIMH; NIH/NIDA; Biospecimen Collection Source Site—NDRI; Biospecimen Collection Source Site—RPCI; Biospecimen Core Resource—VARI; Brain Bank Repository—University of Miami Brain Endowment Bank; Leidos Biomedical—Project Management; ELSI Study; Genome Browser Data Integration &Visualization—EBI; Genome Browser Data Integration &Visualization—UCSC Genomics Institute, University of California Santa Cruz; Lead analysts; Laboratory, Data Analysis &Coordinating Center (LDACC); NIH program management; Biospecimen collection; Pathology; eQTL manuscript working group; Battle A., Brown C. D., Engelhardt B. E., Montgomery S. B., Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017).29022597 [Google Scholar]
- 62.Knowlton K. U., Pathogenesis of SARS-CoV-2 induced cardiac injury from the perspective of the virus. J. Mol. Cell. Cardiol. 147, 12–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biswas S., et al. , Blood clots in COVID-19 patients: Simplifying the curious mystery. Med. Hypotheses 146, 110371 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rapkiewicz A. V., et al. , Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 24, 100434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taliun D., et al. , Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Auton A., et al. ; 1000 Genomes Project Consortium, A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLaren W., et al. , The ensembl variant effect predictor. Genome Biol. 17, 122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ENCODE Project Consortium, An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chadwick L. H., The NIH Roadmap Epigenomics Program data resource. Epigenomics 4, 317–324 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chèneby J., et al. , ReMap 2020: A database of regulatory regions from an integrative analysis of Human and Arabidopsis DNA-binding sequencing experiments. Nucleic Acids Res. 48, D180–D188 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basile A. O., et al. , Knowledge driven binning and phewas analysis in marshfield personalized medicine research project using Biobin. Pac. Symp. Biocomput. 21, 249–260 (2016). [PMC free article] [PubMed] [Google Scholar]
- 72.Wu M. C., et al. , Rare-variant association testing for sequencing data with the sequence kernel association test. Am. J. Hum. Genet. 89, 82–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan R., et al. , Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367, 1444–1448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Humphrey W., Dalke A., Schulten K., VMD: Visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996). [DOI] [PubMed] [Google Scholar]
- 75.Leigh J. W., Bryant D., POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and/or supporting information.