Abstract
Pine seeds are considered as nonwood forest products (NWFP) with regularly increasing market's demand. They can be eaten in various ways such as roasted or raw. In addition, they are included in various traditional dishes like in cookies, sauces, candies, cakes, breads, and other bakery items and, moreover, for medicinal purposes. GC-MS study is performed to analyze the phytochemical compounds present in the seed extracts of Pinus roxburghii (Chir) and Pinus gerardiana (Chilgoza). In total, 25 compounds were identified each in Chir and Chilgoza. In Chir seeds, abundantly present compounds were 2,4-di-tert-butylphenol (16.6%), followed by ç-Terpinene (9.9%) and cyclohexanol, 4-ethenyl-4-methyl-3-(1-methylethenyl)-, (1à,3à,4á) (9.8%), whereas in Chilgoza seeds, the maximum amount of compound was 1-hexyl-1-nitrocyclohexane (17.3%), followed by phenol, 2,6-bis(1,1-dimethylethyl) (15.4%), and heptadecane, 2-methyl (8.4%). The total phenolic content of Chir seed sample was 1536 ± 4.35 (mg GAE/100 g), whereas in the Chilgoza seed extract was 642.66 ± 2.08 (mg GAE/100 g). The application of RP-HPLC-DAD system revealed that Chir and Chilgoza seeds have maximum quantity of catechin (15.77 ± 0.16 μg/mg and 17.49 ± 0.32 μg/mg, respectively). Both Chir and Chilgoza seed extracts exhibited significant antioxidant (radical scavenging) potential, through H2O2 (618.94 ± 21.45 μg/mL and 575.16 ± 19.88 μg/mL) and DPPH (552.60 ± 13.03 μg/mL and 429.15 ± 3.80 μg/mL) assays, respectively. Additionally, a well-known antibacterial potential was also found in both plants' dichloromethane extracts, with 64 to 256 μg/mL of minimum inhibitory concentrations. As a whole, result shows the importance of both plants as a naturally occurring phytochemical source with significant antibacterial and antioxidant activity.
1. Introduction
Pine trees are one of the most common and widely grown species in the Himalayan region. Categorized under the Pinus genus and Pinaceae family, they are the largest conifer's families in the world and always remain evergreen woody conifer trees [1]. In the Himalayan region (HR), five species of pine are considered indigenous, distributed at different elevation such as Pinus gerardiana (Chilgoza), Pinus wallichiana (Kail), Pinus roxburghii (Chir), Pinus merkusii (Merkus), and Pinus kesiya syn. Insularis (khasi) as shown in Table 1 [2]. Among these, Chilgoza tree nuts have highly incomparable nutritious value therefore, commercialized for edible use and consumed in roasted form; these seeds are added as an ingredient in different dishes [3]. Whereas Chir trees are abundantly present and have ethno medicinal value, nuts are traditionally consumed in India and Pakistan [4, 5].
Table 1.
Geographical allocation of various pines species in HR [2].
| Pine species | Habitat and distribution | Altitude (m) |
|---|---|---|
| P. gerardiana | Occur in drier rocky slope in some part of J&K and Kinnaur region of Himachal Pradesh | 2000-3350 |
| P. wallichiana | Found in the higher altitude of Himalayas drier region along with J&K up to Arunachal Pradesh north-eastern region of India | Till 2700 |
| P. roxburghii Sarg. | Grows up and found in outer Himalayan valleys drier region of J&K to the Arunachal Pradesh | 400-2300 |
| P. merkusii | Found some regions in high moisture area of Indo-Myanmar border | 1500 |
| P. kesiya or insularis | Grows up in high moisture regions of Meghalaya north-eastern part of India | Up to 3000 |
P. roxburghii, known as Chir pine trees, is generally 55 m tall and over 100 cm diameter breadth height (dbh). Tree bark is thick in dark reddish-brown while winter buds are brown, ovoid, small, and nonresinous. The tree leaves are needle-shaped in flabellate-triangular arranged in 3 per bundle and cylindrical in cross-section. Cones are pedunculate, short, ovoid, range in 10‐15 × 6‐9 cm, while seeds are small, about 8-12 mm in length with a long wing of 25 mm, and normally ripen in April [6].
P. gerardiana is traditionally called Chilgoza trees to a medium height (17 to 27 m) and 2-4 m in dbh. The branches are small and horizontal with glabrous bark and silver grey [7]. Leaves are needle-like shaped and dark green, arranged three per cluster. However, the male cones are longer than female ones, which are ovoid with hard woody scales. These seeds are in dark brown colored, pointed from top and cylindrical, and normally ripen in October [8].
Data available to date demonstrate that these trees are composed of different types of phenolic compounds, at varying amounts depending on the geographical origin, harvesting time and storage, with distinct biological activities being also reported [9]. In past two decades, massive studies were carried out on essential oils and extracts of several parts of pine trees. Studies generally focused on nutritional value, chemical composition, food supplements, and in drug formulation [10]. The natural bioactive compounds, including phenols, terpenes, flavonoids, alkaloids, and saponin obtained from different Pinus spp., have reported for their potency against several diseases, e.g., asthma, diabetes, neurodegenerative diseases, cancer, oxidative stress-related diseases, cardiovascular-related problems, liver and kidney disorders, and various pathogenic infections [2]. Among the phytochemicals present, these pine nuts usually contain inherent antioxidants that help in reducing the oxidation rate, namely, flavonoids, e.g., flavonols, and flavanones in various glycoside and aglycone form [11]. For instance various phenolic group constituents like as catechin, gallic acid and quercetin show antioxidant, antiallergic, antimicrobial, anti-inflammatory, UV protection, and anticancer activity [12, 13]. Ellagic acid has potency to inhibit the oxidation of low-density lipoprotein [14]. Vanillic acid is highly efficient to reduce oxidative stress and Aβ1-42-induced cognitive impairment, therefore very effective in Alzheimer's and other neuron-related disease [15].
Till now, studies have been carried out to assess the phytochemical composition and biological potential of Chilgoza seeds [3, 16]. In case of P. roxburghii except seed, the chemical composition and biological activities have been studied on its various parts, viz., needles and bark [4]. In this sense, the present study is aimed at evaluating the total phenolic content of P. gerardiana and P. roxburghii seed extracts and their bioactive compounds and at investigating the antioxidant and antibacterial roles. In addition, chemical composition and quantification of phenolic compounds were assessed by GC-MS and HPLC-DAD.
2. Materials and Methods
2.1. Chemical, Reagents, and Apparatus
2,2-Diphenyl-1-picrylhydrazyl (DPPH), Folin-Ciocalteu phenol reagent, L-ascorbic acid, di-sodium hydrogen phosphate de-hydrate, and sodium dihydrogen phosphate dehydrate were purchased from HiMedia Laboratories Pvt. Ltd. (India). HPLC grade water, sodium carbonate, and hydrogen peroxide were procured from Loba Chemie Pvt. Ltd. (India). Gallic acid, quercetin, ellagic acid, vanillic acid, catechin, epigallocatechin gallate (EGCG), and DCM were procured from Sigma-Aldrich (USA). LC–MS grade acetonitrile, methanol, and formic acid (of 98% purity) were procured from Fisher Chemicals (Hampton, NH, USA). Colistin was procured from HiMedia Laboratories Pvt. Ltd. (India). Labeled, CHROMAFIL Xtra poly ether sulfone (PES), 25 mm, and 0.20 μm syringe filters were purchased from Macherey-Nagel (Düren, Germany). UV-vis spectrophotometer (Evolution 201) was procured from Thermo Fisher Scientific- Shanghai (China). ORBITEK shaker was purchased from Scigenics Biotech Pvt. Ltd. (India). Refrigerated centrifuge C-24 Plus was from REMI Sales and Engineering Ltd. (India). Analytical balance Aczet, CY2202 was purchased from Mettler-Toledo Pvt. Ltd. (India). Rotary vacuum evaporator-RE-52 was purchased from SONAR (India). Micropipette (20-200, 100-1000, and 0.5-10 μL) of variable volume purchased from HiMedia Laboratories Pvt. Ltd. (India).
2.2. Preparation of Seed Extracts
At room temperature, de-shelled Chilgoza and Chir samples were grounded into powder with the help of commercial mixer grinder. Powdered seed samples of 50 g were added to 500 mL conical flask containing 250 mL dichloromethane (DCM) solvent each and kept in incubator cum shaker for 48 h at 37°C. Each sample was strained through Whatman no. 1 filter paper. After the extraction process, the liquid extracts were collected and then concentrated at 40°C by using a rotary vacuum evaporator. The prepared extracts were collected and stored at 4°C in refrigerator for further analysis [17].
2.3. Total Phenolic Content
The total phenolic content (TPC) of Chilgoza and Chir was determined by using Folin-Ciocalteau reagent [18]. Seed extracts (about 20 μg) were separately taken and made volume up to 1 mL by adding distilled water. Then, Folin-phenol reagent (500 μL) was added into that, and 2.5 mL sodium carbonate Na2CO3 (20%) was also added. It was mixed properly and kept away from light for 40 min for incubation and color development. Postincubation, the absorbance was taken at 725 nm. Gallic acid calibration curve was constructed, and linearity was found in 5-25 μg/mL range. Seed extract TPC was stated in mg of gallic acid equivalent (mgGAE/100 g seed extracts) by using the standard curve.
2.4. GC-MS Study of the Seed Extracts
Each seed extract sample was diluted by adding DCM (1:10), and their fraction components were analyzed by GC-MS (TRIPLE QUAD GC-MS/MS) (Thermo Fisher, USA), equipped with an autosampler (TriPlusRSH) and DB 5 column (40 m × 0.15 mm i.d., film thickness of 0.15 μm). For analysis of two different seed extracts, the following GC-MS operating conditions were followed with slightly modification as described by Al-Owaisi et al. [19]: initial temperature was kept at 80°C and time held for a min, thereafter with 10°C/min ramping rate reached up to 180°C by holding 2 min, and finally with same ramping increased to 260°C and was held for 15 minutes. Transfer line temperature, 250°C; carrier gas, He at constant flow rate 0.7 mL/min; split ratio, 71 : 4; 1 μL of injection volume; component ionization, electron impact (70 eV) mode; EI source temperature, 230°C; m/z range, 45-450. The respected component relative concentrations were expressed as percent area on basis evolved in chromatograph. The identity of their components was done on the basis of visual interpretation and compared based on probability and literature search input as per NIST library (v. 2.2, 2014) with different types of compounds identified.
2.5. HPLC Analysis of the Seed Extracts
RP-HPLC system (Capcellpak) of Shimadzu (Kyoto, Japan) equipped well with a C-18 (2) column of phenomenex Luna (4.6 mm i.d. ×25 cm, 5 μm) and a detector of diode array (DA) (SPD-M20A, Shimadzu, Japan) were taken for the phenolic compound quantification, including gallic acid, ellagic acid, vanillic acid, epicatechin, quercetin, catechin, and EGCG isomers [3]. Solvents used to run were as follows: solvent A: aqueous formic acid of 8% and solvent B: 10 : 90, v/v (acetonitrile/methanol). The flow rate was 0.9 mL/min. Injection volume was 20 μL. The gradient was as given: 0 min, 20% B; 7 min, 35% B; 14 min, 45% B; 21 min, 65% B; 25 min, 85% B; and 32 min, 95% B. To monitor all the phenolic compounds, the DA detector wavelengths were fixed at 260, 280, or 320 nm. Standard solution preparation was done as described by Lee et al. [20]. From the stock, 0.01 mL was taken and diluted up to 1 mL diluents to make necessary dilutions and filtered through 0.22 μ PES membrane filters and injected in HPLC system.
2.6. Antioxidant Activities
2.6.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Free Radical Scavenging Assay
The free radical scavenging potential of two seeds' extract was evaluated following the method followed by Bhatti et al. [21] with slightly modifications. Stock solutions of both seeds' extracts (1 mg) were prepared in methanol (MeOH), and further different methanolic solution concentrations (20-640 μg/mL) were prepared. From each concentration, 300 μL was added to methanolic solution 2700 μL of DPPH (4 mg/100 mL). The mixture solution was incubated in absence of light at 37°C room temperature for one hour. Free radical scavenging efficacy of extracts was based on the initial purple color disappearance. Absorbance of solution was taken at 517 nm. For positive control, ascorbic acid was used [21]. Scavenging capacity of DPPH was determined using the formula below given:
| (1) |
where the IC50 of DPPH radical was calculated from the line regression of the percentage of remaining DPPH radical against the sample concentration.
2.6.2. Hydrogen Peroxide Scavenging Assay (H2O2)
The capacity of seed extract to scavenge H2O2 was estimated following the procedure of Bhatti et al. [21]. Briefly, 0.1 mL extract aliquots (20-640 μg/mL) were added into an Eppendorf tubes to made volume up to 0.4 mL with addition of 50 mM (pH 7.4) phosphate buffer and (2 mM) H2O2 solution (0.6 mL). Mixture was properly vortexed and kept for 10 min, and then, absorbance was read at 230 nm. Ascorbic acid was used as positive control [21]. The extracts' ability to scavenge the H2O2 was evaluated by using the following equation:
| (2) |
where A0 is the absorbance of blank and A1 is the absorbance of sample.
2.7. Antibacterial Screening by Minimum Inhibitory Concentration (MIC)
Gram-negative bacteria viz., Salmonella typhimurium MTCC 3224, Klebsiella pneumonia MTCC 109, and Escherichia coli MTCC 443 were used to study the antibacterial potential of both seeds' extracts, using the broth macrodilution technique [22]. DCM extracts were prepared in Mueller Hinton broth, and serial dilutions were obtained, ranging between 0.5 and 256 μg/mL. Bacteria (1‐2 × 108 CFU/mL) were transferred to test tubes and incubated at 37°C for 24 h. Minimum inhibitory concentrations (MIC) were determined, being considered as the lowest concentrations without visible turbidity. Colistin was used as a positive antibacterial control, while DCM was used as negative control [22].
2.8. Statistical Evaluation
Results obtained are expressed as mean value and standard deviation (x ± SD) with three times repeated trials for all experiments. The IC50 values were obtained by plotting inhibition-concentration curves by nonlinear regression analysis. Statistical analysis was performed using the Microsoft Excel.
3. Results and Discussion
3.1. Total Phenolic Content
The TPC of Chilgoza DCM seed extract was 642.66 ± 2.08 mg GAE/100 g, and TPC of Chir seed sample was 1536 ± 4.35 mg GAE/100 g. Hoon et al. reported that the maximum TPC in Chilgoza seeds was highest in water extract, followed by DCM, ethanol (EtOH), hexane (HEX), and ethyl acetate (EtOAc) as well as MeOH extracts [3]. Mahdhi et al. studied the high quantity of total phenols in P. halepensis methanolic-aqueous seed extracts 14.63 ± 0.05 mg/g gallic acid equivalent weight (GAE) [23]. Zulfqar et al. found higher TPC (77.2 ± 1.41 mg GAE/g) in methanolic extract of P. gerardiana dry nuts than in EtOAc extract (52.5 ± 2.9 mg GAE/g) [16]. Valero-Galván et al. found that Pinus cembroides grown in five states of Mexico presented different amounts of TPC in the methanol seed extract [24]. Bolling et al. report that TPC of pine nut was 68 mg GAE/100 mg; however, as per Phenol-Explorer database reports 58 mg GAE/100 mg [25]. Kadri et al. reported that Algerian pine species, viz., Pinus pinea, Pinus halepensis, Pinus canariensis, and Pinus pinaster seed extract, TPC differ within species [26]. Su et al. reported that Pinus koraiensis seed (PKS) ethanolic extract contained higher total phenolic content of 264 mg GAE/g [27].
3.2. GC-MS Analysis of the Seed Extracts
Data obtained from the GC-MS analysis of DCM extracts of Chilgoza and Chir seeds revealed the presence of terpenoids, alcohols, alkenes, aromatic hydrocarbons, etc. (Figures 1 and 2). A total of 25 compounds were detected in Chilgoza and Chir, as shown in Tables 2 and 3. In Chilgoza seeds, the most abundant compound was 1-hexyl-1-nitrocyclohexane (17.3%), followed by phenol, 2,6-bis(1,1-dimethylethyl) (15.4%), and heptadecane, 2-methyl (8.4%), while in Chir seeds, 2,4-di-tert-butylphenol (16.6%), followed by ç-Terpinene (9.9%), cyclohexanol, 4-ethenyl-4-methyl-3-(1-methylethenyl)-, (1à,3à,4á) (9.8%) were the most commonly identified.
Figure 1.
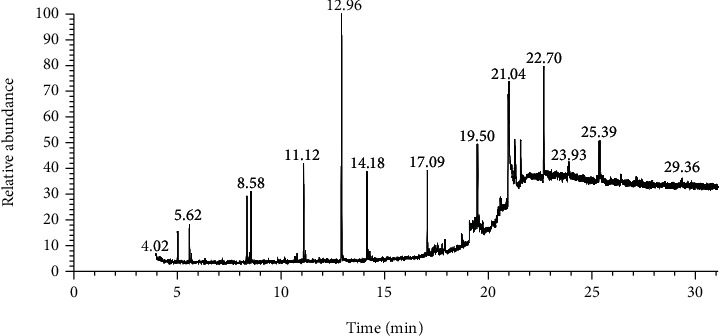
GC-MS profile of the DCM extract of Chilgoza with their retention time and peak assignment as in Table 2.
Figure 2.
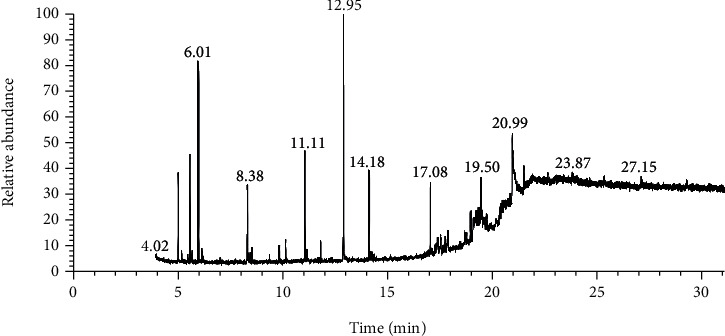
GC-MS profile of the DCM extract of Chir with their retention time and peak assignment as in Table 3.
Table 2.
List of phytocompounds identified in Chilgoza by GC-MS.
| Peak no. | Compound | RT | Area % | Mol. weight | Molecular formula | CAS. no. |
|---|---|---|---|---|---|---|
| 1. | 3-Carene | 5.06 | 1.53 | 136 | C10H16 | 13466-78-9 |
| 2. | 2-Propyn-1-ol, acetate | 5.62 | 2.27 | 98 | C5H6O2 | 627-09-8 |
| 3. | 1-Undecanol | 8.38 | 3.14 | 172 | C11H24O | 112-42-5 |
| 4. | Naphthalene | 8.58 | 3.81 | 128 | C10H8 | 91-20-3 |
| 5. | 8-Heptadecene | 11.12 | 4.75 | 238 | C17H34 | 2579-04-6 |
| 6. | Phenol, 2,6-bis(1,1-dimethylethyl) | 12.96 | 15.42 | 206 | C14H22O | 128-39-2 |
| 7. | 1-Hexadecanol | 14.18 | 5.40 | 242 | C16H34O | 36653-82-4 |
| 8. | 10-Heneicosene | 17.09 | 4.77 | 294 | C21H42 | 95008-11-0 |
| 9. | (2S,4R)-p-Mentha-[1(7),8]-diene 2-hydroperoxide | 17.59 | 1.42 | 168 | C10H16O2 | NA |
| 10. | 2R-Acetoxymethyl-1,3,5-trimethyl-4c-(3-methyl-2-buten-1-yl)-1c-cyclohexanol | 17.93 | 1.43 | 282 | C17H30O3 | NA |
| 11. | Tetradecanoic acid, 10,13-dimethyl-, methyl ester | 18.75 | 1.31 | 270 | C17H34O2 | 267650-23-7 |
| 12. | Tetradecanoic acid, 12- methyl-, methyl ester, (S) | 19.12 | 2.58 | 256 | C16H32O2 | 62691-05-8 |
| 13. | Acetophenone, 2-[(p-nitrophenyl)imino] | 19.21 | 1.60 | 254 | C14H10N2O3 | 6394-60-1 |
| 14. | 2-Methyl-3-(2,2-dimethylpropyl)-butadiene | 19.41 | 1.20 | 138 | C10H18 | 90822-87-0 |
| 15. | 1-Nonadecene | 19.50 | 6.08 | 266 | C19H38 | C19H38 |
| 16. | 8-Isopropyl-5-methyl-5,6,7,8-tetrahydro-2,4-quinazolinedione | 20.21 | 1.28 | 222 | C12H18N2O2 | 63498-93-1 |
| 17. | 4-tert-Octylphenol, TMS derivative | 20.62 | 1.20 | 278 | C17H30OSi | 8721-87-6 |
| 18. | 1-Hexyl-1-nitrocyclohexane | 21.03 | 17.30 | 213 | C12H23NO2 | 118252-09-8 |
| 19. | 5,10-Pentadecadienoic acid, (E,E)- | 21.29 | 2.34 | 238 | C15H26O2 | 64275-68-9 |
| 20. | 1,1,1,3,5,5,5-Heptamethyltrisiloxane | 21.34 | 2.31 | 222 | C7H22O2Si3 | 1873-88-7 |
| 21. | 1-Hexyl-2-nitrocyclohexane | 21.57 | 3.51 | 213 | C12H23NO2 | 118252-04-3 |
| 22. | Heptadecane, 2-methyl | 22.70 | 8.49 | 254 | C18H38 | 1560-89-0 |
| 23. | Cyclotrisiloxane, hexamethyl | 23.93 | 1.48 | 222 | C6H18O3Si3 | 541-05-9 |
| 24. | 4,4-Dipropylheptane | 25.39 | 4.20 | 184 | C13H28 | 17312-72-0 |
| 25. | 1-propanone, 1-[5-ethyl-3-(5-nitro-2-furanyl)-1H-1,2,4-triazol-1-yl] | 27.16 | 1.16 | 264 | C11H12N4O4 | 35732-74-2 |
NA: not applicable.
Table 3.
List of compounds identified in Chir by GC-MS.
| Peak no. | Compound | RT | Area % | Mol. weight | Molecular formula | CAS no |
|---|---|---|---|---|---|---|
| 1. | α-Pinene | 5.06 | 4.59 | 136 | C10H16 | 80-56-8 |
| 2. | 3-Carene | 5.62 | 6.22 | 136 | C10H16 | 13466-78-9 |
| 3. | ç-Terpinene | 6.01 | 9.99 | 136 | C10H16 | 99-85-4 |
| 4. | 1-Undecanol | 8.38 | 4.11 | 172 | C11H24O | 112-42-5 |
| 5. | Oxirane, 2-(chloromethyl)-2-cyclopropyl | 10.20 | 1.43 | 132 | C6H9ClO | 121505-35-9 |
| 6. | 1-Hexadecanol | 11.11 | 5.46 | 242 | C16H34O | 36653-82-4 |
| 7. | 2,4-Di-tert-butylphenol | 12.95 | 16.67 | 206 | C14H22O | 96-76-4 |
| 8. | 10-Heneicosene | 14.18 | 5.74 | 294 | C21H42 | 95008-11-0 |
| 9. | 1-Eicosanol | 17.08 | 4.30 | 298 | C20H42O | 629-96-9 |
| 10. | 5,10-Pentadecadienoic acid, (E,E)- | 17.43 | 2.03 | 238 | C15H26O2 | 64275-68-9 |
| 11. | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-9 (phenylsulfonyl)-, (E,E) | 17.58 | 2.82 | 362 | C21H30O3S | 57683-67-7 |
| 12. | (2S,4R)-p-Mentha-[1(7),8]-diene 2-hydroperoxide | 17.93 | 2.35 | 168 | C10H16O2 | NA |
| 13. | 2,6-Dimethyl-3,5,7-octatriene-2-ol | 18.75 | 1.77 | 152 | C10H16O | 29414-56-0 |
| 14. | 10,10-Dimethyl-2,6-dimethylenebicyclo[7.2.0]undecan-5á-ol | 19.02 | 1.99 | 220 | C15H24O | 19431-80-2 |
| 15. | 4-tert-Octylphenol, TMS derivative | 19.14 | 2.01 | 278 | C17H30OSi | 78721-87-6 |
| 16. | 2,3-Dimethylamphetamine | 19.21 | 2.20 | 163 | C11H17N | 75659-60-8 |
| 17. | 10-Pentadecen-5-yn-1-ol | 19.29 | 1.43 | 222 | C15H26O | 64275-59-8 |
| 18. | 1,1,1,3,5,5,5-Heptamethyltrisiloxane | 19.40 | 1.48 | 222 | C7H22O2Si3 | 1873-88-7 |
| 19. | 1-Hexyl-2-nitrocyclohexane | 19.50 | 4.56 | 213 | C12H23NO2 | 118252-04-3 |
| 20. | 1,2,4-Benzenetricarboxylic acid, 1,2-dimethyl ester | 19.77 | 1.61 | 238 | C11H10O6 | 54699-35-3 |
| 21. | 3,4-Nonadien-6-yne, 5-ethyl-3-methyl | 20.51 | 2.28 | 162 | C12H18 | 61227-88-1 |
| 22. | 4(3H)-Quinolinone, 3-hydroxy | 20.57 | 1.75 | 161 | C9H7NO2 | 55759-82-5 |
| 23. | Cyclohexanol, 4-ethenyl-4-methyl-3-(1-methylethenyl)-, (1à,3à,4á) | 21.00 | 9.86 | 180 | C12H20O | 56298-45-4 |
| 24. | 1-Hexyl-1-nitrocyclohexane 2-tert-butyl-3-(tert-butylimino)-4-phenyl | 21.56 | 2.06 | 213 | C12H23NO2 | 118252-09-8 |
| 25. | Thieno[2,3-b]pyridine, 5-ethyl-3-nitro | 27.15 | 1.30 | 208 | C9H8N2O2S | 51043-51-7 |
NA: not applicable.
Kadri et al. reported α-pinene only in P. pinaster. Tables 4 and 5 display the biological activities of some compounds present in Chilgoza and Chir extracts, as reported in the various literatures [26]. However, GC-MS studies on pine nuts extracts were not updated in the literature; researchers emphasized on the essential oil analysis attained by extraction or emission from various tree parts, viz., branches, bark, cones, and needles [28].
Table 4.
Biological activities of Chilgoza seed compounds.
| Compound name | Nature of compound | Structure | Biological activities | Ref |
|---|---|---|---|---|
| 3-Carene | Monoterpenes |
|
Antiacetylcholinesterase and antimicrobial | [29] |
| 2,4-Di-tert-butylphenol or phenol, 2,6-bis(1,1-dimethylethyl) | Phenylpropanes |
|
Antimicrobial, antioxidant, anti-inflammatory, cytotoxic, nematicidal, insecticidal, and allelopathic effect | [30] |
| 1-Hexyl-1-nitrocyclohexane | Ketone |
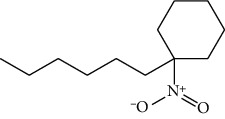
|
Antioxidant, antimicrobial, anti-inflammatory | [31] |
| 1-Nonadecene | Alkene |

|
Antifungal, anticancer | [32] |
| Naphthalene | Aromatic hydrocarbon |
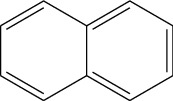
|
Anticancer, antiviral, antimicrobial, antidepressant, antineurodegenerative antidiabetic, anti-inflammatory, antitubercular, antihypertensive, antipsychotic, anticonvulsant | [33] |
Table 5.
Biological activities of Chir seed compounds.
| Compound name | Nature of compound | Structure | Biological activities | Ref |
|---|---|---|---|---|
| α-Pinene | Monoterpene |
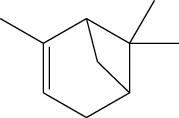
|
Antibiotic resistance modulation, antimicrobial, anti-Leishmania, antitumor, analgesic, antioxidant, anti-inflammatory, and antimalarial | [34] |
| ç-Terpinene | Monoterpene |
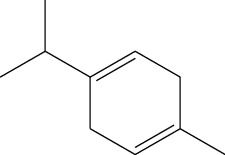
|
Antibacterial | [35] |
| 9-Hexacosene | Alkene |
|
Analgesic and anti-inflammatory | [36] |
| 1-Undecanol | Aliphatic alcohol |
|
Bactericidal, larvicidal, and membrane-damaging activity | [37] |
| 1-Eicosanol | Primary alcohols |
|
Antitumor and antibacterial activity | [38] |
3.3. HPLC Analysis of the Seed Extracts
Six compounds were detected by HPLC-DAD system based on available standards (Supplementary Table S1). The highest quantity was of catechin (10.49 ± 0.32 μg/mg), followed by gallic acid (5.39 ± 0.39 μg/mg), ellagic acid (5.21 ± 0.15 μg/mg), vanillic acid (1.6 ± 0.12 μg/mg), quercetin (0.84 ± 0.04 μg/mg), and EGCG (0.15 ± 0.04 μg/mg) were found in Chilgoza seeds. On the other hand, Chir seeds contained catechin (1.57 ± 0.16 μg/mg), followed by ellagic acid (1.47 ± 0.06 μg/mg), gallic acid (1.31 ± 0.08 μg/mg), quercetin (1.28 ± 0.09 μg/mg), and vanillic acid (0.27 ± 0.02 μg/mg). EGCG was not detected in the Chir sample.
Hoon et al. also reported the highest quantity of catechin in Chilgoza DCM seed extract, but our data on EGCG contradict that obtained by this author [3]. Zulfqar et al. found that the maximum quantity of gallic acid (11.41 ppm) in methanolic extract of P. gerardiana dry nuts and in ethyl acetate extract quercetin was highest (165.33 ppm) [16]. Sadeghi et al. found maximum amounts of epicatechin (10.3 ± 0.18 μg/mg), followed by catechin (10.1 ± 0.18 μg/mg) in Pinus eldarica seeds grown in different regions of the Tehran province in Iran [39]. Mahdhi et al. compared eleven different phenolic compounds identified from P. halepensis methanolic-aqueous seed extract by convection-drying method and sun-drying method and reported that cirsiliol was chief flavonoid component, i.e., (0.761 and 1.916), than luteolin (0.589 and 1.760), followed by catechin (+) (0.569 and 0.888) and luteolin-7-O-glucoside (0.017 and 0.148) mg/100 g of dry weight, respectively [23].
3.4. Antioxidant Activities
The results of the antioxidant activity of Chilgoza and Chir seed extracts are shown in Table 6. Ascorbic acid showed stronger antioxidant activities in both DPPH (326.70 ± 9.64 μg/mL) and H2O2 (375.73 ± 11.73 μg/mL) assays as compared to both seed powder extracts tested. Comparing both extracts, the antioxidant potential of Chilgoza seed extracts in both DPPH and H2O2 assays was higher compared to that of Chir seed sample extract, which seems to be attributed to the presence of the high amount of antioxidant phytocompounds in Chilgoza samples as detected through the HPLC-DAD system, although the TPC in Chir seed samples was almost double than Chilgoza seeds.
Table 6.
Antioxidant activity of Chilgoza and Chir seed extracts on DPPH and H2O2 assays.
| Plant name | Plant part extract | Mean IC50 value μg/mL ± standard deviation | |
|---|---|---|---|
| DPPH | H2O2 | ||
| Pinus gerardiana (Chilgoza) | Seed | 429.15 ± 3.80 | 575.16 ± 19.88 |
| Pinus roxburghii (Chir) | Seed | 552.60 ± 13.03 | 618.94 ± 21.45 |
| Ascorbic acid (control) | DPPH (326.70 ± 9.64 μg/mL) | H2O2 (375.73 ± 11.73 μg/mL) | |
However, earlier studies reported on pine nuts' phytochemical composition analyzes the presence of tocopherols, carotenoids, phytosterols, linoleic acids, and vitamin C, all of them revealing strong antioxidant potential, being their concentration higher than that in phenolic compounds [3, 9, 25]. The results obtained by DPPH assay to Chilgoza seeds DPPH were found accordingly with Hoon et al., who reported that an IC50 value of DCM seed extract was as good as compared to EtOAc, EtOH, HEX, and MeOH extracts. Moreover, Chilgoza water extract results were much better [3]. Zulfqar et al. reported that the percentage of DPPH inhibition of both MeOH and EtOAc extract of P. gerardiana dry nuts was 76.33 ± 2.51% and 73.67 ± 2.75% at concentration of 10 mg/mL and was statistically insignificant [16]. Valero-Galván et al. reported that methanol seed extract of P. cembroides grown in the five states of Mexico revealed different antioxidant activity assessed by DPPH assay [24]. P. halepinsis found in Palestine region displayed that methanolic extract by maceration and Soxhlet extraction method showed IC50 of 0.12 mg/mL and 0.43 mg/mL, respectively [10]. Mahdhi et al. studied maximum antioxidant activities from P. halepensis methanolic-aqueous seed extracts by using DPPH at concentration of 0.08 mg/mL [23]. Su et al. reported that Pinus koraiensis seed (PKS) ethanol extract displayed significant scavenging activity on 2,2-diphenyl-picrylhydrazyl (DPPH) (EC50, 0.023 ± 0.004 mg/mL) and significant suppressive effect on lipid peroxidation in liver as well as enhance the glutathione (GSH) and superoxide dismutase (SOD) antioxidant enzyme levels and reduce malondialdehyde (MDA) content in the brain and liver of rat [27]. Stem bark hydro alcoholic extract of P. roxburghii, P. wallichiana, and P. gerardiana showed significant IC50 value (μg/mL) against DPPH at concentrations 97.54, 111.40, and 102.86, and ascorbic acid showed value at 18, respectively, and H2O2 (μg/mL) showed IC50 value at 86.90, 84.18, and 81.83 while ascorbic acid showed 16.72, respectively [40].
3.5. Antibacterial Activity
The DCM extract of both Chilgoza and Chir seeds was found effective against Gram negative bacteria, viz., S. typhimurium MTCC. 3224, K. pneumonia MTCC. 109, and E. coli MTCC. 443. Results of seed extracts of Chilgoza and Chir antibacterial activity are shown in Table 7. Colistin was used as a positive control (MIC value was 8 μg/mL).
Table 7.
Antibacterial activities of Chilgoza and Chir seed extracts.
| Plant name | Plant part extract/MIC μg/mL | Bacteria |
| Pinus gerardiana (Chilgoza) | Seed/128 | Salmonella typhimurium MTCC 3224 |
| Pinus gerardiana (Chilgoza) | Seed/128 | Klebsiella pneumonia MTCC 109 |
| Pinus gerardiana (Chilgoza) | Seed/256 | Escherichia coli MTCC 443 |
| Plant name | Plant part extract/MIC μg/mL | Bacteria |
| Pinus roxburghii (Chir) | Seed/128 | Salmonella typhimurium MTCC 3224 |
| Pinus roxburghii (Chir) | Seed/64 | Klebsiella pneumonia MTCC 109 |
| Pinus roxburghii (Chir) | Seed/128 | Escherichia coli MTCC 443 |
Both Chilgoza and Chir seed antibacterial potentials were expected due to the occurrence of the antimicrobial compounds 3-carene, 2,4-di-tert-butylphenol, 1-hexyl-1-nitrocyclohexane, naphthalene, α-pinene, γ-terpinene, 1-undecanol, and 1-eicosanol, as reported in Tables 4 and 5. The possible target sites of phytocompounds in microbes are cell membrane, cell wall, and different enzymes. Salim et al. found that the antibacterial activities of P. halepinsis ethanolic seed extracts displayed good inhibition percentage against bacteria Staphylococcus aureus, E. coli, and Shigella at range of 0.02 g/mL [10]. Sharma et al. reported the antibacterial activity against Pseudomonas aeruginosa, K. pneumonia, and E. coli to the bark hydroalcoholic extract of three types of pine species, viz., P. roxburghii (Chir), P. wallichiana (kail), and P. gerardiana (Chilgoza) by well diffusion method, despite P. wallichiana displayed the most prominent antibacterial activity [40].
4. Conclusion and Future Perspectives
Secondary metabolites present in pine seed extracts are coated with excellent biological properties. In this study, the DCM seed extract of P. gerardiana and P. roxburghii revealed to be a rich source of molecules with interesting antioxidant and antimicrobial effects. The obtained results are positive and, if supported by in vivo studies, may be further proposed to be used for therapeutic purposes. In the near future, deeper studies on this field should be done, and other biological effects of such trees' extracts should also be carried out in experimental trials. Other studies should also be done to a better understanding of the impact of seed collection from different regions with altitudinal variation, in addition to correlation with climate, soil, and regional geographic data in chemical composition. Equally important will be to perform a combined analysis of protein, amino acid, minerals, and lipid profiles to reach a more clear understanding on the real potentialities of these less investigated trees.
However, the Chilgoza seeds are eaten in roasted form in several countries, but still Chilgoza and Chir seeds are not utilized in functional food development. Recently, our group has developed the cookies having Chilgoza and Chir seeds used in decorated form to enhance its nutritional value [41]. Still there is a need to develop functional/nutraceutical foods using Chilgoza and Chir seeds which ultimately gives new employment horizon to the hilly area people.
The limitation of the current study is the selection of extraction solvent. We believe that DCM solvent has not much compatibility with our seed samples; that was the reason we got antioxidants and antibacterial activity at higher concentration.
Acknowledgments
We gratefully acknowledge the open access funding by the Scientific Grant Agency (VEGA Project 1/0482/20), the Excellence Project PrF UHK 2011/2021-2022, and the MH CZ-DRO (UHHK, 00179906) for the financial support.
Contributor Information
Kamil Kuča, Email: kamil.kuca@uhk.cz.
Prerna Bhardwaj, Email: prernabhardwaj135@gmail.com.
Data Availability
The data used to support the findings of this study are available from the corresponding authors from request.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Authors' Contributions
PB and KK conceptualized and designed the project. KB performed all experiments and wrote the main manuscript text. RS analyzed the data. NC-M, MV, and NKU critically analyzed the data and drafted the final manuscript. MV and KK arranged the funds.
Supplementary Materials
Table S1: retention time of different flavonoids.
References
- 1.Richardson D. M., Rundel P. W., Jackson S. T., et al. Human impacts in pine forests: past, present and future. Annual Review of Ecology, Evolution, and Systematics . 2007;38(1):275–297. doi: 10.1146/annurev.ecolsys.38.091206.095650. [DOI] [Google Scholar]
- 2.Kanchan B., Prerna B., Simran K. Medicinal value of secondary metabolites of pines grown in Himalayan region of India. Research Journal of Biotechnology . 2020;15:131–140. [Google Scholar]
- 3.Hoon L. Y., Choo C., Watawana M. I., Jayawardena N., Waisundara V. Y. Evaluation of the total antioxidant capacity and antioxidant compounds of different solvent extracts of Chilgoza pine nuts (Pinus gerardiana) Journal of Functional Foods . 2015;18:1014–1021. doi: 10.1016/j.jff.2014.07.009. [DOI] [Google Scholar]
- 4.Sharma A., Sharma L., Goyal R. A review on Himalayan pine species: ethnopharmacological, phytochemical and pharmacological aspects. Pharmacognosy Journal . 2018;10(4):611–619. doi: 10.5530/pj.2018.4.100. [DOI] [Google Scholar]
- 5.Awan H. U. M., Pettenella D. Pine nuts: a review of recent sanitary conditions and market development. Forests . 2017;8(10):p. 367. doi: 10.3390/f8100367. [DOI] [Google Scholar]
- 6.Kaushik D., Aggarwal A., Kaushik P., Mehra R., Rana A. C. Pinus roxburghii- incredible gift in the lap of Himalayas. International Journal of Pharmacognosy and Phytochemical Research . 2010;2:29–35. [Google Scholar]
- 7.Kumar R., Shamet G. S., Chaturvedi O. P., Avasthe R. K., Singh C. Ecology of Chilgoza pine (Pinus gerardianaWall.) in dry temperate forests of North West Himalaya. Ecology, Environment & Conservation . 2013;19:1063–1066. [Google Scholar]
- 8.Peltier R., Dauffy V. The Chilgoza of Kinnaur. Influence of the Pinus gerardiana edible seed market chain organization on forest regeneration in the Indian Himalayas. Fruits . 2009;64(2):99–110. doi: 10.1051/fruits/2009005. [DOI] [Google Scholar]
- 9.Lutz M., Álvarez K., Loewe V. Chemical composition of pine nut (Pinus pinea L.) grown in three geographical macrozones in Chile. CyTA Journal of Food . 2016;15:284–290. doi: 10.1080/19476337.2016.1250109. [DOI] [Google Scholar]
- 10.Salim H., Rimawi W. H., Shahin S. Phytochemical analysis and antibacterial activity of extracts from Palestinian Aleppo pine seeds, bark and cones. Asian Journal of Chemistry . 2019;31(1):143–147. doi: 10.14233/ajchem.2019.21633. [DOI] [Google Scholar]
- 11.Miraliakbari H., Shahidi F. Oxidative stability of tree nut oils. Journal of Agricultural and Food Chemistry . 2008;56(12):4751–4759. doi: 10.1021/jf8000982. [DOI] [PubMed] [Google Scholar]
- 12.Shah D., Gandhi M., Kumar A., Cruz-Martins N., Sharma R., Nair S. Current insights into epigenetics, noncoding RNA interactome and clinical pharmacokinetics of dietary polyphenols in cancer chemoprevention. Critical Reviews in Food Science and Nutrition . 2021;26:1–37. doi: 10.1080/10408398.2021.1968786. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R., Martins N., Kuca K., et al. Chyawanprash: a traditional Indian bioactive health supplement. Biomolecules . 2019;9(5):p. 161. doi: 10.3390/biom9050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson K. J., Teuber S. S., Gobeille A., Cremin P., Waterhouse A. L., Steinberg F. M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. The Journal of Nutrition . 2001;131(11):2837–2842. doi: 10.1093/jn/131.11.2837. [DOI] [PubMed] [Google Scholar]
- 15.Sharma N., Tiwari N., Vyas M., Khurana N., Muthuraman A., Utreja P. An overview of therapeutic effects of vanillic acid. Plant Archives . 2020;20:3053–3059. [Google Scholar]
- 16.Zulfqar F., Akhtar M. F., Saleem A., Akhtar B., Sharif A., Saleem U. Chemical characterization, antioxidant evaluation, and antidiabetic potential of Pinus gerardiana (pine nuts) extracts. Journal of Food Biochemistry . 2020;44(6, article e13199) doi: 10.1111/jfbc.13199. [DOI] [PubMed] [Google Scholar]
- 17.Morini G., Maga J. A. Volatile compounds in roasted and boiled Chinese chestnuts (Castanea molissima) LWT - Food Science and Technology . 1995;28(6):638–640. doi: 10.1016/0023-6438(95)90014-4. [DOI] [Google Scholar]
- 18.Senguttuvan J., Paulsamy S., Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pacific Journal of Tropical Biomedicine . 2014;4:S359–S367. doi: 10.12980/APJTB.4.2014C1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Owaisi M., Al-Hadiwi N., Khan S. H. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pacific Journal of Tropical Biomedicine . 2014;4(12):964–970. doi: 10.12980/APJTB.4.201414B295. [DOI] [Google Scholar]
- 20.Lee B. L., New A. L., Ong C. N. Simultaneous determination of tocotrienols, tocopherols, retinol and major carotenoids in human plasma. Clinical Chemistry . 2003;49:2065–2066. doi: 10.1373/clinchem.2003.022681. [DOI] [PubMed] [Google Scholar]
- 21.Bhatti M. Z., Ali A., Ahmad D., Saeed A., Malik S. A. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Research Notes . 2015;8(1):p. 279. doi: 10.1186/s13104-015-1228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews J. M. Determination of minimum inhibitory concentrations. The Journal of Antimicrobial Chemotherapy . 2001;48(suppl_1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 23.Mahdhi A., Ghazghazi H., El Aloui M., Ben Salem R., Rigane G. Identification and quantification of phenolic and fatty acid profiles in Pinus halepensis mill. seeds by LC-ESI-MS and GC: effect of drying methods on chemical composition. Food Science & Nutrition . 2021;9(4):1907–1916. doi: 10.1002/fsn3.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valero-Galván J., Reyna-González M., Chico-Romero P. A., et al. Seed characteristics and nutritional composition of pine nut from five populations of P. cembroides from the states of Hidalgo and Chihuahua, Mexico. Molecules . 2019;24(11):p. 2057. doi: 10.3390/molecules24112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolling B. W., Chen O. C.-Y., McKay D. L., Blumberg J. B. Tree nut phytochemicals; composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutrition Research Reviews . 2011;24(2):244–275. doi: 10.1017/S095442241100014X. [DOI] [PubMed] [Google Scholar]
- 26.Kadri N., Khettal B., Aid Y., Kherfellah S., Sobhi W., Barragan-Montero V. Some physicochemical characteristics of pinus (Pinus halepensis Mill., Pinus pinea L., Pinus pinaster and Pinus canariensis) seeds from North Algeria, their lipid profiles and volatile contents. Food Chemistry . 2015;188:184–192. doi: 10.1016/j.foodchem.2015.04.138. [DOI] [PubMed] [Google Scholar]
- 27.Su X. Y., Wang Z. Y., Liu J. R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chemistry . 2009;117(4):681–686. doi: 10.1016/j.foodchem.2009.04.076. [DOI] [Google Scholar]
- 28.Dob T., Berramdane T., Chelgoum C. Chemical composition of essential oil of Pinus halepensis Miller growing in Algeria. Comptes Rendus Chimie . 2005;8(11-12):1939–1945. doi: 10.1016/j.crci.2005.05.007. [DOI] [Google Scholar]
- 29.Znati M., Jabrane A., Hajlaoui H., et al. Chemical composition and in vitro evaluation of antimicrobial and anti-acetylcholinesterase properties of the flower oil of Ferula lutea. Natural Product Communications . 2012;7(7):947–950. [PubMed] [Google Scholar]
- 30.Zhao F., Wang P., Lucardi R. D., Su Z., Li S. Natural sources and bioactivities of 2, 4-di-tert-butylphenol and its analogs. Toxins (Basel) . 2020;12(1):p. 35. doi: 10.3390/toxins12010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvamangai G., Bhaskar A. GC-MS analysis of phytocomponents in the methanolic extract of Eupatorium triplinerve. Asian Pacific Journal of Tropical Biomedicine . 2012;2(3):S1329–S1332. doi: 10.1016/S2221-1691(12)60410-9. [DOI] [Google Scholar]
- 32.Premathilaka R., Silva M. Bioactive compounds and antioxidant activity of Bunchosia armenica. World Journal of Pharmaceutical Sciences . 2016;5:1237–1247. [Google Scholar]
- 33.Makar S., Saha T., Singh S. K. Naphthalene, a versatile platform in medicinal chemistry: sky-high perspective. European Journal of Medicinal Chemistry . 2019;161:252–276. doi: 10.1016/j.ejmech.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Salehi B., Upadhyay S., Orhan E. I., et al. Therapeutic potential of α-and β-pinene: a miracle gift of nature. Biomolecules . 2019;9(11):p. 738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sbayou H., Ababou B., Boukachabine K., Manresa A., Zerouali K., Amghar S. Chemical composition and antibacterial activity of Artemisia herba-alba and Mentha pulegium essential oils. Journal of Life Sciences . 2014;8:35–41. [Google Scholar]
- 36.Githinji C. G., Mbugua P. M., Kanui T. I., Kariuki D. K. Analgesic and anti-inflammatory activities of 9-hexacosene and stigmasterol isolated from Mondia whytei. Phytopharm . 2012;2:212–223. [Google Scholar]
- 37.Togashi N., Shiraishi A., Nishizaka M., et al. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules . 2007;12(2):139–148. doi: 10.3390/12020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee S., Karmakar A., Azmi S. A., Barik A. Antibacterial activity of long-chain primary alcohols from Solena amplexicaulis leaves. Proceedings of the Zoological Society (Calcutta) . 2018;71(4):313–319. doi: 10.1007/s12595-017-0208-0. [DOI] [Google Scholar]
- 39.Sadeghi A. M., Fallah H. H., Tejalizadekhoob Y., et al. Determination of phenolic compounds in Pinus eldarica by HPLC. Journal of Medicinal Plants . 2014;13:1–12. [Google Scholar]
- 40.Sharma A., Goyal R., Sharma L. Potential biological efficacy of Pinus plant species against oxidative, inflammatory and microbial disorders. BMC Complementary and Alternative Medicine . 2016;16:1–11. doi: 10.1186/s12906-016-1011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhardwaj K., Bhardwaj P., Reddy R., Pathera A. Formulation of Chir and Chilgoza nuts cookies. Indian patent filing No-202211026889 . Intellectual Property of India; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: retention time of different flavonoids.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors from request.


