Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible and progressive airflow limitation and encompasses a spectrum of diseases, including chronic obstructive bronchitis and emphysema. Pyroptosis is a unique form of inflammatory cell death mediated by the activation of caspase-1 and inflammasomes. The long non-coding RNA (lncRNA) growth arrest-specific 5 (GAS5) is a well-documented tumor suppressor, which is associated with cell proliferation and death in various diseases. The aim of the present study was to evaluate whether lncRNA GAS5 is associated with the pyroptosis in COPD. To create a COPD cell model, MRC-5 cells were treated with 10 µg/ml lipopolysaccharide (LPS) for 48 h. Then the level of pro-caspase 1, caspase 1, IL-1β, IL-18, NLRP3 and cleaved gasdermin D (GSDMD) was examined by western blotting. GAS5 mRNA level was detected by qualitative PCR following LPS treatment in MRC-5 cells. Subsequently, IL-2, IL-6, IL-10 and TNF-α in MRC-5 cells was measured by ELISA. Then the proliferation ability of MRC-5 cells was detected by CCK-8. Cell death was detected by TUNEL assay. LDH release was measured using an LDH Cytotoxicity Assay kit. The Magna RIP kit was used to validate the interaction between GAS5 and miR-223-3p. The present study revealed that increased expression levels of caspase-1, IL-1β, IL-18 and cleaved GSDMD were observed in LPS-treated MRC-5 cells, indicating that pyroptosis is involved in COPD progression. Additionally, LPS induced the increase in GAS5 mRNA expression levels and the release of inflammatory factors (IL-2, IL-6, IL-10 and TNF-α), suggesting that GAS5 is implicated in pyroptosis in COPD. Furthermore, upregulation of GAS5 promoted cell death and inhibited proliferation in the MRC-5 cell line. Additionally, increased GAS5 expression significantly promoted the production of caspase-1, IL-1β, IL-18, cleaved GSDMD and NLR pyrin domain containing protein 3 (NLRP3). A dual-luciferase assay demonstrated that GAS5 could directly bind to microRNA-223-3p (miR-223-3p), and NLRP3 is a direct target of miR-223-3p. Furthermore, GAS5 reduced the expression levels of miR-223-3p, while it increased the expression levels of NLRP3. The present study concluded that lncRNA GAS5 promoted pyroptosis in COPD by targeting the miR-223-3p/NLRP3 axis, implying that GAS5 could be a potential target for COPD.
Keywords: chronic obstructive pulmonary disease, human lung fibroblast cell line, pyroptosis, microRNA-223-3p, NLR family pyrin domain containing 3
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disorder that causes the death of >3 million individuals worldwide every year (1). COPD will be the third leading cause of death in the world by 2030 (2). COPD is characterized by persistent irreversible airflow limitation due to the loss of elastic fibers from small airways and alveolar walls (3). The limitation is related to the chronic inflammatory response of the respiratory tract and lungs to harmful particles or gases (4). There is increasing evidence that persistent airflow limitation is closely related to airway remodeling, which occurs mainly in small airways with an inner diameter of <2 mm (5–7).
Pulmonary fibroblasts are closely related to COPD airway remodeling. Fibrosis-related factors produced by pulmonary fibroblasts, such as collagen fibers, elastic fibers, reticular fibers and constantly secreted matrix collagen, are increased during COPD airway remodeling, which affects the protease-antiproteinase balance (8). Various cytokines and inflammatory mediators produced by fibroblasts also enhance the tissue inflammation (9). Pulmonary fibroblasts have been shown to contribute to airway remodeling in COPD through synthesis and secretion of the main components of the extracellular matrix (9). Pulmonary fibroblasts are transformed into pulmonary myofibroblasts activated by TGF-β1 (10,11). Myofibroblast differentiation, characterized by ECM production and α-smooth muscle actin expression, is one of the primary molecular mechanisms of airway remodeling in COPD (12). However, the mechanisms of fibroblasts in COPD remain poorly understood.
Cell death may serve a vital role in the pathogenesis of COPD (13). Increased cell death can be observed during destruction of lung tissue in both humans and mice (14,15). Pyroptosis, mediated by the activation of caspase-1 and the inflammasome, is a unique form of inflammatory cell death (16). Activated caspase-1 proteolytically cleaves inactive pro-IL-1β and pro-IL-18 into the mature inflammatory cytokines IL-1β and IL-18, which induce the inflammasome activation-dependent pyroptotic cell death, which results in the production of inflammatory chemokines and TNF-α (17). Gasdermin D (GSDMD), a substrate of caspase-1, has been identified as the pyroptosis executioner (18). Inflammasomes, important products of the inflammatory reaction, serve a vital role in pyroptosis. NLR pyrin domain containing protein 3 (NLRP3) is one of the most widely studied inflammasomes and contains a caspase recruitment domain (19). Inflammasome activators, including extracellular ATP, reactive oxygen species and damage-associated molecular patterns, are also increased in the airways of patients with COPD (20). Pyroptosis induced by triggering receptor expressed on myeloid cells 1-mediated activation of the NLRP3 inflammasome aggravates COPD development (21). In addition, cigarette smoke extract-induced pyroptosis is also involved in the progression of COPD (22). These results suggest a potential critical role of pyroptosis in COPD progression.
Long non-coding RNAs (lncRNAs) are noncoding RNAs longer than 200 nucleotides with limited coding potential. They serve crucial roles in various biological processes, including cell apoptosis, invasion, proliferation, migration and therapeutic responsiveness, by regulating gene expression (23). Importantly, lncRNAs drive multiple important disease phenotypes in different ways (24), such as RNA decay, transcription regulation, microRNA (miRNA/miR) sponging and epigenetic modification (25). One of the major mechanisms of lncRNAs is functioning as competing endogenous RNAs (ceRNAs) in a regulatory network, including lncRNAs, miRNAs and target mRNAs (26). ceRNAs compete for miRNA binding and block miRNA-mediated target gene silencing (27).
lncRNAs are widely involved in the pathogenesis of COPD. The lncRNA small nuclear RNA host gene 5 (SNHG5) ameliorates the effects of cigarette smoke extract on the proliferation, apoptosis and inflammation of a COPD cell model via the miR-132/PTEN axis in COPD (28), while lncRNA MIR155HG promotes the polarization of M1 macrophage and the release of pro-inflammatory cytokines in COPD (29). Furthermore, long intergenic non-protein coding RNA 987 mitigates COPD by regulating lipopolysaccharide (LPS)-caused cell apoptosis, oxidative stress, inflammation and autophagy via the Let-7b-5p/sirtuin1 axis (30). Growth arrest-specific 5 (GAS5), a well-documented lncRNA, is an important factor associated with cell proliferation and death in various diseases (31,32). GAS5 acts as a ceRNA to sponge miR-223-3p in certain diseases. For example, GAS5 promotes the microglial inflammatory response in Parkinson's disease by targeting the miR-223-3p/NLRP3 pathway, and low expression levels of GAS5 injure myocardial cells and are associated with the progression of chronic heart failure by regulating miR-223-3p (33,34). Previous studies suggest that GAS5 serves a vital role in cell death (35–38). Nevertheless, it has not been elucidated if lncRNA GAS5 is involved in cell death in COPD.
The present study investigated the effect of lncRNA GAS5 on a cell model of COPD, as well as whether lncRNA GAS5 is involved in pyroptosis and the underlying molecular mechanisms.
Materials and methods
Cell culture
The MRC-5 human lung fibroblast cell line (American Type Culture Collection) was cultured in DMEM (cat. no. 12430054; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (cat. no. 10100147; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (cat. no. 15070063; Thermo Fisher Scientific, Inc.). The cells were grown in 75-cm2 flask at 37°C in a humidified atmosphere with 5% CO2 and were passaged 1:3 using 0.25% trypsin (cat. no. 1672868; Thermo Fisher Scientific, Inc.) when they reached 80–90% confluence. LPS was purchased from Sigma-Aldrich; Merck KGaA (cat. no. L2880). MRC-5 cells were stimulated with 10 µg/ml LPS for 48 h at 37°C (39) in the present study. Cells not treated with LPS were used as Control.
Western blotting
Proteins were extracted from MRC-5 cells by RIPA lysis buffer (Beyotime Institute of Biotechnology; cat. no. P0013B) and quantified using BCA. Proteins (30 µg per lane) were separated by SDS-PAGE on a 10% gel and subsequently transferred onto a PVDF membrane, which were blocked with 5% skimmed milk for 1 h at room temperature. The following primary antibodies were employed overnight at 4°C: Rabbit anti-caspase-1 (1:500 dilution; cat. no. BM4291; Boster Biological Technology), rabbit anti-pro-caspase-1 (1:1,000 dilution; cat. no. ab179515; Abcam), rabbit anti-IL-18 (1:500 dilution; cat. no. M00124; Boster Biological Technology), rabbit anti-IL-1β (1:500 dilution; cat. no. A1112; ABclonal Biotech Co., Ltd.), rabbit anti-cleaved N-terminal GSDMD (1:1,000 dilution; cat. no. ab215203; Abcam) and mouse anti-GAPDH (1:5,000 dilution; cat. no. A00227-1; Boster Biological Technology). Horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG (1:5,000 dilution; cat. no. BM2002 and BA1070; Boster Biological Technology) was used as a secondary antibody for 1 h at room temperature. Immunoreactive protein bands were detected by ECL hypersensitive chemiluminescence kit (cat. no. P0018M; Beyotime Institute of Biotechnology) with an Odyssey Scanning System (version 3.0, LI-COR Biosciences).
The third-generation lentiviral vector construction and lentiviral infection
pcDNA3.1 (+) GAS5 overexpression vector (OE-GAS5) were designed and constructed based on human GAS5 full-length coding protein sequences (GenBank accession number NC_000001) by Shanghai GeneChem Co., Ltd. Then, 20 µM OE-GAS5 or overexpression negative control lentivirus (NC) vectors were transfected into MRC-5 cells with Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. MRC-5 cells were transfected at 37°C for 8 h and were used in subsequent experiments 48 h post-transfection.
Quantitative PCR (qPCR)
Total RNA was isolated from MRC-5 cells using TRIzol® (cat. no. 15596026; Thermo Fisher Scientific, Inc.). The concentration and quality of RNA were determined using an ND-2000 Spectrophotometer. The extracted RNA was reverse-transcribed into cDNA using a HiScript Reverse Transcriptase kit (Vazyme Biotech Co., Ltd, China). Thermocycling conditions for RT-PCR were 25°C for 5 min, 50°C for 45 min and 85°C for 2 min, followed by holding at 4°C. quantitative PCR (qPCR) was performed with the KAPA SYBR FAST qPCR kit (Kapa Biosystems) using a SimpliAmp™ PCR System (Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: 95°C for 5 min, followed by 40 cycles at 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec. The primers for qPCR were designed using Primer 5.0 (Premier Biosoft International). The primer pairs used were as follows: GAPDH (gene accession no. AF275320) forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′; GAS5 (gene accession no. NR_152533) forward, 5′-GTGTCCCCAAGGAAGGATGA-3′ and reverse, 5′-GTAGTCAAGCCGACTCTCCA-3′; NLRP3 (gene accession no. AB120959) forward, 5′-GCTGGCATCTGGATGAGGAA-3′ and reverse, 5′-GTGTGTCCTGAGCCATGGAA-3′; hsa-miR-223-3p-RT, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGGGGT-3′; hsa-miR-223-3p forward, 5′-TTGTCAGTTTGTCAA-3′ and reverse, 5′-CAGTGCAGGGTCCGAGGT-3′; hsa-miR-223-3p mimic, 5′-UGUCAGUUUGUCAAAUACCCC-3′; hsa-miR-223-3p inhibitor, 5′-GGGGUAUUUGACAAACUGACA-3′; hsa-miRNA mimic NC, 5′-UUUGUACUACACAAAAGUACUG-3′; hsa-miRNA inhibitor NC, 5′-CAGUACUUUUGUGUAGUACAAA-3′; hsa-U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. GAPDH and small nuclear RNA U6 were used as internal references to normalize the expression of mRNA and miRNA, respectively. The samples were analyzed in triplicate. The data were quantified using the 2−ΔΔCq method (40).
Immunofluorescence analyses
Immunofluorescence staining of MRC-5 cells was performed as previously described (41). MRC-5 cells plated onto cover glasses were fixed with 4% paraformaldehyde for 15 min and then blocked in 5% BSA for 1 h both at room temperature. Fixed MRC-5 cells were incubated at 4°C overnight with rabbit anti-caspase-1, followed by incubation with anti-rabbit IgG 594 secondary antibodies (1:200 dilution; cat. no. ab150080; Abcam) for 1 h at 37°C. The images were acquired using a fluorescence microscope (Nikon E800; Nikon Corporation) equipped with a digital camera (1200F; Nikon Corporation) and image acquisition software (NIS-Elements AR 3.2; Nikon Corporation). A total of three independent samples in each group were included for quantitative analysis in the present study.
Apoptosis analysis
MRC-5 cells were treated with 10 µg/ml LPS for 48 h. The cells were subjected to a TUNEL assay according to the manufacturer's instructions (Dead End Fluorometric TUNEL System; Promega Corporation). Briefly, treated MRC-5 cells were fixed with 4% paraformaldehyde phosphate at 4°C for 10 min. Subsequently, the cells were rinsed briefly with PBS, and then permeabilized with 0.1% Triton X-100 for 2 min on ice. Then, the cells were incubated with TUNEL reagent in the dark for 1 h at 37°C. The cells were rinsed with PBS and counterstained with 10 µg/ml DAPI at 37°C for 5 min. After the sections were sealed with neutral resin (cat. no. 96949-21-2; Sinopharm Chemical Reagent Co., Ltd.), fluorescence in three random fields of view was measured using a fluorescence microscope (Nikon E800; Nikon Corporation) equipped with a digital camera (1200F; Nikon Corporation) and image acquisition software (NIS-Elements AR 3.2, Nikon Corporation).
Cell Counting kit-8 (CCK-8)
MRC-5 cells were routinely cultured in 96-well plates (4×104 cells/well) for 24 h. Subsequently, 10 µl Cell Counting kit-8 (CCK-8) solution (Dojindo Laboratories, Inc.) was added to the medium and the cells were incubated for 2 h at 37°C with 5% CO2. The optical density value was measured at a wavelength of 450 nm with a Spectrafluor microreader plate (Molecular Devices, LLC). These experiments were repeated three times.
Bioinformatics analysis
StarBase database v2.0 (https://starbase.sysu.edu.cn/index.php/) was used to predict the binding site of GAS5 and miR-223-3p. miR-223-3p and NLRP3 target site was predicted using RNAhybrid v2.2 (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid).
Luciferase activity assay
The binding of GAS5 with miR-223-3p was validated using dual-luciferase reporter assay. Hsa-miR-223-3p mimics and hsa-miR-223-3p inhibitor were designed and synthesized by Shanghai GenePharma Co., Ltd. as follows: Mimics, 5′-UGUCAGUUUGUCAAAUACCCC-3′; mimic NC, 5′-UUUGUACUACACAAAAGUACUG-3′; miR-223-3p inhibitor, 5′-GGGGUAUUUGACAAACUGACA-3′ and inhibitor NC, 5′-CAGUACUUUUGUGUAGUACAAA-3′. Wild-type (WT) and mutant (MUT) GAS5 or NLRP3 3′ untranslated regions were overexpressed and then cloned into the luciferase reporter vector pmirGLO (Promega Corporation). 293T cells were cultured at 37°C overnight to 70–80% confluence, then ~2×105 cells were seeded into 24-well plates. After culturing for 24 h, 293T cells were co-transfected with luciferase plasmids and miR-223-3p mimics using Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.). After 48 h, luciferase activity was determined using a Dual-Luciferase Reporter Assay kit (cat. no. FR201-01; TransGen Biotech Co., Ltd.) by Eppendorf BioSpectrometer fluorescence (BioPhotometer® D30; Eppendorf), and firefly luciferase activity was normalized against Renilla luciferase activity.
Lactate dehydrogenase (LDH) assay
LDH release was measured using an LDH Cytotoxicity Assay kit (cat. no. C0017; Beyotime Institute of Biotechnology) as previously described (42). Supernatants from Control, NC and OE-GAS5 MRC-5 cells were collected and 10% (vol/vol) LDH release reagent was added. LDH release was measured at an optical density of 490 nm using the Eppendorf BioSpectrometer fluorescence. Cytotoxicity was calculated as % × (experimental LDH release-cell spontaneous LDH release)/(maximum LDH release-cell spontaneous LDH release).
ELISA
Suitably treated MRC-5 cells were cultured for 24 h in DMEM with 10% FBS. The supernatant was collected to detect the levels of IL-2, IL-6, IL-10 and TNF-α using their respective kits according to the manufacturer's instructions. IL-2 Human ELISA kit (cat. no. EH2IL22), IL-6 Human ELISA kit (cat. no. EH2IL6), IL-10 Human ELISA kit (cat. no. EHIL10) and TNF-α Human ELISA kit (cat. no. BMS223HS) were purchased from Thermo Fisher Scientific, Inc. The calibration curves were plotted on semi-log papers, and the optical density values of samples were calculated from the standard curve for three assays.
RNA immunoprecipitation (RIP)
To validate the interaction between GAS5 and miR-223-3p binding proteins, the Magna RIP kit (cat. no. 17–700; Millipore) was used as previously described (43). MRC-5 cells (1×107 in each group) were washed with cold PBS and harvested with cell scrapers. MRC-5 cells were lysed using RIP Lysis Buffer (150 mM KCl, 25 mM Tris pH 7.4, 5 mM EDTA, 0.5% NP40, 100 µl for per IP reaction) supplemented with RNase and protease cocktail inhibitor, followed by centrifugation at 12,000 × g and 4°C for 1 min. The supernatants were collected and incubated with 5 µg Argonaute-2 antibody (1:1,000, cat. no. 233727; Abcam) or control IgG to capture the RNAs used for qPCR. Magnetic beads for immunoprecipitation were prepared according to the kit instructions. The RNA binding protein-RNA complexes were immunoprecipitated with premade magnetic beads at 4°C overnight with agitation. Then the immunocomplex was precipitated using protein G Dynabeads (40 µl for per IP reaction). After washing the beads with ice-cold RIP Wash Buffer from the Magna RIP kit, the RNA binding proteins were digested with proteinase K at 55°C for 30 min with shaking. The purified RNA was isolated with TRIzol® reagent (cat. no. 15596018; Thermo Fisher Scientific, Inc.) and reverse transcribed, and the relative gene expression of GAS5 and miR-223-3p was measured by qPCR, which was performed as described above.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (8.0; GraphPad Software, Inc.). Each experiment was repeated three times. Data are presented as the mean ± standard deviation. Unpaired Student's t-test was used for two group comparisons and one-way ANOVA followed by Duncan's post hoc test was used for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
MRC-5 cells display characteristic features of pyroptosis after LPS treatment
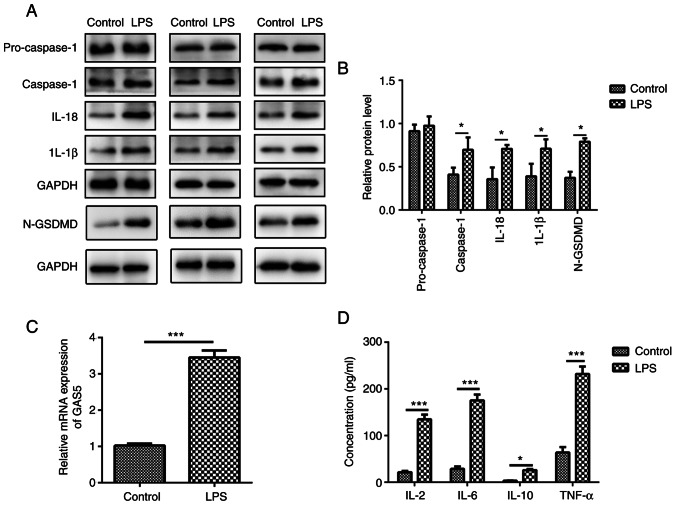
Pyroptosis is a unique form of inflammatory cell death, which depends on the activation of caspase-1, IL-1β and IL-18 (16). To elucidate the relationship between COPD and pyroptosis, the present study revealed that after 10 µg/ml LPS treatment, the expression levels of caspase-1, IL-1β, IL-18 and cleaved N-terminal GSDMD were increased in MRC-5 human lung fibroblast cells (Fig. 1A and B).
Figure 1.
LPS treatment induces pyroptosis in MRC-5 cells. MRC-5 cells were treated with 10 µg/ml LPS for 48 h. (A) Protein expression levels of pro-caspase-1, caspase-1, IL-1β, IL-18 and N-GSDMD in LPS-treated MRC-5 cells were measured using western blotting; three sets of representative blots are shown. (B) Semi-quantification of gene expression normalized to GAPDH. (C) GAS5 mRNA expression levels in LPS-treated MRC-5 cells. (D) Quantitative measurement of IL-2, IL-6, IL-10 and TNF-α in LPS-treated MRC-5 cells using ELISA. The results are presented as the mean ± SD (n=3; *P<0.05, ***P<0.001 vs. control). LPS, lipopolysaccharide; GAS5, growth arrest-specific 5; N-GSDMD, cleaved N-terminal gasdermin D.
LPS induces GAS5 expression
lncRNA GAS5, a well-known tumor suppressor lncRNA, aggravates apoptosis and inflammation in several human diseases, including myocardial infarction and multiple sclerosis (7,44,45). However, whether lncRNA GAS5 is involved in LPS-induced pyroptosis of MRC-5 cells is not fully understood. The level of GAS5 was significantly increased (3.31±0.48) after LPS treatment in MRC-5 cells compared with that in the control group (1.05±0.09) (Fig. 1C; P<0.001), indicating that GAS5 is involved in the pyroptosis of MRC-5 cells. Additionally, LPS exposure markedly increased the levels of pro-inflammatory factors IL-2, IL-6, IL-10 and TNF-α in MRC-5 cells (Fig. 1D). The results demonstrated that lncRNA GAS5 might be associated with the release of inflammatory factors that induce pyroptosis.
Upregulation of lncRNA GAS5 induces pyroptosis
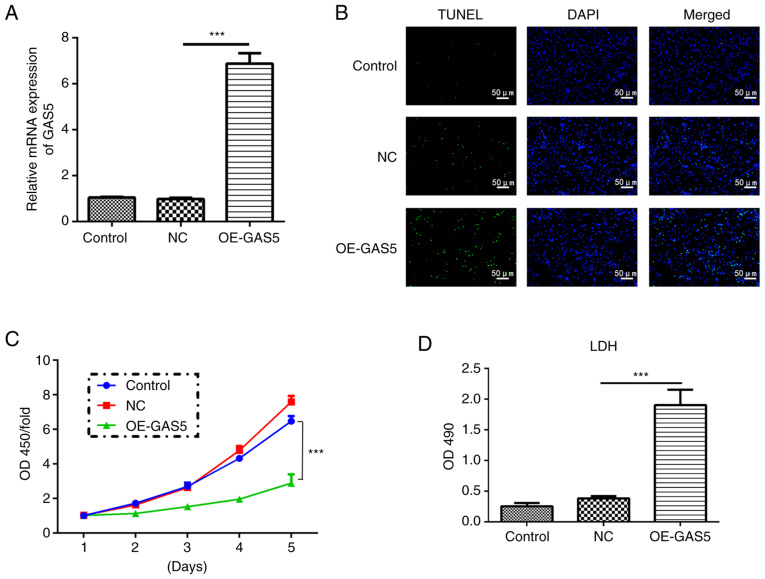
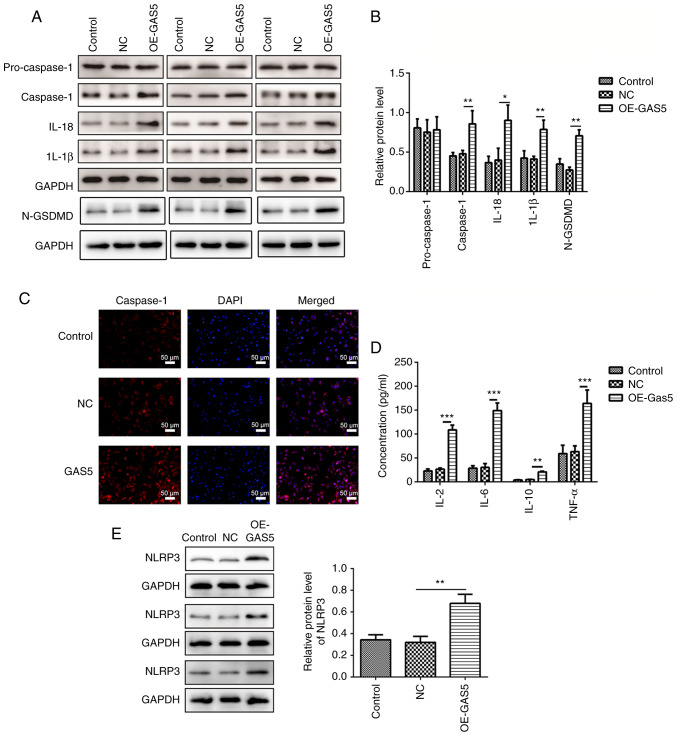
To explore the role of lncRNA GAS5 in pyroptosis, lncRNA GAS5 was first overexpressed (OE-GAS5) and it was demonstrated that the mRNA expression level of the lncRNA GAS5 group was 7-fold higher than that of the control and negative control (NC) groups (Fig. 2A). Upregulation of GAS5 efficiently induced cell death assessed by a TUNEL assay, while it significantly inhibited cell proliferation at the fifth day as examined by a CCK-8 assay in MRC-5 cells (Fig. 2B and C). In addition, highly expressed GAS5 significantly enhanced the release of LDH, an inflammatory response marker, suggesting an increased inflammatory response (Fig. 2D). Overexpression of lncRNA GAS5 significantly increased the protein levels of caspase-1, IL-1β, IL-18 and cleaved N-terminal GSDMD (Fig. 3A and B). Immunofluorescence analyses revealed that upregulation of lncRNA GAS5 promoted the expression of caspase-1 in MRC-5 cells (Fig. 3C). Furthermore, overexpression of GAS5 significantly promoted the expression of the pro-inflammatory factors IL-2, IL-6, IL-10 and TNF-α (Fig. 3D). Additionally, overexpression of lncRNA GAS5 notably increased the level of NLRP3 (Fig. 3E), one of the key elements in the NLRP3 inflammasome complex (17).
Figure 2.
GAS5 induces cell death and inhibits the proliferation of MRC-5 cells. (A) mRNA expression levels of GAS5 were evaluated after transfection of lentivirus vectors containing human GAS5 full-length. (B) GAS5 overexpression in MRC-5 cells induced apoptosis as measured using a TUNEL assay. Scale bar, 50 µm. (C) Cell proliferation quantified in GAS5-overexpressing MRC-5 cells. (D) Cell death was assessed by lactate dehydrogenase release. The results are presented as the mean ± SD (n=3; ***P<0.001 vs. control). GAS5, growth arrest-specific 5; LDH, lactate dehydrogenase; OD, optical density; OE, overexpression; NC, negative control.
Figure 3.
Overexpression of lncRNA GAS5 induces characteristic features of pyroptosis in MRC-5 cells. (A) Protein expression levels of pro-caspase-1, caspase-1, IL-1β, IL-18 and N-GSDMD in GAS5-overexpressing MRC-5 cells were measured using western blotting and three sets of representative blots are shown. (B) Semi-quantification of gene expression normalized to GAPDH. (C) Immunofluorescence staining showing the levels of caspase-1 in GAS5-overexpressing MRC-5 cells. Scale bar, 50 µm. (D) Quantitative measurement of IL-2, IL-6, IL-10 and TNF-α in GAS5-overexpressing MRC-5 cells using ELISA. (E) Protein expression levels of NLRP3 in GAS5-overexpressing MRC-5 cells were measured using western blotting. Semi-quantification of NLRP3 expression normalized to GAPDH and three sets of representative blots are shown. The results are presented as the mean ± SD (n=3; *P<0.05, **P<0.01, ***P<0.001 vs. control). N-GSDMD, cleaved N-terminal gasdermin D; GAS5, growth arrest-specific 5; lncRNA, long non-coding RNA; NLRP3, NLR family pyrin domain containing 3; NC, negative control; OE, overexpression.
GAS5 positively regulates NLRP3 expression by sponging miR-223-3p
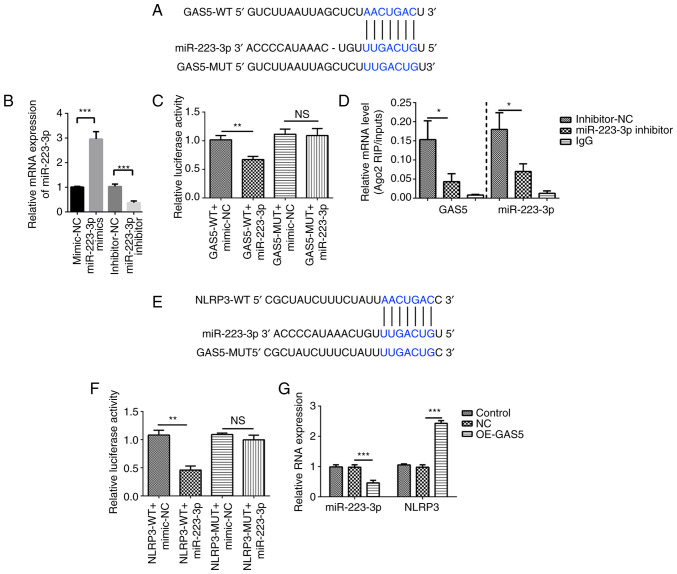
To examine the mechanisms by which GAS5 positively regulated NLRP3 expression, it was predicted that GAS5 could serve as a miRNA sponge. The StarBase database identified that GAS5 can bind to miR-223-3p (Fig. 4A). The miRNA mimics and inhibitors of miR-223-3p were transfected into MRC-5 cells to achieve ectopic miRNA expression, and the results revealed that miR-223-3p expression was increased by miR-223-3p mimics and suppressed by miR-223-3p inhibitors (Fig. 4B). Luciferase reporter assays were used to detect whether GAS5 binds miR-223-3p directly. A decrease in luciferase reporter activity was determined in 293T cells when NLRP3 was co-transfected with miR-223-3p mimics, but not with the negative control (Fig. 4C). Subsequently, the RIP assay using an antibody against argonaute RISC catalytic component 2 or IgG antibody showed that GAS5-WT directly interacted with miR-223-3p (Fig. 4D).
Figure 4.
GAS5 targets the miR-223-3p/NLRP3 axis. (A) Bioinformatics analysis was used to predict the binding site of GAS5 to miR-223-3p. (B) Transfection efficiency of miR-223-3p mimics and inhibitor was analyzed using qPCR. (C) GAS5 binds to the putative target sites of miR-223-3p as verified using a luciferase assay. (D) RIP assay followed by qPCR was used to determine GAS5 and miR-223-3p expression. (E) Bioinformatics analysis was used to predict the binding site of miR-223-3p to NLRP3. (F) miR-223-3p binds to the putative target sites of NLRP3 as verified using a luciferase assay. (G) Expression levels of miR-223-3p and NLRP3 in GAS5-overexpressing MRC-5 cells were measured using qPCR. The results are presented as the mean ± SD (n=3; *P<0.05, **P<0.01, ***P<0.001 vs. control). GAS5, growth arrest-specific 5; NLRP3, NLR family pyrin domain containing 3; miR-223-3p, microRNA 223-3p; 3′ UTR, 3′ untranslated region; Ago2, argonaute RISC catalytic component 2; OE, overexpression; NC, GAS5 overexpression negative control lentivirus vectors; RIP, RNA immunoprecipitation; qPCR, quantitative PCR; WT, wild-type; MUT, mutant; NS, no significant difference.
Furthermore, RNAhybrid 2.2 showed that miR-223-3p targets NLRP3 (Fig. 4E). A dual luciferase reporter assay showed decreased luciferase activity in 293T cells co-transfected with miR-223-3p and NLRP3-WT compared with that in cells co-transfected with miR-223-3p and NLRP3-MUT, which verified the binding between NLRP3 and miR-223-3p. However, the relative luciferase activity of the NLRP3-MUT co-transfected with miR-223-3p group was not markedly changed compared with that of the NLRP3-MUT co-transfected with miRNA-NC group (Fig. 4F). Furthermore, overexpression of GAS5 significantly inhibited miR-223-3p expression and increased that of NLRP3 as confirmed by qPCR (Fig. 4G). Thus, it was concluded that GAS5 induced pyroptosis in MRC-5 cells by targeting miR-223-3p/NLRP3.
Discussion
Aberrant expression of lncRNAs is involved in several pulmonary disorders, revealing the association of lncRNAs with the pathogenesis of these diseases (46). GAS5, a well-studied lncRNA, is involved in the progression and prognosis of multiple cancers (47–49). It has been demonstrated that GAS5 expression is notably increased in asthma to control the regulatory T/T helper 17 cells balance and glucocorticoid activity, while it is decreased in non-small cell lung cancer (NSCLC) to regulate the PTEN/PI3K/AKT signaling pathway (50–52). Nonetheless, to the best of our knowledge, no studies on the expression or function of GAS5 in COPD have been published. In the present study, increased GAS5 expression was verified in COPD model cells. Furthermore, it was observed that the overexpression of GAS5 induced MRC-5 cell death that was caused by the activation of caspase-1 and inflammasomes. Thus, it was concluded that GAS5 is involved in the regulation of pyroptosis in COPD.
COPD is a multifactorial, multi-mechanical disease with high morbidity and mortality (53), the pathogenesis of which involves numerous pathophysiological processes, such as the inflammatory response, oxidative stress, apoptosis, protease/antiproteinase imbalance, production of autoantibodies, alteration in cell proliferation, and cellular aging (54). Pyroptosis, characterized by DNA damage and membrane rupture, is known as a unique form of caspase-1-dependent cell death (16). Caspase-1 regulates the splicing of inactive pro-IL-1β and pro-IL-18 into mature inflammatory cytokines IL-1β and IL-18 to mediate pyroptosis (55). In the present study, it was revealed that LPS treatment of MRC-5 cells significantly induced caspase-1, IL-18 and IL-1β gene expression, indicating that pyroptosis is involved in the development of COPD. However, the specific mechanisms remain unclear.
GAS5, one of the most well-established tumor-suppressive lncRNAs, is associated with cell death in multiple human malignancies (35,56,57). GAS5 regulates cell growth arrest and induces apoptosis through p53- or E2 factor 1-dependent pathways in NSCLC (58). GAS5 promotes vascular smooth muscle cell growth arrest and apoptosis in vascular remodeling (44). Exosomal GAS5 silencing reduces the apoptosis of macrophages and vascular endothelial cells in atherosclerosis (57). Overexpression of GAS5 decreases the level of IL-18 and induces the apoptosis of fibroblast-like synoviocytes (59). Additionally, GAS5 overexpression decreases cell inflammatory responses and apoptosis in LPS-treated MLE-12 cells (60). To the best of our knowledge, at present, there is no evidence illustrating the exact role of GAS5 in COPD.
In the present study, it was revealed that GAS5 expression was significantly increased in LPS-treated MRC-5 cells, suggesting that GAS5 might be associated with the pathogenesis of COPD. At the same time, upregulation of GAS5 induced the release of IL-2, IL-6, IL-10 and TNF-α, which is different from the function of GAS5 in MLE-12 cells and fibroblast-like synoviocytes, in which overexpression of GAS5 decreases cell inflammatory responses (57,59). The aforementioned results indicated that GAS5 served opposite roles in inflammatory responses in different diseases. IL-10 exhibits diverse effects in the immune response. On the one hand, IL-10 is an anti-inflammatory cytokine that suppresses the expression or function of multiple inflammatory cytokines, including IFN-γ, TNF-α and IL-6. On the other hand, IL-10 can enhance immune events, such as immunoglobulin production by B cells, the cytotoxicity of natural killer cells and CD8+ T cells (61,62). An increased IL-10 concentration has been identified in the blood samples of patients with COPD (63), which might lead to immunosuppression and reduce the inflammatory response in COPD progression. Additionally, the present study also revealed that overexpression of GAS5 promoted death and inhibited proliferation by activating caspase-1, IL-1β, IL-18 and NLRP3 in MRC-5 cells. Caspase-1 activation is mediated by inflammasomes, including NLRP3, NLR family pyrin domain containing 1b (NLRP1b) and NLR family card domain containing 4 (NLRC4) (64). In the present study, it was only demonstrated that overexpression of GAS5 increased the level of NLRP3 without further investigating if lncRNA GAS5 promotes pyroptosis by regulating NLRP1b and NLRC4 inflammasomes, which should be investigated in the future.
lncRNAs have been demonstrated to regulate gene expression as ceRNAs (65). This means lncRNAs can regulate the target gene expression by competitively binding to the response elements within miRNA. For example, lncRNA H19 promotes acute lymphoblastic leukemia by sponging miR326 to reduce BCL-2 expression (66). Long intergenic non coding RNA 1184 increases the proliferation and invasion of colorectal cancer by targeting the miR-331/HER2-p signaling pathway (67). Furthermore, ceRNA networks have also been reported to regulate the progression of COPD. lncRNA SNHG5, cancer susceptibility 2 and RP11-86H7.1 have all been reported to serve as miRNA sponges and keep miRNA away from its target genes through ceRNA networks to serve important roles in the progression of COPD (28,68,69). However, to the best of our knowledge, at present, no study has reported the role of GAS5 in COPD. It needs to be explored whether GAS5 can serve as a ceRNA in COPD. In the present study, a series of experiments demonstrated that GAS5 could directly bind to miR-223-3p. At present, there is only one report that miR-223-3p is increased in patients with COPD (70). In contrast to this conclusion, the present results are in line with existing knowledge suggesting that miR-223-3p acts as a tumor suppressor in lung squamous cell carcinoma (71), and colon (72), prostate (73) and ovarian cancer (74). This divergence may be due to the difference of in vitro and in vivo experimental environments and the influence of the qualitative difference across cell types. Additionally, it was also revealed that miR-223-3p could directly bind to NLRP3, which is in line with previous reports regarding the role of miR-223-3p (75). Therefore, it was concluded that GAS5 could regulate COPD progression by targeting the miR-223-3p/NLRP3 axis.
These findings provide knowledge regarding the pathogenesis of COPD. The present study provided evidence that lncRNA GAS5 may function as a regulator of pyroptosis in COPD by targeting the miR-223-3p/NLRP3 axis. GAS5 overexpression promoted cell death via the regulation of caspase-1, IL-18 and IL-1β expression in MRC-5 cells. To the best of our knowledge, the present study was the first to validate the role of GAS5 during COPD progression. However, the present study only upregulated lncRNA GAS5 in in vitro experiment to support the above conclusion. More evidence is required to validate the predictions and conclusions of the present study, for example, in in vivo as well as clinical studies.
There are certain limitations of the present study. For example, the in vitro experiments were only performed in MRC-5 cells, and thus, further experiments should be performed in more cell lines to confirm the findings reported in the present study. Additionally, LPS-treated MRC-5 cells can also be used as a model for other diseases, such as pneumonia and acute respiratory distress syndrome (46,76). Thus, the results in the present study need to be verified in improved COPD models. Due to the limited experimental conditions, the role and mechanisms of GAS5 in COPD have not been confirmed in any animal model or human tissue samples. Thus, there are still some deficiencies in the present study, and a lot of work is required in the future.
In conclusion, the results of the present study suggested that lncRNA GAS5 expression was significantly increased in a COPD cell model. Overexpression of GAS5 promoted pyroptosis by targeting miR-223-3p/NLRP3 in MRC-5 cells. These findings suggest that GAS5 serves an important role in the progression of COPD.
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant nos. 81660013 and 81860015).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YC and YD were responsible for the conception of the present study. RM, YC and YD confirm the authenticity of all the raw data. RM and JL conducted the experiments, and analyzed and interpreted the data. JL performed the statistical analysis. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mouronte-Roibas C, Leiro-Fernandez V, Fernandez-Villar A, Botana-Rial M, Ramos-Hernandez C, Ruano-Ravina A. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett. 2016;382:240–244. doi: 10.1016/j.canlet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21:14–23. doi: 10.1111/resp.12660. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931–1940. doi: 10.1016/S0140-6736(17)31222-9. [DOI] [PubMed] [Google Scholar]
- 4.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur Respir J. 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, Tao Y, Li H, Niu Y, Li L, Kan H, Xie J, Chen R. Acute effects of fine particulate matter constituents on cardiopulmonary function in a panel of COPD patients. Sci Total Environ. 2021;770:144753. doi: 10.1016/j.scitotenv.2020.144753. [DOI] [PubMed] [Google Scholar]
- 6.Hirano T, Matsunaga K, Oishi K, Doi K, Harada M, Suizu J, Murakawa K, Chikumoto A, Ohteru Y, Matsuda K, et al. Abundant TNF-LIGHT expression in the airways of patients with asthma with persistent airflow limitation: Association with nitrative and inflammatory profiles. Respir Investig. 2021;59:651–660. doi: 10.1016/j.resinv.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Bettoncelli G, Blasi F, Brusasco V, Centanni S, Corrado A, De Benedetto F, De Michele F, Di Maria GU, Donner CF, Falcone F, et al. The clinical and integrated management of COPD. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31((Suppl 1)):S3–S21. [PubMed] [Google Scholar]
- 8.Salazar LM, Herrera AM. Fibrotic response of tissue remodeling in COPD. Lung. 2011;189:101–109. doi: 10.1007/s00408-011-9279-2. [DOI] [PubMed] [Google Scholar]
- 9.Krimmer DI, Burgess JK, Wooi TK, Black JL, Oliver BG. Matrix proteins from smoke-exposed fibroblasts are pro-proliferative. Am J Respir Cell Mol Biol. 2012;46:34–39. doi: 10.1165/rcmb.2010-0426OC. [DOI] [PubMed] [Google Scholar]
- 10.Di T, Yang Y, Fu C, Zhang Z, Qin C, Sai X, Liu J, Hu C, Zheng M, Wu Y, Bian T. Let-7 mediated airway remodelling in chronic obstructive pulmonary disease via the regulation of IL-6. Eur J Clin Invest. 2021;51:e13425. doi: 10.1111/eci.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spanjer AI, Baarsma HA, Oostenbrink LM, Jansen SR, Kuipers CC, Lindner M, Postma DS, Meurs H, Heijink IH, Gosens R, Königshoff M. TGF-beta-induced profibrotic signaling is regulated in part by the WNT receptor frizzled-8. FASEB J. 2016;30:1823–1835. doi: 10.1096/fj.201500129. [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ. Small airway fibrosis in COPD. Int J Biochem Cell Biol. 2019;116:105598. doi: 10.1016/j.biocel.2019.105598. [DOI] [PubMed] [Google Scholar]
- 13.Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2006;3:713–717. doi: 10.1513/pats.200605-104SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conlon TM, John-Schuster G, Heide D, Pfister D, Lehmann M, Hu Y, Ertuz Z, Lopez MA, Ansari M, Strunz M, et al. Inhibition of LTbetaR signalling activates WNT-induced regeneration in lung. Nature. 2020;588:151–156. doi: 10.1038/s41586-020-2882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T, Fanning KV, Nyunoya T, Chen Y, Zou C. Cigarette smoke extract induces airway epithelial cell death via repressing PRMT6/AKT signaling. Aging (Albany NY) 2020;12:24301–24317. doi: 10.18632/aging.202210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, Wiesner U, Bradbury MS, Niethammer P, Zaritsky A, Overholtzer M. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22:1042–1048. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SJ, Hong KS, Jeong JH, Lee M, Choi AMK, Stout-Delgado HW, Moon JS. DROSHA-dependent AIM2 inflammasome activation contributes to lung inflammation during idiopathic pulmonary fibrosis. Cells. 2019;8:938. doi: 10.3390/cells8080938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Cao R, Fang D, Wang J, Yu Y, Ye H, Kang P, Li Z, Wang H, Gao Q. ALDH2 overexpression alleviates high glucose-induced cardiotoxicity by inhibiting nlrp3 inflammasome activation. J Diabetes Res. 2019;2019:4857921. doi: 10.1155/2019/4857921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Chen Q, Yu Q, Xiao J, Zhao H. TREM-1 aggravates chronic obstructive pulmonary disease development via activation NLRP3 inflammasome-mediated pyroptosis. Inflamm Res. 2021;70:971–980. doi: 10.1007/s00011-021-01490-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang MY, Jiang YX, Yang YC, Liu JY, Huo C, Ji XL, Qu YQ. Cigarette smoke extract induces pyroptosis in human bronchial epithelial cells through the ROS/NLRP3/caspase-1 pathway. Life Sci. 2021;269:119090. doi: 10.1016/j.lfs.2021.119090. [DOI] [PubMed] [Google Scholar]
- 23.Xue C, Chen C, Gu X, Li L. Progress and assessment of lncRNA DGCR5 in malignant phenotype and immune infiltration of human cancers. Am J Cancer Res. 2021;11:1–13. [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong F, Ren D, Ye X, Li C, Wang Y, et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer. 2018;17:79. doi: 10.1186/s12943-018-0827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song YX, Sun JX, Zhao JH, Yang YC, Shi JX, Wu ZH, Chen XW, Gao P, Miao ZF, Wang ZN. Non-coding RNAs participate in the regulatory network of CLDN4 via ceRNA mediated miRNA evasion. Nat Commun. 2017;8:289. doi: 10.1038/s41467-017-00304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Q, Zheng J, Wang X, Hu W, Jiang Y, Jiang Y. LncRNA SNHG5 regulates cell apoptosis and inflammation by miR-132/PTEN axis in COPD. Biomed Pharmacother. 2020;126:110016. doi: 10.1016/j.biopha.2020.110016. [DOI] [PubMed] [Google Scholar]
- 29.Li N, Liu Y, Cai J. LncRNA MIR155HG regulates M1/M2 macrophage polarization in chronic obstructive pulmonary disease. Biomed Pharmacother. 2019;117:109015. doi: 10.1016/j.biopha.2019.109015. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Chen J, Chen W, Liu L, Dong M, Ji J, Hu D, Zhang N. LINC00987 ameliorates COPD by regulating LPS-induced cell apoptosis, oxidative stress, inflammation and autophagy through Let-7b-5p/SIRT1 axis. Int J Chron Obstruct Pulmon Dis. 2020;15:3213–3225. doi: 10.2147/COPD.S276429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye K, Wang S, Zhang H, Han H, Ma B, Nan W. Long noncoding RNA GAS5 suppresses cell growth and epithelial-mesenchymal transition in osteosarcoma by regulating the miR-221/ARHI pathway. J Cell Biochem. 2017;118:4772–4781. doi: 10.1002/jcb.26145. [DOI] [PubMed] [Google Scholar]
- 32.Lyu K, Xu Y, Yue H, Li Y, Zhao J, Chen L, Wu J, Zhu X, Chai L, Li C, et al. Long noncoding RNA GAS5 acts as a tumor suppressor in laryngeal squamous cell carcinoma via miR-21. Cancer Manag Res. 2019;11:8487–8498. doi: 10.2147/CMAR.S232421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu W, Zhang L, Geng Y, Liu Y, Zhang N. Long noncoding RNA GAS5 promotes microglial inflammatory response in parkinson's disease by regulating NLRP3 pathway through sponging miR-223-3p. Int Immunopharmacol. 2020;85:106614. doi: 10.1016/j.intimp.2020.106614. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Du P, Qiang X, Jin D, Liu H, Li B, Guo J. Low-expressed GAS5 injure myocardial cells and progression of chronic heart failure via regulation of miR-223-3P. Exp Mol Pathol. 2020;117:104529. doi: 10.1016/j.yexmp.2020.104529. [DOI] [PubMed] [Google Scholar]
- 35.He X, Wang S, Li M, Zhong L, Zheng H, Sun Y, Lai Y, Chen X, Wei G, Si X, et al. Long noncoding RNA GAS5 induces abdominal aortic aneurysm formation by promoting smooth muscle apoptosis. Theranostics. 2019;9:5558–5576. doi: 10.7150/thno.34463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazar J, Rosado A, Shelley J, Marchica J, Westmoreland TJ. The long non-coding RNA GAS5 differentially regulates cell cycle arrest and apoptosis through activation of BRCA1 and p53 in human neuroblastoma. Oncotarget. 2017;8:6589–6607. doi: 10.18632/oncotarget.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li D, He S. MAGE3 and Survivin activated dendritic cell immunotherapy for the treatment of non-small cell lung cancer. Oncol Lett. 2018;15:8777–8783. doi: 10.3892/ol.2018.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Yang H, Xiao Y, Tang X, Li Y, Han Q, Fu J, Yang Y, Zhu Y. LncRNA GAS5 is a critical regulator of metastasis phenotype of melanoma cells and inhibits tumor growth in vivo. Onco Targets Ther. 2016;9:4075–4087. doi: 10.2147/OTT.S98203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu H, Qu X, Wang F, Chang J, Cheng R, Song X, Chen T, Zhang G. MicroRNA-206 promotes lipopolysaccharide-induced inflammation injury via regulation of IRAK1 in MRC-5 cells. Int Immunopharmacol. 2019;73:590–598. doi: 10.1016/j.intimp.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Wang F, Wang B, Wu T, Wang Y, Huo W, Zhang S, Su Y, Liu J, Liu Y, Yu J. Pseudolaric acid B induces apoptosis in human rhabdomyosarcoma RD cells. Oncol Lett. 2020;20:358. doi: 10.3892/ol.2020.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lian L, Xue J, Li W, Ren J, Tang F, Liu Y, Xue F, Dai J. VscF in T3SS1 helps to translocate VPA0226 in vibrio parahaemolyticus. Front Cell Infect Microbiol. 2021;11:652432. doi: 10.3389/fcimb.2021.652432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoll L, Rodriguez-Trejo A, Guay C, Brozzi F, Bayazit MB, Gattesco S, Menoud V, Sobel J, Marques AC, Veno MT, et al. A circular RNA generated from an intron of the insulin gene controls insulin secretion. Nat Commun. 2020;11:5611. doi: 10.1038/s41467-020-19381-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang R, Mei X, Wang YC, Cui XB, Zhang G, Li W, Chen SY. LncRNA GAS5 regulates vascular smooth muscle cell cycle arrest and apoptosis via p53 pathway. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2516–2525. doi: 10.1016/j.bbadis.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie MY, Hou LJ. LncRNA GAS5 aggravates myocardial infarction by sponging miR-26b. Int J Cardiol. 2021;331:210. doi: 10.1016/j.ijcard.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Tang X, Wang T, Qiu C, Zheng F, Xu J, Zhong B. Long non-coding RNA (lncRNA) CRNDE regulated lipopolysaccharides (LPS)-induced MRC-5 inflammation injury through targeting MiR-141. Med Sci Monit. 2020;26:e920928. doi: 10.12659/MSM.920928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L, Li J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18:143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filippova EA, Fridman MV, Burdennyy AM, Loginov VI, Pronina IV, Lukina SS, Dmitriev AA, Braga EA. Long noncoding RNA GAS5 in breast cancer: Epigenetic mechanisms and biological functions. Int J Mol Sci. 2021;22:6810. doi: 10.3390/ijms22136810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Xie Z, Lei X, Gan R. Long non-coding RNA GAS5 in human cancer. Oncol Lett. 2020;20:2587–2594. doi: 10.3892/ol.2020.11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu YY, Wu Y, Lin MJ, Bian T, Xiao YL, Qin C. LncRNA-MEG3 functions as a competing endogenous RNA to regulate Treg/Th17 balance in patients with asthma by targeting microRNA-17/RORgammat. Biomed Pharmacother. 2019;111:386–394. doi: 10.1016/j.biopha.2018.12.080. [DOI] [PubMed] [Google Scholar]
- 51.Keenan CR, Schuliga MJ, Stewart AG. Pro-inflammatory mediators increase levels of the noncoding RNA GAS5 in airway smooth muscle and epithelial cells. Can J Physiol Pharmacol. 2015;93:203–206. doi: 10.1139/cjpp-2014-0391. [DOI] [PubMed] [Google Scholar]
- 52.Cao L, Chen J, Ou B, Liu C, Zou Y, Chen Q. GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomed Pharmacother. 2017;93:570–579. doi: 10.1016/j.biopha.2017.06.089. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Jin X, Jiao X, Li J, Liang L, Ma Y, Liu R, Li Z. Advances in pharmacological actions and mechanisms of flavonoids from traditional chinese medicine in treating chronic obstructive pulmonary disease. Evid Based Complement Alternat Med. 2020;2020:8871105. doi: 10.1155/2020/8871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol. 2011;254:72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F, Xu D, Hou K, Gou X, Lv N, Fang W, Li Y. Pretreatment of indobufen and aspirin and their combinations with clopidogrel or ticagrelor alleviates inflammasome mediated pyroptosis via inhibiting NF-κB/NLRP3 pathway in ischemic stroke. J Neuroimmune Pharmacol. 2021;16:835–853. doi: 10.1007/s11481-020-09978-9. [DOI] [PubMed] [Google Scholar]
- 56.Fawzy MS, Toraih EA, Ageeli EA, Al-Qahtanie SA, Hussein MH, Kandil E. Noncoding RNAs orchestrate cell growth, death and drug resistance in renal cell carcinoma. Epigenomics. 2020;12:199–219. doi: 10.2217/epi-2019-0120. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Yang W, Guo Y, Chen W, Zheng P, Zeng J, Tong W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS One. 2017;12:e0185406. doi: 10.1371/journal.pone.0185406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F, Song Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol Carcinog. 2015;54((Suppl 1)):E1–E12. doi: 10.1002/mc.22120. [DOI] [PubMed] [Google Scholar]
- 59.Ma C, Wang W, Li P. LncRNA GAS5 overexpression downregulates IL-18 and induces the apoptosis of fibroblast-like synoviocytes. Clin Rheumatol. 2019;38:3275–3280. doi: 10.1007/s10067-019-04691-2. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Liu S. LncRNA GAS5 suppresses inflammatory responses and apoptosis of alveolar epithelial cells by targeting miR-429/DUSP1. Exp Mol Pathol. 2020;113:104357. doi: 10.1016/j.yexmp.2019.104357. [DOI] [PubMed] [Google Scholar]
- 61.Nagata K, Nishiyama C. IL-10 in mast cell-mediated immune responses: Anti-inflammatory and proinflammatory roles. Int J Mol Sci. 2021;22:4972. doi: 10.3390/ijms22094972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castellucci M, Rossato M, Calzetti F, Tamassia N, Zeminian S, Cassatella MA, Bazzoni F. IL-10 disrupts the Brd4-docking sites to inhibit LPS-induced CXCL8 and TNF-alpha expression in monocytes: Implications for chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;136:781–791. doi: 10.1016/j.jaci.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 63.Wei B, Li CS. Changes in Th1/Th2-producing cytokines during acute exacerbation chronic obstructive pulmonary disease. J Int Med Res. 2018;46:3890–3902. doi: 10.1177/0300060518781642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng W, Chu Q, Xu T. The long noncoding RNA NARL regulates immune responses via microRNA-mediated NOD1 downregulation in teleost fish. J Biol Chem. 2021;296:100414. doi: 10.1016/j.jbc.2021.100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mofidi M, Rahgozar S, Pouyanrad S. Increased level of long non coding RNA H19 is correlated with the downregulation of miR-326 and BCL-2 genes in pediatric acute lymphoblastic leukemia, a possible hallmark for leukemogenesis. Mol Biol Rep. 2021;48:1531–1538. doi: 10.1007/s11033-021-06161-y. [DOI] [PubMed] [Google Scholar]
- 67.Sui YX, Zhao DL, Yu Y, Wang LC. The role, function, and mechanism of long intergenic noncoding RNA1184 (linc01184) in colorectal cancer. Dis Markers. 2021;2021:8897906. doi: 10.1155/2021/8897906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu P, Zhang H, Zeng H, Meng Y, Gao H, Zhang M, Zhao L. LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis. Ther Adv Respir Dis. 2021;15:17534666211028072. doi: 10.1177/17534666211028072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao J, Pu J, Hao B, Huang L, Chen J, Hong W, Zhou Y, Li B, Ran P. LncRNA RP11-86H7.1 promotes airway inflammation induced by TRAPM2.5 by acting as a ceRNA of miRNA-9-5p to regulate NFKB1 in HBECS. Sci Rep. 2020;10:11587. doi: 10.1038/s41598-020-68327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roffel MP, Maes T, Brandsma CA, van den Berge M, Vanaudenaerde BM, Joos GF, Brusselle GG, Heijink IH, Bracke KR. MiR-223 is increased in lungs of patients with COPD and modulates cigarette smoke-induced pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. 2021;321:L1091–L1104. doi: 10.1152/ajplung.00252.2021. [DOI] [PubMed] [Google Scholar]
- 71.Luo P, Wang Q, Ye Y, Zhang J, Lu D, Cheng L, Zhou H, Xie M, Wang B. MiR-223-3p functions as a tumor suppressor in lung squamous cell carcinoma by miR-223-3p-mutant p53 regulatory feedback loop. J Exp Clin Cancer Res. 2019;38:74. doi: 10.1186/s13046-019-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chai B, Guo Y, Cui X, Liu J, Suo Y, Dou Z, Li N. MiR-223-3p promotes the proliferation, invasion and migration of colon cancer cells by negative regulating PRDM1. Am J Transl Res. 2019;11:4516–4523. [PMC free article] [PubMed] [Google Scholar]
- 73.Wei Y, Yang J, Yi L, Wang Y, Dong Z, Liu Z, Ou-yang S, Wu H, Zhong Z, Yin Z, et al. MiR-223-3p targeting SEPT6 promotes the biological behavior of prostate cancer. Sci Rep. 2014;4:7546. doi: 10.1038/srep07546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang G, Liu J, Wang Q, Huang X, Yang R, Pang Y, Yang M. MicroRNA-223-3p regulates ovarian cancer cell proliferation and invasion by targeting SOX11 expression. Int J Mol Sci. 2017;18:1208. doi: 10.3390/ijms18061208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long FQ, Kou CX, Li K, Wu J, Wang QQ. MiR-223-3p inhibits rTp17-induced inflammasome activation and pyroptosis by targeting NLRP3. J Cell Mol Med. 2020;24:14405–14414. doi: 10.1111/jcmm.16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie Y, Qian Y, Wang Y, Liu K, Li X. Mechanical stretch and LPS affect the proliferation, extracellular matrix remodeling and viscoelasticity of lung fibroblasts. Exp Ther Med. 2020;20:5. doi: 10.3892/etm.2020.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.